Abstract
Blunt cerebrovascular injury (BCVI) is a relatively rare but potentially devastating finding in patients with high-energy blunt force trauma or direct cervical and/or craniofacial injury. The radiologist plays an essential role in identifying and grading the various types of vascular injury, including minimal intimal injury, dissection with raised intimal flap or intraluminal thrombus, intramural hematoma, pseudoaneurysm, occlusion, transection, and arteriovenous fistula. Early identification of BCVI is important, as treatment with antithrombotic therapy has been shown to reduce the incidence of postinjury ischemic stroke. Patients with specific mechanisms of injury, particular imaging findings, or certain clinical signs and symptoms have been identified as appropriate and cost-effective for BCVI screening. Although digital subtraction angiography was previously considered the standard examination for screening, technologic improvements have led to its replacement with computed tomographic angiography. Of note, although not appropriate for screening, improvements in magnetic resonance angiography with vessel wall imaging hold promise as supplemental imaging studies that may improve diagnostic specificity for vessel wall injuries. Understanding the screening criteria, imaging modalities of choice, imaging appearances, and grading of BCVI is essential for the radiologist to ensure fast and appropriate diagnosis and treatment. This article details the imaging evaluation of BCVI and discusses the clinical and follow-up imaging implications of specific injury findings.
©RSNA, 2018
SA-CME LEARNING OBJECTIVES
After completing this journal-based SA-CME activity, participants will be able to:
■ Understand the screening criteria for and imaging evaluation of BCVI in the trauma setting.
■ Describe the imaging appearance and grading of BCVI.
■ Discuss the clinical and follow-up imaging implications of specific findings.
Introduction
Blunt cerebrovascular injury (BCVI) is a collective term used to describe blunt force injuries to the cervical carotid and vertebral arteries. These injuries are most commonly identified in trauma patient populations, with high-energy injury mechanisms to the head, neck, and thorax as predisposing factors. These injuries are rare, even within the trauma population. When left untreated, they are of high clinical significance because of their association with adverse neurologic events, including cerebral infarction and death.
The diagnostic radiologist plays an essential role in both identifying these lesions and guiding management decisions. As such, a firm understanding of the appropriate screening criteria, imaging modalities of choice, and grading schema is necessary to ensure that BCVI is identified quickly and treated appropriately. The objectives of this article are to describe the appropriate BCVI screening criteria within the acute trauma setting, discuss modality-specific imaging guidelines and radiologic evaluation of BCVI in trauma patients, demonstrate the appearance and grading of BCVI, and examine clinical and follow-up imaging implications of specific injury findings.
BCVI Epidemiology
Historically, the true prevalence of BCVI has been difficult to ascertain, as many of these lesions are clinically asymptomatic at the time of initial imaging workup, overshadowed by more severe traumatic injuries or masked by traumatic brain injuries. Early radiologic studies suggested a prevalence as low as 0.08% (1); however, improvements in computed tomographic (CT) technology paired with trends toward more comprehensive imaging workups of trauma patients (including asymptomatic patients in the emergency department setting) have led to an increase in BCVI detection. Recent studies suggest a BCVI prevalence of 1.0%–1.6% within the trauma patient population (2–4) and up to 2.7% when constrained to patients with higher injury scores (5).
When left untreated, BCVI is associated with increased morbidity and mortality, most commonly due to cerebral infarction (3,6). The increases in morbidity and mortality are dependent on both BCVI location and severity. Carotid artery injuries carry higher rates of downstream infarction than vertebral artery injuries, and high-grade injuries (grades III, IV, and V) carry higher rates than low-grade injuries (grade I and II). Published data suggest that untreated blunt carotid artery injuries possess a morbidity rate of 32%–67% and a mortality rate of 17%–38% (7–11), whereas untreated blunt vertebral artery injuries possess a morbidity rate of 14%–24% (9,10) and a mortality rate of 8%–18% (6,11).
Mechanisms of Injury
Trauma is the most common cause of BCVI, and high-energy injury mechanisms are associated with an increased risk of underlying BCVI. On average, high-speed motor vehicle collisions account for more than 50% of BCVIs (4,12). Other injury mechanisms associated with BCVI include falls from heights greater than standing, pedestrian-versus-car accidents, assaults, suicide attempts that involve hanging, and other direct head, neck, and facial trauma.
On rare occasions, cervical arterial injuries can be seen in the context of low-energy injury mechanisms, commonly referred to as trivial trauma. Unlike high-energy mechanisms, these injury mechanisms are generally not associated with additional substantial traumatic injuries, other than those sustained by the cervical vasculature.
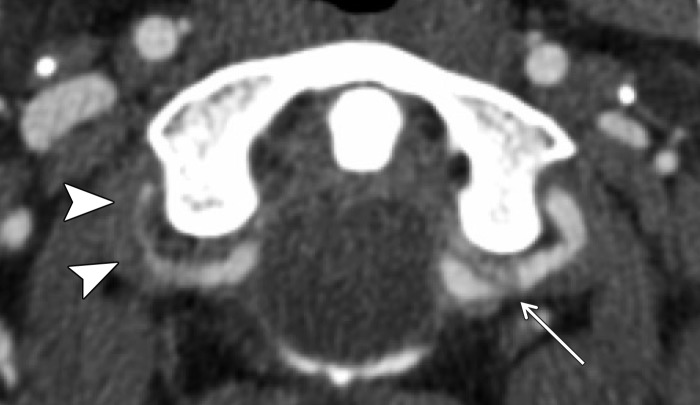
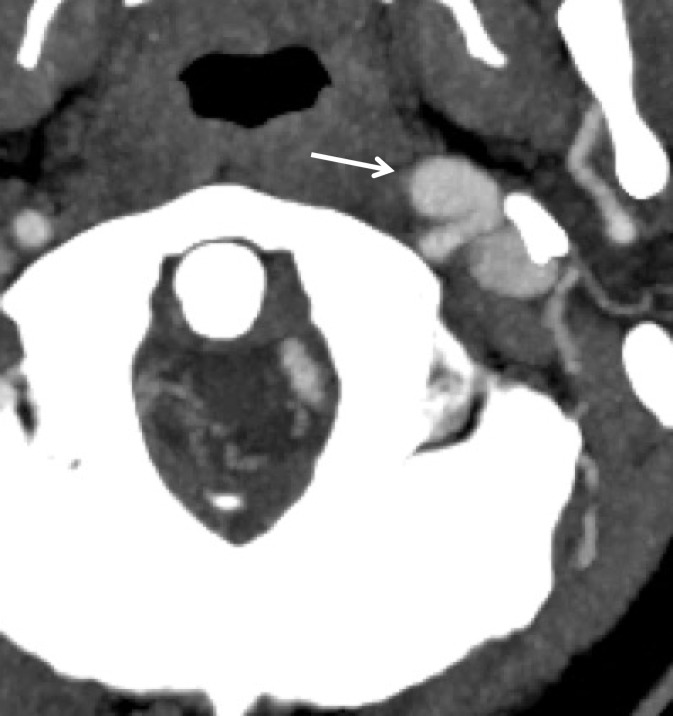
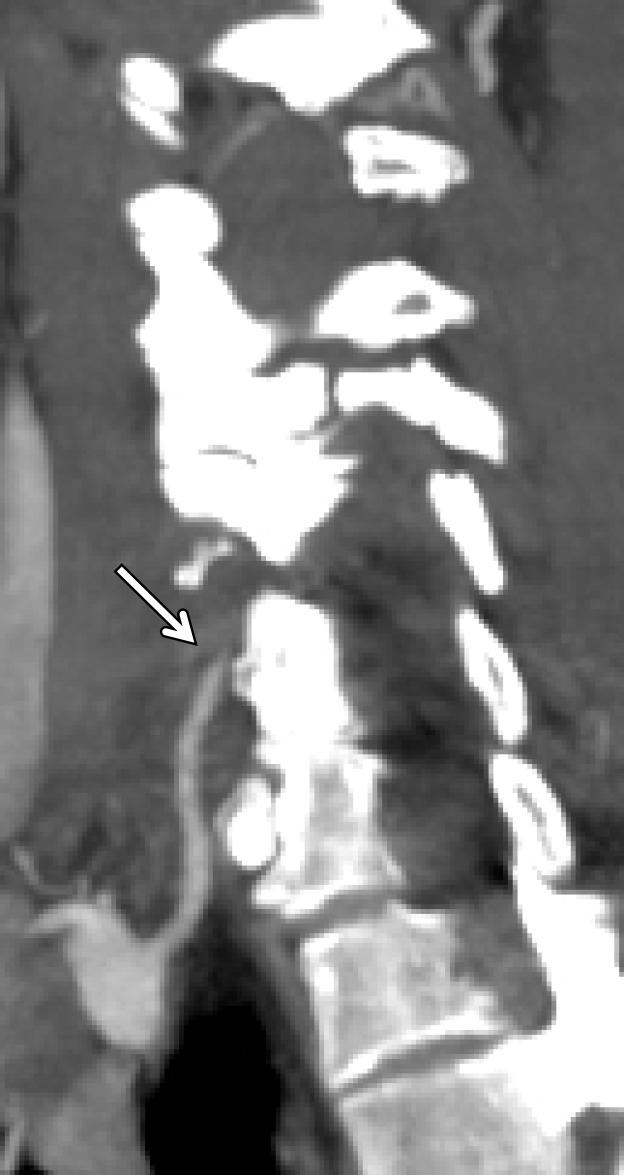
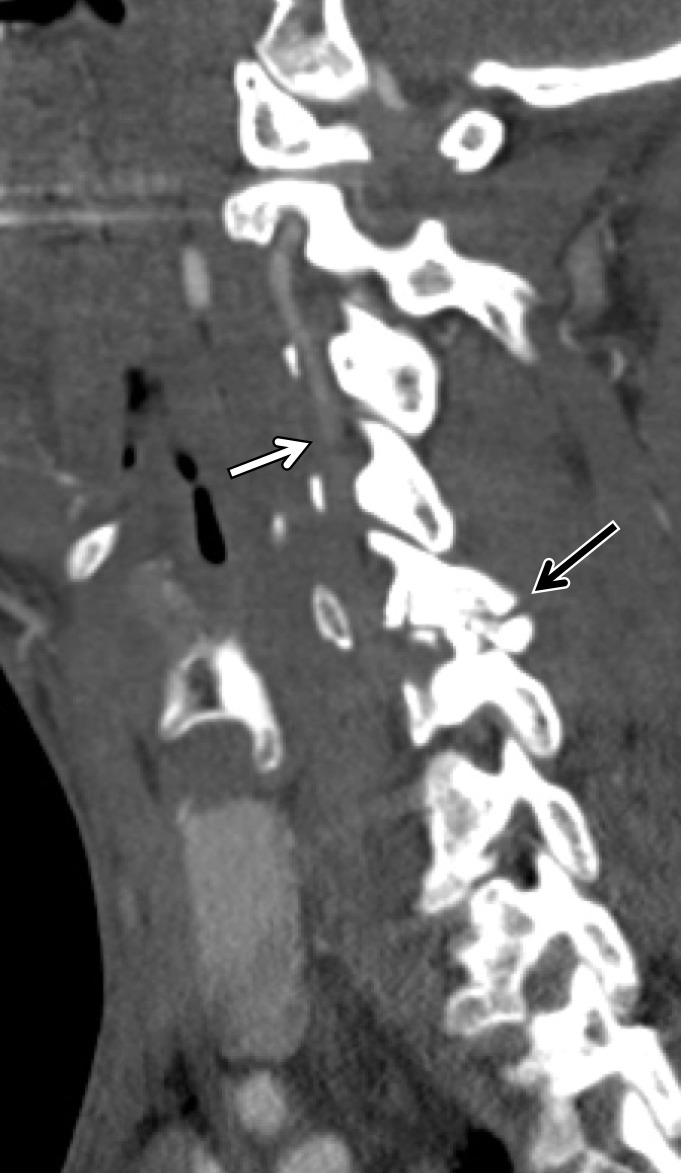
A classic example of a trivial trauma mechanism is chiropractic manipulation of the neck (13) (Fig 1). Patients with these injuries characteristically present with neck pain or vague neurologic complaints before being diagnosed with underlying cerebrovascular injury. Nontraumatic spontaneous cerebrovascular injuries have also been reported in mechanisms that would not typically raise suspicion for injury, including certain athletic activities and yoga positions; daily activities including coughing, nose-blowing, shaving, and vomiting; and other activities that include rapid head movement (13).
Figure 1.
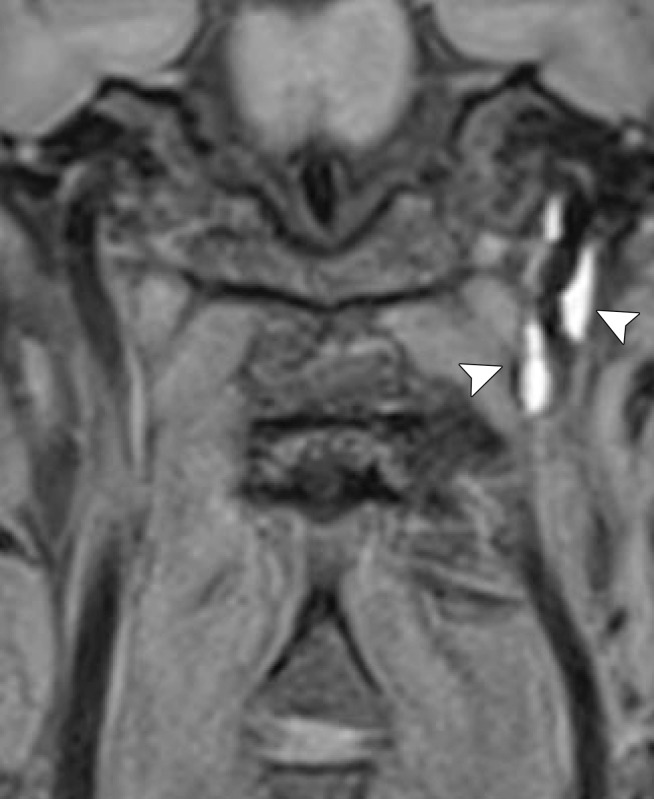
Cerebrovascular injury from trivial trauma in a 35-year-old man who presented with left posterior cerebral artery territory stroke after chiropractic manipulation. Axial CT angiogram shows bilateral vertebral artery dissections, with right vertebral artery intramural hematoma narrowing the lumen (arrowheads), as well as left vertebral artery dissection and intraluminal thrombus (arrow). An abrupt cutoff in the left posterior cerebral artery was also seen (not shown), representing a thromboembolic occlusion.
The pathophysiology underlying these injuries is not clearly understood. In such patients, various connective tissue disorders may manifest, including Marfan syndrome, Ehlers-Danlos syndrome, and even unrecognized minor connective tissue disorders, predisposing the patient to spontaneous arterial injury.
Cervical spine fractures have the strongest association with BCVI (14,15), with injuries of the upper cervical spine, ligamentous injuries, and traumatic subluxations placing patients at the highest risk (16). The prevalence of BCVI in trauma patients with a C1–C3 spinal fracture or dislocation has been reported to be as high as 8%, whereas the prevalence in patients with a C4–C7 injury is markedly lower at approximately 2% (17). In addition to cervical spine injuries, other traumatic injuries are associated with an increased risk of BCVI. These include complex skull fractures, basilar skull fractures, occipital condyle fractures, displaced midface fractures, and mandible fractures, particularly those involving the mandibular condyles (18).
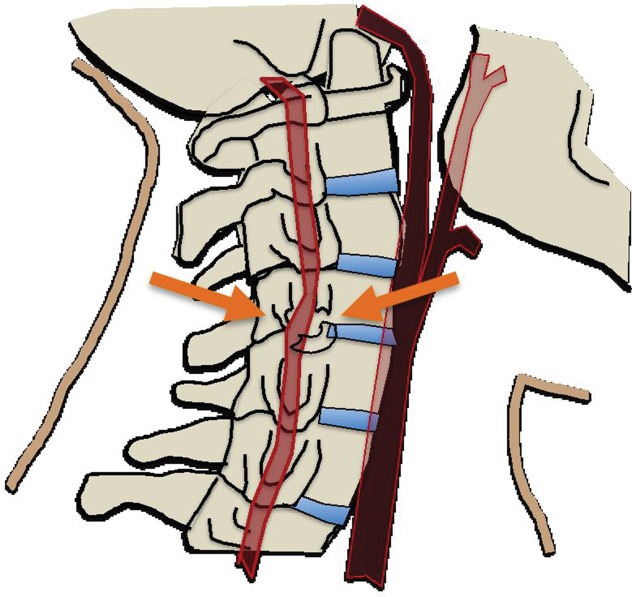
During blunt force head and neck trauma, the arteries of the neck may experience longitudinal stretching, twisting, or compressive forces (19–21). In the context of a carotid artery injury, it is thought that cervical hyperextension and contralateral rotation lead to stretching of the internal carotid artery (ICA) over the C1–C3 transverse processes, precipitating a vessel wall injury. In the case of vertebral artery injury, bone fracture, subluxation, or ligamentous injury may cause stretching of the vertebral artery between adjacent structures of the cervical spine or at the dural margins as the artery enters the skull (22–24).
In addition to stretching and twisting injuries, direct blows to the neck may directly injure or compress an artery against an adjacent osseous structure. This may be seen when a steering wheel impacts an unrestrained driver’s upper thorax or neck, or when a compressive force is applied to the neck, as in cases of strangulation. Such episodes of vascular impingement are also seen with cervical hyperflexion injuries and displaced mandibular fractures, where the ICA is crushed between the mandible and the spine.
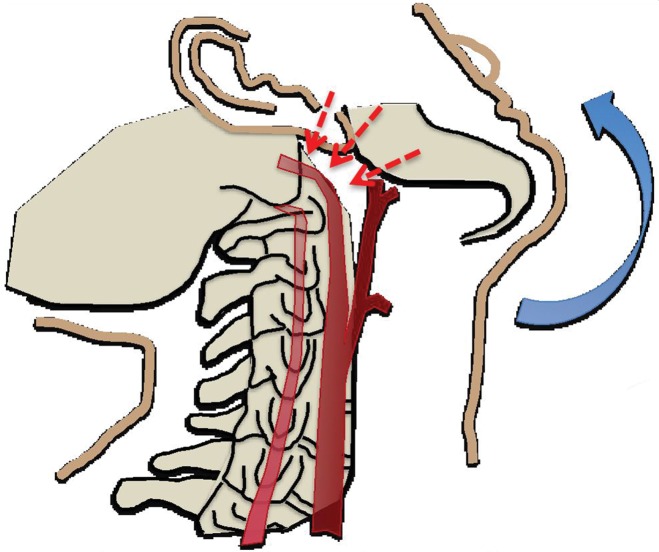
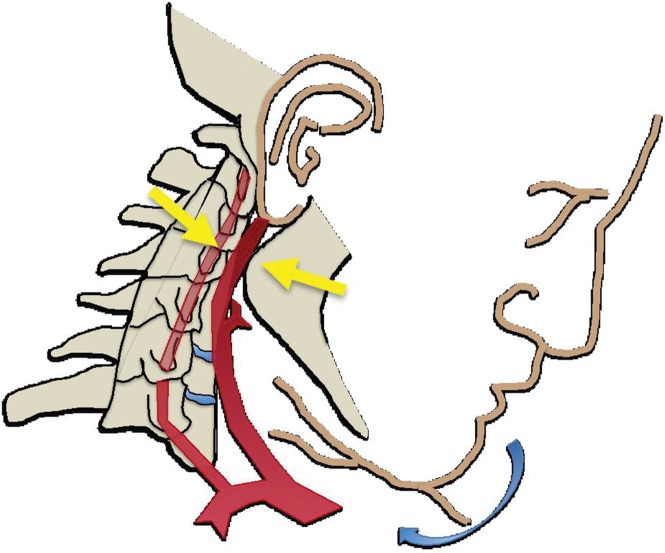
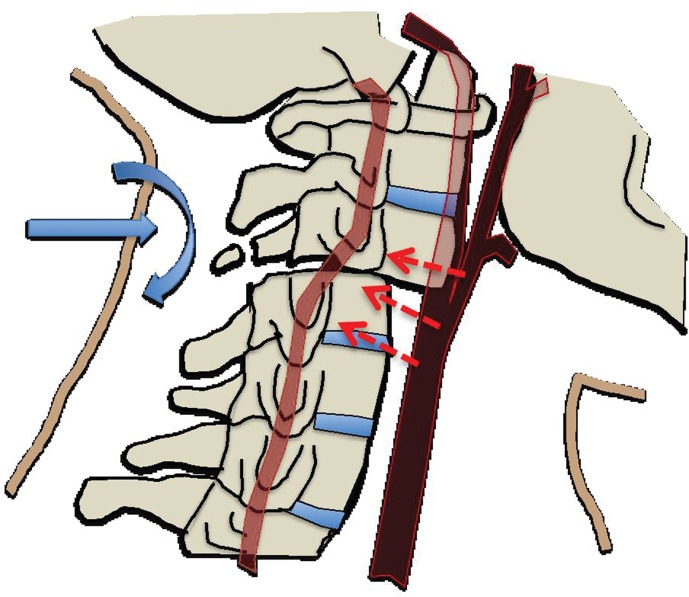
In cases of skull base or dislocated occipital condyle fractures, the vertebral arteries may become impinged between the fractured skull base and the ring of the atlas. Finally, sharp bone fragments may directly lacerate cervical vessels during traumatic cervical spine or skull base fractures. The risk is especially high when fractures extend through the transverse foramina of the cervical vertebrae or into the carotid canal of the skull base (Fig 2).
Figure 2a.
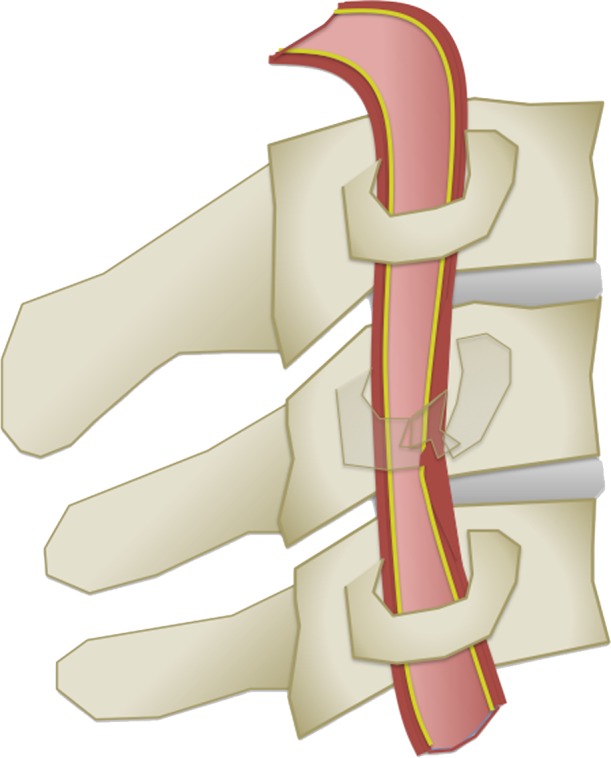
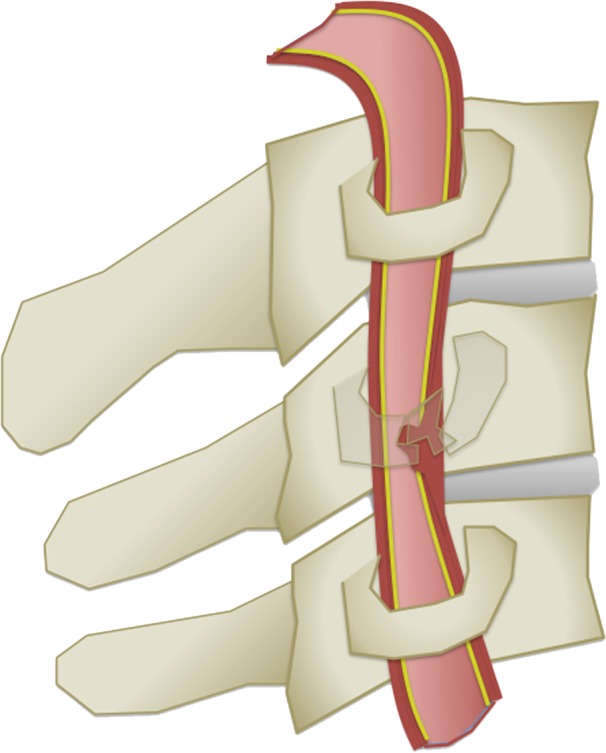
(a) Longitudinal stretching and/or twisting of the vessel. Illustration depicts hyperextension and contralateral rotation, with stretching of the ICA over the C1–C3 transverse processes (red dashed arrows). Blue arrows in a–c = direction of movement. (b) Vessel impingement. Illustration depicts compression of the ICA between the mandible and spine (yellow arrows), which may result from hyperflexion or displaced mandible fracture. Impingement may also result from skull base or cervical spine fracture. Likewise, a blow to the neck or strangulation may directly compress or impinge a vessel against an adjacent structure. (c) Stretching of the vessel. Illustration depicts stretching of the vertebral artery between subluxated or dislocated bone structures (red dashed arrows), which may occur in cases of cervical fracture, dislocation, or ligamentous injury. (d) Direct laceration. Illustration depicts a fracture with direct injury to the vertebral artery by an adjacent bone fragment (orange arrows). This is especially relevant in cases of vertebral transverse foramen or carotid canal fracture.
Figure 2b.
(a) Longitudinal stretching and/or twisting of the vessel. Illustration depicts hyperextension and contralateral rotation, with stretching of the ICA over the C1–C3 transverse processes (red dashed arrows). Blue arrows in a–c = direction of movement. (b) Vessel impingement. Illustration depicts compression of the ICA between the mandible and spine (yellow arrows), which may result from hyperflexion or displaced mandible fracture. Impingement may also result from skull base or cervical spine fracture. Likewise, a blow to the neck or strangulation may directly compress or impinge a vessel against an adjacent structure. (c) Stretching of the vessel. Illustration depicts stretching of the vertebral artery between subluxated or dislocated bone structures (red dashed arrows), which may occur in cases of cervical fracture, dislocation, or ligamentous injury. (d) Direct laceration. Illustration depicts a fracture with direct injury to the vertebral artery by an adjacent bone fragment (orange arrows). This is especially relevant in cases of vertebral transverse foramen or carotid canal fracture.
Figure 2c.
(a) Longitudinal stretching and/or twisting of the vessel. Illustration depicts hyperextension and contralateral rotation, with stretching of the ICA over the C1–C3 transverse processes (red dashed arrows). Blue arrows in a–c = direction of movement. (b) Vessel impingement. Illustration depicts compression of the ICA between the mandible and spine (yellow arrows), which may result from hyperflexion or displaced mandible fracture. Impingement may also result from skull base or cervical spine fracture. Likewise, a blow to the neck or strangulation may directly compress or impinge a vessel against an adjacent structure. (c) Stretching of the vessel. Illustration depicts stretching of the vertebral artery between subluxated or dislocated bone structures (red dashed arrows), which may occur in cases of cervical fracture, dislocation, or ligamentous injury. (d) Direct laceration. Illustration depicts a fracture with direct injury to the vertebral artery by an adjacent bone fragment (orange arrows). This is especially relevant in cases of vertebral transverse foramen or carotid canal fracture.
Figure 2d.
(a) Longitudinal stretching and/or twisting of the vessel. Illustration depicts hyperextension and contralateral rotation, with stretching of the ICA over the C1–C3 transverse processes (red dashed arrows). Blue arrows in a–c = direction of movement. (b) Vessel impingement. Illustration depicts compression of the ICA between the mandible and spine (yellow arrows), which may result from hyperflexion or displaced mandible fracture. Impingement may also result from skull base or cervical spine fracture. Likewise, a blow to the neck or strangulation may directly compress or impinge a vessel against an adjacent structure. (c) Stretching of the vessel. Illustration depicts stretching of the vertebral artery between subluxated or dislocated bone structures (red dashed arrows), which may occur in cases of cervical fracture, dislocation, or ligamentous injury. (d) Direct laceration. Illustration depicts a fracture with direct injury to the vertebral artery by an adjacent bone fragment (orange arrows). This is especially relevant in cases of vertebral transverse foramen or carotid canal fracture.
Notably, blunt injury can also affect the venous structures of the head and neck. Although rare, bone fragments may lacerate or external forces may compress the cervical jugular veins, leading to hemorrhage and/or luminal thrombosis. Calvarial or skull base fractures involving the jugular bulb or dural venous sinuses may cause venous lacerations and epidural hematomas; mass effect on dural venous sinuses from an epidural hematoma may precipitate a dural venous sinus thrombosis (DVST).
BCVI Pathophysiology
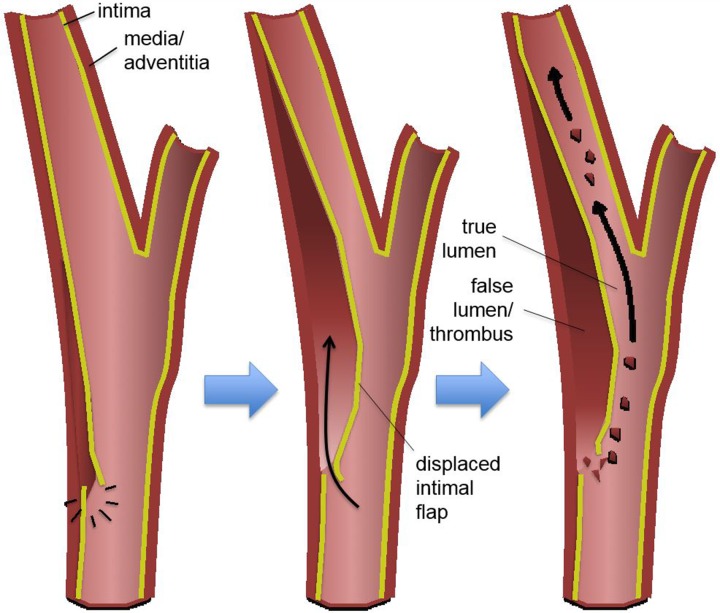
In the setting of BCVI, pathologic stretching, twisting, or shearing forces most commonly result in intimal injury to the vessel, regardless of the mechanism. Blood flowing through the vessel lumen may then dissect into the vessel wall at the site of an intimal defect. This dissection can then propagate cranially, in the direction of bulk intravascular blood flow. If severe enough, a vessel wall dissection may lead to substantial flow-limiting luminal stenosis or occlusion. Importantly, intimal injuries expose intravascular blood to the thrombogenic subendothelial extracellular matrix, leading to platelet activation, aggregation, and subsequent downstream embolization with vascular occlusion (Fig 3a).
Figure 3a.
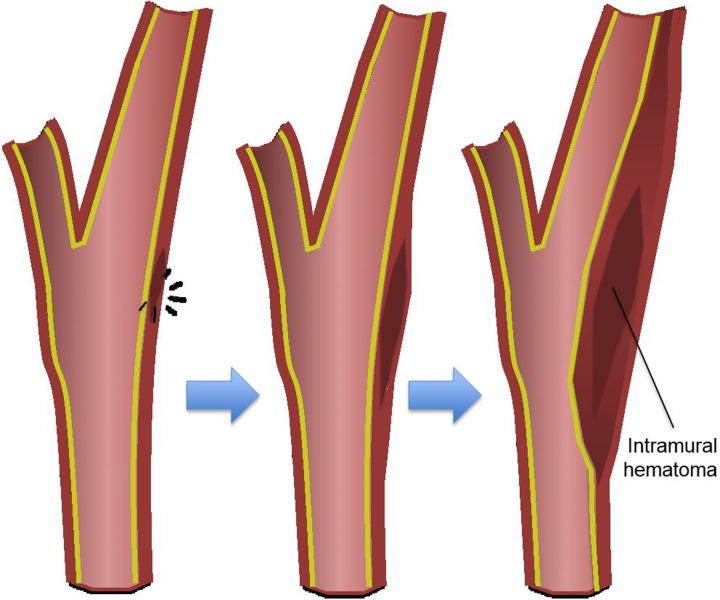
Blunt arterial injury pathophysiology. Blue arrows = injury progression. (a) Illustration depicts an intimal injury with dissection. An intimal tear may dissect, progress cranially, and cause luminal narrowing or occlusion. Thrombus may form in the false lumen and at the site of injury, leading to embolization and downstream occlusion. Black arrows = direction of blood flow. (b) Illustration depicts an intramural hematoma, an adventitiomedial injury, in which the intima may remain intact. An intramural hematoma forms within the vessel wall due to bleeding from the vasa vasorum. Blood can then dissect through the media and/or adventitia, causing luminal stenosis and/or occlusion.
Adventitiomedial and adventitial injuries are distinct from arterial intimal injuries in that the intimal surface may remain intact. Instead of intravascular blood dissecting into the arterial wall through an intimal defect, an intramural hematoma forms within the vessel wall, as a result of injury to the vasa vasorum. Blood then dissects through the media and/or adventitia, leaving the intimal lining of the vessel intact. Like arterial intimal injuries, dissecting intramural hematomas may cause luminal stenosis and/or occlusion, depending on the size and extent of the hematoma (Fig 3b).
Figure 3b.
Blunt arterial injury pathophysiology. Blue arrows = injury progression. (a) Illustration depicts an intimal injury with dissection. An intimal tear may dissect, progress cranially, and cause luminal narrowing or occlusion. Thrombus may form in the false lumen and at the site of injury, leading to embolization and downstream occlusion. Black arrows = direction of blood flow. (b) Illustration depicts an intramural hematoma, an adventitiomedial injury, in which the intima may remain intact. An intramural hematoma forms within the vessel wall due to bleeding from the vasa vasorum. Blood can then dissect through the media and/or adventitia, causing luminal stenosis and/or occlusion.
A traumatic pseudoaneurysm is a focal outpouching of an arterial wall, in which all the layers of the vessel wall do not remain intact. Blood dissects through a breach in the vessel wall but is contained by incomplete mural layers (at minimum by adventitia or surrounding perivascular soft tissue). This results in a saccular or fusiform outpouching seen on vascular images. Like dissecting arterial intimal injuries and dissecting wall hematomas, pseudoaneuryms may also cause vessel stenosis and/or occlusion, as the aneurysmal pouch may compress the adjacent lumen. Additionally, abnormal flow dynamics within the aneurysm in conjunction with exposed subendothelial materials within the sac may promote blood coagulation, thrombus formation, and downstream clot embolization. Although there is a theoretical risk of rupture, reports of devastating rupture in the literature remain anecdotal (25).
Arterial transection is the most severe form of BCVI. These injuries manifest with arterial hemorrhage into the soft tissues of the neck or face and can result in massive stroke or rapid exsanguination if not quickly treated. In some instances, a fistulous connection may form between the injured cervical artery and adjacent venous structures, resulting in a high-flow arteriovenous fistula (AVF). These lesions, especially traumatic cervical AVFs, are infrequently encountered in most clinical practices; many such patients are less likely to reach the hospital and survive to the point of imaging.
Caroticocavernous fistulas (CCFs) represent a specific form of AVF that may be seen in cases of traumatic skull base fracture or ruptured cavernous ICA injury, characterized by an abnormal communication between the internal carotid circulation and the cavernous sinus.
Venous mural injuries may also occur, although perhaps more commonly in penetrating injuries. A venous pseudoaneurysm is a contained rupture in which the wall of the vein is partially or focally disrupted. A venous transection occurs when the entire wall is lacerated and/or disrupted. In cases of calvarial or skull base fracture involving the dural venous sinuses, injury to the dural lining or flow-altering compression on the sinus may precipitate thrombosis and occlusion.
Clinical Manifestations
The clinical manifestations of BCVI are highly variable, depending on both the artery injured and the vascular distribution affected within the brain. New focal or lateralizing neurologic deficits should raise suspicion for underlying BCVI and can offer valuable insight into which cervical artery is affected. Additionally, neurologic symptoms that are incongruous with nonangiographic cross-sectional imaging findings at the time of admission are worrisome for evolving or overlooked BCVI and warrant a more thorough imaging evaluation. Other clinical signs suspicious for more serious BCVI include acute arterial hemorrhage from the neck, mouth, nose, and ears; expanding cervical hematomas; or a cervical bruit, especially in patients younger than 50 years of age.
Arterial hemorrhage or expanding hematoma are worrisome for an underlying transection or AVF. The potential complications of traumatic AVF, especially those not initially detected and treated in a delayed fashion, depend on the vascular compartment involved and the lesion flow dynamics. In the special case of CCFs, symptoms may also include pulsatile exophthalmos, conjunctival chemosis, tinnitus, and cranial nerve palsies. Venous congestion from the high-flow communication results in orbital symptoms that can rapidly progress to vision loss (26); rarely, plegia may develop secondary to brainstem congestion (27). Given the high-flow nature of both cervical and intracranial AVF, back-pressure with retrograde cortical venous drainage may develop, which can lead to cerebral subarachnoid or parenchymal hemorrhage. For cervical fistulas, a recurrent focal rupture with associated hemorrhage can also occur, manifesting with neurologic symptoms, hematoma, or arterial hemorrhage from the neck, mouth, nose, or ears.
Notably, in as many as 80% of patients with BCVI, there is a latent period of variable length between the time of injury and when neurologic symptoms begin to manifest (4). This latent period may last anywhere from hours to days or longer; however, on average, it lasts approximately 72 hours before the onset of clinically identifiable infarction (2,12,28–30). It is during this latent period that vascular imaging is of highest value in terms of identifying asymptomatic BCVI and guiding clinical management decisions.
BCVI Screening
Screening Guidelines
Over the past 20 years, various BCVI screening guidelines have emerged from several professional organizations and research groups to identify more of these injuries in trauma patients while simultaneously reducing the number of unnecessary imaging studies performed in patients at low risk for BCVI (Table 1). These guidelines include the so-called Denver criteria and modified Denver criteria, described in a series of articles from 1999 to 2004 (9,15,22,30–32), and later the Western Trauma Association (WTA) screening recommendations, published in 2009, which were based on the Denver criteria (33).
Table 1:
Screening Recommendations for BCVI
The Eastern Association for the Surgery of Trauma (EAST) established guidelines in 2010, including recommendations based on the level of evidence (34). Recommendations were based on a literature review from 1965 to 2005, categorizing articles as class I (randomized controlled trials), class II (prospective and retrospective studies, including cohort studies and case-control studies), and class III (uncontrolled retrospective studies). EAST recommendations were defined as level I if based on class I or strong class II data; level II if justified by class II evidence or a preponderance of class III data; and level III if supported by a more limited quantity of class III data only. Of note, EAST makes no level I recommendations on the subject of BCVI screening.
According to the Denver criteria and WTA guidelines, clinical signs that should elicit emergent screening include arterial hemorrhage from the neck, mouth, nose, or ears; expanding cervical hematoma; a cervical bruit in a patient under 50 years of age; focal neurologic deficits; a stroke at secondary imaging; or any neurologic deficit inconsistent with imaging findings. The presence of seat belt abrasions was originally included in the screening criteria but later found not to be an isolated risk factor for BCVI (35). Screening for BCVI was given a level II recommendation by EAST only in the event of unexplained neurologic symptoms or arterial epistaxis after trauma.
According to the Denver criteria and WTA guidelines, radiologic risk factors associated with BCVI that should precipitate urgent screening in neurologically asymptomatic patients include high-energy injury mechanisms with Le Fort II or III fracture patterns; basilar skull fracture with carotid canal involvement; cervical vertebral body or transverse foramen fracture, subluxation, or ligamentous injury; any fracture at C1 through C3; closed head injuries with diffuse axonal injury and a Glasgow Coma Score of less than 6; clothesline-type injuries with associated swelling and/or pain; or near-hanging with anoxia.
EAST guidelines give a level III recommendation for screening in asymptomatic patients in the event of a Glasgow Coma Score less than or equal to 8; a diffuse axonal injury; petrous bone fracture; a cervical spine fracture at C1 through C3, involving the transverse foramen or with subluxation or a rotational component; and Le Fort II or III fracture. Of note, it has been shown that the presence of multiple risk factors further increases the probability of BCVI (15).
Over the past 2 decades, the number of clinical signs and radiologic risk factors associated with BCVI has increased, resulting in more BCVIs captured at screening cross-sectional imaging. For example, Burlew et al (18) published an article that suggested additional risk factors be added to previous screening criteria, including patients with mandible fractures, complex skull fractures, traumatic brain injury with thoracic injuries, scalp degloving, and thoracic vascular injuries (Table 1). Of note, despite these improvements in BCVI detection, it is still believed that a nonnegligible number of these lesions are overlooked; up to 30% of BCVIs detected at CT angiography have no associated signs or risk factors for such injuries (36).
Screening by Modality
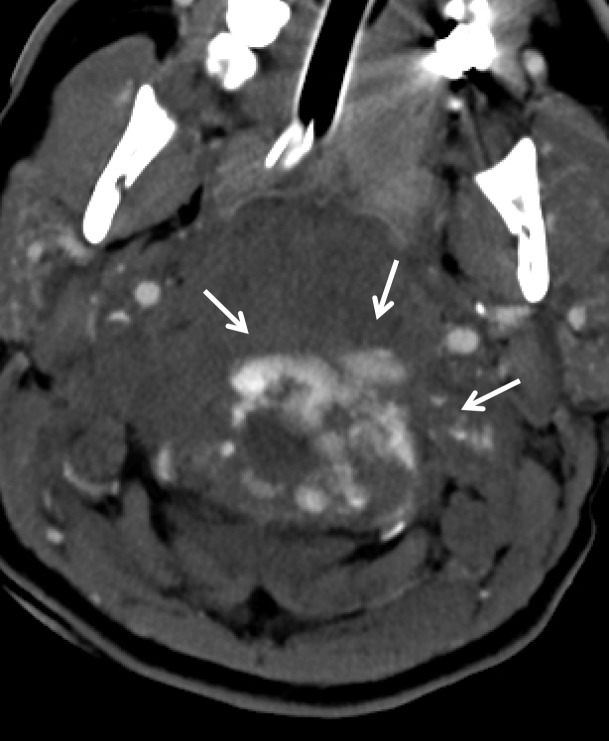
Digital Subtraction Angiography.—Digital subtraction angiography (DSA) has historically been considered the standard imaging modality for the detection of BCVI, possessing the highest sensitivity while simultaneously allowing for the evaluation of collateral circulation. Cothren et al (11) showed that DSA is a cost-effective tool for screening BCVI in high-risk populations, as the cost of screening was roughly equivalent to the cost of averted strokes. EAST guidelines give a level II recommendation for DSA as a screening tool for BCVI (34). However, DSA is invasive (requiring arterial puncture) and puts patients at risk for access-site complications, iatrogenic vessel wall injury, and embolic stroke. Additionally, DSA relies on intraluminal opacification for blood vessel visualization but does not allow direct visualization of the vessel wall, limiting detection of nonstenotic intramural hematoma (37). At most institutions, DSA is reserved for the evaluation of patients who have equivocal or negative results at CT angiography but for whom high clinical suspicion for BCVI persists (Fig 4).
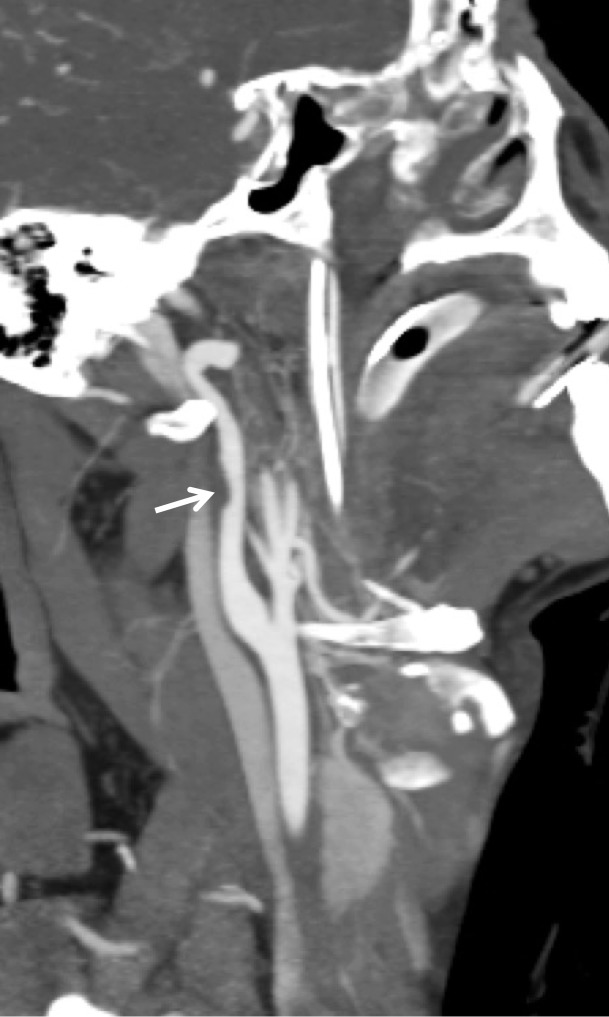
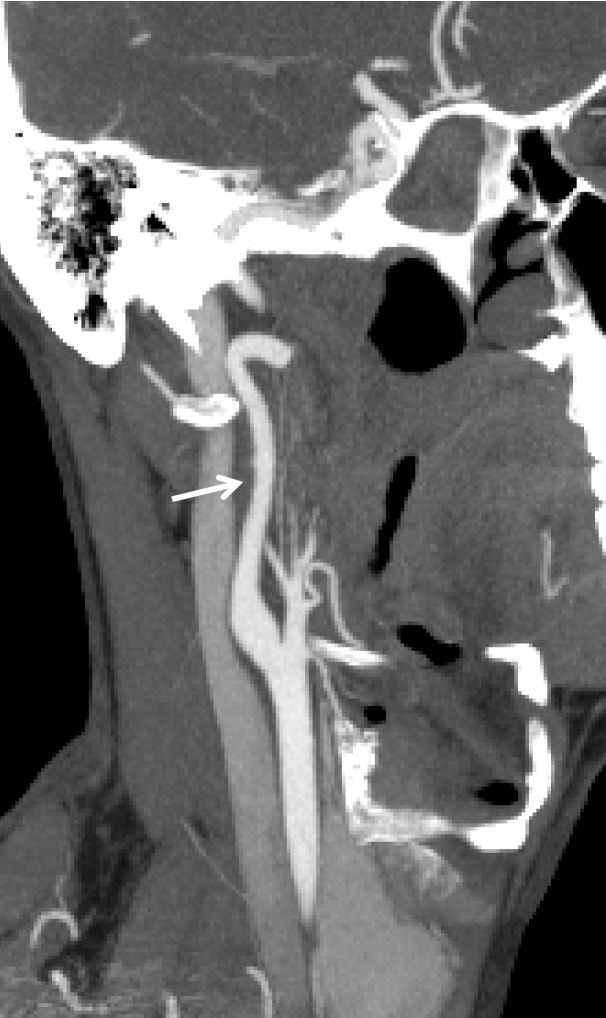
Figure 4a.
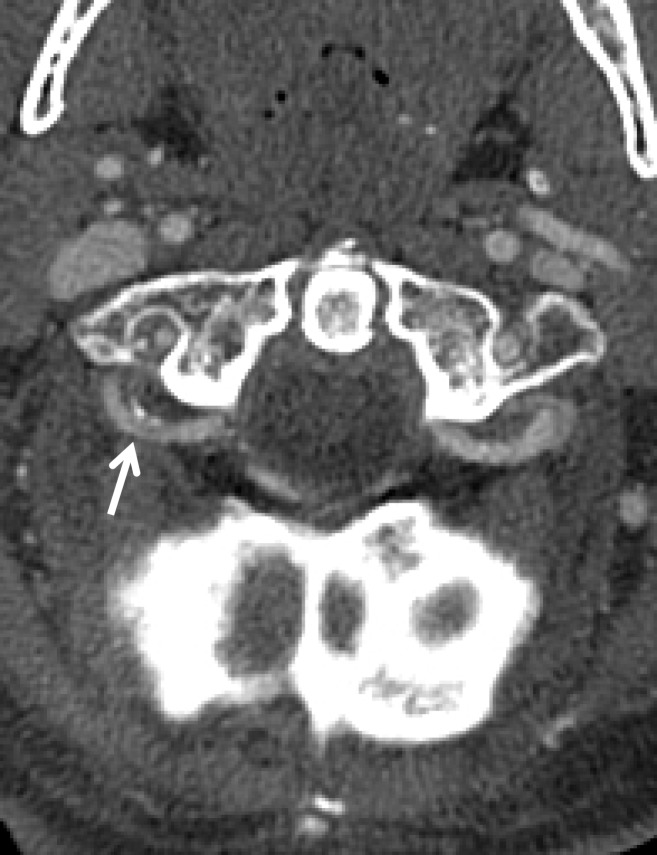
BCVI in a young patient who underwent strangulation. (a, b) Axial (a) and sagittal (b) CT angiographic images show focal irregularity of the right ICA with wall thickening, causing mild narrowing (arrows), proximal to the skull base. DSA was performed to further define the injury. (c) Digital subtraction angiographic image shows a focal filling defect (arrow) at the site of injury, compatible with intraluminal thrombus versus intramural hematoma.
Figure 4b.
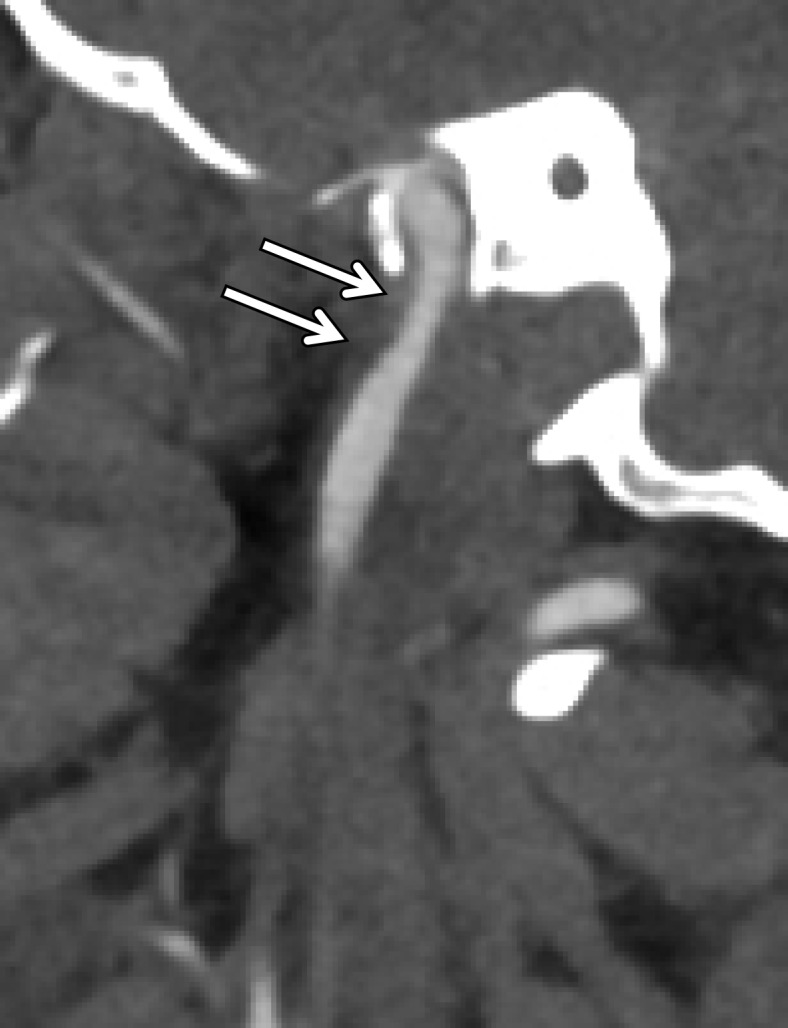
BCVI in a young patient who underwent strangulation. (a, b) Axial (a) and sagittal (b) CT angiographic images show focal irregularity of the right ICA with wall thickening, causing mild narrowing (arrows), proximal to the skull base. DSA was performed to further define the injury. (c) Digital subtraction angiographic image shows a focal filling defect (arrow) at the site of injury, compatible with intraluminal thrombus versus intramural hematoma.
Figure 4c.
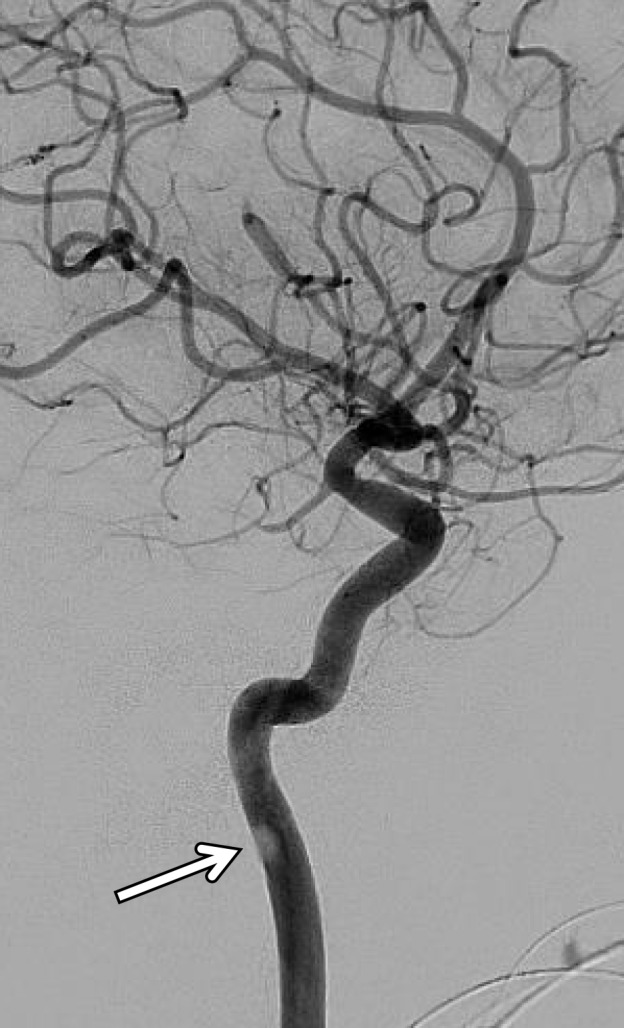
BCVI in a young patient who underwent strangulation. (a, b) Axial (a) and sagittal (b) CT angiographic images show focal irregularity of the right ICA with wall thickening, causing mild narrowing (arrows), proximal to the skull base. DSA was performed to further define the injury. (c) Digital subtraction angiographic image shows a focal filling defect (arrow) at the site of injury, compatible with intraluminal thrombus versus intramural hematoma.
CT Angiography.—With the widespread availability of 32-channel and higher multidetector CT scanners, CT angiography has largely supplanted DSA as the first-line screening modality for BCVI. Historically, one- to four-section CT angiography demonstrated an unacceptably low sensitivity and specificity for the detection of BCVI. Indeed, EAST level II guidelines specifically state that four-section CT angiography (as well as duplex ultrasonography) are inadequate as tools for BCVI screening. However, as CT scanner technology has improved (incorporating more detectors into the scanner design to allow for rapid acquisition and improved spatial resolution), so too has the sensitivity and specificity for BCVI detection using CT angiography.
Recent studies have shown that, against the DSA imaging standard, CT angiography performed on 16- to 64-channel multidetector CT scanners has a sensitivity of 66%–98% and specificity of 92%–100% (38–40), with the majority of missed BCVIs representing low-grade grade I injuries. EAST gives a level III recommendation for the use of eight+-section multidetector CT angiography as a screening modality. Furthermore, a study published by Kaye et al (41) demonstrated that CT angiography is a cost-effective screening tool in high-risk populations, ultimately preventing the most strokes at a reasonable cost among all screening modalities (41).
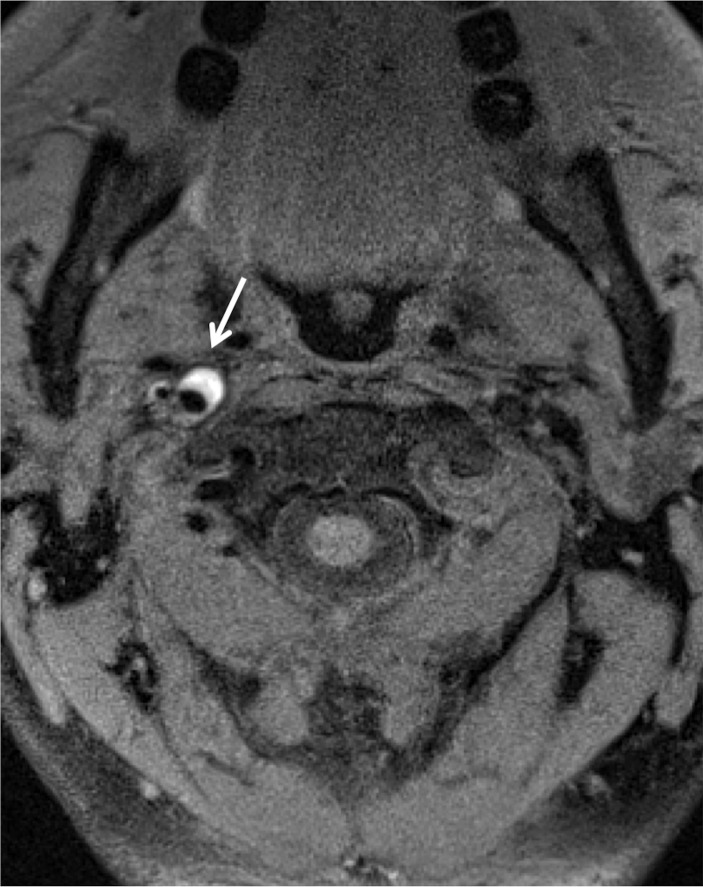
MR Imaging.—Although MR imaging has currently not been considered an appropriate tool for BCVI screening, quality proton-density–weighted or fat-saturated T1-weighted sequences are useful in delineating arterial dissection (Fig 5). Indeed, abnormalities of vessel flow voids may also be incidentally noted on standard T1-, T2-, and proton-density–weighted images at cervical spine MR imaging. Dissections usually show a crescent of hyperintense or isointense signal about the flow void of cervical arteries, likely within the false lumen. A complete loss of the vessel flow void may represent occlusion or slow flow through a dissected vessel.
Figure 5a.
ICA dissection. (a) Axial fat-saturated T1-weighted MR image shows a hyperintense crescent (arrow) about the right ICA flow void, representing the false lumen. (b) Surface-rendered three-dimensional (3D) reconstruction of DSA data following right ICA injection shows irregularity and narrowing of the distal cervical right ICA (arrows).
Figure 5b.
ICA dissection. (a) Axial fat-saturated T1-weighted MR image shows a hyperintense crescent (arrow) about the right ICA flow void, representing the false lumen. (b) Surface-rendered three-dimensional (3D) reconstruction of DSA data following right ICA injection shows irregularity and narrowing of the distal cervical right ICA (arrows).
The advantages of MR imaging include a lack of ionizing radiation (which is of particular importance in young patients), a lower incidence of gadolinium-based contrast agent allergies when compared with those of iodinated contrast agents, and lack of skull base streak artifacts seen at CT. Additionally, MR imaging is far superior to DSA and CT angiography in the evaluation of acute cerebral infarction. Unfortunately, MR angiography requires long image acquisition times and is more susceptible to motion artifact when compared with CT angiography.
MR angiography historically has had poor sensitivities and specificities for BCVI detection (compared with the DSA standard), measuring 50%–75% and 67%, respectively (9,42,43), making it inferior to CT angiography as an appropriate BCVI screening modality (44). In recent years, the use of MR angiography complemented by dedicated high-resolution vessel wall imaging for detecting and defining BCVI has been explored. Although standard time-of-flight MR angiography offers no diagnostic benefit over CT angiography/DSA, high-resolution vessel wall imaging holds promise as a valuable imaging correlate in conjunction with luminal imaging (CT angiography and MR angiography).
Blood suppression techniques limit flow artifacts and allow visualization of wall disease adjacent to flowing blood in the lumen. In particular, this technique may be useful as a tool for follow-up and problem solving in equivocal cases, especially when wall hematoma and dissection are in the differential diagnosis. Importantly, recent studies demonstrate good accuracy of vessel wall MR imaging in detecting BCVI when compared with that of CT angiography, and implementation of vessel wall MR imaging for follow-up BCVI evaluation should be considered in many cases (45).
CT Venography.—When there is concern for venous injury, whether intracranially or in the neck, CT venography should be performed, as CT angiography may not adequately opacify venous structures in the dural sinuses and neck. Although no official recommendations by the WTA or EAST exist for the evaluation of venous injury, any calvarial or skull base fracture that extends to the dural venous sinus or jugular bulb should raise suspicion and prompt consideration for performing CT venography. In a retrospective study of 140 blunt trauma patients with skull fractures extending to a dural sinus or jugular bulb, approximately 41% had thrombosis, 55% of which were occlusive; 7% of patients with DVST had venous infarction (46).
Dedicated MR venography sequences may be useful in cases of skull fracture involving dural sinuses, as they are highly sensitive to the presence of DVST. Although each MR imaging sequence has varying strengths and weaknesses in the detection of DVST, a combination of conventional MR imaging sequences performs best, with sensitivity of 79% and specificity of 90% in one study (47) and a sensitivity greater than 99% in another study (although with low specificity) (48).
Imaging Findings
There is a wide spectrum of BCVI severity, and accurate radiologic grading plays an essential role in the clinical prognostication and management of these injuries. Over the years, multiple grading systems for evaluating the severity of BCVI have been developed and clinically tested.
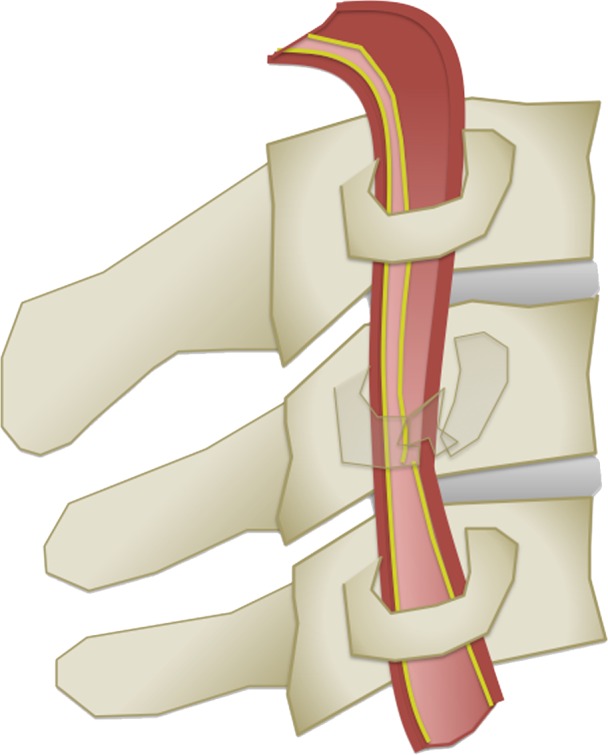
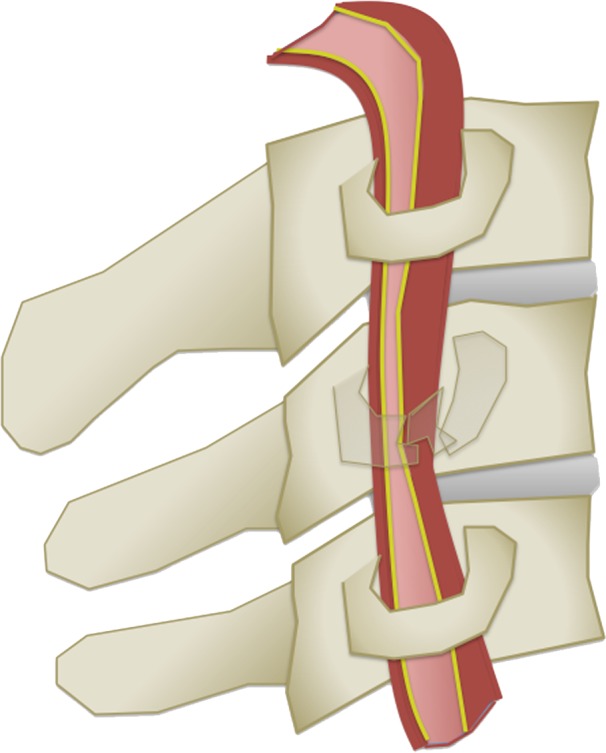
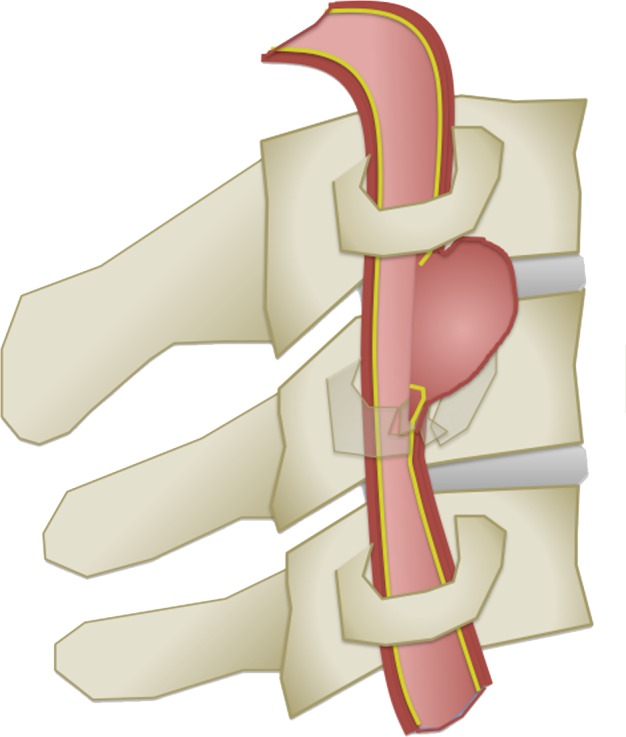
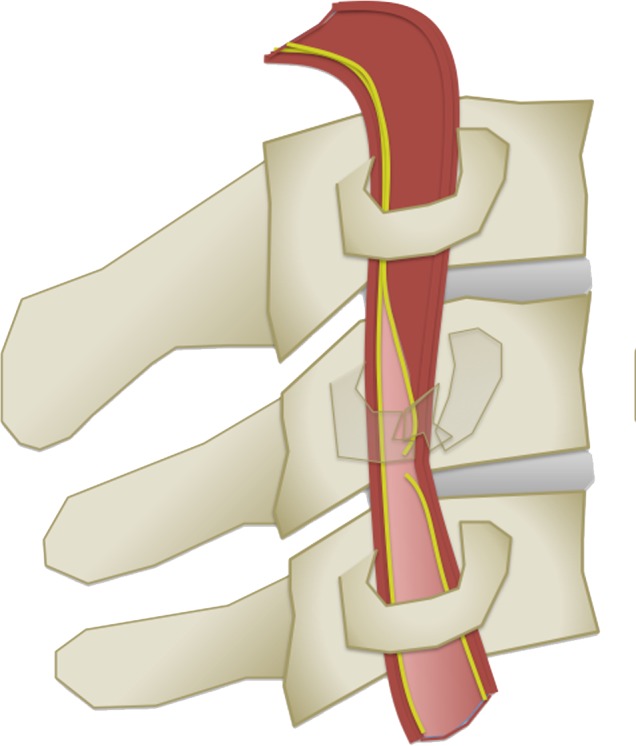
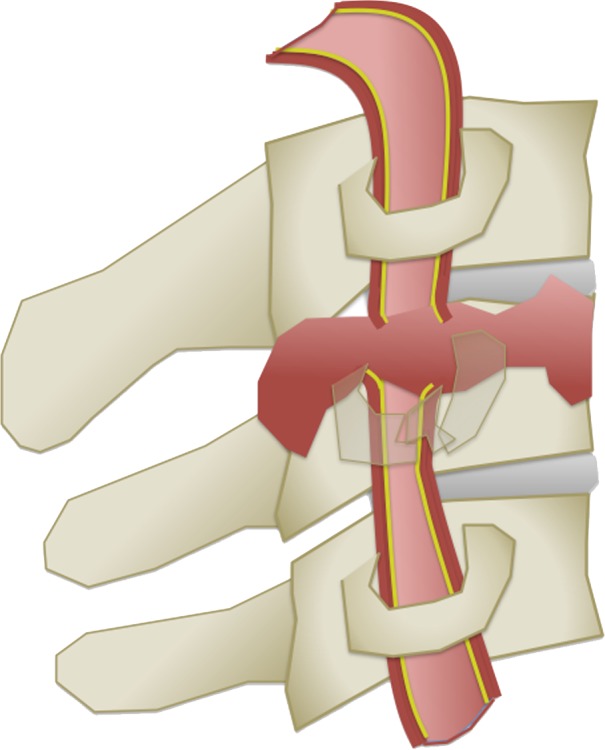
The Denver scale (also referred to as the Biffl scale) is a widely accepted and commonly used system that assigns a grade to a lesion on a one- to five-point scale, with increasing grades corresponding to more severe vascular injuries (30,39). Grade I injury is defined as vessel wall irregularity, dissection, or intramural hematoma with less than 25% stenosis. Grade II injury is defined as either a dissection or intramural hematoma with greater than 25% stenosis but less than complete vascular occlusion. Additionally, any injury demonstrating a raised intimal flap or distinct intraluminal thrombus is defined as grade II. Grade III injury corresponds to a traumatic pseudoaneurysm. Grade IV injury represents complete vessel occlusion. Finally, grade V injury is defined as arterial transection and/or AVF formation (Table 2, Fig 6).
Table 2:
Grading BCVI
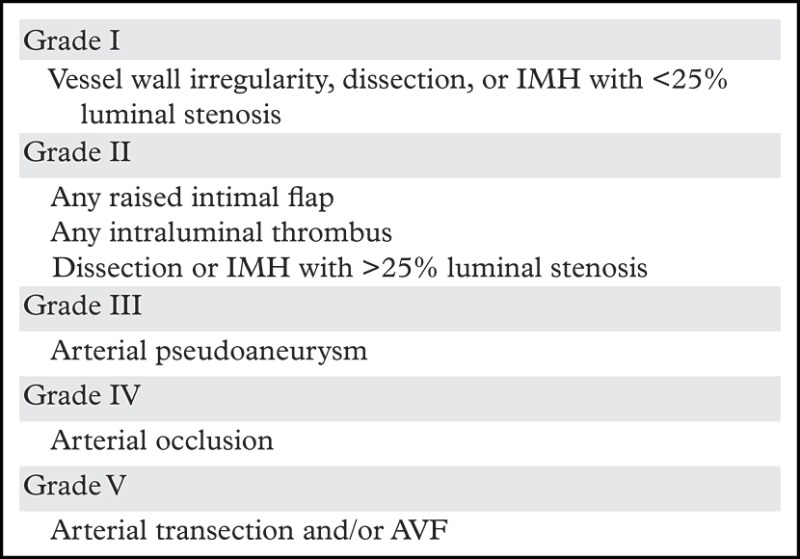
Source.—Reference 28.
Note.—IMH = intramural hematoma.
Figure 6a.
BCVI grading scale. (a) Illustration depicts a grade I injury, characterized by minimal intimal injury with vessel wall irregularity, dissection, or intramural hematoma, with less than 25% luminal stenosis. (b–d) Illustrations depict grade II injuries with intraluminal thrombus (b), dissection with a raised/displaced intimal flap (c), and intramural hematoma (d), with greater than 25% stenosis. (e) Illustration depicts a grade III injury pseudoaneurysm, seen as a focal outpouching/dilatation through a disrupted vessel wall. (f) Illustration depicts a grade IV injury, with dissection or thrombus causing vessel occlusion. (g) Illustration depicts a grade V injury, with complete vessel transection and extravasation.
Figure 6b.
BCVI grading scale. (a) Illustration depicts a grade I injury, characterized by minimal intimal injury with vessel wall irregularity, dissection, or intramural hematoma, with less than 25% luminal stenosis. (b–d) Illustrations depict grade II injuries with intraluminal thrombus (b), dissection with a raised/displaced intimal flap (c), and intramural hematoma (d), with greater than 25% stenosis. (e) Illustration depicts a grade III injury pseudoaneurysm, seen as a focal outpouching/dilatation through a disrupted vessel wall. (f) Illustration depicts a grade IV injury, with dissection or thrombus causing vessel occlusion. (g) Illustration depicts a grade V injury, with complete vessel transection and extravasation.
Figure 6c.
BCVI grading scale. (a) Illustration depicts a grade I injury, characterized by minimal intimal injury with vessel wall irregularity, dissection, or intramural hematoma, with less than 25% luminal stenosis. (b–d) Illustrations depict grade II injuries with intraluminal thrombus (b), dissection with a raised/displaced intimal flap (c), and intramural hematoma (d), with greater than 25% stenosis. (e) Illustration depicts a grade III injury pseudoaneurysm, seen as a focal outpouching/dilatation through a disrupted vessel wall. (f) Illustration depicts a grade IV injury, with dissection or thrombus causing vessel occlusion. (g) Illustration depicts a grade V injury, with complete vessel transection and extravasation.
Figure 6d.
BCVI grading scale. (a) Illustration depicts a grade I injury, characterized by minimal intimal injury with vessel wall irregularity, dissection, or intramural hematoma, with less than 25% luminal stenosis. (b–d) Illustrations depict grade II injuries with intraluminal thrombus (b), dissection with a raised/displaced intimal flap (c), and intramural hematoma (d), with greater than 25% stenosis. (e) Illustration depicts a grade III injury pseudoaneurysm, seen as a focal outpouching/dilatation through a disrupted vessel wall. (f) Illustration depicts a grade IV injury, with dissection or thrombus causing vessel occlusion. (g) Illustration depicts a grade V injury, with complete vessel transection and extravasation.
Figure 6e.
BCVI grading scale. (a) Illustration depicts a grade I injury, characterized by minimal intimal injury with vessel wall irregularity, dissection, or intramural hematoma, with less than 25% luminal stenosis. (b–d) Illustrations depict grade II injuries with intraluminal thrombus (b), dissection with a raised/displaced intimal flap (c), and intramural hematoma (d), with greater than 25% stenosis. (e) Illustration depicts a grade III injury pseudoaneurysm, seen as a focal outpouching/dilatation through a disrupted vessel wall. (f) Illustration depicts a grade IV injury, with dissection or thrombus causing vessel occlusion. (g) Illustration depicts a grade V injury, with complete vessel transection and extravasation.
Figure 6f.
BCVI grading scale. (a) Illustration depicts a grade I injury, characterized by minimal intimal injury with vessel wall irregularity, dissection, or intramural hematoma, with less than 25% luminal stenosis. (b–d) Illustrations depict grade II injuries with intraluminal thrombus (b), dissection with a raised/displaced intimal flap (c), and intramural hematoma (d), with greater than 25% stenosis. (e) Illustration depicts a grade III injury pseudoaneurysm, seen as a focal outpouching/dilatation through a disrupted vessel wall. (f) Illustration depicts a grade IV injury, with dissection or thrombus causing vessel occlusion. (g) Illustration depicts a grade V injury, with complete vessel transection and extravasation.
Figure 6g.
BCVI grading scale. (a) Illustration depicts a grade I injury, characterized by minimal intimal injury with vessel wall irregularity, dissection, or intramural hematoma, with less than 25% luminal stenosis. (b–d) Illustrations depict grade II injuries with intraluminal thrombus (b), dissection with a raised/displaced intimal flap (c), and intramural hematoma (d), with greater than 25% stenosis. (e) Illustration depicts a grade III injury pseudoaneurysm, seen as a focal outpouching/dilatation through a disrupted vessel wall. (f) Illustration depicts a grade IV injury, with dissection or thrombus causing vessel occlusion. (g) Illustration depicts a grade V injury, with complete vessel transection and extravasation.
Minimal Intimal Injury
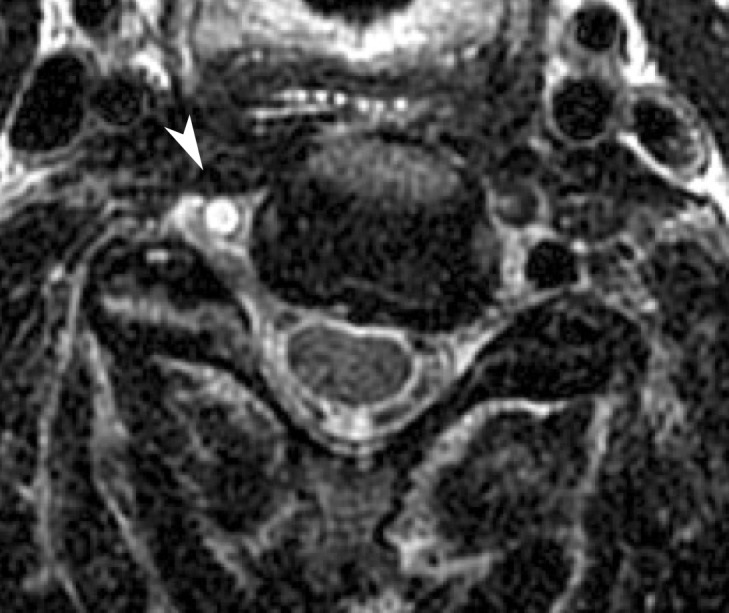
Minimal intimal injuries correspond to grade I BCVI. At CT angiography and DSA, these injuries demonstrate nonstenotic (<25% narrowing) luminal irregularity and may appear as a minimal contour undulation or subtle vessel wall thickening (Fig 7). They are sometimes best depicted on multiplanar or three-dimensional (3D) reformatted images. Radiologically, these injuries are difficult to distinguish from posttraumatic vasospasm. Time-of-flight MR angiography findings are similar to those seen at CT angiography; however, MR angiography is less sensitive than CT angiography for detecting these lesions. Of note, dedicated vessel wall MR imaging sequences may improve intramural hematoma detection and increase MR angiography sensitivity. Equivocal luminal irregularities and wall thickening seen at CT angiography may be further evaluated with black-blood vessel wall imaging techniques; in these cases, normal vessels, vasospasms, or chronic vascular diseases (ie, atherosclerosis) can be confidently distinguished from a T1 hyperintense intramural hematoma (Fig 8).
Figure 7.
Minimal intimal injury in a trauma patient with multiple calvarial and facial fractures. Oblique maximum intensity projection (MIP) CT angiogram demonstrates mild wall thickening and irregularity with less than 25% luminal narrowing (arrows), compatible with a grade I injury.
Figure 8a.
Minimal intimal injury in a trauma patient. (a) Coronal CT angiogram shows what appears to be minimal luminal irregularity (arrows), compatible with a grade I injury. (b, c) Axial (b) and coronal (c) fat-saturated T1-weighted MR images show normal signal intensity in the vessel walls (arrows). Lack of intramural hematoma or wall thickening suggests that the CT angiographic findings (a) likely represent artifact or chronic irregularity.
Figure 8b.
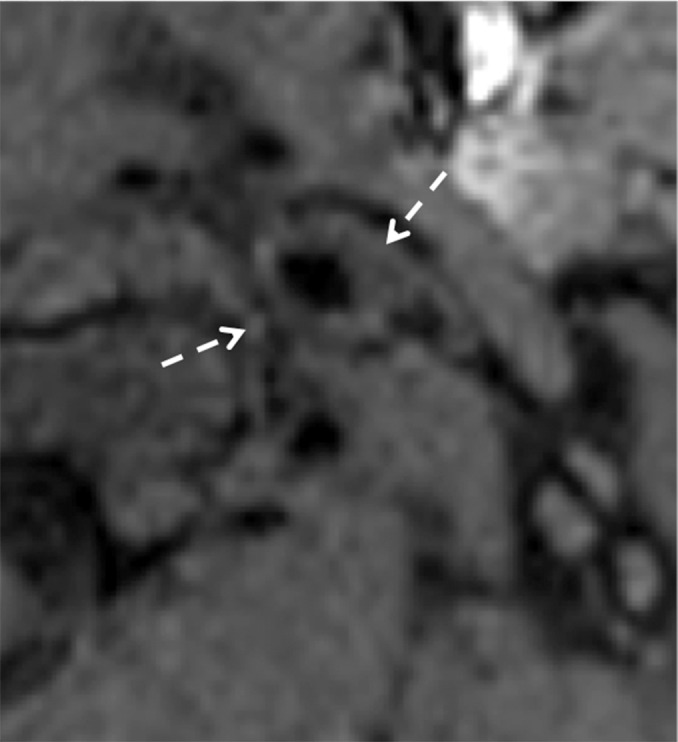
Minimal intimal injury in a trauma patient. (a) Coronal CT angiogram shows what appears to be minimal luminal irregularity (arrows), compatible with a grade I injury. (b, c) Axial (b) and coronal (c) fat-saturated T1-weighted MR images show normal signal intensity in the vessel walls (arrows). Lack of intramural hematoma or wall thickening suggests that the CT angiographic findings (a) likely represent artifact or chronic irregularity.
Figure 8c.
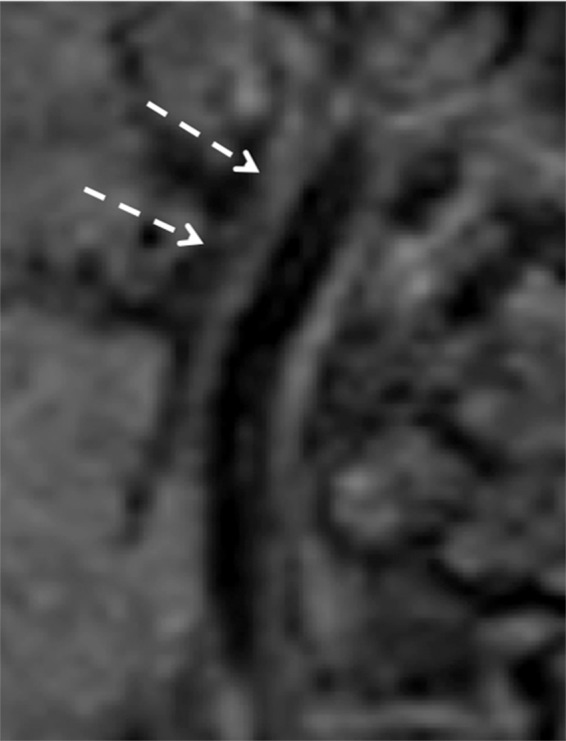
Minimal intimal injury in a trauma patient. (a) Coronal CT angiogram shows what appears to be minimal luminal irregularity (arrows), compatible with a grade I injury. (b, c) Axial (b) and coronal (c) fat-saturated T1-weighted MR images show normal signal intensity in the vessel walls (arrows). Lack of intramural hematoma or wall thickening suggests that the CT angiographic findings (a) likely represent artifact or chronic irregularity.
Intramural Hematoma
Dissecting intramural hematoma typically represents grade I or II BCVI, unless it causes full occlusion of the vessel, in which case it would be a grade IV injury. At all cross-sectional imaging modalities, these lesions appear as long segments of mural thickening in either an eccentric or circumferential distribution. Lesions demonstrating less than 25% luminal stenosis are classified as grade I injuries, whereas lesions demonstrating more than 25% luminal stenosis are classified as grade II injuries (Figs 9, 10). In theory, the vessel intima may be intact, although in practice this is not possible to resolve.
Figures 9.
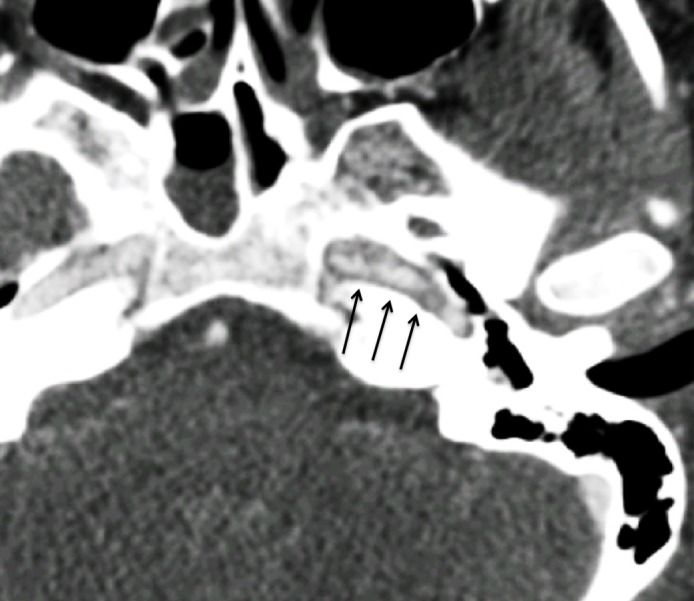
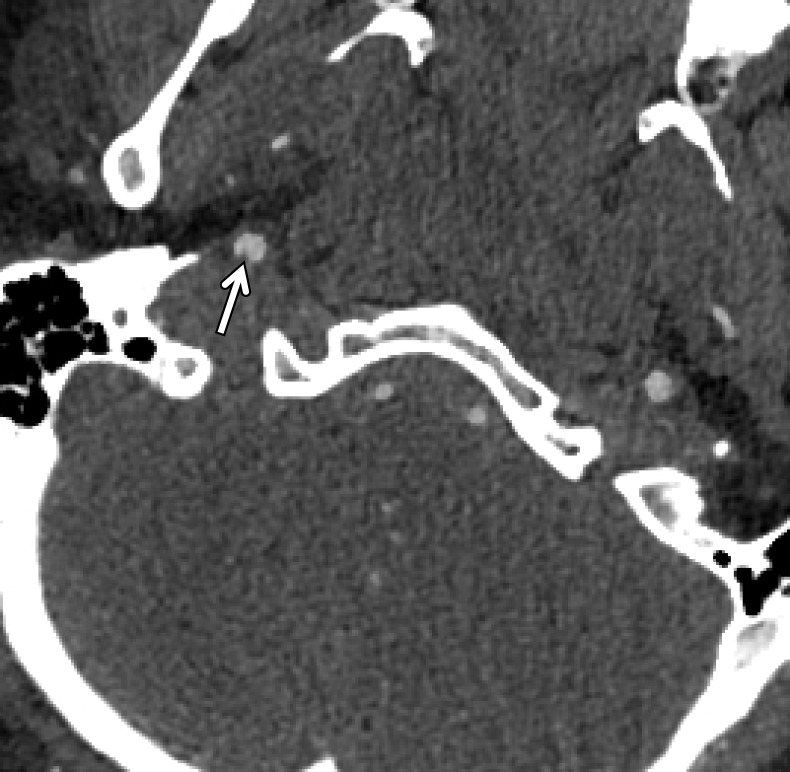
Intramural hematoma in a polytrauma patient with skull fractures after a motor vehicle collision. Axial CT angiogram shows luminal irregularity with greater than 25% narrowing of the petrous/lacerum segment of the left ICA (arrows), compatible with a grade II injury. No definite displaced intimal flap or false lumen was noted. The findings likely represent intramural hematoma.
Figures 10a.
Intramural hematoma and intraluminal thrombus. Axial (a) and sagittal (b) CT angiographic images at the C1 level demonstrate left V3 segment vertebral artery wall thickening and luminal narrowing (>25%) (black arrows), representing intramural hematoma, as well as a focal luminal thrombus (white arrow in b), compatible with grade II injury.
Figures 10b.
Intramural hematoma and intraluminal thrombus. Axial (a) and sagittal (b) CT angiographic images at the C1 level demonstrate left V3 segment vertebral artery wall thickening and luminal narrowing (>25%) (black arrows), representing intramural hematoma, as well as a focal luminal thrombus (white arrow in b), compatible with grade II injury.
On MR images, the intramural hematoma can be more completely defined. In the subacute stage, intramural hematoma will generally be hyperintense on proton-density–weighted and fat-saturated T1-weighted images (Fig 5). With improved blood suppression in areas of recirculation or slow flow, dedicated vessel wall MR imaging sequences can eliminate flow artifacts that may be confused with arterial injuries at routine MR imaging sequences. Of note, the signal intensity characteristics of a vessel wall hematoma are dependent on the age of injury, suggesting that vessel wall imaging could be helpful for cases in which the age of injury is not known (49). Intramural hematoma typically exhibits T1 isointensity in the acute phase (1–3 days), subsequently demonstrating different levels of T1 hyperintensity in the early and late subacute phase (3–14 days), and T1 isointensity in the chronic phase (>14 days).
Dissection with Raised Intimal Flap
Cervical artery dissections with raised intimal flaps represent grade II injuries. At cross-sectional imaging, a linear filling defect is seen within the vessel lumen. Classically, a “double-lumen” sign with contrast material opacifying the false lumen is pathognomonic for these injuries (Figs 11, 12). In clinical practice, however, the false lumen may or may not fully opacify. Multiplanar imaging is helpful in defining the full length of these lesions. On MR images, the false lumen will be hyperintense on proton-density–weighted and fat-saturated T1-weighted images owing to slow-flowing blood products or thrombus formation (Fig 5).
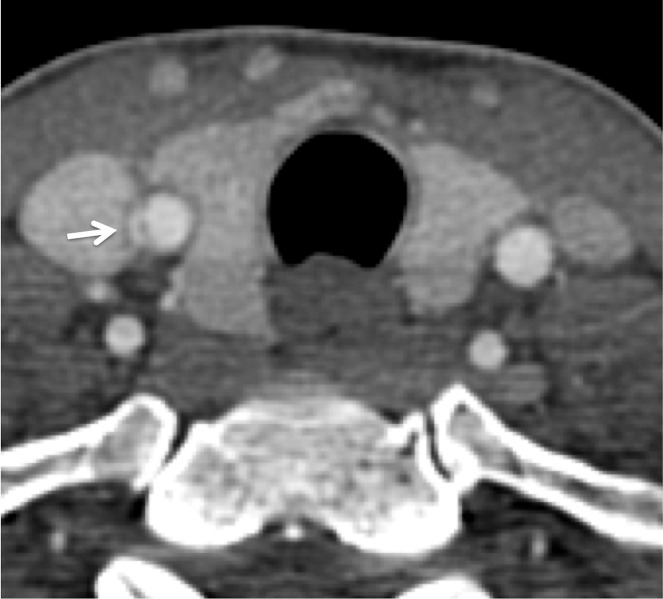
Figure 11a.
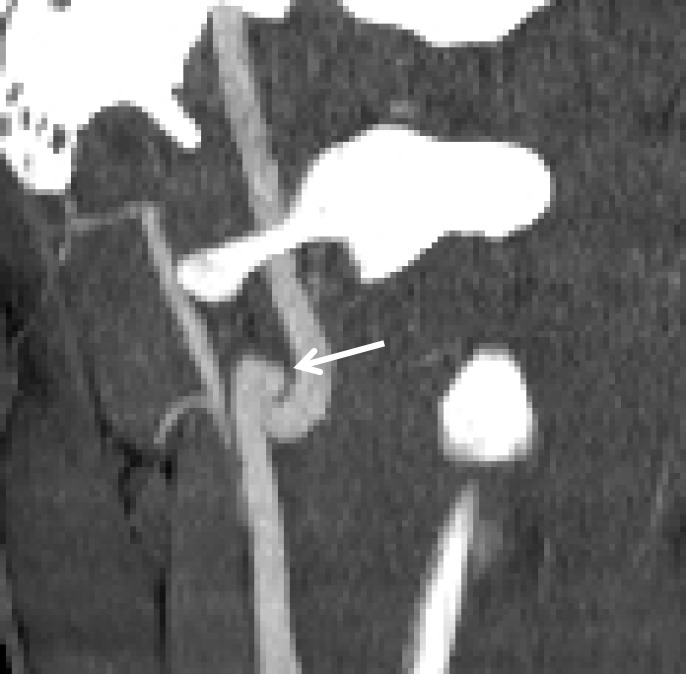
Displaced intimal flap of the common carotid artery in a 30-year-old man who presented with Le Fort II fractures after being hit in the face with a boulder. Axial (a) and coronal MIP (b) CT angiographic images show a displaced intimal flap of the right common carotid artery (arrow), compatible with grade II injury. Of note, BCVI is relatively rare in the common carotid arteries compared with the internal carotid and vertebral arteries.
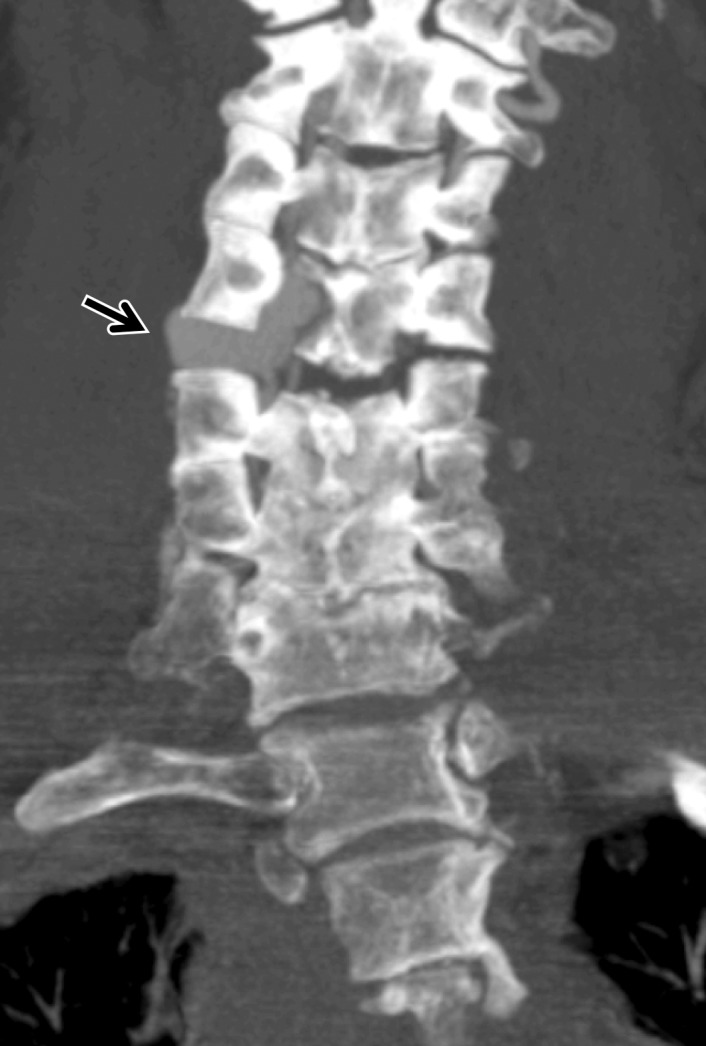
Figure 12a.
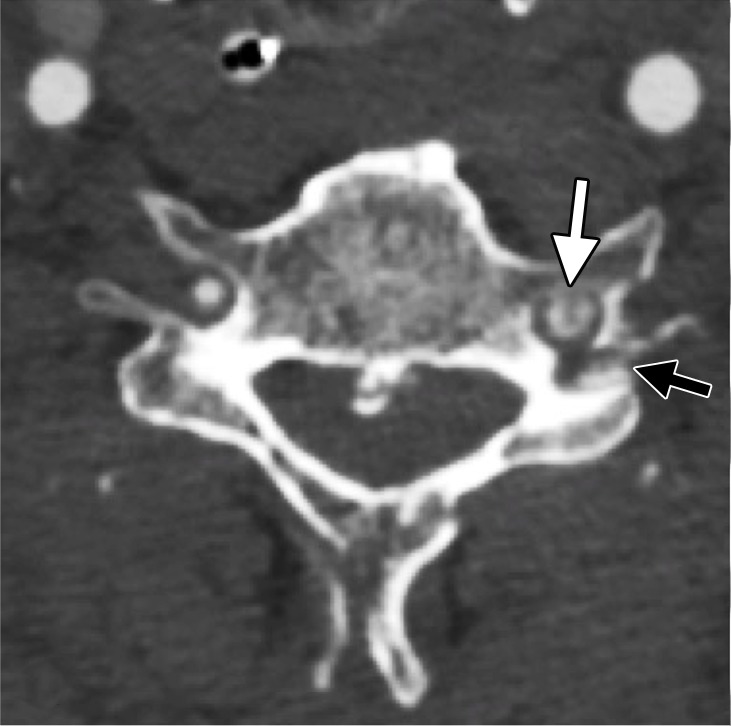
Dissection with raised intimal flap in a patient with Le Fort III facial fractures and fracture of a cervical transverse foramen (black arrow in a) after a motorcycle collision. Axial (a) and sagittal (b) CT angiographic images demonstrate a mural thrombus with raised intimal flap of the left vertebral artery within the transverse foramen (white arrow), adjacent to the fracture.
Figure 11b.
Displaced intimal flap of the common carotid artery in a 30-year-old man who presented with Le Fort II fractures after being hit in the face with a boulder. Axial (a) and coronal MIP (b) CT angiographic images show a displaced intimal flap of the right common carotid artery (arrow), compatible with grade II injury. Of note, BCVI is relatively rare in the common carotid arteries compared with the internal carotid and vertebral arteries.
Figure 12b.
Dissection with raised intimal flap in a patient with Le Fort III facial fractures and fracture of a cervical transverse foramen (black arrow in a) after a motorcycle collision. Axial (a) and sagittal (b) CT angiographic images demonstrate a mural thrombus with raised intimal flap of the left vertebral artery within the transverse foramen (white arrow), adjacent to the fracture.
Intraluminal Thrombus
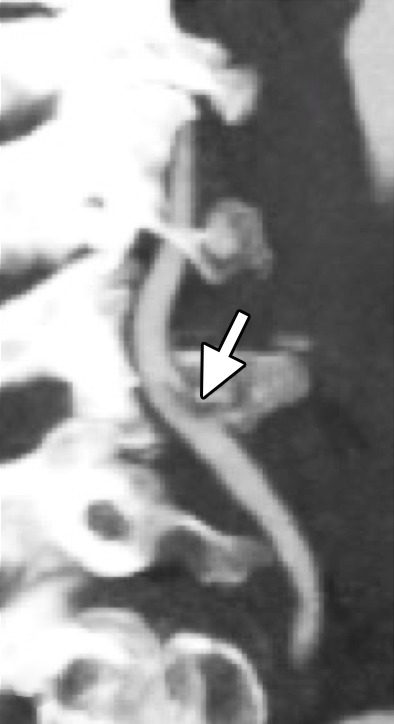
Because intimal injury exposes luminal blood to thrombogenic subendothelial components, focal intraluminal filling defects may form at the site of injury, corresponding to intraluminal thrombus. When present, a focal intraluminal thrombus corresponds to a grade II injury (Figs 1, 10, 13).
Figure 13a.
Focal intramural/intraluminal thrombus in a patient with cervical spine fractures after a high-speed motor vehicle collision. Axial (a) and sagittal (b) CT angiographic images show a mural thrombus (arrow) with focal narrowing greater than 25%, a grade II injury.
Figure 13b.
Focal intramural/intraluminal thrombus in a patient with cervical spine fractures after a high-speed motor vehicle collision. Axial (a) and sagittal (b) CT angiographic images show a mural thrombus (arrow) with focal narrowing greater than 25%, a grade II injury.
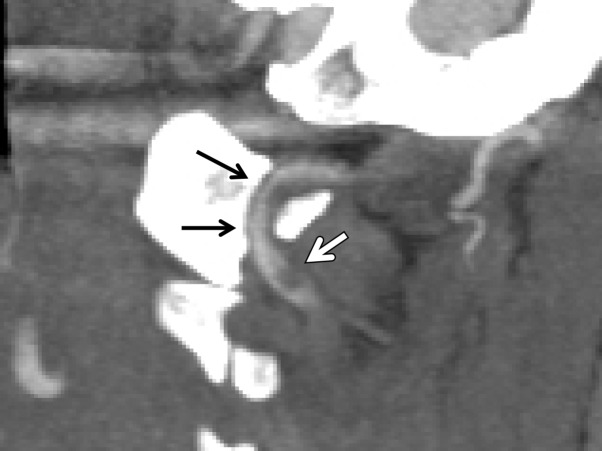
Pseudoaneurysm
A traumatic pseudoaneurysm represents a contained partial rupture of the vessel and corresponds to a grade III injury. At CT angiography and DSA, these lesions demonstrate variable focal ballooning of the arterial wall, with eccentric outpouching of the arterial lumen (Figs 14, 15). There is some variability in appearance of these injuries; while some may manifest with minimal fusiform contour enlargement, others may manifest with large saccular outpouchings. When large enough, the pseudoaneurysm may generate mass effect on the adjacent vessel wall, causing native luminal stenosis (Fig 15). Vessel wall MR imaging sequences may be helpful in defining the mural injury, including extent of associated intramural hematoma, dissection, and pseudoaneurysm margins.
Figure 14a.
Pseudoaneurysm in a patient with minimal trauma but who had a family history of dissections. Axial (a) and sagittal (b) MIP CT angiographic images and lateral neck digital subtraction angiographic image following a left common carotid artery injection (c) show a large saccular outpouching of contrast material from the left ICA (arrow), representing a pseudoaneurysm and compatible with grade III injury.
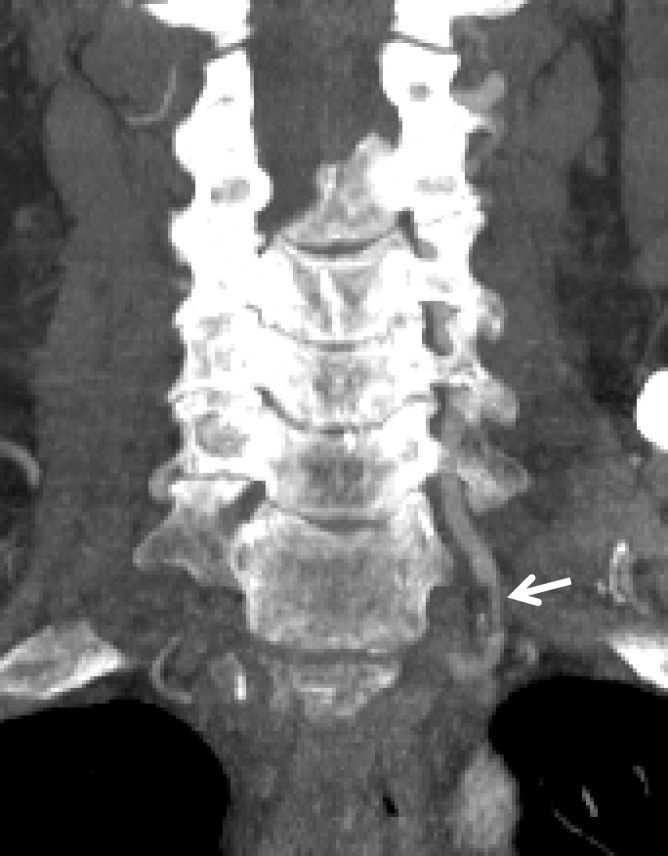
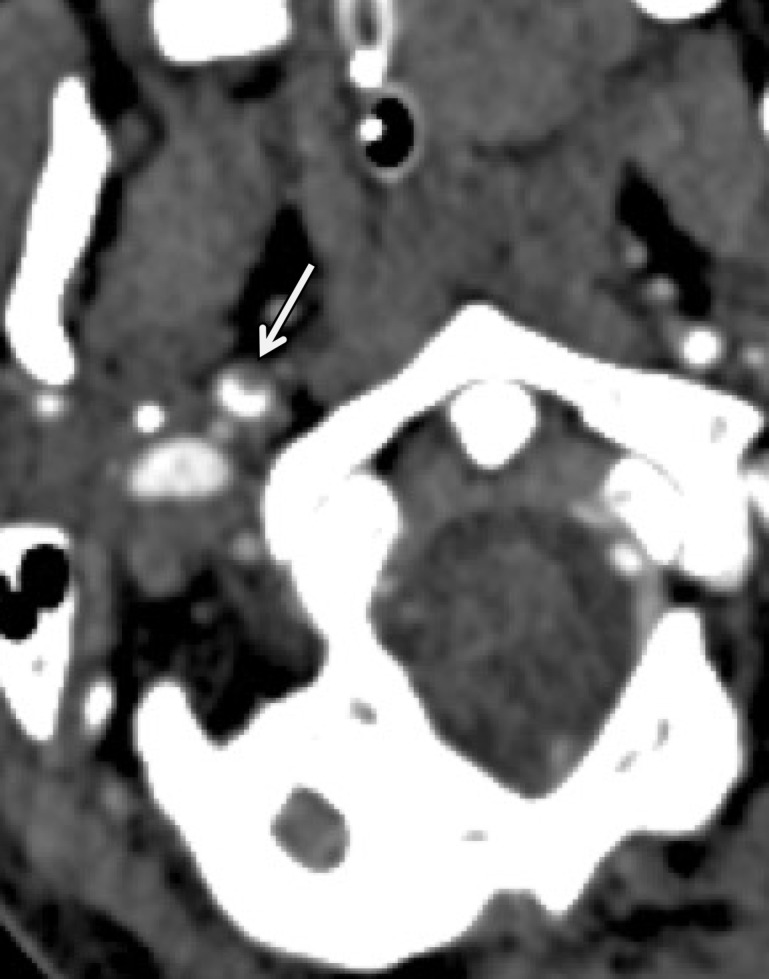
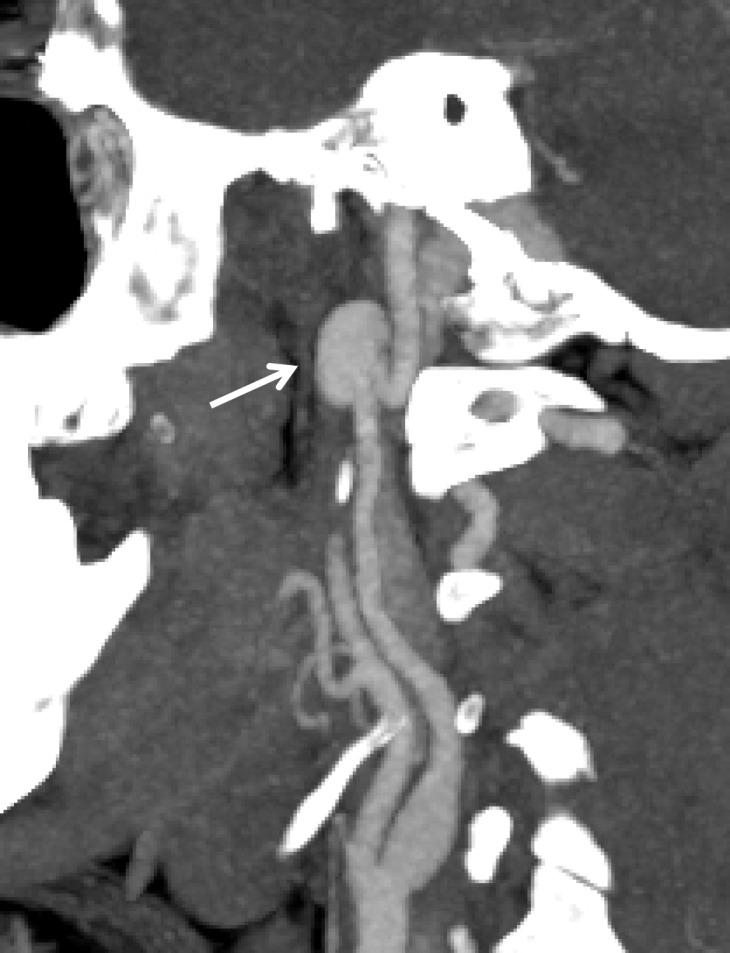
Figure 15a.
Pseudoaneurysm with enlargement at follow-up imaging in a polytrauma patient with cervical spine fractures. (a) Sagittal oblique MIP CT angiogram shows a pseudoaneurysm of the right cervical ICA (arrow), representing a grade III injury. The pseudoaneurysm and associated mural hematoma narrow the adjacent lumen (arrowhead). (b) Sagittal oblique CT angiogram obtained 7 days later shows slight interval enlargement of the pseudoaneurysm (arrow).
Figure 14b.
Pseudoaneurysm in a patient with minimal trauma but who had a family history of dissections. Axial (a) and sagittal (b) MIP CT angiographic images and lateral neck digital subtraction angiographic image following a left common carotid artery injection (c) show a large saccular outpouching of contrast material from the left ICA (arrow), representing a pseudoaneurysm and compatible with grade III injury.
Figure 14c.
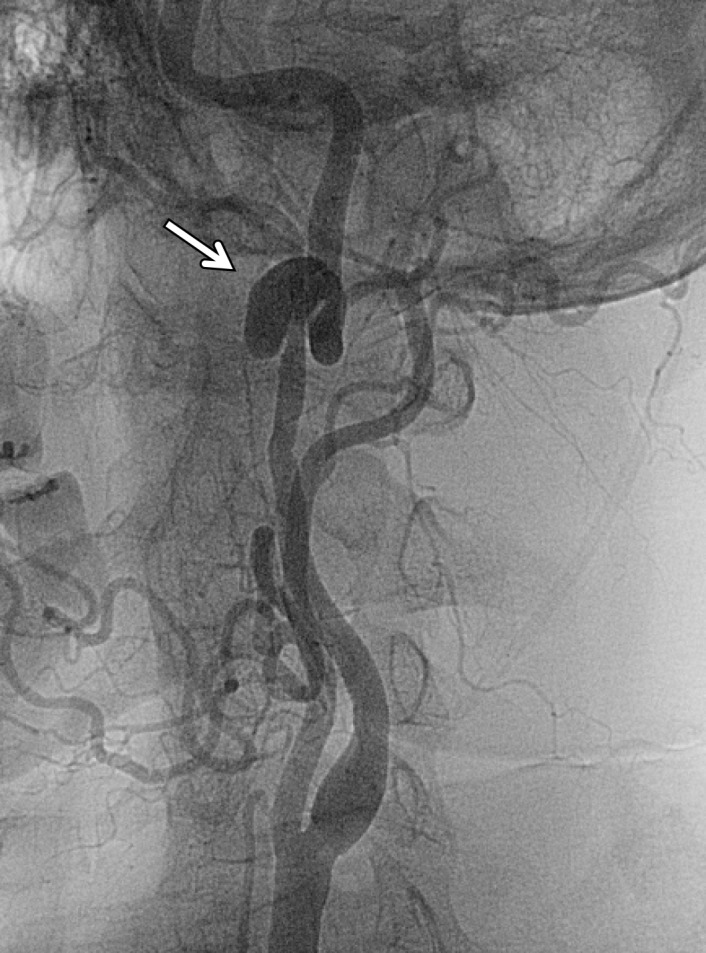
Pseudoaneurysm in a patient with minimal trauma but who had a family history of dissections. Axial (a) and sagittal (b) MIP CT angiographic images and lateral neck digital subtraction angiographic image following a left common carotid artery injection (c) show a large saccular outpouching of contrast material from the left ICA (arrow), representing a pseudoaneurysm and compatible with grade III injury.
Figure 15b.
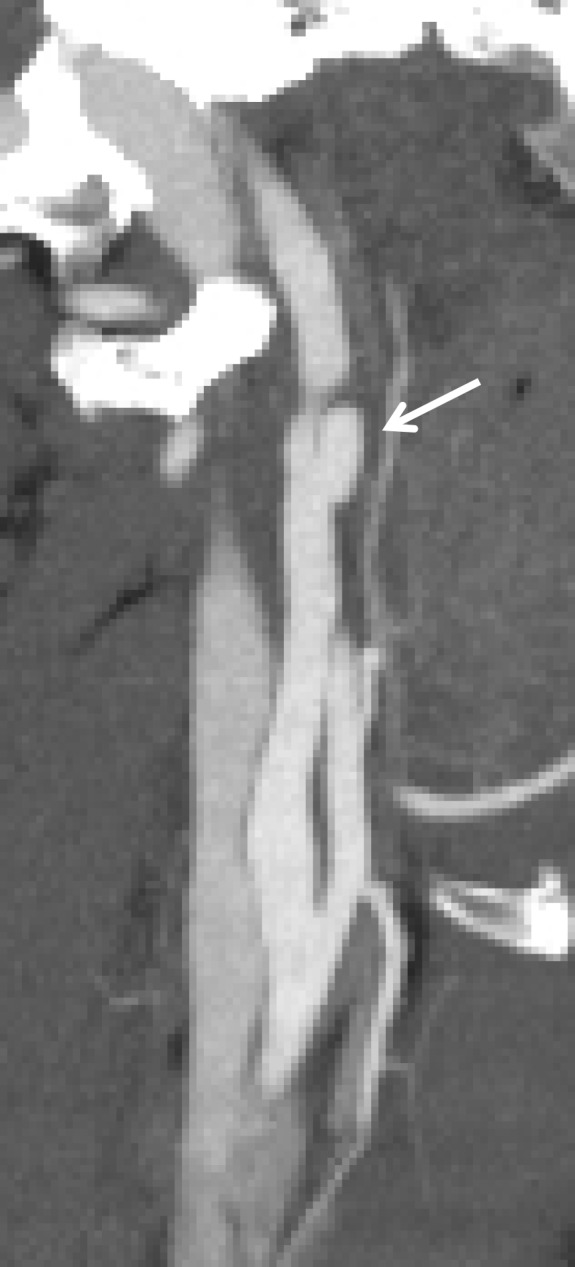
Pseudoaneurysm with enlargement at follow-up imaging in a polytrauma patient with cervical spine fractures. (a) Sagittal oblique MIP CT angiogram shows a pseudoaneurysm of the right cervical ICA (arrow), representing a grade III injury. The pseudoaneurysm and associated mural hematoma narrow the adjacent lumen (arrowhead). (b) Sagittal oblique CT angiogram obtained 7 days later shows slight interval enlargement of the pseudoaneurysm (arrow).
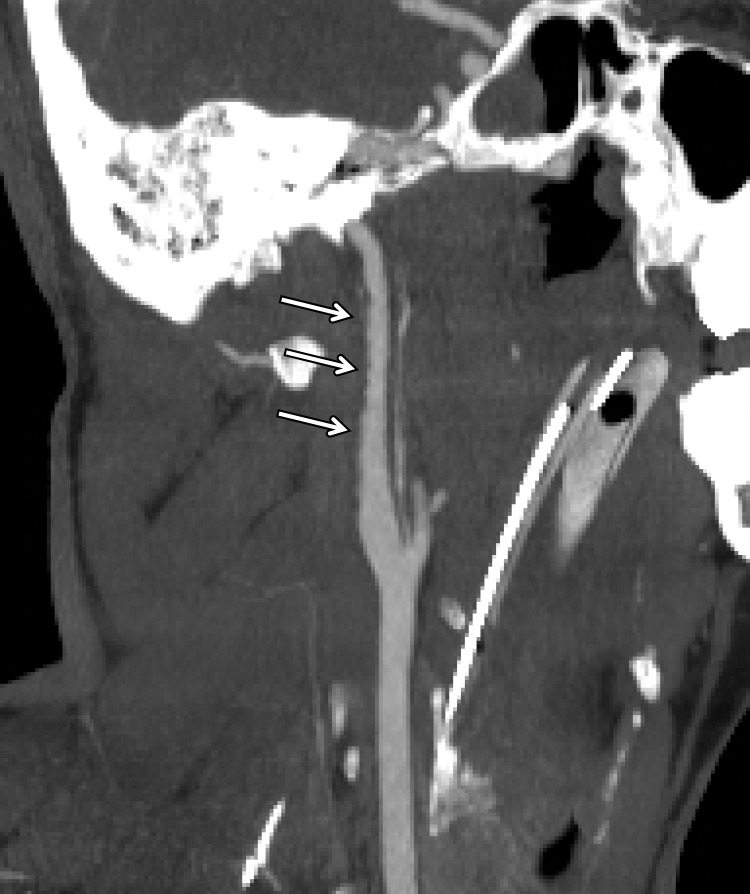
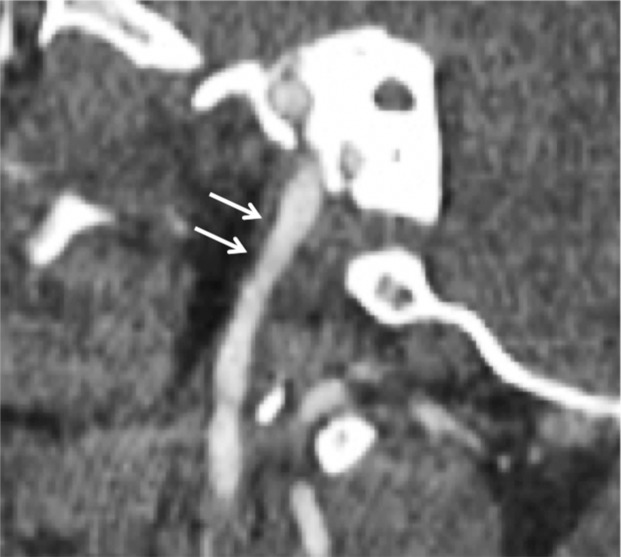
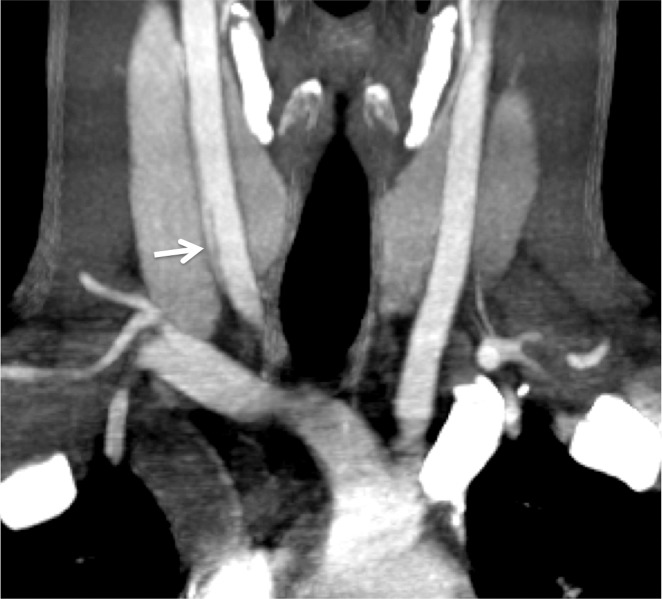
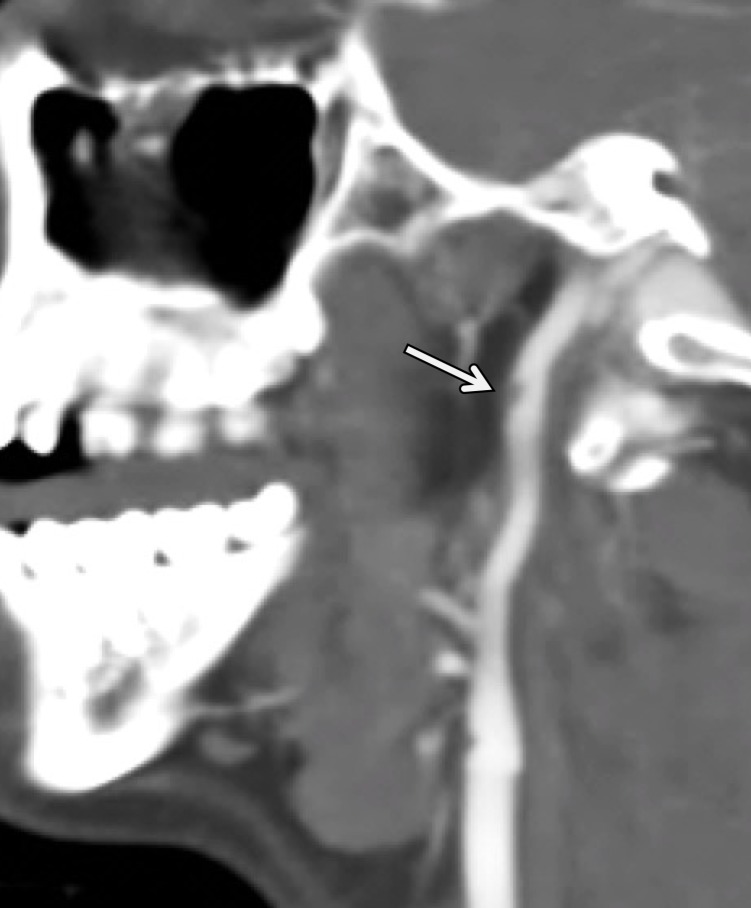
Arterial Occlusion
Arterial occlusion represents a grade IV injury. In the cervical carotid arteries, acute injuries occurring in the proximal cervical ICA often demonstrate tapering before complete occlusion at CT angiography/DSA (Fig 16). In contrast, vertebral artery occlusions more commonly exhibit abrupt arterial cutoffs when occluded (Fig 17). The length of vertebral artery occlusion is highly variable and is likely attributable to differences in collateral flow. At MR imaging, the occluded vessel will be isointense or hyperintense on proton-density–weighted and fat-saturated T1-weighted images, with loss of the dark vessel flow void. Time-of-flight MR angiography will demonstrate an abrupt or tapered cutoff of flow-related enhancement. Vessel wall MR imaging sequences are helpful when differentiating chronic atherosclerotic lesions causing high-grade narrowing or occlusion from acute dissections or intramural hematoma.
Figure 16a.
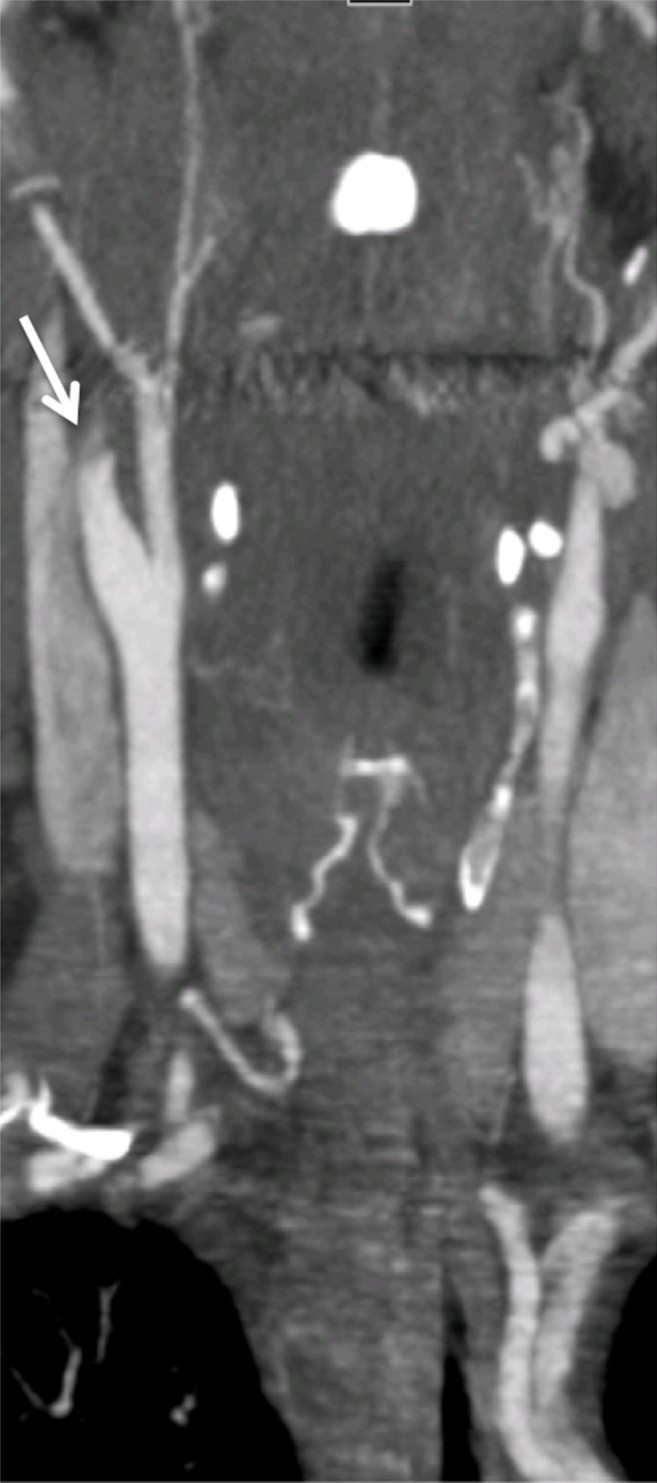
ICA occlusion in a patient who presented with polytrauma, including bilateral skull base fractures, after being struck by a motor vehicle. Coronal CT angiogram (a) and oblique digital subtraction angiographic image obtained after a right common carotid artery injection (b) show dissection with tapering and complete occlusion of the proximal cervical ICA (arrow), representing a grade IV injury.
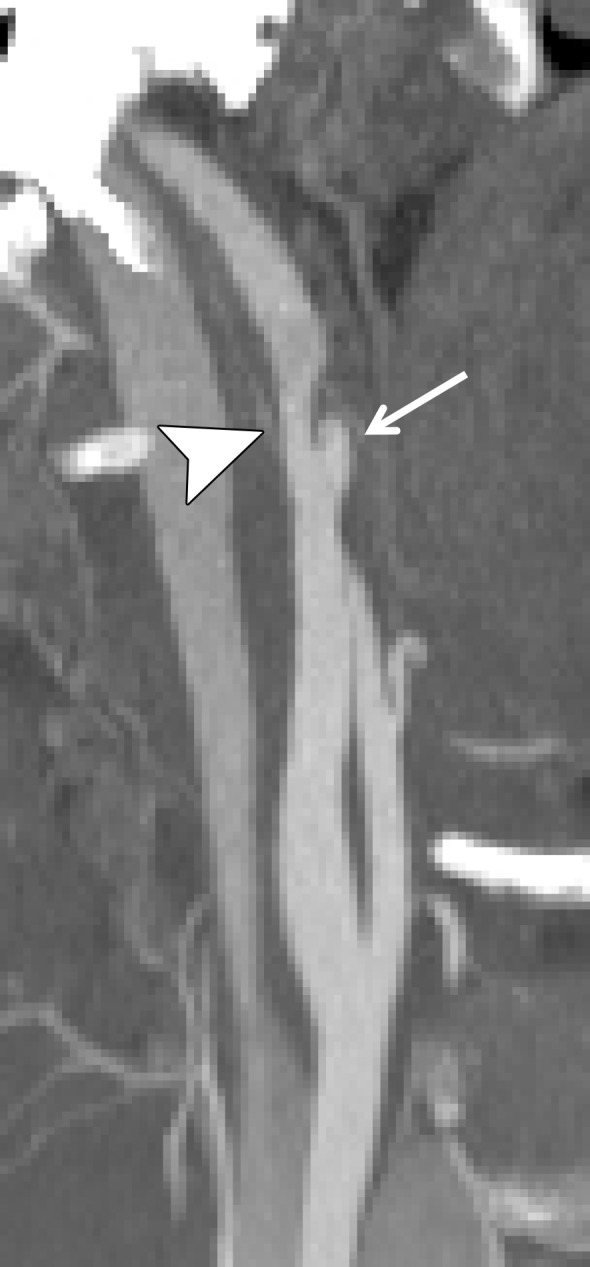
Figure 17a.
Vertebral artery occlusion. (a, b) Sagittal oblique (a) and sagittal (b) CT angiographic images show occlusion of the proximal right vertebral artery (arrow in a), with reconstitution distally at the C3–C4 vertebral level (white arrow in b), compatible with a grade IV injury. Fracture of the C5 articular facet (black arrow in b) is also seen. (c, d) Axial CT angiographic (c) and axial T2-weighted MR (d) images demonstrate occlusion (arrow in c) and loss of right vertebral artery flow void (arrowhead in d).
Figure 16b.
ICA occlusion in a patient who presented with polytrauma, including bilateral skull base fractures, after being struck by a motor vehicle. Coronal CT angiogram (a) and oblique digital subtraction angiographic image obtained after a right common carotid artery injection (b) show dissection with tapering and complete occlusion of the proximal cervical ICA (arrow), representing a grade IV injury.
Figure 17b.
Vertebral artery occlusion. (a, b) Sagittal oblique (a) and sagittal (b) CT angiographic images show occlusion of the proximal right vertebral artery (arrow in a), with reconstitution distally at the C3–C4 vertebral level (white arrow in b), compatible with a grade IV injury. Fracture of the C5 articular facet (black arrow in b) is also seen. (c, d) Axial CT angiographic (c) and axial T2-weighted MR (d) images demonstrate occlusion (arrow in c) and loss of right vertebral artery flow void (arrowhead in d).
Figure 17c.
Vertebral artery occlusion. (a, b) Sagittal oblique (a) and sagittal (b) CT angiographic images show occlusion of the proximal right vertebral artery (arrow in a), with reconstitution distally at the C3–C4 vertebral level (white arrow in b), compatible with a grade IV injury. Fracture of the C5 articular facet (black arrow in b) is also seen. (c, d) Axial CT angiographic (c) and axial T2-weighted MR (d) images demonstrate occlusion (arrow in c) and loss of right vertebral artery flow void (arrowhead in d).
Figure 17d.
Vertebral artery occlusion. (a, b) Sagittal oblique (a) and sagittal (b) CT angiographic images show occlusion of the proximal right vertebral artery (arrow in a), with reconstitution distally at the C3–C4 vertebral level (white arrow in b), compatible with a grade IV injury. Fracture of the C5 articular facet (black arrow in b) is also seen. (c, d) Axial CT angiographic (c) and axial T2-weighted MR (d) images demonstrate occlusion (arrow in c) and loss of right vertebral artery flow void (arrowhead in d).
Arterial Transection and AVF
Arterial transection and posttraumatic AVF represent grade V injuries. At CT angiography/DSA, a transection appears as an irregular collection of extravascular contrast material surrounding the parent vessel (Figs 18, 19). On delayed images, the contrast material collection disperses owing to active extravasation. There may be no filling or minimal intravascular contrast material opacification downstream from the site of injury.
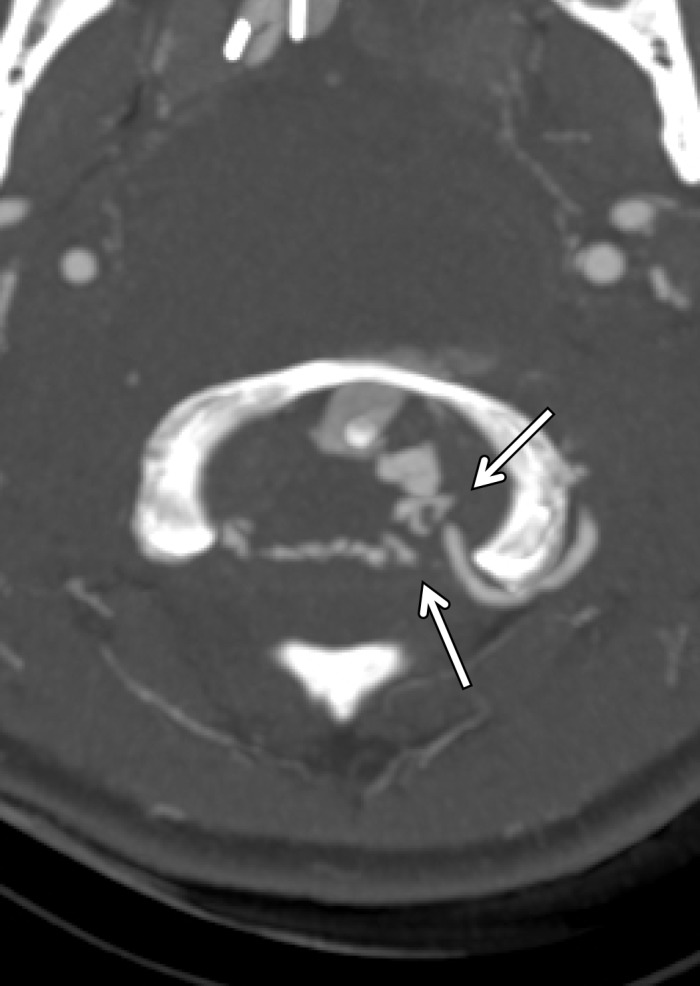
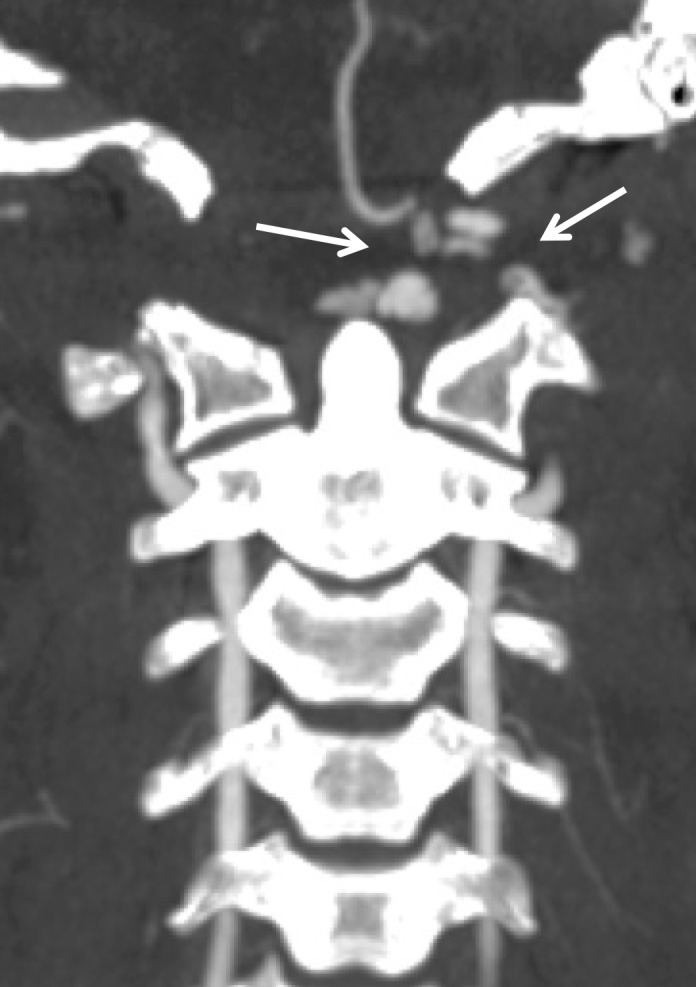
Figure 18a.
Vertebral artery transection in a polytrauma patient with atlanto-occipital dissociation. (a) Axial CT image shows active extravasation of contrast material within a large hematoma at the widened craniocervical junction (arrows). (b, c) Axial (b) and coronal (c) MIP images from CT angiography demonstrate hemorrhage from the transected left V3–V4 segment vertebral artery at the C1 level (arrows), representing grade V injury.
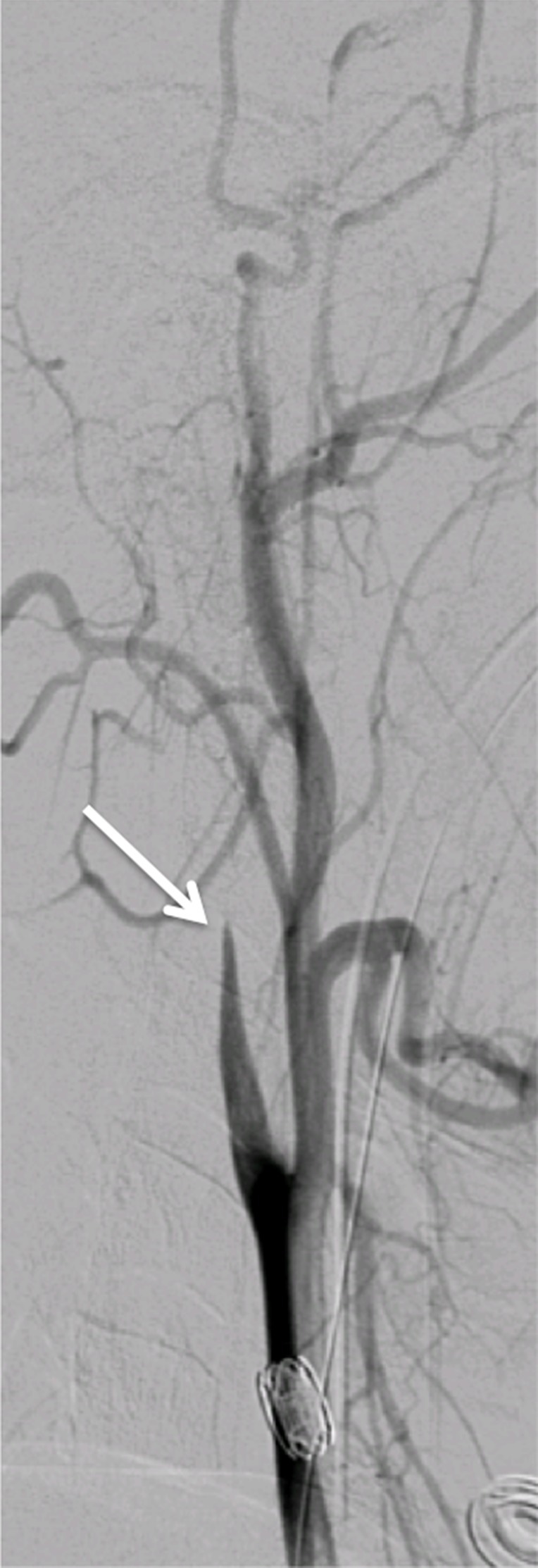
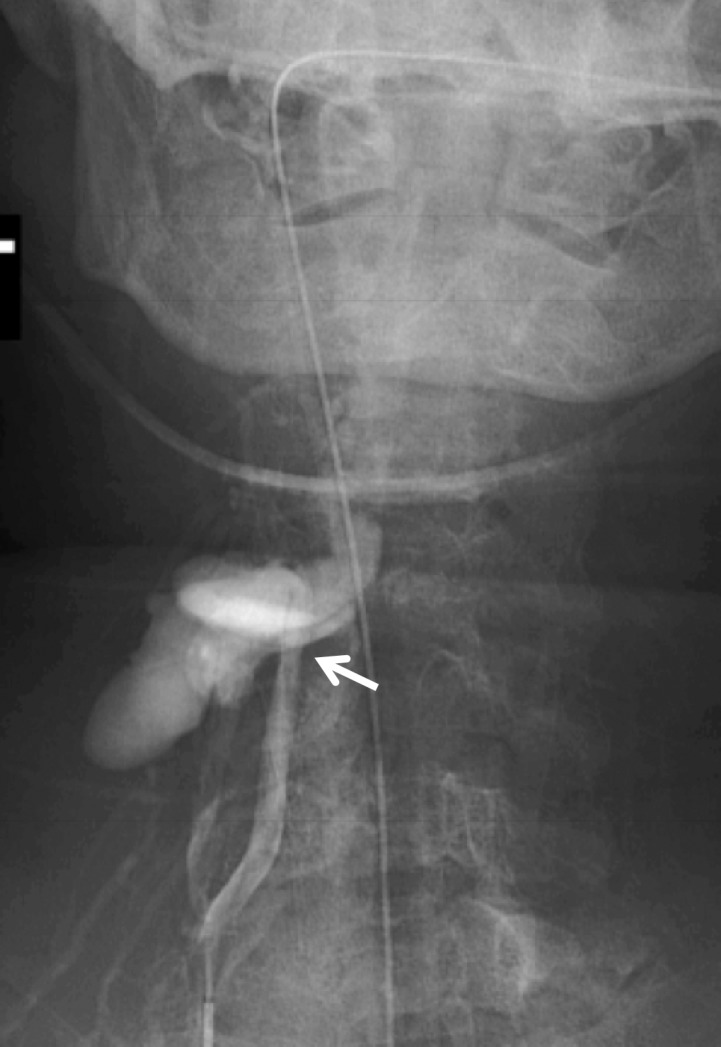
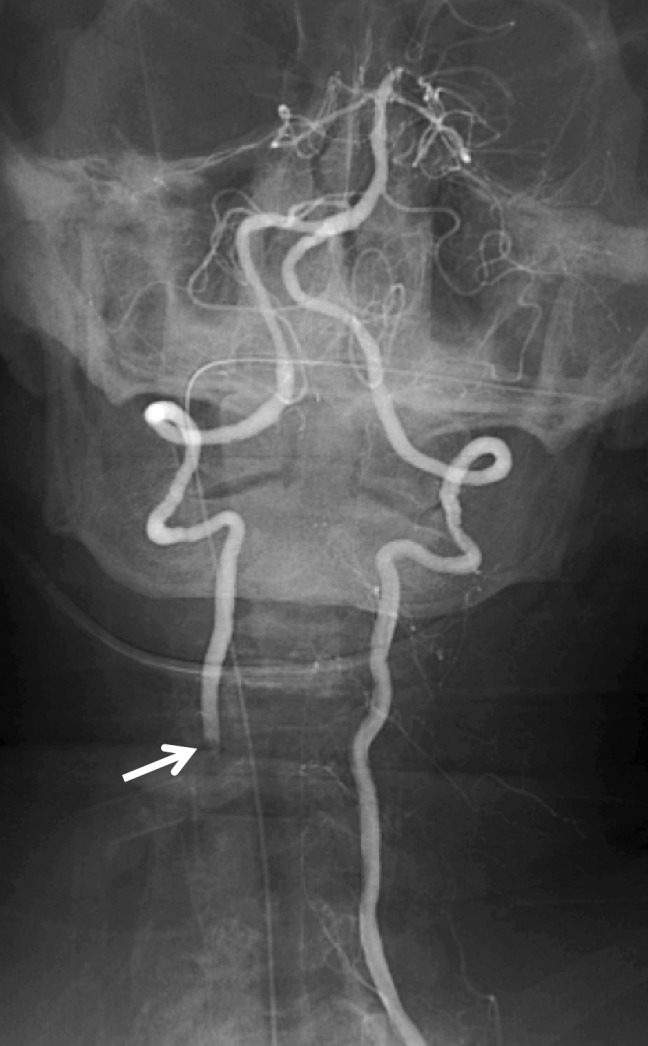
Figure 19a.
Vertebral artery transection and AVF in a polytrauma patient with C4–C5 fracture dislocation. (a) Coronal MIP image from CT angiography shows active extravasation with a hematoma (arrow) centered in the right C4 transverse foramen at the level of the fracture-dislocation. (b) Right vertebral artery injection catheter angiogram shows active extravasation, representing transection of the right vertebral artery (arrow), compatible with grade V injury. (c) Left vertebral artery angiogram shows retrograde flow into the right vertebral artery, to the level of the transection (arrow). (d–f) Right vertebral artery injection digital subtraction angiographic images in three subsequent phases show early venous drainage, compatible with AVF.
Figure 18b.
Vertebral artery transection in a polytrauma patient with atlanto-occipital dissociation. (a) Axial CT image shows active extravasation of contrast material within a large hematoma at the widened craniocervical junction (arrows). (b, c) Axial (b) and coronal (c) MIP images from CT angiography demonstrate hemorrhage from the transected left V3–V4 segment vertebral artery at the C1 level (arrows), representing grade V injury.
Figure 18c.
Vertebral artery transection in a polytrauma patient with atlanto-occipital dissociation. (a) Axial CT image shows active extravasation of contrast material within a large hematoma at the widened craniocervical junction (arrows). (b, c) Axial (b) and coronal (c) MIP images from CT angiography demonstrate hemorrhage from the transected left V3–V4 segment vertebral artery at the C1 level (arrows), representing grade V injury.
Figure 19b.
Vertebral artery transection and AVF in a polytrauma patient with C4–C5 fracture dislocation. (a) Coronal MIP image from CT angiography shows active extravasation with a hematoma (arrow) centered in the right C4 transverse foramen at the level of the fracture-dislocation. (b) Right vertebral artery injection catheter angiogram shows active extravasation, representing transection of the right vertebral artery (arrow), compatible with grade V injury. (c) Left vertebral artery angiogram shows retrograde flow into the right vertebral artery, to the level of the transection (arrow). (d–f) Right vertebral artery injection digital subtraction angiographic images in three subsequent phases show early venous drainage, compatible with AVF.
Figure 19c.
Vertebral artery transection and AVF in a polytrauma patient with C4–C5 fracture dislocation. (a) Coronal MIP image from CT angiography shows active extravasation with a hematoma (arrow) centered in the right C4 transverse foramen at the level of the fracture-dislocation. (b) Right vertebral artery injection catheter angiogram shows active extravasation, representing transection of the right vertebral artery (arrow), compatible with grade V injury. (c) Left vertebral artery angiogram shows retrograde flow into the right vertebral artery, to the level of the transection (arrow). (d–f) Right vertebral artery injection digital subtraction angiographic images in three subsequent phases show early venous drainage, compatible with AVF.
Figure 19d.
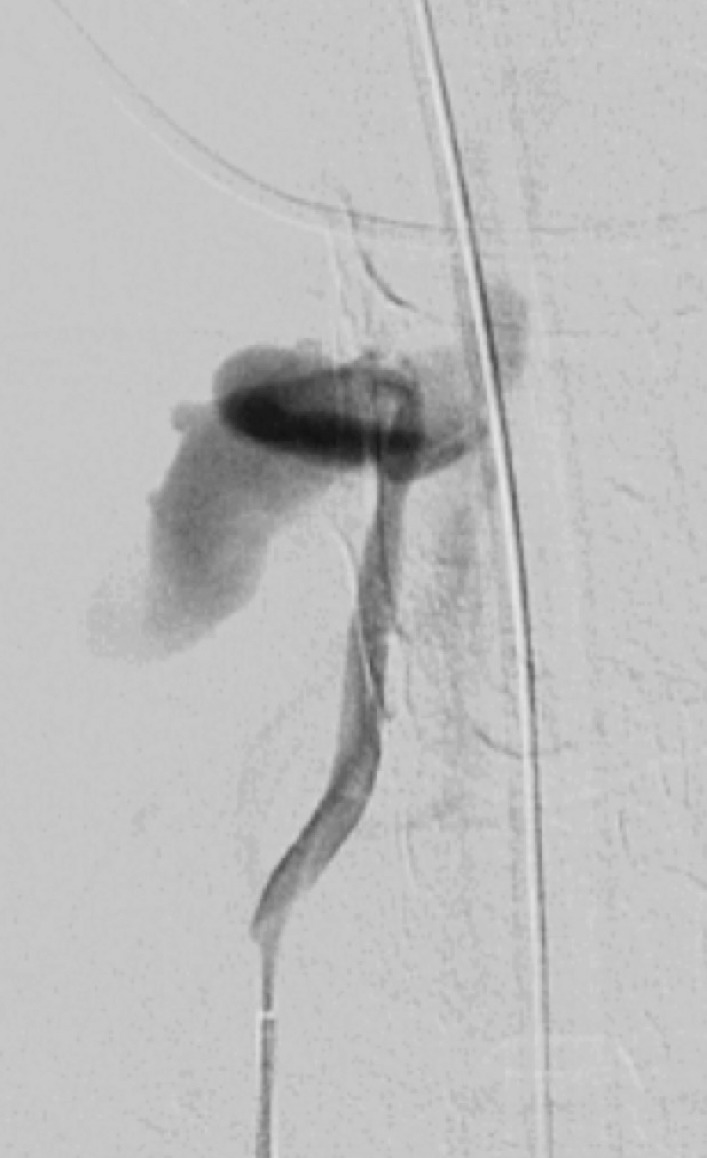
Vertebral artery transection and AVF in a polytrauma patient with C4–C5 fracture dislocation. (a) Coronal MIP image from CT angiography shows active extravasation with a hematoma (arrow) centered in the right C4 transverse foramen at the level of the fracture-dislocation. (b) Right vertebral artery injection catheter angiogram shows active extravasation, representing transection of the right vertebral artery (arrow), compatible with grade V injury. (c) Left vertebral artery angiogram shows retrograde flow into the right vertebral artery, to the level of the transection (arrow). (d–f) Right vertebral artery injection digital subtraction angiographic images in three subsequent phases show early venous drainage, compatible with AVF.
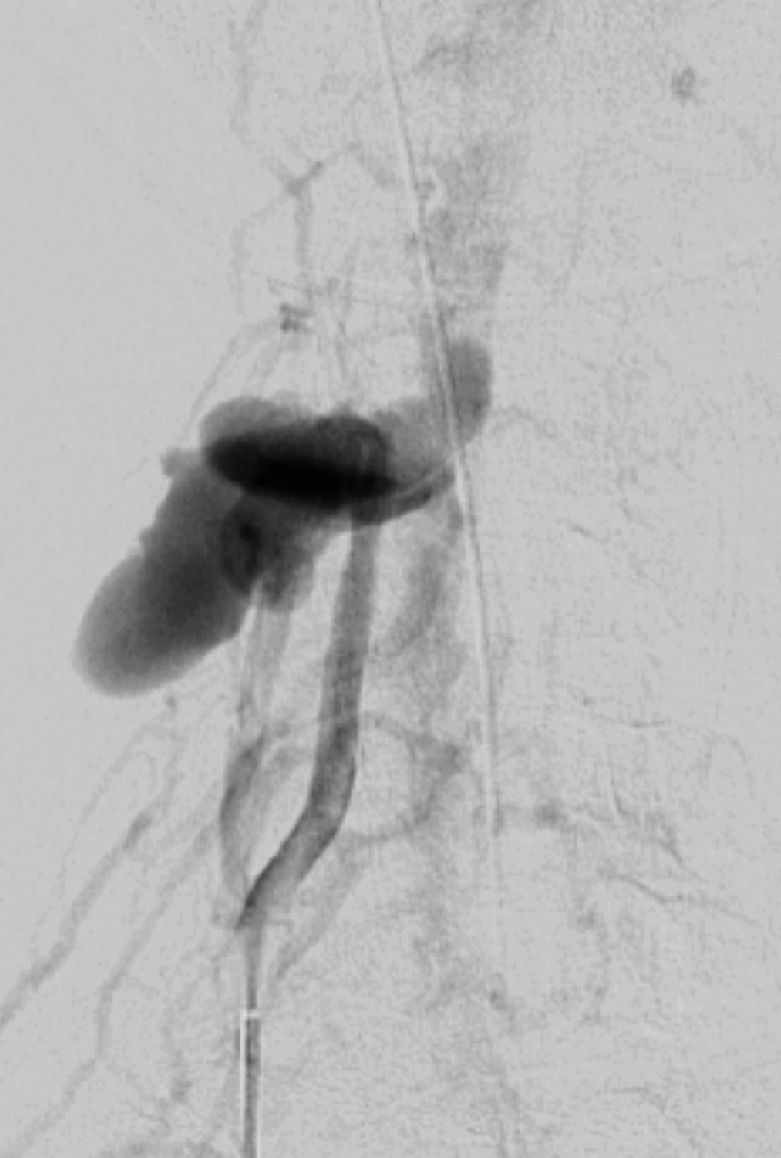
Figure 19e.
Vertebral artery transection and AVF in a polytrauma patient with C4–C5 fracture dislocation. (a) Coronal MIP image from CT angiography shows active extravasation with a hematoma (arrow) centered in the right C4 transverse foramen at the level of the fracture-dislocation. (b) Right vertebral artery injection catheter angiogram shows active extravasation, representing transection of the right vertebral artery (arrow), compatible with grade V injury. (c) Left vertebral artery angiogram shows retrograde flow into the right vertebral artery, to the level of the transection (arrow). (d–f) Right vertebral artery injection digital subtraction angiographic images in three subsequent phases show early venous drainage, compatible with AVF.
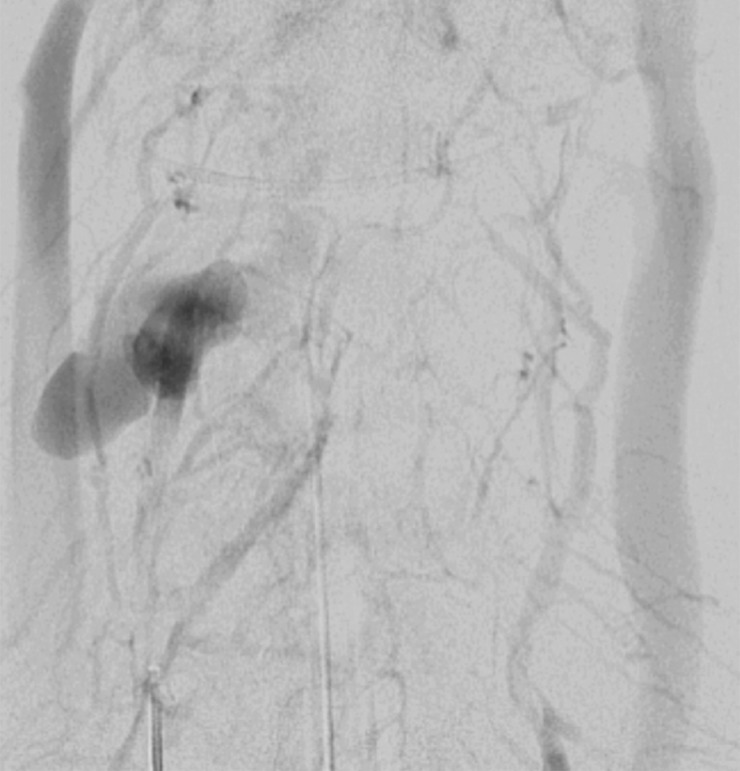
Figure 19f.
Vertebral artery transection and AVF in a polytrauma patient with C4–C5 fracture dislocation. (a) Coronal MIP image from CT angiography shows active extravasation with a hematoma (arrow) centered in the right C4 transverse foramen at the level of the fracture-dislocation. (b) Right vertebral artery injection catheter angiogram shows active extravasation, representing transection of the right vertebral artery (arrow), compatible with grade V injury. (c) Left vertebral artery angiogram shows retrograde flow into the right vertebral artery, to the level of the transection (arrow). (d–f) Right vertebral artery injection digital subtraction angiographic images in three subsequent phases show early venous drainage, compatible with AVF.
AVF may occur when an abnormal high-flow connection is made between an injured artery and draining veins. This may be difficult to detect with single-phase CT angiography, but with DSA it is easily identified as early venous filling during the arterial phase (Fig 19). Additionally, there may be enlargement of the involved draining veins in response to increased flow. In cases of CCFs, proptosis with enlarged superior ophthalmic veins and orbital edema may bring attention to an asymmetrically opacified cavernous sinus in the arterial phase at CT angiography. Time-resolved MR angiography with high temporal resolution may also be useful in the diagnosis and characterization of AVF, characterized by early venous filling (50).
Venous Injury
Although not included in standard grading and imaging schemes for the evaluation of BCVI, venous injuries may also occur in high-energy blunt force trauma and may be discovered incidentally when evaluating for arterial injury at screening examinations. At nonenhanced CT, hyperintense signal of the dural sinus above that of normal sinus blood may indicate DVST. At CT venography, a flow void within the dural sinus is diagnostic. Associated gyral enhancement and prominent intramedullary veins are also suggestive of DVST owing to venous backflow.
MR imaging including MR venography may be useful in equivocal cases. MR venography would show a lack of flow-related enhancement within the affected sinus. T1- and T2-weighted MR imaging will show an absent flow void, with variable intensity depending on the age of the clot. T2*-weighted images may show blooming of susceptibility artifact within the clot. Of note, an enhanced T1-weighted 3D volumetric gradient-echo sequence has been shown to be the single most sensitive and specific MR imaging sequence for the detection of DVST, demonstrating a filling defect at the site of the clot (48).
Mimics and Pitfalls
Differentiating BCVI from other nontraumatic vascular causes and normal vascular variants can be difficult. Understanding the findings of alternate diagnoses that mimic BCVI and the imaging pitfalls that prevent its visualization and identification are important to ensure an accurate and timely diagnosis, and to prevent a wrong diagnosis and inappropriate treatment.
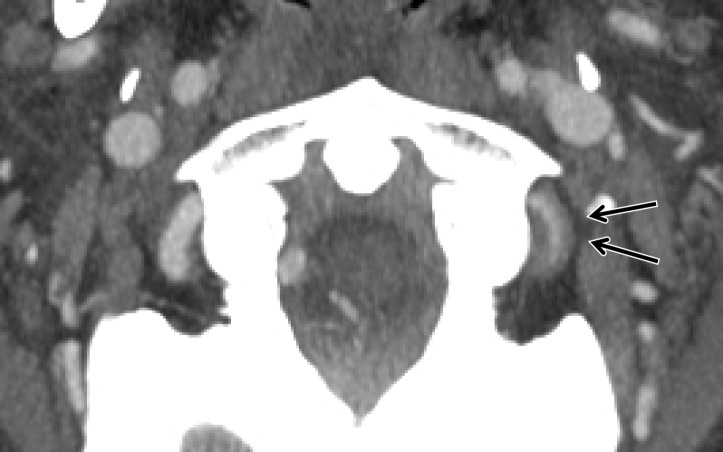
Atherosclerosis is an extremely common condition that can easily be confused with BCVI. As atherosclerosis is more common in older patients, age and the presence of multiple vascular risk factors are useful pieces of clinical information to differentiate atherosclerosis from BCVI. The presence of calcification and lesion location at the common carotid artery origin, vertebral artery origin, or within the carotid bulb are suggestive of atherosclerosis (Fig 20). Vessel wall MR imaging using a fat-saturated T1-weighted sequence can help differentiate atherosclerotic plaque from an acute injury. In the case of BCVI, intramural hematoma may be bright on T1-weighted images, whereas atherosclerosis usually will not be.
Figure 20a.
Atherosclerosis mimicking BCVI in a polytrauma patient at high risk for BCVI. (a) Coronal MIP image from CT angiography shows a mural lesion causing narrowing of the proximal left vertebral artery (arrow). Associated calcification is present, compatible with atherosclerotic plaque. (b) Axial CT angiographic image at the C1 level shows a calcified atherosclerotic plaque of the right V3 vertebral artery, causing narrowing and wall thickening (arrow), mimicking a stenotic BCVI (ie, intramural hematoma).
Figure 20b.
Atherosclerosis mimicking BCVI in a polytrauma patient at high risk for BCVI. (a) Coronal MIP image from CT angiography shows a mural lesion causing narrowing of the proximal left vertebral artery (arrow). Associated calcification is present, compatible with atherosclerotic plaque. (b) Axial CT angiographic image at the C1 level shows a calcified atherosclerotic plaque of the right V3 vertebral artery, causing narrowing and wall thickening (arrow), mimicking a stenotic BCVI (ie, intramural hematoma).
In addition to atherosclerosis, carotid fibromuscular dysplasia and vasospasm are relatively common BCVI mimics. More rarely, vasculitis can give a similar appearance. In the case of carotid fibromuscular dysplasia, the carotid arteries may demonstrate a “string of beads” appearance, with alternating segments of vessel stenosis and dilatation. Multifocal and bilateral vascular involvement, as well as involvement of the renal arteries, are suggestive of fibromuscular dysplasia. Vasospasm is a commonly encountered phenomenon after severe trauma. It may be impossible to distinguish transient vasospasm from low-grade BCVI at initial cross-sectional imaging. Repeat imaging often allows distinction of vasospasm from BCVI, with vasospasm resolving over several hours and BCVI usually lasting longer or even persisting indefinitely.
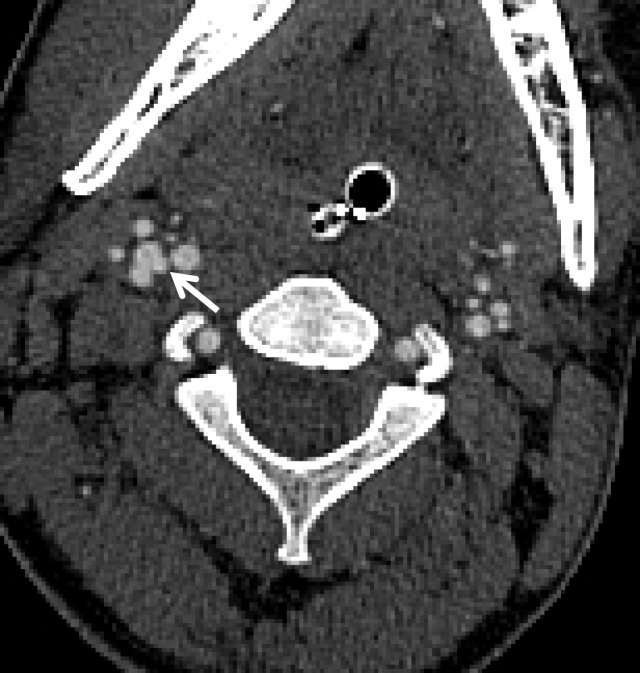
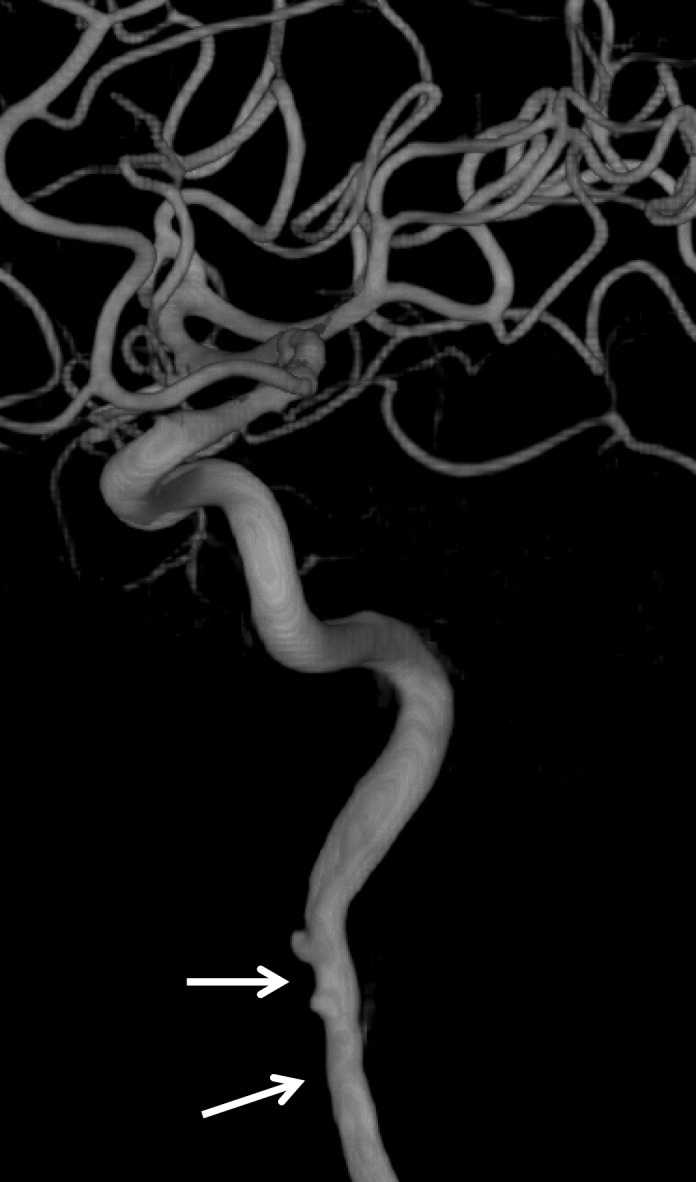
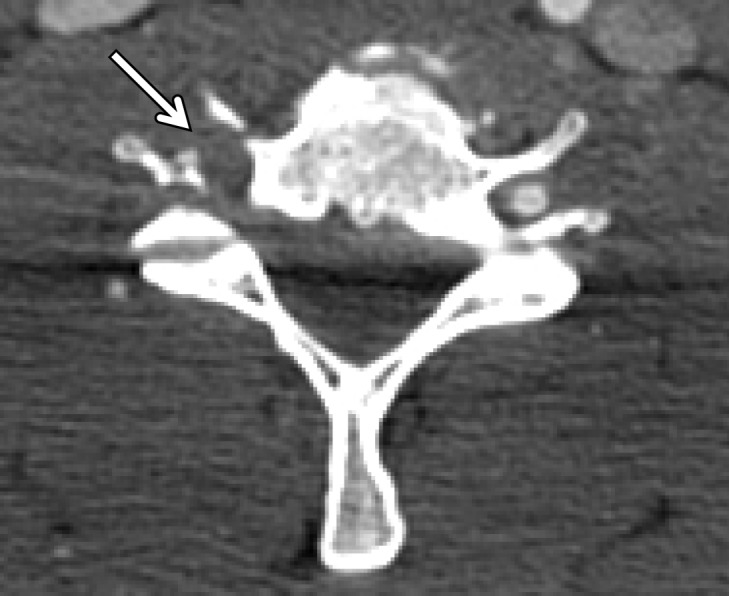
Normal vascular variants can both mimic and mask vascular injuries. Coiled or looped segments of redundant cervical ICA are common areas of injury and can mask or mimic low-grade BCVI or a small focal outpouching (Fig 21). Congenitally hypoplastic cervical arteries can sometimes be mistaken for dissection or occlusion. Hypoplastic ICAs are rare; the presence of an asymmetrically small carotid canal would be highly suggestive of this congenital variant. Hypoplastic vertebral arteries are far more common and are usually uniformly narrow along the length of the vessel. In these cases, the V2 segment of the vertebral artery traverses asymmetrically small transverse foramina.
Figure 21a.
BCVI involving coiled or looped vascular segments. Coiled or looped vascular segments make evaluation for luminal and wall abnormalities difficult. Axial (a) and coronal (b) MIP images from CT angiography show a small medially directed pseudoaneurysm (arrow), which is easily ignored as it subtly projects from the coiled segment.
Figure 21b.
BCVI involving coiled or looped vascular segments. Coiled or looped vascular segments make evaluation for luminal and wall abnormalities difficult. Axial (a) and coronal (b) MIP images from CT angiography show a small medially directed pseudoaneurysm (arrow), which is easily ignored as it subtly projects from the coiled segment.
A common pitfall for accurate and timely diagnosis of BCVI is the presence of imaging artifacts. This is particularly true where the vessels course behind the mandibles and enter the skull base. Motion can both obscure disease or create the illusion of it; step-off artifacts may falsely create irregular luminal margins. Streak artifact from dental amalgam or spinal hardware is a common problem, found in nearly 5% of CT scans (51). Unfortunately, streak artifacts from dental amalgam occur at sites commonly affected by BCVI—the skull base and posterior to the mandible—and may either obscure or create the illusion of vascular injury (Fig 22).
Figure 22.
Streak artifact. Axial CT angiogram shows extensive streak artifact emanating from the dental amalgam, which makes the evaluation of arterial contours difficult. A grade II BCVI of the right vertebral artery (arrow) is difficult to appreciate. Additionally, one may imagine the presence of linear filling defects in the normal bilateral ICAs (arrowheads); the artifactual streaks mimic subtle dissection flaps.
Poor arterial enhancement due to suboptimal bolus timing and venous contamination can obscure critical vascular findings, producing poor arterial opacification or allowing for confounding opacification of venous structures surrounding the vertebral arteries in the transverse foramina.
When imaging with time-of-flight MR angiography, flow artifacts may create the illusion of a dissection flap, intraluminal thrombus, or stenosis. When imaging the neck with proton-density–weighted and fat-saturated T1-weighted MR imaging sequences, the appearance of a mural hematoma is dependent on the time course of imaging. The time dependency of these sequences may give rise to false negatives, as blood within the vessel wall may not appear bright in the hyperacute (<24 hours) and acute (1–3 days) phases (49). False positives may also arise when altered flow dynamics cause peripheral intraluminal hyperintense signal abnormalities. These artifacts may be mitigated by black-blood flow suppression techniques used at vessel wall imaging.
BCVI location also plays a role in how easily these injuries are identified. Injuries involving the ICA at the skull base and within the carotid canal are more easily overlooked than elsewhere in the head and neck. Tortuous V3 segments of the vertebral arteries are notorious for masking BCVI. Source axial images, off-axis multiplanar reconstructions, and MIP reconstructions can help eliminate uncertainty when evaluating these segments.
BCVI Treatment
Early identification and treatment of BCVI with antithrombotic therapy is essential for minimizing the risk of BCVI-related stroke and/or death. Antithrombotic therapy is a term that collectively refers to antiplatelet and anticoagulation therapies. The use of antithrombotic therapy for the treatment of BCVI in trauma patients has been shown to be safe, without increasing the risk of adverse bleeding events when compared with those of trauma patients who did not receive antithrombotic therapy (52).
Currently, the standard of care is to treat all grade I–IV injuries with antithrombotic therapy unless a patient has a contraindication to such therapy (EAST level II recommendation) (4,29,34,53,54). Some advocate for heparin use in hospitalized patients who may be undergoing surgical interventions owing to its short half-life and the ease with which it is reversed (33).
Although no randomized controlled trials exist that compare the use of antiplatelet and anticoagulation therapies in the management of BCVI, several studies suggest that the use of aspirin is at least as effective as heparin for stroke prevention (29,53,54). Furthermore, the use of aspirin has been associated with fewer bleeding-related complications in comparison with those in anticoagulated patients (55).
In grade V injuries where there are “hard signs” of active bleeding or hemodynamic instability suggestive of major vascular injury (shock, pulsatile or expanding hematoma, or bleeding from mouth, ears, nose, shock, etc), surgical or endovascular treatment must be considered, as immediate treatment of active hemorrhage is imperative for improving patient outcome. Direct pressure should be applied to an expanding cervical hematoma until surgical or endovascular intervention can be accomplished. If there is bleeding from the mouth, ears, or nose, surgical ligation or targeted angioembolization of the affected vessel can be performed. In cases of AVF (including CCFs), embolization or direct repair of the fistulous connection may be carried out to decrease retrograde flow and alleviate high intracranial pressure.
Of note, approaches to surgical accessibility are based on the zone system for penetrating injuries. In this system, the neck is divided into zones I–III. Zone I extends from the sternal notch and clavicles to the cricoid cartilage, zone II extends from the cricoid cartilage to the angle of the mandible, and zone III extends from the angle of the mandible to the base of the skull. An injury to zone II is considered a surgically explorable lesion. However, owing to difficult surgical access, exploration of injuries in zones I and III is limited, and injuries there should prompt consideration of endovascular control and/or definitive treatment if possible (56).
Failure of medical therapy may also be an indication for surgical or endovascular intervention. EAST guidelines give a level III recommendation for consideration of operative or interventional treatment in cases with early neurologic deficits and an accessible carotid lesion. Typically, this is not necessary for grade I injuries, as they do not possess substantial flow-limiting potential or thromboembolic complications. Rarely, however, intervention may be necessary for the treatment of grade II injuries if they progress in extent or degree of stenosis or continue to cause thromboembolic events refractory to therapy.
Additionally, intervention may be warranted for grade III pseudoaneurysms, as these lesions rarely resolve with observation alone (34). Intervention should be considered if the lesion becomes symptomatic despite optimal medical management or if the pseudoaneurysm grows to 1.0–1.5 cm in diameter. In these cases, endovascular stent placement or, less commonly, resection with patch interposition graft placement or coil embolization may be considered to definitively treat the pseudoaneurysm (Fig 23). Notably, the role of a carotid artery stent for the treatment of a BCVI pseudoaneurysm remains to be defined owing to high occlusion rates (25), and should be reserved for cases with refractory symptoms or with a rapid increase in size (57). No data exist to show any benefit of surgical or endovascular intervention for grade IV injuries. Not surprisingly, surgical or endovascular intervention is the first-line therapy for the treatment of grade V injuries. In the case of surgically accessible carotid artery transections, primary repair, interposition graft placement, or surgical ligation should be considered. If surgical access to the site of injury is not possible, endovascular stent placement or angioembolization can be pursued (33,34).
Figure 23a.
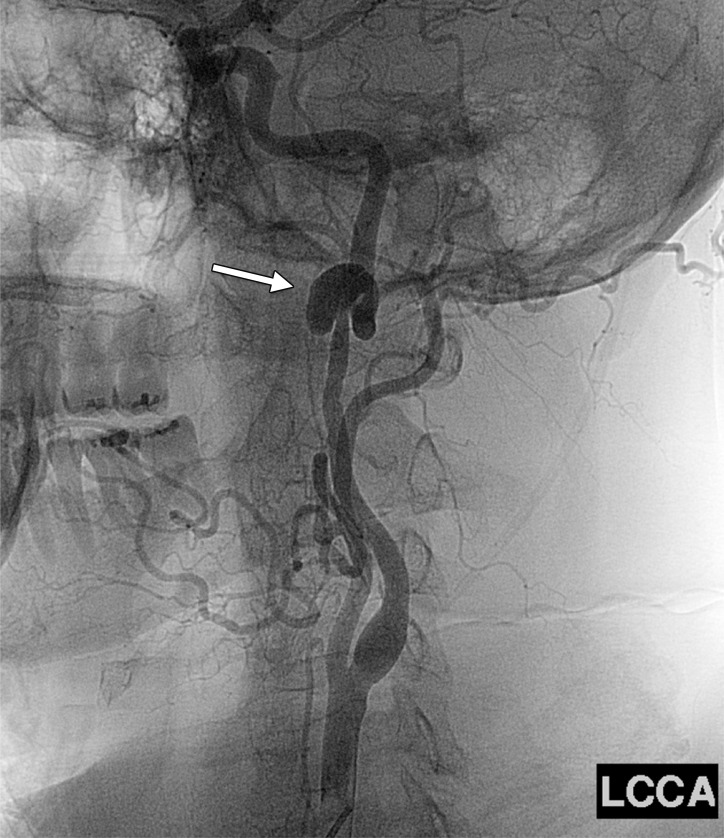
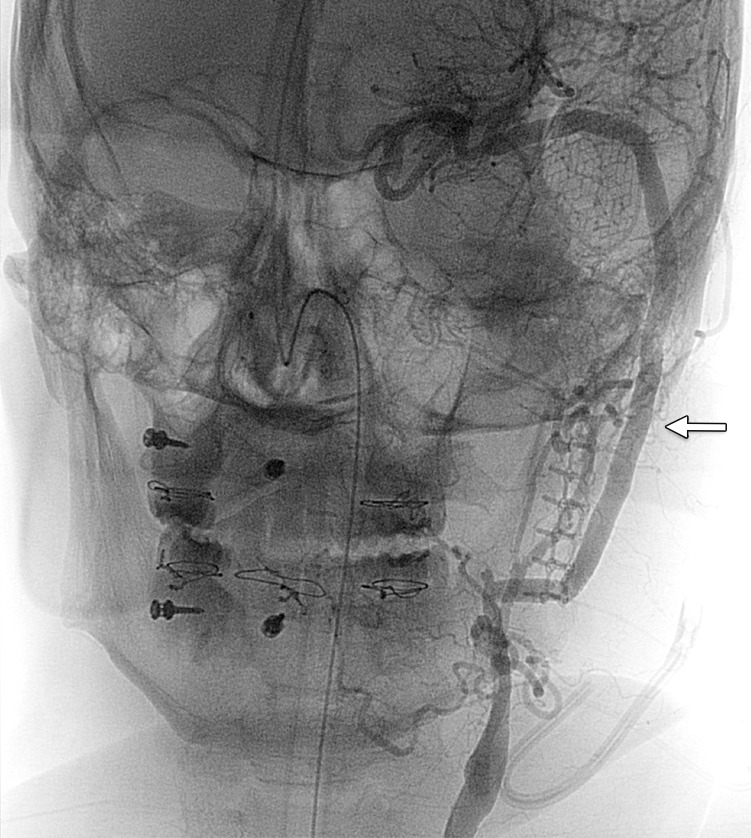
Definitive treatment of a large pseudoaneurysm. (a) Lateral projection angiogram of the left common carotid artery shows a large left cervical ICA aneurysm (arrow). (b) Postoperative frontal projection angiogram of the left common carotid artery shows the patent proximal ICA–to–middle cerebral artery bypass graft (arrow).
Figure 23b.
Definitive treatment of a large pseudoaneurysm. (a) Lateral projection angiogram of the left common carotid artery shows a large left cervical ICA aneurysm (arrow). (b) Postoperative frontal projection angiogram of the left common carotid artery shows the patent proximal ICA–to–middle cerebral artery bypass graft (arrow).
The duration of medical BCVI treatment depends on the grade of the injury and the degree to which it heals. Grade I injuries are treated with daily aspirin therapy until the lesion resolves; this may take approximately 3–6 months. Grade II and III BCVIs require indefinite antiplatelet therapy for stable lesions. In the case that these injuries do heal and resolve, antithrombotic therapy can be discontinued.
Grade IV injuries require lifetime antiplatelet therapy, with aspirin therapy as the usual first-line agent. If neurologic symptoms persist or the lesion increases in severity, clopidogrel can be added to the treatment regimen or a transition to anticoagulation therapy can be made. In this case, warfarin is most commonly used with a target international normalized ratio of 2–3 (34). In cases of grade V injury, the risks and benefits of long-term therapy must be evaluated after stent placement or embolization, and no specific data exist to guide therapy.
Follow-up Imaging
In general, follow-up imaging is used to guide antithrombotic treatment duration. CT angiography is the preferred follow-up imaging modality, given that it is fast, noninvasive, and cost-effective. It has been proposed by both the WTA and EAST that repeat CT angiography be performed 7–10 days following initial identification of grade I–III BCVIs to evaluate for injury progression or resolution (33,34). If the injury has healed, antithrombotic therapy can be discontinued. If the injury persists, therapy should be continued with interval follow-up imaging to evaluate for stability.
The WTA recommends 3 months of additional antithrombotic therapy with subsequent reimaging if the lesion persists at the initial follow-up examination. If the lesion is found to increase in severity, consideration should be given to surgical or endovascular intervention. Note that routine follow-up imaging of grade IV and V injuries in asymptomatic patients is unnecessary given that these lesions are unlikely to change in a manner that will alter treatment (58).
Injury evolution is largely dependent on the initial injury grade. As a general rule, low-grade injuries (grade I or II) are more likely to heal or improve than high-grade injuries (grade III, IV, or V) (Fig 24). Up to 75% of grade I injuries will heal over the course of weeks to months, while 8% of grade II injuries will completely resolve and 30% will improve to grade I in the same time frame. Injury progression is highly unlikely for grade I injuries, with only 8% of these lesions increasing in severity at follow-up imaging.
Figure 24a.
Healing of a low-grade injury in a polytrauma patient. (a) Oblique sagittal MIP image from CT angiography shows a focal intramural hematoma in the right cervical ICA (arrow) causing 25%–50% narrowing, representing a grade II injury. (b) Oblique sagittal MIP image from follow-up CT angiography obtained after 3 months of antiplatelet therapy demonstrates near-complete healing of the grade II injury in the cervical ICA (arrow).
Figure 24b.
Healing of a low-grade injury in a polytrauma patient. (a) Oblique sagittal MIP image from CT angiography shows a focal intramural hematoma in the right cervical ICA (arrow) causing 25%–50% narrowing, representing a grade II injury. (b) Oblique sagittal MIP image from follow-up CT angiography obtained after 3 months of antiplatelet therapy demonstrates near-complete healing of the grade II injury in the cervical ICA (arrow).
Injury progression is more common in grade II injuries, with 40%–43% showing interval progression in grade at follow-up CT angiography (Fig 25). While grade III injuries heal or improve only 11% of the time, they worsen approximately 25% of the time (Fig 15). Up to 40% of grade IV BCVIs recanalize, while the majority do not change (4,53,59). In all instances, injury progression to complete occlusion or pseudoaneurysm should prompt strong consideration for endovascular or surgical intervention.
Figure 25a.
Worsening of a low-grade injury in a polytrauma patient with right occipital condyle fracture. (a) Oblique CT angiogram of the right vertebral artery at the time of injury shows an intramural hematoma (arrow) with 25%–50% narrowing, representing grade I–II injury. (b, c) Oblique (b) and frontal projection 3D-rendered (c) images from CT angiography obtained 7 days later show development of a small pseudoaneurysm (arrow), which represents progression to a grade III injury.
Figure 25b.
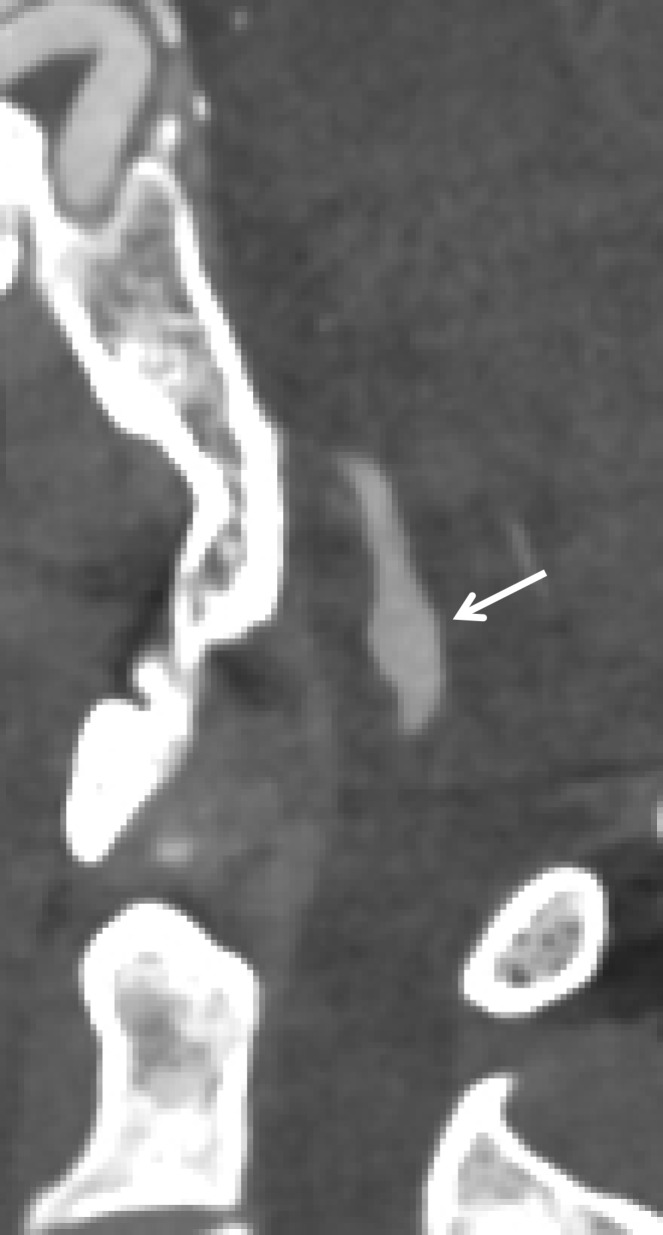
Worsening of a low-grade injury in a polytrauma patient with right occipital condyle fracture. (a) Oblique CT angiogram of the right vertebral artery at the time of injury shows an intramural hematoma (arrow) with 25%–50% narrowing, representing grade I–II injury. (b, c) Oblique (b) and frontal projection 3D-rendered (c) images from CT angiography obtained 7 days later show development of a small pseudoaneurysm (arrow), which represents progression to a grade III injury.
Figure 25c.
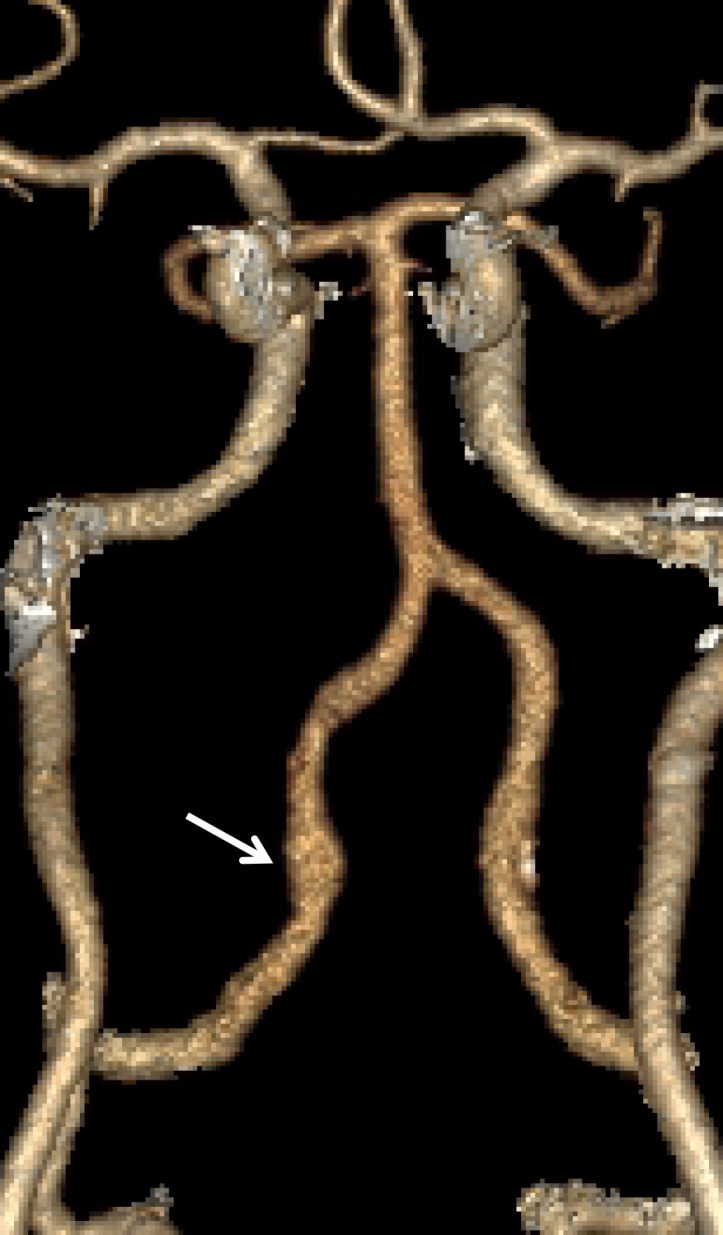
Worsening of a low-grade injury in a polytrauma patient with right occipital condyle fracture. (a) Oblique CT angiogram of the right vertebral artery at the time of injury shows an intramural hematoma (arrow) with 25%–50% narrowing, representing grade I–II injury. (b, c) Oblique (b) and frontal projection 3D-rendered (c) images from CT angiography obtained 7 days later show development of a small pseudoaneurysm (arrow), which represents progression to a grade III injury.
As an advanced imaging technique, vessel wall MR imaging supplements luminal time-of-flight MR angiography and represents a useful follow-up imaging examination for problem solving vascular findings, including differentiating intraluminal thrombus from intramural hematoma, atherosclerotic plaque, or normal vessel wall. Studies have demonstrated that vessel wall MR imaging is feasible and superior to conventional MR imaging for detection of arterial dissection (60–63). Vessel wall MR imaging may be most useful in evaluating patients who have undergone recent cross-sectional imaging studies with equivocal results for the presence of BCVI. In these patients, vessel wall imaging often effectively allows BCVI to be ruled out or ruled in (Fig 8). Additionally, the ability of vessel wall imaging to demonstrate subtle vascular injuries is supported by recent research demonstrating its superior ability to allow identification of intimal flaps, vascular wall thickening, and intramural hematoma (43) (Fig 26).
Figure 26a.
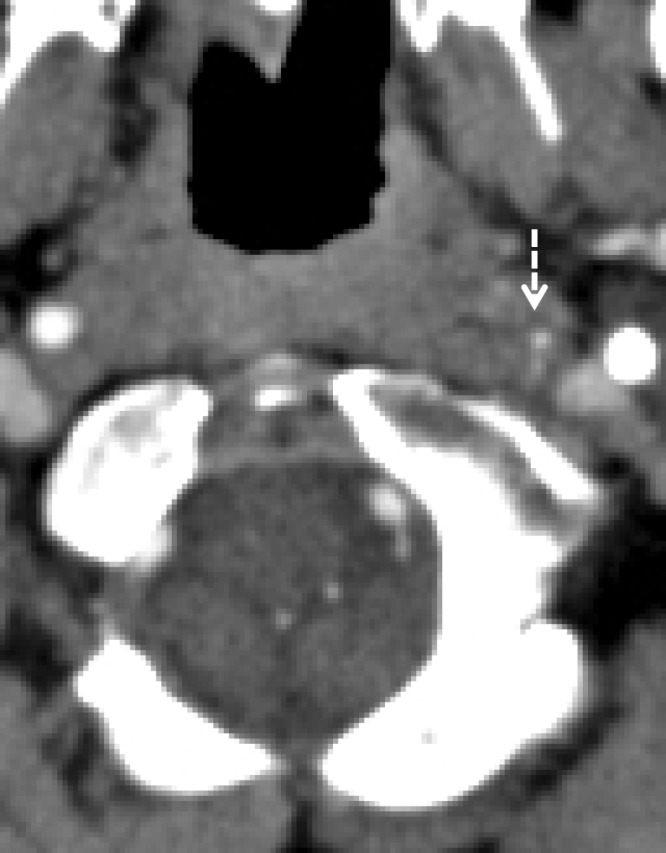
Vessel wall imaging of left ICA dissection. (a, b) Axial (a) and oblique (b) images from initial CT angiography show a high-grade stenosis, with a thin string of contrast material opacification (arrow), proximal to the skull base. Vessel wall MR imaging was performed 5 weeks later. (c) Three-dimensional time-of-flight MIP image shows improvement of the stenosis, with only minimal remaining luminal narrowing (arrow). (d, e) Axial (d) and coronal (e) black-blood fat-saturated T1-weighted MR images show persistent hyperintensity (arrowheads) within a thickened arterial wall, compatible with a subacute intramural hematoma. Note that, unlike the luminal time-of-flight MR angiogram, the vessel wall MR imaging sequences delineate the extent of true vessel wall disease.
Figure 26b.
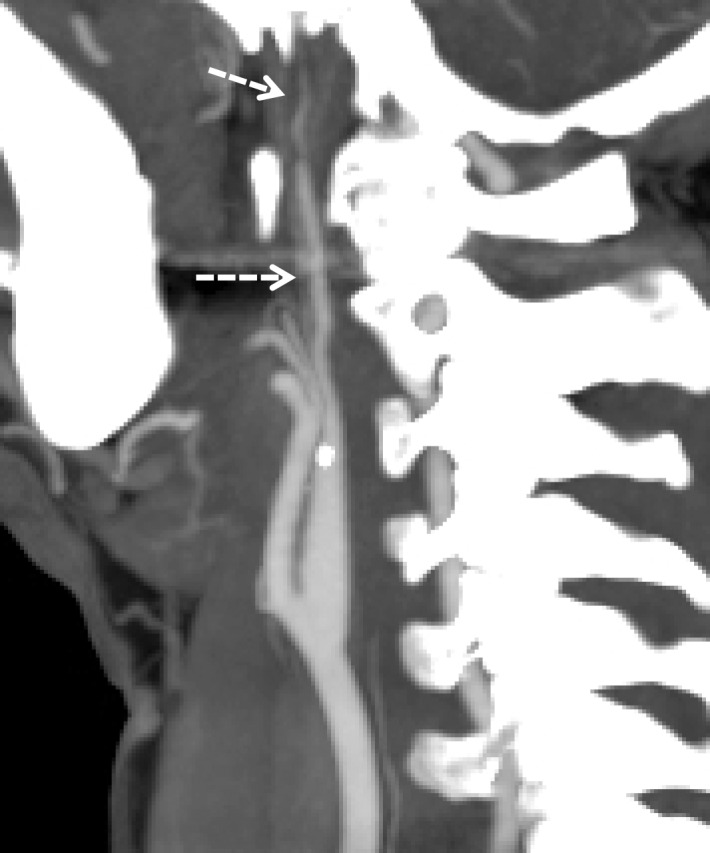
Vessel wall imaging of left ICA dissection. (a, b) Axial (a) and oblique (b) images from initial CT angiography show a high-grade stenosis, with a thin string of contrast material opacification (arrow), proximal to the skull base. Vessel wall MR imaging was performed 5 weeks later. (c) Three-dimensional time-of-flight MIP image shows improvement of the stenosis, with only minimal remaining luminal narrowing (arrow). (d, e) Axial (d) and coronal (e) black-blood fat-saturated T1-weighted MR images show persistent hyperintensity (arrowheads) within a thickened arterial wall, compatible with a subacute intramural hematoma. Note that, unlike the luminal time-of-flight MR angiogram, the vessel wall MR imaging sequences delineate the extent of true vessel wall disease.
Figure 26c.
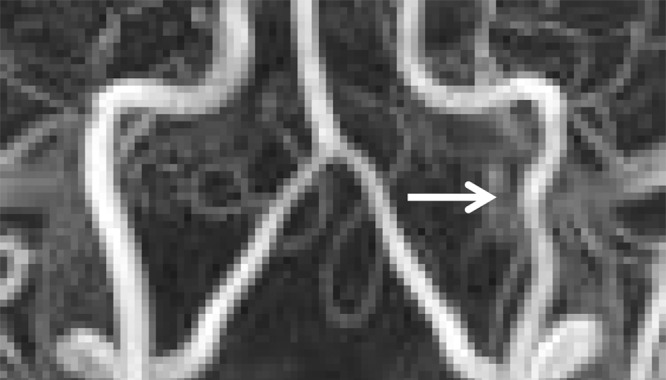
Vessel wall imaging of left ICA dissection. (a, b) Axial (a) and oblique (b) images from initial CT angiography show a high-grade stenosis, with a thin string of contrast material opacification (arrow), proximal to the skull base. Vessel wall MR imaging was performed 5 weeks later. (c) Three-dimensional time-of-flight MIP image shows improvement of the stenosis, with only minimal remaining luminal narrowing (arrow). (d, e) Axial (d) and coronal (e) black-blood fat-saturated T1-weighted MR images show persistent hyperintensity (arrowheads) within a thickened arterial wall, compatible with a subacute intramural hematoma. Note that, unlike the luminal time-of-flight MR angiogram, the vessel wall MR imaging sequences delineate the extent of true vessel wall disease.
Figure 26d.
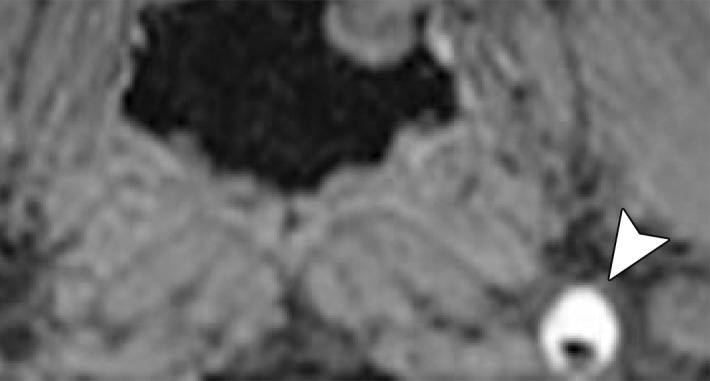
Vessel wall imaging of left ICA dissection. (a, b) Axial (a) and oblique (b) images from initial CT angiography show a high-grade stenosis, with a thin string of contrast material opacification (arrow), proximal to the skull base. Vessel wall MR imaging was performed 5 weeks later. (c) Three-dimensional time-of-flight MIP image shows improvement of the stenosis, with only minimal remaining luminal narrowing (arrow). (d, e) Axial (d) and coronal (e) black-blood fat-saturated T1-weighted MR images show persistent hyperintensity (arrowheads) within a thickened arterial wall, compatible with a subacute intramural hematoma. Note that, unlike the luminal time-of-flight MR angiogram, the vessel wall MR imaging sequences delineate the extent of true vessel wall disease.
Figure 26e.
Vessel wall imaging of left ICA dissection. (a, b) Axial (a) and oblique (b) images from initial CT angiography show a high-grade stenosis, with a thin string of contrast material opacification (arrow), proximal to the skull base. Vessel wall MR imaging was performed 5 weeks later. (c) Three-dimensional time-of-flight MIP image shows improvement of the stenosis, with only minimal remaining luminal narrowing (arrow). (d, e) Axial (d) and coronal (e) black-blood fat-saturated T1-weighted MR images show persistent hyperintensity (arrowheads) within a thickened arterial wall, compatible with a subacute intramural hematoma. Note that, unlike the luminal time-of-flight MR angiogram, the vessel wall MR imaging sequences delineate the extent of true vessel wall disease.
BCVI Outcomes
In the era before screening, untreated BCVIs possessed a mortality rate of 23%–28% and a stroke-induced morbidity rate of 48%–58%, as these injuries were generally not diagnosed until the neurologic event (64). BCVI-related stroke rates are dependent on the artery injured; injuries involving the carotid arteries are more likely to lead to infarction than injuries involving the vertebral arteries. More recent studies have defined overall mortality rates of 7%–21% for carotid artery injury and 4%–8% for vertebral artery injury; overall stroke rate has been defined as approximately 26%–41% for carotid artery injury and 14%–24% for vertebral artery injury (4,10,30,65–68). Additionally, for carotid artery injury, stroke rate increases with increasing injury grade, while stroke rate in vertebral artery injury is not significantly influenced by injury grade.
A study from the Denver group (69) demonstrated a stroke rate of 38% in grade II injury, 27% in grade III injury, and 28% in grade IV injury. It is worth mentioning that recent reports call into question the previously reported stroke rate as overestimations, suggesting that the true stroke rate may be closer to 5% (59,70). A lower calculated rate may result from increased discovery of asymptomatic low-grade lesions at screening.
Regardless, early injury identification and antithrombotic therapy have been shown to dramatically decrease stroke rate. Retrospective reviews by Cothren et al (11,32) demonstrate a reduction in stroke rate with antithrombotic therapy from 21%–46% in untreated patients to 0%–0.5% in treated patients. Another retrospective study showed stroke rate in untreated carotid artery and vertebral artery injuries of 64% and 54%, respectively, with antithrombotic treatment stroke rate of 6.8% in carotid artery injury and 2.6% in vertebral artery injury (10).
Conclusion
Early identification of BCVI in trauma patients is essential for reducing injury-associated morbidity and mortality, with CT angiography serving as an effective BCVI screening test in patients who demonstrate specific clinical signs and imaging risk factors. In patients with BCVI, treatment with antithrombotic therapy is the standard of care, reducing the incidence of postinjury ischemic stroke and death. Repeat follow-up imaging further guides treatment decisions, with lesion resolution serving as an indication to discontinue antithrombotic therapy and lesion worsening suggesting a consideration of interventional therapies.
Although CT angiography plays a central role in evaluating for BCVI, new techniques including vessel wall MR imaging hold promise as supplemental imaging studies that may improve diagnostic specificity for vessel wall injury and decrease radiation exposure for patients. The radiologist plays an essential role in identifying and grading BCVI, as well as interpreting results of follow-up imaging studies and thus guiding subsequent treatment.
Recipient of a Certificate of Merit award for an education exhibit at the 2016 RSNA Annual Meeting.
For this journal-based SA-CME activity, the authors, editor, and reviewers have disclosed no relevant relationships.
M.M.B. supported by a GE Radiology Research Academic Fellowship grant from the Association of University Radiologists and by National Institutes of Health grants (R21AR068009 and R01NS092207 01A1).
Abbreviations:
- AVF
- arteriovenous fistula
- BCVI
- blunt cerebrovascular injury
- CCF
- caroticocavernous fistula
- DSA
- digital subtraction angiography
- DVST
- dural venous sinus thrombosis
- EAST
- Eastern Association for the Surgery of Trauma
- ICA
- internal carotid artery
- MIP
- maximum intensity projection
- 3D
- three-dimensional
- WTA
- Western Trauma Association
References
- 1.Davis JW, Holbrook TL, Hoyt DB, Mackersie RC, Field TO, Jr, Shackford SR. Blunt carotid artery dissection: incidence, associated injuries, screening, and treatment. J Trauma 1990;30(12):1514–1517. [PubMed] [Google Scholar]
- 2.Berne JD, Reuland KS, Villarreal DH, McGovern TM, Rowe SA, Norwood SH. Sixteen-slice multi-detector computed tomographic angiography improves the accuracy of screening for blunt cerebrovascular injury. J Trauma 2006;60(6):1204–1209; discussion 1209–1210. [DOI] [PubMed] [Google Scholar]
- 3.Schneidereit NP, Simons R, Nicolaou S, et al. Utility of screening for blunt vascular neck injuries with computed tomographic angiography. J Trauma 2006;60(1):209–215; discussion 215–216. [DOI] [PubMed] [Google Scholar]
- 4.Biffl WL, Ray CE, Jr, Moore EE, et al. Treatment-related outcomes from blunt cerebrovascular injuries: importance of routine follow-up arteriography. Ann Surg 2002;235(5):699–706; discussion 706–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mutze S, Rademacher G, Matthes G, Hosten N, Stengel D. Blunt cerebrovascular injury in patients with blunt multiple trauma: diagnostic accuracy of duplex Doppler US and early CT angiography. Radiology 2005;237(3):884–892. [DOI] [PubMed] [Google Scholar]
- 6.McKevitt EC, Kirkpatrick AW, Vertesi L, Granger R, Simons RK. Blunt vascular neck injuries: diagnosis and outcomes of extracranial vessel injury. J Trauma 2002;53(3):472–476. [DOI] [PubMed] [Google Scholar]
- 7.Carrillo EH, Osborne DL, Spain DA, Miller FB, Senler SO, Richardson JD. Blunt carotid artery injuries: difficulties with the diagnosis prior to neurologic event. J Trauma 1999;46(6):1120–1125. [DOI] [PubMed] [Google Scholar]
- 8.Fabian TC, Patton JH, Jr, Croce MA, Minard G, Kudsk KA, Pritchard FE. Blunt carotid injury: importance of early diagnosis and anticoagulant therapy. Ann Surg 1996;223(5):513–522; discussion 522–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biffl WL, Ray CE, Jr, Moore EE, Mestek M, Johnson JL, Burch JM. Noninvasive diagnosis of blunt cerebrovascular injuries: a preliminary report. J Trauma 2002;53(5):850–856. [DOI] [PubMed] [Google Scholar]
- 10.Miller PR, Fabian TC, Bee TK, et al. Blunt cerebrovascular injuries: diagnosis and treatment. J Trauma 2001;51(2):279–285; discussion 285–286. [DOI] [PubMed] [Google Scholar]
- 11.Cothren CC, Moore EE, Ray CE, Jr, et al. Screening for blunt cerebrovascular injuries is cost-effective. Am J Surg 2005;190(6):845–849. [DOI] [PubMed] [Google Scholar]
- 12.Cogbill TH, Moore EE, Meissner M, et al. The spectrum of blunt injury to the carotid artery: a multicenter perspective. J Trauma 1994;37(3):473–479. [DOI] [PubMed] [Google Scholar]
- 13.Biffl WL, Moore EE, Elliott JP, Brega KE, Burch JM. Blunt cerebrovascular injuries. Curr Probl Surg 1999;36(7):505–599. [DOI] [PubMed] [Google Scholar]
- 14.Biffl WL, Moore EE, Offner PJ, et al. Optimizing screening for blunt cerebrovascular injuries. Am J Surg 1999;178(6):517–522. [DOI] [PubMed] [Google Scholar]
- 15.Franz RW, Willette PA, Wood MJ, Wright ML, Hartman JF. A systematic review and meta-analysis of diagnostic screening criteria for blunt cerebrovascular injuries. J Am Coll Surg 2012;214(3):313–327. [DOI] [PubMed] [Google Scholar]
- 16.Cothren CC, Moore EE, Biffl WL, et al. Cervical spine fracture patterns predictive of blunt vertebral artery injury. J Trauma 2003;55:811. [DOI] [PubMed] [Google Scholar]
- 17.Kopelman TR, Leeds S, Berardoni NE, et al. Incidence of blunt cerebrovascular injury in low-risk cervical spine fractures. Am J Surg 2011;202(6):684–688; discussion 688–689. [DOI] [PubMed] [Google Scholar]
- 18.Burlew CC, Biffl WL, Moore EE, Barnett CC, Johnson JL, Bensard DD. Blunt cerebrovascular injuries: redefining screening criteria in the era of noninvasive diagnosis. J Trauma Acute Care Surg 2012;72(2):330–335; discussion 336–337; quiz 539. [DOI] [PubMed] [Google Scholar]
- 19.Moar JJ. Traumatic rupture of the cervical carotid arteries: an autopsy and histopathological study of 200 cases. Forensic Sci Int 1987;34(4):227–244. [DOI] [PubMed] [Google Scholar]
- 20.Mulloy JP, Flick PA, Gold RE. Blunt carotid injury: a review. Radiology 1998;207(3):571–585. [DOI] [PubMed] [Google Scholar]
- 21.Crissey MM, Bernstein EF. Delayed presentation of carotid intimal tear following blunt craniocervical trauma. Surgery 1974;75(4):543–549. [PubMed] [Google Scholar]
- 22.Cothren CC, Moore EE. Blunt cerebrovascular injuries. Clinics (Sao Paulo) 2005;60(6):489–496. [DOI] [PubMed] [Google Scholar]
- 23.Schneider RC, Crosby EC. Vascular insufficiency of brain stem and spinal cord in spinal trauma. Neurology 1959;9:643–656. [DOI] [PubMed] [Google Scholar]
- 24.Carpenter S. Injury of neck as cause of vertebral artery thrombosis. J Neurosurg 1961;18:849–853. [DOI] [PubMed] [Google Scholar]
- 25.Cothren CC, Moore EE, Ray CE, Jr, et al. Carotid artery stents for blunt cerebrovascular injury: risks exceed benefits. Arch Surg 2005;140(5):480–485; discussion 485–486. [DOI] [PubMed] [Google Scholar]
- 26.Fattahi TT, Brandt MT, Jenkins WS, Steinberg B. Traumatic carotid-cavernous fistula: pathophysiology and treatment. J Craniofac Surg 2003;14(2):240–246. [DOI] [PubMed] [Google Scholar]
- 27.Shintani S, Tsuruoka S, Shiigai T. Carotid-cavernous fistula with brainstem congestion mimicking tumor on MRI. Neurology 2000;55(12):1929–1931. [DOI] [PubMed] [Google Scholar]
- 28.Fabian TC, George SM, Jr, Croce MA, Mangiante EC, Voeller GR, Kudsk KA. Carotid artery trauma: management based on mechanism of injury. J Trauma 1990;30(8):953–961; discussion 961–963. [DOI] [PubMed] [Google Scholar]
- 29.Cothren CC, Biffl WL, Moore EE, Kashuk JL, Johnson JL. Treatment for blunt cerebrovascular injuries: equivalence of anticoagulation and antiplatelet agents. Arch Surg 2009;144(7):685–690. [DOI] [PubMed] [Google Scholar]
- 30.Biffl WL, Moore EE, Offner PJ, Brega KE, Franciose RJ, Burch JM. Blunt carotid arterial injuries: implications of a new grading scale. J Trauma 1999;47(5):845–853. [DOI] [PubMed] [Google Scholar]
- 31.Cothren CC, Moore EE, Ray CE, Jr, Johnson JL, Moore JB, Burch JM. Cervical spine fracture patterns mandating screening to rule out blunt cerebrovascular injury. Surgery 2007;141(1):76–82. [DOI] [PubMed] [Google Scholar]
- 32.Cothren CC, Moore EE, Biffl WL, et al. Anticoagulation is the gold standard therapy for blunt carotid injuries to reduce stroke rate. Arch Surg 2004;139(5):540–545; discussion 545–546. [DOI] [PubMed] [Google Scholar]
- 33.Biffl WL, Cothren CC, Moore EE, et al. Western Trauma Association critical decisions in trauma: screening for and treatment of blunt cerebrovascular injuries. J Trauma 2009;67(6):1150–1153. [DOI] [PubMed] [Google Scholar]
- 34.Bromberg WJ, Collier BC, Diebel LN, et al. Blunt cerebrovascular injury practice management guidelines: the Eastern Association for the Surgery of Trauma. J Trauma 2010;68(2):471–477. [DOI] [PubMed] [Google Scholar]
- 35.Rozycki GS, Tremblay L, Feliciano DV, et al. A prospective study for the detection of vascular injury in adult and pediatric patients with cervicothoracic seat belt signs. J Trauma 2002;52(4):618–623; discussion 623–624. [DOI] [PubMed] [Google Scholar]
- 36.Bruns BR, Tesoriero R, Kufera J, et al. Blunt cerebrovascular injury screening guidelines: what are we willing to miss? J Trauma Acute Care Surg 2014;76(3):691–695. [DOI] [PubMed] [Google Scholar]
- 37.Lum C, Chakraborty S, Schlossmacher M, et al. Vertebral artery dissection with a normal-appearing lumen at multisection CT angiography: the importance of identifying wall hematoma. AJNR Am J Neuroradiol 2009;30(4):787–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eastman AL, Chason DP, Perez CL, McAnulty AL, Minei JP. Computed tomographic angiography for the diagnosis of blunt cervical vascular injury: is it ready for primetime? J Trauma 2006;60(5):925–929; discussion 929. [DOI] [PubMed] [Google Scholar]
- 39.Roberts DJ, Chaubey VP, Zygun DA, et al. Diagnostic accuracy of computed tomographic angiography for blunt cerebrovascular injury detection in trauma patients: a systematic review and meta-analysis. Ann Surg 2013;257(4):621–632. [DOI] [PubMed] [Google Scholar]
- 40.Paulus EM, Fabian TC, Savage SA, et al. Blunt cerebrovascular injury screening with 64-channel multidetector computed tomography: more slices finally cut it. J Trauma Acute Care Surg 2014;76(2):279–283; discussion 284–285. [DOI] [PubMed] [Google Scholar]
- 41.Kaye D, Brasel KJ, Neideen T, Weigelt JA. Screening for blunt cerebrovascular injuries is cost-effective. J Trauma 2011;70(5):1051–1056; discussion 1056–1057. [DOI] [PubMed] [Google Scholar]
- 42.Lévy C, Laissy JP, Raveau V, et al. Carotid and vertebral artery dissections: three-dimensional time-of-flight MR angiography and MR imaging versus conventional angiography. Radiology 1994;190(1):97–103. [DOI] [PubMed] [Google Scholar]
- 43.Miller PR, Fabian TC, Croce MA, et al. Prospective screening for blunt cerebrovascular injuries: analysis of diagnostic modalities and outcomes. Ann Surg 2002;236(3):386–393; discussion 393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vertinsky AT, Schwartz NE, Fischbein NJ, Rosenberg J, Albers GW, Zaharchuk G. Comparison of multidetector CT angiography and MR imaging of cervical artery dissection. AJNR Am J Neuroradiol 2008;29(9):1753–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hunter MA, Santosh C, Teasdale E, Forbes KP. High-resolution double inversion recovery black-blood imaging of cervical artery dissection using 3T MR imaging. AJNR Am J Neuroradiol 2012;33(11):E133–E137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Delgado Almandoz JE, Kelly HR, Schaefer PW, Lev MH, Gonzalez RG, Romero JM. Prevalence of traumatic dural venous sinus thrombosis in high-risk acute blunt head trauma patients evaluated with multidetector CT venography. Radiology 2010;255(2):570–577. [DOI] [PubMed] [Google Scholar]
- 47.Patel D, Machnowska M, Symons S, et al. Diagnostic performance of routine brain MRI sequences for dural venous sinus thrombosis. AJNR Am J Neuroradiol doi: 10.3174/ajnr.A4843. Published online June 16, 2016. Accessed July 2016. [DOI] [PMC free article] [PubMed]
- 48.Sadigh G, Mullins ME, Saindane AM. Diagnostic performance of MRI sequences for evaluation of dural venous sinus thrombosis. AJR Am J Roentgenol 2016;206(6):1298–1306. [DOI] [PubMed] [Google Scholar]
- 49.Habs M, Pfefferkorn T, Cyran CC, et al. Age determination of vessel wall hematoma in spontaneous cervical artery dissection: a multi-sequence 3T cardiovascular magnetic resonance study. J Cardiovasc Magn Reson 2011;13:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seeger A, Kramer U, Bischof F, et al. Feasibility of noninvasive diagnosis and treatment planning in a case series with carotid-cavernous fistula using high-resolution time-resolved MR-angiography with stochastic trajectories (TWIST) and extended parallel acquisition technique (ePAT 6) at 3 T. Clin Neuroradiol 2015;25(3):241–247. [DOI] [PubMed] [Google Scholar]
- 51.Borisch I, Boehme T, Butz B, Hamer OW, Feuerbach S, Zorger N. Screening for carotid injury in trauma patients: image quality of 16-detector-row computed tomography angiography. Acta Radiol 2007;48(7):798–805. [DOI] [PubMed] [Google Scholar]
- 52.Shahan CP, Magnotti LJ, McBeth PB, Weinberg JA, Croce MA, Fabian TC. Early antithrombotic therapy is safe and effective in patients with blunt cerebrovascular injury and solid organ injury or traumatic brain injury. J Trauma Acute Care Surg 2016;81(1):173–177. [DOI] [PubMed] [Google Scholar]
- 53.Edwards NM, Fabian TC, Claridge JA, Timmons SD, Fischer PE, Croce MA. Antithrombotic therapy and endovascular stents are effective treatment for blunt carotid injuries: results from longterm followup. J Am Coll Surg 2007;204(5):1007–1013; discussion 1014–1015. [DOI] [PubMed] [Google Scholar]
- 54.Chowdhury MM, Sabbagh CN, Jackson D, Coughlin PA, Ghosh J. Antithrombotic treatment for acute extracranial carotid artery dissections: a meta-analysis. Eur J Vasc Endovasc Surg 2015;50(2):148–156. [DOI] [PubMed] [Google Scholar]
- 55.Wahl WL, Brandt MM, Thompson BG, Taheri PA, Greenfield LJ. Antiplatelet therapy: an alternative to heparin for blunt carotid injury. J Trauma 2002;52(5):896–901. [DOI] [PubMed] [Google Scholar]
- 56.Sperry JL, Moore EE, Coimbra R, et al. Western Trauma Association critical decisions in trauma: penetrating neck trauma. J Trauma Acute Care Surg 2013;75(6):936–940. [DOI] [PubMed] [Google Scholar]
- 57.Burlew CC, Biffl WL, Moore EE, et al. Endovascular stenting is rarely necessary for the management of blunt cerebrovascular injuries. J Am Coll Surg 2014;218(5):1012–1017. [DOI] [PubMed] [Google Scholar]
- 58.Wagenaar AE, Burlew CC, Biffl WL, et al. Early repeat imaging is not warranted for high-grade blunt cerebrovascular injuries. J Trauma Acute Care Surg 2014;77(4):540–545; quiz 650. [DOI] [PubMed] [Google Scholar]
- 59.Scott WW, Sharp S, Figueroa SA, et al. Clinical and radiographic outcomes following traumatic grade 3 and 4 carotid artery injuries: a 10-year retrospective analysis from a level 1 trauma center—the Parkland Carotid and Vertebral Artery Injury Survey. J Neurosurg 2015;122(3):610–615. [DOI] [PubMed] [Google Scholar]
- 60.Edjlali M, Roca P, Rabrait C, Naggara O, Oppenheim C. 3D fast spin-echo T1 black-blood imaging for the diagnosis of cervical artery dissection. AJNR Am J Neuroradiol 2013;34(9):E103–E106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cuvinciuc V, Viallon M, Momjian-Mayor I, et al. 3D fat-saturated T1 SPACE sequence for the diagnosis of cervical artery dissection. Neuroradiology 2013;55(5):595–602. [DOI] [PubMed] [Google Scholar]
- 62.Natori T, Sasaki M, Miyoshi M, et al. Detection of vessel wall lesions in spontaneous symptomatic vertebrobasilar artery dissection using T1-weighted 3-dimensional imaging. J Stroke Cerebrovasc Dis 2014;23(9):2419–2424. [DOI] [PubMed] [Google Scholar]
- 63.Bachmann R, Nassenstein I, Kooijman H, et al. High-resolution magnetic resonance imaging (MRI) at 3.0 Tesla in the short-term follow-up of patients with proven cervical artery dissection. Invest Radiol 2007;42(6):460–466. [DOI] [PubMed] [Google Scholar]
- 64.Biffl WL, Moore EE, Ryu RK, et al. The unrecognized epidemic of blunt carotid arterial injuries: early diagnosis improves neurologic outcome. Ann Surg 1998;228(4): 462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Spaniolas K, Velmahos GC, Alam HB, de Moya M, Tabbara M, Sailhamer E. Does improved detection of blunt vertebral artery injuries lead to improved outcomes? Analysis of the National Trauma Data Bank. World J Surg 2008;32(10):2190–2194. [DOI] [PubMed] [Google Scholar]
- 66.Ramadan F, Rutledge R, Oller D, Howell P, Baker C, Keagy B. Carotid artery trauma: a review of contemporary trauma center experiences. J Vasc Surg 1995;21(1):46–55; discussion 55–56. [DOI] [PubMed] [Google Scholar]
- 67.Alterman DM, Heidel RE, Daley BJ, et al. Contemporary outcomes of vertebral artery injury. J Vasc Surg 2013;57(3):741–746; discussion 746. [DOI] [PubMed] [Google Scholar]
- 68.Biffl WL, Moore EE, Elliott JP, et al. The devastating potential of blunt vertebral arterial injuries. Ann Surg 2000;231(5):672–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burlew CC, Biffl WL. Imaging for blunt carotid and vertebral artery injuries. Surg Clin North Am 2011;91(1):217–231. [DOI] [PubMed] [Google Scholar]
- 70.Scott WW, Sharp S, Figueroa SA, et al. Clinical and radiographic outcomes following traumatic grade 1 and 2 carotid artery injuries: a 10-year retrospective analysis from a level I trauma center—the Parkland Carotid and Vertebral Artery Injury Survey. J Neurosurg 2015;122(5):1196–1201. [DOI] [PubMed] [Google Scholar]