Abstract
Introduction:
Little is known about hysterectomy and bilateral salpingo-oophorectomy (BSO), which are associated with both health risks and benefits, among women Veterans.
Purpose of the Study:
To compare the prevalence of hysterectomy with or without BSO, and early hysterectomy, between postmenopausal Veterans and non-Veterans.
Design and Methods:
We used baseline data from the Women’s Health Initiative Clinical Trial and Observational Study. Multinomial logistic regression models examined differences in the prevalence of hysterectomy (neither hysterectomy nor BSO, hysterectomy without BSO, and hysterectomy with BSO) between Veterans and non-Veterans. Generalized linear models were used to determine whether early hysterectomy (before age 40) differed between Veterans and non-Veterans. Analyses were stratified by birth cohort (<65, ≥65 years at enrollment).
Results:
The unadjusted prevalence of hysterectomy without BSO was similar among Veterans and non-Veterans in both birth cohorts (<65: 22% vs 21%; ≥65: 22% vs 21%). The unadjusted prevalence of hysterectomy with BSO was equivalent among Veterans and non-Veterans in the >65 cohort (21%), but higher among Veterans in the <65 cohort (22% vs 19%). In adjusted analyses, although no differences were observed in the >65 cohort, Veterans in the <65 cohort had higher odds of hysterectomy without BSO (odds ratio [OR] 1.18, 95% confidence interval [CI] 1.03, 1.36) and with BSO (OR 1.26, 95% CI 1.10, 1.45), as well as elevated risk of early hysterectomy (relative risk 1.32, 95% CI 1.19, 1.47), compared with non-Veterans.
Implications:
Aging women Veterans may have higher prevalence of hysterectomy and BSO than non-Veterans. This information contributes to understanding the health needs and risks of women Veterans and can inform clinical practice and policy for this population.
Keywords: Women Veterans, Hysterectomy, Bilateral salpingo-oophorectomy
Hysterectomy is the second most frequently performed major surgery after cesarean delivery among U.S. women of reproductive age (American Congress of Obstetricians and Gynecologists [ ACOG], 2011 ; National Center for Health Statistics [ NCHS], 2012 ). Factors that increase the likelihood of hysterectomy include older age, obesity, lower socioeconomic status, African American race/ethnicity, and psychosocial factors affecting patient or physician preferences ( Brett, Marsh, & Madans, 1997 ; Powell et al., 2005 ; Qi et al., 2013 ; Sievert, Murphy, Morrison, Reza, & Brown, 2013 ). Approximately 50% of women who undergo hysterectomy will simultaneously undergo a bilateral salpingo-oophorectomy (BSO; Whiteman et al., 2008 ). Hysterectomy can improve quality of life and reduce pain and other related complaints among women with benign gynecologic conditions, and nearly 90% of hysterectomies in the United States are performed to treat such conditions ( Davies & Doyle, 2002 ; Merrill, 2008 ). Hysterectomy and BSO may also be used to treat or prevent ovarian and other cancers ( Carlson, Nichols, & Schiff, 1993 ).
In addition to these benefits, however, hysterectomy with or without BSO carries short- and long-term health risks. In contrast to less invasive options to treat benign gynecologic conditions, hysterectomy is associated with perioperative risks, including intra- and postoperative hemorrhage, wound infections, injury to adjacent organs, urinary tract infection, and thromboembolic disease ( Spilsbury, Hammond, Bulsara, & Semmens, 2008 ). Additionally, a growing body of epidemiologic literature suggests long-term health consequences associated with hysterectomy and BSO, including increased risk of cardiovascular disease (CVD), urinary incontinence, and dementia ( Brown, Sawaya, Thom, & Grady, 2000 ; Howard et al., 2005 ; Jacoby et al., 2011 ; Phung et al., 2010 ; Rocca, Grossardt, Shuster, & Stewart, 2012 ). Moreover, hysterectomy with or without BSO before age 40 (early hysterectomy) is associated with increased incidence of all-cause and coronary heart disease mortality ( Gierach et al., 2014 ).
The potential long-term health consequences of these procedures may be particularly relevant to aging women Veterans, as two prior studies suggest that women Veterans report higher prevalence of hysterectomy and undergo these procedures at younger ages than their non-Veteran counterparts ( Gardella, Johnson, Dobie, & Bradley, 2005 ; Lehavot, Hoerster, Nelson, Jakupcak, & Simpson, 2012a ). These previous studies, however, had several important limitations. First, differentiation was not made between hysterectomy alone and hysterectomy with BSO, the latter of which has been associated with more significant long-term health risks than hysterectomy alone. Second, only one of these studies directly compared women Veterans with non-Veterans ( Lehavot, Hoerster, Nelson, Jakupcak, & Simpson, 2012b ). Lastly, these studies examined hysterectomy prevalence in women across all ages rather than by birth cohort, which may obscure variations related to secular trends in military policies and gynecologic practice. Additional epidemiologic study of this question, using alternative data sources and analyses, is therefore warranted.
Conceptual Model
We used the Andersen Behavioral Model of Health Services Use as the conceptual framework to understand potential mechanisms underlying expected differences in the prevalence of hysterectomy between Veterans and non-Veterans ( Andersen, 1995 ). The Andersen model is a multilevel conceptual model that proposes a pathway between individual and contextual factors—including predisposing, enabling, and need factors—and health care utilization and outcomes ( Andersen, 1995 , 2008 ; Babitsch, Gohl, & von Lengerke, 2012 ). This model was previously adapted to understand patterns and determinants of health care utilization among women Veterans ( Washington, Davis, Der-Martirosian, & Yano, 2013 ). Drawing on this previous work, we hypothesized that Veteran status would be associated with higher prevalence of individual and contextual factors that could lead to a differential risk of hysterectomy or early hysterectomy with or without BSO. Predisposing factors that could differ between Veterans and non-Veterans include demographic characteristics (e.g., age, race/ethnicity, and education), lifetime history of sexual trauma, and preferences and beliefs about hysterectomy. Enabling factors such as access to health care, insurance status, geography, and availability of alternatives to hysterectomy for benign gynecologic conditions could also differ between Veterans and non-Veterans, both during their military service and once they transition to Veteran status. Lastly, differences in physical and mental health status ( Blosnich, Dichter, Cerulli, Batten, & Bossarte, 2014 ; California’s women Veterans and military sexual trauma , 2012 ; Lehavot et al., 2012b ) could result in varying need for hysterectomy with or without BSO, including both “evaluated need” by providers and “perceived need” by patients ( Andersen, 1995 ).
We also considered the role of birth cohort as a modifying factor in the association between Veteran status and prevalence of hysterectomy with or without BSO and early hysterectomy. Characteristics of women Veterans, military policies, and standards of care in medical practice have changed over time ( Washington, Bean-Mayberry, Hamilton, Cordasco, & Yano, 2013 ). For example, prior to the 1970s, pregnancy, marriage, or dependent children were grounds for military discharge and rank attainment and percentage of women serving in the military were restricted ( Devilbiss, 1990 ). The end of these restrictions during the late 1960s and 1970s likely influenced who chose to join the military, both in terms of their characteristics and reproductive goals. In addition to changes in military policy, general standards of care for benign gynecologic conditions changed over time ( Cheong, Smotra, & Williams, 2014 ; Marjoribanks, Lethaby, & Farquhar, 2006 ). The approval of the birth control pill in 1957 for menstrual disorders and in 1960 for contraception, as well as resulting shifts in the timing of childbearing, affected care for benign gynecologic conditions and women’s decision making regarding hysterectomy ( Poston, 2010 ). The rise of multiple less invasive treatment options for benign gynecologic conditions in the 1980s and 1990s also led to a significant decline in hysterectomy rates as well as an increase in age at hysterectomy ( Turner, Shepherd, Wang, Bunker, & Lowder, 2013 ; Wright et al., 2013 ).
In this analysis, we therefore investigate the association of Veteran status and hysterectomy with and without BSO, and whether differences vary across birth cohorts in order to better understand the health risks and needs of aging women Veterans. We hypothesized that (a) women Veterans would have a higher prevalence of hysterectomy with or without BSO, as well as early hysterectomy before age 40, compared with non-Veterans and (b) these associations would differ by birth cohort.
Methods
We conducted a cross-sectional analysis using baseline data from the Women’s Health Initiative (WHI) Clinical Trial (CT) and Observational Study (OS). Postmenopausal women ages 50–79 who were willing and able to provide informed consent and intended to reside in the area for at least 3 years were recruited into WHI from 40 clinical centers across the United States between 1993 and 1998. Exclusion criteria included any medical condition with predicted survival of <3 years; alcohol or drug dependency; mental illness, including severe depression; dementia; or active participation in one or more other randomized intervention trials ( Anderson et al., 2003 ; Hays et al., 2003 ). Additional details on eligibility criteria for each study component were previously published ( Anderson et al., 2003 ). If eligible, women could enroll in one or both of Hormone Therapy (HT) and Dietary Modification (DM) Trials where they were randomized to the intervention or control arms. Women who did not meet eligibility criteria for either trial or who were unwilling to be randomized to either the HT or DM trials were able to join the OS ( Hays et al., 2003 ). In total, 161,808 women were enrolled in the WHI (CT and OS) at baseline. Baseline data were collected via self-administered forms, in-person or telephone interviews, and clinical measurements. The IRB at each participating site approved the study, and all participants provided written informed consent.
Study Variables
Veteran Status
Participants at baseline responded to the question, “Have you served in the U.S. armed forces on active duty for a period of 180 days or more,” a standard question for evaluating Veteran status in national Centers for Disease Control and Prevention studies ( Hoerster et al., 2012 ; Koepsell, Reiber, & Simmons, 2002 ; Lehavot et al., 2014 ). Participants with a missing response were excluded.
Hysterectomy, BSO, and Early Hysterectomy
During screening for study eligibility, participants self-reported whether they had ever had a hysterectomy (no/yes); and if yes, the age at which they had the hysterectomy (in categories). Participants also reported whether they had an oophorectomy (no; yes, one ovary removed [unilateral]; yes, both removed [bilateral]; yes, unknown number removed; yes, part of an ovary removed [partial], don’t know). Based on responses to these questions, we created a hysterectomy variable with three levels: no hysterectomy, hysterectomy without BSO, and hysterectomy with BSO. Those with partial or unilateral oophorectomy but an intact uterus were classified as “no hysterectomy.” We excluded participants who responded “don’t know” or who had missing data on hysterectomy or oophorectomy; we also excluded women with an unknown number of ovaries removed. Among women with a history of hysterectomy, we also created a dichotomous early hysterectomy variable, defined as hysterectomy with or without BSO prior to age 40 (yes/no). The definition for early hysterectomy was chosen based on findings from Gierach et al., which demonstrated a rise in mortality risk among women who had hysterectomy alone or hysterectomy with concurrent BSO prior to age 40 ( Gierach et al., 2014 ).
Birth Cohort
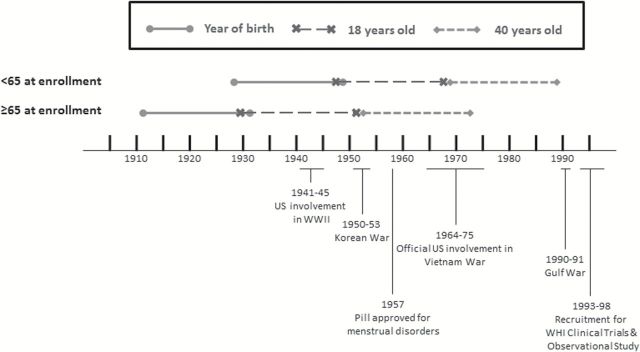
We created two birth cohorts to capture the potential impact of changes in surgical/gynecologic practice and military policy on prevalence of hysterectomy and BSO among women Veterans and non-Veterans during different eras ( Figure 1 ). Birth cohorts were defined based on year of birth as estimated from age and year of study enrollment. Those <65 years of age at enrollment represent a cohort of women who mostly turned 18 (enlistment eligible) following the Women’s Armed Services Integration Act (1948), which authorized regular and reserve status for women in the military; and who would have turned 40 as around the time nonsurgical treatments for menstrual disorders and irregular bleeding became more readily available ( Munro, 2007 ; Tyrer, 1999 ). Those women <65 years of age at enrollment likely served in the Vietnam War or later conflicts. In contrast, women ≥65 years of age at enrollment likely came of age prior to these events and were most likely to have served in either World War II or the Korean War. Specific data on age at enlistment was not available in WHI; however, sources on military history indicate that most women have enrolled before age 30 ( Bellafair, 2005 ; “Women in Vietnam,” 2015 ).
Figure 1.
Timeline for birth cohorts as estimated from age at enrollment.
Reproductive Cancers
We identified women with a history of any reproductive cancer, which was defined as self-report of a physician diagnosis of any current or prior cervical, endometrial, or ovarian cancer during eligibility screenings.
Additional Covariates
Additional baseline demographic and reproductive variables included age, race/ethnicity, educational attainment, pack-years of smoking, age at menarche, parity, and HT use. Body mass index (BMI) was calculated from in-person measurements of height and weight at the time of study enrollment.
Data Analysis
We first examined baseline demographic, behavioral, and reproductive characteristics stratified by Veteran status and birth cohort. To examine differences in the prevalence of hysterectomy and BSO between Veterans and non-Veterans, we fit multinomial logistic regression models separately by birth cohort to estimate odds ratios (ORs) and 95% confidence intervals (CIs). To determine whether the prevalence of early hysterectomy differed between Veterans and non-Veterans, we fit a generalized linear model with a log-link and Poisson distribution to obtain estimates of relative risk (RR) and 95% CIs, separately by birth cohort. Women with a hysterectomy but no reported age at hysterectomy were excluded from this analysis.
Potential confounding variables were measured in WHI only at study baseline, and details of timing regarding military service were not collected. Therefore, it was not possible to specify temporality of confounders, the outcome (hysterectomy/BSO), and exposure (military service). Given the possible bidirectional influence of many potential confounding variables, we therefore sequentially adjusted for potential confounders in two separate models. Model 1 only included age (modeled continuously); parity (modeled nominally; never pregnant, no term pregnancies, 1, 2, and 3+ term pregnancies); and race/ethnicity (non-white, white). Although the relationship of parity and hysterectomy was potentially bidirectional, we opted to include parity in Model 1 given that the majority of hysterectomies occur either at the end of or after the childbearing years ( Turner et al., 2013 ) and that prior evidence suggests higher parity is an important risk factor for hysterectomy ( Brett et al., 1997 ; Gardella et al., 2005 ). Model 2 included Model 1 variables as well as other potential confounders with possible bidirectional effect including: education (modeled ordinally; no school, 1–4 years, 5–8 years, 9–11 years, high school diploma or GED, vocational or training school, some college or Associate Degree, college graduate, some postgraduate or professional school, Master’s Degree, and Doctoral Degree); BMI (continuous); pack-years of smoking (continuous); age at menarche (modeled nominally; <11, 11–12, 13–14, 15+ years of age); and HT use (modeled nominally; never, former, current).
We repeated our analyses excluding women with unilateral or partial oophorectomy from the no hysterectomy group, as these women were suspected to differ from those without hysterectomy and with fully intact ovaries. Additionally, we repeated all analyses excluding women with a history of reproductive cancers to determine whether any observed associations, or lack thereof, reflected differences in reproductive cancers commonly treated by hysterectomy and/or oophorectomy. In order to facilitate interpretation of results, we also calculated the marginal effect of Veteran status on hysterectomy, which provided the difference in probability of procedures between Veterans and non-Veterans.
All analyses were conducted using Stata 13.0 (StataCorp, College Station, TX).
Results
We identified 1,319 Veteran women and 78,122 non-Veteran women <65 years of age who had data on hysterectomy and oophorectomy. Among those 65+ years of age, the study sample included 2,307 Veterans and 60,206 non-Veterans ( Figure 2 ). Compared with non-Veterans, Veterans across both cohorts were more likely to have at least some college or vocational education, to have smoked, and to have never been pregnant ( Table 1 ). Veterans also had slightly higher prevalence of reproductive cancers compared with non-Veterans, although the percentages were low in all women across both birth cohorts. Veterans and non-Veterans within each birth cohort had similar distributions of BMI at the time of study enrollment, age at menarche, and HT use.
Figure 2.
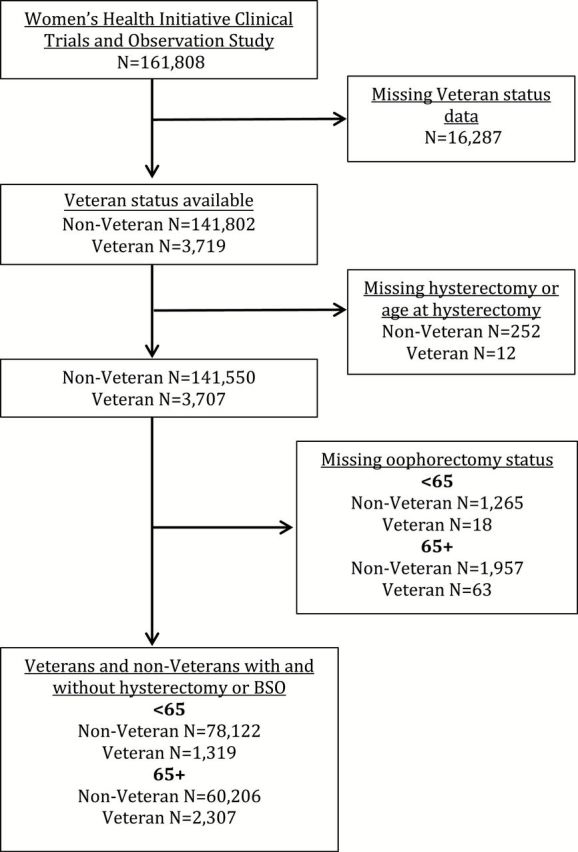
Exclusions and final analytic sample.
Table 1.
Baseline Demographic, Behavioral, and Reproductive Characteristics of Women Veterans and Non-Veterans, Stratified by Birth Cohort (Age at Enrollment)
| <65 at enrollment | ≥65 at enrollment | |||||||
|---|---|---|---|---|---|---|---|---|
| Non-Veterans | Veterans | Non-Veterans | Veterans | |||||
| N a | % a | N a | % a | N a | % a | N a | % a | |
| Total | 78,122 | 1,319 | 60,206 | 2,307 | ||||
| Race/ethnicity | ||||||||
| American Indian/Alaskan Native | 363 | 0.5 | 15 | 1.1 | 204 | 0.3 | 8 | 0.3 |
| Asian/Pacific Islander | 2,220 | 2.8 | 29 | 2.2 | 1,678 | 2.8 | 17 | 0.7 |
| Black/African American | 8,106 | 10.4 | 171 | 13.0 | 3,917 | 6.5 | 82 | 3.6 |
| Hispanic/Latino | 4,012 | 5.1 | 49 | 3.7 | 1,469 | 2.4 | 30 | 1.3 |
| White | 62,327 | 79.8 | 1,033 | 78.3 | 52,072 | 86.5 | 2,133 | 92.5 |
| Other | 923 | 1.2 | 20 | 1.5 | 692 | 1.1 | 26 | 1.1 |
| Education | ||||||||
| High school diploma/GED or less | 15,274 | 19.6 | 180 | 13.6 | 15,256 | 25.3 | 243 | 10.5 |
| At least some college or vocational/training school | 62,281 | 79.7 | 1,133 | 85.9 | 44,625 | 74.1 | 2,056 | 89.1 |
| BMI | ||||||||
| Underweight | 601 | 0.8 | 14 | 1.1 | 621 | 1.0 | 30 | 1.3 |
| Normal | 26,440 | 33.8 | 381 | 28.9 | 21,248 | 35.3 | 836 | 36.2 |
| Overweight | 25,750 | 33.0 | 462 | 35.0 | 21,625 | 35.9 | 827 | 35.8 |
| Obese | 24,591 | 31.5 | 448 | 34.0 | 16,176 | 26.9 | 591 | 25.6 |
| Pack-years of smoking | ||||||||
| Never | 38,159 | 48.8 | 533 | 40.4 | 31,676 | 52.6 | 1,058 | 45.9 |
| <5 | 12,259 | 15.7 | 187 | 14.2 | 7,371 | 12.2 | 291 | 12.6 |
| 5–<20 | 11,417 | 14.6 | 215 | 16.3 | 7,727 | 12.8 | 305 | 13.2 |
| 20+ | 13,755 | 17.6 | 328 | 24.9 | 11,324 | 18.8 | 543 | 23.5 |
| Age at menarche | ||||||||
| <11 | 6,175 | 7.9 | 106 | 8.0 | 3,046 | 5.1 | 92 | 4.0 |
| 11–12 | 33,279 | 42.6 | 545 | 41.3 | 24,182 | 40.2 | 890 | 38.6 |
| 13–14 | 31,657 | 40.5 | 522 | 39.6 | 26,420 | 43.9 | 1,060 | 45.9 |
| 15+ | 6,880 | 8.8 | 139 | 10.5 | 6,398 | 10.6 | 262 | 11.4 |
| Parity | ||||||||
| Never pregnant | 7,252 | 9.3 | 238 | 18.0 | 5,301 | 8.8 | 369 | 16.0 |
| No term pregnancies | 2,367 | 3.0 | 63 | 4.8 | 1,259 | 2.1 | 70 | 3.0 |
| 1 | 7,417 | 9.5 | 146 | 11.1 | 4,722 | 7.8 | 230 | 10.0 |
| 2 | 21,443 | 27.4 | 341 | 25.9 | 13,493 | 22.4 | 438 | 19.0 |
| 3+ | 39,321 | 50.3 | 524 | 39.7 | 35,142 | 58.4 | 1,190 | 51.6 |
| Hormone therapy | ||||||||
| Never | 30,145 | 38.6 | 480 | 36.4 | 29,919 | 49.7 | 1,115 | 48.3 |
| Past | 10,664 | 13.7 | 208 | 15.8 | 10,952 | 18.2 | 498 | 21.6 |
| Current | 37,246 | 47.7 | 630 | 47.8 | 19,283 | 32.0 | 691 | 30.0 |
| Reproductive cancer b | ||||||||
| No | 75,781 | 97.0 | 1,266 | 96.0 | 57,813 | 96.0 | 2,190 | 94.9 |
| Yes | 1,930 | 2.5 | 42 | 3.2 | 1,860 | 3.1 | 89 | 3.9 |
| Hysterectomy | ||||||||
| No | 47,184 | 60.4 | 740 | 56.1 | 34,861 | 57.9 | 1,335 | 57.9 |
| Yes | 30,938 | 39.6 | 579 | 43.9 | 25,345 | 42.1 | 972 | 42.1 |
| Age at hysterectomy c | ||||||||
| <40 | 12,427 | 40.2 | 271 | 46.8 | 6,345 | 25.0 | 199 | 20.5 |
| 40–49 | 13,425 | 43.4 | 217 | 37.5 | 11,045 | 43.6 | 445 | 45.8 |
| 50–59 | 3,473 | 11.2 | 65 | 11.2 | 3,614 | 14.3 | 138 | 14.2 |
| 60+ | 1,613 | 5.2 | 26 | 4.5 | 4,341 | 17.1 | 190 | 19.5 |
Notes: BMI = body mass index.
a Numbers may not add to totals and percents to missing due to missing data.
b Ovarian, endometrial, or cervical cancer.
c Among women with a hysterectomy.
Prevalence of Hysterectomy With or Without BSO
Among women <65 years old at enrollment, Veterans had a similar prevalence of hysterectomy without BSO compared with non-Veterans (21.5% Veterans vs. 20.9% non-Veterans) but were more likely to have had a hysterectomy with BSO (Veterans 22.3% vs. non-Veterans 18.7%; Table 2 ). Among women ≥65 years old at enrollment, Veterans and non-Veterans had similar prevalence of hysterectomy without BSO (Veterans 21.7% vs. non-Veterans 21.0%) and hysterectomy with BSO (Veterans 20.6% vs. non-Veterans 21.1%). Adjusting for a limited set of confounders (Model 1), Veterans <65 years old had 1.2 times higher odds of hysterectomy without BSO (OR 1.18, 95% CI 1.03, 1.36) and 1.3 times higher odds of hysterectomy with BSO (OR 1.26, 95% CI 1.10, 1.45), compared with non-Veterans. Among those >65 at enrollment, no differences between Veterans and non-Veterans were apparent, adjusting for a limited set of confounders (Model 1). Results in both birth cohorts remained unchanged with additional adjustment for health characteristics in Model 2 and when individuals with any oophorectomy but no hysterectomy ( n = 3,936) or those with a history of reproductive cancers ( n = 3,899) were excluded ( Supplementary Tables 1 and Supplementary Data ).
Table 2.
Association Between Veteran Status and Hysterectomy, Stratified by Birth Cohort (Age at Enrollment)
| Birth cohort | Non-Veterans a | Veterans a | Model 1 b | Model 2 c | ||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | OR (95% CI) | p value | OR (95% CI) | p value | |
| <65 at enrollment | ||||||||
| None d | 46,867 | 60.4 | 737 | 56.3 | 1.00 | — | 1.00 | — |
| Hysterectomy without BSO | 16,221 | 20.9 | 281 | 21.5 | 1.18 (1.03, 1.36) | .02 | 1.18 (1.02, 1.37) | .03 |
| Hysterectomy with BSO | 14,544 | 18.7 | 292 | 22.3 | 1.26 (1.10, 1.45) | <.001 | 1.28 (1.10, 1.48) | .001 |
| ≥65 at enrollment | ||||||||
| None d | 34,607 | 57.9 | 1,320 | 57.7 | 1.00 | — | 1.00 | — |
| Hysterectomy without BSO | 12,558 | 21.0 | 496 | 21.7 | 1.07 (0.97, 1.20) | .19 | 1.09 (0.97, 1.22) | .15 |
| Hysterectomy with BSO | 12,582 | 21.1 | 470 | 20.6 | 0.98 (0.88, 1.10) | .76 | 0.97 (0.87, 1.10) | .67 |
Notes: BMI = body mass index; BSO = bilateral salpingo-oophorectomy; CI = confidence interval; OR = odds ratio.
a Numbers and percents reflect those from Model 1.
b Adjusted for age (continuous), race (white, non-white), and parity (nominal).
c Adjusted for age (continuous), race (white, non-white), parity (nominal), education (ordinal), and age at menarche (nominal), BMI (continuous), pack-years of smoking (continuous), hormone therapy use (nominal); due to additional adjustments, sample sizes are smaller than in Adjustment 1 models due to missing information.
d Including women without hysterectomy but with partial, unilateral, or bilateral oophorectomy or unknown number of ovaries removed.
Prevalence of Early Hysterectomy
Among those <65 years old at enrollment, Veterans had a higher prevalence of early hysterectomy compared with non-Veterans (Veterans 21.1% vs. non-Veterans 16.4%; Table 3 ). Among women ≥65 years old at enrollment, the prevalence of early hysterectomy was similar in Veterans and non-Veterans (8.9% and 11.0%, respectively). After minimal adjustment (Model 1), Veterans <65 years old had a 32% higher risk of early hysterectomy, compared with non-Veterans (RR 1.32, 95% CI 1.19, 1.47). This translates to a 5% increase in the probability of early hysterectomy (95% CI 2.9%, 6.3%, data not shown). No differences in RR of early hysterectomy were detected between those Veterans and non-Veterans >65 years old, adjusting Model 1 confounders (RR 0.895, 95% CI 0.78, 1.01). Results in both cohorts were not substantially altered when adjusting for additional potential confounders in Model 2 or when women with a history of reproductive cancers were excluded ( n = 40,039; Supplementary Table 3 ).
Table 3.
Association Between Veteran Status and Early Hysterectomy (Age < 40), Stratified by Birth Cohort (Age at Enrollment)
| Birth cohort | Non-Veterans a | Veterans a | Model 1 b | Model 2 c | ||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | RR (95% CI) | p value | RR (95% CI) | p value | |
| <65 at enrollment | 12,879 | 16.4 | 280 | 21.1 | 1.32 (1.19, 1.47) | <.001 | 1.29 (1.16, 1.44) | <.001 |
| ≥65 at enrollment | 6,758 | 11.0 | 209 | 8.9 | 0.89 (0.78, 1.01) | .08 | 0.90 (0.79, 1.03) | .13 |
Notes: BMI = body mass index; BSO = bilateral salpingo-oophorectomy; CI = confidence interval; RR = relative risk.
a Numbers and percents reflect those from Adjustment 1 models.
b Adjusted for age (continuous), race (white, non-white), and parity (nominal).
c Adjusted for age (continuous), race (white, non-white), parity (nominal), education (ordinal), age at menarche (nominal), BMI (continuous), pack-years of smoking (continuous), and hormone therapy use (nominal); due to additional adjustments, sample sizes are smaller than in Adjustment 1 models due to missing information.
Discussion
In this analysis of over 140,000 postmenopausal women, we found that, among women <65 years old at WHI enrollment, Veterans were more likely to have experienced prior hysterectomy with or without BSO and early hysterectomy before age 40, compared with non-Veterans. Such differences in hysterectomy with or without BSO or early hysterectomy were not evident among WHI participants who enrolled at >65 years of age.
Drawing on the Anderson Model of Healthcare Utilization as a conceptual framework ( Andersen, 1995 ; Washington, Davis, et al., 2013 ), we hypothesize that the higher likelihood of hysterectomy and BSO among Veterans versus non-Veterans in the <65 birth cohort reflects differences across a variety of measured and unmeasured predisposing, enabling, and need factors. In our adjusted models, we were able to control for a limited number of known predisposing risk factors for hysterectomy including age, race, and parity. Additional unmeasured predisposing factors, however, may play an important role in the observed associations. For example, differences in preferences and beliefs related to hysterectomy and differences in rates of sexual trauma between Veterans and non-Veterans may be important mediators that we were unable to capture in our data. Although we controlled for education and race, which are both associated with health care access, it is likely that there were additional unmeasured enabling factors that varied between Veterans and non-Veterans. These unmeasured factors may have contributed to our findings through their association with general health care access or access to less-invasive alternatives for treatment of benign gynecologic conditions, such as hormonal contraception, endometrial ablation, or uterine artery embolization ( Stewart, Shuster, & Rocca, 2012 ). Lastly, differences in “evaluated need” and “perceived need” for hysterectomy, driven by a higher prevalence of mental and physical health conditions among Veterans ( Lehavot et al., 2012b ), may mediate the observed associations. Although we were able to adjust for several health factors such as BMI and smoking, we did not have data regarding other health factors or indication for hysterectomy or BSO. Differences in mental health or medical risk factors for benign gynecologic conditions or reproductive cancers may also contribute to our findings.
The presence of an association in the younger but not the older birth cohort of women may reflect changes in medical practice and military policy over time. As roles for women in the military expanded and eligibility requirements became less restrictive, the characteristics of those who chose military careers changed, potentially resulting in differences in underlying risk factors for benign gynecologic conditions between Veterans and non-Veterans in the younger versus older birth cohorts ( Washington, Bean-Mayberry, et al., 2013 ). For example, differences in the prevalence of trauma and pelvic pain between Veterans and non-Veterans may be greatest among Vietnam-era women due to their greater likelihood of prior sexual abuse ( Blanton, 2012 ). These differences may have in turn influenced the likelihood of hysterectomy among those <65 years at enrollment, many of whom served in Vietnam ( Lamvu, 2011 ; Latthe, Mignini, Gray, Hills, & Khan, 2006 ). However, given that data regarding trauma, pelvic pain, and health care preferences and access at the time of hysterectomy were unavailable, we can only speculate as to whether or how differences in these factors may have influenced our results.
Our findings should be considered in light of several additional limitations. We were unable to ascertain the temporality of military service and hysterectomy, which limited our ability to draw causal inferences. However, the vast majority of women Veterans reported being over 30 years of age at the time of hysterectomy and sources on military history indicate that most women likely enrolled in the military before age 30; therefore, we expect that the majority of hysterectomies occurred during or after military service. We also relied on self-report of both military service and hysterectomy, which could potentially lead to misclassification. However, information on military service was ascertained with a standard question routinely used across large, national studies, including the Center for Disease Control and Prevention’s Behavioral Risk Factor Surveillance System ( Hoerster et al., 2012 ; Reiber, Koepsell, Maynard, Haas, & Boyko, 2004 ). Furthermore, a number of studies have confirmed the high validity of self-reported hysterectomy (96%–100%; Gentry-Maharaj et al., 2014 ; Green et al., 1997 ; Lucas, Azevedo, & Barros, 2008 ; Phipps & Buist, 2009 ) as well as oophorectomy (73%–100% depending on if unilateral or bilateral oophorectomy; Lucas et al., 2008 ; Phipps & Buist, 2009 ). Residual confounding is another possible source of bias, as potential confounders (e.g., BMI, smoking, hormone use) were only measured at baseline, precluding our ability to adjust for status of these variables prior to hysterectomy. Nevertheless, baseline status of these confounders may be at least partially correlated with status preceding hysterectomy, and further adjustment for these variables in Model 2 did not appreciably change our results. Lastly, the population included in the WHI was a select group that is not necessarily representative of the general population or the current population of women Veterans, which potentially limits the generalizability of our findings. Nevertheless, our study provides new data on variations in prevalence of hysterectomy with and without BSO by history of military service and birth cohort and utilizes one of few national data sets that contains detailed reproductive health information for both women Veterans and non-Veterans.
Practice and Policy Implications
Our finding of a higher prevalence of both hysterectomy alone and hysterectomy with BSO among Veterans compared with non-Veterans has important implications with respect to the health of aging women Veterans. Hysterectomy alone carries potential risks of incontinence ( Kudish et al., 2014 ) and earlier menopause ( Moorman et al., 2011 ). Concomitant hysterectomy and BSO have additionally been associated with CVD and dementia, as well as all-cause and coronary heart disease mortality in women who undergo hysterectomy and BSO before age 40, although it is unclear whether this is due to underlying risks among women who undergo these procedures or the procedures themselves ( Gierach et al., 2014 ; Jacoby, Grady, & Sawaya, 2009 ; Parker et al., 2009 ; Rocca et al., 2012 ).
The higher than expected prevalence of hysterectomy both with and without BSO among women Veterans suggests that aging women Veterans may be at greater risk than their non-Veteran counterparts for these conditions. Additional research is needed to better understand the potential role of military service in reproductive health outcomes such as hysterectomy and BSO, and how this may affect the health of women Veterans. Given the increasing number of women Veterans using Department of Veterans Affairs (VA) health care, these data also suggest a need for the VA to continue to build capacity to address the health concerns of older women. Additionally, given that the majority of women Veterans receive some or all of their health care outside VA, non-VA health care providers should be aware of women’s history of military service and potential associated health risks.
Funding
The Women’s Health Initiative programs are funded by the NationalHeart, Lung, and Blood Institute; National Institutes of Health; U.S. Department of Health and Human Services through contracts, HHSN268201100046C, HHSN268201100001C, HHSN268201 100002C, HHSN268201100003C, HHSN268201100004C. WHI Investigators: Program Office: (National Heart, Lung, and Blood Institute, Bethesda, MD) Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller. Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg. Investigators and Academic Centers: (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker. Women’s Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker. Drs. Callegari and Gray were supported by VA Health Services Research and Development (HSR&D) Postdoctoral Fellowships (TPM 61-041 and TPP 61-029, respectively). Dr. Katon was supported by the U.S. Department of Veterans Affairs; Office of Patient Care Services; Women’s Health Services and the VA Puget Sound Center of Innovation for Veteran-Centered and Value-Driven Care. The research reported here was also supported by Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service FOP14-439 and the VA Office of Women’s Health.
Supplementary Material
Acknowledgments
The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
References
- American Congress of Obstetrics & Gynecology (ACOG) . ( 2011. ). Women’s Health Stats and Facts . Washington, DC: . Retrieved from http://www.acog.org/-/media/NewsRoom/MediaKit.pdf [Google Scholar]
- Andersen R. M . ( 1995. ). Revisiting the behavioral model and access to medical care: Does it matter? Journal of Health and Social Behavior , 36 , 1 – 10 . Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7738325 [PubMed] [Google Scholar]
- Andersen R. M . ( 2008. ). National health surveys and the behavioral model of health services use . Medical Care , 46 , 647 – 653 . doi: 10.1097/MLR.0b013e31817a835d [DOI] [PubMed] [Google Scholar]
- Anderson G. L. Manson J. Wallace R. Lund B. Hall D. Davis S. , … Prentice R. L . ( 2003. ). Implementation of the Women’s Health Initiative study design . Annals of Epidemiology , 13 ( 9 Suppl .), S5 – S17 . Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/14575938 [DOI] [PubMed] [Google Scholar]
- Babitsch B. Gohl D. , & von Lengerke T . ( 2012. ). Re-revisiting Andersen’s Behavioral Model of Health Services Use: a systematic review of studies from 1998–2011 . Psychosocial Medicine , 9 , Doc11 . doi: 10.3205/psm000089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellafair J. A . ( 2005. ). The Women’s Army Corps: A Commemoration of World War II Service . Retrieved from http://www.history.army.mil/brochures/wac/wac.htm [Google Scholar]
- Blanton R. E . ( 2012. ). California’s women Veterans and military sexual trauma . Retrieved from California: http://www.library.ca.gov/crb/12/S-12-004.pdf [Google Scholar]
- Blosnich J. R. Dichter M. E. Cerulli C. Batten S. V. , & Bossarte R. M . ( 2014. ). Disparities in adverse childhood experiences among individuals with a history of military service . JAMA Psychiatry , 71 , 1041 – 1048 . doi: 10.1001/jamapsychiatry.2014.724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett K. M. Marsh J. V. , & Madans J. H . ( 1997. ). Epidemiology of hysterectomy in the United States: Demographic and reproductive factors in a nationally representative sample . Journal of Women’s Health , 6 , 309 – 316 . Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9201665 [DOI] [PubMed] [Google Scholar]
- Brown J. S. Sawaya G. Thom D. H. , & Grady D . ( 2000. ). Hysterectomy and urinary incontinence: A systematic review . Lancet , 356 , 535 – 539 . doi: 10.1016/S0140-6736(00)02577-0 [DOI] [PubMed] [Google Scholar]
- Carlson K. J. Nichols D. H. , & Schiff I . ( 1993. ). Indications for hysterectomy . The New England Journal of Medicine , 328 , 856 – 860 . doi: 10.1056/NEJM199303253281207 [DOI] [PubMed] [Google Scholar]
- Cheong Y. C. Smotra G. , & Williams A. C . ( 2014. ). Non-surgical interventions for the management of chronic pelvic pain . Cochrane Database of Systematic Reviews , 3 , CD008797 . doi: 10.1002/14651858.CD008797.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. E. , & Doyle P. M . ( 2002. ). Quality of life studies in unselected gynaecological outpatients and inpatients before and after hysterectomy . Journal of Obstetrics and Gynaecology , 22 , 523 – 526 . doi: 10.1080/0144361021000003681 [DOI] [PubMed] [Google Scholar]
- Devilbiss M . ( 1990. ). Women and military service: A history, analysis, and overview of key issues . Retrieved from http://www.dtic.mil/dtic/tr/fulltext/u2/a229958.pdf [Google Scholar]
- Gardella C. Johnson K. M. Dobie D. J. , & Bradley K. A . ( 2005. ). Prevalence of hysterectomy and associated factors in women Veterans Affairs patients . Journal of Reproductive Medicine , 50 , 166 – 172 . Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15841928 [PubMed] [Google Scholar]
- Gentry-Maharaj A. Taylor H. Kalsi J. Ryan A. Burnell M. Sharma A. , … Menon U . ( 2014. ). Validity of self-reported hysterectomy: A prospective cohort study within the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) . BMJ Open , 4 , e004421 . doi: 10.1136/bmjopen-2013-004421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierach G. L. Pfeiffer R. M. Patel D. A. Black A. Schairer C. Gill A. , … Sherman M. E . ( 2014. ). Long-term overall and disease-specific mortality associated with benign gynecologic surgery performed at different ages . Menopause , 21, 592 – 601 . doi: 10.1097/GME.0000000000000118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A. Purdie D. Green L. Dick M. L. Bain C. , & Siskind V . ( 1997. ). Validity of self-reported hysterectomy and tubal sterilisation. The Survey of Women’s Health Study Group . Australian and New Zealand Journal of Public Health , 21 , 337 – 340 . Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/ 9270164 [DOI] [PubMed] [Google Scholar]
- Hays J. Hunt J. R. Hubbell F. A. Anderson G. L. Limacher M. Allen C. , & Rossouw J. E . ( 2003. ). The Women’s Health Initiative recruitment methods and results . Annals of Epidemiology , 13 ( 9 Suppl .), S18 – S77 . Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/14575939 [DOI] [PubMed] [Google Scholar]
- Hoerster K. D. Lehavot K. Simpson T. McFall M. Reiber G. , & Nelson K. M . ( 2012. ). Health and health behavior differences: U.S. Military, veteran, and civilian men . American Journal of Preventive Medicine , 43 , 483 – 489 . doi: 10.1016/j.amepre.2012.07.029 [DOI] [PubMed] [Google Scholar]
- Howard B. V. Kuller L. Langer R. Manson J. E. Allen C. Assaf A. , … Trevisan M . ( 2005. ). Risk of cardiovascular disease by hysterectomy status, with and without oophorectomy: The Women’s Health Initiative Observational Study . Circulation , 111 , 1462 – 1470 . doi: 10.1161/01.CIR.0000159344.21672.FD [DOI] [PubMed] [Google Scholar]
- Jacoby V. L. Grady D. , & Sawaya G. F . ( 2009. ). Oophorectomy as a risk factor for coronary heart disease . American Journal of Obstetrics & Gynecology , 200 , 140.e141 – 140.e149 . doi: 10.1016/j.ajog.2008.08.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby V. L. Grady D. Wactawski-Wende J. Manson J. E. Allison M. A. Kuppermann M. , … Stefanick M. L . ( 2011. ). Oophorectomy vs ovarian conservation with hysterectomy: Cardiovascular disease, hip fracture, and cancer in the Women’s Health Initiative Observational Study . Archives of Internal Medicine , 171 , 760 – 768 . doi: 10.1001/archinternmed.2011.121 [DOI] [PubMed] [Google Scholar]
- Koepsell T. Reiber G. , & Simmons K. W . ( 2002. ). Behavioral risk factors and use of preventive services among veterans in Washington State . Preventive Medicine , 35 , 557 – 562 . Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12460523 [DOI] [PubMed] [Google Scholar]
- Kudish B. I. Shveiky D. Gutman R. E. Jacoby V. Sokol A. I. Rodabough R. , … Iglesia C. B . ( 2014. ). Hysterectomy and urinary incontinence in postmenopausal women . International Urogynecology Journal , 25 , 1523 – 1531 . doi: 10.1007/s00192-014-2422-x [DOI] [PubMed] [Google Scholar]
- Lamvu G . ( 2011. ). Role of hysterectomy in the treatment of chronic pelvic pain . Obstetrics and Gynecology , 117 , 1175 – 1178 . doi: 10.1097/AOG.0b013e31821646e1 [DOI] [PubMed] [Google Scholar]
- Latthe P. Mignini L. Gray R. Hills R. , & Khan K . ( 2006. ). Factors predisposing women to chronic pelvic pain: systematic review . British Medical Journal , 332 , 749 – 755 . doi: 10.1136/bmj.38748.697465.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehavot K. Hoerster K. D. Nelson K. M. Jakupcak M. , & Simpson T. L . ( 2012a. ). Health indicators for military, veteran, and civilian women . American Journal of Preventive Medicine , 42 , 473 – 480 . doi: 10.1016/j.amepre.2012.01.006 [DOI] [PubMed] [Google Scholar]
- Lehavot K. Hoerster K. D. Nelson K. M. Jakupcak M. , & Simpson T. L . ( 2012b. ). Health indicators for military, veteran, and civilian women . American Journal of Preventive Medicine , 42 , 473 – 480 . doi: 10.1016/j.amepre.2012.01.006 [DOI] [PubMed] [Google Scholar]
- Lehavot K. Katon J. G. Williams E. C. Nelson K. M. Gardella C. M. Reiber G. E. , & Simpson T. L . ( 2014. ). Sexual behaviors and sexually transmitted infections in a nationally representative sample of women veterans and nonveterans . Journal of Women's Health , 23 , 246 – 252 . doi: 10.1089/jwh.2013.4327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas R. Azevedo A. , & Barros H . ( 2008. ). Self-reported data on reproductive variables were reliable among postmenopausal women . Journal of Clinical Epidemiology , 61 , 945 – 950 . doi: 10.1016/j.jclinepi.2007.11.001 [DOI] [PubMed] [Google Scholar]
- Marjoribanks J. Lethaby A. , & Farquhar C . ( 2006. ). Surgery versus medical therapy for heavy menstrual bleeding . Cochrane Database of Systematic Reviews , ( 2 ), CD003855 . doi: 10.1002/14651858.CD003855.pub2 [DOI] [PubMed] [Google Scholar]
- Merrill R. M . ( 2008. ). Hysterectomy surveillance in the United States, 1997 through 2005 . Medical Science Monitor , 14 , CR24 – CR31 . Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/18160941 [PubMed] [Google Scholar]
- Moorman P. G. Myers E. R. Schildkraut J. M. Iversen E. S. Wang F. , & Warren N . ( 2011. ). Effect of hysterectomy with ovarian preservation on ovarian function . Obstetrics and Gynecology , 118 , 1271 – 1279 . doi: 10.1097/AOG.0b013e318236fd12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro M. G . ( 2007. ). Management of heavy menstrual bleeding: Is hysterectomy the radical mastectomy of gynecology? , 50 , 324 – 353 . doi: 10.1097/GRF.0b013e31804a82e2 [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics (NCHS) . ( 2012. ) Health, United States, 2011: With special feature on socioeconomic status and health . Hyattsville, MD: . Retrieved from http://www.cdc.gov/nchs/data/hus/hus11.pdf [PubMed] [Google Scholar]
- Parker W. H. Broder M. S. Chang E. Feskanich D. Farquhar C. Liu Z. , … Manson J. E . ( 2009. ). Ovarian conservation at the time of hysterectomy and long-term health outcomes in the nurses’ health study . Obstetrics and Gynecology , 113 , 1027 – 1037 . doi: 10.1097/AOG.0b013e3181a11c64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipps A. I. , & Buist D. S . ( 2009. ). Validation of self-reported history of hysterectomy and oophorectomy among women in an integrated group practice setting . Menopause , 16 , 576 – 581 . doi: 10.1097/gme.0b013e31818ffe28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phung T. K. Waltoft B. L. Laursen T. M. Settnes A. Kessing L. V. Mortensen P. B. , & Waldemar G . ( 2010. ). Hysterectomy, oophorectomy and risk of dementia: A nationwide historical cohort study . Dementia and Geriatric Cognitive Disorders , 30 , 43 – 50 . doi: 10.1159/000314681 [DOI] [PubMed] [Google Scholar]
- Poston D . ( 2010. ). Population and society: An introduction to demography . New York: : Cambridge University Press; . [Google Scholar]
- Powell L. H. Meyer P. Weiss G. Matthews K. A. Santoro N. Randolph J. F. Jr. , … Sutton-Tyrrell K . ( 2005. ). Ethnic differences in past hysterectomy for benign conditions . Womens Health Issues , 15 , 179 – 186 . doi: 10.1016/j.whi.2005.05.002 [DOI] [PubMed] [Google Scholar]
- Qi L. Nassir R. Kosoy R. Garcia L. Waetjen L. E. Ochs-Balcom H. M. , … Seldin M. F . ( 2013. ). Relationship between hysterectomy and admixture in African American women . American Journal of Obstetrics & Gynecology , 208 , 279.e271 – 279.e277 . doi: 10.1016/j.ajog.2013.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiber G. E. Koepsell T. D. Maynard C. Haas L. B. , & Boyko E. J . ( 2004. ). Diabetes in nonveterans, veterans, and veterans receiving Department of Veterans Affairs health care . Diabetes Care , 27 ( Suppl. 2 ), B3 – B9 . Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15113776 [DOI] [PubMed] [Google Scholar]
- Rocca W. A. Grossardt B. R. Shuster L. T. , & Stewart E. A . ( 2012. ). Hysterectomy, oophorectomy, estrogen, and the risk of dementia . Neurodegenerative Diseases , 10 , 175 – 178 . doi: 10.1159/000334764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievert L. L. Murphy L. Morrison L. A. Reza A. M. , & Brown D. E . ( 2013. ). Age at menopause and determinants of hysterectomy and menopause in a multi-ethnic community: The Hilo Women’s Health Study . Maturitas , 76 , 334 – 341 . doi: 10.1016/j.maturitas.2013.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilsbury K. Hammond I. Bulsara M. , & Semmens J. B . ( 2008. ). Morbidity outcomes of 78,577 hysterectomies for benign reasons over 23 years . BJOG , 115 , 1473 – 1483 . doi: 10.1111/j.1471-0528.2008.01921.x [DOI] [PubMed] [Google Scholar]
- Stewart E. A. Shuster L. T. , & Rocca W. A . ( 2012. ). Reassessing hysterectomy . Minnesota Medicine , 95 , 36 – 39 . [PMC free article] [PubMed] [Google Scholar]
- Turner L. C. Shepherd J. P. Wang L. Bunker C. H. , & Lowder J. L . ( 2013. ). Hysterectomy surgical trends: a more accurate depiction of the last decade? American Journal of Obstetrics and Gynecology , 208 , 277.e271 – 277.e277 . doi: 10.1016/j.ajog.2013.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrer L . ( 1999. ). Introduction of the pill and its impact . Contraception , 59 ( 1 Suppl .), 11S – 16S . Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10342090 [DOI] [PubMed] [Google Scholar]
- Washington D. L. Bean-Mayberry B. Hamilton A. B. Cordasco K. M. , & Yano E. M . ( 2013. ). Women veterans’ healthcare delivery preferences and use by military service era: Findings from the national survey of women veterans . Journal of General Internal Medicine , 28 ( Suppl. 2 ), 571 – 576 . doi: 10.1007/s11606-012- 2323-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington D. L. Davis T. D. Der-Martirosian C. , & Yano E. M . ( 2013. ). PTSD risk and mental health care engagement in a multi-war era community sample of women veterans . Journal of General Internal Medicine , 28 , 894 – 900 . doi: 10.1007/s11606-012-2303-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteman M. K. Hillis S. D. Jamieson D. J. Morrow B. Podgornik M. N. Brett K. M. , & Marchbanks P. A . ( 2008. ). Inpatient hysterectomy surveillance in the United States, 2000–2004 . American Journal of Obstetrics & Gynecology , 198 , 34.e31 – 34.e37 . doi: 10.1016/j.ajog.2007.05.039 [DOI] [PubMed] [Google Scholar]
- Women in Vietnam . ( 2015. ). Retrieved from http://www.vietnamveteransplaza.com/the-vietnam-war/women-in-vietnam/
- Wright J. D. Herzog T. J. Tsui J. Ananth C. V. Lewin S. N. Lu Y. S. , … Hershman D. L . ( 2013. ). Nationwide trends in the performance of inpatient hysterectomy in the United States . Obstetrics and Gynecology , 122 ( 2 Pt 1 ), 233 – 241 . doi: 10.1097/AOG.0b013e318299a6cf [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.