Abstract
Purpose of the Study:
To compare the prevalence and cardiometabolic health impact of sleep disturbance among postmenopausal Veteran and non-Veteran participants in the Women’s Health Initiative (WHI).
Design and Methods:
The prevalence of five categories of sleep disturbance—medication/alcohol use for sleep; risk for insomnia; risk for sleep disordered breathing [SDB]; risk for comorbid insomnia and SDB (insomnia + SDB); and aberrant sleep duration [SLD]—was compared in 3,707 Veterans and 141,354 non-Veterans using logistic or multinomial regression. Cox proportional hazards models were used to evaluate the association of sleep disturbance and incident cardiovascular disease (CVD) and Type 2 diabetes in Veterans and non-Veterans.
Results:
Women Veterans were more likely to have high risk for insomnia + SDB relative to non-Veteran participants. However, prevalence of other forms of sleep disturbance was similar across groups. Baseline sleep disturbance was not differentially associated with cardiometabolic health outcomes in Veteran versus non-Veteran women. Risks for SDB and insomnia + SDB were both linked to heightened risk of CVD and diabetes; SLD was consistently linked with greater risk of CVD and diabetes in non-Veterans but less strongly and consistently in Veterans.
Implications:
Efforts to identify and treat sleep disturbances in postmenopausal women are needed and may positively contribute to the attenuation of cardiometabolic morbidity risk. Increased awareness of women Veterans’ vulnerability to postmenopausal insomnia + SDB may be particularly important for health care providers who treat this population.
Keywords: Women, Veterans, Sleep, Postmenopausal Health
Cardiometabolic morbidity and mortality are salient health concerns among postmenopausal women, including Veterans, whose risk may be further impacted by their high prevalence of smoking ( Hoerster et al., 2012 ), posttraumatic stress disorder (PTSD; Hughes, Jouldjian, Washington, Alessi, & Martin, 2013 ), and sleep disturbances ( Hoerster et al., 2012 ). Given the fact that there are nearly 1 million women Veterans (WVs) aged 50 or older currently living in the United States (U.S. Department of Veterans Affairs, 2014), it is surprising that no extant research has examined the association of sleep and postmenopausal cardiometabolic morbidity in this population. The present work is a first step toward addressing this literature gap.
Cardiometabolic Risk and Morbidity Among Military Populations
Several prior studies document a higher prevalence of cardiometabolic risk factors, including tobacco use, obesity, and metabolic abnormalities in both military and Veteran populations relative to non-Veterans ( Hoerster et al., 2012 ; McKinney, McIntire, Carmody, & Joseph, 1997 ; Weitlauf et al., 2015 ). Moreover, gender-specific research on this topic suggests a 42% prevalence of hypertension, a 39% prevalence of hyperlipidemia, and an 18% prevalence of diabetes among postmenopausal WVs aged 55–64 years (c.f., Vimalananda et al., 2013 ). Military occupational hazards, including war zone deployment, combat, or military sexual trauma, may also impact cardiometabolic morbidity via sleep disruption ( Frayne et al., 1999 ; Frayne, Skinner, Sullivan, & Freund, 2003 ). Specifically, PTSD symptoms, including hyperarousal and the experience of nightmares, may increase nocturnal awakenings or arousals from sleep, resulting in sleep fragmentation. This may both generally decrease sleep quality and ultimately compound risk for chronic illness. Specifically, PTSD may contribute to increased risk of metabolic syndrome in Veterans, including women, via neuro-endocrine-immune abnormalities ( Heppner et al., 2009 ). Sleep fragmentation may also increase upper airway collapsibility and contribute to the increased risk of SDB in individuals with PTSD ( Krakow, Ulibarri, Moore, & McIver, 2015 ). These potential mechanisms underscore the importance of research that addresses modifiable risk factors that may improve cardiometabolic health in individuals exposed to military occupational hazards (c.f., Rosenbaum et al., 2015 ).
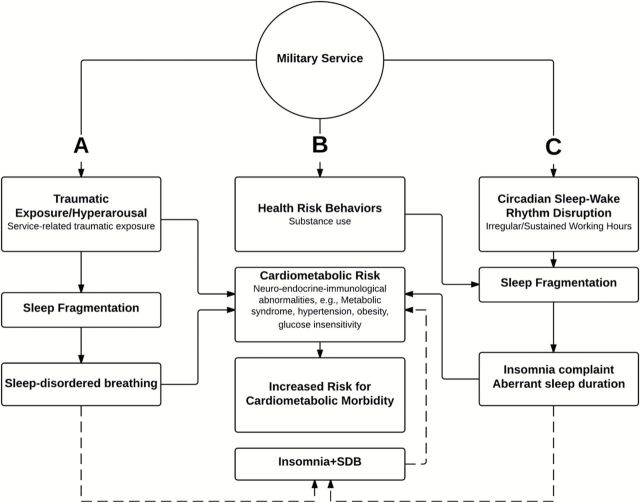
Figure 1 presents a conceptual model of three pathways from military service to cardiometabolic risk, morbidity and mortality based, in part, on Seeman and Crimmins’ biopsychosocial model of aging (2001).
Figure 1.
Conceptual framework adapted from biopsychosocial model of healthy aging ( Seeman & Crimmins, 2001 ). We propose three pathways from military service to cardiometabolic morbidity via sleep disturbance in postmenopausal women veterans: ( A ) traumatic exposure/hyperarousal and associated sleep-disordered breathing (SDB); ( B ) health risk behaviors; and ( C ) circadian sleep–wake rhythm disruption and associated insomnia complaint or aberrant sleep duration. The dashed line represents the hypothesized association of comorbid insomnia and SDB on cardiometabolic morbidity.
Military Service and Sleep Disturbance Risk
Military personnel and Veterans have four times greater risk for sleep disturbance than the general population (Armed Forces Health Surveillance Center, 2010a, 2010b; Faestel, Littell, Vitiello, Forsberg, & Littman, 2013 ). Although most prior research has focused on men, early work describes an alarming prevalence of insomnia complaints among military (Armed Forces, 2010a) and Veteran women ( Fung et al., 2013 ; Hughes et al., 2013 ). High rates of aberrant sleep duration (SLD) have also been documented in Veterans ( Swinkels, Ulmer, Beckham, Buse, & Registry, 2013 ). This is cause for concern as aberrant SLD, particularly very short sleep, is strongly associated with increased cardiometabolic risk and has been linked with smoking, obesity, hypertension, diabetes, and mental health conditions (e.g., PTSD and depression), all of which are prevalent among Veterans, including women ( Stranges et al., 2008 ). The prevalence of use of sleep aids (e.g., medication or alcohol for sleep) among Veterans has not been fully investigated; however, a sharp uptick in use of sleep aids among postmenopausal women in general is well documented ( Kaneita et al., 2007 ; Quera-Salva, Orluc, Goldenberg, & Guilleminault, 1991 ).
Military occupational risks, such as prolonged deployment or duty assignments that severely restrict sleep, are a primary precursor to circadian rhythm dysfunction, sleep fragmentation, sleep dissatisfaction, insomnia, or aberrant SLD in military and Veteran populations ( Figure 1 ; Dijk & Czeisler, 1994 ; Faestel et al., 2013 ). High rates of sleep-interfering health risk behaviors, for example, tobacco, alcohol ( Hoerster et al., 2012 ; Swinkels et al., 2013 ), and military-related traumatic exposures and resultant PTSD may also interfere with sleep ( Jordan et al., 1991 ; Lavie, 2001 ). As mentioned previously, sleep fragmentation is a candidate mechanism for the higher prevalence of sleep disordered breathing (SDB) in individuals with PTSD ( Figure 1 ; Krakow et al., 2015 ). Taken together, the number of sleep-interfering exposures of Veteran women may increase risk for insomnia, SDB, and their combination: comorbid insomnia + SDB.
Sleep Disturbance and Cardiometabolic Risk
Poor sleep associated with insomnia or SDB has been empirically linked to many cardiometabolic consequences including obesity, hypertension, and impaired glucose metabolism ( Grandner, Sands-Lincoln, Pak, & Garland, 2013 ; Knutson, 2012 ) as well as accelerated morbidity ( Figure 1 ; Cappuccio, Cooper, D’Elia, Strazzullo, & Miller, 2011 ; Knutson, 2012 ; Loke, Brown, Kwok, Niruban, & Myint, 2012 ; Sands et al., 2013 ; Sands-Lincoln et al., 2013 ). When SDB and insomnia co-occur—insomnia + SDB—these health risks may be intensified ( Fung et al., 2013 ; Luyster et al., 2014 ; Ong & Crawford, 2013 ). Given the heightened prevalence of insomnia, SDB, and health risk behaviors that compound sleep and cardiometabolic health problems, comorbid insomnia + SDB may be a particularly important health risk among postmenopausal WVs.
To address this possibility, the present study will utilize data drawn from the Women’s Health Initiative (WHI) to comparatively examine the prevalence and consequences of five categories of sleep disturbance, including use of medication/alcohol for sleep, aberrant SLD, risk for insomnia, risk for SDB, and comorbid insomnia + SDB risk, on the postmenopausal cardiometabolic health among nearly 4,000 postmenopausal WV participants and more than 140,000 non-Veteran participants in the WHI. We hypothesize that WVs will have greater likelihood of all five sleep disturbance categories at baseline relative to their non-Veteran counterparts. We will also examine whether the association of sleep disturbance on incident cardiovascular disease (CVD) and Type 2 diabetes during the 20-year follow-up vary as a function of Veteran status.
Design and Methods
Study Population
The study population was a subset of the 161,808 Veteran and non-Veteran participants from the Clinical Trial (CT) and Observational Study of the WHI, a longitudinal study of postmenopausal health and mortality risks in women aged 50–79 years at enrollment ( Anderson et al., 2003 ; Hays et al., 2003 ; The Women’s Health Initiative Study Group, 1998). The study population of the WHI was recruited nationally between 1993 and 1998 through 40 clinical centers and is representative of the racial/ethnic and geographic diversity of the U.S. women population ( Anderson et al., 2003 ; Hays et al., 2003 ; The Women’s Health Initiative Study Group, 1998). If eligible, participants in the CT could enroll in one or more of the three trial components, including the Hormone Therapy Trials (estrogen or estrogen + progesterone), the Dietary Modification Trial, or the Calcium/Vitamin D Trial, and were randomized to the intervention or control groups of each ( Hays et al., 2003 ). Institutional Review Boards from all sites approved the study, and all participants provided written informed consent.
Data were collected using self-administered forms, in-person or by phone interviews, and clinical measurements. The WHI follow-up efforts are ongoing; the present work includes data through 2013.
Study Variables
Veteran Status
Participants were self-identified as Veterans if they responded affirmatively to the baseline question, “Have you served in the U.S. armed forces on active duty for a period of 180 days or more.” This standard measure of Veteran status has been used in several national surveys, including those conducted by the Centers for Disease Control and Prevention ( Hoerster et al., 2012 ; Koepsell, Reiber, & Simmons, 2002 ; Lehavot et al., 2014 ). Those who responded negatively were classified as non-Veterans, whereas those with missing information were excluded.
Self-Reported Sleep Disturbances Encompassed Five Different Baseline Variables
Medication(s) or Alcohol for Sleep (No/Yes)
This was affirmatively defined as baseline self-reported use of medication or alcohol for sleep or verified use of a sedative/hypnotic medication. Use of sedative/hypnotic medications was identified by matching the participant’s prescription medications brought in during in-person visits to the Master Drug Data Base (Medi-Span, Indianapolis, IN) which includes a therapeutic hypnotic class code (600000–610000) provided by the American Hospital Formulary Service. Endorsement of medication or alcohol as a sleep aid may suggest a measure of sleep dissatisfaction or complaint not captured by the remaining sleep disturbance measures.
Risk of Insomnia
This was defined based on the Women’s Health Initiative Insomnia Rating Scale (WHIIRS) score (Levine, Kaplan, et al., 2003 ; Levine, Lewis, et al., 2003 ), a 5-item scale assessing self-reported sleep initiation insomnia (or sleep latency), sleep maintenance insomnia, early morning awakening, and sleep quality. Each item was coded 0 to 4 for frequency or quality resulting in a total score range of 0–20, with higher scores indicating poorer sleep. We defined scores ≥9 as indicating high risk for insomnia and <9 as low risk (Levine, Kaplan, et al., 2003 ).
High Risk for SDB
This addressed risk factors for sleep apnea adapted from the Berlin Questionnaire ( Netzer, Stoohs, Netzer, Clark, & Strohl, 1999 ), including snoring history, tiredness, and history of high blood pressure or obesity ( Mustafa, Erokwu, Ebose, & Strohl, 2005 ). One point was awarded for each of the following items: (1) self-reported snoring ≥3 times a week; (2) self-reported falling asleep during quiet activities ≥3 times a week; and (3) either hypertension (self-reported physician diagnosis or measured systolic blood pressure [BP] ≥ 140mm Hg or diastolic BP ≥ 90mm Hg) or measured obesity (body mass index [BMI] > 30kg/m 2 ). Scores ranged from 0 to 3 points, with scores ≥2 defined as high risk for SDB and <2 as low risk.
Comorbid Insomnia and SDB Risk
High risk for insomnia + SDB was assigned to participants who were designated as high risk for both conditions, as defined earlier. Conversely, low risk of insomnia + SDB was assigned to participants with either low risk for both or high risk for only one.
Sleep Duration
SLD was based on self-report and defined as very short (≤5 hours), short (6 hours), average (7–8 hours), and long (≥9 hours; Swinkels et al., 2013 ). By definition, all categories of SLD outside of average were considered aberrant for this study.
Adjudicated Cardiometabolic Health Outcomes
Cardiovascular Disease
CVD was defined as physician-adjudicated report of one of the following: clinical myocardial infarction (MI), definite silent MI (definite evolving Q wave MI), possible silent MI (possible evolving Q wave MI), definite or possible coronary heart disease (CHD) death, angina, coronary artery bypass graft (CABG), percutaneous transluminal coronary angioplasty, carotid artery disease (CAD), congestive heart failure (CHF), stroke, or peripheral vascular disease (PVD). CVD events were initially identified through annually mailed follow-up contacts and regularly scheduled study-related follow-up medical examinations and were confirmed through local and central adjudication. CVD-related deaths were confirmed via the National Death Index Plus ( Curb et al., 2003 ).
Diabetes
Diabetes was defined as self-reported treatment (pills or insulin injections) for diabetes. A validity study conducted in a subset of the WHI participants suggested that self-report was a reasonably good indicator of diagnosed diabetes when compared with medication inventories and fasting glucose ( Margolis et al., 2008 ), as well as medical records ( Jackson et al., 2014 ).
Additional Variables
Baseline Prevalent Disease
Either diabetes or CVD (i.e., self-reported physician diagnosis of stroke, MI, CHF, angina, CABG, percutaneous transluminal coronary angioplasty, CAD, or PVD) was used to identify participants to be excluded from each of the respective cardiometabolic outcome analyses.
Covariates
Covariates included demographic characteristics such as age, self-reported race/ethnicity, education, and whether living with a partner (married or in a marriage-like relationship); health status indices, including body mass index (BMI); presence of vasomotor symptoms (i.e., hot flashes or night sweats); and health risk behaviors, including smoking status (current, former, or never), and physical activity (MET-hours per week; Ainsworth et al., 1993 ). Self-reporting napping is a symptom associated with several of our key exposure variables, including insomnia, aberrant SLD and daytime sleepiness due to SDB. To address this confound, self-reported napping was included as a covariate. Assignment to the WHI study arms was also included as a covariate, as was baseline prevalence of depression, based on the Burnam Scale ( Burnam, Wells, Leake, & Landsverk, 1988 ). Consistent with prior WHI work, we used a cutoff of ≥0.06 to identify women with symptoms consistent with depressive disorders ( Bertone-Johnson et al., 2011 ; Spangler et al., 2008 ).
Data Analysis
We first examined the baseline demographic and health behavior characteristics of the study sample, stratified by Veteran status.
We next examined baseline prevalence of medication or alcohol use for sleep, high risk for insomnia, high risk for SDB, high risk for insomnia + SDB, and aberrant SLD in WV and non-Veteran participants. We further examined whether WVs and non-Veterans differed in sleep disturbance at baseline for all dichotomous variables using generalized linear models with a log link (Poisson distribution) to obtain estimates of the prevalence ratio (PR) and 95% confidence intervals (CIs) using robust standard errors ( Zou, 2004 ). For SLD, which had more than two categories, we employed multinomial logistic regression with average SLD as the reference group. In order to aid in interpretation, we calculated the difference in the probability of being within each category between Veterans and non-Veterans (the marginal effect) along with 95% CIs. Refer to Tables 2–4 for sequential adjustment of covariates in each analysis.
Finally, to estimate the association between each of the sleep disturbance exposures and incident CVD and diabetes, we employed Cox proportional hazards models to obtain estimates of the hazard ratio (HR) and 95% CIs. We included an interaction term between Veteran status and sleep disturbance in order to determine whether associations differed between WVs and non-Veterans. Disease occurrence time was defined as days from enrollment to each of CVD and diabetes, and censoring time for non-events as days from enrollment to the earlier of the date of last follow-up contact or September 20, 2010 for diabetes and September 20, 2013 for CVD. We excluded participants with CVD or diabetes at baseline from their respective models and adjusted for all variables in Adjustment 3 as earlier, excluding depression.
All analyses were conducted in Stata 13.0 ( StataCorp, 2013 ).
Results
A total of 145,061 women had baseline data on Veteran status and at least one measure of sleep disturbance, 3,707 of whom identified as Veterans (2.6%, Table 1 , Figure 2 ). WVs were older (49.8% Veterans 70–79 years old vs 21.7% non-Veterans), more educated (46.9% Veterans at least college graduates vs 39.5% non-Veterans), and more likely to be former or current smokers (54.1% vs 48.2% non-Veterans). WVs were less likely to have vasomotor symptoms (26.0% vs 32.7%).
Table 1.
Characteristics of non-Veteran and Veteran Women in the Women’s Health Initiative With At Least One Measure of Sleep Disturbance at Baseline
| Total | Non-Veteran | Veteran | ||
|---|---|---|---|---|
| N a | % a | N a | % a | |
| 141,354 | 100.0 | 3,707 | 100.0 | |
| Age (years) | ||||
| <50–59 | 46,222 | 32.7 | 783 | 21.1 |
| 60–69 | 64,455 | 45.6 | 1,077 | 29.1 |
| 70–79 | 30,677 | 21.7 | 1,847 | 49.8 |
| Race/ethnicity | ||||
| American Indian/Alaskan Native | 601 | 0.4 | 25 | 0.7 |
| Asian/Pacific Islander | 3,978 | 2.8 | 45 | 1.2 |
| Black/African American | 12,790 | 9.0 | 261 | 7.0 |
| Hispanic/Latino | 5,573 | 3.9 | 85 | 2.3 |
| White | 116,373 | 82.3 | 3,231 | 87.2 |
| Other | 1,669 | 1.2 | 47 | 1.3 |
| Education | ||||
| Less than high school | 7,412 | 5.2 | 62 | 1.7 |
| High school diploma/GED | 24,148 | 17.1 | 376 | 10.1 |
| Some college or vocational/training school | 53,046 | 37.5 | 1,516 | 40.9 |
| College graduate or greater | 55,839 | 39.5 | 1,739 | 46.9 |
| Living with a partner | ||||
| No | 116,640 | 82.5 | 2,865 | 77.3 |
| Yes | 24,238 | 17.1 | 832 | 22.4 |
| Smoking status | ||||
| Never | 71,416 | 50.5 | 1,624 | 43.8 |
| Former | 58,731 | 41.5 | 1,693 | 45.7 |
| Current | 9,537 | 6.7 | 317 | 8.6 |
| Physical activity | ||||
| Inactive (0–1.7 METS/week) | 31,212 | 22.1 | 778 | 21.0 |
| Low (>1.7 to <8.4 METS/week) | 39,765 | 28.1 | 995 | 26.8 |
| Medium (8.4 to <20 METS/week) | 39,254 | 27.8 | 1,062 | 28.6 |
| High (≥20 METS/week) | 30,871 | 21.8 | 866 | 23.4 |
| BMI | ||||
| Underweight | 1,244 | 0.9 | 44 | 1.2 |
| Normal | 48,528 | 34.3 | 1,243 | 33.5 |
| Overweight | 48,336 | 34.2 | 1,319 | 35.6 |
| Obese | 41,948 | 29.7 | 1,062 | 28.6 |
| Vasomotor symptoms b | ||||
| No | 94,329 | 66.7 | 2,719 | 73.3 |
| Yes | 46,180 | 32.7 | 964 | 26.0 |
| Depression | ||||
| No | 122,923 | 87.0 | 3,288 | 88.7 |
| Yes | 15,333 | 10.8 | 341 | 9.2 |
| Napping ≥3 times per week | ||||
| No | 122,237 | 86.5 | 3,096 | 83.5 |
| Yes | 18,498 | 13.1 | 597 | 16.1 |
| Cardiovascular disease at baseline | ||||
| No | 125,241 | 88.6 | 3,157 | 85.2 |
| Yes | 13,601 | 9.6 | 461 | 12.4 |
| Diabetes history at baseline | ||||
| No | 132,807 | 94.0 | 3,487 | 94.1 |
| Yes | 8,449 | 6.0 | 216 | 5.8 |
| Study assignment | ||||
| Observational study | 85,319 | 60.4 | 2,297 | 62.0 |
| Clinical Trials c | ||||
| Estrogen intervention | 4,435 | 3.1 | 111 | 3.0 |
| Estrogen control | 4,428 | 3.1 | 136 | 3.7 |
| Estrogen + progestin intervention | 6,925 | 4.9 | 204 | 5.5 |
| Estrogen + progestin control | 6,923 | 4.9 | 192 | 5.2 |
| Dietary modification intervention | 13,348 | 9.4 | 333 | 9.0 |
| Dietary modification control | 19,976 | 14.1 | 434 | 11.7 |
Notes: BMI = body mass index; GED = General Educational Development; METS = Metabolic Equivalents.
a Numbers may not add to totals and percents to 100 due to missing information.
b Defined as hot flashes and night sweats
c Women could also be randomized to the calcium and vitamin D intervention or control arms, which overlapped with the other clinical trials and occurred 12–24 months after initial randomization.
Figure 2.
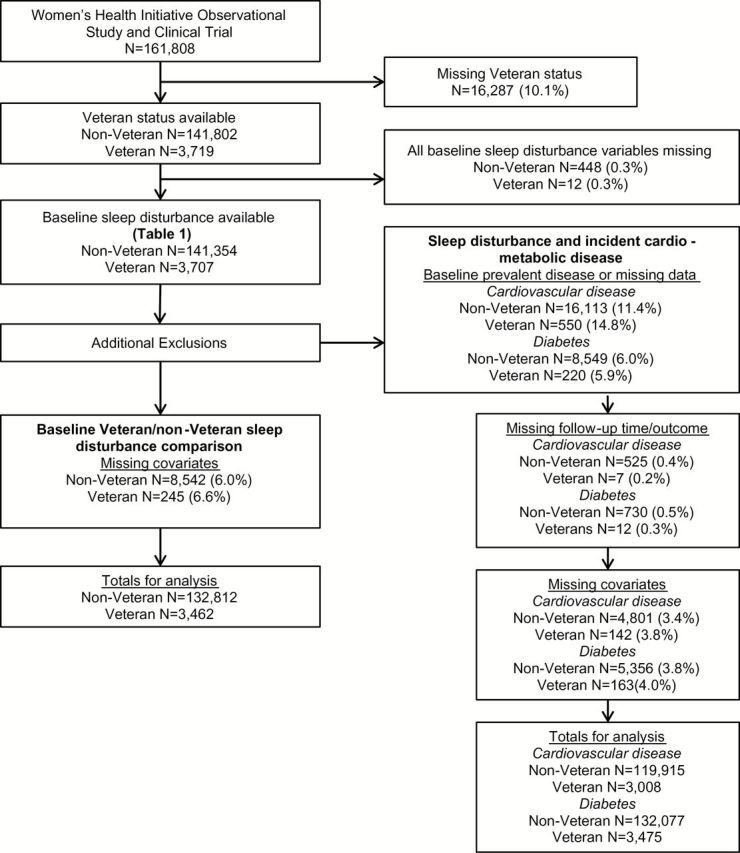
Flow diagram for study inclusion and exclusion. A total of 141,354 non-Veterans and 3,707 Veterans had baseline data on sleep disturbance, which reflects the study population described in Table 1 . Additional exclusions were made for each study question.
Prevalence of Sleep Disturbance
At baseline, the unadjusted prevalence of the various sleep disturbances among the 145,061 included women was comparable between WVs and non-Veterans ( Tables 2 and 3 ); medication or alcohol for sleep was 24.7% and 24.6% for WVs and non-Veterans, respectively. Similarly, insomnia risk was 30.5% and 30.8%, SDB risk was 25.1% and 24.9%, and insomnia + SDB risk was 10.3% and 9.5% for WVs and non-Veterans, respectively. SLD was also similar between WVs and non-Veterans; very short SLD was 8.5% and 8.3%, short SLD was 26.2% and 27.5%, and average SLD was and 60.2% and 59.9% for non-Veterans and WVs, respectively. WVs reported significantly higher unadjusted prevalence of long sleep (5.1%) compared with non-Veterans (4.3%).
Table 2.
Association Between Veteran Status and Sleep Disturbance At Baseline
| Non-Veterans a | Veterans a | Veterans compared with non-Veterans | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjustment 1 b | Adjustment 2 c | ||||||||
| N | % | N | % | PR (95% CI) | p Value | PR (95% CI) | p Value | PR (95% CI) | p Value | |
| Medication or alcohol for sleep | 32,303 | 24.6 | 845 | 24.7 | 1.02 (0.96, 1.08) | .51 | 1.00 (0.94, 1.06) | .98 | 1.00 (0.94, 1.06) | .88 |
| High risk for insomnia | 40,717 | 30.8 | 1,049 | 30.5 | 1.00 (0.96, 1.05) | .90 | 1.00 (0.95, 1.05) | .99 | 1.00 (0.95, 1.05) | .91 |
| High risk for SDB | 32,990 | 24.9 | 864 | 25.1 | 1.00 (0.95, 1.06) | .92 | 1.02 (0.97, 1.08) | .41 | 1.02 (0.97, 1.08) | .46 |
| High risk for insomnia and SDB | 12,540 | 9.5 | 355 | 10.3 | 1.09 (0.99, 1.19) | .10 | 1.13 (1.02, 1.24) | .02 | 1.13 (1.02, 1.24) | .02 |
Notes: CI = confidence interval; BMI = body mass index; PR = prevalence ratio; SDB = sleep disordered breathing.
a Numbers and percents reflect those from the fully adjusted model (Adjustment 2), thus sample sizes from unadjusted and Adjustment 1 models are at least as large as those reported in the table.
b Adjusted for age (continuous), race (White/non-White), education (nominal), BMI (continuous), and current smoker (no/yes).
c Adjusted for age (continuous), race (White/non-White), education (nominal), BMI (continuous), current smoker (no/yes), living with a partner (no/yes), physical activity (MET-hours per week, continuous), vasomotor symptoms (no/yes), depression (no/yes), napping at baseline (<3 times per week or 3+ times per week), and study arm (nominal).
Table 3.
Association Between Veteran Status and Sleep duration At Baseline
| Non-Veteran a | Veteran a | Veterans compared with non-Veterans | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjustment 1 b | Adjustment 2 c | ||||||||
| N a | % | N a | % | Diff (95% CI) | p Value | Diff (95% CI) | p Value | Diff (95% CI) | p Value | |
| Very short | 10,956 | 8.3 | 293 | 8.5 | 0.59 (−0.35, 1.52) | .22 | 1.12 (0.12, 2.11) | .03 | 0.98 (−0.01, 1.97) | .05 |
| Short | 36,558 | 27.5 | 907 | 26.2 | −1.11 (−2.55, 0.33) | .13 | −0.83 (−2.31, 0.66) | .28 | −0.93 (−2.43, 0.57) | .22 |
| Average | 79,440 | 59.9 | 2,084 | 60.2 | −0.22 (−1.82, 1.39) | .79 | −0.81 (−2.44, 0.81) | .33 | −0.51 (−2.14, 1.11) | .54 |
| Long | 5,746 | 4.3 | 175 | 5.1 | 0.74 (0.02, 1.45) | .04 | 0.52 (−0.18, 1.23) | .14 | 0.47 (−0.24, 1.17) | .20 |
Notes: BMI = body mass index; CI = confidence interval; Diff = difference in percent (percentage points).
a Numbers and percents reflect those from the fully adjusted model (Adjustment 2), thus sample sizes from unadjusted and Adjustment 1 models are at least as large as those reported in the table.
b Adjusted for age (continuous), race (White/non-White), education (nominal), BMI (continuous), and current smoker (no/yes).
c Adjusted for age (continuous), race (White/non-White), education (nominal), BMI (continuous), current smoker (no/yes), living with a partner (no/yes), physical activity (MET-hours per week, continuous), vasomotor symptoms (no/yes), depression (no/yes), napping at baseline (<3 times per week/3+ times per week), and study arm (nominal).
In fully adjusted analyses, there were no differences between the 3,462 WVs and 132,812 non-Veterans in baseline use of medication or alcohol for sleep ( Table 2 ; PR = 1.00, 95% CI = 0.94, 1.06), high risk for insomnia (PR = 1.00, 95% CI = 0.95, 1.05), or high risk for SDB (PR = 1.02, 95% CI = 0.97, 1.08). Prevalence of high risk for insomnia + SDB in WVs was 1.13 times that of non-Veterans (95% CI = 1.02, 1.24). There were no differences between WVs and non-Veterans in the probabilities for very short or long SLD ( Table 3 ).
Sleep Disturbance and CVD
Among participants who did not have prevalent CVD but did report at least one sleep disturbance at the WHI baseline, the incidence of CVD was 9.4 per 1,000 person-years of follow-up among 124,716 non-Veterans and 13.1 per 1,000 person-years among the 3,150 WVs. In fully adjusted analyses, there were no differences in the association between sleep disturbance and CVD by Veteran status among the 3,008 Veterans and 119,915 non-Veterans with all covariates ( p values for interaction: .14 to .94; Table 4 ). Almost all markers of poor sleep health were associated with incident CVD during the 20-year follow-up among non-Veterans, with mostly positive effects of similar magnitude observed in WVs ( Table 4 ). WVs who reported using medication or alcohol for sleep at baseline were 26% more likely to develop CVD than nonusers (95% CI = 1.03, 1.54) and non-Veterans were 17% more likely (95% CI = 1.13, 1.21). We observed an almost 30% increased risk of CVD for women at high risk for SDB among both WVs (HR = 1.28, 95% CI = 1.05, 1.56) and non-Veterans (HR = 1.29, 95% CI = 1.24, 1.34), as well as for high risk for insomnia + SDB among both WVs (HR = 1.24, 95% CI = 0.94, 1.64) and non-Veterans (HR = 1.27, 95% CI = 1.21, 1.34). Among non-Veterans, there were weak associations between high risk for insomnia and CVD (HR = 1.08, 95% CI = 1.05, 1.12) as well as very short SLD and CVD (HR = 1.15, 95% CI = 1.09, 1.23), whereas no significant associations were detected in WVs.
Table 4.
Adjusted Association Between Sleep Disturbance At Baseline and Incident Health Outcomes, by Veteran Status
| Non-Veterans | Veterans | p Value for interaction | |||
|---|---|---|---|---|---|
| HR a (95% CI) | p Value | HR a (95% CI) | p Value | ||
| Cardiovascular disease b | |||||
| (Incident cases: non-Veterans = 14,166; Veterans = 474) | |||||
| Medication or alcohol for sleep | 1.17 (1.13, 1.21) | <.001 | 1.26 (1.03, 1.54) | .02 | .47 |
| High risk for insomnia | 1.08 (1.04, 1.12) | <.001 | 1.04 (0.85, 1.26) | .73 | .66 |
| High risk for SDB | 1.29 (1.24, 1.34) | <.001 | 1.28 (1.05, 1.56) | .02 | .95 |
| High risk for insomnia and SDB | 1.27 (1.20, 1.34) | <.001 | 1.24 (0.94, 1.63) | .13 | .87 |
| Sleep duration | |||||
| Very short | 1.15 (1.08, 1.22) | <.001 | 1.12 (0.81, 1.55) | .50 | .88 |
| Short | 1.07 (1.03, 1.11) | .001 | 0.90 (0.73, 1.12) | .36 | .14 |
| Average | 1.00 (Ref) | — | 1.00 (Ref) | — | — |
| Long | 1.07 (0.99, 1.16) | .08 | 0.87 (0.56, 1.36) | .55 | .37 |
| Diabetes c | |||||
| (Incident cases: non-Veterans = 12,003; Veterans = 309) | |||||
| Medication or alcohol for sleep | 1.04 (0.99, 1.08) | .10 | 1.12 (0.86, 1.44) | 0.40 | .58 |
| High risk for insomnia | 1.04 (0.99, 1.08) | .06 | 1.16 (0.92, 1.47) | 0.21 | .36 |
| High risk for SDB | 1.37 (1.32, 1.43) | <.001 | 1.26 (0.99, 1.59) | 0.06 | .47 |
| High risk for insomnia and SDB | 1.30 (1.23, 1.37) | <.001 | 1.49 (1.11, 2.00) | .01 | .35 |
| Sleep duration | |||||
| Very short | 1.11 (1.05, 1.19) | <.001 | 1.22 (0.85, 1.75) | .28 | .63 |
| Short | 1.03 (0.99, 1.07) | .20 | 1.08 (0.84, 1.40) | .54 | .68 |
| Average | 1.00 (Ref) | — | 1.00 (Ref) | — | — |
| Long | 1.12 (1.03, 1.22) | .01 | 1.05 (0.62, 1.79) | .84 | .82 |
Notes: CI = confidence interval; HR = hazard ratio; SDB = sleep disordered breathing.
a Adjusted for baseline age (continuous), race (White/non-White), education (nominal), BMI (continuous), living with partner (no/yes), physical activity (continuous, MET-hours per week), vasomotor symptoms (no/yes), napping (<3 times per week/3+ times per week), current smoker (no/yes), and study arm (nominal).
b Defined as adjudicated clinical myocardial infarction (MI), evolving Q wave MI (definite silent MI), possible evolving Q wave MI (possible silent MI), definite or possible coronary heart disease death, angina, coronary artery bypass graft, percutaneous transluminal coronary angioplasty, carotid artery disease, congestive heart failure, stroke, and peripheral artery disease.
c Defined as first time receiving treatment for diabetes.
Sleep Disturbance and Diabetes
Incident diabetes development during follow-up occurred at a rate of 8.7 per 1,000 person-years follow-up for both the 132,077 WVs and 3,475 non-Veterans without prevalent diabetes and with at least one sleep disturbance measure at baseline.
Associations between sleep disturbance and incident diabetes did not vary by Veteran status ( p values for interaction: .36 to .82; Table 4 ). Among the 3,312 WVs and 126,721 non-Veterans with all covariates, there were no statistically significant associations between incident diabetes and use of medication or alcohol for sleep or high risk for insomnia ( Table 4 ). There was, however, a 26% and 37% increase in risk of diabetes associated with high risk for SDB in WVs (95% CI = 0.99, 1.59) and non-Veterans (95% CI = 1.32, 1.43). Even larger increases were associated with high risk for insomnia + SDB, with a 49% increase in WVs (95% CI = 1.11, 2.00) and 30% increase in non-Veterans (95% CI = 1.23, 1.37; Table 4 ). Very short SLD, relative to average SLD, was associated with increased risk of diabetes in non-Veterans (HR = 1.12, 95% CI = 1.05, 1.19), with a nonsignificant trend for an effect in WVs (HR = 1.22, 95% CI = 0.85, 1.75). Among non-Veterans, long SLD relative to average was also positively associated with diabetes (HR = 1.12, 95% CI = 1.03, 1.22).
Discussion
Our study characterized the prevalence of sleep disturbance in 3,707 postmenopausal women Veteran and non-Veteran participants from the WHI, specifically prospectively examining the association of sleep disturbances with incident CVD and diabetes. Results revealed a significantly heightened risk for comorbid insomnia + SDB among Veterans, relative to non-Veterans. However, risk of other forms of sleep disturbance (i.e., medication/alcohol use for sleep, risk for insomnia, risk for SDB, or aberrant SLD) was similar across groups. Moreover, sleep disturbances were not associated with greater risk of cardiometabolic morbidity among WVs compared with non-Veterans; rather, poor sleep health at baseline was associated with increased risk of cardiometabolic morbidity in both groups.
Findings related to the greater prevalence of comorbid insomnia and sleep-disordered breathing (insomnia + SDB) risk among WVs are noteworthy as it may signal heightened risk for inflammation and its associated negative physical health consequences ( Gupta & Arnedt, 2012 ) as well diminished cognitive functioning ( Zimmerman & Aloia, 2012 ). Thus, further research that examines the association of insomnia + SDB and depression, cancer, and risk for mild cognitive impairment, and dementia, among older postmenopausal WVs may be fruitful.
Though the reasons underlying their greater vulnerability to comorbid insomnia + SDB remain unknown, the heightened prevalence of key health risk behaviors (e.g., smoking) among WV participants is likely a salient contributing factor. Lifetime prevalence of PTSD was not measured in the WHI; however, the significant prevalence of this disorder among WVs is well documented ( Dobie et al., 2004 ; Turner, Turse, & Dohrenwend, 2007 ) and may contribute to the observed heightened rates of high risk for insomnia + SDB ( Krakow et al., 2015 ). That we did not find a differential relationship between sleep disturbance and cardiometabolic morbidity by Veteran status is perhaps surprising in this light. Nevertheless, this may reflect the fact that fully adjusted models accounted for many salient demographic and health risk behaviors that varied by Veteran status. It may also reflect the higher prevalence (and thus greater risk of exclusion from analyses) of cardiometabolic disease among WVs at the WHI baseline.
Findings depart from the prior literature in several important ways. In contrast to several prior studies, we did not find an overall heightened prevalence of sleep disturbances in Veterans relative to non-Veterans ( Fung et al., 2013 ; Hughes et al., 2013 ). However, important differences in our study methodology and population may explain this difference. In addition, Sands-Lincoln and colleagues (2013) reported that postmenopausal women in the WHI Observational Study with a combined high risk for insomnia and long SLD (≥10 hours) had higher incident CHD than postmenopausal women without these problems. In contrast, our findings suggest that high risk for insomnia, short SLD, and very short SLD are associated with higher risk of CVD in the non-Veteran group. Failure to replicate Sands-Lincoln’s (2013) work may be due to slightly different methodology, that is, a more liberal definition of long SLD (≥9 vs ≥10 hours) or broader inclusion of the WHI participants.
Limitations include use of an observational design, which means our findings represent associations, not causal linkages, among our core variables of Veteran status, sleep disturbance, CVD, and diabetes. Second, Veteran status and all measures of sleep disturbance (with the exception of some components of SDB risk) were self-reported symptoms. Measures of insomnia and SDB were not diagnostic, and all self-reported sleep disturbances may be subject to reporting bias—increasing the risk of misclassification (e.g., assigned a sleep disturbance when none existed). However, it is unlikely that any misclassification differed between Veterans and non-Veterans, and this type of nondifferential measurement error would be expected to bias associations toward null values rather than a true effect. Third, there were, undoubtedly, important but unmeasured confounding factors that were not accounted for in our findings. Specifically, the lack of contextual details about Veterans’ prior military service and military-related exposures (e.g., trauma) that might confound results is a notable limitation. Finally, generalizability of our findings may be limited as the study population was voluntarily recruited, not derived from a population-based sample, and reflects a unique cadre of WVs who are age consistent with military service during and before the Vietnam War. Given the tremendous evolution in roles and opportunities for military women since that time, findings may not generalize to more contemporary Veteran cohorts. Methodological strengths of our study include the use of a large, nationally recruited group of postmenopausal women in WHI (The Women’s Health Initiative Study Group, 1998), a strong rate of participant retention, and the inclusion of rigorously adjudicated health outcomes (cardiometabolic morbidity variables).
Conclusion
This work represents the first large-scale effort to delineate the prevalence of sleep disturbance in postmenopausal WVs and examines the potential synergistic effects of Veteran status and sleep disturbance on postmenopausal cardiometabolic outcomes. Findings of WVs’ heightened prevalence of risk for comorbid insomnia and SDB offer important implications for clinical practice and policy—illuminating the value of routine screening for sleep disturbances, particularly insomnia, sleep disordered breathing, and their co-occurrence in postmenopausal WVs. Treatment of insomnia + SDB may require a multidisciplinary team (i.e., combination cognitive behavioral therapy for insomnia and positive airway pressure therapy) underscoring the importance of WVs’ access to both medical and behavioral health care providers. Further research that replicates and confirms our findings is needed, as is research that investigates the etiologic factors associated with Veterans’ heightened prevalence of postmenopausal sleep disturbances, and addresses optimal treatment strategies, is also needed.
Funding
The Women’s Health Initiative program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN2 68201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C. Wyeth Pharmaceuticals provided the study drug and the placebo to the WHI trial. ClinicalTrials.gov Identifiers: NCT00000611. This research is supported, in part, by the Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment and the Department of Veterans Affairs Sierra-Pacific Mental Illness Research, Education, and Clinical Center (MIRECC), the Women’s Mental Health and Aging Core of the Sierra Pacific MIRECC, and VA Health Services Research and Development FOP 14-439. Dr. Ulmer was supported by a Veterans Affairs Research Career Development Award (CDA 09-218) while working on this manuscript. The research reported here was also supported by Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service FOP14-439 and the VA Office of Women’s Health.
Acknowledgments
The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
References
- Ainsworth B. E., Haskell W. L., Leon A. S., Jacobs D. R., Jr, Montoye H. J., Sallis J. F., Paffenbarger R. S., Jr . ( 1993. ). Compendium of physical activities: Classification of energy costs of human physical activities . Medicine and Science in Sports and Exercise , 25 , 71 – 80 . doi: 10.1249/00005768-199301000-00011 [DOI] [PubMed] [Google Scholar]
- Anderson G. L. Manson J. Wallace R. Lund B. Hall D. Davis S. … Prentice R. L . ( 2003. ). Implementation of the Women’s Health Initiative study design . Annals of Epidemiology , 13 ( 9 Suppl .), S5 – S17 . doi: 10.1016/s1047-2797(03)00043-7 [DOI] [PubMed] [Google Scholar]
- Armed Forces Health Surveillance Center . ( 2010a. ). Insomnia, active component, U.S. Armed Forces, January 2000–December 2009 . Medical Surveillance Monthly Report , 17 , 12 – 15 . [Google Scholar]
- Armed Forces Health Surveillance Center . ( 2010b. ). Obstructive sleep apnea, active component, U.S. Armed Forces, January 2000–December 2009 . Medical Surveillance Monthly Report , 17 , 8 – 11 . [Google Scholar]
- Bertone-Johnson E. R. Powers S. I. Spangler L. Brunner R. L. Michael Y. L. Larson J. C. … Manson J. E . ( 2011. ). Vitamin D intake from foods and supplements and depressive symptoms in a diverse population of older women . The American Journal of Clinical Nutrition , 94 , 1104 – 1112 . doi: 10.3945/ajcn.111.017384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnam M. A., Wells K. B., Leake B., Landsverk J . ( 1988. ). Development of a brief screening instrument for detecting depressive disorders . Medical Care , 26 , 775 – 789 . doi: 10.1097/00005650-198808000-00004 [DOI] [PubMed] [Google Scholar]
- Cappuccio F. P., Cooper D., D’Elia L., Strazzullo P., Miller M. A . ( 2011. ). Sleep duration predicts cardiovascular outcomes: A systematic review and meta-analysis of prospective studies . European Heart Journal , 32 , 1484 – 1492 . doi: 10.1093/eurheartj/ehr007 [DOI] [PubMed] [Google Scholar]
- Curb J. D. McTiernan A. Heckbert S. R. Kooperberg C. Stanford J. Nevitt M. … Daugherty S .; WHI Morbidity and Mortality Committee . ( 2003. ). Outcomes ascertainment and adjudication methods in the Women’s Health Initiative . Annals of Epidemiology , 13 ( 9 Suppl .), S122 – S128 .doi: 10.1016/s1047-2797(03)00048-6 [DOI] [PubMed] [Google Scholar]
- Dijk D. J., Czeisler C. A . ( 1994. ). Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans . Neuroscience Letters , 166 , 63 – 68 . doi: 10.1016/0304-3940(94)90841-9 [DOI] [PubMed] [Google Scholar]
- Dobie D. J., Kivlahan D. R., Maynard C., Bush K. R., Davis T. M., Bradley K. A . ( 2004. ). Posttraumatic stress disorder in female veterans: Association with self-reported health problems and functional impairment . Archives of Internal Medicine , 164 , 394 – 400 . doi: 10.1001/archinte.164.4.394 [DOI] [PubMed] [Google Scholar]
- Faestel P. M., Littell C. T., Vitiello M. V., Forsberg C. W., Littman A. J . ( 2013. ). Perceived insufficient rest or sleep among veterans: Behavioral Risk Factor Surveillance System 2009 . Journal of Clinical Sleep Medicine: JCSM , 9 , 577 – 584 . doi: 10.5664/jcsm.2754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frayne S. M., Skinner K. M., Sullivan L. M., Freund K. M . ( 2003. ). Sexual assault while in the military: Violence as a predictor of cardiac risk? Violence and Victims , 18 , 219 – 225 . doi: 10.5664/jcsm.2754 [DOI] [PubMed] [Google Scholar]
- Frayne S. M., Skinner K. M., Sullivan L. M., Tripp T. J., Hankin C. S., Kressin N. R., Miller D. R . ( 1999. ). Medical profile of women Veterans Administration outpatients who report a history of sexual assault occurring while in the military . Journal of Women’s Health & Gender-based Medicine , 8 , 835 – 845 . doi: 10.1089/152460999319156 [DOI] [PubMed] [Google Scholar]
- Fung C. H., Martin J. L., Dzierzewski J. M., Jouldjian S., Josephson K., Park M., Alessi C . ( 2013. ). Prevalence and symptoms of occult sleep disordered breathing among older veterans with insomnia . Journal of Clinical Sleep Medicine: JCSM , 9 , 1173 – 1178 . doi: 10.5664/jcsm.3162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandner M. A., Sands-Lincoln M. R., Pak V. M., Garland S. N . ( 2013. ). Sleep duration, cardiovascular disease, and proinflammatory biomarkers . Nature and Science of Sleep , 5 , 93 – 107 . doi: 10.2147/NSS.S31063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S. , & Arnedt J. T . ( 2012. ). Sleep and cardiovascular disease . In Riba M. Wulsin L. , & Rubenfire M. (Eds.), Psychiatry and Heart Disease: The Mind, Brain, and Heart (pp. 135 – 152 ). Chichester, UK: : John Wiley; . doi: 10.1002/9780470975138.ch11 [Google Scholar]
- Hays J., Hunt J. R., Hubbell F. A., Anderson G. L., Limacher M., Allen C., Rossouw J. E . ( 2003. ). The Women’s Health Initiative recruitment methods and results . Annals of Epidemiology , 13 ( 9 Suppl .), S18 – S77 . doi: 10.1016/s1047-2797(03)00042-5 [DOI] [PubMed] [Google Scholar]
- Heppner P. S. Crawford E. F. Haji U. A. Afari N. Hauger R. L. Dashevsky B. A. … Baker D. G . ( 2009. ). The association of posttraumatic stress disorder and metabolic syndrome: A study of increased health risk in veterans . BMC Medicine , 7 , 1 . doi: 10.1186/1741-7015-7-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerster K. D., Lehavot K., Simpson T., McFall M., Reiber G., Nelson K. M . ( 2012. ). Health and health behavior differences: U.S. Military, veteran, and civilian men . American Journal of Preventive Medicine , 43 , 483 – 489 . doi: 10.1016/j.amepre.2012.07.029 [DOI] [PubMed] [Google Scholar]
- Hughes J., Jouldjian S., Washington D. L., Alessi C. A., Martin J. L . ( 2013. ). Insomnia and symptoms of post-traumatic stress disorder among women veterans . Behavioral Sleep Medicine , 11 , 258 – 274 . doi: 10.1080/15402002.2012.683903 [DOI] [PubMed] [Google Scholar]
- Jackson J. M. DeFor T. A. Crain A. L. Kerby T. J. Strayer L. S. Lewis C. E. … Margolis K. L . ( 2014. ). Validity of diabetes self-reports in the Women’s Health Initiative . Menopause (New York, N.Y.) , 21 , 861 – 868 . doi: 10.1097/GME.0000000000000189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan B. K., Schlenger W. E., Hough R., Kulka R. A., Weiss D., Fairbank J. A., Marmar C. R . ( 1991. ). Lifetime and current prevalence of specific psychiatric disorders among Vietnam veterans and controls . Archives of General Psychiatry , 48 , 207 – 215 . doi: 10.1001/archpsyc.1991.01810270019002 [DOI] [PubMed] [Google Scholar]
- Kaneita Y. Uchiyama M. Takemura S. Yokoyama E. Miyake T. Harano S. … Ohida T . ( 2007. ). Use of alcohol and hypnotic medication as aids to sleep among the Japanese general population . Sleep Medicine , 8 , 723 – 732 . doi: 10.1016/j.sleep.2006.10.009 [DOI] [PubMed] [Google Scholar]
- Knutson K. L . ( 2012. ). Does inadequate sleep play a role in vulnerability to obesity? American Journal of Human Biology: The Official Journal of the Human Biology Council , 24 , 361 – 371 . doi: 10.1002/ajhb.22219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepsell T., Reiber G., Simmons K. W . ( 2002. ). Behavioral risk factors and use of preventive services among veterans in Washington State . Preventive Medicine , 35 ( 6 ), 557 – 562 .doi: 10.1006/pmed.2002.1121 [DOI] [PubMed] [Google Scholar]
- Krakow B. J., Ulibarri V. A., Moore B. A., McIver N. D . ( 2015. ). Posttraumatic stress disorder and sleep-disordered breathing: A review of comorbidity research . Sleep Medicine Reviews , 24 , 37 – 45 . doi: 10.1016/j.smrv.2014.11.001 [DOI] [PubMed] [Google Scholar]
- Lavie P . ( 2001. ). Sleep disturbances in the wake of traumatic events . The New England Journal of Medicine , 345 , 1825 – 1832 . doi: 10.1056/NEJMra012893 [DOI] [PubMed] [Google Scholar]
- Lehavot K., Katon J. G., Williams E. C., Nelson K. M., Gardella C. M., Reiber G. E., Simpson T. L . ( 2014. ). Sexual behaviors and sexually transmitted infections in a nationally representative sample of women veterans and nonveterans . Journal of Women’s Health , 23 , 246 – 252 . doi: 10.1089/jwh.2013.4327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine D. W. Kaplan R. M. Kripke D. F. Bowen D. J. Naughton M. J. , & Shumaker S. A . ( 2003. ). Factor structure and measurement invariance of the Women’s Health Initiative Insomnia Rating Scale . Psychological Assessment , 15 , 123 – 136 . doi: 10.1037/1040-3590.15.2 [DOI] [PubMed] [Google Scholar]
- Levine D. W. Lewis M. A. Bowen D. J. Kripke D. F. Kaplan R. M. Naughton M. J. , & Shumaker S. A . ( 2003. ). Reliability and validity of Women’s Health Initiative Insomnia Rating Scale . Psychological Assessment , 15 , 137 – 148 . doi: 10.1037/1040-3590.15.2.137 [DOI] [PubMed] [Google Scholar]
- Loke Y. K., Brown J. W., Kwok C. S., Niruban A., Myint P. K . ( 2012. ). Association of obstructive sleep apnea with risk of serious cardiovascular events: A systematic review and meta-analysis . Circulation: Cardiovascular Quality and Outcomes , 5 , 720 – 728 . doi: 10.1161/CIRCOUTCOMES.111.964783 [DOI] [PubMed] [Google Scholar]
- Luyster F. S., Kip K. E., Buysse D. J., Aiyer A. N., Reis S. E., Strollo P. J., Jr . ( 2014. ). Traditional and nontraditional cardiovascular risk factors in comorbid insomnia and sleep apnea . Sleep , 37 , 593 – 600 . doi: 10.5665/sleep.3506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis K. L. Lihong Qi Brzyski R. Bonds D. E. Howard B. V Kempainen S. , … Phillips L. S . ( 2008. ). Validity of diabetes self-reports in the Women’s Health Initiative: Comparison with medication inventories and fasting glucose measurements . Clinical Trials , 5 , 240 – 247 . doi: 10.1177/1740774508091749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney W. P. McIntire D. D. Carmody T. J. , & Joseph A . ( 1997. ). Comparing the smoking behavior of veterans and nonveterans . Public Health Reports , 112 , 212 – 218 . [PMC free article] [PubMed] [Google Scholar]
- Mustafa M. Erokwu N. Ebose I. , & Strohl K . ( 2005. ). Sleep problems and the risk for sleep disorders in an outpatient veteran population . Sleep & Breathing , 9 , 57 – 63 . doi: 10.1007/s11325-005-0016-z [DOI] [PubMed] [Google Scholar]
- Netzer N. C. Stoohs R. A. Netzer C. M. Clark K. , & Strohl K. P . ( 1999. ). Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome . Annals of Internal Medicine , 131 , 485 – 491 . doi: 10.7326/0003-4819-131-7-199910050-00002 [DOI] [PubMed] [Google Scholar]
- Ong J. C., Crawford M. R . ( 2013. ). Insomnia and obstructive sleep apnea . Sleep Medicine Clinics , 8 , 389 – 398 . doi: 10.1016/j.jsmc.2013.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quera-Salva M. A., Orluc A., Goldenberg F., Guilleminault C . ( 1991. ). Insomnia and use of hypnotics: Study of a French population . Sleep , 14 , 386 – 391 . [DOI] [PubMed] [Google Scholar]
- Rosenbaum S. Stubbs B. Ward P. B. Steel Z. Lederman O. , & Vancampfort D . ( 2015. ). The prevalence and risk of metabolic syndrome and its components among people with posttraumatic stress disorder: A systematic review and meta-analysis . Metabolism , 64 , 926 – 33 . doi: 10.1016/j.metabol.2015.04.009 [DOI] [PubMed] [Google Scholar]
- Sands M. Loucks E. B. Lu B. Carskadon M. A. Sharkey K. Stefanick M. … Eaton C. B . ( 2013. ). Self-reported snoring and risk of cardiovascular disease among postmenopausal women (from the Women’s Health Initiative) . The American Journal of Cardiology , 111 , 540 – 546 . doi: 10.1016/j.amjcard.2012.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands-Lincoln M. Loucks E. B. Lu B. Carskadon M. A. Sharkey K. Stefanick M. L. … Eaton C. B . ( 2013. ). Sleep duration, insomnia, and coronary heart disease among postmenopausal women in the Women’s Health Initiative . Journal of Women’s Health , 22 , 477 – 486 . doi: 10.1089/jwh.2012.3918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman T. E. , & Crimmins E . ( 2001. ). Social environment effects on health and aging: Integrating epidemiologic and demographic approaches and perspectives . Annals of the New York Academy of Sciences , 954 , 88 – 117 . doi: 10.1111/j.1749–6632.2001.tb02749.x [DOI] [PubMed] [Google Scholar]
- Spangler L. Scholes D. Brunner R. L. Robbins J. Reed S. D. Newton K. M. … Lacroix A. Z . ( 2008. ). Depressive symptoms, bone loss, and fractures in postmenopausal women . Journal of General Internal Medicine , 23 , 567 – 574 . doi: 10.1007/s11606-008-0525-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp . ( 2013. ). Stata Statistical Software: Release 13 . College Station, TX: : StataCorp LP; . [Google Scholar]
- Stranges S. Dorn J. M. Shipley M. J. Kandala N.-B. Trevisan M. Miller M. A. , … Cappuccio F. P . ( 2008. ). Correlates of short and long sleep duration: A cross-cultural comparison between the United Kingdom and the United States . American Journal of Epidemiology , 168 , 1353 – 1364 . doi: 10.1093/aje/kwn337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinkels C. M. Ulmer C. S. Beckham J. C. Buse N. , & Registry M. M . ( 2013. ). The association of sleep duration, mental health, and health risk behaviors . Sleep , 36 , 1019 – 1025 . doi: 10.5665/sleep.2800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Women’s Health Initiative Study Group . ( 1998. ). Design of the Women’s Health Initiative Clinical Trial and Observational Study—Examples from the Women’s Health Initiative . Controlled Clinical Trials , 19 , 61 – 109 . doi: 10.1016/s0197-2456(97)00078-0 [DOI] [PubMed] [Google Scholar]
- Weitlauf J. C. Lacroix A. Z. Bird C. E. Woods N. F. Washington D. L. Katon J. G. , … Stefanick M. L . ( 2015. ). Prospective analysis of health and mortality risk in veteran and non-veteran participants in the Women’s Health Initiative . Women’s Health Issues , 25 , 648 – 656 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J. B., Turse N. A., Dohrenwend B. P . ( 2007. ). Circumstances of service and gender differences in war-related PTSD: Findings from the National Vietnam Veteran Readjustment Study . Journal of Traumatic Stress , 20 , 643 – 649 . doi: 10.1002/jts.20245 [DOI] [PubMed] [Google Scholar]
- U.S. Department of Veterans Affairs, & National Center for Veterans Analysis and Statistics . ( 2014. ). Veteran Population . The Veteran Population Projection Model 2014 (VetPop2014). Retrieved November 22, 2014, from http://www.va.gov/vetdata/veteran_population.asp
- Vimalananda V. G. Miller D. R. Christiansen C. L. Wang W. Tremblay P. , & Fincke B. G . ( 2013. ). Cardiovascular disease risk factors among women veterans at VA medical facilities . Journal of General Internal Medicine , 28 , 517 – 523 . doi: 10.1007/s11606-013-2381-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman M. E., Aloia M. S . ( 2012. ). Sleep-disordered breathing and cognition in older adults . Current Neurology and Neuroscience Reports , 12 , 537 – 546 . doi: 10.1007/s11910-012-0298-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou G . ( 2004. ). A modified poisson regression approach to prospective studies with binary data . American Journal of Epidemiology , 159 , 702 – 706 . doi: 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]