Abstract
The aim of the present study was to determine the effect of implanted neural stem cells (NSCs) on the functional recovery of tree shrews (TSs) subjected to hemi-sectioned spinal cord injury (hSCI), and to investigate the possible mechanism involved. NSCs (passage 2), derived from the hippocampus of TSs (embryonic day 20), were labeled with Hoechst 33342 and transplanted intraspinally into the hSC of TSs at thoracic level 10 in the acute (immediately after injury) and chronic (day 9 post-injury) stages. The Basso-Beattie-Bresnahan (BBB) score was recorded from days 1 to 16 post-injury, and the survival, migration, differentiation and neurotrophic factor (NTF) expression in vivo were detected. In vitro and in vivo, the expanded NSCs were able to differentiate into neurons and astrocytes, and secreted a variety of NTFs, including ciliary NTF, transforming growth factor-β1, glial cell line-derived NTF, nerve growth factor (NGF), brain-derived NTF and insulin-like growth factor. Following transplantation, the BBB score in the TSs with chronic-stage transplantation exhibited a statistically significant increase, while there was no significant difference in the acute group, compared with the control group. This corresponded with the marked upregulation of NGF indicated by reverse transcription-quantitative polymerase chain reaction. In conclusion, the transplantation of NSCs into the hSC in the chronic phase, but not the acute stage, of hSCI in non-human primate TSs is effective and associated with upregulated NGF expression. These findings may provide novel strategies for the treatment of SCI in clinical patients.
Keywords: neural stem cells, hemi-sectioned spinal cord injury, neural behavior, neurotrophic factors, cell transplantation
Introduction
Spinal cord injury (SCI) is recognized as a catastrophic threat to human health, with a gradually increasing morbidity rate worldwide (1,2). It is known that hemi-sectioned SCI (hSCI) can lead to spastic paralysis on the injured side, as well as flaccid paralysis on the other side, and its injury degree lies between that of contusion injury and transection injury. hSCI results in marked motor impairments that harm the daily quality of life (3), however, stem cell therapy could offer an effective treatment solution (4-7).
It is known that stem cells are a type of multi-potent cell with the ability to replicate (self-renewal). Under certain conditions, they can differentiate into various functional cells (8,9). Neural stem cells (NSCs), an important type of stem cell, possess the potential for giving rise to cells that are identical to themselves (self-renewal) (10) and producing neurons, astrocytes and oligodendrocytes (pluripotency) (11). NSC transplantation may shed new light on the practical study of central nervous system injury (4,12), and NSCs could serve as the ideal source of transplanted cells. Moreover, NSCs are able to restore defective nerve tissues by self-proliferation and differentiation (13), and secrete a variety of neurotrophic factors (NTFs), including ciliary NTF (CNTF), transforming growth factor-β1 (TGF-β1), glial cell line-derived NTF (GDNF), nerve growth factor (NGF), brain-derived NTF (BDNF) and insulin-like growth factor (IGF) (14). As grafted cells, the expression of these NTFs from NSCs may support the survival and proliferation of host neural cells by improving the microenvironment, promoting expression of regeneration-related genes and accelerating local spinal cord restoration (15-17). However, the extensive evidence concerning the recovery of motor function following NSC transplantation after SCI has mainly been reported in rodents (18,19). Primates are so scarce and valuable, while rodents including rats and mice are greatly different from primates. Therefore, tree shrews are the best choice for the source of NSCs. Crab-eating monkeys have such disadvantages as a high cost, lower availability and difficulty in breeding. However, for human embryos, ethical issues exist (20-23). Thus, a novel animal model for NSC transplantation is required. With the successful isolation of NSCs in the adult rodent, the study of NSCs in tree shrews (TS), a non-human primate, is becoming a focus, as they present with more advanced development than rats, have a lower economic cost and are a more convenient resource than monkeys, and the closest relative to primates (24,25).
Furthermore, TSs have successfully been turned into an animal model for the study of hepatitis B virus infection (26-28), myopia (29), depression (30) and bacterial infection (31). However, TSs in the hSCI model and TS NSC transplantation into the hSC have not previously been studied, and the molecular factors of TS NSC grafts remain to be decided. Therefore, in the present study, a TS hSCI model was established to investigate the effect of TS NSC transplantation on the motor function improvement in the acute and chronic stages, and the possible mechanisms involved in associated features such as proliferation, differentiation and NTF secretion were examined.
Materials and methods
Animals and ethics statement
The present study used 3 female TSs who were 20 days pregnant and 50 healthy adult female TSs, weighing 120±20 g, 6 months old, which were obtained from the Experimental Animal Center of Kunming Medical University (Kunming, Yunnan, China) and were housed in the Laboratory Animal Center of Sichuan University (Chengdu, Sichuan, China). Animal experimental protocols were approved and performed according to the guidelines of the Institutional Medical Experimental Animal Care Committee of Sichuan University, West China Hospital (Sichuan, China). Guidelines for Laboratory Animal Care and Safety from the Unites States National Institutes of Health (Bethesda, MD, USA) were also followed. Animals were raised in separated cages in a room with a temperature of 20±5°C, 40-60% humidity and using a 12:12-h light/dark cycle, with free access to pellet chow and water. The breeding cages were plastic, equipped with stainless steel cup with lid cover and a plastic water jar. Following hSCI experiments, body temperatures were maintained, and the bladders of the TSs were manually massaged three times daily to enhance their function.
Isolation of NSCs
The pregnant TSs were euthanized with a mixture of 70% CO2 and 30% O2. Next, the embryonic TSs were harvested under sterile conditions and immersed in an incubator filled with D-Hank's solution (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Under an anatomical microscope, the hippocampi of the embryonic TSs were harvested and placed in a centrifuge tube for later use. To isolate the NSCs, the hippocampi were sectioned into small pieces and digested with 0.25% trypsin (1:250; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 20 min, and then Dulbecco's modified Eagle's medium (DMEM)/F12 (1:1; Gibco; Thermo Fisher Scientific, Inc.) containing serum was added to stop the digestion. Following centrifugation at 560 × g (4°C) for 5 min, the supernatant was discarded, and 100 ml DMEM/F12 medium (1:1) was added, with addition of 2 ml 1% B27 (both Gibco; Thermo Fisher Scientific, Inc.), 2 mmol/l glutamine, 2 μg basic fibroblast growth factor (basic FGF; PeproTech, Inc., Rocky Hill, NJ, USA), 1% N2 (Gibco; Thermo Fisher Scientific, Inc.), 10,000 U/l penicillin and 10 mg/l streptomycin. Next, the cell suspension was harvested and the cells were inoculated onto culture plates or bottles at a density of 5×105/ml, then kept in an incubator containing 5% CO2 at 37°C. The culture medium was half replaced every other day.
NSC passaging
At 7 days post-culture, when the cells had grown to near confluency, they were passaged two to four times. Briefly, following centrifugation at 560 × g (4°C) for 5 min in a 15-ml centrifuge tube, the supernatant was discarded. The cell suspension was re-suspended into DMEM/F12 containing FGF (20 ng/ml) and epidermal growth factor (20 ng/ml). The cellular density was adjusted to 1.5-2.5×106/ml and inoculated into the culture bottles (25 ml in volume). Subsequent to lightly swaying the culture bottle for well distribution, the cultured NSCs were incubated in an 37°C incubator.
Cultured NSCs at passage two (P2) were collected for later use: A small quantity of NSCs was used for cell identification, some of them were used to identify their differentiation ability, some were used to detect their secretory functions and a large number were marked by Hoechst 33342 for transplantation in the hSC.
Morphological observation
During the primary and secondary culture of cells derived from the hippocampus of the TSs, an inverted phase-contrast microscope (Leica Microsystems GmbH, Wetzlar, Germany) was employed to observe and record the morphology and growth situation, including size, diameter and shape of NSCs at day 1-7 of P0, P1 and P2, respectively. The formation of neurospheres was also observed.
Experimental groups
A total of 50 female TSs were randomly divided into the sham group, the acute hSCI with medium (MEM) (acute control) group, the acute hSCI with NSC implantation (acute) group, the chronic hSCI with NSC implantation (chronic) group and the chronic hSCI with MEM (chronic) group, with 10 TSs in each group (Table I). TSs in the acute or acute control groups underwent hSCI and were treated with NSC suspension or MEM immediately after injury. TSs in the chronic or chronic control groups were subjected to hSCI and received the NSC suspension or MEM at day 9 post-injury. TSs in the sham group underwent neither hSCI nor transplant injections (Table I).
Table I.
Animals and grouping (n=50).
| Groups | n | BBB | RT-qPCR | IF |
|---|---|---|---|---|
| Sham | 10 | 10 | 5 | 5 |
| Acute hSCI with NSCs | 10 | 10 | 5 | 5 |
| Acute hSCI with MEM | 10 | 10 | 5 | 5 |
| Chro hSCI with NSCs | 10 | 10 | 5 | 5 |
| Chro hSCI with MEM | 10 | 10 | 5 | 5 |
hSCI, hemi-sectioned spinal cord injury; NSCs, neural stem cells; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; IF, immunofluorescence; Chro, chronic; MEM, minimal essential medium; BBB, Basso-Beattie-Bresnahan motor score.
hSCI model
All TSs were anesthetized intraperitoneally with by 2% sodium pentobarbital (30 mg/kg) and placed in the prone position. A midline skin incision was made at the thoracic area (T8-T12) and then the paravertebral muscles and supraspinal ligaments were separated. TSs then underwent a T10 laminectomy and received an hSCI. The surgical wounds were closed with 3-0 silk sutures, and the TSs were injected with normal saline (0.9%; 5 ml) and received cefotaxime sodium (2.5%; 0.5 ml) for 7 days. The bladders of the TSs were manually compressed three times a day until the recovery of the micturition reflex.
NSC labeling and transplantation
Hoechst 33342, a nuclear dye was used for NSC transplantation labeling. To label implanted cells, 3 mg/ml Hoechst (Sigma-Aldrich; Merck KGaA) was added to the medium at 72 h prior to transplantation. For tracing cells after transplantation, NSCs labeled with Hoechst 33342 could be observed under a fluorescence microscope (Leica Microsystems GmbH). For transplantation, cells were isolated by treatment with 0.25% trypsin (Sigma-Aldrich; Merck KGaA) and 0.5 mm EDTA (Sigma-Aldrich; Merck KGaA) at room temperature for 10 min. The digestion was stopped by adding 1 ml fetal bovine serum. The cells were then washed five times with phosphate-buffered saline (PBS). Nucleated NSCs were counted using a cytometer to guarantee sufficient NSC numbers for transplantation. In total, ~6 μl cell suspension (1×107/ml) or equivalent normal MEM was slowly transplanted into the spinal cord around the injury site, and at day 9 post-injury, respectively. Following transplantation, the T10 spinous process was tightened with 1-0 silk suture to prevent kyphosis and to obtain contact between the graft and spinal cord stumps. Muscle and skin were sutured layer to layer, and the TSs were placed in warm cages overnight. Food and water were provided ad libitum. Manual bladder expression was performed twice a day until recovery of the bladder reflex.
Behavior evaluation
To compare the effect of NSC transplantation on the injured spinal cord, locomotor functional recovery was examined in the TSs at days 1-16 following hSCI, as determined by the Basso-Beattie-Bresnahan motor score (BBB score), which was graded from 0 points (absence of any hind limb movement) to 21 points (normal mobility) (32). Subjects were acclimated to the open enclosure (99 cm in diameter, 23 cm deep) for 3 days prior to detection for 5 min/day, as TSs often remain motionless when introduced to a new apparatus. Each subject was then observed for 4 min. All measurements were conducted in a double-blind manner. The mean score was determined from 3 individual researchers.
Immunohistochemistry
Immunohistochemical staining was performed to confirm the cultured NSCs and their differentiation ability, as well as to detect the expression of the trophic factors in vitro. Meanwhile, the survival, migration and differentiation of NSCs in vivo after transplantation were also evaluated.
For in vitro detection, the cell suspension of P2 was moved to the 6-well plates and dropped onto sterile cover slips. The 6-well plates containing the NSCs were incubated at 37°C and the purified NSCs were fixed with 4% paraformaldehyde for 20 min. For in vivo detection, at 16 days post-injury, the TSs were anesthetized with 2% pentobarbital sodium 30 mg/kg [intraperitoneal (i.p.)] and underwent transcardiac perfusion with heparinized 0.9% saline, followed by 4% formaldehyde in 0.1 ml ice-cold phosphate buffer (pH 7.4; Shanghai Haoran Biotechnology Co., Ltd., Shanghai, China). Next, the samples collected from 20-mm long spinal cord segments containing the injury and injection sites, were post-fixed for 5 h at 4°C. Once the spinal cords from the different groups had been embedded in the same paraffin, respectively, the blocks were cut into 4-μm sections for later use. After routine de-paraf-finization and rehydration, immunohistochemistry analysis was performed on the sections of spinal cord tissue.
Rinsed with 0.01 M PBS, the glass slides of the cultured NSCs and spinal cord sections were incubated with 5% goat serum for 30 min at 37°C to quench non-specific binding. Next, they were incubated overnight at 4°C with primary antibodies of nestin (a marker of NSCs), neuron-specific nuclear protein (NeuN; a marker of neurons), glial fibrillary acidic protein (GFAP; marker of astrocytes) and trophic factors (CNTF, TGF-β1, GDNF, NGF, BDNF and IGF) (Table II). As for the control group, the primary antibody was substituted with 0.01 M PBS. Thereafter, the glass slides and tissue sections were washed with 0.01 M PBS three times, each for 2 min. The slides and sections were then incubated with secondary antibodies (Table II) at 37°C for 30 min. Sections were observed under an immunofluorescence microscope (Leica Microsystems GmbH). Furthermore, 4′,6-diamidino-2-phenylindole (DAPI) was used to counterstain the nuclei. Finally, in order to determine the positive number stained in vitro, cells from five fields in each well were collected, each detection in vitro was prepared for 6 plates (6-pore plate) of cells and each pore was put into one sterile cover slip.
Table II.
Antibody list.
| Primary antibody | Company | Species | Concentration | Secondary antibody | Company | Species | Concentration | Cat. nos. |
|---|---|---|---|---|---|---|---|---|
| IGF | ZSGB-BIOa | Rabbit | 1:50 | Cy3 | Jacksonb | Goat anti-rabbit | 1:200 | 111-165-003 |
| BDNF | ZSGB-BIO | Rabbit | 1:50 | Cy3 | Jackson | Goat anti-rabbit | 1:200 | 111-165-003 |
| NGF | ZSGB-BIO | Rabbit | 1:50 | Cy3 | Jackson | Goat anti-rabbit | 1:200 | 111-165-003 |
| GDNF | ZSGB-BIO | Rabbit | 1:100 | Cy3 | Jackson | Goat anti-rabbit | 1:200 | 111-165-003 |
| TGF-β1 | Abcamc | Rabbit | 1:100 | Cy3 | Jackson | Goat anti-rabbit | 1:200 | 111-165-003 |
| CNTF | Biossd | Rabbit | 1:50 | Cy3 | Jackson | Goat anti-rabbit | 1:200 | 111-165-003 |
| GFAP | ZSGB-BIO | Rabbit | 1:50 | Cy3 | Jackson | Goat anti-rabbit | 1:200 | 111-165-003 |
| NeuNe | Bioss | Rabbit | 1:100 | Cy3 | Jackson | Goat anti-mouse | 1:200 | 111-165-003 |
| NeuNf | Bioss | Rabbit | 1:100 | 488 | Invitrogeng | Goat anti-rabbit | 1:100 | A-11034 |
| Nestin | ZSGB-BIO | Rabbit | 1:50 | Cy3 | Jackson | Goat anti-rabbit | 1:200 | 111-165-003 |
ZSGB-BIO; Origene Technologies, Inc., Beijing, China;
Jackson,;
Abcam, Cambridge, MA, USA;
Bioss, Woburn, MA, USA;
NeuN detection in vitro;
NeuN detection in vivo;
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA. Cell identification, Nestin. Differentiation identification: GFAP and NeuN. NTF: IGF, BDNF, NT-3, GDNF, TGF-β1 and CNTF. NeuN, neuron-specific nuclear protein; IGF, insulin-like growth factor; BDNF, brain-derived neurotrophic factor; NGF, nerve growth factor; GDNF, glial cell line-derived neurotrophic factor; TGF-β1, transforming growth factor-β1, CNTF, ciliary neurotrophic factor; GFAP, glial fibrillary acidic protein.
For detection in vivo, five fields for each section and five sections for each detection were selected randomly. All the detections were evaluated by 3 investigators blinded to the experimental information using Image-Pro Plus 6.0 software (Media Cybernetics, Silver Spring, MD, USA), and then the mean rates of positive cells for each detection were calculated.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
At 16 days post-injury, the spinal cord caudal to the injury site (20-mm long containing the injury and injection sites) was harvested after 2% pentobarbital sodium (30 mg/kg; i.p.) anesthesia and placed into a 1.5-ml Eppendorf tube without RNase, then stored at −80°C. Afterwards, the mRNA of the NTFs, including NGF, CNTF, BDNF, GDNF, TGF-β1 and IGF, were detected by RT-qPCR. Briefly, total RNA from the spinal cord tissues was isolated using TRIzol reagent (SuperfecTRI™) according to the manufacturer's protocol (Invitrogen; Thermo Fisher Scientific, Inc.), and reverse transcribed to cDNA with the RevertAid™ First Strand cDNA synthesis kit (Thermo Fisher Scientific, Inc.). Single-stranded cDNAs were synthesized by incubating template RNA (2.5 μg) with oligo(dT)18 primer (1 μl) and nuclease-free water (to 12 μl) at 65°C for 5 min in a volume of 12 μl, followed by mixing RevertAid M-MuLV Reverse Transcriptase (200 μ/μl, 1 μl) with 5X reaction buffer (4 μl), RiboLock RNase Inhibitor (20 μ/μl, 1 μl) and 10 mM dNTP Mix (2 μl) with incubation for 60 min at 42°C in a final volume of 20 μl. The reaction was terminated by heating to 70°C for 5 min. PCR was performed using s T100™ Thermal Cycler (Bio-Rad Laboratories, Inc., Hercules, CA, USA) to validate the level of NTFs; 5 μl of 5-fold diluted template cDNA was added in each system, with a final volume of 25 μl. Subsequently, RT-qPCR of cDNA was performed using the forward and reverse primer sequences as shown in Table III. PCR amplification was performed as follows: i) Initial denaturation (1 cycle, 95°C for 3 min); ii) denaturation (40 cycles, 95°C for 15 sec); and iii) amplification (40 cycles, 53°C for 30 sec and 60°C for 40 sec). The PCR products were verified by 1% agarose gel electrophoresis, visualized by GoldView (Wolsen) staining (Xi'an Wolsen Bio-Technology Co., Ltd, Xi'an, China). The gels were scanned using an AlphaImager gel documentation system (Bio-Rad Laboratories, Inc.), and bands were quantified using ImageJ software (NIH, Bethesda, MD, USA).
Table III.
Information of primer sequences.
| Gene | Upstream | Downstream | Annealing temperature, °C |
|---|---|---|---|
| CNTF | 5′-AGGAAGATTCGTTCAGACCT-3′ | 5′-GTTCTCTTGGAGTCGCTCTG-3′ | 53 |
| TGF-β1 | 5′-GGCAGCTGTACATTGACTT-3′ | 5′-AGGGCAAGGACCTTGCTGT-3′ | 53 |
| GDNF | 5′- TCTGCCTGGTGTTGCTCC-3′ | 5′-CCTCTGCGACCTTTCCCT-3′ | 52 |
| NGF | 5′-GAAGCCCACTGGACTAAACT-3′ | 5′-ACAGTGATGTTGCGGGTCTG-3′ | 54 |
| BDNF | 5′-GGTGTCGTAAAGTTCCACCA-3′ | 5′-GCCAAGTTGCCTTGTCCGT-3′ | 54 |
| IGF | 5′-GATACACATCATGTCGTCTT-3′ | 5′-GCCTGTGGGCTTGTTGAAGT-3′ | 50 |
| β-actin | 5′-GAAGATCAAGATCATTGCTCCT-3′ | 5′-TACTCCTGCTTGCTGATCCA-3′ | 52 |
CNTF, ciliary neurotrophic factor; TGF-β1, transforming growth factor-β1; GDNF, glial cell line-derived neurotrophic factor; NGF, nerve growth factor; BDNF, brain-derived neurotrophic factor; IGF, insulin-like growth factor.
Statistical analysis
SPSS software (version 17.0; SPSS Inc., Chicago, IL, USA) was used to process data. The experimental results are expressed as the mean ± standard deviation, and were analyzed by Student's t-test with a two-tailed distribution. For multiple group comparisons, analysis of variance with Tukey's post hoc test was applied. P<0.05 was considered to indicate a statistically significant difference.
Results
Morphology of the TS NSCs
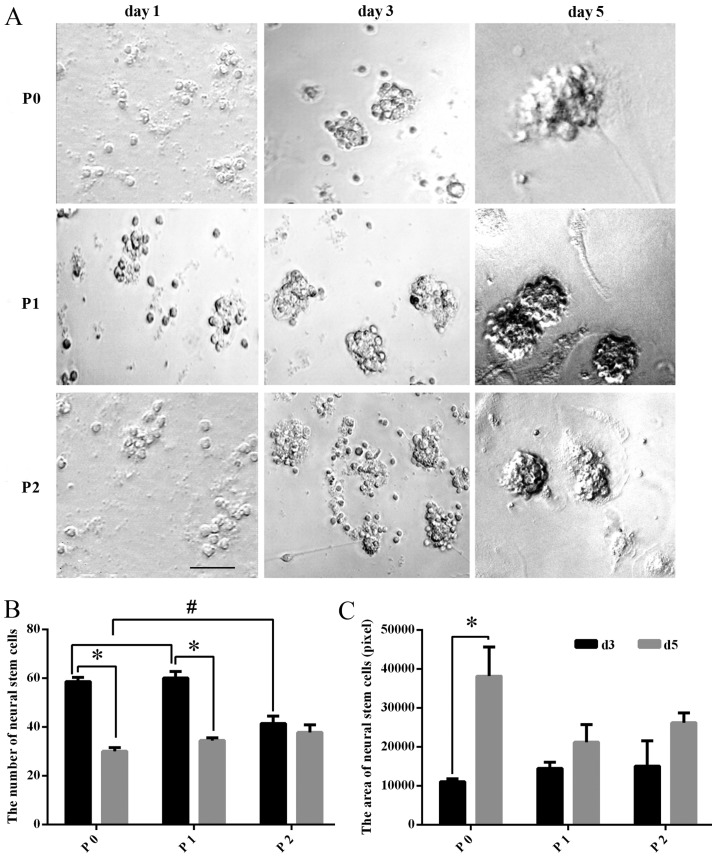
At day 1 in primary culture, the cells were small and round, 5-10 μm in diameter, with no cell processes under a microscope. The distribution appeared scattered in cell suspension, but with excellent refraction. At day 3 in primary culture, small cell spheres arose in the liquid and grew in a clustered and floating manner. At day 5 in primary culture, spherical colonies formed by dozens of cells could be observed. With the development of time, the number and the diameter of the spherical colonies markedly increased. Following continuous passaging, at P1 and P2, greater numbers of NSCs were obtained, whose growth characteristics were similar to that of the original generation (Fig. 1A).
Figure 1.
Morphology of TS NSCs cultured in vitro. (A) TS NSCs were observed under a microscope without dye processing on days 1, 3 and 5 of primary culture (P0), and subculture (P1 and P2). Scale bar, 100 μm. (B and C) The number of NSCs in the different culture stages (P0, P1 and P2) was compared at different culture times (days 3 and 5). Data are presented as the mean ± SD (n=5). The area of NSCs in the different culture stages (P0, P1 and P2) was compared at different times (days 3 and 5). Data are presented as the mean ± SD (n=6). TS, tree shrew; NSC, neural stem cell; P, passage; d, day; SD, standard deviation.
Proliferation of NSCs cultured in vitro
The number of NSCs decreased significantly in the P0 and P1 groups (P<0.01), while in P2, the difference in the number of NSC exhibited no statistical significance (P>0.05) between days 3 and 5. Among all groups, the number of the cultured NSCs at day 3 in P2 was significantly fewer than that in P0 or P1 (P<0.01), while for the cultured NSCs at day 5, there was no significant difference among P0, P1 and P2 (P>0.05) (Fig. 1B). Meanwhile, the size of the cultured NSCs was also calculated, and the comparison between days 3 and 5 of P0, P1 and P2 showed that the mean size of the NSCs (neurospheres) at day 5 was significantly larger than that at day 3 in P0, whereas the size changes between days3 and 5 in P1 and P2 exhibited no statistical significance (P>0.05). In addition, comparison among P0, P1 and P2 groups revealed no significance (P>0.05) (Fig. 1C).
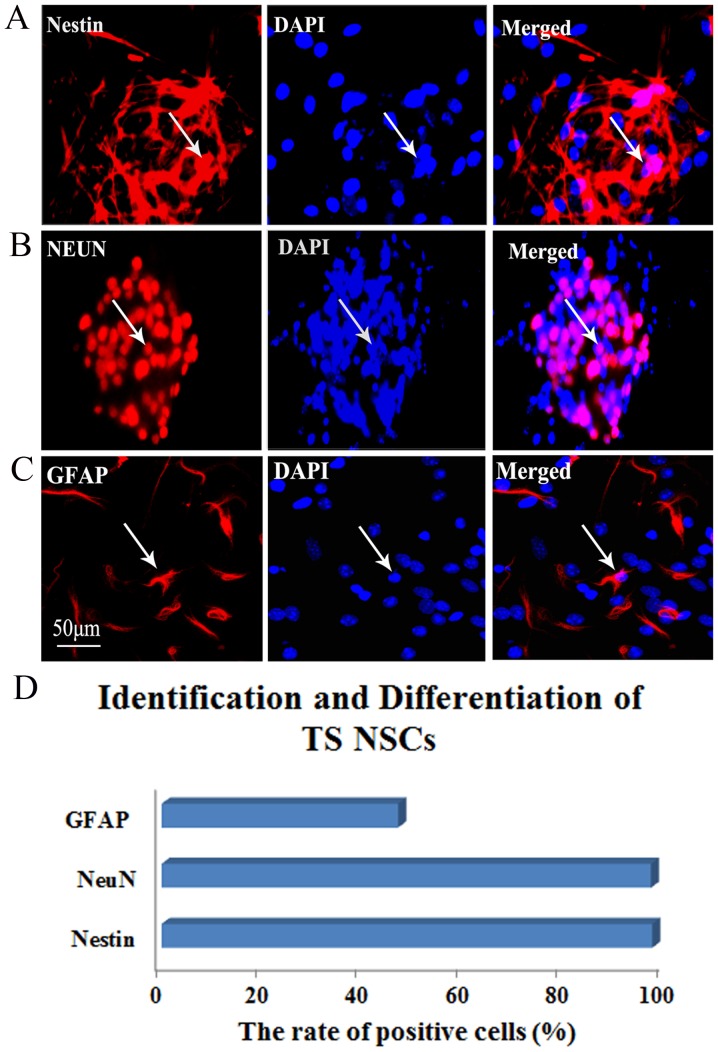
Identification and differentiation of TS NSCs
To identify the cultured TS NSCs and evaluate the differentiation phenotype of the grafted cells in vitro, immunohistochemical staining of Nestin, NeuN and GFAP was performed at P2 after culture. In close approximation of our expectations, the rate of Nestin-positive expression was 99.58%, which confirmed the purity of the cultured NSCs. Almost all NSCs were NeuN-positive (97.23%), which implied that the majority of the cultured TS NSCs differentiated into neurons. The GFAP-positive rate was 46.99% in NSCs, which explained why a small quantity of NSCs differentiated into astrocytes (Fig. 2).
Figure 2.
Identification and differentiation of the cultured TS NSCs. (A) Immunofluorescent staining of Nestin (red, left) for identifying the TS NSCs, DAPI with blue staining was shown in middle and the merge picture showed Nestin positive rate was 99.58% (right). (B and C) Immunofluorescent staining of NeuN and GFAP for confirmation of the differentiation of the cultured NSCs into neurons and astrocytes (red, left). DAPI stained the nuclei with blue florescence (middle). Merged images show the NeuN- and GFAP-positive cells, from which expression rates of 97.23 and 46.99%, respectively, were obtained (right). White arrows represent the positive cells. Scale bar, 50 μm. Cells from five fields in each well were collected, and each detection in vitro was prepared for 6 plates (6-pore plate) of cells. (D) Representative bar graph for the rate of positive cells. TS, tree shrew; NSC, neural stem cell; DAPI, 4′,6-diamidino-2-phenylindole; GFAP, glial fibrillary acidic protein; NeuN, neuron-specific nuclear protein.
Expression of NTFs in the cultured TS NSCs
Using the double-labeled immunofluorescence staining of BDNF CNTF, GDNF, IGF, TGF-β1 and NGF with DAPI, the expression of these NTFs expressed in the cultured TS NSCs were detected at P2 following culture. The results showed that the cultured TS NSCs exhibited positive immunoreactivity for the aforementioned NTFs. Moreover, quantitative analysis showed that the positive rates of CNTF, GDNF and IGF were 100%, and that the positive rate of TGF-β1 was 96.67%. NGF had a positive rate of 89.96%. Furthermore, BDNF was observed with a positive rate of 50%. This outcome indicated that NSCs can secrete CNTF, GDNF, IGF, TGF-β1, NGF and a small amount of BDNF (Fig. 3).
Figure 3.
Expression of NTFs in the NSCs of tree shrews. Immunofluorescent staining of CNTF, TGF-β1, GDNF, NGF, BDNF and IGF is shown in the left of (A-F) (red), and cell nuclei were redyed by 4′,6-diamidino-2-phenylindole (DAPI) (blue) in vitro (A-F, middle). The merged images show positive CNTF (B; right), GDNF (C; right) and IGF (D; right) expression, with a determined mean rate of 100%, BDNF (A, right) was 50%, NGF was 89.96% (E, right) and TGF-β1 was 96.67% (F, right). Scale bar, 50 μm. White arrows represented the positive cells. Cells from five fields in each well were collected, each detection in vitro was prepared for six plates (six-pore plate) of cells. NTF, neurotrophic factors; NSCs, neural stem cells; CNTF, ciliary NTF; TGF-β1, transforming growth factor-β1; GDNF, glial cell line-derived NTF; NGF, nerve growth factor; BDNF, brain-derived NTF; IGF, insulin-like growth factor.
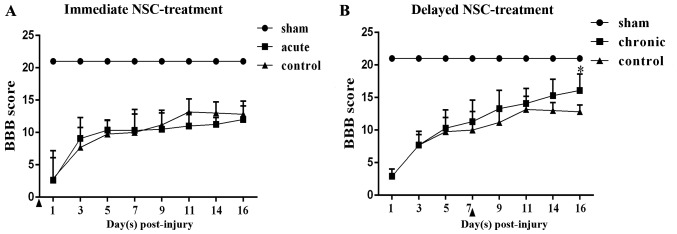
BBB score
The changes of hind-limb function were assessed using the BBB scores from days 1 to 16 post-hSCI. hSCI resulted in spastic paralysis on the injury side of the TSs, as well as flaccid paralysis on the other side, with a BBB score of 3 at day 1 post-injury in the four groups. Over time, the locomotor function could be partially restored. At day 7 post-injury, the hind-limb locomotor functions had recovered spontaneously to an approximate BBB score of 10. Moreover, in the chronic group, significantly greater functional recovery was observed compared with that in the chronic control group at day 16 post-injury (P<0.05). By contrast, the acute group did not show any functional recovery compared with the acute control group (Fig. 4).
Figure 4.
Analysis of functional recovery measured by BBB scores following NSC transplantation. (A) The BBB scores in hSCI tree shrews treated with NSCs immediately after hSCI did not differ from those of the controls. (B) The chronic group exhibited significantly better functional recovery than the chronic control group at day 16 post-injury. Data are presented as the mean ± standard deviation (n=10). *P<0.01 vs. control group. Arrowheads indicate the transplantation time. Chronic, NSC transplantation at day 9 post-injury; acute, NSC transplantation immediately after injury; TS, tree shrew; BBB, Basso-Beattie-Bresnahan; NSC, neural stem cell; hSCI, hemi-sectioned spinal cord injury.
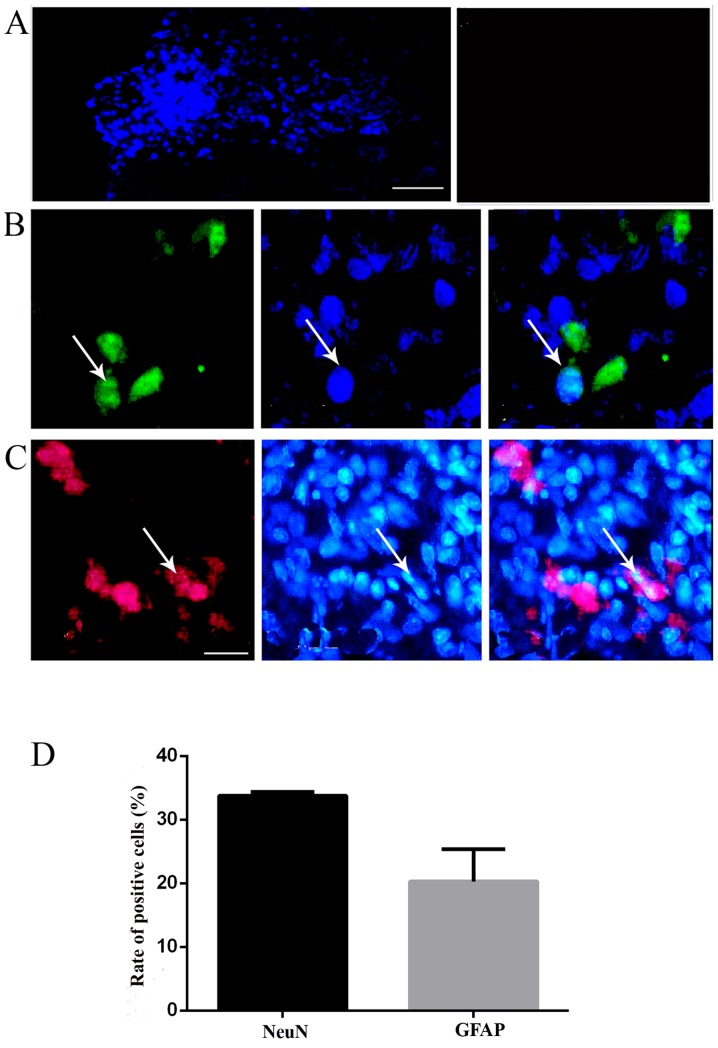
Survival, migration and differentiation of the transplanted NSCs in vivo
At day 16 post-injury in the chronic group, a large number of surviving NSCs with blue nuclei staining labeled by Hoechst 33342 could be found in the spinal cord of the NSC-transplanted TSs (near the needle passage), whereas there were no Hoechst-positive cells in the control group; the results indicated that NSCs could survive and migrate in the spinal cord around the injection site (Fig. 5A). In order to detect the differentiation of NSCs in the host spinal cord, the immunohistochemical staining of specific markers was performed to recognize neurons and astrocytes, as performed in vitro. The implanted NSCs with blue nuclei staining were found in the host spinal cord, confirming the survival of the NSCs (Fig. 5B and C). Simultaneously, positive staining of neuron marker NeuN confirmed the differentiation of NSCs into neurons (Fig. 5B). In addition, a few NSCs exhibited GFAP-positive staining, a marker of astrocytes, demonstrating the differentiation of the transplanted NSCs into astrocytes (Fig. 5C). The merged images revealed that in the chronic group, certain grafted cells differentiated into NeuN-positive cells (33.8±0.6%), followed by GFAP-positive astrocytes (20.3±5.1%) (Fig. 5B-D).
Figure. 5.
Transplanted TS NSCs survived and differentiated into neurons and glia-like cells in vivo. (A) NSCs labeled with Hoechst (blue fluorescence, left) survived and migrated at day 16 post-injury in vivo. The control exhibited no positive reactivity (right). (B) Hoechst+ grafted NSCs (blue florescence, middle) differentiated into NeuN+ neurons (green florescence, left), and GFAP+ astrocytes (red, left) in the chronic groups, with merged images shown in the right panels. Scale bar, (A) 100 μm and (B and C) 50 μm. White arrows indicate the positive cells. The images were captured 1 cm below the lesion. (D) Representative bar graph for the rate of NeuN- and GFAP-positive cells. NSC, neural stem cell; TS, tree shrew; NeuN, neuron-specific nuclear protein; GFAP, glial fibrillary acidic protein.
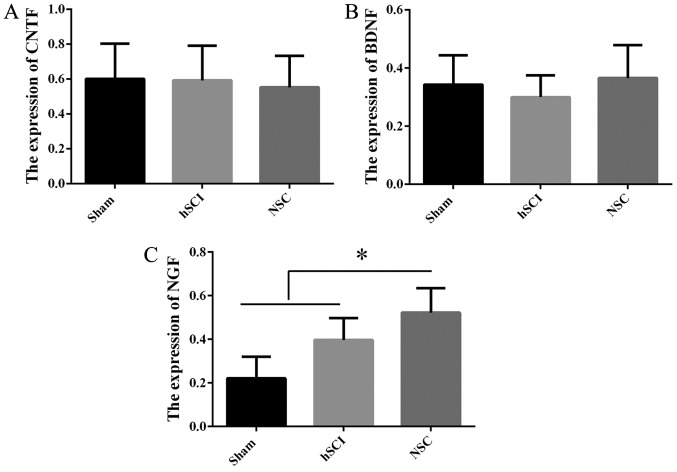
Expression of NTFs in vivo following NSC transplantation
In order to detect the mechanism of the functional recovery at day 16 post-injury in the chronic group, RT-qPCR was employed to confirm NTF gene expression in vivo among the sham, hSCI and NSCs transplantation groups. Compared with the mRNA of NSCs from the sham group, NGF gene expression was increased markedly in the chronic group (P<0.05), while there was no significant difference for CNTF, BDNF (Fig. 6), IGF, TGF-β1 and GDNF (data not shown) among the above three groups.
Figure. 6.
Expression of NTFs in vivo after tree shrew NSC transplantation. (A) Analysis of CNTF mRNA expression in the spinal cord tissues caudal to the injured site (20-mm long containing the injury and injection sites) among the sham, hSCI and NSC transplantation groups in the chronic phase at day 16 post-injury. (B) Analysis of BDNF mRNA expression in the tissues aforementioned. (C) Analysis of NGF mRNA expression in the tissues aforementioned. *P<0.05 vs. NSC group. Data are represented as the mean ± SD (n=10). NSC, neural stem cell; hSCI, hemi-sectioned spinal cord injury; NTF, neurotrophic factor; CNTF, ciliary NTF; BDNF, brain-derived NTF; NGF, nerve growth factor.
Discussion
In the present study, it was demonstrated in vitro and in vivo that the NSCs from TSs possessed the potential for self-renewal, pluripotency into neurons and astrocytes, and the production of different types of NTFs, including CNTF, TGF-β1, GDNF, NGF, BDNF and IGF. Following transplantation in the chronic phase of hSCI, the BBB score revealed that the locomotor function in the hind-limbs was improved, but that there was no statistical significance in the acute-phase transplantation, when compared with the control group. To the best of our knowledge, these findings, for the first time, indicate that the transplantation of NSCs from TSs is available for neurological function improvement following hSCI, but only in the acute phase, and that the expression of multiple NTFs linked with the upregulation of NGF is probably involved in the underlying mechanism. In the experiments assessing the proliferation of NSCs from TSs, it was confirmed that the NSCs possessed the capacity to proliferate in vitro and in vivo. In vitro, the number of NSCs in P0 and P1 reached the growth peak in culture on day 3. The area of neurosphere formation kept increasing as well throughout the culture, which innovatively illustrated that NSCs from TSs can successfully proliferate in vitro. These results suggested that NSCs from TSs could be considered as an available cell source for the treatment of disease. In vivo, via local injections of NSCs, transplanted NSCs from TSs were verified to survive and proliferate in the hSC of other TSs, which is useful for understanding the functional repair occurring following hSCI in non-human primates.
The majority of NSC experimental models have been focused on rodents and few studies have involved the use of primates or humans due to the associated ethical issues and the lack of availability (33-37). Compared with rats, TSs exhibit biological characteristics and gross anatomy that are more similar to those of humans (24,38). Furthermore, TSs have a lower economic cost and are a more convenient resource than other animals. Therefore, TSs have more advantages as translational research on NSCs than rodents.
The present study verified that NSCs from TSs could differentiate into neurons and astrocytes in vivo and in vitro, which may replace the damaged nerve cells to restore the structure of the injured spinal cord following transplantation. Over the past decades, NSCs have been reported to exhibit multi-directional differentiation to neurons and astrocytes (13,21,36,39). However, the differentiation ability of NSCs in non-human primates, such as TSs, was previously unknown. To the best of our knowledge, the present study was the first to demonstrate the differentiation characteristics of NSCs from TSs. The present experiments therefore provided crucial evidence that NSCs can differentiate into neurons and glial cells, which assist in reconstructing neural injury.
In the present study, the BBB score exhibited no significant difference in terms of varying observation points in the acute phase group compared with those in the acute control group; this indicated that NSC implantation in the chronic phase could contribute to the recovery of nervous function in TSs, but not in the acute phase. A number of studies found that the degenerative degree of the spinal cord tissue near the spinal cord transection or contusion was reduced significantly following NSC transplantation in the chronic phase (40,41), but associated mechanisms involved in the NTFs were not mentioned, let alone the non-human primate tree shrew model. Therefore, the present study obtained novel findings that NSC transplantation into TSs in the chronic phase could effectively improve nervous function, which may be linked to the secretion of NTFs in the chronic group.
The results of IHC and RT-qPCR showed that NSCs could secrete CNTF, TGF-β1, GDNF, NGF, BDNF and IGF in vitro, while NGF gene expression increased markedly in vivo. The upregulation of NGF may contribute to neuronal growth, development and functional integrity, therefore improving the BBB scores in the hSCI TSs with NSC transplantation. According to previous studies, there are two main hypotheses for the effectively promotion of neural functional recovery by NSCs: The alternative theory and the nutrition theory. Previous studies have demonstrated that NSC transplantation can differentiate into neurons and glial cells to repair the neuronal necrosis, but little evidence shows that grafted NSCs can integrate into the neural networks of the host. Therefore, whether grafted NSCs can integrate into host neural network has become important for research, as this may help to further interpret the role of NSCs transplantation in the restoration of nerve function (14-17,42-44). The present results showed that the upregulated NGF expression expressed by NSCs directly or caused by NSCs indirectly could promote survival and proliferation, and ultimately improve motor function following hSCI. These results will aid in understanding the molecular mechanisms for stem cell therapy in diseases of non-human primates, which may ultimately become available to future patients in the clinic.
Acknowledgments
This study was supported by a grant from the Key Grant of Natural Science Foundation from Yunnan Province (2014FA009) and the National Key Technology Research and Development Program of the Ministry of Science and Technology of China (no. 2014BAI01B10) and by the Program Innovative Research Team In Science and Technology in Yunnan Province.
Footnotes
Competing interests
The authors declare that they have no competing interests.
References
- 1.Jain NB, Ayers GD, Peterson EN, Harris MB, Morse L, O'Connor KC, Garshick E. Traumatic spinal cord injury in the United States, 1993-2012. JAMA. 2015;313:2236–2243. doi: 10.1001/jama.2015.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Löfvenmark I, Norrbrink C, Nilsson-Wikmar L, Hultling C, Chakandinakira S, Hasselberg M. Traumatic spinal cord injury in Botswana: Characteristics, aetiology and mortality. Spinal Cord. 2015;53:150–154. doi: 10.1038/sc.2014.203. [DOI] [PubMed] [Google Scholar]
- 3.Rognoni C, Fizzotti G, Pistarini C, Quaglini S. Quality of life of patients with spinal cord injury in Italy: Preliminary evaluation. Stud Health Technol Inform. 2014;205:935–939. [PubMed] [Google Scholar]
- 4.Dooley D, Vidal P, Hendrix S. Immunopharmacological intervention for successful neural stem cell therapy: New perspectives in CNS neurogenesis and repair. Pharmacol Ther. 2014;141:21–31. doi: 10.1016/j.pharmthera.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Whittemore SR. Neuronal replacement strategies for spinal cord injury. J Neurotrauma. 1999;16:667–673. doi: 10.1089/neu.1999.16.667. [DOI] [PubMed] [Google Scholar]
- 6.Park KI, Liu S, Flax JD, Nissim S, Stieg PE, Snyder EY. Transplantation of neural progenitor and stem cells: Developmental insights may suggest new therapies for spinal cord and other CNS dysfunction. J Neurotrauma. 1999;16:675–687. doi: 10.1089/neu.1999.16.675. [DOI] [PubMed] [Google Scholar]
- 7.Neirinckx V, Agirman G, Coste C, Marquet A, Dion V, Rogister B, Franzen R, Wislet S. Adult bone marrow mesenchymal and neural crest stem cells are chemoattractive and accelerate motor recovery in a mouse model of spinal cord injury. Stem Cell Res Ther. 2015;6:211. doi: 10.1186/s13287-015-0202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnabé-Heider F, Frisén J. Stem cells for spinal cord repair. Cell Stem Cell. 2008;3:16–24. doi: 10.1016/j.stem.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 9.Enzmann GU, Benton RL, Talbott JF, Cao Q, Whittemore SR. Functional considerations of stem cell transplantation therapy for spinal cord repair. J Neurotrauma. 2006;23:479–495. doi: 10.1089/neu.2006.23.479. [DOI] [PubMed] [Google Scholar]
- 10.Gross CG. Neurogenesis in the adult brain: death of a dogma. Nat Rev Neurosci. 2000;1:67–73. doi: 10.1038/35036235. [DOI] [PubMed] [Google Scholar]
- 11.Doetsch F. The glial identity of neural stem cells. Nat Neurosci. 2003;6:1127–1134. doi: 10.1038/nn1144. [DOI] [PubMed] [Google Scholar]
- 12.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 13.Bonaguidi MA, Wheeler MA, Shapiro JS, Stadel RP, Sun GJ, Ming GL, Song H. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell. 2011;145:1142–1155. doi: 10.1016/j.cell.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu P, Jones LL, Snyder EY, Tuszynski MH. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol. 2003;181:115–129. doi: 10.1016/S0014-4886(03)00037-2. [DOI] [PubMed] [Google Scholar]
- 15.Blesch A, Tuszynski MH. GDNF gene delivery to injured adult CNS motor neurons promotes axonal growth, expression of the trophic neuropeptide CGRP, and cellular protection. J Comp Neurol. 2001;436:399–410. doi: 10.1002/cne.1076. [DOI] [PubMed] [Google Scholar]
- 16.Grill R, Murai K, Blesch A, Gage FH, Tuszynski MH. Cellular delivery of neurotrophin-3 promotes corticospinal axonal growth and partial functional recovery after spinal cord injury. J Neurosci. 1997;17:5560–5572. doi: 10.1523/JNEUROSCI.17-14-05560.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McTigue DM, Horner PJ, Stokes BT, Gage FH. Neurotrophin-3 and brain-derived neurotrophic factor induce oligodendrocyte proliferation and myelination of regenerating axons in the contused adult rat spinal cord. J Neurosci. 1998;18:5354–5365. doi: 10.1523/JNEUROSCI.18-14-05354.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karimi-Abdolrezaee S, Eftekharpour E. Stem cells and spinal cord injury repair. Adv Exp Med Biol. 2012;760:53–73. doi: 10.1007/978-1-4614-4090-1_4. [DOI] [PubMed] [Google Scholar]
- 19.Wang D, Zhang J. Effects of hypothermia combined with neural stem cell transplantation on recovery of neurological function in rats with spinal cord injury. Mol Med Rep. 2015;11:1759–1767. doi: 10.3892/mmr.2014.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu YR, Chen G, Wei Y, Wan H, Li JH, Chang WJ, Zhang XL, Liu R, Sun ZZ. In vitro culture, induction and differentiation of neural stem cells from rat embryo. J Clin Rehabilitative Tissue Eng Res. 2009;13:3651–3655. [Google Scholar]
- 21.Hu YY, Duan XH, Chen M, Wang D. Proliferation and differentiation of neural stem cells from newborn mouse hippocampi in vitro. Chin J Tissue Eng Res. 2013;17:79–85. [Google Scholar]
- 22.Yan SX, Liu XM, Xiang P, Wang P, Wang D. Culture and differentiation of cynomolgus monkey neural stem cells. J Clin Rehabilitative Tissue Eng Res. 2009;13:8821–8824. [Google Scholar]
- 23.Razavi S, Razavi MR, Ahmadi N, Kazemi M. Estrogen treatment enhances neurogenic differentiation of human adipose derived stem cells in vitro. Iran J Basic Med Sci. 2015;18:799–804. [PMC free article] [PubMed] [Google Scholar]
- 24.Janecka JE, Miller W, Pringle TH, Wiens F, Zitzmann A, Helgen KM, Springer MS, Murphy WJ. Molecular and genomic data identify the closest living relative of primates. Science. 2007;318:792–794. doi: 10.1126/science.1147555. [DOI] [PubMed] [Google Scholar]
- 25.Fan Y, Huang ZY, Cao CC, Chen CS, Chen YX, Fan DD, He J, Hou HL, Hu L, Hu XT, et al. Genome of the Chinese tree shrew. Nat Commun. 2013;4:1426. doi: 10.1038/ncomms2416. [DOI] [PubMed] [Google Scholar]
- 26.Ruan P, Yang C, Su J, Cao J, Ou C, Luo C, Tang Y, Wang Q, Yang F, Shi J, et al. Histopathological changes in the liver of tree shrew (Tupaia belangeri chinensis) persistently infected with hepatitis B virus. Virol J. 2013;10:333. doi: 10.1186/1743-422X-10-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Q, Yang C, Su JJ, Cao J, Ou C, Yang F, Zhang JJ, Shi JL, Wang DP, Wang XJ, et al. Factors influencing long-term hepatitis B virus infection of the tree shrew (Tupaia belangeri chinensis) as an in vivo model of chronic hepatitis B. Zhonghua Gan Zang Bing Za Zhi. 2012;20:654–658. doi: 10.3760/cma.j.issn.1007-3418.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Xu G, Gao Z, He W, Ma Y, Feng X, Cai T, Lu F, Liu L, Li W. microRNA expression in hepatitis B virus infected primary treeshrew hepatocytes and the independence of intracellular miR-122 level for de novo HBV infection in culture. Virology. 2014;448:247–254. doi: 10.1016/j.virol.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 29.Grytz R, Siegwart JT., Jr Changing material properties of the tree shrew sclera during minus lens compensation and recovery. Invest Ophthalmol Vis Sci. 2015;56:2065–2078. doi: 10.1167/iovs.14-15352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, Zhou QX, Tian M, Yang YX, Xu L. Tree shrew models: A chronic social defeat model of depression and a one-trial captive conditioning model of learning and memory. Dongwuxue Yanjiu. 2011;32:24–30. doi: 10.3724/SP.J.1141.2011.01024. [DOI] [PubMed] [Google Scholar]
- 31.Li SA, Lee WH, Zhang Y. Two bacterial infection models in tree shrew for evaluating the efficacy of antimicrobial agents. Dongwuxue Yanjiu. 2012;33:1–6. doi: 10.3724/SP.J.1141.2012.01001. [DOI] [PubMed] [Google Scholar]
- 32.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 33.Coumans JV, Lin TT, Dai HN, MacArthur L, McAtee M, Nash C, Bregman BS. Axonal regeneration and functional recovery after complete spinal cord transection in rats by delayed treatment with transplants and neurotrophins. J Neurosci. 2001;21:9334–9344. doi: 10.1523/JNEUROSCI.21-23-09334.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu S, Suzuki Y, Kitada M, Kitaura M, Kataoka K, Takahashi J, Ide C, Nishimura Y. Migration, integration, and differentiation of hippocampus-derived neurosphere cells after transplantation into injured rat spinal cord. Neurosci Lett. 2001;312:173–176. doi: 10.1016/S0304-3940(01)02219-4. [DOI] [PubMed] [Google Scholar]
- 35.Yi BR, Kim SU, Choi KC. Development and application of neural stem cells for treating various human neurological diseases in animal models. Lab Anim Res. 2013;29:131–137. doi: 10.5625/lar.2013.29.3.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 37.Vroemen M, Aigner L, Winkler J, Weidner N. Adult neural progenitor cell grafts survive after acute spinal cord injury and integrate along axonal pathways. Eur J Neurosci. 2003;18:743–751. doi: 10.1046/j.1460-9568.2003.02804.x. [DOI] [PubMed] [Google Scholar]
- 38.Fan Y, Yu D, Yao YG. Tree shrew database (TreeshrewDB): A genomic knowledge base for the Chinese tree shrew. Sci Rep. 2014;4:7145. doi: 10.1038/srep07145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dromard C, Guillon H, Rigau V, Ripoll C, Sabourin JC, Perrin FE, Scamps F, Bozza S, Sabatier P, Lonjon N, et al. Adult human spinal cord harbors neural precursor cells that generate neurons and glial cells in vitro. J Neurosci Res. 2008;86:1916–1926. doi: 10.1002/jnr.21646. [DOI] [PubMed] [Google Scholar]
- 40.Nishimura S, Yasuda A, Iwai H, Takano M, Kobayashi Y, Nori S, Tsuji O, Fujiyoshi K, Ebise H, Toyama Y, et al. Time-dependent changes in the microenvironment of injured spinal cord affects the therapeutic potential of neural stem cell transplantation for spinal cord injury. Mol Brain. 2013;6:3. doi: 10.1186/1756-6606-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsuji O, Miura K, Fujiyoshi K, Momoshima S, Nakamura M, Okano H. Cell therapy for spinal cord injury by neural stem/progenitor cells derived from iPS/ES cells. Neurotherapeutics. 2011;8:668–676. doi: 10.1007/s13311-011-0063-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamei N, Tanaka N, Oishi Y, Hamasaki T, Nakanishi K, Sakai N, Ochi M. BDNF, NT-3, and NGF released from transplanted neural progenitor cells promote corticospinal axon growth in organotypic cocultures. Spine. 2007;32:1272–1278. doi: 10.1097/BRS.0b013e318059afab. [DOI] [PubMed] [Google Scholar]
- 43.Weishaupt N, Blesch A, Fouad K. BDNF: The career of a multifaceted neurotrophin in spinal cord injury. Exp Neurol. 2012;238:254–264. doi: 10.1016/j.expneurol.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 44.Maisonpierre PC, Belluscio L, Friedman B, Alderson RF, Wiegand SJ, Furth ME, Lindsay RM, Yancopoulos GD. NT-3, BDNF, and NGF in the developing rat nervous system: Parallel as well as reciprocal patterns of expression. Neuron. 1990;5:501–509. doi: 10.1016/0896-6273(90)90089-X. [DOI] [PubMed] [Google Scholar]