Abstract
Background and Aims
Randomised trials have described the benefits of adalimumab [ADA] for ulcerative colitis [UC]; however, few data are available on health-related quality of life [HRQL] and health care costs in clinical practice.
Methods
InspirADA, a multicentre, prospective study, evaluated the effect of ADA in patients with moderate to severe UC treated according to usual clinical practice. Outcomes assessed were: Simple Clinical Colitis Activity Index [SCCAI] response/remission rates; changes in HRQL; all-cause direct costs; and UC-related direct and indirect costs from baseline to Week 26.
Results
Data from 463 patients were analysed. At Week 26, 67% (95% confidence interval [CI]: 62%, 71%) of patients achieved response; 48% [95% CI: 44%, 53%] were in remission. For the overall population, significant [all p < 0.001] improvements from baseline to Week 26 were observed for the Short Inflammatory Bowel Disease Questionnaire [SIBDQ] (mean change ± standard deviation [SD]: 17.4 ± 14.5) and the European Quality of Life—5 Dimensions—5 Level [EQ-5D-5L] (index: 0.1 ± 0.2; visual analogue scale [VAS]: 19.5 ± 25.8). Parallel improvements were seen in work productivity [11% absolute decrease in absenteeism; 25% absolute decrease in impairment while working; and 27% absolute decrease in impairment of ability to perform daily activities, all p < 0.001]. Among study completers, cumulative all-cause medical costs and UC-related medical costs were significantly [both p < 0.001] reduced by 59% and 77%, respectively, 6 months after initiation of therapy compared with the preceding 6 months. The safety profile of ADA was consistent with that observed in previous clinical trials.
Conclusions
ADA therapy in usual clinical practice is effective at improving and maintaining symptomatic control, improving HRQL, and decreasing costs of medical care among patients with UC.
Keywords: Ulcerative colitis, patient outcomes, health care costs
1. Introduction
Ulcerative colitis [UC] is characterised by bloody diarrhoea, abdominal cramps, and faecal urgency and incontinence with periods of exacerbation and remission.1–4 Consequently, health-related quality of life [HRQL] is substantially lower among patients with UC than in the general population.5–8 The total economic burden of UC has been estimated at $8.1–14.9 billion annually in the USA and €12.5–29.1 billion in Europe.9 In the past, hospitalisations accounted for 41–55% of medical costs in UC, but more recently, the costs of biologic therapy [31%] now appear to exceed those of hospitalisation [23%].9,10 Indirect costs are estimated to account for a third of the total economic burden.9,10
Adalimumab [ADA] is approved in Europe, North America, Japan, Australia, and Latin America for the treatment of moderate-to-severely active UC in adults who have had an inadequate response to conventional therapy including corticosteroids and/or thiopurines, or who are intolerant to or have medical contraindications to these therapies. Randomised clinical trials [RCTs] have demonstrated that treatment with ADA results in higher rates of clinical remission and mucosal healing, and improvement in HRQL, than observed in patients who received placebo.11,12 ADA is also associated with a reduced risk of hospitalisation in patients with UC.13
However, these data were obtained from RCTs designed primarily to evaluate the efficacy and safety of ADA,11,12,14 and patients with inflammatory bowel disease entering into clinical trials are not necessarily representative of those in usual practice.15 Limited data are available regarding the effects of ADA on clinical outcomes,16–20 HRQL, treatment satisfaction, health care utilisation, and health care costs in usual clinical practice. The objectives of the present multinational study were to provide such data.
2. Materials and Methods
2.1. Study design and participants
This was a Phase 3b, prospective, multicentre, multinational, open-label, 26-week study [ClinicalTrials.gov Identifier: NCT01550965] to evaluate clinical outcomes, HRQL, treatment satisfaction, health care utilisation, and health care costs among UC patients treated with ADA following usual clinical practice. The ranked primary effectiveness endpoints of the InspirADA study were: [1] change from baseline in Short Inflammatory Bowel Disease Questionnaire [SIBDQ] at Week 26; and [2] change [6 months after initiating ADA vs 6 months before initiating ADA] in costs of UC-related medical care excluding ADA costs. Secondary outcomes included assessment of Simple Clinical Colitis Activity Index [SCCAI] response and remission, change in HRQL measures, change in health care use, and change in health care costs. Patients with UC were enrolled at 92 sites located in Austria [2], Belgium [7], Canada [9], Czech Republic [2], Denmark [2], France [5], Germany [5], Greece [3], Ireland [4], Israel [5], Italy [5], Poland [5], Portugal [4], Russia [8], Slovakia [4], Spain [9], Sweden [1], Switzerland [1], Turkey [3], and the UK [8]. The study was conducted between May 2012 and April 2015.
Patients [18 to 75 years old] with moderate to severely active UC (defined as a Physician’s Global Assessment [PGA] ≥2) and SIBDQ ≤ 45 at baseline, who had experienced rectal bleeding within 7 days of baseline, were enrolled in the study. Patients had to be receiving concurrent treatment defined by at least one of the following: stable oral corticosteroid [prednisolone ≥ 20 mg/day or equivalent] for at least 14 days prior to baseline, or stable oral corticosteroid dose [prednisolone < 20 mg/day or equivalent] for at least 21 days prior to baseline, and/or at least a consecutive 12-week course of azathioprine or mercaptopurine prior to baseline. Concurrent therapy was not required for patients previously treated with corticosteroid or immunosuppressant drugs who had failed to respond to or could not tolerate their treatment. Consistent with clinical practice, concomitant medications could be introduced, stopped or changed at any time at the investigator’s discretion. Patients or their physicians had to be able to provide complete medical care resource utilisation information for the previous 6 months. Patients who had previously used any tumour necrosis factor [TNF] antagonist were eligible unless they had been treated within the past 56 days or had been a primary non-responder. Patients who had previously experienced a treatment-limiting adverse reaction to a TNF antagonist before being able to demonstrate response were not considered primary non-responders and could participate in the study.
Participants received 160/80 mg ADA at Week 0/2 followed by 40 mg ADA every other week [eow] from Week 4 through Week 24. Patients who did not respond to ADA by Week 8 [defined as PGA ≥ 2 and not achieving a decrease in SCCAI ≥ 2 points compared with baseline] were to discontinue ADA. Patients who lost response or had a flare at or after Week 8 could escalate to 40 mg ADA weekly dosing.
2.2. Clinical outcomes
Clinical outcomes were assessed using the SCCAI, a validated measure of disease activity in UC.21–24 The SCCAI questionnaire was completed by the physician, based on an interview and examination of the patient. Variables assessed included bowel frequency [day and night], defaecation urgency, blood in stool, general well-being, and extraintestinal manifestations [EIMs]. Higher SCCAI scores [range of 0–19] indicate greater disease activity. Clinical response was defined as a SCCAI decrease of ≥ 2 points from baseline. Remission was defined as SCCAI ≤ 2. Absence of rectal bleeding, which has been shown to be associated with mucosal healing,25 was also assessed by evaluating the proportion of patients reporting ‘no blood in stool’ on the SCCAI. The presence of EIMs [arthritis, pyoderma gangrenosum, erythema nodosum, or uveitis] were collected in the SCCAI from baseline through Week 26. EIM resolution was assessed at Weeks 2, 8, and 26 and was defined as ‘no reported EIM’ at the respective visit. Durable resolution from Week 2 to 26 and from Week 8 to 26 was defined as no EIM at the respective and subsequent visits. Physicians made an overall assessment of the severity of a patient’s UC (Physician’s Global Assessment [PGA]) using the following ordinal scale: 0 = normal, 1 = mild disease, 2 = moderate disease, and 3 = severe disease.
2.3. Health-related quality of life and treatment satisfaction
HRQL, work productivity, and treatment satisfaction were assessed using validated questionnaires that were completed by patients. The SIBDQ is a disease-specific HRQL instrument that consists of 10 questions to measure the effect of inflammatory bowel disease on social, emotional, and physical well-being.26,27 Total scores range from 10 [worst health] to 70 [best health]. A 9-point change in the SIBDQ is considered a minimal clinically important difference [MCID].28
The European Quality of Life—5 Dimensions—5 Level [EQ-5D-5L] is a generic HRQL instrument that provides a standardised measure of health status, composed of the following five dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression.29,30 The total index score ranges from –0.594 to 1.000, where higher scores indicate better HRQL. The EQ-5D-5L visual analogue scale [VAS] ranges from 0–100, where 0 represents the worst imaginable health state and 100 the best imaginable health state. Lower index and VAS scores indicate worse HRQL.
For those employed, the Work Productivity and Activity Impairment [WPAI] questionnaire was used to assess time missed from work, impairment while working, overall work impairment and, for all patients, impairment of daily activities.31,32 WPAI outcomes were expressed as percentage of impairment/productivity loss [range 0–100% impairment] with higher values indicating greater impairment and less productivity. A decrease of 7% in WPAI outcome score for an individual patient is considered a MCID.33
Satisfaction with medication was assessed using the Treatment Satisfaction Questionnaire for Medication [TSQM].34 TSQM has 14 items across four domains: effectiveness, side effects, convenience, and global satisfaction. The TSQM items are answered on 2-, 5-, or 7-point Likert-type scales. The score for each domain is the sum of the items corresponding to each domain, which is then transformed into a 100-point scale. Higher scores indicate greater satisfaction with medication, greater perceived effectiveness, greater convenience, and lower burden associated with side effects.
2.4. Resource utilisation
Health care resource utilisation was assessed by the study investigators and recorded on case report forms. Study investigators asked patients about their medications and previous/recent visits to other physicians. Any therapies that patients received and/or discontinued within the 6 months before baseline were recorded, along with whether or not the therapies were UC-related. All-cause and UC-related health care resource utilisation data during the 6-month period before baseline were collected retrospectively at baseline. During the 6-month period after baseline, data were collected prospectively at each follow-up visit. Health care resource utilisation assessment included the number of hospitalisations, days in hospital, and outpatient visits/procedures, such as unscheduled physician consultations and emergency department visits, as well as surgical and diagnostic procedures including radiology and endoscopy appointments. Investigators recorded whether hospitalisations or outpatient visits/procedures were UC-related on case report forms. Any missing resource use data were assumed to be zero. Any therapies that patients received and/or discontinued within the 6 months preceding baseline were recorded, along with whether the therapies were UC-related or not.
2.5. Safety
Treatment-emergent adverse events were documented to assess safety during the study. A structured questionnaire was designed and used to assess adverse events in this study. The items in the questionnaire included diagnosis or symptoms of the adverse event, onset date and duration of adverse event, severity of the adverse event [hospitalization, medical intervention, disability, death, congenital anomaly, miscarriage, spontaneous/elective abortion], investigator opinion of the relationship of the adverse event to adalimumab, action taken for adverse event, and other cause of adverse event. If it was a serious adverse event, a set of questionnaires was used to collect more detailed clinical information about the adverse event, laboratory data, and medication use.
2.6. Data analyses
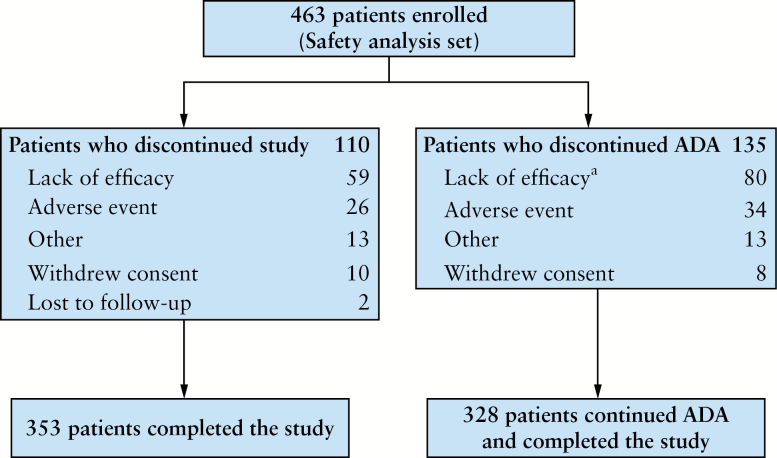
The safety analysis set [N = 463] consisted of all enrolled patients who received at least one dose of ADA during the study [Figure 1]. The intent-to-treat analysis set [N = 461] consisted of all patients in the safety analysis set who had data for at least one post-baseline assessment of any effectiveness measurement. Analyses of clinical outcomes, HRQL, treatment satisfaction, health care utilisation, and health care costs were performed using the intent-to-treat analysis set.
Figure 1.
Patient disposition. aA total of 80/463 patients discontinued adalimumab [ADA] owing to a lack of efficacy; of these, 66 patients discontinued ADA by Week 8.
2.6.1. Clinical outcomes
The proportion of patients achieving SCCAI response/remission was determined at Weeks 2, 8, and 26. Non-responder imputation [NRI] was used for response/remission when SCCAI data were missing, regardless of dose-escalation status, in the primary analysis. In a sensitivity analysis, patients who dose-escalated were assumed to be non-responders/non-remitters at Week 26. The proportion of patients reporting ‘no blood in stool’ on the SCCAI was determined at Weeks 2, 8, and 26; missing data were imputed using last observation carried forward [LOCF]. The point estimates for these outcomes were calculated with their 95% confidence intervals [CIs].
The proportions of patients with EIMs at baseline and Weeks 2, 8, and 26 were calculated. Missing data were imputed using LOCF, regardless of dose-escalation status. McNemar’s test was used to compare the presence of any EIM at Weeks 2, 8, and 26 with baseline. Resolution at Weeks 2, 8, and 26 [no reported EIM at the respective visit] and durable resolution [no EIM at the respective and subsequent visits] of any EIM from Week 2 to 26 and from Week 8 to 26 was assessed in the subset of patients with EIMs at baseline.
2.6.2. HRQL and treatment satisfaction
Mean change from baseline to Weeks 2, 8, and 26 in SIBDQ and mean change from baseline to Week 26 in EQ-5D-5L scores, WPAI domain scores, and TSQM domain scores were assessed for statistical significance using paired t tests, and their associated 95% CIs were calculated. Missing data were imputed using LOCF, regardless of dose-escalation status. No adjustments for multiple comparisons were made.
2.6.3. Resource use and costs
All-cause and UC-related resource use was determined by calculating the number of hospitalisations, days in hospital, and number of outpatient visits/procedures from data collected on case report forms. Direct costs were defined as medical costs associated with hospitalisations and outpatient visits/procedures and costs of all medications including ADA.35 Direct costs were standardised using UK National Health Service [NHS] reference costs36 for all participating countries. All costs were obtained from the UK NHS with the exception of outpatient primary care costs, which were obtained from the Personal Social Services Research Unit.37 The average cost of ADA included eow and weekly treatment and was based on the average doses taken during the study at the 2014 hospital price38 in the UK; it should be noted that these costs may vary depending on country, contracts, and tenders. Costs associated with resource utilisation were determined by multiplying the unit costs obtained from the UK NHS or the Personal Social Services Research Unit by the number of times each resource was used. Indirect costs were defined as costs associated with work loss resulting from absenteeism and disability because of UC and were based on the WPAI questionnaire using average weekly earnings from the 2014 UK Annual Salary Survey of Hours and Earnings39 for all participating countries. All-cause [for any reason] direct costs, all-cause medical costs, all-cause pharmacy costs, UC-related direct costs, UC-related medical costs, UC-related pharmacy costs and UC-related total costs were calculated for the 353 patients who completed the study. Total costs were the sum of direct and indirect costs. Change [defined as 6 months after initiating ADA vs 6 months previously] in resource utilisation and costs associated with health care use was determined. Comparisons of costs and resource utilisation before and after the onset of ADA were made using paired t tests. All costs were adjusted to 2014 British pounds sterling.
2.6.4. Treatment-emergent adverse events
Treatment-emergent adverse events were summarised by frequency and percentage. The treatment-emergent adverse event rate per 100 patient years of exposure to ADA was also calculated. The numerator of the rate was the total number of adverse events reported. The denominator of the rate was the total number of days exposed to ADA summed across all treated patients divided by 365.25. The adverse event rate per 100 patient-years [PY] of exposure was calculated as the numerator divided by the denominator multiplied by 100.
2.7. Ethics approval
The study was approved by an independent ethics committee and conducted in accordance with applicable regulations and guidelines governing clinical study conduct, and with the ethical principles that have their origin in the Declaration of Helsinki. All patients who participated in the study gave informed consent before any study-related screening procedures were performed.
3. Results
3.1. Study population
Of the 463 study participants, 72 [16%] received a TNF antagonist before enrolment in the study. A total of 66 [14%] patients discontinued ADA at Week 8 because of lack of efficacy as defined by the protocol; 397 [86%] continued into the maintenance phase. Of the 461 patients who comprised the intent-to-treat population, 129 [28%] patients moved to ADA weekly. The average age of the study population was 41.8 years [Table 1]. Among patients who did not use immunosuppressant drugs at baseline [n = 278], only 14 [5%] added immunosuppressants during the follow-up. Of those who were free from corticosteroids at baseline [n = 201], 47 [23%] initiated corticosteroid during the follow-up. Slightly more than half [55%] of the patients were men and most [97%] of the population were White.
Table 1.
Demographic and baseline characteristics.
| Variable | N | |
|---|---|---|
| Age [years], mean ± SD | 461 | 41.8 ± 13.8 |
| Male, n [%] | 461 | 255 [55.3] |
| White, n [%] | 461 | 447 [97.0] |
| UC duration [years], mean ± SD | 459 | 7.3 ± 7.0 |
| Prior TNF antagonist use, n [%] | 463 | 72 [15.6] |
| UC-related concomitant medications,an [%] | ||
| Mesalazine | 463 | 304 [65.7] |
| Azathioprine | 463 | 168 [36.3] |
| Prednisolone | 463 | 122 [26.3] |
| Prednisone | 463 | 91 [19.7] |
| Methylprednisone | 463 | 70 [15.1] |
| Sulphasalazine | 463 | 36 [7.8] |
| Mercaptopurine | 463 | 28 [6.0] |
| Physician’s Global Assessment [PGA], n [%] | 460 | |
| PGA of 2 | 386 [83.9] | |
| PGA of 3 | 74 [16.1] | |
| SCCAI, mean ± SD | 461 | 7.7 ± 2.4 |
| SIBDQ total score, mean ± SD | 460 | 30.9 ± 8.7 |
| Bowel | 461 | 9.5 ± 3.1 |
| Social | 460 | 6.0 ± 2.5 |
| Systemic | 461 | 6.5 ± 2.4 |
| Emotional | 461 | 8.9 ± 3.1 |
| EQ-5D-5L total score, mean ± SD | 454 | 0.6 ± 0.2 |
| EQ-5D-5L VAS, mean ± SD | 451 | 50.9 ± 19.4 |
| TSQM, mean ± SD | ||
| Effectiveness | 452 | 35.2 ± 18.5 |
| Side effects | 449 | 70.3 ± 34.3 |
| Convenience | 451 | 68.3 ± 20.4 |
| Global satisfaction | 449 | 38.0 ± 20.5 |
| WPAI, mean ± SD | ||
| Work time missed [%]b | 223 | 20.4 ± 30.1 |
| Impairment while working [%]b | 229 | 50.2 ± 24.8 |
| Overall work impairment [%]b | 221 | 58.5 ± 26.7 |
| Activity impairment [%] | 446 | 59.0 ± 23.5 |
EQ-5D-5L, The European Quality of Life—5 Dimensions—5 Level; PGA, Physician’s Global Assessment; SCCAI, Simple Clinical Colitis Activity Index; SD, standard deviation; SIBDQ, Short Inflammatory Bowel Disease Questionnaire TNF, tumour necrosis factor; TSQM, Treatment Satisfaction Questionnaire for Medication; UC, ulcerative colitis; VAS, visual analogue scale; WPAI, Work Productivity and Activity Impairment.
aConcomitant UC-related medications administered to ≥ 5% of patients. A concomitant medication was defined as any medication that started before the first dose of adalimumab [ADA] and continued after the first dose of study drug or any medication that started on or after the first dose of ADA, but was not started later than 14 days after the last dose of ADA.
bOnly patients who were employed were assessed.
3.2. SCCAI response and remission
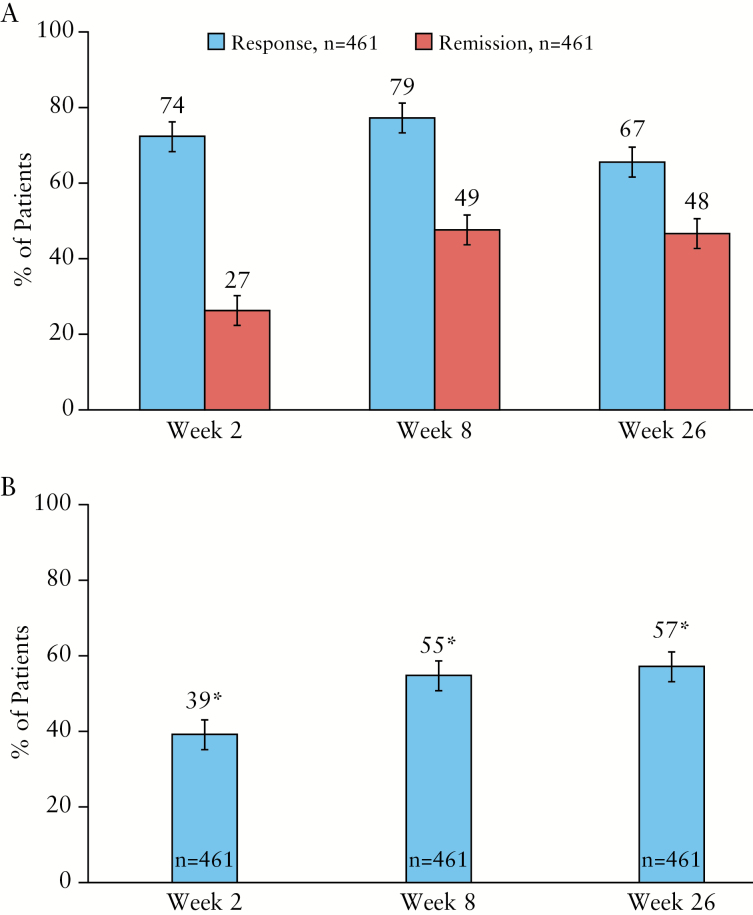
Improvements in clinical outcomes as assessed by SCCAI were seen as early as Week 2. At Week 8, most patients had a clinical response and almost half achieved SCCAI remission (rates [95% CI]: 79% [75%, 82%] for response; 49% [44%, 54%] for remission). Clinical response and SCCAI remission were sustained at Week 26 (67% [62%, 71%] response; 48% [44%, 53%] remission) [Figure 2A]. At Week 26, SCCAI response and remission rates [95% CI] were 49% [44%, 53%] and 39% [34%, 43%], respectively, when patients who dose-escalated were counted as non-responders. For the 129 patients who moved to ADA weekly, response and remission rates were 64% [55%, 72%] and 33% [25%, 41%], respectively, at Week 26. Clinical improvements were similar by previous TNF antagonist exposure (SCCAI response rates [95% CI]: 68% [57%, 79%] for TNF antagonist experienced, and 66% [62%, 71%] for TNF antagonist-naïve patients; SCCAI remission rates [95% CI]: 42% [30%, 53%] and 49% [44%, 54%] among TNF antagonist-experienced and TNF antagonist-naïve patients, respectively). ‘No blood in stool’ rates [95% CI] were reported by 39% [35%, 44%] and 57% [52%, 61%] of patients at Weeks 2 and 26, respectively [Figure 2B].
Figure 2.
[A] Percentage of patients who achieved SCCAI response and SCCAI remission after ADA therapy, and 95% CI. Clinical response was defined as a decrease of ≥ 2 points from baseline in SCCAI. Remission was defined as SCCAI ≤ 2. Missing data were imputed using NRI. [B] Percentage of patients with no blood in their stool after ADA therapy and 95% CI. Missing data were imputed using LOCF. Significant change [denoted by asterisk] from baseline [p < 0.001] using McNemar’s test was seen at Weeks 2, 8, and 26. SCCAI, Simple Clinical Colitis Activity Index; ADA, adalimumab; CI, confidence interval; NRI, non-responder imputation; LOCF, last observation carried forward.
3.3. Extraintestinal manifestations
At baseline, 88 [19%] patients reported an EIM. The most commonly reported EIM was arthritis [84/88 patients], which included arthralgia. Pyoderma gangrenosum, erythema nodosum, and uveitis were each reported in < 1% of patients at baseline and at Weeks 2, 8, and 26. The overall percentage of patients with any EIM decreased significantly [p < 0.001] from baseline over time: 13%, 12%, and 11% at Weeks 2, 8, and 26, respectively. Among those with any EIM at baseline, resolution of EIMs increased over time: 40%, 52%, and 64% at Weeks 2, 8, and 26, respectively; durable resolution was 24% from Week 2 to 26 and 44% from Week 8 to 26. Among those with arthritis at baseline, resolution rates were 37%, 50%, and 62% at Weeks 2, 8, and 26, respectively; durable resolution was 20% from Week 2 to 26 and 42% from Week 8 to 26.
3.4. Health-related quality of life
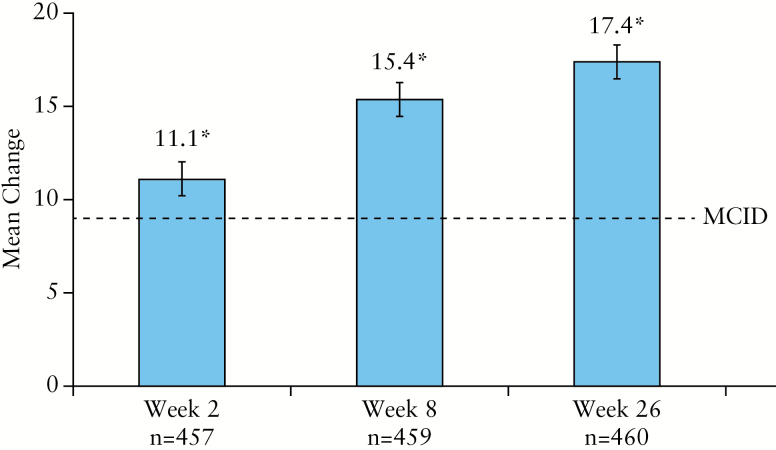
As shown in Figure 3, treatment with ADA was associated with a statistically significant and clinically meaningful improvement in SIBDQ from baseline to Week 26 (mean change ± standard deviation [SD]: 17.4 ± 14.5; 95% CI: 16.1, 18.7, p < 0.001). A statistically significant improvement in SIBDQ total score was seen as early as Week 2 after initiating ADA therapy and was maintained through Week 26; 55% [253/457], 68% [310/459], and 69% [316/460] of patients exceeded MCID for SIBDQ at Weeks 2, 8, and 26, respectively. Similarly, significant [all p < 0.001] increases [mean change ± SD] from baseline to Week 26 were observed for each of the individual domains of SIBDQ: bowel [5.1 ± 4.7; 95% CI: 4.7, 5.5], social [4.0 ± 3.7; 95% CI: 3.7, 4.3], systemic [3.1 ± 3.1; 95% CI: 2.8, 3.4], and emotional [5.2 ± 4.9; 95% CI: 4.7, 5.6].
Figure 3.
Mean change from baseline in total SIBDQ score and 95% CI. Missing data were imputed using LOCF. A nine-point change in the SIBDQ is considered an MCID.28 Significant change [denoted by asterisk] from baseline [p < 0.001] using paired t test was seen at Weeks 2, 8, and 26. CI, confidence interval; MCID, minimal clinically important difference; SD, standard deviation; SIBDQ, Short Inflammatory Bowel Disease Questionnaire; LOCF, last observation carried forward.
By Week 26, patients reported improvement in general health status as shown by significant increases [mean change ± SD] from baseline in EQ-5D-5L index [0.1 ± 0.2; 95% CI: 0.1, 0.2, p < 0.001] and VAS scores [19.5 ± 25.8; 95% CI: 17.1, 21.9, p < 0.001].
3.5. Work productivity
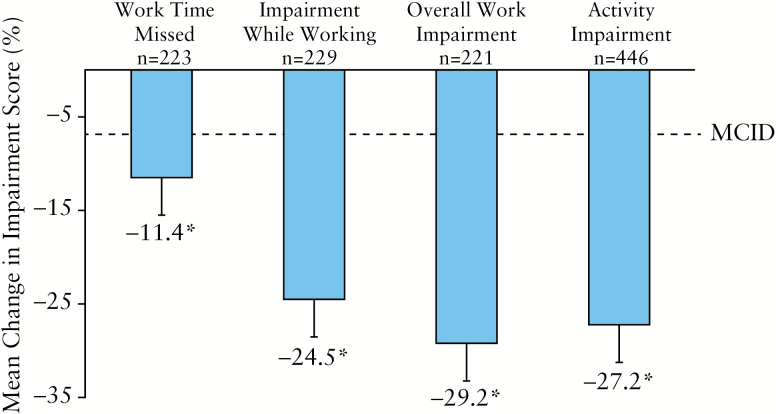
Improvements in work productivity and ability to perform daily activities were seen as early as Week 2 and were maintained through Week 26. As shown in Figure 4, significant reductions [all p < 0.001] were seen from baseline to Week 26 in absenteeism [an absolute decrease of 11%], impairment while working [an absolute decrease of 25%], and overall work impairment [an absolute decrease of 29%]. Patients also experienced significant improvement in their ability to perform daily activities with ADA therapy [decrease in impairment of 27%, p < 0.001]. At Week 26, patients exceeded the MCID for work time missed [41%; 91/223], impairment while working [74%; 170/229], overall work impairment [75%; 166/221], and activity impairment [73%; 325/446].
Figure 4.
Mean change from baseline to week 26 in WPAI and 95% CI. A decrease of 7% in WPAI outcome score for an individual patient is considered a MCID.33 Missing data were imputed using LOCF. Significant change [denoted by asterisk] from baseline [p < 0.001] using paired t test was observed for work time missed, impairment while working, overall work impairment, and activity impairment. Except for activity impairment, which was assessed for all patients, WPAI outcomes were assessed for employed patients only. MCID, minimal clinically important difference; SD, standard deviation; WPAI, Work Productivity and Activity Impairment; CI, confidence interval; LOCF, last observation carried forward.
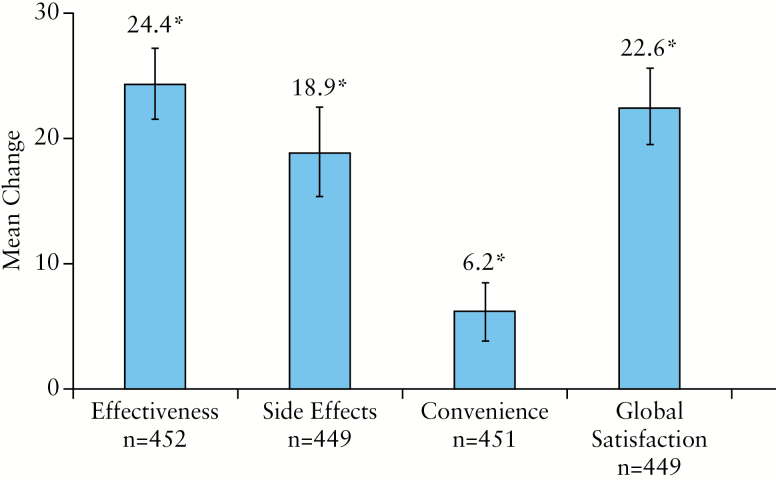
3.6. TSQM
Overall, mean patient satisfaction scores [± SD] increased significantly [all p < 0.001, Figure 5] from baseline to Week 26 for all four domains of the TSQM: effectiveness [35.2 ± 18.5 vs 59.5 ± 29.2], side effects [70.3 ± 34.3 vs 89.3 ± 21.5], convenience [68.3 ± 20.4 vs 74.5 ± 18.2], and global satisfaction [38.0 ± 20.5 vs 60.6 ± 29.2].
Figure 5.
Mean change from baseline to Week 26 in TSQM domain scores and 95% CI. Missing data were imputed using LOCF. Significant change [denoted by asterisk] from baseline [P < 0.001] was seen for all four TSQM domains using paired t test. SD, standard deviation; TSQM, Treatment Satisfaction Questionnaire for Medication; CI, confidence interval; LOCF, last observation carried forward.
3.7. Health care resource use and costs
Among patients who completed the study, a significant [all p < 0.001] decrease in health care resource use was observed 6 months after initiating ADA compared with 6 months before the onset of ADA therapy. Reductions in resource use [mean ± SD] included a decrease of 4.6 ± 6.3 UC-related outpatient visits/procedures, a decrease of 0.3 ± 0.8 UC-related hospitalisations, a decrease of 0.3 ± 0.8 hospitalisations for any reason, as well as a decrease of 3.3 ± 10.0 days hospitalised for any reason.
Mean costs [95% CI] during the 6 months before vs the 6 months after initiation of ADA were £1785 [1619, 1950] vs £727 [539, 915] for all-cause medical costs, £754 [634, 874] vs £6684 [6515, 6852] for all-cause pharmacy costs, and together £2539 [2330, 2747] vs £7411 [7160, 7662] for all-cause direct [medical + pharmacy] costs. These results show that all-cause medical costs were significantly [p < 0.001] reduced by £1058/patient [59% decrease], and all-cause pharmacy costs and all-cause direct costs were significantly increased by £5930 and £4872, respectively. Mean costs [95% CI] before vs after initiation of ADA were £1560 [1417, 1703] vs £365 [238, 492] for UC-related medical costs, £683 [576, 790] vs £6563 [6433, 6694] for UC-related pharmacy costs, and together £2243 [2055, 2430] vs £6928 [6749, 7108] for UC-related direct [medical + pharmacy] costs, and £5941 [5153, 6730] vs £1974 [1546, 2403] for UC-related indirect costs. The total UC-related costs [direct plus indirect] were £8184 [7378, 8989] vs £8903 [8417, 9388] 6 months before vs after ADA initiation. The UC-related direct costs were significantly increased by £4685/patient [p < 0.001], mainly due to the increase of UC-related pharmacy costs, whereas UC-related medical costs and UC-related indirect costs were significantly reduced following initiation of ADA, by £1195 [77% decrease] and £3967 [67% decrease] [both p < 0.001] per patient. The overall increase in costs for UC-related direct and indirect components was not statistically significant [£719, p = 0.054] during the 6 months following ADA initiation compared with the 6 months preceding.
3.8. Safety
An overview of treatment-emergent adverse events and rates of adverse events per 100 PYs of exposure are provided in Table 2. Overall, 74% of patients had at least 1 treatment-emergent adverse event and 39% had an adverse event assessed as at least possibly related to ADA by the study investigator; 14% of patients experienced serious adverse events and 4% of patients had serious adverse events that were assessed as at least possibly-related to ADA by the study. There was one treatment-emergent death reported during this study. The patient, a 42-year-old man, died in a motorcycle accident; this event was considered to be not related to treatment with ADA.
Table 2.
Treatment-emergent adverse events [AE]a: safety analysis set.
| Adverse event | Adalimumab | |
|---|---|---|
| N = 463 | ||
| PYs = 199.0 | ||
| n [%] | Events [per 100 PY] | |
| Any AE | 344 [74.3] | 1474 [740.7] |
| Any AE at least possibly drug-relatedb | 182 [39.3] | 444 [223.1] |
| Severe AE | 57 [12.3] | 78 [39.2] |
| Serious AE | 65 [14.0] | 81 [40.7] |
| AE leading to discontinuation of ADA | 53 [11.4] | 62 [31.2] |
| Any infection | 137 [29.6] | 213 [107.0] |
| Serious infection | 8 [1.7] | 10 [5.0] |
| Opportunistic infection [excluding oral candidiasis and TB] | 1 [0.2] | 1 [0.5] |
| Oral candidiasis | 1 [0.2] | 1 [0.5] |
| Active TB | 0 | 0 |
| Parasitic infection | 3 [0.6] | 3 [1.5] |
| Any malignancy | 3 [0.6] | 3 [1.5] |
| Any melanoma | 1 [0.2] | 1 [0.5] |
| Allergic reaction | 16 [3.5] | 17 [8.5] |
| Lupus-like reactions and systemic lupus erythematosus | 0 | 0 |
| Myocardial infarction | 1 [0.2] | 1 [0.5] |
| Cerebrovascular accident | 1 [0.2] | 2 [1.0] |
| Worsening/new onset of psoriasis | 3 [0.6] | 3 [1.5] |
| Haematological disorders including pancytopenia | 25 [5.4] | 27 [13.6] |
| Injection site reaction | 46 [9.9] | 69 [34.7] |
| Death | 1 [0.2] | 1 [0.5] |
ADA, adalimumab; PY, patient-years; TB, tuberculosis.
aTreatment-emergent adverse events were defined as any events with an onset date on/after the date of first dose of ADA and not later than 70 days after the last dose of ADA.
bAssessed by the investigator.
Infections were reported by 30% of patients [107 events/100 PY], including 2% with serious infections [5.0 events/100 PY]. Eight patients had 10 serious infections. One patient each had rectal abscess, cytomegalovirus infection, appendicitis, wound sepsis, herpes zoster, and pyelonephritis; one patient had osteomyelitis and cellulitis, and one patient had otitis media and sinusitis; all 10 serious infections resolved. Three patients (1% [1.5 events/100 PY]) had malignancies. One patient each had malignant melanoma of the eyelid, prostate cancer, and testicular seminoma.
4. Discussion
The present study evaluated clinical outcomes, HRQL, treatment satisfaction, health care resource utilisation, and associated costs among patients with moderate to severe UC who were treated with ADA according to usual clinical practice. Following initiation of ADA, patients with moderate to severely active UC had significant improvements in clinical symptoms as early as Week 2, and by Week 26 most patients had responded, with nearly half achieving SCCAI remission. Consistent with previously reported results,17,20,40 remission rates were lower in patients with previous exposure to TNF antagonists [42%] compared with TNF antagonist-naïve patients [49%]. Significant and clinically meaningful changes in HRQL were also observed as early as Week 2 and were maintained through Week 26. Perhaps as a result of disease control and corresponding improvements in quality of life and work productivity, patients receiving ADA reported high treatment satisfaction. Significant and clinically relevant reductions in time missed from work and impairment while working, as well as improvement in ability to perform daily activities, were also observed as a result of treatment with ADA. Not only was rapid and sustained effectiveness of ADA seen for all parameters, but all-cause medical costs were halved and UC-related medical costs were reduced by three-quarters. Although the UC-related direct and indirect costs reported in this study do not offset the estimated costs of ADA therapy, the benefit to the patient is substantial, with nearly half of the patients in remission and almost 70% achieving a response by Month 6, fewer hospitalisations and surgeries, and sustained improvements in work productivity and overall quality of life, for an incremental UC-related cost of £719 over 6 months.
Consistent with data obtained in RCTs, data from InspirADA demonstrated that ADA is an effective treatment for UC patients.11,12,14,40,41 Higher rates of clinical response and remission reported in the InspirADA study compared with the ULTRA trials might well be explained by methodological differences.11,12 Similar to clinical practice, ADA dosage and concomitant medications could be adjusted any time in the study; thus, patients in the InspirADA study could continue treatment in the face of a temporary flare, as opposed to RCTs where failure rules are strictly enforced, concomitant mediation changes are generally prohibited, and patients who dose-escalate are considered non-responders. Moreover, the use of different disease activity indices may have played a role in observed differences. In most registration clinical trials, Mayo scores were calculated using mean values obtained from the patient’s diary over several days; however, in ULTRA 1 and ULTRA 2,11,12 the Mayo score was calculated using the worst-rank [ie highest] of three daily scores, which is likely to have resulted in a lower estimate of therapeutic effect. However, as the Mayo score is not often used in clinical practice, the SCCAI was employed in the InspirADA study. The higher response and remission rates seen in the present study are aligned with results recently reported in a real-world assessment of the effectiveness of TNF antagonists.18 It is also possible that the higher responses were influenced by the fact that InspirADA was an open-label study for a drug that patients know is effective for the treatment of UC.
The strength of this study is that data were obtained in the context of the clinical practice setting, which may provide results that are more generalisable to a broader patient population than data obtained in a more restrictive, controlled clinical trial setting. Limitations of this study include the open-label, single treatment arm design; the short-term [26 weeks] nature of the study; absence of endoscopy and pharmacokinetic data; and the retrospective collection of data about previous treatment. The retrospective collection of health care utilisation data before baseline may be subject to recall bias; thus the true cost-saving effect of ADA treatment may have been underestimated in this study. In the current analysis, data were not available to comprehensively evaluate the long-term economic benefits of ADA treatment for UC patients. In the short term [within 6 months], the costs of ADA were found to be a major component of the direct costs of UC care; however, substantial benefits were also observed from reduced health care utilizations and work productivity gained. Previous studies have shown that long-term use of ADA is not only associated with substantial clinical benefits, but is also cost-effective compared with standard of care using both clinical trial data42,43 and real-life data.44
Data were collected from 20 countries and, since the unit price for health care utilization varies by country, the cost results may not be generalisable to individual countries. Assumptions were made for the cost analysis by extracting cost information associated with resource utilization and drugs for the UK, because the UK contributed the largest number of patients in this study [n = 90] and due to the availability of the data necessary [from the UK NHS] to perform meaningful analysis. The MCID values of 9 points for SIBDQ and 7 points for WPAI are assumed from the literature on Crohn’s disease28,33 and have not been validated in patients with UC.
Overall, in clinical practice, achievement of response and remission and improvements in HRQL among patients treated with ADA for UC were rapid, sustained, and clinically meaningful. Improvements in work productivity and performance of daily activities probably resulted in patient satisfaction with ADA therapy. ADA therapy significantly reduced use of health care resources and their associated costs, as well as costs of work loss resulting from absenteeism and disability among patients with moderate to severely active UC. ADA was well tolerated and no new safety signals were identified.
Funding
This work was supported by AbbVie. The study sponsor participated in the interpretation of data, review, and approval of the article.
Conflict of Interest
ST was an adviser to, and received educational or research grants from, or was an invited lecturer for AbbVie, Asahi, Boehringer Ingelheim, BMS, Cosmo, Elan, Ferring, FPRT Bio, Genentech/Roche, Genzyme, Glenmark, GW Pharmaceuticals, Lilly, Merck, Novartis, Novo Nordisk, Ocera, Pfizer, Shire, Santarus, SigmoidPharma, Synthon, Takeda, Tillotts, Topivert, Trino Therapeutics with Wellcome Trust, UCB Pharma, Vertex, VHsquared, Vifor, Warner Chilcott, and Zeria. All advisory boards were suspended Q1 2012–2014 while President of ECCO. BF received financial support for research from Centocor, Merck, UCB, and Abbott; lecture fee[s] from Centocor, Merck, Abbott; and was a consultant for Centocor, Merck, UCB, Abbott, Millenium/Takeda, Genentech/Hoffman LaRoche, Neovacs, Merck/Serono, Bristol Myers Squibb, Robarts, Tillotts, Pfizer, and Falk Pharma. LP-B received consulting fees from Merck, AbbVie, Janssen, Genentech, Mitsubishi, Ferring, Norgine, Tillots, Vifor, Therakos, Pharmacosmos, Pilège, BMS, UCB-pharma, Hospira, Celltrion, Takeda, Biogaran, Boerhinger-Ingelheim, Lilly, Pfizer, HAC-Pharma, Index Pharmaceuticals, Amgen, Sandoz; and lecture fees from Merck, AbbVie, Takeda, Janssen, Takeda, Ferring, Norgine, Tillots, Vifor, Therakos, Mitsubishi, and HAC-pharma. RP received consultant and/or lecture fees from AbbVie, Amgen, AstraZeneca, Axcan Pharma [now Aptalis], Biogen Idec, Bristol-Myers Squibb, Centocor, ChemoCentryx, Eisai Medical Research, Elan Pharmaceuticals, Ferring, Genentech, GlaxoSmithKline, Janssen, Merck Sharp & Dohme, Millennium Pharmaceuticals [now Takeda], Ocera Therapeutics, Otsuka America Pharmaceutical, Pfizer, Shire Pharmaceuticals, Prometheus Laboratories, Schering-Plough, Synta Pharmaceuticals, Teva, UCB Pharma, and Warner Chilcott. SD received fees for board membership from Merck Sharp & Dohme, consulting fees from Schering Plough, AstraZeneca, Abbott Laboratories, AbbVie, and Takeda Millennium, and lecture fees including fees for service on speakers’ bureaus from UCB Pharma, Ferring, and Merck Sharp & Dohme. AL, AMR, JP, BLP, MB, MS, NC, and RBT are employees and stock holders of AbbVie. SW and JC are former employees of AbbVie.
Author Contributions
All authors made a substantial contribution to the concept and design of the work and the analysis and interpretation of the data. All authors made critical revisions for important intellectual content and they all approved the final version of the article and are accountable for its content.
Acknowledgments
Medical writing support was provided by Joann Hettasch, Fishawack Group of Companies, Conshohocken, PA, and was funded by AbbVie.
References
- 1. Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med 2011;365:1713–25. [DOI] [PubMed] [Google Scholar]
- 2. Dignass A, Eliakim R, Magro F et al. . Second European evidence-based consensus on the diagnosis and management of ulcerative colitis. Part 1: definitions and diagnosis. J Crohns Colitis 2012;6:965–90. [DOI] [PubMed] [Google Scholar]
- 3. Dignass A, Lindsay JO, Sturm A et al. . Second European evidence-based consensus on the diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis 2012;6:991–1030. [DOI] [PubMed] [Google Scholar]
- 4. Van Assche G, Dignass A, Bokemeyer B et al. ; European Crohn’s and Colitis Organisation. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis. Part 3: special situations. J Crohns Colitis 2013;7:1–33. [DOI] [PubMed] [Google Scholar]
- 5. Janke KH, Raible A, Bauer M et al. . Questions on life satisfaction [FLZM] in inflammatory bowel disease. Int J Colorectal Dis 2004;19:343–53. [DOI] [PubMed] [Google Scholar]
- 6. Janke KH, Klump B, Gregor M, Meisner C, Haeuser W. Determinants of life satisfaction in inflammatory bowel disease. Inflamm Bowel Dis 2005;11:272–86. [DOI] [PubMed] [Google Scholar]
- 7. Bernklev T, Jahnsen J, Lygren I, Henriksen M, Vatn M, Moum B. Health-related quality of life in patients with inflammatory bowel disease measured with the short form-36: psychometric assessments and a comparison with general population norms. Inflamm Bowel Dis 2005;11:909–18. [DOI] [PubMed] [Google Scholar]
- 8. Irvine EJ. Quality of life of patients with ulcerative colitis: past, present, and future. Inflamm Bowel Dis 2008;14:554–65. [DOI] [PubMed] [Google Scholar]
- 9. Cohen RD, Yu AP, Wu EQ, Xie J, Mulani PM, Chao J. Systematic review: the costs of ulcerative colitis in Western countries. Aliment Pharmacol Ther 2010;31:693–707. [DOI] [PubMed] [Google Scholar]
- 10. van der Valk ME, Mangen MJ, Leenders M et al. ; COIN study group and the Dutch Initiative on Crohn and Colitis. Health care costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti-TNFα therapy: results from the COIN study. Gut 2014;63:72–9. [DOI] [PubMed] [Google Scholar]
- 11. Reinisch W, Sandborn WJ, Hommes DW et al. . Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut 2011;60:780–7. [DOI] [PubMed] [Google Scholar]
- 12. Sandborn WJ, van Assche G, Reinisch W et al. . Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2012;142:257–65.e1–3. [DOI] [PubMed] [Google Scholar]
- 13. Feagan BG, Sandborn WJ, Lazar A et al. . Adalimumab therapy is associated with reduced risk of hospitalization in patients with ulcerative colitis. Gastroenterology 2014;146:110–8.e3. [DOI] [PubMed] [Google Scholar]
- 14. Reinisch W, Sandborn WJ, Panaccione R et al. . 52-week efficacy of adalimumab in patients with moderately to severely active ulcerative colitis who failed corticosteroids and/or immunosuppressants. Inflamm Bowel Dis 2013;19:1700–9. [DOI] [PubMed] [Google Scholar]
- 15. Ha C, Ullman TA, Siegel CA, Kornbluth A. Patients enrolled in randomized controlled trials do not represent the inflammatory bowel disease patient population. Clin Gastroenterol Hepatol 2012;10:1002–7; quiz e78. [DOI] [PubMed] [Google Scholar]
- 16. Bálint A, Farkas K, Palatka K et al. . Efficacy and safety of adalimumab in ulcerative colitis refractory to conventional therapy in routine clinical practice. J Crohns Colitis 2016;10:26–30. [DOI] [PubMed] [Google Scholar]
- 17. Iborra M, Pérez-Gisbert J, Bosca-Watts MM et al. ; Spanish Working Group on Crohn’s Disease and Ulcerative Colitis [GETECCU]. Effectiveness of adalimumab for the treatment of ulcerative colitis in clinical practice: comparison between anti-tumour necrosis factor-naïve and non-naïve patients. J Gastroenterol 2017;52:788–99. [DOI] [PubMed] [Google Scholar]
- 18. Sandborn WJ, Sakuraba A, Wang A et al. . Comparison of real-world outcomes of adalimumab and infliximab for patients with ulcerative colitis in the United States. Curr Med Res Opin 2016;32:1233–41. [DOI] [PubMed] [Google Scholar]
- 19. García-Bosch O, Gisbert JP, Cañas-Ventura A et al. . Observational study on the efficacy of adalimumab for the treatment of ulcerative colitis and predictors of outcome. J Crohns Colitis 2013;7:717–22. [DOI] [PubMed] [Google Scholar]
- 20. Taxonera C, Iglesias E, Muñoz F et al. . Adalimumab maintenance treatment in ulcerative colitis: outcomes by prior anti-TNF use and efficacy of dose escalation. Dig Dis Sci 2017;62:481–90. [DOI] [PubMed] [Google Scholar]
- 21. Walsh AJ, Bryant RV, Travis SP. Current best practice for disease activity assessment in IBD. Nat Rev Gastroenterol Hepatol 2016;13:567–79. [DOI] [PubMed] [Google Scholar]
- 22. Higgins PD, Leung J, Schwartz M, Mapili J, Wren PA, Zimmermann EM. The quantitative validation of non-endoscopic disease activity indices in ulcerative colitis. Aliment Pharmacol Ther 2007;25:333–42. [DOI] [PubMed] [Google Scholar]
- 23. Higgins PD, Schwartz M, Mapili J, Krokos I, Leung J, Zimmermann EM. Patient defined dichotomous end points for remission and clinical improvement in ulcerative colitis. Gut 2005;54:782–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Walmsley RS, Ayres RC, Pounder RE, Allan RN. A simple clinical colitis activity index. Gut 1998;43:29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jharap B, Sandborn WJ, Reinisch W et al. . Randomised clinical study: discrepancies between patient-reported outcomes and endoscopic appearance in moderate to severe ulcerative colitis. Aliment Pharmacol Ther 2015;42:1082–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Irvine EJ, Zhou Q, Thompson AK. The Short Inflammatory Bowel Disease Questionnaire: a quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT Investigators. Canadian Crohn’s Relapse Prevention Trial. Am J Gastroenterol 1996;91:1571–8. [PubMed] [Google Scholar]
- 27. Jowett SL, Seal CJ, Barton JR, Welfare MR. The short inflammatory bowel disease questionnaire is reliable and responsive to clinically important change in ulcerative colitis. Am J Gastroenterol 2001;96:2921–8. [DOI] [PubMed] [Google Scholar]
- 28. Lichtiger S, Binion DG, Wolf DC et al. . The CHOICE trial: adalimumab demonstrates safety, fistula healing, improved quality of life and increased work productivity in patients with Crohn’s disease who failed prior infliximab therapy. Aliment Pharmacol Ther 2010;32:1228–39. [DOI] [PubMed] [Google Scholar]
- 29. EuroQol. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 30. EuroQol Group. EQ-5D-5L User Guide. Version 2.1 April 2015. http://www.euroqol.org/about-eq-5d/publications/user-guide.html Accessed December 2, 2015.
- 31. Reilly MC, Gerlier L, Brabant Y, Brown M. Validity, reliability, and responsiveness of the work productivity and activity impairment questionnaire in Crohn’s disease. Clin Ther 2008;30:393–404. [DOI] [PubMed] [Google Scholar]
- 32. Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics 1993;4:353–65. [DOI] [PubMed] [Google Scholar]
- 33. Binion DG, Louis E, Oldenburg B et al. . Effect of adalimumab on work productivity and indirect costs in moderate to severe Crohn’s disease: a meta-analysis. Can J Gastroenterol 2011;25:492–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Atkinson MJ, Sinha A, Hass SL et al. . Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication [TSQM], using a national panel study of chronic disease. Health Qual Life Outcomes 2004;2:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Department of Health. Drugs and Pharmaceutical Electronic Market Information [eMit] https://www.gov.uk/government/publications/drugs- and-pharmaceutical-electronic-market-information-emit Accessed September 8, 2016.
- 36.Department of Health. UK National Schedule of Reference Costs 2012–13 for NHS Trusts and NHS Foundation Trusts 2013. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/260405/2012-13_national_schedule_of_reference_costs.xls Accessed October 2, 2014.
- 37. Personal Social Services Research Unit. Unit Costs of Health and Social Care. 2013 http://www.pssru.ac.uk/project-pages/unit-costs/2013/ Accessed October 2, 2014.
- 38. Buckinghamshire Healthcare NHST Guideline 738FM.2. Adalimumab, Etanercept, Infliximab and Ustekinumab for the Treatment of Adults With Psoriasis. 2012 http://www.bucksformulary.nhs.uk/docs/Guideline_738FM.pdf Accessed January 30, 2017.
- 39. Office of National Statistics. Statistical Bulletin: Annual Survey of Hours and Earnings, 2014 Provisional Results 2014. http://www.ons.gov.uk/ons/ dcp171778_385428.pdf Accessed December 7, 2014.
- 40. Sandborn WJ, Colombel JF, D’Haens G et al. . One-year maintenance outcomes among patients with moderately-to-severely active ulcerative colitis who responded to induction therapy with adalimumab: subgroup analyses from ULTRA 2. Aliment Pharmacol Ther 2013;37:204–13. [DOI] [PubMed] [Google Scholar]
- 41. Colombel JF, Sandborn WJ, Ghosh S et al. . Four-year maintenance treatment with adalimumab in patients with moderately to severely active ulcerative colitis: Data from ULTRA 1, 2, and 3. Am J Gastroenterol 2014;109:1771–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ali T, Skup M, Yang M et al. . Cost-effectiveness of adalimumab in moderately to severely active ulcerative colitis. Am J Gastroenterol 2012;107:S641 http://www.nature.com/ajg/journal/v107/n1s/index.html [Google Scholar]
- 43. Ghosh S, Desjardins O, Skup M et al. . Cost-effectiveness of adalimumab for the treatment of moderately to severely active ulcerative colitis in Canada. Value in Health 2014;17:A37. [Google Scholar]
- 44. Beilman CL, Thanh NX, Ung V et al. . Real-life treatment paradigms show adalimumab is cost-effective for the management of ulcerative colitis. Can J Gastroenterol Hepatol 2016;2016:5315798. [DOI] [PMC free article] [PubMed] [Google Scholar]