Abstract
Background and Aims
Progressive multifocal leukoencephalopathy [PML], a brain infection associated with anti-integrin drugs that inhibit lymphocyte translocation from bloodstream to tissue, can be fatal. Decreased central nervous system [CNS] immune surveillance leading to this infection has been reported in patients with multiple sclerosis or Crohn’s disease treated with anti-integrin antibody natalizumab. PF-00547659 is an investigational human monoclonal antibody for inflammatory bowel disease, targeted against α4β7-mucosal addressin cell-adhesion molecule-1 [the integrin ligand selectively expressed in the gut]. We hypothesised that this selective agent would not affect central nervous system immune surveillance.
Methods
Cerebrospinal fluid from five healthy volunteers, and from 10 patients with Crohn’s disease previously treated with immunosuppressants, was evaluated to assess the feasibility of the study. Subsequently, 39 patients with active Crohn’s disease and previous immunosuppression were evaluated over 12 weeks of PF-00547659-induction therapy. We measured total lymphocytes, T cell subsets in cerebrospinal fluid, and circulating β7+ memory cells. Disease activity was assessed using the Harvey–Bradshaw Index.
Results
Patients treated with PF-00547659 had no reduction of cerebrospinal fluid lymphocytes, T-lymphocyte subsets, or CD4:CD8 ratio, whereas circulating β7+ memory cells increased significantly. A total of 28/35 [80%] patients had a clinical response and 27/34 [79%] had disease remission. Treatment-related adverse events, none serious, were reported in 23/49 [47%] patients.
Conclusions
In patients with active Crohn’s disease, natalizumab therapy increases the risk for PML, and the increased risk is thought to be associated with iatrogenic leukopenia within the CNS. PML under PF-00547659 may be a lesser concern, as this agent did not reduce lymphocytes or T cell subsets in the cerebrospinal fluid.
Keywords: Crohn’s disease, inflammatory bowel disease, MAdCAM-1, PF-00547659, immune surveillance
1. Introduction
Inhibition of lymphocyte trafficking from blood vessels into tissue offers the promise of treatment for a variety of inflammatory diseases. Lymphocyte trafficking is a complex process that is mediated in part by binding of integrins, such as LFA-1 and α4β7, to cell adhesion molecules, such as ICAM-1 and VCAM-1.1,2 However, this category of drugs has significantly lost momentum due to the high incidence of progressive multifocal leukoencephalopathy [PML]. The first two anti-integrin agents reported to inhibit lymphocyte trafficking were: 1] efalizumab [indicated for the treatment of psoriasis3] which targets the integrins of the LFA-1-intercellular adhesion molecule-1 [ICAM-1]; and 2] natalizumab (indicated for the treatment of both multiple sclerosis [MS]4 and Crohn’s disease [CD]5) which targets the integrins of the α4β1-vascular cell adhesion molecule-1 [VCAM-1] and the α4β7-mucosal addressin cell-adhesion molecule-1 [MAdCAM-1]. Both drugs are associated with an increased risk of PML, a serious and potentially fatal opportunistic central nervous system [CNS] infection caused by the John Cunningham virus [JCV].6–8 The mechanism by which natalizumab is thought to cause PML includes diminished CNS immune surveillance due to inhibition of the α4β1-VCAM-1 pathway.9
In patients treated with natalizumab, three clinical risk factors for PML have been identified: 1] treatment duration [ie, 25–48 months]; 2] previous exposure to JCV, measured by the presence of anti-JCV antibodies; and 3] history of previous immunosuppressant therapy. More than 1% of patients who had all three risk factors developed PML.10 Analysis of cerebrospinal fluid [CSF] from patients with MS as early as 6 weeks after the first dose of natalizumab showed a reduction in CD4+ T cell count and inversion of the CD4:CD8 ratio, a finding consistent with impaired immune surveillance.11 As a result, therapies with greater selectivity have been developed especially for inflammatory bowel disease [IBD], in the belief that they would reduce or eliminate the risk of PML while maintaining anti-inflammatory potency in the gut.12
Crohn’s disease is a relapsing systemic IBD that mainly affects the gastrointestinal [GI] tract; it is triggered by environmental factors in genetically susceptible individuals, resulting in a disturbed mucosal immune response leading to chronic ulcerations in the GI tract.13 A key feature of this inflammatory process is transmural inflammation, with a massive presence of leukocytes recruited from the circulation into the gut mucosa.14
Interference with mechanisms regulating lymphocyte adhesion and trafficking into inflamed gut tissue is an attractive target for therapeutic intervention.15 The integrin α4β7 mediates the homing of memory T cells to the intestinal mucosa via binding to its endothelial ligand, MAdCAM-1.10,16 MAdCAM-1 is upregulated in the presence of inflammation and is expressed at high levels on high endothelial venules in gut and gut-associated lymphoid tissue of patients with IBD.17 Although it can also be induced by inflammation,18 MAdCAM-1 is not constitutively expressed in the CNS19; therefore, drugs that selectively target this translocation axis may reduce inflammation in the gut without affecting immune surveillance in the CNS.
PF-00547659 is a human monoclonal antibody that binds selectively and with high affinity to mouse and human MAdCAM-1; it potently inhibits adhesion of α4β7+ leukocytes to MAdCAM-1, while having no effect on the adhesion of α4β1 to VCAM-1.20 The purpose of our study was to investigate the effects of 8 weeks of induction therapy with high-dose PF-00547659 on the cellular elements of CNS immune surveillance in patients with active CD and a history of immunosuppressive therapy.
2. Methods
2.1. Study design
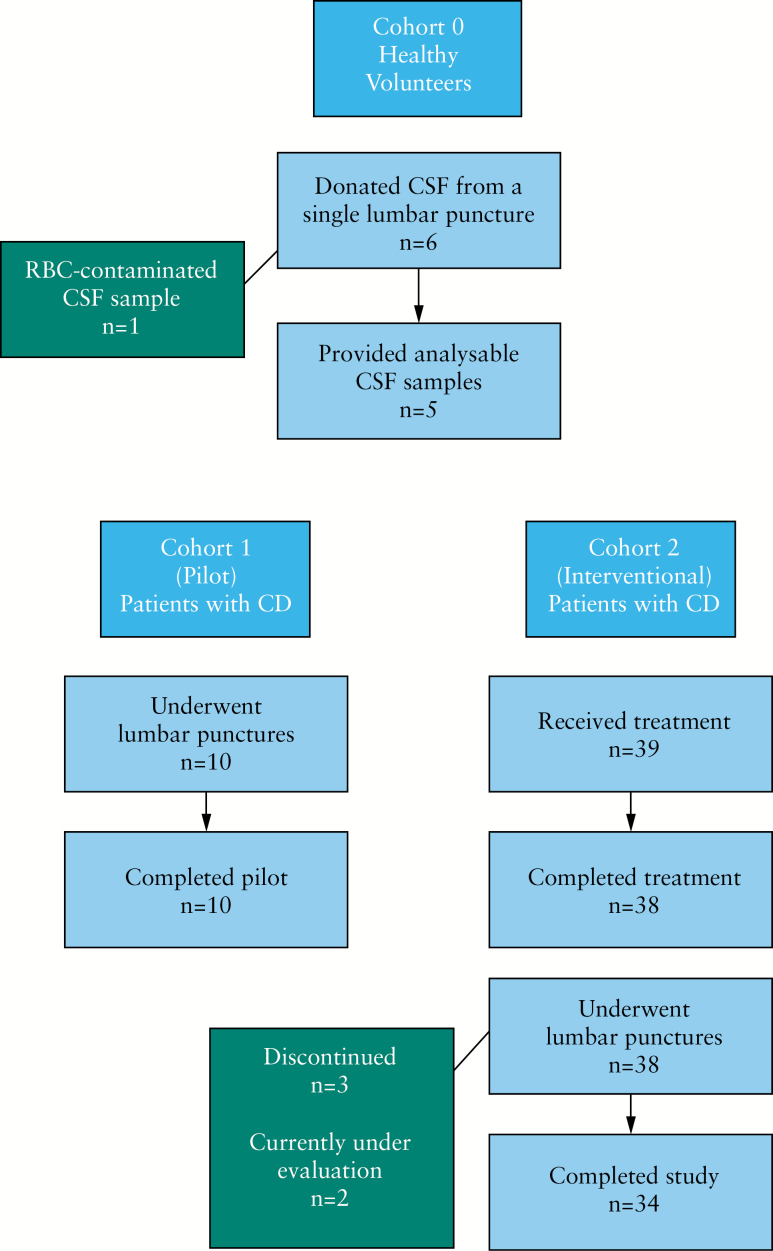
The study was executed in three parts under two protocols [Figure 1]:
Figure 1.
Design of the TOSCA study─Cohorts 0, 1, and 2.
CSF from an initial group of six healthy volunteers [designated Cohort 0, in the first protocol] was studied to determine whether TransFix® could stabilise CSF lymphocytes and their surface markers in order to permit use of a central flow cytometry laboratory.
Two cohorts were evaluated in the second protocol. Cohort 1 included 10 volunteers with CD who underwent two lumbar punctures [LP] with a 14-day interval, and then received treatment with PF-00547659. In this cohort we addressed two questions: i] do patients with CD who received previous immunosuppressant therapy have a sufficient number of CSF lymphocytes to provide reliable flow cytometry data; and ii] is the intra–individual variability low enough to permit conclusions regarding treatment effect?
After review of the results of Cohort 1, Cohort 2 included 39 additional patients with CD. CSF was collected via LP before and after three 225-mg doses of PF-00547659. The drug was administered subcutaneously at least 24 h after the first LP, and then every 28 days. The study was conducted in compliance with the Declaration of Helsinki, good clinical practice guidelines, and national regulations for protection of human subjects. The research protocol and subject compensation were approved by the relevant institutional review board or ethics committee at each centre, and all human participants gave written informed consent. Patients were compensated for their participation. Data were analysed by the sponsor according to a statistical analysis plan approved by the authors.
2.2. Patient populations
Subjects in Cohort 0 were healthy volunteers aged 18–55 years, with BMI 18–30 kg/m2 and body mass > 50 kg. Patients in Cohorts 1 and 2 were adults with moderate to severe CD (Harvey–Bradshaw Index [HBI] > 8) and high-sensitivity C-reactive protein [hs-CRP] > 5 mg/L or—in patients with stomas or hsCRP < 50 mg/L—active disease present on endoscopy or other imaging studies [as determined by the investigator], who had previously failed or been intolerant to treatment with anti-tumour necrosis factor [anti-TNF] agents and immunosuppressants [azathioprine, 6-mercaptopurine or methotrexate]. We excluded patients with evidence of active enteric infection, tuberculosis, heart failure, demyelinating disease, HIV infection, prednisone dose > 20 mg/day [or equivalent], or previous therapy with natalizumab, vedolizumab, or other anti-integrin therapy.
The presence of active CD was confirmed by endoscopy that showed mucosal ulceration > 0.5 cm, cobblestoning, or at least five aphthous ulcers—documented by photograph. Patients with stomas were included if they demonstrated active disease on endoscopy or appropriate imaging, eg. computed tomography/magnetic resonance enterography. Those who were receiving therapy with azathioprine, 6-mercaptopurine, or methotrexate were allowed to enter the study provided they terminated such therapy between Weeks 8 and 12 of the study. We permitted stable doses of prednisolone ≤ 20 mg/day or equivalent oral systemic corticosteroid dose, or oral budesonide ≤ 6 mg/day.
2.3. Lumbar puncture and CSF
Patients who met inclusion criteria underwent LP using a 22G 90-mm atraumatic spinal anaesthesia needle with introducer [http://www.imd-inc.com/spinalanesthesia.php]. In healthy volunteers, 10 mL of CSF were withdrawn and preserved in TransFix®. In patients with CD, 14 mL of CSF were withdrawn; 10 mL preserved with TransFix® were transferred to a central laboratory for flow cytometry; 3 mL were analysed at the local laboratory for culture, protein, glucose, and cell count; and 1 mL was evaluated for JCV DNA and anti-JCV antibody.
2.4. Study drug
PF-00547659 was administered by subcutaneous injection every 4 weeks. The 225-mg dose was selected as the highest repeated dose given in the Phase I trial.21
2.5. Study visits
Healthy volunteers had a single visit. Patients with CD returned every 4 weeks for treatment, assessment of disease activity with HBI,22 evaluation of adverse events, physical examination, electrocardiogram [ECG], and laboratory tests. All patients who underwent the first LP were permitted to receive the study drug. At each LP visit, venous blood was also withdrawn for analysis by flow cytometry. In Cohort 1, the second LP was performed before the administration of the study drug and 14 ± 4 days after the first LP. In Cohort 2, the second LP was performed 2 ± 1 weeks after the latest dose of study drug [ie. at Week 9–11 after baseline]. The final study visit was performed 4 weeks after the third dose of study drug, at which time patients who had a clinical response to the study drug were allowed to enrol in an open-label extension study.
2.6. Study endpoints
The primary endpoint was the percentage change in absolute lymphocyte count in the CSF of patients with CD after receiving three monthly doses of PF-00547659. Secondary endpoints included clinical response (defined as a reduction in disease activity [HBI score] from baseline by ≥ 3 points) and remission [defined as an HBI score < 5 points]. Additional analyses included characterisation of lymphocyte subpopulations in both CSF and blood before and after treatment with PF-00547659.
2.7. Pharmacodynamic, efficacy, and safety evaluations
2.7.1. Flow cytometry
Flow cytometry data were analysed by WinList® 7.0 [Verity Software, ME]. All CSF and blood samples were analysed with a BD FACSCanto-II® [Becton Dickinson, San Jose, CA] by LabCorp® [Mechelen, Belgium] in a regulated environment. Assays were validated for analysis of CSF and blood samples.
2.7.2. Cerebrospinal fluid
CSF samples were collected in TransFix®-containing tubes. In Cohort 0, they were analysed within 2 h, then stored at room temperature for 24 and 48 h for repeat studies. In Cohorts 1 and 2, they were transported to a central laboratory within 24 h of LP. Details are presented in the Supplementary material, available at ECCO-JCC online.
2.8. Study oversight
The protocol for this study was developed by the authors and approved by ethics committees and, where appropriate, national or regional authorities. A data-monitoring committee adjudicated any patients with unexplained neurological findings or suspected potential PML. All authors had full access to all data. All the authors reviewed and approved the final manuscript, and made the decision to submit the manuscript for publication and all vouch for the veracity and completeness of the data, the analyses and the fidelity of the study to the protocol.
2.9. Statistical analysis
The analysis was designed to show that the percentage decrease from baseline in absolute lymphocyte count in CSF is < 50% if the true percentage change is approximately 10%. Assuming a standard deviation of the paired differences [post-dose vs baseline] on the log scale of 0.99, approximately 15 patients were required to yield approximately 83% power with α = 0.10 [one-sided]. Assuming a dropout rate of 25%, enrolment of 20 patients was planned to yield approximately 15 paired samples for analysis. The primary end point—change from baseline in absolute lymphocyte count—was tested using a paired t-test on the log-transformed data. The estimates and the confidence intervals [CI] were constructed for the geometric mean ratio [GMR]. For results to be deemed statistically significant, the 10% lower confidence limit of the percentage decrease from baseline of the GMR had to be > 0.5. The data reported were obtained from a cleaned locked database for inclusion in this manuscript.
3. Results
As shown in Table 1, healthy volunteers in Cohort 0 were similar in age to patients with CD. One volunteer was replaced due to a traumatic LP. CD patients had long-term, active disease, and a significant subset had stomas. All 49 CD patients from both Cohorts 1 and 2 had previous treatment with both immunosuppressants and anti-TNF agents. In Cohort 2, 17/23 [74%] patients tested had positive anti-JCV serology [Table 1].
Table 1.
Demographic and baseline characteristics.
| Characteristic | Cohort 0 [n = 6] | Cohort 1 [n = 10] | Cohort 2 [n = 39] |
|---|---|---|---|
| Age [years] | 37.7 [12.1] | 40.9 [15.9] | 37.4 [10.6] |
| Gender [male/female] | 5 /1 | 8/2 | 13/26 |
| Weight [kg] | 81.2 [9.1] | 67.8 [12.6] | 69.6 [17.0] |
| Duration of Crohn’s disease [years] | N/A | 13.3 [6.3] | 12.6 [6.3] |
| Anti-JCV antibody seropositivea [n] | N/A | 5 | 17 |
| Intestinal stoma present [n] | N/A | 3 | 11 |
| Harvey–Bradshaw Index total score, median [min, max] | N/A | 10.0 [8, 12] | 9.0 [5, 10] |
| High sensitivity C-reactive protein, mg/L, median [min, max] | N/A | 16.4 [0.7, 67.2] | 5.0 [0.5, 79.5] |
| Duration anti-TNF use [years] | N/A | 3.2 [1.6] | 3.6 [2.9] |
| Ongoing immunosuppressant [n] | N/A | 1 | 9b |
| Ongoing glucocorticoids [n] | N/A | 7 | 12 |
All values are mean [standard deviation] values, unless otherwise noted. CV, coefficient of variation; JCV, John Cunningham virus; N/A, not applicable; TNF, tumour necrosis factor.
aOnly 23 patients tested [in Cohort 2].
bThree patients each continued to receive azathioprine, 6-mercaptopurine, and methotrexate during the study.
3.1. CSF/TransFix® stability─cohort 0
The absolute counts of lymphocytes and subpopulations were unaffected by storage for up to 48 h after collection in four of the five donors; in one donor [Volunteer #3] absolute counts decreased by 26.0% to 51.9% at 24 h and 72.6% to 81.8% at 24 h after collection. Post-collection storage of CSF samples did not influence the frequency of lymphocyte subpopulations in CSF in any of the five volunteers [Supplementary Table S1 and Supplementary Figure S1, available at ECCO-JCC online].
3.2. Pre-interventional CSF characteristics─cohort 1
In patients with CD, the numbers of lymphocytes and lymphocyte subpopulations were within published reference ranges.23,24 Pre-treatment CSF lymphocyte and lymphocyte subset counts were similar between LP 1 and 2 for most patients evaluated in Cohort 1 [Supplementary Table S2 and Supplementary Figure S2, available as Supplementary data at ECCO-JCC online]. The mean fold change in lymphocytes using a mixed model from LP1 to LP2 [90% CI] was 0.94 [0.71, 1.25].
3.3. Effects of PF-00547659─Cohort 2
3.3.1. Immunological effects
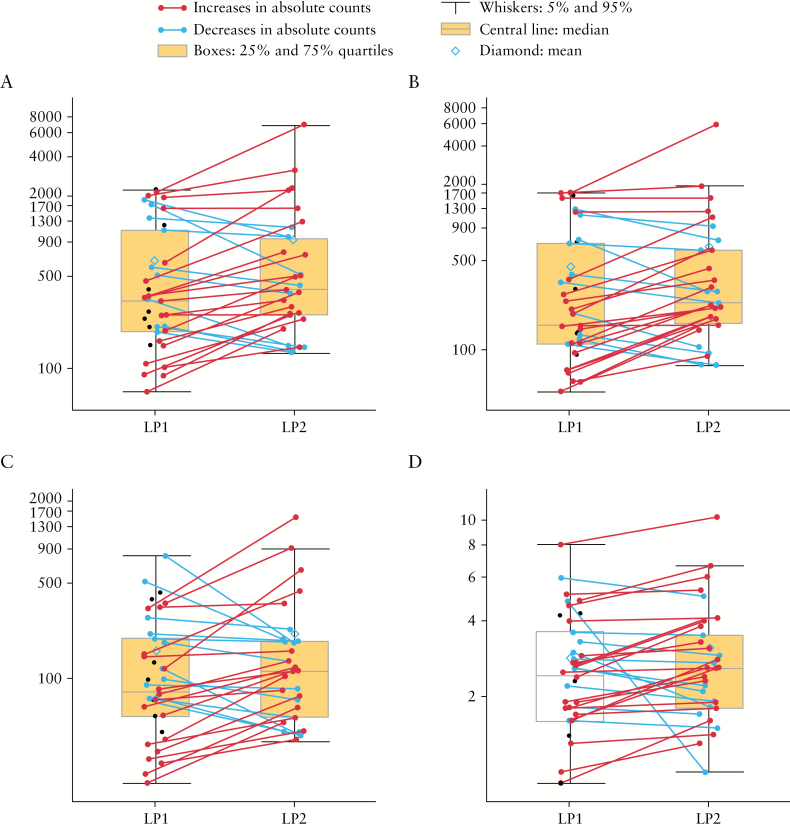
After treatment, there was a small increase in the numbers of overall CSF lymphocytes across several subsets [CD3+; CD4+; CD8+]; the CD4:CD8 ratio was similar before and after treatment [Figure 2; Supplementary Table S3, available at ECCO-JCC online]. Among the nine patients who continued to receive immunosuppressant therapy during the study, baseline numbers of T-lymphocytes and subsets were about 20% lower than in the other 28 patients, but the differences were neither significant nor below the range seen in healthy volunteers [Table 1]. Concurrent use of systemic glucocorticoids was associated with lower pre-treatment CSF CD4+, but not CDF8+ T-lymphocytes, in a dose-related manner [data not shown].
Figure 2.
Changes in CSF lymphocytes and lymphocyte subpopulations after treatment–Cohort 2. A: total lymphocytes [cells/mL]; B: CD3+CD4+ lymphocytes [cells/mL]; C: CD3+CD8+ lymphocytes [cells/mL]; D: CD4:CD8. Vertical axis is log10 CSF cell count in cells/mL, except for D, where it is the CD4+:CD8+ ratio. Lines represent data for individual patients before and after treatment. Unpaired black dots at LP1 are data from patients who did not undergo repeat LP. CSF, cerebrospinal fluid; LP, lumbar puncture.
CSF CD4:CD8 ratios were similar both before and after treatment, and in patients receiving or not receiving concomitant immunosuppressants. As in healthy subjects, very few NK and B cells were seen. Post-treatment changes were similar in magnitude regardless of ongoing immunosuppressant treatment [Table 2]. No JCV DNA was detected in 20 pre-treatment or 19 post-treatment samples of CSF.
Table 2.
Changes in cerebrospinal fluid T cell subsets by concurrent immunosuppressant use–Cohort 2 [geometric mean, 95% confidence interval].
| Concurrent immunosuppressant use | ||||
|---|---|---|---|---|
| No | Yes | |||
| Population | Pre-treatment | Post-treatment | Pre-treatment | Post-treatment |
| N | 28 | 22 | 9 | 8 |
| CD3+ | 387 [262, 570] | 512 [313, 837] | 299 [118, 759] | 397 [202, 783] |
| CD3+/CD4+ | 260 [173, 393] | 348 [211, 576] | 207 [79, 542] | 293 [143, 602] |
| CD3+/CD8+ | 107 [75, 153] | 134 [84, 213] | 82 [34, 199] | 96 [52, 175] |
| CD4:CD8 | 2.43 [1.96, 3.01] | 2.58 [2.03, 3.28] | 2.53 [1.77, 3.62] | 3.03 [2.11, 4.36] |
| CD3–/CD16+CD56+ | 7 [4, 11] | 7 [4, 11] | 6 [2, 20] | 7 [2, 21] |
| CD3–/CD19+ | 4 [2, 6] | 3 [2, 5] | 7 [2, 24] | 7 [3, 18] |
Values are cells/mL [apart from CD4:CD8].
In peripheral blood, the only change associated with PF-00547659 treatment was a 1.45-fold mean increase [90% CI: 1.31, 1.61] in β7+ central memory T-lymphocytes between baseline and Week 9 [Table 3; Supplementary Table S4, available at ECCO-JCC online].
Table 3.
Blood lymphocytes by flow cytometry before and after treatment with 225 mg PF-00547659–Cohort 2 [geometric mean, 95% CI].
| Population | Pre-treatment [n = 39] | Post-treatment [n = 32] | Mean fold change |
|---|---|---|---|
| Lymphocytes | 1482 [1273, 1724] | 1670 [1423, 1961] | 1.10 [0.99, 1.23] |
| CD3+/CD4+ | 715 [591, 865] | 806 [653, 995] | 1.11 [0.97, 1.27] |
| CD3+/CD8+ | 305 [246, 377] | 326 [260, 410] | 1.05 [0.92, 1.20] |
| CD4:CD8 | 2.01 [1.91, 2.89] | 2.47 [1.97, 3.10] | 1.05 [0.95, 1.17] |
| CD3+CD4+CD45RO+CD27+/β7+ | 61 [51, 73] | 91 [71, 116] | 1.44 [1.28, 1.64] |
Values are cells/mL [apart from CD4:CD8].
3.4. Clinical effects
3.4.1. Clinical response
All treated patients [n = 49] were evaluated for clinical effects. Of the 35 patients in Cohorts 1 and 2 with an evaluable HBI [ie. patients without stoma], 28 [80%] achieved a clinical response and 27 [77%] achieved clinical remission at Week 12. Enrolment with hsCRP ≤ 5.0 mg/L vs > 5.0 mg/L had no impact on rates of remission or response. The mean HBI decreased significantly from 8.7 [1.7] to 3.6 [2.7] points [p < 0.001]. The reduction in HBI was –6.6 ± 1.72 for patients who continued to receive immunosuppressants, and –4.8 ± 3.02 for those who did not. The HBI without the points assessed for stool count was evaluated in patients with stomas [Supplementary Table S5, available as Supplementary data at ECCO-JCC online]. The improvement in this partial HBI was similar in patients with intact GI tracts and those with stomas. The only disease biomarker assessed was hs-CRP, the geometric mean value for which decreased 29.8% in all patients.
3.4.2. Safety
Treatment-emergent adverse events were reported by 46 [94%] patients. No patient withdrew from the study due to an adverse event. The most common adverse events are listed in Table 4; 23 patients had adverse events considered related to study treatment, including four injection-site reactions. The most common events considered related to study treatment were arthralgia and nasopharyngitis, reported in four patients each. One patient who did not experience clinical response discontinued from the study; two other patients withdrew consent; and one was lost to follow-up.
Table 4.
Treatment-emergent adverse events reported for > 4 patients─Cohorts 1 and 2.
| Event | Number of patients [%] |
|---|---|
| Any adverse event | 46 [93.9] |
| Gastrointestinal | 17 [34.7] |
| Abdominal pain | 5 [10.2] |
| General/site | 15 [30.6] |
| Fatigue | 6 [12.2] |
| Infections and infestations | 21 [42.9] |
| Nasopharyngitis | 8 [16.3] |
| Musculoskeletal and connective tissue | 16 [32.7] |
| Arthralgia | 9 [18.4] |
| Nervous system | 9 [18.4] |
| Headache | 6 [12.2] |
Five serious adverse events [SAEs] were reported in four patients. One had enterocutaneous fistula and fatigue, one had nephrolithiasis and two patients reported exacerbations of CD. All events resolved and none was considered to be related to study treatment.
3.4.3. Clinical laboratory parameters
None of the laboratory abnormalities was reported as an AE by the investigators. No significant elevations in liver tests were observed [ie. bilirubin was always < 2 × ULN and transaminases were always < 3 × ULN], and no cases met the criteria of Hy’s Law.
4. Discussion
The selective anti-MAdCAM monoclonal antibody PF-00547659 was developed to mitigate the risk of PML seen with natalizumab. We hypothesised that its lack of binding to VCAM-1 would result in preserved lymphocyte translocation into the CSF, thereby minimising the risk of PML. Of the known risk factors for PML in natalizumab-treated patients,10 only the mechanism by which previous immunosuppressant exposure increases risk is not understood.
We were concerned that previous immunosuppression might lower total lymphocyte count or the number of CD4+ T-lymphocytes in CSF, which would make our results uninterpretable. Because of inter-laboratory variability in flow cytometry data, we used a central laboratory, with up to 24-h transport time for this critical test. For this study to be valid, we needed to ensure that CSF samples would be stable and results would be interpretable in our patient population. The first part of the study demonstrated that: 1] CSF T-lymphocyte surface markers were stabilised with TransFix®, a stabilizer for blood lymphocytes,25,26 for ≥ 24 h; 2] patients with CD and previous immunosuppressant exposure had CSF lymphocyte counts and subpopulations similar to healthy subjects; and 3] within-subject variability in CSF lymphocyte counts was low enough to evaluate the primary objective.
We then demonstrated that a 12-week induction course of anti-MAdCAM-1 monoclonal antibody PF-00547659 did not affect the number or nature of lymphocytes in the CSF. The increase in circulating β7+ central memory T-lymphocytes in PF-00547659-treated patients likely reflects pharmacodynamic activity in drug-mediated reduction of integrin-mediated lymphocyte extravasation into the gut, as previously reported in animal models.20 A recent publication27 suggests that vedolizumab treatment may result in accumulation of T-reg cells in the circulation of ulcerative colitis patients, which may limit expansion of T-effector cells in the peripheral blood and favour suppression of systemic inflammation.
Natalizumab was the first anti-adhesion molecule to be approved for treatment of patients with CD. As mentioned earlier, its acceptance has been limited by the increased risk of PML, a life-threatening opportunistic infection caused by the John Cunningham virus that attacks the CNS.6 Natalizumab blocks the α4-integrins on leukocytes, which target both MAdCAM-1 and VCAM-1 the gut, and VCAM-1 in the CNS, and therefore can result in profound depression of CSF lymphocytes, especially CD4+ T-lymphocytes.11 This effect can persist for 6 months after treatment with natalizumab.28 Interestingly, no case of PML has been reported more than 6 months after cessation of natalizumab therapy unless another immunosuppressive agent was given.29
The emergence of PML has underscored the importance of CNS immune surveillance of latent JCV reactivation. Previous evidence suggests that VCAM-1 plays a central role in the CNS immune response to viruses.30 In contrast, our results suggest that MAdCAM-1 does not mediate leukocyte migration into the CNS compartment in patients with CD.
Although MAdCAM-1 is considered mostly gut-selective, it was found to be upregulated in choroid plexus epithelium during experimental autoimmune encephalomyelitis [EAE], an autoimmune animal model of MS.19 Monoclonal antibodies against MAdCAM-1 have been shown to effectively prevent the development of actively induced EAE and decrease the number of CNS leukocytes in this model,31 although conflicting evidence has been reported.32 It was therefore important to confirm that MAdCAM inhibition would have no impact on CNS lymphocytes.
Vedolizumab, an anti-integrin monoclonal antibody targeted against the α4β7 integrin, is effective in the treatment of IBD.33,34 It was the first of the next generation of integrin antibodies with a more selective mode of action—it specifically targets the α4β7 integrin on circulating lymphocytes and blocks its interaction with MAdCAM-1, but does not inhibit α4β1 binding to VCAM-1. Thus, vedolizumab prevents leukocyte migration to the intestinal mucosa. Vedolizumab did not affect T-lymphocyte populations in the CSF of healthy volunteers 5 weeks after a single administration,35 nor did it inhibit immune surveillance of the CNS in primates.36 However, the effect of vedolizumab in IBD patients’ CNS immune surveillance has not yet been studied.
In conclusion, as measured by the numbers of overall CSF lymphocytes, lymphocyte subsets [CD3+, CD4+, CD8+] and the CD4:CD8 ratio, selective binding of MAdCAM-1 with PF-00547659 does not impair normal CNS immune surveillance in patients with CD and previous immunosuppression, regardless of anti-JC virus antibody seropositivity. Whether the long-term risk of CNS opportunistic infections with high cumulative doses of PF-00547659 is increased cannot be categorically ruled out with the data generated in this study. However, our data provide a good biological rationale to suggest that PF-00547659 treatment of IBD should have a lower risk of PML than do non-selective anti-integrin therapies.
Funding
This work was supported by Pfizer Inc. Medical writing support was provided by John Bilbruck and Donna McGuire of Engage Scientific Solutions, which was funded by Pfizer Inc.
Conflict of Interest
GD’H: research support: AbbVie, Covidien, Dr Falk Pharma, Ferring, Johnson & Johnson and Prometheus Laboratories/Nestlé; consulting fees: AbbVie, Ablynx, Amakem, AM-Pharma, Bristol-Meiers Squibb, Boerhinger Ingelheim, Celgene, Celltrion, Covidien, Ferring, Galapagos, Gilead, GlaxoSmithKline, Hospira, Medimetrics, Millenium/Takeda, Mitsubishi Tanabe Pharma, MSD, Mundipharma, Novo Nordisk, Pfizer, Prometheus Laboratories/Nestlé, Receptos, Robarts Clinical Trials, Sandoz, SetPoint Medical, Shire, and Topivert; speaker fees: AbbVie, Ferring, Johnson & Johnson, Mundipharma, Millenium/Takeda, MSD, Pfizer, Shire, Takeda, Tillotts, UCB and Vifor Pharma. SV: research support: AbbVie, MSD, Pfizer, and Takeda; consulting fees: AbbVie, MSD, Takeda, Ferring, Genentech/Roche, Shire, Pfizer, Galapagos, Mundipharma, Hospira, Celgene, Second Genome, and Janssen; speaker fees: AbbVie, MSD, Takeda, Ferring, Dr Falk Pharma, Hospira, and Tillotts. HV: speaker fees: AbbVie, Astro-Pharma, Ferring, Merck, MSD, Takeda. MA; speaker fees: AbbVie, Ferring, Genentech, GSK, Hospira, Janssen, MSD, Novartis, Novo Nordisk, Pfizer, UCB. PD: research support: Astra-Zeneca, Danisco France SAS, Danone France, Ferring, Giuliani SpA, Lesaffre, Marcq en Baroeul, Ocera Therapeutics, Roquette, Sanofi-Synthelabo, UCB, Yoplait, and Omega Pharma; consulting fees: BioFortis, Danisco France SAS, Danone France, Ferring, Giuliani SpA, Lesaffre, Marcq en Baroeul, Ocera Therapeutics, Roquette, UCB, Txcell, MSD, Abbott, Norgine, Genfit, Omega Pharma International, PPM Pharma, Kitozyme, and LFB Biotechnologies; speaker fees: Procter and Gamble, Ferring, Schering Plough, Shire, UCB, MSD, Norgine, Abbott, and PiLeJe Group. AVG: research support: Medtronics, Shire, MSD, Atlantic Pharmaceuticals, and FreseniusKabi. WJS: grant support, personal fees, and non-financial support from Pfizer during the conduct of the study; grant support from Pfizer, Exact Sciences, Amgen, the American College of Gastroenterology, and the Eli and Edythe Broad Foundation; grant support and personal fees from Prometheus Laboratories, AbbVie, Boehringer Ingelheim, Takeda, Atlantic Pharmaceuticals, Janssen, Bristol-Myers Squibb, Genentech, and Nutrition Science Partners; and personal fees from Kyowa Hakko Kirin, Millennium Pharmaceuticals, Celgene Cellular Therapeutics, Santarus, Salix Pharmaceuticals, Catabasis Pharmaceuticals, Vertex Pharmaceuticals, Warner Chilcott, Gilead Sciences, Cosmo Pharmaceuticals, Ferring, Sigmoid Biotechnologies, Tillotts Pharma, Am Pharma BV, Dr. August Wolff, Avaxia Biologics, Zyngenia, Ironwood Pharmaceuticals, InDex Pharmaceuticals, Nestlé, Lexicon Pharmaceuticals, UCB, Orexigen Therapeutics, Luitpold Pharmaceuticals, Baxter Healthcare, Ferring Research Institute, Amgen, Novo Nordisk, Mesoblast Inc., Shire, Ardelyx Inc., Actavis, Seattle Genetics, MedImmune [AstraZeneca], Actogenix NV, Lipid Therapeutics GmbH, Eisai, Qu Biologics, Toray Industries Inc., Teva Pharmaceutical Industries, Eli Lilly, Chiasma, TiGenix, Adherion Therapeutics, Immune Pharmaceuticals, Celgene, Arena Pharmaceuticals, Ambrx Inc., Akros Pharma, Vascular Biogenics, Theradiag, Forward Pharma, Regeneron, Galapagos, Seres Health, Ritter Pharmaceuticals, Theravance Biopharma, Palatin Technologies, Biogen, and the University of Western Ontario [owner of Robarts Clinical Trials], outside the submitted work. DCB: research support: Abbott, Astellas [formerly Fujisawa], Biocodex, Facet Biotech [formerly Protein Design Labs], Hitachi, and Shire; consulting fees: Abbott, AstraZeneca, Bayer Schering Pharma [BSP], Cellerix, [TiGenix], Celgene, Foreward, Genentech [Roche group], Medac Pharma Inc., Merck, MSD [formerly Essex Pharma], Pfizer, Otsuka, Facet Biotech [formerly Protein Design Labs], Takeda, UCB, Vifor Pharma; speaker fees: Abbott, AstraZeneca, Dr Falk Pharma, Ferring, MSD [formerly Essex Pharma], Medac Pharma Inc., Merck, Otsuka, Pfizer, Shire, Takeda, UCB. RMR is an employee of Biogen, Inc., and holds stock. GMC, AA, FC, JC, RC, KJG, AK, VP, SR, MOS, YZ, and MH-Z were employees of Pfizer during the TOSCA study. WR: research support: Abbott Laboratories, AbbVie, Aesca, Centocor, Dr Falk Pharma GmbH, Immundiagnsotik, and MSD; consulting fees: Abbott Laboratories, Abbvie, Aesca, Amgen, AM Pharma, Astellas, Astra Zeneca, Avaxia, Bioclinica, Biogen IDEC, Boehringer-Ingelheim, Bristol-Myers Squibb, Cellerix, Chemocentryx, Celgene, Centocor, Celltrion, Covance, Danone Austria, Elan, Falk Pharma GmbH, Ferring, Galapagos, Genentech, Gilead, Grünenthal, ICON, Index Pharma, Inova, Janssen, Johnson & Johnson, Kyowa Hakko Kirin Pharma, Lipid Therapeutics, MedImmune, Millenium, Mitsubishi Tanabe Pharma Corporation, MSD, Nestlé, Novartis, Ocera, Otsuka, PDL, Pharmacosmos, Pfizer, Procter and Gamble, Prometheus Laboratories, Robarts Clinical Trial, Schering-Plough, Second Genome, Setpointmedical, Takeda, Therakos, Tigenix, UCB, Vifor Pharma, Zyngenia, and 4S; speaker fees: Abbott Laboratories, AbbVie, Aesca, Aptalis, Centocor, Celltrion, Danone Austria, Elan, Dr. Falk Pharma GmbH, Ferring, Immundiagnostik, Mitsubishi Tanabe Pharma Corporation, MSD, Otsuka, PDL, Pharmacosmos, Schering-Plough, Shire, Takeda, Therakos, Vifor Pharma, and Yakult. OS: research support: Teva Pharmaceuticals and Opexa Therapeutics; consulting fees: Novartis, Genentech, Sanofi-Aventis, Huron Life Sciences, Navigant Consulting; travel support: Pfizer; advisory board member: Abbott Laboratories, Abbvie, Aesca, Amgen, AM Pharma, Astellas, Astra Zeneca, Avaxia, Biogen IDEC, Boehringer-Ingelheim, Bristol-Myers Squibb, Cellerix, Chemocentryx, Celgene, Centocor, Celltrion, Danone Austria, Elan, Ferring, Galapagos, Genentech, Grünenthal, Inova, Janssen, Johnson & Johnson, Kyowa Hakko Kirin Pharma, Lipid Therapeutics, MedImmune, Millenium, Mitsubishi Tanabe Pharma Corporation, MSD, Nestlé, Novartis, Ocera Therapeutics, Otsuka, PDL, Pharmacosmos, Pfizer, Procter and Gamble, Prometheus Laboratories, Schering-Plough, Second Genome, Setpointmedical, Takeda, Therakos, Tigenix, UCB, Zyngenia, and 4SC.
Author Contributions
The following chart identifies the roles of each of the co-authors.
| Author | Author rolesa | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Geert D’Haens | X | X | X | X | X | ||||
| Severine Vermeire | X | X | X | X | X | ||||
| Harald Vogelsang | X | X | X | X | X | ||||
| Matthieu Allez | X | X | X | X | X | ||||
| Pierre Desreumaux | X | X | X | X | X | ||||
| Andre Van Gossum | X | X | X | X | X | ||||
| William J. Sandborn | X | X | X | X | |||||
| Daniel C. Baumgart | X | X | X | X | X | ||||
| Richard M. Ransohoff | X | X | X | X | X | ||||
| Gail M. Comer | X | X | X | X | X | X | |||
| Alaa Ahmad | X | X | X | X | X | ||||
| Fabio Cataldi | X | X | X | X | X | X | |||
| John Cheng | X | X | X | X | X | ||||
| Robert Clare | X | X | X | X | |||||
| Kenneth J. Gorelick | X | X | X | X | X | ||||
| Annamarie Kaminski | X | X | X | X | |||||
| Vivek Pradhan | X | X | X | X | X | X | |||
| Sunday Rivers | X | X | X | X | |||||
| Matthew O. Sikpi | X | X | X | X | X | X | |||
| Yanhua Zhang | X | X | X | X | |||||
| Mina Hassan-Zahraee | X | X | X | X | X | ||||
| Walter Reinisch | X | X | X | X | X | ||||
| Olaf Stuve | X | X | X | X | |||||
a1: study concept and design; 2: acquisition of data; 3: analysis and interpretation of data; 4: drafting of the manuscript; 5: critical revision of the manuscript for important intellectual content; 6: statistical analysis; 7: obtained funding; 8: technical or material support; 9: study supervision.
Supplementary Data
Supplementary data are available at ECCO-JCC online.
Supplementary Material
Acknowledgments
The authors would like to thank the members of the data-monitoring committee: Dr Colm O’Morain [Chair], Dr David Clifford, Dr Paola Cinque, Dr Glen Cooke, Dr Lauren Kruppe, and Dr Dan Anbar.
References
- 1. Ransohoff RM, Kivisäkk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol 2003;3:569–81. [DOI] [PubMed] [Google Scholar]
- 2. Sigmundsdottir H, Butcher EC. Environmental cues, dendritic cells and the programming of tissue-selective lymphocyte trafficking. Nat Immunol 2008;9:981–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lebwohl M, Tyring SK, Hamilton TK et al. ; Efalizumab Study Group. A novel targeted T cell modulator, efalizumab, for plaque psoriasis. N Engl J Med 2003;349:2004–13. [DOI] [PubMed] [Google Scholar]
- 4. Polman CH, O’Connor PW, Havrdova E et al. ; AFFIRM Investigators. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2006;354:899–910. [DOI] [PubMed] [Google Scholar]
- 5. Sandborn WJ, Colombel JF, Enns R et al. ; International Efficacy of Natalizumab as Active Crohn’s Therapy [ENACT-1] Trial Group; Evaluation of Natalizumab as Continuous Therapy [ENACT-2] Trial Group. Natalizumab induction and maintenance therapy for Crohn’s disease. N Engl J Med 2005;353:1912–25. [DOI] [PubMed] [Google Scholar]
- 6. Weber T, Major EO. Progressive multifocal leukoencephalopathy: molecular biology, pathogenesis and clinical impact. Intervirology 1997;40:98–111. [DOI] [PubMed] [Google Scholar]
- 7. Ransohoff RM, Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol 2012;12:623–35. [DOI] [PubMed] [Google Scholar]
- 8. Dong-Si T, Gheuens S, Gangadharan A et al. . Predictors of survival and functional outcomes in natalizumab-associated progressive multifocal leukoencephalopathy. J Neurovirol 2015;21:637–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carson KR, Focosi D, Major EO et al. . Monoclonal antibody-associated progressive multifocal leucoencephalopathy in patients treated with rituximab, natalizumab, and efalizumab: a review from the Research on Adverse Drug Events and Reports [RADAR] Project. Lancet Oncol 2009;10:816–24. [DOI] [PubMed] [Google Scholar]
- 10. Bloomgren G, Richman S, Hotermans C et al. . Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med 2012;366:1870–80. [DOI] [PubMed] [Google Scholar]
- 11. Stüve O, Marra CM, Bar-Or A et al. . Altered CD4+/CD8+ T cell ratios in cerebrospinal fluid of natalizumab-treated patients with multiple sclerosis. Arch Neurol 2006;63:1383–7. [DOI] [PubMed] [Google Scholar]
- 12. Bravatà I, Allocca M, Fiorino G, Danese S. Integrins and adhesion molecules as targets to treat inflammatory bowel disease. Curr Opin Pharmacol 2015;25:67–71. [DOI] [PubMed] [Google Scholar]
- 13. Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet 2012;380:1590–605. [DOI] [PubMed] [Google Scholar]
- 14. Eksteen B, Liaskou E, Adams DH. Lymphocyte homing and its role in the pathogenesis of IBD. Inflamm Bowel Dis 2008;14:1298–312. [DOI] [PubMed] [Google Scholar]
- 15. Thomas S, Baumgart DC. Targeting leukocyte migration and adhesion in Crohn’s disease and ulcerative colitis. Inflammopharmacology 2012;20:1–18. [DOI] [PubMed] [Google Scholar]
- 16. Ghosh S, Panaccione R. Anti-adhesion molecule therapy for inflammatory bowel disease. Ther Adv Gastroenterol 2010;3:239–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Briskin M, Winsor-Hines D, Shyjan A et al. . Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol 1997;151:97–110. [PMC free article] [PubMed] [Google Scholar]
- 18. Steffen BJ, Breier G, Butcher EC, Schulz M, Engelhardt B. ICAM-1, VCAM-1, and MAdCAM-1 are expressed on choroid plexus epithelium but not endothelium and mediate binding of lymphocytes in vitro. Am J Pathol 1996;148:1819–38. [PMC free article] [PubMed] [Google Scholar]
- 19. Allavena R, Noy S, Andrews M, Pullen N. CNS elevation of vascular and not mucosal addressin cell adhesion molecules in patients with multiple sclerosis. Am J Pathol 2010;176:556–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pullen N, Molloy E, Carter D et al. . Pharmacological characterization of PF-00547659, an anti-human MAdCAM monoclonal antibody. Br J Pharmacol 2009;157:281–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vermeire S, Ghosh S, Panes J et al. . The mucosal addressin cell adhesion molecule antibody PF-00547,659 in ulcerative colitis: a randomised study. Gut 2011;60:1068–75. [DOI] [PubMed] [Google Scholar]
- 22. Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet 1980;1:514. [DOI] [PubMed] [Google Scholar]
- 23. de Graaf MT, de Jongste AH, Kraan J, Boonstra JG, Sillevis Smitt PA, Gratama JW. Flow cytometric characterization of cerebrospinal fluid cells. Cytometry B Clin Cytom 2011;80:271–81. [DOI] [PubMed] [Google Scholar]
- 24. de Graaf MT, Smitt PA, Luitwieler RL et al. . Central memory CD4+ T cells dominate the normal cerebrospinal fluid. Cytometry B Clin Cytom 2011;80:43–50. [DOI] [PubMed] [Google Scholar]
- 25. Canonico B, Zamai L, Burattini S et al. . Evaluation of leukocyte stabilisation in TransFix-treated blood samples by flow cytometry and transmission electron microscopy. J Immunol Methods 2004;295:67–78. [DOI] [PubMed] [Google Scholar]
- 26. Plate MM, Louzao R, Steele PM et al. . Evaluation of the blood stabilizers TransFix and Cyto-Chex BCT for low-cost CD4 T cell methodologies. Viral Immunol 2009;22:329–32. [DOI] [PubMed] [Google Scholar]
- 27. Fischer A, Zundler S, Atreya R et al. . Differential effects of α4β7 and GPR15 on homing of effector and regulatory T cells from patients with UC to the inflamed gut in vivo. Gut 2016;65:1642–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stüve O, Marra CM, Jerome KR et al. . Immune surveillance in multiple sclerosis patients treated with natalizumab. Ann Neurol 2006;59:743–7. [DOI] [PubMed] [Google Scholar]
- 29. Fine AJ, Sorbello A, Kortepeter C, Scarazzini L. Progressive multifocal leukoencephalopathy after natalizumab discontinuation. Ann Neurol 2014;75:108–15. [DOI] [PubMed] [Google Scholar]
- 30. Ou R, Zhang M, Huang L, Flavell RA, Koni PA, Moskophidis D. Regulation of immune response and inflammatory reactions against viral infection by VCAM-1. J Virol 2008;82:2952–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kanwar JR, Kanwar RK, Wang D, Krissansen GW. Prevention of a chronic progressive form of experimental autoimmune encephalomyelitis by an antibody against mucosal addressin cell adhesion molecule-1, given early in the course of disease progression. Immunol Cell Biol 2000;78:641–5. [DOI] [PubMed] [Google Scholar]
- 32. Döring A, Pfeiffer F, Meier M et al. . TET inducible expression of the α4β7-integrin ligand MAdCAM-1 on the blood-brain barrier does not influence the immunopathogenesis of experimental autoimmune encephalomyelitis. Eur J Immunol 2011;41:813–21. [DOI] [PubMed] [Google Scholar]
- 33. Sandborn WJ, Feagan BG, Rutgeerts P et al. ; GEMINI 2 Study Group. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2013;369:711–21. [DOI] [PubMed] [Google Scholar]
- 34. Feagan BG, Rutgeerts P, Sands BE et al. ; GEMINI 1 Study Group. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013;369:699–710. [DOI] [PubMed] [Google Scholar]
- 35. Milch C, Wyant T, Xu J et al. . Vedolizumab, a monoclonal antibody to the gut homing α4β7 integrin, does not affect cerebrospinal fluid T-lymphocyte immunophenotype. J Neuroimmunol 2013;264:123–6. [DOI] [PubMed] [Google Scholar]
- 36. Haanstra KG, Hofman SO, Lopes Estêvão DM et al. . Antagonizing the α4β1 integrin, but not α4β7, inhibits leukocytic infiltration of the central nervous system in rhesus monkey experimental autoimmune encephalomyelitis. J Immunol 2013;190:1961–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.