Abstract
Many injectable drugs require delivery strategies for enhancing their pharmacokinetics due to rapid loss via renal filtration if possess low molecular weight (<60–70 kDa) and/or clearance by the body's components (e.g., proteases, antibodies, high-efficiency receptors) in their native form. FDA-approved polyethylene glycol (PEG) is a vehicle for improving therapeutics, but artificial polymers have potential biocompatibility and immunogenicity liabilities. Here, we utilized a natural vertebrate carbohydrate, heparosan (HEP), the biosynthetic precursor of heparan sulfate and heparin, to enhance performance of a biologic drug. The HEP polysaccharide was stable with a long half-life (~8 days for 99-kDa chain) in the nonhuman primate bloodstream, but was efficiently degraded to very short oligosaccharides when internalized by cells, and then excreted into urine and feces. Several HEP-modified human granulocyte-colony stimulating factor (G-CSF) conjugates were synthesized with defined quasi-monodisperse HEP polysaccharide chains. Single dosing of 55- or 99-kDa HEP-G-CSF in rats increased blood neutrophil levels comparable to PEG-G-CSF conjugates. Repeated dosing of HEP-G-CSF or HEP alone for 2 weeks did not cause HEP-specific toxic effects in rats. HEP did not possess the anticoagulant behavior of its daughter, heparin, based on testing in rats or clinical diagnostic assays with human plasma. Neither anti-HEP IgG nor IgM antibodies were detected in a long-term (9 doses over 7 months) immunogenicity study of the HEP-drug conjugate with rats. These proof-of-concept experiments with HEP-G-CSF indicate that it is a valid drug candidate for neutropenia and suggest the potential of this HEP-based platform as a safe alternative delivery vehicle for other therapeutics.
Keywords: chemoenzymatic synthesis, glycosaminoglycans, heparosan/heparan sulfate, pharmacokinetics, polyethylene glycol
Introduction
Polypeptides or nucleic acids with therapeutic activity are becoming ever increasingly useful modern medicines, but can have problems due to their short plasma half-life and/or negative interactions with the body's various systems. Covalent modification with a polyethylene glycol (PEG) polymer vehicle, called PEGylation, which provides surface shielding to the drugs and/or reduce its rapid elimination, was one of the early successful attempts to rectify these issues resulting in multiple FDA-approved therapeutics (Pasut and Veronese 2012; Kolate et al. 2014). For drugs that are cleared by the action of receptors and/or enzymes (e.g., regulatory proteases, nucleases), the polymer chain sterically hinders access to the drug surface allowing persistence in the bloodstream for extended times. Conjugation also prevents smaller drugs from being lost via renal filtration by increasing the hydrodynamic size of the therapeutic (i.e. typically molecules <60–70 kDa are excreted rapidly). However, PEG employed in drug conjugates has potential liabilities including lack of a safe degradation pathway, accumulation in some tissues, emerging immunogenicity, and potential triggering of the complement system (Verhoef et al. 2014; Zhang et al. 2014). Therefore, PEGylation alternatives have been explored although all have their caveats (Qi and Chilkoti 2015).
Heparosan (HEP; [-4-N-acetylglucosamine-α1,4-glucuronic acid-β1-]n) is the initial unsulfated, unepimerized polysaccharide backbone precursor of heparan sulfate (HS) and heparin, two structurally related essential carbohydrates in animals involved in phenomena including proliferation, inflammation, and hemostasis. HEP should be biocompatible in the body because it is an intermediate in the HS/heparin biosynthetic pathway (DeAngelis 2015). Due to incomplete modification of the sugar chain, stretches of HEP naturally exist in the mature human HS molecules. Certain pathogenic bacteria exploit the “self” nature of this polymer by using an HEP capsule to help evade the immune system during infection (DeAngelis 2002). The immature HEP precursor does not possess the sulfate groups known to be key in the interactions with the plethora of known HS- or heparin-active proteins, therefore, the HEP chain should not be degraded, bound, or cleared thus have an extended lifetime in extracellular spaces and plasma compared to the mature forms (DeAngelis 2015). However, the lysosomal degradation pathway will readily break down any glycosaminoglycan including HEP that is internalized by the cell (DeAngelis 2015); in contrast, PEG is poorly metabolized (with some toxic metabolites) and is known to accumulate in the body (Bendele et al. 1998; Rudmann et al. 2013; Zhang et al. 2014).
We predicted that HEP-drug conjugates would possess larger hydrodynamic sizes and longer plasma half-lives than the free drug plus be more biocompatible and biodegradable than therapeutics with artificial polymers. Unlike most other polysaccharides found in Nature and/or used currently being explored in drug delivery (e.g., polysialic acid, hydroxyethylstarch) (Qi and Chilkoti 2015), the HEP chain can be produced in a very defined, targeted fashion via chemoenzymatic synthesis. This control of molecular weight (MW) and polymer size distribution, and precise installation of drug-coupling sites should allow better control of pharmacokinetics as well as facilitate drug manufacture and regulatory review.
We employed the enzyme PmHS1, a bacterial HEP synthase, to polymerize the HEP molecule in vitro using the two donor uridine-diphospho-sugars, UDP-glucuronic acid (GlcA) and UDP-N-acetylglucosamine (GlcNAc) (Sismey-Ragatz et al. 2007). Recombinant PmHS1 elongates short HEP oligosaccharides in a synchronous fashion to synthesize HEP polymers of very narrow size distribution or “quasi-monodisperse.” Furthermore, by adjusting the stoichiometric ratio of the oligosaccharide acceptor and donor sugars in a reaction, HEP with any desired chain length between ~10 and ~800 kDa (~50 to 4000 monosaccharides) can be produced. As a bonus, when this acceptor is chemically modified at the reducing end with certain functional groups (e.g., amine, aldehyde, azide, etc.), all of the resulting HEP polymers also possess these moieties and thus are readily coupled to drug cargo via common conjugation techniques (U.S. Patent # 9,603,945). In contrast, most other polysaccharides are (i) polydisperse in size, (ii) available and/or useful in a rather limited range of MWs and/or (iii) not completely or uniformly activated for coupling to drug cargo.
Granulocyte-colony stimulating factor (G-CSF) is a hematopoietic growth hormone that regulates proliferation and differentiation of neutrophilic granulocytes and enhances their immunological functions against bacterial infection (Metcalf 1986). Filgrastim, a recombinant human G-CSF (Neupogen®; Amgen), was FDA-approved to treat chemotherapy-induced neutropenia (abnormally low neutrophil count) in cancer patients, but due to its short half-life in circulation, this drug requires multiple daily injections to achieve the desired boost in white blood cells (WBCs). G-CSF, a small ~20 kDa protein, is eliminated from the human body mainly via renal filtration. To overcome this problem, G-CSF was PEGylated with a 20-kDa PEG molecule forming a conjugate with larger hydrodynamic size, Pegfilgrastim (Neulasta®; Amgen), which possesses prolonged half-life and thus requires just a single injection for effect (Arvedson et al. 2015).
Here we report the intrinsic behavior of the HEP polysaccharide in the mammalian body as well as the characterization of efficacy, toxicology, and immune response of HEP-G-CSF conjugates in a rat model.
Results and discussion
HEP pharmacokinetics in nonhuman primates
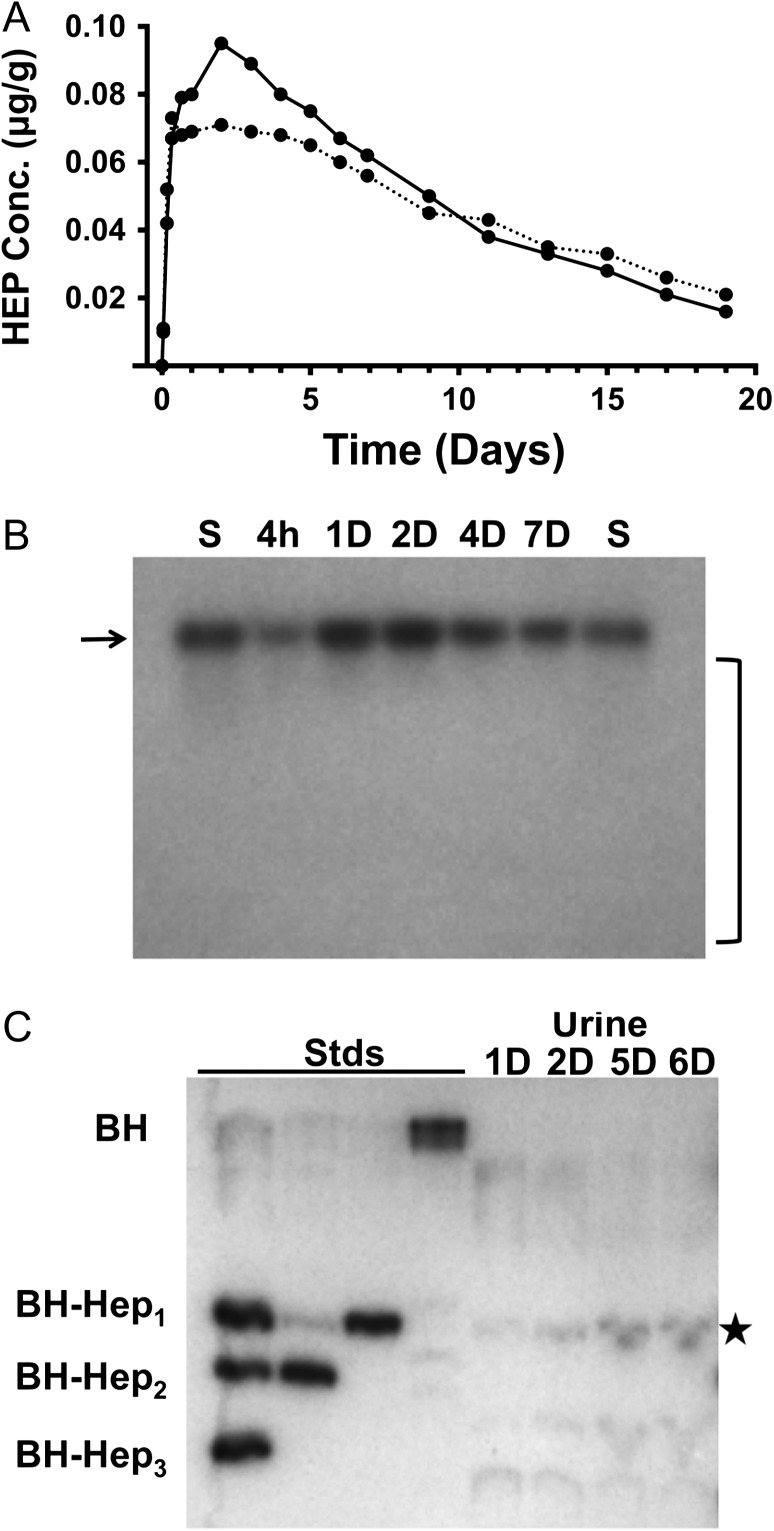
The in vivo behavior of HEP was tested by subcutaneously injecting Cynologus monkeys with radioactive polysaccharide (99 kDa = ~522 monosaccharide units). HEP efficiently transited from the tissue to bloodstream and possessed a long plasma half-life (t1/2 = 192 h or ~8 days; Figure 1A); in contrast, the tiny 125I-Bolton-Hunter tag itself would be excreted within minutes without the polymer. The HEP polymer efficiently migrated from the injection site based on (i) comparing the initial dose to the maximum plasma concentration, Cmax, (0.084 μg/g) values with respect to the animal's estimated blood volume and (ii) the radioactive mass balance (after 456 h = ~2.4 half-lives, ~79% of 125I was excreted versus the 82% predicted mathematically). In addition, in pilot studies with rats with similar 125I-HEP probes, after a few days, there was no detectable radioactivity at the intramuscular injection site in postmortem measurements (unpublished). Gel electrophoresis showed that the size of the injected HEP in the circulation for days and the starting polymer was equivalent (Figure 1B), thus HEP is very stable in the extracellular spaces, lymph, and blood.
Fig. 1.
Behavior of the heparosan (HEP) polysaccharide in nonhuman primates. (A) Plasma pharmacokinetics of radioactive Bolton-Hunter (BH) tagged HEP (99-kDa or ~500 sugars units long) in Cynologus macaques (n = 2) after subcutaneous injection; a ~8-day half-life is observed. (B) Agarose gel (1%, 1× TAE) and autoradiographic analysis of blood samples from Panel A. The size of starting probe (S) and circulating polymers at different times (hours, h; days, D) were equivalent (marked with arrow); no substantial degradation products accumulate in the blood (absence of signal in the bracketed area). (C) Thin-layer chromatography of urine metabolites of the radioactive HEP probe; the major excreted species migrated as one GlcA monosaccharide attached to the BH probe residue (BH-Hep1) and is marked with a star (Stds, synthetic oligosaccharide standards; two or three sugars attached to BH = BH-Hep2 or BH-Hep3, respectively).
If HEP is internalized into cells (pinocytosis, etc.) and transported to lysosomes, it should be degraded by resident glucuronidase and hexosaminidase exoglycosidases, as seen for heparin or hyaluronan (DeAngelis 2015). We observed that the original ~522-sugar long 125I-probe was indeed broken down into very small fragments composed of ~1 to 3 sugars (Figure 1C) that were efficiently excreted into the urine and feces. In fact, this resulting GlcA-modified residue is structurally akin to the metabolites of the vertebrate glucuronidation pathway used to increase solubility and to facilitate excretion of various drugs, hormones, and pollutants. Another advantage is that the HEP backbone's degradation products, GlcNAc and GlcA, normal monosaccharides in animals, are nontoxic and either recycled or metabolized by cells. Therefore, this HEP-based drug-conjugate configuration is a virtually “zero-residue” system. On the other hand, PEG can yield aldehyde and ketone metabolites as well as accumulate in tissues, even creating vacuoles due to the build-up (Bendele et al. 1998; Rudmann et al. 2013).
HEP-G-CSF conjugate synthesis and characterization in vitro and in silico
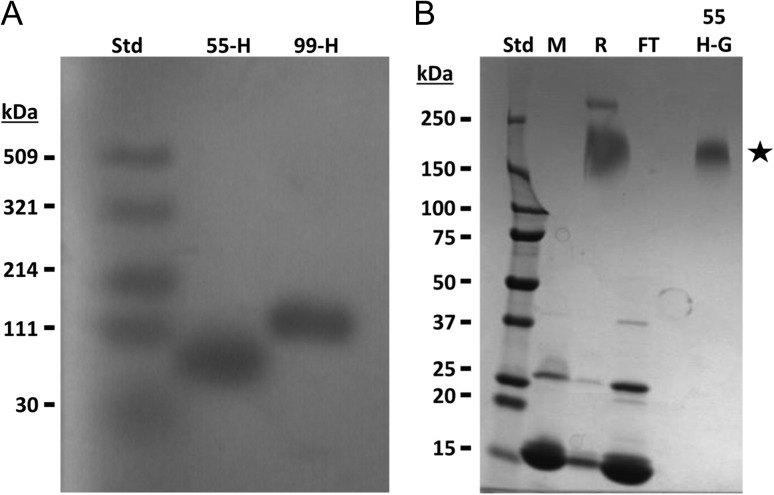
The 55-kDa and 99-kDa HEP-amine (HEP-NH2) polymers were produced via in vitro polymerization with HEP-amine trisaccharide acceptor (Figure 2A; Supplementary Fig. 1). These sizes, larger than Neulasta's 20-kDa PEG chain, were selected in an effort to more effectively shield the G-CSF surface; the practical upper limit for PEG in injectable drugs is typically ~20–40 kDa because larger MW PEG are excreted very slowly with increased propensity to accumulate in the body. The HEP-NH2 polydispersity (Mw/Mn), an indication of size distribution, was 1.005–1.007 (“1” is the value for a perfect ideal monodisperse polymer) by multiangle light scattering analyses (Baggenstoss and Weigel 2006). These HEP-NH2 polymers were then converted to HEP-aldehyde and used to conjugate to the protein drug's N-terminus via reductive alkylation in analogy to the PEG-G-CSF drug synthesis (Kinstler et al. 2002).
Fig. 2.
Electrophoretic analyses of HEP-amine and HEP-G-CSF conjugate. (A) HEP-amine polymers were run on an agarose gel (1%, 1× TAE) with Stains-All detection. Quasi-monodisperse polymers were produced via chemoenzymatic reaction. Std, Select-HA® Ladder (note: hyaluronan standards do not run equivalently to HEP); 55-H, 55-kDa HEP-NH2; 99-H, 99-kDa HEP-NH2. (B) Samples from the HEP-G-CSF preparation process were separated on a 4–20% SDS-PAGE gradient gel and Coomassie Blue stained (notes: HEP, a carbohydrate, does not stain here; migration of the conjugate is not simply additive of the HEP and protein MWs due to differences in the components’ charge density and shape). Std, Precision Plus® protein standard; M, free G-CSF protein; R, conjugation reaction; FT, uncoupled protein in column flow-through; 55H-G, purified 55-kDa HEP-G-CSF conjugate (star). Addition of HEP polymer to G-CSF retards the mobility of the protein during electrophoresis compared to the free protein.
The HEP-G-CSF conjugates (shown schematically in Figure 3) were characterized by electrophoresis (Figure 2B), chromatography, and a cell proliferation assay (Matsuda et al. 1989) (Supplementary Figs. 2, 4); new larger MW species with bioactivity were detected. As a benchmark control and to assess processing of the drug in our hands, a 20-kDa PEG-G-CSF conjugate (same size as the commercial Neulasta) was also prepared in-house (Supplementary Fig. 3).
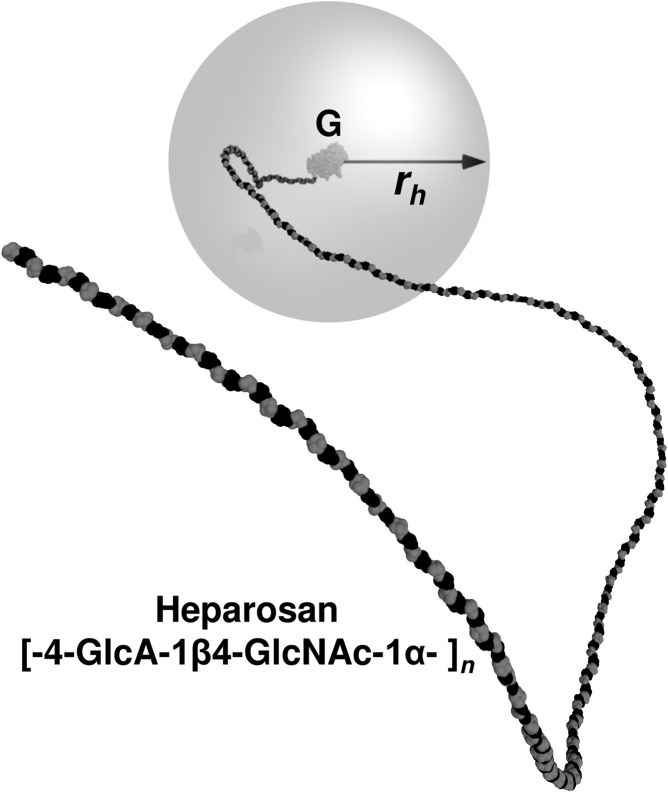
Fig. 3.
Molecular simulation-based model of HEP-modified G-CSF. The covalently attached HEP sugar chain increases the hydrodynamic radius (rh) and partially shields the surface of a drug such as a biologic (e.g., polypeptide, aptamer, etc.). In this work, the amino terminus of the G-CSF protein (G; rh ~2 nm) was attached via reductive amination to the reducing end of the HEP polymer (chain with black GlcA and gray GlcNAc sugars). In this molecular simulation model of the 55-kDa HEP-G-CSF, the gray sphere at 20 nm radius is roughly coincident with the predicted average rh. The HEP conformation depicted was a simulation snapshot with the average rh.
Heparin lysase III and tryptic peptide mapping of HEP-G-CSF yielded a major species corresponding to the drug's N-terminal peptide “MTPLGPASSLPQSFLLK” with an additional 715.22 Da sugar “stub” (equivalent to the HEP trisaccharide digestion product plus linker post-reductive amination; see Supplementary Figs. 1, 5) indicating that the HEP was indeed attached to the drug as designed. As a negative control, unmodified G-CSF treated and analyzed identically did not yield species corresponding to the stub-peptide mass. It is interesting to note that such mapping experiments to localize polymer attachment sites are not possible with PEG-conjugates as there is no facile, gentle method to remove the artificial polymer from the biologic; this drawback is a current problematic issue for quality control and manufacture in industry.
In order to predict the relative shielding of the G-CSF drug (hydrodynamic radius, rh = ~2 nm) conferred by various lengths of the HEP polymer chain, coarse-grained molecular simulations were employed. These models calculate that the rh is ~7, 18 or 32 nm for the 20-, 55- or 99-kDa HEP-G-CSF conjugates, respectively (Table I, Supplementary Fig. 6). Therefore, it is expected that access of the pharmacophore (binding site) on the drug to the G-CSF receptor would be blocked part of the time due to steric hindrance imparted by the HEP chain, thus allowing longer circulation times and extended pharmacokinetics. In addition, the size of these HEP conjugates should be above the renal filtration threshold thus preventing or slowing excretion into the urine.
Table I.
Relationship of heparosan (HEP) chain size and hydrodynamic radius (rh)
| Mw (kDa) | Chain size (monosaccharides) | rh (nm) | Average # of sugars within rh (monosaccharides) |
|---|---|---|---|
| 20 | 106 | 6.5 | 12b |
| 55 | 290 | 17.8 ± 0.1a | 56b |
| 99 | 522 | 31.6 ± 0.9a | 99b |
A series of 800 ns molecular simulations (including a 40 ns equilibration period that was discarded from calculations) were employed to estimate the hydrodynamic radius of a single HEP chain (20-, 55- or 99-kDa) attached to a G-CSF protein. The average number of sugars residing within a sphere with dimensions of the rh is also presented. The HEP chain generates a more substantial barrier around the central drug as the polymer length increases.
aPrediction errors estimated from the range of the cumulative moving average rh over the last 200 ns of the respective simulation, where they were appreciable.
bAverage value rounded to the nearest whole number.
The HEP-G-CSF preparations were also tested for endotoxin (derived from Gram-negative bacterial surface molecules present in small amounts in the recombinant PmHS1 enzyme as well as the environment). This potential contaminant has been reported to influence neutrophil levels in mice (Copeland et al. 2005; ~2-fold perturbation with ~10 ng dose) as well as trigger G-CSF production in certain human cells (Tazi et al. 1991; alteration from baseline when more than ~10 ng/mL). The 55-kDa and 99-kDa HEP conjugates and the PEG-conjugates had <1.7, <6 or <1 endotoxin units/mg (1 EU = ~0.2 ng endotoxin per FDA), respectively. Most of the endotoxin originated from the Escherichia coli-derived recombinant G-CSF drug itself as supplied by the vendor. Therefore, these conjugates were suitable for the efficacy studies (described in the next paragraph) as the endotoxin load/dose was roughly >200-fold less than the level needed to stimulate the above potential artifacts as well as far below the recommended rat endotoxin dosing limit of <~90 EU/mg (Malyala & Singh 2008).
HEP-G-CSF conjugate efficacy testing in rats
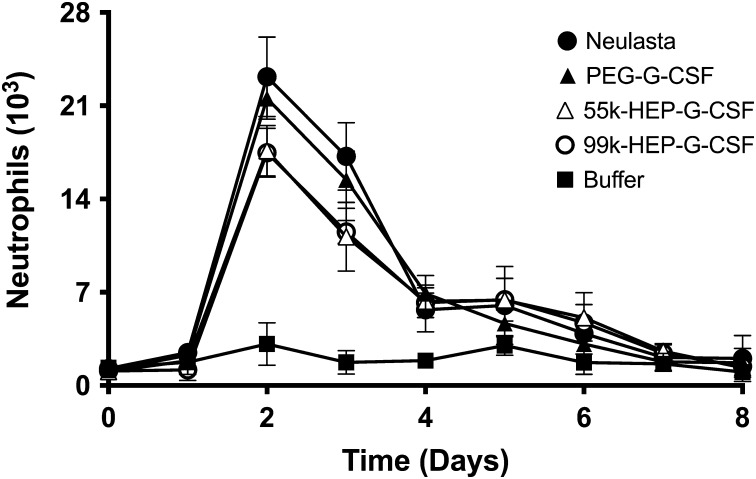
The therapeutic effect of 55-kDa and 99-kDa HEP-G-CSF in healthy rats was evaluated using Neulasta® (commercial 20-kDa PEGylated G-CSF) and in-house prepared 20-kDa PEG-conjugates as benchmarks for dosing and performance (Crobu et al. 2014). Changes in hematology in all four of the treatment groups were observed (Supplementary Table 1 and Supplementary Fig. 7). WBC counts were statistically higher compared to the buffer control for all G-CSF conjugates on Days 2–3 and increases in the absolute neutrophils were observed (Figure 4). HEP-G-CSF is predicted to share the same mechanism as PEG-G-CSF for achieving its therapeutic effects, i.e. by slowing down the receptor-mediated clearance of the protein thus allowing continued stimulation of the bone marrow to produce new WBC. The PEG-conjugates stimulated somewhat higher WBC levels in rats than HEP conjugates at early times, but the HEP-G-CSF conjugates had slightly higher or similar levels later (Days 5–6). The PEG-versions, optimized for years in comparison to our new HEP-based system, can actually cause very high WBC proliferation that induces a debilitating bone pain side effect (i.e. too many cells in confined bone marrow space). The two HEP-G-CSF samples used in this efficacy test were discovery-stage preparations that have not been extensively optimized for formulation (including HEP chain size), stability or potency.
Fig. 4.
Neutrophil counts in blood of rats treated with various G-CSF conjugates. The HEP conjugates (white symbols) increased the level of neutrophils in a similar fashion as the PEG benchmarks (black circles, triangles) indicating utility for treating neutropenia.
Additional hypotheses for the small efficacy differences observed between the HEP- and PEG-conjugates include: (i) varying rates of transport through the body (PEG is neutral while HEP is highly negatively charged), and/or (ii) potentially higher HEP-mediated shielding of the G-CSF surface (i.e. less access to the receptor docking site due to greater steric hindrance) as indicated by lower in vitro potencies in the M-NFS-60 cell proliferation assays (Supplementary Fig. 4).
In summary, these data demonstrate HEP-G-CSF-mediated stimulation of the growth and differentiation of neutrophils, a pharmaceutical marker reflecting the clinical benefit for neutropenic patients.
Safety testing of HEP and HEP-G-CSF
A repeated dosing toxicological study in rats based on the Neulasta preclinical work (Crobu et al. 2014) was performed with 55-kDa HEP-G-CSF, and its two components injected alone, either 55-kDa HEP-amine or G-CSF. Typically Neulasta is dosed once every ~2–3 weeks, but here the test articles were tested every other day for 2 weeks at a 5-fold higher dosage than used in treatment. In general, all compounds were well tolerated in comparison to the vehicle control; all rats survived until the scheduled termination. No toxicological response was observed in terms of clinical signs, and changes in body weight or in food consumption. There were no adverse hematology, coagulation, or blood chemistry changes for HEP, HEP-G-CSF conjugate or G-CSF groups. General hematology changes observed in HEP-G-CSF and G-CSF groups were similar between the two groups and were considered to be due to the known pharmacological action of G-CSF and were microscopically correlated with increased hematopoiesis observed in bone marrow, spleen and liver. In regard to histopathology, except for minimal and sporadic individual cell death in liver, granulopoiesis observed in bone marrow and extramedullary hematopoiesis observed in liver and spleen were considered to be due to pharmacologic effects of the G-CSF in these test articles. Vacuoles or inclusion bodies were neither noted in kidneys nor choroid plexus of brain (side effects observed in some PEG treatments; Rudmann et al. 2013) in any of the treatment groups.
HEP was also tested in the prothrombin time diagnostic assay with human plasma because we had some initial concerns that the coagulation pathway could potentially be affected by this precursor of HS and heparin. Fortunately, even at a dosage of 15,000-fold higher than the therapeutic heparin control, HEP had no inhibitory effect on coagulation, thus alleviating another concern for potential side effects with this drug delivery system.
Immunogenicity testing of HEP-G-CSF in rats
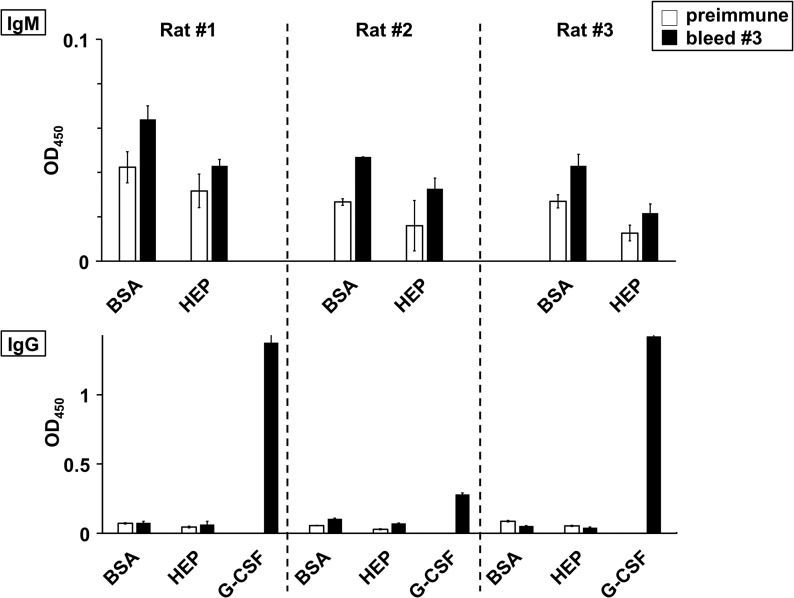
To further evaluate safety, a long-term immunological study of 55-kDa HEP-G-CSF was performed in rats (schedule in Supplementary Fig. 8). While antibodies against human G-CSF started to appear as early as in the first postinjection bleed (the rat and the human proteins are nonidentical at the amino acid sequence level and the attachment of a single HEP chain is not expected to completely shield the drug surface from the immune system), no IgM or IgG antibody directed against HEP was detected even after nine injections of HEP-G-CSF over the 7-month span (Figure 5). Both preimmune and immunized sera had comparable signals for the wells with BSA alone, the negative control for assessing assay background, and the wells with HEP-BSA conjugate.
Fig. 5.
HEP-G-CSF long-term immunological study in rats by ELISA. Healthy rats were repeatedly challenged with HEP-GSF (nine injections in ~7 months) and several bleeds were collected (see Supplementary Fig. 7 for study design) for testing by ELISA (0.5 or 5 μL sera/well for IgM or IgG, respectively, in anti-HEP tests with 30 min colorimetric development; 1 μL sera/well for IgG in anti-G-CSF tests with 15 min development). Representative data from averaged triplicate wells from the preimmune (white bars) or the third, final (black bars) bleeds are shown. There were no anti-HEP-specific immunoglobulin IgM (top) or IgG (bottom) detected because the signals from wells coated with HEP-BSA (HEP) were not higher than the signal from the negative control BSA-coated wells (BSA). On the other hand, an anti-G-CSF response (G-CSF) was detected in all the three rats (due to the nonidentical sequence of the human protein drug). Overall, this data indicates that HEP itself is not immunogenic.
In addition to the HEP chain itself, we also considered issues with the potential immunogenicity of the linker. The predicted in vivo residual sugar-linker-amino acid metabolite (GlcA-alkyl/benzyl-methionine derived from the action of human lysosomal exoglycosidases and protease enzymes) is very small and is expected to be excreted quickly because it structurally mimics a typical glucuronidation metabolic pathway product. Work with polysaccharide-protein antigens used in bacterial capsular vaccines suggested that longer glycans (>~8 repeat units) on the peptide are needed to stimulate high-level antibody production (Avci et al. 2011); the predicted HEP-conjugate metabolite is much smaller than this threshold.
Industry has been looking for PEGylation alternatives to address its potential limitations and/or bring additional benefits to drug conjugates. PEG lacks a natural, efficient degradation system and thus has been associated with the accumulation of intracellular vacuoles in animals (Bendele et al. 1998; Rudmann et al. 2013), which could become a concern for long-term pediatric or genetic disease treatments. PEG has also been reported to induce anti-PEG antibodies (Richter and Akerblom 1984; Veronese 2001; Armstrong et al. 2007; Garay et al. 2012; Hershfield et al. 2014; Ganson et al. 2015). Studies correlate the existence of preexisting or induced anti-PEG with poor or nonresponsiveness to some PEGylated drugs. A significant percentage (~22–25%) of normal blood donors show evidence of anti-PEG antibodies (Richter and Akerblom 1984), while this number was only 0.2% about two decades earlier (Hershfield et al. 2014), suggesting that anti-PEG antibodies may result from exposure of “free” PEG which has been used evermore as excipients or ingredients in cosmetics, toothpaste, laxatives, and other over-the-counter products. Recently, a phase III clinical study testing a PEG-aptamer was halted because one patient died from an anaphylactic reaction. Researchers postulated that high levels of preexisting anti-PEG found in this patient was a major, although not the sole, factor necessary for triggering the “first-exposure” allergic reaction (Ganson et al. 2015).
Conclusions
HEP is a natural molecule in human body and has a known robust degradation pathway. It is reasonable to predict that HEP is a safe molecule, and indeed, our toxicological and immunological studies did not raise concerns. Therefore, HEP-modified drugs may be able to rescue patients with preexisting PEG antibodies and/or be used for life-long therapies where multiple drug exposures are likely.
Future work will focus on more size-ranging tests (i.e. use different HEP chain lengths) in rats as well as expand to other animal model systems. As the polymer size and hydrodynamic radius is increased, the steric hindrance or shielding effect on the cargo also increases, thus can lead to longer plasma half-lives. However, after a certain polymer size, a plateau of half-life and efficacy is typically observed (unpublished studies). In addition, these larger polymers display more viscosity and can be more difficult to handle during processing or patient administration. Balancing these factors and empirical testing is needed to tune the “sweet spot” of the HEP chain for each therapeutic.
In summary, the combination of the intrinsic behavior of HEP and our synthetic strategy for HEP-based delivery vehicles yields a promising platform (trademarked HEPtune®) for upgrading current PEGylated therapeutics as well as boosting performance of emerging drug candidates. In addition, this system is new intellectual property (US Patent # 9,603,945 and others) thus allowing innovative options for lifecycle management and expanding markets.
Methods
Materials
The highest grade chemicals and buffer components were purchased from Sigma-Aldrich, Amresco, or Thermo Fisher unless otherwise indicated. Neulasta® (Amgen) was purchased from a pharmacy.
Preparation of HEP-NH2 and HEP-aldehyde polysaccharides
First, HEP-amine (HEP-NH2) was polymerized by the action of PmHS1, an HEP synthase enzyme, building onto a modified trisaccharide acceptor, HEP3-NH2 (Supplementary Fig. 1). The MW of the polymerization products was controlled by the molar ratio of UDP-sugar precursors to acceptor (Sismey-Ragatz et al. 2007). Typically, in vitro HEP polymerization reactions contain 0.2–0.5 mg/mL of PmHS1 (Sismey-Ragatz et al. 2007), 40 mM UDP-GlcNAc (Roche), 40 mM UDP-GlcA (Sigma or Roche), 50 mM HEPES, pH 7.2, 1–25 mM MnCl2 and variable amounts of acceptor.
After the reactions were incubated at 30°C for 16–40 h, the polymer was purified by strong anion exchange chromatography (SAX) with a linear NaCl gradient (0.1 to 1 M) on Q Sepharose resin (GE) with monitoring at 215 nm (from carbonyls of GlcA and GlcNAc) and ultrafiltration against water to concentrate and de-salt the target-containing fractions. The amount of polysaccharide was quantitated by the carbazole assay with a glucuronic acid standard (Bitter and Muir 1962); typical yields were ~85–95% based on UDP-sugar input. The MW was assessed by agarose gel electrophoresis with Stains-all detection (Lee and Cowman 1994) using hyaluronan standards (LoLadder; Hyalose, LLC, Oklahoma City, OK) and multiangle laser light scattering (Baggenstoss and Weigel 2006) (MALLS; service provided by Department of Biochemistry and Molecular Biology of University of Oklahoma Health Sciences Center). The endotoxin level was measured by the Limulus amebocyte lysate (LAL) assay following the manufacturer's protocol (Lonza Pyrogent Gel Clot assay; Basel, Switzerland).
Second, the HEP-NH2 polysaccharide was converted to HEP-aldehyde (HEP-CHO) by reaction with a ~40- to 50-fold molar excess of succinimidyl-p-formylbenzoate (Chem-Impex International Inc; Wood Dale, IL) (Supplementary Fig. 1). The latter reagent in dimethylsulfoxide (DMSO) was slowly added into a gently mixing solution of HEP-NH2 in 100 mM HEPES, pH 7.2 (final DMSO concentration ~20–30%). After incubation at room temperature for 2–3 h with gentle stirring, the polymer was precipitated by the addition of NaCl (0.2 M final) and 2.5 volumes of ethanol. After cooling on ice for ~0.5 h, polymer was collected by centrifugation and then re-dissolved in water. Traces of the aldehyde reagent were further removed by SAX on Q Sepharose as above. The eluate fractions containing HEP-CHO were alcohol precipitated again and re-dissolved in water, and stored frozen at −80°C. The polymer amount, size and endotoxin levels were measured as above.
Preparation of radioactive 125I-HEP probe
A portion of the 99-kDa HEP-NH2 was labeled with Bolton-Hunter-N-hydroxysuccinimide (BH-NHS; Perkin Elmer NEX 120). The BH-NHS stock in benzene (250 μCi; 2200 Ci/mmol) was dried down in the shipping vial with a gentle stream of air (via a syringe needle in a fume hood with a charcoal filter on the outlet port to capture any potential stray radio-iodine), then re-dissolved in 30 μL dry DMSO before the addition of a solution of HEP-NH2 (200 μg) in HEPES, pH 8.4 buffer (50 mM final) with vigorous mixing. After 80 min at room temperature, to remove any free BH reagent, 100 μL water was added to the reaction, mixed, and then extracted with an equal volume of n-butanol followed by centrifugation. The aqueous phase with target BH-HEP probe was removed and the lower phase was back-extracted with water and n-butanol again. The aqueous phases were pooled and then applied to a Sephadex G-50 (PD-10 column; GE) run in 0.2 M ammonium formate (operation performed in fume hood). The void volume fractions with radioactive probe were lyophilized twice from water to remove the volatile buffer salts (note: vial with a charcoal filter lid was employed to trap any volatile radio-iodine), re-dissolved in PBS, and filter-sterilized (0.45 μm) before injection into animals (next section). The final radiochemical specific activity of the probe was ~86 Ci/mmol.
HEP pharmacokinetics in nonhuman primates
The in vivo efficacy study was performed by a contract research organization (Xenometrics, LLC, Stilwell, Kansas; protocol approved by IACUC) according to the principles of Good Laboratory Practice (GLP) in an AAALAC-accredited facility. Two young adult male Cynologus macaques (5–8 years old; 5.6–6 kg) were dosed with the 125I-HEP probe (each 63 μCi in 1 mL; ~11 μg/kg) by subcutaneous (SC) injection between the shoulder blades in a shaved area. Samples of blood, urine (from collectors of metabolic cages and cage rinses), and feces were collected periodically over 19 days. Animal health, weight, and food consumption were monitored daily.
Plasma was obtained from whole blood by centrifugation (1811 × g, 10 min at 4°C) and stored frozen. Urine was stored frozen. Feces was homogenized with three parts water by weight to facilitate dispensing and frozen. Portions of the samples were subjected to radioanalysis; plasma radioactivity values were subjected to pharmacokinetic (PK) analysis for determination of simple PK parameters including half-life by the noncompartmental method using WinNonlin Professional ver 5.2 (Pharsight Corp, Mountain View, CA). Radioactivity in blood and plasma were measured in duplicate while urine and feces were in triplicate. The animals showed no evidence of distress or adverse clinical observations and were returned to the colony at the study conclusion after quarantine.
Urine metabolites were analyzed by normal phase thin-layer chromatography on high performance silica plates with a loading zone (Whatman Partisil LHPKDF). Radioactive BH-labeled HEP monosaccharide, disaccharide or trisaccharide standards (containing the same aglycone) were tagged in a similar fashion as the 99-kDa HEP probe, except that the unincorporated probe was removed using n-butanol extraction. After spotting the urine and drying without heat, the plate was prerun with 5:1 n-butanol/acetic acid and dried; this step swept some small salts away from the sample to allow better analyte migration. The plate was then developed with n-butanol/acetic acid/water solvent (1.5:1:1). After the run, the plate was dried and exposed to X-ray film (Biomax MR; Kodak, Rochester, NY) at −80°C with an enhancing screen.
Preparation of HEP-G-CSF conjugates
Reductive amination was employed to couple the HEP-CHO polysaccharide to the amino terminus of the G-CSF (Supplementary Fig. 1) drug in analogy to the manufacture of Neulasta®. In general, the reactions contained a 5:1 to 8:1 molar ratio of HEP-CHO reagent and E. coli-derived recombinant human G-CSF (ProSpec; Ness-Ziona, Israel) protein (final ~20–35 and 0.9–3 mg/mL, respectively). A solution of G-CSF at 1–5 mg/mL in 100 mM sodium acetate, pH 5.0, was added to a vial containing HEP-CHO solution in the same buffer. The mixture was incubated for ~30 min at room temperature with gentle mixing by rolling to allow the initial step of the conjugation, Schiff base formation, to occur. Then sodium cyanoborohydride in water was added to the solution (final 75–100 mM) and the reaction was incubated for ~16 h at room temperature with gentle rolling.
After incubation, the reaction mixture was diluted with water to 0.5 mg/mL G-CSF and the solution was loaded onto HiTrap Q Sepharose HP SAX column (GE; prepacked 5 mL column or self-packed in GE XK column, depending on the scale) preequilibrated with 20 mM sodium acetate, pH 5.0 (buffer A) at a flow rate of ~30 cm/h. The column was washed with five column volumes (CVs) of buffer A and the conjugate was eluted with a step gradient to 50% of buffer B (1 M NaCl in buffer A) over 20 CVs. The fractions with absorbance at 280 nm (corresponding to protein) were analyzed by SDS-PAGE and the target conjugate-containing fractions (~0.3–0.45 M NaCl, depending on the MW of the conjugates) were concentrated and exchanged into “G-CSF Storage Buffer” (10 mM sodium acetate, pH 4.0–4.2, 5% sorbitol) with Amicon-15 ultrafiltration spin units (Millipore) with molecular weight cut off (MWCO) of 3 kDa or 10 kDa, depending on the MW of the conjugates. The conjugate solutions were then filter-sterilized through 0.2 μm Costar spin filter units (Corning), aliquoted, and stored at −80°C.
The LAL assay was used to measure endotoxin levels. All conjugate concentrations indicated in this study are based on the concentration of protein (i.e. G-CSF) determined via the Bradford assay (BioRAD; Hercules, CA) with a bovine serum albumin (BSA) standard (Bradford 1976). HEP-conjugate purity (typically ~79–97%) was analyzed by HPLC size exclusion chromatography on a BioSep-SEC-S 2000 column (Phenomenex; Torrance, CA) eluted with phosphate-buffered saline using detection at 280 nm; no free G-CSF was detected. Overall conjugate yields (based on the starting protein) were ~27% for the 55-kDa HEP or ~7% for the 99-kDa conjugates.
Preparation of PEG-G-CSF
The modification of G-CSF with 20-kDa mPEG-butyraldehyde (Laysan Bio Inc., Arab, AL) was done in a similar fashion as for HEP-G-CSF except that the purification employed cation exchange chromatography rather than SAX. The conjugation reaction mixture was diluted with 20 mM Na acetate, pH 4.0, (buffer C) so the final concentration of G-CSF was no more than 0.5 mg/mL and the pH was adjusted to about 4.0 with 100 mM HCl. The solution was loaded onto HiTrap SP Sepharose HP column (GE) preequilibrated with buffer C at a flow rate of ~150 cm/h. The column was washed with 5 CVs of buffer C and the PEG-G-CSF conjugate was eluted with a step gradient of 10%, 15%, 20%, 25%, 35% and 50% of Buffer D (1 M NaCl in buffer C). The fractions having an absorbance at 280 nm were analyzed by SDS-PAGE and the conjugate eluate (~0.15 M NaCl) was concentrated and exchanged into G-CSF Storage Buffer in Amicon-15 spin units with 3 kDa MWCO. The conjugate solution was then sterile filtered through 0.2 μm Costar spin filter units, aliquoted, and stored at −80°C. The yield (~20% based on starting protein) and purity (~60%; no free G-CSF was detected) were estimated as for HEP-G-CSF.
Gel electrophoresis of conjugates
SDS-PAGE of intact conjugates was performed using premade 4–20% TGX gradient gels (BioRAD) and samples were diluted with reducing buffer. The gels were run in Tris/Glycine/SDS buffer (BioRAD) at 100 V and were stained with Coomassie Blue. The HEP- and PEG-drug conjugates both ran with slower migration as expected for molecules with a higher MW than the parental drug. In addition, the relative staining intensity is also reduced in both cases upon polymer attachment.
Agarose gel electrophoresis with Stains-All detection (Lee and Cowman 1994) was employed to confirm that a single HEP chain was attached to the G-CSF protein. In this mode of electrophoresis, the addition of a small protein (especially the ~20 kDa globular drug used here) does not significantly affect the mobility of the long polysaccharide (as noted in Jing et al. 2006). The starting HEP-CHO polymers and the HEP-G-CSF conjugate products tested in this work displayed roughly equivalent mobility in this gel system (not shown).
Peptide mapping of HEP chain location on G-CSF conjugate by liquid chromatography-mass spectrometry
The location of the HEP chain on the protein was determined by digesting the sugar chain to a short 3-sugar “stub” using an HEP-specific bacterial glycosidase, fragmenting the protein by protease digestion, and identifying the modified peptide by mass spectroscopy (Supplementary Fig. 1, structure “D”). The HEP-G-CSF preparations (5 μg of protein) were treated with heparin lyase III (gift from Dr. Robert Linhardt, RPI) in 20 mM Tris, pH 7.2, overnight at 30°C. A parallel sample of unmodified G-CSF was treated identically. The mixtures were run on a 15% SDS-PAGE gel, stained with Coomassie Blue, destained (5:5:90 acetic acid/methanol/water), and the relevant protein bands excised from the gel for analysis by the University of Oklahoma Health Sciences Laboratory for Molecular Biology and Cytometry Research.
The gel pieces were fully destained with 25 mM ammonium bicarbonate in 50% acetonitrile, reduced with 55 mM Tris[2-carboxyethyl]phosphine in 25 mM ammonium bicarbonate, alkylated with 100 mM iodoacetamide in 25 mM ammonium bicarbonate, dried, and then digested with 100 ng trypsin in 25 mM ammonium bicarbonate at 37°C overnight. The digest solutions were collected, dried, and then reconstituted in 10 mM ammonium formate, pH 10.0.
Liquid chromatography tandem mass spectrometry was performed by coupling a nanoAcquity ultraperformance liquid chromatograph (UPLC) (Waters Corp., Manchester, UK) to a Q-TOF SYNAPT G2S instrument (Waters). Each protein digest (~100 ng of peptide) was delivered to a trap column (300 μm × 50 mm nanoAcquity UPLC NanoEase Column, 5 μm BEH C18; Waters) at a flow rate of 2 μL/min in 99.9% solvent A (A = 10 mM ammonium formate, pH 10, in HPLC grade water). After 3 min of loading and washing, peptides were transferred to another trap column (180 μm × 20 mm nanoAcquity UPLC 2G-V/MTrap, 5 μm Symmetry C18; Waters) using a gradient from 1–60% solvent B (B = 100% acetonitrile).
The peptides were then eluted and separated on an analytical column (7.5 μm × 150 mm nanoAcquity UPLC, 1.8 μm HSST3, Waters) equilibrated in 99% buffer C (C = 0.1% formic acid in water)/1% buffer D (D = 0.1% formic acid in acetonitrile) at a flow rate of 200 nL/min using a gradient from 1–40% buffer D for 60 min. The eluent was sprayed via PicoTip Emitters (Waters; spray voltage, 3.0 kV; sampling cone voltage, 30 V; source offset, 60 V; source temperature 70°C; cone gas flow, off; nano flow gas pressure, 0.3 bar; purge gas flow, 750 mL/h). The SYNAPT G2S instrument was operated in data-independent mode with ion mobility (HDMSe). Full scan MS and MS2 spectra (m/z 50-2000) were acquired in resolution mode (20,000 resolution full width at half maximum at m/z 400). Tandem mass spectra were generated in the trapping region of the ion mobility cell by using a collisional energy ramp from 20 V (low mass, start/end) to 35 V (high mass, start/end). A variable ion mobility spectrometry wave velocity was used (ramped from 300 m/s to 600 m/s (start to end) over the full cycle). A manual release time of 500 μs was set for the mobility trapping and a trap height of 15 V with an extract height of 0 V. The pusher/ion mobility synchronization for the HDMSe method was performed using MassLynx V4.1 and DriftScope v2.4. LockSpray of Glufibrinopeptide-B (m/z 785.8427) was acquired every 60 s and lock mass correction was applied post acquisition.
Raw MS data were processed by the ProteinLynx Global Server (Waters) for peptide and protein identification. MS/MS spectra were searched against a FASTA file containing CSF3_HUMAN and common contaminants of interest with the following search parameters: full tryptic specificity (up to two missed cleavage sites), carbamidomethylation of cysteine residues were set as fixed modifications, and N-terminal protein acetylation, methionine oxidation, and the Hep3-linker stub modification (+715.2216 Da) as variable modifications.
M-NFS-60 cell proliferation assay for G-CSF activity
The in vitro assay was performed as described previously (Cox et al. 2004) except a different live cell counting stain method was employed. In brief, murine M-NFS-60 cells (ATCC # CRL-1838; Manassas, VA) were plated at 5–10 × 103 cells/well in RPMI-1640 (Gibco) with 10% fetal bovine serum (Atlanta Biologicals) after a thorough wash with PBS. The cells were treated with either: media alone, 30 ng/mL M-CSF, or PEG-/HEP-G-CSF in serial dilutions ranging from 50 ag/mL to 50 ng/mL at 37°C, 5% CO2 for 3–4 days. Each concentration was tested in triplicate wells. CellTiter 96® AQueous One solution (Promega) was added and incubated for 3 h according to the manufacturer's instructions. Absorbance was measured at 490 nm and triplicate data averaged. The effective concentration for 50% growth stimulation (EC50) was measured as the midpoint between the average background absorbance (no G-CSF control) and the average maximal signal (peak of the titrations).
Theoretical modeling of HEP-G-CSF conjugates in silico
Computer simulations investigated the relationship between HEP chain size and the conjugate hydrodynamic size as in the accompanying paper (Lane et al. 2017). Briefly, coarse-grained representations of a single 20-, 55- or 99-kDa HEP polymer chain (defined as 106, 290 or 522 monosaccharide units, respectively) anchored onto an impenetrable sphere with the dimensions of the G-CSF molecule (2 nm radius, based on structural dataset 1GNC in the Protein Data Bank; www.rcsb.org) and simulated for 840 ns; the assemblies were allowed to equilibrate for the first 40 ns and this data was discarded (i.e. not used in any subsequent analyses). Molecular configurations were output every 25 ps and appended to the coordinates of 1GNC, which was moved and orientated such that: (i) it was completely within the modeled impenetrable sphere and (ii) its N-terminus was adjacent the chain anchor. These coordinates were used to calculate the radius of gyration of the conjugated system as a function of simulated time. It was assumed that the combined system behaved as a flexible linear chain and thus the hydrodynamic radius (rh) is approximately 1.5 times the calculated radius of gyration (Konishi et al. 1991), allowing it to be estimated. The predicted hydrodynamic radii were averaged over the latter 800 ns of simulated time for each HEP polymer conjugate (Table I, Supplementary Fig. 6).
To assess the proportion of the HEP chain within the conjugate's hydrodynamic radius (i.e. the more shielded volume around the drug) at each time point in the respective simulations, the combined HEP-G-CSF molecule was moved such that the origin was placed at the protein component's center of mass. Counting from the reducing end attachment site, each monosaccharide residue was sequentially tested to see if it was wholly contained within the precalculated rh distance and the number of the last sugar within rh was recorded. These numbers were averaged over each respective simulation to arrive at the average number of monosaccharides within the rh.
Subcutaneous single-dose comparison study with HEP-G-CSF and PEG-G-CSF in healthy rats
The in vivo efficacy study was performed by a contract research organization (Xenometrics LLC) according to the principles of GLP and the protocol was IACUC-approved and conducted in an AAALAC-accredited facility. Male Sprague-Dawley rats (n= 5–7) were randomized and dosed by subcutaneous injection (SC) into a shaved region in the dorsal area with either control vehicle (G-CSF Storage Buffer), or one of the four test articles of 55-kDa HEP-G-CSF, 99-kDa HEP-G-CSF, in-house-made 20-kDa PEG-G-CSF, or Neulasta (commercially prepared 20-kDa PEGylated G-CSF) at 0.1 mg/kg (based on protein content) in a volume of 1.5 mL/kg. These rats were observed for 7 days followed by termination on Day 8.
Blood was collected from the retro-orbital sinus for hematology studies before dosing and once daily after injection. Clinical observations, body weights, food consumption, and clinical pathology data were collected. The test article dose groups were compared to the vehicle control group. Mean and standard deviations were calculated for all quantitative data. Continuous group mean data were examined and evaluated for equality or homogeneity of variance using the Decision Tree statistical structure. This included analysis of variance (ANOVA) and/or covariance (ACOVA), pairwise tests by the Dunnett's Test, and simple t-tests for parametric data and the Bartlett's Test for homogeneity of variance. When appropriate, the parametric data were analyzed for a dose-related trend using the Williams Test. Nonhomogenous data were analyzed using a stepwise Dunnett's Test (parametric data). In general, statistical tests were performed as two-sided tests with results taken as significant with probability (P) levels of ≤0.05 or ≤0.01, with the exception of trend tests (Williams and Shirley), where only the top dose was analyzed using a two-sided test.
Toxicology study in rats
The toxicology study was performed by Xenometrics, LLC according to the principles of GLP and the protocol was IACUC-approved. To evaluate any potential toxicity of HEP polymer itself and HEP-G-CSF conjugate, male Sprague-Dawley rats (n = 5–7) were given 55-kDa HEP-NH2 at 30 mg/kg (in phosphate-buffered saline, PBS) or 55-kDa HEP-G-CSF conjugate at 0.5 mg/kg (~5-fold higher than human therapeutic dose; in G-CSF Storage Buffer) every two days over 14 days via the subcutaneous route in the dorsal area (similar to the repeated dosing schedule used in preclinical testing of Neulasta). For comparison, another two groups of rats received either G-CSF at 0.5 mg/kg or G-CSF Storage Buffer as vehicle control. Examined parameters included: clinical observations, mortality and moribundity checks, body weights, food consumption, serum chemistry, blood enzymes panel, hematology, coagulation, urinalysis, gross pathology, organ weights, and microscopic pathology. Statistical methods were the same as the efficacy study described above. Sections of the liver, kidney, and brain (choroid plexus areas) tissues, all regions with potential PEG accumulation based on past literature, were stained with hematoxylin and eosin and examined by an accredited pathologist.
Coagulation test with human plasma
HEP was tested in the activated partial thromboplastin time (APTT) assay because it is used to monitor the effect of heparin dosage on clotting in the clinic. The human diagnostic assay on an automated analyzer (Diagnostica Stago STA Compact Coagulometer; Veterans Administration Hospital; Oklahoma City, OK) was performed with the standard clinical protocol with the normal, healthy pooled human control plasma as the sample for testing. The effect of the addition of HEP or the heparin standard on the time to clot was compared. Briefly, each sample-plasma mixture (100 μL) was added to 100 μL of APTT reagent (Diagnostica Stago PTT reagent). After 4 min incubation at 37°C, 100 μL of CaCl2 (30 mM) is added and the time to clot formation determined. All controls (normal and abnormal heparin controls) were within the established statistical values. In these tests, HEP was inactive as an inhibitor of coagulation (i.e. no delay in clotting time) even at 15,000-fold higher doses than heparin.
Immunological challenge study in rats
To verify the predictions that HEP, a “self” molecule, would not be immunogenic, the HEP-drug conjugate was tested in a rat model. Three male Sprague-Dawley rats were injected SC in the dorsal region with HEP-G-CSF in saline every three weeks at a level of 0.4–0.7 mg/kg (contract research organization SDIX; Newark, DE) in an IACUC-approved protocol. Blood for production of serum were collected before the first injection (Week zero, preimmune bleed) and at Week 9, 15 and 27 (rat #3) or at Week 9, 15 and 30 (rats #1 and #2) (postimmunization bleeds 1, 2, and 3) (Supplementary Fig. 8). The bleeds were frozen and shipped backed to Caisson Biotech, LLC in Oklahoma City, Oklahoma for testing for the potential presence of anti-HEP-antibodies by ELISA with goat anti-rat IgG or IgM as the secondary antibodies (horseradish peroxidase conjugates; Thermo Scientific). The presence of anti-G-CSF antibodies was also measured (the human drug was used in a rat subject thus nonidentical G-CSF sequences). All steps were performed at room temperature.
Amine-binding maleic anhydride 96-well plates (Thermo Scientific) were coated with 200 μL of antigen solution: either G-CSF at 0.01 mg/mL or 100 mM Tris, pH 7.2 or BSA (negative controls), or 0.1 mg/mL HEP-BSA (an HEP conjugate produced via reductive amination of 55-kDa HEP to bovine serum albumin in analogy to HEP-G-CSF). The immobilization of HEP-BSA to the well surface was verified and validated by radiochemical tests of tritiated UDP-sugar incorporation mediated by the HEP synthase, PmHS1; the radioactive sugars were indeed added to the HEP chain in the wells (not shown).
After 4–6 h incubation at room temperature, the plates were washed with PBST and blocked overnight with 1% bovine serum albumin (Promega) in PBST (phosphate-buffered saline with 0.05% Tween). The test sera (0.5–5 μL/well) diluted in PBST with 1% BSA were added to the wells for 2–3 h of incubation. After thoroughly washing wells with PBST, horseradish peroxidase-conjugated goat polyclonal anti-rat IgG or IgM (Thermo Scientific) detection agents diluted in PBST with 1% BSA were added to the wells and the plates were incubated for 2 h. After final washing with PBST, a peroxidase substrate solution (TMB Substrate Kit, Thermo Scientific) for color development was added. After 30 min for BSA or HEP-BSA wells (or 15 min for G-CSF wells), the reactions were stopped with 2 M H2SO4 and the intensity of the color was measured by a spectrophotometric plate reader at 450 nm. The data from triplicate wells was averaged and shown with standard deviation. The ELISA tests were repeated as two to three independent assays on different days.
Supplementary Material
Acknowledgements
We thank Dr. F. Michael Haller for assistance with HEP chemistry, Amanda Gilliam for assistance with pilot protein coupling reactions, Liz Bullen for assistance with cell culture, Dr. Virginie Sjoelund for mass spectrometry, Dr. Rachel S. Lane for assistance with figure preparation, Jana Gausman for coagulation assays, and Dr. Robert J. Linhardt for the gift of heparin lyase.
Supplementary data
Funding
This work was supported in part by a National Institutes of Health, Small Business Innovation Research (SBIR) grant (1R43CA159494-01) to W.J. and P.L.D. and an OCAST grant from the Oklahoma Center for the Advancement of Science and Technology to P.L.D.
Conflict of interest statement
W.J. and P.L.D. have a financial stake in Caisson Biotech, LLC.
Abbreviations
GAG, glycosaminoglycan; G-CSF, granulocyte colony stimulating factor; GlcA, glucuronic acid; GlcNAc, N-acetylglucosamine; HEP, heparosan; HS, heparan sulfate; MS, mass spectrometry; PEG, polyethylene glycol; UPLC, ultraperformance liquid chromatography.
References
- Armstrong JK, Hempel G, Koling S, Chan LS, Fisher T, Meiselman HJ, Garratty G. 2007. Antibody against poly(ethylene glycol) adversely affects PEG-asparaginase therapy in acute lymphoblastic leukemia patients. Cancer. 110:103–111. [DOI] [PubMed] [Google Scholar]
- Arvedson T, O'Kelly J, Yang B-B. 2015. Design rationale and development approach for pegfilgrastim as a long-acting granulocyte colony-stimulating factor. BioDrugs. 29:185–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avci FY, Li X, Tsuji M, Kasper DL. 2011. A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nat Med. 17:1602–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggenstoss BA, Weigel PH. 2006. Size exclusion chromatography-multiangle laser light scattering analysis of hyaluronan size distributions made by membrane-bound hyaluronan synthase. Anal Biochem. 352:243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendele A, Seely J, Richey C, Sennello G, Shopp G. 1998. Short communication: renal tubular vacuolation in animals treated with polyethylene-glycol-conjugated proteins. Toxicol Sci. 42:152–157. [DOI] [PubMed] [Google Scholar]
- Bitter T, Muir HM. 1962. A modified uronic acid carbazole reaction. Anal Biochem. 4:330–334. [DOI] [PubMed] [Google Scholar]
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 72:248–254. [DOI] [PubMed] [Google Scholar]
- Copeland S, Warren HS, Lowry SF, Calvano SE, Remick D; Inflammation and the Host Response to Injury Investigators. 2005. Acute inflammatory response to endotoxin in mice and humans. Clin Diagn Lab Immunol. 12(1):60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox GN, Smith DJ, Carlson SJ, Bendele AM, Chlipala EA, Doherty DH. 2004. Enhanced circulating half-life and hematopoietic properties of a human granulocyte colony-stimulating factor/immunoglobulin fusion protein. Exp Hematol. 32:441–449. [DOI] [PubMed] [Google Scholar]
- Crobu D, Spinetti G, Schrepfer R, Tonon G, Jotti G, Onali P, Dedoni S, Orsini G, Di Stefano A. 2014. Preclinical and clinical phase I studies of a new recombinant Filgrastim (BK0023) in comparison with Neupogen®. BMC Pharmacol Toxicol. 15:7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeAngelis PL. 2002. Microbial glycosaminoglycan glycosyltransferases. Glycobiology. 12:9R–16R. [DOI] [PubMed] [Google Scholar]
- DeAngelis PL. 2015. Heparosan, a promising ‘naturally good’ polymeric conjugating vehicle for delivery of injectable therapeutics. Expert Opin Drug Deliv. 12:349–352. [DOI] [PubMed] [Google Scholar]
- Ganson NJ, Povsic TJ, Sullenger BA, Alexander JH, Zelenkofske SL, Sailstad JM, Rusconi CP, Hershfield MS. 2015. Pre-existing anti-polyethylene glycol antibody linked to first-exposure allergic reactions to pegnivacogin, a PEGylated RNA aptamer. J Allergy Clin Immunol. 137:1610–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garay RP, El-Gewely R, Armstrong JK, Garratty G, Richette P. 2012. Antibodies against polyethylene glycol in healthy subjects and in patients treated with PEG-conjugated agents. Expert Opin Drug Deliv. 9:1319–1323. [DOI] [PubMed] [Google Scholar]
- Hershfield MS, Ganson NJ, Kelly SJ, Scarlett EL, Jaggers DA, Sundy JS. 2014. Induced and pre-existing anti-polyethylene glycol antibody in a trial of every 3-week dosing of pegloticase for refractory gout, including in organ transplant recipients. Arthritis Res Ther. 16:R63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing W, Haller FM, Almond A, DeAngelis PL. 2006. Defined megadalton hyaluronan polymer standards. Anal Biochem. 355:183–188. [DOI] [PubMed] [Google Scholar]
- Kinstler O, Molineux G, Treuheit M, Ladd D, Gegg C. 2002. Mono-N-terminal poly(ethylene glycol)-protein conjugates. Adv Drug Deliv Rev. 54:477–485. [DOI] [PubMed] [Google Scholar]
- Kolate A, Baradia D, Patil S, Vhora I, Kore G, Misra A. 2014. PEG—a versatile conjugating ligand for drugs and drug delivery systems. J Control Release. 192:67–81. [DOI] [PubMed] [Google Scholar]
- Konishi T, Yoshizaki T, Yamakawa H. 1991. On the “Universal Constants” ρ and Φ of flexible polymers. Macromolecules. 24:5614–5622. [Google Scholar]
- Lane RS, Haller FM, Chavaroche AAE, Almond A, DeAngelis PL. 2017. Heparosan-coated liposomes for drug delivery. Glycobiology. 27(11):1053–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HG, Cowman MK. 1994. An agarose gel electrophoretic method for analysis of hyaluronan molecular weight distribution. Anal Biochem. 219:278–287. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Shirafuji N, Asano S. 1989. Human granulocyte colony-stimulating factor specifically binds to murine myeloblastic NFS-60 cells and activates their guanosine triphosphate binding proteins/adenylate cyclase system. Blood. 74:2343–2348. [PubMed] [Google Scholar]
- Malyala P, Singh M. 2008. Endotoxin limits in formulations for preclinical research. J Pharm Sci. 97:2041–2044. [DOI] [PubMed] [Google Scholar]
- Metcalf D. 1986. The molecular biology and functions of the granulocyte-macrophage colony-stimulating factors. Blood. 67:257–267. [PubMed] [Google Scholar]
- Pasut G, Veronese FM. 2012. State of the art in PEGylation: The great versatility achieved after forty years of research. J Control Release. 161:461–472. [DOI] [PubMed] [Google Scholar]
- Qi Y, Chilkoti A. 2015. Protein-polymer conjugation-moving beyond PEGylation. Curr Opin Chem Biol. 28:181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter AW, Akerblom E. 1984. Polyethylene glycol reactive antibodies in man: Titer distribution in allergic patients treated with monomethoxy polyethylene glycol modified allergens or placebo, and in healthy blood donors. Int Arch Allergy Appl Immunol. 74:36–39. [DOI] [PubMed] [Google Scholar]
- Rudmann DG, Alston JT, Hanson JC, Heidel S. 2013. High molecular weight polyethylene glycol cellular distribution and PEG-associated cytoplasmic vacuolation is molecular weight dependent and does not require conjugation to proteins. Toxicol Pathol. 41:970–983. [DOI] [PubMed] [Google Scholar]
- Sismey-Ragatz AE, Green DE, Otto NJ, Rejzek M, Field RA, DeAngelis PL. 2007. Chemoenzymatic synthesis with distinct Pasteurella heparosan synthases: Monodisperse polymers and unnatural structures. J Biol Chem. 282:28321–28327. [DOI] [PubMed] [Google Scholar]
- Tazi A, Nioche S, Chastre J, Smiéjan JM, Hance AJ 1991. Spontaneous release of granulocyte colony-stimulating factor (G-CSF) by alveolar macrophages in the course of bacterial pneumonia and sarcoidosis: endotoxin-dependent and endotoxin-independent G-CSF release by cells recovered by bronchoalveolar lavage. Am J Respir Cell Mol Biol. 4(2):140–147. [DOI] [PubMed] [Google Scholar]
- Verhoef JJ, Carpenter JF, Anchordoquy TJ, Schellekens H. 2014. Potential induction of anti-PEG antibodies and complement activation toward PEGylated therapeutics. Drug Discov Today. 19:1945–1952. [DOI] [PubMed] [Google Scholar]
- Veronese FM. 2001. Peptide and protein PEGylation: A review of problems and solutions. Biomaterials. 22:405–417. [DOI] [PubMed] [Google Scholar]
- Zhang F, Liu M, Wan H. 2014. Discussion about several potential drawbacks of PEGylated therapeutic proteins. Biol Pharm Bull. 37:335–339. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.