Abstract
Background and Aims
Individuals with familial adenomatous polyposis (FAP) may undergo a total proctocolectomy with ileal pouch-anal anastomosis (IPAA) to surgically treat their disease. Inflammation of the ileal pouch, termed pouchitis, is uncommon in FAP patients but prevalent in patients who received IPAA for ulcerative colitis, a type of inflammatory bowel disease (IBD).
Methods and Results
We report on two FAP siblings, living in the same household, who underwent IPAA surgery within one week of each other. Their mother also had an IPAA for FAP. One sibling developed pouchitis while his brother and mother have remained pouchitis-free. We investigated the genetic and microbial factors that might explain the development of pouchitis in the one sibling. We surveyed DNA isolated from the two brothers and their parents for NOD2 IBD risk variants by Sanger sequencing. The composition of mucosa-associated bacteria was analyzed by 16S rRNA gene sequencing on terminal ileum and rectal tissue collected at the time of surgical resection from the two brothers. The sibling with pouchitis inherited the IBD-associated risk alleles for NOD2 (rs17221417 and rs2076756) from his healthy father. Both the mother and unaffected brother lacked these variants. Microbiome sequencing of the terminal ileum and rectum found reduced levels of potentially ‘beneficial’ bacteria (Faecalibacterium prausnitzii, Bacteroides, and Ruminococcaceae) in the sibling with pouchitis relative to his brother.
Conclusion
These findings suggest that the NOD2 signaling pathway may contribute to intrinsic bacterial dysbiosis which is pre-existing and which may then predispose individuals to pouchitis after IPAA surgery.
Keywords: 16S rRNA, single nucleotide polymorphism, pouchitis, microbiota
1. Introduction
Familial adenomatous polyposis (FAP) is an autosomal dominant disorder which leads to the formation of hundreds to thousands of polyps throughout the colon.1 Although initially benign, if left untreated, these individuals will develop colon cancer prior to the age of 50 years.1 FAP is caused by various germline mutations in the adenomatous polyposis coli (APC) gene, the majority of which lead to expression of a truncated protein.2 APC is a member of a cytoplasmic ‘destruction complex’ of proteins which suppresses Wnt/β-catenin signaling by targeting the β-catenin transcriptional co-activator for degradation.3 In cells harboring APC mutations, deregulated β-catenin translocates into the nucleus and associates with T-cell factor/lymphoid enhancer binding factor (TCF/LEF) transcription factors to aberrantly drive expression of Wnt/β-catenin target genes.4 Expression of these targets, including the MYC proto-oncogene, contributes to transformation by promoting cellular proliferation and growth.4
To prophylactically remove the risk of colorectal cancer, FAP patients undergo a surgical procedure involving total proctocolectomy with ileal pouch-anal anastomosis (IPAA). This procedure is also common for patients with ulcerative colitis (UC), a type of inflammatory bowel disease (IBD). Up to 50% of UC patients with an IPAA develop inflammation of the ileal pouch, known as pouchitis.5 Symptoms of pouchitis may include urgency, increased stool frequency, abdominal pain, and rectal bleeding. Like UC patients, FAP individuals with IPAA develop pouchitis, although much less frequently (0–14%).6 The underlying inflammatory disease associated with UC is potentially one mechanism to explain why pouchitis is more prevalent in UC patients rather than FAP patients.7 Recent studies suggest that bacterial dysbiosis may be contributory to pouchitis development in UC patients.8,9 Furthermore, a multicenter genetic association study implicated the nucleotide oligomerization domain 2 (NOD2) signaling pathway in pouchitis risk,10 providing further evidence that alterations in bacterial homeostasis may predispose IPAA patients to pouchitis.
2. Case Report
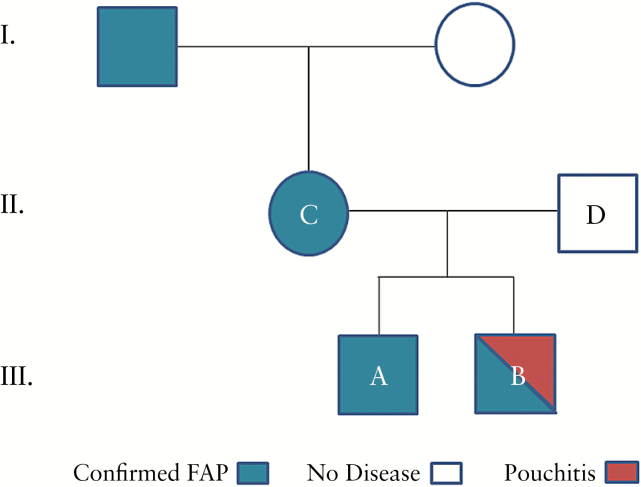
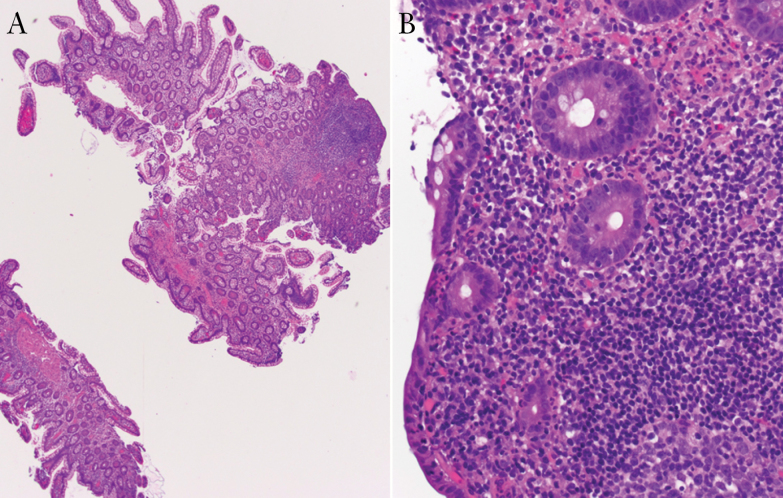
We report on an African-American family with multiple generations afflicted by FAP (Figure 1) confirmed by the presence of a single nucleotide polymorphism (SNP) in APC (R232X). Four immediate family members were recruited and are designated patients A, B, C, and D. Siblings A and B had a total proctocolectomy with IPAA scheduled within one week of each other. A J-pouch was constructed in both siblings of similar lengths (Table 1). Patient B had a one-stage surgery while patient A required a two-stage surgery, incorporating a temporary ileostomy. At the time of surgery, neither was on antibiotics. Pre- and post-operatively, both were non-smoking and lived in the same household. Patient B developed his first episode of pouchitis three months after surgery. Pouchitis was confirmed by symptoms of frequency, tenesmus, and proctoscopy and biopsy showing severely inflamed pouch mucosa (Figure 2). The mother (patient C) also had FAP and received IPAA surgery at an outside institution. To date, she has not developed pouchitis. The father (patient D) has no family history of IBD nor has been diagnosed with FAP or IBD. Since surgical and environmental factors were similar between the two siblings, we hypothesized that IBD-associated genetic variants may contribute to the differential pouchitis susceptibility seen in the two siblings.
Figure 1.
Pedigree denoting the family history of familial adenomatous polyposis. FAP is denoted in blue with pouchitis in red. Mother and father are designated C and D, respectively. Brothers are designated A and B, with patient B developing pouchitis.
Table 1.
Clinical indices and demographics.
| Patient A | Patient B | |
|---|---|---|
| Pouchitis | No | Yes |
| FAP genetic mutation | APC R232X | APC R232X |
| Age at surgery | 18 | 16 |
| Race | African-American | African-American |
| Sex | Male | Male |
| IPAA surgery year | 2009 | 2009 |
| Pouch type | J | J |
| Pouch length (cm) | 22 | 20 |
| IPAA surgery stages | 2 stage | 1 stage |
| Medication usage pre-op | None | None |
| Smoking status | Never smoked | Never smoked |
Figure 2.
Biopsy of the ileal pouch from patient B. (A) Under low power, the small intestinal type mucosa shows focal villous blunting and a lymphoid follicle (original magnification x 40). (B) High power view shows focally decreased mucin and neutrophilic infiltration in the lamina propria and epithelium (original magnification x 400).
The NOD2 signaling pathway was previously implicated in a predisposition to pouchitis.10 To determine the potential role of this pathway in the pedigree, we genotyped the parents and siblings for SNPs associated with the NOD2 locus. DNA from all four family members was Sanger sequenced for NOD2 SNPs (rs2066844, rs2066847, rs2076756, and rs17221417). We found that only rs17221417 and rs2076756 segregated by pouchitis diagnosis (Table 2). These two SNPs are in moderate linkage disequilibrium (D’ = 1.0, R2 = 0.592). Patient B, the sibling that developed pouchitis, was heterozygous for both SNPs. His brother, patient A, did not have either risk allele. Genotyping of the parents indicated that patient B inherited the NOD2 risk alleles from his healthy father, patient D. These data indicate that patient B had two variants found in the NOD2 signaling pathway that play a role in enteric host defense.
Table 2.
NOD2 genotypes.
| Sibling 1 (A) | Sibling 2 (B) | Mother (C) | Father (D) | |
|---|---|---|---|---|
| Pouchitis | No | Yes | No | N/A |
| rs17221417 | CC | CG | CC | CG |
| rs2076756 | AA | AG | AA | AG |
| rs2066844 | CC | CC | CC | CC |
| rs2066847 | DD | DD | DD | DD |
Crohn’s disease risk allele for each variant is italicized.
DD indicates that the frameshift C insertion was not present.
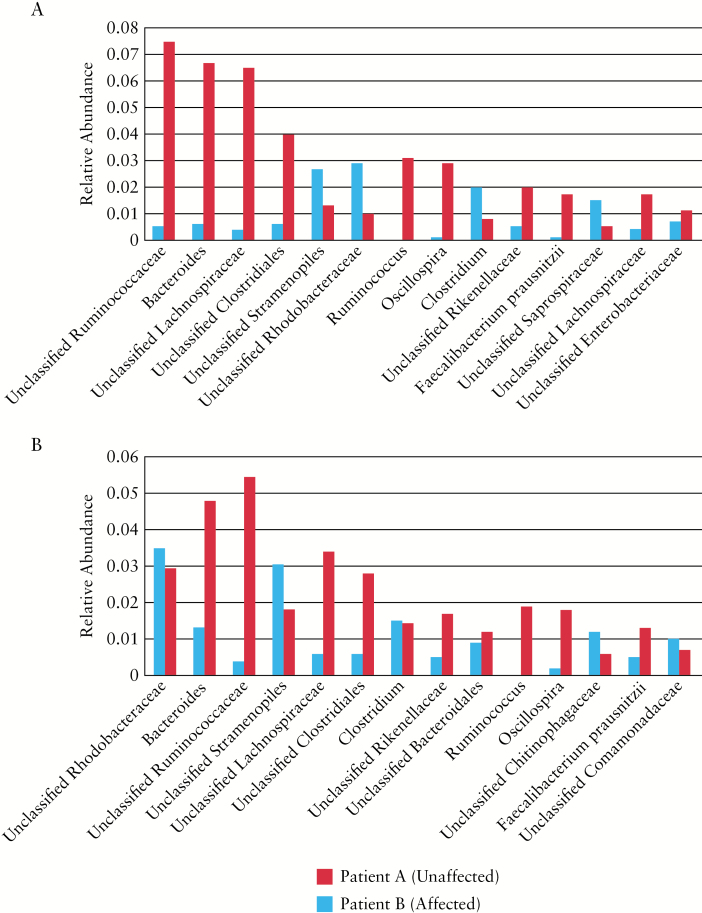
To discern whether bacterial dysbiosis could account for why one sibling developed pouchitis whereas the other did not, we performed 16S rRNA gene sequencing from the terminal ileum and rectum at the time of IPAA surgery. Full thickness tissue sections were obtained from a polyp-free area immediately after resection from both terminal ileum and rectum. DNA was extracted from the full thickness tissue using the DNeasy Blood and Tissue kit (Qiagen, Germantown, MD) and an aliquot of the DNA sent for 16S rRNA gene sequencing. Bioinformatic analysis was performed using QIIME 1.9.0.11 and open reference operational taxonomic units (OTUs) were assigned using the USEARCH7 algorithm and taxonomy assigned using Greengenes 16S rRNA gene database.12 Since the ileal pouch was constructed from the terminal ileum and connected to the rectum, we surveyed both tissue types at the time of surgery to gain a better understanding of how the intrinsic microbial composition differed between the two siblings. Moreover, analyzing these two tissues was imperative since the microbiome temporally shifts to a phenotype more similar to the colon as the ileal pouch matures.13 Within the rectum, we identified diminished levels of OTUs associated with Faecalibacterium prausnitzii, Bacteroides, Ruminococcaceae, and Lachnospiraceae in patient B, who developed pouchitis, compared to levels in patient A (Figure 3A). Additionally, patient B demonstrated enrichment of Rhodobacteraceae, Stramenopiles, and Clostridium taxa relative to levels in patient A. Similar patterns were seen in the terminal ileum of both individuals, with the most drastic differences described as a reduction in Ruminococcaceae, Lachnospiraceae, and Clostridiales taxa in patient B (Figure 3B). Together, these findings indicate that the bacterial dysbiosis, and specifically that associated with pouchitis, was present prior to the IPAA surgery.
Figure 3.
Bacterial dysbiosis is pre-existing at the time of IPAA surgery. 16S rRNA gene sequencing was performed on tissue samples to analyze the mucosa-associated bacteria at the time of IPAA surgery. Relative abundance was calculated and compared between siblings A (red) and B (blue). Patient B subsequently developed pouchitis while patient A did not. (A) Mucosa-associated bacteria from rectal tissue at the time of IPAA. (B) Mucosa-associated bacteria from terminal ileum tissue at the time of IPAA.
3. Discussion
Pouchitis is a relatively common medical condition in individuals who receive IPAA surgery for UC. However, pouchitis is rare in FAP patients who receive the same surgery.6 Based on this dichotomy, it is thought that the genetics and microbial dysbiosis associated with IBD may increase risk for pouchitis in the UC population.7 One difficulty with cohort studies is properly controlling for genetic variation and differences in environmental exposures. In this study, we reported on a family in which the mother and her two sons had FAP and underwent IPAA surgery. As environmental factors would be largely comparable in this family, we addressed whether genetic and microbial factors could potentially explain why pouchitis developed in only one of the two siblings.
Our analysis found that patient B inherited NOD2 intronic variants, rs17221417 and rs2076756, from his asymptomatic father. NOD2 was the first gene found to be associated with Crohn’s disease (CD).14,15 The NOD2 signaling pathway recognizes muramyl dipeptide (MDP) on the cell surface of intracellular bacteria to stimulate an innate immune response and clearance of pathogens.16 These SNPs were found to increase the risk for developing CD in two large European cohorts.17,18 The NOD2 1007fsCins variant (rs2066847) confers high risk for developing CD (OR: 3.99).19 Interestingly, this polymorphism is not commonly found in UC patients.20,21 However, rs2066847 was associated with chronic pouchitis in UC patients (OR: 3.21 [95% CI: 1.38–7.47]) in a large multicenter study,10 confirming two smaller studies.22,23 IPAA is contraindicated in CD patients so the association between NOD2 variants and pouchitis, which commonly occurs in UC patients, suggests that dysregulation of the NOD2 signaling pathway, and therefore defective bacterial recognition, may be involved in the pathogenesis of pouchitis.
Since both brothers were living in the same household at surgery and exposed to the same environments, the aforementioned genetic variants in the NOD2 signaling pathway may have been involved in intrinsic bacterial dysbiosis present prior to IPAA construction. This dysbiosis may have therefore predisposed patient B to subsequent development of pouchitis. F. prausnitzii is probably the most well-defined bacterial taxa associated with IBD.24,25 Microbiome analysis identified reduced levels of F. prausnitzii in the terminal ileum and rectum at the time of IPAA surgery in patient B, the sibling who subsequently developed pouchitis. These bacteria are commonly considered beneficial due to their production of the short chain fatty acid butyrate,26 which provides a fuel source to the ileal pouch enterocytes.27 These bacteria also demonstrate anti-inflammatory properties,28,29 partially through the secretion of metabolites that block the NF-κB pathway and IL-8 production.30 Lower levels F. prausnitzii OTUs in patient B correlates with previous findings in patients with acute/recurrent pouchitis whether operated on for UC or FAP compared to those with healthy UC and FAP pouches.8 Moreover, reduction of mucosa-associated F. prausnitzii was seen in CD patients homozygous for the NOD2 SNP13 variant,31 suggesting that genetics plays a role in the host microbial composition.
In summary, we analyzed genetic and microbial factors to evaluate the role of the NOD2 signaling pathway in pouchitis pathogenesis in one of two brothers with FAP who lived in the same household. Two genetic variants in the NOD2 gene (rs17221417 and rs2076756) were inherited by the sibling with pouchitis. Moreover, the sibling who subsequently developed pouchitis had a dysbiotic ileal and rectal microbiome that was pre-existing at the time of surgery, which included reduced levels of butyrate-producing bacteria, such as F. prausnitzii. These findings suggest that genetics may contribute to intrinsic bacterial dysbiosis which subsequently predisposes to the development of pouchitis.
Funding
This work was supported by the Carlino Fund for IBD Research and in part by the National Center for Advancing Translational Sciences (NCATS) [grant numbers UL1 TR000127, TL1 TR000125 to K.M.S.].
Conflict of Interest
None to disclose.
Author Contributions
Study design: KMS, GSY, WAK
Performed experiments: KMS, JRW, LRH, SD, ZY, RL
Data analysis and interpretation: KMS, JRW, ZY, RL, GSY, WAK
Drafting the manuscript: KMS, GSY, WAK
Critical revision of manuscript for intellectual content and approval of final version: all authors.
Acknowledgments
Thank you to the patients for their involvement in the study.
References
- 1. Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology 2010;138:2044–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hagen CE, Setia N, Lauwers GY. Familial adenomatous polyposis: A review of gastrointestinal manifestations. Diagn Histopathol 2015;21:152–60. [Google Scholar]
- 3. Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell 2012;149:1192–205. [DOI] [PubMed] [Google Scholar]
- 4. Fearon ER. Molecular genetics of colorectal cancer. Annu Rev Pathol 2011;6:479–507. [DOI] [PubMed] [Google Scholar]
- 5. Shen B, Lashner BA. Diagnosis and treatment of pouchitis. Gastroenterol Hepatol (N Y) 2008;4:355–61. [PMC free article] [PubMed] [Google Scholar]
- 6. McLaughlin SD, Clark SK, Tekkis PP, Nicholls RJ, Ciclitira PJ. The bacterial pathogenesis and treatment of pouchitis. Therap Adv Gastroenterol 2010;3:335–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schieffer K, Williams E, Yochum G, Koltun W. Review article: the pathogenesis of pouchitis. Aliment Pharmacol Ther 2016;44:817–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reshef L, Kovacs A, Ofer A, et al. Pouch inflammation is associated with a decrease in specific bacterial taxa. Gastroenterology 2015;149:718–27. [DOI] [PubMed] [Google Scholar]
- 9. McLaughlin SD, Walker AW, Churcher C, et al. The bacteriology of pouchitis: a molecular phylogenetic analysis using 16S rRNA gene cloning and sequencing. Ann Surg 2010;252:90–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tyler AD, Milgrom R, Stempak JM, et al. The NOD2insC polymorphism is associated with worse outcome following ileal pouch-anal anastomosis for ulcerative colitis. Gut 2013;62:1433–9. [DOI] [PubMed] [Google Scholar]
- 11. Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 2010;7:335–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. DeSantis TZ, Hugenholtz P, Larsen N, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 2006;72:5069–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Young VB, Raffals LH, Huse SM, et al. Multiphasic analysis of the temporal development of the distal gut microbiota in patients following ileal pouch anal anastomosis. Microbiome 2013;1:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hugot JP, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 2001;411:599–603. [DOI] [PubMed] [Google Scholar]
- 15. Ogura Y, Bonen DK, Inohara N, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 2001;411:603–6. [DOI] [PubMed] [Google Scholar]
- 16. Philpott DJ, Sorbara MT, Robertson SJ, Croitoru K, Girardin SE. NOD proteins: regulators of inflammation in health and disease. Nat Rev Immunol 2014;14:9–23. [DOI] [PubMed] [Google Scholar]
- 17. Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 2007;447:661–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Franke A, Hampe J, Rosenstiel P, et al. Systematic association mapping identifies NELL1 as a novel IBD disease gene. PLoS One 2007;2:e691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barrett JC, Hansoul S, Nicolae DL, et al. ; NIDDK IBD Genetics Consortium; Belgian-French IBD Consortium; Wellcome Trust Case Control Consortium. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat Genet 2008;40:955–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Waterman M, Xu W, Stempak JM, et al. Distinct and overlapping genetic loci in Crohn’s disease and ulcerative colitis: correlations with pathogenesis. Inflamm Bowel Dis 2011;17:1936–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Glas J, Seiderer J, Tillack C, et al. The NOD2 single nucleotide polymorphisms rs2066843 and rs2076756 are novel and common Crohn’s disease susceptibility gene variants. PLoS One 2010;5:e14466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Meier CB, Hegazi RA, Aisenberg J, et al. Innate immune receptor genetic polymorphisms in pouchitis: is CARD15 a susceptibility factor? Inflamm Bowel Dis 2005;11:965–71. [DOI] [PubMed] [Google Scholar]
- 23. Sehgal R, Berg A, Hegarty JP, et al. NOD2/CARD15 mutations correlate with severe pouchitis after ileal pouch-anal anastomosis. Dis Colon Rectum 2010;53:1487–94. [DOI] [PubMed] [Google Scholar]
- 24. Cao Y, Shen J, Ran ZH. Association between Faecalibacterium prausnitzii reduction and inflammatory bowel disease: a meta-analysis and systematic review of the literature. Gastroenterol Res Pract 2014;2014:872725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Halfvarson J, Brislawn CJ, Lamendella R, et al. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat Microbiol 2017;2:17004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett 2009;294:1–8. [DOI] [PubMed] [Google Scholar]
- 27. Chapman MA, Hutton M, Grahn MF, Williams NS. Metabolic adaptation of terminal ileal mucosa after construction of an ileoanal pouch. Br J Surg 1997;84:71–3. [PubMed] [Google Scholar]
- 28. Macfarlane GT, Macfarlane S. Fermentation in the human large intestine: its physiologic consequences and the potential contribution of prebiotics. J Clin Gastroenterol 2011;45:S120–7. [DOI] [PubMed] [Google Scholar]
- 29. Plöger S, Stumpff F, Penner GB, et al. Microbial butyrate and its role for barrier function in the gastrointestinal tract. Ann N Y Acad Sci 2012;1258:52–9. [DOI] [PubMed] [Google Scholar]
- 30. Sokol H, Pigneur B, Watterlot L, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A 2008;105:16731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rehman A, Sina C, Gavrilova O, et al. Nod2 is essential for temporal development of intestinal microbial communities. Gut 2011;60:1354–62. [DOI] [PubMed] [Google Scholar]