Abstract
The Toll-like receptor 4 (TLR4) signal pathway-induced inflammation is considered to be a crucial link to myocardial ischemia reperfusion injury (MIRI). Our previous study proved that radioprotective 105 kDa protein (RP105), a negative regulator of TLR4, performed a protective role in MIRI by anti-apoptosis approach. However, the mechanism of RP105 cardioprotection of anti-inflammation is still unclear. This study aimed to explore the underlying mechanism of RP105 anti-inflammation effect in MIRI. We established a rat model of MIRI induced by ligation of the left anterior descending coronary artery for 30 min followed by 2 h reperfusion. Animals were pre-infected with Ad-EGFP-RP105, Ad-EGFP or saline at the apex of the heart. All rats were sacrificed to collect blood samples and myocardial tissue and assessed by immunofluorescence, blood biochemical analysis, Evans blue/triphenyltetrazolium chloride (TTC), hematoxylin and eosin (H&E) staining, enzyme-linked immuno sorbent assay (ELISA), western blot analysis, quantitative PCR and electrophoretic mobility shift assay (EMSA). RP105 overexpression with adenovirus vectors reduced serum myocardial enzyme (CK-MB and LDH) activities, decreased myocardial infarct size, mitigated inflammatory factors interferon-β and tumor necrosis factor-α during MIRI. We also found that Ad-RP105 group exerted distinct repression of TLR4/TRIF signal pathway related proteins and mRNAs (TRIF, TBK-1, IRF3 and p-IRF3) with a low transcriptional activity of IRF3. These findings first expounded that RP105 could alleviate the ischemia reperfusion induced inflammatory status in heart via inhibiting TLR4/TRIF signaling pathway and provided a theoretical foundation of RP105 gene in MIRI.
Keywords: radioprotective 105 kDa protein, anti-inflammation, Toll-like receptor 4, TIR domain containing adaptor inducing interferon β, interferon regulating factor 3
Introduction
Ischemic heart disease (IHD) is a serious health problem in the world with growing morbidity and mortality (1). Reconstruction of blood supply is not only the most effective treatment for IHD, but also the arch-criminal for myocardial ischemia reperfusion injury (MIRI) and reducing the therapeutic benefit (2). We could not abandon the profit of percutaneous coronary intervention, coronary artery bypass grafting or thrombolytic agents but rescue reperfusion injury. The procedure of MIRI refers to a series of complicated pathological processes, including inflammatory response (3), calcium overload (4), complement activation (5), cell autophagy (6) and apoptosis (7). The inflammatory response with Toll-like receptor (TLR) signaling is considered to be a pivotal link to myocardial ischemia/reperfusion induced tissue injury (8,9). Toll-like receptor 4 (TLR4) transducted signal with an extracellular leucine rich repeat (LRR) domain and a Toll/IL-1 receptor (TIR) domain plays a critical role in the induction of the inflammatory response during MIRI (10). TLR4 was the first discovered mammalian TLR and the only one of the TLRs family member that can activate the myeloid differentiation factor 88 (MyD88)-dependent pathways and TIR domain containing adapter inducing interferon β (TRIF) dependent-pathway (11). TLR4/MyD88 and TLR4/TRIF activated the transcriptional activity of nuclear factor-κB (NF-κB) and interferon regulating factor 3 (IRF3) respectively, inducing a series of inflammatory factors.
A variety of studies have demonstrated that radioprotective 105 kDa protein (RP105) is a specific inhibitor of the TLR4-triggered inflammatory response in dendritic cells, macrophages and monocytes (12-14). RP105 is the specific homologue of TLR4 of TLRs family containing an LRR domain, but TIR domain, which could not transmit signaling alone (15). Surface expression and signal transmission of TLR4 depends upon co-expression of myeloid differential protein-2 (MD-2), while surface expression and TLR4 signaling inhibition of RP105 relies on co-localization of MD-1 homogenized to MD1 (15). The physiological combination of RP105/MD-1 complex and TLR4/MD-2 complex contributes to the specific inhibition of LPS-induced TLR4 inflammation signaling by RP105 (16).
RP105 suppresses the MIRI-induced TLR4 signal in cardiomyocytes in vivo. Our previous study proved that overexpression of RP105 in cardiomyocytes resulted in de-activation of the TLR4/P38MAPK/AP-1 signaling pathways, and was followed by repressing myocardial cell apoptosis to protect MIRI (17). In addition, our unpublished data show that RP105 transmission into cardiac myocytes markedly repressed the pro-inflammatory action and remitted myocardial damage during MIRI via TLR4/MyD88 signaling pathway. Nevertheless, the mechanism of RP105 cardioprotection is complicated and still incomplete. In this study, we utilized adenoviral transfection of RP105 into cardiomyocytes to explore and consummate underlying anti-inflammation mechanism further in an animal model of MIRI. Our data illustrates that RP105 efficiently alleviated ischemia reperfusion induced myocardial damage by negative regulation of TLR4/TRIF signal pathway.
Materials and methods
Animal care and adenovirus vector construction
All experiments were approved by the Insitutional Animal Care Committee of the Faculty of Medicine, China Three Gorges University, Yichang, China. Sprague-Dawley rats weighing 220-250 g were obtained from the Animal Center, China Three Gorges University, Yichang, China. All the animals were housed in specific pathogen-free (SPF) barrier environment during the procedure of feeding and surgery.
Targeting gene RP105 was obtained by PCR and linked to the shuttle vector Gv135 (CMv-MCS-EGFP) to form the linker production. The recombinant vector CMv-RP105-MCS-EGFP was transformed to E. coli DH5a cells to obtain a large number of positive clones and confirmed by enzyme digestion and DNA sequencing. AdMax virus packaging system was used for the cotransfection of HEK293 cells by RP105 recombinant shuttle vector and auxiliary packaging plasmid. By means of Cre/loxP recombinant enzyme system, we acquired recombinant adenovirus Ad-RP105-EGFP and its negative control Ad-EGFP. After the packaging, amplification and purification of recombinant adenovirus vector, we obtained the virus titer of 1×109 PFU/ml according to the manufacturer's protocol.
Rats were randomly divided into four equal groups (n=10): i) sham, normal non-ischemic group; ii) IR, myocardial ischemia reperfusion group infected with saline; iii) Ad-RP105, myocardial ischemia reperfusion infected with Ad-EGFP-RP105; and iv) Ad-EGFP, myocardial ischemia reperfusion infected with Ad-EGFP.
Surgical procedure of adenovirus transfection and MIRI model establishment
All the surgical procedures of adenovirus transfer and MIRI model establishment proceeded as previously described (17,18) in the SPF animal laboratory, avoiding the post-operation infections and other disturbing elements of rats before their sacrifice. The animals were anesthetized by intraperitoneal injection sodium pentobarbital (40 mg/kg) and fastened to the animal operating table. Rats were ventilated via tracheal intubation of venous indwelling needle (24 G) with room air from the small animal ventilator at the rate of 80 breath/min and the inspiratory/expiratory ratio of 2:1. The heart was exposed through a left thoracotomy between the fourth and fifth ribs and transfected with 100 μl normal saline, Ad-RP105-EGFP and Ad-EGFP at the heart apex of three separate sites in respective group. Three days later, the second anesthetization, ventilation and thoracotomy in the same manner was performed. After the heart re-exposed, the left anterior descending coronary artery (LAD) was ligated by 6-0 silk suture with medical latex tubing (inner diameter, 1.5 mm, socket) to permit reversible occlusion of LAD. Myocardial ischemia was monitored by lead II ECG with ST segment elevation and ischemic region with pallor. Animals underwent 30 min of myocardial ischemia, followed by 120 min of reperfusion. Sham-operated rats were subjected the same procedures without the blockage of the LAD or repatency. After surgery of ischemic and reperfusion, all rats were sacrificed to collect blood samples and myocardial tissue for the next step.
Immunofluorescent assay
We assessed the expression of RP105 successful transfection in cardiac muscle tissue via immunofluorescent assay. Myocardial tissues were fixed in 4.0% paraformaldehyde, embedded in paraffin, and sectioned into 4 μm slices. After sections were dewaxed and antigen fixing, slices were sealed by 1% bovine serum albumin (BSA) for 30 min at room temperature, incubated with anti-RP105 antibody (dilution 1:80; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) overnight at 4°C, hatched with Fluor Cy3 (red)-conjugated rabbit anti-bovine IgG (dilution 1:100; Boster Biotechnology, Wuhan, China) for 60 min at 20-37°C in a humidified box and stained with 4′,6′-diamidino-2-phenylindole (DAPI) under the conditions of protection from light. Immunofluorescent assay was executed with fluorescence microscopy (BX51; Olympus America, Melville, NY, USA) in the dark.
Biochemical analysis
The blood serum lactate dehydrogenase (LDH) and creatine kinase MB (CK-MB) were measured using a commercially available analytical kit (Beijing Kemeidongya Biotechnology Ltd., Wuhan, China).
Assessment of myocardial infarct size
Myocardial infarct area was measured by the Evans blue/triphenyltetrazolium chloride (TTC) double-staining as previously described (17,18). At the end of 2 h reperfusion, 1 ml 2.0% Evans blue dye (Sigma-Aldrich, St. Louis, MO, USA) was injected via jugular vein with LAD re-ligated. The heart was isolated from in vivo, frozen at −80°C for 30 sec, sliced into 5 sections (2 mm) and stained in 1.5% TTC (Sigma Chemicals) at 37°C for 15 min. There are three colors in the sections, including blue representing the viable non-ischemic area, red expressing the myocardium area at risk (AAR) and white presenting infarct area (IA). The assessment of myocardial infarct size is presented by the percentage of IA/AAR that was calculated in Image-Pro Plus 5.0 software.
Hematoxylin and eosin (H&E) staining
Hearts were fixed with 4% buffered formaldehyde for 24 h at room temperature, embedding in paraffin wax, and cutting into slices (5 μm). Subsequently, sections were stained with H&E and observed under a light microscope.
Enzyme-linked immunosorbent assay (ELISA)
The levels of interferon-β (IFN-β) and tumor necrosis factor-α (TNF-α) in myocardial tissue were detected by ELISA following the manufacturer's instructions, using ELISA kits (Abcam, Cambridge, MA, USA).
Western blot analysis
We processed western blot analysis to examine the protein levels of TRL4/TRIF signaling pathway downstream in myocardial tissue. Protein extraction was exerted by the RIPA Lysis Buffer and concentration detection was determined using a bicinchoninic acid protein assay kit (both from Beyotime, Jiangsu, China). Equal amounts of protein were separated by 12% sodium dodecyl sulfate-poly-acrylamide gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose membrane by electroblotting. The membrane was blocked by 5% defatted milk with Tris-buffered saline Tween-20 (TBST) for 2 h at room temperature in case of nonspecific binding. Then, the rinsed membrane was incubated with the respective specific primary antibody overnight at 4°C, followed by a peroxidase conjugated secondary antibodies. We used anti-TRIF (dilution 1:600), anti-TBK1 (dilution 1:1,000) (both from Santa Cruz Biotechnology, Inc.), anti-IRF3 (dilution 1:1,000) and anti-p-IRF3 (dilution 1:600) (both from Cell Signaling Technology, Danvers, MA, USA). horseradish peroxidase glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was counted as a housekeeping gene to normalize the intensity of different samples. Band was measured with an enhanced chemiluminescence (ECL) reagent (Thermo Fisher Scientific, Waltham, MA, USA) and a analyzed with BandScan 5.0 software.
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
Total RNA was extracted from myocardial tissue with TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) and reverse-transcribed into complementary DNA (cDNA) with commercial cDNA synthesis kit (Thermo Fisher Scientific). qRT-PCR was conducted with the ABI Prism 7500 system using SYBR-Green/flourescein qPCR Master Mix kit (Thermo Fisher Scientific). Amplification conditions were: 50°C (2 min), 95°C (10 min), followed by 40 cycles of 95°C (30 sec), 60°C (30 sec). Primers used to amplify TRL4/TRIF signaling pathway relevant gene fragments are listed in Table I. Target gene mRNA expression was normalized by their ratios to the reference gene β-actin.
Table I.
Primers used for qRT-RCR.
| Gene | Sense | Antisense |
|---|---|---|
| β-actin | CACGATGGAGGGGCCGGACTCATC | TAAAGACCTCTATGCCAACACAGT |
| TRIF | TGAGGAGGGTTTCTGGTAGC | GGATGCCCAGAAGAACTTGT |
| TBK-1 | AGATGTGGTGGGCGGAATGA | CCGTGGCTGCGTGGTAGAAT |
| IRF3 | ATGGCTGACTTTGGCATCTT | GCTAATCGCAACACTTCTTTCC |
qRT-RCR, quantitative reverse transcription-polymerase chain reaction; TRIF, TIR domain containing adapter inducing interferon β; IRF3, interferon regulating factor 3.
Electrophoretic mobility shift assay (EMSA)
EMSA for the DNA-binding activity of IRF3 in cardiomyocytes was performed. IRF3 binding consensus oligonucleotides containing the ISREs in the rat IFN-β promoter (5′-GAAAACTGAAAGGGAGAACTGAAAGTGGG-3′) were annealed and end-labeled with biotin. Nucleoprotein extracted from myocardial cell of respective group was incubated for 20 min on ice with binding buffer before probe accretion. The mixture with addition of the probe was further incubated for 15 min at 16°C, using LightShift Chemiluminescent EMSA kit (viagene Biotech, Ningbo, China). Unlabeled probe (5′-GAACCTGACCAGGGAGCCTGACCAGTGGG-3′) was used for the competition assays. Protein with labeled or unlabeled probe mixture was separated by electrophoresis on a 5.5% polyacrylamide gel. Subsequently, gel was dried and exposed to X-ray film after electrophoresis.
Statistical analysis
Statistical analyses were performed using SPSS software (version 19.0). Data are expressed as mean ± SD. comparisons between two groups were performed using the Student's t-test, while multiple comparisons were conducted by one-way analysis of variance with a Bonferroni post hoc test. The value of P<0.05 was considered to be statistically significant.
Results
Successful delivery of RP105 into rat cardiac muscle tissue
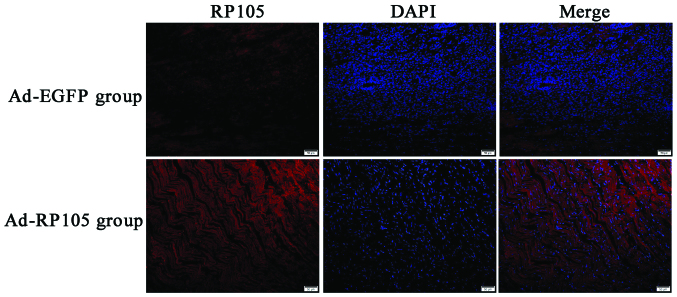
We injected the adenoviral vector Ad-EGFP-RP105 into three separate sites at the apex of the rat heart, exploring the anti-inflammatory mechanism of RP105 cardioprotection in MIRI model. RP105 transduction was obviously visible in Ad-EGFP-RP105 group, whereas only very low levels of endogenous RP105 were detected in Ad-EGFP group (Fig. 1). The DAPI-labeled nuclei density was higher in the latter group, which was considered to be the nuclei of inflammatory cells.
Figure 1.
Efficiency of radioprotective 105 kDa protein (RP105) transfection into rat cardiac muscle tissue. Either Ad-EGFP group (upper row) or Ad-RP105 group (down row) were subjected to myocardial ischemia for 30 min and reperfusion 2 h with injected Ad-EGFP or Ad-EGFP-RP105 three days earlier. Cardiomyocytes were observed by fluorescent microscopy for RP105 (red) and 4′,6′-diamidino-2-phenylindole (DAPI)-labeled nuclei (blue). Scale bar, 50 μm.
Upregulation of RP105 reduces serum myocardial enzyme activities during MIRI
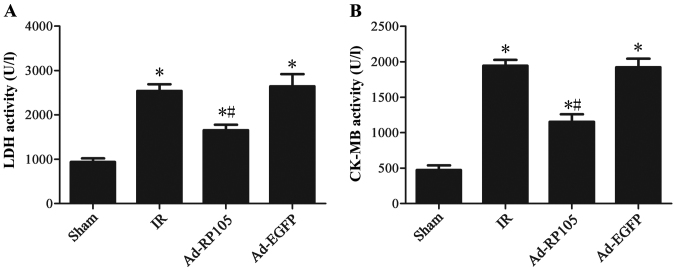
Fig. 2 illustrates that 30 min ischemia followed by 2 h reperfusion resulted in a significant increase in the activities of serum myocardial enzyme CK-MB and LDH compared to the sham group (sham group vs. IR group, P<0.05). After RP105 transduction into myocardium, the CK-MB and LDH level decreased significantly compared to IR group or Ad-EGFP group (Ad-RP105 group vs. IR group, P<0.05; Ad-RP105 group vs. Ad-EGFP group, P<0.05).
Figure 2.
Upregulation of radioprotective 105 kDa protein (RP105) reduces serum myocardial enzyme activities during myocardial ischemia reperfusion injury (MIRI). (A) The activity of CK-MB in blood serum. (B) The activity of lactate dehydrogenase (LDH) in blood serum t. values are mean ± SD. *P<0.05 as compared with sham group; #P<0.05 as compared with IR or Ad-EGFP group.
Upregulation of RP105 decreases myocardial infarction during MIRI
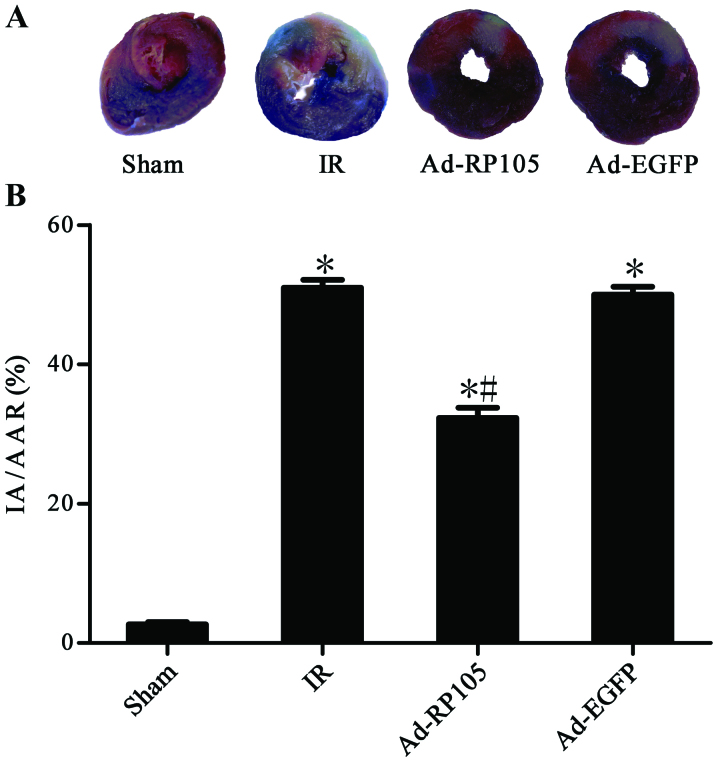
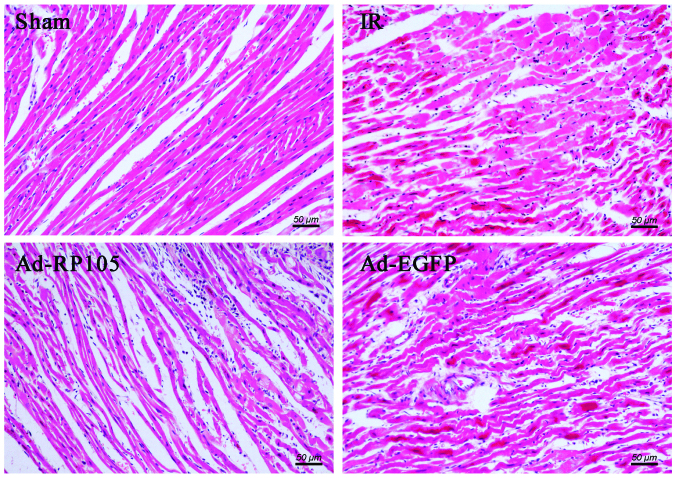
Evans blue/TTC double-staining showed obvious white infarct area after MIRI (Fig. 3A) and myocardial infarct size was 51.20±1.21% in IR group and 50.43±1.21% in Ad-EGFP group (Fig. 3B) (IR group vs. Ad-EGFP group, P>0.05). Interestingly, the myocardium transduced with Ad-EGFP-RP105 exerted cardioprotective effect and had less infarct tissue 32.71±1.51% (Ad-RP105 group vs. IR group, P<0.05; Ad-RP105 group vs. Ad-EGFP group, P<0.05). In light microscopy, we found that infarcted myocardium performed myocardial fiber deformation and disorganization, myocardial cell swelling and rupturing, and inflammatory cell infiltration was distinct in IR group and Ad-EGFP group (Fig. 4). Whereas, myocardial tissues from sham group were arranged regularly in clear striations without apomorphosis or necrosis. However, delivery of RP105 partially rescued myocardium with approximately normal structure, slight edema and a tiny amount of inflammatory cell infiltration.
Figure 3.
Upregulation of radioprotective 105 kDa protein (RP105) decreases myocardial infarct size during myocardial ischemia reperfusion injury (MIRI). (A) Evans blue/triphenyltetrazolium chloride (TTC) double-staining showed the myocardial infarction with blue (the viable non-ischemic area), red (AAR) and white (IA). (B) The infarct size is presented by the percentage of IA/AAR. values are means ± SD. As compared with sham group; #P<0.05 as compared with IR or Ad-EGFP group.
Figure 4.
Upregulation of radioprotective 105 kDa protein (RP105) decreases myocardial infarction during myocardial ischemia reperfusion injury (MIRI) under a light microscope. Myocardial tissues from sham group were well arranged in clear striations with normal cell morphology. In the IR group and Ad-EGFP group, infarcted myocardium showed myocardial fiber deformation and disorganization, myocardial cell swelling and rupturing, and inflammatory cell infiltration. The Ad-RP105 group displayed partial remission of myocardium with approximate normal structure, slight edema and a tiny amount of inflammatory cell infiltration.
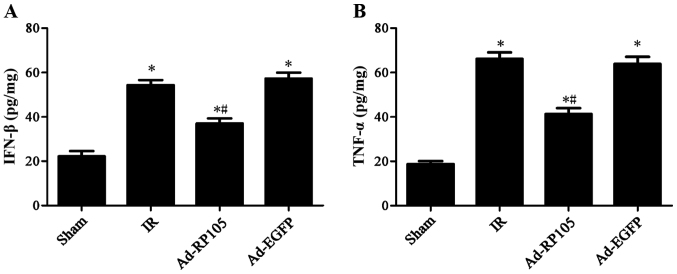
Upregulation of RP105 mitigates inflammatory factors IFN-β and TNF-α during MIRI
Rats subjected to MIRI exhibited a remarkable increase in inflammatory cytokine IFN-β by 54.37±1.30 and TNF-α by 66.16±2.91 in IR group, as compared to the sham group (Fig. 5) (sham group vs. IR group, P-value both <0.05). Notably, upregulation of RP105 in cardiac muscle cells mitigated IFN-β to 37.01±1.31 and TNF-α to 41.33±2.73 (Ad-RP105 group vs. IR group, both P<0.05). Adenoviral transfection of Ad-EGFP showed no significant difference on the two cytokines comparing to IR group (IR group vs. Ad-EGFP group, P>0.05).
Figure 5.
Upregulation of radioprotective 105 kDa protein (RP105) mitigates inflammatory factors IFN-β and TNF-α during myocardial ischemia reperfusion injury MIRI). (A) The level of IFN-β in myocardial tissue. (B) The level of TNF-α in myocardial tissue. values are mean ± SD. *P<0.05 as compared with sham group; #P<0.05 as compared with IR or Ad-EGFP group.
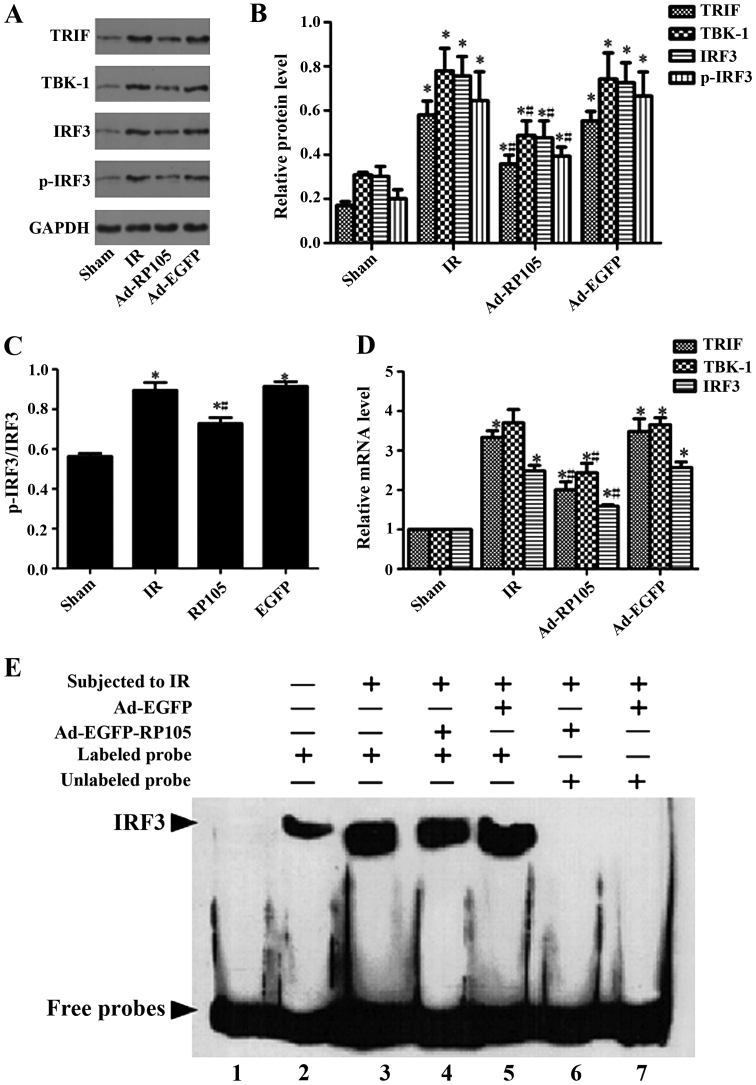
Upregulation of RP105 suppresses inflammation during MIRI via inhibiting TLR4/TRIF signaling pathway
We verified the relative protein and corresponding mRNA in TLR4/TRIF/ IRF3 signaling pathway, as well as IRF3 transcription activity, to explore the specific cardioprotection mechanism of RP105 during MIRI. Comparing with sham group, the expression of relative protein TRIF, TBK-1, IRF3, p-IRF3 and the ratio of p-IRF3 and IRF3 were obviously upregulated after 30 min ischemia and 2 h reperfusion (Fig. 6A–C) (sham group vs. IR group, P<0.05). Transduction of RP105 into myocardium markedly decreased above protein expression (Ad-RP105 vs. IR group, P<0.05). qRT-PCR confirmed the above analogical results in mRNA level. The mRNA levels of TRIF, TBK-1, IRF3 and p-IRF3 were notably higher in IR group than in the sham group (Fig. 6D, sham group vs. IR group, P<0.05). Similarly, upregulation of RP105 reduced the mRNA expression after delivering RP105 (Ad-RP105 vs. IR group, P<0.05). Ad-EGFP did not affect IR-induced increase in protein or mRNA level of TLR4/TRIF pathway (Ad-EGFP vs. IR group, P-value both >0.05). We implemented EMSA assay to assess the transcriptional activity of IRF3 in cardiomyocytes. Myocardial ischemia reperfusion markedly increased the DNA-binding and transcriptional activity of IRF3 (Fig. 6E) (sham group vs. IR group, P<0.05), yet transfection of RP105 could attenuate it to some degree (Ad-RP105 vs. IR group, P<0.05).
Figure 6.
Upregulation of radioprotective 105 kDa protein (RP105) suppresses inflammation during myocardial ischemia reperfusion injury (MIRI) via inhibiting Toll-like receptor 4 (TLR4)/TIR domain containing adaptor inducing interferon β (TRIF) signaling pathway. (A and B) Western blot assays for the expression of relative protein TRIF, TBK-1, interferon regulating factor 3 (IRF3) and p-IRF3 in cardiomyocytes. (C) The ratio of IRF3 and p-IRF3. (D) PCR for the mRNA levels of TRIF, TBK-1, IRF3 and p-IRF3 in cardiomyocytes. (E) Electrophoretic mobility shift assay (EMSA) for the DNA-binding activity of IRF3 in cardiomyocytes. Line 1 in EMSA assay only contained the labeled probe without protein. Line 2 to 7 incorporated labeled or unlabeled probes with respective group protein. values are mean ± SD. *P<0.05 as compared with sham group; #P<0.05 as compared with IR or Ad-EGFP group.
Discussion
Although myocardial ischemia and reperfusion occurred in an aseptic environment, cell debris and endogenous molecules like fibronectin-EDA and heat shock protein released from myocardium, the so-called danger-associated molecular patterns (DAMPs), would activate abacterial myocardial innate immune response and injure cardiac muscle tissue via TLRs signaling excitation (19). TLR4 plays a vital role in the regulation of immune response and the activation of inflammatory response during MIRI, as a result of pro-inflammatory cytokine induction and chemokine factor infiltration (11). In the present study, we demonstrated that RP105 exerted a dramatic inhibition of TLR4 inflammatory signaling pathway, developing potent protective effect against myocardial ischemia and reperfusion induced inflammation. RP105 overexpression with adenovirus vectors reduced serum myocardial enzyme (CK-MB and LDH) activities, decreased myocardial infarct size and mitigated inflammatory factors IFN-β and TNF-α during MIRI, and TLR4/TRIF signal pathway relative proteins and mRNAs (TRIF, TBK-1, IRF3 and p-IRF3) were repressed with a low transcriptional activity of IRF3. These findings first expounded that RP105 suppressed the ischemia reperfusion induced inflammatory status in the heart via inhibiting TLR4/ TRIF signaling pathway.
RP105 performes disparate regulatory roles in diverse diseases owing to different cell types. RP105 was initially discovered in the proliferation and radioactivation of murine B cells (20). The proliferation and activation, dependent upon TLR4 signaling, were distinctly reduced during the LPS- induced humoral immune response in the B cells derived from RP105 knockout mice (21). In other words, RP105 carried out a promotional function in B cell activated inflammatory reponse. Contrarily, the role of RP105 in myeloid cells (monocyte and macrophagocyte) was the opposite. RP105-/- monocytes increased the mean fluorescence intensity (MFI) of monocyte activated marker CD11b and promoted the production of pro-inflammation factors TNF-α and IL-6 stimulated by LPS, compared to WT mice (22). Pre-incubated with inhibitory anti-MD-1 antibody used to block the function of the RP105/ MD-1 complex in macrophages, there was increased secretion of pro-inflammatory cytokines TNF-α and IL-12p40 derived from TLR4/MyD88 pathway and dreased anti-inflammatory cytokine IL-10 (23). RP105 developed a physiological mediator in myeloid cell inflammatory response induced by TLR4 signal negatively. There is a hypothesis to explain this phenomenon. Dichotomous roles of RP105 on TLR4 signaling in B cells and myeloid cells are on account of disparate expression of TLR4 in these different cell types, leading to the diverse interactions with cell surface molecular partners (13). TLR4/MD-2 has a higher affinity for homodimerization with itself than for heterodimerization with RP105-MD-1 (13). High expression of TLR4/MD-2 [e.g., on macrophages (24)] induced lower affinity heterodimeric interactions to inhibit TLR4 multimerization and signaling, while low expression of TLR4/MD-2 [e.g., on B cells (21)] promoted heterodimeric interactions to facilitate further TLR4 recruitment and signaling. The fact that RP105 displays promotion role in B cell activation and inhibition role in myeloid cells activation, may decipher the RP105 controversial regulation in diverse conditions. The roles of RP105 in the heart are very few and the mechanism remains unclear and imperfect. The study of Louwe et al proved that deficiency of the RP105 caused a boost in the inflammatory status and a remarkable cardiac dilatation after induction of myocardial infarction by amplifying TLR4 signaling (25). Our data confirmed that RP105 contributed to cardioprotection and negatively regulated the TLR4/TRIF- induced inflammatory signal pathway.
TRIF was discovered as a TIR domain-containing adaptor protein of TLR3 and TLR4 for signal transmission, inducing type I IFNs strongly (26). Stimulation of TRIF-dependent signaling pathway resulted in the revitalization of the the transcriptional regulator IRF3, the late-phase activation of NF-κB, and the activation of mitogen-activated protein kinase (MAPK) (27,28). As TLR4 excitation, TRIF-related adaptor molecule (TRAM) mediated the interaction between TIR domain and TRIF to facilitate the conformation changes of TRIF and recruitment of the downstream TNF receptor-associated factor 3 (TRAF3) and TRAF6 (29,30). TRAF6 promoted the recruitment and ubiquitylation of receptor interacting protein 1 (RIP1) that compound with transforming growth factor-β-activated kinase 1 (TAK1) and MAPKs, leading to the nuclear translocation of NF-κB and AP-1, respectively. TRAF3 is the trigger of kinase TBK1 activation, as a result of IRF3 phosphorylation and translocation into the nucleus. The TLR4/TRIF/IRF3 signaling pathway induces type I interferon and IFN-inducible genes (31,32). IFN-β in turn is recognized by the surface receptor and triggers the activation and transduction of JAK/STAT1 signaling, inducing the expression of inducible genes, such as iNOS (33), IL-6 (34) TNF-α (35) and CXCL10 (33). Pro-inflammatory cytokines transcribed by TLR4/TRIF/IRF3 signal pathway damage various organisms. Several studies reported that inhibition of TRIF signaling cascade exerted a protective role during ischemia reperfusion injury in the liver, intestines and retina (36-41). Kang et al (36) and Kang and Lee (37) suggested that melatonin ameliorated the liver ischemia reperfusion damage via suppressing the type I IFN signaling pathway downstream of TLR4/TRIF/IRF3. Further studies indicated that ischemia reperfusion induced liver damage could be improved by inhibiting TLR4-triggered MyD88 and TRIF- dependent pathways (38,39). In the analysis of intestinal and retinal ischemia reperfusion injury, TRIF signaling contributed more significantly to disease development than MyD88 signaling, and TRIF-/- had an obviously reduced pro-inflammatory status after IR (40,41). This study demonstrated that TLR4/TRIF/IRF3 signaling played a crucial role in ischemia reperfusion-induced myocardial damage and inhibition of it could develop cardioprotection through RP105 recombinant adenovirus transfection.
RP105 delivery into myocardium via recombinant adenovirus vector to alleviate MIRI is a kind of gene therapy in our present study. The basic concept of gene therapy is to transfer functional genetic material with viral vectors or non-viral vectors into human organs, tissues, or cells to supply therapeutic effects in order to prevent or treat disease (42). Genetics has become a promising therapeutic approach to treat and heal cardiovascular diseases and able to recover the heart from dysfunctional to a functional state (43). There is a successful case. Intracoronary administration of an adenovirus vector encoding the human HGF gene (Ad-HGF) in patients with coronary heart disease, led to high expression of HGF and its receptor (c-met), augmented serum concentration of HGF, vEGF, MCP-1 and IL-10 and reduced serum concentration of IL-8, and increased the percentage of CD34+ and CD117+ cells in peripheral blood (44). However, gene therapy efficacy is mainly restricted by insufficient transfection efficiency of the target gene, negative immunoreaction to the treatment and weak of therapeutic effect over time (45). Thus, the key point of gene therapy is an appropriate targeting gene and an efficient and safe gene delivery vector. We have confirmed the cardioprotection of RP105 in MIRI, but its clinical application remains uncertain and needs further study.
In conclusion, the present study demonstrated that adenovirus transmission of RP105 into cardiocytes decreased serum myocardial enzyme activities and pro-inflammatory cytokines via inhibiting TLR4/TRIF signaling pathway to alleviate heart from ischemia reperfusion injury. Our findings provide a theoretical foundation of RP105 gene in MIRI.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (grant nos. 81170133, 81200088 and 81470387), the Hubei Province's Science Technology Support program (grant no. 2015BKA340) and the Hubei Province's OutstandingMedical Academic Leader program (grant no. 201304), China.
Footnotes
Competing interests
The authors declare that they have no competing interests.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Executive summary: Heart disease and stroke statistics - 2014 update: a report from the American Heart Association. Circulation. 2014;129:399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- 2.Fang Y, Hu J. Toll-like receptor and its roles in myocardial ischemic/reperfusion injury. Med Sci Monit. 2011;17:RA100–RA109. doi: 10.12659/MSM.881709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang M, Chen J, Zhao J, Meng M. Etanercept attenuates myocardial ischemia/reperfusion injury by decreasing inflammation and oxidative stress. PLoS One. 2014;9:e108024. doi: 10.1371/journal.pone.0108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma HJ, Li Q, Ma HJ, Guan Y, Shi M, Yang J, Li DP, Zhang Y. Chronic intermittent hypobaric hypoxia ameliorates ischemia/reperfusion-induced calcium overload in heart via Na/Ca2 exchanger in developing rats. Cell Physiol Biochem. 2014;34:313–324. doi: 10.1159/000363001. [DOI] [PubMed] [Google Scholar]
- 5.Ilczuk T, Wasiutynski A, Wilczek E, Gornicka B. The study of the protein complement in myocardial infarction. Immunol Lett. 2014;162:262–268. doi: 10.1016/j.imlet.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 6.Xu J, Qin X, Cai X, Yang L, Xing Y, Li J, Zhang L, Tang Y, Liu J, Zhang X, et al. Mitochondrial JNK activation triggers autophagy and apoptosis and aggravates myocardial injury following ischemia/reperfusion. Biochim Biophys Acta. 2015;1852:262–270. doi: 10.1016/j.bbadis.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Kim MY, Lim SH, Lee J. Intake of hot water-extracted apple protects against myocardial injury by inhibiting apoptosis in an ischemia/reperfusion rat model. Nutr Res. 2014;34:951–960. doi: 10.1016/j.nutres.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31–47. doi: 10.1016/S0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 9.Chao W. Toll-like receptor signaling: A critical modulator of cell survival and ischemic injury in the heart. Am J Physiol Heart Circ Physiol. 2009;296:H1–H12. doi: 10.1152/ajpheart.00995.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding HS, Yang J, Gong FL, Yang J, Ding JW, Li S, Jiang YR. High mobility group [corrected] box 1 mediates neutrophil recruitment in myocardial ischemia-reperfusion injury through toll like receptor 4-related pathway. Gene. 2012;509:149–153. doi: 10.1016/j.gene.2012.07.072. [DOI] [PubMed] [Google Scholar]
- 11.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 12.Divanovic S, Trompette A, Atabani SF, Madan R, Golenbock DT, visintin A, Finberg RW, Tarakhovsky A, Vogel SN, Belkaid Y, et al. Inhibition of TLR-4/MD-2 signaling by RP105/MD-1. J Endotoxin Res. 2005;11:363–368. doi: 10.1177/09680519050110061201. [DOI] [PubMed] [Google Scholar]
- 13.Divanovic S, Trompette A, Atabani SF, Madan R, Golenbock DT, visintin A, Finberg RW, Tarakhovsky A, Vogel SN, Belkaid Y, et al. Negative regulation of Toll-like receptor 4 signaling by the Toll-like receptor homolog RP105. Nat Immunol. 2005;6:571–578. doi: 10.1038/ni1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wezel A, van der velden D, Maassen JM, Lagraauw HM, de vries MR, Karper JC, Kuiper J, Bot I, Quax PH. RP105 deficiency attenuates early atherosclerosis via decreased monocyte influx in a CCR2 dependent manner. Atherosclerosis. 2015;238:132–139. doi: 10.1016/j.atherosclerosis.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 15.Ohto U, Miyake K, Shimizu T. Crystal structures of mouse and human RP105/MD-1 complexes reveal unique dimer organization of the toll-like receptor family. J Mol Biol. 2011;413:815–825. doi: 10.1016/j.jmb.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 16.Kimoto M, Nagasawa K, Miyake K. Role of TLR4/MD-2 and RP105/MD-1 in innate recognition of lipopolysaccharide. Scand J Infect Dis. 2003;35:568–572. doi: 10.1080/00365540310015700. [DOI] [PubMed] [Google Scholar]
- 17.Yang J1, Guo X, Yang J, Ding JW, Li S, Yang R, Fan ZX, Yang CJ. RP105 protects against apoptosis in ischemia/reperfusion-induced myocardial damage in rats by suppressing TLR4-mediated signaling pathways. Cell Physiol Biochem. 2015;36:2137–2148. doi: 10.1159/000430180. [DOI] [PubMed] [Google Scholar]
- 18.Yang J, Chen L, Yang J, Ding J, Li S, Wu H, Zhang J, Fan Z, Dong W, Li X. MicroRNA-22 targeting CBP protects against myocardial ischemia-reperfusion injury through anti-apoptosis in rats. Mol Biol Rep. 2014;41:555–561. doi: 10.1007/s11033-013-2891-x. [DOI] [PubMed] [Google Scholar]
- 19.vilahur G, Badimon L. Ischemia/reperfusion activates myocardial innate immune response: The key role of the toll-like receptor. Front Physiol. 2014;5:496. doi: 10.3389/fphys.2014.00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyake K, Yamashita Y, Hitoshi Y, Takatsu K, Kimoto M. Murine B cell proliferation and protection from apoptosis with an antibody against a 105-kD molecule: Unresponsiveness of X-linked immunodeficient B cells. J Exp Med. 1994;180:1217–1224. doi: 10.1084/jem.180.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogata H, Su I, Miyake K, Nagai Y, Akashi S, Mecklenbräuker I, Rajewsky K, Kimoto M, Tarakhovsky A. The toll-like receptor protein RP105 regulates lipopolysaccharide signaling in B cells. J Exp Med. 2000;192:23–29. doi: 10.1084/jem.192.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bastiaansen AJ, Karper JC, Wezel A, de Boer HC, Welten SM, de Jong RC, Peters EA, de vries MR, van Oeveren-Rietdijk AM, van Zonneveld AJ, et al. TLR4 accessory molecule RP105 (CD180) regulates monocyte-driven arteriogenesis in a murine hind limb ischemia model. PLoS One. 2014;9:e99882. doi: 10.1371/journal.pone.0099882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoebe K, Du X, Georgel P, Janssen E, Tabeta K, Kim SO, Goode J, Lin P, Mann N, Mudd S, et al. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature. 2003;424:743–748. doi: 10.1038/nature01889. [DOI] [PubMed] [Google Scholar]
- 24.Akashi S1, Shimazu R, Ogata H, Nagai Y, Takeda K, Kimoto M, Miyake K. Cutting edge: cell surface expression and lipopolysaccharide signaling via the toll-like receptor 4-MD-2 complex on mouse peritoneal macrophages. J Immunol. 2000;164:3471–3475. doi: 10.4049/jimmunol.164.7.3471. [DOI] [PubMed] [Google Scholar]
- 25.Louwe MC, Karper JC, de vries MR, Nossent AY, Bastiaansen AJ, van der Hoorn JW, Willems van Dijk K, Rensen PC, Steendijk P, Smit JW, et al. RP105 deficiency aggravates cardiac dysfunction after myocardial infarction in mice. Int J Cardiol. 2014;176:788–793. doi: 10.1016/j.ijcard.2014.07.086. [DOI] [PubMed] [Google Scholar]
- 26.Liu S, Cai X, Wu J, Cong Q, Chen X, Li T, Du F, Ren J, Wu YT, Grishin NV, et al. Phosphorylation of innate immune adaptor proteins MAvS, STING, and TRIF induces IRF3 activation. Science. 2015;347:aaa2630. doi: 10.1126/science.aaa2630. [DOI] [PubMed] [Google Scholar]
- 27.Covert MW, Leung TH, Gaston JE, Baltimore D. Achieving stability of lipopolysaccharide-induced NF-kappaB activation. Science. 2005;309:1854–1857. doi: 10.1126/science.1112304. [DOI] [PubMed] [Google Scholar]
- 28.Fitzgerald KA, McWhirter SM, Faia KL, Rowe DC, Latz E, Golenbock DT, Coyle AJ, Liao SM, Maniatis T. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. 2003;4:491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 29.Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol. 2008;9:361–368. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 31.Bowie AG, Haga IR. The role of Toll-like receptors in the host response to viruses. Mol Immunol. 2005;42:859–867. doi: 10.1016/j.molimm.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Perry AK, Chen G, Zheng D, Tang H, Cheng G. The host type I interferon response to viral and bacterial infections. Cell Res. 2005;15:407–422. doi: 10.1038/sj.cr.7290309. [DOI] [PubMed] [Google Scholar]
- 33.Toshchakov V, Jones BW, Perera PY, Thomas K, Cody MJ, Zhang S, Williams BR, Major J, Hamilton TA, Fenton MJ, et al. TLR4, but not TLR2, mediates IFN-beta-induced STAT1alpha/beta-dependent gene expression in macrophages. Nat Immunol. 2002;3:392–398. doi: 10.1038/ni774. [DOI] [PubMed] [Google Scholar]
- 34.Samavati L, Rastogi R, Du W, Hüttemann M, Fite A, Franchi L. STAT3 tyrosine phosphorylation is critical for interleukin 1 beta and interleukin-6 production in response to lipopolysaccharide and live bacteria. Mol Immunol. 2009;46:1867–1877. doi: 10.1016/j.molimm.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 35.Hui KP1, Lee SM, Cheung CY, Ng IH, Poon LL, Guan Y, Ip NY, Lau AS, Peiris JS. Induction of proinflammatory cytokines in primary human macrophages by influenza A virus (H5N1) is selectively regulated by IFN regulatory factor 3 and p38 MAPK. J Immunol. 2009;182:1088–1098. doi: 10.4049/jimmunol.182.2.1088. [DOI] [PubMed] [Google Scholar]
- 36.Kang JW, Koh EJ, Lee SM. Melatonin protects liver against ischemia and reperfusion injury through inhibition of toll-like receptor signaling pathway. J Pineal Res. 2011;50:403–411. doi: 10.1111/j.1600-079X.2011.00858.x. [DOI] [PubMed] [Google Scholar]
- 37.Kang JW, Lee SM. Melatonin inhibits type 1 interferon signaling of toll-like receptor 4 via heme oxygenase-1 induction in hepatic ischemia/reperfusion. J Pineal Res. 2012;53:67–76. doi: 10.1111/j.1600-079X.2012.00972.x. [DOI] [PubMed] [Google Scholar]
- 38.Huang HF, Zeng Z, Wang KH, Zhang HY, Wang S, Zhou WX, Wang ZB, Xu WG, Duan J. Heme oxygenase-1 protects rat liver against warm ischemia/reperfusion injury via TLR2/TLR4-triggered signaling pathways. World J Gastroenterol. 2015;21:2937–2948. doi: 10.3748/wjg.v21.i10.2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahmoud MF, Gamal S, El-Fayoumi HM. Limonin attenuates hepatocellular injury following liver ischemia and reperfusion in rats via toll-like receptor dependent pathway. Eur J Pharmacol. 2014;740:676–682. doi: 10.1016/j.ejphar.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 40.Dvoriantchikova G, Santos AR, Danek D, Dvoriantchikova X, Ivanov D. The TIR-domain-containing adapter inducing interferon-β-dependent signaling cascade plays a crucial role in ischemia-reperfusion-induced retinal injury, whereas the contribution of the myeloid differentiation primary response 88-dependent signaling cascade is not as pivotal. Eur J Neurosci. 2014;40:2502–2512. doi: 10.1111/ejn.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang J, He GZ, Wang YK, Zhu QK, Chen W, Guo T. TLR4-HMGB1-, MyD88- and TRIF-dependent signaling in mouse intestinal ischemia/reperfusion injury. World J Gastroenterol. 2015;21:8314–8325. doi: 10.3748/wjg.v21.i27.8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lyon AR, Sato M, Hajjar RJ, Samulski RJ, Harding SE. Gene therapy: Targeting the myocardium. Heart. 2008;94:89–99. doi: 10.1136/hrt.2007.116483. [DOI] [PubMed] [Google Scholar]
- 43.Mason D, Chen YZ, Krishnan HV, Sant S. Cardiac gene therapy: Recent advances and future directions. J Control Release. 2015;215:101–111. doi: 10.1016/j.jconrel.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 44.Yang ZJ, Xu SL, Chen B, Zhang SL, Zhang YL, Wei W, Ma DC, Wang LS, Zhu TB, Li CJ, et al. Hepatocyte growth factor plays a critical role in the regulation of cytokine production and induction of endothelial progenitor cell mobilization: A pilot gene therapy study in patients with coronary heart disease. Clin Exp Pharmacol Physiol. 2009;36:790–796. doi: 10.1111/j.1440-1681.2009.05151.x. [DOI] [PubMed] [Google Scholar]
- 45.Sasano T, Kikuchi K, McDonald AD, Lai S, Donahue JK. Targeted high-efficiency, homogeneous myocardial gene transfer. J Mol Cell Cardiol. 2007;42:954–961. doi: 10.1016/j.yjmcc.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]