Abstract
Background and Aims:
To investigate the efficacy and safety of three different dosages of embryonated, viable eggs of Trichuris suis [TSO] versus placebo for induction of remission in mildly-to-moderately active ileocolonic, uncomplicated Crohn’s disease [CD].
Methods:
Adults with active CD [n = 252] randomly received six fortnightly doses of 250, 2500, or 7500 TSO/15 ml suspension/day [TSO 250, TSO 2500, TSO 7500], or 15 ml placebo solution/day, in a double-blind fashion, with 4 weeks’ follow-up. Primary endpoint was the rate of clinical remission [Crohn’s Disease Activity Index [CDAI] < 150] at end of treatment, ie at Week 12 or withdrawal. Secondary endpoints included the course of clinical remission, rate of clinical response, change in CDAI, change in markers of inflammation, mucosal healing, and Physician’s Global Assessment.
Results:
Clinical remission at Week 12 occurred in 38.5%, 35.2%, and 47.2% of TSO 250, TSO 2500, and TSO 7500 patients, respectively, and in 42.9% of placebo recipients. TSO induced a dose-dependent immunological response. There was no response regarding laboratory markers of inflammation. Other secondary efficacy variables also showed no advantage of TSO over placebo for treatment of active CD. Administration of TSO did not result in any serious adverse drug reaction. Review of non-serious suspected adverse drug reactions following TSO did not reveal any safety concerns.
Conclusions:
Administration of 250–7500 TSO fortnightly over 12 weeks was safe and showed a dose-dependent immunological response, but no TSO dose showed a clinically relevant effect over placebo for induction of clinical remission or response in mildly-to-moderately active, ileocolonic CD.
Keywords: Crohn’s disease, randomized, Trichuris suis ova [TSO]
1. Introduction
Despite significant improvements in understanding genetic susceptibility, pathways of inflammation, and the biology of inflammatory bowel disease [IBD], treatment is still unsatisfactory. Most approaches target pro-inflammatory signals and pathways, and include broad anti-inflammatory drugs such as 5-aminosalicylic acid or steroids, more specific immunosuppressants such as thiopurines, and antibodies to tumour necrosis factor or to integrins. Many other attempts to target components of the immune system have failed.1,2
Some, but not all, epidemiological data 3–6 suggest that the absence of some ‘old friends’, including helminths and other microbiota, and intensified hygiene during the first years of life, lead to increased manifestation of immune-related diseases such as IBD. 7–10 In particular, the association between an absence of helminth infections and the increased occurrence of immune-related disorders has led to many experimental studies.11,12 In animal models, helminths modulate the microbiota,13 ameliorate dextran sodium sulphate-induced colitis,14 exert a protective effect on IL-10-mediated dinitrobenzene sulphonic acid-induced colitis,15 and improve IL-10 knockout colitis.16 They induce IL-I0 production in lamina propria dendritic cells while decreasing IL-12p40,17 and filarial cystatin induces IL-I0-producing macrophages.18 More recently, it has been found that products of Trichuris suis reduce the barrier function and suppress inflammatory cytokine production in intestinal epithelial cells.19 Based on these data, it has been suggested that helminths may influence immunoregulatory systems.
Accordingly, a number of clinical studies have been performed. In particular, a detailed case report of remission induction in severe ulcerative colitis by Trichuris trichiuria has received attention.20 Currently, more than 20 clinical trials of helminth therapy have been planned, started, or completed. 21 A decade ago, Summers et al. pioneered clinical studies in IBD using non-pathogenic, embryonated, viable eggs [ova] of Trichuris suis [TSO].22,23 In a placebo-controlled trial in ulcerative colitis, improvement was significantly better in the TSO treatment arm,23 and an open-label study of patients with Crohn’s disease [CD] showed a 79% clinical response to TSO with clinical remission in 72% of patients.22 More recently, the safety and tolerability of TSO have been described in a randomised clinical trial, but efficacy data were not reported.24
Therefore, a prospective, randomised, double-blind, placebo-controlled, multicentre trial comparing the efficacy and safety of fortnightly administration of 250, 2500, and 7500 TSO/15 ml suspension/day versus placebo for induction of remission in mildly-to-moderately active ileocolonic, uncomplicated CD was undertaken. The aim of the study was to confirm the preliminary efficacy results observed in the uncontrolled study22 and to demonstrate that TSO is superior to placebo for induction of clinical remission in CD by Week 12.
2. Methods
2.1. Study design and setting
This was a randomised, double-blind, placebo-controlled, multicentre Phase II study undertaken to evaluate the efficacy and safety of three different dosages of TSO versus placebo in mildly-to-moderately active CD patients. The study was performed during November 2010 to February 2014 at 53 hospitals or private practices in Austria [n = 1], Czech Republic [n = 5], Denmark [n = 2], Germany [n = 43], and Switzerland [n = 2] [ClinicalTrials.gov number, NCT01279577; EudraCT number, 2006-000720-13]. The study was conducted in accordance with the International Conference on Harmonisation [ICH] Guideline for Good Clinical Practice and was approved by independent ethics committees for each centre.
The study comprised a 7–10-day screening phase, a 12-week double-blind treatment phase with eight visits: Weeks 0 [baseline], 1, 2, 4, 6, 8, 10, and 12 (end of treatment/withdrawal [EoT]), and a follow-up visit, which took place 4 weeks after the EoT visit.
The study was conducted with four treatment groups in the form of a parallel group comparison. The primary objective was to demonstrate the superiority of any of the three TSO dosing groups compared with placebo for the induction of clinical remission in CD.
2.2. Study population
For this Phase II trial, we included male and female patients between 18 and 75 years of age with a confirmed diagnosis of mildly-to-moderately active uncomplicated CD [Crohn’s disease activity index25,26 [CDAI] ≥ 220 and ≤ 350], localised either in the terminal ileum [L1], the colon [L2], or ileocolon [L3]. Patients had to have elevated markers for inflammation at baseline (C-reactive protein [CRP] ≥ 2 x upper limit of normal [ULN; 5 mg/l] or stool calprotectin > ULN [50 µg/g stool]) and could not be receiving any CD-specific concomitant treatment [except stable mesalazine].
Patients were not eligible for the study if any of the following major exclusion criteria was present: known Crohn’s lesions in the upper gastrointestinal tract [up to and including the jejunum] with present symptoms; evidence of infectious diarrhoea, abscess, perforation, active fistulas, or active perianal lesions; clinical signs of stricturing disease; treatment with immunosuppressants or biologics (anti-tumour necrosis factor [TNF]-α agents), anti-integrin agents, thiopurine [ie, azathioprine, 6-mercaptopurine, or 6-thioguanine], methotrexate, tacrolimus, cyclophosphamide, or cyclosporine] within the past 3 months prior to baseline; treatment with antibiotics [eg, metronidazole or ciprofloxacin]; anti-parasitic medications within the past 2 weeks prior to baseline; or treatment with topical or systemic glucocorticosteroid within the past 4 weeks prior to baseline, or within the past 8 weeks if patients had been treated for longer than 3 months. In addition, patients known to be steroid-dependent or refractory, and patients who had been unresponsive both to treatment with a biologic [eg, anti-TNF-α agents or anti-integrin agents] and a thiopurine were to be excluded.
2.3. Randomisation and study medication
2.3.1. Allocation to treatment
At baseline, eligible patients were allocated to treatment by a computer-generated list of random numbers, using randomly permuted blocks with a flexible block size of 4 or 8. The list was generated and held by staff at a contract research organisation who were not involved in the planning, conduct, or analysis of the study. Patients were centrally randomised without any stratification in a 1:1:1:1 ratio via an interactive web response system to receive double-blind treatment with six fortnightly dosages of 250, 2500, or 7500 TSO/15 ml suspension/day [TSO 250, TSO 2500, TSO 7500], or 15 ml placebo solution.
2.3.2. Study medication
All trial medications were marked with an individual treatment kit number on each bottle. At Weeks 0, 2, 4, 6, 8, and 10 the treatment kit number of the bottle of the TSO/placebo suspension for one patient was provided to the investigator via the interactive web response system. The content of the corresponding bottle was gently shaken and administered by the patient directly at the study site under supervision of the study personnel, so that 100% single dose compliance could be guaranteed. Each bottle contained 15 ml suspension of 250, 2500, or 7500 embryonated, viable TSO as the active ingredient, or a placebo solution, manufactured by Ovamed GmbH, Barsbüttel, Germany under good manufacturing practice conditions. The appearance and taste of the placebo solution were indistinguishable from those of the TSO suspension.
2.4. Evaluation schedule and assessments
At baseline and all subsequent visits, the CDAI [based on daily diary cards] for the visit was calculated, vital signs and patient’s quality of life (using the Short Health Scale [SHS]27) were recorded, and central laboratory assessments were performed (stool samples analysed only at baseline and Weeks 4, 8, 12 [EoT visit] and follow-up visit). Physician’s Global Assessment [PGA] according to Hanauer et al.28 was performed at the EoT visit.
Optionally, ileocolonoscopy was undertaken at baseline and EoT visit, in which case the Simple Endoscopic Score for Crohn’s Disease [SES-CD]29 was to be used to rate any endoscopic finding.
Since helminths can induce eosinophilia, the results of blood eosinophil counts and eosinophil derived neutoxin [EDN] levels in stools were kept blinded to the investigators during the study.
To evaluate a potential immunological effect of TSO administration, the presence of T. suis-specific excretory/secretory [E/S] antigen-specific antibodies was measured by an enzyme-linked immunosorbent assay [ELISA; total IgG]. The ELISA method, originally developed for pig serum,30 was adapted and validated for human serum by Parasite Technologies A/S, Horsholm, Denmark [validation data on file]. Serum samples obtained in a subset of patients [ie, six remitters and six non-remitters in each of the four treatment groups; a total of 48 patients] were analysed in order to confirm that the embryonated eggs of T. suis were viable and had hatched, by determining whether a TSO-specific immunological response had been induced.
2.5. Study endpoints
The primary efficacy endpoint was the proportion of patients achieving clinical remission, defined as a CDAI < 150 at EoT, ie at Week 12 or withdrawal visit (last observation carried forward [LOCF]). The main secondary efficacy endpoints included the proportion of patients with clinical remission [CDAI < 150] in the course of the study, the proportion of patients with a reduction of ≥ 100 points in total CDAI at EoT compared with baseline, the mean change from baseline to EoT in total CDAI score, ‘treatment success’ and ‘treatment benefit’ based on the PGA score at EoT,28 the mean change from baseline to EoT in CRP, stool calprotectin and stool lactoferrin, and the mean change from baseline to EoT in the four dimensions of the SHS. The proportion of patients with mucosal healing at EoT, with mucosal healing defined as a SES-CD 1 subscore of ‘0’, was calculated only for patients who had a baseline SES-CD 1 subscore of ‘≥ 1’.
Immunological endpoints were values for blood eosinophil counts and stool EDN and change from baseline [for each visit], and the optional E/S antigen ELISA [total IgG] results, which were obtained in a subset of patients showing the highest and lowest improvement in CDAI, respectively, and presented for each visit and standardised as percentage change from baseline.
Safety endpoints included vital signs and adverse events [clinical and laboratory] and laboratory test results [including faeces analysis]. At the EoT visit, tolerability was independently assessed by the patient and the physician as very good, good, satisfactory, or poor.
2.6. Statistics
The study was performed using a two-stage group sequential adaptive design with possible sample size adjustment and optional stopping of one or two of the active treatment arms after a prospectively planned interim analysis, conducted by an independent data monitoring committee [IDMC].
Assuming rates of clinical remission of 50% in any of the TSO groups and 20% in the placebo group at EoT, a sample size of 53 patients in each group in the intention-to-treat [ITT] analysis [120 patients up to the interim analysis, 92 patients subsequently] would confer 80% power to yield a statistically significant result [one-sided α = 0.008] [see Supplementary Appendix 2, available as Supplementary data at ECCO-JCC online].
Evaluation of the primary efficacy variable was performed for the full analysis set [ITT analysis] and for the per-protocol [PP] set [PP analysis]. The primary analysis for confirmatory testing was the ITT analysis. For confirmatory hypothesis testing at the interim analysis as well as the final analysis, the inverse normal method of combining the p-values of the normal approximation tests for the comparison of rates was used [Supplementary Appendix 2]. If a patient discontinued the study prematurely, the last CDAI value recorded under study medication was included [LOCF method]. Patients without a post-baseline CDAI value were regarded as not having shown a response to treatment. All p-values resulting from further statistical tests, as well as for analyses of secondary efficacy or safety endpoints, were interpreted in the exploratory sense and therefore, no correction of p-values for multiplicity was performed.
Statistical testing of the primary endpoint was done using the ADDPLAN 5 MC software [licensed by Addplan GmbH, an ICON company, Cologne, Germany]. All other analyses were conducted using the SAS V.9.2 statistical package for Windows [SAS Institute, Cary, NC, USA].
The safety and ITT population comprised all randomised patients who received at least one dose of study medication. The PP population included all ITT patients without major protocol violations who were adequately compliant to study medication intake and study procedures, and provided at least one post-baseline CDAI value under study medication.
3. Results
3.1. Study population
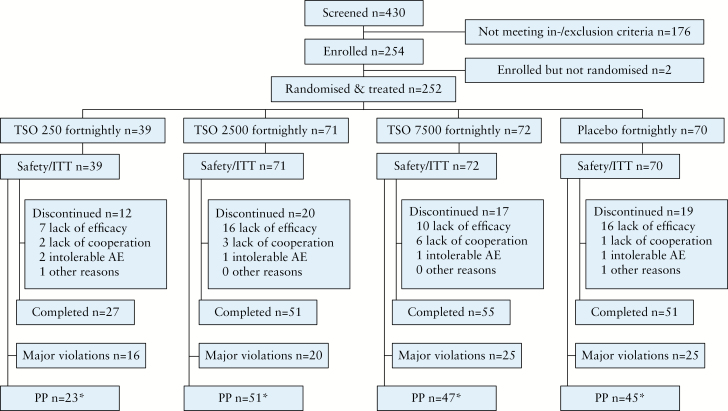
In total, 430 patients were screened, of whom 254 met all eligibility criteria and were enrolled into the study. Of these, two patients were not randomised and did not receive any study treatment, and thus were excluded from the ITT analysis. A total of 252 patients were thus randomised and received study treatment and were included into the safety and ITT analysis sets; 184 completed the 12-week study visit [Figure 1]. In total, 86 ITT patients were excluded from the PP population, most frequently due to due to major protocol deviations [n = 62] [Figure 1].
Figure 1.
Patient disposition. AE, adverse event; ITT, intention-to-treat; PP, per protocol; TSO, embryonated, viable eggs [ova] of Trichuris suis. * 86 ITT patients were excluded from the PP population (62 protocol deviations, 22 received <3 doses of study medication,18 discontinued the study prematurely, 6 end of treatment/withdrawal visit >21 days after last study drug administration [≥1 reason was possible]).
Based on the results of the first interim analysis of 120 observed patients [30 patients in each group], the IDMC recommended a further interim analysis after recruitment of 240–250 patients. In a blinded manner, the IDMC also recommended discontinuation of the TSO 250 dosing group due to futility, as assessed by an unblinded statistician via the central randomisation list, such that the investigators and the operational study team of the sponsor remained blinded. At the time of the IDMC’s recommendation, 156 patients were already enrolled. Therefore, stage 1 of the study includes all four treatment groups and consisted of 156 patients. At the second interim analysis, conducted after 239 patients had completed the study, the IDMC recommended the termination of further study recruitment due to futility. Since recruitment continued during the interim analysis, a total of 252 patients were evaluable for the final ITT analysis set, of whom 96 patients were analysed in stage 2 of the study.
The baseline demographic and clinical characteristics of the ITT population were similar between treatment groups [Table 1].Objective parameters for inflammation confirmed the presence of inflammation in the patient population.
Table 1.
Baseline demographics and clinical characteristics [ITT]
| n [%] / mean [SD] | TSO 250 [n = 39] | TSO 2500 [n = 71] | TSO 7500 [n = 72] | Placebo [n = 70] | All [n = 252] | |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 19 [48.7%] | 29 [40.8%] | 24 [33.3%] | 26 [37.1%] | 98 [38.9%] | |
| Female | 20 [51.3%] | 42 [59.2%] | 48 [66.7%] | 44 [62.9%] | 154 [61.1%] | |
| Race | ||||||
| White | 39 [100.0%] | 70 [98.6%] | 70 [97.2%] | 70 [100.0%] | 249 [98.8%] | |
| Age [years] | 37.8 [9.5] | 37.8 [11.0] | 34.8 [11.0] | 37.7 [12.8] | 36.9 [11.3] | |
| BMI [kg/m2] | 23.1 [4.2] | 24.5 [4.6] | 24.9 [5.9] | 24.2 [4.4] | 24.3 [4.9] | |
| Smoking habits | ||||||
| Current | 14 [35.9%] | 20 [28.2%] | 21 [29.2%] | 18 [25.7%] | 73 [29.0%] | |
| Former | 10 [25.6%] | 23 [32.4%] | 18 [25.0%] | 22 [31.4%] | 73 [29.0%] | |
| Never | 15 [38.5%] | 28 [39.4%] | 33 [45.8%] | 30 [42.9%] | 106 [42.1%] | |
| Case history | ||||||
| Established disease | 29 [74.4%] | 41 [57.7%] | 51 [70.8%] | 46 [65.7%] | 167 [66.3%] | |
| Chronic course | 8 [20.5%] | 19 [26.8%] | 18 [25.0%] | 23 [32.9%] | 68 [27.0%] | |
| New diagnosis | 2 [5.1%] | 11 [15.5%] | 3 [4.2%] | 1 [1.4%] | 17 [6.7%] | |
| Duration of disease since first diagnosis of CD [years] | 8.0 [6.8] | 7.9 [7.8] | 6.4 [6.4] | 9.5 [7.4] | 7.9 [7.2] | |
| Median [range] | 5.9 [0.3–29.1] | 5.6 [0.0–34.3] | 4.1 [0.1–30.2] | 7.2 [0.5–30.7] | 5.9 [0.0–34.3] | |
| < 5 years | 16 [41.0%] | 33 [46.5%] | 39 [54.2%] | 24 [34.3%] | 112 [44.4%] | |
| ≥ 5 years | 23 [59.0%] | 38 [53.5%] | 33 [45.8%] | 46 [65.7%] | 140 [55.6%] | |
| Localisation of disease | ||||||
| Ileocoecala | 18 [46.2%] | 34 [47.9%] | 38 [52.8%] | 37 [52.9%] | 127 [50.4%] | |
| Ileocolonicb | 12 [30.8%] | 16 [22.5%] | 16 [22.2%] | 14 [20.0%] | 58 [23.0%] | |
| Colonicc | 9 [23.1%] | 13 [18.3%] | 12 [16.7%] | 12 [17.1%] | 46 [18.3%] | |
| Concomitant treatment | ||||||
| Mesalazine | 9 [23.1%] | 16 [22.5%] | 17 [23.6%] | 12 [17.1%] | 63 [23.8%] | |
| Extraintestinal manifestations at baseline | ||||||
| Arthralgia | 15 [38.5%] | 24 [33.8%] | 18 [25.0%] | 21 [30.0%] | 78 [31.0%] | |
| Arthritis | 8 [20.5%] | 4 [5.6%] | 10 [13.9%] | 15 [21.4%] | 37 [14.7%] | |
| Iritis | 4 [10.3%] | 3 [4.2%] | 2 [2.8%] | 4 [5.7%] | 13 [5.2%] | |
| Erythema nodosum | 2 [5.1%] | 1 [1.4%] | 1 [1.4%] | 1 [1.4%] | 5 [2.0%] | |
| Aphthous stomatitis | 2 [5.1%] | 2 [2.8%] | 3 [4.2%] | 3 [4.3%] | 10 [4.0%] | |
| Uveitis | 2 [5.1%] | --- | 1 [1.4%] | 2 [2.9%] | 5 [2.0%] | |
| Pyoderma gangrenosum | 1 [2.6%] | 2 [2.8%] | --- | 1 [1.4%] | 4 [1.6%] | |
| CDAI at baseline | 267 [40.0] | 266 [38.8] | 271 [46.8] | 271 [46.5] | 269 [43.3] | |
| Calprotectin at baseline [µg/g] | 1073 [1473] n = 38 |
1614 [2115] n = 71 |
1452 [2226] n = 71 |
1146 [1846] n = 70 |
1355 [1991] n = 250 |
|
| Median [range] | 551 [77–6322] | 892 [33–12 000] | 557 [56–12 000] | 503 [48–12 000] | 592 [33–12 000] | |
| > ULN | 38 [97.4%] | 70 [98.6%] | 71 [98.6%] | 69 [98.6%] | 248 [98.4%] | |
| > 5 x ULN | 29 [74.4%] | 58 [81.7%] | 50 [69.4%] | 49 [70.0%] | 186 [73.8%] | |
| CRP at baseline [mg/l] | 15.3 [22.4] | 18.0 [25.7] | 20.4 [26.1] | 18.9 [24.2] | 18.5 [24.8] | |
| Median [range] | 6.9 [0.1–99.9] | 8.8 [0.1–135.5] | 12.8 [0.1–121.2] | 10.8 [0.1–150.8] | 10.6 [0.1–150.8] | |
| > ULN | 22 [56.4%] | 43 [60.6%] | 48 [66.7%] | 47 [67.1%] | 160 [63.5%] | |
| > 2 x ULN | 17 [43.6%] | 32 [45.1%] | 41 [56.9%] | 38 [54.3%] | 128 [50.8%] | |
| Calprotectin > 5 x ULN and CRP > 2 x ULN | 9 [39.1%] | 21 [41.2%] | 19 [40.4%] | 17 [37.8%] | 66 [39.8%] | |
| SES-CD at baseline | 4.3 [0.6] n = 3 |
12.7 [7.0] n = 15 |
11.0 [6.1] n = 16 |
10.1 [7.4] n = 8 |
11.0 [6.6] n = 42 |
|
| Median [range] | 4 [4–5] | 11 [4–24] | 9.5 [3–20] | 7.5 [3–20] | 9 [3–24] | |
| Previous treatment with azathioprine | 3 [7.7] | 5 [7.0] | 4 [5.6] | 7 [10.0] | 19 [7.5] | |
| Previous treatment with α-TNF | 1 [2.6] | 5 [7.0] | 5 [6.9] | 5 [7.1] | 16 [6.3] |
aInflammation only in terminal ileum, neoterminal ileum, and/or caecum.
bInflammation in terminal ileum, neoterminal ileum, and/or caecum and in at least one of the following segments: ascending colon, transverse colon, descending colon, sigmoid and/or rectum.
cInflammation only in at least one of the following segments: ascending colon, transverse colon, descending colon, sigmoid and/or rectum
BMI, body mass index; CD, Crohn’s disease; CDAI, Crohn’s Disease Activity Index; CRP, C-reactive protein; ITT, intention-to-treat; SD, standard deviation; SES-CD, Simple Endoscopic Score for Crohn’s Disease; TNF, tumour necrosis factor; TSO, embryonated, viable eggs [ova] of Trichuris suis; ULN, upper limit of normal.
3.2. Clinical efficacy
3.2.1. Primary efficacy endpoint
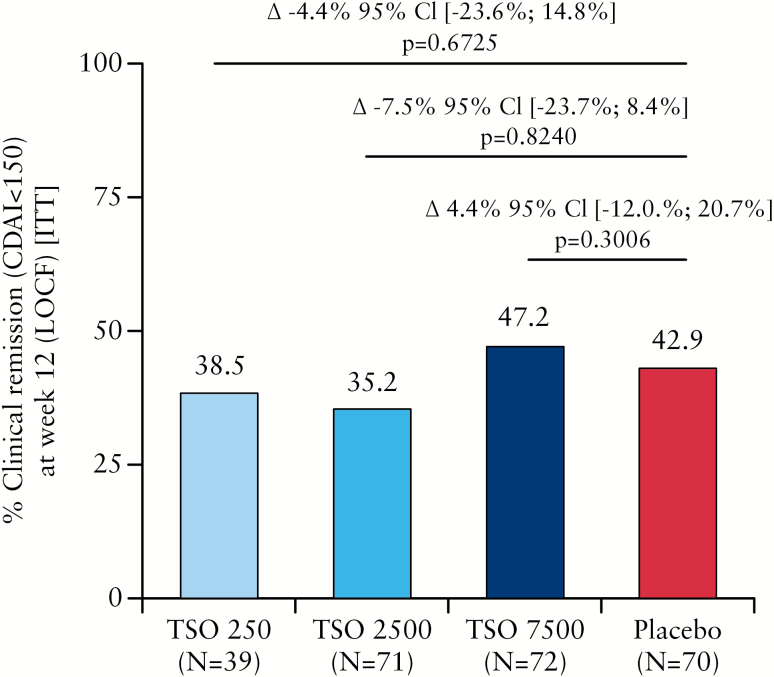
A summary of clinical remission rates at Week 12 [LOCF] in the final analysis [ITT] is presented in Figure 2. Significant superiority over placebo was not observed in any of the TSO treatment groups. This finding was confirmed in a robustness analysis performed in the PP population [Supplementary Table 1, available as Supplementary data at ECCO-JCC online].
Figure 2.
Clinical remission (Crohn’s disease activity index [CDAI] < 150) at Week 12 (last observation carried forward [LOCF]] in the intention-to-treat [ITT] population). CI, confidence interval; TSO, embryonated, viable eggs [ova] of Trichuris suis.
In several pre-planned subgroup analyses of the primary endpoint, the proportions of patients with clinical remission at EoT showed a large variability between treatment groups and between subgroups. No consistent difference in the proportions of patients with clinical remission at EoT between TSO and placebo treatment was detected in any subgroup [Supplementary Table 2, available as Supplementary data at ECCO-JCC online].
In a post hoc analysis, baseline characteristics of remitters versus non-remitters were evaluated in order to find any reason which could explain the observed high placebo response [Supplementary Table 3, available as Supplementary data at ECCO-JCC online]. However, no obvious difference or explanation was observed. Interestingly, the clinical remission rates were not consistent between the two stages of the study [Supplementary Table 4, available as Supplementary data at ECCO-JCC online].
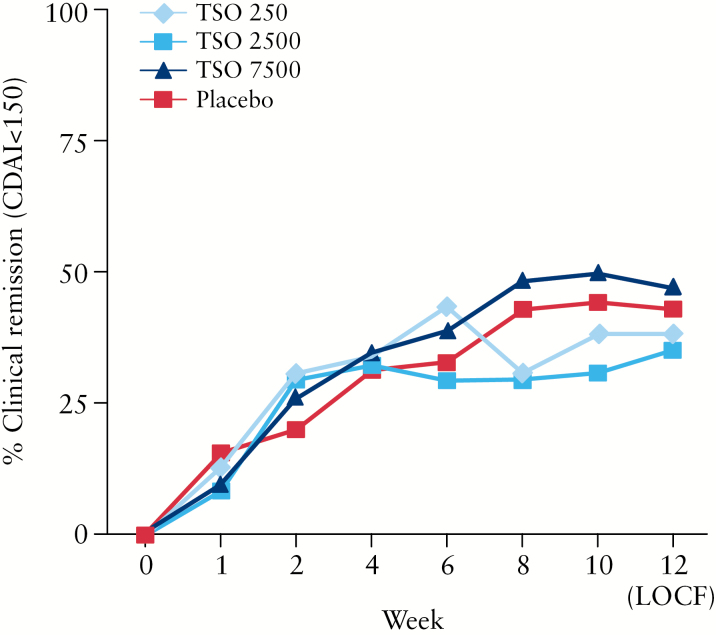
Remission rates in both the placebo group and the TSO 2500 group increased towards the end of the study [Supplementary Table 4].
3.2.2. Secondary efficacy endpoints
The course of clinical remission showed no clinically relevant or statistically significant difference between the active treatment groups compared with placebo [Figure 3]. In line with this, the major secondary efficacy endpoints also revealed no signs of clinically relevant or statistically significant difference between the TSO groups and placebo [Table 2].
Figure 3.
Course of clinical remission (Crohn’s disease activity index [CDAI] < 150) in the intention-to-treat [ITT] population. LOCF, last observation carried forward; TSO, embryonated, viable eggs [ova] of Trichuris suis.
Table 2.
Major secondary efficacy endpoints [ITT].
| TSO 250 [n = 39] | TSO 2500 [n = 71] | TSO 7500 [n = 72] | Placebo [n = 70] | ||
|---|---|---|---|---|---|
| ≥ 100 points drop in CDAI | n [%] | 16/39 [41.0%] | 31/71 [43.7%] | 36/72 [50.0%] | 32/70 [45.7%] |
| Change in total CDAI | Mean [SD] 95% CI |
-67 [100.6] [-99.9‚ -34.6] |
-83 [111.6] [-109.7‚ -56.8] |
-102 [111.4] [-127.7‚ -75.4] |
-83 [127.0] [-112.8‚ -52.2] |
| CRP [mg/l] | |||||
| Screening | Mean [SD] | 16.1 [20.5] n = 38 | 16.8 [20.5] n = 71 | 17.0 [21.6] n = 71 | 17.8 [21.7] n = 70 |
| Week 12 [LOCF] | Mean [SD] | 19.7 [30.0] n = 39 | 15.6 [24.1] n = 71 | 19.5 [25.6] n = 71 | 18.6 [23.9] n = 70 |
| Change | Mean [SD] | 3.9 [26.4] n = 39 | -1.2 [15.7] n = 71 | 2.6 [23.8] n = 71 | 0.8 [18.8] n = 70 |
| Calprotectin [µg/g] | |||||
| Screening | Mean [SD] | 1073 [1473.0] n = 38 | 1614 [2115.3] n = 71 | 1452 [2225.9] n = 71 | 1146 [1846.3] n = 70 |
| Week 12 [LOCF] | Mean [SD] | 1375 [2003.4] n = 39 | 1112 [1420.3] n = 68 | 1559 [2556.9] n = 69 | 952 [1086.6] n = 62 |
| Change | Mean [SD] | 325 [1907.8] n = 38 | -465 [2401.0] n = 68 | 183 [2394.2] n = 68 | 26 [1352.6] n = 62 |
| Lactoferrin [µg/ml] | |||||
| Screening | Mean [SD] | 124 [220.9] n = 36 | 147 [293.7] n = 71 | 136 [228.7] n = 72 | 141 [296.2] n = 70 |
| Week 12 [LOCF] | Mean [SD] | 134 [243.5] n = 39 | 105 [171.5] n = 68 | 167 [356.9] n = 69 | 100 [200.6] n = 62 |
| Change | Mean [SD] | 14 [294.9] n = 36 | -34 [325.3] n = 68 | 37.4 [340.2] n = 69 | -5 [250.4] n = 62 |
| PGA Week 12 [LOCF] | |||||
| Treatment successa | n [%] | 9 [23.1%] | 20 [28.2%] | 19 [26.4%] | 16 [22.9%] |
| Treatment benefitb | n [%] | 22 [56.4%] | 39 [54.9%] | 43 [59.7%] | 40 [57.1%] |
| Short Health Scales: change from baseline | |||||
| Symptom burden | Mean [SD] | -9.7 [27.6] n = 38 | -14.1 [26.0] n = 71 | -18.7 [29.1] n = 68 | -15.1 [27.0] n = 70 |
| Social function | Mean [SD] | -11.1 [26.2] n = 38 | -17.1 [28.4] n = 71 | -20.0 [28.2] n = 68 | -16.3 [31.6] n = 70 |
| Disease-related worry | Mean [SD] | -11.2 [26.6] n = 38 | -15.2 [26.3] n = 71 | -17.2 [28.0] n = 68 | -17.7 [29.4] n = 70 |
| General well-being | Mean [SD] | -7.1 [29.5] n = 38 | -8.0 [24.8] n = 71 | -17.7 [26.5] n = 68 | -14.8 [27.9] n = 70 |
aPatients with either complete relief of symptoms or marked improvement of symptoms.
bPatients with either complete relief of symptoms, or marked improvement of symptoms, or moderate improvement of symptoms, or slight improvement of symptoms.
CDAI, Crohn’s disease activity index; CI, confidence interval; CRP, C-reactive protein; ITT. Intention-to-treat; LOCF, last observation carried forward; PGA, Physician’s Global Assessment; SD, standard deviation; TSO, embryonated, viable eggs [ova] of Trichuris suis.
Of note, despite a clinical response in all treatment groups, there was no clinically relevant change from baseline in markers for inflammation.
The four scores of the SHS questionnaire showed a mean decrease from baseline to EoT in all treatment groups, indicating an improvement in patients’ quality of life.
The total SES-CD could be calculated for only 42 patients at baseline and 37 patients at the EoT/withdrawal visit. Due to the low number of patients with evaluable values for total SES-CD, no descriptive analyses of total SES-CD and changes in total SES-CD were performed. The proportions of patients with mucosal healing at the EoT/withdrawal visit were calculated on the basis of SES-CD 1. Mucosal healing was defined as change in SES-CD 1 from values ‘≥ 1’ at baseline to ‘0’ at the EoT/withdrawal visit in any location. Two patients each in the TSO 250 and placebo groups, and one patient each in the TSO 2500 and TSO 7500 groups, showed mucosal healing at the EoT/withdrawal visit.
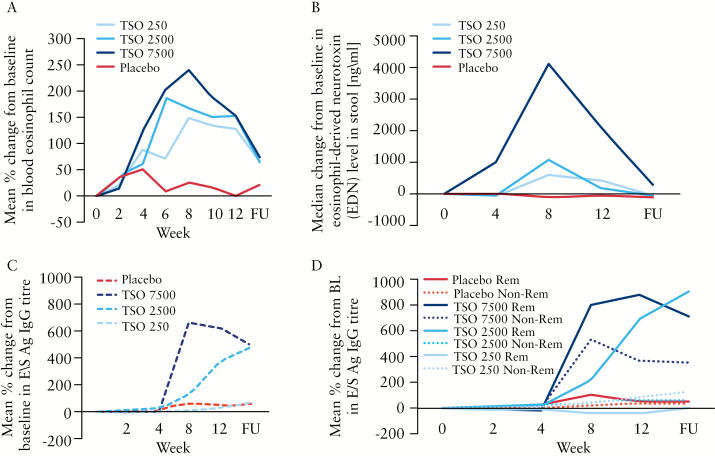
3.3. Immunological response
The course of the relative percentage change in blood eosinophil counts from baseline is presented in Figure 4a. A difference between the placebo and TSO groups was observed from Week 4 onwards, with a dose-dependent increase in the TSO treatment groups which peaked between Week 6 and Week 8 of treatment. The course of the absolute change from baseline in EDN levels in stools is depicted in Figure 4b. A similar pattern was observed, with a clear dose-dependent response in the TSO groups.
Figure 4.
Course of unspecific and specific immunological response to embryonated, viable eggs [ova] of Trichuris suis [TSO]. [A] Relative change from baseline in blood eosinophil counts [%]. [B] Absolute change from baseline in eosinophilic-derived neurotoxin levels in stool. [C] Relative change from baseline in T. suis-specific excretory/secretory antigen immunoglobulin G [E/S Ag IgG] titre. [D] Relative change from baseline in E/S Ag IgG titre in clinical remitter [Rem] and non-remitter [Non-Rem] at Week 12. FU, 4-week follow-up visit.
Since the primary and major secondary endpoints were not met, a T. suis-specific humoral response was measured in only 12 patients per treatment group instead of in all patients as originally planned. Six patients in each group were selected who were in remission at EoT and showed the highest decrease in the total CDAI score from baseline, as well as six patients who were non-remitters at EoT and showed the lowest decrease or even an increase from baseline in the total CDAI score. Results of the analysis of T. suis E/S antigen-specific total IgG, expressed as ‘% of reference serum’ are displayed in Figure 4c as the relative overall change from baseline. A clear dose-dependent immunological response was observed after three administrations [ie, after week 4] of TSO. Moreover, the immunological response was somewhat delayed in the TSO 2500 group compared with the TSO 7500 group. Stratified analysis by remitter versus non-remitter showed that the non-remitters also showed a relevant immunological response only in the TSO 7500 group [Figure 4d]. In all other TSO groups, only the remitters showed a relevant immunological response.
3.4. Safety
The proportion of patients with treatment-emergent adverse events was similar in the treatment groups: 72% TSO 250, 72% TSO 2500, 76% TSO 7500, 73% placebo [see Supplementary Table 5, available as Supplementary data at ECCO-JCC online, for details].
Suspect adverse drug reactions [ADRs, ie at least possibly related to study medication] were reported in 13%, 23%, 8%, and 19% of patients on TSO 250, TSO 2500, TSO 7500, and placebo, respectively. ADRs following TSO administration were most often gastrointestinal disorders [Table 3]. A detailed overview of ADRs is given in Supplementary Table 6, available as Supplementary data at ECCO-JCC online. There were 23 serious adverse events reported [TSO 250: 2, TSO 2500: 3, TSO 7500: 7, placebo: 11]. Apart from a hypersensitivity reaction following administration of placebo solution, no other serious adverse event was assessed by the investigator as at least possibly related to study medication.
Table 3.
Number of adverse drug reactions by system organ class.
| TSO 250 | TSO 2500 | TSO 7500 | Placebo | |
|---|---|---|---|---|
| Gastrointestinal disorders | 3 | 9 | 4 | 7 |
| Investigations [changes in laboratory parameter] | 1 | 8 | 5 | 12 |
| General disorders and administration site conditions | --- | 5 | --- | 2 |
| Nervous system disorders | 3 | 1 | 1 | 1 |
| Skin and subcutaneous tissue disorders | --- | 3 | --- | 1 |
| Musculoskeletal and connective tissue disorders | --- | 1 | --- | 1 |
| Infections and infestations | --- | 2 | --- | --- |
| Blood and lymphatic system disorders | --- | --- | --- | 1 |
| Immune system disorders | --- | --- | --- | 1 |
| Psychiatric disorders | --- | 1 | --- | --- |
| Respiratory, thoracic and mediastinal disorders | 1 | --- | --- | --- |
TSO, embryonated, viable eggs [ova] of Trichuris suis.
Withdrawal due to an adverse event was recorded in 23%, 21%, 18%, and 20% of patients in the TSO 250, TSO 2500, TSO 7500, and placebo groups, respectively. The majority of patients were withdrawn from the study due to lack of efficacy, but no patient was withdrawn due to an intolerable suspected ADR.
Laboratory results raised no safety concerns. As expected, TSO treatment resulted in a marked increase in eosinophils in the blood. In two patients in the TSO 7500 group, eggs of T. suis were detected microscopically in faeces once at Week 12, with a negative result at follow-up.
Despite use of contraception [oral contraceptive; diaphragm/condom] two patients on TSO 7500 became pregnant during the course of the study, resulting in the birth of a healthy baby in one case and spontaneous abortion in the other. Abortion was not classified as an ADR by the supervising investigator.
Tolerability of TSO was assessed by both the investigators and patients as very good or good in the vast majority of patients.
4. Discussion
This is the first randomised, double-blind, placebo-controlled, multicentre Phase II proof-of-concept study to evaluate the efficacy and safety of three different dosages of TSO for induction of remission in mildly-to-moderately active, ileocolonic, uncomplicated CD. The trial failed to show superiority of TSO treatment versus placebo for the primary endpoint, induction of clinical remission at Week 12 [LOCF] in patients with active CD. Nor did the secondary efficacy variables show any advantage of TSO over placebo for the treatment of active CD. Safety results were favourable, similar to those described in previous study reports.24,31,32
The absence of a clinically relevant benefit for TSO treatment does not appear to have been due a lack of embryonated, viable T. suis eggs or hatched larvae. Administration of TSO led to a dose-dependent increase in the percentage of blood eosinophils and stool EDN levels, as would be expected in patients affected with T. suis. Additionally, a dose-dependent specific humoral immunological response to E/S antigen of T. suis, with seroconversion to IgG, was recorded in a subgroup of patients in each treatment group. Thus, the pharmacodynamic assessments and immunological tests clearly indicate that TSO administration exerted an effect on the host, but had no influence on rates of clinical remission. There are other possible reasons for this disappointing result. First, the duration of treatment has been insufficient considering the long-established disease in this cohort of patients [median 4–7 years]. Treatment for 6–12 months may be required to achieve a clinical effect. 21 Second, induction of remission may be the wrong outcome measure. Maintenance of quiescent disease over a prolonged period could have been a better approach. Third, the hygiene hypothesis may be incorrect. The inverse relationship between the presence of helminths and the manifestation of immune-related disease may only be a marker for more general lifestyle changes. 21 The fact that some other trials of TSO in other indications also failed33 points in this direction. In the future, it might be worthwhile to use helminth products in an appropriate form systemically or locally in order to achieve a more pronounced effect and possibly reduce the placebo response.34,35 The very high placebo response in this study, in spite of the clear proof of ongoing inflammation at entry into the trial with no change in inflammatory parameters, points to a strong mental signal36 which may be associated with the idea of swallowing a ‘living drug’ such as helminth eggs.
A wide range of post hoc analyses did not reveal a reason for the high response rate in the placebo arm. Patients opting for this type of treatment may have been recruited by accepting the belief of physicians to a large extent. The increased remission rate in the second stage of the study in most groups may support this view.
The typical side effects of TSO are gastrointestinal disorders, probably due to attachment of larvae to the mucosal epithelium. In studies of other diseases, such as allergic rhinitis32 and multiple sclerosis,37 transient gastrointestinal adverse events occurred in approximately 50% of patients treated with TSO. This high frequency of adverse events was not apparent in this study, probably due to overlap with IBD-related symptoms.
Following high doses of TSO, very small numbers of ova might be detected in stools. This was a rare observation in our trial. In accordance with other authors, it was classified as a sign that eggs were being passed through rather than infection with new egg production.37 Nevertheless, it is prudent to bear in mind the possibility of larval invasiveness,38 and careful safety monitoring is therefore mandatory in any patient receiving TSO.
In conclusion, this study provides no evidence for a positive effect of different doses of TSO over 12 weeks for the induction of remission in CD patients with proven active inflammatory disease. The application of TSO or placebo induced a relatively high subjective response, as measured by the CDAI and the PGA, but had no impact on objective parameters of inflammation such as CRP or calprotectin, in spite of evidence for an immunological effect indicating arrival and hatching of T. suis larvae in the digestive tract.
Funding
This work was supported by Dr Falk Pharma GmbH, Freiburg, Germany. The sponsor contributed to the design and conduct of the study, data management, analysis and interpretation of the data, and review and approval of the manuscript. Copy-editing support was provided by a freelance writer funded by Dr Falk Pharma GmbH.
Conflict of Interest
JS has received speaker’s fees from Falk Foundation and AbbVie. KF has received speaker’s fees from AbbVie and Falk Foundation. GR has received speaker’s fees from Falk Foundation and grant support from Dr Falk Pharma GmbH. JL has received speaker’s fees from Falk Foundation, Repha GmbH, and Techlab Inc., and research funding from Techlab Inc. GN has received speaker’s fees from AbbVie, Merck, MSD, Takeda, and Ferring and is a consultant for AbbVie and MSD. OB has received speaker’s fees from Falk Foundation. NT has received speaker’s fees from AbbVie, Falk Foundation, Ferring, MSD, Takeda, and Vifor, and is a consultant for Jansen, MSD, Mundipharma, Pfizer, and Takeda. JK has received speaker’s fees from Dr Falk, AbbVie, MSD, and Takeda, and is a member of advisory boards for MSD and Takeda. CO has received speaker’s fees from Falk Foundation. KD, RG, and RM are employees of Dr Falk Pharma GmbH. All remaining authors disclose no conflicts.
Author Contributions
The study concept and design was developed by JS in collaboration with the sponsor, Dr Falk Pharma GmbH. Recruitment of study patients: all authors except KD, RG, and RM. Generation, analysis, and interpretation of data: JS, KD, RG and RM. Drafting of the manuscript: JS, KD, and RM. JS had full access to all of the data in the study and takes responsibility for its integrity and the accuracy of the data analysis. Critical revision of the manuscript for important intellectual content: JS, KD, RG, and RM. All authors critically reviewed the manuscript and approved it for publication.
Supplementary Material
Acknowledgments
The authors would like to thank all patients and investigators for their participation and contribution to the study, as well as the members of the sponsor-independent data monitoring committee: Prof. Dr Walter Lehmacher [statistician], Prof. Dr Volker Groß [gastroenterologist], and Prof. Dr Wolfgang Kruis [gastroenterologist]. Grateful thanks also go to Dr Christian Kappel and Dr Helene Kringel [both of Parasite Technologies A/S, Horsholm, Denmark] for ELISA measurement for the presence of T. suis E/S antigen-specific total IgG antibodies in human patient serum [funded by Dr Falk Pharma GmbH], as well as to Caroline Dunstall [freelance] for providing proofreading support [funded by Dr Falk Pharma GmbH], and Dr Silke Jörgens [Aptiv Solutions GmbH, an ICON company, Cologne, Germany] for her statistical expertise.
Collaborators: Members of the International TRUST-2 Study Group are listed in Supplementary Appendix 1, available as Supplementary data at ECCO-JCC online.
References
- 1. Danese S. New therapies for inflammatory bowel disease: from the bench to the bedside. Gut 2012;61:918–32. [DOI] [PubMed] [Google Scholar]
- 2. Scharl M, Vavricka SR, Rogler G. Review: new anti-cytokines for IBD: what is in the pipeline? Curr Drug Targets 2013;14:1405–20. [DOI] [PubMed] [Google Scholar]
- 3. Bager P, Vinkel HA, Wohlfahrt J, et al. Helminth infection does not reduce risk for chronic inflammatory disease in a population-based cohort study. Gastroenterology 2012;142:55–62. [DOI] [PubMed] [Google Scholar]
- 4. Fumagalli M, Pozzoli U, Cagliani R, et al. Parasites represent a major selective force for interleukin genes and shape the genetic predisposition to autoimmune conditions. J Exp Med 2009;206:1395–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hafner S, Timmer A, Herfarth H, et al. The role of domestic hygiene in inflammatory bowel diseases: hepatitis A and worm infestations. Eur J Gastroenterol Hepatol 2008;20:561–6. [DOI] [PubMed] [Google Scholar]
- 6. Kabeerdoss J, Pugazhendhi S, Subramanian V, et al. Exposure to hookworms in patients with Crohn’s disease: a case-control study. Aliment Pharmacol Ther 2011;34:923–30. [DOI] [PubMed] [Google Scholar]
- 7. Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med 2002;347:911–20. [DOI] [PubMed] [Google Scholar]
- 8. Elliott DE, Weinstock JV. Helminth-host immunological interactions: prevention and control of immune-mediated diseases. Ann NY Acad Sci 2012;1247:83–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rook GA. Hygiene and other early childhood influences on the subsequent function of the immune system. Dig Dis 2011;29:144–53. [DOI] [PubMed] [Google Scholar]
- 10. Yazdanbakhsh M, Matricardi PM. Parasites and the hygiene hypothesis: regulating the immune system? Clin Rev Allergy Immunol 2004;26:15–24. [DOI] [PubMed] [Google Scholar]
- 11. Elliott DE, Weinstock JV. Where are we on worms? Curr Opin Gastroenterol 2012;28:551–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scholmerich J. Trichuris suis ova, lecithin and other fancy molecules. Dig Dis 2014;32Suppl 1:67–73. [DOI] [PubMed] [Google Scholar]
- 13. Walk ST, Blum AM, Ewing SA, et al. Alteration of the murine gut microbiota during infection with the parasitic helminth Heligmosomoides polygyrus. Inflamm Bowel Dis 2010;16:1841–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cancado GG, Fiuza JA, de Paiva NC, et al. Hookworm products ameliorate dextran sodium sulfate-induced colitis in BALB/c mice. Inflamm Bowel Dis 2011;17:2275–86. [DOI] [PubMed] [Google Scholar]
- 15. Hunter MM, Wang A, Hirota CL, et al. Neutralizing anti-IL-10 antibody blocks the protective effect of tapeworm infection in a murine model of chemically induced colitis. J Immunol 2005;174:7368–75. [DOI] [PubMed] [Google Scholar]
- 16. Elliott DE, Setiawan T, Metwali A, et al. Heligmosomoides polygyrus inhibits established colitis in IL-10-deficient mice. Eur J Immunol 2004;34:2690–8. [DOI] [PubMed] [Google Scholar]
- 17. Hang L, Setiawan T, Blum AM, et al. Heligmosomoides polygyrus infection can inhibit colitis through direct interaction with innate immunity. J Immunol 2010;185:3184–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schnoeller C, Rausch S, Pillai S, et al. A helminth immunomodulator reduces allergic and inflammatory responses by induction of IL-10-producing macrophages. J Immunol 2008;180:4265–72. [DOI] [PubMed] [Google Scholar]
- 19. Hiemstra IH, Klaver EJ, Vrijland K, et al. Excreted/secreted Trichuris suis products reduce barrier function and suppress inflammatory cytokine production of intestinal epithelial cells. Mol Immunol 2014;60:1–7. [DOI] [PubMed] [Google Scholar]
- 20. Broadhurst MJ, Leung JM, Kashyap V, et al. IL-22+ CD4+ T cells are associated with therapeutic trichuris trichiura infection in an ulcerative colitis patient. Sci Transl Med 2010;2:60–88. [DOI] [PubMed] [Google Scholar]
- 21. Fleming JO, Weinstock JV. Clinical trials of helminth therapy in autoimmune diseases: rationale and findings. Parasite Immunol 2015;37:277–92. [DOI] [PubMed] [Google Scholar]
- 22. Summers RW, Elliott DE, Urban JF, et al. Trichuris suis therapy in Crohn’s disease. Gut 2005;54:87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Summers RW, Elliott DE, Urban JF, et al. Trichuris suis therapy for active ulcerative colitis: A randomized controlled trial. Gastroenterology 2005;128:825–32. [DOI] [PubMed] [Google Scholar]
- 24. Sandborn WJ, Elliott DE, Weinstock J, et al. Randomised clinical trial: the safety and tolerability of Trichuris suis ova in patients with Crohn’s disease. Aliment Pharmacol Ther 2013;38:255–63. [DOI] [PubMed] [Google Scholar]
- 25. Best WR, Becktel JM, Singleton JW, et al. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology 1976;70:439–44. [PubMed] [Google Scholar]
- 26. Best WR, Becktel JM, Singleton JW. Rederived values of the eight coefficients of the Crohn’s Disease Activity Index [CDAI]. Gastroenterology 1979;77:843–6. [PubMed] [Google Scholar]
- 27. Stjernman H, Grännö C, Järnerot G, et al. Short Health Scale: A valid, reliable, and responsive instrument for subjective health assessment in Crohn’s disease. Inflamm Bowel Dis 2008;14:47–52. [DOI] [PubMed] [Google Scholar]
- 28. Hanauer SB, Schwartz J, Robinson M, et al. Mesalamine capsules for treatment of active ulcerative colitis: results of a controlled trial. Pentasa Study Group. Am J Gastroenterol 1993;88:1188–97. [PubMed] [Google Scholar]
- 29. Daperno M, D’Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: The SES-CD. Gastrointest Endosc 2004;60:505–12. [DOI] [PubMed] [Google Scholar]
- 30. Kringel H, Roepstorff A. Trichuris suis excretory/secretory antigen-specific antibodies in serum from single-inoculated pigs. Parasite Immunol 2007;29:327–30. [DOI] [PubMed] [Google Scholar]
- 31. Summers RW, Elliott DE, Weinstock JV. Why Trichuris suis should prove safe for use in inflammatory bowel diseases [correspondence]. Inflamm Bowel Dis 2005;11:783–4. [DOI] [PubMed] [Google Scholar]
- 32. Bager P, Kapel C, Roepstorff A, et al. Symptoms after ingestion of pig whipworm Trichuris suis eggs in a randomized placebo-controlled double-blind clinical trial. PLoS One 2011;6:e22346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bager P, Arnved J, Ronborg S, et al. Trichuris suis ova therapy for allergic rhinitis: A randomized double-blind placebo controlled clinical trial. J Allergy Clin Immunol 2010;125:123–30. [DOI] [PubMed] [Google Scholar]
- 34. Ghedin E. Panning for molecular gold in whipworm genomes. Nat Genet 2014;46:661–3. [DOI] [PubMed] [Google Scholar]
- 35. Harnett W, Harnett MM. Molecular basis of worm-induced immunomodulation. Parasite Immunol 2006;28:535–43. [DOI] [PubMed] [Google Scholar]
- 36. Kaptchuk TJ, Miller FG. Placebo effects in medicine. N Engl J Med 2015;373:8–9. [DOI] [PubMed] [Google Scholar]
- 37. Fleming JO, Isaak A, Lee JE, et al. Probiotic helminth administration in relapsing-remitting multiple sclerosis: a phase 1 study. Mult Scler 2011;17:743–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. van Kruiningen HJ, West AB. Potential danger in the medical use of Trichuris suis for the treatment of inflammatory bowel disease. Inflamm Bowel Dis 2005;11:515. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.