Abstract
Background and Aims:
M cells associated with organised lymphoid tissues such as intestinal Peyer’s patches provide surveillance of the intestinal lumen. Inflammation or infection in the colon can induce an M cell population associated with lymphoid infiltrates; paradoxically, induction is dependent on the inflammatory cytokine tumour necrosis factor [TNF]-α. Anti-TNFα blockade is an important therapeutic in inflammatory bowel disease, so understanding the effects of TNFα signalling is important in refining therapeutics.
Methods:
To dissect pro-inflammatory signals from M cell inductive signals, we used confocal microscopy image analysis to assess requirements for specific cytokine receptor signals using TNF receptor 1 [TNFR1] and 2 [TNFR2] knockouts [ko] back-crossed to the PGRP-S-dsRed transgene; separate groups were treated with soluble lymphotoxin β receptor [sLTβR] to block LTβR signalling. All groups were treated with dextran sodium sulphate [DSS] to induce colitis.
Results:
Deficiency of TNFR1 or TNFR2 did not prevent DSS-induced inflammation nor induction of stromal cell expression of receptor activator of nuclear factor kappa-B ligand [RANKL], but absence of TNFR2 prevented M cell induction. LTβR blockade had no effect on M cell induction, but it appeared to reduce RANKL induction below adjacent M cells.
Conclusions:
TNFR2 is required for inflammation-inducible M cells, indicating that constitutive versus inflammation-inducible M cells depend on different triggers. The inducible M cell dependence on TNFR2 suggests that this specific subset is dependent on TNFα in addition to a presumed requirement for RANKL. Since inducible M cell function will influence immune responses, selective blockade of TNFα may affect colonic inflammation.
Keywords: Colitis, M cell, DSS, TNF alpha, lymphotoxin beta
1. Introduction
Mucosal immune surveillance is largely performed by organised lymphoid tissues such as Peyer’s patches [PP], isolated lymphoid follicles [ILF], and colonic patches [CP].1–3 The epithelium associated with these lymphoid tissues features specialised epithelial cells called M cells, which provide transcytosis of microparticles to underlying dendritic cells and lymphocytes to trigger mucosal immunity.1,4 The development of these tissues is dependent on lymphoid tissue inducer cells [LTi], which produce inducing cytokines such as lymphotoxin α1β2, a trimeric protein that binds to the lymphotoxin-β receptor [LTβR/TNFRSF3]; LTi cytokines induce stromal cells to develop specialised phenotypes, including mesenchymal cells producing organising cytokines such as CXCL13 and CCL21.3,5–9
M cells in organised lymphoid tissues are additionally thought to be dependent on the cytokine receptor activator of nuclear factor kappa-B ligand [RANKL]/TNFSF11 produced by fibroblast/mesenchymal-type cells in the lamina propria.10,11 Moreover, interactions between B lymphocytes and committed M cells are important in the acquisition of M cell transcytosis function.12–15 The genetic programme that distinguishes M cells from neighbouring enterocytes may require one or more of the above mentioned cytokines; for example, addition of TNFα and LTβR -ligand antibodies induces expression of M cell genes in Caco-2BBe cells in vitro.16
The cytokines mentioned above are also associated with inflammatory responses, and accordingly, models of inflammatory bowel disease [IBD] were shown to induce new M cells in the colon.17. The induction of colonic M cells in the dextran sodium sulphate [DSS] model appeared to be specifically dependent on TNFα, as blockade with anti-TNFα antibodies abrogated M cell induction. Analysis of tissue mRNAs also confirmed the induction of RANKL. The induced colonic M cells were capable of microparticle transcytosis, suggesting full surveillance function. However, these M cells were associated with variable mononuclear infiltrates in the lamina propria, and there was only poor expression of organised stromal cell markers including ER-TR7 and CXCL13. Induction of M cells without fully organised mucosal lymphoid tissues raises the question of whether the associated transcytosis function might in fact promote ongoing colonic inflammation by increasing microbial access to the lamina propria. Would anti-TNFα blockade provide some of its therapeutic effects by preventing M cell induction?
In this study, we examine the specific requirements for cytokine signalling for inflammation-induced colonic M cells. The role of TNFα in M cell induction may be differentially dependent on the two receptors for TNFα: TNFR1/TNFRSF1A and TNFR2/TNFRSF1B. Differences in signalling by these receptors may have important consequences; in the central nervous system, inflammatory syndromes such as multiple sclerosis [MS] and cuprizone-induced demyelination show differential dependence on TNFR1 versus TNFR2. For example, in cuprizone-induced demyelination, TNFR2 signalling appears to be critical to promoting oligodendrocyte proliferation and restoration of axonal myelination; yet interestingly, TNFR2 is not required for normal myelination during early development.18,19 Thus in clinical settings, global blockade of TNFα can exacerbate MS symptoms, or even induce new-onset MS in patients treated with TNFα blockade.20–24 As cited above, M cells may also depend on lymphotoxin α1β2 or RANKL, so the contributions of each of these cytokines in the inflammation-induced induction of colonic M cells needs to be examined. Our findings suggest that normal development of ‘constitutive’ organised lymphoid tissues and associated M cells may depend on signals rather different from those involved in inflammation-inducible colonic M cell populations. These differences suggest that in clinical IBD, carefully targeted therapeutic strategies may significantly affect the induction of M cells and their impact on inflammation and immunity in the intestine.
2. Materials and Methods
2.1. Animals
Animal studies were performed in accordance with institutional IACUC and NIH guidelines, with approved protocols. All mice were housed in specific pathogen-free conditions in microisolator cages. The peptidoglycan recognition protein-S [PGRP-S]-dsRed transgene25 was back-crossed to at least two generations to TNFR1ko [Jax strain #003242] and TNFR2ko [Jax strain #003246] both on the C57BL/6 background; in some experimental replicates, PGRP-S-dsRed/CX3CR1-EGFP mice on a partial C57BL/6 back-cross were used as controls and in others, TNFR2ko heterozygous [‘TNFR2het’] mice on the C57BL/6 background were used as indicated. Data shown here present results using the latter control group, though nearly identical results were obtained in all experimental replicates. A separate group of PGRP-S-dsRed mice were back-crossed more than five generations to the BALB/c background in the LTβR blockade studies. All mice used were 2–4 months old.
2.2. Colitis induction
All animals were given 5% [w/v] dextran sodium sulphate [DSS] [M.W. 36,0000-50,000 MP Biomedicals, Solon, OH] in drinking water ad libitum for 6 days; control mice were given regular drinking water. It is known that different mouse strains [C57BL/6 versus BALB/c] respond differently to DSS,26,27 and back-crosses to C57BL/6 and BALB/c might not have been far enough to present the strict strain-specific response., Thus 5% was used as a dose giving uniform pathology in all groups, as preliminary studies found that inconsistent results were obtained using a 3% dose. Mice were weighed every day, and at the end of the study, intestines were excised for histological studies.
2.3. Lymphotoxin β receptor [LTβR] blockade
PGRP-S-dsRed mice [BALB/c backcross] were given three intraperitoneal [i.p.] injections containing 100 µg mouse LTβR-mIgG1 [soluble LTβR, or ‘sLTβR’] or an IgG1 isotype control antibody [MOPC-21 clone 113] [Biogen Idec, San Diego, CA] at Days 0, 3, and 5 of DSS treatment.28
2.4. Histology and morphometrics
M cell counts were performed as follows. Colons were excised and opened longitudinally along the mesenteric side and pinned villus side up onto Whatman paper. Tissue was fixed in 4% PFA [Electron Microscopy Sciences, Hatfield, PA], 30% sucrose [Fisher Scientific, Waltham, MA] in PBS solution for 2 h at 4°C. The fixed, flattened colon was cut into 0.5 cm pieces and placed on end in OCT [Sakura Finetek, Torrance, CA] and frozen before 16-µm cryostat sections were taken; 4% PFA/30% sucrose/PBS-fixed Peyer’s patches from each mouse were mounted into the same OCT mould as their colon pieces. Cryostat sections were treated with 0.5% Tween-20 in PBS [Fisher Scientific, Waltham, MA], washed 3 times in 0.1%Tween/PBS and then blocked using 0.1% Tween-20 in blocker casein solution [Thermo Fisher Scientific, Rockford, IL]. For biotin-labelled antibodies, the Avidin/Biotin Blocking Kit [Vector Labs, Burlingame, CA] was used per manufacturer’s instructions before blocking with casein-Tween.
Primary antibodies, B220/CD45R [BD Pharmingen, 553086, 1:200 dilution] and RANKL/CD254 [eBioscience, 14-5952-82, 1:100 dilution] were added to fresh casein blocking solution and incubated for 1 h at room temperature. Samples were again washed three times in 0.1% Tween/PBS. Secondary antibodies, Streptavidin 647 [Molecular Probes, S32357, 1:500] and Donkey Anti-Rat 647 [Jackson ImmunoResearch, 712-606-153, 1:500 dilution] were added to fresh casein blocking solution and incubated for 30 min at room temperature. Samples were washed three times in 0.1% Tween/PBS before mounting with Prolong Gold antifade reagent containing DAPI [Thermo Fisher Scientific, Rockford, IL]. Tissues were allowed to cure overnight and imaged. For M cell counts, 24 random images of full-thickness colon sections at 40X magnification were taken for each animal, and dsRed+ M cells were counted by visual inspection to include cells in the epithelial layer and excluding small dsRed+ neutrophils in the lamina propria using Volocity software v6.1 [Perkin Elmer, Waltham, MA].
Data figures show total M cell counts summed from all 24 images for each animal. For RANKL:M cell ratios, M cell dsRed+ cross-sectional areas and RANKL+ cross-sectional areas in both Peyer’s patches and colon were measured with Volocity software using common thresholds for black point and white point settings. For each image, these areas were used to calculate a RANKL:M cell ratio, and data presented show ratios for individual images. All images were acquired on a BD CARV II spinning-disk confocal imager [BD Biosystems] attached to a Zeiss Axio Observer inverted microscope. Hardware, including the confocal microscope and digital camera [Qimaging Rolera EMC2], was controlled by Metamorph imaging software. Images were further optimised by using Volocity deconvolution software [v 6.1; PerkinElmer].
2.5. Statistics analysis
All statistics analysis was performed using GraphPad Prism software [v 6.0h]. For DSS colitis weights, groups were compared by standard unpaired t tests. M cell counts were analysed using unpaired t tests, and RANKL:M cell ratios were analysed using the unpaired Mann-Whitney test [*, p < 0.05; **, p < 0.005; ***, p < 0.0005; ****, p < 0.0001].
3. Results
3.1. DSS induces similar colonic inflammatory disease in both TNFR1- and TNFR2-deficient mice
In studies on colonic inflammation induced by DSS treatment and Citrobacter rodentium infection, we found significant induction of M cells associated with loosely organised lymphocyte infiltrates in the lamina propria.17 Detection of the M cells was dependent on the use of a reporter transgene with the PGRP-S promoter driving expression of the fluorescent protein dsRed [‘PGRP-S-dsRed’] transgenic mice.25,29 These induced cells were shown to possess the capability to transcytose fluorescent bacteria across the mucosal barrier, consistent with their identification as M cells, despite the absence of well-organised compact lymphoid tissues. The induction of M cells was found to be dependent on TNFα, as anti-TNFα antibody treatment completely abrogated M cell induction in the colon, though inflammation was not markedly reduced. Additional factors in the M cell induction were not tested.
Due to the very low percentage of M cells among intestinal enterocytes, histological methods were used to provide quantification of M cell numbers. Indeed, although histological methods could identify as much as 20–25% M cells among Peyer’s patch follicle epithelium [not shown], induced colonic M cells were found to be below 1% of total epithelial nuclei by histological methods, even in DSS-induced tissues. Differences between flow cytometric analysis and histological assays may suggest significant under-counting of M cells by flow cytometry; indeed, whereas we estimated 20–25% M cells in Peyer’s patch follicle epithelium, one analysis using flow cytometric methods30 only counted approximately 7% M cells. Similar differences would apply to detection of M cells in DSS-induced colon. This difference may be due to the observation that M cells have dramatic morphological differences compared with conventional enterocytes, such as the inclusion of one or more basolateral pocket B lymphocytes. Such morphological differences may lead to M cell-specific loss due to cell fragility, or exclusion due to light scatter characteristics during cytometry gating. The histological approach also ensures that our counts are specific to epithelial M cells and not infiltrating dsRed-expressing neutrophils known to infiltrate the inflamed lamina propria of DSS-induced mice.17
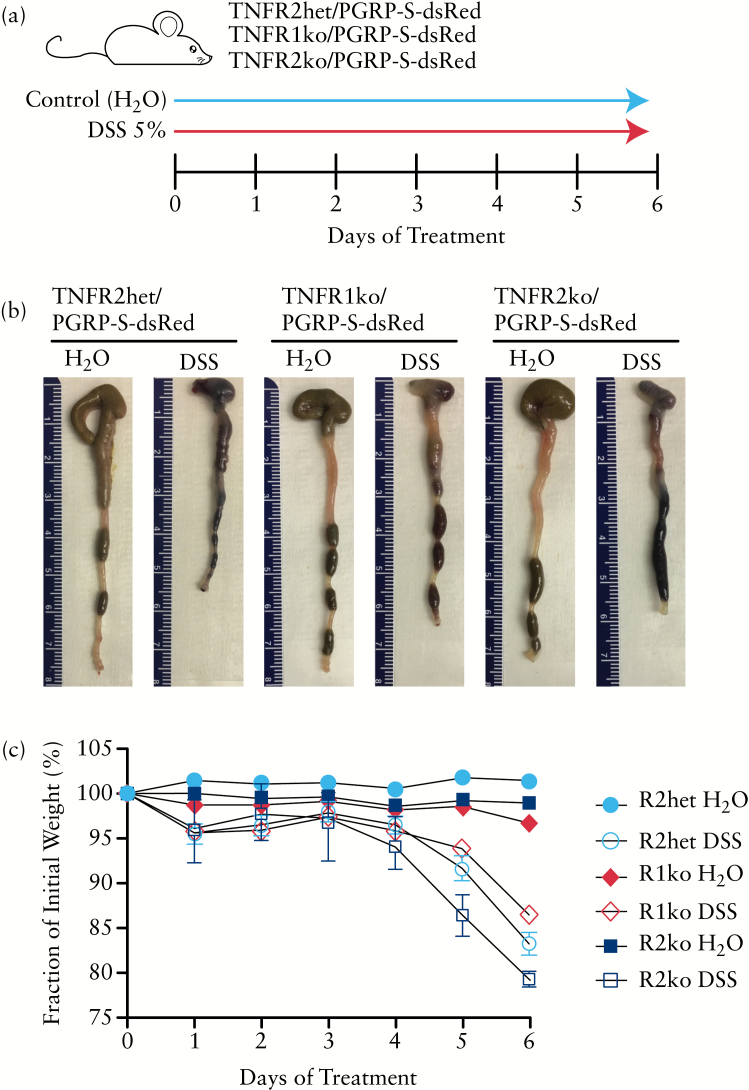
To dissect the requirements for TNFR signalling in the induction of colonic M cells, in the present study we back-crossed the PGRP-S-dsRed transgene in both TNFR1 and TNFR2 knockout mice on the C57BL/6 genetic background, and assessed the response to DSS. Figure 1 shows that in both sets of knockout mice, colonic inflammation is triggered by DSS in drinking water, with shortening of the colon and progressive weight loss. These results are consistent with previous reports that both types of knockout mice are susceptible to DSS colitis.31–34
Figure 1.
TNFR1 and TNFR2 knockout mice are susceptible to dextran sodium sulphate [DSS] colitis. a. Treatment protocol used in this study. b. Colonic shortening is evident in both TNFR1 and TNFR2 knockout mice. Representative images of colons are shown next to centimetre ruler. c. Weight loss is uniformly seen in both TNFR1 and TNFR2 knockout mice in parallel with controls [TNFR2 heterozygous mice]. Results were similar in three replicate studies.
3.2. TNFR1 and TNFR2 are not required for development of constitutive Peyer’s patch and colonic patch lymphoid tissues
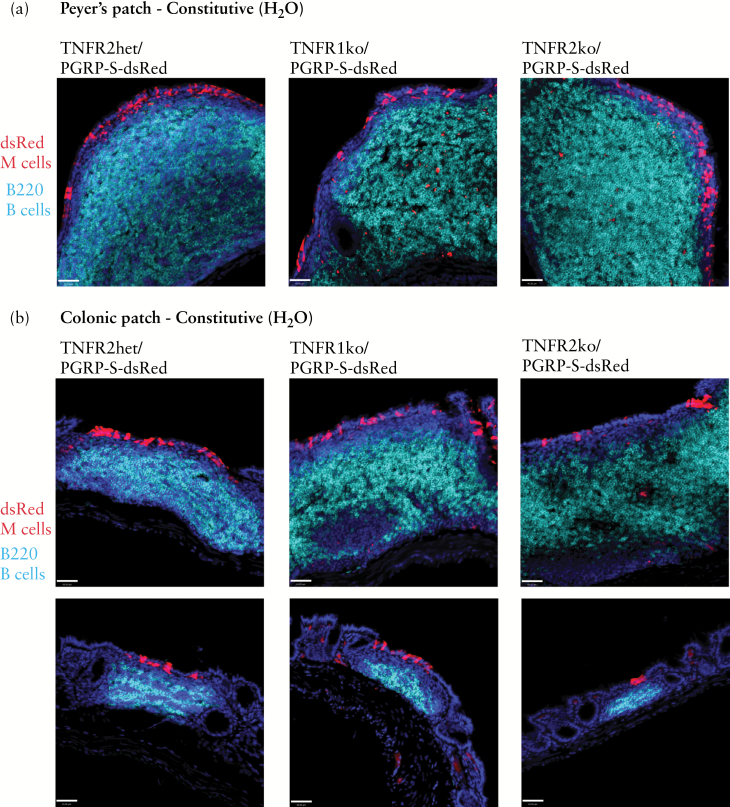
Since M cells are most commonly associated with organised lymphoid tissues in the intestine, we first confirmed that conventional Peyer’s patches and colonic patches were normally present in both TNFR1 and TNFR2 knockout mice. Figure 2 shows that compact B lymphocyte follicles and the associated M cells are evident in a normal number and distribution in the follicle-associated epithelium [expressing the PGRP-S-dsRed reporter] of both Peyer’s patches [Figure 2a] and colonic patches [Figure 2b]. From these results, it appears that these ‘constitutive’ mucosal lymphoid tissues are not dependent on either TNFR1 or TNFR2 during normal development.
Figure 2.
Constitutive Peyer’s patch and colonic patch M cell development is intact in TNFR1 and TNFR2 knockout mice. a. Expression of PGRP-S-dsRed transgene reporter [red] in M cells, with immunostaining for B cells [cyan] shows normal development and organisation of Peyer’s patches in TNFR1 and TNFR2 knockout mice. Scale bar: 40 µm. b. Constitutive colonic patch B cell follicle [cyan] organisation and associated M cells [red] are intact in TNFR1 and TNFR2 knockout mice. Scale bar: 40 µm.
3.3. TNFR2, but not TNFR1, is required for DSS-induced colonic M cells
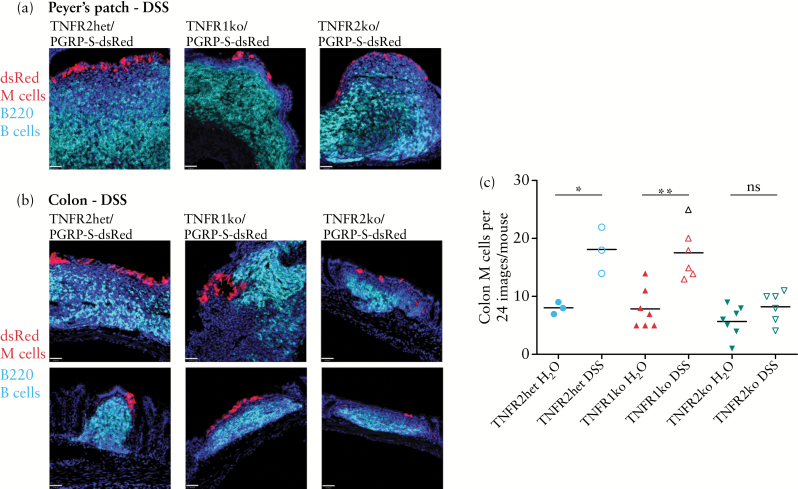
We next studied the influence of the TNFR1 and TNFR2 knockouts on DSS-induced colonic M cells. In similar fashion as in our previous study on M cell induction, we performed counts of M cells using surveys of histological sections of colon, though these surveys were extended to include greater areas of tissue. As previously reported17 and shown in Figure 3, although there were no significant differences in the Peyer’s patches [Figure 3a], the colon showed mononuclear infiltrates in the lamina propria [Figure 3b] that were often only loosely organised with weaker expression of stromal cell markers compared with Peyer’s patches, though consistently associated with induced dsRed-expressing M cells in the overlying epithelium. As noted above, constitutive colonic patch M cells are present in all knockout mice, so they will be counted among the induced M cells, as we have no way to distinguish these cells by phenotype. Due to the small size of colonic patches [compared with Peyer’s patches], they could not be dissected out from the colonic tissues before preparation of tissues for histological analysis. However, DSS-induced M cells, whether associated with colonic patches or new lymphoid infiltrates, should show the same TNFα dependence previously reported.
Figure 3.
Colonic M cell induction by dextran sodium sulphate [DSS] is absent in TNFR2 but not TNFR1 knockout mice. a. DSS treatment has no marked effect on the development and organisation of Peyer’s patch B cell follicles [cyan] and associated M cells [red] in TNFR1 and TNFR2 knockout mice. Scale bar: 40 µm. b. DSS treatment induces infiltrates in the colon with variable organisation of B cells [cyan]. M cells [red] are found associated with infiltrates although there are fewer M cells found in TNFR2 knockout mice. Scale bar: 40 µm. c. M cell counts in a histological survey of colon [24 images from each mouse] show significant increases in M cell counts in control and TNFR1 knockout mice, but no significant increase above background in TNFR2 knockout mice. Results are representative of three independent replicate studies. [*P < 0.05; **P < 0.005; ns, not significant].
With respect to the TNFR1 knockout mice, DSS treatment induced a significant increase in overall M cell numbers in the colon, similar to that seen in our previous study. However, by contrast, TNFR2 knockout mice showed no significant increase in the overall number of colonic M cells above background counts [Figure 3c]. Even in areas where B cell infiltrates were found associated with M cells, the number of M cells was low relative to similar infiltrates in wild-type and TNFR1 knockouts. Thus, it appears that in accordance with our previous study, ‘inducible’ M cells in the colon are strictly dependent on TNFα cytokine signalling; moreover, they are specifically dependent on signalling through TNFR2 but not TNFR1.
3.4. RANKL induction in the colon is independent of both TNFR1 and TNFR2 signalling
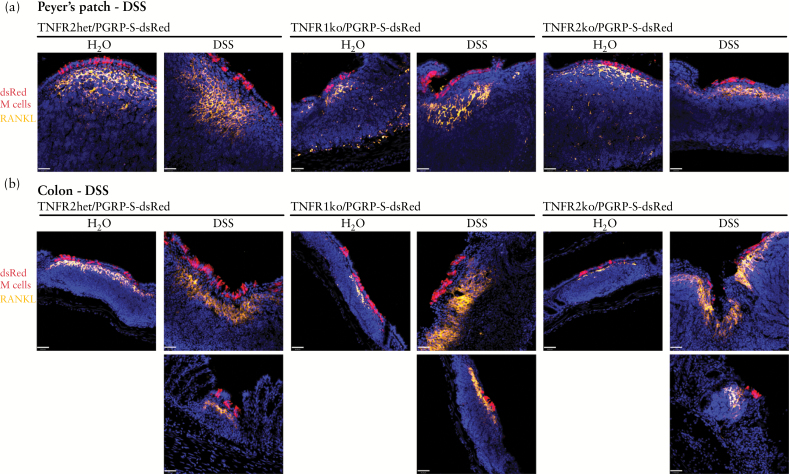
Studies have suggested that the cytokine RANKL is important for Peyer’s patch M cell development; blockade of RANKL in vivo reduced the number of Peyer’s patch M cells,10 and addition of exogenous RANKL in organoid cultures11,35 also promoted M cell development. To assess the potential involvement of RANKL in the induction of M cells in our studies, we stained Peyer’s patch and colonic tissues for RANKL [Figure 4]. The rationale for histological analysis of RANKL is that although general tissue levels of RANKL transcripts may increase overall in DSS-induced inflammation,17 the actual measurable induction by quantitative polymerase chain reaction [qPCR] within whole tissue extracts was only about 2-fold in some groups of mice. This was likely due to pre-existing background levels, dilution of induced RANKL mRNA transcripts by whole tissue extracts, compounded by the specific inhibitory effects of DSS on mRNA extraction and cDNA synthesis.36 In this context, the dynamic range in detecting overall tissue levels of RANKL would potentially mask the more critical effect of local inflammation-induced RANKL in their effect on adjacent epithelial M cell precursors. Therefore, a histological analysis to assess the specific expression of RANKL by cells adjacent to induced M cells is more indicative of any relationship between these cells. Accordingly, RANKL was predictably seen in highly localised arrangements of fibroblast-like stromal cells in Peyer’s patches directly below the follicle epithelium M cells [Figure 4a], and there was an apparent increase in the extent of RANKL staining in the Peyer’s patches of DSS-treated mice.
Figure 4.
Dextran sodium sulphate [DSS] induces lamina propria RANKL expression in PP and colon. a. Immunostaining of Peyer’s patches [PP] for receptor activator of nuclear factor kappa-B ligand [RANKL] [orange] shows expression in stromal cells, with apparent increases in DSS-treated mice, though no apparent effect on M cells [red]. Scale bar: 40 µm. b. DSS-induced colonic infiltrates show induction of lamina propria stromal cell RANKL expression [orange], with apparent increase in RANKL signal relative to constitutive colonic patch levels. M cells in red. Scale bar: 40 µm.
In the lamina propria infiltrates below DSS-induced colonic M cells, RANKL was also induced in the lamina propria, again in a highly localised pattern suggestive of stromal fibroblasts [Figure 4b]. This induction of RANKL was expected, based on our previous analysis of gene transcript induction in intestinal tissue extracts in the DSS model17; however, immunostaining demonstrates that most RANKL signal is very strongly induced but specifically localised to areas with infiltrates and adjacent induced M cells, rather than uniformly induced across the tissue. Interestingly, induction was seen in both TNFR1 and TNFR2 knockout mice treated with DSS [Figure 4b] despite the striking deficit in M cell induction in TNFR2 knockout mice. These results indicate that RANKL induction may be independent of either TNFR1 and TNFR2 signalling, so other as yet unknown factors may instead be more critical to the induction of stromal fibroblast expression of RANKL. It should be noted that in one recent study, Salmonella infection appeared to induce de novo M cell development through autocrine expression of RANKL by intestinal epithelial cells.37 However, our staining for RANKL in DSS-treated tissues only detected signal in the lamina propria stromal cells, and did not show any evidence for induction in colonic epithelium.
Finally, since TNFR2 knockout mice were unable to induce new colonic M cells with DSS treatment, the induction of RANKL in the lamina propria of TNFR2 knockout mice is also apparently not by itself sufficient to induce new inflammation-associated colonic M cells. This suggests that constitutive mucosal lymphoid tissue M cells require different factors compared with inducible M cells. In this scenario, RANKL would be more strictly considered as both necessary and sufficient for development of constitutive M cells. By contrast, TNFR2 signalling, along with probable coordination with RANKL, is a requirement specifically applied to development of inflammation-inducible colonic M cells.
3.5. DSS induction of colonic M cells is independent of LTβR blockade
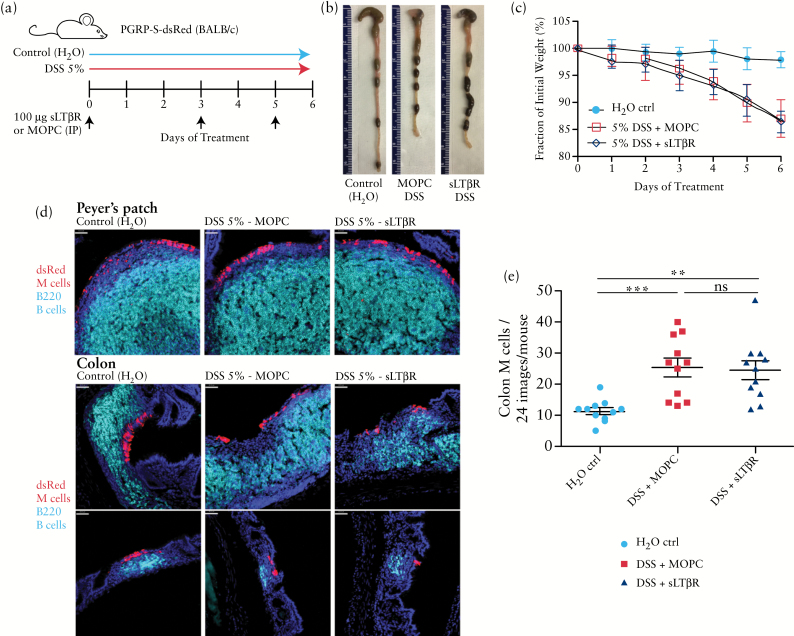
M cell development is usually associated with organised lymphoid tissues such as the constitutive Peyer’s patches and colonic patches, as well as nasopharyngeal associated lymphoid tissues [NALT] in the upper airway. These organised lymphoid tissues are thought to be dependent on induction by lymphoid tissue inducer cells, recently identified as a subset of innate lymphoid cells. These cells provide a variety of signals to induce lymphoid stromal cell development, including the trimeric cytokine lymphotoxin α1β2, known to be the ligand for the lymphotoxin beta receptor [LTβR]. To test the potential for LTβR involvement in DSS-inducible colonic M cells, we treated PGRP-S-dsRed mice with soluble LTβR [sLTβR] or isotype control antibody during DSS induction [Figure 5a]. Treatment with sLTβR had no effect on DSS-induced clinical symptoms [Figure 5b, c], nor on the induction of colonic M cells [Figure 5d, e].
Figure 5.
Dextran sodium sulphate [DSS] M cell induction is unaffected by LTβR blockade. a. Treatment protocol shown indicating timing of injection of soluble LTβR [sLTβR] or isotype control antibody on Days 0, 3, and 5. b. Representative colon images show that mice remain susceptible to DSS colitis despite LTβR blockade. c. LTβR blockade has no effect on DSS-induced weight loss. d. LTβR blockade did not significantly alter the B cell follicle organisation [cyan] in constitutive Peyer’s patches, nor did it affect M cells [red] in the follicle epithelium. Similarly, it did not affect colonic infiltrates or associated M cells. Scale bar: 40 µm. e. Colonic M cell induction was not affected by LTβR blockade. Results are representative of three independent replicate studies [**P < 0.005; ***P < 0.0005; ns, not significant].
3.6. LTβR blockade reduces DSS induction of RANKL relative to M cells in Peyer’s patch and colon
As with our studies on TNFR1 and TNFR2 knockout mice, immunostaining for RANKL also showed induction in stromal fibroblasts in both Peyer’s patches and among colonic infiltrates, but not in epithelial cells [Figure 6a]. Interestingly however, it appeared that in both the Peyer’s patches and colonic infiltrates, sLTβR treatment appeared to significantly reduce the overall level of RANKL signal compared with isotype control treatment, despite overall consistency in the numbers of induced M cells. Morphometric analysis of RANKL immunostaining signal and M cell PGRP-S-dsRed reporter signal confirmed this impression. Here, we used fluorescence signals for both RANKL and dsRed-expressing M cells, and calculated a ratio of the local fluorescence areas rather than attempt to perform specific M cell counts; with this method, the comparisons can be performed for any image regardless of the absolute RANKL or M cell induction. Since results are calculated as a local ratio of signals, the quantification is internally controlled, and would not be subject to the great variation from random sampling of sections lacking M cell and RANKL induction.
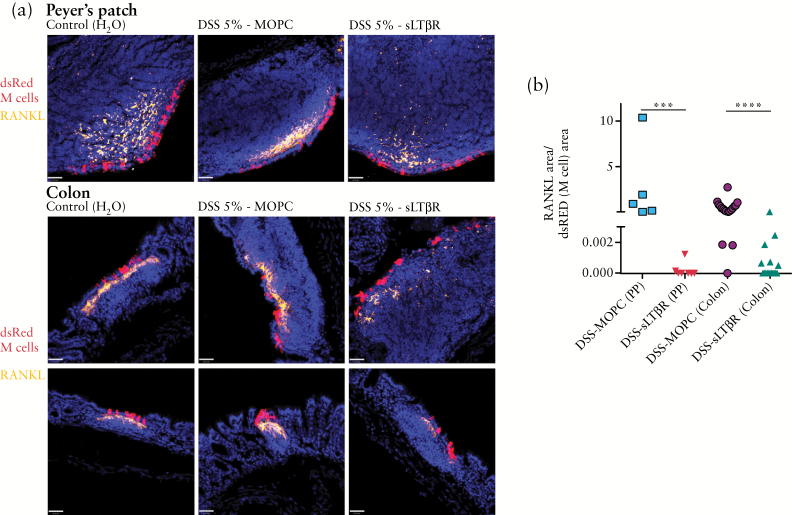
Figure 6.
Dextran sodium sulphate DSS induction of receptor activator of nuclear factor kappa-B ligand RANKL in PP and colon is reduced by LTβR blockade. a. Immunostaining of Peyer’s patches [PP] and colonic infiltrates for RANKL [orange] show expression in stromal cells, with apparent decreased signal in DSS/sLTβR-treated mice, though no apparent effect on M cells [red]. Scale bar: 40 µm. b. Although colonic M cell induction was not affected by LTβR blockade [Figure 5e], morphometric analysis of RANKL:M cell ratios shows alteration of ratios indicating decrease in RANKL signal relative to M cell signal in both Peyer’s patches and colonic infiltrates. Each data point reflects a calculated RANKL:M cell ratio from a single microscopy image; multiple images were acquired from each of two different mice per experimental condition. Note that the y-axis is split, in order to show more clearly that among the LTβR blockade group, many of the RANKL:M cell ratios were zero [***P < 0.0005; ****P < 0.0001].
The results of our morphometric analysis showed that the calculated ratio of RANKL immunofluorescence to M cell cross-sectional dsRed fluorescence was significantly decreased in both Peyer’s patches and colonic M cell-associated infiltrates [Figure 6b]. Note that overall M cell numbers were unchanged by sLTβR treatment, so this shift in the ratio is actually indicative of specific relative loss of the RANKL signal in each section where M cells and RANKL appeared together. Thus, whereas blockade of LTβR had no effect on colonic M cell induction by DSS, it did appear to affect RANKL induction. An implication of this effect is that RANKL may be in part dependent on LTβR signalling in both constitutive organised lymphoid tissues [e.g. Peyer’s patches] and inflammation-associated stromal cell changes [e.g. in the colon].
The apparent regulation of RANKL by LTβR signalling is consistent with reports that LTβR and RANKL are both specifically associated with development of constitutive M cells,5,9–11,38 although those previous studies did not distinguish an inflammation-inducible colonic M cell population as reported in the present study. Moreover, it also reinforces the distinction reported here of inflammation-inducible M cells being dependent on TNFR2 signalling while less dependent on ‘constitutive’ signals for mucosal lymphoid tissues such as LTβR signalling. Interestingly, based on recent in vitro enteroid studies, there appears to be a synergistic effect of TNFα signalling with RANKL39 though that study might only reflect constitutive M cell development, and differential roles of TNFR1 versus TNFR2 were not examined.
4. Discussion
M cell immune surveillance in the intestine is a double-edged sword. M cell-mediated transcytosis of bacteria and viruses from the intestinal lumen to organised immune tissues is a key step in initiation of mucosal immunity, but it is also the route of entry for many pathogens including Reovidiae, Yersinia, Salmonella, and anthrax.40–47 Thus, in the case of inflammatory bowel disease, inflammation-inducible M cells in the colon raise the question of whether these cells promote ongoing microbe-triggered inflammation or whether they enhance mucosal immune defence. Since the induced M cells are associated with variably organised lymphoid aggregates, it is not known whether they are providing effective responses to luminal microbes. Thus, it is possible that therapeutic approaches to colonic inflammation should take into account the induction of new M cells that may essentially provide a major breach in mucosal barrier function.
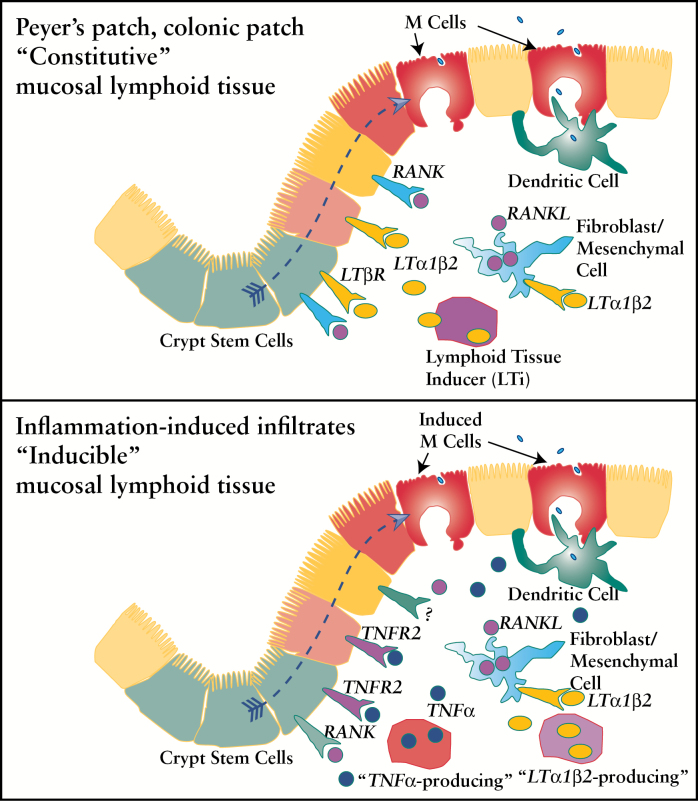
The present report shows that we can identify specific signals that can differentially affect the constitutively generated organised lymphoid tissues such as Peyer’s patches and colonic patches, versus the inflammation-inducible M cells with their poorly organised infiltrates. Specifically, our results suggest that whereas previous studies implicate LTβR and RANKL in constitutive mucosal lymphoid tissue development, our studies have identified a distinct inflammation-inducible M cell population that is dependent on TNFR2 signalling but not TNFR1 nor LTβR. Distinct factor requirements for early development versus inflammation-triggered tissue responses may also apply to RANKL induction: during constitutive development of mucosal organised lymphoid tissues, LTβR blockade appeared to have no effect on TRANCE/RANKL expression by stromal cells,48 though specific quantitation was not performed. The model in Figure 7 is a model with speculative details on interacting cells and cytokines that attempts to incorporate our results; the top part of the figure shows the dependence of constitutive M cells on LTβR and RANKL [Figure 7 top], and the bottom part of the figure indicates the role of TNFR2 [and RANKL] in the inducible population in M cell induction and speculative additional possible role of LTβR in the regulation of RANKL on stromal cells [Figure 7 bottom].
Figure 7.
Speculative model for constitutive versus inducible M cell subsets. Top, constitutive mucosal lymphoid tissues such as Peyer’s patches and colonic patches are shown with speculative involvement of LTα1β2/LTβR and receptor activator of nuclear factor kappa-B ligand [RANKL]/RANK signalling in M cell development. Bottom, inducible mucosal lymphoid tissues are shown with speculative involvement of TNFα/TNFR2 and possible additional signals in M cell induction, and LTα1β2/LTβR signalling on stromal cells to induce RANKL expression.
Overall, our results are consistent with a more generalisable hypothesis that there are distinct factor requirements in development of constitutive mucosal lymphoid tissues versus factors involved in inflammation-induced lymphoid structures. For example, in the airways, whereas LTβR signalling is required for most constitutive mucosal lymphoid tissue development, it appears not to be required for inducible bronchus-associated lymphoid tissue [iBALT], and cytokines such as IL-22 may be more important.49,50 However, it is not yet clear whether iBALT-associated M cells51 are TNFR2-dependent.
Finally, returning to the case of intestinal inflammation, the specific cellular interactions associated with the production of the signals for each distinct M cell subset remain to be identified; but once target signalling pathways and cellular actors are established, more focused therapeutic approaches can be developed that may significantly alter the inflammatory process in inflammatory bowel disease. In addition, a dissection of the distinct requirements and functions of inducible M cells and associated mucosal immune tissues will be important in understanding their role in chronic inflammation; it is not yet clear whether these induced cells help promote continuing inflammation, or whether they are critical to initiating immunoregulatory mechanisms. Knowing these roles should lead to more specifically targeted therapies that will promote the regulation and resolution of chronic intestinal inflammation.
Funding
This work was supported by the National Institute for Allergy and Infectious Disease of the National Institutes of Health [under award R01AI63426].
Conflict of Interest
The authors have no conflicting financial interests.
Author Contributions
EP designed and performed the experiments and helped with data analysis; EW performed histological morphometric analysis on RANKL; and DDL helped with the design of the experiments and data analysis, helped assemble the data figures, and wrote the manuscript.
Acknowledgments
The authors thank Elma Frias for management of the animal colony, and Olivia Sakhon for helpful comments on the manuscript and assistance with experiment preparation. We thank Biogen Inc. for their generous gift of soluble lymphotoxin beta receptor [sLTβR].
References
- 1. Neutra MR, Pringault E, Kraehenbuhl JP. Antigen sampling across epithelial barriers and induction of mucosal immune responses. Annu Rev Immunol 1996;14:275–300. [DOI] [PubMed] [Google Scholar]
- 2. Kraehenbuhl JP, Neutra MR. Epithelial M cells: differentiation and function. Annu Rev Cell Dev Biol 2000;16:301–32. [DOI] [PubMed] [Google Scholar]
- 3. Vondenhoff MF, Kraal G, Mebius RE. Lymphoid organogenesis in brief. Eur J Immunol 2007;37[Suppl 1]:S46–52. [DOI] [PubMed] [Google Scholar]
- 4. Neutra MR, Frey A, Kraehenbuhl JP. Epithelial M cells: gateways for mucosal infection and immunization. Cell 1996;86:345–8. [DOI] [PubMed] [Google Scholar]
- 5. Matsumoto M, Iwamasa K, Rennert PD, et al. Involvement of distinct cellular compartments in the abnormal lymphoid organogenesis in lymphotoxin-alpha-deficient mice and alymphoplasia [aly] mice defined by the chimeric analysis. J Immunol 1999;163:1584–91. [PubMed] [Google Scholar]
- 6. Spits H, Artis D, Colonna M, et al. Innate lymphoid cells–a proposal for uniform nomenclature. Nat Rev Immunol 2013;13:145–9. [DOI] [PubMed] [Google Scholar]
- 7. Molenaar R, Greuter M, van der Marel AP, et al. Lymph node stromal cells support dendritic cell-induced gut-homing of T cells. J Immunol 2009;183:6395–402. [DOI] [PubMed] [Google Scholar]
- 8. Roozendaal R, Mebius RE. Stromal cell-immune cell interactions. Annu Rev Immunol 2011;29:23–43. [DOI] [PubMed] [Google Scholar]
- 9. Rennert PD, Browning JL, Mebius R, Mackay F, Hochman PS. Surface lymphotoxin alpha/beta complex is required for the development of peripheral lymphoid organs. J Exp Med 1996;184:1999–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Knoop KA, Kumar N, Butler BR, et al. RANKL is necessary and sufficient to initiate development of antigen-sampling M cells in the intestinal epithelium. J Immunol 2009;183:5738–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Lau W, Kujala P, Schneeberger K, et al. Peyer’s patch M cells derived from Lgr5[+] stem cells require SpiB and are induced by RankL in cultured ‘miniguts.’ Mol Cell Biol 2012;32:3639–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kernéis S, Bogdanova A, Kraehenbuhl JP, Pringault E. Conversion by Peyer’s patch lymphocytes of human enterocytes into M cells that transport bacteria. Science 1997;277:949–52. [DOI] [PubMed] [Google Scholar]
- 13. Hsieh EH, Fernandez X, Wang J, et al. CD137 is required for M cell functional maturation but not lineage commitment. Am J Pathol 2010;177:666–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Golovkina TV, Shlomchik M, Hannum L, Chervonsky A. Organogenic role of B lymphocytes in mucosal immunity. Science 1999;286:1965–8. [DOI] [PubMed] [Google Scholar]
- 15. Jarry A, Robaszkiewicz M, Brousse N, Potet F. Immune cells associated with M cells in the follicle-associated epithelium of Peyer’s patches in the rat. An electron- and immuno-electron-microscopic study. Cell Tissue Res 1989;255:293–8. [DOI] [PubMed] [Google Scholar]
- 16. Wang J, Lopez-Fraga M, Rynko A, Lo DD. TNFR and LTbetaR agonists induce follicle-associated epithelium and M cell specific genes in rat and human intestinal epithelial cells. Cytokine 2009;47:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bennett KM, Parnell EA, Sanscartier C, et al. Induction of colonic M cells during intestinal inflammation. Am J Pathol 2016;186:1166–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Patel JR, Williams JL, Muccigrosso MM, et al. Astrocyte TNFR2 is required for CXCL12-mediated regulation of oligodendrocyte progenitor proliferation and differentiation within the adult CNS. Acta Neuropathol 2012;124:847–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, Ting JP. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci 2001;4:1116–22. [DOI] [PubMed] [Google Scholar]
- 20. van Oosten BW, Barkhof F, Truyen L, et al. Increased MRI activity and immune activation in two multiple sclerosis patients treated with the monoclonal anti-tumor necrosis factor antibody cA2. Neurology 1996;47:1531–4. [DOI] [PubMed] [Google Scholar]
- 21. TNF neutralization in MS: results of a randomized, placebo-controlled multicenter study. The Lenercept Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group. Neurology 1999;53:457–65. [PubMed] [Google Scholar]
- 22. Sicotte NL, Voskuhl RR. Onset of multiple sclerosis associated with anti-TNF therapy. Neurology 2001;57:1885–8. [DOI] [PubMed] [Google Scholar]
- 23. Kaltsonoudis E, Voulgari PV, Konitsiotis S, Drosos AA. Demyelination and other neurological adverse events after anti-TNF therapy. Autoimmun Rev 2014;13:54–8. [DOI] [PubMed] [Google Scholar]
- 24. Theibich A, Dreyer L, Magyari M, Locht H. Demyelinizing neurological disease after treatment with tumor necrosis factor alpha-inhibiting agents in a rheumatological outpatient clinic: description of six cases. Clin Rheumatol 2014;33:719–23. [DOI] [PubMed] [Google Scholar]
- 25. Wang J, Gusti V, Saraswati A, Lo DD. Convergent and divergent development among M cell lineages in mouse mucosal epithelium. J Immunol 2011;187:5277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Whittem CG, Williams AD, Williams CS. Murine colitis modeling using Dextran Sulfate Sodium [DSS]. J Vis Exp 2010, Jan 19. doi: 10.3791/1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 1990;98:694–702. [DOI] [PubMed] [Google Scholar]
- 28. Rennert PD, Browning JL, Mebius R, Mackay F, Hochman PS. Surface lymphotoxin alpha/beta complex is required for the development of peripheral lymphoid organs. J Exp Med 1996;184:1999–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lo D, Tynan W, Dickerson J, et al. Peptidoglycan recognition protein expression in mouse Peyer’s Patch follicle associated epithelium suggests functional specialisation. Cell Immunol 2003;224:8–16. [DOI] [PubMed] [Google Scholar]
- 30. Terahara K, Yoshida M, Igarashi O, et al. Comprehensive gene expression profiling of Peyer’s patch M cells, villous M-like cells, and intestinal epithelial cells. J Immunol 2008;180:7840–6. [DOI] [PubMed] [Google Scholar]
- 31. Wang F, Schwarz BT, Graham WV, et al. IFN-gamma-induced TNFR2 expression is required for TNF-dependent intestinal epithelial barrier dysfunction. Gastroenterology 2006;131:1153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stillie R, Stadnyk AW. Role of TNF receptors, TNFR1 and TNFR2, in dextran sodium sulfate-induced colitis. Inflamm Bowel Dis 2009;15:1515–25. [DOI] [PubMed] [Google Scholar]
- 33. Mizoguchi E, Mizoguchi A, Takedatsu H, et al. Role of tumor necrosis factor receptor 2 [TNFR2] in colonic epithelial hyperplasia and chronic intestinal inflammation in mice. Gastroenterology 2002;122:134–44. [DOI] [PubMed] [Google Scholar]
- 34. Corredor J, Yan F, Shen CC, et al. Tumor necrosis factor regulates intestinal epithelial cell migration by receptor-dependent mechanisms. Am J Physiol Cell Physiol 2003;284:C953–61. [DOI] [PubMed] [Google Scholar]
- 35. Rouch JD, Scott A, Lei NY, et al. Development of functional Microfold [M] cells from intestinal stem cells in primary human enteroids. PloS One 2016;11:e0148216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Viennois E, Chen F, Laroui H, Baker MT, Merlin D. Dextran sodium sulfate inhibits the activities of both polymerase and reverse transcriptase: lithium chloride purification, a rapid and efficient technique to purify RNA. BMC Res Notes 2013;6:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tahoun A, Mahajan S, Paxton E, et al. Salmonella transforms follicle-associated epithelial cells into M cells to promote intestinal invasion. Cell Host Microbe 2012;12:645–56. [DOI] [PubMed] [Google Scholar]
- 38. Honda K, Nakano H, Yoshida H, et al. Molecular basis for hematopoietic/mesenchymal interaction during initiation of Peyer’s patch organogenesis. J Exp Med 2001;193:621–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wood MB, Rios D, Williams IR. TNF-α augments RANKL-dependent intestinal M cell differentiation in enteroid cultures. Am J Physiol Cell Physiol 2016;311:C498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wolf JL, Rubin DH, Finberg R, et al. Intestinal M cells: a pathway for entry of reovirus into the host. Science 1981;212:471–2. [DOI] [PubMed] [Google Scholar]
- 41. Clark MA, Hirst BH, Jepson MA. M-cell surface beta1 integrin expression and invasin-mediated targeting of Yersinia pseudotuberculosis to mouse Peyer’s patch M cells. Infect Immun 1998;66:1237–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schulte R, Kerneis S, Klinke S, et al. Translocation of Yersinia entrocolitica across reconstituted intestinal epithelial monolayers is triggered by Yersinia invasin binding to beta1 integrins apically expressed on M-like cells. Cell Microbiol 2000;2:173–85. [DOI] [PubMed] [Google Scholar]
- 43. Fotopoulos G, Harari A, Michetti P, Trono D, Pantaleo G, Kraehenbuhl JP. Transepithelial transport of HIV-1 by M cells is receptor-mediated. Proc Natl Acad Sci U S A 2002;99:9410–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hase K, Kawano K, Nochi T, et al. Uptake through glycoprotein 2 of FimH[+] bacteria by M cells initiates mucosal immune response. Nature 2009;462:226–30. [DOI] [PubMed] [Google Scholar]
- 45. Martinez-Argudo I, Jepson MA. Salmonella translocates across an in vitro M cell model independently of SPI-1 and SPI-2. Microbiology 2008;154:3887–94. [DOI] [PubMed] [Google Scholar]
- 46. Glomski IJ, Piris-Gimenez A, Huerre M, Mock M, Goossens PL. Primary involvement of pharynx and peyer’s patch in inhalational and intestinal anthrax. PLoS Pathog 2007;3:e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dersch P, Isberg RR. A region of the Yersinia pseudotuberculosis invasin protein enhances integrin-mediated uptake into mammalian cells and promotes self-association. EMBO J 1999;18:1199–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Taylor RT, Patel SR, Lin E, et al. Lymphotoxin-independent expression of TNF-related activation-induced cytokine by stromal cells in cryptopatches, isolated lymphoid follicles, and Peyer’s patches. J Immunol 2007;178:5659–67. [DOI] [PubMed] [Google Scholar]
- 49. Hwang JY, Randall TD, Silva-Sanchez A. Inducible bronchus-associated lymphoid tissue: taming inflammation in the lung. Front Immunol 2016;7:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Barone F, Nayar S, Campos J, et al. IL-22 regulates lymphoid chemokine production and assembly of tertiary lymphoid organs. Proc Natl Acad Sci U S A 2015;112:11024–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tango M, Suzuki E, Gejyo F, Ushiki T. The presence of specialised epithelial cells on the bronchus-associated lymphoid tissue [BALT] in the mouse. Arch Histol Cytol 2000;63:81–9. [DOI] [PubMed] [Google Scholar]