Abstract
Objective
Recent studies have suggested that the enteric nervous system can modulate gut immunity. Ecto-nucleoside triphosphate diphosphohydrolases [E-NTPDases] regulate purinergic signalling by sequential phosphohydrolysis of pro-inflammatory extracellular adenosine 5'-triphosphate [ATP]. Herein, we test the hypothesis that E-NTPDases modulate gut inflammation via neuro-immune crosstalk.
Design
We determined expression patterns of NTPDase2 and NTPDase3 in murine and human colon. Experimental colitis was induced by dextran sodium sulphate [DSS] in genetically engineered mice deficient in NTPDase2 or NTPDase3. We compared plasma adenosine diphosphatase [ADPase] activity from Crohn’s patients and healthy controls, and linked the enzyme activity to Crohn’s disease activity.
Results
NTPDase2 and -3 were chiefly expressed in cells of the enteric nervous system in both murine and human colon. When compared with wild type, DSS-induced colitis was exacerbated in Entpd2, and to a lesser extent, Entpd3 null mice as measured by disease activity score and histology, and marked anaemia was seen in both. Colonic macrophages isolated from Entpd2 null mice displayed a pro-inflammatory phenotype compared with wild type. In human plasma, Crohn’s patients had decreases in ADPase activity when compared with healthy controls. The drop in ADPase activity was likely associated with changes in NTPDase2 and -3, as suggested by inhibitor studies, and were correlated with Crohn’s disease activity.
Conclusions
NTPDase2 and -3 are ecto-enzymes expressed in the enteric nervous system. Both enzymes confer protection against gut inflammation in experimental colitis and exhibit alterations in Crohn’s disease. These observations suggest that purinergic signalling modulated by E-NTPDases governs neuro-immune interactions that are relevant in Crohn’s disease.
Keywords: enteric nervous system, Crohn’s disease, ectonucleotidase
1. Introduction
Crohn’s disease is a relapsing inflammatory condition that can be influenced by genetics, dysbiosis, mucosal barrier integrity and alterations in innate and adaptive immunity.1,2 Emerging evidence suggests that the enteric nervous system plays an important role in gut inflammation, and could be relevant in Crohn’s disease.3
A recent study by Gabanyi and coworkers reported that enteric neurons can directly influence the phenotype of macrophages in the lamina propria and muscularis externa.4 It has also been shown that glial cells in the gut can perpetuate the release of adenosine triphosphate [ATP] into the extracellular space, which mediates inflammation via purinergic signalling.5 Extracellular ATP [eATP], a pro-inflammatory signalling molecule, is rapidly hydrolysed via cell surface-located ecto-nucleoside triphosphate diphosphohydrolases [E-NTPDases] and ecto-5’-nucleotidase to ultimately produce adenosine, which in turn has mostly anti-inflammatory properties.6,7 E-NTPDases therefore often mediate an anti-inflammatory response in immune regulation.
CD39, also known as NTPDase1, the prototype member of the E-NTPDase family, is expressed on immune cells and endothelial cells.8 CD39/ENTPD1 deletion exacerbates colitis in a dextran sodium sulphate [DSS]-induced experimental murine model.9,10 Individuals with a single nucleotide polymorphism [SNP] variant associated with low levels of CD39 expression exhibit increased susceptibility to Crohn’s disease in case-control studies.9 Recent genome-wide association studies [GWAS] suggest strong associations between genotype variants of CD39 and immunophenotype of regulatory T cells.11,12 We have shown that CD39 can affect Crohn’s disease in part via modulation of the number and function of Th17 cells.13–15 This effect is in part also mediated by an increase in the levels of adenosine via CD39, which mediates an anti-inflammatory response through adenosine 2A [A2A] receptors.16 The role of adenosine in colitis is emphasised by studies that showed amelioration of experimental colitis by administration of adenosine 2A [A2A] receptor agonists.17,18
Within the gut, ATPase activity is predominantly localised in blood vessels, areas of the smooth muscle layers, and the enteric nervous system.19 NTPDase2 and NTPDase3 are two cell membrane-located E-NTPDases that share significant structural homology and functional similarity to CD39.7,20 Both ectonucleotidases are known to be expressed in nerve tissues.21,22 NTPDase2 expression has been associated with immature and non-myelinating Schwann cells in peripheral nerves, satellite glial cells in the dorsal root ganglia and sympathetic ganglia.23 In the gut, NTPDase2 and NTPDase3 expression has been described on cells of the enteric nervous system, with NTPDase2 on glial cells and NTPDase3 on both glia and neurons.19,23,24 Nonetheless, any roles NTPDase2 and NTPDase3 might play in gut inflammation are unknown.
Herein, we test the hypothesis that purinergic signalling modulated by NTPDase2 and NTPDase3 may regulate intestinal neuro-immune interaction and affect gut inflammation. We further investigate the relevance of this mechanism in human Crohn’s disease.
2. Methods and Reagents
2.1 Animals
All animal care and experiments were carried out under the guidelines and protocols approved by the animal care and use committee at Beth Israel Deaconess Medical Center [BIDMC]. We generated a global Entpd2 [encoding NTPDase2] null mouse line as described previously.25 The strategy for generating global Entpd3 [encoding NTPDase3] null mice is illustrated in Supplementary Figure 1, available as Supplementary data at ECCO-JCC online. Male animals between 8–12 weeks of age were used in all experiments. Age-matched C57/B6 wild type mice were used as controls.
2.2 Induction and assessment of experimental colitis
Age-matched Entpd2 null, Entpd3 null and wild type mice [n ≥ 5 per group] were treated with 3% DSS in drinking water that they received without restriction for 7 consecutive days. Control mice received standard drinking water. Every 24 h, body weights were measured and stools were examined. A disease activity score was calculated for each day using weight loss, degree of bleeding, and stool consistency as detailed in Supplementary Table 1, available as Supplementary data at ECCO-JCC online. After 7 days, mice were euthanised to retrieve colon tissue for histological analysis or cell isolation. Blood was sampled in 10% sodium citrate and haematocrit was measured from whole blood using microhaematocrit capillary tubes [Fisherbrand, Pittsburgh, PA]. Colons were opened longitudinally, cleaned, and embedded in OCT freezing medium [Fisher Scientific, Waltham, MA]. The tissues were immediately snap-frozen in pre-cooled isopentane and stored at -80°C until use. The experiment was conducted three times, with n ≥ 5 mice per group each time. Results from one representative experiment are shown. The disease activity is also assessed histologically. A gastrointestinal pathologist with experience in animal models, who was blinded to the study design, reviewed one full set of colon tissue with haematoxylin and eosin [H&E] stain. Two histological scores were assigned using scoring systems described by Kim and Wirtz, respectively.26,27 The statistical difference among these three groups was analysed by analysis of variance [ANOVA].
2.3 Immunohistochemistry
Formalin-fixed, paraffin-embedded [FFPE] human colon tissue or frozen murine colon tissue were cut into 6-µm sections. One slide from each block was stained by haematoxylin and eosin for morphological analysis. For immunohistochemistry, sections of murine colon tissue were fixed in acetone and sections of human colon tissue were cut from FFPE cassettes and blocked with 7% horse serum [Vector Labs, Burlingame, CA] for 30 min. The tissues were first incubated with primary antibodies overnight at 4°C. A complete list of the primary and secondary antibodies used for immunohistochemistry is shown in Supplementary Table 2, available as Supplementary data at ECCO-JCC online. We used tissues from Entpd2 and Entpd3 null mice to control for the specificity of mouse NTPDase2 and -3 antibodies. Every set of the experiment was controlled by the use of secondary antibody alone in the absence of primary antibodies, to control for the nonspecific binding from secondary antibody. After peroxidase and biotin activity blocking, sections were incubated with the biotinylated secondary antibody for 1 h, continued with Avidin Biotin complex HRP and visualised with ImmPACT DAB [Vector Labs]. All slides were mounted on Cytoseal, and examined and recorded on a Nikon microscope. For fluorescent double staining, we used the respective fluorescent secondary antibodies or streptavidin conjugated with Alexa Fluor 594 [Jackson ImmunoResearch, Wet Grove, PA]. Sections were co-stained with Hoechst and covered with polyvinyl alcohol mounting medium [Sigma-Aldrich] and examined on a Nikon MultiPhoton Fluorescent Microscope.
2.4 Isolation of colon macrophages and flow cytometry
Immune cells were isolated from freshly harvested colon tissue as described by Gabanyi et al. with modifications.4 In short, colons were washed, briefly incubated with 1mM DTT, and rinsed. The tissues were cut into small pieces and resuspended in digestion buffer [HBSS with Ca2+ and Mg2+, 0.5mg/ml Collagenase IV] [Worthington, Lakewood, NJ], 0.05mg/ml DNase I [Roche Diagnostics, Indianapolis, IN], 5% FBS, 1mM NaPyr, 25mM HEPES. After 40 min incubation at 37°C under constant shaking, tissues were mechanically homogenised, filtered through a 100-µm cell strainer, and centrifuged. Cell pellets were then resuspended in 40% percoll solution, layered over an 80% percoll solution [GE Healthcare, Uppsala, Sweden], and centrifuged at 2000 rpm for 20 min with no brake during deceleration. The interphase was collected and washed, then resuspended in PBS to test cell viability using the Zombie AcquaTM fixable viability kit [Biolegend, San Diego, CA]. After washing, cells were resuspended in PBS with 2% fetal bovine serum, and incubated with antibodies against CD45-Pacific Blue, CD11b-PE-Cy7, F4/80-APC, MHCII-APC-Cy7, or CD86-APC-Cy7, Ly6C-Alexa Fluor 700 [Biolegend] and CD39 [PE, eBioscience, San Diego, CA] for 20 min, washed, and then analysed on a Gallios Flow Cytometer [Beckman Coulter, Danvers, MA].
2.5 Human plasma and tissue samples
Plasma samples from 14 healthy volunteers and 28 patients with Crohn’s disease were used in the study. Crohn’s disease patients had an established diagnosis based on history, endoscopic findings, and pathology. Blood samples were obtained through standard phlebotomy, after which plasmas were isolated and stored at -80°C before analysis. Patient history, Harvey-Bradshaw index [HBI] for Crohn’s disease activity, and routine laboratory tests were collected at the time of blood sampling.28 The FFPE colonic tissues from five patients who underwent ileocaecectomy due to Crohn’s disease were also obtained; mean age was 39 [range 24–59, three females and two males]. All five patients had ileocolonic disease and were treatment-experienced with two or more immunomodulatory therapies, including thiopurines and anti-tumour necrosis factor alpha [TNF]α, before the surgery. Cancer-free colon tissues from five patients who underwent resection for colorectal cancer, without history of inflammatory bowel disease, were used as controls. The study has been approved by the institutional review board at Beth Israel Deaconess Medical Center [BIDMC].
2.6 ADPase activity assay
ADPase activity assay was performed with 14C-ADP as substrate and analysed by thin layer chromatography [TLC], as described previously.29 Briefly, human plasmas were centrifuged at 2500 g for 10 min at room temperature before use. Each reaction contained 5 μl of 200 μM 14C-ADP [American Radiolabeled Chemicals, St Louis, MO], 10 mM calcium chloride, 10 mM magnesium chloride in phosphate-buffered saline [PBS] at pH 7.5 supplemented by inhibitors. The inhibitor concentrations were chosen based on previous literature or 10-fold that of the Ki: erythro-9-[2-hydroxy-3-nonyl]adenine [EHNA, Sigma Aldrich, St Louis, MO] at 150 μM, polyoxometalate 1 [POM1, Tocris Bioscience, Bristol, UK] at 26 μM, polyoxometalate 6 [POM6] at 40 μM, P1,P5-di-[adenosine-5’]pentaphosphate [Ap5A, Sigma Aldrich] at 80 μM. Inhibitors were incubated with plasma for 15 min before the addition of ADP substrate. For quantitation, recombinant potato apyrase [New England Biolabs, Ipswich, MA] at 0, 50, 100, 150, 200, 300, 400, 1000 mU/μl was included as standard. One unit of ATPase activity is defined as the amount of enzyme that catalyses the conversion of 1 µmol of ATP to ADP in 1 min at 30°C in a total reaction volume of 50 µl, and equals 12 times that of the ADPase activity. The reaction mixtures were incubated at 30°C for 2 h, and subsequently terminated by EDTA at 100 mM. A total of 1 μl sample was loaded onto a pre-casted silica TLC plate [Sigma Aldrich]. The TLC was performed using 150 ml buffer with isopropanol, ammonia, and water at a ratio of 4:2:1. as previously described.30 After drying, the isotope-labelled nucleotides were detected by auto-exposure on HyBlot CT films [Denville Scientific, Metuchen, NJ] for 24–72 h. The image was subsequently scanned, digitised to 32 bit BW format, and background-corrected in Image J.31 The standard curve was fitted with the following sigmoidal function:
where y is ADP conversion [defined by the ratio between adenosine and total nucleotide density], x is ADPase activity in the standards, A is the maximal ADP conversion, B is the log of inflection point, C is hillslope, and D is a correction factor for asymmetry of the sigmoidal curve. The ADPase activity in plasma samples was calculated with constants derived from the above equation using a least square deviation regression method. The inhibitor specific activity was calculated by subtracting the ADPase activity in the presence and absence of inhibitors, and reported in both absolute values and percentage activity of the total.
2.7 Statistical analysis
We compared total and inhibitor-specific ADPase activity in healthy controls and Crohn’s patients by two-tailed Student’s t test with unequal variance. Two-tailed Student’s t test with equal variance was used to compare the total and inhibitor-specific ADPase activities between inactive vs active Crohn’s disease characterised by HBI. The relationships between HBI and inhibitor-specific ADPase activity were analysed by linear regression. All statistical analyses were performed STATA/IC version 13.0 [Stata Corp., College Station, TX].
3. Results
3.1 Expression of NTPDase2 and -3 in murine intestine under normal conditions and colitis
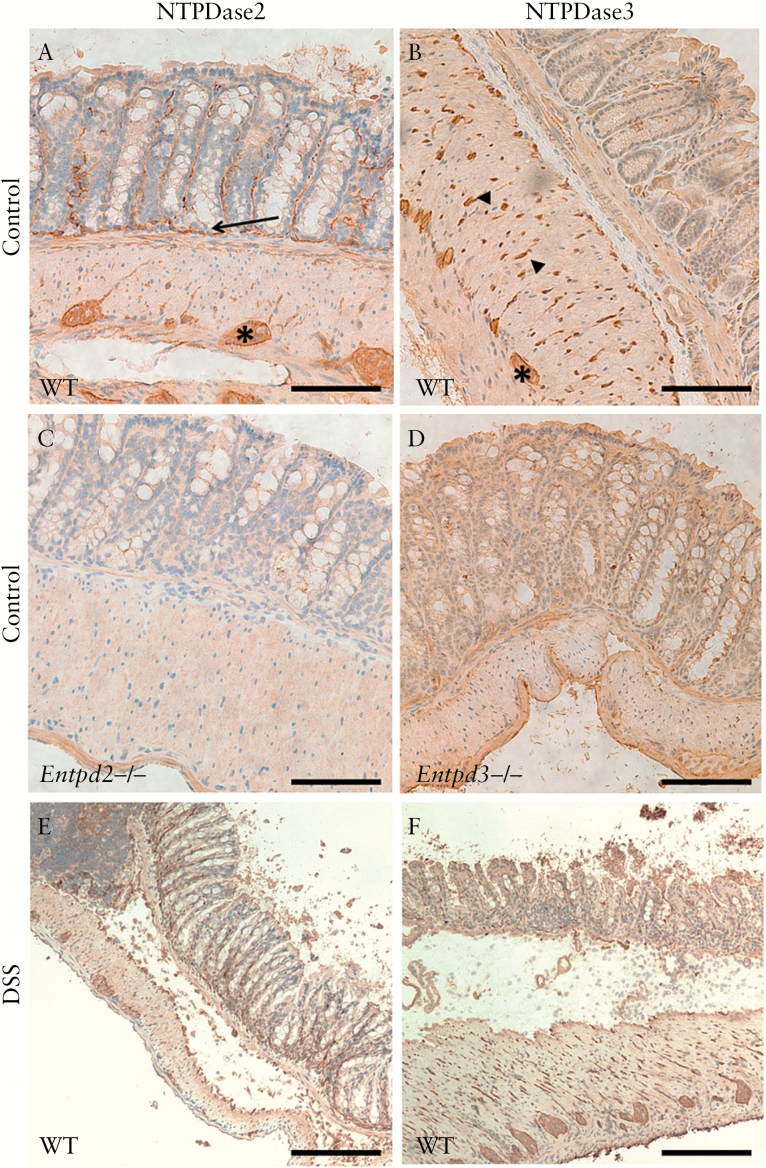
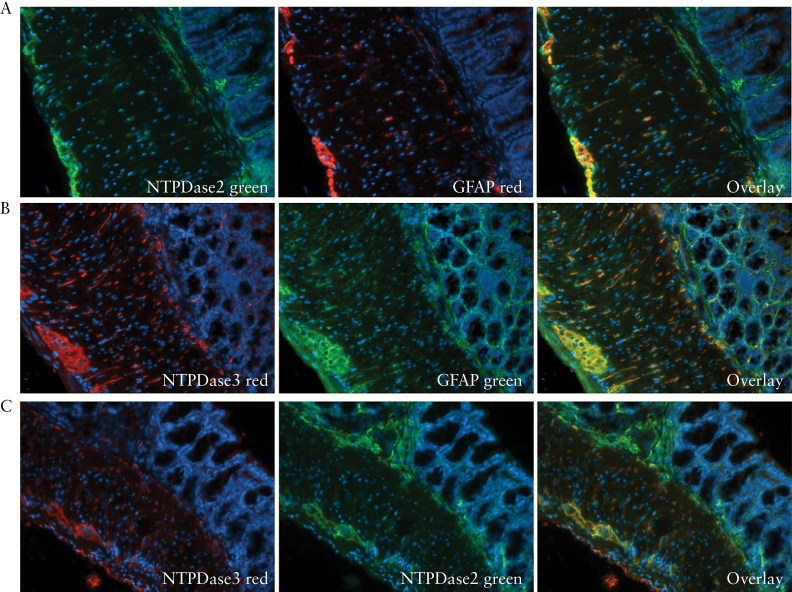
We first examined the expression of NTPDase2 and -3 using frozen murine intestinal tissues. Both E-NTPDases were expressed predominantly in the enteric nervous system [Figure 1A, B]. The specificity of the staining by NTPDase2 and -3 was confirmed by immunohistochemistry using intestinal tissues from Entpd2 and Entpd3 null mice [Figure 1C, D]. NTPDase3 showed a strong expression on nerve/glial cells that penetrate the smooth muscle layers, whereas NTPDase2 expression was scanty there. When co-stained using fluorescent microscopy, both enzymes demonstrated substantial overlap with glial fibrillary acidic protein [GFAP], a glial cell marker [Figure 2A, B]. Notably, the expression of NTPDase2 appeared to extend further into the submucosa and lamina propria, whereas the expression of NTPDase3 was mostly limited to the muscularis externa [Figure 2C].
Figure 1.
NTPDase2 and -3 expression in normal murine colon and experimental colitis. A. NTPDase2 is expressed in the ganglia of the myenteric plexus [*], scarcely in fibres of the muscularis propria, and more strongly in thin elongated structures of the submucosa and lamina propria [arrows]. B. NTPDase3 expression is also localised in the ganglion cells, with an abundant expression in cells and fibres throughout the smooth muscle layer [arrow heads] and little expression in the submucosa and lamina propria. C, D. NTPDase2 and -3 expression were absent in Entpd2 [C] and Entpd3 [D] null mice, respectively. After 7 days of dextran sodium sulphate [DSS]induction. E, F. NTPDase2 and -3 expression in the colon after DSS induction. The scale bar represents 200 µm.
Figure 2.
Expression of NTPDase2 and -3 in the murine enteric nervous system. A, B. Co-immunostaining of GFAP as a marker of glial cells with NTPDase2 [A] and NTPDase3 [B]. C. Co-immunostaining of NTPDase2 [in green] and NTPDase3 [in red].
After induction of experimental colitis by DSS treatment over 7 days, the overall expression pattern of NTPDase2 and -3 remained largely unchanged [Figure 1E, F]. NTPDase2 appeared more prominent in the submucosa and lamina propria when compared with control mice, whereas no significant difference between NTPDase3 expression was noted, which might be an impression attributed to the widening of the submucosa as a result of inflammation and oedema. The integrity of the muscularis externa in the colon was largely maintained in DSS-treated mice [Figure 1E, F]. There were no apparent changes in NTPDase2 or 3 expression in this layer.
3.2 Genetic deletion of NTPDase2 exacerbates DSS-induced colitis in mice
To validate the observations of increased NTPDase2 expression in the submucosa and lamina propria in DSS-induced colitis, we further tested the roles of NTPDase2 and -3 in inflammation by comparing the severity of DSS-induced colitis between mice deficient in NTPDase2 [Entpd2 null] or NTPDase3 [Entpd3 null] and wild type C57BL6 mice.
Under normal conditions, both NTPDase2- and -3-deficient mice developed normally, without clinical signs of spontaneous colitis or malabsorption. Both knockout strains had a similar baseline haematocrit compared with wild type [Supplementary Figure 3A, available as Supplementary data at ECCO-JCC online]. Intestinal histology showed normal morphology under baseline conditions despite the absence of enzymes in the enteric nervous system [Supplementary Figure 3B].
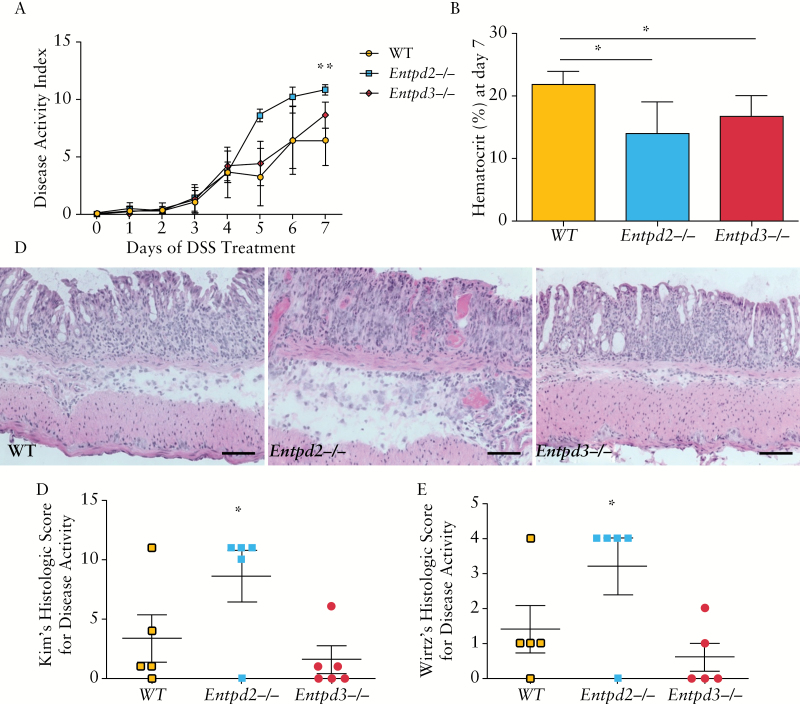
We subjected age-matched wild type, Entpd2 null, and Entpd3 null mice to 7 days of treatment with 3% DSS. A clinical disease activity index, taking into account weight loss, rectal bleeding, and stool consistency, was calculated every day for the duration of treatment [Figure 3A]. Starting on Day 5, Entpd2 null mice showed a significantly higher disease activity index when compared with wild type either on Day 7 [10.8 ± 0.4 vs 6.4 ± 2.2, p = 0.001] or by comparing the area under the curve [AUC; 35.2 ± 4.1 vs 21.0 ± 6.8, p = 0.007]. The disease activity of Entpd3 null mice was not significantly different from that of the wild type mice. Individual scores for weight loss rectal bleeding and stool showed similar trends [Supplementary Figure 4, available as Supplementary data at ECCO-JCC online]. Of note, Entpd2 null mice had 20.2% weight loss compared with 10.4% among the wild type mice [p = 0.009]. When we combined results from three independent sets of experiments, the AUC of disease activity score over 7 days among Entpd2 null mice was significantly higher than that of the wild type [30.7 ± 1.9 vs 19.3 ± 1.4, p < 0.001], whereas the Entpd3 and wild type mice had no significant difference [20.4 ± 1.5 vs 19.3 ± 1.4, p = 0.6]. We also measured haematocrit after 7 days of DSS treatment as a more objective marker for the extent of colitis-induced blood loss. Both Entpd2 null and Entpd3 null mice had a significantly lower haematocrit when compared with wild type controls [14.0 ± 5.1% vs 16.8 ± 3.3% vs 21.8 ± 2.2%, p = 0.02 and 0.04, respectively] [Figure 3B]. The difference between Entpd2 and Entpd3 was not significant.
Figure 3.
NTPDase2-deficient mice develop exacerbated inflammation in dextran sodium sulphate [DSS]-induced colitis. A. Daily clinical disease activity index in DSS induced colitis. This index was calculated from individual scores for weight loss, bleeding and stool consistency as described in Supplementary Table 1, available as Supplementary data at ECCO-JCC online. DSS-induced colitis is most severe in Entpd2 null mice when compared with wild type and Entpd3 null mice [n = 5 mice per group, this figure represents one out of three independent experiments that showed the same trends]. B. Haematocrit was measured after 7 days of treatment with 3% DSS. C. Representative haematoxylin and eosin [H&E]-stained tissue sections of mouse colon after 7 days of treatment with 3% DSS. Scale bar represents 200 µm. D, E. Histology scores of colitis using Kim’s [D] and Wirtz’s [E] methods. Error bars show standard deviations in all panels; p-values are calculated using one-way analysis of variance [ANOVA] referencing to the wild type group [*p < 0.05, **p < 0.005]. Area under the curve [AUC] of panel A was used for comparison.
In H&E-stained histological sections, we observed an increase in mucosal destruction and infiltration by mononuclear cells in Entpd2 null mice when compared with the wild type, whereas the histological changes in Entpd3 null mice after DSS treatment were similar to those in wild type mice [Figure 3C]. These observations were supported by significant differences [p = 0.04–0.05] in the histology score between Entpd2 null and wild type mice calculated using either the Kim or the Wirtz method [Figure 3D, E].
3.3 Genetic deletion of NTPDase2 alters the macrophage phenotype in DSS-induced colitis
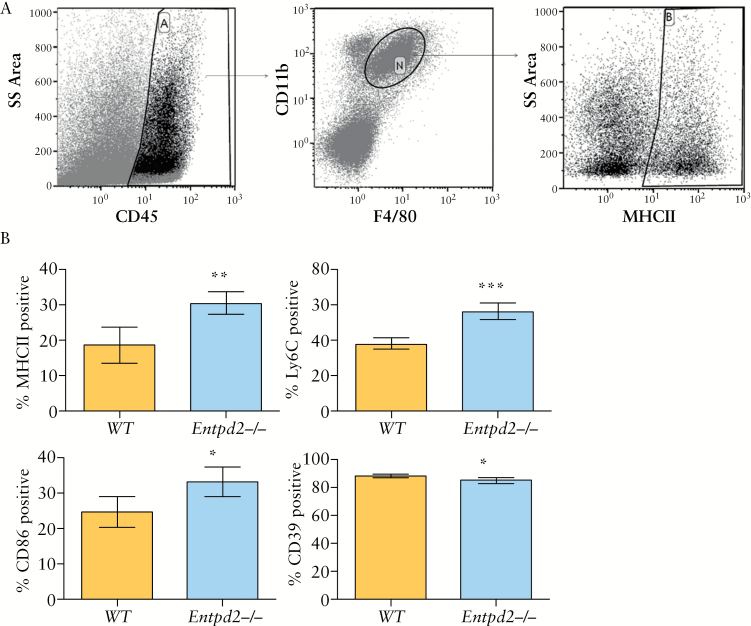
Macrophages are essential in the inflammation caused by DSS-induced colitis, and have been shown to be under the direct influence of enteric nervous system.4,32 To test whether the more severe colitis seen in Entpd2 null mice was a result of changes in the phenotype of macrophages, we isolated macrophages from colon tissues after 7 days of colitis induction with DSS. Flow cytometric analysis of markers of macrophage activation showed a distinct profile in Entpd2 null mice when compared with wild type mice [Figure 4A, B]. These macrophages [live, CD45+ CD11b+ F4/80+ cells] had significantly higher expressions of MHCII [30 ± 3% vs 19 ± 5%, p = 0.002], CD86 [33 ± 4% vs 25 ± 4%, p = 0.01], and Ly6C [56 ± 5% vs 38 ± 3%, p < 0.0001], as well as lower expression of CD39 [85 ± 2% vs 88 ± 1%, p = 0.01], which is consistent with a more pro-inflammatory polarisation. In comparison with Entpd2 null animals, we did not observe a significant difference in the macrophage phenotype between Entpd3 null and wild type mice, which is in keeping with the milder phenotype seen in Entpd3 mice [data not shown]. To determine whether DSS activates extra-intestinally derived macrophages, we performed immunohistochemistry of the spleen using markers of macrophages. Interestingly, in comparison with wild type and Entpd3 null mice, the spleen of the Entpd2 null mice demonstrated a loss of normal architecture, with red and white pulps upon DSS stimulation [Supplementary Figure 5A, available as Supplementary data at ECCO-JCC online]. This was accompanied by a decrease in F480, Ly6C, and CD39 positive cells [Supplementary Figure 5B-F].
Figure 4.
NTPDase2-deficient mice exhibit pro-inflammatory macrophage phenotype in dextran sodium sulphate [DSS]-induced colitis. A. Gating strategy of macrophages after isolation from colon tissue. After gating of live, single cells [not shown], CD45+CD11b+F4/80+ cells were gated as shown and defined as macrophages [Gate ‘N’]. Exemplary gating for MHCII positivity. B. Comparison of macrophage markers [in % of CD45+CD11b+F4/80+ cells as shown in A] between wild type [WT] and Entpd2 null mice [n = 5 per group]. Error bars show standard deviation; p-values are calculated using Student’s t test [*p < 0.05, **p < 0.005, ***p < 0.001].
3.4 Expression of NTPDase2 and NTPDase3 in human colon under normal conditions and in Crohn’s disease
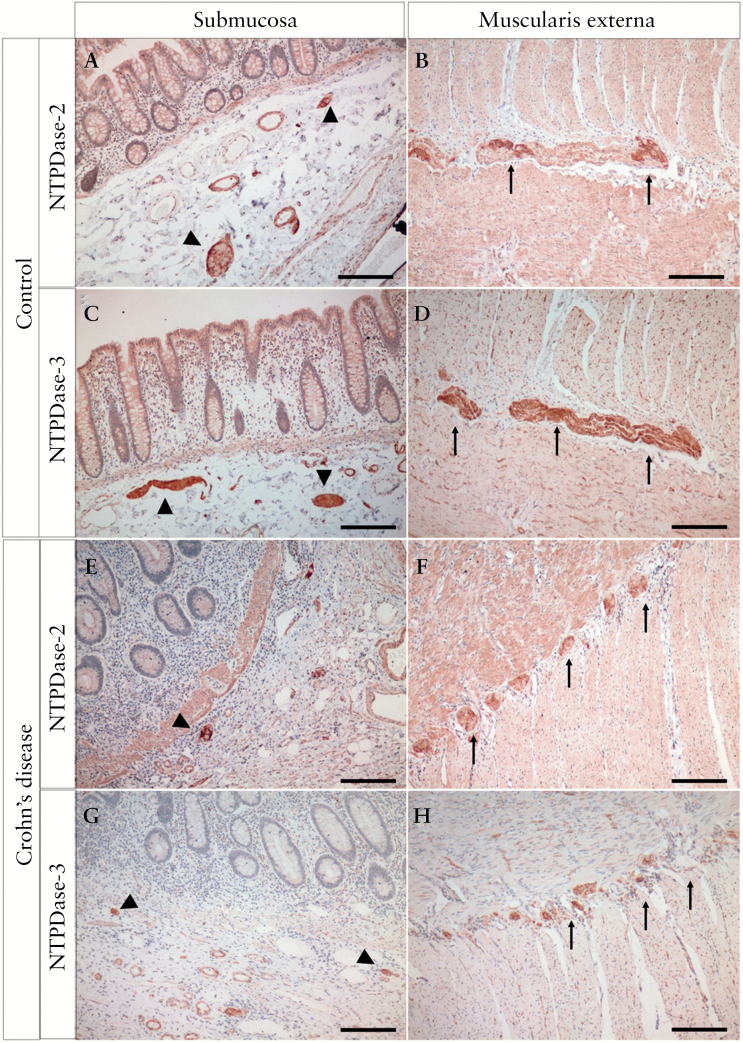
To determine the relevance of our findings in human disease, we examined the expression of NTPDase2 and -3 in human colon tissue. The expression of NTPDase2 and -3 had similar features in the human colon as compared with mice. Both NTPDase2 and NTPDase3 are expressed predominantly in the enteric nervous system and some perivascular cells [Figure 5A–D]. Whereas both ecto-enzymes can be observed in ganglia of the submucosal [Meissner’s] plexus and myenteric [Auerbach’s] plexus, nerve fibres within the smooth muscle layers as well as bigger nerve bundles stain intensely for NTPDase3, and to a lesser extent for NTPDase2 [Figure 5B, D, arrows]. NTPDase2 expression was also noted in the subendothelial cells of most blood vessels, whereas NTPDase3 expression was restricted to subendothelial cells of small venules.
Figure 5.
Colonic expression of NTPDase2 and -3 in human enteric nervous system. Representative images of immunohistochemistry highlighting the expression of NTPDase2 [A, B, E, F] and NTPDase3 [C, D, G, H] in the human colon from controls [A-D] and Crohn’s patients [E, F]. Arrow heads, submucosal plexus; arrows, myenteric plexus. The scale bar represents 500 µm.
Compared with controls, the overall expression of both NTPDase2 and -3 showed similar patterns in Crohn’s patients. However, in Crohn’s patients, the submucosal plexus appeared to be smaller compared with controls [Figure 5E, G, arrowheads]. Furthermore, the myenteric plexus in Crohn’s patients was disintegrated into smaller fragments in diseased tissues, each of which was surrounded and sometimes fully encircled by infiltrating mononuclear cells [Figure 5F, H, arrows; Supplementary Figure 6E, F, available as Supplementary data at ECCO-JCC online]. In the absence of inflammation, macrophages [CD68+ cells] were present either within or adjacent to the myenteric plexus, whereas few lymphocytes [CD3+ cells] were noted there [Supplementary Figure 6C, D]. In Crohn’s disease, there was an increase in the number of both macrophages and lymphocytes, whereas macrophages were still the dominant immune cell population in the area surrounding the myenteric plexus [Supplementary Figure 6G, H].
3.5 Changes in ectonucleotidase activity in the plasma of patients with Crohn’s disease
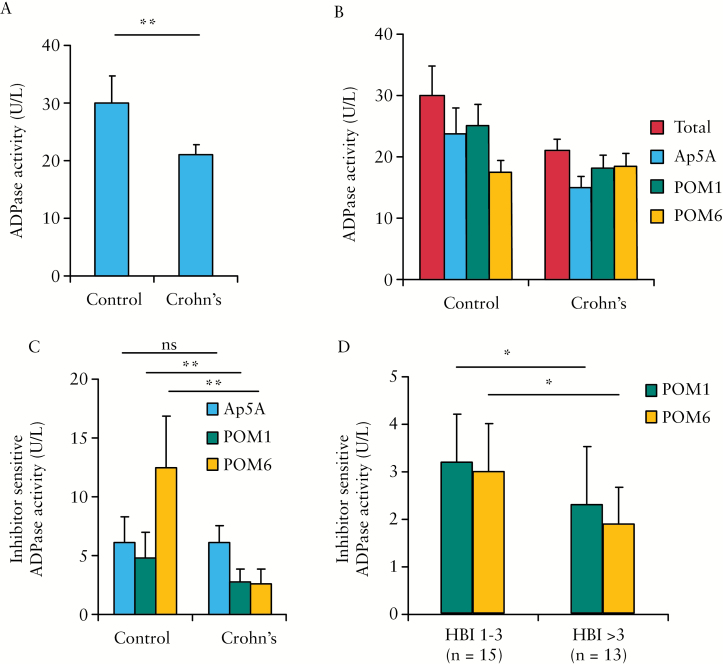
We have previously shown that cell membrane-localised E-NTPDases are present on microparticles circulating in the blood stream and contribute to the ectonucleotidase activity in the plasma.29,33 As microparticles are derived from cells, circulating ectonucleotidase activity may serve as a surrogate marker for cellular ecto-enzyme expression that cannot be easily sampled in humans. We compared ADPase activity in the plasma of both Crohn’s patients and controls. The plasmas of a total of 28 patients with Crohn’s disease were collected for the study. Their background characteristics are summarised in Table 1. A control group was composed of 14 healthy volunteers without inflammatory bowel disease, with an average age of 51, 64% females. The overall ADPase activity in Crohn’s patients was 21.2 ± 1.7 U/L, significantly less than in controls [30.4 ± 4.9 U/L, p < 0.0001] [Figure 6A; Supplementary Table 3, available as Supplementary data at ECCO-JCC online].
Table 1.
Background characteristics of Crohn’s disease patients.
| Background characteristics, % | |
|---|---|
| Age [years] | |
| 17–40 | 42.9% |
| 41+ | 57.1% |
| Gender | |
| % female | 42.9% |
| Duration [years] | 15 [5.5, 22] |
| Type of disease | |
| Inflammatory | 28.6% |
| Stricturing | 21.4% |
| Penetrating | 50.0% |
| Location of disease | |
| Ileal | 32.1% |
| Colonic | 25.0% |
| Ileocolonic | 42.9% |
| Disease activity | |
| HBI | 3 [2, 5] |
| WBC [× 103 /µL] | 7 [5.7, 8.4] |
| CRP [ng/dL, mg/L] | 2.7 [1.2, 13.9] |
HBI, Harvey-Bradshaw Index; WBC, white blood cells; CRP, C-reactive protein.
Figure 6.
Decreases in circulating ectonucleotidase activity in Crohn’s disease. A. A comparison of total plasma ectonucleotidase activities. Samples from 28 Crohn’s [CD] patients and 14 healthy controls were analysed. B. The ectonucleotidase activity in the presence of inhibitors Ap5A [adenylate kinase 1], POM1 [CD39 and NTPDase3], and POM6 [NTPDase2, and -3]. C. A comparison of POM1- and POM6-sensitive ectonucleotidase activities between controls and Crohn’s patients. D. A comparison of POM1- and POM6-sensitive ectonucleotidase activities between Crohn’s patient with HBI 1–3 and with HBI > 3; p-values are calculated using Student’s t test and labelled with asterisks: *p < 0.05, **p < 0.01, ***p < 0.001.
We then measured the ADPase activity in the presence of three inhibitors: Ap5A [inhibits adenylate kinase 1], POM1 [inhibits CD39 and NTPDase3], and POM6 [inhibits NTPDase2 and -3] [Figure 6B]. The POM6-sensitive ectonucleotidase activity, defined by the difference between ADPase activity with and without POM6, was substantially lower among Crohn’s patients [2.5 ± 1.2 U/L] when compared with healthy controls [13.0 ± 4.5 U/L, p < 0.0001] [Figure 6C; Supplementary Table 3]. A similar, but smaller difference was seen in POM1-sensitive activity. No difference in Ap5A-sensitive activities were noticed between Crohn’s and control groups. As POM6 has a similar Ki for NTPDase2 and -3, but does not inhibit CD39, non-CD39 E-NTPDases are likely the major contributor to the difference in ADPase activity between Crohn’s patients and controls.
To further test the specificity of our findings, we investigated whether the plasma ADPase activity correlates with Crohn’s disease activity. Patients with Harvey-Bradshaw index [HBI] values of 4 or higher had significantly lower levels of POM1 and POM6-sensitive ADPase activity than patients with an HBI of 3 or less [2.3 ± 1.0 vs 3.2 ± 1.0 U/L, p = 0.02 and 1.9 ± 0.8 vs 3.0 ± 1.2 U/L, p = 0.005, respectively] [Figure 6D; Supplementary Table 4, available as Supplementary data at ECCO-JCC online]. Both POM1- and POM6-sensitive ADPase activities in the plasma demonstrated a near-inverse linear relationship with the HBI of Crohn’s patients [Supplementary Figure 7A, B, available as Supplementary data at ECCO-JCC online].
4. Discussion
Gut inflammation affects the integrity and function of the enteric nervous system, as supported by both mechanistic investigations in animals and empirical observations of prolonged bowel dysfunction during recovery of flares in inflammatory bowel disease.34 Emerging evidence suggests that neuronal modulation can in turn impact on the intestinal immune system.3,4 However, the molecular mechanisms that underlie this neuro-immune crosstalk are not fully understood. It is also unclear whether such interactions are clinically relevant.
Our study suggests that purinergic signalling may be a crucial pathway that potentially regulates neuro-immune interaction in the gut. We noted that deletions of defined E-NTPDases with primary expression in the enteric nerve system affect gut inflammation in models of colitis, likely via altered purinergic signalling. Plasma ADPase activity in part derived from E-NTPDases associated with microvesicles is lower among patients with Crohn’s disease, and exhibits an inverse linear relationship with Crohn’s disease activity.
Ectonucleotidases of the CD39 family catalyse the phosphohydrolysis of extracellular nucleotides, and hence modulate purinergic signalling towards a more anti-inflammatory pathway.20 CD39 is expressed on endothelial and immune cells and has been implicated in human Crohn’s disease as well as in experimental colitis.9,10 The protective effect of CD39 in colitis has been ascribed to its direct effect on the phenotype of T cells.11,14 However, it is not expressed on neurons or glial cells and cannot explain the finding of heightened ATPase activity in areas of the enteric nervous system.19 Our present study thus focuses on NTPDase2 and NTPDase3, two ecto-enzymes of the CD39 family that are expressed primarily by cells of the enteric nervous system.24
Since the discoveries by Burnstock and others, purinergic signalling has been found to play a unique role in intracellular communication on several levels in the gut.35 Extracellular nucleotides transmit signals both among neurons in myenteric and submucosal ganglia via P2X2 and P2Y1 receptors and between nerve and smooth muscle cells via P2Y1 receptors, exerting an inhibitory effect on the muscularis.36–38 In addition to these implications of purinergic signalling in ganglionic and neuromuscular transmission, extracellular ATP [eATP] also functions as a potent pro-inflammatory signalling molecule. eATP activates ionotropic P2X7 receptors in macrophages, dendritic cells, and neutrophils, which in turn induce NLRP3 inflammasome assembly and the release of interleukin 1β and 18.39 eATP has also been shown to mediate the communication between neurons and glia and thus contribute to the maintenance of intestinal homeostasis and the mucosal barrier.40,41 Glial cells can perpetuate the release of pro-inflammatory eATP in the setting of intestinal inflammation via the activation of P2Y1 receptors, which in turn mediates neuronal cell death via P2X7 receptors.5 Furthermore, enteric glial cells have been shown to express pro-inflammatory cytokines such as interleukin-1β and interleukin-6.42
Here we observe that the deletion of NTPDase2 or NTPDase3 exacerbates gut inflammation in DSS-induced colitis. The increased severity of colitis in NTPDase2 deficiency is associated with a change in intestinal macrophages toward a more pro-inflammatory phenotype, as defined by increased surface expression of MHCII, CD86, and Ly6C and lower expression of CD39.43,44 Enteric neurons in the muscularis externa have been shown to mediate the polarisation of tissue-resident macrophages toward a tissue-protective phenotype.4 It is plausible that NTPDase2 in the enteric nervous system contributes to this immune-regulatory mechanism by decreasing the local eATP concentration and thus modulating inflammasome activation of intestinal macrophages and other immune cells. Consequently, the absence of NTPDase2 results in a local accumulation of eATP that can lead to stronger pro-inflammatory macrophage activation through P2X7 receptors as well as activation of glial cells via P2Y1 receptors.5,39
In humans, we found macrophages to be the dominant inflammatory cells surrounding the myenteric plexus under both normal and inflammatory states secondary to Crohn’s disease, supporting the concept of macrophages as central players in intestinal neuro-immune crosstalk. Human NTPDase2 and -3 are expressed predominantly in cells of the enteric nervous ganglia, in a pattern similar to that in mice. In Crohn’s colitis, macrophages infiltrate NTPDase2- and NTPDase3-positive ganglia in the muscularis and seem to impair their histological integrity. The spatial proximity between the enteric nerve system and macrophages in humans, and dynamic changes in response to inflammation, are in support of this neuro-immune crosstalk in Crohn’s disease. An increase in the numbers of CD86- and MHC II-positive macrophages in Entpd2 null mice may boost macrophage antigen presentation and activation of T cells. In addition, we showed that plasma ADPase activity in humans corresponding to NTPDase2 and -3 is lower among patients with Crohn’s disease compared with controls, and inversely correlates with Crohn’s disease activity, suggesting that alteration in ectonucleotidases may be associated with Crohn’s disease. We have previously described the presence of E-NTPDases on the surface of circulating microparticles, which accounts for a significant proportion of plasma ADPase activity.29 Although it is not easy to confirm the origin of these microparticles, our data suggest that this quantifiable enzyme activity may serve as a surrogate marker of tissue ecto-enzyme activity, which is not easily accessible in humans, and can represent a useful biomarker for diagnosis and disease staging. We have previously shown that luminal extracellular vesicles affect the function of epithelial cells and macrophages.45 Whether the E-NTPDase-containing microparticles are functionally active needs to be explored.
In human plasma, both POM1- and POM6-sensitive ADPase activities inversely correlate with Crohn’s disease activity. POM6, an inhibitor for NTPDase2 and -3, shows a much larger impact on plasma ADPase activity than POM1, an inhibitor for CD39 and NTPDase3.46 This suggests that the substantial decrease in total ADPase activity in disease is caused, at least in part, by decrease of NTPDase3 in addition to decrease in NTPDase2. In our animal model, mice deficient in NTPDase2 exhibit more severe experimental colitis. Interestingly, the impact of NTPDase3 deletion is less dramatic, despite a broad overlap in the expression of these two related enzymes. This might be explained by slight differences in expression between NTPDase2 and 3. In mice, NTPDase3 is largely expressed in the muscularis externa, whereas NTPDase2 expression extends to the mucosa and submucosa. The fact that DSS-induced colitis spares the muscularis externa may account for the stronger phenotype in Entpd2 null mice. In contrast, the inflammation in Crohn’s disease is transmural. In addition, NTPDase2 has a significantly lower ADPase activity compared with NTPDase3, which could lead to local accumulation of ADP and a relative lack of adenosine.20 It is possible that the specific enzyme kinetics of NTPDase2 and modified purinergic signalling mediated by eADP may contribute to the differences in experimental colitis.
Our study validates previous observations on the expression patterns of NTPDase2 and -3 in the murine enteric nervous system and confirms a similar expression in human intestine.19 Lavoie and colleagues reported that in mice, NTPDase3 is limited to neuronal cells in the myenteric ganglia, based on its co-immunostaining with PGP9.519 Here, we observe co-immunostaining of NTPDase3 with glial cell marker GFAP, suggesting co-expression in enteric glial cells. NTPDase3 is the predominant E-NTPDase in intramuscular fibres that are also positive for GFAP in both murine and human intestine. In comparison, the expression of NTPDase2 is largely limited to areas of the submucosal and myenteric plexus of the enteric nervous system.
Although the expression of NTPDase2 and -3 in the enteric nervous system suggests a link between neuronal signalling and inflammation, other possible mechanisms should be considered. Immunohistochemical staining for both enzymes can also be observed in the perivasculature.19 However, we did not observe thrombosis, platelet activation, or vascular inflammation associated with inflammation in either Entpd2 or Entpd3 null animals. CD39, the E-NTPDase expressed on endothelial cells, seems to be the dominant regulator of eATP and eADP in the vasculature. Furthermore, although the exacerbated colitis in Entpd2 null mice suggests a role of this enzyme in the pathophysiology of colitis, the causal relationship between NTPDase2 and -3 activities and Crohn’s disease severity in humans needs to be further studied. It is also possible that the lower ADPase activity in Crohn’s disease is a reflection of damage to the enteric nervous system, as gut inflammation is known to cause plexitis and neuronal loss.34 Another potential impact of these ectonucleotidases is on platelet function, as extracellular ADP is a major regulator of platelet activation. Crohn’s disease is associated with platelet dysfunction and a prothrombotic state.47 It should also be noted that we only used male mice in the study. Whether there is a gender difference in this regulation needs to be explored in the future.
In summary, we show that global deletion of NTPDase2, and to a lesser extent NTPDase3, exacerbates DSS-induced colitis in mice. In addition, we demonstrate a decrease in plasma ADPase activity among Crohn’s patients along with alterations in the integrity of the enteric nervous system. Taken together, these observations support the concept of a neuro-immune crosstalk that appears to be, at least in part, mediated by purinergic signalling. Further work is needed to fully understand the mechanism, including identification of cells and purinergic receptors involved in this pathway, and to explore the potential of NTPDase2 and NTPDase3 as new diagnostic biomarkers for inflammation and therapeutic targets in inflammatory bowel disease.
Funding
This work is in part supported by: a DFG grant from the German Research Foundation to LF [FE 1434/1-1]; National Institute of Health [NIH] grants to SCR [P01HL107152, R21CA164970] and ACM [K23DK084338, R03DK105161]; a grant from the Canadian Institutes of Health Research [CIHR] and an award from the Fonds de recherche du Québec–Santé [FRQS] to JS; a Clinical Research Award from the American College of Gastroenterology [ACG] and the Alan Hofmann Clinical and Translational Research Award from the American Association for the Study of Liver Diseases [AASLD] to ZGJ.
Conflict of Interest
The authors declare no competing interests in relation to this study.
Author Contributions
LF, SM, EUY, ZGJ were involved in the study design, experimentation, data analysis; ACM and ASC were involved in clinical sample collection; HS and CEM provided key support regarding the enzyme activity assay; JS provided key antibodies; MSL, WY, SCR participated in data analysis; all authors were involved in manuscript preparation and revision.
Supplementary Data
Supplementary data are available at ECCO-JCC online.
Supplementary Material
References
- 1. Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet 2012;380:1590–605. [DOI] [PubMed] [Google Scholar]
- 2. Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007;448:427–34. [DOI] [PubMed] [Google Scholar]
- 3. Di Giovangiulio M, Verheijden S, Bosmans G, Stakenborg N, Boeckxstaens GE, Matteoli G. The neuromodulation of the intestinal immune system and its relevance in inflammatory bowel disease. Front Immunol 2015;6:590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gabanyi I, Muller PA, Feighery L, et al. Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell 2016;164:378–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown IA, McClain JL, Watson RE, et al. Enteric glia mediate neuron death in colitis through purinergic pathways that require connexin-43 and nitric oxide. Cell Mol Gastroenterol Hepatol 2016;2:77–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eltzschig HK, Sitkovsky MV, Robson SC. Purinergic signaling during inflammation. N Engl J Med 2012;367:2322–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zimmermann H, Zebisch M, Sträter N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal 2012;8:437–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Antonioli L, Pacher P, Vizi ES, et al. CD39 and CD73 in immunity and inflammation. Trends Mol Med 2013;19:355–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Friedman DJ, Künzli BM, A-Rahim YI, et al. From the cover: CD39 deletion exacerbates experimental murine colitis and human polymorphisms increase susceptibility to inflammatory bowel disease. Proc Natl Acad Sci U S A 2009;106:16788–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Künzli BM, Berberat PO, Dwyer K, et al. Variable impact of CD39 in experimental murine colitis. Dig Dis Sci 2011;56:1393–403. [DOI] [PubMed] [Google Scholar]
- 11. Orrù V, Steri M, Sole G, et al. Genetic variants regulating immune cell levels in health and disease. Cell 2013;155:242–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roederer M, Quaye L, Mangino M, et al. The genetic architecture of the human immune system: a bioresource for autoimmunity and disease pathogenesis. Cell 2015;161:387–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Longhi MS, Moss A, Bai A, et al. Characterization of human CD39+ Th17 cells with suppressor activity and modulation in inflammatory bowel disease. PLoS One 2014;9:e87956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bai A, Moss A, Kokkotou E, et al. CD39 and CD161 modulate Th17 responses in Crohn’s disease. J Immunol 2014;193:3366–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bai A, Robson S. Beyond ecto-nucleotidase: CD39 defines human Th17 cells with CD161. Purinergic Signal 2015;11:317–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Naganuma M, Wiznerowicz EB, Lappas CM, et al. Cutting edge: Critical role for A2A adenosine receptors in the T cell-mediated regulation of colitis. J Immunol 2006;177:2765–9. [DOI] [PubMed] [Google Scholar]
- 17. Odashima M, Bamias G, Rivera-Nieves J, et al. Activation of A2A adenosine receptor attenuates intestinal inflammation in animal models of inflammatory bowel disease. Gastroenterology 2005;129:26–33. [DOI] [PubMed] [Google Scholar]
- 18. Pallio G, Bitto A, Pizzino G, et al. Adenosine receptor stimulation by polydeoxyribonucleotide improves tissue repair and symptomology in experimental colitis. Front Pharmacol 2016;7:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lavoie EG, Gulbransen BD, Martín-Satué M, Aliagas E, Sharkey KA, Sévigny J. Ectonucleotidases in the digestive system: focus on NTPDase3 localization. Am J Physiol Gastrointest Liver Physiol 2011;300:G608–20. [DOI] [PubMed] [Google Scholar]
- 20. Robson SC, Sévigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal 2006;2:409–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wink MR, Braganhol E, Tamajusuku AS, et al. Nucleoside triphosphate diphosphohydrolase-2 [NTPDase2/CD39L1] is the dominant ectonucleotidase expressed by rat astrocytes. Neuroscience 2006;138:421–32. [DOI] [PubMed] [Google Scholar]
- 22. Belcher SM, Zsarnovszky A, Crawford PA, et al. Immunolocalization of ecto-nucleoside triphosphate diphosphohydrolase 3 in rat brain: implications for modulation of multiple homeostatic systems including feeding and sleep-wake behaviors. Neuroscience 2006;137:1331–46. [DOI] [PubMed] [Google Scholar]
- 23. Braun N, Sevigny J, Robson SC, et al. Association of the ecto-ATPase NTPDase2 with glial cells of the peripheral nervous system. Glia 2004;45:124–32. [DOI] [PubMed] [Google Scholar]
- 24. Cardoso AM, Schetinger MR, Correia-de-Sa P, et al. Impact of ectonucleotidases in autonomic nervous functions. Auton Neurosci 2015;191:25–38. [DOI] [PubMed] [Google Scholar]
- 25. Gampe K, Haverkamp S, Robson SC, et al. NTPDase2 and the P2Y1 receptor are not required for mammalian eye formation. Purinergic Signal 2015;11:155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim JJ, Shajib MS, Manocha MM, et al. Investigating intestinal inflammation in DSS-induced model of IBD. J Vis Exp 2012, Feb 1. doi: 10.3791/3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wirtz S, Neufert C, Weigmann B, et al. Chemically induced mouse models of intestinal inflammation. Nat Protoc 2007;2:541–6. [DOI] [PubMed] [Google Scholar]
- 28. Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet 1980;1:514. [DOI] [PubMed] [Google Scholar]
- 29. Jiang ZG, Wu Y, Csizmadia E, et al. Characterization of circulating microparticle-associated CD39 family ecto-nucleotidases in human plasma. Purinergic Signal 2014;10:611–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu Y, Sun X, Kaczmarek E, et al. RanBPM associates with CD39 and modulates ecto-nucleotidase activity. Biochem J 2006;396:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012;9:671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bain CC, Mowat AM. The monocyte-macrophage axis in the intestine. Cell Immunol 2014;291:41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Banz Y, Beldi G, Wu Y, et al. CD39 is incorporated into plasma microparticles where it maintains functional properties and impacts endothelial activation. Br J Haematol 2008;142:627–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brierley SM, Linden DR. Neuroplasticity and dysfunction after gastrointestinal inflammation. Nat Rev Gastroenterol Hepatol 2014;11:611–27. [DOI] [PubMed] [Google Scholar]
- 35. Burnstock G, Campbell G, Bennett M, et al. Inhibition of the smooth muscle on the Taenia Coli. Nature 1963;200:581–2. [DOI] [PubMed] [Google Scholar]
- 36. Gallego D, Gil V, Martinez-Cutillas M, et al. Purinergic neuromuscular transmission is absent in the colon of P2Y[1] knocked out mice. J Physiol 2012;590:1943–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Galligan JJ. Ligand-gated ion channels in the enteric nervous system. Neurogastroenterol Motil 2002;14:611–23. [DOI] [PubMed] [Google Scholar]
- 38. Gulbransen BD, Bashashati M, Hirota SA, et al. Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat Med 2012;18:600–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gombault A, Baron L, Couillin I. ATP release and purinergic signaling in NLRP3 inflammasome activation. Front Immunol 2012;3:414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fields RD, Stevens B. ATP: an extracellular signaling molecule between neurons and glia. Trends Neurosci 2000;23:625–33. [DOI] [PubMed] [Google Scholar]
- 41. Rühl A. Glial cells in the gut. Neurogastroenterol Motil 2005;17:777–90. [DOI] [PubMed] [Google Scholar]
- 42. Khan I, Collins SM. Expression of cytokines in the longitudinal muscle myenteric plexus of the inflamed intestine of rat. Gastroenterology 1994;107:691–700. [DOI] [PubMed] [Google Scholar]
- 43. Cipriani G, Gibbons SJ, Kashyap PC, Farrugia G. Intrinsic gastrointestinal macrophages: their phenotype and role in gastrointestinal motility. Cell Mol Gastroenterol Hepatol 2016;2:120–30.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front Immunol 2014;5:514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mitsuhashi S, Feldbrugge L, Csizmadia E, et al. Luminal extracellular vesicles [EVs] in inflammatory bowel disease [IBD] exhibit proinflammatory effects on epithelial cells and macrophages. Inflamm Bowel Dis 2016;22:1587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Muller CE, Iqbal J, Baqi Y, et al. Polyoxometalates—a new class of potent ecto-nucleoside triphosphate diphosphohydrolase [NTPDase] inhibitors. Bioorg Med Chem Lett 2006;16:5943–7. [DOI] [PubMed] [Google Scholar]
- 47. Danese S, Motte Cd Cde L, Fiocchi C. Platelets in inflammatory bowel disease: clinical, pathogenic, and therapeutic implications. Am J Gastroenterol 2004;99:938–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.