Abstract
Inflammatory bowel diseases, such as ulcerative colitis and Crohn’s disease, are disabling conditions characterised by chronic, relapsing inflammation of the gastrointestinal tract. Current treatments are not universally effective or, in the case of therapeutic antibodies, are hampered by immune responses. Janus kinase inhibitors are orally delivered small molecules that target cytokine signalling by preventing phosphorylation of Janus kinases associated with the cytokine receptor. Subsequently, phosphorylation of signal transducers and activators of transcription that relay Janus kinase signalling and transcription of cytokines in the nucleus will be diminished. Key cytokines in the pathogenesis of inflammatory bowel diseases are targeted by Janus kinase inhibitors. Several Janus kinase inhibitors are in development for the treatment of inflammatory bowel diseases. Tofacitinib, inhibiting signalling via all Janus kinase family members, was effective in phase 2 and 3 trials in moderate-severe ulcerative colitis. GSK2586184, a Janus kinase 1 selective inhibitor, induced clinical and endoscopic response in ulcerative colitis; however, the study was discontinued at an early stage due to liver toxicity observed in systemic lupus patients receiving the drug. Filgotinib, a Janus kinase 1 selective inhibitor investigated in treatment of Crohn’s disease, was superior to placebo. As adverse events associated with the broad immunological effect of these agents have been reported, the future application of these drugs is potentially limited. We will discuss the treatment efficacy of Janus kinase inhibition in inflammatory bowel diseases, how current Janus kinase inhibitors available target immune responses relevant in inflammatory bowel disease, and whether more specific kinase inhibition could be effective.
Keywords: Kinase inhibitors, tofacitinib, ulcerative colitis, Crohn’s disease, filgotinib
1. Introduction
Inflammatory bowel diseases [IBD], such as ulcerative colitis [UC] and Crohn’s disease [CD], are chronic intermittent diseases that lead to structural damage of the bowel wall. In UC, the inflammation is limited to the mucosa and extends from the rectum proximally. CD can be located in any part of the gastrointestinal tract and is characterised by transmural inflammation and complications.1,2 The aetiology of IBD remains unknown; however, the current paradigm states that genetic susceptibility and environmental factors play roles in triggering an inappropriate mucosal immune response.3
The current treatment of IBD includes glucocorticoids, 5-aminosalicylic acids, immunosuppressants, anti-tumour necrosis factor [anti-TNF] agents, the anti-integrin antibody vedolizumab, and the interleukin [IL] 12/23 antibody ustekinumab.4 Although the introduction of anti-TNF agents has improved IBD treatment options importantly, loss of response is frequent and the need for surgery remains high.5,6 Promising new drugs, such as antibodies against the α4β7 integrin [vedolizumab] and against mucosal addressin cell adhesion molecule-1 [MadCam-1], are examples of new therapeutic opportunities aimed at inhibiting leukocyte recruitment in the intestine.7 Ustekinumab, a human monoclonal antibody targeting the p40 subunit shared by IL12 and IL23, has demonstrated efficacy in recent phase 3 studies in CD.8 The antibody has been approved recently by the Food and Drug Administration [FDA] and the European Medicines Agency [EMA] for the treatment of CD. However, despite these therapeutic developments, immunogenicity will continue to be a downside of therapeutic antibodies, besides the fact that they are currently only given by parenteral administration.
Janus kinase [JAK] inhibitors are small molecules that are currently under development for the treatment of several immune diseases including psoriasis, rheumatoid arthritis [RA], alopecia areata, and IBD.9–12 In 2012, tofacitinib [Xeljanz©, CP690,550, Pfizer, New York] was the first JAK inhibitor which was granted marketing authorisation by the FDA for the treatment of RA. The benefit/risk evaluation led to a non-approval of tofacitinib for RA and psoriasis by the EMA, but the application is currently being reviewed. Tofacitinib is a pan-JAK inhibitor, targeting all JAK family members. This has raised concerns regarding side effects and safety profile, and more specific inhibitors only targeting single JAKs are currently under development for IBD. These include filgotinib [GLPG0634, Galapagos, Mechelen, Belgium], JNJ-54781532 [Johnson and Johnson, New Brunswick, NJ, USA], and ABT-494 [Abbvie, Chicago, IL, USA].13,14 The efficacy of both the pan-JAK inhibitor tofacitinib and the selective inhibitors in the treatment of IBD is currently under investigation in clinical trials.
In order to understand the potential of JAK inhibitors as novel therapeutic agents for IBD, it is important to understand how they target cytokine signalling. We will review the available evidence on in vitro and in vivo data and clinical trial data in order to address two relevant research questions: 1] how does JAK inhibition affect the immune process relevant to the pathogenesis of IBD; and 2] is there a rationale for specific JAK inhibition in the treatment of UC and/or CD?
2. The JAK-STAT signalling pathway
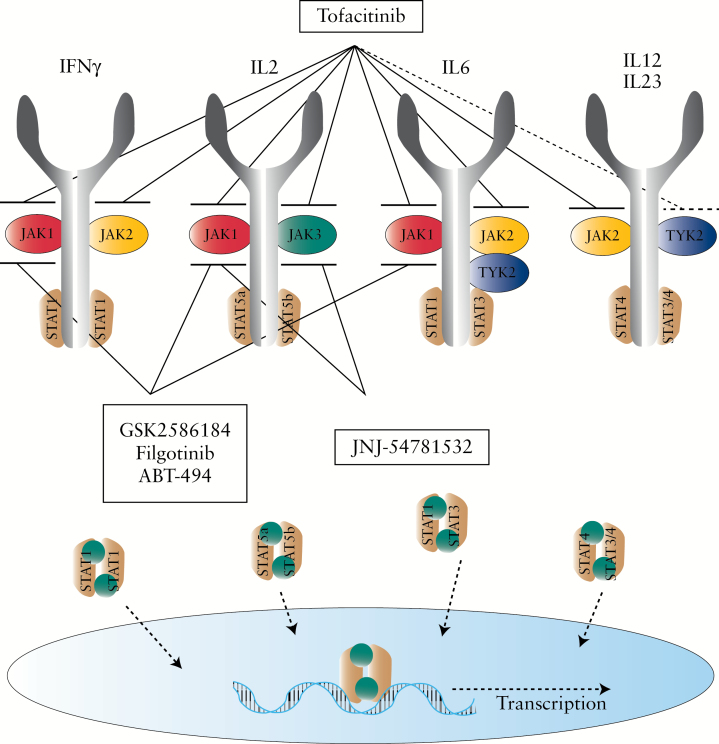
The Janus kinase family is constituted by four distinct members: JAK1, JAK2, JAK3, and tyrosine kinase 2 [TYK2]. The kinases were identified between 1989 and 1993 and named after Janus, the two-faced Roman god of gates and doorways, because these molecules have both a catalytic and a pseudo-kinase domain.15–18 JAKs are located at the cytoplasmic tail of various cytokine receptors, and are activated upon receptor-ligand interaction. Due to the kinase activity, JAK activation results in auto-phosphorylation as well as phosphorylation of the cytokine receptor chains, and the combination of JAKs and the receptor form specific binding sites for one or more members of the STAT [signal transducer and activator of transcription] family.13,19 In turn, the STAT molecules are activated, form homo- or heterodimers, and translocate to the nucleus where they bind to DNA at the level of promoter regions that modulate transcription [Figure 1].20 Different cytokines signal through different receptors, although many share common subunits. The composition of the receptor determines the specific JAKs activated upon cytokine binding, and in combination the receptor and JAK define which of the seven described STAT family members are activated, resulting in different JAK-STAT activation patterns in response to each cytokine. These activation patterns have been reviewed extensively elsewhere.21–23
Figure 1.
The JAK-STAT signalling pathway. Many cytokines important in the pathogenesis of inflammatory bowel disease [IBD] signal via the JAK-STAT pathway. Cytokine signalling induces phosphorylation of JAKs, which phosphorylate STAT proteins. STAT proteins form homo- or heterodimers and migrate into the nucleus where they activate transcription of inflammatory cytokines. JAK inhibitors are new therapeutic agents currently being investigated in clinical trials in IBD. First-generation JAK inhibitors [e.g. tofacitinib] target multiple JAKs, whereas second-generation JAK inhibitors [e.g. filgotinib] selectively target one JAK.
3. IBD-related cytokines targeted by JAK inhibition
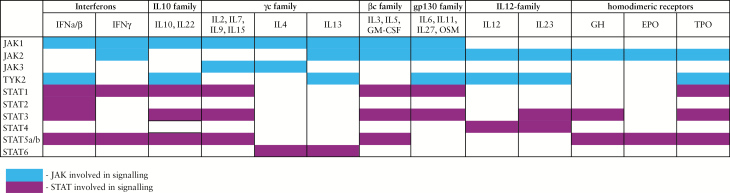
JAK inhibitors interfere with a number of key pro-inflammatory cytokines involved in the pathogenesis of IBD [Figure 2]. In CD, the pathogenic cytokine excess is attributed to activation of antigen-presenting cells and T helper [Th] 1 cells, an interaction that is strongly regulated by IL12 and IL27.24–26 IL12 plays a role in a myriad of immune signalling routes, including those involved in differentiation of naïve CD4+ T cells into interferon [IFN]γ-producing Th1 cells. Under Th1 polarising conditions, IL27 activates IL2-mediated clonal expansion of Th1 cells, contributing to the magnitude of this immune response. IL12 signals via JAK2-TYK2 activating STAT4, whereas IL27 signals via JAK1-JAK2-TYK2 activating STAT1-STAT3.
Figure 2.
Overview of cytokines signalling via the JAK-STAT pathway. JAK inhibitors interfere with a number of key pro-inflammatory cytokines involved in the pathogenesis of inflammatory bowel disease [IBD]. IFN, interferon; IL, interleukin; GM-CSF, granulocyte macrophage colony-stimulating factor; OSM oncostatin M; GH growth hormone; EPO erythropoietin; TPO thrombopoietin. Adapted with permission from Rawlings et al., J Cell Sci 2004;117:1281–3.
In UC, lamina propria T cells produce the Th2-type cytokines IL4, IL5, and IL13. The differentiation of naïve T cells into Th2 cells is mediated by IL4, signalling via JAK1–JAK3 and activating STAT6. IL5; signalling via JAK2 and activating STAT3-STAT5-STAT6, is a key mediator in eosinophil activation and promotes B cell growth. IL13 promotes fibrosis and induces apoptosis and degradation of tight junctions in intestinal epithelial cells, leading to ulceration.27,28 IL13 signals via JAK1-JAK2-TYK2 leading to activation of STAT6. In both CD and UC, increased production of Th17-cell associated cytokines is evident. Naïve T cells differentiate into Th17 cells in presence of cytokines such as IL6 and tumour growth factor β [TGFβ] and are further stabilised by IL21 and IL23. Th17 cells may play a pathogenic role in colitis, producing IFNγ as well as IL17A, IL17F, and IL22. In the context of JAK inhibition, it is important to realise that IL6, IL21, and IL23 all signal via the JAK-STAT pathway. Hence, inhibiting JAK2-TYK2-mediated IL23 signalling could hypothetically prevent pathogenic Th17 differentiation.29
The role of the cytokines produced by Th17 cells in colitis is not clear-cut and may depend on the tissue environment. IL17A increases the release of pro-inflammatory chemokines, cytokines, and other mediators such as matrix metalloproteinases and granulocyte-colony stimulating factor.30 Conversely, a protective role for IL17A in colitis has been described, demonstrated by the development of an aggressive inflammatory disease after adoptive transfer of IL17A knockout CD45RBhigh T cells in a T cell transfer model for colitis.31 Indeed, previous trials using IL17-blocking antibodies for the treatment of Crohn’s disease showed disease worsening rather than improvement.32,33 Data on the effect of JAK inhibition on IL17 production are scarce and mainly stem from murine studies. In a more tolerant environment containing IL6 and TGFβ, JAK inhibition resulted in an increase in IL17A-producing Th17 cells. In contrast, under more pathogenic conditions [addition of IL6 and IL23], the percentage of IL17A-producing Th17 cells decreased dramatically. This suggests that, depending on the micro environment, JAK inhibition can exert different effects.34
Another dual role has been described for IL22, signalling via JAK1-TYK2. Inhibition of IL22 activity could have anti-inflammatory effects by decreasing transcription of IL8 and TNFα via STAT3. In contrast, inhibiting IL22 via JAK1-TYK2 might also interfere with intestinal epithelial cell homeostasis and tissue healing.35 Altogether, it is evident that inhibition of JAKs affects a variety of immune processes relevant to the initiation and perpetuation of IBD, from tissue destruction by Th17-asscociated cytokines to the secretion of pro-inflammatory cytokines produced by lamina propria T cells.
4. Lessons learned from animal and human mutations in JAK-STAT proteins
Genome-wide association studies have identified single-nucleotide polymorphisms [SNP] coding for JAKs and STATs, associated with genetic susceptibility for IBD. Examples are polymorphisms in JAK2, TYK2, STAT1, STAT3, and STAT4. For TYK2, an SNP resulting in a loss of function is associated with a decreased risk for the development of UC.36,37 Conversely, a JAK2 SNP associated with increased risk for development of both UC and CD has recently been shown to be a gain of function polymorphism. 38 In addition many SNPs in cytokines signalling via the JAK-STAT pathway have been identified, such as IL12B and IL23R. This indicates the importance of the JAK-STAT pathway in the pathogenesis of IBD and supports the rationale of targeting this pathway.39 An overview of human gain- and loss-of-function mutations has been described elsewhere.40 The immune response deficits associated with those mutations show phenotypic similarity among murine and human abnormalities, indicating that the JAK-STAT pathway is largely conserved.
Murine JAK1 deficiency is characterised by a general unresponsiveness to type II cytokine signalling and impaired lymphoid and neuronal development, leading to perinatal death.41 In humans, loss- or gain-of-function JAK1 mutations have not been described. JAK2-deficient mice fail to respond to erythropoietin, interleukin 3 and thrombopoietin, also leading to embryonic mortality. However in an inducible Jak2V617F knock-in mouse, all major features of human polycythaemia vera were observed.42 In humans, a loss-of-function JAK2 mutation has not been described; however, a gain-of-function mutation accelerating JAK2 signalling causes myeloproliferative disorders such as polycythaemia vera, essential thrombocytosis, and primary myelofibrosis.43 JAK3 deficiency causes a reduction of thymocytes as well as severe B and T cell lymphopenia in mice.44 Human mutations in the gene encoding the γc or JAK3 causes X-linked severe combined immunodeficiency syndrome [X-SCID], a disease characterised by a decreased number and impaired function of T cells and natural killer cells, with preserved numbers of defective B cells.45,46
TYK2-deficient mice are known to have an increased susceptibility to bacterial and viral infections.47 Eight cases of human TYK2 deficiency have been described. The main clinical features of TYK2 deficiency in the first patient were severe atopic dermatitis and elevated serum immunoglobulin E, also known as hyper-immunoglobulin E syndrome [HIES].48 All other patients suffered from intracellular bacterial and viral infections attributed to impaired IL12 and IFNα/β signalling, but no clinical signs of HIES were observed in these patients. All eight patients displayed impaired but not abolished cellular responses to IL23, with normal proportions of circulating IL17+ T cells. In addition, an impaired but not abolished response to IL10 was observed in all patients, without any clinical features of IBD.49,50
The overlap in phenotypes described in mice and humans is important because it reflects the conserved nature of most JAK signalling pathways across species, and highlights the potential risk of treating IBD patients with non-selective JAK inhibitors. JAK1 and TYK2 inhibition could lead to an increased susceptibility to bacterial and viral infections, already demonstrated in clinical trials. JAK2 inhibition interferes with haematopoiesis and will likely result in low blood cell counts, whereas JAK3 inhibition could give rise to lymphopenia. Every JAK inhibitor is characterised by interference of all the four pathways to a variable degree. Evidently, more selective JAK inhibitors reducing signalling via one or two JAK family members only are a more attractive alternative option for pan-JAK inhibition and are currently being developed. The balance between anti-inflammatory effects and toxicity, however, needs to be carefully monitored and investigated for every individual compound.
5. Preclinical data: efficacy of pan-JAK inhibitors and selective JAK inhibitors
In murine and human T cells, tofacitinib was shown to inhibit STAT phosphorylation induced both by cytokines signalling via the common gamma chain [including IL2, -4, -7, -15, and -21] and by non-gamma chain-dependent cytokines [including IFNƳ, IL6, and IL12]. As a result, tofacitinib blocks differentiation of Th1 and Th2 cells in murine cells.34,51 In collagen-induced arthritis, a murine model for RA, oral administration of tofacitinib 50 mg/kg b.d. reduced plasma inflammatory cytokine concentrations within 4 h and reduced arthritis symptoms within 48 h.34 For induction of Th17 cells, the data are less clear. Th17 cells induced in the presence of IL-23 are strongly inhibited upon addition of tofacitinib, whereas induction of Th17 in the absence of IL-23 is stimulated.52 As a result, tofacitinib accelerated the onset of experimental autoimmune encephalitis, a murine model for multiple sclerosis, suggesting that the effects may differ between specific pathologies. Experience with tofacitinib in murine colitis is limited, but oral administration did prevent the development of oxazolone-induced colitis.53
Filgotinib is a JAK inhibitor that was shown to be more JAK1-selective than tofacitinib and was investigated in a phase 2 trial for CD.54 In vitro, filgotinib decreased transcriptional markers for human Th1 and Th2 polarisation and, albeit to a lesser extent, markers for Th17 polarisation. Furthermore, in a collagen-induced arthritis model, oral administration of filgotinib 3 mg/kg once daily for 14 days after onset of arthritis resulted in dose-dependent reduction of clinical scores and prevention of disease progression in rats.55
Inhibition of JAK3 appears to be an attractive alternative for pan-JAK inhibition, as it mainly exerts its effector function in immune cells, thus limiting potential side effects. JANEX-1, a selective JAK3 inhibitor, was investigated in a trinitibenzene sulphonic acid [TNBS] colitis model, in which mice received 25 mg/kg of JANEX-1 intraperitoneally daily. JANEX-1 administration prevented TNBS-induced diarrhoea and wasting and reduced colon wall thickness and colon weight.56 In contrast, a study investigating the course of 2.5% dextran sulphate sodium [DSS]-induced colitis in JAK3 knockout mice revealed earlier and more severe disease. However, loss of JAK3 in this model was constitutive, resulting in decreased enterocyte differentiation and aberrant barrier function. Consequently, animals were likely more sensitive to DSS-induced damage, explaining the accelerated course of disease. Targeted JAK3 inhibition in wild type mice after onset of DSS colitis has not been evaluated.57
Interleukin 12 and 23, both signalling via JAK2-TYK2, play a central role in intestinal inflammation. Preclinical studies on TYK2 inhibition in colitis show contrasting results. In a DSS colitis model, TYK2 knockout mice displayed a slower onset of disease and less disease activity. In addition, TYK2 knockout mice were protected against a decrease in body weight, and colon shortening was not as severe as in TYK2 sufficient mice. In a TNBS colitis model, the mortality rate was 100% in the TYK2-sufficient mice, whereas the mortality rate of the TYK2 knockout mice was 50%.58 Conversely, an independent study reported increased sensitivity to DSS in TYK2-deficient animals. In this study, this was attributed to a decrease in phosphorylation of STAT3 by IL22.35 The explanation for these contrasting data is not clear, but may be related to the difference in genetic background [C57Bl/6 vs Balb/c animals] or differences in microbiological status between the two institutes. Data on pharmacological TYK2 inhibition in colitis models are not available to date.
6. JAK inhibitors in IBD: where are we?
6.1. Tofacitinib in CD
Tofacitinib was investigated in a phase 2a trial including 139 patients with moderately to severely active Crohn’s disease, defined as a Crohn’s Disease Activity Index [CDAI] of 220–450. 59 Three different doses of tofacitinib were given for 4 weeks. The primary endpoint, a decrease in CDAI by 70 points, was observed in 36.1% [p = 0.467], 57.6% [p = 0.466], and 45.7% at 1, 3, and 15 mg tofacitinib b.d. vs in 47.1% of the patients receiving placebo [p = > 0.1].60 The placebo response and remission rates were unexpectedly high, possibly due to the lack of stringent inclusion criteria for active disease such as the presence of endoscopic ulcerations. However, patients receiving 15 mg b.d. had evidence of a biochemical response as shown by a stronger decrease in serum C-reactive protein [CRP], mean change from baseline in tofacitinib-treated patients -0.93 mg/l (95% confidence interval [CI] -1.53 to -0.33) vs -0.07 mg/l [95% CI -0.43 to 0.28) in the placebo group and in faecal calprotectin -0.70 mg/l [95% CI -1.11 to −0.29] in tofacitinib-treated vs -0.08 mg/l [95% CI -0.38 to 0.55] in placebo.
In a more recent phase 2b induction study, the efficacy of tofacitinib 5 and 10 mg b.d. for 8 weeks was evaluated in 280 patients with moderately to severely active CD.61 The observed proportions of patients in remission [CDAI < 150] were 43.0% and 43.5% for the 10 and 5 mg doses, respectively, vs 36.7% of patients in the placebo group. The proportions of patients with a decrease in CDAI by 100 points at Week 8 were 14–16% higher for tofacitinib 5 and 10 mg b.d. vs placebo. A mean change from baseline CRP of -0.42 and -0.73 mglL was observed for the 5 and 10 mg doses, whereas in the placebo group CRP increased from baseline with 0.12 mg/l. Faecal calprotectin levels were reduced from baseline, but not statistically significantly.
This phase 2b study then re-randomised patients who achieved a CDAI drop of at least 100 points at Week 8 in order to investigate the effects of maintenance therapy.62 Tofacitinib 5 or 10 mg b.d. or placebo b.d. was studied in 180 patients [CDAI ≥ 220 to ≤ 450 at the time of re-randomisation] for 26 weeks. Clinical response and remission rates with 5 or 10 mg of tofacitinib b.d. were higher for the 10 mg b.d. treatment arm, but this was not statistically superior to placebo [39.5% and 55.8% vs 38.1%, respectively]. Further development of tofacitinib in CD has been discontinued, although higher doses, better patient selection, and longer induction treatment may have led to better results.
6.2. JAK1 selective inhibition in CD
Whereas tofacitinib inhibits cytokines signalling via all JAK family members, filgotinib selectively targets the JAK1 signalling. Filgotinib was investigated in a 20-week phase 2 study [FITZROY] in 174 patients with moderate to severe CD, defined by a CDAI of 220–450 and endoscopic evidence of disease.54 Both anti-TNF naive and exposed patients were included in this study. Patients were randomised to receive either 200 mg or placebo daily for up to 10 weeks. Based on the Week 10 response, patients continued use of filgotinib [200 or 100 mg] or placebo for an additional 10 weeks. The primary endpoint, clinical remission at Week 10 [CDAI < 150], was achieved in 47% of patients in the filgotinib arm vs 23% in the placebo arm [p = 0.0077]. In patients treated with filgotinib, a significant decrease in histopathology scores was observed, as compared with patients treated with placebo [p = 0.0359]. Quality of life as measured by the Inflammatory Bowel Disease Questionnaire [IBDQ] improved in the treatment arm [p = 0.0046]. Normalisation of CRP [< 8 mg/l] was observed in a greater proportion of patients on filgotinib [27%] than in patients on placebo [14%]. In addition, in 25% of the patients receiving filgotinib vs 14% of the patients on placebo, the Simple Endoscopic Score for Crohn’s Disease [SES-CD] improved by at least 50%, although statistical significance was not reached [p > 0.16].Similar incidences in early discontinuations and [serious] adverse events [SAEs] including infections were observed, with the majority of the SAEs related to worsening of Crohn’s disease. The efficacy of a second JAK1 selective inhibitor [ABT-494, AbbVie] is currently being investigated in a phase 2 study for the treatment of CD. An overview of non-selective and selective JAK inhibitors investigated in CD is indicated in Table 1. Despite the disappointing results of tofacitinib in the treatment of CD, JAK 1 selective inhibitors appear to hold promise.
Table 1.
Janus kinase inhibitors in development for Crohn’s disease.
| Crohn’s disease | |||||||
|---|---|---|---|---|---|---|---|
| Clinical trial no. | Start | Drug | Developer | Specificity | Type | Duration | Status |
| NCT00615199 | 2008 [1] | Tofacitinib [CP-690,550] | Pfizer | JAK1, JAK2, JAK3, TYK2 | Safety | 4 weeks | Phase 2, completed |
| NCT01393626 | 2011 [10] | Tofacitinib [CP-690,550] | Pfizer | JAK1, JAK2, JAK3, TYK2 | Induction | 8 weeks | Phase 2, completed |
| NCT01393899 | 2012 [3] | Tofacitinib [CP-690,550] | Pfizer | JAK1, JAK2, JAK3, TYK2 | Maintenance | 26 weeks | Phase 2, completed |
| NCT01470599 | 2012 [4] | Tofacitinib [CP-690,550] | Pfizer | JAK1, JAK2, JAK3, TYK2 | Open-label | 52 weeks | Phase 2, completed |
| NCT02048618 | 2014 [2] | Filgotinib [GLPG0634] | Galapagos, AbbVie | JAK1 | Safety | 20 weeks | Phase 2, completed |
| NCT02365649 | 2015 [3] | ABT-494 | AbbVie | JAK1 | Induction | 16 weeks | Phase2, active, not recruiting |
| NCT02782663 | 2016 [5] | ABT-494 | AbbVie | JAK1 | Long-term | 24 months | Phase 2, completed |
6.3. Tofacitinib in UC
A first phase 2 trial with tofacitinib was conducted in 194 patients with moderate to severe UC in whom conventional therapy had failed.63 Tofacitinib was given b.d. at doses of 0.5, 3, 10, or 15 mg for 8 weeks. Clinical response was defined as a decrease in the MAYO score of at least three points and a relative decrease by at least 30% accompanied by a decrease in rectal bleeding subscore of at least one point or an absolute rectal bleeding subscore of 0 or 1. This endpoint was reached in 32% [p = 0.39], 48% [p = 0.55], 61% [p = 0.10], and 78% [p < 0.001] of patients on the 0.5, 3, 10 and 15 mg dose, respectively, vs 42% of patients on placebo. Clinical remission, defined as a total Mayo score of 0–2 with no subscore exceeding 1, was observed in 13% [p = 0.76], 33% [p = 0.01], 48% [p = < 0.001, and 41% [p = < 0.001] of patients receiving 0.5, 3, 10, and 15 mg b.d., respectively, compared with 10% of the patients receiving placebo. Endoscopic remission, stringently defined as an endoscopic subscore of 0, was observed in 10% [p = 0.14], 18% [p = 0.01], 30% [p < 0.001], and 27% [p = < 0.001] of patients receiving 3, 10, and 15 mg b.d., respectively, and in 2% in the placebo arm. Both the 10-mg and 15-mg dose decreased baseline calprotectin levels significantly after eight weeks of treatment.63
The OCTAVE 1 and OCTAVE 2 induction studies were two identical phase 3 studies in which patients with moderate to severe UC received tofacitinib 10 mg b.d. or placebo for 8 weeks.12 Initially, a higher dose of 15 mg b.d. was also studied, but this dose arm was discontinued early in the course of the trial as a corporate decision due to safety concerns including hyperlipidaemia and viral infections. In the OCTAVE 1 induction study, 598 patients were included, all of whom failed one or more previous treatments. The primary endpoint, remission at Week 8, was achieved in 18.5% of patients receiving tofacitinib vs 8.2% receiving placebo [p = < 0.01]. Mucosal healing [Mayo endoscopic subscore ≤ 1] was observed in 31.3% of patients receiving tofacitinib vs 15.6% of patients receiving placebo [p = < 0.001]. Clinical response, defined in the same way as in the phase 2 studies, was achieved in 59.9% of patients receiving tofacitinib vs 32.8% in the placebo group [p = < 0.001]. The treatment effect of tofacitinib 10 mg b.d. was similar between anti-TNF naïve and anti-TNF exposed patients. In the OCTAVE 2 induction study, 541 patients were included, of whom 55% had been exposed to anti-TNF previously. Clinical remission was observed in 16.6% of patients receiving tofacitinib vs 3.6% in the placebo group [p = < 0.001]. Mucosal healing was achieved in 28.4% of patients in the tofacitinib group vs 11.16% in the placebo arm [p = < 0.001], whereas clinical response was obtained in 55.0% vs 28.6% [p = < 0.001]. As in the OCTAVE 1 study, the efficacy in anti-TNF experienced patients and in anti-TNF naive patients was similar.
6.4. JAK1 selective inhibition in UC
Two selective JAK inhibitors targeting JAK1 have been investigated in UC patients. The study investigating an oral JAK1 selective inhibitor GSK25856184 was terminated at an early stage due to reports of liver toxicity in patients treated with this agent for systemic lupus erythematosus.64 GSK2586184 was administered at 400 mg b.d. to two patients with moderate to severely active UC for 7 and 2 weeks, respectively. The Total Mayo score decreased from 7 and 11 to 3 in both patients with mucosal healing at early withdrawal sigmoidoscopy. The agent was well tolerated by these two UC patients. A second JAK1 inhibitor, JNJ-54781532 [ASPK015K] developed by Janssen, was investigated in a phase 2b trial in UC, but the results have not yet been released [NCT01959282]. An overview of non-selective and selective JAK inhibitors investigated in UC is presented in Table 2. Further clinical data are needed before the real potential of JAK1 inhibition for UC can be assessed.
Table 2.
Janus kinase inhibitors in development for ulcerative colitis.
| Ulcerative colitis | |||||||
|---|---|---|---|---|---|---|---|
| Clinical trial no. | Start | Drug | Developer | Specificity | Type | Duration | Status |
| NCT00787202 | 2008 [10] | Tofacitinib [CP-690,550] | Pfizer | JAK1, JAK2, JAK3, TYK2 | Safety | 8 weeks | Phase 2, completed |
| NCT01465763 | 2012 [4] | Tofacitinib [CP-690,550] | Pfizer | JAK1, JAK2, JAK3, TYK2 | Induction | 8 weeks | Phase 3, completed |
| NCT01458951 | 2012 [6] | Tofacitinib [CP-690,550] | Pfizer | JAK1, JAK2, JAK3, TYK2 | Induction | 8 weeks | Phase 3, completed |
| NCT01458574 | 2012 [7] | Tofacitinib [CP-690,550] | Pfizer | JAK1, JAK2, JAK3, TYK2 | Maintenance | 52 weeks | Phase 3, completed |
| NCT01470612 | 2012 [10] | Tofacitinib [CP-690,550] | Pfizer | JAK1, JAK2, JAK3, TYK2 | Long-term | 52 weeks | Phase 3, recruiting |
| NCT02000453 | 2013 [11] | GSK2586184 | Galapagos, GSK | JAK1 | Induction | 8 weeks | Phase 1, terminated |
| NCT01959282 | 2013 [11] | JNJ-54781532 | Janssen | JAK3/JAK1 | Safety | 8 weeks | Phase 2b, completed |
| NCT02819635 | 2016 [9] | ABT-494 | AbbVie | JAK1 | Induction | 8 weeks | Phase 2, recruiting |
| NCT02819635 | 2016 [9] | ABT-494 | AbbVie | JAK1 | Maintenance | 44 weeks | Phase 2, recruiting |
7. Safety of JAK inhibitors
7.1. Safety of tofacitinib
Most safety data on tofacitinib are derived from randomised controlled trials [RCTs] conducted in patients with rheumatoid arthritis. A systematic review and meta-analysis assessed incidence rates of serious infections in patients with moderate to severe RA treated with biologic disease-modifying antirheumatic drugs [DMARDs] or with tofacitinib. Tofacitinib data were collected from five phase 2 RCTs, six phase 3 RCTs, and two long-term extension studies, only including the 5- and 10-mg b.d. doses. For infliximab, adalimumab, and golimumab, the incidence rates for serious infections were 6.11, 5.04, and 5.31 events per 100 patient-years, respectively, compared with 3.00 events per 100 patient-years for tofacitinib 10 mg b.d. For the long-term extension studies [10 mg b.d.], the incidence rate of serious infections was 3.19 patients with events per 100 patient-years. Risk differences for serious infections during treatment with biologic DMARDs and tofacitinib vs placebo across RCTs were 0.52, 1.16, and 0.68 [95% CI] for infliximab, adalimumab and golimumab, respectively, vs 0.40 [95% CI] for the patients receiving tofacitinib 10 mg b.d.65 In the phase 3 and open-label long-term extension [LTE] tofacitinib studies in RA, the incidence rate for opportunistic infections was 0.46 per 100 patient-years [95% CI]. Tuberculosis was the most common infection [0.21 per 100 patient-years] during tofacitinib treatment, but was rare in regions with low and medium incidence of tuberculosis.66
In the OCTAVE 1 and OCTAVE 2 induction studies, headache and nasopharyngitis were the most common adverse events observed. In these studies, serious infections were observed in 1.3% and 0.2% of the patients receiving tofacitinib vs no serious infections in the placebo groups. These serious infections included pneumonia, anal abscess, and Clostridium difficile infection. Herpes zoster occurred in 0.6% and 0.5% in the OCTAVE 1 and OCTAVE 2 studies vs 0.8% and 0.0% in both placebo groups.12 In the most recent maintenance study in CD, higher rates of infections were observed in 48.3% and 44.5% of the patients on the 5- and 10-mg b.d. dose, respectively, vs 33.9% of patients on placebo. Two cases of non-serious herpes zoster occurred in the 10-mg b.d. arm and one intestinal perforation in the 5-mg b.d. arm.62 Inhibition of multiple JAK proteins by tofacitinib appears to induce immune suppression, and it remains to be clarified what the effect on cytokine signalling is when only a single JAK protein is targeted.
An important observation in the IBD and RA trials was the increase in the serum lipid levels. In the phase 2a CD trial, an increase from baseline in mean total cholesterol, high-density lipoprotein [HDL], and low-density lipoprotein [LDL] was observed in patients receiving 5 mg and 15 mg b.d. Triglyceride levels also increased in patients receiving 5 mg b.d.59 In the phase 2 UC trial, a dose-dependent increases of HDL and LDL were observed at 8 weeks of treatment. However, this was reversed after discontinuation, consistent with observations regarding serum cholesterol levels as seen in RA trials.63 Exposure to filgotinib 200 mg resulted in an increase of mean HDL and LDL by respectively 11% and 12% at 20 weeks, whereas in patients receiving placebo only an LDL increase was observed, resulting in an improved atherogenic index.54 Patients with RA have lower serum HDL, LDL, and total cholesterol levels in comparison with healthy individuals, possibly driven by an increased cholesterol ester metabolism. In a phase I open-label-mechanistic study, patients with RA received tofacitinib 10 mg b.d. for 6 weeks. Tofacitinib treatment attenuated the cholesterol ester catabolism, increasing cholesterol levels towards those in healthy individuals.67 Similar changes in lipid levels were observed in an RA trial, in which patients received an IL6 receptor antagonist, tocilizumab, suggesting that IL6 signalling might play a role in the alteration of serum lipid levels.68
Since tofacitinib inhibits erythropoietin and GM-CSF signalling mediated via JAK2 in vitro, concerns were raised regarding the induction of anaemia, neutropenia, and thromboctyopenia.69 This problem did not occur in the phase 2 CD trial.59 In the phase 2 UC trial, absolute neutrophil counts dropped below 1.5*109 cells/l in three patients, although never below 1*109 cells/l.63 In Pfizer’s summary safety data presented to the FDA,70 LTE studies of RA patients who had received tofacitinib describe most cases of neutropenia as being mild. In patients who received 5 mg b.d., 4.2% of 1220 patients had an absolute neutrophil count between 1.5*109 cells/l and 2*109 cells/l. In the 10-mg b.d. group, this was observed in 1.9% of 1900 patients. This difference was likely due to longer exposure to the study drug in the 5-mg group. There was no association between the occurrence of serious infections and low neutrophil counts in patients treated with tofacitinib in phase 3 and LTE studies. Most cases of anaemia in the LTE studies were mild. Confirmed anaemia was observed in 8.2% of 1900 RA patients who had received 10 mg b.d. In patients who had received 5 mg b.d., 12.4% of 1319 RA patients had confirmed anaemia. Most cases of lymphopenia in the LTE studies were moderate to severe. A lymphocyte count between 0.5 and 1.5*109 cells/l was observed in 60.2% of 732 patients who had received the 5-mg b.d. dose. In the 10-mg b.d. group, 30.6% of 1258 patients had a moderate to severe lymphopenia during treatment with tofacitinib. There was no observable association between confirmed lymphopenia with lymphocyte levels between 0.5 and 2*109 cells/l and the occurrence of serious infections. Of note, no data are available on patients concomitantly using azathioprine or 6-mercaptopurine, and this combination appears undesirable at present.
Pfizer’s safety report on the use of tofacitinib states a slight increase in the incidence of malignancies. In Phase 3 and LTE studies, of 4791 RA patients treated with tofacitinib, 65 were diagnosed with a malignancy. Most commonly reported were lymphoma, breast cancer, and lung cancer, similar to data reported in RA trials using anti-TNF agents or immune modulators. The report indicated an estimated risk for lymphoma of 0.07 per 100 patient-years.
7.2. Safety of selective JAK inhibitors
In the FITZROY study, similar incidences of infectious adverse events were observed in the CD patients receiving the JAK1 selective inhibitor filgotinib vs the patients receiving placebo in the first 10 weeks. Serious infections were reported in four out of 152 (3%) patients treated with filgotinib up to week 20 vs none in the placebo group. Early discontinuations and SAEs were similar in treated patients vs patients receiving placebo.54 The only safety data available on JAK1 selective inhibition in UC indicated that GSK2586184 was well tolerated in two patients.64
8. Future prospects
Targeting the JAK-STAT pathway, and thus inhibiting signalling of multiple cytokines, appears to have great potential for the treatment of IBD. The main advantages of these agents are that they are orally delivered small molecules, without a risk of immunogenicity. In CD, treatment with tofacitinib showed limited clinical efficacy in both phase 2 trials. Possibly the trial designs were suboptimal with a short duration of treatment and no selection of patients based on endoscopy. Of note, dose-related decreases in serum CRP and faecal calprotectin in actively treated patients suggested a biological treatment effect.59,61 Unfortunately, and partly due to the lack of endoscopic endpoints in the trials, firm conclusions on the efficacy of tofacitinib in CD remain impossible.
Some limitations of the pan-JAK inhibitor tofacitinib [such as broad immune suppression] may be overcome by a more specific blockade of a single JAK. One of the agents selectively blocking JAK1 is filgotinib. In the FITROY study, CD patients were included using clinical and endoscopic endpoints. The results showed that treatment with filgotinib improved SES-CD scores by at least 50% in a larger proportion of treated patients than in patients on placebo. Only one dose of filgotinib was tested [200 mg/day] so it remains somewhat unclear whether the optimal dose has been selected at this point.54 The results of ABT-494, a second JAK1 selective inhibitor investigated in CD, are eagerly awaited [NCT02365649].
In UC, tofacitinib was more effective than placebo in inducing clinical and endoscopic response and remission after 8 weeks of treatment.12,63 Interestingly, in both phase 3 trials efficacy was similar in anti-TNF naïve patients compared with patients that had previously failed TNF inhibitors Also, all patients had failed conventional therapy and half of the patients were using corticosteroids at baseline, indicating the potential of tofacitinib in the treatment of severe UC. Conventional immunosuppression was stopped when patients entered the trial, which means that tofacitinib can be used as single treatment in this population.
The question arises as to where tofacitinib will eventually find its place in the therapeutic armamentarium of UC, given the safety and efficacy of aminosalicylates. It is unlikely that tofacitinib will replace this class as first-line therapy for active UC. After aminosalicylates, most patients currently receive corticosteroids. Tofacitinib could be a treatment option for patients who need corticosteroids, either intermittently or chronically, and perhaps replace thiopurines which are currently being used for this indication without much clinical trial evidence. Notably, the clinical effect of tofacitinib becomes apparent in the first 2 weeks of treatment, whereas thiopurines usually need more than 8 weeks to exert their effect. Whether tofacitinib will finally end up as second-line agent for ulcerative colitis also depends on the outcome of ongoing phase 3 trials with the S1P1 inhibitor ozanimod, yet another oral agent with potential for second-line treatment of UC.71 Based on the trial data, tofacitinib is equally effective in biological-exposed as in biological-naïve patients. As a consequence, even after failure of these agents tofacitinib remains an attractive treatment alternative.
Much of the positioning will eventually also depend on the safety profile of the JAK inhibitors. Since opportunistic infections have been observed, it appears desirable that the drugs be used without other concomitant immunosuppressives and that corticosteroids be weaned as soon as clinically possible. The atherogenic risk associated with elevation of cholesterol levels will need further study. Finally, the safety of long-term use will need to be assessed in follow-up studies and safety registries.
Funding
No funding has been received for this work.
Conflict of Interest
LV has received research funding from GlaxoSmithKline. MW has served as a speaker for Takeda and Ferring. WJ has received research grants from Mead Johnson Pediatric Nutrition Institute, GlaxoSmithKline, Setpoint Medical, and Schwabe GmbH; he has served as a speaker for Takeda. GH has served as speaker, consultant, and principal investigator for Abbott/Abbvie, AM Pharma, Centocor/Jansen Biologics, Engene, Photopill, Setpoint, Novonordisk, MSD, UCB, Takeda, TEVA, Millenium, Boerhinger Ingelheim, Elan, Ferring, DrFALK Pharma, Shire, Cosmo, AstraZeneca, GlaxoSmithKLine, and PDL.
Author Contributions
All authors made substantial contributions to the concept, design, and writing of this manuscript. The final version of the manuscript was approved by all authors.
References
- 1. Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet 2012;380:1606–19. [DOI] [PubMed] [Google Scholar]
- 2. Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet 2012;380:1590–605. [DOI] [PubMed] [Google Scholar]
- 3. Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet 2007;369:1627–40. [DOI] [PubMed] [Google Scholar]
- 4. Danese S. New therapies for inflammatory bowel disease: from the bench to the bedside. Gut 2012;61:918–32. [DOI] [PubMed] [Google Scholar]
- 5. Gisbert JP, Panés J. Loss of response and requirement of infliximab dose intensification in Crohn’s disease: a review. Am J Gastroenterol 2009;104:760–7. [DOI] [PubMed] [Google Scholar]
- 6. Filippi J, Allen PB, Hébuterne X, Peyrin-Biroulet L. Does anti-TNF therapy reduce the requirement for surgery in ulcerative colitis? A systematic review. Curr Drug Targets 2011;12:1440–7. [DOI] [PubMed] [Google Scholar]
- 7. Lam MC, Bressler B. Vedolizumab for ulcerative colitis and Crohn’s disease: results and implications of GEMINI studies. Immunotherapy 2014;6:963–71. [DOI] [PubMed] [Google Scholar]
- 8. Feagan BG, Sandborn WJ, Gasink C, et al. ; UNITI–IM-UNITI Study Group. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2016;375:1946–60. [DOI] [PubMed] [Google Scholar]
- 9. Papp KA, Krueger JG, Feldman SR, et al. Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: Long-term efficacy and safety results from 2 randomized phase-III studies and 1 open-label long-term extension study. J Am Acad Dermatol 2016;74:841–50. [DOI] [PubMed] [Google Scholar]
- 10. Charles-Schoeman C, Burmester G, Nash P, et al. Efficacy and safety of tofacitinib following inadequate response to conventional synthetic or biological disease-modifying antirheumatic drugs. Ann Rheum Dis 2016;75:1293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xing L, Dai Z, Jabbari A, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med 2014;20:1043–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sandborn WJ, Sands BE, D’Haens G, et al. Presentation OP019 efficacy and safety of oral tofacitinib as induction therapy in patients with moderate-to-severe ulcerative colitis: results from 2 phase 3 randomised controlled trials. Eleventh Congress of theEuropean Crohn’s and Colitis Organisation, March 16–19, 2016, RAI Amsterdam, The Netherlands. [Google Scholar]
- 13. O’Shea JJ, Kontzias A, Yamaoka K, Tanaka Y, Laurence A. Janus kinase inhibitors in autoimmune diseases. Ann Rheum Dis 2013;72[Suppl 2]:ii111–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kontzias A, Kotlyar A, Laurence A, Changelian P, O’Shea JJ. Jakinibs: a new class of kinase inhibitors in cancer and autoimmune disease. Curr Opin Pharmacol 2012;12:464–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wilks AF. Two putative protein-tyrosine kinases identified by application of the polymerase chain reaction. Proc Natl Acad Sci U S A 1989;86:1603–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wilks AF, Harpur AG, Kurban RR, Ralph SJ, Zürcher G, Ziemiecki A. Two novel protein-tyrosine kinases, each with a second phosphotransferase-related catalytic domain, define a new class of protein kinase. Mol Cell Biol 1991;11:2057–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Johnston JA, Kawamura M, Kirken RA, et al. Phosphorylation and activation of the Jak-3 Janus kinase in response to interleukin-2. Nature 1994;370:151–3. [DOI] [PubMed] [Google Scholar]
- 18. Rane SG, Reddy EP. JAK3: a novel JAK kinase associated with terminal differentiation of hematopoietic cells. Oncogene 1994;9:2415–23. [PubMed] [Google Scholar]
- 19. Stark GR, Darnell JE., Jr The JAK-STAT pathway at twenty. Immunity 2012;36:503–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Darnell JE., Jr STATs and gene regulation. Science 1997;277:1630–5. [DOI] [PubMed] [Google Scholar]
- 21. Villarino AV, Kanno Y, Ferdinand JR, O’Shea JJ. Mechanisms of Jak/STAT signaling in immunity and disease. J Immunol 2015;194:21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. O’Shea JJ, Plenge R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity 2012;36:542–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol 2005;5:375–86. [DOI] [PubMed] [Google Scholar]
- 24. Monteleone G, Biancone L, Marasco R, et al. Interleukin 12 is expressed and actively released by Crohn’s disease intestinal lamina propria mononuclear cells. Gastroenterology 1997;112:1169–78. [DOI] [PubMed] [Google Scholar]
- 25. Trinchieri G, Pflanz S, Kastelein RA. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity 2003;19:641–4. [DOI] [PubMed] [Google Scholar]
- 26. Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol 2005;5:521–31. [DOI] [PubMed] [Google Scholar]
- 27. Fuss IJ, Heller F, Boirivant M, et al. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J Clin Invest 2004;113:1490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heller F, Florian P, Bojarski C, et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology 2005;129:550–64. [DOI] [PubMed] [Google Scholar]
- 29. Muranski P, Restifo NP. Essentials of Th17 cell commitment and plasticity. Blood 2013;121:2402–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brand S. Crohn’s disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn’s disease. Gut 2009;58:1152–67. [DOI] [PubMed] [Google Scholar]
- 31. O’Connor W, Jr, Kamanaka M, Booth CJ, et al. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol 2009;10:603–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hueber W, Sands BE, Lewitzky S, et al. ; Secukinumab in Crohn’s Disease Study Group. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 2012;61:1693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Targan SR, Feagan B, Vermeire S, et al. A randomized, double-blind, placebo-controlled phase 2 study of brodalumab in patients with moderate-to-severe Crohn’s disease. Am J Gastroenterol 2016;111:1599–607. [DOI] [PubMed] [Google Scholar]
- 34. Ghoreschi K, Jesson MI, Li X, et al. Modulation of innate and adaptive immune responses by tofacitinib [CP-690,550]. J Immunol 2011;186:4234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hainzl E, Stockinger S, Rauch I, et al. Intestinal epithelial cell tyrosine kinase 2 transduces IL-22 signals to protect from acute colitis. J Immunol 2015;195:5011–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li Z, Gakovic M, Ragimbeau J, et al. Two rare disease-associated Tyk2 variants are catalytically impaired but signaling competent. J Immunol 2013;190:2335–44. [DOI] [PubMed] [Google Scholar]
- 37. Can G, Tezel A, Gürkan H, et al. Tyrosine kinase-2 gene polymorphisms are associated with ulcerative colitis and Crohn’s disease in Turkish population. Clin Res Hepatol Gastroenterol 2015;39:489–98. [DOI] [PubMed] [Google Scholar]
- 38. Hedl M, Proctor DD, Abraham C. JAK2 disease-risk variants are gain of function and JAK signaling threshold determines innate receptor-induced proinflammatory cytokine secretion in macrophages. J Immunol 2016;197:3695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jostins L, Ripke S, Weersma RK, et al. ; International IBD Genetics Consortium [IIBDGC]. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012;491:119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. O’Shea JJ, Holland SM, Staudt LM. JAKs and STATs in immunity, immunodeficiency, and cancer. N Engl J Med 2013;368:161–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rodig SJ, Meraz MA, White JM, et al. Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell 1998;93:373–83. [DOI] [PubMed] [Google Scholar]
- 42. Akada H, Yan D, Zou H, Fiering S, Hutchison RE, Mohi MG. Conditional expression of heterozygous or homozygous Jak2V617F from its endogenous promoter induces a polycythemia vera-like disease. Blood 2010;115:3589–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Baxter EJ, Scott LM, Campbell PJ, et al. ; Cancer Genome Project. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 2005;365:1054–61. [DOI] [PubMed] [Google Scholar]
- 44. Nosaka T, van Deursen JM, Tripp RA, et al. Defective lymphoid development in mice lacking Jak3. Science 1995;270:800–2. [DOI] [PubMed] [Google Scholar]
- 45. Casanova JL, Holland SM, Notarangelo LD. Inborn errors of human JAKs and STATs. Immunity 2012;36:515–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Notarangelo LD, Mella P, Jones A, et al. Mutations in severe combined immune deficiency [SCID] due to JAK3 deficiency. Hum Mutat 2001;18:255–63. [DOI] [PubMed] [Google Scholar]
- 47. Shimoda K, Kato K, Aoki K, et al. Tyk2 plays a restricted role in IFN alpha signaling, although it is required for IL-12-mediated T cell function. Immunity 2000;13:561–71. [DOI] [PubMed] [Google Scholar]
- 48. Minegishi Y, Saito M, Tsuchiya S, et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature 2007;448:1058–62. [DOI] [PubMed] [Google Scholar]
- 49. Kilic SS, Hacimustafaoglu M, Boisson-Dupuis S, et al. A patient with tyrosine kinase 2 deficiency without hyper-IgE syndrome. J Pediatr 2012;160:1055–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kreins AY, Ciancanelli MJ, Okada S, et al. Human TYK2 deficiency: mycobacterial and viral infections without hyper-IgE syndrome. J Exp Med 2015;212:1641–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Piscianz E, Valencic E, Cuzzoni E, et al. Fate of lymphocytes after withdrawal of tofacitinib treatment. PLoS One 2014;9:e85463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yoshida H, Kimura A, Fukaya T, et al. Low dose CP-690,550 [tofacitinib], a pan-JAK inhibitor, accelerates the onset of experimental autoimmune encephalomyelitis by potentiating Th17 differentiation. Biochem Biophys Res Commun 2012;418:234–40. [DOI] [PubMed] [Google Scholar]
- 53. Shen F, Brassil P, Hegde S, Beattie D.Poster presentation. P036 Colon-targeted delivery of tofacitinib inhibits oxazolone-induced colitis in mice, despite low systemic exposure. Eleventh Congress of theEuropean Crohn’s and Colitis Organisation, March 16–19, 2016, RAI Amsterdam, The Netherlands. [Google Scholar]
- 54. Vermeire S, Schreiber S, Petryka R, et al. Clinical remission in patients with moderate-to-severe Crohn’s disease treated with filgotinib [the FITZROY study]: results from a phase 2, double-blind, randomised, placebo-controlled trial . Lancet, published online Dec 14 2016. http://dx.doi.org/10.1016/S0140-6736[16]32538-7. [DOI] [PubMed] [Google Scholar]
- 55. Van Rompaey L, Galien R, van der Aar EM, et al. Preclinical characterization of GLPG0634, a selective inhibitor of JAK1, for the treatment of inflammatory diseases. J Immunol 2013;191:3568–77. [DOI] [PubMed] [Google Scholar]
- 56. Uckun FM, Tibbles H, Ozer Z, Qazi S, Vassilev A. Anti-inflammatory activity profile of JANEX-1 in preclinical animal models. Bioorg Med Chem 2008;16:1287–98. [DOI] [PubMed] [Google Scholar]
- 57. Mishra J, Verma RK, Alpini G, Meng F, Kumar N. Role of Janus kinase 3 in mucosal differentiation and predisposition to colitis. J Biol Chem 2013;288:31795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ishizaki M, Akimoto T, Muromoto R, et al. Involvement of tyrosine kinase-2 in both the IL-12/Th1 and IL-23/Th17 axes in vivo. J Immunol 2011;187:181–9. [DOI] [PubMed] [Google Scholar]
- 59. Sandborn WJ, Ghosh S, Panes J, Vranic I, Wang W, Niezychowski W. A phase 2 study of tofacitinib, an oral Janus kinase inhibitor, in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2014;12:1485–93. [DOI] [PubMed] [Google Scholar]
- 60. Best WR, Becktel JM, Singleton JW, Kern F., Jr Development of a Crohn’s disease activity index. National cooperative Crohn’s disease study. Gastroenterology 1976;70:439–44. [PubMed] [Google Scholar]
- 61. Panés J, Sandborn WJ, Schreiber S, et al. Presentation OP021. Efficacy and safety of oral tofacitinib for induction therapy in patients with moderate-to-severe Crohn’s disease: results of a Phase 2b randomised placebo-controlled trial. Eleventh Congress of theEuropean Crohn’s and Colitis Organisation. March 1619, 2016, RAI Amsterdam, The Netherlands. [Google Scholar]
- 62. D’Haens G, Pannaccione R, Higgins PDR, et al. Presentation OP022. Efficacy and safety of oral tofacitinib for maintenance therapy in patients with moderate-to-severe Crohn’s disease: results of a Phase 2b randomised placebo-controlled trial. Eleventh Congress of theEuropean Crohn’s and Colitis Organisation, March 16–19, 2016, RAI Amsterdam, The Netherlands. [Google Scholar]
- 63. Sandborn WJ, Ghosh S, Panes J, et al. ; Study A3921063 Investigators. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med 2012;367:616–24. [DOI] [PubMed] [Google Scholar]
- 64. De Vries LCS, Hilbers-van Leeuwen FWM, Verseijden C, et al. Poster presentation P601. The efficacy of GSK2586184, a novel selective Janus kinase 1 inhibitor, in patients with moderate to severely active ulcerative colitis. Eleventh Congress of theEuropean Crohn’s and Colitis Organisation, March 16–19, 2016, RAI Amsterdam, The Netherlands. [Google Scholar]
- 65. Strand V, Ahadieh S, French J, et al. Systematic review and meta-analysis of serious infections with tofacitinib and biologic disease-modifying antirheumatic drug treatment in rheumatoid arthritis clinical trials. Arthritis Res Ther 2015;17:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Winthrop KL, Park SH, Gul A, et al. Tuberculosis and other opportunistic infections in tofacitinib-treated patients with rheumatoid arthritis. Ann Rheum Dis 2015;75:1133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Charles-Schoeman C, Fleischmann R, Davignon J, et al. Potential mechanisms leading to the abnormal lipid profile in patients with rheumatoid arthritis vs healthy volunteers and reversal by tofacitinib. Arthritis Rheumatol 2015;67:616–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Maini RN, Taylor PC, Szechinski J, et al. ; CHARISMA Study Group. Double-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis Rheum 2006;54:2817–29. [DOI] [PubMed] [Google Scholar]
- 69. Broxmeyer HE. Erythropoietin: multiple targets, actions, and modifying influences for biological and clinical consideration. J Exp Med 2013;210:205–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pfizer Advisory Committee Meeting. Tofacitinib for Treatment of Rheumatoid Arthritis [NDA 203214] May 9, 2012. edition. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/ArthritisAdvisoryCommittee/UCM302960 Accessed February 14, 2014.
- 71. Sandborn WJ, Feagan BG, Wolf DC, et al. ; TOUCHSTONE Study Group. Ozanimod induction and maintenance treatment for ulcerative colitis. N Engl J Med 2016;374:1754–62. [DOI] [PubMed] [Google Scholar]