Abstract
Purpose of the Study:
Women’s military roles, exposures, and associated health outcomes have changed over time. However, mortality risk—within military generations or compared with non-Veteran women—has not been assessed. Using data from the Women’s Health Initiative (WHI), we examined all-cause and cause-specific mortality by Veteran status and military generation among older women.
Design and Methods:
WHI participants (3,719 Veterans; 141,802 non-Veterans), followed for a mean of 15.2 years, were categorized into pre-Vietnam or Vietnam/after generations based on their birth cohort. We used cox proportional hazards models to examine the association between Veteran status and mortality by generation.
Results:
After adjusting for sociodemographic characteristics and WHI study arm, all-cause mortality hazard rate ratios (HRs) for Veterans relative to non-Veterans were 1.16 (95% CI: 1.09–1.23) for pre-Vietnam and 1.16 (95% CI: 0.99–1.36) for Vietnam/after generations. With additional adjustment for health behaviors and risk factors, this excess mortality rate persisted for pre-Vietnam but attenuated for Vietnam/after generations. After further adjustment for medical morbidities, across both generations, Veterans and non-Veterans had similar all-cause mortality rates. Relative to non-Veterans, adjusting for sociodemographics and WHI study arm, pre-Vietnam generation Veterans had higher cancer, cardiovascular, and trauma-related morality rates; Vietnam/after generation Veterans had the highest trauma-related mortality rates (HR = 2.93, 1.64–5.23).
Implications:
Veterans’ higher all-cause mortality rates were limited to the pre-Vietnam generation, consistent with diminution of the healthy soldier effect over the life course. Mechanisms underlying Vietnam/after generation Veteran trauma-related mortality should be elucidated. Efforts to modify salient health risk behaviors specific to each military generation are needed.
Keywords: Women Veterans, Mortality, Cohort effect, Longitudinal study
U.S. military troops put their lives on the line, and the health consequences play out not only during their years of service but often over the subsequent decades ( Washington, Farmer, Mor, Canning, & Yano, 2015 ). However, there have been little data available to directly compare the health and mortality of women Veterans and non-Veterans. Most prior work has focused upon Vietnam era Veterans, with particular emphasis on comparisons between deployed and nondeployed women ( Cypel & Kang, 2008 ; Kang et al., 2014 ; Thomas, Kang, & Dalager, 1991 ). Ascertaining mortality rates and causes among older generations of women Veterans (i.e., pre-Vietnam era, e.g., those who served during World War II (WWII) or Korea War) and comparisons by military generation with mortality seen in women non-Veterans are critical to understanding and addressing the health and health care needs of women Veterans ( Bean-Mayberry et al., 2011 ). Yet, this topic has received limited empirical attention to date.
This literature gap is problematic for several reasons, the most pressing being the need for evidence-guided health care for the oldest group of women Veterans alive today. Indeed, more than 300,000 living women Veterans are 65 years and older, the oldest of which represent the last of the pre-Vietnam generation of military women (U.S. Department of Veterans Affairs, 2014; Washington, Bean-Mayberry, Hamilton, Cordasco, & Yano, 2013 ). Knowledge of causes of mortality in women Veterans, particularly if they differ by military generation or differ from non-Veteran peers, can inform primary, secondary, and tertiary prevention for individual women, and policy and practice in serving this population across VA and non-VA care.
Though these Veteran women undoubtedly share similarities in health concerns and mortality risk with members of their birth cohort ( Der-Martirosian, Cordasco, & Washington, 2013 ), there are also many compelling reasons to suspect that their concerns and risks might be distinctive from that of the general population. Specifically, a substantial literature documents the robust good health and decreased mortality risk of Veterans, including women, relative to the general population ( McLaughlin, Nielsen, & Waller, 2008 ). However, this suggested “healthy soldier effect” may in fact reflect a significant health selection bias among individuals who are selected into military service based upon good health at entry. As such, concern has been raised regarding comparisons with the general population, for whom no such selection bias exists, as they may promote erroneous conclusions about Veterans’ true health and mortality risk ( Bross & Bross, 1987 ; Weitlauf et al., 2015 ). The relationship between health and mortality risk of women Veterans’ relative to similar non-Veteran peers may also vary as a function of the era of military service. Women’s sociodemographic characteristics and their military roles and exposures, and associated physical and mental health risks, have changed over time. For example, women Veterans of Vietnam and more recent eras have a greater prevalence of military sexual trauma, hazardous alcohol use, and posttraumatic stress disorder (PTSD) than women Veterans of earlier eras ( Hoggatt, Williams, Der-Martirosian, Yano, & Washington, 2015 ; Murdoch et al., 2006 ; Washington, Bean-Mayberry, et al., 2013 ; Washington, Davis, Der-Martirosian, & Yano, 2013 ).
U.S. cultural norms with respect to health-promoting behavior and health risk behavior have also changed (Centers for Disease Control and Prevention, 1999; Robert Wood Johnson Foundation, 2011 ). For example, smoking, once a socially accepted behavior, is now declining after public health efforts launched in response to the risk of smoking described by the Surgeon General in 1964—the year of the start of the Vietnam War (Centers for Disease Control and Prevention, 1999). Though this broader cultural or environmental context would be expected to influence both Veterans and non-Veterans, military service experiences could moderate these influences, for example, with promotion of tobacco use during wartime ( Offen, Smith, & Malone, 2013 ; Sharkansky, King, King, Wolfe, Erickson, & Stokes, 2000 ; Smith & Malone, 2009 ). Therefore, we hypothesize that the mortality risk of women Veterans, relative to non-Veteran women, varies by military generation.
The Women’s Health Initiative (WHI) offers a unique opportunity to comparatively evaluate mortality risk among a large sample of U.S. postmenopausal women Veterans and non-Veterans. Indeed, Weitlauf and colleagues (2015) recently found an elevated risk of all-cause mortality among WHI women Veteran participants, relative to non-Veteran participants, even after adjustment for the effects of age and common health risk confounders, such as smoking. We propose to build upon that work by evaluating older women’s mortality rates and causes across Veteran status and generation. Specifically, our primary objective is to examine whether Veteran status was associated with higher rates of all-cause mortality within two military generations of older women. Our secondary objective is to compare cause-specific mortality by Veteran status within each generation.
Per the logic of the healthy soldier effect, we hypothesize that the excess mortality would begin to occur in the oldest group of Veterans, who will have outlived their health advantage. Following this logic, one might anticipate reduced mortality risk among the younger, Vietnam/after generation Veteran women relative to their non-Veteran peers. However, we propose that the Vietnam/after generation of Veterans may in fact depart from the traditional trajectory predicted by the healthy solider effect, for example, related to differential behaviors between generations such as smoking.
Conceptual Model
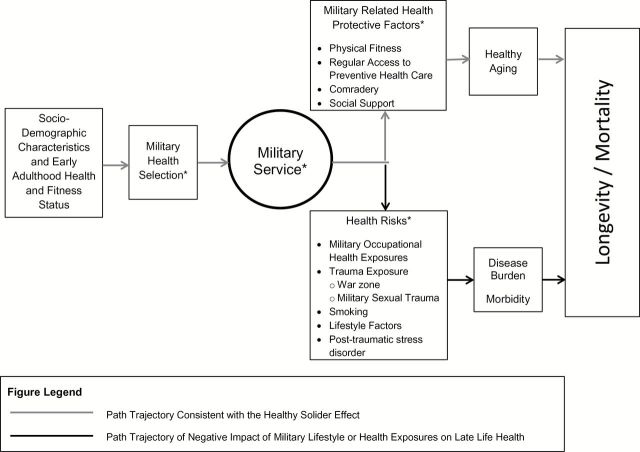
Our conceptual model for the influence of premilitary health and military service on mortality, depicted in Figure 1 , is informed by the biopsychosocial model of health and aging ( Seeman & Crimmins, 2001 ), the constrained choice model ( Bird & Rieker, 2008 ), and the scientific literature on the health effects of military service on women Veterans ( Batuman et al., 2011 ; Bean-Mayberry et al., 2011 ; Washington, Farmer, et al., 2015 ). The biopsychosocial model of health and aging postulates that sociodemographic characteristics interact to influence psychological characteristics and behavioral factors. These relationships are influenced by broader effects of the sociocultural and physical environments across each individual’s life span, and these domains ultimately influence health outcomes, including mortality. Constrained choice emphasizes the impact of decision making at organizational levels beyond the individual in shaping individuals’ opportunity to pursue a healthy life. The constraints shaped by decisions at other levels, including family, work place (in this case both during military service and afterward in part through related policies and programs), community and society, shape health behaviors and other exposures to health risks as well as the protective or detrimental impact of each.
Figure 1.
Conceptual model of influence of military health selection and military service on late life health and longevity/mortality for women Veteran. *Domains hypothesized to vary by military generation, resulting in variations by military generation in healthy aging, disease burden/morbidity, and longevity.
Applied to women Veterans, sociodemographic characteristics and early adulthood health and fitness status would be expected to influence entry into military service, which is depicted in our conceptual model as military health selection ( Figure 1 ). This early adulthood health and fitness, and military health selection, initiates the trajectory for the path that is consistent with the healthy soldier effect. Military service can be represented by Veteran status, where Veteran status is a proxy for the broader effects of the sociocultural and physical military environment that are associated with a number of both health protective factors and health risks. Two recent systematic reviews on women Veterans’ health ( Bean-Mayberry et al., 2011 ) and the health effects of military service on women Veterans ( Batuman et al., 2011 ) describe the life course experiences associated with military influences, exposures, and stressors. For example, military-related health protective factors include behavioral factors such as physical activity, and health risks include behavioral factors such as smoking.
The positive and negative health effects of military service are depicted as separate pathways in our conceptual model ( Figure 1 ), in order to illustrate the hypothesized directionality of military health selection, military-related health protective factors, and health risks, on late life health and longevity or mortality. The key health outcome of interest in our conceptual model is longevity/mortality. Viewed from a life-span/cumulative-effects framework, there are several domains that are hypothesized to vary by military generation, resulting in variations in healthy aging, disease burden/morbidity, and longevity/mortality. Specifically, military selection criteria for women, women’s military roles and exposures, and the broader influences on health risks and preventive health factors in the United States have all changed over time. Thus, our conceptual model illustrates the potential for both positive and negative influences of women’s military generation on their longevity/mortality.
Design and Methods
Study Population
The WHI clinical trials (CT) and observational study (OS) have been described in detail elsewhere ( Hays et al., 2003 ; Women’s Health Initiative Study Group, 1998). In brief, between 1993 and 1998, postmenopausal women aged 50 to 79 years were recruited from 40 clinical centers around the United States. The main study collected follow-up data on 161,808 CT and OS participants until 2005. Main study participants could consent to additional follow-up during Extension Study 1 (ES1), with data collection between 2005 and 2010 (76.9% of main study participants); consenting ES1 participants could also participate in Extension Study 2 (ES2), with data collection between 2010 and 2015 (86.8% of ES1 participants).
Data used in the present investigation were obtained from women who participated in either the CT or OS arms at baseline. Participant data were collected via self-administered forms, interviews (in-person or by telephone), and in-person clinical measurements by trained staff at the clinical centers. Frequency of data collection postbaseline and the specific information collected varied by study enrollment ( Anderson et al., 2003 ). Written informed consent was obtained from all study participants. Procedures and protocols were approved by institutional review boards at all participating institutions.
Veteran Status
Veteran status was determined from the baseline question, “Have you ever served in the U.S. armed forces on active duty for a period of 180 days or more?” Those responding affirmatively were classified as Veterans ( n = 3,719), those responding negatively were classified as non-Veterans ( n = 141,802), and those missing a response ( n = 16,287) were excluded from analyses. This approach to determining Veteran status is used in national surveys conducted by the Centers for Disease Control and Prevention ( Lehavot, Hoerster, Nelson, Jakupcak, & Simpson, 2012 ).
Generation
The considerable age diversity of the WHI study population suggested that participants span multiple birth cohorts, or generations, with the oldest participants representing the “Greatest Generation,” or women who were coming of age during the era of WWII, the Korean War, and the commencement of the cold war. The younger participants represent the “Baby-Boomers”, or those whose coming of age coincided with the Vietnam War. These distinct generations would be expected to impact long-term health and mortality risks, particularly among Veteran participants whose period of military service coincided with different U.S. conflicts (e.g., WWII, the Korean War, or the Vietnam war), each of which brought about varied risk of military-related health exposures, changes to women’s military roles and length of service. To facilitate an examination of the association of Veteran status (compared with non-Veterans) and postmenopausal mortality rates and causes of mortality with respect to each woman’s generation, we assigned all participants to one of the two generations (pre-Vietnam generation or Vietnam/after generation) based upon their year of birth. Because of our interest in Veteran women, generations were characterized by the military conflicts pertinent to each generation. Both Veterans and non-Veterans who were born 1913–1931 were assigned to the pre-Vietnam generation, which coincided with the military service periods (WWII and the Korean War) in which, based upon their age, they would have been first eligible for military service (the average age of women’s military entry being 22 years at that time). Those born 1932–1948 were assigned to the Vietnam and after (Vietnam/after) generation, based on being age consistent with eligibility for military service during the Vietnam War ( Washington, Bean-Mayberry, et al., 2013 ).
Death Ascertainment and Adjudication
Outcome ascertainment procedures, including those used for local and central adjudication, have been described extensively in prior work ( Curb et al., 2003 ). Briefly, follow-up for clinical outcomes was performed at 6-month intervals for women in the CT arm and annually for women in the OS arm. Deaths were identified as part of routine participant follow-up that included reports from family, obituaries, and National Death Index searches. From baseline through 2010, all participant deaths were confirmed by physician adjudication of hospital or medical records, coroner or autopsy reports, or death certificates. After 2010, some deaths were identified via personal notification by the decedent’s family, friends, or personal physician and were not physician adjudicated. National Death Index searches were executed periodically during study follow-up in order to also identify deaths from participants lost to follow-up or who did not consent to the Extension studies. The last National Death Index search was completed in 2013 to identify deaths that occurred through 2011. For this analysis, 55% of the deaths were physician adjudicated, 33% were identified from a National Death Index search, and 12% were identified from personal notification. The underlying causes of death were coded according to the International Classification of Diseases, Ninth Revision (Centers for Disease Control and Prevention).
Covariates
Selection of covariates was informed by the conceptual model. All covariates were assessed at baseline. Demographic and social relationship characteristics included age; race/ethnicity (American Indian/Alaskan Native, Asian/Pacific Islander, Black/African American, Hispanic/Latino, White, other); highest level of education completed; category of current employment; and marital status.
Health behavior practices included self-reported cigarette smoking status (never, former, and current), number of pack years tobacco use, and alcohol intake (gram standardized drinks per week). Recreational physical activity level is reported in metabolic equivalent hours per week, which was computed based on responses to a self-administered questionnaire about frequency, duration, and intensity of exercise and walking. Body mass index (BMI) was calculated from clinically measured weight in kilograms divided by clinically measured height in meters squared. Depression was determined from the 8-item Burnam scale of the Center for the Epidemiologic Study of Depression (CESD) scale ( Burnam, Wells, Leake, & Landsverk, 1988 ), in which scores range from 0 to 0.99, with scores of 0.06 or greater representing symptoms consistent with depressive disorders ( Bertone-Johnson et al., 2012 ). Physician-diagnosed hypertension at baseline was self-reported. Comorbidity was calculated as an absolute count of the self-reported history of diagnosis of 12 chronic conditions (coronary disease, stroke, cancer, diabetes, hip fracture, osteoarthritis, depression, chronic obstructive pulmonary disease, cognitive impairment, sensory impairment, frequent faller, and urinary incontinence) recognized to be prevalent and burdensome in older women (Rillamas-Sun et al., in press). Coronary disease, stroke, cancer, and hip fractures were primary outcomes during follow-up in the main WHI study and were confirmed via local and central physician adjudication. Self-reported history of physician diagnosis of diabetes also included confirmation of treatment (pills or insulin) by medication inventory at baseline.
To account for bias due to variation in eligibility criteria across the CT studies and between the CT and the OS, study assignment was included as a covariate (Estrogen-Alone trial, Estrogen plus Progestin trial, Dietary Modification trial, and OS).
Statistical Analyses
The analytic sample was comprised of 145,521 WHI participants with non-missing data on Veteran status. Baseline characteristics by generation for Veterans and non-Veterans were summarized using means and standard deviations (SD) for continuous variables and frequencies for categorical variables.
Time to event was defined as the interval between baseline and the end of the last known follow-up date (censorship) or date of death (event), whichever occurred first. Age-adjusted all-cause mortality rates per 1,000 person-years by generation and Veteran status were calculated using the direct method. The age distribution in 5-year intervals of the whole WHI cohort was used as the standard population. Cox proportional hazards regression models that included an interaction term for Veteran status and military generation were used to examine the multivariable-adjusted association between Veteran status and all-cause mortality within each generation. Three separate models were executed to estimate hazard rate ratios (HRs) and 95% confidence intervals (CIs) for Veterans compared with non-Veterans within each generation. Model 1 adjusted for sociodemographic characteristics (age, race/ethnicity, and education) and WHI study membership. Model 2 adjusted for Model 1 covariates as well as health behaviors and health risks—smoking (pack years), physical activity, alcohol use, BMI, and depression. Model 3 adjusted for the Model 2 covariates as well as hypertension and number of comorbidities. Only women with complete non-missing data were included in the models. The percent of women excluded from the three models was approximately 1%, 8%, and 47%, respectively, with missing morbidity counts accounting for most of the Model 3 missing data. The proportionality assumption was examined using Martingale residuals, and no violations in the assumptions were noted ( Therneau, Grambsch, & Fleming, 1990 ).
Causes of death that were examined included cancer, cardiovascular disease, and trauma (accidents, suicide, homicide, and other injury). Crude and age-adjusted cause-specific mortality rates per 1,000 person-years by generation and Veteran status were calculated using the direct method of age adjustment for each cause. Within each generation, associations between Veteran status and the cause-specific mortalities were tested using Cox proportional hazards regression models, in an approach similar to the one used to examine all-cause mortality. Hypothesis tests were two sided at alpha .05, and all analyses were conducted in SAS version 9.3 (SAS Inc., Cary, NC).
Results
Sample Characteristics
Baseline characteristics of the 145,521 WHI participants included in this analysis (3,719 Veterans; 141,802 non-Veterans) differed by both Veteran status and generation ( Table 1 ). Among participants born in the pre-Vietnam generation, Veterans tended to be older and more likely to be non-Hispanic White, college educated, employed as a professional, never married, and a current or former smoker compared with non-Veterans. Among the Vietnam/after generation, compared with non-Veterans, Veterans tended to have a higher likelihood of being never married and they were more likely to smoke or have smoked, whereas the differences in race/ethnicity, education, and employment status present in the pre-Vietnam generation were attenuated.
Table 1.
Baseline Characteristics of WHI Sample by Generation and Veteran Status ( n = 145,521)
| Generation a | ||||
|---|---|---|---|---|
| Pre-Vietnam | Vietnam/After | |||
| Veteran n = 2,404 (1.7%) | Non-Veteran n = 64,209 (44.1%) | Veteran n = 1,315 (0.9%) | Non-Veteran n = 77,593 (53.3%) | |
| Age, Mean ( SD ) | 72.2 (3.7) | 69.8 (3.9) | 57.7 (4.3) | 57.8 (4.1) |
| Race/Ethnicity, n (%) | ||||
| American Indian or Alaskan Native | 9 (0.4) | 221 (0.3) | 16 (1.2) | 385 (0.5) |
| Asian or Pacific Islander | 19 (0.8) | 1,771 (2.8) | 27 (2.1) | 2,215 (2.9) |
| Black or African American | 92 (3.8) | 4,443 (6.9) | 171 (13.0) | 8,431 (10.9) |
| Hispanic/Latino | 34 (1.4) | 1,561 (2.4) | 52 (4.0) | 4,110 (5.3) |
| White | 2,213 (92.1) | 55,293 (86.1) | 1,026 (78.0) | 61,324 (79.0) |
| Other | 26 (1.1) | 730 (1.1) | 21 (1.6) | 945 (1.2) |
| Education, n (%) | ||||
| College degree or higher | 1,147 (47.7) | 23,119 (36.0) | 593 (45.1) | 32,856 (42.3) |
| Less than college degree | 1,249 (52.0) | 40,739 (63.4) | 716 (54.4) | 44,166 (57.0) |
| Employment, n (%) | ||||
| Professional | 1,191 (49.5) | 23,913 (37.2) | 637 (48.4) | 34,367 (44.3) |
| Technical | 583 (24.3) | 18,518 (28.8) | 356 (27.1) | 22,828 (29.4) |
| Service | 326 (13.6) | 11,758 (18.3) | 225 (17.1) | 13,020 (16.8) |
| Homemaker only | 244 (10.2) | 8,270 (12.9) | 69 (5.3) | 6,095 (7.9) |
| Marital status, n (%) | ||||
| Married or equivalent relationship | 1,080 (44.9) | 36,047 (56.1) | 727 (55.3) | 52,142 (67.2) |
| Widowed | 721 (30.0) | 17,587 (27.4) | 114 (8.7) | 6,771 (8.7) |
| Divorced/separated | 367 (15.3) | 7,883 (12.3) | 319 (24.3) | 14,826 (19.1) |
| Never married | 230 (9.6) | 2,470 (3.9) | 151 (11.5) | 3,593 (4.6) |
| Smoking status, n (%) | ||||
| Never | 1,094 (45.5) | 33,666 (52.4) | 537 (40.8) | 37,907 (48.9) |
| Former | 1,108 (46.1) | 26,383 (41.1) | 585 (44.5) | 32,455 (41.8) |
| Current | 146 (6.1) | 3,162 (4.9) | 171 (13.0) | 6,390 (8.2) |
| Smoking pack years, mean ( SD ) | 13.4 (22.4) | 10.5 (19.9) | 13.4 (20.9) | 9.5 (17.4) |
| Alcohol intake (drinks/week), mean ( SD ) | 2.62 (5.03) | 2.38 (4.87) | 2.34 (5.01) | 2.37 (4.91) |
| Physical activity (MET-hours/week), mean ( SD ) | 13.4 (13.7) | 12.7 (13.5) | 12.8 (14.9) | 12.3 (13.9) |
| Body mass index, n (%) | ||||
| Overweight (25.0–29.9kg/m 2 ) | 865 (36.0) | 22,994 (35.8) | 456 (34.7) | 25,530 (32.9) |
| Obese (≥30kg/m 2 ) | 606 (25.2) | 17,329 (27.0) | 462 (35.1) | 24,754 (31.9) |
| Depressive symptoms, mean ( SD ) b | 0.03 (0.10) | 0.03 (0.11) | 0.05 (0.16) | 0.05 (0.14) |
| Self-reported hypertension, n (%) | 989 (41.1) | 25,398 (39.6) | 378 (28.8) | 22,374 (28.8) |
| Morbidity count (range: 1–12), median (IQR) | 2 (1–3) | 1 (1–2) | 1 (0–2) | 1 (0–2) |
| WHI Study membership, n (%) | ||||
| E-only | 157 (6.5) | 4,239 (6.6) | 93 (7.1) | 4,656 (6.0) |
| E+P | 256 (10.7) | 6,334 (9.9) | 141 (10.7) | 7,570 (9.8) |
| DM | 438 (18.2) | 12,961 (20.2) | 332 (25.3) | 20,433 (26.3) |
| OS | 1,553 (64.6) | 40,675 (63.4) | 749 (57.0) | 44,934 (51.1) |
Notes: DM = Dietary Modification trial; E+P = Estrogen plus Progestin trial; E-only = Estrogen-Alone trial; IQR = inter-quartile range; MET = metabolic equivalent; OS = observational study; SD = standard deviation; WHI = Women’s Health Initiative.
a Numbers may not add to totals or percents to 100 due to missing data.
b Range 0–0.99, with higher scores indicating greater depressive symptomology, and score ≥0.06 indicative of meeting criteria for depressive symptoms.
All-cause Mortality
Over a mean ( SD ) follow-up period of 15.2 (3.4) years, there were 29,589 deaths ( Table 2 ). In the pre-Vietnam generation, the age-adjusted all-cause mortality rate was 21.7 per 1,000 person-years for Veterans and 18.9 for non-Veterans. Adjusting for age, sociodemographics, and WHI study membership, the rate of mortality was 16% higher for Veterans compared with non-Veterans (Model 1: HR = 1.16, 95% CI 1.09–1.23). Additional adjustment for health behaviors and health risks did not change these findings (Model 2: HR = 1.14, 95% CI 1.07–1.21); however, further adjustment for hypertension and comorbidity eliminated Veterans’ excess mortality difference (Model 3: HR = 1.08, 95% CI 0.98–1.19). In the Vietnam/after generation, the age-adjusted all-cause mortality rate was 9.2 per 1,000 person-years for Veterans and 7.9 for non-Veterans. Adjusting for age, sociodemographics, and WHI study membership, Veterans had an excess mortality of 16% that was not statistically significant (Model 1: HR = 1.16, 95% CI 0.99–1.36). Further adjustment attenuated this mortality difference (Models 2 and 3).
Table 2.
All-cause Mortality Rates by Generation and Veteran status
| Generation | Veteran status | Number of deaths | Total person-years | Rate per 1,000 person-years | Age-adjusted rate | Model 1 | Model 2 | Model 3 |
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||||||
| Pre-Vietnam | Veteran | 1,051 | 32,829.81 | 32.01 | 21.71 | 1.16 (1.09–1.23) | 1.14 (1.07–1.21) | 1.08 (0.98–1.19) |
| Non-Veteran | 20,430 | 919,014.53 | 22.23 | 18.92 | Ref | Ref | Ref | |
| Vietnam/After | Veteran | 155 | 20,595.51 | 7.53 | 9.18 | 1.16 (0.99–1.36) | 1.08 (0.92–1.27) | 1.09 (0.86–1.37) |
| Non-Veteran | 7,953 | 1,217,955.23 | 6.53 | 7.91 | Ref | Ref | ref |
Notes: BMI = body mass index; CI = confidence interval; HR = hazard ratio; WHI = Women’s Health Initiative.
Only women with complete, non-missing data were included in the models. p > .05 for interaction of military generation and Veteran status in all models.
Model 1 (sociodemographics and WHI study membership) adjusted for age, race/ethnicity, education, and WHI study membership.
Model 2 (+ risk factors and behaviors) adjusted for all variables in Model 1, plus smoking (pack years), physical activity, alcohol use, BMI, and depression.
Model 3 (+ comorbidities) adjusted for all variables in Model 2, plus hypertension, and medical comorbidities.
Cause-specific Mortality
Cancer, cardiovascular disease, and trauma accounted for 64% of the observed deaths in the sample ( Table 3 ). In the pre-Vietnam generation, adjusting for age, sociodemographics, and WHI study membership, the mortality rate due to cancer was 13% higher for Veterans compared with non-Veterans (Model 1: HR = 1.13, 95% CI 1.00–1.29). This difference was attenuated after adjustment for health behaviors and health risks (Model 2) and with further adjustment (Model 3). The mortality rate due to cardiovascular disease was 12% higher for pre-Vietnam generation Veterans compared with non-Veterans (Model 1: HR = 1.12, 95% CI 1.00–1.25), and this difference persisted with adjustment for health behaviors and health risks (Model 2) but not with further adjustment for comorbidity (Model 3). The mortality rate due to trauma was 45% higher for Veterans compared with non-Veterans (Model 1: HR = 1.45, 95% CI 1.03–2.06), and this difference also persisted with adjustment for health behaviors and health risks but not with further adjustment for comorbidity. Vietnam/after generation Veterans and non-Veterans did not differ in their cause-specific cancer or cardiovascular mortality rates. By contrast, trauma-related mortality was greater in Vietnam/after generation Veterans than in non-Veterans (Model 1: HR = 2.93, 95% CI 1.64–5.23). In the fully adjusted model, Veterans’ excess mortality was increased further (Model 3: HR = 3.93, 95% CI 1.83–8.43).
Table 3.
Prevalent Sources of Mortality and Cause-specific Mortality Rates by Generation and Veteran Status
| Generation and cause of death | Veteran status | Number of deaths | Total person-years | Rate per 1,000 person-years | Age-adjusted rate | Model 1 | Model 2 | Model 3 |
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||||||
| Pre-Vietnam generation | ||||||||
| Cancer | Veteran | 254 | 32,829.81 | 7.74 | 6.82 | 1.13 (1.00–1.29) | 1.09 (0.96–1.25) | 1.02 (0.84–1.24) |
| Non-Veteran | 5,501 | 919,014.53 | 5.99 | 5.57 | Ref | Ref | Ref | |
| CVD | Veteran | 347 | 32,829.81 | 10.57 | 6.89 | 1.12 (1.00–1.25) | 1.13 (1.01–1.27) | 1.11 (0.94–1.30) |
| Non-Veteran | 6,629 | 919,014.53 | 7.21 | 5.84 | Ref | Ref | Ref | |
| Trauma | Veteran | 34 | 32,829.81 | 1.04 | 0.83 | 1.45 (1.03–2.06) | 1.46 (1.02–2.10) | 1.01 (0.54–1.91) |
| Non-Veteran | 546 | 919,014.53 | 0.59 | 0.50 | Ref | Ref | Ref | |
| Vietnam / After Generation | ||||||||
| Cancer | Veteran | 65 | 20,595.51 | 3.16 | 2.90 | 1.07 (0.84–1.37) | 1.00 (0.78–1.29) | 0.98 (0.69–1.38) |
| Non-Veteran | 3,604 | 1,217,955.23 | 2.96 | 3.41 | Ref | Ref | Ref | |
| CVD | Veteran | 29 | 20,595.51 | 1.41 | 2.57 | 0.98 (0.68–1.42) | 0.81 (0.54–1.22) | 0.82 (0.47–1.46) |
| Non-Veteran | 1,739 | 1,217,955.23 | 1.43 | 1.84 | Ref | Ref | Ref | |
| Trauma | Veteran | 12 | 20,595.51 | 0.58 | 0.43 | 2.93 (1.64–5.23) | 2.90 (1.58–5.31) | 3.93 (1.83–8.43) |
| Non-Veteran | 244 | 1,217,955.23 | 0.20 | 0.25 | Ref | Ref | Ref | |
Notes : BMI = body mass index; CI = confidence interval; CVD = cardiovascular disease; HR = hazard ratio; WHI = Women’s Health Initiative.
Only women with complete, non-missing data were included in the models. p > .05 for interaction of military generation and Veteran status in all cancer and CVD models; for trauma models, p values for interaction were .04, .06, and .007 for Models 1, 2, and 3, respectively.
Model 1 (sociodemographics and WHI study membership) adjusted for age, race/ethnicity, education, and WHI study membership.
Model 2 (+ risk factors and behaviors) adjusted for all variables in Model 1, plus smoking (pack years), physical activity, alcohol use, BMI, and depression.
Model 3 (+ comorbidities) adjusted for all variables in Model 2, plus hypertension, and medical comorbidities.
Discussion
Pre-Vietnam Generation Mortality Differences by Veteran Status
Within each military generation, we found distinct associations between Veteran status and cause-specific mortality rates. Among women of the older, pre-Vietnam generation, Veterans had an all-cause mortality rate that was 16% higher than that of non-Veterans, even after adjusting for differences in age, other sociodemographic characteristics, and WHI study membership. This excess mortality rate persisted after further adjustment for health behaviors and health risks, for example, Veteran women’s higher prevalence of smoking, but not after additional adjustment for baseline hypertension and medical comorbidities. Thus, the higher mortality rates of Veterans compared with non-Veterans of the pre-Vietnam generation were explained by hypertension and compounding medical comorbidities later in life.
We found excess cancer-specific, cardiovascular, and trauma-related mortality for women Veterans in the pre-Vietnam generation. The higher education level of pre-Vietnam era women Veterans was not protective. Compared with non-Veterans, women Veterans of that generation smoked more, and their excess cancer-specific mortality rate was attenuated with adjustment for health behaviors and health risks, whereas cardiovascular and trauma-related mortality rates were attenuated only with further comorbidity adjustment.
Our findings for the older generation women are consistent with prior work ( Bross & Bross, 1987 ; Weitlauf et al., 2015 ) which suggests a diminution of the healthy soldier effect over time. Specifically, as rigorous health standards associated with military selection are not applied to the general population, findings associated with the robust good health of Veterans relative to non-Veterans in early and midlife may be the result of a healthy selection bias—in which a health selected military population is compared with the general public for whom no such selection bias exists ( Bross & Bross, 1987 ). There is evidence in the literature based on studies of men that the healthy soldier effect attenuates with time such that mortality rates in Veterans increase in middle-to-late older adulthood, eventually surpassing (crossing over) that of their non-Veteran counterparts ( Waller, & McGuire, 2011 ; Wilmoth, London, & Parker, 2010 ). Our finding of elevated all-cause and selected cause-specific mortality rates in pre-Vietnam generation women Veterans is particularly striking, because pre-Vietnam generation women Veterans, compared with non-Veterans, had sociodemographic characteristics that are typically associated with a more favorable health profile (White race, college degree, employment as a professional). It had not been tested whether the attenuation of the healthy soldier effect observed in those prior studies also occurred among women in these military generations. Thus, our findings complement and extend the literature characterizing and explaining Veterans’ mortality, specifically in women Veterans.
An alternate explanation for our findings is that, independent of a potential healthy soldier effect, there is an independent effect of Veteran status on mortality. Findings from other WHI studies suggest that the WHI sample may be disproportionately weighted toward healthier older women ( Ma et al., 2013 ), therefore our comparison of postmenopausal women Veterans with similar age non-Veteran peers who were health selected into WHI may reflect a more accurate picture of women Veterans’ mortality rate than has been presented in studies that did not include health-matched non-Veterans.
Vietnam/After Generation Mortality Differences by Veteran Status
Conversely, among women in the younger Vietnam/after generation, Veterans had an all-cause mortality rate that was also 16% higher than non-Veterans, after adjusting for age, other sociodemographics, and WHI study membership, however, this difference was not statistically significant. Additional adjustment for health behaviors and health risks attenuated the excess mortality rate, which remained statistically similar between Veterans and non-Veterans. Further adjustment for baseline medical comorbidities and hypertension did not alter this finding.
Though trauma was not a common cause of death, there was a strikingly higher trauma-related mortality rate among Vietnam/after generation Veterans. Adjusting for the greater health risk behaviors and comorbidities in Vietnam/after women Veterans compared with non-Veterans unmasked an even greater excess trauma-related mortality rate. This finding reflects what others have found in studies of Vietnam era women Veterans ( Cypel & Kang, 2008 ; Kang et al., 2014 ; Thomas, Kang, & Dalager, 1991 ). Military occupational roles were greatly expanded for women serving during the Vietnam era and later, increasing opportunities for long-term (career military) service ( Gruhzit-Hogt, 1999 ; Steinman, 2000 ), which increased women’s opportunities for overseas deployment, warzone exposure, and other occupational health risks associated with military service ( Washington, Davis, et al., 2013 ). Though not measured in the WHI, this generation of military women was the first to report significant prevalence of exposure to military sexual trauma, and not surprisingly, high prevalence of comorbid depression, PTSD, and substance use disorders ( Cypel & Kang, 2008 ; Hoggatt, et al., 2015 ; Washington, Davis, et al., 2013 ). Some postulate injury mortality as a consequence of psychiatric conditions developed after the war, and postwar adoption of behaviors such as heavy drinking that may increase injury risk, as proposed pathways for excess trauma in Vietnam/after generation Veterans ( Bell, Amoroso, Wegman, & Senier, 2001 ). Using a life-span perspective on combat exposure and PTSD symptoms in later life, the VA Normative Aging Study found that prewar, warzone, and postwar factors all had an effect on later life PTSD symptoms ( Kang, Aldwin, Choun, & Spiro, 2015 ). Using WHI data, others have found a heightened prevalence of sleep disturbances, including combined insomnia and sleep-disordered breathing in women Veterans relative to non-Veterans, which would be expected to be directly linked to increased risk of accidents and trauma ( Rissling et al, in press ). Understanding how to reduce this increased trauma-related mortality risk could be very important in the care of this and future cohorts of older women Veterans.
Our findings in the Vietnam/after generation on the association of Veteran status with all-cause mortality differ from what others have found. Prior literature on female Vietnam cohorts showed diminished mortality rates relative to non-Veterans ( Kang et al., 2014 ). We found equivalence, with a trend in the opposite direction. However, we examined Vietnam/after generation women Veterans at an older age than that reported on in most other studies. If diminution of the healthy soldier effect was in play for the Vietnam/after generation, as we postulate it is for the older generation, then we would expect higher mortality rates to persist after adjusting for health behaviors and health risks, but this was not the case. This study had a health selected comparison group, rather than historical, U.S. general population controls. In contrast to prior studies, this study also accounted for a wider range of potential confounding variables (including the higher prevalence of smoking and overweight or obesity in Vietnam/after generation Veterans compared with non-Veterans). We found variability across generations in the effect of Veteran status on mortality rates, which underscores the value of appropriate comparisons.
Limitations
Several limitations must be considered in the interpretation of the results. Because of the age of women at the time of enrollment into WHI, there is likely an inherent selection bias that favors survival of non-Veterans, compared with what may have been found if women were examined at an earlier life stage. Studies that have examined women at an earlier life stage found a higher mortality rate among non-Veterans prior to age 50 ( Kang et al., 2014 ). However, there is a health selection bias into the military, so the current study’s age-related selection bias may have created cohorts that are more appropriately comparable. Nonetheless, the associations we found between Veteran status and mortality are applicable to postmenopausal women and do not necessarily generalize to women in earlier life phases.
Although this analysis is centered on the assumption that military generation is a meaningful way to categorize Veteran participants within WHI, our strategy for assigning military generation is imperfect, but it reflects our best estimate of Veteran participants’ era of service possible. In the National Survey of Women Veterans (NSWV), a large population-based cross-sectional study, there were differences in women Veterans’ military factors and health behaviors by military era ( Washington, Farmer, et al., 2015 ), though Korea era women Veterans shared many similarities with WWII era women. Due to the overlapping nature of service in the WWII and Korea, the current analysis uses two military generations in place of the corresponding three military eras (WWII, Korea, and Vietnam) that the NSWV used for these birth cohorts. Given that there are no contextual details about women’s military service provided in WHI, our findings should be interpreted within the limitation that military generation was not determined based upon primary knowledge of when, where, or in what capacity Veteran participants participated in military service. Though this study’s Vietnam/after generation includes those who are age consistent with Vietnam era military service, some Veterans may have served post-Vietnam, limiting comparisons with studies of Vietnam era cohorts. We did not have detailed information about women Veterans’ military exposures and associated health outcomes and therefore could not ascertain the influence of military sexual trauma and PTSD on mortality rates. Given their smaller sample size, we may have been limited in our ability to detect small differences in mortality rates in the Vietnam/after generation, with 95% CI’s containing potentially clinically relevant differences. However, we did detect more robust Veteran/non-Veteran differences, specifically for trauma-related mortality.
Balancing these limitations, the present study has several strengths. It included a large national sample of postmenopausal women whose health has been closely assessed over an average of 15.2 years as well as the largest cohort of older women Veterans identified in a national study of women’s health. The prospective design and many adjudicated outcomes enabled an examination of mortality differences by Veteran status and generation, with appropriate adjustment for potential confounders.
Implications for Policy and Practice
This study is one of the few assessments of women Veterans’ mortality rates compared with non-Veterans, and it provides new information on women Veterans’ all-cause and cause-specific mortality within military generations. Younger (Vietnam/after generation) women had a higher prevalence of unhealthy behaviors—more smoking, less physical activity, and higher BMI—than older (pre-Vietnam generation) women. It is unclear whether these represent postmilitary generational differences that persisted into postmenopausal life, an aging effect (because people tend to quit smoking with age), or a survival effect, where older women with these behaviors died before being included in the WHI. Whichever the cause, there were different associations between being a Veteran and mortality rates between the two generations. By comparing health selected samples, we were able to directly assess whether women Veterans are at this age experiencing a lingering selection advantage. Our findings support the theory of a healthy soldier effect that attenuates with time for older military generations, such that Veterans have higher mortality rates later in life. Our findings are also suggestive of an independent risk conferred by Veteran status. Per our conceptual model, military-related factors, health risks, and comorbidity are all in the causal pathways affecting mortality. Adjusting for these factors (as we did in Models 2 and 3) attenuated the differences as expected and helps to provide the context for the main effect of Veteran status we found (in Model 1). Although it is not clear whether Veterans’ worse health is attributable to military service or their lives in subsequent years, it is clear that any health advantage had waned even among the younger Vietnam/after generation group and that there are particular causes of mortality for which these women have experienced excess mortality compared with the non-Veteran women.
Military service may have both positive and negative effects. This study demonstrates the importance of examining health protective factors and health risks within the context of military generation. Future research should examine trajectories in health behaviors and the association of these trajectories with women Veterans’ higher mortality rates. Our findings reinforce the need to address health risk behaviors for women Veterans of all military generations and highlight the need to target these health screenings and preventive efforts to each generation’s health risks.
Funding
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201 100003C, HHSN268201100004C, and HHSN271201100004C. The research reported here was supported by Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service FOP14-439 and the VA Office of Women’s Health.
Acknowledgments
The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
References
- Anderson G. L. Manson J. Wallace R. Lund B. Hall D. Davis S. , & Prentice R. L . ( 2003. ). Implementation of the Women’s Health Initiative study design . Annals of Epidemiology , 13 ( 9 Suppl .), S5 – S17 . doi: 10.1016/S1047-2797(03)00043-7 [DOI] [PubMed] [Google Scholar]
- Batuman F. Bean-Mayberry B. Goldzweig C. Huang C. Miake-Lye I. M. Washington D. L. , … Shekelle P. G . ( 2011. ) Health Effects of Military Service on Women Veterans (VA Evidence-based Synthesis Program Reports 05-226). Washington, DC: : Department of Veterans Affairs; . [PubMed] [Google Scholar]
- Bean-Mayberry B. Yano E. M. Washington D. L. Goldzweig C. Batuman F. Huang C. Miake-Lye I. , & Shekelle P. G . ( 2011. ). Systematic review of Women Veterans’ Health: Update on gaps and successes . Women’s Health Issues , 21 ( 4 Suppl .), S84 – S97 . doi: 10.1016/j.whi.2011.04.022 [DOI] [PubMed] [Google Scholar]
- Bell N. Amoroso P. Wegman D. , & Senier L . ( 2001. ). Proposed explanations for excess injury among veterans of the Persian Gulf War and a call for greater attention from policymakers and researchers . Injury Prevention , 7 , 4 – 9 . doi: 10.1136/ip.7.1.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertone-Johnson E. R. Powers S. I. Spangler L. Larson J. Michael Y. L. Millen A. E. , … Manon J. E . ( 2012. ). Vitamin D supplementation and depression in the Women’s Health Initiative Calcium and Vitamin D Trial . American Journal of Epidemiology , 176 , 1 – 13 . doi: 10.1093/aje/kwr482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bross I. D. , & Bross N. S . ( 1987. ). Do atomic Veterans have excess cancer? New results correcting for the healthy soldier bias . American Journal of Epidemiology , 126 , 1042 – 1050 . [DOI] [PubMed] [Google Scholar]
- Bird, C. E., & Rieker, P. P. (2008). Gender and health: the effects of constrained choices and social policies . New York, NY: Cambridge University Press. ISBN-13: 978-0-5218-6415-2 [Google Scholar]
- Burnam M. A. Wells K. B. Leake B. , & Landsverk J . ( 1988. ). Development of a brief screening instrument for detecting depressive disorders . Med Care , 26 , 775 – 789 . [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) . Tobacco use—United States, 1900–1999 . ( 1999. ). Morbidity and Mortality Weekly Report , 48 , 986 – 993 . [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) . Classification of diseases, functioning, and disability . www.cdc.gov/nchs/icd.htm . Accessed May 22, 2015 .
- Curb J. D. McTiernan A. Heckbert S. R. Kooperberg C. Stanford J. Nevitt M. , … Daugherty S . ( 2003. ). Outcomes ascertainment and adjudication methods in the Women’s Health Initiative . Annals of Epidemiology , 13 ( 9 Suppl .), S122 – S128 . doi: 10.1016/S1047-2797(03)00048-6 [DOI] [PubMed] [Google Scholar]
- Cypel Y. , & Kang H . ( 2008. ). Mortality patterns among women Vietnam-era Veterans: Results of a retrospective cohort study . Annals of Epidemiology , 18 , 244 – 252 . doi: 10.1016/j.annepidem.2007.11.009 [DOI] [PubMed] [Google Scholar]
- Der-Martirosian C. Cordasco K. M. , & Washington D. L . ( 2013. ). Health-related quality of life and comorbidity among older women Veterans in the United States . Quality of Life Research , 22 , 2749 – 2756 . doi: 10.1007/s11136-013-0424-7 [DOI] [PubMed] [Google Scholar]
- Gruhzit-Hogt M . ( 1999. ). A time remembered: American women in the Vietnam War . New York, NY: : Presidio Press; . [Google Scholar]
- Hays J. Hunt J. R. Hubbell F. A. Anderson G. L. Limacher M. Allen C. , & Rossouw J. E . ( 2003. ). The Women’s Health Initiative recruitment methods and results . Annals of Epidemiology , 13 ( 9 Suppl .), S18 – S77 . doi: 10.1016/S1047-2797(03)00042-5 [DOI] [PubMed] [Google Scholar]
- Hoggatt K. J. Williams E. C. Der-Martirosian C. Yano E. M. , & Washington D. L . ( 2015. ). National prevalence and correlates of alcohol misuse in women Veterans . Journal of Substance Abuse Treatment , 52 , 10 – 16 . doi: 10.1016/j.jsat.2014.12.003 [DOI] [PubMed] [Google Scholar]
- Kang H. K. Cypel Y. Kilbourne A. M. Magruder K. M. Serpi T. Collins J. F. , … Spiro A. III . ( 2014. ). Mortality study of female US Vietnam Era Veterans, 1965–2010 . American Journal of Epidemiology , 15 , 721 – 730 . doi: 10.1093/aje/kwt319 [DOI] [PubMed] [Google Scholar]
- Kang S. Aldwin C. M. Choun S. , & Spiro A. III ( 2015. ). A life-span perspective on combat exposure and PTSD symptoms in later life: Findings from the VA Normative Aging Study . The Gerontologist . Advance online publication. doi: 10.1093/geront/ gnv120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehavot K. Hoerster K. D. Nelson K. M. Jakupcak M. , & Simpson T. L . ( 2012. ). Health indicators for military, veteran, and civilian women . American Journal of Preventive Medicine , 42 , 473 – 480 . doi: 10.1016/j.amepre.2012.01.006 [DOI] [PubMed] [Google Scholar]
- Ma Y. Hébert J. R. Balasubramanian R. Wedick N. M. Howard B. V. Rosal M. C. , … Manson J. E . ( 2013. ). All-cause, cardiovascular, and cancer mortality rates in postmenopausal white, black, Hispanic, and Asian women with and without diabetes in the United States: The Women’s Health Initiative, 1993–2009 . American Journal of Epidemiology , 178 , 1533 – 1541 . doi: 10.1093/aje/kwt177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin R. Nielsen L. , & Waller M . ( 2008. ). An evaluation of the effect of military service on mortality: Quantifying the healthy soldier effect . Annals of Epidemiology , 18 , 928 – 936 . doi: 10.1016/j.annepidem.2008.09.002 [DOI] [PubMed] [Google Scholar]
- Murdoch M. Bradley A. Mather S. H. Klein R. E. Turner C. L. , & Yano E. M . ( 2006. ). Women and war. What physicians should know . Journal of General Internal Medicine , 21 ( Suppl. 3 ), S5 – S10 . doi: 10.1111/j.1525-1497.2006.00368.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offen N. Smith E. A. , & Malone R. E . ( 2013. ). “They’re going to die anyway”: smoking shelters at Veterans’ facilities . American Journal of Public Health , 103 , 604 – 612 . doi: 10.2105/AJPH.2012.301022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rillamas-Sun E. LaCroix A. Z. Bell C. L. Ryckman K. Ockene J. K. , & Wallace R. B . (in press). The impact of multimorbidity and coronary disease comorbidity on physical function in women aged 80 years and older: The Women’s Health Initiative . Journal of Gerontology: Medical Sciences . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissling, M. B., Gray, K. E., Ulmer, C., Martin, J., Zaslavsky, O., Gray, S. L., . . ., & Weitlauf, J. C. (in press). The Gerontologist , 56 , S54 – S56 . doi: 10.1093/geront/gnv668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert Wood Johnson Foundation . ( 2011. ). Social norms and attitudes about smoking 1991–2010 . Princeton, NJ: : Robert Wood Johnson Foundation; . [Google Scholar]
- Seeman T. E. , & Crimmins E . ( 2001. ). Social environment effects on health and aging: integrating epidemiologic and demographic approaches and perspectives . Annals of the New York Academy of Sciences , 954 , 88 – 117 . doi: 10.1111/j.1749–6632.2001.tb02749.x [DOI] [PubMed] [Google Scholar]
- Sharkansky E. J. King D. W. King L. A. Wolfe J. Erickson D. J. , & Stokes L. R .( 2000. ). Coping with Gulf War combat stress: mediating and moderating effects . Journal of Abnormal Psychology , 109 , 188 – 197 . doi: 10.1037/0021-843X.109.2.188 [PubMed] [Google Scholar]
- Smith E. A. , & Malone R. E . ( 2009. ). “Everywhere the soldier will be”: Wartime tobacco promotion in the US military . American Journal of Public Health , 99 , 1595 – 1602 . doi: 10.2105/AJPH.2008.152983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R . ( 2000. ). Women in the Vietnam war . New York, NY: : Simon and Schuster (TV books) . [Google Scholar]
- Therneau T. M. Grambsch P. M. , & Fleming T. R . ( 1990. ). Martingale-based residuals for survival models . Biometrika , 77 , 147 – 160 . [Google Scholar]
- Thomas T. L. Kang H. K. , & Dalager N. A . Mortality among women Vietnam Veterans, 1973–1987 . ( 1991. ). American Journal of Epidemiology , 134 , 973 – 980 . [DOI] [PubMed] [Google Scholar]
- U.S. Department of Veterans Affairs, National Center for Veterans Analysis and Statistics . ( 2014. ). Veteran Population. The Veteran Population Projection Model 2014 (VetPop2014) . Retrieved November 07, 2014 , from http://www.va.gov/vetdata/veteran_population.asp [Google Scholar]
- Waller M. , & McGuire A. C. L . ( 2011. ). Changes over time in the “healthy solider effect.” Population Health Metrics , 9 , 7 . doi: 10.1186/1478-7954-9-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington D. L. Bean-Mayberry B. Hamilton A. B. Cordasco K. M. , & Yano E. M . ( 2013. ). Women Veterans’ healthcare delivery preferences and use by military service era: Findings from the National Survey of Women Veterans . Journal of General Internal Medicine , 28 ( Suppl. 2 ), S571 – S576 . doi: 10.1007/s11606-012-2323-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington D. L. Davis T. D. Der-Martirosian C. , & Yano E. M . ( 2013. ). PTSD risk and mental health care engagement in a multi-war era community sample of women Veterans . Journal of General Internal Medicine , 28 , 894 – 900 . doi: 10.1007/s11606-012-2303-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington D. L. Farmer M. M. Mor S. S. Canning M. , & Yano E. M . ( 2015. ). Assessment of the health care needs and barriers to VA use experienced by women Veterans: Findings from the National Survey of Women Veterans . Medical Care , 53 ( 4 Suppl. 1 ), S23 – S31 . doi: 10.1097/MLR.0000000000000312 [DOI] [PubMed] [Google Scholar]
- Weitlauf J. C. LaCroix A. Z. Bird C. E. Woods N. F. Washington D. L. Katon J. G. , … Stefanick M. L . ( 2015. ). Prospective analysis of health and mortality risk in Veteran and non-Veteran participants in the Women’s Health Initiative . Women’s Health Issues , 25 , 648 – 656 . doi: 10.1016/j.whi.2015.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmoth J. M. London A. S. , & Parker W. M . ( 2010. ). Military service and men’s health trajectories in later life . The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences , 65 , 744 – 755 . doi: 10.1093/geronb/gbq072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Women’s Health Initiative Study Group . ( 1998. ). Design of the Women’s Health Initiative clinical trial and observational study . Controlled Clinical Trials , 19 , 61 – 109 . doi: 10.1016/S0197-2456(97)00078-0 [DOI] [PubMed] [Google Scholar]