Abstract
BACKGROUND
In prior work involving older persons, the reported associations of spirometric impairments with cardiovascular outcomes may have been confounded by age-related changes in lung function. Hence, using more age-appropriate spirometric criteria from the Global Lung Function Initiative (GLI), we have evaluated the associations of spirometric impairments, specifically restrictive-pattern and airflow-obstruction, with cardiovascular death (CV-death) and hospitalization (CV-hospitalization). In these analyses, we also evaluated the competing outcome of noncardiovascular death (nonCV-death) and calculated measures of relative and absolute risk.
METHODS
Our study sample was drawn from the Cardiovascular Health Study (CHS), including 4,232 community-dwelling white persons aged ≥65 years. Multivariable regression models included the following baseline predictors: GLI-defined restrictive-pattern and airflow-obstruction, age, male gender, obesity, waist circumference, current smoker status, ≥10 pack-years of smoking, hypertension, dyslipidemia, diabetes, and cardiovascular and cerebrovascular disease. Outcomes included adjudicated CV-death, CV-hospitalization, and nonCV-death, ascertained over 10 years of follow-up. Measures of association included hazard ratios (HRs), rate ratios (RRs), and average attributable fraction (AAF), each with 95% confidence intervals.
RESULTS
Restrictive-pattern and airflow-obstruction were associated with CV-death (adjusted HRs: 1.57 [1.18, 2.09] and 1.29 [1.04, 1.60]) and with nonCV-death (adjusted HRs: 2.10 [1.63, 2.69] and 1.79 [1.51, 2.12]), respectively. Airflow-obstruction, but not restrictive-pattern, was also associated with CV-hospitalization (adjusted RRs: 1.18 [1.02, 1.36] and 1.20 [0.96, 1.50], respectively). The adjusted AAFs of restrictive-pattern and airflow-obstruction were 1.68% (0.46, 3.06) and 2.35% (0.22, 4.72) for CV-death, and 3.44% (1.97, 5.08) and 7.77% (5.15, 10.60) for nonCV-death, respectively.
CONCLUSION
Assessment of GLI-defined spirometric impairments contributes to broad geriatric risk stratifications for both cardiovascular and non-cardiovascular outcomes.
INTRODUCTION
In prior work involving older persons, the reported associations of spirometric impairments with cardiovascular outcomes may have been confounded by age-related changes in lung function.1–8 Specifically, prior work defined spirometric impairments based on percentile distributions within a study sample, e.g., quintiles, or on percent predicted (%Pred) values, i.e., [measured/predicted] x 100%.1–8 We note that, to distinguish spirometric impairments from normal-for-age spirometry, percentile distributions should be based on comparisons with a reference population of healthy never-smokers.9,10 We also note that %Pred assumes incorrectly that a given value is equivalent for all persons.11 To illustrate the effect of age in a white male of average height, the same value of 80%Pred for the forced expiratory volume in 1-second (FEV1) will correspond to the 6th and 14th percentile distribution of the reference population at ages 40 and 70 years, respectively.11
To better establish age-appropriate spirometric impairments, an alternative approach was introduced in 2008, termed Lambda-Mu-Sigma (LMS).9 The LMS approach uses spirometric Z-scores to rigorously account for age-related changes in lung function, with a Z-score of −1.64 defining the lower limit of normal (LLN) as the 5th percentile distribution of the reference population.9 In 2012, using data from large populations of asymptomatic lifelong nonsmokers and the LMS approach, the Global Lung Function Initiative (GLI) published reference equations that expanded the availability of spirometric Z-scores, additionally including an age range of up to 95 years.10 However, spirometric impairments as defined by GLI-calculated Z-scores have not yet been evaluated as risk factors for cardiovascular outcomes.
Accordingly, the aim of the current study is to evaluate the associations between GLI-defined spirometric impairments and cardiovascular outcomes, wherein we additionally account for the competing outcome of noncardiovascular death (nonCV-death) and a broad array of potential confounders. Specifically, using multivariable regression models, we have evaluated the associations of GLI-defined restrictive-pattern and airflow-obstruction with cardiovascular death (CV-death), cardiovascular hospitalization (CV-hospitalization), and nonCV-death, respectively, over 10 years of follow-up. In these analyses, we have calculated measures of relative risk (e.g., hazard ratios) and absolute risk (i.e., average attributable fraction [AAF]).12 Our study sample is drawn from the Cardiovascular Health Study (CHS), including persons aged ≥65 years (as older age is associated with reduced lung function and adverse outcomes).9,10,13,14 Because the current study provides a more age-appropriate and comprehensive evaluation, our results may further inform the role of spirometry in geriatric risk stratification, including both cardiovascular and noncardiovascular outcomes.
MATERIALS AND METHODS
Study Population
CHS is a longitudinal study of persons aged ≥65 years, identified from a random sample of Medicare eligibility lists in four communities in the United States.14 For our analytical sample, we included participants from the initial 1989–1990 CHS cohort, as only this group completed clinical and spirometric evaluations at the same visit (study entry). Moreover, consistent with prior work from CHS and other cohorts involving older persons,15–18 we required that participants achieve a spirometric quality control (QC) grade C or higher, i.e., having at least two acceptable forced vital capacity (FVC) maneuvers and FEV11 values matching within 200 mL.15
We note that mandating only two acceptable FVC maneuvers for inclusion in our analytical sample is not consistent with the 2005 spirometric guidelines from the American Thoracic Society (ATS) and European Respiratory Society (ERS),19 wherein the requirement was for at least three acceptable FVC maneuvers and more stringent repeatability criteria. However, the ATS/ERS guidelines also state that: “no spirogram or test result should be rejected solely on the basis of its poor repeatability.”19 Therefore, although greater spirometric repeatability is recommended, the ATS/ERS criteria allow for clinical judgment, as otherwise older persons who are physically frail and at increased risk of adverse health outcomes would be potentially excluded from the current analyses (despite achieving two acceptable FVC maneuvers).15,20,21 Importantly, the CHS criteria that defined the performance of an FVC maneuver as acceptable are consistent with ATS/ERS guidelines.19
Lastly, since the proportion of African-Americans was too small to support our analyses (5.3%), we selected only white participants. Based on our inclusion criteria, the final analytical sample thus included 4232 white participants, representing 81.4% of the 1989–1990 cohort.
The institutional review boards from the Veterans Affairs Connecticut Healthcare System and Yale University approved the current study. We note that the CHS dataset used in the current study had been previously deidentified and was publicly available.
Baseline Demographic and Clinical Characteristics
Baseline characteristics included age, gender, body mass index (BMI), waist circumference, smoking history (current smoker status and pack-years), and cardiovascular conditions and risk factors. A BMI ≥30 kg/m2 (including measured weight and standing height) defined obesity,14 and a waist circumference ≥110 cm in males and ≥95 cm in females defined high risk values,22 based on previously published associations with mortality (adjusted hazard ratios of 1.52 [1.45, 1.59] and 1.79 [1.70, 1.89], respectively, in a pooled analysis of 11 studies [age range 25–83 years]).22 In a supplemental analysis, we evaluated the alternative waist circumference thresholds of >100 cm in males and >90 cm in females as intermediate risk values, based on previously published associations with mortality (adjusted hazard ratios of 1.19 [1.15, 1.24] and 1.50 [1.42, 1.58], respectively, in a pooled analysis of 11 studies [age range 25–83 years]).22
Cardiovascular conditions and risk factors were defined according to CHS criteria, as follows:14 hypertension (systolic ≥160 mm Hg, diastolic ≥95 mm Hg, or history of hypertension requiring antihypertensive medication); dyslipidemia (low-density lipoprotein cholesterol ≥160 mg/dL or high-density lipoprotein cholesterol <40 mg/dL); diabetes mellitus (taking insulin or oral hypoglycemic, or fasting glucose ≥126 mg/dL); and adjudicated coronary heart disease (myocardial infarction or angina), heart failure, claudication, and cerebrovascular disease (stroke or transient ischemic attack).
Spirometric Impairment
Participants underwent spirometry in the seated position, using a water-sealed, Collins Survey II spirometer.14,15 The testing protocol included FVC maneuvers, repeated up to eight times, with the goal of achieving at least three acceptable and two repeatable FVC maneuvers (as per contemporary ATS criteria).15 For reasons discussed earlier, we established our spirometric analytical sample based on a QC grade C or higher, defined by participants having at least two acceptable FVC maneuvers and FEV11 values matching within 200 mL.14,15 Notably, CHS did not specifically evaluate spirometry after administering a bronchodilator.14,15
Spirometric results were reviewed at the CHS Pulmonary Function Reading Center,15 which included flow and volume grades for the forced expiratory volume in 1-second (FEV1) and FVC, respectively. The largest FEV1 and FVC values from acceptable FVC maneuvers were reported, with FEV1/FVC calculated from the largest FEV1 and FVC values.
Using GLI-2012 reference equations for whites (Caucasians),10 which included the predictor variables of age, gender, and the measured standing height, we calculated Z-scores for FEV1/FVC and FVC. With the LLN set at a Z-score of −1.64,10 and applying spirometric categories as described by the ATS/ERS,23 we defined normal spirometry by FEV1/FVC and FVC ≥LLN, restrictive-pattern by FEV1/FVC ≥LLN but FVC <LLN, and airflow-obstruction by FEV1/FVC <LLN.16–18 For those with airflow-obstruction, we also calculated the average FEV1 Z-score: as per prior work,17 FEV1 Z-scores ≥ −1.64 denote mild, < −1.64 but ≥ −2.55 denote moderate, and < −2.55 denote severe airflow-obstruction.
Prior work has established a strong mathematical, clinical, and physiological rationale for GLI-defined spirometric impairments in aging populations,9,10,16–18,24 including restrictive-pattern as representing a restrictive ventilatory defect.24 In addition, prior work has shown that applying a minimum spirometric QC grade C is clinically meaningful when evaluating the phenotypes of normal spirometry and spirometric impairments in aging populations.16–18
Longitudinal Outcomes
Our outcomes of interest were vital status and hospitalization. These were adjudicated centrally by CHS committees through the use of standardized diagnostic criteria, ICD-9-CM codes (International Classification of Diseases, 9th revision, clinical modification), medical records, proxy interviews, obituaries, and death certificates.14,25
Vital status was available on all participants, ascertained over a 10-year follow-up period (mean 8.6 years [standard deviation 2.5 years]). CV-death was adjudicated according to underlying cause, as follows: atherosclerotic coronary heart disease (included heart failure), cerebrovascular disease (stroke), other atherosclerotic disease (such as aortic aneurysm), and other vascular disease (such as valvular heart disease or pulmonary embolism).14,25 All other causes of death were classified as nonCV-death. A CV-hospitalization included any for myocardial infarction, angina, heart failure, claudication, stroke, or transient ischemic attack,14 ascertained over the same 10-year follow-up period.
Statistical Analysis
Using multivariable proportional hazards models and cause-specific models, hazard ratios (HRs) with 95% confidence intervals were calculated as measures of the associations between baseline predictors and the outcome of CV-death and the competing risk of nonCV-death. Baseline predictors included spirometric restrictive-pattern and airflow-obstruction (each relative to normal spirometry), age (per additional year), male gender, obesity, waist circumference, current smoking, ≥10 pack-years, diabetes, hypertension, dyslipidemia, cardiovascular disease (myocardial infarction, angina, heart failure, or claudication), and cerebrovascular disease (stroke or transient ischemic attack). The proportional hazards assumption was tested by using interaction terms between the time-to-event variable and each variable in the model. Goodness-of-fit was assessed by analysis of residuals. Because the amount of missing data was modest (5.1%), a complete case analysis was conducted.
The AAF of each baseline predictor for CV-death and nonCV-death was also calculated across the 10-year follow-up period.12 Unlike other measures of attributable fraction, the AAF is additive in that the sum of the contribution of each predictor to the outcome will not exceed 100%.12 The AAF is also symmetric, with the probability of the outcome based on all combinations of the predictors observed in the data and with final values for the individual AAFs obtained by averaging across these observed combinations.12 In addition, for the point estimate of each AAF, ten-thousand bootstrap samples were generated to establish the 95% confidence intervals. Notably, because the AAF calculation requires a dichotomous variable (Yes vs. No) and because CV-death occurs more commonly in persons aged ≥75 years,13 the AAF calculation for age was based on ≥75 vs. <75 years.
Rate ratios (RRs) with 95% confidence intervals for CV-hospitalization were additionally calculated over a 10-year follow-up period, using multivariable, negative binomial regression models (with the logarithm of time of follow-up as the offset). Baseline predictors were the same as those described earlier for the outcome of death.
SAS® v9.4 (SAS Institute; Cary, NC) was used for all analyses, with p<0.05 (two-sided) interpreted as statistically significant.
RESULTS
Table 1 reports the baseline characteristics of the analytical sample (N=4232): mean age was 72.6 years, 43.1% were male, 17.5% were obese, and 10.8% of males and 36.1% of females had waist circumferences ≥110 cm and ≥95 cm, respectively. In addition, 11.3% were current smokers and 43.0% had smoked ≥10 pack-years. Cardiovascular conditions and related risk factors included: hypertension (41.3%), dyslipidemia (36.7%), coronary heart disease (19.3%), diabetes mellitus (14.7%), cerebrovascular disease (5.3%), heart failure (4.2%), and claudication (2.6%). Spirometric impairments were established in 24.2%, including 5.9% with restrictive-pattern and 18.3% with airflow-obstruction. For those with airflow-obstruction, the FEV1 Z-score averaged −2.26 (i.e., moderately severe). Table 1 also shows outcomes over the 10-year follow-up period, with CV-death adjudicated in 13.9%, nonCV-death in 20%, and CV-hospitalization in 41.1%.
Table 1.
Baseline characteristics and longitudinal health outcomes of the analytical sample
| Variable | N=4,232 a |
|---|---|
| Baseline Characteristic b | |
| Age (years), mean (± SD) | 72.6 ± 5.3 |
| ≥75 years | 1411 (33.3) |
| Males, No. (%) | 1825 (43.1) |
| BMI (kg/m2), mean (± SD) | 26.2 ± 3.9 |
| BMI ≥ 30, No. (%) | 740 (17.5) |
| Waist circumference (cm), mean (± SD) | 93.5 ± 11.5 |
| Males with waist circumference ≥ 110 cm, No. (%) c | 196 (10.8) |
| Females with waist circumference ≥ 95 cm, No. (%) c | 865 (36.1) |
| Smoking status, No. (%) | |
| Never | 1925 (45.5) |
| Former | 1827 (43.2) |
| Current | 477 (11.3) |
| ≥10 pack-years | 1771 (43.0) |
| Cardiovascular conditions and risk factors, No. (%) | |
| Hypertension d | 1746 (41.3) |
| Dyslipidemia e | 1540 (36.7) |
| Coronary heart disease f | 818 (19.3) |
| Diabetes mellitus g | 618 (14.7) |
| Cerebrovascular diseaseh | 225 (5.3) |
| Heart failure | 178 (4.2) |
| Claudication | 108 (2.6) |
| GLI-defined spirometry, No. (%) | |
| Normal | 3207 (75.8) |
| Restrictive-pattern | 252 (5.9) |
| Airflow-obstruction | 773 (18.3) |
| FEV1 Z-score, mean (± SD) | −2.26 ± 1.09 i |
| Health Outcomes, No. (%) | |
| Deaths | |
| CV-death j | 590 (13.9) |
| NonCV-death k | 848 (20.0) |
| Cardiovascular hospitalization l | 1738 (41.1) |
Abbreviations: BMI, body mass index; CV, cardiovascular; FEV1, forced expiratory volume in 1-second; GLI, Global Lung Initiative; SD, standard deviation.
Of the analytical sample of 4,232 participants who completed spirometry, 214 (5.1%) had missing data on baseline characteristics.
Determined concurrently at the baseline visit.
Defined as high risk, based on prior work showing these thresholds as being associated with increased mortality.
Systolic ≥160 mm Hg, diastolic ≥95 mm Hg, or history of hypertension requiring antihypertensive medication.
Low-density lipoprotein cholesterol ≥160 mg/dl or high-density lipoprotein cholesterol <40 mg/dl.
If taking insulin or oral hypoglycemic, or fasting glucose ≥126 mg/dL.
Myocardial infarction, angina, heart failure, or claudication.
Stroke or transient ischemic attack.
Mean value is consistent with moderate airflow-obstruction (see methods).
Death from atherosclerotic coronary heart disease (includes heart failure), cerebrovascular disease (stroke), other atherosclerotic disease (such as aortic aneurysm), or other vascular disease (such as valvular heart disease or pulmonary embolism).
All other deaths that did not meet criteria for CV-death.
Hospitalization for myocardial infarction, angina, heart failure, claudication, stroke, or transient ischemic attack.
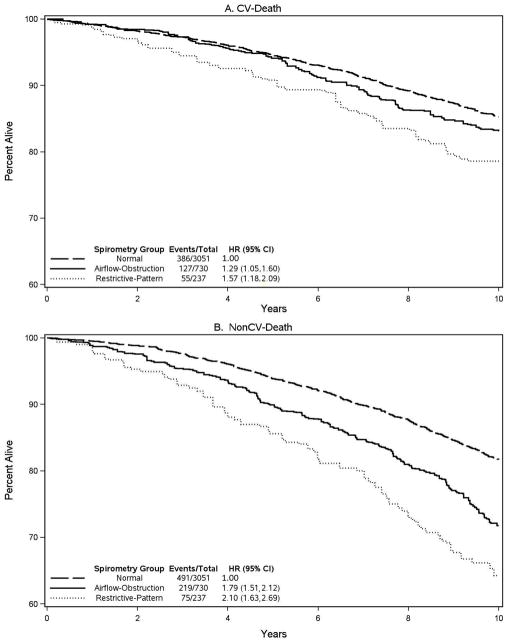
Table 2 reports adjusted hazard ratios (HRs) for CV-death over the 10-year follow-up period, according to baseline predictor. In the multivariable model and relative to normal spirometry, results showed that restrictive-pattern and airflow-obstruction were both associated with CV-death (adjusted HRs 1.57 [1.18, 2.09] and 1.29 [1.04, 1.60], respectively). Associations with CV-death were additionally found for age, male gender, current smoker, hypertension, diabetes, and cardiovascular and cerebrovascular disease, but not for obesity, waist circumference, ≥10 pack-years, and dyslipidemia. In addition, survival curves for CV-death are shown for the three GLI-defined spirometric categories in Figure 1A. The survival curves demonstrate that, relative to normal spirometry, the occurrence of CV-death increases throughout the 10-year follow-up period in restrictive-pattern, but increases after five years of follow-up in airflow-obstruction.
Table 2.
Adjusted hazard ratios for CV-death and nonCV-death over a 10-year follow-up period, according to baseline predictor (N=4,018)a
| Baseline Predictor b | CV-Death c | NonCV-Death d |
|---|---|---|
| Adjusted Hazard Ratio (95% CI) e | ||
| Spirometric restrictive-pattern f | 1.57 (1.18, 2.09) | 2.10 (1.63, 2.69) |
| Spirometric airflow-obstruction f | 1.29 (1.04, 1.60) | 1.79 (1.51, 2.12) |
| Age g | 1.11 (1.10, 1.13) | 1.12 (1.11, 1.14) |
| Male gender | 1.62 (1.34, 1.95) | 1.46 (1.25, 1.71) |
| Obesity (BMI ≥ 30) | 0.87 (0.66, 1.14) | 1.03 (0.81, 1.30) |
| Waist circumference h | 1.13 (0.88, 1.44) | 0.98 (0.80, 1.22) |
| Current smoker | 1.75 (1.34, 2.29) | 1.68 (1.36, 2.09) |
| ≥10 pack-years | 1.10 (0.91, 1.32) | 1.31 (1.11, 1.54) |
| Hypertension i | 1.48 (1.25, 1.76) | 1.11 (0.96, 1.28) |
| Dyslipidemia j | 1.08 (0.91, 1.28) | 0.94 (0.81, 1.10) |
| Diabetes mellitus k | 2.07 (1.71, 2.51) | 1.34 (1.11, 1.62) |
| Cardiovascular Disease l | 2.36 (1.99, 2.81) | 1.05 (0.89, 1.24) |
| Cerebrovascular Disease m | 2.01 (1.56, 2.59) | 1.54 (1.18, 2.00) |
Abbreviation: BMI, body mass index (kg/m2); CI, confidence interval; CV, cardiovascular; GLI, Global Lung Function Initiative.
Of the analytical sample of 4,232 participants who completed spirometry, 214 (5.1%) had missing data on non-spirometric predictors.
Determined concurrently at the baseline visit.
Death from atherosclerotic coronary heart disease (includes heart failure), cerebrovascular disease (stroke), other atherosclerotic disease (such as aortic aneurysm), or other vascular disease (such as valvular heart disease or pulmonary embolism).
All other deaths that did not meet criteria for CV-death.
Estimated using a cause-specific proportional hazards model and adjusted for the baseline predictors shown in the table.
GLI-defined and relative to normal spirometry.
For each additional year of baseline age.
Waist circumference ≥110 cm in males and ≥95 cm in females was defined as high risk, based on prior work showing these thresholds as being associated with increased mortality.
Systolic ≥160 mm Hg, diastolic ≥95 mm Hg, or history of hypertension requiring antihypertensive medication.
Low-density lipoprotein cholesterol ≥160 mg/dl or high-density lipoprotein cholesterol <40 mg/dl.
If taking insulin or oral hypoglycemic, or fasting glucose ≥126 mg/dL.
Myocardial infarction, angina, heart failure, or claudication.
Stroke or transient ischemic attack.
Figure 1.
Survival curves for CV-death (A) and nonCV-death (B) over a 10-year follow-up period, using the three GLI-defined categories of normal spirometry, restrictive-pattern, and airflow-obstruction, respectively a
aIncludes adjusted hazard ratios for CV-death and nonCV-death over the same 10-year follow-up period, wherein GLI-defined restrictive-pattern and airflow obstruction are compared with GLI-defined normal spirometry (see also Table 2).
Table 2 also reports adjusted HRs for the competing risk of nonCV-death over the 10-year follow-up period, according to baseline predictor. In the multivariable model and relative to normal spirometry, results showed that restrictive-pattern and airflow-obstruction were associated with nonCV-death (adjusted HRs 2.10 [1.63, 2.69] and 1.79 [1.51, 2.12], respectively). Associations were additionally found for age, male gender, current smoker, ≥10 pack-years, diabetes, and cerebrovascular disease, but not for obesity, waist circumference, hypertension, dyslipidemia, and cardiovascular disease. In addition, survival curves for nonCV-death are shown for the three GLI-defined spirometric categories in Figure 1B. The survival curves demonstrate that, relative to normal spirometry, the occurrence of nonCV-death increases throughout the 10-year follow-up period in both restrictive-pattern and airflow-obstruction.
Table 3 reports the AAF of each baseline predictor for CV-death and nonCV-death across the 10-year follow-up period. Results showed that the AAFs of restrictive-pattern and airflow-obstruction were 1.68% (0.46, 3.06) and 2.35% (0.22, 4.72) for CV-death, and 3.44% (1.97, 5.08) and 7.77% (5.15, 10.60) for nonCV-death, respectively. In contrast, the baseline predictor with the highest AAF for CV-death and nonCV-death was age ≥75 years (20.33% and 26.32%, respectively). The total AAF contribution of all baseline predictors was 73.70% (67.33, 80.68) for CV-death and 65.00% (58.33, 72.06) for nonCV-death.
Table 3.
Average attributable fraction (AAF) of each baseline predictor for CV-death and nonCV-death, expressed as a percentage, across a 10-year follow-up period (N=4,018)a
| Baseline Predictor b | CV-Death c | NonCV-Death d |
|---|---|---|
| Average Attributable Fraction (95% CI) e | ||
| Spirometric restrictive-pattern f | 1.68 (0.46, 3.06) | 3.44 (1.97, 5.08) |
| Spirometric airflow-obstruction f | 2.35 (0.22, 4.72) | 7.77 (5.15, 10.60) |
| Age g | 20.33 (16.15, 24.83) | 26.32 (22.04, 30.81) |
| Male gender | 11.77 (7.28, 16.51) | 11.27 (6.63, 16.07) |
| Obesity (BMI ≥ 30) | −1.62 (−3.70, 0.55) | −0.33 (−2.46, 1.97) |
| Waist circumference h | 1.40 (−1.48, 4.23) | −0.23 (−3.06, 2.62) |
| Current smoker | 2.98 (1.22, 4.86) | 3.95 (2.03, 6.09) |
| ≥10 pack-years | 1.08 (−3.02, 5.08) | 6.46 (1.57, 11.26) |
| Hypertension i | 9.63 (5.56, 13.84) | 3.28 (−0.38, 7.10) |
| Dyslipidemia j | 1.33 (−2.00, 4.78) | −1.69 (−4.87, 1.54) |
| Diabetes mellitus k | 7.13 (4.78, 9.79) | 2.85 (0.88, 5.04) |
| Cardiovascular Disease l | 12.91 (9.55, 16.65) | 0.36 (−1.97, 2.79) |
| Cerebrovascular Disease m | 2.74 (1.43, 4.24) | 1.50 (0.39, 2.77) |
| Total for baseline predictors | 73.70 (67.33, 80.68) | 65.00 (58.33, 72.06) |
Abbreviation: BMI, body mass index (kg/m2); CI, confidence interval; CV, cardiovascular; GLI, Global Lung Function Initiative.
Of the analytical sample of 4,232 participants who completed spirometry, 214 (5.1%) had missing data on non-spirometric predictors.
Determined concurrently at the baseline visit.
Death from atherosclerotic coronary heart disease (includes heart failure), cerebrovascular disease (stroke), other atherosclerotic disease (such as aortic aneurysm), or other vascular disease (such as valvular heart disease or pulmonary embolism).
All other deaths that did not meet criteria for CV-death.
The fractional (percentage) contribution of each predictor to the respective outcome, based on estimates from pooled logistic regression models.
GLI-defined and calculated based on comparison with normal spirometry.
Age ≥75 vs. <75 years (CV-death is most prevalent in persons aged ≥75 years).
Waist circumference ≥110 cm in males and ≥95 cm in females was defined as high risk, based on prior work showing these thresholds as being associated with increased mortality.
Systolic ≥160 mm Hg, diastolic ≥95 mm Hg, or history of hypertension requiring antihypertensive medication.
Low-density lipoprotein cholesterol ≥160 mg/dl or high-density lipoprotein cholesterol <40 mg/dl.
If taking insulin or oral hypoglycemic, or fasting glucose ≥126 mg/dL.
Myocardial infarction, angina, heart failure, or claudication.
Stroke or transient ischemic attack.
Table 4 reports adjusted rate ratios (RRs) for CV-hospitalization over the 10-year follow-up period, according to baseline predictor. In the multivariable model and relative to normal spirometry, results showed that airflow-obstruction, but not restrictive-pattern, was associated with CV-hospitalization (adjusted RRs 1.18 [1.02, 1.36] and 1.20 [0.96, 1.50], respectively). Associations were additionally found for age, male gender, current smoker, hypertension, dyslipidemia, diabetes mellitus, and cardiovascular and cerebrovascular disease, but not for obesity or waist circumference.
Table 4.
Adjusted rate ratio for CV-hospitalization over a 10-year follow-up period, according to baseline predictor (N=4,018)a
| Baseline Predictor b | CV-Hospitalization c Adjusted Rate Ratio (95% CI) d |
|---|---|
| Spirometric restrictive-pattern e | 1.20 (0.96, 1.50) |
| Spirometric airflow-obstruction e | 1.18 (1.02, 1.36) |
| Age f | 1.07 (1.06, 1.08) |
| Male gender | 1.38 (1.22, 1.55) |
| Obesity (BMI ≥ 30) | 1.10 (0.94, 1.30) |
| Waist circumference g | 1.00 (0.86, 1.17) |
| Current smoker | 1.45 (1.21, 1.74) |
| ≥10 pack-years | 1.09 (0.96, 1.23) |
| Hypertension h | 1.49 (1.34, 1.66) |
| Dyslipidemia i | 1.28 (1.15, 1.43) |
| Diabetes mellitus j | 1.87 (1.62, 2.15) |
| Cardiovascular Disease k | 2.95 (2.62, 3.32) |
| Cerebrovascular Disease l | 1.92 (1.56, 2.37) |
Abbreviations: BMI, body mass index (kg/m2); CI, confidence interval; CV, cardiovascular; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GLI, Global Lung Function Initiative.
Of the analytical sample of 4,232 participants who completed spirometry, 214 (5.1%) had missing data on non-spirometric predictors.
Determined concurrently at the baseline visit.
Hospitalization for myocardial infarction, angina, heart failure, claudication, stroke, or transient ischemic attack.
Estimated from a negative binomial regression model (with the logarithm of time of follow-up as the offset) and adjusted for the baseline predictors shown in the table.
GLI-defined and relative to normal spirometry.
For each additional year of baseline age.
Waist circumference ≥110 cm in males and ≥95 cm in females was defined as high risk, based on prior work showing these thresholds as being associated with increased mortality.
Systolic ≥160 mm Hg, diastolic ≥95 mm Hg, or history of hypertension requiring antihypertensive medication.
Low-density lipoprotein cholesterol ≥160 mg/dl or high-density lipoprotein cholesterol <40 mg/dl.
If taking insulin or oral hypoglycemic, or if fasting glucose ≥126 mg/dL
Myocardial infarction, angina, heart failure, or claudication.
Stroke or transient ischemic attack.
As a supplemental analysis, associations with CV-death and nonCV-death were again evaluated in multivariable regression models, using instead intermediate risk values for waist circumference of >100 cm in males and >90 cm in females. As shown in the Appendix, the results of this supplemental analysis did not change appreciably from those of Tables 2 and 3 (wherein waist circumference thresholds were ≥110 cm in males and ≥95 cm in females).
DISCUSSION
In a large sample of community-dwelling white persons aged ≥65 years, we found that GLI-defined spirometric impairments, specifically restrictive-pattern and airflow-obstruction, increased the risk of CV-death, even after adjusting for multiple confounders (Table 2). Hence, our results support the use of GLI-defined spirometric impairments in geriatric cardiovascular risk stratification.
Although the mechanisms that underlie the associations between spirometric impairments and CV-death are not established in the current study, coronary heart disease (CHD) may have been a major factor. In particular, since 54.5% of our study participants were former or current smokers, spirometric airflow-obstruction was likely due to chronic obstructive pulmonary disease (COPD), and both smoking history and COPD are known to increase the risk of death due to CHD.26,27 Similarly, spirometric restrictive-pattern is associated with CHD and the cardiovascular risk factor of the metabolic syndrome.5,28,29 Other mechanisms that may lead to CV-death, and are also associated with a spirometric impairment, include heart failure and pulmonary hypertension.16,30
GLI-defined spirometric impairments, including restrictive-pattern and airflow-obstruction, were additionally shown to increase the risk of nonCV-death (Table 2). As to mechanisms underlying the associations between spirometric impairments and nonCV-death, these were not established in the current study but may include chronic lower respiratory diseases (COPD and asthma) and lung cancer. Chronic lower respiratory diseases rank as the 3rd leading cause of death in those aged ≥65 years, and 47% of patients with lung cancer are aged ≥70 years.31,32
Additional observations merit discussion regarding four non-spirometric predictors and their associations with CV-death and nonCV-death (Table 2). First, obesity was not associated with CV-death or nonCV-death. These results are consistent with prior work wherein the association between obesity and mortality was not significant in older age.33,34 Second, waist circumference was not associated with CV-death or nonCV-death. This is in contrast to prior studies showing an association between waist circumference and all-cause mortality.22,35 The latter studies, however, did not adjust for cardiovascular conditions and risk factors.22,35 Third, dyslipidemia was not associated with CV-death or nonCV-death. This may reflect an age-dependent survival bias, as prior work suggests that dyslipidemia is more likely to increase the risk of CV-death in middle-age.36,37 Fourth, only current smoker status, but not a smoking history of ≥10 pack-years, was associated with CV-death, whereas both smoking predictors were associated with nonCV-death. Since smoking cessation improves cardiovascular outcomes more promptly than non-cardiovascular outcomes,27,38 we posit that the high rate of smoking cessation in our older CHS sample (43.2% were former smokers) attenuated the association of a smoking history of ≥10 pack-years with CV-death (adjusted HR 1.10), as compared with nonCV-death (adjusted HR 1.31).
Beyond what is measured by relative risk (e.g. hazard ratios), the current study further informs the epidemiology of CV-death and nonCV-death by calculating the AAF (Table 3). For example, applying our AAF results to national death statistics for the year 2014 in Americans aged ≥65 years,31 spirometric restrictive-pattern and airflow-obstruction contributed to 8,227 and 11,508 CV-deaths (calculated as AAF of 0.0168 and 0.0235 x total CV-deaths [489,722], respectively) and 49,280 and 111,309 nonCV-deaths (calculated as AAF of 0.0344 and 0.0777 x total nonCV-deaths [1,432,549], respectively). In comparison, hypertension contributed to 47,160 CV-deaths (calculated as AAF of 0.0963 x 489,722) but was otherwise not associated with nonCV-death. Hence, the sum of CV- and nonCV- deaths potentially attributed to a spirometric impairment (restrictive-pattern and airflow-obstruction) is nearly 4-fold higher than those attributed to baseline hypertension (180,374 vs. 47,160 deaths). Importantly, when using the AAF to estimate the number of CV-deaths and nonCV-deaths that are due to spirometric impairments, these estimates may be unique to a given country, due to differences in the prevalence of spirometric impairments (including how they are defined)39 and potential confounders (i.e., co-existing, non-respiratory cardiovascular risk factors).
We additionally report results showing that the epidemiology of CV-hospitalization differs from that of CV-death. We consider two reasons. First, sudden cardiac death can occur as the first manifestation of cardiovascular disease and, thus, affected individuals will not experience a CV-hospitalization. Such a scenario is supported by prior work in which sudden cardiac death often occurred as the first manifestation of CHD, and the peak prevalence of sudden cardiac death occurred in those aged ≥65 years.40,41 Second, the reported measures of association for nonCV-death were higher with spirometric impairments, especially restrictive-pattern. Accordingly, the occurrence of sudden cardiac death or nonCV-death may have censored (excluded) participants who had a spirometric impairment from the analysis of CV-hospitalization and, in turn, attenuated the rate ratios reported in Table 4, especially for restrictive-pattern.
Our results have implications for public health policy. Factors which reduce the health of the respiratory system, such as exposures to tobacco smoking, allergens, ambient indoor and outdoor air pollution, occupational dusts, and respiratory infections,16 may lead to spirometric impairments and these, in turn, may identify individuals at risk of CV-death and nonCV-death. Given that the stated exposures are often cumulative in older persons, and are also modifiable, our results inform the importance of instituting preventive measures across the lifespan.
Our results also have implications regarding the public health burden of CV-death as a complication of airflow-obstruction (i.e., COPD). Specifically, the survival curves in Figure 1 demonstrate that, relative to normal spirometry, the occurrence of CV-death in participants with airflow-obstruction increased after five years of follow-up. Thus, studies may underestimate the occurrence of CV-death in COPD when based on less than five years of follow-up. This was otherwise not the case with restrictive-pattern, where the increase in CV-death occurred throughout the ten years of follow-up.
The current study has several strengths. We have evaluated a large number of older persons from a well-established population-based study (CHS) that provided concurrent baseline data on spirometry and a broad array of baseline predictors, including adjudicated cardiovascular conditions and risk factors. The duration of follow-up was for 10 years, with vital status available on all participants and with longitudinal outcomes also adjudicated. We have also applied age-appropriate, Z-score based definitions of spirometric impairments, accounted for the competing risk of nonCV-death, and reported results that uniquely included the AAF.
Additional strengths of the current study extend from our avoidance of potentially flawed approaches when defining spirometric impairments and when calculating absolute risk. Specifically, we did not define spirometric impairments based on diagnostic thresholds from the Global initiative for chronic Obstructive Lung Disease (GOLD)42 for two reasons.9–11,16,18,43–45 First, because normal aging impairs respiratory mechanics, the FEV1/FVC is often <0.70 in healthy never-smokers, especially in those aged ≥65 years;9,10 hence, the use of GOLD criteria has serious age-related limitations given that GOLD defines airflow-obstruction based on FEV1/FVC <0.70.9,10,16,18,43–45 Second, because normal aging is associated with greater variability in spirometric performance, diagnostic thresholds for FVC that use %Pred assume incorrectly that a given value is equivalent for all persons;11 hence, the use of GOLD criteria has serious age-related limitations given that GOLD uses an FVC of 80%Pred to distinguish restrictive-pattern from normal-for-age spirometry.11,18,45 Regarding absolute risk, a prior study2 which included only five baseline predictors had calculated the population attributable risk (PAR) for CV-death as exceeding 100% (145.4% for women and 114.7% for men) in participants with a spirometric impairment. In contrast to the total PAR, our use of the AAF is additive in that the sum of the contribution of each predictor to the outcome cannot exceed 100%.12
We acknowledge, however, several potential limitations to our current study. First, we did not evaluate a mixed restrictive-obstructive ventilatory defect. This requires measurement of static lung volumes (i.e., total lung capacity [TLC]), which was not undertaken in CHS. As per ATS/ERS guidelines,23 a mixed restrictive-obstructive ventilatory defect is defined by the FEV1/FVC and TLC, both <LLN. Second, spirometry in CHS was not specifically evaluated after a bronchodilator. Nonetheless, we note that older persons have a reduced capacity to perform multiple FVC maneuvers (pre- and post-bronchodilator) and may have an adverse response to a bronchodilator, and additionally note that post-bronchodilator values may have limited clinical relevance in distinguishing COPD from asthma and low reproducibility over time.17 Third, inclusion in our analytical sample required that spirometry meet a QC grade C or higher, in contrast to the more stringent 2005 ATS/ERS guidelines.19 However, as noted earlier, the more stringent ATS/ERS requirements would potentially exclude older participants who are physically frail and at increased risk of adverse health outcomes.15,20,21,46 Fourth, the total contribution of our baseline predictors yielded an AAF for CV-death of only 73.70% and an AAF for nonCV-death of only 65.00%. Fifth, we did not establish the mechanisms that underlie the associations between spirometric impairments and health outcomes. Lastly, our results applied to white persons only.
To address the above limitations, future work should expand the list of potential baseline predictors of health outcomes, including: a mixed restrictive-obstructive ventilatory defect; alternative diagnostic tests (and protocols) for establishing respiratory impairments that are easier to administer in older persons; more severe levels of hypertension, dyslipidemia, and smoking exposure; and geriatric risk factors such as medication use (polypharmacy) and injurious falls (hip fractures). Future work should also consider mediating factors, including those relating to echocardiography and cardiac biomarkers, and intervening events such as COPD/asthma exacerbations, pneumonia, lung cancer, and respiratory failure, as well as enroll cohorts with more substantial representation of non-white populations.
CONCLUSION
Our results support the use of GLI-defined spirometric impairments in geriatric cardiovascular risk stratification, evidenced by statistically significant associations with cardiovascular outcomes, even after adjusting for multiple confounders. Moreover, establishing a GLI-defined spirometric impairment provides broad geriatric risk stratification, evidenced by the additional statistically significant associations with nonCV-death, also after adjusting for multiple confounders.
Supplementary Material
Highlights.
GLI-defined spirometric impairments increase the risk of CV- and nonCV-deaths.
GLI-defined spirometric impairments account for 4.03% of CV-deaths.
GLI-defined spirometric impairments account for 11.21% of nonCV-deaths.
In 2014 (USA), GLI-defined spirometric impairments accounted for 180,374 total deaths.
Acknowledgments
Funding: United States National Institutes of Health (P30AG021342). The funding source had no role in the design of the study.
The views expressed in this article do not communicate an official position of the Yale University School of Medicine, the United States Department of Veterans Affairs, or the United States National Institutes of Health.
Abbreviations
- AAF
average attributable fraction
- BMI
body mass index
- CV
cardiovascular
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- GLI
Global Lung Function Initiative
- HR
hazard ratio
- nonCV
noncardiovascular
- RR
rate ratio
Footnotes
Author Contributions: CAVF had full access to study data and takes responsibility for data integrity and accuracy of data analysis. Author contributions included: conception and design — CAVF, PHVN, TEM, GJM; analysis and interpretation — CAVF, PHVN, TEM, GJM; and drafting the manuscript for intellectual content CAVF, PHVN, TEM, GJM.
Conflicts of interest: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marcus EB, Curb JD, MacLean VJ, et al. Pulmonary function as a predictor of coronary heart disease. Am J Epidemiol. 1989;129(1):97–104. doi: 10.1093/oxfordjournals.aje.a115128. [DOI] [PubMed] [Google Scholar]
- 2.Hole DJ, Watt GCM, Davey-Smith G, et al. Impaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population study. BMJ. 1996;313(7059):711–716. doi: 10.1136/bmj.313.7059.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Palen J, Rea TD, Manolio TA, et al. Respiratory muscle strength and the risk of incident cardiovascular events. Thorax. 2004;59(12):1063–1067. doi: 10.1136/thx.2004.021915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scarlata S, Pedone C, Fimognari FL, et al. Restrictive pulmonary dysfunction at spirometry and mortality in the elderly. Respir Med. 2008;102:1349–1354. doi: 10.1016/j.rmed.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 5.Lee HM, Chung SJ, Lopez VA, Wong ND. Association of FVC and total mortality in US adults with metabolic syndrome and diabetes. Chest. 2009;136(1):171–176. doi: 10.1378/chest.08-1901. [DOI] [PubMed] [Google Scholar]
- 6.Min KB, Min JY. Reduced lung function, C-reactive protein, and increased risk of cardiovascular mortality. Circ J. 2014;78(9):2309–2316. doi: 10.1253/circj.cj-14-0308. [DOI] [PubMed] [Google Scholar]
- 7.Lee HM, Liu MA, Barrett-Connor E, Wong ND. Association of lung function with coronary heart disease and cardiovascular disease outcomes in elderly: the Rancho Bernardo study. Respir Med. 2014;108(12):1779–1785. doi: 10.1016/j.rmed.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rasmussen T, Køber L, Pedersen JH, et al. Relationship between chronic obstructive pulmonary disease and subclinical coronary artery disease in long-term smokers. Eur Heart J Cardiovascular Imaging. 2013;14:1159–1166. doi: 10.1093/ehjci/jet057. [DOI] [PubMed] [Google Scholar]
- 9.Stanojevic S, Wade A, Stocks J, et al. Reference ranges for spirometry across all ages. Am J Respir Crit Care Med. 2008;177:253–260. doi: 10.1164/rccm.200708-1248OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95 year age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller MR. Does the use of per cent of predicted have any evidence base? Eur Respir J. 2015;45(2):322–323. doi: 10.1183/09031936.00199414. [DOI] [PubMed] [Google Scholar]
- 12.Murphy TE, McAvay G, Carriero NJ, et al. Deaths observed in Medicare beneficiaries: average attributable fraction and its longitudinal extension for many diseases. Statist Med. 2012;31(27):3313–3319. doi: 10.1002/sim.5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rich MW, Chyun D, Skolnick AH, et al. Knowledge gaps in cardiovascular care of older adults: a scientific statement from the American Heart Association, American College of Cardiology, and American Geriatrics Society: executive summary. J Amer Geriatr Soc. 2016;64:2185–2192. doi: 10.1111/jgs.14576. [DOI] [PubMed] [Google Scholar]
- 14.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 15.Griffith KA, Sherrill DL, Siegel EM, et al. Predictors of loss of lung function in the elderly: the cardiovascular health study. Am J Respir Crit Care Med. 2001;163:61–68. doi: 10.1164/ajrccm.163.1.9906089. [DOI] [PubMed] [Google Scholar]
- 16.Vaz Fragoso CA, Gill TM. Respiratory impairment and the aging lung: a novel paradigm for assessing pulmonary function. J Gerontol A Biol Sci Med Sci. 2012;67(3):264–275. doi: 10.1093/gerona/glr198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaz Fragoso CA, McAvay G, Van Ness PH, et al. Phenotype of spirometric impairment in an aging population. Am J Respir Crit Care Med. 2016;193(7):727–735. doi: 10.1164/rccm.201508-1603OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaz Fragoso CA, McAvay G, Van Ness PH, et al. Phenotype of normal spirometry in an aging population. Am J Respir Crit Care Med. 2015;192(7):817–825. doi: 10.1164/rccm.201503-0463OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 20.Allen SC, Yeung P. Inability to draw intersecting pentagons as a predictor of unsatisfactory spirometry technique in elderly hospital inpatients. Age and Ageing. 2006;35:304–316. doi: 10.1093/ageing/afj090. [DOI] [PubMed] [Google Scholar]
- 21.Redlich CA, Tarlo SM, Hankinson JL, et al. Official American Thoracic Society technical standards: spirometry in the occupational setting. Am J Respir Crit Care Med. 2014;189(8):984–994. doi: 10.1164/rccm.201402-0337ST. [DOI] [PubMed] [Google Scholar]
- 22.Cerhan JR, Moore SC, Jacobs EJ, et al. A pooled analysis of waist circumference and mortality in 650,000 adults. Mayo Clin Proc. 2014;89(3):335–345. doi: 10.1016/j.mayocp.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 24.Vaz Fragoso CA, Cain HC, Casaburi R, et al. Spirometry, static lung volumes, and diffusing capacity. Respir Care. 2017;62(9):1137–1147. doi: 10.4187/respcare.05515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newman AB, Sachs MC, Arnold AM, et al. Total and cause-specific mortality in the Cardiovascular Health Study. J Gerontol Med Sci. 2009;64(12):1251–1261. doi: 10.1093/gerona/glp127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huiart L, Ernst P, Suissa S. Cardiovascular morbidity and mortality in COPD. Chest. 2005;128:2640–2646. doi: 10.1378/chest.128.4.2640. [DOI] [PubMed] [Google Scholar]
- 27.Burns DM. Epidemiology of smoking-induced cardiovascular disease. Prog Cardiovasc Dis. 2003;46(1):11–29. doi: 10.1016/s0033-0620(03)00079-3. [DOI] [PubMed] [Google Scholar]
- 28.Friedman GD, Klatsky AL, Siegelaub AB. Lung function and risk of myocardial infarction and sudden cardiac death. N Engl J Med. 1976;294:1071–1075. doi: 10.1056/NEJM197605132942001. [DOI] [PubMed] [Google Scholar]
- 29.Lee HM, Le H, Lee BT, et al. Forced vital capacity paired with Framingham Risk Score for prediction of all-cause mortality. Eur Respir J. 2010;36:1002–1006. doi: 10.1183/09031936.00042410. [DOI] [PubMed] [Google Scholar]
- 30.Sun X-G, MD, Hansen JE, Oudiz RJ, et al. Pulmonary function in primary pulmonary hypertension. J Am Coll Cardiol. 2003;41:1028–1035. doi: 10.1016/s0735-1097(02)02964-9. [DOI] [PubMed] [Google Scholar]
- 31.Heron M. National vital statistics reports. 5. Vol. 65. Hyattsville, MD: National Center for Health Statistics; 2016. Deaths: Leading causes for 2014. [PubMed] [Google Scholar]
- 32.Owonikoko TK, Ragin CC, Belani CP, et al. Lung cancer in elderly patients: an analysis of the surveillance, epidemiology, and end results database. J Clin Oncol. 2007;25:5570–5577. doi: 10.1200/JCO.2007.12.5435. [DOI] [PubMed] [Google Scholar]
- 33.Flegal KM, Kit BK, Oprana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309(1):71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grabowski DC, Ellis JE. High body mass index does not predict mortality in older people: analysis of the longitudinal study of aging. J Am Geriatr Soc. 2001;49:968–979. doi: 10.1046/j.1532-5415.2001.49189.x. [DOI] [PubMed] [Google Scholar]
- 35.Jacobs EJ, Newton CC, Wang Y, et al. Waist circumference and all-cause mortality in a large US cohort. Arch Intern Med. 2010;170(15):1293–1301. doi: 10.1001/archinternmed.2010.201. [DOI] [PubMed] [Google Scholar]
- 36.Lehto S, Ronnemaa T, Haffher SM, et al. Dyslipidemia and hyperglycemia predict coronary heart disease events in middle-aged patients with NIDDM. Diabetes. 1997;46:1354–1359. doi: 10.2337/diab.46.8.1354. [DOI] [PubMed] [Google Scholar]
- 37.Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;316(19):1997–2007. doi: 10.1001/jama.2016.15450. [DOI] [PubMed] [Google Scholar]
- 38.Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328(6):1519–1533. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Backman H, Eriksson B, Hedman L, et al. Restrictive spirometric pattern in the general adult population: methods of defining the condition and consequences on prevalence. Respir Med. 2016;120:116–123. doi: 10.1016/j.rmed.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 40.Zipes DP, Wellen HJ. Sudden cardiac death. Circulation. 1998;98:2334–2351. doi: 10.1161/01.cir.98.21.2334. [DOI] [PubMed] [Google Scholar]
- 41.Chugh SS, Reinier K, Teodorescu C, et al. Epidemiology of sudden cardiac death: clinical and research implications. Prog Cardiovasc Dis. 2008;51(3):213–228. doi: 10.1016/j.pcad.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vogelmeier CF, Criner GJ, Martinez FJ, et al. GOLD executive summary. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease 2017 report. Am J Respir Crit Care Med. 2017;195(5):557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 43.Hansen JF, Sun X-G, Wasserman K. Spirometric criteria for airway obstruction. Chest. 2007;131:349–355. doi: 10.1378/chest.06-1349. [DOI] [PubMed] [Google Scholar]
- 44.Vaz Fragoso CA, Concato J, McAvay G, et al. The ratio of the forced expiratory volume in 1-second to forced vital capacity in establishing chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181:446–451. doi: 10.1164/rccm.200909-1366OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vaz Fragoso CA, Magnussen H, Miller MR, Brusasco V. Spirometry-based diagnostic criteria that are not age-appropriate lack clinical relevance. Am J Respir Crit Care Med. 2017 doi: 10.1164/rccm.201709-1789LE. In press. [DOI] [PubMed] [Google Scholar]
- 46.Milne JS, Williamson J. Respiratory function tests in older people. Clin Sci. 1972;42(3):371–381. doi: 10.1042/cs0420371. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.