Abstract
Many biodemographic studies use biomarkers of inflammation to understand or predict chronic disease and aging. Inflamm-aging, i.e. chronic low-grade inflammation during aging, is commonly characterized by pro-inflammatory biomarkers. However, most studies use just one marker at a time, sometimes leading to conflicting results due to complex interactions among the markers. A multidimensional approach allows a more robust interpretation of the various relationships between the markers. We applied principal component analysis (PCA) to 19 inflammatory biomarkers from the InCHIANTI study. We identified a clear, stable structure among the markers, with the first axis explaining inflammatory activation (both pro- and anti-inflammatory markers loaded strongly and positively) and the second axis innate immune response. The first but not the second axis was strongly correlated with age (r = 0.56, p < 0.0001, r = 0.08 p = 0.053), and both were strongly predictive of mortality (hazard ratios per PCA unit (95% CI): 1.33 (1.16–1.53) and 0.87 (0.76–0.98) respectively) and multiple chronic diseases, but in opposite directions. Both axes were more predictive than any individual markers for baseline chronic diseases and mortality. These results show that PCA can uncover a novel biological structure in the relationships among inflammatory markers, and that key axes of this structure play important roles in chronic disease.
Keywords: Inflammation, Biomarker, Multivariate, Aging, Chronic disease
1. Introduction
Inflammation is known to be important in aging and age-related diseases, including heart disease (Strandberg and Tilvis, 2000), diabetes (Barzilay et al., 2001), cancer (Il’yasova et al., 2005), and Alzheimer’s disease (Akiyama et al., 2000), among others, and is sometimes suggested as a principal aging mechanism (Finch, 2010). We often refer to this phenomenon as “inflamm-aging” (Franceschi et al., 2000) to indicate a chronic low-grade inflammation that occurs with advancing age. It is provoked by a continuous antigenic load and stress, with the persistence of inflammatory stimuli over time representing a biological back-ground creating a predisposition to age-related diseases/disabilities. Most epidemiological studies of inflammation have relied on a single marker as a measure of inflammatory state, often C-reactive protein (CRP) (Strandberg and Tilvis, 2000), interleukin-6 (IL-6) (Bruunsgaard, 2002), or tumor necrosis factor-alpha (TNF-α) (Bruunsgaard et al., 1999). However, as the inflammatory system is known to be complex and involve multiple feedback mechanisms, focusing on only one inflammatory marker may explain conflicting results observed in the literature (Scheller et al., 2011; Yudkin et al., 2000). In one of the few studies to take a multivariate approach, Bandeen-Roche et al. (2009) showed that a single axis of variation was not sufficient to summarize seven common markers, and that there appear to be separate up- and down-regulation components to the system (i.e. simultaneous increases or decreases of multiple biomarkers to regulate the system at higher or lower levels of activity). Accordingly, multivariate approaches can contradict the need to incorporate multiple markers. Similarly, Hsu et al. (2009) showed that calculation of summary variables using a principal component approach does not strengthen associations between inflammation and physical function compared with a single biomarker.
While many of the direct regulatory relationships among inflammatory markers are known (Cesari et al., 2004; Hansson, 2005; Singh and Newman, 2011; Tracy, 2002), this information cannot always be translated into an understanding of how markers co-vary in populations or across long timescales, and thus of how to interpret different inflammatory profiles in a clinical or public health context. An understanding of the multivariate relationships among inflammatory markers thus has the potential to provide clinically relevant interpretations of changing inflammatory markers, and to help understand the underlying (unobservable) biological processes that govern organisation of the inflammatory system at longer timescales. Our goal was to identify stable groups of key inflammatory markers through multivariate tools that provide a better understanding of changes in the inflammatory system during aging.
Here, we applied principal components analysis (PCA) to a set of 19 inflammatory biomarkers in the InCHIANTI database, a cohort of mostly elderly Italians (Ferrucci et al., 2000). PCA identifies key “axes” that summarize the ways in which individuals differ across the ensemble of variables (Jolliffe, 2005). The axes are expressed as linear combinations of the original variables and the coefficients can thus be used to arrive at a biological interpretation of each axis. PCA is a multivariate, data- driven approach that lets the data speak for themselves; a related method, factor analysis, tests the correspondence between the axes and a priori hypotheses. We opted for PCA in this case because we were not certain that there was a sufficient understanding of inflammatory system function and feedback mechanisms at time scales of years to generate robust a priori hypotheses. Axes identified through PCA were validated using population subsets as independent samples, and scores on these axes were then tested for associations with health outcomes, including mortality, and 13 chronic diseases (Cohen et al., 2010; Dusseault-Belanger et al., 2013). Our results do not support simple increases in inflammation with age, but it is possible to identify key axes of variation in inflammatory markers that predict health outcomes independently of age.
2. Methods
2.1. Data
This study uses data from “Invecchiare in Chianti” (Aging in the Chianti area, InCHIANTI), a prospective population-based study of the elderly, developed by the Laboratory of Clinical Epidemiology of the Council of Italian National Research on Aging (INRCA), Florence, Italy. The study population for these analyses included 1453 participants aged between 20 and 102 years old, of which 75% were aged 65 and over, randomly selected from residents in two towns in the Chianti area (Greve in Chianti and Bagno a Ripoli, Tuscany, Italy) using a multi-level stratified sampling method. Initial data collection started in September 1998 and was completed in March 2000. Three and 6-year follow-up assessments of the InCHIANTI study population were performed in the years 2001–2003 and 2004–2006. A detailed description of the sampling procedure and the method of data collection have been published elsewhere (Ferrucci et al., 2000). The ethics committee (INRCA) approved the entire study protocol. For PCA, logistic regression and survival analysis, we used 1010 participants aged 21–96 having full biomarker data at baseline. Only the first visit was used for PCA and logistic regression, due to limited inflammatory biomarker data at later visits. Participants with biomarker or comorbidity measurements that were missing were excluded. The main characteristics of the participants are shown in Table 1.
Table 1.
Main characteristics of the sample population at baseline.
| Mean ± SSD or % (N = 1010) | |
|---|---|
| Sociodemographic characteristics | |
| Age (year) | 67.7 ± 16 |
| Gender (female) | 57.6 |
| Site (Greve) | 41.9 |
| BMI | 27.2 ± 4.1 |
| Smoking (Current) | 18.6 |
| Comorbidities | % |
| Mortality during follow-up | 14.1 |
| Comorbiditya | 30.1 |
| Cardiovascular disease | 12.0 |
| Congestive heart failure | 2.91 |
| Stroke | 2.48 |
| Kidney disease | 2.05 |
| Diabetes | 5.24 |
| Liver disease | 1.90 |
| Arthritis | 7.94 |
| Cancer | 1.83 |
| High blood pressure | 21.5 |
| Myocardial infarction | 3.52 |
| Angina | 2.01 |
| Lung disease | 5.92 |
Note: SD = Standard deviation.
Any of the comorbid conditions that follow.
2.2. Biomarkers
We studied 19 inflammatory biomarkers selected based on their availability and relevance. Details of the methods of measurement of these biomarkers can be found in previous studies (Bandeen-Roche et al., 2009; Cesari et al., 2004; Ferrucci et al., 2000; Varadhan et al., 2014). Included inflammatory markers are as follows: Among the cytokines, interleukin (IL)-1β, which causes a number of different auto-inflammatory syndromes; IL-1RA, which is a member of the IL-1 family that binds to IL-1 receptors but does not induce any intracellular response; IL-6, which could act as a pro- and anti-inflammatory cytokine; IL-8, which is a chemokine; IL-10, which is anti-inflammatory and inhibits the synthesis of IFN-γ and TNF-α; IL-12 and IL-18, which are pro-inflammatory; and IL-15, which is a cytokine derived from T cells that stimulates T cell proliferation and natural killer cell activation.
The receptors included in our analyses were: SGP130, which prevents IL-6 from binding to the membrane receptor, and soluble (s)IL-6R, which forms a ligand–receptor complex with IL-6 that is capable of stimulating a variety of cellular responses. Interferon IFN-γ is critical for innate and adaptive immunity. We also measured transforming growth factor TGF-β1, tumor necrosis factor TNF-α, and TRAIL. Two of their receptors, STNF-RI and STNF-RII, were also included.
We had data on two chemokines MCP and MIP, the latter of which induces the production of IL-6 and TNF-α. In addition, we used high sensitivity C-reactive protein (hsCRP), which is a clinical marker of systemic inflammatory state. The main characteristics of the markers used are shown in Table 2.
Table 2.
Main characteristics of the inflammatory markers.
| Mean ± SD (N = 1010) | Age correlationa | |
|---|---|---|
| Inflammatory markers | ||
| hsCRP (μg/ml) | 4.32 ± 6.65 | 0.15** |
| IFN-γ (pg/ml) | 23.14 ± 267.83 | 0.01 |
| IL-1β (pg/ml) | 0.28 ± 1.33 | 0.02 |
| IL-1RA (pg/ml) | 147.72 ± 101.12 | 0.07 * |
| IL-6 (pg/ml) | 3.25 ± 2.33 | 0.31** |
| sIL-6R (ng/ml) | 104.74 ± 58.71 | 0.04 |
| IL-8 (pg/ml) | 11.06 ± 147.93 | 0.02 |
| IL-10 (pg/ml) | 90.59 ± 355.44 | −0.13** |
| IL-12 (pg/ml) | 14.05 ± 166.36 | 0.01 |
| IL-15 (pg/ml) | 2.50 ± 0.59 | 0.19** |
| IL-18 (pg/ml) | 383.60 ± 140.54 | 0.21** |
| MCP (pg/ml) | 53.11 ± 328.30 | 0.02 |
| MIP (pg/ml) | 88.63 ± 159.07 | 0.09 * |
| SGP130 (ng/ml) | 305.32 ± 60.40 | 0.25** |
| STNF-RI (pg/ml) | 1375.8 ± 643.05 | 0.48** |
| STNF-RII (pg/ml) | 2620 ± 779.97 | 0.51** |
| TGF-β1 (pg/ml) | 12,063 ± 7342.67 | 0.02 |
| TNF-α (pg/ml) | 6.35 ± 46.19 | 0.02 |
| TRAIL (pg/ml) | 75.5 ± 40.59 | 0.01 |
Pearson’s correlation with age at baseline.
SD, standard deviation; hsCRP, high sensitivity C-reactive protein; IFN-γ, interferon-γ; IL, interleukin; IL-1RA, interleukin-1 receptor antagonist; sIL-6R, soluble IL-6 receptor; MCP, monocyte chemoattractant protein-1; MIP, macrophage inflammatory protein-1b; SGP130, soluble glycoprotein 130; STNF-R, soluble TNF receptor; TGF, transforming growth factor; TNF-α, tumor necrosis factor-alpha; TRAIL, TNF-related apoptosis-inducing ligand.
p < 0.05.
p < 0.01.
2.3. Comorbidities
We selected 13 chronic conditions based on sufficient prevalence for meaningful analysis. Each was assessed at baseline and at follow-up visits. The conditions are: high blood pressure, lung, kidney, liver and cardiovascular disease, stroke, angina, congestive heart failure, diabetes, arthritis, cancer and myocardial infarction. Detailed data were available from which to evaluate the presence or absence of chronic diseases; when possible we coded each patient as 0 (no disease), 1 (having the disease), or 0.5 (ambiguous). Details are available in the online supplement. We excluded the participants with an intermediate state to facilitate model interpretation; alternative models counting them as positive or negative did not substantially change results (data not shown). From the 13 comorbidities we created a new variable named “comorbidity” which has a value of 1 if an individual has one or more of the 13 diseases mentioned above and 0 otherwise. Information on baseline prevalence of the comorbidities is shown in Table 1. Percentages for the comorbidities represent the proportion of individuals that had the chronic disease at baseline (with the exception of mortality) or developed it in one of the follow-ups, for a total of 2785 visits with 1010 individuals.
2.4. Statistical analyses
We first transformed our data to meet the assumption of normality needed for the PCA. We used a logarithmic transformation for all variables except SGP130 and TNF-α, which were already normally distributed. Subsequently, variables were standardized by subtracting their mean and dividing by their standard deviation. Thereafter, we performed a PCA on the 19 biomarkers. The stability of each of the axes under random sampling was then tested using bootstrap methods based on Daudin’s algorithm (Daudin et al., 1988). This method produces random samples of the same size where each individual can be selected more than once in the same sample. From the original database, we thus synthesized 5000 other databases and performed a PCA on each of them. This allowed us to construct confidence intervals and to verify the variation of the components in each axis as well as the proportion of variance explained. In addition, a similar approach using non-random sampling was performed to determine whether axis stability and interpretation were conserved even across mutually exclusive and potentially biologically different sub-populations. The groups used for this included men, women, residents of Greve in Chianti or Bango a Ripoli, and age groups (<65, 65+ years). Finally, PCA results were verified using a “sparse PCA,” which acts much like a factor analysis to identify axes that align strongly with the original variables based on pre-defined weights (Zou et al., 2006) This can aid in the interpretation by limiting the number of variables strongly associated with each axis, but has the disadvantage of depending to a certain extent on the weights, which are arbitrary.
For the two axes found to be stable, we computed a “score” for each individual. This score represents the position of an individual on the principal axis. We then assessed the correlation between participant age and score on each of these two main axes. These scores were then divided by their respective standard deviations so that they would have the same scales (and thus comparable effect sizes) as individual markers in regression analyses (below).
Relationships between PCA scores and baseline comorbidities were assessed using logistic regression controlling for age as a cubic spline. Splines were fit using the bs function in the fda package in R. The same model was fit for individuals who died before the first follow-up to assess mortality. Odds ratios (ORs) per unit score were then estimated for the scores of the two main axes. All models were run with and without control for smoking status, BMI, site, and sex. BMI and smoking may be important confounders; for example increased visceral adiposity and smoking are potent triggers of inflammatory mediators (Prospective Studies Collaboration, 2009; US Department of Health and Human Services, 2004); but may also affect health state through pathways other than inflammation. Models were also assessed with stratified sex.
Using longitudinal data on chronic diseases and excluding individuals with the respective baseline chronic disease, we fitted survival analyses using a Cox proportional hazard function controlling for age as a cubic spline. Due to limited inflammatory biomarker data at later visits, only the baseline scores of the two main axes were included in the models. The survival models indicated whether a high baseline score increases or reduces the likelihood of incidence. Hazard ratios (HRs) per unit score were estimated.
3. Results
3.1. Principal component analysis
We performed PCA on 19 inflammatory biomarkers transformed and standardized on 1010 individuals aged 21–96 years. The first principal axis (PCA1) explained 19% of the total variance among the inflammatory markers and the second (PCA2) explained 10% for a cumulative of 29% (Fig. 1). Only the first two axes were stable across 5000 random (bootstrapped) samples.
Fig. 1.
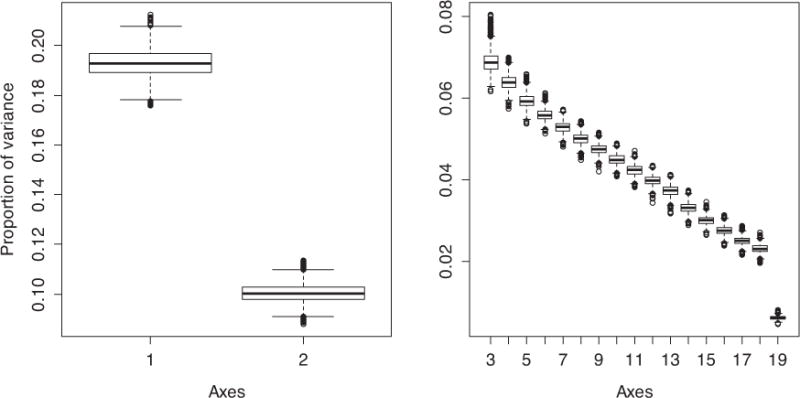
Boxplot of variance explained for each of the 19 axes using 5000 random samples from the bootstrap. The first two axes (PCA1 and PCA2) are presented separately to allow appropriate y-axis scaling.
Using these 5000 iterations, we calculated the correlation between the original scores and those created by the bootstrap samples. A strong correlation for the same axis across bootstrap samples would indicate that the axis interpretation is robust to fluctuations in sample composition. The correlation coefficient (r) varies between 0.990 and 0.9999 for PCA1 and varies between 0.91 and 0.9999 for PCA2 with 95% confidence (Fig. 2).
Fig. 2.
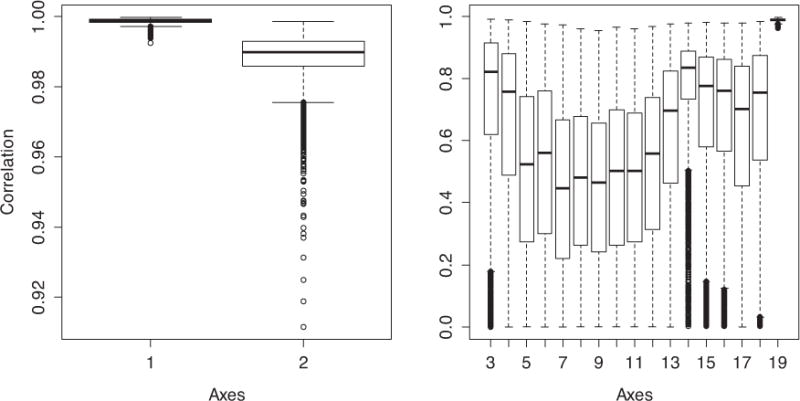
Boxplot of correlations between the original scores and those created by the 5000 random samples from the bootstrap for each of the 19 axes.
Starting with the 3rd axis, axis order is occasionally inversed in bootstrap samples, leading to very low correlations, but even among the non-inversed samples it is clear that the correlations are much weaker than for the first two axes, which need to be shown with a separate y-axis scale to indicate variation.
The stability of the main axis loadings across non-random samples is shown in Fig. 3. The order and importance of the axis loadings is essentially unchanged even in mutually exclusive subsamples.
Fig. 3.
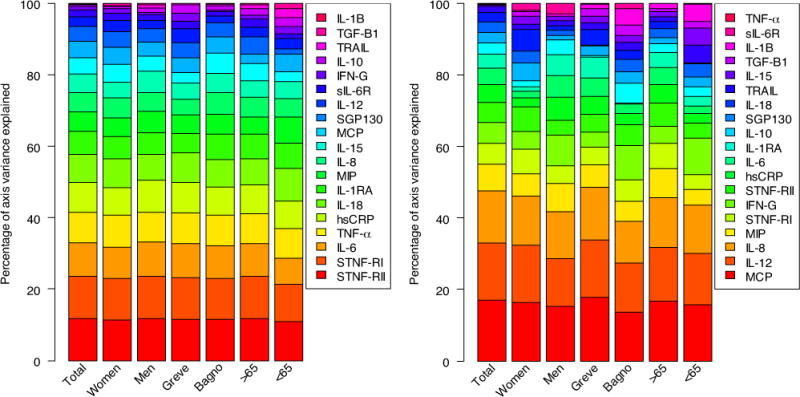
Strength and stability of axis loadings for PCA1 (left) and PCA2 (right) across non-random, often mutually exclusive population subsamples (the entire population, women, men, those from Greve in Chianti, those from Bagno a Ripoli, those aged less than 65, those aged more than 65). Here, each color represents one of the 19 markers, ordered from bottom to top by their importance in the full population analysis, represented by the height of the color. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
A particular group of markers comprising STNF-RI, STNF-RII, IL6, TNF-α, hsCRP, IL-18, IL1-RA appears to be dissociated from all others, as graphically represented PCA1 (Fig. 4). A similar observation was made for MCP, IL-8 and IL-12 on PCA2 (Fig. 4). Since each group is the most representative in its respective axis, they drive the interpretation of the axes, though the markers explaining PCA2 also loaded moderately on PCA1. Note that the markers that load most heavily on PCA1 include both pro- and anti-inflammatory markers, and that they load in the same direction. In other words, PCA1 explains the degree to which an individual has simultaneously high (or low) levels of both pro- and anti-inflammatory markers. Results were confirmed with sparse PCA, which showed that loadings were similarly distributed among the axes (see Supplementary content).
Fig. 4.
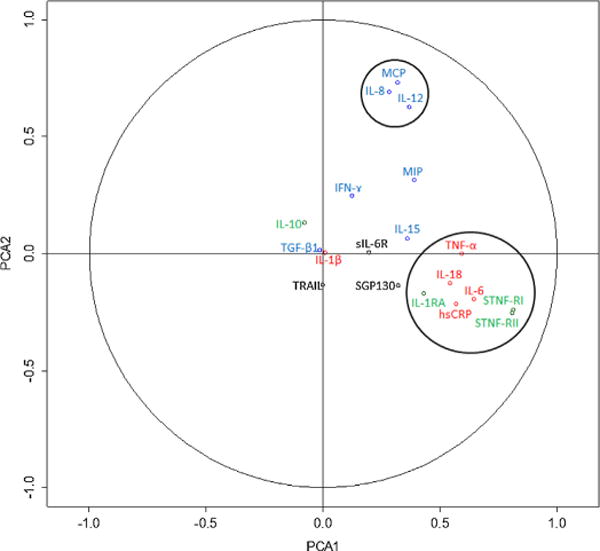
Biplot of loadings for the first two PCA axes. A loading far from zero on a given axis indicates that the variable in question plays an important role in determining the axis. Green names are anti-inflammatory markers, red names are pro-inflammatory, blue names are part of the innate immune system and black names are the rest. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Based on key markers that compose the two stable groups, we conducted a PCA and assessed the scores for participants for the first two axes. PCA1 showed a strong correlation with age (r = 0.56, p < 0.0001, Fig. 5-left), whereas PCA2 showed a very weak and only marginally significant association with age (r = 0.08, p = 0.053, Fig. 5-right). Note that hsCRP, IL-1RA, IL-6, IL-10, IL-15, IL-18, MIP, SGP130, STNF-RI, and STNF-RII were significantly correlated with age (Table 2), but by incorporating the correlation structure of the PCA, we obtain a higher correlation than any measure alone.
Fig. 5.
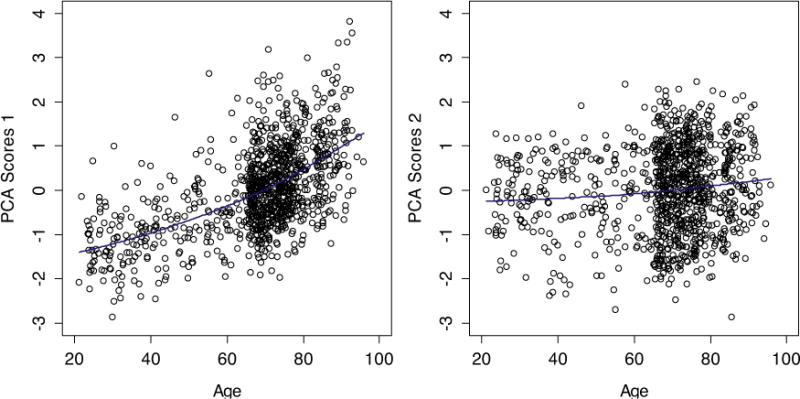
Correlations between age and the standardized scores for PCA1 (left), PCA2 (right). Blue lines indicated cubic spline fits.
3.2. Logistic regression models
We used logistic regression to calculate the ORs of the 13 comorbidities and mortality for PCA scores after adjusting for age. The ORs and respective 95% confidence intervals are shown in Table 3. Note that ORs are per unit PCA; the scale of PCA1 (after being standardized for comparison purposes) goes from roughly −3 to +3, so the OR between the individual with the lowest score and the highest score is the OR to the sixth power, 2.346 ≈ 164 for mortality, for example. ORs for key individual markers associated with the main axes were also included in the table for comparison. As expected from the unchanged order and importance of the axis loadings between genders, no significant differences were observed between men and women in the logistic models (data available in the supplementary content). Models were also adjusted for age, sex, site, BMI and smoking; results are shown in Table 3.
Table 3.
Results from the logistic regressions for baseline comorbidities.
| Comorbidity conditions | n | Joint Model, OR (95% CI)
|
Separate models, OR
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PCA1a | PCA2a | STNF-RI | IL-6 | hsCRP | TNF-α | IL-12 | IL8 | MCP | ||
| Model A | ||||||||||
| Mortality | 135 | 2.34** (1.72–3.25) | 0.63** (0.48–0.82) | 2.00 ** | 1.98 ** | 1.98** | – | – | – | – |
| Comorbidity | 686 | 1.50** (1.23–1.84) | 0.78** (0.66–0.90) | 1.33** | 1.32 ** | 1.28** | – | – | – | – |
| Cardiovascular disease | 184 | 1.57** (1.26–1.96) | 0.69** (0.57–0.83) | 1.43** | 1.52 ** | 1.26** | 1.23* | – | 0.83* | – |
| Congestive heart failure | 48 | 2.02** (1.42–2.91) | 0.43** (0.30–0.61) | 1.90** | 1.75 ** | 1.41* | 1.35* | – | – | 0.64* |
| Stroke | 46 | 2.05** (1.49–2.86) | 0.74* (0.55–0.98) | 1.88** | – | 1.69** | 1.28* | – | – | – |
| Kidney disease | 26 | 2.84** (1.41–5.81) | 0.79 (0.40–1.37) | 2.49** | 2.36 * | – | 1.88* | – | – | – |
| Diabetes | 112 | 1.61** (1.28–2.04) | 0.95 (0.79–1.15) | 1.25* | 1.42 ** | 1.17* | – | – | – | 1.22* |
| Liver disease | 41 | 1.28 (0.88–1.85) | 0.66* (0.47–0.90) | 1.62* | – | – | – | – | – | 0.62* |
| Arthritis | 91 | 1.04 (0.80–1.36) | 0.74* (0.58–0.93) | – | – | – | – | – | 0.77* | – |
| Cancer | 16 | 1.68′ (0.97–2.83) | 0.82 (0.50–1.32) | – | – | – | – | – | – | – |
| High blood pressure | 422 | 1.11 (0.94–1.29) | 0.94 (0.83–1.07) | – | – | – | – | – | – | – |
| Myocardial Infarction | 31 | 1.15 (0.74–1.75) | 0.96 (0.66–1.38) | – | – | – | – | – | – | – |
| Angina | 43 | 1.32 (0.92–1.87) | 0.87 (0.79–1.18) | – | – | – | – | – | – | – |
| Lung disease | 130 | 1.16 (0.93–1.45) | 0.87 (0.72–1.05) | – | 1.44 ** | 1.36** | – | – | – | – |
| Model B | ||||||||||
| Mortality | 135 | 1.97** (1.42–2.74) | 0.66** (0.50–0.87) | 1.77** | 1.81 ** | 1.80** | – | – | – | – |
| Comorbidity | 686 | 1.33** (1.08–1.65) | 0.82** (0.70–0.97) | – | 1.30 ** | 1.25* | – | – | – | – |
| Cardiovascular disease | 184 | 1.53** (1.21–1.93) | 0.66** (0.54–0.81) | 1.48** | 1.45 ** | 1.27* | 1.22* | – | 0.81* | – |
| Congestive heart failure | 48 | 1.85** (1.27–2.72) | 0.43** (0.29–0.62) | 1.74** | 1.58 * | – | 1.31* | – | – | 0.60 ** |
| Stroke | 46 | 2.04** (1.40–2.96) | 0.74* (0.53–0.98) | 1.76** | – | 1.68** | – | – | – | – |
| Kidney disease | 26 | 9.78** (3.22–43.1) | 0.48* (0.20–0.98) | 8.24** | 3.19 ** | 2.79* | – | – | – | – |
| Diabetes | 112 | 1.52** (1.19–1.94) | 0.97 (0.79–1.19) | – | 1.37 * | – | – | – | – | – |
| Liver disease | 41 | 1.17 (0.77–1.74) | 0.68* (0.48–0.95) | – | – | – | – | – | – | 0.66* |
| Arthritis | 91 | 1.01 (0.74–1.37) | 0.84 (0.66–1.08) | – | – | – | – | – | – | – |
| Cancer | 16 | 1.90* (0.97–2.83) | 0.67 (0.38–1.13) | 1.88* | – | – | – | – | – | – |
| High blood pressure | 422 | 1.02 (0.86–1.21) | 0.98 (0.88–1.17) | – | – | – | – | – | – | – |
| Myocardial Infarction | 31 | 1.22 (0.78–1.90) | 0.95 (0.64–1.39) | – | – | – | – | – | – | – |
| Angina | 43 | 1.29 (0.88–1.87) | 0.86 (0.62–1.18) | – | – | – | – | – | – | – |
| Lung disease | 130 | 1.00 (0.79–1.29) | 0.94 (0.77–1.15) | – | – | – | – | – | – | – |
Odds ratios are per unit change in the scores of PCA1 and PCA2.
OR, Odds ratio; CI, Confidence intervals.
p < 0.10.
p < 0.05.
p < 0.01.
The joint model included both PCA1 and PCA2 as simultaneous predictors of chronic diseases. Each inflammatory marker was modeled separately for its effect on each chronic disease. Model A controlled for age only and Model B controlled for age, sex, site, BMI and smoking.
3.3. Survival analysis
From the scores assessed at baseline with PCA, we used a survival analysis to assess the long-term effect of a high or low score on the two main axes for the 13 comorbidities and mortality after adjusting for age. The hazard ratios (HRs) and 95% confidence intervals are shown in Table 4. Stratification by sex did not meaningfully change the results (data available in the supplementary content). We also adjusted the models for age, sex, site, BMI and smoking; results are shown in Table 4.
Table 4.
Results from the survival analysis for comorbidities.
| Comorbidity conditions | n | Joint Model, HR (95% CI)
|
Separate models, HR
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PCA1a | PCA2a | STNF-RI | IL-6 | hsCRP | TNF-α | IL-12 | IL8 | MCP | ||
| Model A | ||||||||||
| Mortality | 257 | 1.33** (1.16–1.53) | 0.87* (0.76–0.98) | 1.30 ** | 1.28 ** | 1.21 ** | – | – | – | – |
| Comorbidity | 153 | 1.23* (1.03–1.49) | 0.87′ (0.74–1.02) | – | – | 1.33 ** | – | – | – | – |
| Cardiovascular disease | 151 | 1.10 (0.92–1.32) | 1.04 (0.89–1.22) | – | 1.32 ** | 1.35 ** | 0.81 * | – | – | – |
| Congestive heart failure | 33 | 1.16 (0.78–1.73) | 0.85 (0.59–1.22) | – | 1.53 * | 1.47 * | – | – | – | – |
| Stroke | 23 | 1.26 (0.80–1.99) | 0.99 (0.68–1.47) | – | 1.63 * | – | – | – | – | – |
| Kidney disease | 31 | 1.74** (1.16–2.61) | 1.14 (0.81–1.62) | 2.76 ** | – | – | – | – | – | 1.54 * |
| Diabetes | 34 | 1.26 (0.86–1.86) | 1.07 (0.78–1.49) | – | – | – | – | – | – | – |
| Liver disease | 12 | 0.72 (0.35–1.49) | 0.57 (0.29–1.12) | – | – | – | – | – | – | – |
| Arthritis | 130 | 1.09 (0.88–1.34) | 0.95 (0.81–1.13) | – | – | – | – | – | – | – |
| Cancer | 35 | 0.90 (0.61–1.34) | 1.24 (0.90–1.69) | – | – | – | – | – | – | – |
| High blood pressure | 178 | 1.25* (1.05–1.49) | 0.86* (0.75–0.99) | – | – | 1.19 * | – | – | – | – |
| Myocardial Infarction | 67 | 0.74* (0.55–0.99) | 1.08 (0.85–1.36) | 0.71* | – | – | 0.77 * | – | – | – |
| Angina | 13 | 0.88 (0.46–1.68) | 1.89* (1.06–3.36) | – | – | – | – | – | – | – |
| Lung disease | 35 | 1.24 (0.86–1.81) | 1.09 (0.80–1.48) | – | 1.53 * | – | 1.58 * | – | – | – |
| Model B | ||||||||||
| Mortality | 257 | 1.27** (1.10–1.47) | 0.89′ (0.78–0.98) | 1.26 ** | 1.24 ** | 1.18 ** | – | – | – | – |
| Comorbidity | 153 | 1.17* (1.02–1.42) | 0.90 (0.76–1.08) | – | – | 1.35 ** | – | – | – | – |
| Cardiovascular disease | 151 | 1.03 (0.85–1.25) | 1.11 (0.94–1.32) | – | 1.25 * | 1.33 ** | 0.81 * | – | – | – |
| Congestive heart failure | 33 | 1.03 (0.68–1.57) | 0.94 (0.64–1.39) | – | – | – | – | – | – | – |
| Stroke | 23 | 1.21 (0.76–1.92) | 1.09 (0.73–1.64) | – | – | – | – | – | – | – |
| Kidney disease | 31 | 1.76** (1.15–2.70) | 1.13 (0.78–1.64) | 3.49 ** | – | – | – | – | – | 1.57 * |
| Diabetes | 34 | 1.04 (0.69–1.59) | 1.19 (0.84–1.71) | – | – | – | – | – | – | – |
| Liver disease | 12 | 0.61(0.27–1.36) | 0.57 (0.29–1.13) | – | – | – | – | – | – | – |
| Arthritis | 130 | 1.06 (0.85–1.32) | 0.95 (0.81–1.13) | – | – | – | – | – | – | – |
| Cancer | 35 | 0.84 (0.55–1.28) | 1.31 (0.93–1.83) | – | – | – | – | – | – | – |
| High blood pressure | 178 | 1.21* (1.04–1.44) | 0.88* (0.76–0.99) | – | – | 1.20 * | – | – | – | – |
| Myocardial Infarction | 67 | 0.73* (0.54–0.99) | 1.10 (0.86–1.42) | 0.69* | – | – | 0.75 * | – | – | – |
| Angina | 13 | 0.85 (0.44–1.64) | 1.82* (1.01–3.03) | – | – | – | – | – | – | – |
| Lung disease | 35 | 1.24 (0.83–1.84) | 1.09 (0.79–1.52) | – | – | – | – | – | – | – |
Hazard ratios are per unit change in the scores of PCA1 and PCA2.
HR, Hazard ratio; CI, Confidence intervals.
p < 0.10.
p < 0.05.
p < 0.01.
The joint model included both PCA1 and PCA2 as simultaneous predictors of chronic diseases. Each inflammatory marker was modeled separately for its effect on each chronic disease. Model A controlled for age only and Model B controlled for age, sex, site, BMI and smoking.
4. Discussion
The present study used a multivariate approach to assess the relationship between inflammatory markers, age, chronic diseases, and mortality in an elderly population. A traditional understanding of “Inflamm-aging” suggests that low-grade inflammation increases during aging and can be measured by levels of pro-inflammatory markers (Singh and Newman, 2011). Contrary to this idea, our results showed that the main axis of variation we detected – which can clearly be interpreted as a measure of Inflamm-aging given its associations with individual markers, age, and health outcomes – implied simultaneous changes in both pro- and anti-inflammatory markers (STNF-RI, STNF-RII, IL-6, TNF-α, hsCRP, IL-18 and IL-1 RA). Individuals thus varied in terms of the overall activation of their inflammatory systems much more than in terms of the pro- vs. anti-inflammatory balance, with individuals that showed high levels of pro-inflammatory markers also tending to show high levels of anti-inflammatory markers. We did not detect an axis representing pro- versus anti-inflammatory balance, indicating that such a balance does not explain important variation at the population level.
Overall, we detected two predominant, highly stable axes of variation in the inflammatory system. Together, these axes explained 29% of the total variance among the inflammatory markers, enough to indicate their importance, but far less than 100%. The remaining axes were unstable, suggesting that complex system dynamics determine a large part of the variance in ways that cannot be easily characterized by approaches such as PCA. Nonetheless, the two axes identified have clear, interesting biological interpretations. Obviously, had we included more markers, we might have obtained a different axis structure, perhaps detecting other important axes, or other markers associated with PCA1 and PCA2. However, given the stability of our results in subpopulations and the concordance of this study with others (Bandeen-Roche et al., 2009; Varadhan et al., 2014; Hsu et al., 2009), the core interpretations of PCA1 and PCA2 would almost certainly remain unchanged. This was also confirmed by the sparse PCA, which reduces the number of meaningful loadings from the axes to simplify interpretation.
The first axis was driven largely by STNF-RI, STNF-RII, IL-6, TNF-α, hsCRP, IL-18 and IL-1 RA and was strongly correlated with age. As STNF-RI, STNF-RII and IL-6 are individually correlated with age and known to be associated with health outcomes (Diniz et al., 2010; Fernandez-Real et al., 2001; Il’yasova et al., 2005; Safranow et al., 2009) the result is not unexpected. However, the combination of these variables through PCA leads to a stronger correlation than any variable alone. As noted above, the loadings for PCA1 indicate that it is not a simple measure of more inflammation: it is driven by higher levels of both pro- and anti-inflammatory markers, indicating a more activated (but not necessarily more inflamed) inflammatory system. One interpretation would be that increasing levels of pro-inflammatory markers with age stimulate a corresponding augmentation in anti-inflammatory markers, with varying outcomes depending on the nature of the stimulation, the pre-existing physiological reserve, and the current immune background.
Inflammation is well known to be associated with many chronic diseases such as diabetes, atherosclerosis and cardiovascular disease (Hansson, 2005; Schmidt et al., 1999), and this was confirmed here. The ORs indicating the baseline presence of chronic diseases based on the first axis were surprisingly large (considering that the ORs are per unit PCA, and the PCAs range from −3 to 3), and were significant for mortality, presence of any comorbidity, and individual chronic diseases such as cardiovascular disease, congestive heart failure, stroke, kidney disease and diabetes. These findings remain unchanged even after controlling for age, sex, site, BMI and smoking. The effect sizes decreased slightly but all remained significant; the small magnitude of the decrease suggests that results are unlikely to be attributable to residual confounding. For most of the remaining chronic diseases, sample sizes were too small to be conclusive. The majority of these diseases are related to the cardiovascular system. The causes of cardiovascular diseases are diverse but atherosclerosis and/or hypertension are the most common (Epstein and Ross, 1999; Sowers et al., 2001). Interestingly the relationship was not significant for hypertension, despite a large sample, suggesting an independent contribution of inflammation to cardiovascular disease. This supports the study of Pearson et al. (2003) who showed that in the case of inflammatory markers, the association with cardiovascular disease might reflect a response to other, established risk factors (e.g., obesity, diabetes, hyperlipidemia, cigarette smoking) or due to inflammatory processes as part of atherosclerotic disease. Furthermore, PCA1 generally predicted outcomes more strongly than the individual markers, particularly for prevalence rather than incidence.
The strong associations between PCA1 and health outcomes at baseline shown via logistic regression do not address causality. Indeed, it is likely that diseased states feed back into inflammatory systems, and that the elevated levels of PCA1 are as much consequence as cause of chronic diseases. This is borne out by the survival analyses, where effect sizes were generally much smaller and rarely significant, though still almost always positive. The lack of significance, but not the smaller effect sizes, is also probably due to the often much smaller sample sizes for incidence rather than prevalence for many diseases. Nonetheless, there were still clear associations for mortality and kidney disease, and, unlike in the logistic regressions, hypertension incidence was associated with high PCA1 scores. Together, these results are consistent with biological impacts of PCA1 on chronic diseases and mortality, but also with effects of chronic diseases on PCA1, forming positive feedback loops. These effects appear to be heterogeneous across diseases, with, for example, PCA1 having a potentially large effect on kidney disease, and cardiovascular disease having a potentially large effect on PCA1.
Given these results with chronic diseases, we should hesitate to interpret PCA1 as a measure of aging or part of the aging process. Although the correlation between PCA1 and age is strong, it is possible that changes in PCA1 reflect chronic disease processes in feedback loops, and that the age-PCA1 correlation is due to age-related increases in chronic disease risk. Just as no older individual (80+) in our sample had a very low score on PCA1, no individual at these ages was completely free from chronic diseases, and we have very little statistical power to address the possibility of disease-independent Inflamm-aging. However, PCA2 was strongly associated with chronic diseases but not age, so we cannot exclude the possibility that PCA1 is linked to aging as well as to chronic diseases. In any case, PCA1 suggests that Inflamm-aging is a generalized dysregulation of the inflammatory system, but that this dysregulation proceeds in a relatively predictable fashion and results in a coherent restructuration of the levels of multiple key inflammatory markers.
Hsu et al. (2009) recently conducted a PCA on eight inflammatory markers (STNF-RI, STNF-RII, IL-6, TNF-α, hsCRP, sIL-2R, sIL-6R and plasminogen activator inhibitor-1) in the Health ABC study, a large cohort study of aging. They identified two principal components and showed that both were associated with physical function measures. Interestingly, they appear to measure the same PCA1 we do (TNF-α, STNF-RI, STNF-RII, sIL-6R, sIL-2R). However, the PCA2 we describe was not detected in their study, probably because the main innate immune markers that underlie it were not available in that data set. The findings are thus highly complementary.
Another recent study applied survival analysis and PCA to biomarkers of inflammation from InCHIANTI to develop clinical predictors of mortality up to 10 years (Varadhan et al., 2014). They used a weighted summary score (WSS) and principal component summary score (PCS) based on five markers chosen for their association with mortality (IL-6, STNF-RI, hsCRP, IL-18 and IL1-RA), as well as an inflammatory index score (IIS) based on the two best markers (IL-6 and STNF-R1). IIS had the strongest predictive power for 1-, 2- and 10-year mortality. The most important markers that drive the interpretation of our main axis are STNF-RI, STNF-RII and IL-6. Table 5 shows the correlations between PCA1 and the measures in the study of Varadhan et al. (2014) as well as the HRs both assessed with the InChianti participants (n = 1010) for mortality. HRs of IIS and WSS perform slightly better than PCA1, but the overall effects are comparable considering the confidence intervals.
Table 5.
Correlation matrix and Hazard ratios comparison for mortality.
| Measure | PCA1 | PCS | IIS | WSS | HR(95% CI) |
|---|---|---|---|---|---|
| PCA1 | 1 | – | – | – | 1.33 (1.16–1.53) |
| PCS | 0.88 | 1 | – | – | 1.18 (1.04–1.34) |
| IIS | 0.86 | 0.84 | 1 | – | 1.47 (1.26–1.72) |
| WSS | 0.91 | 0.93 | 0.92 | 1 | 1.54 (1.26–1.88) |
Notes: PCA1, scores from the first axis of PCA with 19 biomarkers; PCS, scores from the first axis of PCA with 5 biomarkers; ISS, inflammatory index score was calculated as follows ISS, 1/3 IL-6 + 2/3 STNF-RI; WSS, weighted summary score was calculated as a weighted sum of the 5 biomarkers.
While the approaches of the two studies are similar, Varadhan et al. (2014) has clinical prediction as a primary goal, whereas we emphasize biological understanding. In this sense the two studies are confirmatory and complementary, despite some potentially important methodological differences (e.g. variable selection criteria). We provide evidence of two stable biological processes; they provide a clinically relevant measure of the first.
In addition, a previous analysis using the Leiden 85-plus study showed that an unopposed pro-inflammatory response is beneficial for survival in the oldest (Wijsman et al., 2011). Furthermore, low-pro and low-anti-inflammatory markers showed an increase in mortality, contrasting with our results. However, their result did not apply in a subsample of individuals aged 90+, and our study cohort is composed mainly of younger individuals, suggesting a possibility strong non-linear interaction of these effects with age.
The second biological process we identified is explained largely by MCP, IL-12 and IL-8. This axis can be interpreted as reflecting aspects of the innate immune response. The innate immune system comprises the cells and mechanisms that defend the body against immediate infectious agents regardless of prior exposure. Innate immune cells include natural killer cells, granulocytes (mast cells, eosinophils and basophils) and phagocytes (macrophages, neutrophils and dendritic cells), and the main role of MCP, IL-12 and IL-8 is to attract and stimulate these cells. While PCA2 is highly stable and appears to represent important variation in the state of the inflammatory system, it does not appear to be directly related to the aging process. As MCP, IL-12 and IL-8 were not significantly correlated with age, the weak correlation with age was not unexpected. It may reflect genetic variation in the structure of the immune system or transient variation in inflammatory state based on minor, short-term immune challenges occurring through all physiological systems independent of age.
However, PCA2 was associated with roughly the same chronic diseases as PCA1, but in the opposite direction (i.e. OR/HR less than 1), meaning that an increase in PCA2 is protective for the chronic diseases. Perhaps this counterintuitive result indicates that high levels of innate immune markers are associated with a robust innate capacity rather than with a current immune challenge. Additionally, while effect sizes for PCA1 and individual markers moved in parallel, PCA2 was often a stronger predictor (e.g. congestive heart failure) or weaker predictor (e.g. kidney disease) than PCA1 and the individual markers, confirming its complementary role in chronic disease. We can see from Fig. 4 that MCP, IL-12 and IL-8 also loaded positively on the first axis. This means that Inflamm-aging seems to be incorporating part of the innate immune activation in our dataset, despite the opposing predictive values of the two for chronic diseases. Other studies suggest that extrinsic (environmental) factors become increasingly important in the elderly, and as age is associated with a breakdown of the epithelial barriers of the skin, lung and gastrointestinal tract, the innate immune system is more challenged (Gomez et al., 2005), perhaps needed as a counterbalance to age-related changes in the more sophisticated components of the first axis. This might explain the stability of the axis in our data.
4.1. Limitations
We showed that PCA was stable for the two main axes across InCHIANTI, but analyses need to be replicated in other data sets to look for a general trend across populations. The absence of longitudinal data on the full set of inflammatory markers also obliged us to use a single time point estimate for PCA1 and PCA2. Given the substantial short-term fluctuations of inflammatory markers, this could have introduced a substantial noise component and decreased our statistical power to predict health outcomes. The small sample sizes for some conditions like cancer or liver disease (1.83% and 1.9% of the sample respectively) make the logistic regression results sensitive to perturbations. Also, there were substantial missing values among the 13 chronic diseases, and we were unable to incorporate medication status into our models; still, results agree with the studies of Varadhan et al. (2014) and Hsu et al. (2009). The availability of the visits (a maximum of four visits with a 3-year space between each) and the missing values of several markers at follow-ups is a constraint when using the Cox proportional hazards model. Lastly, the circulating inflammatory markers we used spill over from the tissues and thus represent the complex signalling pathways that take place in the cells (Chaturvedi et al., 2013). It is not clear if these inflammatory mediators are causal factors or just byproducts of pathologies. As our study focuses on biological understanding rather than clinical prediction, the PCA axes we detect can be interpreted as representing key variation in inflammatory state, including the tissue processes to which they are only indirectly linked. However, caution should be used in interpreting the axes based on the loadings, since it is not clear to what extent the tissue processes are truly reflected by circulating levels of individual markers.
5. Conclusions
Multivariate analysis of inflammatory biomarkers appears to show an augmentation in overall activation of inflammatory systems with aging, but not necessarily in levels of inflammation per se, as is generally supposed. In this sense, simple use of IL-6 or CRP as inflammatory markers may be a bit misleading, even though they correlate well with overall activation and predict chronic diseases. When considered altogether, the markers STNF-RI, STNF-RII, IL-6, TNF-α, hsCRP, IL-18 and IL-1 RA are, as a group, strongly associated with chronic diseases and mortality in this elderly population. A second key axis, hitherto unappreciated in the scientific literature, describes innate immune system activation and may be protective against many chronic diseases and mortality. The combination of the two axes suggests that Inflamm-aging is not simply an increase in pro-inflammatory markers but may be a fine balance between them. Future studies of inflammation should continue to use multiple markers and take systems-based approaches to estimate the relevant underlying biological processes.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute on Aging.
Funding
Alan A. Cohen is a member of the FRQ-S-supported Centre de recherche sur le vieillissement and Centre de recherche clinique du CHUS and is a funded Research Scholar of the FRQ-S. This research was supported by CIHR grant #s 110789, 120305, 119485 and by NSERC Discovery Grant # 402079-2011.
Abbreviations
- PCA
principle component analysis
- PCA1
first principal axis
- PCA2
second principal axis
- OR
odds ratio
- HR
hazard ratio
- AIC
Akaike information criterion
- hsCRP
high sensitivity C-reactive protein
- IFN-γ
interferon-γ
- IL
interleukin
- MCP
monocyte chemoattractant protein-1
- MIP
macrophage inflammatory protein-1b
- SGP130
soluble glycoprotein 130
- TNF-α
tumor necrosis factor-alpha
- STNF-R
soluble TNF receptor
- TGF
transforming growth factor
- TRAIL
TNF-related apoptosis-inducing ligand
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.mad.2014.06.005.
References
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WST, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O’Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandeen-Roche K, Walston JD, Huang Y, Semba RD, Ferrucci L. Measuring systemic inflammatory regulation in older adults: evidence and utility. Rejuvenat Res. 2009;12:403–410. doi: 10.1089/rej.2009.0883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilay JI, Abraham L, Heckbert SR, Cushman M, Kuller LH, Resnick HE, Tracy RP. The relation of markers of inflammation to the development of glucose disorders in the elderly: the cardiovascular health study. Diabetes. 2001;50:2384–2389. doi: 10.2337/diabetes.50.10.2384. [DOI] [PubMed] [Google Scholar]
- Bruunsgaard H. Effects of tumor necrosis factor-alpha and interleukin-6 in elderly populations. Eur Cytokine Netw. 2002;13:389–391. [PubMed] [Google Scholar]
- Bruunsgaard H, Andersen-Ranberg K, Jeune B, Pedersen AN, Skinhøj P, Pedersen BK. A high plasma concentration of TNF-α is associated with dementia in centenarians. J Gerontol Ser A: Biol Sci Med Sci. 1999;54:M357–M364. doi: 10.1093/gerona/54.7.m357. [DOI] [PubMed] [Google Scholar]
- Cesari M, Penninx BWJH, Pahor M, Lauretani F, Corsi AM, Williams GR, Guralnik JM, Ferrucci L. Inflammatory markers and physical performance in older persons: The InCHIANTI Study. J Gerontol Ser A: Biol Sci Med Sci. 2004;59:M242–M248. doi: 10.1093/gerona/59.3.m242. [DOI] [PubMed] [Google Scholar]
- Chaturvedi AK, Moore SC, Hildesheim A. Invited commentary: circulating inflammation markers and cancer risk – implications for epidemiologic studies. Am J Epidemiol. 2013;177:14–19. doi: 10.1093/aje/kws357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AA, Dhingra N, Jotkar RM, Rodriguez PS, Sharma VP, Jha P. The Summary Index of Malaria Surveillance (SIMS): a stable index of malaria within India. Popul Health Metr. 2010;8 doi: 10.1186/1478-7954-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daudin J, Duby C, Trecourt P. Stability of principal component analysis studied by the bootstrap method. Stat: J Theor Appl Stat. 1988;19:241–258. [Google Scholar]
- Diniz BS, Teixeira AL, Ojopi EB, Talib LL, Mendonca VA, Gattaz WF, Forlenza OV. Higher serum sTNFR1 level predicts conversion from mild cognitive impairment to Alzheimer’s disease. J Alzheimers Dis. 2010;22:1305–1311. doi: 10.3233/JAD-2010-100921. [DOI] [PubMed] [Google Scholar]
- Dusseault-Belanger F, Cohen AA, Hivert M, Courteau J, Vanasse A. Validating metabolic syndrome through principal component analysis in a medically diverse. Realistic Cohort Metab Syndr Relat Disord. 2013;11:21–28. doi: 10.1089/met.2012.0094. [DOI] [PubMed] [Google Scholar]
- Epstein FH, Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Fernandez-Real J, Vayreda M, Richart C, Gutierrez C, Broch M, Vendrell J, Ricart W. Circulating interleukin 6 levels, blood pressure, and insulin sensitivity in apparently healthy men and women. J Clin Endocrinol Metab. 2001;86:1154–1159. doi: 10.1210/jcem.86.3.7305. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Bandinell S, Benvenuti E, Di Iorio A, Macchi C, Harris T, Guralnik JM. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- Finch CE. The Biology of Human Longevity: Inflammation, Nutrition, and Aging in the Evolution of Lifespans. Academic Press; Burlington, Mayriland, USA: 2010. [Google Scholar]
- Franceschi C, Bonafè M, Valensin S, Olivieri F, de Luca M, Ottaviani E, de Benedictis G. Inflamm-aging: an evolutionary perspective on immunesenescence. Ann NY Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Gomez CR, Boehmer ED, Kovacs EJ. The aging innate immune system. Curr Opin Immunol. 2005;17:457–462. doi: 10.1016/j.coi.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- Hsu FC, Kritchevsky SB, Liu Y, Kanaya A, Newman AB, Perry SE, Visser M, Pahor M, Harris TB, Nicklas BJ, Health, A.B.C., Study Association between inflammatory components and physical function in the health, aging, and body composition study: a principal component analysis approach. J Gerontol Ser A: Biol Sci Med Sci. 2009;64:581–589. doi: 10.1093/gerona/glp005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Il’yasova D, Colbert LH, Harris TB, Newman AB, Bauer DC, Satterfield S, Kritchevsky SB. Circulating levels of inflammatory markers and cancer risk in the health aging and body composition cohort. Cancer Epidemiol Biomarkers Prevent. 2005;14:2413–2418. doi: 10.1158/1055-9965.EPI-05-0316. [DOI] [PubMed] [Google Scholar]
- Jolliffe I. Principal Component Analysis. Wiley Online Library; Verlag, New Yok, USA: 2005. [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F, Centers for Disease Control and Prevention, American Heart Association Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- Prospective Studies Collaboration. Body-mass index and cause-specific mortality in 900,000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safranow K, Dziedziejko V, Rzeuski R, Czyz˙ycka E, Wojtarowicz A, Bińczak-Kuleta A, Jakubowska K, Olszewska M, Ciechanowicz A, Kornacewicz-Jach Z. Plasma concentrations of TNF-α and its soluble receptors sTNFR1 and sTNFR2 in patients with coronary artery disease. Tissue Antigens. 2009;74:386–392. doi: 10.1111/j.1399-0039.2009.01332.x. [DOI] [PubMed] [Google Scholar]
- Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta (BBA) – Mol Cell Res. 2011;1813:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- Schmidt MI, Duncan BB, Sharrett AR, Lindberg G, Savage PJ, Offenbacher S, Azambuja MI, Tracy RP, Heiss G. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. Lancet. 1999;353:1649–1652. doi: 10.1016/s0140-6736(99)01046-6. [DOI] [PubMed] [Google Scholar]
- Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011;10:319–329. doi: 10.1016/j.arr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers JR, Epstein M, Frohlich ED. Diabetes, hypertension, and cardiovascular disease: an update. Hypertension. 2001;37:1053–1059. doi: 10.1161/01.hyp.37.4.1053. [DOI] [PubMed] [Google Scholar]
- Strandberg TE, Tilvis RS. C-reactive protein, cardiovascular risk factors, and mortality in a prospective study in the elderly. Arterioscler Thromb Vasc Biol. 2000;20:1057–1060. doi: 10.1161/01.atv.20.4.1057. [DOI] [PubMed] [Google Scholar]
- Tracy RP. Inflammation in cardiovascular disease: cart, horse or both – revisited. Arterioscler Thromb Vasc Biol. 2002;22:1514–1515. doi: 10.1161/01.atv.0000035403.39442.db. [DOI] [PubMed] [Google Scholar]
- Department of Health, U.S., Human Services. The Health Consequences of Smoking: A Report of the Surgeon General. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: 2004. p. 62. [Google Scholar]
- Varadhan R, Yao W, Matteini A, Beamer BA, Xue QL, Yang H, Manwani B, Reiner A, Jenny N, Parekh N, Fallin MD, Newman A, Bandeen-Roche K, Tracy R, Ferrucci L, Walston J. Simple biologically informed inflammatory index of two serum cytokines predicts 10 year all-cause mortality in older adults. J Gerontol Ser A: Biol Sci Med Sci. 2014;69:165–173. doi: 10.1093/gerona/glt023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijsman CA, Maier AB, de Craen AJ, van den Biggelaar AH, Westendorp RG. An unopposed proinflammatory response is beneficial for survival in the oldest old. Results of the Leiden 85-plus Study. J Gerontol Ser A: Biol Sci Med Sci. 2011;66:393–399. doi: 10.1093/gerona/glq212. [DOI] [PubMed] [Google Scholar]
- Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148:209–214. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- Zou H, Hastie T, Tibshirani R. Sparse principal component analysis. J Comput Graph Stat. 2006;15:265–286. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


