Summary
A low but increasing risk of invasive Mycobacterium chimaera infection was identified in cardiothoracic patients. A mechanism for transmission in surgery was identified and, coupled with genome sequencing, provides new evidence supporting a causal relationship between contaminated heater-coolers and infection.
Keywords: nontuberculous mycobacteria, equipment contamination, aerosol release, disease outbreaks, cardiac surgical procedures.
Abstract
Background.
An urgent UK investigation was launched to assess risk of invasive Mycobacterium chimaera infection in cardiothoracic surgery and a possible association with cardiopulmonary bypass heater-cooler units following alerts in Switzerland and The Netherlands.
Methods.
Parallel investigations were pursued: (1) identification of cardiopulmonary bypass–associated M. chimaera infection through national laboratory and hospital admissions data linkage; (2) cohort study to assess patient risk; (3) microbiological and aerobiological investigations of heater-coolers in situ and under controlled laboratory conditions; and (4) whole-genome sequencing of clinical and environmental isolates.
Results.
Eighteen probable cases of cardiopulmonary bypass–associated M. chimaera infection were identified; all except one occurred in adults. Patients had undergone valve replacement in 11 hospitals between 2007 and 2015, a median of 19 months prior to onset (range, 3 months to 5 years). Risk to patients increased after 2010 from <0.2 to 1.65 per 10000 person-years in 2013, a 9-fold rise for infections within 2 years of surgery (rate ratio, 9.08 [95% CI, 1.81–87.76]). Endocarditis was the most common presentation (n = 11). To date, 9 patients have died. Investigations identified aerosol release through breaches in heater-cooler tanks. Mycobacterium chimaera and other pathogens were recovered from water and air samples. Phylogenetic analysis found close clustering of strains from probable cases.
Conclusions.
We identified low but escalating risk of severe M. chimaera infection associated with heater-coolers with cases in a quarter of cardiothoracic centers. Our investigations strengthen etiological evidence for the role of heater-coolers in transmission and raise the possibility of an ongoing, international point-source outbreak. Active management of heater-coolers and heightened clinical awareness are imperative given the consequences of infection.
(See the Editorial Commentary by Kanamori et al on pages 343–6.)
Hospital clusters of nontuberculous mycobacteria (NTM) are well recognized and usually attributed to direct exposure to hospital water supplies or indirect exposure to contaminated medical devices, including surgical instruments and dialysis machines [1–5]. In the context of cardiac surgery, NTM infections are rare; small outbreaks have been described, usually caused by fast-growing NTM such as Mycobacterium fortuitum and Mycobacterium chelonae [6, 7]. Infections have been attributed to contaminated porcine valves; the source is typically unknown although local contamination is usually considered responsible [8, 9]. Consequences are severe, with surgical debridement frequently required [8].
In 2014, 6 cases of severe infection due to Mycobacterium chimaera, a recently described slow-growing mycobacterium within the Mycobacterium avium complex (MAC; similar to Mycobacterium intracellulare), were reported in cardiac surgery patients in Zurich [10]. Investigators hypothesized that patients were infected by contaminated aerosols from the water tanks of heater-cooler units (HCUs) used during cardiopulmonary bypass. The Netherlands, Germany, and the United States subsequently reported similar cases, raising concerns that risk was not restricted to a single center as such HCUs are in global use [11, 12].
To inform national and international response, including the potential need for altered clinical practice, we used a multistranded approach to rapidly investigate and quantify risk to patients in the United Kingdom.
METHODS
The investigations undertaken included national case finding, aerobiological assessment of a decommissioned HCU, environmental sampling of HCUs in use in hospitals, and whole-genome sequence analysis of patient and environmental isolates. Further details are provided in the Supplementary Materials.
Case Definition
A possible case was defined as an individual with MAC cultured from any clinical specimen who had undergone cardiothoracic surgery involving bypass in the preceding 4 years, the maximum documented latency period [10]. Those with a clinical presentation consistent with intraoperative inoculation (endocarditis, surgical site or disseminated infection), were termed probable cases. Probable cases where additionally the same organism was isolated from the HCU used in that patient’s surgery were termed confirmed cases.
Case Finding
Cases were retrospectively identified using national reference and routine laboratory records (2007–2015) matched to national hospital admissions datasets using unique identifiers. Microbiology laboratories were asked to prospectively report possible cases from 11 March 2015. A standardized clinical dataset was collected on all possible cases by telephone interview with local physicians and review of death certification data. Two medical microbiologists used this to classify cases according to the definitions.
Aerobiological Investigation
A decommissioned 3T HCU (Sorin, Milan, Italy), in hospital service since 2002, was subjected to a 4-week aerobiological investigation, comprising microbiological analysis of water and air samples and particle size assessment.
Environmental Sampling
Water and air samples were taken from HCUs at hospitals with and without probable cases and tested for mycobacteria using standard operating procedures [13]. Machines were sampled between February and August 2015.
Microbiological Characterization
Mycobacterial isolates were submitted for identification and archiving. Isolates from probable cases and environmental sampling, identified as M. intracellulare by line probe assay (GenoType Mycobacterium CM, HAIN Lifescience), underwent internal transcribed spacer (ITS) sequencing for provisional differentiation of M. chimaera [14].
Calculation of Risk
A cohort study was undertaken to assess risk of M. chimaera infection in patients undergoing cardiac valve repair/replacement surgery in England. The number of patients at risk was estimated using Hospital Episode Statistics (HES), with procedures selected using standardized codes. The total person-years (PY) at risk was calculated for each annual cohort of patients (2007–2014), assuming a 5-year risk period, with 95% confidence intervals (CIs) calculated assuming a Poisson distribution. No adjustment was made for loss to follow-up. The residual risk for patients with <5 years of follow-up was calculated to estimate future numbers of cases. An equivalent risk was calculated for the general population and for the population with diagnosed human immunodeficiency virus (HIV) in England based on all invasive MAC infections by linking to the national HIV and AIDS Reporting System.
Whole-Genome Sequencing
Whole-genome sequencing (WGS) was performed on isolates from probable cases, control patients without any history in HES of cardiothoracic surgery, and air and water samples from 3T HCUs. DNA was extracted and sequenced using Illumina (San Diego, California) MiSeq and HiSeq sequencing platforms. PacBio (Pacific Biosciences, Menlo Park, California) sequencing of the M. chimaera type strain (DSM 44623) was performed to generate a reference genome sequence. WGS was also performed for representative strains of an additional 13 MAC species. Maximum likelihood phylogenetic trees were constructed using RAxML (version 8.2) [15] to estimate genetic relatedness of isolates. Branch lengths were adjusted for recombination using ClonalFrameML [16].
RESULTS
Case Finding
Between 1 January 2007 and 3 March 2015, 7092 cultures positive for M. intracellulare or other MAC species were identified from the English reference laboratories’ database. Of these, 84% (5954) contained a valid National Health Service (NHS) number identifying 4263 patients. These records were supplemented by routine laboratory reports of nonrespiratory mycobacterial isolates (542 records, 495 patients), giving a total of 4758 patients (Supplementary Figure 6). From these, 54 patients were identified as possible cases having undergone cardiothoracic surgery requiring cardiopulmonary bypass in England in the 4 years before their mycobacterial diagnosis. Ten met the definition of a probable case and an additional 6 probable cases were notified prospectively. Investigations in Wales identified a further 3 possible cases, 2 of which were probable cases; no probable cases were identified in Scotland or Northern Ireland.
Clinical and Microbiological Characteristics
The 18 cases were diagnosed between 2008 and 2015 with an increase over time (Supplementary Figure 7). All except one were adults (Table 1). Cases had undergone mitral (n = 3) or aortic valve replacement (n = 15) using tissue or mechanical valves in 11 of 41 UK cardiothoracic centers. Time between surgery and presentation ranged from 3 months to 5.1 years with 7 cases presenting within 1 year. There was considerable delay between presentation and the first culture sent for mycobacterial investigation for many cases (median, 85 days). Four cases were diagnosed within 1 year of surgery, 10 cases within 1–2 years, and 4 cases 3–5 years postsurgery. Individuals presented with endocarditis with or without aortic root abscess or dissemination (n = 11), deep surgical site infection (n = 3), spinal osteomyelitis (n = 1), and disseminated disease (n = 3). At the time of investigation, 9 individuals had died, 2 recovered, and 7 remained unwell and on treatment. Endocarditis or other mycobacterial infection was certified as contributory to all deaths.
Table 1.
Clinical Characteristics of Probable Cases of Severe Mycobacterium chimaera Infection Associated With Cardiopulmonary Bypass Surgery, United Kingdom
| Characteristic | All Cases (N = 18) |
|---|---|
| Female sex, No. (%) | 5 (28) |
| Median age (range), y | 63 (7–81) |
| Type of surgery, No. (%) | |
| Aortic valve replacement | 14 (77) |
| Mitral valve replacement | 3 (17) |
| Aortic valve replacement and homograft to pulmonary valve (redo) | 1 (6) |
| Site of infection, No. (%) | |
| Sternal osteomyelitis | 2 (11) |
| Anterior mediastinal abscess | 1 (6) |
| Spinal osteomyelitis and discitis | 1 (6) |
| Endocarditis | 5 (28) |
| Endocarditis, aortic root abscess | 3 (17) |
| Endocarditis, disseminated infection | 3 (17) |
| Disseminated infection | 3 (17) |
| Median time between surgery and presentation (range), y | 1.15 (0.25–5.1) |
| Median time between presentation and first mycobacterial culture (range), d | 85 (6–457) |
| Outcome, No. (%) | |
| Death | 9 (50) |
| Recovered | 2 (11) |
| Remains unwell and on treatment | 7 (38) |
| Median time between culture and death (range), d | 71 (14–567) |
Isolates were available from 16 cases and underwent ITS sequencing. Fourteen were provisionally identified as M. chimaera, and 2 could not be identified.
Aerobiological Investigation
The 3 water tanks of the 3T HCU were filled with sterile water and sampled 10 times over 4 weeks. In the absence of decontamination, the heterotrophic plate count remained stable (mean, 4.3 × 108 colony-forming units [CFU]/L) and comprised a mixed population of waterborne organisms including Sphingomonas paucimobilis, Stenotrophomonas maltophilia, Brevundimonas vesicularis, and M. chimaera.
When the HCU was not circulating water, the mean number of bacteria detected in the air 30 cm from the unit was 10 CFU/m3 (n = 8). Once water was circulating, the number significantly increased (mean, 560 CFU/m3; n = 10; P < .01). Organisms isolated from tank water, including M. chimaera, were also recovered from the air. Spatial analysis indicated the highest level of aerosol was released from the rear of the machine (Supplementary Figure 8).
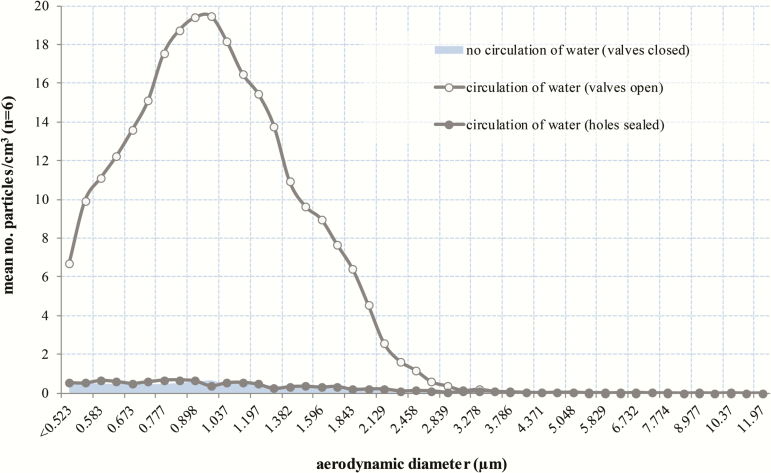
Examination of the HCU identified breaches in the tank covers. An aerodynamic particle sizer detected aerosol release from holes close to the flow and return pipes of both circuits and a gap between the tank sealing plates (Figure 1). When the HCU was not circulating water, the mean number of particles detected 2 cm above the holes and gap was 0.22/cm3 and 2.08/cm3, respectively (n = 6; Figure 2; Supplementary Figure 9). When water was circulating, this significantly increased (mean: 5.84/cm3 [holes] and 7.71/cm3 [gap]; n = 6; P < .01). Particle size distribution peaked at just under 1 µm and clearly indicated aerosol release through construction joints on the tank covers into the body of the HCU. Flow visualization demonstrated movement of particles from these areas to the outside environment via the cooling fan at the rear of the HCU (Supplementary Figure 10). Sealing the holes and gaps significantly reduced the number of particles detected (P < .01), in most cases to baseline levels (Figure 2; Supplementary Figure 9).
Figure 1.
Holes (highlighted by the red circles) close to the flow and return pipes of both heater-cooler circuits and gap between tank sealing plates identified by an aerodynamic particle sizer as areas of aerosol release.
Figure 2.
Mean (n = 6) number of particles released from a series of holes close to the flow and return pipes of the patient circuit. The number of particles detected after the holes had been sealed with adhesive putty is also shown. The mean number of particles detected after the holes had been sealed was similar to that detected when the valves were closed (ie, when the heater-cooler unit was not circulating water), hence an overlapping distribution.
Environmental Sampling
Water was taken from 35 3T HCUs at 10 hospitals in England. Twenty-seven (77%) samples were positive for mycobacteria and 17 (48%) positive for M. chimaera (Supplementary Table 2). Mycobacteria were recovered from air samples taken in the vicinity of 6 of 25 (24%) 3T HCUs during normal operation. Five of these contained M. chimaera. Maquet HCU30 and HCU40 devices (Maquet Cardiopulmonary GmbH, Rastatt, Germany) were also sampled, though a limited number were in use. Three of 6 HCU30 and 4 of 4 HCU40 had mycobacteria in the water, and one of each had M. chimaera. Mycobacteria were not recovered from HCU30 (n = 3) or HCU40 (n = 4) air samples.
With manufacturer and local teams’ permission, the cover and side panels were removed from seven 3T HCUs in situ. There were no visible gaps/holes in the water tanks of 6 HCUs (manufactured 2009–2012), but tank breaches were observed in one 2003 unit.
Risk Assessment
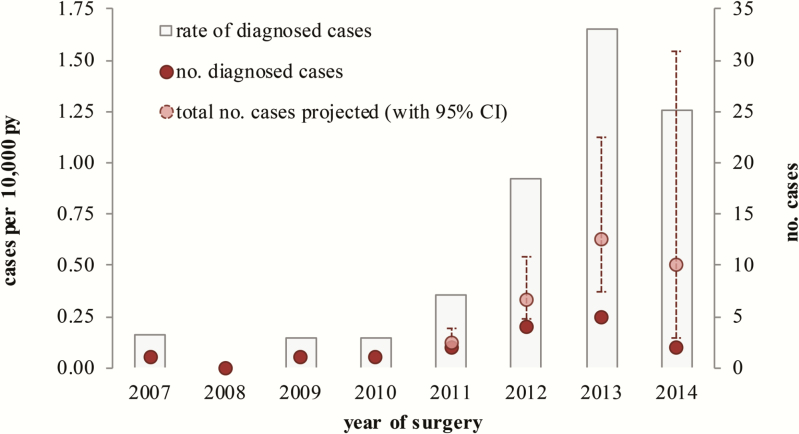
Between 2007 and 2014, 112 644 individuals underwent 115 664 surgical procedures in England involving repair or replacement of cardiac valves. Assuming M. chimaera infection risk was maintained for 5 years (411 141 PY at risk), the 16 cases translates to 0.39 (95% CI, .22–.63) cases per 10000 PY. There was a general increased risk each year after 2010 from <0.2 to 1.65 per 10000 person-years in 2013 (Figure 3). Restricting analyses to infections within 2 years of surgery, given the higher risk in this period, still showed an increasing trend, with 9-fold elevation between 2007–2011 and 2012–2013 (rate ratio, 9.08 [95% CI, 1.81–87.76]). Trust-specific risks for sites with probable cases were generally higher than the national risk estimate, from 0.54 to 3.36 per 10000 PY, with just one Trust having a marginally lower risk (0.38). Based on the 5-year risk period, an additional 19 cases (95% CI, 11–30) can be expected to be diagnosed in the future for individuals who have not completed their period at risk.
Figure 3.
Assessment of risk of Mycobacterium chimaera infection following cardiac valve repair or replacement in England, 2007–2014. Abbreviations: CI, confidence interval; PY, person-years.
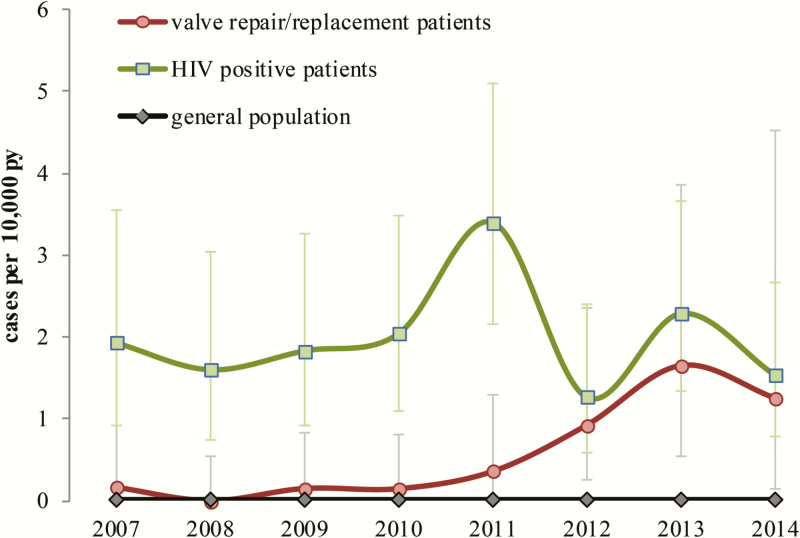
Of 456 patients identified as having invasive MAC infection between 2007 and March 2015, 105 (23%) had a diagnosed HIV infection, a risk of 2.01 per 10000 PY (95% CI, 1.64–2.43) among persons receiving HIV care in England; 97% (99/102) had a CD4 count <200 cells/μL at or around the time of invasive MAC infection. In the general population, individuals not known to be HIV positive or to have undergone valve replacements, the risk of infection was 0.0078 per 10000 PY. From 2012, the risk in valve replacement patients was significantly elevated compared with the general population (P < .001) and approached the risk in HIV-infected patients (Figure 4).
Figure 4.
Comparison of risk of invasive Mycobacterium avium complex disease in persons living with HIV, the general population (defined as individuals not known to be HIV infected or to have undergone cardiac valve repair or replacement), and patients undergoing cardiac valve replacement or repair, England, 2007–2014. Risk of M. avium complex infection was compared for different population groups. For patients undergoing cardiac replacement, these isolates were identified specifically as M. chimaera as part of this investigation. Abbreviations: HIV, human immunodeficiency virus; PY, person-years.
Whole-Genome Sequencing
A total of 274 isolates from 15 probable cases (29 isolates), 159 patient controls (n = 200), and air and water samples from 11 HCUs (n = 45) underwent WGS; 13 were excluded due to suspected contamination with non-MAC DNA. The total length of the M. chimaera genome sequence alignment was 5 534 134 base pairs, of which 1% (55 634) was considered variable. Phylogenetic analysis of sequence data showed that 94% (246/261) of isolates, including 26 from 15 probable cases, 184 from 143 controls, and 36 from 11 HCUs, clustered very closely with the M. chimaera reference strain, confirming the species identification (Supplementary Figure 11). Fifteen isolates fell outside the M. chimaera clade, one from an HCU and one from a probable case, although additional isolates from this case clustered within the M. chimaera clade. Of the 2 probable cases that could not be identified by ITS sequencing, WGS identified one as M. chimaera, while additional isolates from the other case identified it as M. chimaera.
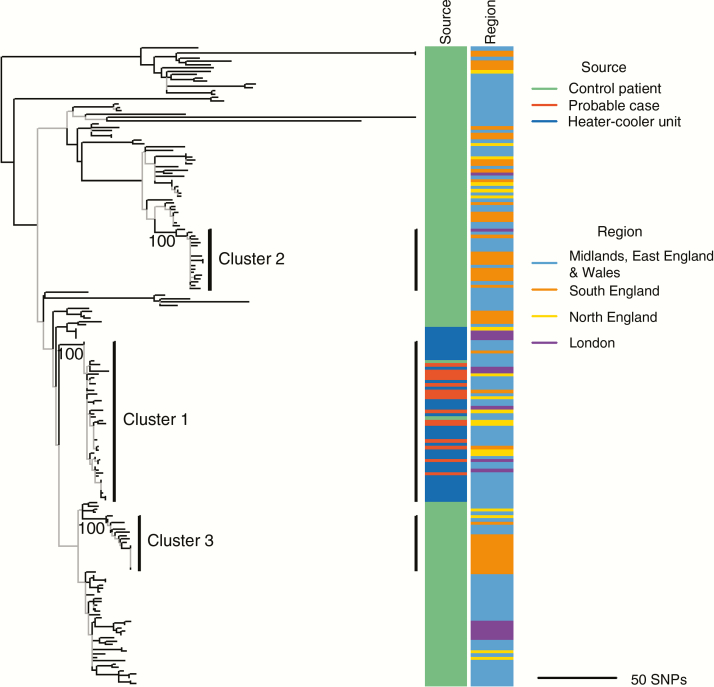
Phylogenetic analysis identified strong clustering of the M. chimaera isolates, which included isolates from all probable cases and 32 of 37 (86%) isolates from Sorin HCUs (cluster 1; Figure 5). Genetic diversity within cluster 1 was much lower than across control isolates with mean pairwise distances of 10 single-nucleotide polymorphisms vs 132. Limited genetic diversity within cluster 1 and across serially sampled patients prevented reliable estimation of the evolutionary rate of M. chimaera (Supplementary Figures 12 and 13).
Figure 5.
Recombination-corrected maximum likelihood phylogenetic tree of Mycobacterium chimaera isolates (n = 191). Data were subsampled to one isolate per patient by including only the first isolate from serially sampled patients or one at random in the absence of any sample date information. The tree is midpoint rooted and branch lengths are scaled in units of the number of single-nucleotide polymorphisms (SNPs) per genome. Branches are colored according to their corresponding bootstrap support value, estimated from 100 bootstrap replicates (gray, <70; black, ≥70). Three strongly supported clusters of closely related isolates are annotated on the tree with the corresponding bootstrap value displayed below the branch (defined as clusters supported by a bootstrap value ≥70, comprising >10 isolates, in which isolates are within 13 SNPs of their closest isolate). Isolates within cluster 1 were sampled over 6.6 years across all 4 National Health Service regions. The 2 panels to the right of the tree show the source and region of each isolate in the tree.
Cluster 1 also included isolates from 2 control patients. Hospital records showed cardiac and respiratory comorbidities for one of these patients, including cardiac investigations in the year before mycobacterial diagnosis, raising the possibility of non-NHS surgery. The other patient received treatment for metastatic cancer at a hospital with a cardiothoracic center, but no procedures likely to require a cardiac operating room were identified.
DISCUSSION
This national investigation identified a significantly elevated and increasing risk of M. chimaera infection after cardiothoracic surgery compared to the general population. By 2012, this approached the risk in persons living with HIV. In the context of other risks experienced by such patients, this was not substantial; of 10 000 patients undergoing such procedures, 120 could expect to experience a surgical site infection, 300–400 to experience endocarditis, and one to develop M. chimaera infection by 5 years postsurgery [17, 18].
Patients undergoing cardiac valve surgery were at particular risk despite cardiopulmonary bypass being commonly used for other procedures, notably coronary artery bypass graft, consistent with other findings [11]. The duration of surgery is similar between these procedures, suggesting that prosthetic valves may predispose to infection [18]. Case fatality was high (50%), comparable to other investigations [11]. This is not unexpected as slow-growing mycobacteria have intrinsic antibiotic resistance, require prolonged treatment, and infect sites challenging for antimicrobial penetration [19, 20]. Furthermore, diagnosis of mycobacterial infection, and thus appropriate treatment, was delayed. Given that the risk approaches that in HIV patients, mycobacterial investigations should be routinely employed or, at minimum, second-line testing in patients with endocarditis and other relevant infections following cardiothoracic surgery should be done.
While other investigators have proposed the HCU as the likely source [10, 21, 22], this study demonstrates for the first time the exact source of the bioaerosol, its low particle size (<1 µm), and release into the operating environment via the rear cooling fan. This significantly strengthens the evidence for an etiological role of HCUs in M. chimaera infection.
Our hospital investigations suggest that HCU contamination is widespread and may not be device specific. This implies a systematic decontamination failure. Other opportunistic pathogens including Legionella species were isolated from water taken from devices. Although review of national surveillance failed to find any cases of legionellosis in healthcare workers with potential occupational exposure to HCUs, transmission of nonmycobacterial infections remains a possibility.
The ability to generate a rapid national quantitative risk assessment was instrumental in allowing safe decision making and avoided unnecessary widespread disruption to cardiothoracic services. This was possible through access to national hospital and microbiology data, from which a preliminary risk quantification was generated in weeks. Through discussions among public health, regulatory, surgical, and perfusion specialists, a proportionate risk management strategy was implemented recognizing that the risk of delaying valve replacement is generally greater than the risk posed by this infection [23].
Immediate mitigation of risk is complex due to the need to maintain cardiothoracic operating capacity. Alternative devices are not readily available; some contain M. chimaera and have not been tested for aerosol generation. Ultraclean operating room ventilation does not appear to provide protection [22]. Local risk management strategies including enhanced HCU decontamination, positioning, and perhaps containment will reduce and may eventually eliminate risk; new HCU models may contribute but require assessment. Given the long latency, further cases should be expected. It is important to maintain physicians’ awareness of the possibility of mycobacterial infection in the context of endocarditis, surgical site infections, and undiagnosed systemic illness following cardiothoracic surgery to ensure prompt and appropriate investigations.
WGS analysis detected very low genetic diversity across probable cases, which were restricted to a single lineage within the M. chimaera species. The absence of any genetically divergent M. chimaera among probable cases and high genetic similarity to HCU isolates is consistent with a role for HCUs in transmission. This shared lineage suggests either a biological advantage to this specific strain or a point-source contamination. International collaborative work is under way to investigate this further [24, 25].
Underascertainment of cases is likely, as mycobacterial investigations are not routinely conducted in suspected endocarditis in the United Kingdom and routine blood cultures have a low sensitivity for mycobacterial growth [26]. Underascertainment may also explain the clustering of cases in some hospitals, reflecting variation in local diagnostic practices, but could also relate to the age of machines in use. Our finding of an increased risk over time may similarly reflect aging of HCUs, should this facilitate increased transmission through a widening of breaches or buildup of biofilm. Improved awareness and testing of patients is also likely to have contributed to the increase.
There are a number of other limitations in the investigation. Nearly a fifth of laboratory records used in case finding in England were missing unique identifiers, preventing us from identifying prior cardiac surgery. We used a 5-year period of risk after surgery based on the observed maximum incubation, but longer latency is possible. As such, our risk estimate is subject to uncertainty. Environmental sampling was by necessity carried out long after any transmission occurred. In-depth aerobiological investigations to date are limited to one machine by one manufacturer and might not be generalizable to other manufacturers or newer HCUs. Of the HCUs visually inspected, breaches in the water tanks were only observed in units manufactured before 2004. Changes to manufacturing processes may have improved the integrity of newer units, but no conclusions can be drawn regarding age or model of device and its potential to release an aerosol given the small number inspected in our study. It was not possible to link individual cases and devices in this investigation. A recommendation to improve traceability of these devices has been made.
In conclusion, our study confirmed a low but continuing and widespread risk of severe M. chimaera infection, a likely result of exposure to bioaerosol produced by HCUs used globally for cardiothoracic surgery. Changes in device management and diagnostics are urgently required to protect patients from this avoidable and potentially fatal infection. In this era of globalized supply of healthcare products, opportunities for widespread dissemination of contaminated devices are ever present, making communication between regulatory and public health authorities essential to minimize delay in identification of patient safety signals.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Supplementary Material
Notes
Acknowledgements. We thank Nick Hinton for his expert querying and linkage of data, the expert advice and assistance provided by the Public Health England (PHE) Surgical Site Infection Surveillance Service (Pauline Harrington, Dr Catherine Wloch, Suzanne Elgohari), the PHE Respiratory Diseases Department (Drs Gavin Dabrera, Dominik Zenner), and the PHE HIV/STI Department (Dr Valerie Delpech). We acknowledge the assistance of Andrew Heggie at Blackpool Teaching Hospitals NHS Foundation Trust, Prof. M. H. Wilcox, and Dr John Paul and staff at Leeds Teaching Hospitals and Brighton and Sussex University Hospitals. We extend our sincere thanks to all NHS Trusts (England), NHS Boards (Scotland), Health Boards (Wales), and Health and Social Care Trusts and Board (Northern Ireland) who assisted with the investigation; the Medicines and Healthcare products Regulatory Agency for advice and direction; the Information Services Division for linkage of data in Scotland; and the PHE Emergency Response Department for administrative support during the investigation. Last, we extend our gratitude to the Health and Social Care Information Centre for provision of Hospital Episode Statistics (© 2015. Reused with the permission of the Health and Social Care Information Centre. All rights reserved) and the Office for National Statistics for supply of Death Registrations.
Disclaimer. The report presents independent research funded by the National Institute for Health Research (NIHR), Wellcome Trust, and the Department of Health. The views expressed in this publication are those of the authors and not necessarily those of the NHS, Wellcome Trust, NIHR, Department of Health, or PHE.
Financial support. This work was supported by Public Health England, the Health Innovation Challenge Fund (a parallel funding partnership between the Wellcome Trust [grant number WT098615/Z/12/Z] and the Department of Health [grant number HICF-T5-358]) and the NIHR Health Protection Research Units at Oxford University (Healthcare Associated Infection and Antimicrobial Resistance [grant number HPRU-2012–10041]) and Imperial College (Respiratory Infections [grant number HPRU-2012–10064]).
Potential conflicts of interest. All authors: No potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Wallace RJ, Jr, Brown BA, Griffith DE. Nosocomial outbreaks/pseudo-outbreaks caused by nontuberculous mycobacteria. Annu Rev Microbiol 1998; 52:453–90. [DOI] [PubMed] [Google Scholar]
- 2. Iroh Tam PY, Kline S, Wagner JE, et al. Rapidly growing mycobacteria among pediatric hematopoietic cell transplant patients traced to the hospital water supply. Pediatr Infect Dis J 2014; 33:1043–6. [DOI] [PubMed] [Google Scholar]
- 3. Tagashira Y, Kozai Y, Yamasa H, Sakurada M, Kashiyama T, Honda H. A cluster of central line-associated bloodstream infections due to rapidly growing nontuberculous mycobacteria in patients with hematologic disorders at a Japanese tertiary care center: an outbreak investigation and review of the literature. Infect Control Hosp Epidemiol 2015; 36:76–80. [DOI] [PubMed] [Google Scholar]
- 4. Lowry PW, Beck-Sague CM, Bland LA, et al. Mycobacterium chelonae infection among patients receiving high-flux dialysis in a hemodialysis clinic in California. J Infect Dis 1990; 161:85–90. [DOI] [PubMed] [Google Scholar]
- 5. Meyers H, Brown-Elliott BA, Moore D, et al. An outbreak of Mycobacterium chelonae infection following liposuction. Clin Infect Dis 2002; 34:1500–7. [DOI] [PubMed] [Google Scholar]
- 6. Kuritsky JN, Bullen MG, Broome CV, Silcox VA, Good RC, Wallace RJ., Jr Sternal wound infections and endocarditis due to organisms of the Mycobacterium fortuitum complex. Ann Intern Med 1983; 98:938–9. [DOI] [PubMed] [Google Scholar]
- 7. Strabelli TM, Siciliano RF, Castelli JB, et al. Mycobacterium chelonae valve endocarditis resulting from contaminated biological prostheses. J Infect 2010; 60:467–73. [DOI] [PubMed] [Google Scholar]
- 8. Unai S, Miessau J, Karbowski P, Bajwa G, Hirose H. Sternal wound infection caused by Mycobacterium chelonae. J Card Surg 2013; 28:687–92. [DOI] [PubMed] [Google Scholar]
- 9. Wallace RJ, Jr, Musser JM, Hull SI, et al. Diversity and sources of rapidly growing mycobacteria associated with infections following cardiac surgery. J Infect Dis 1989; 159:708–16. [DOI] [PubMed] [Google Scholar]
- 10. Sax H, Bloemberg G, Hasse B, et al. Prolonged outbreak of Mycobacterium chimaera infection after open-chest heart surgery. Clin Infect Dis 2015; 61:67–75. [DOI] [PubMed] [Google Scholar]
- 11. Kohler P, Kuster SP, Bloemberg G, et al. Healthcare-associated prosthetic heart valve, aortic vascular graft, and disseminated Mycobacterium chimaera infections subsequent to open heart surgery. Eur Heart J 2015; 36:2745–53. [DOI] [PubMed] [Google Scholar]
- 12. Centers for Disease Control and Prevention. Non-tuberculous Mycobacterium (NTM) infections and heater-cooler devices. Interim practical guidance 2015. Available at: http://www.cdc.gov/HAI/pdfs/outbreaks/CDC-Notice-Heater-Cooler-Units-final-clean.pdf Accessed 6 June 2016.
- 13. Public Health England. Protocol for environmental sampling, processing and culturing of water and air samples for the isolation of slow-growing mycobacteria: standard operating procedure 2015. Available at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/434197/Air_water_environmental_sampling_SoP.pdf Accessed 22 March 2016.
- 14. Tortoli E, Rindi L, Garcia MJ, et al. Proposal to elevate the genetic variant MAC-A, included in the Mycobacterium avium complex, to species rank as Mycobacterium chimaera sp. nov. Int J Syst Evol Microbiol 2004; 54:1277–85. [DOI] [PubMed] [Google Scholar]
- 15. Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014; 30:1312–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Didelot X, Wilson DJ. ClonalFrameML: efficient inference of recombination in whole bacterial genomes. PLoS Comput Biol 2015; 11:e1004041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cahill TJ, Prendergast BD. Infective endocarditis. Lancet 2016; 387:882–93. [DOI] [PubMed] [Google Scholar]
- 18. Public Health England. Surveillance of surgical site infections in NHS hospitals in England 2014/15 Available at: https://www.gov.uk/government/publications/surgical-site-infections-ssi-surveillance-nhs-hospitals-in-england Accessed 6 June 2016.
- 19. van Ingen J, Boeree MJ, van Soolingen D, Mouton JW. Resistance mechanisms and drug susceptibility testing of nontuberculous mycobacteria. Drug Resist Updat 2012; 15:149–61. [DOI] [PubMed] [Google Scholar]
- 20. Griffith DE, Aksamit T, Brown-Elliott BA, et al. ; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007; 175:367–416. [DOI] [PubMed] [Google Scholar]
- 21. Götting T, Klassen S, Jonas D, Benk C, Serr A, Wagner D, et al. Heater-cooler units: contamination of crucial devices in cardiothoracic surgery. J Hosp Infect 2016; 93:223–8. [DOI] [PubMed] [Google Scholar]
- 22. Sommerstein R, Rüegg C, Kohler P, Bloemberg G, Kuster SP, Sax H. Transmission of Mycobacterium chimaera from heater-cooler units during cardiac surgery despite an ultraclean air ventilation system. Emerg Infect Dis 2016; 22:1008–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012; 33:2451–96. [DOI] [PubMed] [Google Scholar]
- 24. Garvey MI, Ashford R, Bradley CW, Bradley CR, Martin TA, Walker J, Jumaa P. Decontamination of heater-cooler units associated with contamination by atypical mycobacteria. J Hosp Infect 2016; 93:229–34. [DOI] [PubMed] [Google Scholar]
- 25. Haller S, Holler C, Jacobshagen A, Hamouda O, Abu SM, Monnet DL, et al. Contamination during production of heater-cooler units by Mycobacterium chimaera potential cause for invasive cardiovascular infections: results of an outbreak investigation in Germany, April 2015 to February 2016. Euro Surveill 2016; 21. pii:30215. [DOI] [PubMed] [Google Scholar]
- 26. Fuller DD, Davis TE, Jr, Denys GA, York MK. Evaluation of BACTEC MYCO/F Lytic medium for recovery of mycobacteria, fungi, and bacteria from blood. J Clin Microbiol 2001; 39:2933–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.