Abstract
Background and Aims
Crohn’s disease-related complications account for a substantial proportion of inflammatory bowel disease-associated health care expenditure. Identifying patients at risk for complications may allow for targeted use of early therapeutic interventions to offset this natural course. We aimed to develop risk prediction models for Crohn’s disease-related surgery and complications.
Methods
Using data from the Randomised Evaluation of an Algorithm for Crohn’s Disease cluster-randomised clinical Trial [REACT], which involved 1898 patients from 40 community practices, separate prediction models were derived and internally validated for predicting Crohn’s disease-related surgery and disease-related complications [defined as the first disease-related surgery, hospitalisation, or complication within 24 months]. Model performance was assessed in terms of discrimination and calibration, decision curves, and net benefit analyses.
Results
There were 130 [6.8%] disease-related surgeries and 504 [26.6%] complications during the 24-month follow-up period. Selected baseline predictors of surgery included age, gender, disease location, Harvey-Bradshaw Index [HBI] score, stool frequency, antimetabolite or 5-aminosalicylate use, and the presence of a fistula, abscess, or abdominal mass. Selected predictors of complications included those same factors for surgery, plus corticosteroid or anti-tumour necrosis factor use, but excluded 5-aminosalicylate use. Discrimination ability, as measured by validated c-statistics, was 0.70 and 0.62 for the surgery and complication models, respectively. Score charts and nomograms were developed to facilitate future risk score calculation.
Conclusions
Separate risk prediction models for Crohn’s disease-related surgery and complications were developed using clinical trial data involving community gastroenterology practices. These models could be used to guide Crohn’s disease management. External validation is warranted.
Keywords: Clinical trials, clinical risk prediction, prognostic model
1. Introduction
Crohn’s disease [CD] is an idiopathic disorder of the gastrointestinal tract, characterised by chronic, segmental inflammation, which typically has a relapsing and remitting course. Despite the use of immunosuppressive maintenance therapies, subclinical transmural inflammation persists in many patients,1 which predisposes them to such complications as strictures and fistulas.2 Recent studies estimate the long-term risk of surgery to be approximately 60–80%,2–5 with the greatest risk in the first few years following diagnosis.
In the past two decades, the advent of biologic therapies and refinement of treatment paradigms have revolutionised the medical management of CD. Specific advances include the introduction of tumour necrosis factor [TNF] antagonists6–13 and integrin inhibitors,14–18 the use of combination therapy,19–22 therapeutic drug monitoring,23,24 and earlier initiation of effective therapies in high-risk patients.12,19–21,25–29 However, one of the greatest challenges in implementing these strategies is determining which patients are most at risk for complicated disease and thus candidates for more intensive monitoring and treatment. This decision is usually based on clinical judgement and heavily weighted by the patient’s disease activity as assessed by symptoms. This approach is practical, but it does not routinely incorporate prognostic factors that drive disease-related complications. Accordingly, identification of patients at highest risk of complications and disease progression, who have the greatest chance of benefiting from early initiation of effective therapy, is an aspirational goal.
In this regard, the approach to therapy in CD has changed dramatically. Formerly, ‘step-care’, in which drugs are used sequentially to attain symptomatic remission, was the preferred paradigm. Whereas this approach is attractive because it avoids over-treating low-risk patients, step-care delays initiation of effective therapy in patients most at risk for adverse outcomes. Recently, attention has turned to early introduction of combination immunosuppression therapy in high-risk patients, to promote mucosal healing and minimise exposure to corticosteroids.19,20 This ‘top-down’ approach requires accurate identification of high-risk patients, to minimise over-treatment in lower-risk patients, with the associated risk of treatment-related adverse events and costs. Conversely, misclassification of high-risk patients delays administration of effective therapies and potentially results in increased risk of complications. Therefore, the ability to accurately risk-stratify patients has garnered considerable interest.30,31
Retrospective analyses of single-centre and population-based cohorts have identified several prognostic factors in CD, including younger age at diagnosis, ileal disease location, perianal disease, stricturing or penetrating phenotype, current smoking, treatment with corticosteroids at diagnosis or corticosteroid dependence, and extensive disease involvement.1,3–5,31–40
Existing clinical prediction models have several limitations. Notably, these models were developed from single centres, referral centres, retrospective samples, small development samples, or cohorts assembled before the widespread use of TNF antagonists, and they often predict excessively long-term risk estimates of outcomes [5–10 years].32,33,41–44 However, to fully utilise this information, prediction models must be established.
Data arising from randomised trials offer a unique ability in developing clinical prediction models, because of their scope, size, multicentre participation and prospective follow-up, thus overcoming some of the aforementioned design limitations. We developed and internally validated clinical prediction models for CD using data from a large cluster randomised trial conducted throughout community practices, comparing two treatment algorithms for CD.21 Our specific aim was to develop separate models for predicting risks of two binary endpoints within 24 months of follow up: [1] occurrence of CD-related surgery; and [2] occurrence of a composite of CD-related surgery, complications or hospitalisation [this composite outcome is henceforth referred to as disease-related complications].
2. Methods
2.1. Data source
The study population was participants from the Randomised Evaluation of an Algorithm for Crohn’s Disease [REACT] trial [NCT01030809].21 Briefly, REACT was a large, cluster-randomised, controlled trial of two distinct algorithms for the management of CD. Forty Canadian and Belgian community-based gastroenterology clinics were randomised, in a 1:1 ratio, to either early combined immunosuppression or step-care. In each clinic, consecutive adult patients with CD were enrolled, regardless of disease activity or concurrent therapy, and followed up to 24 months. The dataset was used to develop separate models to predict the risk over 24 months of having CD-related surgery and disease-related complications.
The reporting of this study follows the Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis [TRIPOD] statement [Supplementary Table 1, available at ECCO-JCC online].45
2.2. Clinical outcomes and definitions
Binary outcomes were defined within 2 years of follow up. The first was the occurrence of CD-related surgery. The second was a CD-related complication. Surgery was considered separately since it is a readily measured event of clinical importance. All events in the REACT trial used in this study were evaluated by an adjudication committee who were blinded to treatment assignment. Disease-related surgeries included resective bowel surgery [ileal resection, ileocaecal resection, proctocolectomy, colectomy, enterectomy, ostomy formation and repair, anastomosis/reanastomosis], stricureplasty, and fistula repair [incision and drainage of abscess, seton placement, fistulotomy, fistulectomy]. Disease-related complications were defined as a composite of disease-related surgery [as defined above], complications [including development of penetrating or stricturing disease, worsening abdominal pain, increased stool frequency, extra-intestinal manifestations, severe perianal disease, fistula, or abscess21] or hospitalisation.
2.3. Selection of predictors
Candidate prognostic factors were selected from the demographic and disease-related variables, using standardised clinical definitions, collected at baseline and before treatment allocation. A search of the literature identified potential prognostic factors of interest that were augmented by clinical judgement. These variables were age at enrolment, age at diagnosis, gender, smoking status, disease location, perianal disease, previous surgical resection for CD, use of each medication at baseline [including 5-aminosalicylates, corticosteroids, immunosuppressives, and TNF antagonists], abdominal pain, abdominal mass, extra-intestinal manifestations, strictures, fistulas, and stool frequency. No laboratory parameters, biomarkers, or cross-sectional imaging items were included. Since the purpose of these models is to predict risks using baseline factors before treatment allocation, treatment assignment was not considered as a predictor in the models. Moreover, coefficients for treatment effect were relatively small compared with other prognostic factors.
2.4. Missing data
No participants had missing outcome data. However, 4.24% [n = 84] of patients had at least one missing variable at baseline, of which it was most common to be missing components of the HBI score. No patterns were observed in the missing values. Only participants with complete baseline data were used for model development.
2.5. Model development
Exploratory univariate data analysis was first conducted to assess adequate event frequency between each outcome and the candidate prognostic factors. Univariable associations between candidate predictors and the outcomes were assessed graphically and using logistic regression. Each model was then constructed using multivariable logistic regression analysis. Predictors with univariate associations p < 0.20 entered the multivariable model. From this full model, unnecessary variables were removed based on a difference in Akaike information criteria [AIC; < 2.0]. In terms of the relative performance of models as estimated from bootstrap replicates, the removal of these variables had a negligible impact on discrimination, the Brier score [< 1% difference in mean difference], and calibration. Although the data arose from a cluster-randomised trial, regression coefficient estimates are not much affected when the degree of clustering is low as in this trial [intraclass correlation coefficient < 0.02].46 Since the focus in prediction studies is on the absolute risk estimate from the combined predictor effects, these analyses were conducted at the patient level without accounting for clustering.
2.6. Predictive performance and model validation
The model development and validation process adhered to recommended guidelines.47,48 Model performance was characterised by the discrimination ability and calibration: the discrimination ability of a model to distinguish between patients with the outcome and patients without the outcome. In the present context, discrimination is measured using the c-statistic, which is identical to the area under the receiver operating characteristic curve for binary outcomes.49 A value of 0.50 for the c-statistic represents the prognostic ability of a coin flip and will therefore correctly differentiate 50% of cases. There are no universal guidelines for describing the quality of discrimination ability, but generally discrimination values below 60% are unacceptable, 60% to 70% may be acceptable and values from 70% to > 90% range from good to excellent. The interpretation of these values is context-dependent. Calibration refers to the agreement between predicted and observed risks.47 This can be assessed by plotting the predicted risks and the observed percentages of patients who had the outcome.
Since model development tends to over-fit the data, commonly referred to as optimism, it is desirable to reduce optimism using internal validation and/or external validation.50 Internal validation of the models was conducted using the bootstrap method with 500 replicates.51 Performance characteristics were calculated for the original [index] and validated models following correction for optimism. Additional details regarding statistical methodology and performance measures are provided in the Supplementary Appendix, available at ECCO-JCC online.
2.7. Sample size
Formal sample size calculation was not conducted. Of the 1982 participants in the REACT trial, 504 had disease-related complications and 130 had surgeries over 2 years of follow-up. According to the guideline of 10 events per variable,52 this dataset is large enough to construct prediction models with at least ten predictors.
2.8. General statistical methods
Exploratory analysis was performed using Stata [version 14.1/IC; StataCorp, College Station, TX]. Model building and evaluation were conducted using package rms47 in R [version 3.3.1; Linux; R Core Team53] through RStudio [version 0.99; RStudio Team54]. Summary statistics are presented as mean ± standard deviation [SD], median, or frequencies and proportions as appropriate.
3. Results
3.1. Patient characteristics and clinical outcomes
Baseline characteristics are outlined in Table 1. A total of 1097 [57.79%] patients were female. Median disease duration was 148.8 months, the mean HBI was 4.1, and almost one-third of patients were taking biologics at baseline. Over the 24-month follow-up period, 6.84% [n = 130] of participants underwent CD-related surgery, and 26.55% [n = 504] of participants experienced a CD-related complication. Univariate associations for each outcome are shown in Table 2.
Table 1.
Overall patient characteristics at baseline [N = 1898].
| Baseline characteristic | Overall N = 1898 | Surgery group N = 130 6.8% | No surgery group N = 1768 93.1% | Complication group N = 504 26.6% | No complication group N = 1394 73.5% |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, years [mean ± SD] | 44.0 ± 14.6 | 42.7 ± 14.0 | 44.1 ± 14.6 | 42.1 ± 14.6 | 44.7 ± 14.5 |
| Female | 1097 [57.78%] | 68 [52.3%] | 1029 [58.2%] | 311 [61.7%] | 786 [56.4%] |
| Smoking status | |||||
| Non-smoker | 939 [49.5%] | 58 [44.6%] | 881 [49.9%] | 241 [47.9%] | 698 [50.1%] |
| Ex-smoker | 550 [29.0%] | 36 [27.7%] | 514 [29.1%] | 405 [29.1%] | 145 [28.8%] |
| Current smoker | 407 [21.44%] | 36 [27.7%] | 371 [21.0%] | 290 [20.8%] | 233 [11.7%] |
| Disease characteristics | |||||
| Duration, months (mean [median]) | 148.8 [119] | 144.8 [111.1] | 149.1 [119.2] | 147.0 [114.0] | 149.5 120.9 |
| HBI score [median; mean ± SD] | 3; 4.1 ± 1.1 | 5; 6.0 ± 3.5 | 3; 4.0 ± 1.1 | 4; 5.0 ± 4.9 | 3; 3.8 ± 3.8 |
| Steroid-free remission [HBI ≤ 4] | 1,065 [56.1%] | 51 [39.2%] | 1014 [57.4%] | 236 [46.8%] | 829 [59.5%] |
| Extra-intestinal manifestations at baseline (n [%]) | 594 [31.3%] | 55 [42.3%] | 539 [30.5%] | 185 [36.7%] | 409 [29.3%] |
| Previous history of disease-related surgery | 864 [45.5%] | 56 [43.1%] | 808 [45.7%] | 244 [48.4%] | 620 [44.5%] |
| Involved intestinal areas | |||||
| Colon | 417 [22.0%] | 17 [13.1%] | 400 [22.6%] | 92 [18.3%] | 325 [23.3%] |
| Small bowel | 654 [34.5%] | 52 [40.0%] | 602 [34.1%] | 169 [33.5%] | 485 [34.8%] |
| Colon and small bowel | 827 [43.6%] | 61 [46.9%] | 766 [43.3%] | 243 [48.2%] | 584 [41.9%] |
| Concurrent medications use | |||||
| Aminosalicylates | 539 [28.4%] | 22 [16.9%] | 517 [29.2%] | 116 [23.0%] | 423 [30.3%] |
| Corticosteroids | 348 [18.3%] | 31 [23.9%] | 317 [17.9%] | 113 [22.4%] | 235 [16.9%] |
| Anti-metabolites | 826 [43.5%] | 48 [36.9%] | 778 [44.0%] | 204 [40.5%] | 622 [44.6%] |
| TNF antagonists | 622 [32.8%] | 53 [40.8%] | 569 [32.2%] | 201 [39.9%] | 421 [30.2%] |
Figures may vary slightly from those published in Khanna et al. [2015], due to exclusion of 84 patients with at least one missing predictor variable.
SD, standard deviation; HBI, Harvey-Bradshaw Index; TNF, tumour necrosis factor.
Table 2.
Univariate associations for Crohn’s disease-related complications or CD-related surgery alone.
| Baseline variable | CD-related surgery | CD-related complicationa | ||
|---|---|---|---|---|
| OR [95% CI] | p-value | OR [95% CI] | p-value | |
| Current age | 0.99 [0.98, 1.00] | 0.27 | 0.99 [0.98, 1.00] | < 0.0001 |
| Gender [male vs. female] | 1.27 [0.89, 1.81] | 0.19 | 0.80 [0.65, 0.99] | 0.04 |
| HBI score [except stool frequency] | 1.36 [1.25, 1.48] | < 0.0001 | 1.18 [1.12, 1.24] | < 0.0001 |
| Stool frequency | 1.08 [1.15, 1.02] | < 0.01 | 1.07 [1.03, 1.11] | < 0.0001 |
| Location of disease involvement | ||||
| Colon only [reference] | 1 | 0.03 | 1 | 0.02 |
| Small bowel and colon | 1.87 [1.08, 3.25] | 1.47 [1.12, 1.94] | ||
| Small bowel only | 2.03 [1.16, 3.57] | 1.23 [0.92, 1.65] | ||
| Presence of new fistula, abscess, or definite abdominal mass | 10.29 [6.23, 16.99] | < 0.0001 | 3.94 [2.48, 6.26] | < 0.0001 |
| Anti-metabolite use | 0.75 [0.52, 1.08] | 0.11 | 0.84 [0.69, 1.04] | 0.10 |
| 5-aminosalicylate use | 0.49 [0.31, 0.79] | < 0.01 | 0.69 [0.54, 0.87] | < 0.01 |
| Corticosteroid use | 1.43 [0.94, 2.18] | 0.10 | 1.43 [1.11, 1.84] | < 0.01 |
| TNF antagonist use | 1.45 [1.01, 2.09] | 0.05 | 1.53 [1.24, 1.89] | < 0.0001 |
| Current smoking vs. ex-/non-smoker | 1.44 [0.96, 2.15] | 0.08 | 1.15 [0.90, 1.47] | 0.26 |
All outcomes are defined within 24 months of follow-up.
CD, Crohn’s disease; OR, odds ratio, CI, confidence interval; HBI, Harvey-Bradshaw Index; TNF, tumour necrosis factor.
aComplication refers to the first occurrence of a CD-related surgery, complication or hospitalisation.
3.2. Model performance and validation
A separate logistic regression model was estimated for each outcome. Table 3 shows the estimates of regression coefficients for the CD-related surgery and CD-related complication models. The baseline predictors included in the surgery model were age, gender, disease location, HBI score, stool frequency, immunosuppressive use, 5-aminosalicylate use, and the presence of a fistula, abscess, or abdominal mass. The baseline predictors for the CD-related complication model also uniquely included the use of corticosteroids and TNF antagonists, in addition to all the variables identified for surgery.
Table 3.
Coefficient estimates of logistic regression models for CD-related surgery and a CD-related complication.
| Baseline predictor | CD-related surgery | CD-related complication a | ||||
|---|---|---|---|---|---|---|
| Regression coefficientb | SE | p-value | Regression coefficientc | SE | p-value | |
| Intercept | -3.607 | 0.327 | -1.6134 | 0.162 | ||
| Age subtract 45 years | -0.0027 | 0.007 | 0.72 | -0.0102 | 0.004 | 0.02 |
| Male vs female | 0.3946 | 0.194 | 0.06 | -0.1848 | 0.110 | 0.13 |
| HBI score [except stool frequency] | 0.2425 | 0.065 | < 0.001 | 0.1139 | 0.042 | 0.01 |
| Stool frequencyd | 0.0741 | 0.060 | 0.25 | 0.0752 | 0.034 | 0.04 |
| Location of disease involvement | ||||||
| Colon only [reference] | 0 | – | – | 0 | – | – |
| Small bowel and colon | 0.3614 | 0.293 | 0.26 | 0.2525 | 0.145 | 0.12 |
| Small bowel only | 0.5167 | 0.297 | 0.11 | 0.1739 | 0.153 | 0.31 |
| Corticosteroid use | – | – | – | 0.2465 | 0.138 | 0.11 |
| Anti-metabolite use | -0.4519 | 0.200 | 0.04 | -0.1598 | 0.110 | 0.19 |
| 5-aminosalicylate use | -0.6015 | 0.253 | 0.03 | – | – | – |
| TNF antagonist use | – | – | – | 0.3887 | 0.114 | < 0.01 |
| Presence of new fistula, abscess, or definite abdominal mass | 1.7019 | 0.306 | < 0.0001 | 1.0840 | 0.261 | < 0.001 |
| Interaction | ||||||
| HBI score × stool frequency | -0.0182 | 0.014 | 0.22 | -0.0136 | 0.009 | 0.18 |
SE, standard error; CD, Crohn’s disease; HBI, Harvey-Bradshaw Index; TNF, tumour necrosis factor.
aComplication refers to the first occurrence of a CD-related surgery, complication, or hospitalisation.
bRegression coefficients are presented before shrinkage [shrinkage factor = 0.92].
cRegression coefficients are presented before shrinkage [shrinkage factor = 0.90].
dStool frequency has a maximum value of 12.
The validated discrimination ability for the CD-related surgery model was reasonably good with a c-statistic of 0.70 (95% confidence interval [CI]: 0.69 to 0.71), whereas the discrimination ability of disease-related complication model was relatively acceptable at 0.62 [95% CI: 0.61 to 0.64] [Supplementary Table 2, available at ECCO-JCC online]. Both models demonstrated low degrees of optimism [shrinkage factors were 0.92 and 0.90, respectively], and excellent calibration and goodness-of-fit statistics [see Supplementary Appendix].
3.3. Computing a risk estimate, score chart, and nomogram
The validated logistic regression model for CD-related surgery within 2 years is shown in Equation 1.
| (1) |
The validation logistic regression model for CD-related complications is shown in Equation 2.
| (2) |
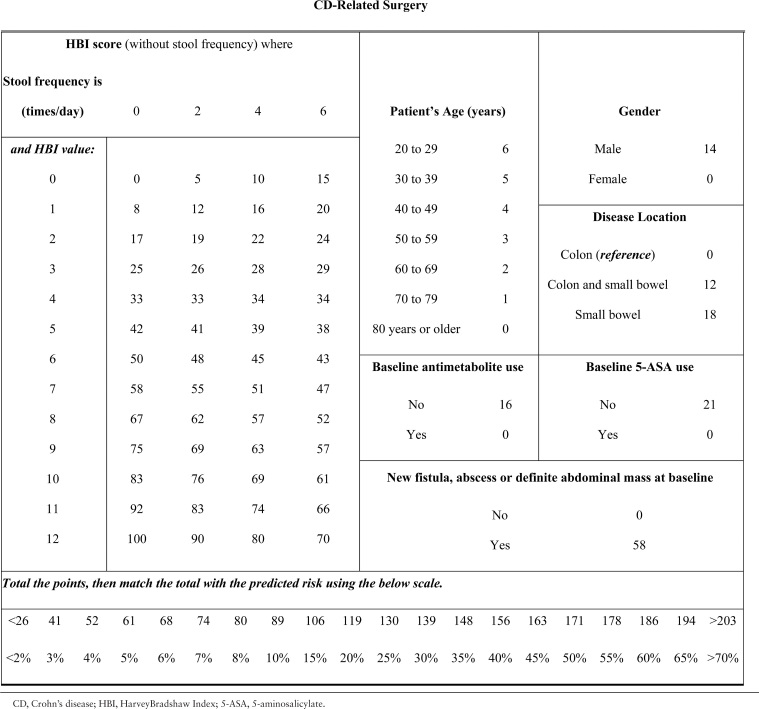
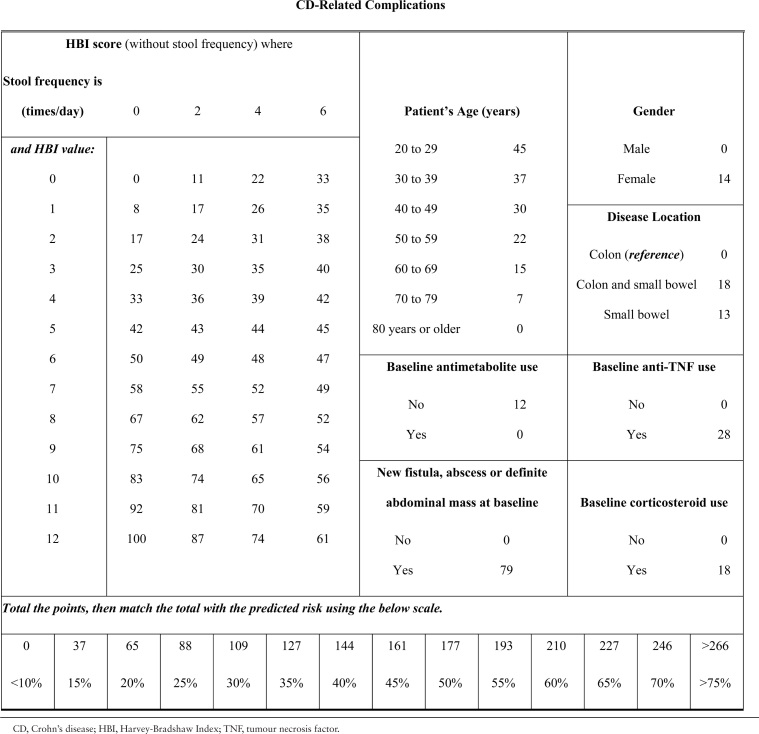
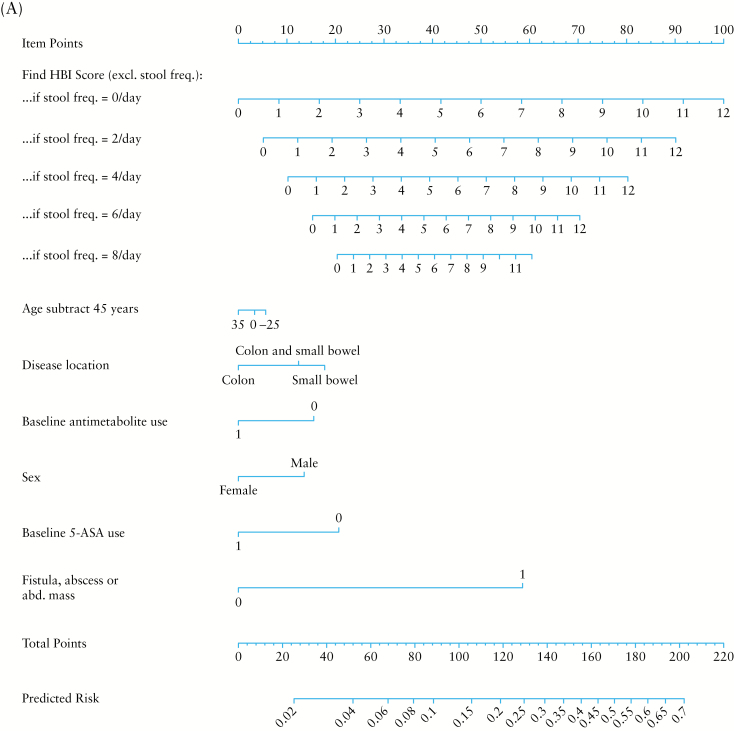
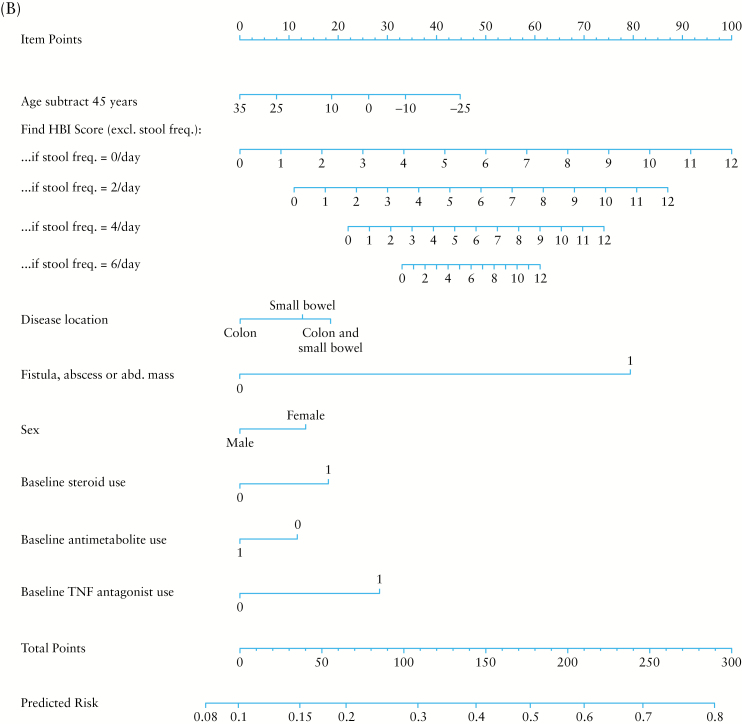
To compute the risk score, multiply the value of each variable with its associated regression coefficient, and then sum all values, producing the linear predictor [lp]. The linear predictor on the log-odds scale may be converted to a risk probability [%] by the inverse log-odds formula: . An example risk calculation is demonstrated in Table 4. A risk score may alternatively be calculated using score charts and converted to a risk estimate [Tables 5 and 6] or computed by the provided nomograms [Figure 1A and B].
Table 4.
Computing the predicted risk score.
| As an example, suppose a 50-year old male patient presents to the clinic with disease confined to the small bowel. His total HBI score is 3 with a stool frequency of two per day, he is currently taking 5-ASA, and does not have a fistula, stricture, or definite abdominal mass. The clinician would now like to predict the risk of surgery within the next 2 years. Start by plugging these values into the equation for the linear predictor. lp = -3.51 [Intercept] + [50 – 45; age, years]*[- 2.5*10–3] + [1; male]*[0.36] + [1 point; HBI subtract stools/day]*[0.22] + [2 stools/day]*[6.8*10–2] + [1; small bowel only]*[0.48] + [0; antimetabolite use]*[-0.42] + [1; 5-ASA use]*[-0.55] + [0; fistula, abscess, abdominal mass]*[1.57] + [1 point; HBI subtract stools/day]*[2 stools/day]*[-1.7*10–2] = -2.8175 ≈ -2.82. Now convert the linear predictor into percent risk. Risk = 100% * 1 / [1 + exp[-lp]] = 5.6% [expected risk is approximately 5.6%]. |
HBI, Harvey-Bradshaw Index; 5-ASA, 5-aminosalicylate.
Table 5.
Score chart for risk of CD-related surgery.
Table 6.
Score chart for risk of CD-related complications.
Figure 1.
Nomogram for the computation of percentage risk of Crohn’s disease-related surgery [A] or complication [B].
For prediction of CD-related surgery, the score ranges from 0 to 233 [Table 5]. A score of 61, 119, or 171 respectively predict a 5%, 20%, or 50% chance of experiencing a disease-related surgery within 24 months of follow-up. For prediction of CD-related complications, the score ranges from 0 to 314 [Table 6]. A score of 88, 177, or 266 respectively predict a 25%, 50%, or 75% chance of experiencing a disease-related complication within 24 months of follow-up.
4. Discussion
We developed two clinical prediction models, one for predicting individual risk of experiencing CD-related surgery, and the other for predicting CD-related complications within 24 months, using data from one of the largest clinical trials conducted in CD to date. These models have been developed using patients from the REACT trial, and are thus intended for use in a broad cross-section of patients found in community gastroenterology clinics and not solely with regard to a specific disease duration or severity. Although both models demonstrated high specificity and negative predictive values, the surgery model had relatively greater predictive ability, likely because surgery is an easily defined and more objective clinical outcome, compared with disease-related complications. Additionally, the development of a score chart using basic clinical variables which are readily calculable facilitates implementation of this score in the outpatient clinic setting, potentially allowing for enhanced decision making between patients and physicians. If validated in an external dataset, the use of this model may help identify patients at higher risk of disease-related complications and especially surgery, who may benefit most from more intensive treatment, combination therapy, and/or closer monitoring for symptoms that require planned surgery [eg. obstructive symptoms in the setting of fibrostenotic disease]. Additionally, all identified baseline predictors in our models were prospectively defined and measured. We believe this is a distinct advantage to retrospective cohort studies in which a greater potential exists for bias during the data acquisition process.
Existing prediction models for CD-related outcomes [summarised in Supplementary Table 5, available at ECCO-JCC online] have been limited by selection or referral centre bias, small sample size, evolving drug treatments and management strategies, long-term time horizons, or broadly inclusive outcome definitions. Furthermore, the time frame in which these models were produced must be considered. Since the greatest risk of complications occurs within the first few years after diagnosis, long-term risk prediction models eventually become confounded or outdated with evolving therapies and treatment strategies. For example, while some models were developed in the pre-biologic era, others define the need for thiopurines as advanced disease; but currently thiopurine monotherapy represents a treatment strategy generally reserved for less severe disease rather than severe disease.55,56 Nevertheless, it is notable that many of the items we identified as independent predictors have previously been shown to have prognostic value in population-based cohorts with longer-term follow up,1,4,57–60 supporting the validity of our results. Prediction of CD-related complications is limited by the use of non-standard or broadly inclusive outcome definitions [Supplementary Table 5], thus preventing meaningful comparisons with our model. This may also explain the lower performance of the model for prediction of CD-related complications.
In the present study, the surgery model has shown promise to accurately predict an adverse outcome which may be useful to guide management strategies and patient care. Such a model could also reasonably be used for risk stratification of patients in clinical studies.
A major strength of the current study is use of data from a large randomised controlled trial [RCT] conducted across community centres, meaning that the endpoints were well defined, complete to 24 months of follow-up, and adjudicated by an independent panel. The pragmatic nature of the trial design meant that consecutive patients were enrolled, regardless of disease activity, duration, phenotype, or treatment, and an algorithm of care applied which is broadly representative of the patient population seen in clinical practice. Thus, we believe these models may be more generalisable to the routine clinical care of adult patients with CD, and can be applied within the community clinic, regardless of disease duration, in keeping with the design of the trial from which the model and data were derived.
It is interesting to point out that smoking is commonly regarded as a poor prognostic factor for Crohn’s disease and especially for postoperative recurrence. In the present study, we found that smoking had limited predictive ability for CD-related complications. Another important consideration with smoking is that there is a degree of subjective reporting, which can lead to inaccurate categorisation of current/recent/past smoking status as well as confounding with the quantity of smoking. Thus, we did not retain this in the final prediction models, which did not appreciably affect predictive performance.
Limitations of this study should be acknowledged. Perhaps the greatest limitation is that these models were validated in the cohort in which they were developed. Thus, independent validation in an external dataset is required before the widespread adoption of any prediction model can be considered. The results presented here encourage seeking such external validation. Participants in the REACT study had a longer average duration of disease, though approximately 15% and 28% were diagnosed within 2 and 5 years, respectively. However, the operating characteristics of these models in only newly diagnosed patients remain to be determined. Indeed, the REACT design was intended to include a broad cross-section of patients within community clinics, so that we may not be limited to specific subsets of disease or demographic characteristics, and applies more generally to a broader patient population.
The CD-related surgery model performed better than the complication model, which may be related to using a more well-defined and objective outcome definition. Although the models use routinely available and clearly defined clinical data collected by trained gastroenterologists, inter-observer variability may influence the validity of the model. Also, the REACT study did not include biomarkers or serological or genetic factors; therefore these factors could not be incorporated into the current models. These factors may provide additional prognostic value beyond routine clinical data.41
Related to this issue of validation is discrimination performance. Prediction of CD-related complications is relatively difficult, as reflected in its low discriminative ability. However, the surgery model performs nominally as well as the model by Siegel and colleagues,43 using completely different modelling approaches and relying solely on readily available clinical variables. Nevertheless, we are cautiously optimistic that the surgery model may prove useful once externally validated.
The treatment allocation was not included in our models, which some may view as a limitation. However, this decision was made a priori for two reasons. First, our primary interest was to develop prediction models using only baseline predictive factors that are available in practice. Second, the effect of treatment to predict these outcomes was found to be relatively small as compared with the factors in our prediction models. This is consistent with prediction literature in other areas, such as cardiovascular disease.61
In summary, we have developed and validated clinical prediction models for CD-related surgery and complications using data from a large randomised clinical trial with good overall performance for predicting the outcome of disease-related surgery within 2 years. We have transformed these models into scoring tools, facilitating their use in the clinic; external validation in independent cohorts with longitudinal follow up is recommended.
Funding
This work was not specifically supported by any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of Interest
RK: honoraria from AbbVie, Janssen, Pfizer, Shire, and Takeda Pharma. PD: research support funding from Takeda and Pfizer, and a training grant through the National Institute of Diabetes and Digestive and Kidney Diseases [NIDDK T32DK007202]. WJS: grant support from Exact Sciences, the American College of Gastroenterology, and the Broad Foundation; grant support and personal fees from Receptos, Amgen, Prometheus Laboratories, AbbVie, Boehringer Ingelheim, Takeda, Atlantic Pharmaceuticals, Janssen, Bristol-Myers Squibb, Genentech, Pfizer, and Nutrition Science Partners; and personal fees from Kyowa Hakko Kirin, Millennium Pharmaceuticals, Celgene Cellular Therapeutics, Santarus, Salix Pharmaceuticals, Catabasis Pharmaceuticals, Vertex Pharmaceuticals, Warner Chilcott, Gilead Sciences, Cosmo Pharmaceuticals, Ferring Pharmaceuticals, Sigmoid Biotechnologies, Tillotts Pharma, Am Pharma BV, Dr. August Wolff, Avaxia Biologics, Zyngenia, Ironwood Pharmaceuticals, Index Pharmaceuticals, Nestle, Lexicon Pharmaceuticals, UCB Pharma, Orexigen, Luitpold Pharmaceuticals, Baxter Health care, Ferring Research Institute, Amgen, Novo Nordisk, Mesoblast Inc., Shire, Ardelyx Inc., Actavis, Seattle Genetics, MedImmune [AstraZeneca], Actogenix NV, Lipid Therapeutics Gmbh, Eisai, Qu Biologics, Toray Industries Inc., Teva Pharmaceuticals, Eli Lilly, Chiasma, TiGenix, Adherion Therapeutics, Immune Pharmaceuticals, Celgene, Arena Pharmaceuticals, Ambrx Inc., Akros Pharma, Vascular Biogenics, Theradiag, Forward Pharma, Regeneron, Galapagos, Seres Health, Ritter Pharmaceuticals, Theravance, Palatin, Biogen, and the University of Western Ontario [owner of Robarts Clinical Trials Inc]. VJ: scientific advisory board fees from Abbvie, Sandoz, Ferring and Janssen; speaker’s fees from Takeda and Ferring; and travel support for conference attendance from Vifor pharmaceuticals. BGF: grant/research support from Millennium Pharmaceuticals, Merck, Tillotts Pharma AG, AbbVie, Novartis Pharmaceuticals, Centocor Inc., Elan/Biogen, UCB Pharma, Bristol-Myers Squibb, Genentech, ActoGenix, and Wyeth Pharmaceuticals Inc.; consulting fees from Millennium Pharmaceuticals, Merck, Centocor Inc., Elan/Biogen, Janssen-Ortho, Teva Pharmaceuticals, Bristol-Myers Squibb, Celgene, UCB Pharma, AbbVie, Astra Zeneca, Serono, Genentech, Tillotts Pharma AG, Unity Pharmaceuticals, Albireo Pharma, Given Imaging Inc., Salix Pharmaceuticals, Novonordisk, GSK, Actogenix, Prometheus Therapeutics and Diagnostics, Athersys, Axcan, Gilead, Pfizer, Shire, Wyeth, Zealand Pharma, Zyngenia, GiCare Pharma Inc., and Sigmoid Pharma; and speaker fees from UCB, AbbVie, and J&J/Janssen.
Author Contributions
LG: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis. GZ: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; study supervision. RK: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; study supervision. PD: critical revision of the manuscript for important intellectual content. WJS: critical revision of the manuscript for important intellectual content. VJ: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; study supervision. BGF: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; study supervision. All authors have approved the final version of this manuscript.
Supplementary Data
Supplementary data are available at ECCO-JCC online.
Supplementary Material
References
- 1. Henriksen M, Jahnsen J, Lygren I et al. ; Ibsen Study Group. Clinical course in Crohn’s disease: results of a five-year population-based follow-up study [the IBSEN study]. Scand J Gastroenterol 2007;42:602–10. [DOI] [PubMed] [Google Scholar]
- 2. Peyrin-Biroulet L, Loftus EV Jr, Colombel JF, Sandborn WJ. The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol 2010;105:289–97. [DOI] [PubMed] [Google Scholar]
- 3. Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011;140:1785–94. [DOI] [PubMed] [Google Scholar]
- 4. Peyrin-Biroulet L, Harmsen WS, Tremaine WJ, Zinsmeister AR, Sandborn WJ, Loftus EV. Surgery in a population-based cohort of Crohn’s disease from Olmsted County, Minnesota [1970–2004]. Am J Gastroenterol 2012;107:1693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Solberg IC, Vatn MH, Høie O et al. ; IBSEN Study Group. Clinical course in Crohn’s disease: results of a Norwegian population-based ten-year follow-up study. Clin Gastroenterol Hepatol 2007;5:1430–8. [DOI] [PubMed] [Google Scholar]
- 6. Colombel JF, Sandborn WJ, Rutgeerts P et al. . Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology 2007;132:52–65. [DOI] [PubMed] [Google Scholar]
- 7. Hanauer SB, Feagan BG, Lichtenstein GR et al. ; ACCENT I Study Group. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet 2002;359:1541–9. [DOI] [PubMed] [Google Scholar]
- 8. Rutgeerts P, Feagan BG, Lichtenstein GR et al. . Comparison of scheduled and episodic treatment strategies of infliximab in Crohn’s disease. Gastroenterology 2004;126:402–13. [DOI] [PubMed] [Google Scholar]
- 9. Sandborn WJ, Feagan BG, Stoinov S et al. ; PRECISE 1 Study Investigators. Certolizumab pegol for the treatment of Crohn’s disease. N Engl J Med 2007;357:228–38. [DOI] [PubMed] [Google Scholar]
- 10. Sandborn WJ, Hanauer SB, Rutgeerts P et al. . Adalimumab for maintenance treatment of Crohn’s disease: results of the CLASSIC II trial. Gut 2007;56:1232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sandborn WJ, Lee SD, Randall C et al. . Long-term safety and efficacy of certolizumab pegol in the treatment of Crohn’s disease: 7-year results from the PRECiSE 3 study. Aliment Pharmacol Ther 2014;40:903–16. [DOI] [PubMed] [Google Scholar]
- 12. Schreiber S, Colombel JF, Bloomfield R et al. ; PRECiSE 2 Study Investigators. Increased response and remission rates in short-duration Crohn’s disease with subcutaneous certolizumab pegol: an analysis of PRECiSE 2 randomised maintenance trial data. Am J Gastroenterol 2010;105:1574–82. [DOI] [PubMed] [Google Scholar]
- 13. Schreiber S, Khaliq-Kareemi M, Lawrance IC et al. ; PRECISE 2 Study Investigators. Maintenance therapy with certolizumab pegol for Crohn’s disease. N Engl J Med 2007;357:239–50. [DOI] [PubMed] [Google Scholar]
- 14. Sandborn WJ, Colombel JF, Enns R et al. ; International Efficacy of Natalizumab as Active Crohn’s Therapy [ENACT-1] Trial Group; Evaluation of Natalizumab as Continuous Therapy [ENACT-2] Trial Group. Natalizumab induction and maintenance therapy for Crohn’s disease. N Engl J Med 2005;353:1912–25. [DOI] [PubMed] [Google Scholar]
- 15. Sandborn WJ, Feagan BG, Rutgeerts P et al. ; GEMINI 2 Study Group. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2013;369:711–21. [DOI] [PubMed] [Google Scholar]
- 16. Sands BE, Feagan BG, Rutgeerts P et al. . Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology 2014;147:618–27.e3. [DOI] [PubMed] [Google Scholar]
- 17. Targan SR, Feagan BG, Fedorak RN et al. ; International Efficacy of Natalizumab in Crohn’s Disease Response and Remission [ENCORE] Trial Group. Natalizumab for the treatment of active Crohn’s disease: results of the ENCORE Trial. Gastroenterology 2007;132:1672–83. [DOI] [PubMed] [Google Scholar]
- 18. Vermeire S, Loftus EV, Colombel J-F et al. . Long-term efficacy of vedolizumab for Crohn’s disease. J Crohns Colitis 2017;11:412–24. [DOI] [PubMed] [Google Scholar]
- 19. Colombel JF, Sandborn WJ, Reinisch W et al. ; SONIC Study Group. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 2010;362:1383–95. [DOI] [PubMed] [Google Scholar]
- 20. D’Haens G, Baert F, van Assche G et al. ; Belgian Inflammatory Bowel Disease Research Group; North-Holland Gut Club. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial. Lancet 2008;371:660–7. [DOI] [PubMed] [Google Scholar]
- 21. Khanna R, Bressler B, Levesque BG et al. ; REACT Study Investigators. Early combined immunosuppression for the management of Crohn’s disease [REACT]: a cluster randomised controlled trial. Lancet 2015;386:1825–34. [DOI] [PubMed] [Google Scholar]
- 22. Vermeire S, Noman M, Van Assche G, Baert F, D’Haens G, Rutgeerts P. Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn’s disease. Gut 2007;56:1226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Scott FI, Lichtenstein GR. Advances in therapeutic drug monitoring of biologic therapies in inflammatory bowel disease: 2015 in review. Curr Treat Options Gastroenterol 2016;14:91–102. [DOI] [PubMed] [Google Scholar]
- 24. Vande Casteele N, Ferrante M, Van Assche G et al. . Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology 2015;148:1320–9.e3. [DOI] [PubMed] [Google Scholar]
- 25. Colombel JF, Reinisch W, Mantzaris GJ et al. . Randomised clinical trial: Deep remission in biologic and immunomodulator nai ve patients with Crohn’s disease - a sonic post hoc analysis. Aliment Pharmacol Ther 2015;41:734–46. [DOI] [PubMed] [Google Scholar]
- 26. Colombel JF, Rutgeerts PJ, Sandborn WJ et al. . Adalimumab induces deep remission in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2014;12:414–22.e5. [DOI] [PubMed] [Google Scholar]
- 27. Feagan BG, Sandborn WJ, Gasink C et al. . UNITI–IM-UNITI Study Group. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2016;375:1946–60. [DOI] [PubMed] [Google Scholar]
- 28. Lémann M, Mary JY, Duclos B et al. . Groupe d’Etude Therapeutique des Affections Inflammatoires du Tube Digestif [GETAID]. Infliximab plus azathioprine for steroid-dependent Crohn’s disease patients: a randomised placebo-controlled trial. Gastroenterology 2006;130:1054–61. [DOI] [PubMed] [Google Scholar]
- 29. Rutgeerts P, Van Assche G, Sandborn WJ et al. . EXTEND Investigators. Adalimumab induces and maintains mucosal healing in patients with Crohn’s disease: data from the EXTEND trial. Gastroenterology 2012;142:1102–11.e2. [DOI] [PubMed] [Google Scholar]
- 30. Benitez JM, Louis E. Can we predict the high-risk patient?Dig Dis 2014;32:328–36. [DOI] [PubMed] [Google Scholar]
- 31. Nasir BF, Griffiths LR, Nasir A et al. . An envirogenomic signature is associated with risk of IBD-related surgery in a population-based Crohn’s disease cohort. J Gastrointest Surg 2013;17:1643–50. [DOI] [PubMed] [Google Scholar]
- 32. Beaugerie L, Seksik P, Nion-Larmurier I, Gendre JP, Cosnes J. Predictors of Crohn’s disease. Gastroenterology 2006;130:650–6. [DOI] [PubMed] [Google Scholar]
- 33. Loly C, Belaiche J, Louis E. Predictors of severe Crohn’s disease. Scand J Gastroenterol 2008;43:948–54. [DOI] [PubMed] [Google Scholar]
- 34. Ramadas AV, Gunesh S, Thomas GAO, Williams GT, Hawthorne AB. Natural history of Crohn’s disease in a population-based cohort from Cardiff [1986–2003]: A study of changes in medical treatment and surgical resection rates. Gut 2010;59:1200–6. [DOI] [PubMed] [Google Scholar]
- 35. Romberg-Camps MJ, Dagnelie PC, Kester AD et al. . Influence of phenotype at diagnosis and of other potential prognostic factors on the course of inflammatory bowel disease. Am J Gastroenterol 2009;104:371–83. [DOI] [PubMed] [Google Scholar]
- 36. Ryan JD, Silverberg MS, Xu W et al. . Predicting complicated Crohn’s disease and surgery: phenotypes, genetics, serology and psychological characteristics of a population-based cohort. Aliment Pharmacol Ther 2013;38:274–83. [DOI] [PubMed] [Google Scholar]
- 37. Tarrant KM, Barclay ML, Frampton CM, Gearry RB. Perianal disease predicts changes in Crohn’s disease phenotype-results of a population-based study of inflammatory bowel disease phenotype. Am J Gastroenterol 2008;103:3082–93. [DOI] [PubMed] [Google Scholar]
- 38. Thia KT, Sandborn WJ, Harmsen WS, Zinsmeister AR, Loftus EV Jr. Risk factors associated with progression to intestinal complications of Crohn’s disease in a population-based cohort. Gastroenterology 2010;139:1147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wolters FL, Russel MG, Sijbrandij J et al. . Phenotype at diagnosis predicts recurrence rates in Crohn’s disease. Gut 2006;55:1124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang CH, Ding J, Gao Y, Chen X, Yang ZB, Xiao SD. Risk factors that predict the requirement of aggressive therapy among Chinese patients with Crohn’s disease. J Dig Dis 2011;12:99–104. [DOI] [PubMed] [Google Scholar]
- 41. Dubinsky MC, Kugathasan S, Kwon S et al. . Multidimensional prognostic risk assessment identifies association between IL12B variation and surgery in Crohn’s disease. Inflamm Bowel Dis 2013;19:1662–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lakatos PL, Sipeki N, Kovacs G et al. . Risk matrix for prediction of disease progression in a referral cohort of patients with Crohn’s disease. J Crohns Colitis 2015;9:891–8. [DOI] [PubMed] [Google Scholar]
- 43. Siegel CA, Horton H, Siegel LS et al. . A validated web-based tool to display individualised Crohn’s disease predicted outcomes based on clinical, serologic and genetic variables. Aliment Pharmacol Ther 2016;43:262–71. [DOI] [PubMed] [Google Scholar]
- 44. Solberg IC, Cvancarova M, Vatn MH, Moum B; IBSEN Study Group Risk matrix for prediction of advanced disease in a population-based study of patients with Crohn’s Disease [the IBSEN Study]. Inflamm Bowel Dis 2014;20:60–8. [DOI] [PubMed] [Google Scholar]
- 45. Collins GS, Reitsma JB, Altman DG, Moons KG; TRIPOD Group Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis [TRIPOD]: the TRIPOD statement. The TRIPOD Group. Circulation 2015;131:211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bouwmeester W, Twisk JW, Kappen TH, van Klei WA, Moons KG, Vergouwe Y. Prediction models for clustered data: comparison of a random intercept and standard regression model. BMC Med Res Methodol 2013;13:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Harrell FE., Jr Regression Modeling Strategies. New York, NY: Springer International Publishing; 2015. [Google Scholar]
- 48. Steyerberg EW. Clinical Prediction Models. New York, NY: Springer; 2009. [Google Scholar]
- 49. Miller ME, Langefeld CD, Tierney WM, Hui SL, McDonald CJ. Validation of probabilistic predictions. Med Decis Making 1993;13:49–58. [DOI] [PubMed] [Google Scholar]
- 50. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15:361–87. [DOI] [PubMed] [Google Scholar]
- 51. Efron B. How biased is the apparent error rate of a prediction rule?J Am Stat Assoc 1986;81:461–70. [Google Scholar]
- 52. Wynants L, Bouwmeester W, Moons KG et al. . A simulation study of sample size demonstrated the importance of the number of events per variable to develop prediction models in clustered data. J Clin Epidemiol 2015;68:1406–14. [DOI] [PubMed] [Google Scholar]
- 53. R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2016. [Google Scholar]
- 54. R Studio Team. Rstudio: Integrated Development Environment for r. Vienna: R Foundation for Statistical Computing; 2015. [Google Scholar]
- 55. Dignass A, Van Assche G, Lindsay JO et al. . European Crohn’s and Colitis Organisation [ECCO]. The second European evidence-based consensus on the diagnosis and management of Crohn’s disease: Current management. J Crohns Colitis 2010;4:28–62. [DOI] [PubMed] [Google Scholar]
- 56. Gomollón F, Dignass A, Annese V et al. . ECCO. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: Part 1: diagnosis and medical management. J Crohns Colitis 2017;11:3–25. [DOI] [PubMed] [Google Scholar]
- 57. Bernell O, Lapidus A, Hellers G. Risk factors for surgery and postoperative recurrence in Crohn’s disease. Ann Surg 2000;231:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Golovics PA, Lakatos L, Mandel MD et al. . Prevalence and predictors of hospitalisation in Crohn’s disease in a prospective population-based inception cohort from 2000–2012. World J Gastroenterol 2015;21:7272–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lovasz BD, Lakatos L, Horvath A et al. . Evolution of disease phenotype in adult and pediatric onset Crohn’s disease in a population-based cohort. World J Gastroenterol 2013;19:2217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nguyen GC, Nugent Z, Shaw S, Bernstein CN. Outcomes of patients with Crohn’s disease improved from 1988 to 2008 and were associated with increased specialist care. Gastroenterology 2011;141:90–7. [DOI] [PubMed] [Google Scholar]
- 61. Steyerberg EW, Vergouwe Y. Towards better clinical prediction models: seven steps for development and an ABCD for validation. Eur Heart J 2014;35:1925–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.