Abstract
Background
Immigrants from certain low- and middle-income countries are more prone to cancers attributed to viral infections in early life. Cervical cancer is caused by human papillomavirus but is highly preventable by regular screening. We assessed participation among immigrants in a population-based cervical screening programme and identified factors that predicted non-adherence within different immigrant groups.
Methods
We used data from several nationwide registries. The study population consisted of 208 626 (15%) immigrants and 1 157 223 (85%) native Norwegians. Non-adherence was defined as no eligible screening test in 2008–12. We estimated prevalence ratios with 95% confidence intervals (CIs) for factors associated with non-adherence by modified Poisson regression.
Results
In total, 52% of immigrants were not screened. All immigrants showed 1.72 times higher non-adherence rates (95% CI 1.71–1.73) compared with native Norwegian women when adjusted for age and parity. The proportion of non-adherent immigrants varied substantially by region of origin and country of origin. Being unemployed or not in the workforce, being unmarried, having low income and having a male general practitioner was associated with non-adherence regardless of region of origin. Living <10 years in Norway was an evident determinant of non-adherence among most but not all immigrant groups.
Conclusions
An increasing proportion of immigrants and low screening participation among them pose new public health challenges in Europe. Immigrants are diverse in terms of their sociodemographic attributes and screening participation. Tailored information and service delivery may be necessary to increase cancer screening among immigrants.
Introduction
Immigrants from certain low- and middle-income countries are more prone to cancers related to infections experienced in early life.1,2 Cervical cancer is caused by human papillomavirus (HPV)3,4 but is highly preventable by regular screening. However, immigrants tend to be less adherent to screening, and this is not only attributable to demographic or socioeconomic factors.5–13
Lack of time is a barrier to screening for all women.12–17 In addition, immigrants face special problems related to poor proficiency in the new language, literacy, perceived discrimination and low cultural sensitivity among health personnel. Lack of information on how to use health services, lack of knowledge or misunderstandings about screening, fear, embarrassment, shame and cultural perceptions are additional reasons why immigrants may not comply with screening.12,14,17–20
Migration poses increasing public health challenges in Europe.19–21 Information on health resource use among immigrants is needed to adapt services. Existing evidence has mainly been derived from self-reported or community-level data, or from subsets of immigrant groups. The aim of this registry-based study was to assess screening participation among immigrants in a nationwide cervical screening programme in Norway. We also wanted to identify which sociodemographic, health care and migration-related factors within different immigrant groups predicted non-adherence.
Methods
The Norwegian Cervical Cancer Screening Programme (NCCSP) is responsible for the registration and monitoring of all cervical screening and diagnostic activity on an individual level. Organization of the screening programme is described elsewhere.22 Briefly, the NCCSP informs women about screening when they reach age 25 or immigrate to Norway after that age. The NCCSP sends a reminder when 3 years have passed since a previous screening test. Another reminder follows if no screening test is registered within 12 months. All women are reminded until they are 69-years old, unless they have personally opted-out from the screening programme. Women make appointments with their general practitioners (GPs) or gynaecologist for smear-taking. Women have to pay a co-payment which is ∼30 € for the consultation at a GP. Some women opt to pay more for the consultation at a gynaecologist.
Study cohort
We identified women aged from 26 to 69 years, who were alive and resident in Norway on the 31 December 2012 from the National Registry (n = 1 403 687). Women can actively reserve themselves against having their direct identifiers registered in the NCCSP when the test result is normal. These women (n = 2489) were excluded from the cohort due to incomplete screening history. An immigrant woman was defined as someone who had immigrated to Norway or was born in Norway to two immigrant parents. A woman was native Norwegian if at least one of her parents was Norwegian. We excluded women who had opted-out (n = 19 697), women with a previous diagnosis of gynaecological cancer (n = 12 152) and women who were under surveillance after cervical abnormalities (n = 3500). We ended up with a study cohort of 1 365 849 women, of which 208 626 were immigrants (Supplementary figure S1).
Outcome
We categorized women in mutually exclusive groups based on each woman’s individual smear history. An adherent woman had a screening test recorded in the NCCSP in 2008–12. The criterion for a screening test was a Pap smear that had not been preceded by cytological or histological changes or HPV testing within the previous 24 months. A non-adherent woman had no eligible screening test. Each woman was given a status date when she attended (the month of the primary screening test) or should have attended, screening (the month of the last reminder) during her last screening interval. If these dates were not available, the status date was set as 1 July 2010, or the date women turned 25 years old.
Explanatory variables
All residents in Norway are entitled to have a GP (https://helsenorge.no/foreigners-in-norway/the-right-to-a-doctor). Women choose their GP by logging in to a website connected to the regular GP registry (fastlegeregisteret). We characterized the GP for each women from this registry as previously explained.23 We defined the GP as foreign if the GP’s country of origin was not Norway. We divided foreign GPs further into those who had the same region of origin as their patients and those who had not. We obtained women’s postal codes and individual sociodemographic data at the status date from the National Registry, Statistics Norway and the Norwegian Labour and Welfare Administration as described earlier.23 The driving distance between a woman’s home and her GP’s office was calculated using ArcGIS software (Esri, Redlands, CA, USA). The study was approved by the Norwegian Data Inspectorate and the South-East Committee for Medical and Health Research Ethics.
Statistical analysis
Several models of modified Poisson regression were fitted using non-adherence as a binary outcome.24,25 First, we assessed the prevalence ratios (PRs) of non-adherence with 95% confidence intervals (CIs) for immigrants overall and by their region of origin relative to native Norwegian women. We adjusted PRs for age and parity as these covariates differed between immigrants and native Norwegians, and because substantially higher participation rates have been observed during pregnancy when free antepartum visits are available.26 To answer our second research question on predictors of non-adherence, we first conducted a backward stepwise regression for all immigrants.27 Then, we included significant explanatory variables into the final regression models which we stratified by women’s region of origin (Supplementary table S1). Missing or unknown data were excluded from the analyses which were done using Stata (version 14.0, StataCorp, College Station, TX, USA).
Results
Immigrants made up 15% of the total screening population. First generation immigrants constituted 97.5% of the immigrant population (Supplementary figure S1). The mean age among immigrants was 39.1 whereas it was 46.0 years among native Norwegians. Immigrants had given birth in Norway fewer times (mean parity 0.2) than native Norwegians (mean parity 1.8). Only 0.5% of native Norwegians had not enrolled in the regular GP scheme, whereas the proportion of immigrants without a regular GP was 5.8%. Women from Eastern Europe had clearly less often registered themselves with a GP (8.2%) than women from other world regions (Supplementary figure S2).
The majority of immigrants was from Eastern Europe, constituting 4.4% of the screening population (table 1). Overall, 52% of immigrants and 32% of native Norwegians were non-adherent. The proportion of non-adherent immigrants differed substantially by region and country of origin. The non-adherence among immigrants ranged from 37% among Danish immigrants up to 78% among Lithuanian immigrants (table 1).
Table 1.
Target population and non-adherence to the Norwegian Cervical Cancer Screening Program in 2008–12 by region of origin and major contributing countries
| Region of origin | Target population (n = 1 365 849) | Non-adherent women | ||
|---|---|---|---|---|
| n | % of total | n | % of targeted | |
| Norway | 1 157 223 | 84.7 | 365 995 | 31.6 |
| Nordic countries | 23 243 | 1.7 | 9051 | 38.9 |
| Sweden | 12 022 | 0.9 | 4512 | 37.5 |
| Denmark | 5831 | 0.4 | 2156 | 37.0 |
| Finland | 2959 | 0.2 | 1201 | 40.6 |
| Eastern Europe | 60 219 | 4.4 | 36 990 | 61.4 |
| Poland | 19 206 | 1.4 | 13 372 | 69.6 |
| Russia | 7762 | 0.6 | 3618 | 46.6 |
| Lithuania | 7645 | 0.6 | 5968 | 78.1 |
| Bosnia-Herzegovina | 5050 | 0.4 | 2184 | 43.3 |
| Kosovo | 3192 | 0.2 | 1370 | 42.9 |
| Romania | 3012 | 0.2 | 1906 | 63.3 |
| Western Europe | 18 381 | 1.4 | 8857 | 48.2 |
| Germany | 7650 | 0.6 | 3801 | 49.7 |
| UK | 3254 | 0.2 | 1385 | 42.6 |
| The Netherlands | 2124 | 0.2 | 852 | 40.1 |
| America, Oceania | 12 845 | 1.0 | 5486 | 42.7 |
| USA | 2707 | 0.2 | 1153 | 42.6 |
| Chile | 2563 | 0.2 | 1060 | 41.4 |
| Brazil | 2142 | 0.2 | 873 | 40.8 |
| Western Asia, North Africa | 16 361 | 1.2 | 7381 | 45.1 |
| Iraq | 5932 | 0.4 | 2384 | 40.2 |
| Turkey | 4222 | 0.3 | 1868 | 44.2 |
| Morocco | 2165 | 0.2 | 1025 | 47.3 |
| Sub-Saharan Africa | 17 584 | 1.3 | 9399 | 53.5 |
| Somalia | 6414 | 0.5 | 3751 | 58.5 |
| Eritrea | 3344 | 0.2 | 1984 | 59.3 |
| South Central Asia | 26 719 | 2.0 | 13 486 | 50.5 |
| Pakistan | 8957 | 0.7 | 4812 | 53.7 |
| Iran | 5528 | 0.4 | 2297 | 41.6 |
| India | 3489 | 0.3 | 1859 | 53.3 |
| Sri Lanka | 3692 | 0.3 | 1857 | 50.3 |
| Afganistan | 2528 | 0.2 | 1239 | 49.0 |
| Eastern Asia | 33 274 | 2.4 | 18 316 | 55.1 |
| Philippines | 10 565 | 0.8 | 6508 | 61.6 |
| Thailand | 10 465 | 0.8 | 5761 | 55.1 |
| Vietnam | 6485 | 0.5 | 2912 | 44.9 |
| China | 3257 | 0.2 | 1763 | 54.1 |
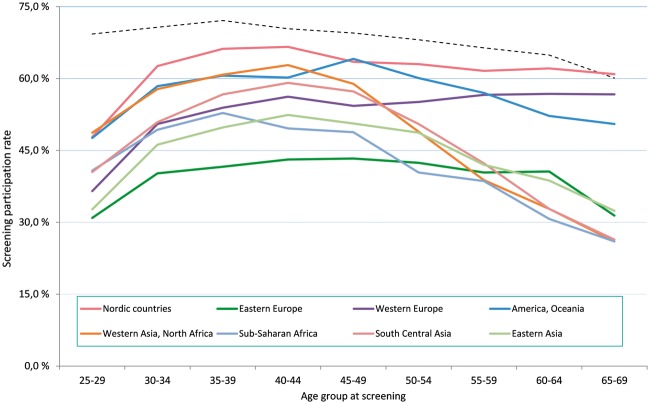
We found lower screening participation among immigrants across all ages but the difference was greatest among women younger than 40 years. In all age groups, Nordic immigrants were the most compliant to screening. We observed different screening participation patterns by age and region of origin (figure 1).
Figure 1.
Screening adherence among native Norwegian (dashed line) and immigrant women (solid lines) to the Norwegian Cervical Cancer Screening Program between 2008 and 2012 by region of origin and age group at screening
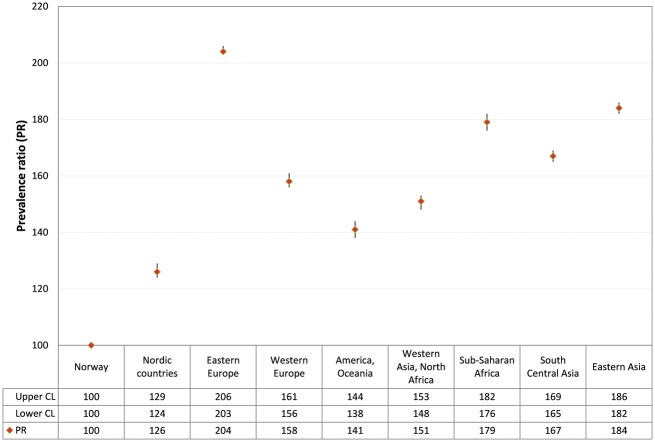
All immigrants combined showed 1.72 times higher non-adherence rates (95% CI 1.71–1.73) compared with native Norwegian women. However, PRs of non-adherence varied substantially by region of origin. Immigrants from Eastern Europe showed the highest non-adherence rates (PR 2.04, 95% CI 2.03–2.06) compared with native Norwegians, as shown in figure 2.
Figure 2.
PR of non-adherence to the Norwegian Cervical Cancer Screening Program in 2008–12 with 95% CIs by region of origin, adjusted for age and parity
Overall, 57% of immigrants had lived in Norway for <10 years. Married women constituted the majority of the immigrants. Immigrants from Western Europe and Nordic countries had the highest educational attainment, and they belonged most often to the top income quintile. Sub-Saharan African women were least likely to have a university degree or to work in white-collar occupations. The majority of immigrants was registered with a male GP. Among Nordic immigrants, 70% had a Norwegian GP, whereas immigrants from other regions more often had a foreign GP. Study characteristics are given in Supplementary table S2a and b.
Stratified regression models indicated that non-adherence increased with increasing age at migration to Norway for all immigrants, except for South Central Asian women (data not shown). Living <10 years in Norway was associated with higher non-adherence rates in most immigrant groups (table 2). However, the length of stay had no effect among immigrants from Western Asia and North Africa and had the opposite effect for Sub-Saharan African immigrants (PR 0.93, 95% CI 0.88–0.99).
Table 2.
Predictors of non-adherence to the Norwegian Cervical Cancer Screening Program in 2008–12 among immigrants by region of origin
| Covariate (reference) | PR (95% CI) for non-adherencea | |||||||
|---|---|---|---|---|---|---|---|---|
| Nordic countries | Eastern Europe | Western Europe | America, Oceania | Western Asia, North Africa | Sub-Saharan Africa | South Central Asia | Eastern Asia | |
| Length of stay (≥10 years) | ||||||||
| <10 years | 1.18 (1.11–1.25) | 1.15 (1.10–1.19) | 1.23 (1.15–1.32) | 1.11 (1.02–1.20) | 1.00 (0.95–1.06) | 0.93 (0.88–0.99) | 1.13 (1.07–1.18) | 1.09 (1.04–1.14) |
| Marital status (married) | ||||||||
| Unmarried | 1.30 (1.24–1.36) | 1.22 (1.20–1.24) | 1.37 (1.31–1.43) | 1.49 (1.40–1.58) | 1.54 (1.46–1.63) | 1.21 (1.16–1.26) | 1.42 (1.36–1.47) | 1.48 (1.44–1.52) |
| Divorced/widowed | 1.18 (1.12–1.25) | 1.02 (1.00–1.05) | 1.17 (1.11–1.24) | 1.12 (1.06–1.20) | 1.15 (1.09–1.20) | 1.11 (1.06–1.16) | 1.10 (1.06–1.14) | 1.10 (1.06–1.13) |
| Employment (white-collar job) | ||||||||
| Unemployed | 1.20 (1.13–1.27) | 1.18 (1.14–1.21) | 1.26 (1.19–1.34) | 1.21 (1.11–1.31) | 1.16 (1.07–1.26) | 1.24 (1.14–1.34) | 1.16 (1.10–1.22) | 1.12 (1.07–1.18) |
| Blue-collar job | 1.13 (1.08–1.18) | 1.17 (1.14–1.21) | 1.12 (1.07–1.17) | 1.04 (0.96–1.12) | 1.10 (1.01–1.19) | 1.05 (0.97–1.14) | 1.08 (1.03–1.14) | 1.07 (1.03–1.12) |
| Annual income (high) | ||||||||
| Low | 1.39 (1.30–1.48) | 1.32 (1.27–1.38) | 1.30 (1.22–1.39) | 1.32 (1.20–1.46) | 1.27 (1.15–1.42) | 1.23 (1.13–1.34) | 1.26 (1.18–1.34) | 1.19 (1.12–1.26) |
| Medium | 1.10 (1.04–1.15) | 1.07 (1.03–1.11) | 1.05 (1.00–1.11) | 0.99 (0.91–1.09) | 1.03 (0.93–1.14) | 1.04 (0.96–1.13) | 1.02 (0.97–1.09) | 1.02 (0.97–1.07) |
| Distance to GP (1–9.9 km) | ||||||||
| <1 km | 1.09 (1.05–1.14) | 1.05 (1.04–1.07) | 1.07 (1.03–1.11) | 1.06 (1.00–1.12) | 1.01 (0.97–1.05) | 1.02 (0.99–1.06) | 0.99 (0.96–1.03) | 1.01 (0.98–1.03) |
| >10km | 1.11 (1.06–1.17) | 1.08 (1.06–1.10) | 1.12 (1.07–1.17) | 1.12 (1.05–1.19) | 1.03 (0.98–1.09) | 1.10 (1.05–1.15) | 1.05 (1.01–1.08) | 1.09 (1.06–1.12) |
| GP’s gender (female) | ||||||||
| male | 1.21 (1.16–1.26) | 1.13 (1.11–1.15) | 1.14 (1.09–1.18) | 1.12 (1.06–1.18) | 1.14 (1.10–1.18) | 1.07 (1.03–1.11) | 1.11 (1.08–1.14) | 1.16 (1.14–1.19) |
| GP’s origin (Norway) | ||||||||
| Foreign, different from woman | 1.08 (1.04–1.13) | 1.02 (1.01–1.04) | 1.01 (0.96–1.07) | 1.01 (0.96–1.07) | 1.04 (1.01–1.09) | 1.06 (1.03–1.10) | 1.05 (1.02–1.08) | 1.01 (0.98–1.03) |
| Foreign, same as woman | 1.05 (0.99–1.12) | 0.96 (0.94–0.99) | 0.99 (0.88–1.12) | 1.00 (0.89–1.13) | 1.04 (0.98–1.12) | 1.06 (0.97–1.16) | 1.09 (1.05–1.13) | 0.99 (0.95–1.04) |
Adjusted for age at migration, age, parity, region of residence in Norway and GP’s age at screening in addition to all covariates in the table.
For all immigrants, the highest PRs of non-adherence were observed for being unmarried. PRs ranged from 1.21 (95% CI 1.16–1.26) among Sub-Saharan African women to 1.54 (1.46–1.63) among women from Western Asia or North Africa. Being unemployed or not in the workforce, having low income and having a male GP was associated with non-adherence across all world regions. Occupation predicted non-adherence for the majority of immigrant groups (table 2).
Distance to the screening site did not predict non-adherence for women from Western Asia and North Africa. For all other immigrant groups, living >10 km away from the GP’s office predicted non-adherence. For European women, also living <1 km away from the GP was associated with higher levels of non-adherence compared with intermediate distance.
Except for women from Western Europe, America, Oceania and Eastern Asia, a foreign GP increased the risk of non-adherence. Eastern European women with a GP of the same origin showed increased adherence (PR of non-adherence 0.96, 95% CI 0.94–0.99), whereas a GP of the same region of origin predicted non-adherence for South Central Asian women (PR 1.09, 95% CI 1.05–1.13). For all other regions, having a GP of the same origin was associated with similar non-adherence rates compared with women with a Norwegian GP (table 2). We did not observe consistent differences in non-adherence rates by GP’s age and different immigrant groups (data not shown).
Discussion
We showed that 52% of immigrants were not screened for cervical cancer in the population-based programme in Norway in 2008–12. The proportion of non-adherent women varied substantially across immigrants based on their age, region of origin and country of origin. Being unemployed or not in workforce, being unmarried, having low income and having a male GP was associated with non-adherence for immigrants from all world regions but effect sizes were generally smaller. Living <10 years in Norway predicted non-adherence among some immigrant groups, whereas there was little or no effect among others.
Non-adherence was positively associated with being an immigrant. However, the risk of non-adherence was not similar across immigrant groups.5–13,28,29 We also observed that among immigrants sharing the same region of origin, non-adherence rates varied substantially by country of origin. Immigrants were diverse in terms of their sociodemographic attributes. Compared with the general screening population, high-income country immigrants had higher socioeconomic status whereas the opposite was true for immigrants from low- and middle-income countries.23 Only two countries have reported higher cervical screening coverage among immigrants compared with the host population. Both studies attributed this finding to higher use of health services outside the screening programme among wealthier individuals.30,31
Up to 6% of immigrants had not registered themselves with a GP, as compared with 0.5% of native Norwegians. This impedes the effectiveness of reminders from the NCCSP. Moreover, the NCCSP currently provides information on screening in Norwegian only. Language barriers undermine both the access and the quality of health services for immigrants.18,19,32 Providing information in several languages and adapted for different cultural backgrounds are means to tailor screening programmes at relatively low cost. Impersonal communication through printed materials may still not work, and community networks may be the most effective way to reach immigrants.18,20
Many immigrant women attend screening as a result of their GP’s advice.14 However, immigrants may have regular contacts with health services but still are never screened.33 Due to communication difficulties, GP appointments can take more time and GPs have little time to raise awareness of screening. Language barriers can be overcome by using easily accessible and free professional interpreting services and training GPs to use them.19 Further, a strategy that does not use GP time, such as an option to see a nurse or midwife to discuss screening, or provision of a video could be useful.8
Several studies among immigrants with different backgrounds have raised the importance of a female smear-taker.6,10,12,14,17,32 In our study, a male GP predicted non-adherence for all immigrant groups. Interestingly, the effect of the GP’s gender was greatest among Nordic immigrants who had the lowest non-adherence rates overall. This might reflect that the GP’s gender comes into play rather late in the process when women are making informed choices for or against screening. Furthermore, Finnish and Swedish immigrants are often screened by trained female midwives in their home countries.
Some studies have demonstrated higher non-adherence rates when a physician and a patient have the same background.6,13 In our study, a foreign GP predicted non-adherence for the majority of immigrant groups. This indicates that the lack of effective communication may be a more important screening barrier than the nationality of the GP. Furthermore, a GP of the same origin facilitated screening among women from Eastern Europe whereas it predicted non-adherence for South Central Asian women. This was a new finding and suggests that cultural factors likely play a role. Future research is needed to study if non-adherence attributed to foreign GPs is due to differences in medical training, due to reluctance to undergo screening by a foreign GP or both.
We observed that living >10 km away from the screening site increased the risk of non-adherence for most immigrant groups. Most likely difficulties scheduling a screening appointment to fit in with daily commitments increases with increasing distance. Very short distance (<1 km) to the screening site was associated with non-adherence only among European immigrants. This may be due to rural–urban differences in screening use, a factor we did not adjust for in this study. A study using the Norwegian Health Economics Administration Database from primary health care reported that women in rural areas had more Pap smears. Although the study material was somewhat selected and included only a small subset of smears taken within the NCCSP, authors suggested that women in rural areas tend to be better integrated in society and rural GP’s have less patients.10
We used length of stay as a proxy for acculturation.34 Several studies have reported that longer time since immigration increases the likelihood of screening.6,32,35–37 Our study confirmed that acculturation is a determinant of non-adherence for most immigrants. A plausible explanation is that labour immigrants travel to their home countries to get health care services during the first years in Norway.10 This observation is supported by the high proportion of Eastern European women lacking a GP in our material. In our study, African and Western Asian immigrants who had stayed ≥ 10 years in Norway were less likely to be screened than those who had more recently arrived. Our results underlie the complexity and diversity of immigrant behaviours and acculturation. Thus, immigrants may need targeted interventions to engage them with cervical screening. A possible means to increase screening participation could be, for instance, screening at well woman clinics which may be considered more convenient than visiting a GP.17 Also, less invasive screening methods such as HPV testing on self-collected samples could remove some of the barriers associated with current screening practice.12,15,16,33
We found an evident decrease in screening participation in older age groups among immigrants from low- and middle-income countries. Old age has been associated with being overdue or never screened also in other studies.7–9,36 In Western countries, health facilities are not easily accessible for elderly immigrants who tend to be particularly prone to poor language proficiency.9,38 Furthermore, a general lack of knowledge about cervical cancer can influence screening participation particularly among older immigrants.17
Consistent with the literature, unmarried immigrants had the highest levels of non-adherence in our study.9,19,35,36 The predictive power of marital status has often been attributed to pregnancy and childbirth which provide a gateway into the health care system. Our estimates were adjusted for parity, and the positive effect of marital status has been evident also for women and men participating in colorectal cancer screening.39 Thus, marriage facilitates screening participation also by other means, such as providing social support. Some ethnic minority women may also perceive cervical screening as a sign of poor health and/or bad sexual behaviour, and thus the practice is only acceptable for married women.12,17,18
Low income has been associated with non-adherence to screening among immigrants.6,7,36 We found that being unemployed or being outside of workforce was associated with higher levels of non-adherence across immigrants from all world regions when adjusted for the length of stay and income. This supports the notion that employment facilitates inclusion into the local culture and health care through language learning.20
Lower screening participation can also result from an informed decision-making process. However, knowledge about screening for cancer in general is rather low and that applies particularly for immigrants.9 Many immigrants do not recognize the terms ‘cervical screening’ or ‘smear’, suggesting that clarification of what cervical screening is should be included in information for ethnic minority women.17
This was entirely a registry-based study using individual level information from comprehensive nationwide registries. We could reliably identify screening participation, and we paid close attention to include only screening visits and not surveillance due to previous abnormal smears. Studies with self-reported screening history suffer from recall bias and selection bias as ‘hard-to-reach’ women, who are most difficult to engage in screening activities, do not respond to surveys.16,40 There may also be ethnic and sociodemographic differences in reporting accuracy.40 A retrospective design can introduce a selection bias by unmeasured factors that have an effect on screening participation and survival also in our study.
We could not confirm whether the smear was taken by the registered GP, by other GP sharing the same office or by a gynaecologist. We do not anticipate high use of gynaecologists among immigrants, and women whose own GP is male and/or foreign may prefer another smear-taker. Thus, our results probably underestimate the true effects of the GP’s characteristics.
Because of their low number, we combined second generation with first generation immigrants. In future studies on health service utilization among immigrants, it would be useful to differentiate between first and second generation immigrants and between labour immigrants and refugees or asylum-seekers.38
Immigrants constitute a substantial proportion of the total population. Making cancer prevention programmes more responsive to immigrants will require special attention. Immigrants are diverse in terms of their sociodemographic attributes and screening participation. To engage immigrants with cancer screening may warrant tailored approaches to information and service delivery.
Supplementary data
Supplementary data are available at EURPUB online.
Supplementary Material
Acknowledgements
The authors thank Ole Klungsøyr for statistical consultancy and Barbara Schwendtner from Geodata AS for her help with the article.
Funding
The study was supported by the Norwegian Cancer Society (grant 5777899) and the Cancer Registry of Norway. The work has been presented orally at 6th EUPHA Conference on Migrant and Ethnic Minority Health 2016 in Oslo, Norway (June 23–26), at ANCR & NCU Symposium 2016 in Tuusula, Finland (30 August–1 September) and at HPV 2017 Conference in Cape Town, South Africa (March 1–4).
Conflicts of interest: None declared.
Key points
Immigrants do not comply with cervical screening but evidence has mainly been derived from self-reported or community-level data, or from subsets of immigrant groups.
Self-reported data tend to overestimate screening utilization and underestimate disparities because people, who are the most difficult to engage with screening, do not respond to surveys.
Screening adherence among immigrants poses new public health challenges in Europe.
Distributions of the sociodemographic attributes and screening participation pattern is different for immigrants coming from different world regions.
Acculturation is an evident determinant of non-adherence for some immigrants, whereas there is no effect among others. To engage all immigrants with cervical screening may warrant adaptation of information and service delivery.
References
- 1. Arnold M, Razum O, Coebergh JW. Cancer risk diversity in non-western migrants to Europe: an overview of the literature. Eur J Cancer 2010;46:2647–59. [DOI] [PubMed] [Google Scholar]
- 2. McDermott S, Desmeules M, Lewis R, et al. Cancer incidence among Canadian immigrants, 1980–1998: results from a national cohort study. J Immigr Minor Health 2011;13:15–26. [DOI] [PubMed] [Google Scholar]
- 3. Li N, Franceschi S, Howell-Jones R, et al. Human papillomavirus type distribution in 30,848 cervical cancers worldwide: variation by geographical region, histological type and year of publication. Int J Cancer 2011;128:927–35. [DOI] [PubMed] [Google Scholar]
- 4. Guan P, Howell-Jones R, Bruni L, et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer 2012;131:2349–59. [DOI] [PubMed] [Google Scholar]
- 5. Moser K, Patnick J, Beral V. Inequalities in reported use of breast and cervical screening in Great Britain: analysis of cross sectional survey data. BMJ 2009;338:b2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lofters AK, Moineddin R, Hwang SW, Glazier RH. Predictors of low cervical cancer screening among immigrant women in Ontario, Canada. BMC Womens Health 2011;11:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rondet C, Lapostolle A, Soler M, et al. Are immigrants and nationals born to immigrants at higher risk for delayed or no lifetime breast and cervical cancer screening? The results from a population-based survey in Paris metropolitan area in 2010. PLoS One 2014;9:e87046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marlow LAV, Wardle J, Waller J. Understanding cervical screening non-attendance among ethnic minority women in England. Br J Cancer 2015;113:833–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brzoska P, Abdul-Rida C. Participation in cancer screening among female migrants and non-migrants in Germany: a cross-sectional study on the role of demographic and socioeconomic factors. Medicine 2016;95:e4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Møen KA, Kumar BN, Qureshi S, Esperanza D. Differences in cervical cancer screening between immigrants and nonimmigrants in Norway –a primary health care register-based study. Eur J Cancer Prev 2016, Oct 4. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Azerkan F, Sparén P, Sandin S, et al. Cervical screening participation and risk among Swedish-born and immigrant women in Sweden. Int J Cancer 2012;130:937–47. [DOI] [PubMed] [Google Scholar]
- 12. Ghebre RG, Sewali B, Osamn S, et al. Cervical cancer: barriers to screening in the Somali community in Minnesota. J Immigr Minor Health 2015;17:722–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lofters AK, Ng R, Lobb R. Primary care physician characteristics associated with cancer screening: a retrospective cohort study in Ontario, Canada. Cancer Med 2015;4:212–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abdullahi A, Copping J, Kessel A, et al. Cervical screening: perceptions and barriers to uptake among Somali women in Camden. Public Health 2009;123: 680–5. [DOI] [PubMed] [Google Scholar]
- 15. Bosgraaf RP, Ketelaars PJW, Verhoef VMJ, et al. Reasons for non-attendance to cervical screening and preferences for HPV self-sampling in Dutch women. Prev Med 2014; 64:108–13. [DOI] [PubMed] [Google Scholar]
- 16. Virtanen A, Nieminen P, Niironen M, et al. Self-sampling experiences among non-attendees to cervical screening. Gynecol Oncol 2014;135:487–94. [DOI] [PubMed] [Google Scholar]
- 17. Marlow LAV, Waller J, Wardle J. Barriers to cervical cancer screening among ethnic minority women: a qualitative study. J Fam Plann Reprod Health Care 2015; 41:248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Scheppers E, van Dongen E, Dekker J, et al. Potential barriers to the use of health services among ethnic minorities: a review. Fam Pract 2006;23:325–48. [DOI] [PubMed] [Google Scholar]
- 19. Rechel B, Mladovsky P, Ingleby D, et al. Migration and health in an increasingly diverse Europe. Lancet 2013;381:1235–45. [DOI] [PubMed] [Google Scholar]
- 20. Simon J, Kiss N, Laszewska A, Mayer S. Public health aspects of migrant health: a review of the evidence on health status for labour migrants in the European Region. Health Evidence Synthesis Network report 43. Copenhagen: WHO Regional Office for Europe, 2015. [PubMed] [Google Scholar]
- 21. Statistics Norway. 2016. Immigrants and Norwegian-born to immigrant parents, 1 January 2016, StatBank, Table 07108. Available at: www.ssb.no/en (28 September 2016, date last accessed).
- 22. Nygård JF, Skare GB, Thoresen SØ. The cervical cancer screening programme in Norway, 1992–2000: changes in Pap smear coverage and incidence of cervical cancer. J Med Screen 2002;9:86–91. [DOI] [PubMed] [Google Scholar]
- 23. Leinonen MK, Campbell S, Klungsøyr O, et al. Personal and provider level factors influence participation to cervical cancer screening: a retrospective register-based study of 1.3 million women in Norway. Prev Med 2017;94:31–9. [DOI] [PubMed] [Google Scholar]
- 24. Barros AJ, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol 2003;3:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702–6. [DOI] [PubMed] [Google Scholar]
- 26. Nygård M, Daltveit AK, Thoresen SO, Nygård JF. Effect of an antepartum Pap smear on the coverage of a cervical cancer screening programme: a population-based prospective study. BMC Health Serv Res 2007;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health 1989;79:340–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Webb R, Richardson J, Pickles A. A population-based study of primary care predictors of non-attendance for cervical screening. J Med Screen 2004;11: 135–40. [DOI] [PubMed] [Google Scholar]
- 29. Gesink D, Mihic A, Antal J, et al. Who are the under- and never-screened for cancer in Ontario: a qualitative investigation. BMC Public Health 2014;14:495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rodvall Y, Kemetli L, Tishelman C, Törnberg S. Factors related to participation in a cervical cancer screening programme in urban Sweden. Eur J Cancer Prev 2005;14:459–66. [DOI] [PubMed] [Google Scholar]
- 31. Rodriquez-Sales V, Roura E, Ibanez R, et al. Coverage of cervical cancer screening in Catalonia for the period 2008–2011 among immigrants and Spanish-born women. Front Oncol 2013;3:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grandahl M, Tyden T, Gottvall M, et al. Immigrant women’s experiences and views on the prevention of cervical cancer: a qualitative study. Health Expect 2012;18:344–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sewali B, Okuyemi KS, Askhir A, et al. Cervical cancer screening with clinic-based Pap test versus home HPV test among Somali immigrant women in Minnesota: a pilot randomized controlled trial. Cancer Med 2015;4:620–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Urquia ML, Gagnon AJ. Glossary: migration and health. J Epidemiol Community Health 2011;65:467e472. [DOI] [PubMed] [Google Scholar]
- 35. Woltman KJ, Newbold KB. Immigrant women and cervical cancer screening uptake: a multilevel analysis. Can J Public Health 2007;98:470–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Khadilkar A, Chen Y. Rate of cervical cancer screening associated with immigration status and number of years since immigration in Ontario, Canada. J Immigr Minor Health 2013;15:244–8. [DOI] [PubMed] [Google Scholar]
- 37. Harcourt N, Ghebre RG, Whembolua GL, et al. Factors associated with breast and cervical cancer screening behavior among African immigrant women in Minnesota. J Immigr Minor Health 2014;16:450–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Diaz E, Kumar BN. Differential utilization of primary health care services among older immigrants and Norwegians: a register-based comparative study in Norway. BMC Health Serv Res 2014;14:623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van Jaarsveld CH, Miles A, Edwards R, Wardle J. Marriage and cancer prevention: does marital status and inviting both spouses together influence colorectal cancer screening participation? J Med Screen 2006;13:172–6. [DOI] [PubMed] [Google Scholar]
- 40. Rauscher GH, Johnson TP, Cho YI, Walk JA. Accuracy of self-reported cancer-screening histories: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2008;17: 748–57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.