Abstract
Purpose of the Study
Older adults in residential care and assisted living (RC/AL) are less healthy than the general elderly population, and some have needs similar to those in nursing homes, making this an important group in which to assess potential overuse or underuse of preventive services. We determined the health status of RC/AL residents and distinguished characteristics between those who may and may not benefit from preventive services requiring a life expectancy ≥5 years.
Design and Methods
Cross-sectional survey of a nationally representative sample of RC/AL residents using 2010 data from the National Survey of Residential Care Facilities. The primary outcome was the weighted frequency distribution of health states using three predictive mortality indices: Charlson Comorbidity Index, 4-year mortality index, and 9-year mortality index.
Results
A total of 666,700 of 733,300 (weighted) residents met criteria for inclusion. Based on the three indices, 10%–15% were in good health, 11%–70% in intermediate health, and 20%–76% in poor health.
Implications
Using triangulation between 3 well-validated mortality indices, 10%–15% of RC/AL residents are in good health and highly likely to benefit from preventive services that require ≥5 year life expectancy. In addition, many residents have uncertain benefit and would benefit from shared decision making.
Keywords: Preventive services, Assisted living, Likelihood of benefit, Mortality indices
As of 2013, almost 15% of the United States (U.S.) population was 65 years of age or older (U.S. Census Bureau, 2013). Approximately 750,000 reside in residential care/assisted living (RC/AL; (Park-Lee et al., 2011). Not surprisingly, a national survey of RC/AL residents found they have medical conditions common among older adults, including high blood pressure (57% of residents) and dementia (42%; Caffrey et al., 2012). However, RC/AL residents’ care needs are highly variable, ranging from few medical care needs to nursing home-level care needs (Zimmerman et al., 2003). Given the range of need among this population, it is important to understand their overall health status and their potential benefit from preventive care.
Among all populations, preventive services are indicated when they reduce mortality from the targeted condition. Most preventive services require some time lag to see benefit, which varies by condition and is based on life expectancy. An older adult needs a 5- to 10-year life expectancy to achieve a survival benefit from colorectal cancer (CRC) or breast cancer screening (Lee et al., 2013), but as little as 1- to 2-year life expectancy to see benefit from asymptomatic abdominal aortic aneurysm screening (Thompson et al., 2012), and a 2- to 3-year life expectancy is needed to benefit from lowering blood pressure to improve cardiovascular outcomes (Holmes, Hayley, Alexander, & Sachs, 2006). The limited evidence of RC/AL residents’ benefit from preventive services revealed that in two RC/AL settings, 32% had never received CRC screening, although over 20% of those in poor health were “up-to-date” with screening (Moore et al., 2014). The overall health status of RC/AL residents can determine their potential benefit from preventive services and inform a gap regarding this important health care issue.
This study used nationally representative data from the National Survey of Residential Care Facilities (NSRCF) to better understand the health of RC/AL residents and their potential benefit from preventive services requiring a 5- to 10-year life expectancy. We used three well-validated mortality indices to triangulate RC/AL residents’ health status, hypothesizing that these indices would clarify the health status of RC/AL residents and also the percentage who might benefit from preventive services.
Methods
The 2010 NSRCF is the first and only nationwide data collection effort to examine RC/AL residents (Moss et al., 2011); its methodology has been reported elsewhere (Caffrey et al., 2012). The analyses reported herein used these data, excluding the 9% of residents who either were younger than 60 years of age (because they tend to differ from older RC/AL residents) or had CRC (because our initial intention was to limit this analysis to CRC screening).
The primary outcome was the distribution of residents’ health state based on three well-validated mortality indices: the Charlson Comorbidity Index (CCI) (Charlson, Szatrowski, Peterson, & Gold, 1994), a 4-year mortality index (Lee, Lindquist, Segal, & Covinsky, 2006), and a 9-year mortality index (Schonberg, Davis, McCarthy, & Marcantonio, 2011). The CCI is the most commonly used measure of comorbidity and mortality (de Groot, Beckerman, Lankhorst, & Bouter, 2003) and has been used in numerous populations. CCI scores range from 0 to 35 and are based on 19 conditions (Charlson et al., 1994). The 4-year index scores range from 0 to 26 based on 12 variables: age, sex, body mass index (BMI), smoking, chronic obstructive pulmonary disease (COPD), cancer, diabetes, congestive heart failure, and the ability to bath, walk several blocks, manage money, and push large objects (Lee et al., 2006). The 9-year index scores range from 0 to 31 based on 11 variables: age, sex, BMI, smoking status, self-reported health, COPD, cancer, diabetes, ability to perform everyday chores, ability to walk several blocks, and recent hospitalizations (Schonberg et al., 2011).
We used each resident’s score on each index to assign a health state that reflects mortality risk and consequently life expectancy and likely benefit from screening. For the CCI health states, based on established criteria, we used a combination of the CCI score and the individual’s age to categorize health states: good health—the youngest and healthiest residents (age 60–79 and CCI = 0, age 60–74 and CCI = 1–3, or age 60–69 and CCI ≥ 4), who were expected to live more than 10 years; intermediate health—the younger residents with increasing comorbidity and the oldest healthiest residents (age 70–74 and CCI ≥ 4, age 75–79 and CCI = 1–3, or age 80–84 and CCI = 0), who were expected to live 5–10 years; and poor health—the sickest and oldest residents (age 75–79 and CCI ≥ 4, age 80–84 and CCI > 0, or age ≥ 85), who were expected to live less than 5 years (Kistler, Kirby, Lee, Casadei, & Walter, 2011). For the 4-year index, if the score was less than 9, residents were assigned good health (<20% risk of 4-year mortality); if it was 10–13, they were assigned intermediate health (20–63% risk of 4-year mortality); and if it was ≥14, they were in poor health (>64% risk of 4-year mortality). If the 9-year index score was less than 8, the resident was assigned good health (<26% risk of 9-year mortality); if it was 9–16, they were assigned intermediate health (26–75% risk of 9-year mortality); and if it was ≥17, they were in poor health (>75% risk of 9-year mortality).
To create each individual’s score on each index, we used public use data dictionaries from the 2010 NSRCF website (http://www.cdc.gov/nchs/nsrcf/nsrcf_questionnaires.htm) and restricted-use data files. Each resident survey included free-text sections to capture discrete elements such as other health conditions not explicitly asked in the NSRCF. The majority of data elements from the indices were found in the dictionaries, but a few elements for each index had to be imputed from free-text responses. Also, when rates were significantly lower than expected, they were imputed based on rates reported in the literature [i.e., for mild or moderate/severe liver disease (Bell et al., 2008), peptic ulcer disease (Munnangi & Sonnenberg, 1997; Sung, Kuipers, & El-Serag, 2009), and connective tissue disease (Helmick et al., 2008; Lawrence et al., 2008)]. For both the 4-year and 9-year indices, we imputed residents’ BMI (Flegal, Carroll, Kit, & Ogden, 2012), frequency of hospitalizations (Wright, 2013), and smoking status (King, Dube, Kaufmann, Shaw, & Pechacek, 2011). Only BMI, smoking status, and connective tissue disease were conditioned on other characteristics (i.e., gender for BMI and smoking status, and gender, age group, and race for connective tissue disease). Otherwise, imputations assigned points randomly (Table 1).
Table 1.
Elements Necessary for the Three Mortality Indices Not Found in the NSRCF and Method of Imputation
| Index | Method of imputation |
|---|---|
| Charlson Comorbidity Index | |
| Mild liver disease | Random imputation by race (0.33 of cases if black; 0.25 if other race) |
| Moderate or severe liver disease | Random imputation of liver disease. If assigned as liver disease in first random imputation, 0.15 then randomly imputed to have moderate or severe by race (result is roughly 0.28 of black participants have mild liver disease and 0.05 have moderate/ severe; 0.21 of other races have mild disease; and 0.04 have moderate/severe disease) |
| Peptic ulcer disease | 0.014 of cases randomly imputed |
| Connective tissue disease | Polymyalgia rheumatica randomly imputed within gender and age group Systemic lupus erythematosus randomly imputed within gender and race |
| Rheumatoid arthritis randomly imputed within gender and age group | |
| 4-Year and 9-year index | |
| Hospital admissions | 0.333 of reported hospitalizations randomly imputed as >2 nights |
| BMI < 25 | For males, randomly imputed 0.235 cases with BMI < 25 |
| For females, randomly imputed 0.265 with BMI < 25 | |
| Tobacco use | For the 4-year index: |
| For females, randomly imputed 0.45 to have ever smoked; of those, randomly imputed 0.333 to be current smokers (1/3 of the 45% who ever smoked are current smokers) | |
| For males, randomly imputed 0.70 to have ever smoked; of those, randomly imputed 0.29 to be current smokers | |
| For the 9-year index: | |
| For females, given ever smokers and current smokers imputed as above, the result is 0.30 of all females are randomly imputed to be former smokers | |
| For males, given ever smokers and current smokers imputed as above, the result is 0.50 of all males are randomly imputed to be former smokers | |
Note: BMI = body mass index.
For each resident, a CCI, 4-year index, and 9-year index score were calculated. Scores were then converted to health states (good, intermediate, or poor), and we examined the correspondence of health states using pairwise cross-tabulations using SAS PROC SURVEYFREQ. We used SAS PROC SURVEYREG to assess the correspondence of the 4- and 9-year indices with the CCI index.
Results
Sample Characteristics and Prevalence of Health States
A total of 666,700 residents (91% weighted frequency) were retained of the 733,300 residents nation wide. Most were female, white, and resided in the south (Table 2). In calculating the CCI health states, 0.7% of the cases were lost due to missing data, so results of this score are based on a weighted frequency of 661,900. Calculations for the 4-year and 9-year indices were based on the full sample (n = 666,700). Overall, based on the CCI-derived health states, 4-year index, and 9-year index, respectively, 13%, 15%, and 10% were in the good health state group; 11%, 44%, and 70% in the intermediate health group; and 76%, 41%, and 20% in the poor health group (Table 3).
Table 2.
Weighted Frequencies of RC/AL Resident Characteristics, N = 666,700
| Characteristic | Number (%) of residents |
|---|---|
| Age | |
| 60–64 | 20,001 (3) |
| 65–69 | 26,668 (4) |
| 70–74 | 33,335 (5) |
| 75–79 | 60,003 (9) |
| 80–84 | 133,340 (20) |
| 85 and older | 386,686 (58) |
| Gender | |
| Female | 477,900 (72) |
| Male | 184,000 (28) |
| Race | |
| White | 611,300 (92) |
| Black | 20,600 (3) |
| Other | 30,000 (5) |
| Region | |
| Northeast | 110,300 (17) |
| Midwest | 156,100 (24) |
| South | 201,300 (30) |
| West | 194,300 (29) |
Notes: Number does not equal 666,700 in any category due to missing data. AL = assisted living; RC = residential care.
Table 3.
Distribution of the Charlson Comorbidity Index-derived Health States by the 4-Year and 9-Year Mortality Index Health States, N = 666,700
| Charlson Comorbidity Index-derived health states, n (%) | Weighted frequencies 4-year and 9-year, n (%)a | ||||
|---|---|---|---|---|---|
| Good | Intermediate | Poor | |||
| 4-Year index health states | Good | 52,800 (62) | 21,800 (29) | 19,000 (4) | 97,300 (13) |
| Intermediate | 27,000 (32) | 42,300 (57) | 224,000 (45) | 295,600 (11) | |
| Poor | 5,300 (6) | 10,400 (14) | 259,000 (51) | 273,900 (76) | |
| 9-Year index health states | Good | 38,400 (45) | 13,500 (18) | 15,300 (3) | 68,100 (10) |
| Intermediate | 45,800 (54) | 56,400 (76) | 357,900 (71) | 464,000 (70) | |
| Poor | 900 (1) | 4,700 (6) | 128,800 (26) | 134,600 (20) | |
| Weighted frequencies Charlson comorbidity | 85,500 (13) | 73,700 (11) | 502,700 (76) | ||
aNumbers do not sum to equal 666,700 in any category due to missing data.
Triangulation Among Health Indices
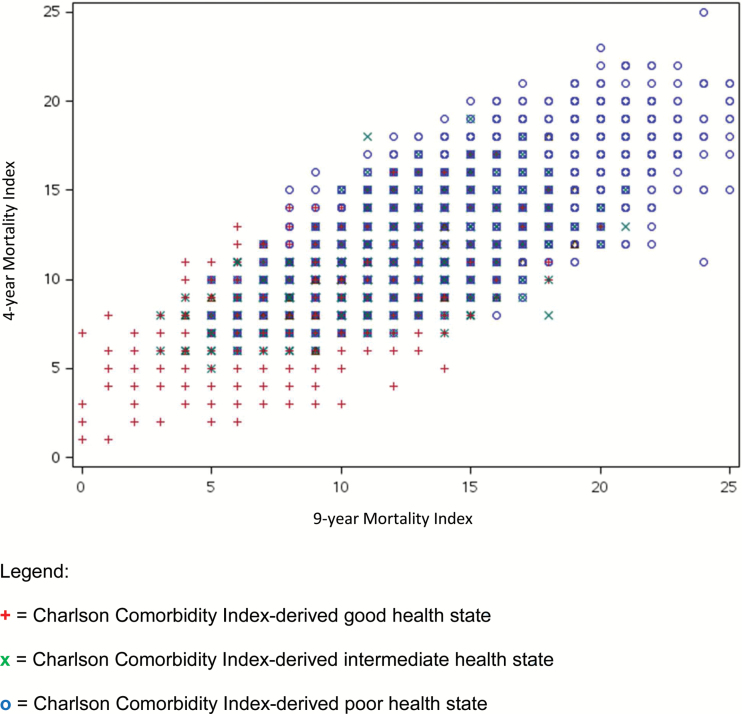
When we triangulated the three indices, we found a general correspondence, such that those in good health, as derived by the CCI, also scored lower on the 4-year and 9-year indices, and vice versa (Figure 1). The correlations of the 4-year and 9-year health status classifications with the CCI-derived health states were moderate (0.54 and 0.43, respectively). However, only 27% of residents were assigned the same health states across all three indices. Of those in the CCI-derived good health state, 6% and 1% were categorized in the poor health state according to the 4-year and 9-year indices, respectively (Table 3). Of those in the CCI-derived poor health state, 4% and 3% were in the good health state according to the 4-year and 9-year indices, respectively. In total, 4%–10% of individuals were highly misclassified.
Figure 1.
Triangulation of the three mortality indices, N = 661,900. + = Charlson Comorbidity Index-derived good health state. × = Charlson Comorbidity Index-derived intermediate health state. o = Charlson Comorbidity Index-derived poor health state.
Conclusion
This study found that a small percentage of RC/AL residents are in good health and highly likely to benefit from preventive services requiring a 5- to 10-year life expectancy to see benefit. Many residents have possible, but uncertain, benefit from such services. For these residents, providers should individualize their discussion around the balance of risks and benefits in the context of the resident’s values (Walter & Covinsky, 2001). Other work examining RC/AL resident health states either have not used representative national samples (Hawes, Phillips, Rose, Holan, & Sherman, 2003; Moore et al., 2014) or have used only counts of comorbidities or functional status (De Gagne, So, Oh, Park, & Palmer, 2013; Wheaton, Ford, Cunningham, & Croft, 2015), but not established mortality indices.
In general, we found RC/AL residents are less likely to benefit from screening than community-dwelling older adults. National Health Interview Survey that used the 9-year mortality index found 30% of adults aged 65 and older in good health, with an additional 32% in intermediate health (Royce, Hendrix, Stokes, Allen, & Chen, 2014). The RC/AL population is therefore less healthy than community-dwelling older adults, but that said, screening requires individualization.
No preventive service is without risk (Pignone, Rich, Teutsch, Berg, & Lohr, 2002); however, studies of more invasive services, such as CRC screening, reveal that although healthy older adults do experience harms from screening (e.g., pain, worry, potential complications), they recover over time (Kistler et al., 2011). Refraining from screening those in poor health will reduce overuse and minimize the harms of these services (Harris, Wilt, & Qaseem, 2015). Although only 10%–15% of RC/AL residents are highly likely to benefit from preventive services requiring a 5- to 10-year life expectancy, for many others (11%–70%, depending on the measure used) some benefit may exist. The standard of care for those individuals for whom the benefit of a preventive service is uncertain is shared decision making (Elwyn et al., 2012). For these residents, providers should individualize their discussion around the balance of risks and benefits in the context of the resident’s values (Walter & Covinsky, 2001).
One limitation to this work was the imputation of variables based on other sources of data that could have resulted in misclassification. Given that smoking, for example, is likely to be correlated with other comorbidities such as coronary heart disease, random assignment of smoking status (after conditioning on gender) could be expected to result in fewer high scores than would result from knowing true smoking status (which would trend the groups toward the null). In addition, we initially intended to focus on CRC screening, and so excluded those with a prior history of CRC, and those younger than 60 years. These exclusions limit our ability to comment on these groups, which represent a minority (9%) of the RC/AL population.
In spite of these limitations, the prevalence data reported herein are useful to inform preventive and other health care needs of the broad population of RC/AL residents. Evidence quantifying the rates of inappropriate screening in RC/AL is scant. Given the variety of health care providers in RC/AL and the case mix of the resident population, inappropriate screening may be particularly high. In two large RC/AL settings, we found both underuse and overuse of screening (Moore et al., 2014). Future research is advised to examine how often appropriate provision of preventive services occur on a larger scale, and whether shared decision making occurs for those who need it. Furthermore, the methodology used in this study can provide an approach to determining appropriateness of care for RC/AL residents as research into this population continues to grow.
Funding
This research was supported, in part, by Award K12 HS19468-01 from the Agency for Healthcare Research and Quality awarded to the UNC Mentored Career Development Program in Comparative Effectiveness Research, and by the UNC Lineberger Comprehensive Cancer Center (to Dr. Kistler). This research also was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant KL2TR000084.
Acknowledgments
This research was conducted as one of the Collaborative Studies of Long-Term Care through the Cecil G. Sheps Center for Health Services Research, University of North Carolina at Chapel Hill. Dr. Kistler had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The content is solely the responsibility of the authors and does not necessarily represent the official views of AHRQ or the NIH. None of the authors have any conflict of interest, either personal or financial, to report.
References
- Bell B. P. Manos M. M. Zaman A. Terrault N. Thomas A. Navarro V. J., & Sofair A. N (2008). The epidemiology of newly diagnosed chronic liver disease in gastroenterology practices in the United States: Results from population-based surveillance. The American Journal of Gastroenterology, 103, 2727–2736; quiz 2737. doi:10.1111/j.1572-0241.2008.02071.x [DOI] [PubMed] [Google Scholar]
- Caffrey C. Sengupta M. Park-Lee E. Moss A. Rosenoff E., & Harris-Kojetin L (2012). Residents living in residential care facilities: United States, 2010. NCHS Data Brief, 1–8. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/22617169 [PubMed]
- Charlson M. Szatrowski T. P. Peterson J., & Gold J (1994). Validation of a combined comorbidity index. Journal of Clinical Epidemiology, 47, 1245–1251. doi:0895-4356(94)90129-5 [pii] [DOI] [PubMed] [Google Scholar]
- De Gagne J. C. So A. Oh J. Park S., & Palmer M. H (2013). Sociodemographic and health indicators of older women with urinary incontinence: 2010 National Survey of Residential Care Facilities. Journal of the American Geriatrics Society, 61, 981–986. doi:10.1111/jgs.12258 [DOI] [PubMed] [Google Scholar]
- de Groot V. Beckerman H. Lankhorst G. J., & Bouter L. M (2003). How to measure comorbidity. A critical review of available methods. Journal of Clinical Epidemiology, 56, 221–229. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12725876 [DOI] [PubMed] [Google Scholar]
- Elwyn G. Frosch D. Thomson R. Joseph-Williams N. Lloyd A. Kinnersley P., … Barry M (2012). Shared decision making: A model for clinical practice. Journal of General Internal Medicine, 27, 1361–1367. doi:10.1007/s11606-012-2077-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal K. M. Carroll M. D. Kit B. K., & Ogden C. L (2012). Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA, 307, 491–497. doi:10.1001/jama.2012.39 [DOI] [PubMed] [Google Scholar]
- Harris R. P. Wilt T. J., & Qaseem A (2015). A Value Framework for Cancer Screening: Advice for High-Value Care From the American College of Physicians. Annals of Internal Medicine, 162, 712–717. doi:10.7326/M14-2327 [DOI] [PubMed] [Google Scholar]
- Hawes C. Phillips C. D. Rose M. Holan S., & Sherman M (2003). A national survey of assisted living facilities. The Gerontologist, 43, 875–882. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/14704387 [DOI] [PubMed] [Google Scholar]
- Helmick C. G. Felson D. T. Lawrence R. C. Gabriel S. Hirsch R. Kwoh C. K., … National Arthritis Data Workgroup. (2008). Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis and Rheumatism, 58, 15–25. doi:10.1002/art.23177 [DOI] [PubMed] [Google Scholar]
- Holmes H. M. Hayley D. C. Alexander G. C., & Sachs G. A (2006). Reconsidering medication appropriateness for patients late in life. Archives of Internal Medicine, 166, 605–609. doi:10.1001/archinte.166.6.605 [DOI] [PubMed] [Google Scholar]
- King B. Dube S. Kaufmann R. Shaw L., & Pechacek T (2011). Vital Signs: Current Cigarette Smoking among Adults Aged ≥18 Years - United States, 2005–2010. Retrieved from http://search.proquest.com/docview/893424518?accountid=14244 [Google Scholar]
- Kistler C. E. Kirby K. A. Lee D. Casadei M. A., & Walter L. C (2011). Long-term outcomes following positive fecal occult blood test results in older adults: Benefits and burdens. Archives of Internal Medicine, 171, 1344–1351. doi:10.1001/archinternmed.2011.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence R. C. Felson D. T. Helmick C. G. Arnold L. M. Choi H. Deyo R. A., … National Arthritis Data Workgroup. (2008). Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis and Rheumatism, 58, 26–35. doi:10.1002/art.23176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. J. Boscardin W. J. Stijacic-Cenzer I. Conell-Price J. O’Brien S., & Walter L. C (2013). Time lag to benefit after screening for breast and colorectal cancer: meta-analysis of survival data from the United States, Sweden, United Kingdom, and Denmark. BMJ, 346, e8441. doi:10.1136/bmj.e8441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. J. Lindquist K. Segal M. R., & Covinsky K. E (2006). Development and validation of a prognostic index for 4-year mortality in older adults. JAMA, 295, 801–808. doi:10.1001/jama.295.7.801 [DOI] [PubMed] [Google Scholar]
- Moore K. L. Lewis C. L. Zimmerman S. Ward K. Reed D. Rodriguez R., & Kistler C. E (2014). A pilot study to examine colorectal cancer screening in two assisted living communities. Journal of the American Geriatrics Society, 62, 386–388. doi:10.1111/jgs.12665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss A. J. Harris-Kojetin L. D. Sengupta M. Park-Lee E. Caffrey C. Rosenoff E., … Greene A. M (2011). Design and operation of the 2010 National Survey of Residential Care Facilities. Vital and Health Statistics. Ser. 1, Programs and Collection Procedures, 1–131. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/22486061 [PubMed]
- Munnangi S., & Sonnenberg A (1997). Time trends of physician visits and treatment patterns of peptic ulcer disease in the United States. Archives of Internal Medicine, 157, 1489–1494. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9224228 [PubMed] [Google Scholar]
- Park-Lee E. Caffrey C. Sengupta M. Moss A. J. Rosenoff E., & Harris-Kojetin L. D (2011). Residential care facilities: A key sector in the spectrum of long-term care providers in the United States. NCHS Data Brief, 1–8. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/22617275 [PubMed]
- Pignone M. Rich M. Teutsch S. M. Berg A. O., & Lohr K. N (2002). Screening for colorectal cancer in adults at average risk: A summary of the evidence for the U.S. Preventive Services Task Force. Annals of Internal Medicine, 137, 132–141. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12118972 [DOI] [PubMed] [Google Scholar]
- Royce T. J. Hendrix L. H. Stokes W. A. Allen I. M., & Chen R. C (2014). Cancer screening rates in individuals with different life expectancies. JAMA Internal Medicine, 174, 1558–1565. doi:10.1001/jamainternmed.2014.3895 [DOI] [PubMed] [Google Scholar]
- Schonberg M. A. Davis R. B. McCarthy E. P., & Marcantonio E. R (2011). External validation of an index to predict up to 9-year mortality of community-dwelling adults aged 65 and older. Journal of the American Geriatrics Society, 59, 1444–1451. doi:10.1111/j.1532-5415.2011.03523.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung J. J. Kuipers E. J., & El-Serag H. B (2009). Systematic review: The global incidence and prevalence of peptic ulcer disease. Alimentary Pharmacology & Therapeutics, 29, 938–946. doi:10.1111/j.1365-2036.2009.03960.x [DOI] [PubMed] [Google Scholar]
- Thompson S. G. Ashton H. A. Gao L. Buxton M. J. Scott R. A. P., & On Behalf of the Multicentre Aneurysm Screening Study Group. (2012). Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. British Journal of Surgery, 99, 1649–1656. doi:10.1002/bjs.8897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Census Bureau. (2013). State and County QuickFacts Retrieved August 5, 2015, from http://quickfacts.census.gov/qfd/states/00000.html
- Walter L. C., & Covinsky K. E (2001). Cancer screening in elderly patients: A framework for individualized decision making. JAMA, 285, 2750–2756. doi:jsc00476 [pii] [DOI] [PubMed] [Google Scholar]
- Wheaton A. G. Ford E. S. Cunningham T. J., & Croft J. B (2015). Chronic obstructive pulmonary disease, hospital visits, and comorbidities: National Survey of Residential Care Facilities, 2010. Journal of Aging and Health, 27, 480–499. doi:10.1177/0898264314552419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. (2013). Memorandum Report: Hospitals’ Use of Observation Stays and Short Inpatient Stays for Medicare Beneficiaries, OEI-02-12-00040. Office of the Inspector General. [Google Scholar]
- Zimmerman S. Gruber-Baldini A. L. Sloane P. D. Eckert J. K. Hebel J. R. Morgan L. A., … Konrad T. R (2003). Assisted living and nursing homes: Apples and oranges? The Gerontologist, 43, 107–117. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12711731 [DOI] [PubMed] [Google Scholar]