Abstract
Background and Aims:
NLRP3 inflammasome is known to be involved in inflammatory bowel diseases. However, it is controversial whether it is pathogenic or beneficial. This study evaluated the roles of NLRP3 inflammasome in the pathogenesis of inflammatory bowel disease in IL-10-/- mice and humans.
Methods:
NLRP3 inflammasome in colonic mucosa, macrophages, and colonic epithelial cells were analysed by western blotting. The NLRP3 inflammasome components were studied by sucrose density gradient fractionation, chemical cross-linking, and co-immunoprecipitation. The role of NLPR3 inflammasome in the pathogenesis of colitis was extensively evaluated in IL-10-/- mice, using a specific NLPR3 inflammasome inhibitor glyburide.
Results:
NLRP3 inflammasome was upregulated in colonic mucosa of both IL-10-/- mice and Crohn’s patients. NLRP3 inflammasome activity in IL-10-/- mice was elevated prior to colitis onset; it progressively increased as disease worsened and peaked as macroscopic disease emerged. NLRP3 inflammasome was found in both intestinal epithelial cells and colonic macrophages, as a large complex with a molecular weight of ≥ 360 kDa in size. In the absence of IL-10, NLRP3 inflammasome was spontaneously active and more robustly responsive when activated by LPS and nigericin. Glyburide markedly suppressed NLRP3 inflammasome expression/activation in IL-10-/- mice, leading to not only alleviation of ongoing colitis but also prevention/delay of disease onset. Glyburide also effectively inhibited the release of proinflammatory cytokines/chemokines by mucosal explants from Crohn’s patients.
Conclusions:
Abnormal activation of NLRP3 inflammasome plays a major pathogenic role in the development of chronic colitis in IL-10-/- mice and humans. Glyburide, an FDA-approved drug, may have great potential in the management of inflammatory bowel diseases.
Keywords: NLRP3 inflammasome, IL-10, inflammatory bowel diseases [IBD]
1. Introduction
Inflammatory bowel diseases [IBD], including ulcerative colitis [UC] and Crohn’s disease [CD], result from dysregulation of the mucosal immunity in the gastrointestinal tract.1 Murine models of IBD, with distinctive phenotypes that in many ways resemble human IBD, are invaluable tools for understanding the molecular and cellular mechanisms leading to IBD.2,3 Interleukin-10 deficient [IL-10-/-] mice, which spontaneously develop colitis under conventional housing or specific pathogen-free [SPF] conditions but not when germ free, are one of the most widely used murine models of IBD.4 The fact that patients with IL-10R mutations spontaneously developed Crohn’s disease at a very young age [< 1 year old],5 further underscores the importance of IL-10 signalling and the relevance of using the IL-10-/- mouse model of colitis in understanding the pathogenesis of IBD, particularly Crohn’s disease.
Nucleotide-binding domain leucine-rich repeat-containing receptors [NLRs] are a family of intracellular innate immune receptors with more than 22 and 33 members in humans and mice, respectively.6 Those receptors, believed to play essential roles in host-microbes and host-environment interactions as well as immune regulation,7 have attracted significant attention in the areas of intestinal mucosal immunity and inflammation.8,9 One of the best-characterised members of the NLRP [NOD-like receptor family, pyrin domain-containing] subfamily is NLRP3.7 NLRP3 has been shown to respond to a large spectrum of pathogens as well as immunogens or endogenous danger signals.10–13 Homotypic interactions between the pyrin domain of NLRP3 and the adaptor protein molecule ASC, an apoptosis-associated speck-like protein containing a caspase-recruitment domain, bridges the association of caspase-1 to NLRP3 in a large protein complex referred to as the NLRP3 ‘inflammasome’.14 Activated caspase-1 converts the cytosolic precursors of proinflammatory cytokine IL-1β into biologically active forms, which plays a vital role in the pathogenesis of several autoinflammatory diseases.15 However, previous studies aimed at characterising the NLRP3 inflammasome complex and its activation have been performed in vitro using cell culture models or cell-free systems.
Abnormal NLRP3 inflammasome activity has been incriminated in the pathogenesis of several autoinflammatory diseases.15 Although current data indicate a potential role of NLRP3 inflammasome in IBD pathogenesis, the molecular mechanism is still elusive. Data from human specimens and from murine colitis models indicate that excessive expression of IL-1β plays a key role in the pathogenesis of IBD.16 Only a few recent reports have examined the potential role of NLRP3 inflammasome in murine models of colitis, and the conclusions are inconsistent.17–20 Whereas the reasons for this inconsistency are unknown, with strong possibilities of either well-recognised variable effects of microbiota at different facilities where experiments were performed or variations of genetic backgrounds, these conflicting data emphasise the need for further investigation of the role of NLRP3 inflammasome in IBD. Moreover, there is a total lack of human-relevant data with regard to the function of NLRP3 inflammasome in human IBD.
In this study, using both IL-10-/- mice and mucosal explant culture of resected colon from IBD patients, we extensively examined the involvement of NLRP3 inflammasome in colonic inflammation. We demonstrated a time-dependent progressive increase in the expression and activity of NLRP3 inflammasome prior to colitis onset in IL-10-/- mice, and this increase peaked when disease phenotypes emerged. NLRP3 inflammasome was expressed and hyperactive in both macrophages and intestinal epithelial cells of IL-10-/- mice. Inhibition of specific NLRP3 inflammasome by glyburide resulted in potent alleviation of ongoing chronic colitis [therapeutic] and marked prevention/delay of the disease onset in IL-10-/- mice, as well as suppression of multiple proinflammatory cytokines/chemokines by mucosal explants of CD patients.
2. Materials and Methods
2.1. Animals
IL-10-/- mice and wild-type [WT] mice on the C57BL/6 background were housed in Helicobacter-positive specific pathogen-free [SPF] conditions at Johns Hopkins Animal Facility under controlled temperature [25°C] and photoperiods [12:12 h light-dark cycle]. Care and experimentation of mice were performed in accordance with institutional guidelines under protocols approved by the Institutional Animal Care and Use Committee.
2.2. Patients
Patients with clinically and histologically proven diagnosis of CD [n = 10; six for mucosal explant culture and four for western blot analysis] and UC [n = 4], as well as healthy controls [n = 4; CTRL: subjects who underwent colonoscopy or surgery because of colon cancer or abdominal problems but showed negative histological findings in the gut], were included in this study at the Meyerhoff IBD Center at Johns Hopkins Hospital. The patients’ demographic information is shown in Supplementary Table S1, available at ECCO-JCC online. The study using human tissues was approved by the Johns Hopkins Medicine Institutional Review Boards [JHM IRBs]. Written informed consent was received from all participants before inclusion in the study.
2.3. Antibodies
Antibodies [Abs] used were purchased from Santa Cruz Biotechnology [Santa Cruz, CA; ASC, NLRP3, TNF-α, IL-1β and IL-4], Cell Signaling [Danvers, MA; caspase-1 and NLRP1], Biolegend [San Diego, CA; IL-6 and IL-23p19], Biosource [Grand Island, NY; IFN-γ], eBioscience, [San Diego, CA; NOD2], Sigma [St Louis, MO; α-Actin], Invitrogen [Carlsbad, CA; Alexa Fluor 488 donkey anti-rabbit, Alexa Fluor 594 donkey anti-goat and Alexa Fluor 568 donkey anti-rat], and Rockland Immunochemicals [Gilbertsville, PA; secondary IRDye antibodies]. Antibody to vacuolar ATPase [V-ATPase] was described previously.21
2.4. Protein extraction from colonic mucosa of mice and human biopsies
Mucosa scraped from the isolated colon of mice at 4°C, or human colonic biopsies, were snap-frozen and stored at −80°C. Protein extraction was performed as we previously described.22
2.5. Evaluation of colitis
Macroscopic disease was defined as the presence of visible signs of any of the following: circumferential colon wall thickening and oedema [mucosal hyperplasia], loose/watery stool, rectal prolapse, or ulceration.23 Colon weights in grams without caecum, which were measured after faeces were flushed out with cold phosphate-buffered saline [PBS], were correlated with macroscopic disease. The sections of proximal and distal colon were stained with haematoxylin and eosin [H&E] according to standard protocols. Histological scoring [HS] was performed as described by Berg et al. (0 to 4 for each colon segment [proximal or distal] and 0 to 8 for a combined score [proximal + distal]).24
2.6. Isolation of peritoneal macrophages and colonic macrophages and epithelial cells
Peritoneal macrophages and colonic epithelial cells [CECs] were prepared as we previously described.22,25 Cells were plated at 2 million per well on six-well plates and stimulated with or without 100 ng/ml of ultra-pure lipopolysaccharide solution [LPS] [Sigma-Aldrich, St Louis, MO] for 18 h and then washed with Hank’s Balanced Salt Solution [HBSS] and treated with ATP [5 mM] in fetal bovine serum [FBS]-free Dulbecco Modified Eagle Medium [DMEM] medium for 4 h, followed by incubation with or without nigericin [20 μM] or z-VAD [20 mM] in FBS-free DMEM medium for 6 h. To test the effects of IL-10, cells in selected wells were incubated with IL-10 [100 ng/ml] 3 h before LPS treatment. Following treatment, culture media and cells were harvested. Colonic macrophages [C-MΦ] were obtained from the colon of 3-month-old IL-10-/- mice, using MACS® CD11b Microbeads [Miltenyi Biotec Inc., San Diego, CA].
2.7. Analysis of colonic NLRP3 inflammasome complexes by sucrose density gradient fractionation, cross-linking, and immunoprecipitation
Preparation and fractionation of sucrose density gradients [10–30% step gradient, 2.5% increment] were performed as we described previously,26,27 except that sucrose, instead of OptiPrep, was used. In cross-linking experiments, scraped colonic mucosa was incubated with 2 mM disuccinimidyl suberate [DSS] [Thermo Scientific, Waltham, MA] in PBS buffer for 15 min at 4°C. Cross-linking was quenched with Tris buffer [by adding 1 M Tris, pH7.8, to a final concentration of 20 mM] for 15 min at room temperature. Immunoprecipitation [IP] of inflammasome components was performed as we described previously.28
2.8. Sodium dodecyl sulfate polyacrylamide gel electrophoresis, western blot analysis, and immunohistochemistry
Protein extraction, western blot and immunohistochemistry were performed as we previously described.21,22,29
2.9. RNA isolation and real-time polymerase chain reaction analysis
Mouse colonic mucosa RNA was extracted with Trizol [Invitrogen, Carlsbad, CA], and reverse-transcribed to cDNA using a high capacity cDNA reverse transcription Kit [Applied Biosystem, Carlsbad, CA]. Polymerase chain reaction [PCR] amplification was carried out using 12.5µl DreamTaq™ Green PCR Master Mix [Fermentas, Glen Burnie, MD] [see primer sequences in Supplementary Table S2, available at ECCO-JCC online].
2.10. Glyburide treatment of IL-10-/- mice
For therapeutic effects, 6-month-old IL-10-/- mice were randomly divided into two groups: glyburide treatment group [n = 28] and control group [n = 20]. Mice were injected intraperitoneally with 400 μl glyburide in PBS [at three different doses: 0.5, 5.0, 50 mg/kg] once a day for 3 consecutive days and then every other day for a total of 2 weeks. Control mice received the formulation vehicle (dimethyl sulphoxide [DMSO]/10% 2-hydroxypropyl-β-cyclodextrin [2.5:97.5]) in 400 μl PBS. Mice were sacrificed on Day 15. For the disease-preventive study, 1-month-old IL-10-/- mice were randomly divided into two groups: glyburide [5 mg/kg] treatment group [n = 16] and control group [n = 30], as described in the therapeutic studies above. Mice were treated for 2.5 months and sacrificed when 3.5 months old. Colitis was evaluated as described above. Mucosal protein extraction and analyses were done as described above. NLRP3, ASC and IL-1β were analysed by western blotting and cytokines/chemokines by multiplex enzyme-linked immunosorbent assay [ELISA] [see Supplementary Methods, available at ECCO-JCC online].
2.11. Culture of colonic mucosa explants from patients with severe Crohn’s disease
Colonic mucosa was isolated from surgically resected colon from patients with severe Crohn’s disease [n = 6]. Explant culture was carried out as described by Baba et al.30 The culture supernatants were collected for cytokines/chemokines analysis by multiplex ELISA [see Supplementary Methods].
2.12. Profiling cytokines/chemokines by multiplex ELISA
The goal for the cytokine study is to determine specifically what immunological pathways have been affected when NLRP3 inflammasome is inhibited by glyburide. The rationale for selection of cytokines/chemokines measured and the lists of the cytokines/chemokines were described in Supplementary Methods.
2.13. Statistical analysis
The data were analysed using SPSS [version 14.0; SPSS, Inc., Cary, NC, USA]. Statistical analyses were done using a Student’s t test for two groups study, and expressed as mean ± standard error of the mean [SEM]. The data were evaluated with one-way analysis of variance [ANOVA] for three groups study and confirmed using Tukey’s test for multiple comparisons; p-values < 0.05 were considered statistically significant.
3. Results
3.1. Morphological and molecular characterisation of chronic colitis in IL-10-/- mice
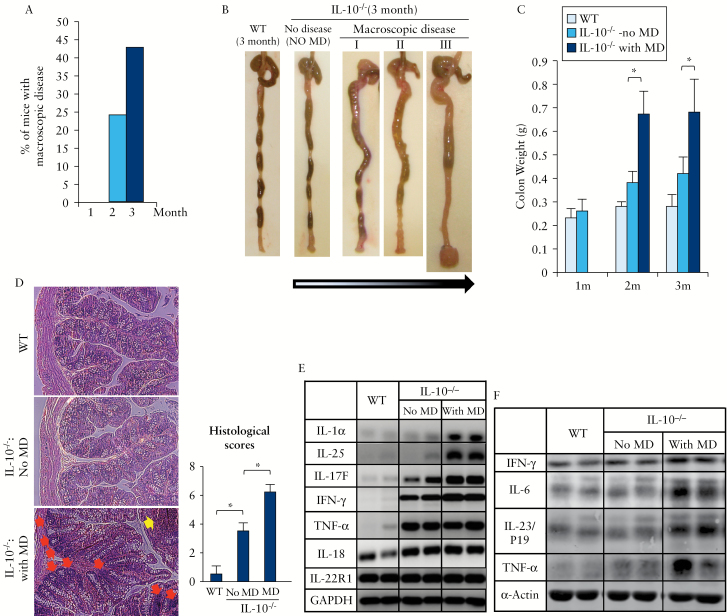
IL-10-/- mice housed at Johns Hopkins MRB Animal Facility exhibited no macroscopic disease at 1 month of age, whereas 24% of these mice had macroscopic colitis at 2 months and 43% by 3 months of age [Figure 1A]. Macroscopic colitis was characterised by colon enlargement and wall thickening, diarrhoea or poorly formed stool pellets, and/or rectal prolapse [Figure 1B]. Based on the presence of macroscopic disease, IL-10-/- mice of the same age were divided into two groups: without or with colitis. We found that fresh colon weight could be used as an accurate indicator of macroscopic disease in IL-10-/- mice [Figure 1C]. The degree of thickness or enlargement of the colon wall in the colitis group was highly correlated with the colon weight [compare Figure 1B with 1C]. Histological analysis supported our macroscopic classification: mice with macroscopic colitis exhibited marked microscopic disease, with shrinkage of goblet cells, loss of crypts, and excessive regenerative hyperplasia or leukocyte infiltration [Figure 1D].
Figure 1.
Characterisation of the age-dependent development of colitis in IL-10 deficient mice. IL-10-/- mice were divided into two phenotypic groups: with macroscopic disease/colitis [MD] and without macroscopic disease/colitis [No MD]. [A] Percentage of IL-10-/- mice with macroscopic disease/colitis at 1, 2, and 3months old, respectively [n > 20 mice per group]. [B] Progressive stages of colitis development in 3-months-old IL-10-/- mice, from no disease to severe colitis with rectal prolapse. [C] Colon weights. [D] Haematoxylin and eosin [H&E] staining [proximal colon] and histological scores in wild-type [WT] and IL-10-/- mice with or without macroscopic colitis [original magnification X 20]. [Leukocyte infiltration: yellow arrow; shrinkage of goblet cells: red arrows.] [E and F] Distinct profiles of proinflammatory cytokines at RNA [E] or protein [F] levels. The two lanes in each pair [each column] are duplicates from two individual mice. They are representative of at least four mice in each group. Glyceraldehyde-3-phosphate dehydrogenase [GAPDH] or actin was used as internal control; *p < 0.05.
Since IL-10-/- mice with or without macroscopic colitis showed a significant difference in histology, we postulated that these two groups could be classified as two progressive stages of chronic colitis. To test this possibility, we measured the expression of multiple cytokines at both RNA and protein levels. In the group without macroscopic colitis, although neither macroscopic nor histological abnormalities were present, the mRNA levels of major proinflammatory cytokines, including IL-18, tumour necrosis factor [TNF]-α, interferon gamma [IFN-γ] and IL-17F, were significantly elevated compared with those in WT mice [Figure 1E]. The disease groups exhibited not only much higher levels of IFN-γ and IL-17F [and IL-17A, data not shown] than those in ‘non-disease’ group, but mice with macroscopic disease also manifested a unique expression of IL-1α and IL-25. As a negative control, no change was found in the expression of IL-22 receptor-1 [IL-22R1] among the three groups. Furthermore, western blot [Figure 1F] showed elevations of three proinflammatory cytokines, IL6, IL-23/p19, and TNF-α, in the group with macroscopic disease compared with those in WT or IL-10-/- mice with no macroscopic disease. Although the mRNA level of TNF-α appeared similar between IL-10-/- mice with and without macroscopic disease [Figure 1E], the protein level of TNF-α was higher in the disease group [Figure 1F]. These histological and biochemical results supported our notion that IL-10-/- mice without macroscopic colitis could be considered as having already an early stage of colitis at the biochemical level.
3.2. NLRP3 inflammasome components in IL-10-/- mice were constitutively expressed at low levels, and upregulated/activated before onset of macroscopic or even microscopic disease
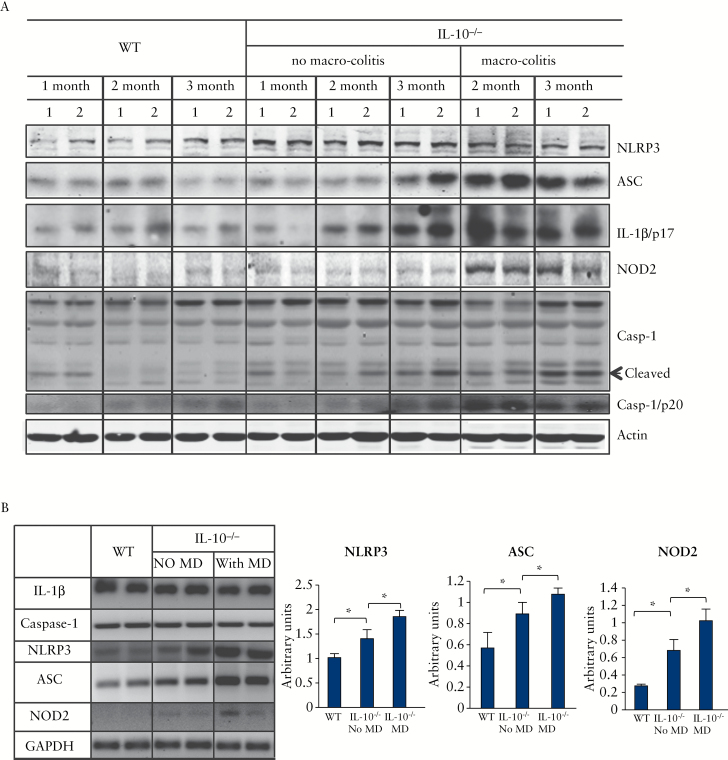
We first analysed the expression of all major components of the NLRP3 inflammasome, including NLRP3, ASC, caspase-1, and IL-1β, as well as the levels of the active forms of caspase-1 [p20] and IL-1β [p17]. The dynamic expression of these components in WT mice and IL-10-/- mice was compared at different developmental stages [1, 2, and 3 months old], with IL-10-/- mice being divided into two distinct groups: with or without macroscopic colitis. As shown in Figure 2A, all major components of the NLRP3 inflammasome were dramatically elevated in the IL-10-/- mice with macroscopic disease. More importantly, even in IL-10-/- mice without macroscopic disease, these components were found to increase gradually with age. The NLRP3 levels in IL-10-/- colon were elevated at a very early stage [1 month] when no microscopic disease had developed, increased continuously with time, and remained at a high plateau throughout the 3-month experimental period. In IL-10-/- mice with macroscopic disease, the levels of ASC, IL-1β-p17, and caspase-1-p20 peaked at 2s month old. Even in IL-10-/- mice without macroscopic disease, these proteins at 3 months old reached to a high level almost comparable to that in the IL-10-/- mice with macroscopic disease. Surprisingly, NOD2, a member of the intracellular NLRP family and one of the best characterised IBD-associated genes,31 was upregulated at a much later stage and remained high throughout the disease course.
Figure 2.
All NLRP3 inflammasome components were constitutively expressed, and upregulated and activated in IL-10-/- mice. [A] Western blot analyses of inflammasome proteins in colonic mucosa from wild type [WT] or IL-10-/- mice with or without macroscopic colitis [MD] at different ages [1, 2, and 3 months old]. Total lysate [60 μg] was analysed by sodium dodecyl sulphate polyacrylamide gel electrophoresis [SDS-PAGE] and western blot with antibodies indicated. The two lanes in pairs in each column [labelled as 1 and 2] are samples from two individual mice. Representatives of at least four mice in each group are shown. [B] Transcriptional regulation of NLRP3 inflammasome. The mRNA of each component was analysed by real-time polymerase chain reaction [RT-PCR] from colonic mucosa of 3-month-old mice. Note: The 1- and 2-month-old no-macroscopic-colitis IL-10-/- mice in this study exhibited neither macroscopic nor microscopic colitis. Casp-1/p20 and IL-1β/p17 are the active forms of caspase-1 and IL-1β. Representatives of data obtained from experiments with 3–5 mice are shown; *p < 0.05.
To determine if regulation occurs at the transcription level, we measured the mRNA expression of NLRP3 inflammasome by RT-PCR [Figure 2B]. The levels of NLRP3 and ASC expression, as well as NOD2, were significantly elevated in IL-10-/- mice with macroscopic disease, compared with the group of WT and IL-10-/- mice without macroscopic disease. These data indicate that NLRP3, ASC, and NOD2 are all upregulated in the colonic mucosa of IL-10-/- mice, both transcriptionally and translationally. In contrast, the mRNA expression of caspase-1 or IL-1β was not changed.
3.3. NLRP3, but not NLRP1 or NLPR6, was present in the same complex with ASC, and caspase-1 in vivo in colonic mucosa
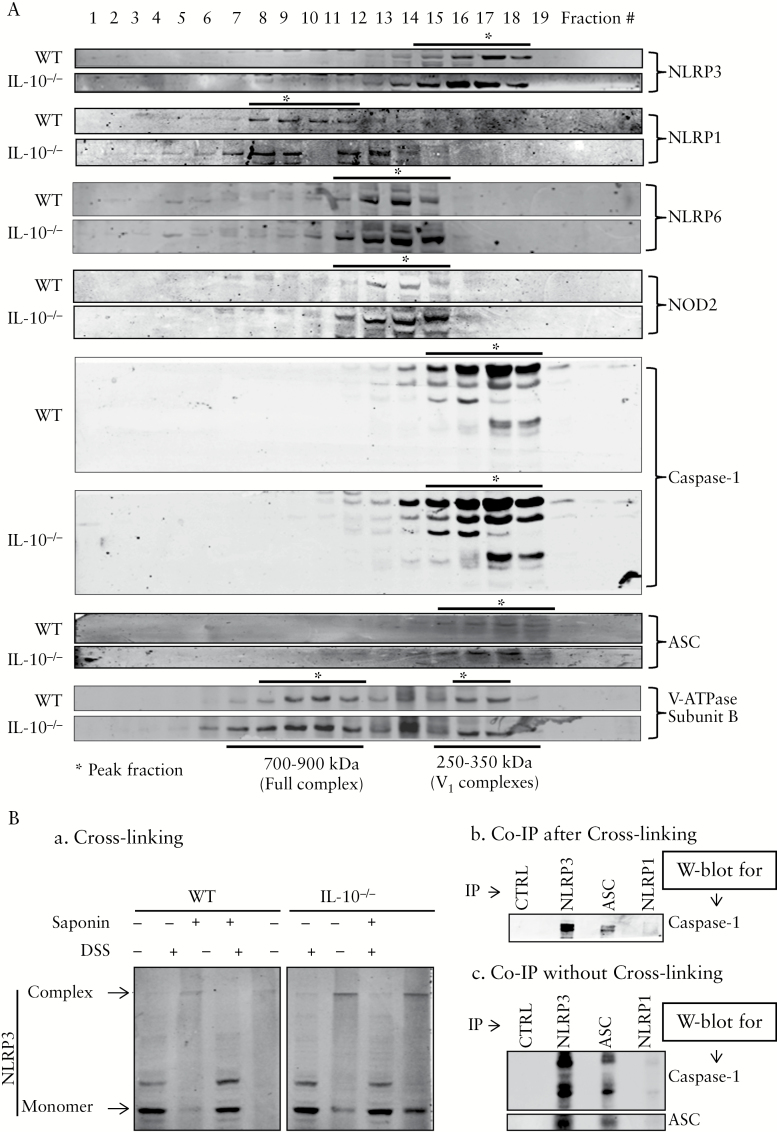
NLRP3 inflammasome complex was first analysed by sucrose density gradient fractionation of the Triton X-100-solubilised colonic mucosa extracts of WT and IL-10-/- mice. NLRP3 [but not NLRP1 or NLRP6], ASC, and caspase-1 were present in identical density fractions, suggesting that they are present in the same multiprotein complex, with a molecular weight of approximately 250–350 kDa [Figure 3A]. NLRP1 and NLRP6 were present in larger complexes [300–700 kDa] than NLRP3. The three NLRP proteins tested were present in different complexes [complex size: NLRP1 > NLRP6 > NLRP3]. NOD2, another member of NLRP family, appeared to be in the same complex with NLRP6. Surprisingly, we found that the NLRP3 complex of the same size was also assembled in WT mice [Figure 3A]. Although the expression of NLRP3 and ASC, and of caspase-1, were much higher in IL-10-/- mice than in WT mice, the size of the complex remained unchanged.
Figure 3.
Elevation of in vivo NLRP3/caspase-1/ASC complexes in IL-10-/- mice. [A] NLRP3, caspase-1, and ASC co-sedimented in the same density fractions, suggesting that they are in the same complex. Total proteins of colonic mucosa [3 mg] from wild-type [WT] or IL-10-/- mice with macroscopic diseases at 3 months old were fractioned into 20 fractions [equal volumes] using a sucrose density gradient. Proteins in each fraction were analysed by sodium dodecyl sulfate polyacrylamide gel electrophoresis [SDS-PAGE] and western blotting. V-ATPase, consisting of the full complex [600–900 kDa] and the V1 peripheral subcomplexes [250–350 kDa], was used as molecular weight markers along the density gradient. [B] In vivo NLRP3 ASC-caspase-1 complex was further confirmed by chemical cross-linking and co-immunoprecipitation [co-IP]. [a] Cross-linked NLRP3 complex by DSS exhibits large protein complexes > 250 kDa, as indicated by the shift of NLRP3 on SDS-PAGE. [b] Cross-linked NLRP3 complex could be immunoprecipitated as a single large complex using antibodies against NLRP3 and adaptor protein ASC. IP complexes were analysed by SDS-PAGE and western blot using anti-caspase-1 antibody. [c] co-IP of NLRP3 complex components without disuccinimidyl suberate [DSS]-cross-linking. Co-IP was performed and analysed as described in [B]. Control IgG [CTRL] and anti-NLRP1 antibody were used as negative controls for IP. Representatives of at least three independent experiments are shown.
We further performed chemical cross-linking and co-immunoprecipitation [co-IP] in order to provide direct evidence for the presence of multiprotein NLRP3 inflammasome in the colonic mucosa of IL-10-/- mice. As shown in Figure 3B-a, after cross-linking with DSS, NLRP3 mobility on SDS-PAGE was severely retarded [molecular weight shifted from 105 kDa to ~ 350 kDa], indicating that it forms a complex of similar size as observed by density gradient fractionation [Figure 3A]. Saponin, which was intended to increase the cross-linking efficiency by permeabilising the cell membranes, did not make any major difference. When the cross-linked mucosal extracts were subjected to co-IP with either anti-NLRP3 or anti-ASC, followed by SDS-PAGE and western blot for anti-caspase-1, both NLPR3 and ASC, but not NLRP1, co-precipitated caspase-1 [Figure 3B-b]. Similar results were observed when co-IP with samples that were not cross-linked [Figure 3B-c]. These data, together with those from density gradient fractionation [Figure 3A], strongly supported our conclusion that the NLRP3/ASC/caspase-1 complex was indeed formed in vivo, with a molecular mass of ~ 350 kDa.
3.4. Activation of NLRP3 inflammasome occurred in both epithelial cells and macrophages in the colon
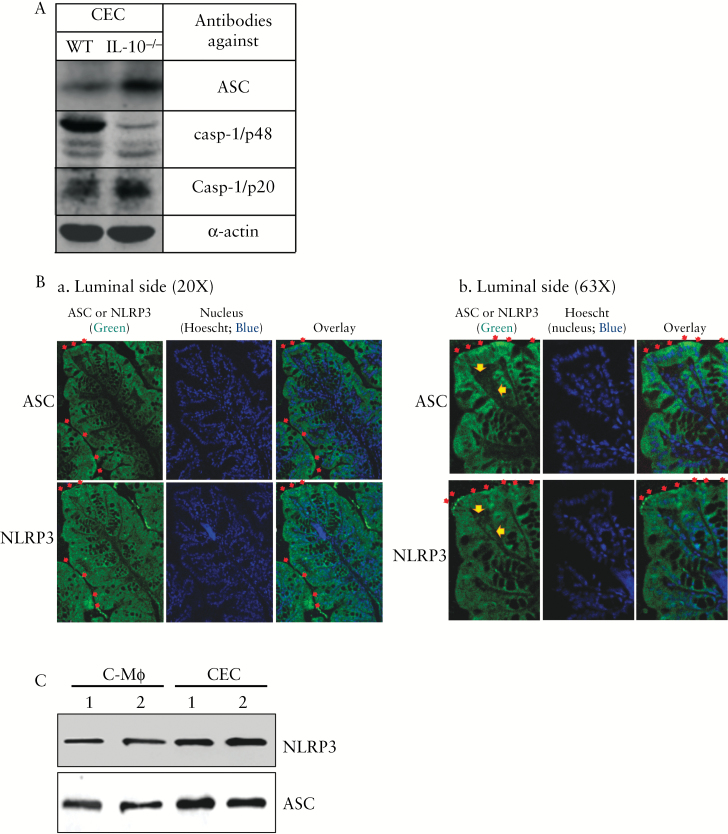
We purified CECs and examined the expression of ASC, a key component of the NLRP3 inflammasome, as well as the activation of caspase-1. As shown in Figure 4A, both ASC and activated caspase-1 [casp-1/p20] were markedly increased in the CECs of IL-10-/- mice compared with those of WT. The presence of the NLRP3 inflammasome in CECs was confirmed by immunofluorescent microscopy [Figure 4B]. Because the available reliable antibodies against NLRP3 and ASC were both generated from the same host [rabbit], immunofluorescent localisation was performed using consecutive sections of paraffin-embedded proximal colon from IL-10-/- mice. Although NLRP3 inflammasome is thought to be most abundantly expressed in macrophages,32–34 we found that the expression of NLRP3 and ASC was found more abundant in the CECs of IL-10-/- mice [Figure 4B] compared with that in lamina propria [Figure 4B-b, indicated by yellow arrows], where immune cells are present and have been reported to express NLRP3 inflammasome.35 The higher expression of NLRP3 and ASC in CECs than in colonic macrophages was further confirmed by western blot [Figure 4C]. Unlike NLRP3/ASC, NOD2 expression had a more dispersed distribution throughout the mucosa, with a marked co-localisation with nuclei [Supplementary Figure S1, available at ECCO-JCC online]. Nevertheless, in IL-10-/- mice, both NLRP3 and ASC expressions were elevated in the colonic macrophages when compared with those in WT mice [Supplementary Figure S2, available at ECCO-JCC online].
Figure 4.
Expression and activation of the NLRP3 inflammasome occur in both colonic macrophages and colonic epithelial cells. [A] Expression and activation of adaptor protein ASC in purified colonic epithelial cells [CECs]. CECs isolated from wild-type [WT] and IL-10-/- mice were analysed by sodium dodecyl sulfate polyacrylamide gel electrophoresis [SDS-PAGE] and western blotted for ASC, casapse-1 pro-enzyme form [casp-1/p48], and activated form of caspase-1 [casp-1/p20]. [B] Immunofluorescent staining for NLRP3 and ASC using consecutive sections of proximal colon from IL-10-/- mice [B-a, b]. [B-b] an enlarged area [top left quarter] of the image in panel [B-a]. Positive staining of NLRP3 and ASC is shown in green; nuclei are stained with Hoescht [blue]. ASC- and NLRP3-positive cells are both epithelial cells and cells in the lamina propria [indicated by yellow arrows; B-b]. Luminal surface expression of ASC or NLRP3 often appears punctate [red arrows]. [C] Comparison of NLRP3 and ASC expressions in colonic macrophages and CECs in IL-10-/- mice. Representatives of at least three independent experiments are shown.
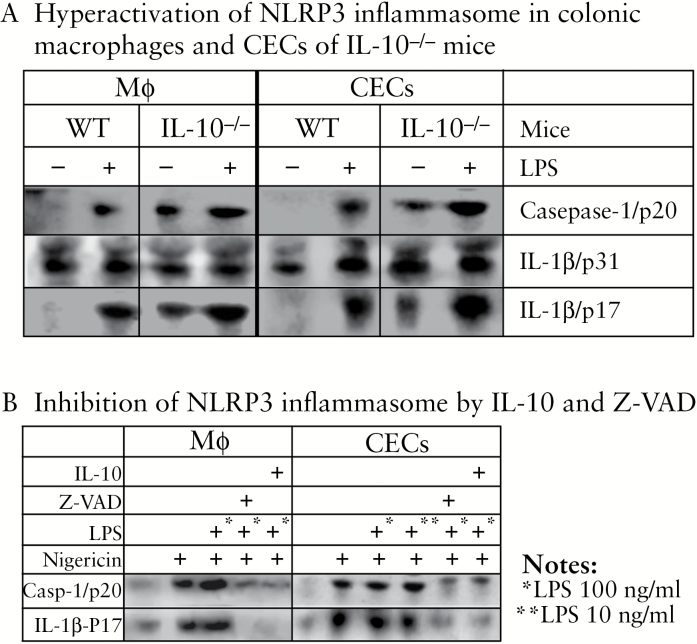
3.5. Activation of NLRP3 inflammasome in both macrophages and colonic epithelial cells was spontaneous and more robust in the absence of IL-10, and could be potently suppressed by IL-10
First, in both macrophages and CECs from IL-10-/- mice, the NLRP3 inflammasome activation was both more spontaneously active and more robust after 18 h of LPS stimulation than in WT mice [Figure 5A]. Second, NLRP3 inflammasome activation could be potently suppressed by IL-10 in both macrophages and CECs [Figure 5B]. NLRP3 inflammasome can be activated by a variety of agents, including the microbial toxins nigericin and adenosine triphosphate [ATP].12 We demonstrated that IL-10 effectively inhibited the LPS-induced production of TNF-α in macrophages [RAW 264.7] [Supplementary Figure S3, available at ECCO-JCC online], and that the morphological changes of macrophages caused by LPS/nigericin-induced activation of NLRP3 inflammasome were dramatically rescued by IL-10 treatment [Supplementary Figure S4, available at ECCO-JCC online]. In both macrophages and CECs isolated from IL-10-/- mice, LPS/nigericin-induced NLRP3 inflammasome activation could be potently dampened, with a similar efficiency, by the pan-caspase inhibitor z-VAD or IL-10 [Figure 5B].
Figure 5.
Activation of NLRP3 inflammasome was constitutive in macrophages and colonic epithelial cells [CECs] isolated from IL-10-/- mice, and its activation could be suppressed by either IL-10 or caspase inhibitor [Z-VAD]. [A] NLRP3 inflammasome was constitutively activated in the macrophages and CECs of IL-10-/- (but not in wild-type [WT]) mice, and more robust responsive to lipopolysaccharide [LPS] activation. Macrophages and CECs isolated from WT and IL-10-/- mice were incubated with or without stimulation with LPS [100 ng/ml] for 18 h. [B] Activation of the NLRP3 inflammasome in macrophages and CECs could be potently suppressed by IL-10 or inflammasome inhibitor Z-VAD. Macrophages from IL-10-/- mice were incubated with or without LPS [100 ng/ml, overnight pretreatment]. After medium change, cells were incubated with inflammasome activator nigericin [20 μM, 4 h] in the presence or absence of Z-VAD [20 μM, 6 h] or IL-10 [100 ng/ml, 3 h] before LPS treatment, as indicated. We demonstrated that both LPS + nigericin (as shown in [B]) and LPS alone (as shown in [A]) were sufficient to activate inflammasome, a result that is consistent with previous reports in which LPS alone was able to activate the inflammasome.43 Representatives of at least three independent experiments are shown.
It is also interesting to note that the NOD2 expression in IL-10-/- macrophages was hypersensitive to LPS [Supplementary Figure S5, available at ECCO-JCC online]. In macrophages isolated from IL-10-/- mice, 1 ng/ml LPS was sufficient to maximally induce NOD2 expression (No NOD2 additional induction at higher concentrations [10 or 100 ng/ml] of LPS), whereas in WT macrophages a similar level of NOD2 could be achieved only when LPS was increased to 100 ng/ml [Figure S5].
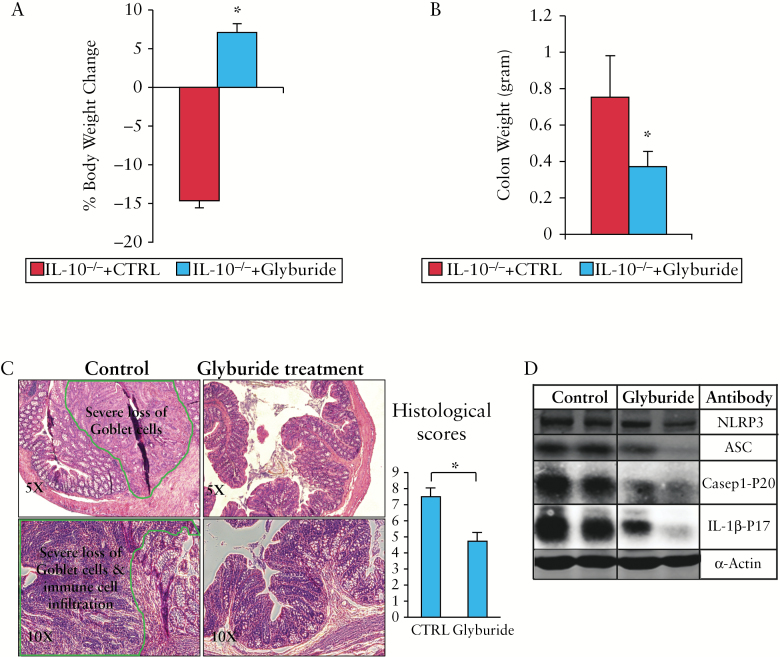
3.6. Glyburide administration effectively inhibited the activation of NLRP3 inflammasome and resulted in alleviation of chronic colitis
Glyburide, an inhibitor of ATP-sensitive potassium [KATP] channels,36 is widely used for the treatment of type 2 diabetes.37 It was recently demonstrated to be a selective and potent inhibitor of the NLRP3 inflammasome in macrophages as well as in a mouse model of LPS-induced endotoxaemia.38,39 In contrast to the control mice which exhibited > 10% body weight loss, glyburide-treated mice showed an average of 7% body weight increase [Figure 6A]. This increase in body weight was accompanied by a significant decrease in colon weight [Figure 6B] and a marked improvement of histological disease [including an increased number of goblet cells, a decreased number of infiltrating inflammatory cells, and dramatically reduced hyperplasia; Figure 6C]. At the molecular level, western blot analysis of colonic mucosa showed that glyburide treatment dramatically decreased the expression of ASC, NLRP3, and activated caspase-1 and IL-1β [Figure 6D]. It is important to note that lower doses of glyburide [0.5 or 5.0 mg/kg] exhibited a similar therapeutic effect [as 50 mg/kg] on the IL-10-/- colitis [Figure S6].
Figure 6.
Inhibition of NLRP3 inflammasome with glyburide resulted in alleviation of chronic colitis. IL-10-/- mice [6 months old] were treated intraperitoneally daily with 50 mg/kg glyburide for 14 days [glyburide group: n = 13; control group: n = 10]. [A] Body weight, [B] colon weight changes were analysed on Day 15. [C] Haematoxylin and eosin [H&E] staining and histological scores. [D] Glyburide dramatically decreased the expression of inflammasome proteins NLRP3 and adaptor protein ASC and the activation of IL-1β. Mucosal extracts from control and glyburide-treated groups were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis [SDS-PAGE] and analysed by western blot with different antibodies as indicated; *p < 0.05 [glyburide-treated vs controls].
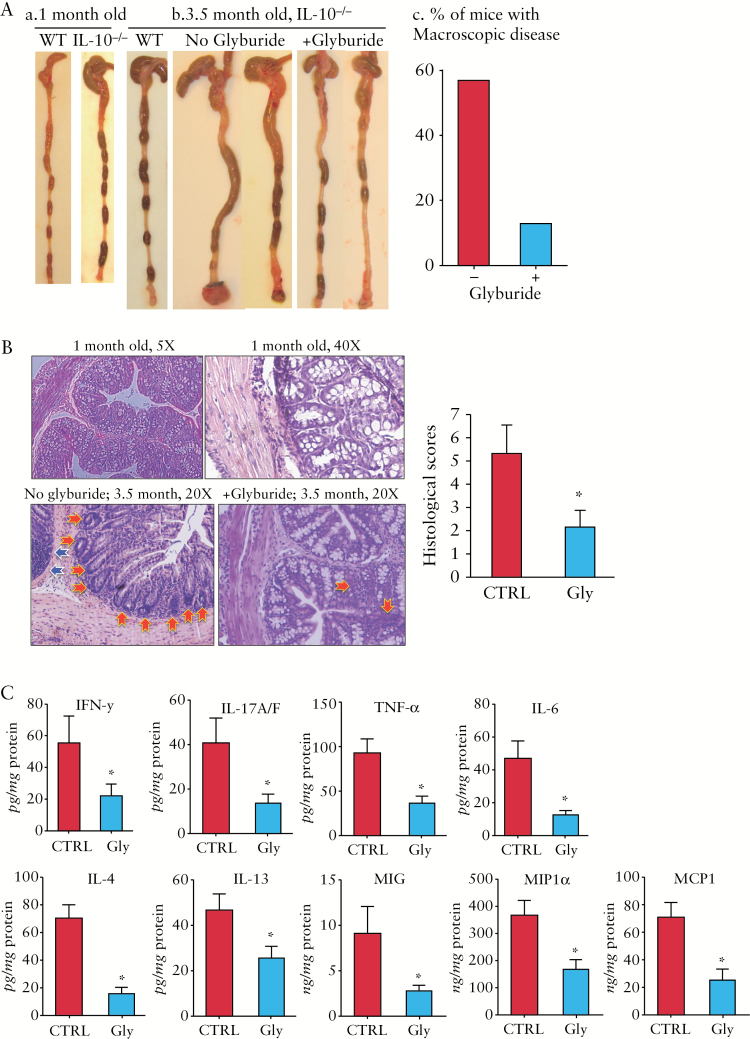
3.7. Glyburide treatment of IL-10-/- mice before disease onset prevented or delayed the spontaneous colitis
We further determined whether the spontaneous colitis could be prevented when giving glyburide to 1-month-old IL-10-/- mice that had not developed the macroscopic disease. Among the 16 1-month-old IL-10-/- mice treated with glyburide every other day for 2.5 months, only two developed mild macroscopic colitis [12.5%] [Figure 7A]. This is in sharp contrast to the age-matched [3.5-month] untreated mice [vehicle controls], 56.7% [17 of 30] of which developed macroscopic colitis. Whereas the colon of the control mice exhibited apparent inflammation [loss of goblet cells and infiltration of inflammatory cells], glyburide-treated IL-10-/- mice exhibited microscopically normal mucosa, with markedly improved histological scores [Figure 7B]. At molecular levels, glyburide-treated IL-10-/- mice exhibited markedly lower levels of multiple major proinflammatory cytokines/chemokines compared with those in vehicle controls, including IL-1β [Figure 6D; western blots], IFN-γ, IL-17A/F, TNF-α, IL-6, MIG, MIP1α, and MCP1 [Figure 7C; multiplex ELISA].
Figure 7.
Inhibition of NLRP3 inflammasome with glyburide before disease onset markedly suppressed multiple major proinflammatory cytokines/chemokines and effectively prevented or delayed spontaneous colitis. IL-10-/- mice [1 month old] were intraperitoneally administrated with glyburide every other day for 2.5 months [the final age of mice: 3.5 months]. [A] Colon and stool morphology of wild-type [WT] and IL-10-/- mice at 1 month [a] and 3.5 months old [b]; percentage of macroscopic colitis [c]. [B] Haematoxylin and eosin [H&E] staining and histological scores of colonic sections of IL-10-/- mice at 1 month old [top two panels], and 3.5 months old [bottom two panels] with or without glyburide treatment. Leukocyte infiltration [blue arrows]; loss of crypt and goblet cells [red arrows]. [C] Expression of multiple proinflammatory cytokines and chemokines, as measured by multiplex enzyme-linked immunosorbent assay [ELISA] [see Supplementary Methods, available at ECCO-JCC online]; *p < 0.05 [glyburide-treated vs controls].
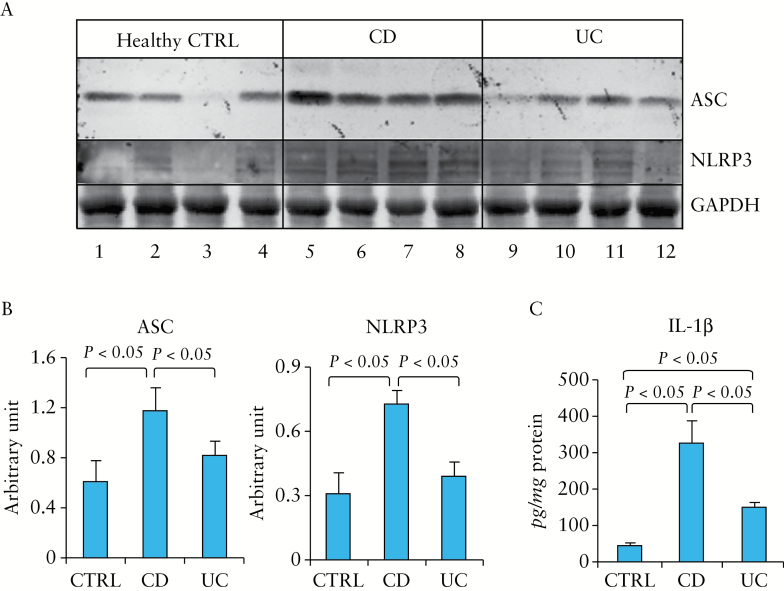
3.8. NLRP3, ASC, and IL-1β were elevated in the colonic mucosa of patients with active IBD
To test the relevance of our mouse findings to the human IBD, we first examined, by western blot, whether the expression of NLRP3 and ASC were also elevated in human IBD. As shown in Figure 8, both proteins were significantly elevated in the colonic mucosa of CD patients, compared with those of healthy controls, but did not reach statistical significance in UC vs healthy controls [Figure 8A, B]. A significant increase of IL-1β expression in the colon of Crohn’s patients was also observed [by ELISA] compared with that in healthy controls [Figure 8C]. UC patients also exhibited a significant increase in IL-1β expression compared with healthy controls [though to a lesser extent relative to Crohn’s vs healthy controls].
Figure 8.
NLRP3 inflammasome components were upregulated in the colonic mucosa of patients with active Crohn’s disease [CD] when compared with those of active ulcerative colitis [UC] and healthy controls [CTRL]. [A] Proteins from colonic mucosal biopsies [n = 4 per group] were analysed by sodium dodecyl sulfate polyacrylamide gel electrophoresis [SDS-PAGE] and western blot for adaptor protein ASC and NLRP3. Glyceraldehyde-3-phosphate dehydrogenase [GAPDH] was used as a loading control. [B] Statistical analysis of the data in [A]. [C] Levels of IL-1β in the colonic mucosa of subjects described above, analysed by enzyme-linked immunosorbent assay [ELISA].
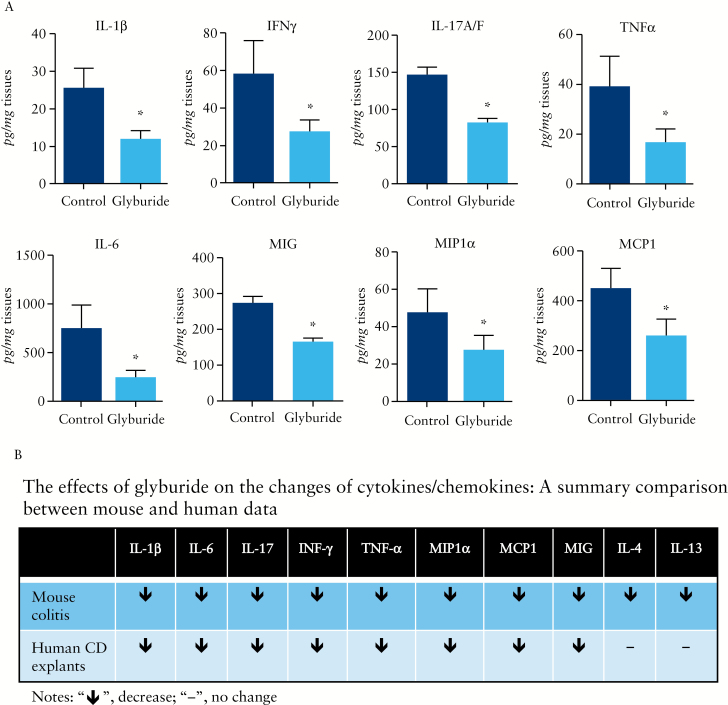
3.9. Glyburide inhibited the release of major proinflammatory cytokines and chemokines by colonic mucosal explants isolated from resected colon of Crohn’s patients
The mucosal explants from patients with severe Crohn’s disease actively secreted multiple proinflammatory cytokines/chemokines during 24-h culture, but glyburide treatment effectively suppressed their release [Figure 9A]. Among 10 cytokines/chemokines which were selected for analysis based on the mouse data [Figure 7C], eight exhibited a similar suppressive response to glyburide in mouse models vs human [as summarised in Figure 9B].
Figure 9.
Glyburide suppressed the release of multiple proinflammatory cytokines and chemokines by colonic mucosal explants from resected colon of Crohn’s patients. Colonic mucosal explants were cultured in the presence or absence [control] of 0.5 mM glyburide for 24 h. [A] Cytokines/chemokines released from explants to the culture medium were analysed by multiplex ELISA [see Supplementary Methods, available at ECCO-JCC online]; *p < 0.05 [glyburide-treated vs controls]. [B] A summary comparison of cytokines/chemokines profiles between human and mouse data.
4. Discussion
We describe here compelling evidence, both in vitro and in vivo, that the NLRP3 inflammasome plays a critically pathogenic role in IBD in both IL-10-/- mice and humans.
We found that both transcriptional and translational levels of NLRP3 and ASC were significantly increased in the colonic mucosa of IL-10-/- mice before the onset of microscopic or macroscopic colitis [Figure 2], compared with those of WT mice. Since the NLRP family of proteins are known to be ‘danger-sensing’ innate immune receptors,40 early elevation of NLRP3 expression would suggest that NLRP3 may act as a ‘first stress-responder’ of the cellular surveillance/defence system to sense and respond to microbial or ‘danger signals’ and transduce the signals downstream to activate the expression of other molecules such as ASC and caspase-1. Although ASC elevation occurs after NLRP3, ASC may function as a rate-limiting factor in the progress of colitis development. Interestingly however, unlike NLRP3 and ASC, the elevation of NOD2 expression in IL-10-/- mice occurs at a much later stage when mice exhibit macroscopic disease. These data suggest that involvement of NLPR3 in the pathogenesis of colitis may occur at the early stage of colitis.
Although Zhang et al. have demonstrated that IL-1β receptor antagonist or caspase-1 inhibitors ameliorated colitis,41 these data could not differentiate which exact NLRP inflammasome was involved, as both IL-1β and caspase-1 are common downstream targets of multiple NLRP inflammasome including NLRP1, NLRP3, and NLRP6, which are all present in the colonic mucosa of IL-10-/- mice [Figure 3]. We demonstrated that NLRP3 was co-sedimented with ASC and caspase-1 [equilibrated in the identical density fractions], demonstrating that it is the NLRP3 inflammasome complex [but not NLRP1 or NLRP6 complex] that is activated in the IL-10-/- mice compared with the WT mice. Chemical cross-linking and co-immunoprecipitation further confirm the assembly of the multi-protein NLRP3 complex in the colonic mucosa of IL-10-/- mice.
Previous in vitro experiments in cell culture models suggested that the NLRP3 inflammasome contains NLRP3, ASC, and caspase-1.42 However, so far there has been no direct evidence that this complex is indeed assembled in vivo. We found the NLRP3 inflammasome complexes from isolated peritoneal macrophages were 400–750 kDa [Figure S7], in the range of the reported sizes [estimated to be 500–700 kDa in size or even larger in the in vitro cell culture system overexpressing ASC32–34]. Interestingly, our colonic in vivo data showed a smaller size [250–300 kDa], which is likely to be the physiological size of the NLRP3 complex in vivo [at least in colonic mucosa]. It is likely that either the in vitro overexpression system gives rise to non-physiological artefacts, or this smaller size is unique to the intestinal mucosa. Assuming a ‘minimal unit’ of NLRP3 inflammasome contains one copy of each of the three major components [NLRP3 = 104 kDa, caspase-1 = 20–50 kDa, and ASC = 24 kDa], its total molecular weight would be approximately 178 kDa [104 + 50 + 24]. Thus, based on our calculated size of the complex by density gradient and DSS cross-linking, we propose that the NLRP3 inflammasome may function in vivo as a complex containing dimers of the ‘minimal units’, including two copies of each major component, with a total molecular weight of 356 kDa in size [178 kDa x 2].
The NLRP3 inflammasome proteins are constitutively expressed at low levels and assembled into multi-protein complexes under the normal physiological conditions of WT mice [Figure 3]. Although it was suggested that the large NLRP3 inflammasome forms only when activated by in vitro cell systems,10,12 we found that the size of the NLRP3 complex appeared to be similar to that at basal [normal] conditions [WT]. This would suggest that the NLRP3 inflammasome is functionally operative under normal conditions. Such observation is surprising, but not totally unexpected, given that previous studies have shown that mice deficient in NLRP3 or caspase-1 manifest greater intestinal injury and inflammation than those occurring in WT mice upon exposure to DSS.17 These findings suggest a protective role of the NLRP3 inflammasome in the development of DSS-induced colitis,17 although other studies indicate a pathogenic role of the NLRP3 inflammasome in both the DSS model19 and the IL-10-/- model of colitis.41 Thus, it is conceivable that ‘baseline’ activity of the NLRP3 inflammasome or its downstream targets [activation of caspase-1 and IL-1β] is necessary for the normal functions of the intestine. Disruption of such an activity, as in the case of gene knockouts, impairs the host’s ability to handle ‘adverse situations’ such as DSS insults or lack of IL-10, leading to pathological consequences. This may explain the results of the gene knockout experiments.
Innate immunity cells, including granulocytes, monocytes, and dendritic cells, express NLRP3 and ASC.35 In terms of epithelial cells, a previous study reported that the NLRP3 inflammasome shows restricted expression that is limited to non-keratinising epithelia such as are present in the oropharynx, oesophagus, and ectocervix.35 Our data demonstrate that NLRP3 inflammasome is expressed in both colonic macrophages and CECs [Figure 4], with a higher expression in CECs. This would indicate that both colonic macrophages and CECs are important in NLRP3 inflammasome-mediated chronic colitis. In the previous study, Zhang et al. reported that IL-10 inhibited NLRP3 inflammasome activation and IL-1β production in bone marrow-derived macrophages.41 We further confirmed that the activation of NLRP3 inflammasome was effectively abolished in peritoneal macrophages by IL-10 [Figure 5B]. A novel part of our study is that the NLRP3 inflammasome in both macrophages and CECs of IL-10-/- mice was spontaneously active and hyperactive when stimulated, compared with in those from WT mice [Figure 5A]. Such a phenomenon appears to be reminiscent of the outcome of gain-of-function NLRP3 mutations that results in the Muckle-Wells syndrome, in which the NLRP3 inflammasome is spontaneously activated in the patient’s monocytes.43
The NLRP3 activation of CECs was also potently inhibited by IL-10, supporting a notion that the IL-10 signalling pathway is involved directly or indirectly in the inflammasome activation of both macrophages and CECs. Therefore, it is conceivable that IL-10 acts as a suppressor of NLRP3 inflammasome at multiple levels in IBD, including both transcription/translation of major components [NLRP3 and ASC] and potential subsequent downstream caspase-1 activation/IL-1β processing. The overall downregulation of multiple proinflammatory cytokines in the gut of IL-10-/- mice may have at least in part resulted from IL-10-mediated suppression of IL-1β activation, which is thought to be able to potently amplify the pathogen recognition signals through MyD88-dependent pathogen-sensing Toll-like receptors [TLRs].44 Detailed molecular mechanisms of IL-10-induced inhibition of NLRP3 and suppression of gut inflammation remains to be determined, including a possibility of IL-1 signal-mediated suppression of Treg cells through enhancement of Th1.44
It is worth noting that NLRP3 inflammasome has been implicated in protecting mice against DSS-induced or Citrobacter-induced colitis, through regulating the functions of intestinal epithelia.17,45 Specifically, Zaki et al. reported that NLRP3 inflammasome protected against intestinal epithelial integrity in the DSS model of colitis.17 Song-Zhao et al. suggested that NLRP3 inflammasome protected against Citrobacter-induced colitis by limiting pathogen colonisation, independently of IL-1β or caspase-1.45
Glyburide is a specific inhibitor of NLRP3 inflammasome downstream of the P2X4 receptor.38,39 We demonstrated for the first time that this specific NLRP3 inflammasome inhibitor had both therapeutic [Figure 6] and preventive [Figure 7] effects on the colitis of IL-10-/- mice. The glyburide studies support our hypothesis that an over-reactive NLRP3 inflammasome is a key component of the pathogenic pathway in IBD. Moreover, the disease-preventive effects of glyburide, together with our data showing a very early increase in the colonic expression and activation of NLRP3 inflammasome before the onset of colitis, provide evidence that an abnormal activation of NLRP3 inflammasome may play a vital role in the early stage of disease development. These data also provide a preclinical proof-of-principle that the NLRP3 inflammasome is a potential therapeutic target in Crohn’s disease.
Considering significant expression of the NLRP3 inflammasome in CECs, as well as suggested effects of NLRP3 inflammasome on barrier function by previous reports using the mouse model with DSS-mediated insult,17,45 we determined whether the suppressive effect of glyburide on colitis is due at least in part to an improved barrier function of CECs. However, experiments with 5-day post-confluent Caco-2/BBE grown on transwell filters showed that glyburide did not significantly affect transepithelial electrical resistance [TEER] under either normal or inflammatory conditions [Figure S8, available at ECCO-JCC online].
Based on our findings, we propose a working model [Figure S9, available at ECCO-JCC online] to illustrate a sequential involvement of NLRP3 inflammasome in the development of colitis in IL-10-/- mice: if IL-10 is lacking, the NLRP3 inflammasome is spontaneously and persistently activated. Such activation initiates and progressively drives the colonic inflammation during the early phase of colitis; NOD2 may further exacerbate the inflammation at the later stage when macroscopic disease or clinical signs emerge. Therefore, in the development of colitis in IL-10-/- mice, we suppose that the NLRP3 inflammasome may play an important role in the early onset of disease, whereas NOD2 may act at the late stage of the disease.
Extending our finding to human disease, we demonstrated that ASC, NLRP3, and IL-1β were all upregulated in the colonic mucosa of patients with Crohn’s compared with that of healthy controls and UC patients [Figure 8]. Moreover, Crohn’s patients’ colonic mucosal explant culture revealed that inhibition of NLRP3 inflammasome by glyburide effectively suppressed the release of major proinflammatory cytokines/chemokines [Figure 9A]. The fact that eight of 10 cytokines/chemokines exhibited a similar suppressive response to glyburide in mouse models and human models [Figure 9B], suggested that data from the mouse model of colitis remarkably recapitulated human study. These data strongly support a pathogenic role of NLRP3 inflammasome in human IBD.
In summary, abnormal activation of the NLRP3 inflammasome appears to be a major pathogenic factor contributing to the development of chronic colitis in IL-10-/- mice. Elevated expression of NLRP3 and ASC in the colonic mucosa of Crohn’s patients suggests a similar role of the NLRP3 inflammasome in patients with Crohn’s disease. Our data not only support the possibility of using glyburide, a Food and Drug Administration [FDA]-approved drug, as a therapeutic agent in Crohn’s disease, but also provide preclinical evidence and a molecular basis for developing new IBD therapies targeting the NLRP3 inflammasome and its downstream signalling molecules.
Funding
The study was supported primarily by Broad Medical Research Program [IBD-0119R]] and the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases R21 [DK077064 and P30DK089502].
Conflict of Interest
None declared.
Author Contributions
LL initiated the project, performed most of the experiments, and wrote the manuscript; YD performed a significant part of experiments. MY and SJ contributed to some specific experiments. JY, MEJ, YS, and JZ helped in some of the experiments; ML, SRB, BS, and MM were involved in providing clinical samples from patients and healthy controls. XL was involved in project inception, design, and supervision, and manuscript writing and revision.
Supplementary Data
Supplementary data to this article can be found at ECCO-JCC online.
Supplementary Material
Acknowledgments
The authors would like to thank the Image Core of the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases-funded Johns Hopkins Digestive Diseases Basic and Translational Research Core Center. The authors would also like to thank Dr Alan Hofmann for his advice, comments, and editing this manuscript.
References
- 1. Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol 2010;28:573–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mizoguchi A. Animal models of inflammatory bowel disease. Prog Mol Biol Transl Sci 2012;105:263–320. [DOI] [PubMed] [Google Scholar]
- 3. Kiesler P, Fuss IJ, Strober W. Experimental models of inflammatory bowel diseases. Cell Mol Gastroenterol Hepatol 2015;1:154–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rennick DM, Fort MM. Lessons from genetically engineered animal models. XII. IL-10-deficient [IL-10[-/-] mice and intestinal inflammation. Am J Physiol Gastrointest Liver Physiol 2000;278:G829–33. [DOI] [PubMed] [Google Scholar]
- 5. Glocker EO, Kotlarz D, Boztug K, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med 2009;361:2033–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Zoete MR, Palm NW, Zhu S, Flavell RA. Inflammasomes. Cold Spring Harb Perspect Biol 2014;6:a016287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Franchi L, Muñoz-Planillo R, Núñez G. Sensing and reacting to microbes through the inflammasomes. Nat Immunol 2012;13:325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Corridoni D, Arseneau KO, Cifone MG, Cominelli F. The dual role of NOD-like receptors in mucosal innate immunity and chronic intestinal inflammation. Front Immunol 2014;5:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davis BK, Philipson C, Hontecillas R, Eden K, Bassaganya-Riera J, Allen IC. Emerging significance of NLRs in inflammatory bowel disease. Inflamm Bowel Dis 2014;20:2412–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dostert C, Pétrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 2008;320:674–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hornung V, Bauernfeind F, Halle A, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol 2008;9:847–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pétrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ 2007;14:1583–9. [DOI] [PubMed] [Google Scholar]
- 13. Duncan JA, Bergstralh DT, Wang Y, et al. Cryopyrin/NALP3 binds ATP/dATP, is an ATPase, and requires ATP binding to mediate inflammatory signaling. Proc Natl Acad Sci U S A 2007;104:8041–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 2002;10:417–26. [DOI] [PubMed] [Google Scholar]
- 15. LaRock CN, Todd J, LaRock DL, et al. Il-1b is an innate immune sensor of microbial proteolysis. Science Immunol 2016;1:eaah3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reinecker HC, Steffen M, Witthoeft T, et al. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn’s disease. Clin Exp Immunol 1993;94:174–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, Kanneganti TD. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity 2010;32:379–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Allen IC, TeKippe EM, Woodford RM, et al. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J Exp Med 2010;207:1045–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bauer C, Duewell P, Mayer C, et al. Colitis induced in mice with dextran sulfate sodium [DSS] is mediated by the NLRP3 inflammasome. Gut 2010;59:1192–9. [DOI] [PubMed] [Google Scholar]
- 20. Hu B, Elinav E, Huber S, et al. Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4. Proc Natl Acad Sci U S A 2010;107:21635–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li X, Su RT, Hsu HT, Sze H. The molecular chaperone calnexin associates with the vacuolar H[+]-ATPase from oat seedlings. Plant Cell 1998;10:119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alex P, Ye M, Zachos NC, et al. Clcn5 knockout mice exhibit novel immunomodulatory effects and are more susceptible to dextran sulfate sodium-induced colitis. J Immunol 2010;184:3988–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McCafferty DM, Sihota E, Muscara M, Wallace JL, Sharkey KA, Kubes P. Spontaneously developing chronic colitis in IL-10/iNOS double-deficient mice. Am J Physiol Gastrointest Liver Physiol 2000;279:G90–9. [DOI] [PubMed] [Google Scholar]
- 24. Berg DJ, Davidson N, Kühn R, et al. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4[+] TH1-like responses. J Clin Invest 1996;98:1010–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu L, Tan Q, Hu B, et al. Somatostatin inhibits the production of interferon-gamma by intestinal epithelial cells during intestinal ischemia-reperfusion in macaques. Dig Dis Sci 2014;59:2423–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li X, Donowitz M. Fractionation of subcellular membrane vesicles of epithelial and nonepithelial cells by OptiPrep density gradient ultracentrifugation. Methods Mol Biol 2008;440:97–110. [DOI] [PubMed] [Google Scholar]
- 27. Li X, Zhang H, Cheong A, et al. Carbachol regulation of rabbit ileal brush border Na+-H+ exchanger 3 [NHE3] occurs through changes in NHE3 trafficking and complex formation and is Src dependent. J Physiol 2004;556:791–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sarker R, Grønborg M, Cha B, et al. Casein kinase 2 binds to the C terminus of Na+/H+ exchanger 3 [NHE3] and stimulates NHE3 basal activity by phosphorylating a separate site in NHE3. Mol Biol Cell 2008;19:3859–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alex P, Zachos NC, Nguyen T, et al. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm Bowel Dis 2009;15:341–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Baba N, Van VQ, Wakahara K, et al. CD47 fusion protein targets CD172a+ cells in Crohn’s disease and dampens the production of IL-1β and TNF. J Exp Med 2013;210:1251–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature 2011;474:307–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hsu LC, Ali SR, McGillivray S, et al. A NOD2-NALP1 complex mediates caspase-1-dependent IL-1beta secretion in response to Bacillus anthracis infection and muramyl dipeptide. Proc Natl Acad Sci U S A 2008;105:7803–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fernandes-Alnemri T, Wu J, Yu JW, et al. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ 2007;14:1590–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Masumoto J, Taniguchi S, Ayukawa K, et al. ASC, a novel 22-kDa protein, aggregates during apoptosis of human promyelocytic leukemia HL-60 cells. J Biol Chem 1999;274:33835–8. [DOI] [PubMed] [Google Scholar]
- 35. Kummer JA, Broekhuizen R, Everett H, et al. Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J Histochem Cytochem 2007;55:443–52. [DOI] [PubMed] [Google Scholar]
- 36. Ashcroft FM. ATP-sensitive potassium channelopathies: focus on insulin secretion. J Clin Invest 2005;115:2047–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Riddle MC. Editorial: sulfonylureas differ in effects on ischemic preconditioning–is it time to retire glyburide? J Clin Endocrinol Metab 2003;88:528–30. [DOI] [PubMed] [Google Scholar]
- 38. Lamkanfi M, Mueller JL, Vitari AC, et al. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J Cell Biol 2009;187:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hirota SA, Ng J, Lueng A, et al. NLRP3 inflammasome plays a key role in the regulation of intestinal homeostasis. Inflamm Bowel Dis 2011;17:1359–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Horvath GL, Schrum JE, De Nardo CM, Latz E. Intracellular sensing of microbes and danger signals by the inflammasomes. Immunol Rev 2011;243:119–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang J, Fu S, Sun S, Li Z, Guo B. Inflammasome activation has an important role in the development of spontaneous colitis. Mucosal Immunol 2014;7:1139–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Manji GA, Wang L, Geddes BJ, et al. PYPAF1, a PYRIN-containing Apaf1-like protein that assembles with ASC and regulates activation of NF-kappa B. J Biol Chem 2002;277:11570–5. [DOI] [PubMed] [Google Scholar]
- 43. Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity 2004;20:319–25. [DOI] [PubMed] [Google Scholar]
- 44. Campbell DJ. MyD88 and IL-1: loosening T[reg] cells’ firm grip. Trends Immunol 2014;35:95–6. [DOI] [PubMed] [Google Scholar]
- 45. Song-Zhao GX, Srinivasan N, Pott J, Baban D, Frankel G, Maloy KJ. Nlrp3 activation in the intestinal epithelium protects against a mucosal pathogen. Mucosal Immunol 2014;7:763–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.