Abstract
Background:
Gastric and duodenal Crohn’s disease [CD]-associated strictures are rare. Evidence on endoscopic balloon dilation [EBD] of upper gastrointestinal [GI] CD strictures is limited, in particular in respect to serial dilations.
Methods:
Prospective short- and long-term outcome data as well as complication rates on a cohort of upper GI CD-associated stricture dilations [stomach and duodenum] were collected from 1999 to 2015. Factors linked with clinical and technical success, long-term efficacy and complication rates were investigated.
Results:
A total of 35 CD patients with symptomatic CD-associated upper GI strictures [20% gastric, 67% duodenal, 11% both; mean age at diagnosis 25 years; mean CD duration to stricture 79.9 months; median post-dilation follow-up 22.1 months] underwent a total of 96 pneumatic dilations [33 gastric and 63 duodenal]. The median maximal dilation diameter was 15 mm. Technical success was achieved in 93% and clinical success in 87%, with a complication rate of 4% per procedure. The mean time to re-dilation was 2.2 months and mean time to stricture-related surgery after first dilation was 2.8 months. There was no difference in short-term efficacy, safety, or long-term outcome between the first and any later dilation procedure in the same patient.
Conclusions:
Pneumatic dilation of upper GI CD-associated strictures has a high rate of short-term technical and clinical success, with moderate long-term efficacy and acceptable complication rates. Serial dilations do not change the efficacy and could be a feasible option to delay or prevent surgical intervention.
Keywords: intestinal fibrosis, therapy, endoscopy
1. Introduction
Crohn’s disease [CD] is a chronic relapsing inflammatory disease that can occur in any segment of the gastrointestinal [GI] tract from the mouth to the anus.1 Within the first 10 years after diagnosis, up to one-third of CD patients develop a fibrostenotic phenotype manifested by narrowing of the bowel lumen and clinical signs of intestinal obstruction.2–4 This drives the ultimate need for surgery in this patient population.5,6 In fact, three-fourths of CD-affected patients have to undergo surgery at least once during their lifetime.2 Stricture location in CD follows the distribution of disease, and hence the vast majority of CD-related strictures are located in the colon or ileocolon.7 Far less common are strictures in the upper GI tract, namely the oesophagus, stomach, or duodenum, with their incidence varying between 0.5% and 4 %.8,9
Endoscopy-based therapeutic techniques are a frequently used first-line approach for treatment of fibrostenosing CD of the lower GI tract, particularly ileocaecal strictures or strictures of the neo-terminal ileum. Endoscopic balloon dilation [EBD] is minimally invasive, preserves the intestinal length and combines robust short- and long-term success with an acceptable complication rate.10–12 Upper GI strictures are also accessible for through-the-scope [TTS] balloon systems using upper endoscopy, but information about the efficacy of this approach is strikingly limited. Only six reports of a combined total of 37 cases have specially discussed EBD of upper GI strictures in CD.13–18 The largest study on safety and efficacy of EBD in CD-induced upper GI strictures indicated a technical success rate of 92.5% with a complication rate of 3%. After 23 months’ follow-up, only 25% of the patients remained symptom-free.13
The aims of this large prospective cohort study were: [1] to evaluate the efficacy, safety, and long-term outcome of EBD in a large cohort of symptomatic CD-associated upper GI strictures; and [2] to investigate the efficacy, safety and long-term outcome of serial dilations in the same patient.
2. Materials and Methods
2.1. Patient population
This is a cohort study using historical data derived from the electronic medical record [EMR] of a tertiary referral centre. The EMR was queried for all CD patients with upper GI involvement undergoing EBD for CD-related primary [not anastomotic] strictures in the stomach or duodenum between 1999 and 2015.
Inclusion criteria were:
[1] the diagnosis of CD, based on clinical, radiographic, endoscopic, serological, and histopathological criteria according to the European Crohn’s and Colitis Organisation Clinical Consensus Guidelines19;
[2] documented upper GI involvement of CD;
[3] the presence of a clinically symptomatic stricture in the stomach or duodenum on imaging or endoscopy in areas that are affected by Crohn’s disease;
[4] naive CD strictures [no previous surgery at the site of the stricture];
[5] performance of an endoscopic TTS EBD.
Special attention was paid to ensure that the upper GI strictures were CD-related, for which endoscopic, radiographic, clinical or histological findings were reviewed. Patients were excluded if: the aetiology of a stricture was in question; in case of any signs of dysplasia or malignancy; or age less than 18 years. Oesophageal strictures were excluded, due to possible overlap with strictures of peptic origin that do not allow a clear distinction. Patient characteristics can be found in Table 1.
Table 1.
Patient characteristics.
| Total [N = 35] | ||
|---|---|---|
| Factor | N | Summary |
| Gender | 35 | |
| Female | 15 [42.9] | |
| BMI | 34 | 23.7 ± 4.2 |
| Family history of Crohn’s disease | 33 | 5 [15.2] |
| Smoking | 34 | |
| Current | 3 [8.8] | |
| Ex-smoker | 8 [23.5] | |
| Never | 23 [67.6] | |
| Age at diagnosis [years] | 33 | 25.0 ± 12.9 |
| Disease location [non-exclusive] | ||
| Upper gastrointestinal | 35 | 35 [100.0] |
| Jejunum/proximal ileum | 35 | 5 [14.3] |
| Ileum | 35 | 17 [48.6] |
| Ileocaecal | 35 | 16 [45.7] |
| Colon | 35 | 13 [37.1] |
| Rectum | 35 | 19 [54.3] |
| Any ileal involvement | 35 | 32 [91.4] |
| Extra-intestinal manifestations [non-exclusive] | ||
| Any | 32 | 19 [59.4] |
| Joint | 32 | 12 [37.5] |
| Skin | 32 | 1 [3.1] |
| Bone | 32 | 3 [9.4] |
| Anaemia | 32 | 9 [28.1] |
| Montreal classification | 35 | |
| B2 | 19 [54.3] | |
| B2p | 8 [22.9] | |
| B3 | 1 [2.9] | |
| B3p | 7 [20.0] | |
| Months from diagnosis to first stricture | 33 | 79.9 [52.1, 251.1] |
| Location of strictures during follow-up | 35 | |
| Stomach | 7 [20.0] | |
| Duodenum | 24 [68.6] | |
| Both | 4 [11.4] | |
| No. of stricture dilations during follow-up | 35 | |
| 1 | 15 [42.9] | |
| 2 | 6 [17.1] | |
| 3 | 4 [11.4] | |
| > = 4 | 10 [28.6] | |
| Surgery for stricture | 32 | 11 [34.4] |
| Strictureplasty | 9 [81.8] | |
| Resection | 2 [18.2] | |
| Follow-up after first dilation [months] | 35 | 15.1 [4.9, 45.7] |
Values presented as mean ± standard deviation, median [P25, P75], or N [%].
BMI, body mass index.
2.2. Demographics and clinical variables
The following variables were obtained: patient’s demographics (gender, body mass index [BMI], family history, smoking history [never, ex-smoker or current], age at diagnosis) were extracted. Clinical data including disease location, extra-intestinal manifestations (presence of arthralgia, skin manifestations, anaemia, primary sclerosing cholangitis [PSC], eye or bone disease), CD phenotype based on the Montreal classification,20 time from diagnosis to first stricture, location of strictures and number of dilations, time to symptom recurrence, re-dilation and surgery after dilation, type of surgery [stricturoplasty, gastro-jejunal bypass, resection], and use of proton pump inhibitors [PPIs] or any CD-related medications. CD medications were categorised into 5-aminosalicylic acid [5-ASA], systemic steroids, immunomodulators [anti-metabolites, methotrexate], anti-tumour necrosis factor antibodies [anti-TNFs], and no therapy, and data were collected for any time up to the first dilation and from there on until end of follow-up. Stricture evaluation was performed based on endoscopy or cross-sectional imaging (computerised tomography enterography [CTE], magnetic resonance enterography [MRE] or barium studies). Upper GI strictures were characterised based on location into fundus, gastric body, antrum, or pylorus as well as duodenum D1, D2, or D3, length of stricture [≤ 5 cm or > 5 cm], presence of pre-stenotic dilation, angulation, type of sedation [conscious vs general], dilation method used [fixed size or graded dilation], dilation time, maximum diameter of balloon used, ability to pass the scope before and after dilation, use of fluoroscopy, presence of abnormal mucosa at the time of dilation [inflammation, ulceration, or friability], use of concomitant therapies [steroid injection, stent placement, anti-TNF injection, or needle knife therapy], complications related to the procedure [defined as pain, bleeding, perforation, surgery, need for hospitalisation, and death]. Once collected, data were transferred and stored in a secure coded anonymised database for analysis [RedCap].
2.3. Endoscopic protocol
Dilations were performed in an outpatient and inpatient settings by interventional endoscopists or IBD specialists. Dilations were performed with a standard 8.8 or 9.9 mm, flexible, single-channel, therapeutic upper endoscope [GIF-180 or 190 series; Olympus Optical, Tokyo, Japan] using graded through-the-scope balloons [CRE balloon, Boston Scientific Microvasive, Natick, MA, USA].
2.4. Outcome measures
The primary outcomes were technical and clinical success as well as long-term efficacy. Technical success was defined as ability to pass the scope beyond stricture after dilation. Clinical success was defined as relief of obstructive symptoms. Long-term efficacy was defined as: [1] clinical efficacy [time to recurrence of clinical symptoms]; [2] re-dilation [time to need for repeat EBD of the same stricture]; or [3] need for surgery [time to surgical intervention at the site of the previously dilated stricture]. Follow-up for a particular patient was terminated at time of surgical intervention. Complications were defined as dilation-related intense abdominal pain leading to physician encounter, minor bleeding, bleeding requiring transfusion, bleeding requiring endoscopic re-intervention, fever, perforation, need for hospitalisation, need for surgery within 1 week after dilation, or death.
2.5. Statistical analysis
Data are presented as mean ± standard deviation, median [25th, 75th percentiles], or frequency [per cent]. Generalised estimating equations were used to assess factors associated with technical success and clinical efficacy. An independent covariance matrix was used to account for correlation between multiple dilations done on the same patient. In this setting only a univariable analysis was done, as < 15 patients did not have data on the outcome. In addition, Cox regression analysis was used to assess factors associated with recurrence of symptoms or need for re-dilation or stricture surgery. For each outcome, follow-up time was defined as months from dilation to event or latest follow-up. Standard errors and p- values are based on a robust [sandwich] variance estimator that accounts for the repetition of patients. Multivariable analysis was also performed for each of the three outcomes. An automated stepwise variable selection method performed on 1000 bootstrap samples was used to choose the final models. Disease duration, stricture duration, stricture length, medications used at time of dilation, abnormal mucosa, diameter of balloon, and use of concomitant therapies were considered for inclusion in each model; the four variables with the highest inclusion rates were included in the final model for each outcome. A p < 0.05 was considered statistically significant. All analyses were performed using SAS [version 9.4, SAS Institute, Cary, NC].
2.6. Ethical considerations
Data were collected using EMR without any direct contact with the patients for the purpose of study. The need for informed consent was waived and the study was approved by the Cleveland Clinic Institutional Review Board [CCF 13-443].
3. Results
3.1. Patient demographics
A total of 96 dilations in 35 unrelated CD subjects were eligible for analysis [Table 1]. Mean age at diagnosis was 25 years, with a median time from diagnosis to stricture of 79.9 months; 91.4% of patients had ileal disease involvement with 42.9% having perianal disease. Of the included strictures, 20% were gastric, 69% duodenal, and 11% in both locations; 71% had one to three dilations and the remainder at least fofur dilations of the same stricture. The median follow-up was 15.1 months.
3.2. Patient and stricture characteristics at time of dilation
At time of stricture dilation, the median time from diagnosis to dilation was 5 months [Table 2]; 69% of strictures were diagnosed during upper endoscopy, 80% were shorter than 5 cm, and only 5% were angulated with 35% of the strictures showing a prestenotic dilation. Totals of 52% of patients were exposed to anti-TNF antibodies and 83% to proton pump inhibitors, before dilation. Half of the patients received conscious sedation and the other half received monitored anaesthesia care. The mucosa was found to be abnormal [inflammation, ulceration, or friability] in 59% of dilations; 57% of strictures were passable with the upper endoscope before dilation. Controlled radial expansion [CRE] balloons were used in all cases. A graded dilation was performed in 89% with a median diameter of 15 mm; 14% of strictures were dilated under fluoroscopy and in only 4% of cases concomitant techniques including steroid injection [3%] and needle knife [1%] were used. Data on inflation time were only available for three dilations and hence this information was omitted. Results separated by gastric or duodenal stricture location can be found in Table 2.
Table 2.
Patient and stricture characteristics at time of dilation.
| Overall | Stomach | Duodenum | ||||
|---|---|---|---|---|---|---|
| [N = 96] | [N = 33] | [N = 63] | ||||
| Factor | N | Summary | N | Summary | N | Summary |
| Months from stricture diagnosis to dilation | 96 | 5.0 [0.35, 22.4] | 33 | 5.0 [0.72, 22.0] | 63 | 5.1[0.23, 22.9] |
| Method of diagnosis | 96 | 33 | 63 | |||
| Barium swallow | 27 [28.1] | 16 [48.5] | 11 [17.5] | |||
| CT enterography | 3 [3.1] | 0 [0.0] | 3 [4.8] | |||
| EGD | 66 [68.8] | 17 [51.5] | 49 [77.8] | |||
| Stricture length | 81 | 31 | 50 | |||
| < 5 cm | 65 [80.2] | 17 [54.8] | 48 [96.0] | |||
| > 5 cm | 16 [19.8] | 14 [45.2] | 2 [4.0] | |||
| Pre-stenotic dilation | 93 | 33 [35.5] | 33 | 10 [30.3] | 60 | 23 [38.3] |
| Angulated | 96 | 5 [5.2] | 33 | 0 [0.0] | 63 | 5 [7.9] |
| Medications before dilation [non-exclusive] | ||||||
| 5-ASA | 96 | 24 [25.0] | 33 | 6 [18.2] | 63 | 18 [28.6] |
| Systemic steroids | 96 | 71 [74.0] | 33 | 25 [75.8] | 63 | 46 [73.0] |
| Anti-metabolite | 96 | 37 [38.5] | 33 | 16 [48.5] | 63 | 21 [33.3] |
| Methotrexate | 96 | 15 [15.6] | 33 | 3 [9.1] | 63 | 12 [19.0] |
| Anti-TNF | 96 | 50 [52.1] | 33 | 15 [45.5] | 63 | 35 [55.6] |
| PPI | 96 | 80 [83.3] | 33 | 28 [84.8] | 63 | 52 [82.5] |
| Type of sedation | 96 | 33 | 63 | |||
| Conscious | 48 [50.0] | 12 [36.4] | 36 [57.1] | |||
| General sedation | 48 [50.0] | 21 [63.6] | 27 [42.9] | |||
| Abnormal mucosa at time of dilation | 95 | 56 [58.9] | 32 | 19 [59.4] | 63 | 37 [58.7] |
| Passage of scope beyond stricture before dilation | 90 | 51 [56.7] | 30 | 22 [73.3] | 60 | 29 [48.3] |
| Dilation method | 96 | 33 | 63 | |||
| CRE balloon | 96 [100.0] | 33 [100.0] | 63 [100.0] | |||
| Graded dilation | 94 | 84 [89.4] | 32 | 31 [96.9] | 62 | 53 [85.5] |
| Maximum diameter of balloon used [mm] | 91 | 15.0 [12.0, 20.0] | 32 | 20.0 [16.5, 20.0] | 59 | 15.0 [12.0, 17.0] |
| Fluoroscopy used | 95 | 13 [13.7] | 33 | 0 [0.0] | 62 | 13 [21.0] |
| Concomitant therapies [non-exclusive] | ||||||
| Any concomitant therapy | 96 | 4 [4.2] | 33 | 2 [6.1] | 63 | 2 [3.2] |
| Steroid injection | 96 | 3 [3.1] | 33 | 1 [3.0] | 63 | 2 [3.2] |
| Needle knife | 96 | 1 [1.0] | 33 | 1 [3.0] | 63 | 0 [0.0] |
Values presented as mean ± standard deviation, median [P25, P75] or N [column %].
CT, computed tomography; EGD, oesophagogastroduodenoscopy; CRE, controlled radial expansion; ASA, aminosalicylic acid; TNF, tumour necrosis factor; PPI, proton pump inhibitor.
3.3. Dilation outcomes
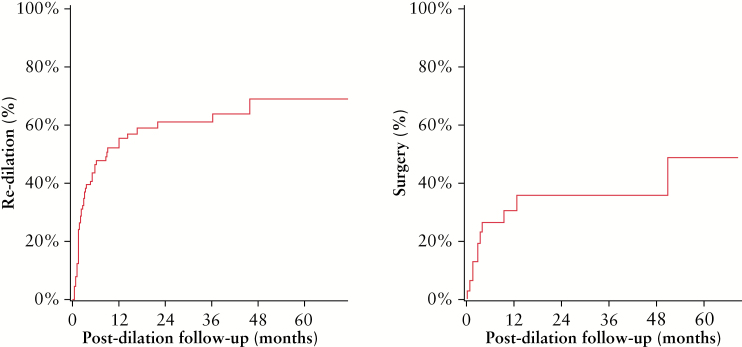
Overall, dilation increased the ability to pass the stricture with the endoscope from 57% to 93% [defined as technical success: passage of scope through stricture after dilation] and 87% had clinical efficacy [relief of symptoms after dilation]. Interestingly, on multivariate analysis, technical success and clinical efficacy were not dependent on the length of the stricture [< 5 cm vs > 5 cm]. A complication occurred in 4% of dilations [Table 3]. The complications occurred at the first dilation in three patients and at the second dilation in one patient [three minor bleeding episodes and one perforation]. All patients were managed conservatively. Minor bleeding episodes did not require any transfusion. The patient who experienced perforation underwent dilation of a pyloric stricture of length less than 5 cm to a maximum diameter of 20 mm. No mucosal inflammation or ulceration was noted in this patient at the time of balloon dilation. Patient subsequently presented with abdominal pain, and perforation was found on imaging. No surgical intervention was required. In addition to immediate complications, we also looked at 30-day mortality and need of hospitalisation or increase in length of hospitalisation within 30 days following the procedure. We did not find any increase in mortality or any further complications requiring hospitalisation. Subsequently, 63% of patients had recurrence of obstructive symptoms after a median of 1.3 months, 56% of subjects underwent re-dilation after a median time of 2.2 months, and 34% needed surgery at the site of the stricture after a median of 2.8 months, after the first dilation.
Table 3.
Dilation outcomes.
| Overall | Stomach | Duodenum | ||||
|---|---|---|---|---|---|---|
| [N = 96] | [N = 33] | [N = 63] | ||||
| Factor | N | Summary | N | Summary | N | Summary |
| Technical success | 89 | 83 [93.3] | 30 | 28 [93.3] | 59 | 55 [93.2] |
| Clinical efficacy | 91 | 79 [86.8] | 32 | 27 [84.4] | 59 | 52 [88.1] |
| Complications [non-exclusive] | ||||||
| Any complication | 90 | 4 [4.4] | 32 | 2 [6.3] | 58 | 2 [3.4] |
| Bleeding | 90 | 3 [3.3] | 32 | 1 [3.1] | 58 | 2 [3.4] |
| Perforation | 90 | 1 [1.1] | 32 | 1 [3.1] | 58 | 0 [0.0] |
| Symptom recurrence during f/u | 75 | 47 [62.7] | 27 | 13 [48.1] | 48 | 34 [70.8] |
| Months to symptom recurrence | 75 | 1.34 [0.72, 4.13] | 13 | 1.2 [0.85, 3.0] | 34 | 1.7 [0.52, 5.1] |
| Re-dilation during f/u | 90 | 50 [55.6] | 32 | 23 [71.9] | 58 | 27 [46.6] |
| Months to re-dilation | 50 | 2.2 [1.4, 5.7] | 23 | 2.0 [1.5, 3.4] | 27 | 3.2 [1.3, 6.2] |
| Surgery during f/u | 32 | 11 [34.4] | — | — | — | — |
| Months to surgery | 11 | 2.8 [1.6, 9.5] | — | — | — | — |
| Post-dilation follow-up [months] | 96 | 22.1 [6.2, 41.1] | 33 | 19.1 [10.7, 29.5] | 63 | 23.1 [5.3, 46.1] |
Values presented as median [P25, P75] or N [column %].
F/u, follow-up.
3.4. Factors associated with short-term efficacy
To evaluate factors linked to technical and clinical efficacy, we performed a Cox regression analysis. After including all the above collected clinical and demographic information, we found that a longer time from stricture diagnosis to dilation decreased technical success (odds ratio [OR] 0.87; 95% confidence interval [CI] 0.76, 0.99; p = 0.0330, but increased clinical efficacy [OR 1.6; 95% CI 1.03, 2.5; p = 0.036]. Every 1-mm increase in maximal balloon diameter increased the odds for clinical efficacy by 20% [OR 1.2; 95% CI 1.00, 1.5; p = 0.045]. We additionally performed an analysis using the specific cut-off for maximum balloon diameter of ≥ 15 mm vs < 15 mm. There was no difference in short-term efficacy when dichotomising the balloon diameters [data not shown]. Any other factor, including disease phenotype, stricture characteristics, inflammation at the site of the stricture, stricture location [stomach versus duodenum], or anti-TNF therapy, did not influence technical or clinical success rate [p < 0.05]. The overall number of recorded complications was too small to allow a factor analysis. No multivariable analysis was performed for technical success or clinical efficacy because of the low number of patients who did not achieve these outcomes.
3.5. Factors associated with long-term efficacy
We next assessed whether patient demographics or phenotype influence time to need for re-dilation or surgery. The Kaplan-Meier curves for the time from dilation to re-dilation or surgery can be found in Figure 1. After adjusting our Cox regression analysis for potential confounders [after considering all variables: disease duration at diagnosis of stricture, medication use, stricture length, abnormal mucosa at time of dilation], no factor was associated with a shorter or longer time to re-dilation. Factors linked to a shorter time to need for surgery at the upper GI stricture were the presence of internal penetrating disease (hazard ratio [HR] 3.6; 95% CI 1.1, 11.9; p = 0.034), anti-TNF antibodies at the time of dilation [HR 3.3; 95% CI 1.1, 9.8], and the use of any concomitant treatment [HR 30; 95% CI 4.4, 206.4; p < 0.001], suggestive of an overall more severe CD course. The maximum balloon diameter used at the initial dilation did not affect long-term efficacy, which was true for analysing the diameter as a continuous variable or dichotomising into ≥ 15 mm vs < 15 mm [data not shown]. Out of the 34% of patients who required surgical intervention during follow-up, 82% had stricturoplasty and 18% underwent resection. We did not assess data on time to surgery in a patient-level analysis [from first stricture/dilation to surgery] as, in patients with multiple strictures, we could not be certain which stricture location prompted the surgery. There was no difference in time to re-dilation or time to surgery comparing strictures in the stomach vs strictures in the duodenum [Supplementary Figure 1, available at ECCO-JCC online].
Figure 1.
Survival analysis depicting the time from first dilation to re-dilation or surgery.
3.6. Efficacy of serial upper GI dilation of the same stricture
Data on the efficacy of serial dilations of symptomatic upper GI strictures are lacking. We followed patients with a single stricture dilated serially at the same stricture location, and assessed differences in short- or long-term efficacy [Table 4]. There was no difference in the technical or clinical success between the first three dilation.s and success was not affected by the location of the stricture [gastric vs duodenum]. The proportion of patients needing re-dilation was not significantly different over time, even though nominally patients after the third dilation in the same location had a higher percentage of repeat endoscopic dilation. Complications over time were not included in this analysis due to their low number. We additionally separated duodenal and gastric strictures and analysed them separately. There was no difference found in any of the tested parameters. These data suggest that short- and long-term efficacies over time do not change.
Table 4.
Outcomes by number of dilations.
| 1st dilation | 2nd dilation | 3rd dilation | |||||
|---|---|---|---|---|---|---|---|
| [N = 47] | [N = 21] | [N = 10] | |||||
| Factor | N | Summary | N | Summary | N | Summary | p-Value |
| Months since previous dilation | 0 | — | 21 | 1.5 [1.3, 3.2] | 10 | 1.6 [1.1, 5.1] | 0.11 |
| Technical success | 46 | 43 [93.5] | 18 | 16 [88.9] | 9 | 8 [88.9] | 0.97 |
| Clinical success | 45 | 39 [86.7] | 19 | 15 [78.9] | 10 | 9 [90.0] | 0.97 |
| Re-dilation | 43 | 21 [48.8] | 21 | 10 [47.6] | 10 | 7 [70.0] | 0.51 |
Values presented as median [P25, P75] or N [column %].
4. Discussion
Data on the efficacy of endoscopic management of CD-induced upper GI strictures are strikingly limited. Almost all publications report about upper GI and lower GI tract EBD combined, without a separate data evaluation. We here present a prospective investigation, including 96 dilations performed in 35 subjects for naïve upper GI strictures. Our study showed a technical success rate of 93%, clinical success rate of 87%, and a complication rate of 4% per procedure. Over a median follow up period of 22 months, symptom recurrence occurred in 67% of the patients. Re-dilation was required in 56% and surgery in 34% of the subjects. Overall, EBD delayed the need of surgery in two-thirds [66%] of patients. Although more than half of patients [56%] required re-dilation during follow-up, re-dilations were found to be of comparable technical and clinical success to the first dilation and had similar efficacy and safety.
Our study is in concordance with previously published small case series. Guo et al.13 reported a total of 67 dilations with an overall complication rate of 3% per procedure. Technical success, defined as immediate passage of scope through the stricture after dilation, was achieved in 93% of dilations. Long-term efficacy was defined as ability to tolerate a normal diet and was seen in 25% of patients during a follow-up of 23 months. No data on serial dilations were reported. Bettenworth et al.12 showed a 5-fold higher risk of earlier surgery after EBD in upper GI strictures as compared with EBD in small bowel or colon, and stricture length of ≤ 5 cm was found to be the only predictor of longer surgery-free interval in ileo-colonic strictures.
De novo strictures as compared with anastomotic strictures, and a higher balloon diameter, were shown to be predictors of increased technical success. The presence of inflammation at the stricture site did not influence the short-term or long-term outcomes or the complication rate. Although this combined analysis showed that subsequent dilations had almost similar technical and clinical success rates without any significant rise in complication rates, 99% of the strictures were ileal and 62% were anastomotic, making any conclusion about de novo upper GI strictures impossible.
Matsui et al.16 followed a mixed cohort of 55 patients [14 small intestinal, 34 ileocolitis, and seven colitis type cases] to investigate efficacy of EBD. A short-term technical success rate of 86% was noticed in 47 patients; only 40 patients had ≥ 6 months of follow-up; and symptom recurrence occurred in 55%, requiring re-dilation. In all, 78% [31/40] remained surgery free after a median follow-up of 37 months, but in this series only six out of 40 examined patients had gastroduodenal strictures and those were not analysed separately. Another case series examined three patients with upper GI strictures [one pyloric and two duodenal]; strictures were dilated using over the wire balloons in one instance and at laparotomy through a small enterotomy in the others. No symptom recurrence was noticed for 5 and 8 months, respectively.21 Endo and colleagues22 showed a clinical success rate of 94% in a cohort of 30 patients, with only 10 CD upper GI strictures; 28% required re-dilation after a median follow-up of 26 months and 64% remained surgery free.
In another retrospective review by Singh et al.18 including 17 patients with 29 CD strictures, only three patients had upper GI strictures, located in the duodenal bulb. Overall success rate in study was 77%, and 100% success rate in UGI strictures, and each of these had received intralesional steroid injections. Both of the abovestudies showed high complication rates of 11% and 10%, respectively, and no separate analysis on CD strictures in the upper GI tract was performed. This manuscript also reported a lower recurrence rate [10%] in the steroid injection group as compared with no steroid injection [31%], but a recent prospective randomised controlled trial by East et al.23 suggested a worse outcome in the steroid injection arm. Comparing upper and lower GI strictures, the complication rates appear similar.24
What does our study add to the literature? To date this is the largest investigation of EBD on CD-associated de novo strictures of the UGI tract and the only study assessing the efficacy of serial dilations. We present clinically relevant endpoints and included a wide range of demographic and clinical factors in our statistical models. With an increasing time between diagnosis of the stricture and EBD the technical success rate decreases, suggesting that delay of intervention may aggravate the stenosis and hence make it more difficult to dilate. At the same time clinical success rate increased, which could be explained by more severe symptoms due to the prolongated presence of the stricture, higher stricture rigidity, and hence a higher proportion of patients experiencing relief after EBD. This is also supported by the fact that a larger maximal balloon dilation increased the clinical success rate.
We did not identify any factors predicting the need for re-dilation. Interestingly, a multivariate analysis revealed a link between internal penetrating disease, exposure to anti-TNF antibody, and the use of concomitant therapy, with an earlier need for surgical intervention. Rather than being the cause for the earlier intervention, these factors likely represent indicators of a more severe disease course in the CD subjects. After excluding CD of the oesophagus and oesophageal strictures, we based our investigation on a well-defined population focusing on CD strictures of only the stomach and duodenum. We assumed that the overlap between peptic strictures, a common complication of gastro-oesophageal reflux disease [GERD], and CD-associated strictures is too difficult to discern.
Peptic strictures are common and—at the same time—typical histological features of CD are infrequently seen in the oesophagus.25 Our inclusion criteria required the documented presence of upper GI Crohn’s disease and the development of an upper GI stricture in the same location, and all post-surgical strictures were excluded. Only one perforation and no increase in mortality or hospitalisation rate after EBD occurred at the first nor at any subsequent dilations, suggesting that EBD is a very safe and effective short-term method to treat CD-induced UGI strictures. To prevent or delay surgery multiple subsequent dilations are likely needed, given the overall short time to re-dilation in our patient cohort. Morbidity and complications in upper GI stricture surgeries can be significant and, despite the need for repeated dilations, dilation of upper GI strictures may still be a treatment option.
This study has several limitations. Given the rarity of upper GI strictures, the data are limited by the number of patients. Our hospital is a regional referral centre for severe CD cases, introducing a possible selection bias. There is a risk of misdiagnosis in attributing to Crohn’s the aetiology of stricture which includes peptic ulcer disease, granulomatous diseases [such as tuberculosis, sarcoidosis], or carcinoma. The available long follow-up times together with a thorough evaluation reduce the risk of this error in our study, and we used the utmost care in the selection of our patients. With regard to outcome of need for surgery, operator-based selection bias may apply when deciding about the therapeutic approach [dilation vs surgery], depending on the experience and ease of the treating physician in performing EBD. The same holds true for the approach chosen for each dilation. Since this is not a randomised placebo-controlled trial, we cannot exclude a placebo effect of EBD on improvement of clinical symptoms. A placebo-controlled trial may be considered unethical, as patients present with symptomatic intestinal obstruction. The possibility of a subgroup analysis is limited by the small number of patients.
Our study shows that EBD is a safe and effective modality to treat CD-induced strictures in the upper GI tract and that it can be used multiple times in the same stricture without any subsequent apparent increase in complication rate or decrease in efficacy. EBD is hence an option in all patients with CD-induced upper GI strictures in which dilation is technically feasible and no abscess, fistula, phlegmon, dysplasia, or malignancy is present. Future studies with larger patient populations, longer follow-up, and possibly direct comparison with surgical interventions, are warranted to confirm our results and further explore EBD in upper GI CD strictures.
Funding
This work was supported by grants from the National Institutes of Health [T32DK083251, P30DK097948 Pilot, K08DK110415] and the European Crohn’s and Colitis Foundation to FR.
Conflict of Interest
FR is on the Advisory Board for AbbVie and UCB, consultant to Samsung, UCB, Thetis and Roche and on the speakers’ bureau of AbbVie. AS, NA, SK, HK, RL, BS, JP, and BL have nothing to disclose.
Author Contributions
Study concept and design: FR, AS; acquisition of data: AS, FR, NA, SK; analysis and interpretation of data: FR, AS, RL; drafting of the manuscript and critical revision of the manuscript for important intellectual content: all authors; statistical analysis: RL, FR; obtained funding: FR; administrative, technical, or material support: BS, KH, BL; study supervision: FR, BL.
Supplementary Data
Supplementary data can be found online at ECCO-JCC online.
Supplementary Material
References
- 1. Mills S, Stamos MJ. Colonic Crohn’s disease. Clin Colon Rectal Surg 2007;20:309–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cosnes J, Cattan S, Blain A, et al. Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis 2002;8:244–50. [DOI] [PubMed] [Google Scholar]
- 3. Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011;140:1785–94. [DOI] [PubMed] [Google Scholar]
- 4. Latella G, Papi C. Crucial steps in the natural history of inflammatory bowel disease. World J Gastroenterol 2012;18:3790–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Graham MF, Diegelmann RF, Elson CO, et al. Collagen content and types in the intestinal strictures of Crohn’s disease. Gastroenterology 1988;94:257–65. [DOI] [PubMed] [Google Scholar]
- 6. Rieder F, Fiocchi C. Intestinal fibrosis in IBD—a dynamic, multifactorial process. Nat Rev Gastroenterol Hepatol 2009;6:228–35. [DOI] [PubMed] [Google Scholar]
- 7. Fukumoto A, Tanaka S, Yamamoto H, et al. Diagnosis and treatment of small-bowel stricture by double balloon endoscopy. Gastrointest Endosc 2007;66:S108–12. [DOI] [PubMed] [Google Scholar]
- 8. Nugent FW, Roy MA. Duodenal Crohn’s disease: an analysis of 89 cases. Am J Gastroenterol 1989;84:249–54. [PubMed] [Google Scholar]
- 9. Van Assche G, Geboes K, Rutgeerts P. Medical therapy for Crohn’s disease strictures. Inflamm Bowel Dis 2004;10:55–60. [DOI] [PubMed] [Google Scholar]
- 10. Asairinachan A, An V, Daniel ES, Johnston MJ, Woods RJ. Endoscopic balloon dilatation of Crohn’s strictures: a safe method to defer surgery in selective cases. ANZ J Surg 2016, Apr 8. doi: 10.1111/ans.13500. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 11. Navaneethan U, Lourdusamy V, Njei B, Shen B. Endoscopic balloon dilation in the management of strictures in Crohn’s disease: a systematic review and meta-analysis of non-randomized trials. Surg Endosc 2016;30:5434–43. [DOI] [PubMed] [Google Scholar]
- 12. Bettenworth D, Gustavsson A, Atreja A, et al. A pooled analysis of efficacy, safety, and long-term outcome of endoscopic balloon dilation therapy for patients with stricturing Crohn’s disease. Inflamm Bowel Dis 2017;23:133–42. [DOI] [PubMed] [Google Scholar]
- 13. Guo F, Huang Y, Zhu W, et al. Efficacy and safety of endoscopic balloon dilation for upper gastrointestinal strictures of Crohn’s disease. Dig Dis Sci 2016;61:2977–85. [DOI] [PubMed] [Google Scholar]
- 14. Kelly SM, Hunter JO. Endoscopic balloon dilatation of duodenal strictures in Crohn’s disease. Postgrad Med J 1995;71:623–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Matsui T, Hatakeyama S, Ikeda K, Yao T, Takenaka K, Sakurai T. Long-term outcome of endoscopic balloon dilation in obstructive gastroduodenal Crohn’s disease. Endoscopy 1997;29:640–5. [DOI] [PubMed] [Google Scholar]
- 16. Matsui T, Ikeda K, Tsuda S, et al. Long-term outcome of endoscopic balloon dilation in obstructive gastrointestinal Crohn’s disease: a prospective long-term study. Diagn Ther Endosc 2000;6:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Murthy UK. Repeated hydrostatic balloon dilation in obstructive gastroduodenal Crohn’s disease. Gastrointest Endosc 1991;37:484–5. [DOI] [PubMed] [Google Scholar]
- 18. Singh VV, Draganov P, Valentine J. Efficacy and safety of endoscopic balloon dilation of symptomatic upper and lower gastrointestinal Crohn’s disease strictures. J Clin Gastroenterol 2005;39:284–90. [DOI] [PubMed] [Google Scholar]
- 19. Gomollón F, Dignass A, Annese V, et al. ; ECCO. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 1: Diagnosis and Medical Management. J Crohns Colitis 2017;11:3–25. [DOI] [PubMed] [Google Scholar]
- 20. Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 2006;55:749–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alexander-Williams J, Allan A, Morel P, Hawker PC, Dykes PW, O’Connor H. The therapeutic dilatation of enteric strictures due to Crohn’s disease. Ann R Coll Surg Engl 1986;68:95–7. [PMC free article] [PubMed] [Google Scholar]
- 22. Endo K, Takahashi S, Shiga H, Kakuta Y, Kinouchi Y, Shimosegawa T. Short and long-term outcomes of endoscopic balloon dilatation for Crohn’s disease strictures. World J Gastroenterol 2013;19:86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. East JE, Brooker JC, Rutter MD, Saunders BP. A pilot study of intrastricture steroid versus placebo injection after balloon dilatation of Crohn’s strictures. Clin Gastroenterol Hepatol 2007;5:1065–9. [DOI] [PubMed] [Google Scholar]
- 24. Bettenworth D, Lopez R, Hindryckx P, Levesque BG, Rieder F. Heterogeneity in endoscopic treatment of Crohn’s disease-associated strictures: an international inflammatory bowel disease specialist survey. J Gastroenterol 2016;51:939–48. [DOI] [PubMed] [Google Scholar]
- 25. Decker GA, Loftus EV, Jr, Pasha TM, Tremaine WJ, Sandborn WJ. Crohn’s disease of the esophagus: clinical features and outcomes. Inflamm Bowel Dis 2001;7:113–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.