Abstract
Juvenile idiopathic arthritis (JIA) encompasses all forms of chronic idiopathic arthritis that arise before age 16. Previous studies have found JIA to be associated with lower Fc galactosylation of circulating IgG, but the overall spectrum of glycan changes and the net impact on IgG function are unknown. Using ultra performance liquid chromatography (UPLC), we compared IgG glycosylation in 54 subjects with recent-onset untreated JIA with 98 healthy pediatric controls, paired to biophysical profiling of affinity for 20 IgG receptors using a high-throughput multiplexed microsphere assay. Patients with JIA exhibited an increase in hypogalactosylated and hyposialylated IgG glycans, but no change in fucosylation or bisection, together with alteration in the spectrum of IgG ligand binding. Supervised machine learning demonstrated a robust capacity to discriminate JIA subjects from controls using either glycosylation or binding data. The binding signature was driven predominantly by enhanced affinity for Fc receptor like protein 5 (FcRL5), a noncanonical Fc receptor expressed on B cells. Affinity for FcRL5 correlated inversely with galactosylation and sialylation, a relationship confirmed through enzymatic manipulation. These results demonstrate the capacity of combined structural and biophysical IgG phenotyping to define the overall functional impact of IgG glycan changes and implicate FcRL5 as a potential cellular sensor of IgG glycosylation.
Keywords: FcRL5, IgG glycan, IgG receptor binding, juvenile idiopathic arthritis, machine learning
Introduction
Juvenile idiopathic arthritis (JIA), encompasses all forms of chronic idiopathic arthritis diagnosed before age 16 (Prakken et al. 2011). While the mechanisms leading to JIA remain incompletely defined, a role for antibodies is suggested by the presence of anti-keratin, anti-nuclear and anti-DEK antibodies in many patients and by characteristic decreases in galactosylation of circulating IgG (Moore et al. 1982; Parekh RP et al. 1988; Sumar et al. 1991; Bond et al. 1996; Hromadnikova et al. 2002; Mor-Vaknin et al. 2011; Ercan et al. 2012a). IgG Fc domain glycosylation is a key post-translational modification that modulates antibody binding to Fc receptors and consequently influences antibody effector function (Raju 2008; Anthony and Ravetch 2010). In humans, each IgG heavy chain is decorated in the Fc region with one of approximately 30 different sugar forms that vary in mannose, bisecting N-acetylglucosamine (GlcNAc), fucose, galactose and sialic acid content. The increase in agalactosylated (G0) IgG evident in JIA (Sumar et al. 1991) is observed also in adult rheumatoid arthritis (RA) (Parekh et al. 1985; Ercan et al. 2010a) systemic lupus erythematosus (Vuckovic et al. 2015), Lambert-Eaton syndrome (Selman et al. 2011), ANCA vasculitis (Holland et al. 2002, 2006), cancer (Kodar et al. 2012), infection (Moore et al. 2005) and aging (Kristic et al. 2014).
Whether hypogalactosylated/sialylated IgG contributes to the pathogenesis of inflammatory arthritis is uncertain. Elevation in G0 glycans correlates with arthritis severity as measured by clinical scores (Ercan et al. 2012b), and can predate overt joint swelling (Ercan et al. 2010b; Rombouts et al. 2015), suggesting a potential causative role. Further, aberrant hypogalactosylation tends to normalize during pregnancy-induced RA remission, recurring at the time of disease relapse (Rook et al. 1991; van de Geijn et al. 2009). The potential of G0 IgG species to contribute to disease is suggested by the observations that both agalactosylated IgG (Rademacher et al. 1994) and blockade of sialylation in B cells (Ohmi et al. 2016) enhance collagen-induced arthritis in mouse models. Recent studies in human RA cohorts have further strengthened these observations by showing that repressed sialyltransferase activity in antibody-producing cells is associated with the progression of active RA (Pfeifle et al. 2017). In sum, such studies favor the possibility that hypogalactosylated/hyposialylated IgG contribute to the pathogenesis of RA.
The mechanistic significance of these glycan changes has been controversial. While early studies found G0 IgG to exhibit enhanced binding to the complement-activating mannose-binding lectin (MBL) (Malhotra et al. 1995; Arnold et al. 2006), this pathway of complement activation appears to play little role in murine IgG-mediated arthritis (Nimmerjahn et al. 2007). Nevertheless, G0 IgG may also activate complement through the classical and especially the alternative pathway, thought to be more directly relevant for disease (Banda et al. 2008; 2012). Highly sialylated IgG is less effective at mediating complement-dependent cytotoxicity (Quast et al. 2015). Further, discovery of the immunosuppressive properties of sialylated IgG Fc (Kaneko et al. 2006) has suggested an additional mechanism, in which reduced IgG sialylation blunts immunosuppressive signaling mediated through a pathway involving DC-SIGN (Anthony et al. 2008, 2011). Finally, highly galactosylated (G2) IgG can block pro-inflammatory chemokine receptor signaling (Karsten et al. 2012). Thus, lack of sialic acid and/or galactose is likely to enhance the pro-inflammatory potential of IgG.
Intrigued by the possibility that differential glycosylation may tune the effector function of IgG and render IgG more inflammatory or less immunosuppressive in the context of JIA, we performed a comparative study of serum IgG purified from healthy and JIA-affected children. IgG glycosylation was defined by ultra-performance liquid chromatography (UPLC). Binding to IgG receptors including Fc receptors, C-type lectins and complement cascade-initiating proteins was characterized using a multiplex microsphere-based assay (Boesch et al. 2014). Machine learning methods were used to model and discover relationships between JIA status, IgG glycosylation and antibody interactions with Fc receptors. Both IgG glycan and Fc receptor binding data could robustly differentiate JIA-affected subjects from controls. JIA IgG demonstrated a reduction in both glycosylation and sialylation, while the Fc binding signature was driven principally by enhanced affinity of JIA IgG for Fc receptor-like protein 5 (FcRL5). These changes were co-linear, and experimental manipulation confirmed that affinity for FcRL5 was mediated through both galactose and sialic acid, identifying FcRL5 as a previously unrecognized cell surface sensor of IgG glycosylation status.
Results
IgG glycosylation in juvenile idiopathic arthritis
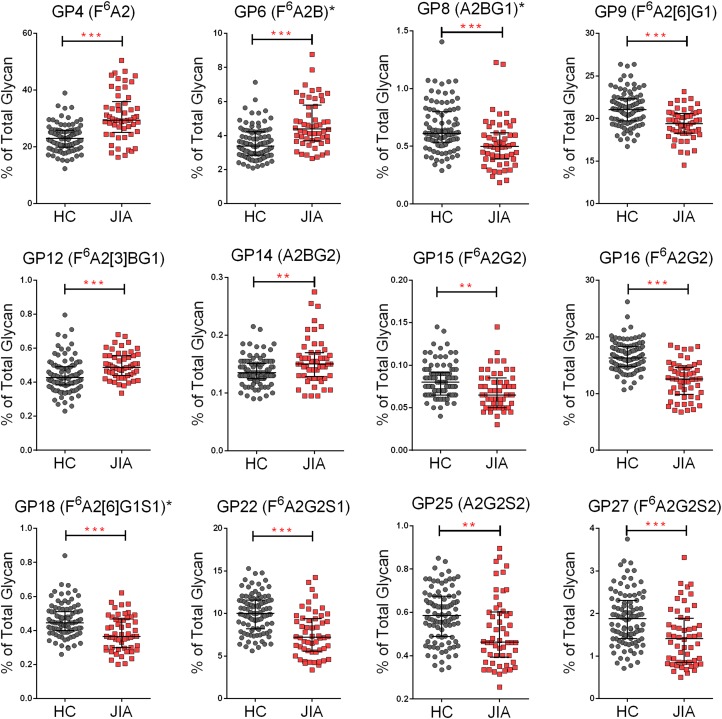
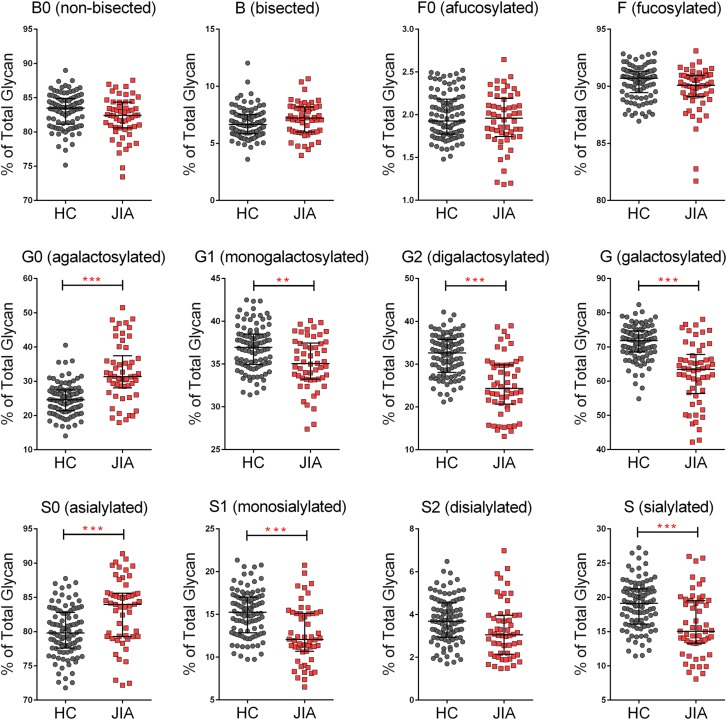
To model antibody glycan-function relationships, we analyzed samples from 98 healthy control children and 54 JIA patients. Antibody glycans were profiled by high-throughput UPLC (Stockmann et al. 2013) (Supplementary data, Figure 1). Antibody binding to a suite of Fc receptors and lectins (Supplementary data, Table 1) was characterized by a customized microsphere array assay (Brown et al. 2012; Boesch et al. 2014). We first sought to compare the IgG glycan profiles in JIA subjects with those observed in healthy controls. For IgG glycan profiles, 28 glycan peaks were automatically integrated and 14 derived aggregated glycan features were calculated based on peak compositions (Supplementary data, Table 2). Of the 28 individual glycan features, 12 showed significant differences between JIA subjects and healthy control children when adjusted for multiple comparisons (Figure 1). IgG from JIA subjects was less likely to bear galactose and sialic acid (NeuNAc) groups, demonstrated by increases in F6A2, F6A2B and F6A2[3]BG1 glycoforms. IgG from healthy controls was more fully galactosylated and sialylated, reflected in increases in F6A2[6]G1, F6A2G2, F6A2[6]G1S1, F6A2G2S1 and F6A2G2S2. Overall, patients with JIA demonstrated reduced IgG galactosylation and sialylation, without alteration in the presence of bisecting GlcNAc or core fucose (Figure 2).
Fig. 1.
IgG glycan compositions in healthy controls and JIA subjects. IgG glycoforms with variable prevalences in healthy control (HC) and JIA subjects. Significant differences were evaluated with a Student t test with a nonpaired, nonequal variance, two-sided hypothesis testing, and a confidence interval of 0.95. Calculated P values were adjusted by the Benjamini Hochberg False Discovery Rate (FDR) method (*P < 0.05, **P < 0.01, ***P < 0.001). This figure is available in black and white in print and in color at Glycobiology online.
Fig. 2.
Comparison of derived glycan compositions in healthy controls and JIA subjects. Derived glycan compositions were calculated and compared between healthy control (HC) and JIA subjects (*P < 0.05, **P < 0.01, ***P < 0.001). This figure is available in black and white in print and in color at Glycobiology online.
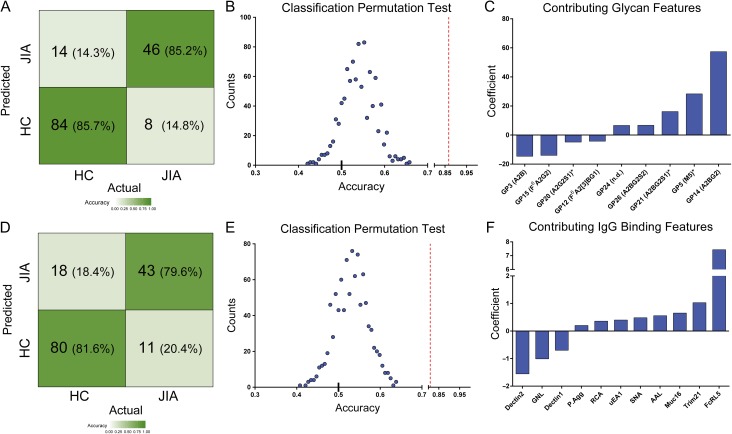
We then sought to determine whether glycosylation differences could distinguish JIA patients from controls. A balanced classifier was trained to discriminate healthy controls from JIA subjects in the setting of 10-fold cross-validation using the elastic net generalized linear algorithm (Friedman et al. 2010). The resulting model successfully classified 85% of subjects (Figure 3A). Permutation tests conducted using the same approach but randomized subject labels demonstrated the robustness of the classifier, indicating that it reliably captured the quantitative contributions of different glycan compositions to meaningful subject group predictions (Figure 3B). In these models, features related to galactose and sialic acid content made strong positive contributions to the healthy phenotype (A2BG2, A2BG2S1, A2BG2S2 and galactosylated glycan) (Figure 3C). Collectively, the generalized classifier further supported the importance of agalactosylated IgG (G0) and lower sialic acid content in defining JIA.
Fig. 3.
Classification of healthy controls and JIA subjects. (A,D) Confusion matrices representing the cross-validated performance of an elastic net classifier trained to discriminate healthy control from JIA subjects using either glycan (A) or IgG phenotypic (D) data. (B,E) The accuracy of classification results in 1000-iterations of 10-fold cross-validation with permuted (blue) as compared to true subject labels (red dashed line) using either glycan (B) or IgG phenotypic (E) data. (C,F) The identity and relative contributions of major glycan (C) or IgG binding (F) features contributing to the classification. Features with coefficients smaller than 5% of the maximum coefficient were omitted. This figure is available in black and white in print and in color at Glycobiology online.
We then tested whether glycan features could differentiate among International League of Associations for Rheumatology (ILAR) subtypes (Petty et al. 2004). Five subgroups of JIA were represented in the sample set: polyarticular rheumatic factor negative (Poly-RF−), polyarticular rheumatic factor positive (Poly-RF+) oligoarticular (Oligo), enthesitis-related arthritis (ERA), and juvenile psoriatic arthritis (JPsA). However, neither individual nor derived aggregated glycan prevalences exhibited statistically significant differences between subjects with different ILAR subtypes (all adjusted P values exceeded 0.05). Although group sizes for these comparisons were small, no trends were observed to suggest that important differences would have emerged through analysis of a larger sample set (Supplementary data, Figure 3).
IgG function in juvenile idiopathic arthritis
To elucidate the functional consequences of differential IgG glycan profiles, the ability of polyclonal IgG to interact with functionally relevant antibody receptors and lectins was characterized using a multiplexed microsphere array. Lectins and Fc receptors were coupled to microspheres, and the interaction between polyclonal IgG and target proteins quantified by detection of bound IgG with a secondary antibody (Boesch et al. 2014).
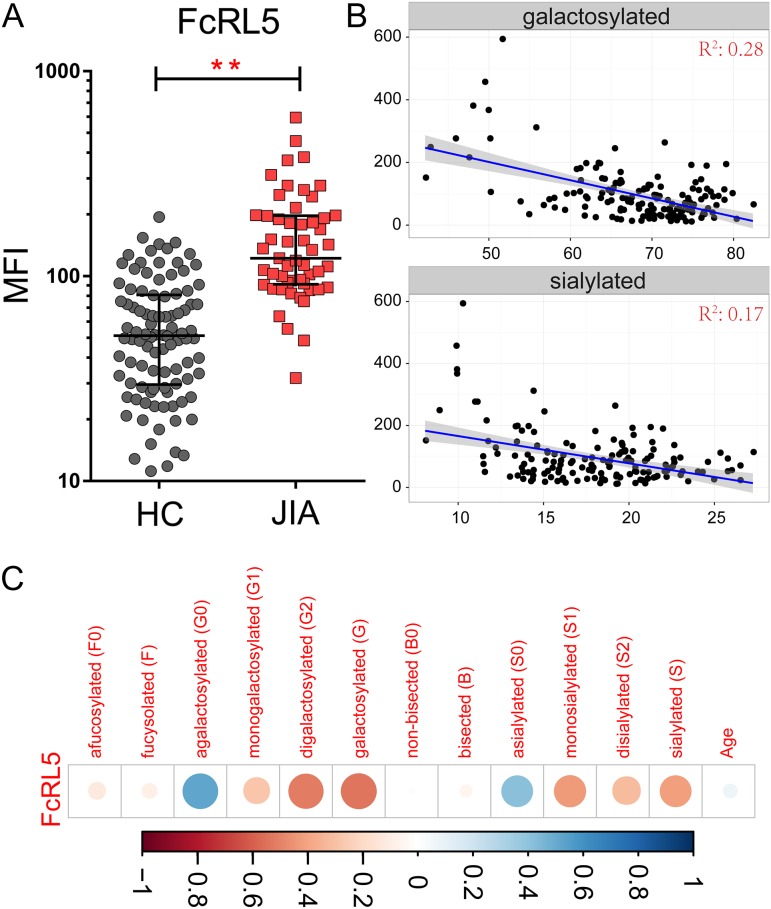
A similar generalized classifier was used to classify JIA status using IgG binding data, resulting in an 80% classification accuracy (Figure 3D), exceeding the essentially random performance observed with permuted data (Figure 3E). Inspection of the features contributing significantly to the classification disclosed heavy reliance on one specific feature, binding to Fc-Receptor Like protein 5 (FcRL5) (Figure 3F). The coefficient weight of FcRL5 was 500% greater than any of the other features. Consistent with this strong contribution, the difference in binding to FcRL5 between subject groups was highly significant, with JIA subjects exhibiting roughly a 2.7-fold increase in recognition by this antibody receptor (Figure 4A).
Fig. 4.
IgG binding to FcRL5 distinguishes subjects and is associated with IgG glycan composition. (A) Median Fluorescent Intensity (MFI) of IgG binding to FcRL5 in healthy controls and JIA subjects (**P < 0.01). (B) Scatter plot between the MFI of IgG binding to FcRL5 (y-axis) and prevalence (% of total IgG glycan) of galactosylated/ sialylated IgG glycan content (x-axis). The linear fit of the scatter plot is presented as a blue line, with the confidence interval colored in gray. (C) Correlation coefficient heatmap presenting the magnitude and direction of correlation (Pearson) between derived antibody glycan compositions and binding to FcRL5. Blue denotes strong positive correlation, and red denotes strong negative correlation. This figure is available in black and white in print and in color at Glycobiology online.
Combining IgG glycan data and IgG binding data did not significantly improve the observed classification accuracy (Classification accuracy: 84.2%). IgG binding was comparable among ILAR subtypes of JIA (Supplementary data, Figure 2). When glycan, IgG binding, or the combination of both data types was used to train a classifier of ILAR subtypes, poor performance was observed. Collectively, these results indicate notable differences between healthy and JIA subjects, but a lack of differentiation among JIA subtypes as classified conventionally.
The binding of IgG to FcRL5 is associated with glycan composition
The significant differences observed between groups and the observed magnitudes and directions of glycan and IgG binding features in classification models suggested that the hypogalactosylated IgG could bind more effectively to FcRL5. Accordingly, we evaluated the correlation between FcRL5 binding data and IgG glycan composition. Interestingly, FcRL5 binding was significantly correlated with the prevalence of both agalactosylated and asialylated species (Figure 4B), and inversely correlated with the prevalence of various galactosylated (monogalactosylated, digalactosylated and galactosylated) and sialylated species (monosialylated, disialylated and sialylated) (Figure 4C). The level of galactosylated species demonstrated a marginally stronger correlation coefficient with FcRL5 binding signal (−0.54, P = 1.34 × 10−4) as compared to the sialylated species (−0.41, P = 1.10 × 10−4), and both observations were robust to the removal of apparent outliers identified by visual inspection. These observations are linked by the fact that the addition of sialic acid requires the presence of galactose. In contrast, no correlative relationships were observed between FcRL5 and glycan bisection or fucosylation (Supplementary data, Figure 3).
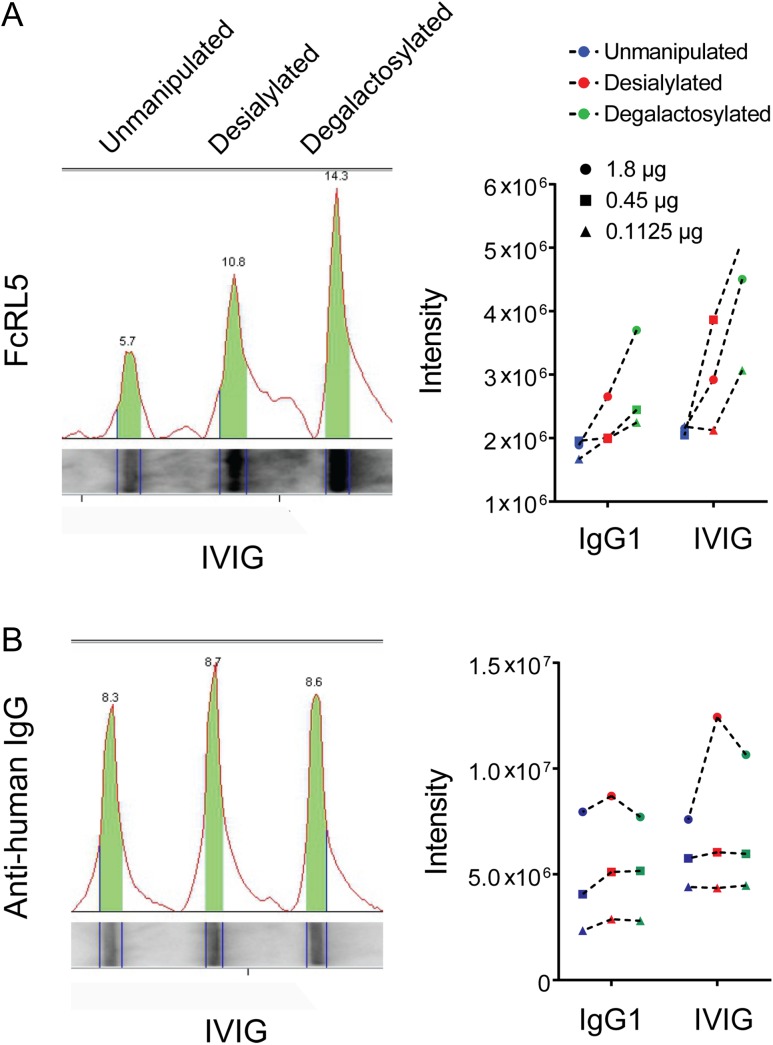
To directly test the role of glycosylation in FcRL5 binding, polyclonal IgG1 and IVIG were treated with neuraminidase to remove sialic acid alone; neuraminidase and galactosidase to remove both sialic acid and galactose; or control (IgG exposed to similar conditions but without enzymes) (Ackerman et al. 2013). Reduction in sialic acid, and reduction in both sialic acid and galactose, potentiated binding to recombinant FcRL5 in a dot-blot assay (Figure 5A and B). Similarly, binding to beads conjugated with FcRL5 increased for both desialylated and degalactosylated antibody pools (Supplementary data, Figure 4). Taken together, these results indicate that IgG binding to FcRL5 is enhanced by reduction in both sialic acid and galactose, potentially implicating this receptor in the functional impact of disease-associated IgG glycan changes associated with conditions such as JIA.
Fig. 5.
IgG binding to FcRL5 is influenced by IgG glycan compositions. (A) Un-manipulated, neuraminidase-treated (desialylated) and neuraminidase- and galactosidase-treated (degalactosylated) IgG pools (IgG1 and IVIG) were slot blotted and the impact of differential glycosylation assessed by detection with FcRL5 (left, IVIG shown). Band intensities were quantified for both polyclonal IgG1 and IVIG at three different antibody concentrations (right). (B) As a test of equivalent antibody loading, the same membrane was stripped and re-blotted to detect (left, IVIG shown) and quantify (right) total IgG via detection with an anti-human IgG secondary. This figure is available in black and white in print and in color at Glycobiology online.
Discussion
JIA is one of the most common autoimmune diseases of children, with a prevalence estimated at 1 in 1000 children (Gare 1998; Manners and Bower 2002) and consequences that may include joint destruction and permanent disability (Bowyer et al. 2003). Previous studies had identified an increase in hypogalactosylated IgG in all JIA subtypes, but additional glycan changes and the resulting consequences for IgG effector functions remain unknown. Here, we show that sialylation is reduced in JIA in addition to galactosylation. We could build robust classifiers that identify distinguishing features between healthy control and JIA-affected subjects. Application of machine learning tools allowed systematic evaluation of the ability of combinations of glycosylation and Fc receptor binding features to classify subjects based on JIA status, and provided specific coefficient weights for each contributing feature. Within the glycosylation data, hypogalactosylated/asialylated IgG glycoforms were the major contributors to successful subject classification. In models learned from Fc receptor binding data, one specific feature, IgG binding to FcRL5, contributed dominantly to the classifier, with a coefficient weight five times greater than any other.
Elevated agalactosylated IgG has been associated with several inflammatory diseases, but the consequences of this increase are poorly understood. In particular, it remains unclear whether elevated expression of hypogalactosylated/asialylated IgG in JIA is a cause or effect of disease. Evidence favoring the former includes the observation that agalactosylated antibodies exhibit greater arthritogenicity in various animal models (Rademacher et al. 1994; Banda et al. 2006, 2007, 2008). Longitudinal studies likewise support a potential role in pathogenesis, since aberrant glycosylation is observed months or years prior to the diagnosis of RA in adults (Ercan et al. 2010b; Rombouts et al. 2015), and RA remission in pregnancy and postpartum recurrence correlate with reduction and then recurrence of aberrant IgG Fc glycosylation, without accompanying changes in Fab glycans (van de Geijn et al. 2009; Bondt et al. 2016). While animal data have implicated IgG sialylation changes in IgG pathogenicity (Kaneko et al. 2006; Ohmi et al. 2016), mechanism(s) associated with this role remain controversial (Crispin et al. 2013; Campbell et al. 2014). In humans, RA- and (per this report) JIA-associated glycan variation encompasses both galactose and sialic acid, whereas in the murine system glycan changes appear relatively restricted to sialylation (Pfeifle et al. 2017). Further, in human pregnancy-induced RA remission, wherein changes to both IgG Fc galactosylation and sialylation levels are observed, disease severity is associated with galactosylation independently of sialylation (Bondt et al. 2013). Thus, whether IgG glyco-variation is a cause or effect of human disease, and whether variation in sialic acid or galactose content is more consequential, remains unresolved.
Aberrant IgG glycosylation can result from environmental exposures, including cytokines and hormones, as well as potentially from differential maturation states of B cells (Omtvedt et al. 2006; Wang et al. 2011; Ercan et al. 2017). How patients with JIA, RA and other inflammatory diseases develop aberrant IgG glycosylation remains to be determined. However, even if IgG glycan changes are driven by the inflamed milieu, it nevertheless is likely that these changes will in turn modulate the inflammatory process through their impact on IgG function. Our binding data implicate a previously unexpected pathway by which such an effect could be mediated: enhanced binding of hyposialylated and/or hypogalacyosylated IgG to FcRL5. FcRL5 is a B cell surface differentiation marker (Davis 2015; Sullivan et al. 2015). FcRL5 contains both immune-receptor tyrosine based inhibition motif (ITIM) and immune-receptor tyrosine based activation motif (ITAM) sequences, and has been shown to interact with the IgG Fc region in a subclass- and glycan-dependent manner (Wilson et al. 2012; Franco et al. 2013). FcRL5 ligation can suppress MAPK activation, calcium mobilization, and whole-cell pTyr in B cells by recruiting SHP-1 and SHP-2 phosphatase (Ehrhardt et al. 2003; Haga et al. 2007; Kochi et al. 2009; Jackson et al. 2010; Zhu et al. 2013). Mutation of the ITIM domain tyrosines in FcRL5 results in enhanced BCR-mediated calcium mobilization (Haga et al. 2007), and cross-linking of TLR9 and FcRL5 enhances B cell proliferation and isotype switching (Dement-Brown et al. 2012). Most previous work has focused on the ITIM, leaving the role of the ITAM as an activator of B cells unclear. Intriguingly, previous studies have found that whole-blood mRNA expression levels of FcRL5 were an effective biomarker of the efficacy of B cell depletion using rituximab for RA treatment (Owczarczyk et al. 2011). Moreover, genetic studies have indicated associations between SNPs in the FCRL locus and chronic immune diseases in different populations, including RA (Kochi et al. 2009), autoimmune Addison’s disease (Owen et al. 2007), multiple sclerosis (Martinez et al. 2007), autoimmune thyroid disease (Burton et al. 2007), and JIA (Ramirez-Bello et al. 2013). While this genetic predisposition is believed to be driven by the augmented expression of a related protein, FcRL3, with at least somewhat overlapping functionality (Kochi et al. 2005; Ehrhardt and Cooper 2011; Bajpai et al. 2012; Li et al. 2014), in combination with our findings these observations highlight the potential relevance of the FcRL family, including FcRL5, in autoimmune diseases such as JIA.
Given the presence of both ITIM and ITAM domains in FcRL5, reduced galactosylation and sialylation in JIA IgG could represent either a negative loop to constrain further B cell activation or a feed-forward loop that promotes B cell activity. The net effect of the interaction of aberrantly glycosylated IgG on FcRL5-expressing cells will help to identify whether this receptor could be an attractive therapeutic target, manipulated for example through depletion of FcRL5+ cells or blockade of FcRL5 binding or signaling. Additional work is needed to determine the relevant signaling pathway(s) and the effect of FcRL5 ligation in specific contexts.
The current ILAR classification divides JIA into six principal subtypes (Petty et al. 2004). Similar IgG Fc glycosylation, assessed as the ratio of G0 to G1 glycans, has been reported among JIA subtypes (Ercan et al. 2012a), but a detailed comparison of IgG-specific glycans as well as antibody receptor binding profiling had not been conducted. In this study, wherein all forms except systemic JIA were represented, neither IgG glycans nor IgG binding profiles exhibited significant variation among JIA subtypes. We also attempted to build two multinomial classifiers to evaluate whether IgG glycan features and IgG binding features segregate with ILAR subcategories of JIA, but could identify no association. Our cohort was too small to suggest another typing schema based on glyco-variation. However, other lines of evidence have also suggested that the classification system is imperfect, including by HLA association data, blood mRNA profiling, and clinical phenotyping (Nigrovic et al. in press). Ongoing efforts are needed to more robustly classify biologically defined JIA subtypes.
Our study has several limitations. First, study subjects ranged in age from 9 months to 16 years. While there was not a significant difference in the age between patients and controls (P = 0.5), healthy controls were not individually matched to JIA patients by age, which might confound study results as age can impact humoral immune responses (LeMaoult et al. 1997), and antibody glycosylation profiles are known to vary with age (de Haan et al. 2016). Second, our microsphere studies measure the effect of glycan changes on binding of free IgG. We cannot exclude the possibility that alternate binding properties might be encountered in antibodies engaged with antigen, or that are part of an immune complex. Finally, UPLC analysis of IgG glycans as employed here measures those liberated from the Fab region as well as from the Fc region. Fab glycans are generally fully developed, often to G2S2 forms, even in the context of inflammation (Holland et al. 2006). Thus Fab glycan contamination would be expected to introduce only conservative bias into our analysis. Moreover, the changes we have identified are consistent with those identified by Fc-specific glycopeptide analyses in RA (Scherer et al. 2009). IgG glycophenotyping findings proved co-linear with those from the microsphere assay, which is Fc specific, confirming experimentally that Fab glycans are unlikely to represent an important unmeasured confounder in our studies.
Conclusion
Taken together, these data demonstrate that JIA is associated with increases in agalactosylated and asialylated IgG, and that these glycan features can be employed to distinguish JIA patients from healthy controls. Further, we show that glycan changes result in altered IgG binding to FcRL5, confirming that glycan aberrancy in JIA translates into a net change in the effector potency of the antibody pool. Together with previous work linking FcRL5 to therapeutic responses in RA (Owczarczyk et al. 2011), our biophysical evidence of a direct impact on IgG glycans in FcRL5 binding identifies a potential new feedback loop in arthritis biology. Additional studies will be required to evaluate whether ligation of FcRL5 by aberrantly glycosylated IgG modulates B cell fate and function in order to play a determinative role in the pathogenesis of JIA and other autoimmune diseases.
Materials and methods
Subjects
Subjects with untreated recent-onset JIA (ICD10 M08.00) (n = 54) were recruited through the Boston Children’s Hospital JIA Registry. Control samples (n = 158) were obtained through the same registry, from discarded samples after routine lead testing, and from a prospective consented study of healthy children (Ercan et al. 2012a). Subjects ranged in age from 9 months to 16 years. The study was reviewed and approved by the Institutional Review Boards of Boston Children’s Hospital and Dartmouth College.
Multiplexed receptor and lectin microarray
In order to assess the ability of each IgG sample to interact with antibody binding proteins including human Fc receptors and lectins, we customized a multivariate microsphere assay from a previously described method (Brown et al. 2012; Boesch et al. 2014). In brief, Fc receptors and lectins (Supplementary data, Table 1) were covalently coupled to fluorescently coded magnetic microspheres as described. IgG was enriched from serum using Melon Gel purification kit (ThermoFisher Scientific, 45206) according to the manufacturer’s instructions, diluted, and incubated with receptor/lectin conjugated microspheres (500 microspheres of each specificity) in a total 50 μL volume of incubation buffer (20 mM Tris pH7.5, 2 mM CaCl2, 150 mM NaCl, and 0.05% (v/v) Tween-20), in black, clear bottom 384-well plates (Greiner Bio One, 781906) covered by micro-plate adhesive film (USA Scientific). Samples were mixed by ultrasonic shaking for 15 s, followed by the incubation on plate shaker (IKA, MTS 2/4) for 4 h at room temperature. Plates were then washed five times with incubation buffer using an automated plate washer (Biotek, 405 Select TS). To detect antibody bound to each receptor/lectin, a volume of 50 μL of anti-human IgG PE-conjugated antibody (Southern Biotech, 9040-09) was added to each well at a concentration of 0.65 μg/mL, followed by incubation at room temperature with shaking for 1 h. Finally, the plates were washed 5 times with and resuspended in Luminex sheath fluid buffer. Plates were sonicated for 15 s prior to data acquisition on a FlexMap 3D (Luminex, Bio-Plex Manager 5.0, Bio-Rad). The net median fluorescence intensity (MFI) was determined by subtracting the background signal (beads in the absence of antibody) from each test sample.
High-throughput glycan analysis
UPLC analysis was performed as described previously (Stockmann et al. 2013). IgG purification, glycan release, labeling and clean-up were performed on the Hamilton STARlet robotic liquid handler (Hamilton, Reno, NV). IgG was purified from serum samples using Protein G plates (ThermoFisher Scientific, 45204). IgG N-glycans were released by digestion with recombinant N-glycosidase F (ProZyme, CA), and then labeled with 2-aminobenzamide (Royle et al. 2008), cleaned-up using solid phase extraction and dried. Purified glycans were then separated by HILIC-UPLC on a Waters H-Class instrument (Waters, Milford, MA) with a Waters BEH glycan column (150 × 2.1 mm, 1.7 μm BEH particles) using separation settings as described previously (Stockmann et al. 2013). Data was processed automatically: chromatograms were integrated into 28 peaks (Pucic et al. 2011), as described in Supplementary data, Table 2, and the prevalence of each glycoform or peak was reported as percentage of total glycan or total integrated peak area. Derived glycans (e.g., total agalactosylated glycans) were calculated as described in Supplementary data, Table 3.
Western blot analysis
Soluble FcRL5 with a C-terminal His6 tag was expressed by HEK293 cells and purified using the standard Ni-NTA chromatography. Soluble FcRL5 was then biotinylated on primary amines (EZ-link Sulfo-NHS-Biotin, Thermo Fisher) according to the manufacturer’s instructions. Human serum IgG1 and intravenous Immunoglobulin (IVIG) were treated by neuraminidase, or both neuraminidase and galactosidase respectively, as previously described (Ackerman et al. 2013). For blotting, 18 μg of each sample in 100 μL PBS was loaded onto a nitrocellulose blotting membrane (GE Healthcare) through the dot-blot microfiltration apparatus (Bio-Rad). The membrane was then blocked in blocking buffer (PBS with 3% BSA) on a shaker at room temperature for 1 h. After that, the membrane was washed three times with blocking buffer and incubated with 100 μg/mL biotinylated-FcRL5 at room temperature for 2 h. The membrane was then washed again, prior to incubation with avidin-HRP conjugate at 2.5 μg/mL (Thermo Fisher) for 1 h. Blots were washed three times and developed by the ECL western blotting substrate (Thermo Fisher) and photographed by the Bio-rad imager. The same membrane was then stripped with 0.1 M glycine-HCL pH 3.0 for 1 h and re-blotted with goat anti-human IgG HRP conjugate (Thermo Fisher) and developed and imaged to quantify total IgG. Band intensities were determined by the Image Lab5.0. The size and range of the band was identified by the “Lanes and Bands” tools from the software. Each band’s intensity was then reported and plotted in the GraphPrism.
Statistical analysis
Age is known to impact IgG glycan compositions (Parekh et al. 1988; Kristic et al. 2014). To reduce the impact of age as a factor in our study, the 98 healthy controls that were best age matched to the 54 JIA subjects (P = 0.5) were selected for statistical analysis. Student’s t test analysis was performed by the Stats R software package, version 3.1.2. Both the glycan and the microarray measurements were compared under nonpaired, nonequal variance and two-sided hypothesis testing with a confidence interval of 0.95. Calculated P values were adjusted by the Benjamini Hochberg False Discovery Rate (FDR) method (Benjamini and Hochberg 1995).
Binomial classification models were built by the Glmnet R package (Friedman et al. 2010). To facilitate comparison of model coefficients, antibody microarray binding data was scaled and centered prior to modeling. Antibody glycan data was measured as the percentage of total glycan, thus no normalization was performed before modeling. Binomial classifiers were trained using either glycan or microarray measurements with the elastic net mixing parameter at 0.4, and balanced observation weights. Coefficient weights of each IgG measurements contributing to the model were evaluated at a specific tuning parameter value (lambda min), which yielded the minimum cross-validated mis-classification error. The contribution of different features to model predictions was generally stable over variable values of lambda (from lambda min to 1 standard deviation of lambda min). Predictive robustness was compared to model performance when group labels were randomly permuted and models trained with 250 iterations of 10-fold cross-validation. Models with prediction accuracy greater than two standard deviations away from the permutation mean (2 SD + Mean) were accepted.
Abbreviations
ERA, enthesitis-related arthritis; ILAR, International League of Associations for Rheumatology; JIA, Juvenile idiopathic arthritis; JPsA, juvenile psoriatic arthritis; MBL, mannose-binding lectin; MFI, median fluorescence intensity; RA, rheumatoid arthritis; UPLC, ultra-performance liquid chromatography.
Supplementary Material
Acknowledgments
We thank Dr. Mark D. Kellogg from Boston Children’s Hospital for providing study samples.
Supplementary data
Funding
This research was supported in part by R01AI102691 from the National Institute of Allergy and Infectious Diseases (NIAID) (M.E.A.), by the Arthritis National Research Foundation (A.E.), by the NIH Office of Research on Women’s Health, National Institute of Child Health and Human Development, Building Interdisciplinary Research Careers in Women’s Health grant K12 HD051959 (A.E.), by the Rheumatology Research Foundation (P.A.N., M.E.A.), by R21AI099435 and P30 AR070253 (P.A.N.), and by the Fundación Bechara (P.A.N.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest statement
The authors declare no competing financial interests.
Authors' contribution
M.E.A. and P.A.N. conceived, planned and secured funding for the project. I.A.H. and P.A.N. provided study samples. H.D.C., H.S., B.A., C.A.M., A.E. performed experiments and analyzed data. H.D.C. created the figures. H.D.C., M.E.A. and P.A.N. wrote the manuscript. All authors critically reviewed, edited and approved the final version.
References
- Ackerman ME, Crispin M, Yu X, Baruah K, Boesch AW, Harvey DJ, Dugast AS, Heizen EL, Ercan A, Choi I et al. . 2013. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J Clin Invest. 123:2183–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. 2011. Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature. 475:110–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony RM, Ravetch JV. 2010. A novel role for the IgG Fc glycan: The anti-inflammatory activity of sialylated IgG Fcs. J Clin Immunol. 30(Suppl 1):S9–S14. [DOI] [PubMed] [Google Scholar]
- Anthony RM, Wermeling F, Karlsson MC, Ravetch JV. 2008. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc Nat Acad Sci USA. 105:19571–19578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold JN, Dwek RA, Rudd PM, Sim RB. 2006. Mannan binding lectin and its interaction with immunoglobulins in health and in disease. Immunol Lett. 106:103–110. [DOI] [PubMed] [Google Scholar]
- Bajpai UD, Swainson LA, Mold JE, Graf JD, Imboden JB, McCune JM. 2012. A functional variant in FCRL3 is associated with higher Fc receptor-like 3 expression on T cell subsets and rheumatoid arthritis disease activity. Arthritis Rheum. 64:2451–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banda NK, Hyatt S, Antonioli AH, White JT, Glogowska M, Takahashi K, Merkel TJ, Stahl GL, Mueller-Ortiz S, Wetsel R et al. . 2012. Role of C3a receptors, C5a receptors, and complement protein C6 deficiency in collagen antibody-induced arthritis in mice. J Immunol (Baltimore, MD: 1950). 188:1469–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banda NK, Takahashi K, Wood AK, Holers VM, Arend WP. 2007. Pathogenic complement activation in collagen antibody-induced arthritis in mice requires amplification by the alternative pathway. J Immunol (Baltimore, MD: 1950). 179:4101–4109. [DOI] [PubMed] [Google Scholar]
- Banda NK, Thurman JM, Kraus D, Wood A, Carroll MC, Arend WP, Holers VM. 2006. Alternative complement pathway activation is essential for inflammation and joint destruction in the passive transfer model of collagen-induced arthritis. J Immunol (Baltimore, MD: 1950). 177:1904–1912. [DOI] [PubMed] [Google Scholar]
- Banda NK, Wood AK, Takahashi K, Levitt B, Rudd PM, Royle L, Abrahams JL, Stahl GL, Holers VM, Arend WP. 2008. Initiation of the alternative pathway of murine complement by immune complexes is dependent on N-glycans in IgG antibodies. Arthritis Rheum. 58:3081–3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Series B (Methodological). 57:289–300. [Google Scholar]
- Boesch AW, Brown EP, Cheng HD, Ofori MO, Normandin E, Nigrovic PA, Alter G, Ackerman ME. 2014. Highly parallel characterization of IgG Fc binding interactions. MAbs. 6:915–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond A, Alavi A, Axford JS, Youinou P, Hay FC. 1996. The relationship between exposed galactose and N-acetylglucosamine residues on IgG in rheumatoid arthritis (RA), juvenile chronic arthritis (JCA) and Sjogren’s syndrome (SS). Clin Exp Immunol. 105:99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondt A, Selman MH, Deelder AM, Hazes JM, Willemsen SP, Wuhrer M, Dolhain RJ. 2013. Association between galactosylation of immunoglobulin G and improvement of rheumatoid arthritis during pregnancy is independent of sialylation. J Proteome Res. 12:4522–4531. [DOI] [PubMed] [Google Scholar]
- Bondt A, Wuhrer M, Kuijper TM, Hazes JM, Dolhain RJ. 2016. Fab glycosylation of immunoglobulin G does not associate with improvement of rheumatoid arthritis during pregnancy. Arthritis Res Ther. 18:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowyer SL, Roettcher PA, Higgins GC, Adams B, Myers LK, Wallace C, Rennebohm R, Moore TL, Pepmueller PH, Spencer C et al. . 2003. Health status of patients with juvenile rheumatoid arthritis at 1 and 5 years after diagnosis. J Rheumatol. 30:394–400. [PubMed] [Google Scholar]
- Brown EP, Licht AF, Dugast AS, Choi I, Bailey-Kellogg C, Alter G, Ackerman ME. 2012. High-throughput, multiplexed IgG subclassing of antigen-specific antibodies from clinical samples. J Immunol Methods. 386:117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton PR, Clayton DG, Cardon LR, Craddock N, Deloukas P, Duncanson A, Kwiatkowski DP, McCarthy MI, Ouwehand WH, Samani NJ et al. . 2007. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat Genet. 39:1329–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IK, Miescher S, Branch DR, Mott PJ, Lazarus AH, Han D, Maraskovsky E, Zuercher AW, Neschadim A, Leontyev D et al. . 2014. Therapeutic effect of IVIG on inflammatory arthritis in mice is dependent on the Fc portion and independent of sialylation or basophils. J Immunol (Baltimore, MD: 1950). 192:5031–5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispin M, Yu X, Bowden TA. 2013. Crystal structure of sialylated IgG Fc: Implications for the mechanism of intravenous immunoglobulin therapy. Proc Nat Acad Sci USA. 110:E3544–E3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RS. 2015. B-1 cell development and function. Ann NY Acad Sci. 1362:110–116. [DOI] [PubMed] [Google Scholar]
- de Haan N, Reiding KR, Driessen G, van der Burg M, Wuhrer M. 2016. Changes in healthy human IgG Fc-glycosylation after birth and during early childhood. J Proteome Res. 15:1853–1861. [DOI] [PubMed] [Google Scholar]
- Dement-Brown J, Newton CS, Ise T, Damdinsuren B, Nagata S, Tolnay M. 2012. Fc receptor-like 5 promotes B cell proliferation and drives the development of cells displaying switched isotypes. J Leukoc Biol. 91:59–67. [DOI] [PubMed] [Google Scholar]
- Ehrhardt GR, Cooper MD. 2011. Immunoregulatory roles for fc receptor-like molecules. Curr Top Microbiol Immunol. 350:89–104. [DOI] [PubMed] [Google Scholar]
- Ehrhardt GR, Davis RS, Hsu JT, Leu CM, Ehrhardt A, Cooper MD. 2003. The inhibitory potential of Fc receptor homolog 4 on memory B cells. Proc Nat Acad Sci USA. 100:13489–13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercan A, Barnes MG, Hazen M, Tory H, Henderson L, Dedeoglu F, Fuhlbrigge RC, Grom A, Holm IA, Kellogg M et al. . 2012. a. Multiple juvenile idiopathic arthritis subtypes demonstrate proinflammatory IgG glycosylation. Arthritis Rheum. 64:3025–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercan A, Cui J, Chatterton DE, Deane KD, Hazen MM, Brintnell W, O’Donnell CI, Derber LA, Weinblatt ME, Shadick NA et al. . 2010. a. Aberrant IgG galactosylation precedes disease onset, correlates with disease activity, and is prevalent in autoantibodies in rheumatoid arthritis. Arthritis Rheum. 62:2239–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercan A, Cui J, Chatterton DEW, Deane KD, Hazen MM, Brintnell W, O’Donnell CI, Derber LA, Weinblatt ME, Shadick NA et al. . 2010. b. IgG galactosylation aberrancy precedes disease onset, correlates with disease activity and is prevalent in autoantibodies in rheumatoid arthritis. Arthritis Rheum. 62:2239–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercan A, Cui J, Hazen MM, Batliwalla F, Royle L, Rudd PM, Coblyn JS, Shadick N, Weinblatt ME, Gregersen P et al. . 2012. b. Hypogalactosylation of serum N-glycans fails to predict clinical response to methotrexate and TNF inhibition in rheumatoid arthritis. Arthritis Res Ther. 14:R43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercan A, Kohrt WM, Cui J, Deane KD, Pezer M, Yu EW, Hausmann JS, Campbell H, Kaiser UB, Rudd PM et al. . 2017. Estrogens regulate glycosylation of IgG in women and men. JCI Insight. 2:e89703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco A, Damdinsuren B, Ise T, Dement-Brown J, Li H, Nagata S, Tolnay M. 2013. Human Fc receptor-like 5 binds intact IgG via mechanisms distinct from those of Fc receptors. J Immunol (Baltimore, MD: 1950). 190:5739–5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J, Hastie T, Tibshirani R. 2010. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 33:1–22. [PMC free article] [PubMed] [Google Scholar]
- Gare BA. 1998. Epidemiology. Bailliere’s Clin Rheumatol. 12:191–208. [DOI] [PubMed] [Google Scholar]
- Haga CL, Ehrhardt GR, Boohaker RJ, Davis RS, Cooper MD. 2007. Fc receptor-like 5 inhibits B cell activation via SHP-1 tyrosine phosphatase recruitment. Proc Nat Acad Sci USA. 104:9770–9775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland M, Takada K, Okumoto T, Takahashi N, Kato K, Adu D, Ben-Smith A, Harper L, Savage CO, Jefferis R. 2002. Hypogalactosylation of serum IgG in patients with ANCA-associated systemic vasculitis. Clin Exp Immunol. 129:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland M, Yagi H, Takahashi N, Kato K, Savage CO, Goodall DM, Jefferis R. 2006. Differential glycosylation of polyclonal IgG, IgG-Fc and IgG-Fab isolated from the sera of patients with ANCA-associated systemic vasculitis. Biochim Biophys Acta. 1760:669–677. [DOI] [PubMed] [Google Scholar]
- Hromadnikova I, Stechova K, Vavrincova P, Hridelova D, Houbova B, Voslarova S, Nekvasilova H, Vavrinec J. 2002. Anti-cyclic citrullinated peptide antibodies in patients with juvenile idiopathic arthritis. Autoimmunity. 35:397–401. [DOI] [PubMed] [Google Scholar]
- Jackson TA, Haga CL, Ehrhardt GR, Davis RS, Cooper MD. 2010. FcR-like 2 inhibition of B cell receptor-mediated activation of B cells. J Immunol (Baltimore, MD: 1950). 185:7405–7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko Y, Nimmerjahn F, Ravetch JV. 2006. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 313:670–673. [DOI] [PubMed] [Google Scholar]
- Karsten CM, Pandey MK, Figge J, Kilchenstein R, Taylor PR, Rosas M, McDonald JU, Orr SJ, Berger M, Petzold D et al. . 2012. Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcgammaRIIB and dectin-1. Nat Med. 18:1401–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochi Y, Myouzen K, Yamada R, Suzuki A, Kurosaki T, Nakamura Y, Yamamoto K. 2009. FCRL3, an autoimmune susceptibility gene, has inhibitory potential on B-cell receptor-mediated signaling. J Immunol (Baltimore, MD: 1950). 183:5502–5510. [DOI] [PubMed] [Google Scholar]
- Kochi Y, Yamada R, Suzuki A, Harley JB, Shirasawa S, Sawada T, Bae SC, Tokuhiro S, Chang X, Sekine A et al. . 2005. A functional variant in FCRL3, encoding Fc receptor-like 3, is associated with rheumatoid arthritis and several autoimmunities. Nat Genet. 37:478–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodar K, Stadlmann J, Klaamas K, Sergeyev B, Kurtenkov O. 2012. Immunoglobulin G Fc N-glycan profiling in patients with gastric cancer by LC-ESI-MS: Relation to tumor progression and survival. Glycoconj J. 29:57–66. [DOI] [PubMed] [Google Scholar]
- Kristic J, Vuckovic F, Menni C, Klaric L, Keser T, Beceheli I, Pucic-Bakovic M, Novokmet M, Mangino M, Thaqi K et al. . 2014. Glycans are a novel biomarker of chronological and biological ages. J Gerontol Series A Biol Sci Med Sci. 69:779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMaoult J, Szabo P, Weksler ME. 1997. Effect of age on humoral immunity, selection of the B-cell repertoire and B-cell development. Immunol Rev. 160:115–126. [DOI] [PubMed] [Google Scholar]
- Li FJ, Won WJ, Becker EJ Jr, Easlick JL, Tabengwa EM, Li R, Shakhmatov M, Honjo K, Burrows PD, Davis RS. 2014. Emerging roles for the FCRL family members in lymphocyte biology and disease. Curr Top Microbiol Immunol. 382:29–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra R, Wormald MR, Rudd PM, Fischer PB, Dwek RA, Sim RB. 1995. Glycosylation changes of IgG associated with rheumatoid arthritis can activate complement via the mannose-binding protein. Nat Med. 1:237–243. [DOI] [PubMed] [Google Scholar]
- Manners PJ, Bower C. 2002. Worldwide prevalence of juvenile arthritis why does it vary so much? J Rheumatol. 29:1520–1530. [PubMed] [Google Scholar]
- Martinez A, Mas A, de Las Heras V, Bartolome M, Arroyo R, Fernandez-Arquero M, de la Concha EG, Urcelay E. 2007. FcRL3 and multiple sclerosis pathogenesis: Role in autoimmunity? J Neuroimmunol. 189:132–136. [DOI] [PubMed] [Google Scholar]
- Moore JS, Wu X, Kulhavy R, Tomana M, Novak J, Moldoveanu Z, Brown R, Goepfert PA, Mestecky J. 2005. Increased levels of galactose-deficient IgG in sera of HIV-1-infected individuals. AIDS. 19:381–389. [DOI] [PubMed] [Google Scholar]
- Moore TL, Sheridan PW, Traycoff RB, Zuckner J, Dorner RW. 1982. Immune complexes in juvenile rheumatoid arthritis: A comparison of four methods. J Rheumatol. 9:395–401. [PubMed] [Google Scholar]
- Mor-Vaknin N, Kappes F, Dick AE, Legendre M, Damoc C, Teitz-Tennenbaum S, Kwok R, Ferrando-May E, Adams BS, Markovitz DM. 2011. DEK in the synovium of patients with juvenile idiopathic arthritis: Characterization of DEK antibodies and posttranslational modification of the DEK autoantigen. Arthritis Rheum. 63:556–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigrovic PA, Raychaudhri S, Thompson SD in press. Genetics and the classification of arthritis in adults and children. Arthritis Rheumatol. doi:10.1002/art.40350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn F, Anthony RM, Ravetch JV. 2007. Agalactosylated IgG antibodies depend on cellular Fc receptors for in vivo activity. Proc Nat Acad Sci USA. 104:8433–8437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmi Y, Ise W, Harazono A, Takakura D, Fukuyama H, Baba Y, Narazaki M, Shoda H, Takahashi N, Ohkawa Y et al. . 2016. Sialylation converts arthritogenic IgG into inhibitors of collagen-induced arthritis. Nat Commun. 7:11205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omtvedt LA, Royle L, Husby G, Sletten K, Radcliffe CM, Harvey DJ, Dwek RA, Rudd PM. 2006. Glycan analysis of monoclonal antibodies secreted in deposition disorders indicates that subsets of plasma cells differentially process IgG glycans. Arthritis Rheum. 54:3433–3440. [DOI] [PubMed] [Google Scholar]
- Owczarczyk K, Lal P, Abbas AR, Wolslegel K, Holweg CT, Dummer W, Kelman A, Brunetta P, Lewin-Koh N, Sorani M et al. . 2011. A plasmablast biomarker for nonresponse to antibody therapy to CD20 in rheumatoid arthritis. Sci Transl Med. 3:101ra192. [DOI] [PubMed] [Google Scholar]
- Owen CJ, Kelly H, Eden JA, Merriman ME, Pearce SH, Merriman TR. 2007. Analysis of the Fc receptor-like-3 (FCRL3) locus in Caucasians with autoimmune disorders suggests a complex pattern of disease association. J Clin Endocrinol Metab. 92:1106–1111. [DOI] [PubMed] [Google Scholar]
- Parekh R, Roitt I, Isenberg D, Dwek R, Rademacher T. 1988. Age-related galactosylation of the N-linked oligosaccharides of human serum IgG. J Exp Med. 167:1731–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh RB, Dwek RA, Sutton BJ, Fernandes DL, Leung A, Stanworth D, Rademacher TW, Mizuochi T, Taniguchi T, Matsuta K et al. . 1985. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 316:452–457. [DOI] [PubMed] [Google Scholar]
- Parekh RB, Roitt IM, Isenberg DA, Dwek RA, Ansell BM, Rademacher TW. 1988. Galactosylation of IgG associated oligosaccharides: Reduction in patients with adult and juvenile onset rheumatoid arthritis and relation to disease activity. Lancet (London, England). 1:966–969. [DOI] [PubMed] [Google Scholar]
- Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, He X, Maldonado-Cocco J, Orozco-Alcala J, Prieur AM et al. . 2004. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: Second revision, Edmonton, 2001. J Rheumatol. 31:390–392. [PubMed] [Google Scholar]
- Pfeifle R, Rothe T, Ipseiz N, Scherer HU, Culemann S, Harre U, Ackermann JA, Seefried M, Kleyer A, Uderhardt S et al. . 2017. Regulation of autoantibody activity by the IL-23-TH17 axis determines the onset of autoimmune disease. Nat Immunol. 18:104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakken B, Albani S, Martini A. 2011. Juvenile idiopathic arthritis. Lancet (London, England). 377:2138–2149. [DOI] [PubMed] [Google Scholar]
- Pucic M, Knezevic A, Vidic J, Adamczyk B, Novokmet M, Polasek O, Gornik O, Supraha-Goreta S, Wormald MR, Redzic I et al. . 2011. High throughput isolation and glycosylation analysis of IgG-variability and heritability of the IgG glycome in three isolated human populations. Mol Cell Prot MCP. 10:M111.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quast I, Keller CW, Maurer MA, Giddens JP, Tackenberg B, Wang LX, Munz C, Nimmerjahn F, Dalakas MC, Lunemann JD. 2015. Sialylation of IgG Fc domain impairs complement-dependent cytotoxicity. J Clin Invest. 125:4160–4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher TW, Williams P, Dwek RA. 1994. Agalactosyl glycoforms of IgG autoantibodies are pathogenic. Proc Nat Acad Sci USA. 91:6123–6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju TS. 2008. Terminal sugars of Fc glycans influence antibody effector functions of IgGs. Curr Opin Immunol. 20:471–478. [DOI] [PubMed] [Google Scholar]
- Ramirez-Bello J, Jimenez-Morales S, Espinosa-Rosales F, Gomez-Vera J, Gutierrez A, Velazquez Cruz R, Baca V, Orozco L. 2013. Juvenile rheumatoid arthritis and asthma, but not childhood-onset systemic lupus erythematosus are associated with FCRL3 polymorphisms in Mexicans. Mol Immunol. 53:374–378. [DOI] [PubMed] [Google Scholar]
- Rombouts Y, Ewing E, van de Stadt LA, Selman MH, Trouw LA, Deelder AM, Huizinga TW, Wuhrer M, van Schaardenburg D, Toes RE et al. . 2015. Anti-citrullinated protein antibodies acquire a pro-inflammatory Fc glycosylation phenotype prior to the onset of rheumatoid arthritis. Ann Rheum Dis. 74:234–241. [DOI] [PubMed] [Google Scholar]
- Rook GA, Steele J, Brealey R, Whyte A, Isenberg D, Sumar N, Nelson JL, Bodman KB, Young A, Roitt IM et al. . 1991. Changes in IgG glycoform levels are associated with remission of arthritis during pregnancy. J Autoimmun. 4:779–794. [DOI] [PubMed] [Google Scholar]
- Royle L, Campbell MP, Radcliffe CM, White DM, Harvey DJ, Abrahams JL, Kim YG, Henry GW, Shadick NA, Weinblatt ME et al. . 2008. HPLC-based analysis of serum N-glycans on a 96-well plate platform with dedicated database software. Anal Biochem. 376:1–12. [DOI] [PubMed] [Google Scholar]
- Scherer HU, Wang J, Toes RE, van der Woude D, Koeleman CA, de Boer AR, Huizinga TW, Deelder AM, Wuhrer M. 2009. Immunoglobulin 1 (IgG1) Fc-glycosylation profiling of anti-citrullinated peptide antibodies from human serum. Proteomics Clin Appl. 3:106–115. [DOI] [PubMed] [Google Scholar]
- Selman MH, Niks EH, Titulaer MJ, Verschuuren JJ, Wuhrer M, Deelder AM. 2011. IgG fc N-glycosylation changes in Lambert-Eaton myasthenic syndrome and myasthenia gravis. J Proteome Res. 10:143–152. [DOI] [PubMed] [Google Scholar]
- Stockmann H, Adamczyk B, Hayes J, Rudd PM. 2013. Automated, high-throughput IgG-antibody glycoprofiling platform. Anal Chem. 85:8841–8849. [DOI] [PubMed] [Google Scholar]
- Sullivan RT, Kim CC, Fontana MF, Feeney ME, Jagannathan P, Boyle MJ, Drakeley CJ, Ssewanyana I, Nankya F, Mayanja-Kizza H et al. . 2015. FCRL5 delineates functionally impaired memory B Cells associated with Plasmodium falciparum exposure. PLoS Pathog. 11:e1004894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumar N, Isenberg DA, Bodman KB, Soltys A, Young A, Leak AM, Round J, Hay FC, Roitt IM. 1991. Reduction in IgG galactose in juvenile and adult onset rheumatoid arthritis measured by a lectin binding method and its relation to rheumatoid factor. Ann Rheum Dis. 50:607–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Geijn FE, Wuhrer M, Selman MH, Willemsen SP, de Man YA, Deelder AM, Hazes JM, Dolhain RJ. 2009. Immunoglobulin G galactosylation and sialylation are associated with pregnancy-induced improvement of rheumatoid arthritis and the postpartum flare: Results from a large prospective cohort study. Arthritis Res Ther. 11:R193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuckovic F, Kristic J, Gudelj I, Teruel M, Keser T, Pezer M, Pucic-Bakovic M, Stambuk J, Trbojevic-Akmacic I, Barrios C et al. . 2015. Association of systemic lupus erythematosus with decreased immunosuppressive potential of the IgG glycome. Arthrit Rheumatol (Hoboken, NJ). 67:2978–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Balog CI, Stavenhagen K, Koeleman CA, Scherer HU, Selman MH, Deelder AM, Huizinga TW, Toes RE, Wuhrer M. 2011. Fc-glycosylation of IgG1 is modulated by B-cell stimuli. Mol Cell Prot. 10:M110.004655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TJ, Fuchs A, Colonna M. 2012. Cutting edge: Human FcRL4 and FcRL5 are receptors for IgA and IgG. J Immunol (Baltimore, MD: 1950). 188:4741–4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Li R, Li H, Zhou T, Davis RS. 2013. FCRL5 exerts binary and compartment-specific influence on innate-like B-cell receptor signaling. Proc Nat Acad Sci USA. 110:E1282–E1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.