Abstract
Purpose
To assess the accuracy of staging positron emission tomography (PET)/computed tomography (CT) in detecting distant metastasis in patients with local-regionally advanced cervical and high-risk endometrial cancer in the clinical trial by the American College of Radiology Imaging Network (ACRIN) and the Gynecology Oncology Group (GOG) (ACRIN 6671/GOG 0233) and to compare central and institutional reader performance.
Materials and Methods
In this prospective multicenter trial, PET/CT and clinical data were reviewed for patients enrolled in ACRIN 6671/GOG 0233. Two central readers, blinded to site read and reference standard, reviewed PET/CT images for distant metastasis. Central review was then compared with institutional point-of-care interpretation. Reference standard was pathologic and imaging follow-up. Test performance for central and site reviews of PET/CT images was calculated and receiver operating characteristic analysis was performed. Generalized estimating equations and nonparametric bootstrap procedure for clustered data were used to assess statistical significance.
Results
There were 153 patients with cervical cancer and 203 patients with endometrial cancer enrolled at 28 sites. Overall prevalence of distant metastasis was 13.7% (21 of 153) for cervical cancer and 11.8% (24 of 203) for endometrial cancer. Central reader PET/CT interpretation demonstrated sensitivity, specificity, positive predictive value (PPV), and negative predictive value of 54.8%, 97.7%, 79.3%, and 93.1% for cervical cancer metastasis versus 64.6%, 98.6%, 86.1%, and 95.4% for endometrial cancer, respectively. By comparison, local institutional review demonstrated sensitivity, specificity, PPV, and negative predictive value of 47.6%, 93.9%, 55.6%, and 91.9% for cervical cancer metastasis and 66.7%, 93.9%, 59.3%, and 95.5% for endometrial cancer, respectively. For central readers, the specificity and PPV of PET/CT detection of cervical and endometrial cancer metastases were all significantly higher compared with that of local institutional review (P < .05). Central reader area under the receiver operating characteristic curve (AUC) values were 0.78 and 0.89 for cervical and endometrial cancer, respectively; these were not significantly different from local institutional AUC values (0.75 and 0.84, respectively; P > .05 for both).
Conclusion
FDG PET/CT demonstrates high specificity and PPV for detecting distant metastasis in cervical and endometrial cancer and should be included in the staging evaluation. Blinded central review of imaging provides improved specificity and PPV for the detection of metastases and should be considered for future oncologic imaging clinical trials.
© RSNA, 2017
Introduction
Uterine cervical and endometrial cancer are two of the most common gynecologic malignancies, responsible for over 65 000 new cancer cases and 14 000 cancer-related deaths each year in the United States (1). ACRIN 6671/GOG 0233, the recent multicenter clinical trial by the American College of Radiology Imaging Network (ACRIN) and the Gynecology Oncology Group (GOG), seeks to evaluate the utility of preoperative fluorine 18 fluorodeoxyglucose (FDG) positron emission tomography (PET)/computed tomography (CT) to help detect retroperitoneal lymph node metastasis in patients with local-regionally advanced cervical and endometrial carcinoma. The primary objectives of this trial were to determine the diagnostic sensitivity and specificity of PET/CT in identifying abdominal and pelvic lymph node metastasis in this patient population. Recently published results from this trial show improved performance of FDG PET/CT compared with CT alone for detection of abdominal and pelvic lymph node metastasis (2,3).
An essential secondary endpoint of this trial was to determine the performance of PET/CT for detecting distant metastatic disease. The presence of distant metastatic disease has important disease implications, including conferring a substantially poorer prognosis for both endometrial (4) and cervical (5) cancer. In addition, distant metastasis guides treatment strategy, triggering initiation of chemotherapy or radiation therapy regimens aimed at controlling hematogenous spread of disease and/or targeting individual metastatic lesions for palliation (6,7). Finally, preoperative detection of distant metastatic disease could potentially avoid morbidity associated with local-regionally–directed procedures and therapies such as surgical lymphadenectomy, which is associated with an increased risk of early and late postoperative complications (8).
The data on diagnostic performance of PET/CT in detection of distant metastases in uterine cancer are sparse. A recent meta-analysis of FDG PET staging of endometrial cancers suggested the sensitivity and specificity of FDG PET for distant metastatic disease are both greater than 90% based on existing literature, but cautioned that these studies are limited by the small number of patients included and the lack of reliable reference standard in most cases (9). A retrospective study evaluating PET/CT detection of distant metastatic disease in cervical cancer reports high sensitivity and specificity (both >90%), although this study was performed in patients in remission undergoing PET/CT for suspected recurrence, rather than initial staging (10). Secondary analysis of the ACRIN 6671/GOG 0233 study population would overcome these limitations because the study has accrued a large number (>350) of patients, all of whom were imaged at the time of initial staging and underwent pathologic, imaging, and clinical follow-up as part of the trial protocol (2,3).
The purpose of this study was to assess the accuracy of staging PET/CT for detecting distant metastasis in patients with local-regionally advanced cervical or high-risk endometrial cancer in the ACRIN 6671/GOG 0233 patient cohort and to compare central and institutional reader performance.
Materials and Methods
Study Population
The patient cohort for this study consisted of patients enrolled in the recent multicenter National Cancer Institute–funded clinical trial sponsored by the ACRIN and the GOG (ACRIN 6671/GOG 0233) evaluating the utility of preoperative FDG PET/CT in detecting abdominopelvic lymph node metastasis in patients with local-regionally advanced cervical and high-risk endometrial carcinoma (2,3). This nonrandomized prospective study was Health Insurance Portability and Accountability Act–compliant and was approved by the institutional review board of each participating institution. All patients gave informed consent to participate in the study. Patients were accrued from March 2007 to June 2013.
All patients in the study had primary, histologically confirmed, untreated local-regionally advanced cervical cancer (stage IB2 tumors, IIA tumors >4 cm, IIB–IVA tumors) or high-risk endometrial cancer (grade 3 endometrioid endometrial carcinoma; clear cell carcinoma, serous papillary carcinoma, or carcinosarcoma [any grade]; or grade 1 or 2 endometrioid endometrial carcinoma with cervical stromal involvement overt at clinical examination or confirmed by endocervical curettage). All patients underwent PET/CT imaging within 2 weeks of surgery. The study schema is summarized in Figure 1. A total of 169 patients with cervical cancer and 215 patients with endometrial cancer from 22 sites were accrued into the study. Five patients with cervical cancer and six patients with endometrial cancer were excluded because diagnostic PET/CT was not available for interpretation, while an additional 11 patients with cervical cancer and six patients with endometrial cancer were excluded for lack of available pathologic or surgical reference for distant metastasis. The final study population consisted of 153 patients with cervical cancer and 203 patients with endometrial cancer with diagnostic PET/CT imaging findings available for site and central review, along with reference standard documentation for presence or absence of distant metastatic disease. The abdominal lymph node PET/CT imaging findings from a subset of patients (129 of 356) enrolled in this trial were previously reported (3) in a study that assessed PET/CT performance for abdominal lymph node detection. Our study focused on PET/CT detection of distant metastatic disease based on the entire study population of ACRIN 6671/GOG 0233.
Figure 1:
Schema of ACRIN 6671/GOG 0233 trial. Bx = biopsy, pts = patients.
Imaging Protocol
All patients underwent FDG PET/CT at the local institution in which they were enrolled, consisting of FDG PET and concurrent diagnostic CT image acquisition from the neck through the upper thigh. PET imaging was performed at 60 minutes ± 10 (standard deviation) after administration of 10–20 mCi (370–740 MBq) (0.14–0.21 mCi [5.18–7.77 MBq]/kg) of FDG. For the diagnostic CT, patients received oral and nonionic intravenous contrast agent unless there was a contraindication to intravenous contrast agent because of allergy, renal dysfunction, or lack of intravenous access. The volume of intravenous contrast agent was administered according to a weight-based protocol of 125–175 mL, and power injected at a rate of 3 mL/sec with a scan delay of 55–85 seconds. Intravenous iodinated contrast agent was used in 144 of 153 (94.1%) PET/CT studies of cervical cancer and 198 of 203 (97.5%) PET/CT studies of endometrial cancer.
PET/CT Image Interpretation for Distant Metastasis
Each imaging study was interpreted by the local site institutional radiologist prior to surgery. As part of the trial, the institutional radiologist was asked to evaluate for the presence of nonregional lymph node or hematogenous metastasis by using a confidence scale of 1–6 as previously described (2), with 1 being definitely negative and 6 being definitely positive. Two independent central readers blinded to the institutional review and the reference standard (M.G. and S.L., both with 11 years of experience interpreting PET/CT studies) also reviewed the images. Distant metastasis was defined according to the International Federation of Gynecology and Obstetrics and included nonregional lymph nodes (including inguinal lymph nodes for endometrial cancer) as well as lesions in the peritoneum, bone, liver, and lung. PET, CT, and fused PET/CT images were reviewed. Each image was scored by using the same 1–6 confidence scale that was used by the institutional readers. For the purpose of test performance calculation, scores of 1–3 were considered negative for presence of distant metastatic disease and scores of 4–6 were considered positive for presence of distant metastatic disease. For each positive score, the location of the lesion(s) was recorded. Both central reader scores for each case were used for the purpose of test performance comparison with local institutional interpretation.
Reference Standard for Metastases
Per study protocol, all imaging findings were jointly reviewed at the point of care by the radiologist and the surgeon at each patient’s local institution prior to surgery. Any lesions suspicious for distant metastases were biopsied, either under imaging guidance preoperatively or at the time of the surgery. For biopsied lesions, the reference standards were the surgical and pathologic reports. Multiple lesions in a single anatomic location were considered a single distant metastatic site. For PET/CT imaging studies interpreted as negative for distant metastatic disease, the 6-month follow-up CT imaging performed after surgery as part of the trial was used to confirm absence of distant metastasis. Any distant metastatic lesions noted on the 6-month follow-up CT images were then compared with PET/CT images obtained before surgery to determine if a corresponding lesion was present in retrospect; if so, this was also considered a positive finding for distant metastasis. If no corresponding lesion was seen on either PET or CT portions of the PET/CT, then such cases were considered PET/CT negative with subsequent progression. For distant metastatic lesions detected retrospectively by central readers but not by the local reader, determination of true or false positivity was made by using a similar protocol. First, a combination of the surgical and pathologic reports were obtained at the time of surgery for lesions that would be visible at surgery. Otherwise, 6-month follow-up CT imaging was used to determine whether findings for distant metastatic disease were true or false-positive by using the same process as defined above.
Statistical Analysis
PET/CT test performance characteristics for detection of distant metastatic lesions, including receiver operating characteristic curves, were calculated for both central and site interpretations on a per-patient basis. For central interpretation, the interpretations of both readers were used for the purpose of test performance calculations, accounting for correlation between the examinations of the same patient. For analysis of sensitivity and specificity, reader scores of 4–6 were considered positive for the presence of distant metastases. 95% confidence intervals for test performance characteristics were constructed by using exact Clopper-Pearson intervals for local institutional data. Nonparametric bootstrap procedure for clustered data was used for evaluating performance characteristics for central data, as well as for evaluating and comparing area under the receiver operating characteristic curves (AUCs) and predictive values for both central and institutional readings. Statistical significance of differences in performance between central and institutional test performance was assessed by using a generalized linear model (Proc Genmod, SAS version 9.4; SAS Institute, Cary, NC).
Results
Patient Population
Among the 356 patients, the mean age ± standard deviation of the cervical cancer cohort was 48.9 years ± 10.6 (range, 24–74 years), whereas the mean age of the endometrial cancer cohort was 62.7 years ± 9.6 (range, 36–81 years).
Prevalence of Distant Metastasis
Overall prevalence of distant metastasis (Table 1) was 12.6% (45 of 356), including 13.7% (21 of 153) for patients with cervical cancer and 11.8% (24 of 203) for patients with endometrial cancer. 37 cases were diagnosed by using biopsy either prior to surgery or at the time of surgery, three cases were diffuse peritoneal metastatic disease noted in the surgical report that did not have a corresponding pathologic report, and five cases were diagnosed after surgery based on follow-up imaging showing growth of a preexisting lesion (followed by biopsy confirmation) interpreted as nonmetastatic at initial PET/CT.
Table 1.
Prevalence and Location of Distant Metastases in the ACRIN 6671/GOG 0233 Cohort Confirmed Pathologically at Surgery or Image-guided Biopsy

*Data in parentheses are percentages, and data in brackets are 95% confidence intervals.
†Data are the number of patients, with percentages in parentheses.
Anatomic sites of distant disease are listed in Table 1. The most common sites included lung (5.2%) and peritoneum (4.6%) for cervical cancer and peritoneum (6.9%) for endometrial cancer. There were four cases with multiple distant sites of metastasis documented in the surgical and pathologic reports; otherwise, a single site of metastasis was noted in each case.
PET/CT Diagnostic Performance for Detection of Distant Metastasis
Combined central reader PET/CT interpretation (Table 2) demonstrated sensitivity, specificity, positive predictive value (PPV), and negative predictive value of 54.8% (23 of 42), 97.7% (258 of 264), 79.3% (23 of 29), and 93.1% (258 of 277) for cervical cancer metastasis versus 64.6% (31 of 48), 98.6% (353 of 358), 86.1% (31 of 36), and 95.4% (353 of 370) for endometrial cancer, respectively. Central reader 1 test performance for sensitivity, specificity, PPV, and negative predictive value was 57.1%, 97.0%, 75.0%, and 93.4% for cervical cancer metastasis versus 70.8%, 98.3%, 85.0%, and 96.2% for endometrial cancer, respectively. By comparison, central reader 2 test performance for sensitivity, specificity, PPV, and negative predictive value was 52.4%, 98.5%, 84.6%, and 92.9% for cervical cancer metastasis and 58.3%, 98.9%, 87.5%, and 94.7% for endometrial cancer, respectively. Examples of distant metastases seen at PET/CT are shown in Figure 2.
Table 2.
Diagnostic Performance of Central Reader and Local Institutional Interpretation of FDG PET/CT for Detecting Distant Metastatic Disease

Note.—Results are based on image interpretation by two central readers blinded to clinical trial data. CR = central reader, NPV = negative predictive value.
*Data in parentheses are 95% confidence intervals.
†Indicate statistical significance of P values comparing local and central performance characteristics. All analyses were performed on a per-patient basis.
Figure 2a:

Images show examples of pathologically confirmed distant metastasis detected by using PET/CT. (a) PET image and (b) CT image in a 34-year-old woman with cervical cancer show pulmonary metastasis (arrows) in right lower lobe that was confirmed at image-guided biopsy. (c) PET image and (d) CT image in a 59-year-old woman with endometrial cancer show multiple FDG-avid peritoneal nodules in cul-de-sac (arrows) confirmed at surgical biopsy. Primary endometrial tumor is also seen (*).
Figure 2b:

Images show examples of pathologically confirmed distant metastasis detected by using PET/CT. (a) PET image and (b) CT image in a 34-year-old woman with cervical cancer show pulmonary metastasis (arrows) in right lower lobe that was confirmed at image-guided biopsy. (c) PET image and (d) CT image in a 59-year-old woman with endometrial cancer show multiple FDG-avid peritoneal nodules in cul-de-sac (arrows) confirmed at surgical biopsy. Primary endometrial tumor is also seen (*).
Figure 2c:

Images show examples of pathologically confirmed distant metastasis detected by using PET/CT. (a) PET image and (b) CT image in a 34-year-old woman with cervical cancer show pulmonary metastasis (arrows) in right lower lobe that was confirmed at image-guided biopsy. (c) PET image and (d) CT image in a 59-year-old woman with endometrial cancer show multiple FDG-avid peritoneal nodules in cul-de-sac (arrows) confirmed at surgical biopsy. Primary endometrial tumor is also seen (*).
Figure 2d:

Images show examples of pathologically confirmed distant metastasis detected by using PET/CT. (a) PET image and (b) CT image in a 34-year-old woman with cervical cancer show pulmonary metastasis (arrows) in right lower lobe that was confirmed at image-guided biopsy. (c) PET image and (d) CT image in a 59-year-old woman with endometrial cancer show multiple FDG-avid peritoneal nodules in cul-de-sac (arrows) confirmed at surgical biopsy. Primary endometrial tumor is also seen (*).
Comparison of Central and Local Reader Diagnostic Performance
Central readers (Table 2) demonstrated significantly higher specificity (97.7% for cervical cancer, 98.6% for endometrial cancer) and PPV (79.3% for cervical cancer, 86.1% for endometrial cancer) for the detection of distant metastatic disease compared with institutional readers (specificity, 93.9% and 93.9%; PPV, 55.6% and 59.3%; P < .01 for specificity and P < .05 for PPV). The central reader interpretation (Fig 3) was slightly more accurate than was the corresponding institutional interpretation (Fig 4) for presence of distant metastasis on a 1–6 scale (AUC, 0.78 vs 0.75; P = .668 for cervical cancer and AUC, 0.89 vs 0.84; P = .488 for endometrial cancer), although the difference was not considered to indicate statistical significance.
Figure 3a:

Images show examples of discordant central and institutional interpretations of PET/CT for detection of distant metastatic disease. (a, d) FDG PET images, (b, e) CT images, and (c, f) fused PET/CT images in two patients. (a–c) Images in 46-year-old woman with cervical cancer show subcentimeter right lower lobe pulmonary nodule (arrows) interpreted as negative at institutional read but positive at central interpretation. Lesion was detected at follow-up imaging when it increased in size and biopsy confirmed metastasis. (d–f) Images in 48-year-old woman with cervical cancer show FDG-avid lesion in cul-de-sac (arrows) interpreted as positive at institutional read but negative at central interpretation; was found to be negative at surgery and (f) sagittal-fused PET/CT image demonstrates lesion to correspond to normal small bowel.
Figure 4a:
Graphs show comparison of receiver operating characteristic (ROC) analysis between central and institutional interpretation of PET/CT for detection of distant metastasis in patients with (a) cervical cancer and (b) endometrial cancer.
Figure 3b:

Images show examples of discordant central and institutional interpretations of PET/CT for detection of distant metastatic disease. (a, d) FDG PET images, (b, e) CT images, and (c, f) fused PET/CT images in two patients. (a–c) Images in 46-year-old woman with cervical cancer show subcentimeter right lower lobe pulmonary nodule (arrows) interpreted as negative at institutional read but positive at central interpretation. Lesion was detected at follow-up imaging when it increased in size and biopsy confirmed metastasis. (d–f) Images in 48-year-old woman with cervical cancer show FDG-avid lesion in cul-de-sac (arrows) interpreted as positive at institutional read but negative at central interpretation; was found to be negative at surgery and (f) sagittal-fused PET/CT image demonstrates lesion to correspond to normal small bowel.
Figure 3c:
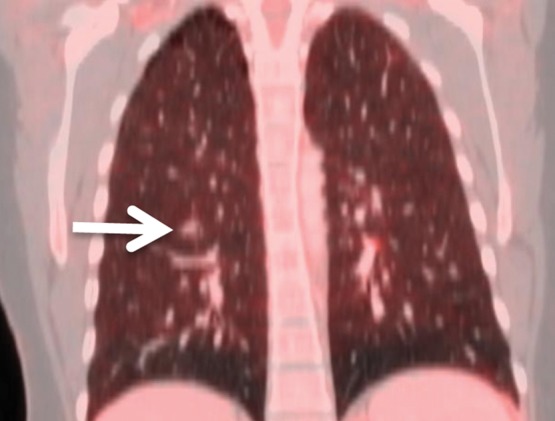
Images show examples of discordant central and institutional interpretations of PET/CT for detection of distant metastatic disease. (a, d) FDG PET images, (b, e) CT images, and (c, f) fused PET/CT images in two patients. (a–c) Images in 46-year-old woman with cervical cancer show subcentimeter right lower lobe pulmonary nodule (arrows) interpreted as negative at institutional read but positive at central interpretation. Lesion was detected at follow-up imaging when it increased in size and biopsy confirmed metastasis. (d–f) Images in 48-year-old woman with cervical cancer show FDG-avid lesion in cul-de-sac (arrows) interpreted as positive at institutional read but negative at central interpretation; was found to be negative at surgery and (f) sagittal-fused PET/CT image demonstrates lesion to correspond to normal small bowel.
Figure 3d:

Images show examples of discordant central and institutional interpretations of PET/CT for detection of distant metastatic disease. (a, d) FDG PET images, (b, e) CT images, and (c, f) fused PET/CT images in two patients. (a–c) Images in 46-year-old woman with cervical cancer show subcentimeter right lower lobe pulmonary nodule (arrows) interpreted as negative at institutional read but positive at central interpretation. Lesion was detected at follow-up imaging when it increased in size and biopsy confirmed metastasis. (d–f) Images in 48-year-old woman with cervical cancer show FDG-avid lesion in cul-de-sac (arrows) interpreted as positive at institutional read but negative at central interpretation; was found to be negative at surgery and (f) sagittal-fused PET/CT image demonstrates lesion to correspond to normal small bowel.
Figure 3e:

Images show examples of discordant central and institutional interpretations of PET/CT for detection of distant metastatic disease. (a, d) FDG PET images, (b, e) CT images, and (c, f) fused PET/CT images in two patients. (a–c) Images in 46-year-old woman with cervical cancer show subcentimeter right lower lobe pulmonary nodule (arrows) interpreted as negative at institutional read but positive at central interpretation. Lesion was detected at follow-up imaging when it increased in size and biopsy confirmed metastasis. (d–f) Images in 48-year-old woman with cervical cancer show FDG-avid lesion in cul-de-sac (arrows) interpreted as positive at institutional read but negative at central interpretation; was found to be negative at surgery and (f) sagittal-fused PET/CT image demonstrates lesion to correspond to normal small bowel.
Figure 3f:

Images show examples of discordant central and institutional interpretations of PET/CT for detection of distant metastatic disease. (a, d) FDG PET images, (b, e) CT images, and (c, f) fused PET/CT images in two patients. (a–c) Images in 46-year-old woman with cervical cancer show subcentimeter right lower lobe pulmonary nodule (arrows) interpreted as negative at institutional read but positive at central interpretation. Lesion was detected at follow-up imaging when it increased in size and biopsy confirmed metastasis. (d–f) Images in 48-year-old woman with cervical cancer show FDG-avid lesion in cul-de-sac (arrows) interpreted as positive at institutional read but negative at central interpretation; was found to be negative at surgery and (f) sagittal-fused PET/CT image demonstrates lesion to correspond to normal small bowel.
Figure 4b:
Graphs show comparison of receiver operating characteristic (ROC) analysis between central and institutional interpretation of PET/CT for detection of distant metastasis in patients with (a) cervical cancer and (b) endometrial cancer.
Discussion
Our results indicate that 12.6% of patients with cervical and endometrial cancer in the ACRIN 6671/GOG 0233 clinical cohort demonstrate unsuspected distant metastases. This high incidence of metastatic disease confirms the need for accurate noninvasive assessment of distant metastasis prior to surgery or other local-regionally–directed therapy in this patient population. Our results show that preoperative PET/CT would be capable of detecting the majority of distant metastases before treatment with few false-positive results. When combined with image-guided or laparoscopic biopsy, this would enable minimally invasive staging and obviate overly aggressive procedures in patients not amenable to curative therapy.
Fusion PET/CT is an ideal imaging modality for treatment planning in local-regionally advanced cervical and high-risk endometrial cancer. The examination combines the sensitivity of FDG PET for detecting extrauterine disease with the spatial resolution of diagnostic CT for anatomic localization and for detection of subcentimeter pulmonary or peritoneal nodules. In addition, investigators in the ACRIN 6671/GOG 0233 trial reported that PET/CT performance for detection of lymph node metastases was high, with an AUC of 0.83 (95% confidence interval: 0.75, 0.91) with patients with cervical cancer and 0.75 (95% confidence interval: 0.63, 0.87) with patients with endometrial cancer (2,3). Preoperative detection of lymphadenopathy allows for planning a minimally invasive lymphadenectomy for pathologic staging. In sum, with capabilities for detection of both retroperitoneal nodal and distant metastases with a high specificity, FDG PET/CT represents a noninvasive tool that would minimize the morbidity of the staging procedures and subsequent therapy.
Few studies in the literature measure the diagnostic accuracy of PET/CT for specific detection of distant metastasis in cervical and endometrial cancer (9–11). Most studies group distant and nodal metastases together. The sensitivity of FDG PET/CT in our study (60% overall) is lower than that observed in a prior single institution study of 30 patients undergoing surveillance PET/CT imaging for detection of recurrent cervical cancer. This study showed 96% sensitivity and 95% specificity for distant metastasis (10) when two nonblinded readers read the imaging examinations retrospectively in consensus. However, our study included larger numbers of patients prospectively enrolled (356 patients) who underwent imaging, surgery, and follow-up under the trial protocol. The image evaluation itself was performed both at the site and centrally with multiple blinded readers, making our results more likely to be generalizable. In addition, similar differences in performance between single and multicenter trials have been observed for FDG PET/CT performance for nodal disease detection (12,13).
Our study also demonstrates that central review of PET/CT imaging has improved performance for distant metastasis assessment compared with local institutional point-of-care interpretation, evidenced by significantly higher specificity and PPV, for both cervical and endometrial cancer. The reason for improved test performance characteristics of blinded independent central review is not clear and likely multifactorial. The increased specificity of central readers compared with local site readers is likely related, at least in part, to differences in the clinical context in which the images were interpreted. Local institutional readers interpreting PET/CT prior to treatment planning likely would interpret these studies with higher sensitivity and lower specificity, knowing that distant metastases would necessitate adjuvant systemic therapy. As these false-positive lesions would be confirmed with biopsy or follow-up imaging, they most likely did not result in overtreatment. In contrast, central readers interpreted images in a research context to establish the diagnostic accuracy of PET/CT, after the patients had already been treated. Minimizing overdiagnosis of distant metastatic disease is important in this patient population because it can lead to selection of chemotherapy or radiation therapy regimens aimed at controlling hematogenous spread of disease or palliation, rather than local-regional therapy with curative intent. Sensitivity was not significantly different between local and central reviewers for either cervical or endometrial cancer. It is also possible that having all of the PET/CT studies read by two central reviewers with specific expertise in PET/CT interpretation and gynecologic cancer imaging is a contributor to higher specificity and PPV (14).
The issue of central versus local interpretation of cancer imaging studies has not been extensively studied. One meta-analysis of imaging biomarker trials showed no significant difference in treatment response assessment by blinded independent central review (measured as progression-free survival) compared with local evaluation in phase III oncologic clinical trials (15). However, in our diagnostic accuracy trial, imaging assessment was performed at a single time point before treatment, rather than repeated imaging assessment of metastatic burden over time. Our results suggest that blinded independent central review of imaging clinical trials may yield improved diagnostic specificity and PPV.
Our study had limitations. We do not have precise data regarding the total number of patients with cervical and endometrial cancer who were evaluated at the 28 participating institutions for the ACRIN 6671/GOG0 233 trial, so there could be potential recruitment bias into terms of the patients who were enrolled. Also, a small number of patients with contraindications to intravenous contrast agent (3.9% in overall cohort) underwent PET/CT without intravenous contrast–material enhancement, which introduced some heterogeneity in the imaging protocol. In addition, as with any multicenter study, there were potential variations in surgical and follow-up imaging protocols that may be confounders. Finally, there was potential for ascertainment bias on the part of local institutional readers interpreting imaging studies at the point of care, as well as central readers interpreting the same studies in a research study, that could possibly affect study results.
In summary, our results demonstrate that a substantial proportion (12.6%) of patients with local-regionally advanced cervical or high-risk endometrial cancer have unsuspected distant metastatic disease. FDG PET/CT is an examination that can help detect the majority of these noninvasively, with minimal false-positive results, and also provides evaluation of regional lymphadenopathy. Thus, FDG PET/CT represents a powerful tool for noninvasive assessment of disease extent and prognosis. Its use in patient evaluation would enable tailoring of the therapy to minimize overly aggressive procedures. Finally, the improved specificity and PPV of blinded central reader PET/CT interpretation compared with local institutional point-of- care evaluation suggests a potential role for blinded central interpretation in future imaging clinical trials related to oncologic staging.
Advances in Knowledge
■ 13.7% (21 of 153) of patients with local-regionally advanced cervical cancer and 11.8% (24 of 203) of patients with high-risk endometrial cancer in the clinical cohort demonstrate unsuspected distant metastases.
■ Preoperative PET/CT detects the majority (23 of 42 [54.8%] for cervical cancer; 31 of 48 [64.6%] for endometrial cancer) of distant metastases before treatment with high specificity and positive predictive value (PPV).
■ The specificity, PPV, and values for central reader PET/CT detection of cervical and endometrial cancer metastases, respectively, were significantly higher compared with local institutional review.
Implications for Patient Care
■ A substantial proportion of patients with local-regionally advanced cervical and high-risk endometrial cancer have unsuspected distant metastatic disease.
■ Use of FDG PET/CT before treatment for detection of distant metastasis in cervical and endometrial cancer would benefit patients by enabling oncologists to tailor therapy in patients with advanced disease to treat metastases while potentially minimizing procedure morbidity.
Acknowledgments
Acknowledgment
The authors thank Adam Opanowski, CNMT, RT, for assistance with study data retrieval and figure construction.
Received May 2, 2017; revision requested June 29; revision received August 15; accepted September 6; final version accepted September 29.
Supported by a Young Investigator Award from the American College of Radiology Imaging Network. The American College of Radiology Imaging Network (U10 CA80098 and U10 CA79778) and Gynecologic Oncology Group (U10 CA27469 and U10 CA37517) receive funding from the National Cancer Institute.
M.S.G. and M.A. contributed equally to this work.
Disclosures of Conflicts of Interest: M.S.G. disclosed no relevant relationships. M.A. disclosed no relevant relationships. A.I.B. disclosed no relevant relationships. R.S.M. disclosed no relevant relationships. M.A.G. disclosed no relevant relationships. S.I.L. Activities related to the present article: reports a grant from the American College of Radiology Imaging Network/National Cancer Institute. Activities not related to the present article: reports personal fees from UpToDate/Wolters Kluwer as a salaried editor. Other relationships: disclosed no relevant relationships.
Abbreviations:
- ACRIN
- American College of Radiology Imaging Network
- AUC
- area under the receiver operating characteristic curve
- FDG
- fluorine 18 fluorodeoxyglucose
- GOG
- Gynecology Oncology Group
- PPV
- positive predictive value
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65(1):5–29. [DOI] [PubMed] [Google Scholar]
- 2.Atri M, Zhang Z, Dehdashti F, et al. Utility of PET/CT to evaluate retroperitoneal lymph node metastasis in advanced cervical cancer: results of ACRIN6671/GOG0233 trial. Gynecol Oncol 2016;142(3):413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atri M, Zhang Z, Dehdashti F, et al. Utility of PET/CT to evaluate retroperitoneal lymph node metastasis in high-risk endometrial cancer: results of ACRIN 6671/GOG 0233 trial. Radiology 2017;283(2):450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thigpen JT, Brady MF, Homesley HD, et al. Phase III trial of doxorubicin with or without cisplatin in advanced endometrial carcinoma: a gynecologic oncology group study. J Clin Oncol 2004;22(19):3902–3908. [DOI] [PubMed] [Google Scholar]
- 5.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 2013;49(6):1374–1403. [DOI] [PubMed] [Google Scholar]
- 6.Bestvina CM, Fleming GF. Chemotherapy for endometrial cancer in adjuvant and advanced disease settings. Oncologist 2016;21(10):1250–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li H, Wu X, Cheng X. Advances in diagnosis and treatment of metastatic cervical cancer. J Gynecol Oncol 2016;27(4):e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst 2008;100(23):1707–1716. [DOI] [PubMed] [Google Scholar]
- 9.Kakhki VR, Shahriari S, Treglia G, et al. Diagnostic performance of fluorine 18 fluorodeoxyglucose positron emission tomography imaging for detection of primary lesion and staging of endometrial cancer patients: systematic review and meta-analysis of the literature. Int J Gynecol Cancer 2013;23(9):1536–1543. [DOI] [PubMed] [Google Scholar]
- 10.Mittra E, El-Maghraby T, Rodriguez CA, et al. Efficacy of 18F-FDG PET/CT in the evaluation of patients with recurrent cervical carcinoma. Eur J Nucl Med Mol Imaging 2009;36(12):1952–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nogami Y, Iida M, Banno K, et al. Application of FDG-PET in cervical cancer and endometrial cancer: utility and future prospects. Anticancer Res 2014;34(2):585–592. [PubMed] [Google Scholar]
- 12.Signorelli M, Guerra L, Buda A, et al. Role of the integrated FDG PET/CT in the surgical management of patients with high risk clinical early stage endometrial cancer: detection of pelvic nodal metastases. Gynecol Oncol 2009;115(2):231–235. [DOI] [PubMed] [Google Scholar]
- 13.Antonsen SL, Jensen LN, Loft A, et al. MRI, PET/CT and ultrasound in the preoperative staging of endometrial cancer: a multicenter prospective comparative study. Gynecol Oncol 2013;128(2):300–308. [DOI] [PubMed] [Google Scholar]
- 14.Dodd LE, Korn EL, Freidlin B, et al. Blinded independent central review of progression-free survival in phase III clinical trials: important design element or unnecessary expense? J Clin Oncol 2008;26(22):3791–3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amit O, Mannino F, Stone AM, et al. Blinded independent central review of progression in cancer clinical trials: results from a meta-analysis. Eur J Cancer 2011;47(12):1772–1778. [DOI] [PubMed] [Google Scholar]





