Abstract
Introduction
The objectives of this study were: 1) to determine the amniotic fluid (AF) microbiology of patients with the diagnosis of clinical chorioamnionitis at term using both cultivation and molecular techniques; and 2) to examine the relationship between intra-amniotic inflammation with and without microorganisms and placental lesions consistent with acute AF infection.
Methods
The AF samples obtained by transabdominal amniocentesis from 46 women with clinical signs of chorioamnionitis at term were analyzed using cultivation techniques (for aerobic and anerobic bacteria as well as genital mycoplasmas) and broad-range polymerase chain reaction (PCR) coupled with electrospray ionization mass spectrometry (PCR/ESI-MS). The frequency of microbial invasion of the amniotic cavity (MIAC), intra-amniotic inflammation [defined as an AF interleukin 6 (IL-6) concentration ≥ 2.6ng/mL], and placental lesions consistent with acute AF infection (acute histologic chorioamnionitis and/or acute funisitis) were examined according to the results of AF cultivation and PCR/ESI-MS as well as AF IL-6 concentrations.
Results
1) Culture identified bacteria in AF from 46% (21/46) of the participants, whereas PCR/ESI-MS was positive formicroorganisms in 59% (27/46) – combining these two tests, microorganisms were detected in 61% (28/46) of patients with clinical chorioamnionitis at term. Eight patients had discordant test results; one had a positive culture and negative PCR/ESI-MS result, whereas seven patients had positive PCR/ESI-MS results and negative cultures. 2) Ureaplasma urealyticum (n = 8) and Gardnerella vaginalis (n = 10) were the microorganisms most frequently identified by cultivation and PCR/ESI-MS, respectively. 3) When combining the results of AF culture, PCR/ESI-MS and AF IL-6 concentrations, 15% (7/46) of patients did not have intra-amniotic inflammation or infection, 6.5% (3/46) had only MIAC, 54% (25/46) had microbial-associated intra-amniotic inflammation, and 24% (11/46) had intra-amniotic inflammation without detectable microorganisms. 4) Placental lesions consistent with acute AF infection were significantly more frequent in patients with microbial-associated intra-amniotic inflammation than in those without intra-amniotic inflammation [70.8% (17/24) vs. 28.6% (2/7); P = 0.04].
Conclusion
Microorganisms in the AF were identified in 61% of patients with clinical chorioamnionitis at term; 54% had microbial-associated intra-amniotic inflammation, whereas 24% had intra-amniotic inflammation without detectable microorganisms.
Keywords: funisitis, Gardnerella vaginalis, histologic chorioamnionitis, intra-amniotic infection/inflammation, microbial invasion of the amniotic cavity (MIAC), PCR/ESI-MS, pregnancy, sterile inflammation, Ureaplasma urealyticum
Introduction
Clinical chorioamnionitis is the most common infection-related diagnosis made in labor and delivery units worldwide [1–4]. The standard clinical definition is based on the studies of Gibbs et al. [5, 6], and refers to the presence of maternal fever associated with clinical signs (i.e., foul-smelling discharge, uterine tenderness, maternal and fetal tachycardia) as well as laboratory abnormalities (i.e., leukocytosis). These signs are thought to be manifestations of both local and systemic maternofetal inflammatory processes initiated in response to microbial invasion of the amniotic cavity (MIAC) [7–13].
The prevalence of clinical chorioamnionitis in term gestations is 5%–12% [3], whereas in preterm gestations with premature rupture of membranes (PROM) it is approximately 20% [2, 14, 15]. Clinical chorioamnionitis at term is associated with a 2- to 4-fold increase in endometritis [16], wound infection [16], septic pelvic thrombophlebitis [3, 16], pelvic abscess [3, 16], maternal admission to the intensive care unit [3, 17], and postpartum hemorrhage [3, 18]. Neonates born to mothers with clinical chorioamnionitis have a high risk of neonatal mortality [1], short-and-long term complications such as neonatal sepsis [19–21], meconium aspiration syndrome [22, 23], stillbirth [24, 25], and neurodevelopmental disorders including cerebral palsy [26–37].
The microbiology of clinical chorioamnionitis was originally described in 1982 using cultivation techniques of amniotic fluid (AF) obtained with transcervical catheters placed in the amniotic cavity [5]. However, retrieval of AF with a transcervical catheter is frequently associated with contamination of the AF with microorganisms that are part of the vaginal ecosystem. Therefore, characterization of the microorganisms associated with clinical chorioamnionitis based on samples obtained by transabdominal amniocentesis is necessary to have an accurate description of the microbiology of this condition. Moreover, the use of molecular techniques to identify microorganisms, which may escape detection with cultivation techniques [38–56], allows adequate classification based upon the sequence of the amplicons and can provide additional information about microbial diversity [57–60].
The objectives of this study were: 1) to determine the AF microbiology of patients with the diagnosis of clinical chorioamnionitis at term using both cultivation and molecular techniques; 2) to assess the frequencies of intraamniotic inflammation that were and were not accompanied by detectable microorganisms in these patients; and 3) to examine the relationship between intra-amniotic inflammation with and without microorganisms and placental lesions consistent with acute AF infection.
Materials and methods
Study population
This retrospective cohort study includes patients with clinical chorioamnionitis at term. Patients were identified by searching the clinical database and Bank of Biological Samples of the Perinatology Research Branch of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). The inclusion criteria were: 1) singleton gestations; 2) gestational age > 37 weeks; and 3) sufficient AF obtained by transabdominal amniocenteses for molecular microbiologic studies. Patients with multiple gestations or fetal malformations were excluded from the study.
Maternal and neonatal data were obtained from clinical chart review, including information about the use of epidural analgesia, intrapartum antibiotic administration, number of vaginal examinations during labor, status of the membranes at the time of amniocentesis (intact or ruptured), and mode of delivery. Patients with the diagnosis of clinical chorioamnionitis were counseled by their treating physicians about the potential value of knowing the precise microorganism involved in the suspected infection. Women who agreed to undergo an amniocentesis were asked to donate additional AF other than that required for clinical studies and allow collection of clinical information for research purposes. Further management of these patients was at the discretion of the attending physician. All patients provided written informed consent and the use of biologic specimens as well as clinical and ultrasound data for research purposes were approved by the Institutional Review Boards of NICHD, Wayne State University and the Sótero del Río Hospital, Santiago, Chile. All patients were enrolled in this protocol at the Sótero del Río Hospital in Santiago, Chile.
Clinical definitions
Gestational age was determined by the last menstrual period and was confirmed by ultrasound examination; the date derived from ultrasound was used if inconsistent with menstrual dating. Clinical chorioamnionitis was diagnosed by the presence of maternal fever (temperature > 37.8°C) accompanied by two or more of the following criteria: 1) uterine tenderness; 2) malodorous vaginal discharge; 3) fetal tachycardia (heart rate > 160 beats/min); 4) maternal tachycardia (heart rate > 100 beats/min); and 5) maternal leukocytosis (leukocyte count > 15,000 cells/mm3) [6, 16]. Spontaneous term labor was defined as the presence of regular uterine contractions with a frequency of at least 1 every 10 min and cervical changes after 37 weeks of gestation.
Microbial invasion of the amniotic cavity was defined according to the results of AF culture and polymerase chain reaction with electrospray ionization mass spectrometry (PCR/ESI-MS) (Ibis® Technology – Athogen, Carlsbad, CA) [51, 55, 61, 62]. Intra-amniotic inflammation was diagnosed when AF interleukin (IL)-6 concentration was ≥ 2.6 ng/mL [63, 64]. Based on the results of AF cultures, PCR/ESI-MS and AF concentration of IL-6, patients were classified as having: 1) no intra-amniotic inflammation/infection (either using AF culture or PCR/ESI-MS); 2) MIAC (identification of microorganisms by either AF cultures or PCR/ESI-MS without intra-amniotic inflammation); 3) microbial-associated intra-amniotic inflammation (combination of MIAC and intra-amniotic inflammation); or 4) intra-amniotic inflammation without detectable microorganisms (an elevated AF IL-6 concentration without evidence of microorganisms using cultivation or molecular methods). Acute histologic chorioamnionitis was diagnosed based on the presence of inflammatory cells in the chorionic plate and/or chorioamniotic membranes [65], and acute funisitis was diagnosed by the presence of neutrophils in the wall of the umbilical vessels and/or Wharton’s jelly, using criteria previously described [65, 66].
Sample collection
Amniotic fluid was transported to the clinical laboratory in a capped sterile syringe and was cultured for aerobic and anaerobic bacteria, including genital mycoplasmas. Evaluation of white blood cell (WBC) count, AF glucose concentration, and Gram stain of AF were also performed shortly after collection. AF not required for clinical assessment was centrifuged for 10 min at 4°C shortly after amniocentesis, and the supernatant was aliquoted and stored at −70°C until analysis. Following delivery, the placenta, umbilical cord, and chorioamniotic membranes were collected and the presence or absence of acute histologic chorioamnionitis and/or funisitis was determined.
Detection of microorganisms with cultivation and molecular methods
Amniotic fluid was analyzed using cultivation techniques (for aerobic and anaerobic bacteria as well as genital mycoplasmas) and with PCR/ESI-MS (Ibis® Technology). Briefly, DNA was extracted from 300 μL of AF using a method that combined bead-beating cell lysis with a magnetic-bead based extraction method [67, 68]. The extracted DNA was amplified on the bacterial artificial chromosome (BAC) spectrum assay according to the manufacturer’s instructions. PCR/ESI-MS can identify 3400 bacteria and 40 Candida spp., which are represented in the platform’s signature database [69–71]. A total of 200 μL of extract was used per sample.
After PCR amplification, 30-μL aliquots of each PCR product were desalted and analyzed by ESI-MS as previously described [70, 72]. The presence of microorganisms was determined by signal processing and triangulation analysis of all base composition signatures obtained from each sample and compared to a database. Along with organism identification, the ESI-MS analysis includes a Q-score and level of detection (LOD). The Q-score, a rating between 0 (low) and 1 (high), represents a relative measure of the strength of the data supporting identification; only Q-scores ≥ 0.90 were reported for the BAC spectrum assay [73]. The LOD describes the amount of amplified DNA present in the sample: this is calculated with reference to an internal calibrant, as previously described [74], and is reported herein as genome equivalents per PCR reaction well (GE/well). The sensitivity (LOD) of PCR/ESI-MS for the detection of bacteria in blood is, on average, 100 CFU/mL (95% CI, 6–600 CFU/mL) [71]. A comparison of detection limits between blood and AF showed that the assays have comparable detection limits (100 CFU/mL) [75].
Determination of IL-6 in amniotic fluid
Amniotic fluid concentrations of IL-6 were determined to assess the magnitude of the intra-amniotic inflammatory response. We used a sensitive and specific enzyme immunoassay obtained from R&D Systems (Minneapolis, MN, USA). The quantitative sandwich enzyme immunoassay technique and the concentrations were determined by interpolation from the standard curves. The inter- and intra-assay coefficients of variation for IL-6 were 8.7% and 4.6%, respectively. The detection limit of the IL-6 assay was 0.09 pg/mL. The AF IL-6 concentrations were determined for research purposes, and such results were not used in patient management. We have previously reported the use of IL-6 for the assessment of intra-amniotic inflammation [44, 51, 61, 63, 75–91].
Maternal and umbilical blood samples
Maternal and umbilical venous blood samples were collected in tubes that contained EDTA. Samples were collected at the time of diagnosis of clinical chorioamnionitis and at delivery from the mother and the neonate, respectively. Blood samples were centrifuged, and supernatants were stored in polypropylene tubes at −70°C. Plasma concentrations of IL-6 were measured with a high sensitivity IL-6 immunoassay (R&D, Minneapolis, MN, USA). The sensitivity of the assay was 0.10 pg/mL. Inter- and intra-assay coefficients of variation were 4.6% and 6.6%, respectively.
Statistical analysis
The Kolmogorov-Smirnov test and visual plot inspection were used to assess the normality of continuous data distributions. Patients were stratified by the status of the membranes (intact or ruptured) at the time of amniocentesis and according to the presence of intraamniotic inflammation or MIAC. Between-group comparisons were performed using the Kruskal-Wallis and the Mann-Whitney U tests to examine the differences in arithmetic variable distributions. The χ2 or Fischer’s exact test was used to test for differences in proportions, as appropriate. A two-tailed P-value of < 0.05 was considered statistically significant. The statistical package used was SPSS v.15.0 (SPSS, Chicago, IL, USA).
Results
Characteristics of the study population
A total of 46 patients with clinical chorioamnionitis diagnosed between 37 and 43 weeks of gestation were included in this study. Demographic and clinical characteristics of the study population are displayed in Table 1. The median [interquartile range (IQR)] gestational age of the study population was 39.8 (IQR: 38.9–40.5) weeks. Upon admission, 83% (38/46) of patients presented with spontaneous labor at term, and 98% (45/46) had intact membranes. Some 65% (30/46) of patients had rupture of membranes during labor. Only 19% (9/46) of the women were admitted with fever; the remainder (81%, 37/46) developed fever after hospital admission. In addition to maternal fever, the most frequent criteria that configured the diagnosis of clinical chorioamnionitis were maternal and fetal tachycardia [91% (42/46) and 76% (35/46), respectively], followed by maternal leukocytosis [72% (33/46)]. Most patients had a vaginal delivery [74% (34/46)], and 26% (12/46) were delivered by cesarean (Table 1).
Table 1.
Clinical characteristics of the study population
| Median (IQR) or % (n/N) | |
|---|---|
| Age (years) | 21 (18 – 25) |
| Body mass index (kg/m2) | 23.7 (21.6 – 24.8) |
| Nulliparity | 65.2% (30/46) |
| Smoking | 10.9 (5/46) |
| Rupture of the membranes at the time of amniocentesis | 65.2 (30/46) |
| GA at amniocentesis (weeks) | 39.8 (38.9 – 40.5) |
| Maternal tachycardia (>100 beats/min) | 91.3 (42/46) |
| Fetal tachycardia (>160 beats/min) | 76.1 (35/46) |
| Uterine tenderness | 8.7 (4/46) |
| Foul-smelling amniotic fluid | 6.5 (3/46) |
| Maternal leukocytosis | 71.7 (33/46) |
| Labor | |
| Spontaneous | 82.6 (38/46) |
| Induced | 15.2 (7/46) |
| C-section | 26.1 (12/46) |
|
| |
| Epidural analgesia before amniocentesis | 76.1 (35) |
|
| |
| AF white blood cells (cells/mm3) | 66.5 (5 – 692.5) |
| AF glucose (mg/dL) | 9 (9 – 9) |
| AF Gram stain positive | 13 (6/46) |
| AF interleukin-6 (ng/mL) | 6.6 (3.0 – 18.0) |
Data presented as median (IQR) or %(n).
IQR: interquartile range; AF: amniotic fluid; GA: gestational age; PCR: polymerase chain reaction; ESI-MS: electrospray ionization mass spectrometry; C-section: cesarean section.
All patients received epidural analgesia during labor. Amniocenteses were performed before the administration of epidural analgesia in 24% (11/46) of patients. Among patients who received antibiotics (n = 43), 88% (38/43) were administered after amniocentesis. Five patients received antibiotics before undergoing amniocentesis (in three cases the amniocentesis was performed 5 minutes after administration of antibiotics and in 2 cases 45 minutes after administration of antibiotics). Ampicillin [83% (38/46)] and gentamicin [85% (39/43)] were the most common antibiotics administered. Three patients did not receive antibiotics.
Prevalence of microbial invasion of the amniotic cavity and microbial diversity
Culture identified bacteria in AF from 46% (21/46) of patients with the diagnosis of clinical chorioamnionitis at term, whereas PCR/ESI-MS was positive for microorganisms in 59% (27/46). When considering positive tests either by culture or PCR/ESI-MS, microorganisms were identified in 61% (28/46) of the study participants. Table 2 shows the microorganisms identified by PCR/ESI-MS for each patient with a positive AF culture and/or PCR/ESI-MS. Eight patients had discordant test results. Veillonella spp. and Lactobacillus spp. were identified in the single patient with a positive AF culture and negative PCR/ESI-MS result, whereas the remaining seven patients had positive PCR/ESI-MS results and negative AF cultures (denoted by “a” in Table 2).
Table 2.
Microorganisms detected in the amniotic fluid of patients with clinical chorioamnionitis at term using cultivation techniques vs. PCR/ESI-MS.
| Case | Microorganisms determined by cultivation | Microorganisms determined by PCR/ESI-MS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| MIAC by culture |
AF germ 1 | AF germ 2 | AF germ 3 | MIAC by PCR/ESI- MS |
PCR germ 1 | PCR germ 2 | PCR germ 3 | PCR germ 4 |
|
| 1 | Yes | Ureaplasma urealyticum | Bacteroides spp | Gardnerella vaginalis | Yes | U. urealyticum | G. vaginalis | Sneathia | |
| 2 | Yes | Ureaplasma urealyticum | Enterococcus spp | G. vaginalis | Yes | U. urealyticum | G. vaginalis | Peptostreptococcus anaerobius | |
| 3 | Yes | U. urealyticum | Mycoplasma hominis | Streptococcus viridans | Yes | Ureaplasma parvum | |||
| 4 | Yes | U. urealyticum | Streptococcus agalactiae | Yes | U. urealyticum | G. vaginalis | |||
| 5 | Yes | U. urealyticum | Streptococcus viridans | Yes | G. vaginalis | Sneathia | |||
| 6 | Yes | U. urealyticum | Peptostreptococcus spp | Yes | Ureaplasma parvum | Lactobacillus species | |||
| 7 | Yes | U. urealyticum | Yes | Ureaplasma parvum | Lactobacillus species | ||||
| 8 | Yes | U. urealyticum | Yes | Firmicute | |||||
| 9 | Yes | M. hominis | Veillonella spp | Bacteroides spp | Yes | Ureaplasma parvum | |||
| 10 | Yes | M. hominis | G. vaginalis | Yes | G. vaginalis | ||||
| 11 | Yes | M. hominis | Yes | Propionibacterium acnes | |||||
| 12 | Yes | S. agalactiae | Staphylococcus aureus | Yes | S. agalactiae | ||||
| 13 | Yes | S. agalactiae | Yes | S. agalactiae | |||||
| 14 | Yes | S. agalactiae | Yes | G. vaginalis | |||||
| 15 | Yes | Porphyromonas spp | Yes | Lactobacillus species | |||||
| 16 | Yes | Porphyromonas spp | Yes | G. vaginalis | Lactobacillus species | ||||
| 17 | Yes | Bacteroides spp | Eubacterium sp | Yes | Sneathia | ||||
| 18 | Yes | Candida albicans | Gram (−) bacilli | Yes | G. vaginalis | ||||
| 19b | Yes | Veillonella spp | Lactobacillus spp | No | Negative | ||||
| 20 | Yes | Peptostreptococcus spp | Yes | G. vaginalis | Abiotrophia defectiva | ||||
| 21 | Yes | Fusobacterium spp | Yes | Fusobacterium nucleatum/periodonticum | |||||
| 22a | No | Yes | Escherichia coli | ||||||
| 23a | No | Yes | G. vaginalis | ||||||
| 24a | No | Yes | Staphylococcus epidermidis | Lactobacillus species | Micrococcus luteus | Candida famata | |||
| 25a | No | Yes | Acinetobacter species | Pseudomonas aeruginosa | |||||
| 26a | No | Yes | Acinetobacter species | P. aeruginosa | |||||
| 27a | No | Yes | Acinetobacter species | ||||||
| 28a | No | Yes | Escherichia coli | ||||||
AF: amniotic fluid; PCR: polymerase chain reaction; ESI-MS: electrospray ionization mass spectrometry; MIAC: microbial invasion of the amniotic cavity.
Positive PCR/ESI-MS and negative AF culture for bacteria
Positive AF culture with negative PCR/ESI-MS
Among patients with a positive AF culture for bacteria, the most frequent microorganism identified was Ureaplasma urealyticum [38% (8/21)], followed by Mycoplasma hominis [19% (4/21)], and Streptococcus agalactiae [19% (4/21)]. Interestingly, more than half of these patients [57% (12/21)] had an AF culture positive for two or more bacteria.
Among patients with a positive AF PCR/ESI-MS, the most frequent microorganism identified was Gardnerella vaginalis [37% (10/27)], followed by U. urealyticum [26% (7/27)] and Lactobacillus spp. [19% (5/27)] (Table 2). Two or more bacteria were identified in 41% (11/27) of these patients.
Among the 28 patients whose AF tested positive by culture or PCR/ESI-MS, 16 bacterial species and 1 fungal species were identified. Of the sixteen bacterial taxa identified, five were detected by both culture and PCR/ESI-MS (Ureaplasma spp., S. agalactiae, Lactobacillus spp., G. vaginalis, and Fusobacterium spp.); four were detected only by AF culture (M. hominis, Porphyromonas sp., Streptococcus viridans, and Veillonella spp.); and seven were detected by only PCR/ESI-MS (Peptostreptococcus anaerobious, Abiotrophia defectiva, Sneathia, Propionibacterium acnes, Acinetobacter species, Pseudomonas aeruginosa, and Escherichia coli). Candida spp. was identified in two cases (Table 2).
Intra-amniotic inflammatory response in patients with clinical chorioamnionitis
Intra-amniotic inflammation (AF IL-6 ≥ 2.6 ng/mL) was identified in 78% (36/46) of the study participants. When combining the results of AF culture, PCR/ESI-MS and AF IL-6 concentrations – 15% (7/46) of the patients did not have intra-amniotic inflammation or infection; 6.5% (3/46) had MIAC; 54% (25/46) had microbial-associated intraamniotic inflammation; and 24% (11/46) had intra-amniotic inflammation without detectable microorganisms.
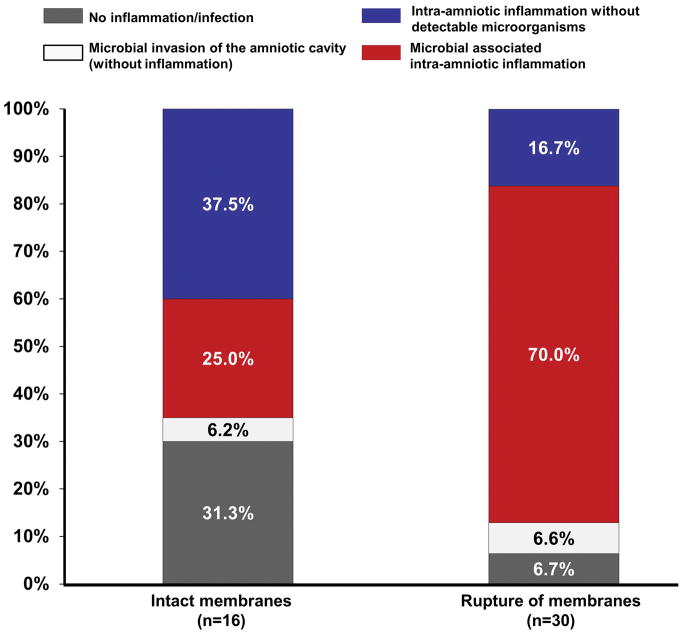
The prevalence of microbial-associated intra-amniotic inflammation and intra-amniotic inflammation without detectable microorganisms differed according to whether the chorioamniotic membranes were intact or ruptured at the time of amniocentesis (Figure 1). Microbial-associated intra-amniotic inflammation was diagnosed in 70% (21/30) of the women with ruptured membranes, and in only 25% (4/16) of those with intact membranes. Likewise, 31% (5/16) of patients who had intact membranes at the time of amniocentesis did not have evidence of intraamniotic inflammation, whereas only 7% of those whose membranes were ruptured did not have evidence of intraamniotic inflammation.
Figure 1.
Prevalence of microbial-associated intra-amniotic inflammation and intra-amniotic inflammation without detectable microorganisms in patients with clinical chorioamnionitis at term according to the status of the membranes at the time of amniocentesis (intact vs. ruptured). Microbial-associated intra-amniotic inflammation was diagnosed in 70% (21/30) of the cases with rupture of the membranes, and in 25% (4/16) of patients with intact membranes.
Table 3 describes differences in distributions of markers of inflammation in AF and maternal blood among the study groups. The median (IQR) AF WBC counts, AF IL-6, and maternal WBC concentrations were each significantly higher in patients with microbial-associated intraamniotic inflammation/infection than in those without intra-amniotic inflammation [AF WBC count: 300 cells/mm3 (39–900) vs. 5 cells/mm3 (0–42); P = 0.003; AF IL-6: 14.1 ng/mL (5.7–36.8) vs. 0.9 ng/mL (0.4–1); P < 0.001; maternal WBC count: 16.7 cells/mm3 (13.1–21) vs. 15 cell/mm3 (10.6–16.8); P = 0.04]. However, there was no significant differences in maternal and umbilical cord blood IL-6 among these groups (P = 0.3 and P = 0.1, respectively; Table 3). Among patients with intra-amniotic inflammation, those with detectable microorganisms had significantly higher median AF WBC count than women without detectable microorganisms (P = 0.03). Moreover, the median AF IL-6 concentrations were marginally higher in microbial-associated intra-amniotic inflammation than in intra-amniotic inflammation without detectable organisms (P = 0.06).
Table 3.
Inflammatory markers in maternal blood, amniotic fluid, and umbilical cord in patients with clinical chorioamnionitis at term according to the results of amniotic fluid cultures and PCR/ESI-MS
| No inflammation/infection (n=7) | p- valuea | Intra-amniotic inflammation without detectable microorganisms (n=11) | p-valueb | Microbial-associated intra-amniotic inflammation (n=25) | p-valuec | |
|---|---|---|---|---|---|---|
| Maternal white blood cell count (103/mm3) | 15 (10.6 – 16.8) | 0.3 | 15.3 (13.7 – 17.8) | 0.2 | 16.7 (13.1 – 21) | 0.04 |
| Maternal blood IL-6 (pg/mL) | 18.8 (11.7 – 37.7) | 0.1 | 4.6 (0.9 – 29) | 0.1 | 9.4 (5.4 – 45.5) | 0.3 |
|
| ||||||
| AF white blood cell count (cells/mm3) | 5 (0 – 42) | 0.6 | 25 (0 – 85) | 0.03 | 300 (39 – 900) | 0.003 |
| AF glucose (mg/dL) | 9 (9 – 12) | 1 | 9 (9 – 10) | 0.04 | 9 (7 – 9) | 0.3 |
| AF IL-6 (ng/mL) | 0.9 (0.4 – 1) | <0.001 | 4.7 (3.2 – 15) | 0.06 | 14.1 (5.7 – 36.8) | <0.001 |
|
| ||||||
| Cord blood IL-6 (pg/mL) | 2.6 (1.9 – 5.8) | 0.4 | 4.3 (2.3 – 6.2) | 0.1 | 6.5 (2.5 – 23.2) | 0.1 |
Data presented as median (interquartile) and percentage and (n); AF: amniotic fluid; IL: interleukin
Comparison between no inflammation/infection and intra-amniotic inflammation without detectable microorganisms.
Comparison between patients with intra-amniotic inflammation without detectable microorganisms and microbial-associated intra-amniotic inflammation.
Comparison between patients with no inflammation and microbial-associated intra-amniotic inflammation.
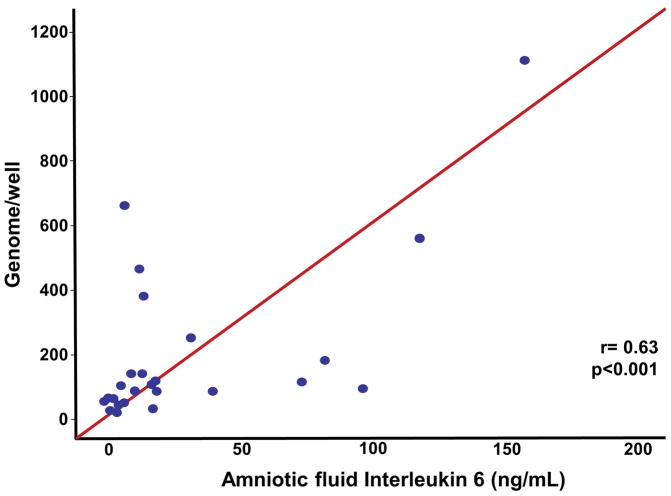
Among patients with a positive PCR/ESI-MS, the median (IQR) AF IL-6 concentration was significantly higher in cases with polymicrobial infection (n = 12) than in those in whom a single microorganism was identified (n = 15) [18.8 ng/mL (9.4–72.6) vs. 6.4 ng/mL (4.0–14.1); P = 0.04]. In addition, the microbial inoculum size, expressed as GE/well, was significantly correlated with AF concentration of IL-6 (Spearman’s rho = 0.63; P < 0.001) (Figure 2). There was no correlation between microbial burden and AF WBC concentration (Spearman’s rho = 0.23; P > 0.05).
Figure 2.
Correlation between amniotic fluid interleukin-6 concentration and the microbial burden (GE/well) in patients with a positive PCR/ESI-MS. The microbial inoculum size was significantly correlated with an AF concentration of IL-6 (Spearman’s ρ = 0.63; P < 0.001).
Relationship between detectable microorganisms in the amniotic fluid and placental lesions consistent with amniotic fluid infection
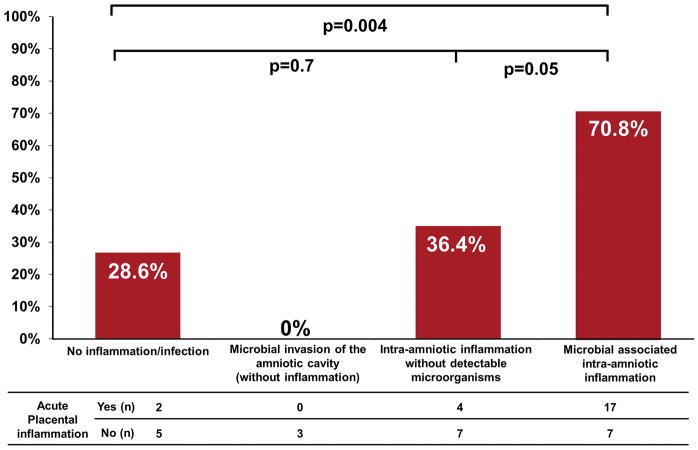
The extraplacental membranes and umbilical cord were examined in 97.8% (45/46) of the study participants; 51% (23/45) had placental lesions consistent with AF infection (acute histologic chorioamnionitis and/or funisitis); 48.8% (22/45) had acute histologic chorioamnionitis; and 28.9% (13/45) had funisitis. Figure 3 shows the frequency of placental lesions consistent with AF infection among the study groups. The prevalence of such placental lesions was significantly higher in patients with microbial-associated intra-amniotic inflammation than in patients with no intra-amniotic inflammation/infection [70.8% (17/24) vs. 28.6% (2/7); P = 0.04], while there was no statistical difference in the frequency of these placental lesions between patients with intra-amniotic inflammation without detectable microorganisms and those without intra-amniotic infection/inflammation [36.4% (4/11) vs. 28.6% (2/7); P = 0.7] (Figure 3). The rate of placental lesions consistent with AF infection was nearly two-fold greater in patients with microbial-associated intra-amniotic inflammation than in those with intra-amniotic inflammation without detectable microorganisms, yet this difference was only marginally significant in light of the sample size in each group [70.8% (17/24) vs. 36.4% (4/11); P = 0.053)] (Figure 3).
Figure 3.
Prevalence of placental acute inflammation (acute histologic chorioamnionitis and/or funisitis) according to the presence or absence of microbial-associated intra-amniotic inflammation, intra-amniotic inflammation without detectable microorganisms, or microbial invasion of the amniotic cavity in patients with clinical signs of chorioamnionitis at term.
Discussion
Principal findings of this study
1) Microorganisms were identified in the AF in 61% (28/46) of women with clinical chorioamnionitis at term; 2) the most common microorganisms identified were G. vaginalis and U. urealyticum; 3) PCR/ESI-MS identified more microorganisms than cultivation of AF, yet some bacterial taxa were preferentially identified by each test, meaning both might be required to determine whether clinical chorioamnionitis at term is microbial-associated; and 4) when combining the results of AF culture, PCR/ESI-MS and AF IL-6 concentrations, 54% of the study participants had microbial-associated intra-amniotic inflammation, whereas 24% had intra-amniotic inflammation without detectable microorganisms.
Microbiology of clinical chorioamnionitis at term
Despite its clinical importance, the microbiology of AF from patients with clinical chorioamnionitis at term has not been adequately characterized. The study of Gibbs et al. used transcervical catheters in 52 patients with clinical signs of chorioamnionitis and 52 patients matched for gestational age [5]. However, AF retrieved by a transcervical catheter is prone to contamination. Approximately, 75% of all samples from patients without clinical chorioamnionitis had >102 colony-forming units/mL in AF cultures [5]. In contrast, when transabdominal amniocentesis was used to assess the amniotic cavity of patients in labor at term without evidence of clinical chorioamnionitis, only 19% (17/90) had positive AF cultures for microorganisms [92]. Transcervical collection of AF for microbiologic studies is associated with a high rate of contamination, making interpretation of microbiologic studies difficult; therefore, this approach has been abandoned in modern studies of the microbiology of the amniotic cavity [93–96]. A study of the microbiology of clinical chorioamnionitis at term using AF retrieved by transabdominal amniocentesis is needed.
The key sign used to diagnose clinical chorioamnionitis is fever, a nonspecific host response to infection or tissue injury. It is now well established that 11%–19% of patients with epidural anesthesia/analgesia develop hyperthermia [23, 97–104]. The mechanism responsible for fever in these cases is unknown, but accumulating evidence suggests it is of an inflammatory nature [103–108]. It is possible that in some cases in which the diagnosis of clinical chorioamnionitis was made after the placement of an epidural, fever is the consequence of the epidural, rather than intra-amniotic inflammation or infection. This differential diagnosis has become a major clinical challenge in labor and delivery rooms worldwide. In our study, 76% (35/46) of patients had clinical signs of chorioamnionitis after the use of epidural analgesia, and of those, 63% (n = 22/35) had microbial-associated intra-amniotic inflammation and 20% (n = 7/35) had intra-amniotic inflammation without demonstrable microorganisms. We propose that analysis of AF can help with a precise diagnosis, and this has implications for both mothers and neonates.
Microorganisms in the AF are present in 61% of patients with clinical chorioamnionitis at term
Clinical chorioamnionitis is diagnosed by the combination of fever and other clinical signs, such as uterine tenderness, foul-smelling odor, fetal and maternal tachycardia, and maternal leukocytosis [109, 110]. The clinical diagnosis at term is rarely confirmed by microbiologic studies [110, 111], yet it is an indication for antibiotic treatment to improve maternal and neonatal outcome [112]. A major finding of our study is that about four out of 10 patients (40%) with the diagnosis of clinical chorioamnionitis did not have any evidence of bacteria in the amniotic cavity identified by culture or molecular methods, and 49% of patients with clinical chorioamnionitis at term did not have any evidence of acute inflammatory placental lesions. Therefore, we conclude that a large fraction of patients with the diagnosis of clinical chorioamnionitis at term do not have bacterial infection. This has clinical implications, as all of these patients and their neonates receive antibiotics [113–120] which can change the neonatal microbiota [121–129] and may have long-term effects in their immune response [130, 131]. It is important to determine which patients require medical intervention, and this can be accomplished through characterization of the AF microbiology for a rational choice of antimicrobial therapy.
Microorganisms in the amniotic cavity in clinical chorioamnionitis at term
Ureaplasma urealyticum was the most common microorganism retrieved from the AF using cultivation techniques, followed by M. hominis, S. agalactiae, and Fusobacterium spp. In contrast, the most common microorganisms detected by PCR/ESI-MS were G. vaginalis, followed by U. urealyticum and Lactobacillus spp. Of three cases in which PCR/ESI-MS identified Acinetobacter spp., AF cultures were negative. In 44% (12/27) of cases with positive AF results by PCR/ESI-MS, multiple organisms were isolated from the same fluid (polymicrobial infection), and in such cases, the magnitude of the intra-amniotic inflammatory response (IL-6) was higher than in cases having only one microorganism.
Gardnerella vaginalis was identified in the AF of 10 patients (Table 2). Cultivation methods identified this microorganism in only three cases; the remainder was identified using only molecular techniques. The number of microbial genomes was similar in both patients with negative culture and those with positive culture. Thus, factors other than the inoculum size may determine microorganism identification by culture. Six patients had Lactobacillus spp., three had Sneathia, and other patients had Veillonella spp. All of these microorganisms have been previously identified in AF [51, 92, 132–135].
For one patient, the PCR/ESI-MS result might have been false negative, as the AF culture was positive for Veillonella spp. and Lactobacillus spp., and this patient also had intra-amniotic inflammation. It is unclear whether results for the seven patients with positive PCR/ESI-MS results and negative AF cultures were affected by contamination (i.e., false-positive PCR/ESI-MS) as three of these patients were positive for Acinetobacter spp., which has occasionally been found in nosocomial infections [136]. However, three of the remaining four patients had intra-amniotic inflammation and/or placental lesions consistent with acute AF infection, suggesting that the discordant results of PCR/ESI-MS were likely to represent true positives. Further studies are warranted to examine the importance and significance of discordant AF culture and PCR/ESI-MS test results in patients with clinical chorioamnionitis at term.
We have previously used PCR/ESI-MS to characterize the microorganisms in the AF in patients with preterm labor and intact membranes [75]. It is noteworthy that the most common organism in preterm labor with intact membranes was Ureaplasma parvum, while in clinical chorioamnionitis, it was Gardnerella, suggesting that the microbiology of the two conditions is somewhat different. Knowledge of the microbiology of the amniotic cavity is important for rational antimicrobial therapy. The observation that genital Mycoplasmas are frequently involved is relevant because these microorganisms are not successfully treated with the antibiotics generally used for the treatment of clinical chorioamnionitis [137], puerperal endometritis [138–145], or neonatal sepsis [146–152]. Ureaplasma has been treated with erythromycin [153], while Mycoplasma requires treatment with other antimicrobial agents [154–156]. Although some may argue that infections with Mycoplasmas can resolve without treatment, an important question is whether this is optimal and always the case. It is not known if infections with genital Mycoplasmas which were not adequately treated may cause endometritis [157], impaired wound healing of the hysterotomy or skin incision, or even secondary infertility [158–161]. Indeed, Ureaplasma spp. is the most common microorganism from incisional wounds after a cesarean delivery [162]. It is unclear if appropriate antimicrobial treatment could reduce the rate of wound complications in patients with clinical chorioamnionitis and intra-amniotic infection due to these microorganisms. The consequences of genital Mycoplasma infection of newborns have also been a subject of study and it remains to be determined whether neonates may benefit from adequate coverage with antimicrobial agents against this microorganism [147, 150].
Intra-amniotic inflammation in clinical chorioamnionitis at term
The AF IL-6 concentrations were used to assess the presence and magnitude of an inflammatory response based upon previous studies conducted by us and others [78–81,85, 90, 96, 163–172], which indicate that AF IL-6 concentrations correlate with the outcome of preterm labor [11,63, 79, 88, 173–179], preterm PROM [173, 180, 181], cervical insufficiency [182], placenta previa [89], and a short cervix [183–186]. The median AF IL-6 concentration in patients with clinical chorioamnionitis and microbial-associated intra-amniotic inflammation was 14 ng/mL, which was higher than that of patients with intra-amniotic inflammation without detectable microorganisms (4.7 ng/mL). However, it is interesting that the median AF concentration of IL-6 in patients with clinical chorioamnionitis and microbial-associated inflammation was substantially lower than that of patients with preterm labor with intact membranes and microbial-associated inflammation, even when most of the latter patients did not have clinical chorioamnionitis (e.g., fever and other signs) (PTL, 96 ng/mL vs. chorioamnionitis at term with microbial-associated intra-amniotic inflammation, 14 ng/mL) [187]. The intensity of the inflammatory response was greater with a larger microbial burden, an observation that is in keeping with our previous findings [51, 55, 75]. We report for the first time that polymicrobial infections are associated with a more intense intra-amniotic inflammatory response. Whether this observation represents engagement of multiple pattern recognition receptors with a more robust inflammatory response remains to be determined [188–191].
Clinical chorioamnionitis without demonstrable bacteria in the amniotic cavity
Intra-amniotic inflammation in the absence of bacteria was identified by cultivation and/or molecular methods in 24% of women with clinical chorioamnionitis at term. What is the cause of intra-amniotic inflammation in these cases? Viruses may play a role; however, our previous studies in preterm labor and other complications of pregnancy indicate that the prevalence of viral invasion of the amniotic cavity is extremely low [75, 192]. Therefore, although possible, we do not believe that this is the most likely cause of nonbacterial intra-amniotic inflammation [187]. Another possibility is that “danger signals” induce an inflammatory response [174–179,193–198]. It is now recognized that cellular damage and stress could lead to release of alarmins [199, 200], which are normal cell constituents released during necrosis or cellular stress capable of inducing an inflammatory response [201–204]. We have previously reported that clinical chorioamnionitis at term is associated with a significant increase in the AF concentrations of high mobility group protein-B1 [179], the prototypic alarmin. One question is whether patients with intra-amniotic inflammation and elevated AF HMGB-1 concentrations correspond to those with intra-amniotic inflammation without detectable microorganisms [187].
Are there consequences of intra-amniotic inflammation without demonstrable microorganisms? The recognition of intra-amniotic inflammation without detectable microorganisms had to await the availability of molecular methods, which excluded the presence of bacteria and a large number of viruses. The current study used molecular microbiologic methods for the detection of bacteria, but not viruses. Therefore, we have not employed the term “sterile inflammation” in this report. The observation that intra-amniotic inflammation without demonstrable microorganisms is accompanied by evidence of a maternal systemic inflammatory response (i.e., acute histologic chorioamnionitis) in 36% (4/11) suggests that the inflammatory process is not confined to the amniotic cavity.
Clinical chorioamnionitis and histologic evidence of placental lesions consistent with amniotic fluid infection
Histologic chorioamnionitis is a maternal host response to the presence of microorganisms in the amniotic cavity or other inducers of inflammation [13, 205]. In this study, 51% (23/45) of patients with clinical chorioamnionitis had placental lesions consistent with AF infection. Our observations are consistent with those of Smulian et al. who reported that 62% (86/139) of patients with clinical chorioamnionitis had histologic confirmation of inflammation in the chorioamniotic membranes [206]. Therefore, a subset of patients with clincial chorioamnionitis does not have acute inflammatory lesions of the placenta. The stimuli for fever and other clinical signs of systemic maternal inflammation in these cases remain to be determined, particularly in those who did not have an epidural.
The frequency of acute histologic chorioamnionitis without evidence of placental or intra-amniotic infection ranged from 30% to more than 50% in prior studies [207–209]. Roberts et al. recently reported the histologic and microbiologic evaluation of 195 placentas from low-risk pregnancies who delivered at term. The authors found that grade 1 or 2 histologic chorioamnionitis was present in 34% (67/195), but microorganisms in the chorioamniotic space were present in only 4% (8/195) [210]. However, Hillier et al. demonstrated that 33% (3/9) of patients with acute histologic chorioamnionitis at term had negative chorioamniotic cultures [208]. Similarly, Zhang et al. reported that microorganisms were cultured in 44% (49/111) of the placentas with histologic evidence of acute chorioamnionitis. They noted that in many instances, “pathogens are not recovered by conventional aerobic and anerobic bacteriologic studies.” Thus, taken together, it is clear that a sizable proportion of patients with acute histologic chorioamnionitis do not have bacteria either in the AF, chorioamniotic membranes, or in the subchorionic fibrin [207].
Strengths and limitations
Strengths of this study include the use of both molecular and cultivation techniques for the identification of microorganisms, blinding of pathologists to obstetrical diagnoses and outcomes, and use of standardized protocols for placental examination. Limitations include those related to sample size, and like all observational studies, causation cannot be inferred from the reported associations.
Conclusions
Microorganisms were identified in the AF in 61% of patients with clinical chorioamnionitis at term; 54% had microbial-associated intra-amniotic inflammation, whereas 24% had intra-amniotic inflammation without detectable bacteria. Despite its frequency and importance, there are major gaps in knowledge about the diagnosis, pathogenesis, microbiology, maternal and fetal immune response, and short-and-long term consequences of clinical chorioamnionitis, as well as optimal treatment. Systems biology can help us make major gains in the understanding of this important condition [211–228].
Acknowledgments
Funding: This research was supported, in part, by the Perinatology Research Branch, Program for Perinatal Research and Obstetrics, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS) and, in part, with Federal funds from NICHD/NIH/DHHS under Contract No. HHSN275201300006C.
References
- 1.Malloy MH. Chorioamnionitis: epidemiology of newborn management and outcome United States 2008. J Perinatol. 2014;34:611–5. doi: 10.1038/jp.2014.81. [DOI] [PubMed] [Google Scholar]
- 2.Tita AT, Andrews WW. Diagnosis and management of clinical chorioamnionitis. Clin Perinatol. 2010;37:339–54. doi: 10.1016/j.clp.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rouse DJ, Landon M, Leveno KJ, Leindecker S, Varner MW, Caritis SN, et al. The maternal-fetal medicine units cesarean registry: chorioamnionitis at term and its duration-relationship to outcomes. Am J Obstet Gynecol. 2004;191:211–6. doi: 10.1016/j.ajog.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Newton ER. Chorioamnionitis and intraamniotic infection. Clin Obstet Gynecol. 1993;36:795–808. doi: 10.1097/00003081-199312000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Gibbs RS, Blanco JD, St Clair PJ, Castaneda YS. Quantitative bacteriology of AF from women with clinical intraamniotic infection at term. J Infect Dis. 1982;145:1–8. doi: 10.1093/infdis/145.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Gibbs RS, Dinsmoor MJ, Newton ER, Ramamurthy RS. A randomized trial of intrapartum versus immediate postpartum treatment of women with intra-amniotic infection. Obstet Gynecol. 1988;72:823–8. doi: 10.1097/00006250-198812000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Gilstrap LC, 3rd, Cox SM. Acute chorioamnionitis. Obstet Gynecol Clin North Am. 1989;16:373–9. [PubMed] [Google Scholar]
- 8.Gibbs RS, Duff P. Progress in pathogenesis and management of clinical intraamniotic infection. Am J Obstet Gynecol. 1991;164:1317–26. doi: 10.1016/0002-9378(91)90707-x. [DOI] [PubMed] [Google Scholar]
- 9.Willi MJ, Winkler M, Fischer DC, Reineke T, Maul H, Rath W. Chorioamnionitis: elevated interleukin-6 and interleukin-8 concentrations in the lower uterine segment. J Perinat Med. 2002;30:292–6. doi: 10.1515/JPM.2002.042. [DOI] [PubMed] [Google Scholar]
- 10.Lee SE, Romero R, Kim CJ, Shim SS, Yoon BH. Funisitis in term pregnancy is associated with microbial invasion of the amniotic cavity and intra-amniotic inflammation. J Matern Fetal Neonatal Med. 2006;19:693–7. doi: 10.1080/14767050600927353. [DOI] [PubMed] [Google Scholar]
- 11.Lee SE, Romero R, Jung H, Park CW, Park JS, Yoon BH. The intensity of the fetal inflammatory response in intraamniotic inflammation with and without microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 2007;197:e291–6. doi: 10.1016/j.ajog.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Gotsch F, Romero R, Kusanovic JP, Mazaki-Tovi S, Pineles BL, Erez O, et al. The fetal inflammatory response syndrome. Clin Obstet Gynecol. 2007;50:652–83. doi: 10.1097/GRF.0b013e31811ebef6. [DOI] [PubMed] [Google Scholar]
- 13.Redline RW. Inflammatory response in acute chorioamnionitis. Semin Fetal Neonatal Med. 2012;17:20–5. doi: 10.1016/j.siny.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Romero R, Quintero R, Oyarzun E, Wu YK, Sabo V, Mazor M, et al. Intraamniotic infection and the onset of labor in preterm premature rupture of the membranes. Am J Obstet Gynecol. 1988;159:661–6. doi: 10.1016/s0002-9378(88)80030-9. [DOI] [PubMed] [Google Scholar]
- 15.Martinelli P, Sarno L, Maruotti GM, Paludetto R. Chorioamnionitis and prematurity: a critical review. J Matern Fetal Neonatal Med. 2012;25:29–31. doi: 10.3109/14767058.2012.714981. [DOI] [PubMed] [Google Scholar]
- 16.Hauth JC, Gilstrap LC, 3rd, Hankins GD, Connor KD. Term maternal and neonatal complications of acute chorioamnionitis. Obstet Gynecol. 1985;66:59–62. [PubMed] [Google Scholar]
- 17.Rojas-Suarez J, Paternina-Caicedo AJ, Miranda J, Mendoza R, Dueñas-Castel C, Bourjeily G. Comparison of severity-of-illness scores in critically ill obstetric patients: a 6-year retrospective cohort. Crit Care Med. 2014;42:1047–54. doi: 10.1097/CCM.0000000000000124. [DOI] [PubMed] [Google Scholar]
- 18.Mark SP, Croughan-Minihane MS, Kilpatrick SJ. Chorioamnionitis and uterine function. Obstet Gynecol. 2000;95:909–12. [PubMed] [Google Scholar]
- 19.Yoder PR, Gibbs RS, Blanco JD, Castaneda YS, St Clair PJ. A prospective, controlled study of maternal and perinatal outcome after intra-amniotic infection at term. Am J Obstet Gynecol. 1983;145:695–701. doi: 10.1016/0002-9378(83)90575-6. [DOI] [PubMed] [Google Scholar]
- 20.Yancey MK, Duff P, Kubilis P, Clark P, Frentzen BH. Risk factors for neonatal sepsis. Obstet Gynecol. 1996;87:188–94. doi: 10.1016/0029-7844(95)00402-5. [DOI] [PubMed] [Google Scholar]
- 21.Ladfors L, Tessin I, Mattsson LA, Eriksson M, Seeberg S, Fall O. Risk factors for neonatal sepsis in offspring of women with prelabor rupture of the membranes at 34–42 weeks. J Perinat Med. 1998;26:94–101. doi: 10.1515/jpme.1998.26.2.94. [DOI] [PubMed] [Google Scholar]
- 22.Rao S, Pavlova Z, Incerpi MH, Ramanathan R. Meconium-stained amniotic fluid and neonatal morbidity in near-term and term deliveries with acute histologic chorioamnionitis and/or funisitis. J Perinatol. 2001;21:537–40. doi: 10.1038/sj.jp.7210564. [DOI] [PubMed] [Google Scholar]
- 23.Greenwell EA, Wyshak G, Ringer SA, Johnson LC, Rivkin MJ, Lieberman E. Intrapartum temperature elevation, epidural use, and adverse outcome in term infants. Pediatrics. 2012;129:e447–54. doi: 10.1542/peds.2010-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moyo SR, Hagerstrand I, Nystrom L, Tswana SA, Blomberg J, Bergström S, et al. Stillbirths and intrauterine infection, histologic chorioamnionitis and microbiological findings. Int J Gynaecol Obstet. 1996;54:115–23. doi: 10.1016/0020-7292(96)02705-1. [DOI] [PubMed] [Google Scholar]
- 25.Han YW, Fardini Y, Chen C, Iacampo KG, Peraino VA, Shamonki JM, et al. Term stillbirth caused by oral Fusobacterium nucleatum. Obstet Gynecol. 2010;115:442–5. doi: 10.1097/AOG.0b013e3181cb9955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hillier SL, Krohn MA, Kiviat NB, Watts DH, Eschenbach DA. Microbiologic causes and neonatal outcomes associated with chorioamnion infection. Am J Obstet Gynecol. 1991;165:955–61. doi: 10.1016/0002-9378(91)90447-y. [DOI] [PubMed] [Google Scholar]
- 27.McGregor JA. Maternal and fetal infection. Curr Opin Obstet Gynecol. 1991;3:15–23. [PubMed] [Google Scholar]
- 28.van Hoeven KH, Anyaegbunam A, Hochster H, Whitty JE, Distant J, Crawford C, et al. Clinical significance of increasing histologic severity of acute inflammation in the fetal membranes and umbilical cord. Pediatr Pathol Lab Med. 1996;16:731–44. [PubMed] [Google Scholar]
- 29.Grether JK, Nelson KB. Maternal infection and cerebral palsy in infants of normal birth weight. J Am Med Assoc. 1997;278:207–11. [PubMed] [Google Scholar]
- 30.Wu YW, Colford JM., Jr Chorioamnionitis as a risk factor for cerebral palsy: a meta-analysis. J Am Med Assoc. 2000;284:1417–24. doi: 10.1001/jama.284.11.1417. [DOI] [PubMed] [Google Scholar]
- 31.Impey L, Greenwood C, MacQuillan K, Reynolds M, Sheil O. Fever in labour and neonatal encephalopathy: a prospective cohort study. Br J Obstet Gynaecol. 2001;108:594–7. doi: 10.1111/j.1471-0528.2001.00145.x. [DOI] [PubMed] [Google Scholar]
- 32.Wu YW, Escobar GJ, Grether JK, Croen LA, Greene JD, Newman TB. Chorioamnionitis and cerebral palsy in term and near-term infants. J Am Med Assoc. 2003;290:2677–84. doi: 10.1001/jama.290.20.2677. [DOI] [PubMed] [Google Scholar]
- 33.Blume HK, Li CI, Loch CM, Koepsell TD. Intrapartum fever and chorioamnionitis as risks for encephalopathy in term newborns: a case-control study. Dev Med Child Neurol. 2008;50:19–24. doi: 10.1111/j.1469-8749.2007.02007.x. [DOI] [PubMed] [Google Scholar]
- 34.Soraisham AS, Singhal N, McMillan DD, Sauve RS, Lee SK Canadian Neonatal Network. A multicenter study on the clinical outcome of chorioamnionitis in preterm infants. Am J Obstet Gynecol. 2009;200:e371–6. doi: 10.1016/j.ajog.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 35.Lee SM, Park JW, Kim BJ, Park CW, Park JS, Jun JK, et al. Acute histologic chorioamnionitis is a risk factor for adverse neonatal outcome in late preterm birth after preterm premature rupture of membranes. PLoS One. 2013;8:e79941. doi: 10.1371/journal.pone.0079941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hagberg H, Wennerholm UB, Savman K. Sequelae of chorioamnionitis. Curr Opin Infect Dis. 2002;15:301–6. doi: 10.1097/00001432-200206000-00014. [DOI] [PubMed] [Google Scholar]
- 37.Nelson KB. The epidemiology of cerebral palsy in term infants. Ment Retard Dev Disabil Res Rev. 2002;8:146–50. doi: 10.1002/mrdd.10037. [DOI] [PubMed] [Google Scholar]
- 38.Blanchard A, Hentschel J, Duffy L, Baldus K, Cassell GH. Detection of Ureaplasma urealyticum by polymerase chain reaction in the urogenital tract of adults, in amniotic fluid, and in the respiratory tract of newborns. Clin Infect Dis. 1993;17:S148–53. doi: 10.1093/clinids/17.supplement_1.s148. [DOI] [PubMed] [Google Scholar]
- 39.Jalava J, Mantymaa ML, Ekblad U, Toivanen P, Skurnik M, Lassila O, et al. Bacterial 16S rDNA polymerase chain reaction in the detection of intra-amniotic infection. Br J Obstet Gynaecol. 1996;103:664–9. doi: 10.1111/j.1471-0528.1996.tb09835.x. [DOI] [PubMed] [Google Scholar]
- 40.Hitti J, Riley DE, Krohn MA, Hillier SL, Agnew KJ, Krieger JN, et al. Broad-spectrum bacterial rDNA polymerase chain reaction assay for detecting amniotic fluid infection among women in premature labor. Clin Infect Dis. 1997;24:1228–32. doi: 10.1086/513669. [DOI] [PubMed] [Google Scholar]
- 41.Yoon BH, Romero R, Kim M, Kim EC, Kim T, Park JS, et al. Clinical implications of detection of Ureaplasma urealyticum in the amniotic cavity with the polymerase chain reaction. Am J Obstet Gynecol. 2000;183:1130–7. doi: 10.1067/mob.2000.109036. [DOI] [PubMed] [Google Scholar]
- 42.Bearfield C, Davenport ES, Sivapathasundaram V, Allaker RP. Possible association between amniotic fluid micro-organism infection and microflora in the mouth. Br J Obstet Gynaecol. 2002;109:527–33. doi: 10.1111/j.1471-0528.2002.01349.x. [DOI] [PubMed] [Google Scholar]
- 43.Gerber S, Vial Y, Hohlfeld P, Witkin SS. Detection of Ureaplasma urealyticum in second-trimester amniotic fluid by polymerase chain reaction correlates with subsequent preterm labor and delivery. J Infect Dis. 2003;187:518–21. doi: 10.1086/368205. [DOI] [PubMed] [Google Scholar]
- 44.Yoon BH, Romero R, Lim JH, Shim SS, Hong JS, Shim JY, et al. The clinical significance of detecting Ureaplasma urealyticum by the polymerase chain reaction in the amniotic fluid of patients with preterm labor. Am J Obstet Gynecol. 2003;189:919–24. doi: 10.1067/s0002-9378(03)00839-1. [DOI] [PubMed] [Google Scholar]
- 45.Jacobsson B, Mattsby-Baltzer I, Andersch B, Bokström H, Holst RM, Nikolaitchouk N, et al. Microbial invasion and cytokine response in amniotic fluid in a Swedish population of women with preterm prelabor rupture of membranes. Acta Obstet Gynecol Scand. 2003;82:423–31. doi: 10.1034/j.1600-0412.2003.00157.x. [DOI] [PubMed] [Google Scholar]
- 46.Jacobsson B, Mattsby-Baltzer I, Andersch B, Bokström H, Holst RM, Wennerholm UB, et al. Microbial invasion and cytokine response in amniotic fluid in a Swedish population of women in preterm labor. Acta Obstet Gynecol Scand. 2003;82:120–8. doi: 10.1034/j.1600-0412.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 47.Kim M, Kim G, Romero R, Shim SS, Kim EC, Yoon BH. Biovar diversity of Ureaplasma urealyticum in amniotic fluid: distribution, intrauterine inflammatory response and pregnancy outcomes. J Perinat Med. 2003;31:146–52. doi: 10.1515/JPM.2003.020. [DOI] [PubMed] [Google Scholar]
- 48.Perni SC, Vardhana S, Korneeva I, Tuttle SL, Paraskevas LR, Chasen ST, et al. Mycoplasma hominis and Ureaplasma urealyticum in midtrimester amniotic fluid: association with amniotic fluid cytokine levels and pregnancy outcome. Am J Obstet Gynecol. 2004;191:1382–6. doi: 10.1016/j.ajog.2004.05.070. [DOI] [PubMed] [Google Scholar]
- 49.Yi J, Yoon BH, Kim EC. Detection and biovar discrimination of Ureaplasma urealyticum by real-time PCR. Mol Cell Probes. 2005;19:255–60. doi: 10.1016/j.mcp.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 50.Leon R, Silva N, Ovalle A, Chaparro A, Ahumada A, Gajardo M, et al. Detection of Porphyromonas gingivalis in the amniotic fluid in pregnant women with a diagnosis of threatened premature labor. J Periodontol. 2007;78:1249–55. doi: 10.1902/jop.2007.060368. [DOI] [PubMed] [Google Scholar]
- 51.DiGiulio DB, Romero R, Amogan HP, Kusanovic JP, Bik EM, Gotsch F, et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One. 2008;3:e3056. doi: 10.1371/journal.pone.0003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Han YW, Shen T, Chung P, Buhimschi IA, Buhimschi CS. Uncultivated bacteria as etiologic agents of intra-amniotic inflammation leading to preterm birth. J Clin Microbiol. 2009;47:38–47. doi: 10.1128/JCM.01206-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oh KJ, Lee KA, Sohn YK, Park CW, Hong JS, Romero R, et al. Intraamniotic infection with genital mycoplasmas exhibits a more intense inflammatory response than intraamniotic infection with other microorganisms in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 2010;203:e211–8. doi: 10.1016/j.ajog.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oh KJ, Lee SE, Jung H, Kim G, Romero R, Yoon BH. Detection of ureaplasmas by the polymerase chain reaction in the amniotic fluid of patients with cervical insufficiency. J Perinat Med. 2010;38:261–8. doi: 10.1515/JPM.2010.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.DiGiulio DB, Romero R, Kusanovic JP, Gómez R, Kim CJ, Seok KS, et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol. 2010;64:38–57. doi: 10.1111/j.1600-0897.2010.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodriguez N, Fernandez C, Zamora Y, Berdasquera D, Rivera JA. Detection of Ureaplasma urealyticum and Ureaplasma parvum in amniotic fluid: association with pregnancy outcomes. J Matern Fetal Neonatal Med. 2011;24:47–50. doi: 10.3109/14767058.2010.482609. [DOI] [PubMed] [Google Scholar]
- 57.Jungkind D. Tech.Sight. Molecular testing for infectious disease. Science. 2001;294:1553–5. doi: 10.1126/science.294.5546.1553. [DOI] [PubMed] [Google Scholar]
- 58.Singh DV. Hexaplex PCR for rapid detection of virulence factors. Expert Rev Mol Diagn. 2003;3:781–4. doi: 10.1586/14737159.3.6.781. [DOI] [PubMed] [Google Scholar]
- 59.Yang S, Rothman RE. PCR-based diagnostics for infectious diseases: uses, limitations, and future applications in acute-care settings. Lancet Infect Dis. 2004;4:337–48. doi: 10.1016/S1473-3099(04)01044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bhattacharya S. Early diagnosis of resistant pathogens: how can it improve antimicrobial treatment? Virulence. 2013;4:172–84. doi: 10.4161/viru.23326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.DiGiulio DB, Gervasi M, Romero R, Mazaki-Tovi S, Vaisbuch E, Kusanovic JP, et al. Microbial invasion of the amniotic cavity in preeclampsia as assessed by cultivation and sequence-based methods. J Perinat Med. 2010;38:503–13. doi: 10.1515/JPM.2010.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.DiGiulio DB, Gervasi MT, Romero R, Vaisbuch E, Mazaki-Tovi S, Kusanovic JP, et al. Microbial invasion of the amniotic cavity in pregnancies with small-for-gestational-age fetuses. J Perinat Med. 2010;38:495–502. doi: 10.1515/JPM.2010.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185:1130–6. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 64.Kim KW, Romero R, Park HS, Park CW, Shim SS, Jun JK, et al. A rapid matrix metalloproteinase-8 bedside test for the detection of intraamniotic inflammation in women with preterm premature rupture of membranes. Am J Obstet Gynecol. 2007;197:e291–5. doi: 10.1016/j.ajog.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 65.Redline RW, Heller D, Keating S, Kingdom J. Placental diagnostic criteria and clinical correlation – a workshop report. Placenta. 2005;26:S114–7. doi: 10.1016/j.placenta.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 66.Pacora P, Chaiworapongsa T, Maymon E, Kim YM, Gomez R, Yoon BH, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med. 2002;11:18–25. doi: 10.1080/jmf.11.1.18.25. [DOI] [PubMed] [Google Scholar]
- 67.Eshoo MW, Crowder CC, Rebman AW, Rounds MA, Matthews HE, Picuri JM, et al. Direct molecular detection and genotyping of Borrelia burgdorferi from whole blood of patients with early Lyme disease. PLoS One. 2012;7:e36825. doi: 10.1371/journal.pone.0036825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shin JH, Ranken R, Sefers SE, Lovari R, Quinn CD, Meng S, et al. Detection, identification, and distribution of fungi in bronchoalveolar lavage specimens by use of multilocus PCR coupled with electrospray ionization/mass spectrometry. J Clin Microbiol. 2013;51:136–41. doi: 10.1128/JCM.01907-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kanneganti TD, Ozoren N, Body-Malapel M, Amer A, Park JH, Franchi L, et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–6. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 70.Ecker DJ, Sampath R, Li H, Massire C, Matthews HE, Toleno D, et al. New technology for rapid molecular diagnosis of bloodstream infections. Expert Rev Mol Diagn. 2010;10:399–415. doi: 10.1586/erm.10.24. [DOI] [PubMed] [Google Scholar]
- 71.Metzgar D, Frinder M, Lovari R, Toleno D, Massire C, Blyn LB, et al. Broad-spectrum biosensor capable of detecting and identifying diverse bacterial and Candida species in blood. J Clin Microbiol. 2013;51:2670–8. doi: 10.1128/JCM.00966-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ecker JA, Massire C, Hall TA, Ranken R, Pennella TT, Agasino Ivy C, et al. Identification of Acinetobacter species and genotyping of Acinetobacter baumannii by multilocus PCR and mass spectrometry. J Clin Microbiol. 2006;44:2921–32. doi: 10.1128/JCM.00619-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brinkman CL, Vergidis P, Uhl JR, Pritt BS, Cockerill FR, Steckelberg JM, et al. PCR-electrospray ionization mass spectrometry for direct detection of pathogens and antimicrobial resistance from heart valves in patients with infective endocarditis. J Clin Microbiol. 2013;51:2040–6. doi: 10.1128/JCM.00304-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hofstadler SA, Sampath R, Blyn LB, Eshoo MW, Hall TA, Jiang Y, et al. TIGER: the universal biosensor. Int J Mass Spectr. 2005;242:23–41. [Google Scholar]
- 75.Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z, et al. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am J Reprod Immunol. 2014;71:330–58. doi: 10.1111/aji.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Romero R, Avila C, Santhanam U, Sehgal PB. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J Clin Invest. 1990;85:1392–400. doi: 10.1172/JCI114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Santhanam U, Avila C, Romero R, Viguet H, Ida N, Sakurai S, et al. Cytokines in normal and abnormal parturition: elevated amniotic fluid interleukin-6 levels in women with premature rupture of membranes associated with intrauterine infection. Cytokine. 1991;3:155–63. doi: 10.1016/1043-4666(91)90037-e. [DOI] [PubMed] [Google Scholar]
- 78.Romero R, Sepulveda W, Kenney JS, Archer LE, Allison AC, Sehgal PB. Interleukin 6 determination in the detection of microbial invasion of the amniotic cavity. Ciba Found Symp. 1992;167:205–20. doi: 10.1002/9780470514269.ch13. discussion 220–3. [DOI] [PubMed] [Google Scholar]
- 79.Romero R, Yoon BH, Kenney JS, Gomez R, Allison AC, Sehgal PB. Amniotic fluid interleukin-6 determinations are of diagnostic and prognostic value in preterm labor. Am J Reprod Immunol. 1993;30:167–83. doi: 10.1111/j.1600-0897.1993.tb00618.x. [DOI] [PubMed] [Google Scholar]
- 80.Romero R, Yoon BH, Mazor M, Gomez R, Diamond MP, Kenney JS, et al. The diagnostic and prognostic value of amniotic fluid white blood cell count, glucose, interleukin-6, and gram stain in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 1993;169:805–16. doi: 10.1016/0002-9378(93)90009-8. [DOI] [PubMed] [Google Scholar]
- 81.Romero R, Yoon BH, Mazor M, Gomez R, Gonzalez R, Diamond MP, et al. A comparative study of the diagnostic performance of amniotic fluid glucose, white blood cell count, interleukin-6, and gram stain in the detection of microbial invasion in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 1993;169:839–51. doi: 10.1016/0002-9378(93)90014-a. [DOI] [PubMed] [Google Scholar]
- 82.Gomez R, Romero R, Galasso M, Behnke E, Insunza A, Cotton DB. The value of amniotic fluid interleukin-6, white blood cell count, and gram stain in the diagnosis of microbial invasion of the amniotic cavity in patients at term. Am J Reprod Immunol. 1994;32:200–10. doi: 10.1111/j.1600-0897.1994.tb01115.x. [DOI] [PubMed] [Google Scholar]
- 83.Chaemsaithong P, Romero R, Korzeniewski SJ, Dong Z, Yeo L, Hassan SS, Kim YM, Yoon BH, Chaiworapongsa T. A point of care test for the determination of amniotic fluid interleukin-6 and the chemokine CXCL-10/IP-10. J Maternal Fetal Neonatal Med. 2015;28:1510–19. doi: 10.3109/14767058.2014.961417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Andrews WW, Hauth JC, Goldenberg RL, Gomez R, Romero R, Cassell GH. Amniotic fluid interleukin-6: correlation with upper genital tract microbial colonization and gestational age in women delivered after spontaneous labor versus indicated delivery. Am J Obstet Gynecol. 1995;173:606–12. doi: 10.1016/0002-9378(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 85.Yoon BH, Romero R, Kim CJ, Jun JK, Gomez R, Choi JH, et al. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol. 1995;172:960–70. doi: 10.1016/0002-9378(95)90028-4. [DOI] [PubMed] [Google Scholar]
- 86.Yoon BH, Romero R, Jun JK, Park KH, Park JD, Ghezzi F, et al. Amniotic fluid cytokines (interleukin-6, tumor necrosis factor alpha, interleukin-1 beta, and interleukin-8) and the risk for the development of bronchopulmonary dysplasia. Am J Obstet Gynecol. 1997;177:825–30. doi: 10.1016/s0002-9378(97)70276-x. [DOI] [PubMed] [Google Scholar]
- 87.Yoon BH, Romero R, Park JS, Chang JW, Kim YA, Kim JC, et al. Microbial invasion of the amniotic cavity with Ureaplasma urealyticum is associated with a robust host response in fetal, amniotic, and maternal compartments. Am J Obstet Gynecol. 1998;179:1254–60. doi: 10.1016/s0002-9378(98)70142-5. [DOI] [PubMed] [Google Scholar]
- 88.Yoon BH, Romero R, Moon JB, Oh SY, Han SY, Kim JC, et al. The frequency and clinical significance of intra-amniotic inflammation in patients with a positive cervical fetal fibronectin. Am J Obstet Gynecol. 2001;185:1137–42. doi: 10.1067/mob.2001.118162. [DOI] [PubMed] [Google Scholar]
- 89.Madan I, Romero R, Kusanovic JP, Mittal P, Chaiworapongsa T, Dong Z, et al. The frequency and clinical significance of intra-amniotic infection and/or inflammation in women with placenta previa and vaginal bleeding: an unexpected observation. J Perinat Med. 2010;38:275–9. doi: 10.1515/JPM.2010.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gervasi MT, Romero R, Bracalente G, Erez O, Dong Z, Hassan SS, et al. Midtrimester amniotic fluid concentrations of interleukin-6 and interferon-gamma-inducible protein-10: evidence for heterogeneity of intra-amniotic inflammation and associations with spontaneous early ( < 32 weeks) and late ( > 32 weeks) preterm delivery. J Perinat Med. 2012;40:329–43. doi: 10.1515/jpm-2012-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Romero R, Kadar N, Miranda J, Korzeniewski SJ, Schwartz AG, Chaemsaithong P, et al. The diagnostic performance of the mass restricted (MR) score in the identification of microbial invasion of the amniotic cavity or intra-amniotic inflammation is not superior to amniotic fluid interleukin-6. J Matern Fetal Neonatal Med. 2014;27:757–69. doi: 10.3109/14767058.2013.844123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Romero R, Nores J, Mazor M, Sepulveda W, Oyarzun E, Parra M, et al. Microbial invasion of the amniotic cavity during term labor. Prevalence and clinical significance. J Reprod Med. 1993;38:543–8. [PubMed] [Google Scholar]
- 93.Bobitt JR, Ledger WJ. Unrecognized amnionitis and prematurity: a preliminary report. J Reprod Med. 1977;19:8–12. [PubMed] [Google Scholar]
- 94.Leigh J, Garite TJ. Amniocentesis and the management of premature labor. Obstet Gynecol. 1986;67:500–6. [PubMed] [Google Scholar]
- 95.Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, et al. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol. 1989;161:817–24. doi: 10.1016/0002-9378(89)90409-2. [DOI] [PubMed] [Google Scholar]
- 96.Hillier SL, Witkin SS, Krohn MA, Watts DH, Kiviat NB, Eschenbach DA. The relationship of amniotic fluid cytokines and preterm delivery, amniotic fluid infection, histologic chorioamnionitis, and chorioamnion infection. Obstet Gynecol. 1993;81:941–8. [PubMed] [Google Scholar]
- 97.Fusi L, Steer PJ, Maresh MJ, Beard RW. Maternal pyrexia associated with the use of epidural analgesia in labour. Lancet. 1989;1:1250–2. doi: 10.1016/s0140-6736(89)92341-6. [DOI] [PubMed] [Google Scholar]
- 98.Camann WR, Hortvet LA, Hughes N, Bader AM, Datta S. Maternal temperature regulation during extradural analgesia for labour. Br J Anaesth. 1991;67:565–8. doi: 10.1093/bja/67.5.565. [DOI] [PubMed] [Google Scholar]
- 99.Lieberman E, Lang JM, Frigoletto F, Jr, Richardson DK, Ringer SA, Cohen A. Epidural analgesia, intrapartum fever, and neonatal sepsis evaluation. Pediatrics. 1997;99:415. doi: 10.1542/peds.99.3.415. [DOI] [PubMed] [Google Scholar]
- 100.Philip J, Alexander JM, Sharma SK, Leveno KJ, McIntire DD, Wiley J. Epidural analgesia during labor and maternal fever. Anesthesiology. 1999;90:1271–5. doi: 10.1097/00000542-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 101.Marmor TR, Krol DM. Labor pain management in the United States: understanding patterns and the issue of choice. Am J Obstet Gynecol. 2002;186:S173–80. doi: 10.1067/mob.2002.121258. [DOI] [PubMed] [Google Scholar]
- 102.Eltzschig HK, Lieberman ES, Camann WR. Regional anesthesia and analgesia for labor and delivery. N Engl J Med. 2003;348:319–32. doi: 10.1056/NEJMra021276. [DOI] [PubMed] [Google Scholar]
- 103.Segal S. Labor epidural analgesia and maternal fever. Anesth Analg. 2010;111:1467–75. doi: 10.1213/ANE.0b013e3181f713d4. [DOI] [PubMed] [Google Scholar]
- 104.Goetzl L. Epidural analgesia and maternal fever: a clinical and research update. Curr Opin Anaesthesiol. 2012;25:292–9. doi: 10.1097/ACO.0b013e3283530d7c. [DOI] [PubMed] [Google Scholar]
- 105.Dashe JS, Rogers BB, McIntire DD, Leveno KJ. Epidural analgesia and intrapartum fever: placental findings. Obstet Gynecol. 1999;93:341–4. doi: 10.1016/s0029-7844(98)00415-3. [DOI] [PubMed] [Google Scholar]
- 106.Goetzl L, Zighelboim I, Badell M, Rivers J, Mastrangèlo MA, Tweardy D, et al. Maternal corticosteroids to prevent intrauterine exposure to hyperthermia and inflammation: a randomized, double-blind, placebo-controlled trial. Am J Obstet Gynecol. 2006;195:1031–7. doi: 10.1016/j.ajog.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 107.Goetzl L. Epidural fever in obstetric patients: it’s a hot topic. Anesth Analg. 2014;118:494–5. doi: 10.1213/ANE.0000000000000112. [DOI] [PubMed] [Google Scholar]
- 108.Goetzl L, Manevich Y, Roedner C, Praktish A, Hebbar L, Townsend DM. Maternal and fetal oxidative stress and intrapartum term fever. Am J Obstet Gynecol. 2010;202:e361–5. doi: 10.1016/j.ajog.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gibbs RS, Castillo MS, Rodgers PJ. Management of acute chorioamnionitis. Am J Obstet Gynecol. 1980;136:709–13. doi: 10.1016/0002-9378(80)90445-7. [DOI] [PubMed] [Google Scholar]
- 110.Curtin WM, Katzman PJ, Florescue H, Metlay LA. Accuracy of signs of clinical chorioamnionitis in the term parturient. J Perinatol. 2013;33:422–8. doi: 10.1038/jp.2012.135. [DOI] [PubMed] [Google Scholar]
- 111.Pankuch GA, Appelbaum PC, Lorenz RP, Botti JJ, Schachter J, Naeye RL. Placental microbiology and histology and the pathogenesis of chorioamnionitis. Obstet Gynecol. 1984;64:802–6. [PubMed] [Google Scholar]
- 112.Lieberman E, Lang J, Richardson DK, Frigoletto FD, Heffner LJ, Cohen A. Intrapartum maternal fever and neonatal outcome. Pediatrics. 2000;105:8–13. doi: 10.1542/peds.105.1.8. [DOI] [PubMed] [Google Scholar]
- 113.Mecredy RL, Wiswell TE, Hume RF. Outcome of term gestation neonates whose mothers received intrapartum antibiotics for suspected chorioamnionitis. Am J Perinatol. 1993;10:365–8. doi: 10.1055/s-2007-994763. [DOI] [PubMed] [Google Scholar]
- 114.Goetzl L, Cohen A, Frigoletto F, Jr, Lang JM, Lieberman E. Maternal epidural analgesia and rates of maternal antibiotic treatment in a low-risk nulliparous population. J Perinatol. 2003;23:457–61. doi: 10.1038/sj.jp.7210967. [DOI] [PubMed] [Google Scholar]
- 115.Botet F, Figueras J, Carbonell-Estrany X, Arca G The Castrillo Study Group. Effect of maternal clinical chorioamnionitis on neonatal morbidity in very-low birthweight infants: a case control study. J Perinat Med. 2010;38:269–73. doi: 10.1515/jpm.2010.029. [DOI] [PubMed] [Google Scholar]
- 116.Heesen M, Klor S, Rossaint R, Straube S, Van de Velde M. Labour epidural analgesia and anti-infectious management of the neonate: a meta-analysis. J Perinat Med. 2012;40:625–30. doi: 10.1515/jpm-2012-0064. [DOI] [PubMed] [Google Scholar]
- 117.Baker CJ, Byington CL, Polin RA. Policy statement-recommendations for the prevention of perinatal group B streptococcal (GBS) disease. Pediatrics. 2011;128:611–6. doi: 10.1542/peds.2011-1466. [DOI] [PubMed] [Google Scholar]
- 118.Polin RA. Management of neonates with suspected or proven early-onset bacterial sepsis. Pediatrics. 2012;129:1006–15. doi: 10.1542/peds.2012-0541. [DOI] [PubMed] [Google Scholar]
- 119.Brady MT, Polin RA. Prevention and management of infants with suspected or proven neonatal sepsis. Pediatrics. 2013;132:166–8. doi: 10.1542/peds.2013-1310. [DOI] [PubMed] [Google Scholar]
- 120.Taylor JA, Opel DJ. Choriophobia: a 1-act play. Pediatrics. 2012;130:342–6. doi: 10.1542/peds.2012-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fanaro S, Chierici R, Guerrini P, Vigi V. Intestinal microflora in early infancy: composition and development. Acta Paediatr Suppl. 2003;91:48–55. doi: 10.1111/j.1651-2227.2003.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 122.Schumann A, Nutten S, Donnicola D, Comelli EM, Mansourian R, Cherbut C. Neonatal antibiotic treatment alters gastrointestinal tract developmental gene expression and intestinal barrier transcriptome. Physiol Genomics. 2005;23:235–45. doi: 10.1152/physiolgenomics.00057.2005. [DOI] [PubMed] [Google Scholar]
- 123.Wall R, Ross RP, Ryan CA, Hussey S, Murphy B, Fitzgerald GF. Role of gut microbiota in early infant development. Clin Med Pediatr. 2009;3:45–54. doi: 10.4137/cmped.s2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Marques TM, Wall R, Ross RP, Fitzgerald GF, Ryan CA, Stanton C. Programming infant gut microbiota: influence of dietary and environmental factors. Curr Opin Biotechnol. 2010;21:149–56. doi: 10.1016/j.copbio.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 125.Madan JC, Salari RC, Saxena D, Davidson L, O’Toole GA, Moore JH. Gut microbial colonisation in premature neonates predicts neonatal sepsis. Arch Dis Child Fetal Neonatal Ed. 2012;97:F456–62. doi: 10.1136/fetalneonatal-2011-301373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Russell SL, Gold MJ, Hartmann M, Willing BP, Thorson L, Wlodarska M. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Reprod. 2012;13:440–7. doi: 10.1038/embor.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Berrington JE, Stewart CJ, Embleton ND, Cummings SP. Gut microbiota in preterm infants: assessment and relevance to health and disease. Arch Dis Child Fetal Neonatal Ed. 2013;98:F286–90. doi: 10.1136/archdischild-2012-302134. [DOI] [PubMed] [Google Scholar]
- 128.Mai V, Torrazza RM, Ukhanova M, Wang X, Sun Y, Li N, et al. Distortions in development of intestinal microbiota associated with late onset sepsis in preterm infants. PLoS One. 2013;8:e52876. doi: 10.1371/journal.pone.0052876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Puiman P, Stoll B, Molbak L, de Bruijn A, Schierbeek H, Boye M, et al. Modulation of the gut microbiota with antibiotic treatment suppresses whole body urea production in neonatal pigs. Am J Physiol Gastrointest Liver Physiol. 2013;304:G300–10. doi: 10.1152/ajpgi.00229.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Romero R, Korzeniewski SJ. Are infants born by elective cesarean delivery without labor at risk for developing immune disorders later in life? Am J Obstet Gynecol. 2013;208:243–6. doi: 10.1016/j.ajog.2012.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cho CE, Norman M. Cesarean section and development of the immune system in the offspring. Am J Obstet Gynecol. 2013;208:249–54. doi: 10.1016/j.ajog.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 132.Lewis JF, Johnson P, Miller P. Evaluation of amniotic fluid for aerobic and anaerobic bacteria. Am J Clin Pathol. 1976;65:58–63. doi: 10.1093/ajcp/65.1.58. [DOI] [PubMed] [Google Scholar]
- 133.Maye DP, Filthuth I, Pugin P, Waldvogel F, Herrman WL. Bacteriological study of amniotic fluid during labor. Acta Obstet Gynecol Scand. 1983;62:603–7. doi: 10.3109/00016348309156257. [DOI] [PubMed] [Google Scholar]
- 134.Romero R, Scioscia AL, Edberg SC, Hobbins JC. Use of parenteral antibiotic therapy to eradicate bacterial colonization of amniotic fluid in premature rupture of membranes. Obstet Gynecol. 1986;67:15S–7S. doi: 10.1097/00006250-198603001-00005. [DOI] [PubMed] [Google Scholar]
- 135.Romero R, Mazor M, Morrotti R, Avila C, Oyarzun E, Insunza A, et al. Infection and labor. VII. Microbial invasion of the amniotic cavity in spontaneous rupture of membranes at term. Am J Obstet Gynecol. 1992;166:129–33. doi: 10.1016/0002-9378(92)91845-2. [DOI] [PubMed] [Google Scholar]
- 136.O’Shea MK. Acinetobacter in modern warfare. Int J Antimicrob Agents. 2012;39:363–75. doi: 10.1016/j.ijantimicag.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 137.Gibbs RS, Blanco JD, St Clair PJ, Castaneda YS. Mycoplasma hominis and intrauterine infection in late pregnancy. Sex Transm Dis. 1983;10:303–6. [PubMed] [Google Scholar]
- 138.Rosene K, Eschenbach DA, Tompkins LS, Kenny GE, Watkins H. Polymicrobial early postpartum endometritis with facultative and anaerobic bacteria, genital mycoplasmas, and Chlamydia trachomatis: treatment with piperacillin or cefoxitin. J Infect Dis. 1986;153:1028–37. doi: 10.1093/infdis/153.6.1028. [DOI] [PubMed] [Google Scholar]
- 139.Williams CM, Okada DM, Marshall JR, Chow AW. Clinical and microbiologic risk evaluation for post-cesarean section endometritis by multivariate discriminant analysis: role of intraoperative mycoplasma, aerobes, and anaerobes. Am J Obstet Gynecol. 1987;156:967–74. doi: 10.1016/0002-9378(87)90369-3. [DOI] [PubMed] [Google Scholar]
- 140.Newton ER, Prihoda TJ, Gibbs RS. A clinical and microbiologic analysis of risk factors for puerperal endometritis. Obstet Gynecol. 1990;75:402–6. [PubMed] [Google Scholar]
- 141.Andrews WW, Shah SR, Goldenberg RL, Cliver SP, Hauth JC, Cassell GH. Association of post-cesarean delivery endometritis with colonization of the chorioamnion by Ureaplasma urealyticum. Obstet Gynecol. 1995;85:509–14. doi: 10.1016/0029-7844(94)00436-H. [DOI] [PubMed] [Google Scholar]
- 142.Andrews WW, Hauth JC, Cliver SP, Savage K, Goldenberg RL. Randomized clinical trial of extended spectrum antibiotic prophylaxis with coverage for Ureaplasma urealyticum to reduce post-cesarean delivery endometritis. Obstet Gynecol. 2003;101:1183–9. doi: 10.1016/s0029-7844(03)00016-4. [DOI] [PubMed] [Google Scholar]
- 143.Chaim W, Horowitz S, David JB, Ingel F, Evinson B, Mazor M. Ureaplasma urealyticum in the development of postpartum endometritis. Eur J Obstet Gynecol Reprod Biol. 2003;109:145–8. doi: 10.1016/s0301-2115(03)00007-1. [DOI] [PubMed] [Google Scholar]
- 144.Andrews WW, Goldenberg RL, Hauth JC, Cliver SP, Conner M, Goepfert AR. Endometrial microbial colonization and plasma cell endometritis after spontaneous or indicated preterm versus term delivery. Am J Obstet Gynecol. 2005;193:739–45. doi: 10.1016/j.ajog.2005.02.128. [DOI] [PubMed] [Google Scholar]
- 145.Patai K, Szilagyi G, Hubay M, Szentmáriay IF, Paulin F. Severe endometritis caused by genital mycoplasmas after caesarean section. J Med Microbiol. 2005;54:1249–50. doi: 10.1099/jmm.0.05457-0. [DOI] [PubMed] [Google Scholar]
- 146.Waites KB, Crouse DT, Philips JB, 3rd, Canupp KC, Cassell GH. Ureaplasmal pneumonia and sepsis associated with persistent pulmonary hypertension of the newborn. Pediatrics. 1989;83:79–85. [PubMed] [Google Scholar]
- 147.Valencia GB, Banzon F, Cummings M, McCormack WM, Glass L, Hammerschlag MR. Mycoplasma hominis and Ureaplasma urealyticum in neonates with suspected infection. Pediatr Infect Dis J. 1993;12:571–3. doi: 10.1097/00006454-199307000-00004. [DOI] [PubMed] [Google Scholar]
- 148.Waites KB, Crouse DT, Cassell GH. Systemic neonatal infection due to Ureaplasma urealyticum. Clin Infect Dis. 1993;17:S131–5. doi: 10.1093/clinids/17.supplement_1.s131. [DOI] [PubMed] [Google Scholar]
- 149.Bonnin F, Petitjean J, Guillois B, Laloum D, Fretignet M, Freymuth F. Prospective study of neonatal genital mycoplasma colonization and infection. Arch Pediatr. 1995;2:636–42. doi: 10.1016/0929-693x(96)81217-6. [DOI] [PubMed] [Google Scholar]
- 150.Wolthers KC, Kornelisse RF, Platenkamp GJ, Schuurman-Van Der Lem MI, van der Schee C, Hartwig NG, et al. A case of Mycoplasma hominis meningo-encephalitis in a full-term infant: rapid recovery after start of treatment with ciprofloxacin. Eur J Pediatr. 2003;162:514–6. doi: 10.1007/s00431-003-1219-6. [DOI] [PubMed] [Google Scholar]
- 151.Morioka I, Fujibayashi H, Enoki E, Yokoyama N, Yokozaki H, Matsuo M. Congenital pneumonia with sepsis caused by intrauterine infection of Ureaplasma parvum in a term newborn: a first case report. J Perinatol. 2010;30:359–62. doi: 10.1038/jp.2009.145. [DOI] [PubMed] [Google Scholar]
- 152.Weisman LE, Leeming AH, Kong L. Appropriate antibiotic therapy improves Ureaplasma sepsis outcome in the neonatal mouse. Pediatr Res. 2012;72:502–6. doi: 10.1038/pr.2012.115. [DOI] [PubMed] [Google Scholar]
- 153.Smorgick N, Frenkel E, Zaidenstein R, Lazarovitch T, Sherman DJ. Antibiotic treatment of intra-amniotic infection with Ureaplasma urealyticum. A case report and literature review. Fetal Diagn Ther. 2007;22:90–3. doi: 10.1159/000097103. [DOI] [PubMed] [Google Scholar]
- 154.Redelinghuys MJ, Ehlers MM, Dreyer AW, Lombaard HA, Kock MM. Antimicrobial susceptibility patterns of Ureaplasma species and Mycoplasma hominis in pregnant women. BMC Infect Dis. 2014;14:171. doi: 10.1186/1471-2334-14-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gomez R, Romero R, Nien JK, Medina L, Carstens M, Kim YM, et al. Antibiotic administration to patients with preterm premature rupture of membranes does not eradicate intra-amniotic infection. J Matern Fetal Neonatal Med. 2007;20:167–73. doi: 10.1080/14767050601135485. [DOI] [PubMed] [Google Scholar]
- 156.Harrison HR, Riggin RM, Alexander ER, Weinstein L. In vitro activity of clindamycin against strains of Chlamydia trachomatis, Mycoplasma hominis, and Ureaplasma urealyticum isolated from pregnant women. Am J Obstet Gynecol. 1984;149:477–80. doi: 10.1016/0002-9378(84)90019-x. [DOI] [PubMed] [Google Scholar]
- 157.Cicinelli E, De Ziegler D, Nicoletti R, Colafiglio G, Saliani N, Resta L, et al. Chronic endometritis: correlation among hysteroscopic, histologic, and bacteriologic findings in a prospective trial with 2190 consecutive office hysteroscopies. Fertil Steril. 2008;89:677–84. doi: 10.1016/j.fertnstert.2007.03.074. [DOI] [PubMed] [Google Scholar]
- 158.Guven MA, Dilek U, Pata O, Dilek S, Ciragil P. Prevalance of Chlamydia trochomatis, Ureaplasma urealyticum and Mycoplasma hominis infections in the unexplained infertile women. Arch Gynecol Obstet. 2007;276:219–23. doi: 10.1007/s00404-006-0279-z. [DOI] [PubMed] [Google Scholar]
- 159.Cohen CR, Mugo NR, Astete SG, Odondo R, Manhart LE, Kiehlbauch JA, et al. Detection of Mycoplasma genitalium in women with laparoscopically diagnosed acute salpingitis. Sex Transm Infect. 2005;81:463–6. doi: 10.1136/sti.2005.015701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Haggerty CL, Totten PA, Astete SG, Lee S, Hoferka SL, Kelsey SF, et al. Failure of cefoxitin and doxycycline to eradicate endometrial Mycoplasma genitalium and the consequence for clinical cure of pelvic inflammatory disease. Sex Transm Infect. 2008;84:338–42. doi: 10.1136/sti.2008.030486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Haggerty CL, Taylor BD. Mycoplasma genitalium: an emerging cause of pelvic inflammatory disease. Infect Dis Obstet Gynecol. 2011;2011:959816. doi: 10.1155/2011/959816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Roberts S, Maccato M, Faro S, Faro S, Pinell P. The microbiology of post-cesarean wound morbidity. Obstet Gynecol. 1993;81:383–6. [PubMed] [Google Scholar]
- 163.Greig PC, Ernest JM, Teot L, Erikson M, Talley R. Amniotic fluid interleukin-6 levels correlate with histologic chorioamnionitis and amniotic fluid cultures in patients in premature labor with intact membranes. Am J Obstet Gynecol. 1993;169:1035–44. doi: 10.1016/0002-9378(93)90050-s. [DOI] [PubMed] [Google Scholar]
- 164.Gravett MG, Witkin SS, Haluska GJ, Edwards JL, Cook MJ, Novy MJ. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol. 1994;171:1660–7. doi: 10.1016/0002-9378(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 165.Negishi H, Yamada H, Mikuni M, Kishida T, Okuyama K, Sagawa T, et al. Correlation between cytokine levels of amniotic fluid and histological chorioamnionitis in preterm delivery. J Perinat Med. 1996;24:633–9. doi: 10.1515/jpme.1996.24.6.633. [DOI] [PubMed] [Google Scholar]
- 166.Gonzalez-Bosquet E, Cerqueira MJ, Dominguez C, Gasser I, Bermejo B, Cabero L. Amniotic fluid glucose and cytokines values in the early diagnosis of amniotic infection in patients with preterm labor and intact membranes. J Matern Fetal Med. 1999;8:155–8. doi: 10.1002/(SICI)1520-6661(199907/08)8:4<155::AID-MFM3>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 167.Kara M, Ozden S, Arioglu P, Cetin A. The significance of amniotic fluid interleukin-6 levels in preterm labour. Aust NZ J Obstet Gynaecol. 1998;38:403–6. doi: 10.1111/j.1479-828x.1998.tb03097.x. [DOI] [PubMed] [Google Scholar]
- 168.Greci LS, Gilson GJ, Nevils B, Izquierdo LA, Qualls CR, Curet LB. Is amniotic fluid analysis the key to preterm labor? A model using interleukin-6 for predicting rapid delivery. Am J Obstet Gynecol. 1998;179:172–8. doi: 10.1016/s0002-9378(98)70269-8. [DOI] [PubMed] [Google Scholar]
- 169.Figueroa R, Garry D, Elimian A, Patel K, Sehgal PB, Tejani N. Evaluation of amniotic fluid cytokines in preterm labor and intact membranes. J Matern Fetal Neonatal Med. 2005;18:241–7. doi: 10.1080/13506120500223241. [DOI] [PubMed] [Google Scholar]
- 170.Massaro G, Scaravilli G, Simeone S, Capuano S, Pastore E, Forte A, et al. Interleukin-6 and Mycoplasma hominis as markers of preterm birth and related brain damage: our experience. J Matern Fetal Neonatal Med. 2009;22:1063–7. doi: 10.3109/14767050903026473. [DOI] [PubMed] [Google Scholar]
- 171.Kim SM, Romero R, Lee J, Mi Lee S, Park CW, Shin Park J, et al. The frequency and clinical significance of intra-amniotic inflammation in women with preterm uterine contractility but without cervical change: do the diagnostic criteria for preterm labor need to be changed? J Matern Fetal Neonatal Med. 2012;25:1212–21. doi: 10.3109/14767058.2011.629256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Combs CA, Gravett M, Garite TJ, Hickok DE, Lapidus J, Porreco R, et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol. 2014;210:e121–5. doi: 10.1016/j.ajog.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 173.Shim SS, Romero R, Hong JS, Park CW, Jun JK, Kim BI, et al. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 2004;191:1339–45. doi: 10.1016/j.ajog.2004.06.085. [DOI] [PubMed] [Google Scholar]
- 174.Friel LA, Romero R, Edwin S, Nien JK, Gomez R, Chaiworapongsa T, et al. The calcium binding protein, S100B, is increased in the amniotic fluid of women with intra-amniotic infection/inflammation and preterm labor with intact or ruptured membranes. J Perinat Med. 2007;35:385–93. doi: 10.1515/JPM.2007.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Romero R, Espinoza J, Hassan S, Gotsch F, Kusanovic JP, Avila C, et al. Soluble receptor for advanced glycation end products (sRAGE) and endogenous secretory RAGE (esRAGE) in amniotic fluid: modulation by infection and inflammation. J Perinat Med. 2008;36:388–98. doi: 10.1515/JPM.2008.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chaiworapongsa T, Erez O, Kusanovic JP, Vaisbuch E, Mazaki-Tovi S, Gotsch F, et al. Amniotic fluid heat shock protein 70 concentration in histologic chorioamnionitis, term and preterm parturition. J Matern Fetal Neonatal Med. 2008;21:449–61. doi: 10.1080/14767050802054550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lee SE, Park IS, Romero R, Yoon BH. Amniotic fluid prostaglandin F2 increases even in sterile amniotic fluid and is an independent predictor of impending delivery in preterm premature rupture of membranes. J Matern Fetal Neonatal Med. 2009;22:880–6. doi: 10.1080/14767050902994648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Romero R, Chaiworapongsa T, Alpay Savasan Z, Xu Y, Hussein Y, Dong Z, et al. Damage-associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: a study of the alarmin HMGB1. J Matern Fetal Neonatal Med. 2011;24:1444–55. doi: 10.3109/14767058.2011.591460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Romero R, Chaiworapongsa T, Savasan ZA, Hussein Y, Dong Z, Kusanovic JP, et al. Clinical chorioamnionitis is characterized by changes in the expression of the alarmin HMGB1 and one of its receptors, sRAGE. J Matern Fetal Neonatal Med. 2012;25:558–67. doi: 10.3109/14767058.2011.599083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Maymon E, Romero R, Chaiworapongsa T, Kim JC, Berman S, Gomez R, et al. Value of amniotic fluid neutrophil collagenase concentrations in preterm premature rupture of membranes. Am J Obstet Gynecol. 2001;185:1143–8. doi: 10.1067/mob.2001.118166. [DOI] [PubMed] [Google Scholar]
- 181.Lee SE, Romero R, Lee SM, Yoon BH. Amniotic fluid volume in intra-amniotic inflammation with and without culture-proven amniotic fluid infection in preterm premature rupture of membranes. J Perinat Med. 2010;38:39–44. doi: 10.1515/JPM.2009.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lee SE, Romero R, Park CW, Jun JK, Yoon BH. The frequency and significance of intraamniotic inflammation in patients with cervical insufficiency. Am J Obstet Gynecol. 2008;198:633e1–8. doi: 10.1016/j.ajog.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 183.Kiefer DG, Keeler SM, Rust OA, Wayock CP, Vintzileos AM, Hanna N. Is midtrimester short cervix a sign of intraamniotic inflammation? Am J Obstet Gynecol. 2009;200:e371–5. doi: 10.1016/j.ajog.2009.01.047. [DOI] [PubMed] [Google Scholar]
- 184.Keeler SM, Kiefer DG, Rust OA, Vintzileos A, Atlas RO, Bornstein E, et al. Comprehensive amniotic fluid cytokine profile evaluation in women with a short cervix: which cytokine(s) correlates best with outcome? Am J Obstet Gynecol. 2009;201:e271–6. doi: 10.1016/j.ajog.2009.05.045. [DOI] [PubMed] [Google Scholar]
- 185.Vaisbuch E, Hassan SS, Mazaki-Tovi S, Nhan-Chang CL, Kusanovic JP, Chaiworapongsa T, et al. Patients with an asymptomatic short cervix ( < or = 15 mm) have a high rate of subclinical intraamniotic inflammation: implications for patient counseling. Am J Obstet Gynecol. 2010;202:433e1–8. doi: 10.1016/j.ajog.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kiefer DG, Keeler SM, Rust O, Chow SS, Craig ME, Peltier MR, et al. Amniotic fluid inflammatory score is associated with pregnancy outcome in patients with mid trimester short cervix. Am J Obstet Gynecol. 2012;206:68e1–6. doi: 10.1016/j.ajog.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 187.Romero R, Miranda J, Chaiworapongsa T, Korzeniewski SJ, Chaemsaithong P, Gotsch F, et al. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol. 2014;72:458–74. doi: 10.1111/aji.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kim YM, Romero R, Chaiworapongsa T, Kim GJ, Kim MR, Kuivaniemi H, et al. Toll-like receptor-2 and -4 in the chorioamniotic membranes in spontaneous labor at term and in preterm parturition that are associated with chorioamnionitis. Am J Obstet Gynecol. 2004;191:1346–55. doi: 10.1016/j.ajog.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 189.Abrahams VM, Bole-Aldo P, Kim YM, Straszewski-Chavez SL, Chaiworapongsa T, Romero R, et al. Divergent trophoblast responses to bacterial products mediated by TLRs. J Immunol. 2004;173:4286–96. doi: 10.4049/jimmunol.173.7.4286. [DOI] [PubMed] [Google Scholar]
- 190.Kim YM, Romero R, Oh SY, Kim CJ, Kilburn BA, Armant DR, et al. Toll-like receptor 4: a potential link between “danger signals,” the innate immune system, and preeclampsia? Am J Obstet Gynecol. 2005;193:921–7. doi: 10.1016/j.ajog.2005.07.076. [DOI] [PubMed] [Google Scholar]
- 191.Abrahams VM, Potter JA, Bhat G, Peltier MR, Saade G, Menon R. Bacterial modulation of human fetal membrane Toll-like receptor expression. Am J Reprod Immunol. 2013;69:33–40. doi: 10.1111/aji.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gervasi MT, Romero R, Bracalente G, Chaiworapongsa T, Erez O, Dong Z, et al. Viral invasion of the amniotic cavity (VIAC) in the midtrimester of pregnancy. J Matern Fetal Neonatal Med. 2012;25:2002–13. doi: 10.3109/14767058.2012.683899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Romero R, Durum S, Dinarello CA, Oyarzun E, Hobbins JC, Mitchell MD. Interleukin-1 stimulates prostaglandin biosynthesis by human amnion. Prostaglandins. 1989;37:13–22. doi: 10.1016/0090-6980(89)90028-2. [DOI] [PubMed] [Google Scholar]
- 194.Romero R, Mazor M, Brandt F, Sepulveda W, Avila C, Cotton DB, et al. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol. 1992;27:117–23. doi: 10.1111/j.1600-0897.1992.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 195.Espinoza J, Romero R, Chaiworapongsa T, Kim JC, Yoshimatsu J, Edwin S, et al. Lipopolysaccharide-binding protein in microbial invasion of the amniotic cavity and human parturition. J Matern Fetal Neonatal Med. 2002;12:313–21. doi: 10.1080/jmf.12.5.313.321. [DOI] [PubMed] [Google Scholar]
- 196.Jacobsson B. Intra-amniotic infection and inflammation in preterm birth – is bacteria always the connection? Commentary on the article by Miralles et al. on page 570. Pediatr Res. 2005;57:473–4. doi: 10.1203/01.PDR.0000156475.50488.B0. [DOI] [PubMed] [Google Scholar]
- 197.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25:21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Christiaens I, Zaragoza DB, Guilbert L, Robertson SA, Mitchell BF, Olson DM. Inflammatory processes in preterm and term parturition. J Reprod Immunol. 2008;79:50–7. doi: 10.1016/j.jri.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 199.Andersson U, Tracey KJ. HMGB1 in sepsis. Scand J Infect Dis. 2003;35:577–84. doi: 10.1080/00365540310016286. [DOI] [PubMed] [Google Scholar]
- 200.Oppenheim JJ, Tewary P, de la Rosa G, Yang D. Alarmins initiate host defense. Adv Exp Med Biol. 2007;601:185–94. doi: 10.1007/978-0-387-72005-0_19. [DOI] [PubMed] [Google Scholar]
- 201.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–37. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–89. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A. HMGB1: endogenous danger signaling. Mol Med. 2008;14:476–84. doi: 10.2119/2008-00034.Klune. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Castiglioni A, Canti V, Rovere-Querini P, Manfredi AA. High-mobility group box 1 (HMGB1) as a master regulator of innate immunity. Cell Tissue Res. 2011;343:189–99. doi: 10.1007/s00441-010-1033-1. [DOI] [PubMed] [Google Scholar]
- 205.Menon R, Taylor RN, Fortunato SJ. Chorioamnionitis – a complex pathophysiologic syndrome. Placenta. 2010;31:113–20. doi: 10.1016/j.placenta.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 206.Smulian JC, Shen-Schwarz S, Vintzileos AM, Lake MF, Ananth CV. Clinical chorioamnionitis and histologic placental inflammation. Obstet Gynecol. 1999;94:1000–5. doi: 10.1016/s0029-7844(99)00416-0. [DOI] [PubMed] [Google Scholar]
- 207.Zhang JM, Kraus FT, Aquino TI. Chorioamnionitis: a comparative histologic, bacteriologic, and clinical study. Int J Gynecol Pathol. 1985;4:1–10. [PubMed] [Google Scholar]
- 208.Hillier SL, Martius J, Krohn M, Kiviat N, Holmes KK, Eschenbach DA. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med. 1988;319:972–8. doi: 10.1056/NEJM198810133191503. [DOI] [PubMed] [Google Scholar]
- 209.Horvath B, Lakatos F, Toth C, Bödecs T, Bódis J. Silent chorioamnionitis and associated pregnancy outcomes: a review of clinical data gathered over a 16-year period. J Perinat Med. 2014;42:441–7. doi: 10.1515/jpm-2013-0186. [DOI] [PubMed] [Google Scholar]
- 210.Roberts DJ, Celi AC, Riley LE, Onderdonk AB, Boyd TK, Johnson LC, et al. Acute histologic chorioamnionitis at term: nearly always noninfectious. PLoS One. 2012;7:e31819. doi: 10.1371/journal.pone.0031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Romero R, Kuivaniemi H, Tromp G. Functional genomics and proteomics in term and preterm parturition. J Clin Endocrinol Metab. 2002;87:2431–4. doi: 10.1210/jcem.87.6.8689. [DOI] [PubMed] [Google Scholar]
- 212.Romero R, Espinoza J, Gotsch F, Kusanovic JP, Friel LA, Erez O, et al. The use of high-dimensional biology (genomics, transcriptomics, proteomics, and metabolomics) to understand the preterm parturition syndrome. Br J Obstet Gynaecol. 2006;113:118–35. doi: 10.1111/j.1471-0528.2006.01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Hassan SS, Romero R, Haddad R, Hendler I, Khalek N, Tromp G, et al. The transcriptome of the uterine cervix before and after spontaneous term parturition. Am J Obstet Gynecol. 2006;195:778–86. doi: 10.1016/j.ajog.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 214.Hassan SS, Romero R, Tarca AL, Draghici S, Pineles B, Bugrim A, et al. Signature pathways identified from gene expression profiles in the human uterine cervix before and after spontaneous term parturition. Am J Obstet Gynecol. 2007;197:250e1–7. doi: 10.1016/j.ajog.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Haddad R, Romero R, Gould BR, Tromp G, Gotsch F, Edwin SS, et al. Angiogenesis gene expression in mouse uterus during the common pathway of parturition. Am J Obstet Gynecol. 2008;198:539e1–8. doi: 10.1016/j.ajog.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 216.Bujold E, Romero R, Kusanovic JP, Erez O, Gotsch F, Chaiworapongsa T, et al. Proteomic profiling of amniotic fluid in preterm labor using two-dimensional liquid separation and mass spectrometry. J Matern Fetal Neonatal Med. 2008;21:697–713. doi: 10.1080/14767050802053289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hassan SS, Romero R, Tarca AL, Nhan-Chang CL, Vaisbuch E, Erez O, et al. The transcriptome of cervical ripening in human pregnancy before the onset of labor at term: identification of novel molecular functions involved in this process. J Matern Fetal Neonatal Med. 2009;22:1183–93. doi: 10.3109/14767050903353216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Romero R, Kusanovic JP, Gotsch F, Erez O, Vaisbuch E, Mazaki-Tovi S, et al. Isobaric labeling and tandem mass spectrometry: a novel approach for profiling and quantifying proteins differentially expressed in amniotic fluid in preterm labor with and without intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med. 2010;23:261–80. doi: 10.3109/14767050903067386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Romero R, Mazaki-Tovi S, Vaisbuch E, Kusanovic JP, Chaiworapongsa T, Gomez R, et al. Metabolomics in premature labor: a novel approach to identify patients at risk for preterm delivery. J Matern Fetal Neonatal Med. 2010;23:1344–59. doi: 10.3109/14767058.2010.482618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Madsen-Bouterse SA, Romero R, Tarca AL, Kusanovic JP, Espinoza J, Kim CJ, et al. The transcriptome of the fetal inflammatory response syndrome. Am J Reprod Immunol. 2010;63:73–92. doi: 10.1111/j.1600-0897.2009.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hassan SS, Romero R, Tarca AL, Nhan-Chang CL, Mittal P, Vaisbuch E, et al. The molecular basis for sonographic cervical shortening at term: identification of differentially expressed genes and the epithelial-mesenchymal transition as a function of cervical length. Am J Obstet Gynecol. 2010;203:472e1–14. doi: 10.1016/j.ajog.2010.06.076. [DOI] [PubMed] [Google Scholar]
- 222.Nhan-Chang CL, Romero R, Tarca AL, Mittal P, Kusanovic JP, Erez O, et al. Characterization of the transcriptome of chorioamniotic membranes at the site of rupture in spontaneous labor at term. Am J Obstet Gynecol. 2010;202:462e1–41. doi: 10.1016/j.ajog.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Mittal P, Romero R, Tarca AL, Gonzalez J, Draghici S, Xu Y, et al. Characterization of the myometrial transcriptome and biological pathways of spontaneous human labor at term. J Perinat Med. 2010;38:617–43. doi: 10.1515/JPM.2010.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Lee DC, Hassan SS, Romero R, Tarca AL, Bhatti G, Gervasi MT, et al. Protein profiling underscores immunological functions of uterine cervical mucus plug in human pregnancy. J Proteomics. 2011;74:817–28. doi: 10.1016/j.jprot.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lee J, Romero R, Chaiworapongsa T, Dong Z, Tarca AL, Xu Y, et al. Characterization of the fetal blood transcriptome and proteome in maternal anti-fetal rejection: evidence of a distinct and novel type of human fetal systemic inflammatory response. Am J Reprod Immunol. 2013;70:265–84. doi: 10.1111/aji.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Chaemsaithong P, Madan I, Romero R, Than NG, Tarca AL, Draghici S, et al. Characterization of the myometrial transcriptome in women with an arrest of dilatation during labor. J Perinat Med. 2013;41:665–81. doi: 10.1515/jpm-2013-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Madan I, Than NG, Romero R, Chaemsaithong P, Miranda J, Tarca AL, et al. The peripheral whole-blood transcriptome of acute pyelonephritis in human pregnancya. J Perinat Med. 2014;42:31–53. doi: 10.1515/jpm-2013-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Romero R, Tarca AL, Chaemsaithong P, Miranda J, Chaiworapongsa T, Jia H, et al. Transcriptome interrogation of human myometrium identifies differentially expressed sense-antisense pairs of protein-coding and long non-coding RNA genes in spontaneous labor at term. J Matern Fetal Neonatal Med. 2014;27:1397–408. doi: 10.3109/14767058.2013.860963. [DOI] [PMC free article] [PubMed] [Google Scholar]