Abstract
Tissue injury can stimulate quiescent cells to proliferate, resulting in metaplasia, to rapidly replace damaged cells and enable regeneration. A new study describes how fully differentiated cells return to proliferation in an autophagy‐ and mTORC1‐dependent manner. Given the striking parallels between this process in mammalian stomach and pancreas, a new term, paligenosis, is proposed for this conserved program.
Subject Categories: Autophagy & Cell Death, Cell Cycle, Development & Differentiation
As well as classical hierarchical differentiation and regulated cell turnover from dedicated stem cell populations, it is now clear that differentiated cells in adult tissues are capable of significant plasticity. In mammals, this plasticity has been revealed mainly through injury models, implying that changes in a previously settled cell fate are an emergency mechanism to repair damage. Although differentiated cells are not necessarily incapable of dividing, in the context of injury and metaplasia, cells lose many of their differentiated features before re‐entering the cell cycle.
Tata and Rajagopal have defined dedifferentiation as “a process of lineage reversion in which differentiated cells acquire the properties of more immature cells within the same lineage hierarchy” (Tata & Rajagopal, 2016). However, some may consider dedifferentiation to imply a return to a completely undifferentiated, multipotent or stem‐like state, and the definition is further complicated when applied to tumour cells (Friedmann‐Morvinski & Verma, 2014; Lin et al, 2017). In the context of regeneration, dedifferentiated cells do not necessarily resemble stem cells, but are proliferative and may give rise to areas of metaplasia. Willet et al (2018) describe the process by which gastric chief and pancreatic acinar cells convert from a differentiated, secretory phenotype to a proliferative, metaplastic one and coin a new term, paligenosis (from the Greek: pali/n/m (backward) + genes (born of) + osis (process)), to describe this program.
The conserved features of the paligenosis program are, firstly, a decrease in mTORC1 signalling and induction of autophagy; secondly, a change in the gene expression program; and lastly, an increase in mTORC1 signalling and onset of proliferation (Fig 1). Willet et al (2018) have investigated the process extensively in the stomach and pancreas and propose that it may be conserved in other regenerative situations.
Figure 1. The paligenosis program.
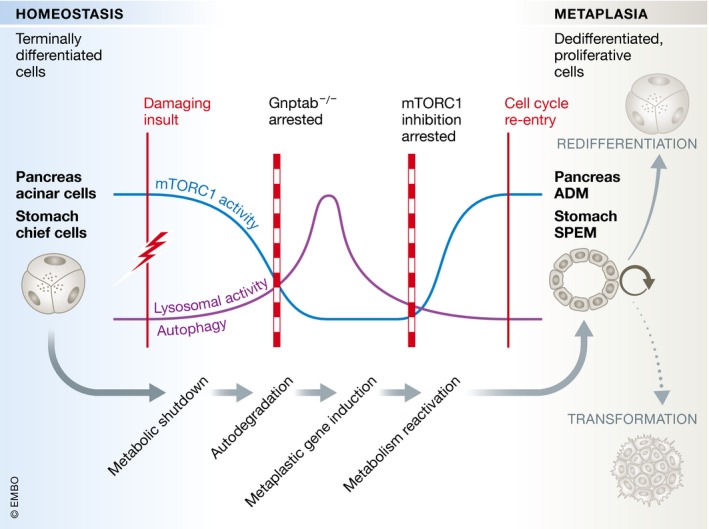
Upon damaging insult, chief cells in the stomach and pancreatic acinar cells decrease metabolic activity whilst temporarily activating lysosomes and autophagic machinery. At later stages, cells induce expression of metaplasia and wound‐healing associated genes including Sox9, Clu and Cd44v, reactivate metabolism and enter the cell cycle. SPEM (spasmolytic polypeptide‐expressing metaplasia) in the stomach and ADM (acinar‐to‐ductal metaplasia) in the pancreas mark the crossroads between tissue regeneration and transformation into precancerous lesions.
Caerulein‐induced acute pancreatitis and high‐dose tamoxifen‐induced gastric injury are well‐described injury models that both result in the temporary occurrence of proliferative cells. In case of the stomach, these are derived from chief cells and form spasmolytic polypeptide‐expressing metaplasia (SPEM), whilst in the pancreas, acinar cells convert into a proliferative duct‐like cell type (acinar‐to‐ductal metaplasia or ADM). Willet et al (2018) find that both cell types decrease their metabolic activity immediately after the damaging insult as judged by the loss of mTORC1‐mediated phosphorylation of ribosomal protein S6. This metabolic shutdown is temporary and recovers fully by the time of maximal metaplasia in both tissues. In addition to the fluctuation in metabolic activity, Willet et al (2018) describe a robust activation of the autodegradative machinery early upon tissue injury as evident by an increase in lysosomes and autodegradative puncta. At the stage of maximal metaplasia, autophagic and lysosomal puncta have decreased, returning to normal levels. The cells have now reached a proliferative state and express the metaplastic signature genes Sox9 and CD44v. Interestingly, although inhibition of autophagy is a key function of mTORC1, inhibition of mTORC1 signalling did not affect autophagy induction by injury, raising the possibility that autophagy may be triggered independently in this context. Inhibition of mTORC1 signalling blocked injury‐induced proliferation, but did not affect genetic reprogramming of cells to the metaplastic phenotype; in turn blocking autophagy by inhibition of lysosomal hydrolase trafficking through Gnptab deletion impaired reprogramming as well as blocking mTORC1 reactivation and proliferation. It will be interesting to unravel the molecular crosstalk between the two pathways and how their activity is linked to the damaging insult. In particular, whether the upstream triggers that induce mTORC1 modulation and induction of autophagy are a conserved part of the paligenosis program or specific to the cell type and type of damage will be pertinent questions to clarify.
Most importantly, it remains to be seen how universal the paligenosis program of metabolic hiatus and autophagy is in different organs and tissues. Willet et al (2018) present findings from hepatectomized liver and tunicamycin‐damaged kidney where, in both cases, the recruitment of terminally differentiated cells into cell cycle involves upregulation of S6 phosphorylation. However, the involvement of autophagy and initial decrease in mTORC1 activity await further investigation. Within the pancreas, fully differentiated duct cells downregulate the E3 ligase Fbw7 and begin to proliferate upon duct ligation (Sancho et al, 2014), and in the colon, inflammatory colitis induces degradation of Fbw7 concomitant with proliferation (Khan et al, 2018). Fbw7 targets mTOR for degradation (Mao et al, 2008), but an involvement of autophagy or metabolic hiatus has not yet been examined in these contexts. Investigation of paligenosis in other injury models, for example glial dedifferentiation following peripheral nerve damage, will also be highly illuminating given the known role of autophagy in degrading myelin (Gomez‐Sanchez et al, 2015).
Another interesting question posed by Willet et al (2018) is the potential biological benefit of paligenosis. There is a low level of “background” proliferation amongst mature chief cells and acinar cells in homeostasis. Furthermore, Troy+ chief cells are a reserve stem cell in the stomach (Stange et al, 2013) and pancreatic acinar cells comprise proliferating sub‐populations with a facultative potential to contribute to other differentiated lineages (Westphalen et al, 2016). This indicates that the complex cellular architecture of secretory cells does not preclude proliferation and raises the question of why cells upon injury opt for paligenosis rather than immediate overproliferation. The Gnptab deletion data from Willet et al (2018) suggest that autophagy is actually a prerequisite for proliferation, at least in the chief cell and acinar contexts analysed. The authors hypothesize that dismantling of the secretory machinery would release amino acids that contribute to the reactivation of mTORC1 and induction of proliferation. A potential second benefit of paligenosis might be that gastric chief and pancreatic acinar cells, which harbour potent cytotoxic enzymes, need to shut down their secretory machinery to prevent enzyme release by dying cells and catastrophic tissue damage. In this scenario, paligenosis using autophagy as a damage control mechanism might be particular to secretory cells.
In recent years, autophagy and mTORC1 have been implicated in both plasticity and proliferation as well as differentiation amongst stem cell populations of different organs (Mizushima & Levine, 2010; Meng et al, 2018). The findings of Willet et al (2018) suggest a shared program for differentiated cells to regain plasticity and proliferative capacity. Notably, both SPEM and ADM must be transient and resolve within a few days, to reduce the risk of progression to dysplasia and cancer (Giroux & Rustgi, 2017). This study should stimulate a wide variety of investigations in different cellular systems, to clarify how unresolved or repeated paligenosis may predispose to cancer, and substantiate the tantalizing hints that autophagy and mTORC1 regulation may be part of a common regeneration mechanism.
The EMBO Journal (2018) 37: e99206
See also: https://doi.org/10.15252/embj.201798311 (April 2018)
References
- Friedmann‐Morvinski D, Verma IM (2014) Dedifferentiation and reprogramming: origins of cancer stem cells. EMBO Rep 15: 244–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroux V, Rustgi AK (2017) Metaplasia: tissue injury adaptation and a precursor to the dysplasia‐cancer sequence. Nat Rev Cancer 17: 594–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez‐Sanchez JA, Carty L, Iruarrizaga‐Lejarreta M, Palomo‐Irigoyen M, Varela‐Rey M, Griffith M, Hantke J, Macias‐Camara N, Azkargorta M, Aurrekoetxea I, De Juan VG, Jefferies HB, Aspichueta P, Elortza F, Aransay AM, Martinez‐Chantar ML, Baas F, Mato JM, Mirsky R, Woodhoo A et al (2015) Schwann cell autophagy, myelinophagy, initiates myelin clearance from injured nerves. J Cell Biol 210: 153–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan OM, Carvalho J, Spencer‐Dene B, Mitter R, Frith D, Snijders AP, Wood SA, Behrens A (2018) The deubiquitinase USP9X regulates FBW7 stability and suppresses colorectal cancer. J Clin Invest https://doi.org/10.1172/JCI97325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B, Coleman JH, Peterson JN, Zunitch MJ, Jang W, Herrick DB, Schwob JE (2017) Injury induces endogenous reprogramming and dedifferentiation of neuronal progenitors to multipotency. Cell Stem Cell 21: 761–774 e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao JH, Kim IJ, Wu D, Climent J, Kang HC, DelRosario R, Balmain A (2008) FBXW7 targets mTOR for degradation and cooperates with PTEN in tumor suppression. Science 321: 1499–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng D, Frank AR, Jewell JL (2018) mTOR signaling in stem and progenitor cells. Development 145: dev152595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Levine B (2010) Autophagy in mammalian development and differentiation. Nat Cell Biol 12: 823–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho R, Gruber R, Gu G, Behrens A (2014) Loss of Fbw7 reprograms adult pancreatic ductal cells into alpha, delta, and beta cells. Cell Stem Cell 15: 139–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange DE, Koo BK, Huch M, Sibbel G, Basak O, Lyubimova A, Kujala P, Bartfeld S, Koster J, Geahlen JH, Peters PJ, van Es JH, van de Wetering M, Mills JC, Clevers H (2013) Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell 155: 357–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata PR, Rajagopal J (2016) Cellular plasticity: 1712 to the present day. Curr Opin Cell Biol 43: 46–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphalen CB, Takemoto Y, Tanaka T, Macchini M, Jiang Z, Renz BW, Chen X, Ormanns S, Nagar K, Tailor Y, May R, Cho Y, Asfaha S, Worthley DL, Hayakawa Y, Urbanska AM, Quante M, Reichert M, Broyde J, Subramaniam PS et al (2016) Dclk1 defines quiescent pancreatic progenitors that promote injury‐induced regeneration and tumorigenesis. Cell Stem Cell 18: 441–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willet SG, Lewis MA, Miao Z, Liu D, Radyk MD, Cunningham RL, Burclaff J, Sibbel G, Lo HG, Blanc V, Davidson NO, Wang Z, Mills JC (2018) Regenerative proliferation of differentiated cells by mTORC1‐dependent paligenosis. EMBO J 37: e98311 [DOI] [PMC free article] [PubMed] [Google Scholar]


