Abstract
BACKGROUND
Little is known about racial-ethnic differences in the distribution of maternal serum levels of angiogenic and antiangiogenic factors and their associations with early-onset preeclampsia.
OBJECTIVE
To investigate the distribution of mid-trimester maternal serum levels of placental growth factor, soluble endoglin, and soluble vascular endothelial growth factor receptor 1 and their associations with early-onset preeclampsia in Whites, Hispanics, and Blacks.
STUDY DESIGN
A population-based nested case-control design was used to identify cases and controls of White, Hispanic, and Black origin from a 2000 to 2007 live-birth cohort in five southern California counties. Cases included 197 women (90 Whites, 67 Hispanics, and 40 Blacks) with early-onset preeclampsia defined as hypertension and proteinuria with onset prior to 32 weeks according to hospital records. Controls included a random sample of 2,363 women without early-onset preeclampsia. Maternal serum specimens collected at 15-20 weeks of gestation as part of routine prenatal screening were tested for placental growth factor, soluble endoglin, and soluble vascular endothelial growth factor receptor 1. Serum levels of the three factors were log-normally distributed. Adjusted natural logarithmic means were compared between cases and controls and between racial-ethnic groups. Odds ratios and 95% confidence intervals derived from logistic regression models were calculated to measure the magnitude of the associations.
RESULTS
Cases showed lower adjusted logarithmic means of placental growth factor but higher adjusted logarithmic means of soluble endoglin than controls across all three groups (P < 0.05). Cases also had higher adjusted means of soluble vascular endothelial growth factor receptor 1 than controls in Whites (7.75 log pg/mL versus 7.52 log pg/mL, P < 0.05) and Hispanics (7.73 log pg/mL versus 7.40 log pg/mL, P < 0.05) but not in Blacks (7.85 log pg/mL versus 7.69 log pg/mL, P = 0.47). Blacks were found to have higher levels of placental growth factor in both cases and controls when compared to Whites and Hispanics (adjusted means: 4.69 log pg/mL and 5.20 log pg/mL in Blacks, 4.08 log pg/mL and 4.78 log pg/mL in Whites, and 3.89 log pg/mL and 4.70 log pg/mL in Hispanics, respectively, P < 0.05). Hispanic cases had the highest adjusted mean of soluble endoglin compared to White and Black cases (9.24 log pg/mL, 9.05 log pg/mL, and 8.93 log pg/mL, respectively, P < 0.05). The weakest association of early-onset preeclampsia with placental growth factor and soluble endoglin was observed in Blacks. The adjusted odds ratio per log pg/mL increase of the two analytes were 0.219 (95% confidence interval, 0.124-0.385)and 5.02 (95% confidence interval, 2.56-9.86) in Blacks in comparison to 0.048 (95% confidence interval, 0.026-0.088) and 36.87 (95% confidence interval, 17.00-79.96) in Whites (P < .05) and 0.028 (95% confidence interval, 0.013-0.060) and 86.68 (95% confidence interval, 31.46-238.81) in Hispanics (P < .05), respectively. As for soluble vascular endothelial growth factor receptor 1, the association was not significantly different among the racial-ethnic groups.
CONCLUSIONS
Racial-ethnic differences were observed in the distribution of midtrimester maternal levels of placental growth factor and soluble endoglin and in the associations with early-onset preeclampsia. These differences should be considered in future studies to improve etiologic and prognostic understanding of early-onset preeclampsia.
Keywords: early-onset preeclampsia, placental growth factor, soluble endoglin, soluble vascular endothelial growth factor receptor-1, racial-ethnic differences
INTRODUCTION
Preeclampsia is one of the most dangerous pregnancy-associated disorders. Affecting 3-5% of pregnancies in the United States, preeclampsia causes 25% of maternal deaths and 25,000-40,000 perinatal deaths each year.1,2 Preeclampsia is heterogeneous in onset and severity of clinical manifestations.3 Preeclampsia occurring at <32 weeks of pregnancy, also known as early-onset preeclampsia with an incidence range of 0.2%-1.4%,4 carries a disproportionately higher risk of maternal and infant mortality and therefore is considered a more severe form of the disease than preeclampsia occurring later in pregnancy.1,3 Angiogenesis is the process of new blood vessel formation and the growth of new blood vessels from existing blood vessels.1,5 These processes are critical to the establishment and functioning of the placenta.1,6 The placenta produces high levels of angiogenic factors, such as placental growth factor (PlGF) in order to maintain an angiogenic balance for normal fetal development.6 Dramatically reduced levels of PlGF and increased levels of antiangiogenic factors, such as soluble endoglin (sEng) and soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) in maternal circulation have been repeatedly reported as important predictors for preeclampsia with more pronounced effects for early-onset compared to late-onset preeclampsia.1,7-15
Black women have been shown to be at higher risk for preeclampsia than White women (4.0% versus 2.8%).16 Hispanics have been found to have lower risk than non-Hispanics (1.3% versus 5.3%)17 although results are not consistent.18,19 While many studies have been published on angiogenic and antiangiogenic factors as screening markers for preeclampsia,1,3 few have examined racial-ethnic differences in the distribution of these factors and in their associations with preeclampsia. To date, no population-based study that we are aware of has concurrently compared the pattern of mid-pregnancy angiogenic and antiangiogenic factors in White, Hispanic, and Black women. A comprehensive study of racial-ethnic differences in maternal angiogenic imbalance is needed to better understand the pathogenesis of preeclampsia within and across racial-ethnic groups.
Utilizing a nested case-control study design, this study aimed to better understand the relationship among maternal serum concentrations of PlGF, sEng, and sVEGFR-1 at 15-20 weeks of gestation in White, Hispanic, and Black women with and without early-onset preeclampsia.
MATERIALS AND METHODS
Case Identification
All early-onset preeclampsia cases (n=197) evaluated in this study were identified by the California Very Preterm Birth Study20 from prenatal screening records and validated via chart review at the hospital of delivery. Detailed study design and methods have been published elsewhere.20 Briefly, very preterm births of White, Hispanic, or Black origin were identified from a population-based cohort created through record linkage of 346,456 mother-baby pairs with stored maternal serum specimens linked to certificates of live births in California from January 2000 to April 2007. White and Hispanic births were defined as both parents being non-Hispanic White and Mexican Hispanic White, respectively, based on birth records. Mexican Hispanic White referred to self-reported White with Hispanic ethnicity origin of Mexican, Mexican-American, or Chicano. Black births were defined as Black mothers of any ethnicity, regardless of father’s race-ethnicity. All of these women participated in routine prenatal screening for aneuploidies and neural tube defects between 15 and 20 weeks through the Genetic Disease Screening Program within the California Department of Public Health and had serum banked after screening.20,21 Study samples were limited to singleton live births delivered in five Southern California counties (Los Angeles, Orange, Riverside, San Bernardino, and San Diego). Within this large cohort, the medical records of 1,117 chronologically identified very preterm births of <32 weeks of gestation (429 Whites, 440 Hispanics, and 248 Blacks) were abstracted to distinguish very preterm births with known causes, including preeclampsia, which formed the case group for this study. Cases with a birth weight > 2,500 g were excluded based on suspected misdating. Pregnancies with structural abnormalities reported on delivery records or identified by the California Birth Defects Monitoring Program registry were also excluded.
Preeclampsia was defined as hypertension accompanied by proteinuria with onset after 20 weeks of gestation in the California Very Preterm Birth Study. Hypertension was defined as blood pressure ≥140 mm Hg systolic or ≥90 mm Hg diastolic on two occasions 6 hours apart. Proteinuria was defined as ≥0.3 grams of protein in a 24-hour urine collection or ≥1+ dipstick on a urine test on two occasions 6 hours apart. Early-onset preeclampsia was defined as preeclampsia with delivery before 32 weeks of gestation. Gestational age estimates for cases were mostly based on ultrasound (61.5%) and date of last menstrual period (29.5%) from the California Prenatal Screening Program with a small part (9.0%) based on maternal hospital chart review.
Hospital chart review identified 207 cases of early-onset preeclampsia with a maternal mid-trimester specimen available for laboratory testing. Ten of the 207 cases were excluded due to maternal specimens collected beyond the gestational window of 15-20 weeks. The final sample included 90 White, 67 Hispanic, and 40 Black cases. Details of the sample selection are listed in the Figure.
FIGURE. Identification flowchart of early-onset preeclampsia cases and controls.
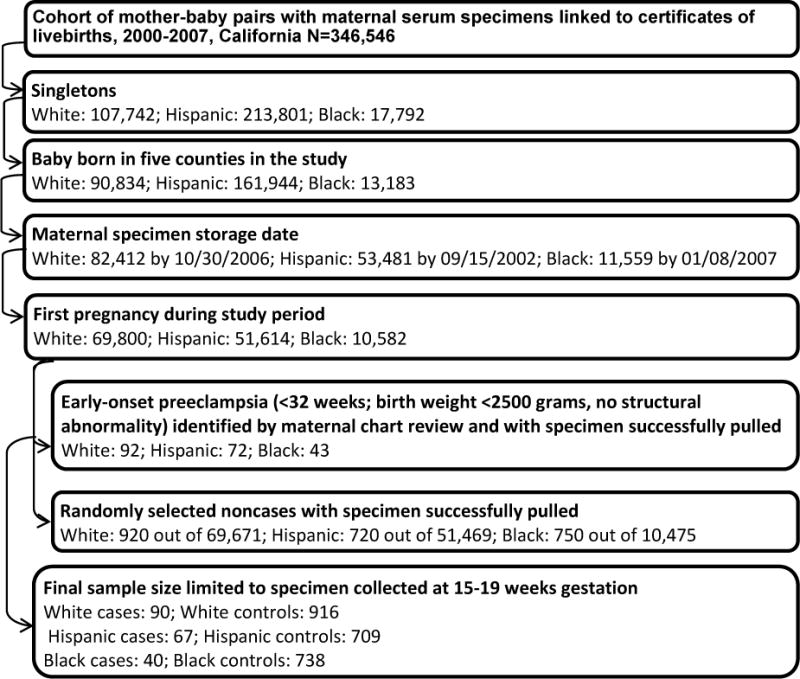
Step-by-step sample selection. Exclusion rates in each step vary between race-ethnicity groups due to different population distributions and exclusion details by race-ethnicity groups.
Control Identification
Controls were non-cases selected at an original sample size of ≥10 times the number of cases before specimen pulling using simple random sampling within the same race-ethnicity specific singleton live-birth cohort as cases. Births with adverse outcomes such as late-onset preeclampsia, preterm birth, and low birth weight were also eligible controls. Controls of White, Hispanic, and Black origin were drawn separately for each racial-ethnic group. For women with multiple pregnancies during the study period, only the first pregnancy was chosen. Control births without banked serum specimens or with structural malformations according to the California Birth Defect Monitoring Program registry were excluded. The final sample included 916 White, 709 Hispanic, and 738 Black controls (Figure). Gestational age estimates for controls were based on ultrasound (55.8%), date of last menstrual period (34.9%), and physical exam (9.3%) from the California Prenatal Screening Program.
Biological Specimens
Banked maternal blood specimens were retrieved from the California Biobank Program.22 As part of the State Prenatal Screening Program, obstetrical care service providers at doctors’ offices or drawing stations collected venous blood from pregnant women who voluntarily enrolled at 15-20 weeks of gestation. The blood, which was collected in serum separator tubes, was sent to one of the seven Newborn and Prenatal Screening Laboratories by mail under ambient conditions. Specimens arrived for triple-marker prenatal testing within seven days of collection (median time = 3 days).20,21 After routine testing, leftover specimens were refrigerated and then processed for long-term storage at −20˚C. Women consented to the routine prenatal screening test in writing and were notified that the blood becomes the property of the State, which allows for a variety of approved research uses of the leftover blood under the highest level of security and confidentiality standards. While women have the option to request the specimen not be used for research purposes, very few assert this right. Specimen vials from cases and controls were intermingled, with vial positions randomly assigned during shipping and laboratory testing. Laboratory personnel were unaware of subjects’ case-control status.
Laboratory methods
Maternal serum levels of PlGF, sEng, and sVEGFR-1 were determined by immunoassays (R&D Systems Inc., Minneapolis, MN) utilizing the quantitative sandwich enzyme immunoassay technique. Laboratory testing was conducted at the research core laboratory of the Perinatology Research Branch, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services. Standard serum specimens were used in each laboratory run and analyte value was determined by interpolation from standard curves. The assays utilized 120 μL, 25 μL, and 25 μL of serum for the PlGF, sEng, and VEGFR-1 assays, respectively. The inter- and intra-assay coefficients of variation obtained in the laboratory were 4.6% and 3.6% for PlGF, 5.3% and 4.3% for sEng, and 2.8% and 2.7% for sVEGFR-1, respectively. The sensitivity of the assays was 5.7 pg/mL for PlGF, 40.0 pg/mL for sEng, and 12.9 pg/mL for sVEGFR-1.
Data analysis
The laboratory levels of PlGF, sEng, and sVEGFR-1 were linked to birth records, prenatal screening program data, and maternal hospital chart data (cases only) for analysis. Maternal characteristics were compared between cases and controls. Gestational weeks at delivery and at preeclampsia diagnosis for cases were compared among the three racial-ethnic groups. The Chi-square test and Student t test were used to estimate the statistical significance of the comparisons for categorical and continuous variables, respectively. Natural logarithmic transformed circulating levels of PlGF, sEng, and sVEGFR-1 were normally distributed in cases and controls. Means (log pg/mL) and standard errors were calculated for cases and controls of each racial-ethnic group and were adjusted by maternal age, education, parity, specimen storage year, gestational age at specimen collection, and maternal weight at or near specimen collection. The adjusted logarithmic means were compared using the Tukey honestly significant difference23 test between cases and controls in each racial-ethnic group and between racial-ethnic groups among cases and controls, respectively. Odds ratios (OR) and 95% confidence intervals (CI) derived from binomial logistic regressions were used to measure effect magnitude of analyte levels in association with case-control status. The logarithmic transformed levels (log pg/mL) of the three analytes were used in the logistic regression to reduce both variance and outlier influence. Race-ethnicity is a significant effect modifier for PlGF and sEng in association with early-onset preeclampsia; therefore, association results were presented separately for each race-ethnicity group. Maternal age, education, parity, specimen storage year, gestational weeks at specimen collection, and maternal weight at specimen collection were included as potential confounders. Standard Z test was conducted to compare ORs between racial-ethnic groups. Given the above-mentioned final sample sizes, this study had adequate power of ≥80% to detect association of moderate or higher effect estimates (OR ≥ 2.6) between cases and controls in each racial-ethnic group under a logistic model with a two-sided test.
All of the statistical analyses were conducted using software (SAS, Version 9.2 for Windows; SAS Institute, Cary, NC). The study protocol was approved by the California Health and Human Services Agency Committee for the Protection of Human Subjects (Project number 10-10-52).
RESULTS
The prevalence of early-onset preeclampsia (<32 weeks of gestation) was 0.13% for Whites (92 of 69,800), 0.14% for Hispanics (72 of 51,614), and 0.41% for Blacks (43 of 10,582) based on the linked birth cohort for this study (Figure), within the lower range previously reported.4 Compared to controls, White cases were more likely to be nulliparous (81.11% versus 50.11%, P < 0.05), and Hispanic cases were older than controls (28.61 years on average versus 26.22 years, P < 0.05), as presented in Table 1. Cases of White and Hispanic origin were heavier at or near the time of specimen collection than controls (76.20 kg on average versus 70.02 kg in Whites and 76.33 kg versus 67.22 kg in Hispanics, P < 0.05, respectively). Maternal education and gestational age at specimen collection were not associated with case-control status in any racial-ethnic group. Gestational age at delivery and at preeclampsia diagnosis among cases did not differ across racial-ethnic groups.
Table 1.
Selected maternal characteristics of early-onset preeclampsia cases and controls by racial-ethnic groups, California births, 2000-2007.
| Characteristics | White | Hispanic | Black | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Controls | Cases | Controls | Cases | Controls | Cases | |
| (N=916) | (N=90) | (N=709) | (N=67) | (N=738) | (N=40) | |
| Maternal age (year, mean ± SD) | 30.00 ± 4.67 | 30.22 ± 5.26 | 26.22 ± 5.72 | 28.61 ± 6.49a | 26.58 ± 5.81 | 27.20 ± 5.60 |
| ≤High school graduate (%) | 23.25 | 18.89 | 85.05 | 82.09 | 53.79 | 50.00 |
| Nulliparity (%) | 50.11 | 81.11b | 35.26 | 41.79 | 36.18 | 45.00 |
| Maternal weight (kilograms) at or near specimen collection (mean ± SD) | 70.02 ± 14.98 | 76.20 ± 17.85a | 67.22 ± 14.40 | 76.33 ± 18.98a | 78.03 ± 20.87 | 80.42 ± 21.18 |
| Gestational age at specimen collection ≥18 weeks of gestationa (%) | 15.61 | 14.44 | 25.81 | 29.85 | 27.51 | 22.50 |
| Delivery < 26 weeks of gestationa (%) | NA | 10.00 | NA | 13.43 | NA | 12.50 |
| Preeclampsia diagnosis < 26 weeks of gestationc (%) | NA | 14.44 | NA | 16.42 | NA | 15.00 |
All data are from birth certificates with the exception of gestational age.
SD, standard deviation; NA, not applicable.
Student t test P < 0.05 comparing cases to controls
Chi-square test P < 0.05 comparing cases to controls
Gestational age based on prenatal screening and hospital charts for cases and prenatal screening only for controls
Cases had lower adjusted logarithmic mean levels of PlGF but higher adjusted logarithmic mean levels of sEng than controls for each of the three racial-ethnic groups (P < 0.05; Table 2). Cases also had higher adjusted mean levels of sVEGFR-1 than controls in Whites (7.78 log pg/mL versus 7.52 log pg/mL, P < 0.05) and Hispanics (7.73 log pg/mL versus 7.40 log pg/mL, P < 0.05) but not in Blacks (7.85 log pg/mL versus 7.69 log pg/mL, P = 0.47). Blacks had larger adjusted logarithmic means of PlGF in both cases and controls and higher adjusted logarithmic means of sVEGFR-1 in controls when compared to Whites and Hispanics (P < 0.05 for all comparisons). Blacks also had larger adjusted means of sEng in controls than Whites (8.58 log pg/mL versus 8.53 log pg/mL, P < 0.05) but smaller adjusted means of sEng in cases than Hispanics (8.93 log pg/mL versus 9.24 log pg/mL, P < 0.05). Analyte levels were similar between Whites and Hispanics, except that Hispanic cases had higher adjusted means of sEng (9.24 log pg/mL versus 9.05 log pg/mL, P < 0.05) and White controls had higher adjusted means of sVEGFR-1 (7.52 log pg/mL versus 7.40 log pg/mL, P < 0.05). The adjusted logarithmic mean levels of sVEGFR1 in cases were similar across all three racial-ethnic groups.
Table 2.
Adjusted1 natural logarithmic mean and standard error of PlGF, sEng, and sVEGFR-1 concentrations (log pg/mL) among cases and controls by racial-ethnic groups, California births, 2000–2007
| Percentiles | White | Hispanics | Black | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Controls (N=916) | Cases (N=90) | Controls (N=709) | Cases (N=67) | Controls (N=738) | Cases (N=40) | |
| PlGF | ||||||
| Mean | 4.78a b | 4.08a c | 4.70a d | 3.89a e | 5.20a b d | 4.69a c e |
| Standard error | 0.04 | 0.06 | 0.04 | 0.07 | 0.03 | 0.09 |
| sEng | ||||||
| Mean | 8.53a b | 9.05a f | 8.54a | 9.24a e f | 8.58a b | 8.93a e |
| Standard error | 0.03 | 0.04 | 0.03 | 0.05 | 0.02 | 0.06 |
| sVEGFR-1 | ||||||
| Mean | 7.52a b g | 7.78a | 7.40a d g | 7.73a | 7.69b d | 7.85 |
| Standard error | 0.04 | 0.07 | 0.04 | 0.07 | 0.04 | 0.09 |
Adjusted by maternal age, education, parity, specimen storage year, maternal weight at or near specimen collection, and gestational week at specimen collection.
PlGF, placental growth factor; sEng, soluble endoglin; sVEGFR-1, soluble vascular endothelial growth factor receptor 1.
P < 0.05 between adjusted means of cases and controls of the same race-ethnicity.
P < 0.05 between adjusted means of White controls and Black controls
P < 0.05 between adjusted means of White cases and Black cases
P < 0.05 between adjusted means of Hispanic controls and Black controls
P < 0.05 between adjusted means of Hispanic cases and Black cases
P < 0.05 between adjusted means of White cases and Hispanic cases
P < 0.05 between adjusted means of White controls and Hispanic controls
Logistic regression results with adjustment for covariates (Table 3) revealed strong associations of midtrimester PlGF, sVEGFR-1, and sEng levels with early-onset preeclampsia. Women with higher PlGF level were at lower risk for early-onset preeclampsia in all three racial-ethnic groups, with the weakest associations observed among Blacks (adjusted OR [AOR], 0.219; 95% CI, 0.124-0.385 per log pg/mL increase [P < 0.05] in comparison to AOR, 0.048; 95% CI, 0.026-0.088 in Whites, and AOR, 0.028; 95% CI, 0.013-0.060 in Hispanics). Elevated levels of sVEGFR-1 and sEng were positively associated with early-onset preeclampsia. Women with a single logarithmic unit increase of sEng levels had a more than five-fold increase in risk for early-onset preeclampsia in all three racial-ethnic groups with the weakest association observed in Blacks (AOR, 5.02; 95% CI, 2.56-9.86 [P < 0.05] in comparison to AOR, 36.87; 95% CI, 17.00-79.96 in Whites, and AOR, 86.68; 95% CI, 31.46-238.81 in Hispanics). There was no significant difference of PlGF and sEng in association with early-onset preeclampsia between Whites and Hispanics. Elevated odds of early-onset preeclampsia associated with higher sVEGFR-1 levels were observed in Whites (AOR, 3.24; 95% CI, 2.04-5.14) and Hispanics (AOR, 3.68; 95% CI, 2.16-6.29) but not in Blacks (AOR, 1.2; 95% CI, 0.7-2.1).
Table 3.
Odds ratios and 95% confidence intervals of PlGF, sEng, and sVEGFR-1 concentrations (per log pg/mL increase) in association with early-onset preeclampsia, by racial-ethnic groups, California births, 2000-2007
| Analytes | White | Hispanics | Black | |||
|---|---|---|---|---|---|---|
|
| ||||||
| OR 95% CI |
OR 95% CI |
OR 95% CI |
||||
|
| ||||||
| Unadjusted | Adjusteda | Unadjusted | Adjusteda | Unadjusted | Adjusteda | |
| PlGF | 0.052b 0.030-0.089 |
0.048b 0.026-0.088 |
0.044c 0.023-0.084 |
0.028c 0.013-0.060 |
0.244b
c 0.143-0.417 |
0.219 b
c 0.124-0.385 |
| sEng | 26.10b 13.33-51.08 |
36.87b 17.00-79.96 |
57.91c 23.72-141.40 |
86.68c 31.46-238.81 |
4.18b
c 2.29-7.64 |
5.02b
c 2.56-9.86 |
| sVEGFR-1 | 2.87 1.88–4.39 |
3.24 2.04–5.14 |
2.66 1.64–4.32 |
3.68 2.16–6.29 |
1.62 0.91–2.89 |
1.61 0.88–2.95 |
Adjusted by maternal age, education, parity, specimen storage year, maternal weight at or near specimen collection, and gestational week at specimen collection.
P < 0.05 between OR of Whites and Blacks
P < 0.05 between OR of Hispanics and Blacks
OR, odds ratio; CI, confidence interval
COMMENT
In this investigation, we observed a number of relationships suggesting that midtrimester angiogenic and antiangiogenic factors are associated with early-onset preeclampsia. We also observed patterns that suggested the strength of these relationships may differ by racial-ethnic group. While low levels of PlGF and high levels of sEng were strongly associated with early-onset preeclampsia across racial-ethnic groups, the associations were stronger in Whites and Hispanics than Blacks (AOR in Whites and Hispanics were more than 4.4 times the AOR in Blacks). Higher sVEGFR-1 levels were only associated with an increased risk of early-onset preeclampsia in women who were Hispanic or White. To our knowledge, this is the first study that describes the racial-ethnic differences of midtrimester angiogenic and antiangiogenic factors in association with early-onset preeclampsia.
The relationships between midtrimester PlGF, sEng, and sVEGFR-1 levels and early-onset preeclampsia have been widely documented.1,3 Previous studies have shown reduced PlGF levels24 in both the first25 and second26 trimesters and elevated sEng24,25,27 and sVEGFR-124,27,28 levels in the second trimester of preeclamptic pregnancies compared to pregnancies without preeclampsia. Results from this study confirmed previous findings and found very strong associations of PlGF and sEng levels and moderately strong associations of sVEGFR-1 levels with early-onset preeclampsia. Differences in the effect magnitude between the three analytes and early-onset preeclampsia have rarely been reported.
Racial-ethnic differences have been well reported in maternal circulation of many proteins such as alpha-fetoprotein29 and tumor necrosis factor alpha (TNF-α)30 but rarely mentioned for angiogenic and antiangiogenic factors. Most previous studies on angiogenic and antiangiogenic factors were conducted in White participants and there is a paucity of information on other ethnic groups.31,32 Black women were found to have slightly higher levels of PlGF, sEng, and sVEGFR-1 than White women and women of other races in one study33 but in another study no difference in PlGF and sVEGFR-1 was found compared to White women.34 A study of sVEGFR-1 levels by parity35 also did not find significant differences in PlGF and sVEGFR-1 levels within the first or second pregnancies of Hispanic women compared to White women. Our study agreed with that of Mijal et al33 indicating higher levels of PlGF and sVEGFR-1 in Black controls than White and Hispanic controls, and with that of Faupel-Badger et al34 and Wolf et al35 showing no PlGF differences between White and Hispanic controls. Our study also found lower levels of sVEGFR-1 in Hispanic than White controls, which is different from the Wolf et al35 study. This study also detected higher sEng levels in Black compared to White controls.
Recent studies have advanced our understanding of etiology of preeclampsia although it remains largely unknown.1,3,36 Preeclampsia is hypothesized as the result of an exaggerated inflammatory response due to maternal constitutional factors, genetic predisposition, and/or immunological maladaptation.3 In the last decade a two-stage pathogenic model has gained acceptance as the underlying pathophysiology of early-onset preeclampsia.31,37-40 In the first stage, inadequate placentation due to shallow cytotrophoblastic invasion restricts blood supply to the placenta and creates a hypoxic placental environment in the first trimester. Oxidative stress then induces ischemic lesions to the placenta, which may promote the release of a variety of molecules including but not limited to PlGF, sEng, and sVEGFR-1 into maternal circulation.1,41-44 In the second stage, the released molecules initiate a cascade of cellular and molecular changes via multiple pathophysiological mechanisms—angiogenesis, exaggerated inflammatory response, endothelial and vascular dysfunction, thrombosis, renin-angiotensin system activation, metabolic and dietary effects, and others such as neuroendocrine system effects16-20— leading to maternal syndromes in the second trimester and later gestation.21 In support of angiogenesis as one of the most important mechanisms44,45 contributing to early-onset preeclampsia, this study detected very strong associations of lower PlGF levels and higher sEng levels with early-onset preeclampsia.
Our novel finding of racial-ethnic differences of PlGF and sEng levels in association with early-onset preeclampsia, if confirmed by other studies to not be due to other factors or mechanisms not studied within the current framework, suggests that there might be different regulations and/or processes of the angiogenic mechanism within racial-ethnic groups. It could also suggest that each racial-ethnic group has its own key pathophysiological mechanisms in the incidence for early-onset preeclampsia, if the racial-ethnic differences can be replicated in future research investigating other pathophysiological mechanisms for early-onset preeclampsia.
Strengths of the present study include its use of a nested case-control design to concurrently compare the distribution of midtrimester PlGF, sEng, and sVEGFR-1 levels in Whites, Hispanics, and Blacks and their associations with early-onset preeclampsia. Racial-ethnic groups were well defined, the outcome was restricted to early-onset preeclampsia, and the population-based sample size was large. Despite these strengths, there were a number of study limitations. First, the study used maternal serum specimens stored at −20˚ C rather than −80˚ C for more than five years by the California Biobank Program22 without immediate refrigeration after routine specimen collection. Although platelets, eosinophils, monocytes, and leukocytes in serum could also release angiogenic factors (i.e., sVEGFR-1) during clotting,46 both serum and plasma have been widely used in previous studies with similar concentration ranges detected from the two specimen types.46 Previous studies have documented the stability of angiogenic factors in specimens stored for more than 12 years27 and have shown that serum levels of angiogenic factors were not affected by storage duration and temperature.3,47 Comparing the adjusted logarithmic means of PlGF, sEng, and sVEGFR-1 levels by storage years, we found no change in PlGF and sVEGFR-1 levels and a slight decrease in sEng levels with >12 storage years among Hispanic controls (Supplementary Table 1). Biomarker stability may be stronger at storage temperature of −80˚ C than at −20˚ C.48 The impact of storage temperature on analyte values in this study is unknown; however, both case and control specimens were handled in a similar way, therefore, differential effect (bias) across case-control and racial-ethnic groups is unlikely. Second, this study tested PlGF, sEng, and sVEGFR-1 concentrations in maternal blood specimens routinely collected from participants in the Prenatal Screening Program during 15-20 weeks. Screening program participants, approximately 70% of women delivering live births in California,20 broadly represent the California birth population in terms of race-ethnicity and education.49 However, women under 20 or over 35 years of age, with late or no prenatal care, or public insurance, are less likely to participate.49 It is not clear how these selection factors may influence the distribution of PlGF, sEng, and sVEGFR-1 and their associations with early-onset preeclampsia. Future studies will need to have more complete coverage of the pregnant population. In addition, the angiogenic and antiangiogenic imbalance in preeclamptic pregnancy is considered to start at the end of first trimester and peak at mid-trimester to term.50 While ready access and availability of banked specimens provided a unique opportunity to investigate the study questions, the one-time-only blood collection limited our ability to fully understand the concentration change over the course of pregnancy and to study PlGF, sEng, and sVEGFR-1 levels in early pregnancy in association with early-onset preeclampsia. Such an investigation represents an important next step.
In summary, this study confirmed the relationship between early-onset preeclampsia and angiogenic and antiangiogenic factors and found that racial-ethnic differences may exist. Our study results point to the need for future research in homogenous population groups stratified by race-ethnicity to potentially improve etiologic and prognostic value of these analytes.
Supplementary Material
Condensation.
Racial-ethnic differences were observed in angiogenic and antiangiogenic factors associated with early-onset preeclampsia.
Acknowledgments
None
SPONSOR/GRANT/FINANCIAL SUPPORT OF THE STUDY: The project described was supported by Health Resources and Services Administration, U.S. Department of Health and Human Services, through grant # R40MC21525.
The work of Dr. Romero was supported by the Perinatology Research Branch, Program for Perinatal Research and Obstetrics, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS) and, in part, with Federal funds from NICHD/NIH/DHHS under Contract No. HHSN275201300006C. Dr. Romero has contributed to this work as part of his official duties as an employee of the United States Federal Government.
Footnotes
CONFLICT OF INTEREST/PERSONAL FINANCIAL DISCLOSURE: The authors report no conflict of interest or personal financial disclosures.
References
- 1.Chaiworapongsa T, Chaemsaithong P, Yeo L, Romero R. Pre-eclampsia part 1: current understanding of its pathophysiology. Nat Rev Nephrol. 2014;10:466–480. doi: 10.1038/nrneph.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ilekis JV, Reddy UM, Roberts JM. Preeclampsia–a pressing problem: an executive summary of a National Institute of Child Health and Human Development workshop. Reprod Sci. 2007;14:508–523. doi: 10.1177/1933719107306232. [DOI] [PubMed] [Google Scholar]
- 3.Forest JC, Charland M, Masse J, et al. Candidate biochemical markers for screening of pre-eclampsia in early pregnancy. Clin Chem Lab Med. 2012;50:973–984. doi: 10.1515/cclm.2011.820. [DOI] [PubMed] [Google Scholar]
- 4.Purde MT, Baumann M, Wiedemann U, et al. Incidence of preeclampsia in pregnant Swiss women. Swiss Med Wkly. 2015;145:w14175. doi: 10.4414/smw.2015.14175. [DOI] [PubMed] [Google Scholar]
- 5.Rogers MS, D’Amato RJ. The effect of genetic diversity on angiogenesis. Exp Cell Res. 2006;312:561–574. doi: 10.1016/j.yexcr.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 6.Faupel-Badger JM, Staff AC, Thadhani R, et al. Maternal angiogenic profile in pregnancies that remain normotensive. Eur J Obstet Gynecol Reprod Biol. 2011;158:189–193. doi: 10.1016/j.ejogrb.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crispi F, Dominguez C, Llurba E, Martin-Gallan P, Cabero L, Gratacos E. Placental angiogenic growth factors and uterine artery Doppler findings for characterization of different subsets in preeclampsia and in isolated intrauterine growth restriction. Am J Obstet Gynecol. 2006;195:201–207. doi: 10.1016/j.ajog.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 8.Wikstrom AK, Larsson A, Eriksson UJ, Nash P, Norden-Lindeberg S, Olovsson M. Placental growth factor and soluble FMS-like tyrosine kinase-1 in early-onset and late-onset preeclampsia. Obstet Gynecol. 2007;109:1368–1374. doi: 10.1097/01.AOG.0000264552.85436.a1. [DOI] [PubMed] [Google Scholar]
- 9.Chaiworapongsa T, Romero R, Kim YM, et al. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern Fetal Neonatal Med. 2005;17:3–18. doi: 10.1080/14767050400028816. [DOI] [PubMed] [Google Scholar]
- 10.Stepan H, Kramer T, Faber R. Maternal plasma concentrations of soluble endoglin in pregnancies with intrauterine growth restriction. J Clin Endocrinol Metab. 2007;92:2831–2834. doi: 10.1210/jc.2006-2774. [DOI] [PubMed] [Google Scholar]
- 11.Wathen KA, Ylikorkala O, Andersson S, Alfthan H, Stenman UH, Vuorela P. Maternal serum endostatin at gestational weeks 16-20 is elevated in subsequent pre-eclampsia but not in intrauterine growth retardation. Acta Obstet Gynecol Scand. 2009;88:593–598. doi: 10.1080/00016340902838293. [DOI] [PubMed] [Google Scholar]
- 12.Kim YN, Lee DS, Jeong DH, Sung MS, Kim KT. The relationship of the level of circulating antiangiogenic factors to the clinical manifestations of preeclampsia. Prenat Diagn. 2009;29:464–470. doi: 10.1002/pd.2203. [DOI] [PubMed] [Google Scholar]
- 13.Staff AC, Harsem NK, Braekke K, Hyer M, Hoover RN, Troisi R. Maternal, gestational and neonatal characteristics and maternal angiogenic factors in normotensive pregnancies. Eur J Obstet Gynecol Reprod Biol. 2009;143:29–33. doi: 10.1016/j.ejogrb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Wikstrom AK, Larsson A, Akerud H, Olovsson M. Increased circulating levels of the antiangiogenic factor endostatin in early-onset but not late-onset preeclampsia. Reprod Sci. 2009;16:995–1000. doi: 10.1177/1933719109339348. [DOI] [PubMed] [Google Scholar]
- 15.Gotsch F, Romero R, Kusanovic JP, et al. Preeclampsia and small-for-gestational age are associated with decreased concentrations of a factor involved in angiogenesis: soluble Tie-2. J Matern Fetal Neonatal Med. 2008;21:389–402. doi: 10.1080/14767050802046069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breathett K, Muhlestein D, Foraker R, Gulati M. Differences in Preeclampsia Rates Between African American and Caucasian Women: Trends from the National Hospital Discharge Survey. J Womens Health (Larchmt) 2014;23:886–893. doi: 10.1089/jwh.2014.4749. [DOI] [PubMed] [Google Scholar]
- 17.Yeo S, Wells PJ, Kieffer EC, Nolan GH. Preeclampsia among Hispanic women in a Detroit health system. Ethn Dis. 2007;17:118–121. [PubMed] [Google Scholar]
- 18.Wolf M, Shah A, Jimenez-Kimble R, Sauk J, Ecker JL, Thadhani R. Differential risk of hypertensive disorders of pregnancy among Hispanic women. J Am Soc Nephrol. 2004;15:1330–1338. doi: 10.1097/01.asn.0000125615.35046.59. [DOI] [PubMed] [Google Scholar]
- 19.Savitz DA, Danilack VA, Engel SM, Elston B, Lipkind HS. Descriptive epidemiology of chronic hypertension, gestational hypertension, and preeclampsia in New York State, 1995–2004. Matern Child Health J. 2014;18:829–838. doi: 10.1007/s10995-013-1307-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kharrazi M, Pearl M, Yang J, et al. California Very Preterm Birth Study: design and characteristics of the population- and biospecimen bank-based nested case-control study. Paediatr Perinat Epidemiol. 2012;26:250–263. doi: 10.1111/j.1365-3016.2011.01252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jelliffe-Pawlowski LL, Shaw GM, Currier RJ, et al. Association of early-preterm birth with abnormal levels of routinely collected first- and second-trimester biomarkers. Am J Obstet Gynecol. 2013;208:492–411. doi: 10.1016/j.ajog.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The California Department of Public H. The California Biobank Program. Available at: http://www.cdph.ca.gov/programs/GDSP/Pages/California%20Biobank%20Program.aspx. Accessed May 24, 2016.
- 23.Tukey JW. The Problem of Multiple Comparison. In: Braun HI, editor. The Collected Works of John W. Tukey. Vol. 8. New York: Chapman & Hall; 1994. [Google Scholar]
- 24.Lim JH, Kim SY, Park SY, Yang JH, Kim MY, Ryu HM. Effective prediction of preeclampsia by a combined ratio of angiogenesis-related factors. Obstet Gynecol. 2008;111:1403–1409. doi: 10.1097/AOG.0b013e3181719b7a. [DOI] [PubMed] [Google Scholar]
- 25.Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt M, Dogan C, Birdir C, et al. Placental growth factor: a predictive marker for preeclampsia. Gynakol Geburtshilfliche Rundsch. 2009;49:94–99. doi: 10.1159/000197908. [DOI] [PubMed] [Google Scholar]
- 27.Rana S, Karumanchi SA, Levine RJ, et al. Sequential changes in antiangiogenic factors in early pregnancy and risk of developing preeclampsia. Hypertension. 2007;50:137–142. doi: 10.1161/HYPERTENSIONAHA.107.087700. [DOI] [PubMed] [Google Scholar]
- 28.Wathen KA, Tuutti E, Stenman UH, et al. Maternal serum-soluble vascular endothelial growth factor receptor-1 in early pregnancy ending in preeclampsia or intrauterine growth retardation. J Clin Endocrinol Metab. 2006;91:180–184. doi: 10.1210/jc.2005-1076. [DOI] [PubMed] [Google Scholar]
- 29.O’Brien JE, Dvorin E, Drugan A, Johnson MP, Yaron Y, Evans MI. Race-ethnicity-specific variation in multiple-marker biochemical screening: alpha-fetoprotein, hCG, and estriol. Obstet Gynecol. 1997;89:355–358. doi: 10.1016/S0029-7844(96)00524-8. [DOI] [PubMed] [Google Scholar]
- 30.Menon R, Dunlop AL, Kramer MR, Fortunato SJ, Hogue CJ. An overview of racial disparities in preterm birth rates: caused by infection or inflammatory response? Acta Obstet Gynecol Scand. 2011;90:1325–1331. doi: 10.1111/j.1600-0412.2011.01135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Govender L, Mackraj I, Gathiram P, Moodley J. The role of angiogenic, anti-angiogenic and vasoactive factors in pre-eclamptic African women: early- versus late-onset pre-eclampsia. Cardiovasc J Afr. 2012;23:153–159. doi: 10.5830/CVJA-2012-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faupel-Badger JM, Wang Y, Staff AC, et al. Maternal and cord steroid sex hormones, angiogenic factors, and insulin-like growth factor axis in African-American preeclamptic and uncomplicated pregnancies. Cancer Causes Control. 2012;23:779–784. doi: 10.1007/s10552-012-9934-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mijal RS, Holzman CB, Rana S, Karumanchi SA, Wang J, Sikorskii A. Midpregnancy levels of angiogenic markers in relation to maternal characteristics. Am J Obstet Gynecol. 2011;204:244–212. doi: 10.1016/j.ajog.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faupel-Badger JM, Wang Y, Karumanchi SA, et al. Associations of pregnancy characteristics with maternal and cord steroid hormones, angiogenic factors, and insulin-like growth factor axis. Cancer Causes Control. 2011;22:1587–1595. doi: 10.1007/s10552-011-9835-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolf M, Shah A, Lam C, et al. Circulating levels of the antiangiogenic marker sFLT-1 are increased in first versus second pregnancies. Am J Obstet Gynecol. 2005;193:16–22. doi: 10.1016/j.ajog.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 36.English FA, Kenny LC, McCarthy FP. Risk factors and effective management of preeclampsia. Integr Blood Press Control. 2015;8:7–12. doi: 10.2147/IBPC.S50641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cudihy D, Lee RV. The pathophysiology of pre-eclampsia: current clinical concepts. J Obstet Gynaecol. 2009;29:576–582. doi: 10.1080/01443610903061751. [DOI] [PubMed] [Google Scholar]
- 38.Yuan HT, Haig D, Ananth KS. Angiogenic factors in the pathogenesis of preeclampsia. Curr Top Dev Biol. 2005;71:297–312. doi: 10.1016/S0070-2153(05)71009-7. [DOI] [PubMed] [Google Scholar]
- 39.Redman CW. Current topic: pre-eclampsia and the placenta. Placenta. 1991;12:301–308. doi: 10.1016/0143-4004(91)90339-h. [DOI] [PubMed] [Google Scholar]
- 40.Roberts JM, Gammill HS. Preeclampsia: recent insights. Hypertension. 2005;46:1243–1249. doi: 10.1161/01.HYP.0000188408.49896.c5. [DOI] [PubMed] [Google Scholar]
- 41.Carty DM, Delles C, Dominiczak AF. Novel biomarkers for predicting preeclampsia. Trends Cardiovasc Med. 2008;18:186–194. doi: 10.1016/j.tcm.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of preeclampsia: linking placental ischemia/hypoxia with microvascular dysfunction. Microcirculation. 2002;9:147–160. doi: 10.1038/sj.mn.7800137. [DOI] [PubMed] [Google Scholar]
- 43.Chelbi ST, Vaiman D. Genetic and epigenetic factors contribute to the onset of preeclampsia. Mol Cell Endocrinol. 2008;282:120–129. doi: 10.1016/j.mce.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 44.Baumwell S, Karumanchi SA. Pre-eclampsia: clinical manifestations and molecular mechanisms. Nephron Clin Pract. 2007;106:c72–c81. doi: 10.1159/000101801. [DOI] [PubMed] [Google Scholar]
- 45.Yliniemi A, Makikallio K, Korpimaki T, Kouru H, Marttala J, Ryynanen M. Combination of PAPPA, fhCGbeta, AFP, PlGF, sTNFR1, and Maternal Characteristics in Prediction of Early-onset Preeclampsia. Clin Med Insights Reprod Health. 2015;9:13–20. doi: 10.4137/CMRH.S21865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chaiworapongsa T, Romero R, Espinoza J, et al. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Young Investigator Award. Am J Obstet Gynecol Jun. 2004;190:1541–1547. doi: 10.1016/j.ajog.2004.03.043. discussion, 1547–1550. [DOI] [PubMed] [Google Scholar]
- 47.Cowans NJ, Alfthan H, Stenman UH, Spencer K. Stability of first trimester placental growth factor in serum and whole blood. Prenat Diagn. 2011;31:1193–1197. doi: 10.1002/pd.2894. [DOI] [PubMed] [Google Scholar]
- 48.Hubel A, Spindler R, Skubitz AP. Storage of human biospecimens: selection of the optimal storage temperature. Biopreservation and biobanking Jun. 2014;12:165–175. doi: 10.1089/bio.2013.0084. [DOI] [PubMed] [Google Scholar]
- 49.Pearl M, Wier ML, Kharrazi M. Assessing the quality of last menstrual period date on California birth records. Paediatr Perinat Epidemiol. 2007;21(Suppl 2):50–61. doi: 10.1111/j.1365-3016.2007.00861.x. [DOI] [PubMed] [Google Scholar]
- 50.Kusanovic JP, Romero R, Chaiworapongsa T, et al. A prospective cohort study of the value of maternal plasma concentrations of angiogenic and anti-angiogenic factors in early pregnancy and midtrimester in the identification of patients destined to develop preeclampsia. J Matern Fetal Neonatal Med. 2009;22:1021–1038. doi: 10.3109/14767050902994754. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


