Abstract
Large alternative donor pools provide the potential for selecting a different donor for a second allogeneic transplant (alloBMT). As HLA disparity may contribute to the graft versus tumor effect, potentially utilizing new mismatched haplotype donors could improve the antitumor activity for relapsed hematologic malignancies despite a previous alloBMT. Data from patients who received a second alloBMT for relapsed hematologic malignancies at Johns Hopkins were analyzed. Outcomes were compared between patients who received a second allograft with the same MHC composition and those who received an allograft with a new mismatched haplotype. Loss of heterozygosity analysis was performed for patients with AML whose first allograft was haploidentical. Between 2005 and 2015, 40 patients received a second BMT for a relapsed hematologic malignancy. The median follow up is 750 (range 26–2950) days. The median OS in the cohort is 928 days (95% CI 602 – NR); median event-free survival (EFS) for the cohort is 500 days (95% CI 355-NR). The 4-year OS is 40% (95% CI 25–64%), and the 4-year EFS is 36% (95% CI 24–55%). The cumulative incidence of non-relapsed mortality NRM by 2 years was 27% (95% CI 13–42%). The cumulative incidence of grade 3–4 aGVHD at 100 days was 15% (95% CI 4–26); the cumulative incidence of extensive cGVHD at 2 years was 22% (95% CI 9–36%). The median survival was 552 days (95% CI 376–2950+) in the group transplanted with a second allograft that did not harbor a new mismatched haplotype, while it was not reached in the group whose allograft contained a new mismatched haplotype (HR 0.36, 95% CI 0.14 – 0.9; p=0.02). EFS was also longer in the group who received an allograft containing a new mismatched haplotype, (NR versus 401 days, HR 0.50, 95% CI .22–1.14, p=0.09). Although the allograft for this patient’s second BMT contained a new mismatched haplotype, AML nevertheless relapsed a second time. Second BMTs are feasible and provide a reasonable chance of long-term survival. An allograft with a new mismatched haplotype may improve outcomes after second BMTs for relapsed hematologic malignancies.
Keywords: haploidentical, post-transplant cyclophosphamide, second transplants, relapse, HLA loss
Introduction
Hematologic malignancies that recur after allogeneic blood or marrow transplantation (alloBMT) typically cannot be cured with chemotherapy or radiation1. Donor lymphocyte infusions can allow the allograft to reestablish disease control and provide long term survival, though with the exception of chronic myelogenous leukemia and post-transplant lymphoproliferative disease, response rates are low and toxicity is high2–8. Long term survival also has been reported after a second allogeneic BMT, as the allograft promotes a sustained graft versus tumor (GVT) effect9–16. Limitations to a second transplant include cumulative toxicities of prior therapy and conditioning, active opportunistic infections, biologically more aggressive disease, and graft-versus-host disease (GVHD)17. Earlier use of BMT, improvements in supportive care, and non-myeloablative conditioning strategies have led to better overall health in many patients who experience relapse. As a result, more patients are eligible to undergo second BMT.
Our group has been performing related HLA-haploidentical BMT (haploBMT) with high-dose post-transplantation cyclophosphamide (PTCy) for GVHD prophylaxis for many years18,19. The average patient has multiple potential related donors that are at least haploidentical20. There are several potential advantages for choosing a different donor when treating relapse with a second allogeneic BMT. Vago and colleagues reported a failure to detect host-specific HLA alleles on relapsed AML cells in five patients who had received haploidentical BMT21. These data suggested that major HLA mismatch provided an important anti-leukemic function. However, many groups have reported no difference when a different donor is used versus the same donor10,13,14,16,22,23, and at least one group reported that using the same donor results in improved outcomes24. These studies typically included very few or no haploidentical allografts. Thus, several years ago, our group decided to choose a different related donor, when possible, for second allogeneic BMT for relapse. We hypothesized that if loss of heterozygosity is a mechanism for relapse after haploBMT, then utilizing a donor allograft that recognized the recipient haplotype shared with the first donor as non-self may improve antitumor activity and thus overall survival (OS). Even in the absence of tumor haplotype loss, utilizing a donor with a different haplotype match might enhance an allogeneic antitumor effect by having a T cell repertoire with a greater potency or efficacy for specific tumor neoantigens. In the case of a failed HLA matched transplant, when haplotype loss would not be favored through selective pressure and thus would not provide a mechanism of relapse, switching to a different haploidentical donor may be beneficial by increasing major histocompatibility mismatch.
Methods
We queried the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins BMT database to identify cases with multiple allogeneic transplants between 2005 and 2014, after approval by the Johns Hopkins IRB for this retrospective review. Inclusion criteria included patients who underwent allogeneic BMT for relapsed hematologic malignancy. Relapse was diagnosed based on published consensus definitions for respective diseases. Second transplants performed for graft failure and those performed for benign diseases were excluded. Data was then collected from clinical charts. Outcomes were censored on 26 Oct 2016.
HLA data was obtained for all BMT recipients and their donors. Three patients received their first transplants at outside institutions and did not have precise HLA typing for their first donor available, but the degree of match could be inferred from clinical documentation. Donors were categorized for the purpose of analyses as matched or haploidentical in discrete categories; matched unrelated donor or umbilical cord grafts with one or two HLA allele mismatched were categorized as “matched”. GVHD scoring was determined by chart review. Acute GVHD is reported as Glucksberg grades 1–425. Several of the patients included were treated before the common adoption of the NIH consensus criteria26; therefore chronic GVHD is reported as limited or extensive.
Statistical methods
Characteristics of patients were summarized and compared by whether the patient received a second transplant with a new haplotype mismatch or not with Wilcoxon rank sum tests for continuous measures and Fisher's exact test for categorical measures. OS was calculated as the time from second transplant to death or last known follow-up. Event-free survival (EFS) was calculated as the time from second transplant to death, relapse, or last known follow-up, whichever came first. OS and EFS were estimated using the Kaplan Meier method. Differences in time to event outcomes between patient groups were estimated using Cox proportional hazards models. Time to relapse was calculated as the time from second transplant to relapse (event), death (competing event), or last known follow-up. Non-relapse mortality (NRM) was calculated as the time from second transplant to death not due to relapse (event), death due to relapse (competing event), or last known follow-up. Comparisons of time to relapse and time to NRM between patient groups were summarized with cumulative incidence estimates and proportional subdistribution hazards calculated using Fine and Gray`s method27.
Loss of heterozygosity analysis
Chimerism samples were obtained from the Johns Hopkins Genomics Laboratory that were banked at the time of chimerism detection. HLA typing was performed by HLA locus specific PCR amplification from genomic DNA followed by reverse sequence specific oligonucleotide probe (rSSOP) hybridization using the LABType® rSSO typing assay, One Lambda, Inc. (Part of Thermo Fisher Scientific).
Results
Patients and first transplant characteristics
Between 2005 and 2014, 40 consecutive patients underwent second allogeneic BMT for a relapsed hematologic malignancy (Table 1). The median age at second transplant was 43.9 (range 1 -to 74). Myeloablative conditioning was used for the first transplant in fifteen patients (38%), and 35 (88%) received (PTCy) as part of their GVHD prophylaxis. The cumulative probability of grade 2–4 aGVHD after the first transplant was 13% (95% CI 2–23%), and limited cGVHD was 13% (95% CI 2–23%). There was no extensive cGVHD. Nineteen patients received matched allografts for their first transplant, and 21 received haploidentical allografts.
Table 1.
Patient, disease, and first transplant characteristics.
| Subjects | 40 | |
|---|---|---|
| median age (range) | 43.9 (1–74) | |
| Female | 14 | |
| Disease type | ||
| AML | 12 | |
| ALL | 12 | |
| HL | 4 | |
| DLBCL | 2 | |
| MDS | 2 | |
| Biphenotypic acute leukemia | 2 | |
| CML | 2 | |
| CMML | 1 | |
| MM | 1 | |
| MCL | 1 | |
| FL | 1 | |
| First Transplant History | ||
| Conditioning Intensity | ||
| Myeloablative | 17 | |
| Reduced intensity | 3 | |
| Non-myeloablative | 20 | |
| Conditioning Regimens | ||
| Flu/Cy/TBI | 19 | |
| Bu/Cy | 11 | |
| Bu/Flu | 4 | |
| Cy/TT/TBI | 3 | |
| Cy/TBI | 2 | |
| Flu/TBI | 1 | |
| Allograft Match | ||
| Haploidentical | 21 | |
| MSD | 9 | |
| MUD | 7 | |
| Umbilical Cord | 2 | |
| MMUD (9/10) | 1 | |
| GVHD Prophylaxis | ||
| PTCy, Tacrolimus, MMF | 24 | |
| PTCy | 10 | |
| MTX, Tacrolimus | 4 | |
| PTCY, Tacrolimus, Sirolimus | 1 | |
| T cell depletion | 1 | |
| Incidence of GVHD* | ||
| Acute | ||
| none or grade 1 | 34 | |
| grades 2–4 | 5 | |
| grades 3–4 | 1 | |
| Chronic | ||
| none | 34 | |
| limited | 5 | |
| extensive | 0 | |
One patient, who received the first transplant at another institution, had GVHD of an unknown type and grade.
MSD – matched sibling donor; MUD – matched unrelated donor; MMUD – mismatched unrelated donor (9/10 HLA matched); PTCy – high-dose posttransplantation cyclophosphamide; MMF – mycophenolate mofetil.
Characteristics of second transplants
The characteristics of salvage therapy and the second transplants are presented in Table 2. Progressive disease was diagnosed before six months in nine patients (23%) and before one year in twenty patients (50%). Complete remission or responsive disease was achieved in 80% of patients prior to receipt of a second allograft. Second transplants were performed a median of 568 (range 146–2335) days from the first BMT.
Table 2.
Second transplant characteristics.
| Characteristic | n | |
|---|---|---|
| Lines of re-induction therapy | ||
| none | 3 | |
| 1 | 18 | |
| 2 | 15 | |
| ≥3 | 4 | |
| Disease state pre-transplant | ||
| CR | 23 | |
| Responsive | 8 | |
| Refractory or PD | 9 | |
| Match of second donor | ||
| Haploidentical | 28 | |
| Full match | 10 | |
| Umbilical Cord | 2 | |
| Same donor first transplant | ||
| Yes | 4 | |
| No | 36 | |
| Conditioning regimens# | ||
| Flu/Cy/TBI | 27 | |
| Bu/Cy | 5 | |
| Cy/TBI | 3 | |
| Flu/Bu | 2 | |
| Flu/Mel | 1 | |
| Mel/TBI | 1 | |
| Bu/Cy/ATG | 1 | |
| GVHD prophylaxis | ||
| PTCy, MMF, Tacrolimus | 30 | |
| MTX/CSA | 4 | |
| Tacrolimus, MMF | 3 | |
| PTCy | 2 | |
| PTCy, CSA, MMF | 1 | |
PTCy – posttransplantation cyclophosphamide; MMF – mycophenolate mofetil.
For umbilical cord grafts (2 total), one patient received Bu/Cy conditioning, and one received Bu/Cy/ATG.
The same donor was selected in four (10%) second transplants. The source of the graft was bone marrow in 23 (58%), peripheral blood stem cells in 15 (38%), and umbilical cord blood in 2 (5%) cases. Most patients received reduced intensity conditioning regimen consisting of fludarabine, cyclophosphamide, and total body irradiation (Table 2). GVHD prophylaxis included PTCy in 83% of cases.
Outcomes, including GVHD
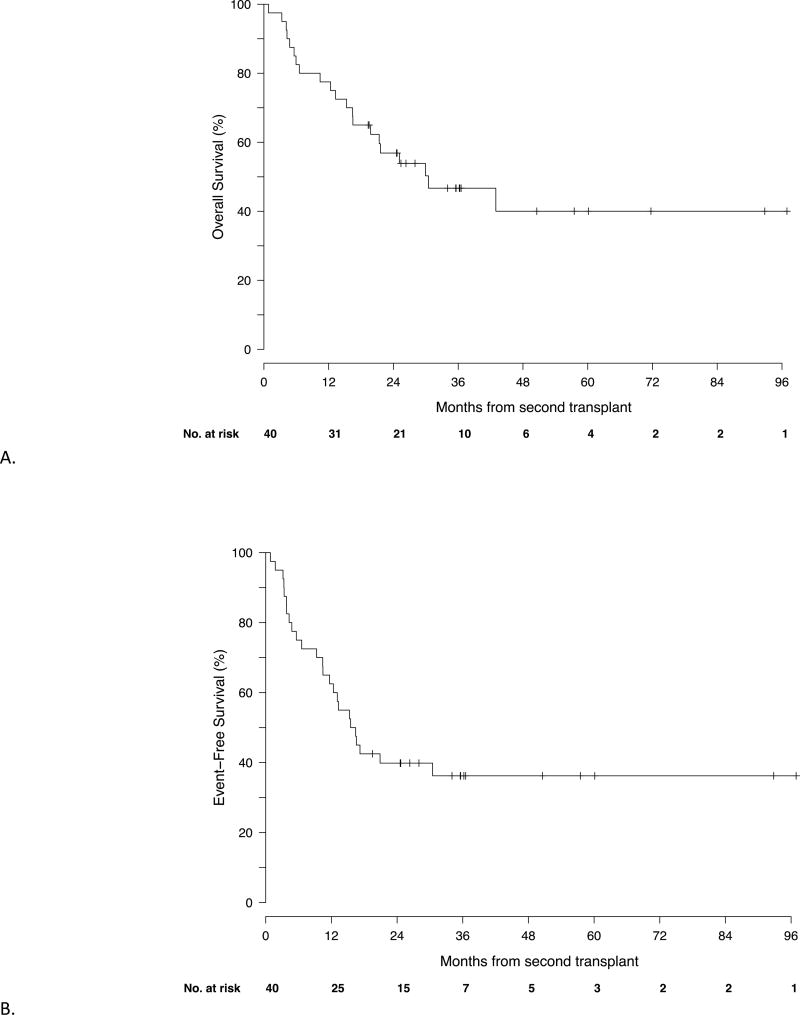
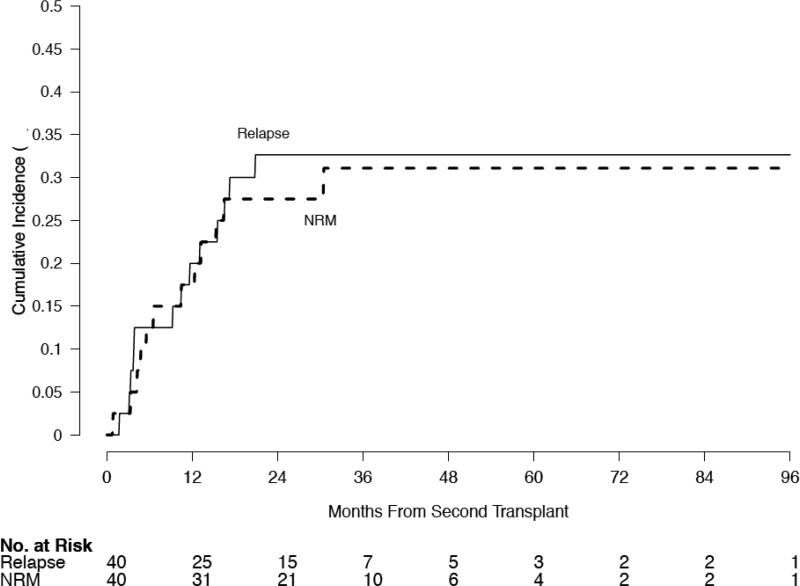
The median follow up is 750 (range 26–2950) days. The median OS in the cohort is 928 days (95% CI 602 – NR); median EFS for the cohort is 500 days (95% CI 355-NR) (Figure 1). The 4 year OS is 40% (95% CI 25–64%), and the 4-year EFS is 36% (95% CI 24–55%). The cumulative incidence of NRM by 2 years was 27% (95% CI 13–42%). The cumulative incidence of grade 3–4 aGVHD at 100 days was 15% (95% CI 4–26); the cumulative incidence of extensive cGVHD at 2 years was 22% (95% CI 9–36%). There was no relationship between the development of acute GVHD and overall survival (data not shown). The cumulative incidences of NRM and relapse are shown in Figure 2. The causes of non-relapse mortality are detailed in Table 3.
Figure 1.
OS (A) and EFS (B). The median OS for the cohort is 928 days (95% CI 602 – NR); median EFS for the cohort is 500 days (95% CI 355-NR).
Figure 2.
Cumulative incidence of relapse at 2 years was 33% (95% CI 18–48%), and the cumulative incidence of NRM by 2 years was 27% (95% CI 13–42%) for the cohort
Table 3.
Causes of non-relapse mortality
| Patient | Disease | 2nd Transplant |
Day of Death |
Cause of Death |
|---|---|---|---|---|
| 1 yo/M | Bi-lineage leukemia | MAC MUD | +130 | PJP pneumonia while receiving high dose corticosteroids for GVHD |
| 9 yo/M | AML | MAC MUD | +101 | pneumonia |
| 12 yo/M | AML | MAC MUD | +404 | GVHD |
| 14 yo M | B-ALL | NMA MUD | +317 | GVHD |
| 32 yo F | NS HL | NMA haplo | +200 | GVHD |
| 36 yo F | AML | NMA haplo | +26 | VOD, Multi-organ failure |
| 45 yo M | MCL | MAC MUD | +145 | graft failure/fungal pneumonia |
| 51 yo M | Follicular lymphoma | NMA haplo | +170 | graft failure/fungal sinusitis |
| 54 yo M | Ph+ B-ALL | NMA haplo | +500 | idiopathic pneumonitis |
| 64 yo M | CMML | NMA haplo | +928 | lung cancer |
| 66 yo F | Ph+ B-ALL | NMA haplo | +376 | Intracranial hemorrhage after a fall |
| 74 yo M | T-PLL | NMA haplo | +466 | pneumonia |
MAC: Myeloablative conditioning; NMA: non-myeloablative; MUD: matched unrelated donor; haplo: haploidentical related donor; GVHD: graft versus host disease; VOD: vasooclusive disease of the liver
HLA matching and outcomes
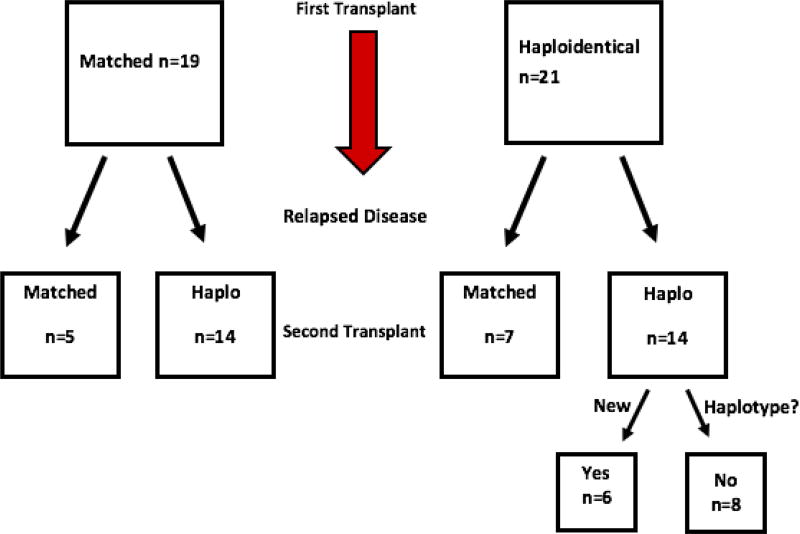
Twenty-one patients received their first allograft from a haploidentical donor. Of those, 14 received a second haploidentical transplant: 8 from a donor sharing the same shared haplotype as the first donor, and 6 from a second donor sharing the other haplotype. Nineteen patients received their first allograft from a fully matched donor. Of those, 14 received a haploidentical allograft at the second transplant, and 5 received a second matched allograft. Thus, 20 of the patients had a new mismatched haplotype in the second allograft: 14 who received a haploidentical donor allograft after a first matched one, and 6 whose second haploidentical donor shared the haplotype that was mismatched in the first transplant. The other 20 second donors were from a second donor that did not harbor a new mismatched haplotype: 10 whose second donor was HLA-matched, 8 sharing the same haplotype as the first donor, and 2 unrelated umbilical cord grafts (Figure 3). There was no significant difference regarding age, gender, disease status, time from first transplant to relapse, lines of therapy, or incidence of acute or chronic GVHD from the first transplant between the groups (Table 4).
Figure 3.
Matching pathway
Table 4.
Characteristics of patients, by receipt of a new mismatched haplotype.
| No New Haplotype (n=20) |
New Mismatched Haplotype (n=20) |
P | ||
|---|---|---|---|---|
| Age – mean (SD) | 38.3 (20.9) | 43.1 (19.8) | 0.46 | |
| Sex – no. (%) | ||||
| Female | 6 (30) | 8 (40) | 0.74 | |
| Male | 14 (70) | 12 (60) | ||
| Disease status– no. (%) | ||||
| Refractory or Progressive | 6 (30) | 2 (10) | 0.24 | |
| Responsive or in Remission | 14 (70) | 18 (90) | ||
| Relapsed < 6 months - no. (%) | ||||
| Yes | 4 (20) | 5 (25) | >0.99 | |
| No | 16 (80) | 15 (75) | ||
| aGVHD Grade 2–4, 1st Transplant - no. (%) | ||||
| No | 14 (70) | 13 (65) | >0.99 | |
| Yes | 6 (30) | 7 (35) | ||
| cGVHD, 1st Transplant - no. (%) | ||||
| No | 16 (80) | 15 (75) | >0.99 | |
| Yes | 4 (20) | 5 (25) | ||
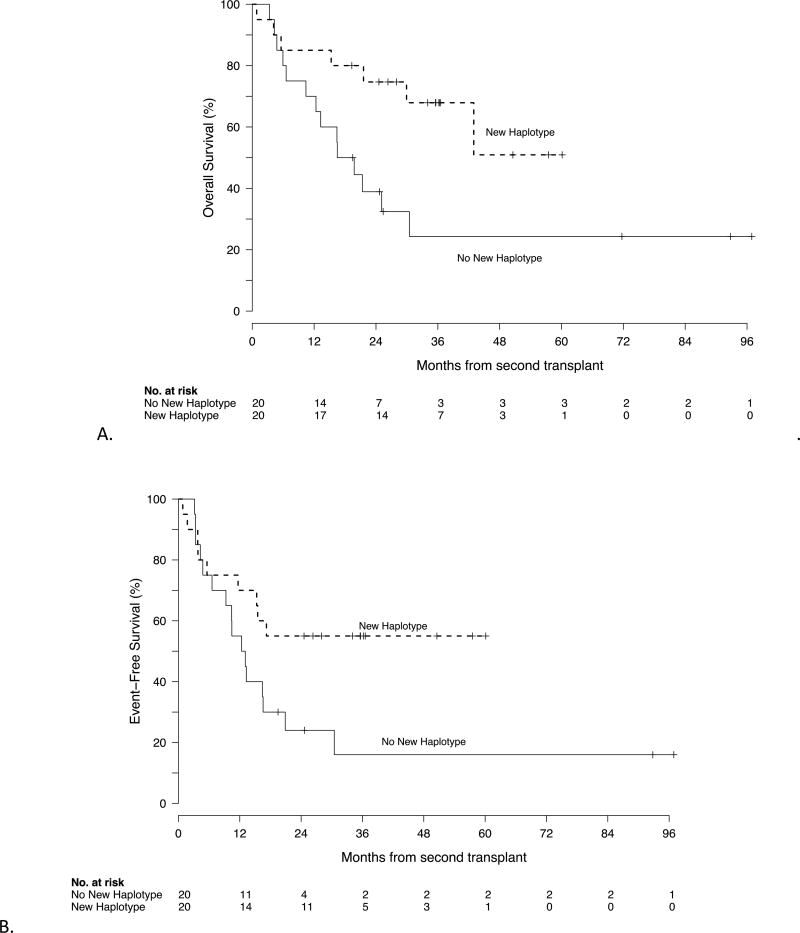
The median survival was 552 days (95% CI 376–2950+) in the group transplanted with a second graft that did not harbor a new mismatched haplotype, while it was not reached in the group whose allograft contained a new mismatched haplotype (HR 0.36, 95% CI 0.14 – 0.9; p=0.02) (figure 4a). Moreover, the same trend held when just looking at the patients who received two haploidentical allografts. The median OS for the 6 patients who were retransplanted with an HLA-haploidentical donor sharing the different haplotype from the first haploidentical donor has not been reached, while the median OS was 502 days (95% CI 317–2950+) among the 8 whose second haplo allograft shared the same haplotype (HR 0.37, 95% CI 0.08–1.7, p=0.16). Median EFS was also longer in the group that received an allograft containing a new mismatched haplotype, (NR versus 401 days, HR 0.50, 95% CI .22–1.14, p=0.09) (figure 4b). We analyzed the effect of choosing a second haploidentical donor. Compared to other graft sources with an OS of 404 days, patients with a haploidentical donor for second BMT had a median overall survival of 1308 days (HR 0.42, 95% CI 0.18–1.0, p=0.05).
Figure 4.
OS (A) and EFS (B) by exposure to new haplotype. The median survival was 552 days (95% CI 376–2950+) in the group transplanted with a second graft that did not harbor a new mismatched haplotype, while it was not reached in the group whose allograft contained a new mismatched haplotype (HR 0.36, 95% CI 0.14 – 0.9; p=0.02). The median event free survival was also longer in the group who received an allograft containing a new mismatched haplotype (NR versus 401 days, HR 0.50, 95% CI .22–1.14, p=0.09)
Univariate predictors of outcome
Univariate predictors of outcome are listed in table 5. Increased risk of mortality was observed when relapse was diagnosed within 6 months of the first transplant (HR 2.49 [1, 6.2]; p=0.07) and with progressive or refractory disease before the start of the second alloBMT(HR 2.56 [1.03 – 6.25]; p=0.06). These effects were mirrored in EFS: relapse within 6 months (HR 2.53 [1.03–6.22]; p=0.06); refractory or progressive disease HR 2.44 (1.0–5.88, p=0.07). There was one long-term survivor among the eight patients who were neither in remission nor had responsive disease at the time of second transplant. This patient had therapy related myeloid neoplasm that persisted after azacitidine and FLAM induction, though blasts were less than 5% at the time of transplant. Acute GVHD diagnosed during the first transplant was associated with an increased risk of NRM (HR 3.44 [1.19–9.94]; p=0.02).
Table 5.
Outcomes of patients receiving second transplant by patient groups
| OS | HR | 95% CI | P | |||
|---|---|---|---|---|---|---|
| Same donor as first | (v. different donor) | 2.31 | 0.77 | 6.92 | 0.16 | |
| Performed 2005–2009 | (v. 2010–2015) | 1.82 | 0.65 | 5.0 | 0.28 | |
| Age > 45 | (v. <45) | 1.8 | 0.73 | 4.42 | 0.19 | |
| Refractory or Progressive Disease | (v. CR or PR) | 2.56 | 1.03 | 6.25 | 0.06 | |
| Relapse diagnosed within 6 months | (v. > 6 months) | 2.49 | 1.0 | 6.2 | 0.07 | |
| Acute GVHD from first transplant | (v. no GVHD) | 2.12 | 0.87 | 5.17 | 0.11 | |
| Second graft contained new haplotype | (v. no new haplotype) | 0.36 | 0.14 | 0.9 | 0.02 | |
| EFS | ||||||
| Same donor as first | (v. different donor) | 1.54 | 0.52 | 4.5 | 0.46 | |
| Performed 2005–2009 | (v. 2010–2015) | 1.89 | 0.2 | 1.44 | 0.25 | |
| Age > 45 | (v. <45) | 2.32 | 0.99 | 5.44 | 0.05 | |
| Refractory or Progressive Disease | (v. CR or PR) | 2.44 | 1 | 5.88 | 0.07 | |
| Relapse diagnosed within 6 months | (v. > 6 months) | 2.53 | 1.03 | 6.22 | 0.06 | |
| Acute GVHD from first transplant | (v. no GVHD) | 1.50 | 0.65 | 3.44 | 0.35 | |
| Second graft contained new haplotype | (v. no new haplotype) | 0.50 | 0.22 | 1.14 | 0.09 | |
| Cumulative Incidence of Relapse | ||||||
| Same donor as first | (v. different donor) | 0.76 | 0.13 | 4.51 | 0.77 | |
| Performed 2005–2009 | (v. 2010–2015) | 1.11 | 0.23 | 5.26 | 0.89 | |
| Age > 45 | (v. <45) | 5.29 | 1.13 | 24.74 | 0.03 | |
| Refractory or Progressive Disease | (v. CR or PR) | 1.67 | 0.45 | 6.25 | 0.44 | |
| Relapse diagnosed within 6 months | (v. > 6 months) | 1.43 | 0.38 | 5.41 | 0.59 | |
| Acute GVHD from first transplant | (v. no GVHD) | 0.44 | 0.1 | 1.87 | 0.27 | |
| Second graft contained new haplotype | (v. no new haplotype) | 0.81 | 0.25 | 2.59 | 0.73 | |
| Cumulative Incidence of Non-Relapse Mortality | ||||||
| Same donor as first | (v. different donor) | 2.62 | 1.02 | 6.71 | 0.05 | |
| Performed 2005–2009 | (v. 2010–2015) | 1.79 | 0.44 | 7.14 | 0.42 | |
| Age > 45 | (v. <45) | 1.09 | 0.38 | 3.18 | 0.87 | |
| Refractory or Progressive Disease | (v. CR or PR) | 2.08 | 0.66 | 6.67 | 0.21 | |
| Relapse diagnosed within 6 months | (v. > 6 months) | 2.09 | 0.61 | 7.2 | 0.24 | |
| Acute GVHD from first transplant | (v. no GVHD) | 3.44 | 1.19 | 9.94 | 0.02 | |
| Second graft contained new haplotype | (v. no new haplotype) | 0.4 | 0.12 | 1.32 | 0.13 | |
Loss of Heterozygosity
Of the twelve patients who received second transplants for AML, five had sufficient tissue from specimens with relapsed disease after their first BMT to test for loss of heterozygosity. Of these five, one patient had detectable loss of heterozygosity (this case was reported previously28). This patient received a second allograft that shared the other haplotype, but nevertheless experienced relapse 524 days after their second transplant.
Discussion
Approximately a quarter of patients relapsing after allogeneic BMT can achieve long-term disease free survival with a second alloBMT10,16,29,30. Most published experience with second alloBMT have reported results using the same HLA-identical donor as the first; moreover, a donor change, usually with a different HLA-matched related or unrelated donor, has not appeared to improve results10,13,14,16,22,23. Our single institution retrospective analysis includes the largest cohort of second haploidentical transplants with PTCy published to date. Our overall results with second allografts are similar to the published experience in other second BMT series: we confirmed the dismal prognosis for those experiencing early relapse; and a graft versus tumor effect did not control progressive disease. Thus, every effort to deliver effective salvage therapy should be made, even if it takes multiple lines of therapy. GVHD rates using PTCy are low and in keeping with the rates seen with first transplants. Acute GVHD observed during the first transplant predicted an increased risk of NRM after second allogeneic BMT, either because of exposure to increased immunosuppressive medication or a predisposition to a GVH reaction to the second allograft.
Although our numbers are small, there appeared to be an advantage to selecting a donor with a new mismatched haplotype, in contrast to previous publications. Confidence intervals are too wide to attribute the beneficial effect to decreased relapse (and therefore a more potent graft versus tumor effect) or decreased NRM. Although the present study was neither prospective nor randomized, systemic bias between patients who have a related haploidentical donor and those who do not is unlikely, as we have not used degree of match as a primary factor in donor choice31. However, patient heterogeneity or statistical chance could play a role in the apparent difference especially in a small, retrospective analysis.
Haplotype loss can be seen in relapsed AML after haploBMT, but it is unlikely to explain why switching to a donor sharing the different haplotype appeared to improve outcome. Not only was haplotype loss after first haplo BMT not common in our series, but the benefit was also seen with second haploBMTs after first HLA-matched allografts. The frequency of T cells specific for a particular tumor neoantigen has been estimated at 1 in 50,000 T cells in peripheral blood, whereas the proportion of T cells reactive against mismatched HLA is as high as 1 in 20.32 Alternatively, there may be other biologic differences between different donors, such as KIR mismatches33, that make switching from the unsuccessful donor attractive.
In summary, second allogeneic haploBMT is a feasible strategy for patients relapsing after their first transplant. In particular, using a second haploidentical donor after failure of a HLA-matched allograft, or sharing a different haplotype in the case of relapse after haploBMT, may be beneficial. For patients who relapse more than 6 months after a bone marrow transplant and who achieve remission with salvage therapy, results are very encouraging. Although a randomized trial to study selecting donors with a new mismatched haplotype may be impractical, larger numbers should provide additional information on the effectiveness of such an approach.
Highlights.
Second BMTs are feasible and provide a reasonable chance of long-term survival.
Allografts with a new mismatched haplotype may improve outcomes after second BMTs for relapsed hematologic malignancies.
Major histocompatibility may be an important driver of the graft versus tumor effect.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Sources Cited
- 1.de Lima M, Porter DL, Battiwalla M, et al. Prevention and treatment of relapse after allogeneic transplantation. Elsevier; 2014. Proceedings from the National Cancer Institute's Second International Workshop on the Biology, Prevention, and Treatment of Relapse After Hematopoietic Stem Cell Transplantation: part III; pp. 4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huff CA, Fuchs EJ, Smith BD, et al. Graft-versus-host reactions and the effectiveness of donor lymphocyte infusions. Biol Blood Marrow Transplant. 2006;12(4):414–21. doi: 10.1016/j.bbmt.2005.11.520. [DOI] [PubMed] [Google Scholar]
- 3.Collins RH, Shpilberg O, Drobyski WR, et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. Journal of Clinical Oncology. 1997;15(2):433–44. doi: 10.1200/JCO.1997.15.2.433. [DOI] [PubMed] [Google Scholar]
- 4.Shimoni A, Gajewski JA, Donato M, et al. Long-Term follow-up of recipients of CD8-depleted donor lymphocyte infusions for the treatment of chronic myelogenous leukemia relapsing after allogeneic progenitor cell transplantation. Biol Blood Marrow Transplant. 2001;7(10):568–75. doi: 10.1016/s1083-8791(01)70017-1. [DOI] [PubMed] [Google Scholar]
- 5.Alyea EP, Canning C, Neuberg D, et al. CD8+ cell depletion of donor lymphocyte infusions using cd8 monoclonal antibody-coated high-density microparticles (CD8-HDM) after allogeneic hematopoietic stem cell transplantation: a pilot study. Bone Marrow Transplant. 2004;34(2):123–8. doi: 10.1038/sj.bmt.1704536. [DOI] [PubMed] [Google Scholar]
- 6.Kolb HJ. Donor leukocyte transfusions for treatment of leukemic relapse after bone marrow transplantation. EBMT Immunology and Chronic Leukemia Working Parties. Vox Sang. 1998;74(Suppl 2):321–9. doi: 10.1111/j.1423-0410.1998.tb05438.x. [DOI] [PubMed] [Google Scholar]
- 7.Zeidan AM, Forde PM, Symons H, et al. HLA-haploidentical donor lymphocyte infusions for patients with relapsed hematologic malignancies after related HLA-haploidentical bone marrow transplantation. Biol Blood Marrow Transplant. 2014;20(3):314–8. doi: 10.1016/j.bbmt.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papadopoulos EB, Ladanyi M, Emanuel D, et al. Infusions of donor leukocytes to treat Epstein-Barr virus-associated lymphoproliferative disorders after allogeneic bone marrow transplantation. N Engl J Med. 1994;330(17):1185–91. doi: 10.1056/NEJM199404283301703. [DOI] [PubMed] [Google Scholar]
- 9.Andreola G, Labopin M, Beelen D, et al. Long-term outcome and prognostic factors of second allogeneic hematopoietic stem cell transplant for acute leukemia in patients with a median follow-up of 10 years. Bone Marrow Transplant. 2015;50(12):1508–12. doi: 10.1038/bmt.2015.193. [DOI] [PubMed] [Google Scholar]
- 10.Ruutu T, de Wreede LC, van Biezen A, et al. Second allogeneic transplantation for relapse of malignant disease: retrospective analysis of outcome and predictive factors by the EBMT. Bone Marrow Transplant. 2015;50(12):1542–50. doi: 10.1038/bmt.2015.186. [DOI] [PubMed] [Google Scholar]
- 11.Vrhovac R, Labopin M, Ciceri F, et al. Second reduced intensity conditioning allogeneic transplant as a rescue strategy for acute leukaemia patients who relapse after an initial RIC allogeneic transplantation: analysis of risk factors and treatment outcomes. Bone Marrow Transplant. 2016;51(2):186–93. doi: 10.1038/bmt.2015.221. [DOI] [PubMed] [Google Scholar]
- 12.Michallet M, Tanguy ML, Socié G, et al. Second allogeneic haematopoietic stem cell transplantation in relapsed acute and chronic leukaemias for patients who underwent a first allogeneic bone marrow transplantation: a survey of the Société Française de Greffe de moelle (SFGM) Br J Haematol. 2000;108(2):400–7. doi: 10.1046/j.1365-2141.2000.01851.x. [DOI] [PubMed] [Google Scholar]
- 13.Christopeit M, Kuss O, Finke J, et al. Second allograft for hematologic relapse of acute leukemia after first allogeneic stem-cell transplantation from related and unrelated donors: the role of donor change. J Clin Oncol. 2013;31(26):3259–71. doi: 10.1200/JCO.2012.44.7961. [DOI] [PubMed] [Google Scholar]
- 14.Shaw BE, Mufti GJ, Mackinnon S, et al. Outcome of second allogeneic transplants using reduced-intensity conditioning following relapse of haematological malignancy after an initial allogeneic transplant. Bone Marrow Transplant. 2008;42(12):783–9. doi: 10.1038/bmt.2008.255. [DOI] [PubMed] [Google Scholar]
- 15.Orti G, Sanz J, Bermudez A, et al. Outcome of Second Allogeneic Hematopoietic Cell Transplantation after Relapse of Myeloid Malignancies following Allogeneic Hematopoietic Cell Transplantation: A Retrospective Cohort on Behalf of the Grupo Español de Trasplante Hematopoyetico. Biol Blood Marrow Transplant. 2016;22(3):584–8. doi: 10.1016/j.bbmt.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Eapen M, Giralt SA, Horowitz MM, et al. Second transplant for acute and chronic leukemia relapsing after first HLA-identical sibling transplant. Bone Marrow Transplant. 2004;34(8):721–7. doi: 10.1038/sj.bmt.1704645. [DOI] [PubMed] [Google Scholar]
- 17.Mielcarek M, Storer BE, Flowers MED, Storb R, Sandmaier BM, Martin PJ. Outcomes among patients with recurrent high-risk hematologic malignancies after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2007;13(10):1160–8. doi: 10.1016/j.bbmt.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 18.O'Donnell PV, Luznik L, Jones RJ, et al. Nonmyeloablative bone marrow transplantation from partially HLA-mismatched related donors using posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2002;8(7):377–86. doi: 10.1053/bbmt.2002.v8.pm12171484. [DOI] [PubMed] [Google Scholar]
- 19.Luznik L, O'Donnell PV, Symons HJ, et al. HLA-Haploidentical Bone Marrow Transplantation for Hematologic Malignancies Using Nonmyeloablative Conditioning and High-Dose, Posttransplantation Cyclophosphamide. Biology of Blood and Marrow Transplantation. 2008;14(6):641–50. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gladstone DE, Zachary AA, Fuchs EJ, et al. Desensitization for Mismatched Hematopoietic Stem Cell Transplantation (HSCT) Blood. 118(21) [Google Scholar]
- 21.Vago L, Perna SK, Zanussi M, et al. Loss of mismatched HLA in leukemia after stem-cell transplantation. N Engl J Med. 2009;361(5):478–88. doi: 10.1056/NEJMoa0811036. [DOI] [PubMed] [Google Scholar]
- 22.Savani BN, Mielke S, Reddy N, Goodman S, Jagasia M, Rezvani K. Management of relapse after allo- SCT for AML and the role of second transplantation. Bone Marrow Transplant. 2009;44(12):769–77. doi: 10.1038/bmt.2009.300. [DOI] [PubMed] [Google Scholar]
- 23.Guardiola P, Kuentz M, Garban F, et al. Second early allogeneic stem cell transplantations for graft failure in acute leukaemia, chronic myeloid leukaemia and aplastic anaemia. French Society of Bone Marrow Transplantation. Br J Haematol. 2000;111(1):292–302. doi: 10.1046/j.1365-2141.2000.02306.x. [DOI] [PubMed] [Google Scholar]
- 24.Menon NN, Jenkins LM, Cui H, et al. Factors associated with improved outcomes after second allogeneic hematopoietic cell transplantation for relapsed pediatric leukemia. Ann Hematol. 2016;95(4):637–44. doi: 10.1007/s00277-016-2599-9. [DOI] [PubMed] [Google Scholar]
- 25.Glucksberg H, Storb R, Fefer A, et al. CLINICAL MANIFESTATIONS OF GRAFT-VERSUS-HOST DISEASE IN HUMAN RECIPIENTS OF MARROW FROM HL-A-MATCHED SIBLING DONOR,S. Transplantation. 1974;18(4):295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–56. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94(446):496. [Google Scholar]
- 28.McCurdy SR, Iglehart BS, Batista DA, et al. Loss of the mismatched human leukocyte antigen haplotype in two acute myelogenous leukemia relapses after haploidentical bone marrow transplantation with post-transplantation cyclophosphamide. Leukemia. 2016;30(10):2102–6. doi: 10.1038/leu.2016.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weisdorf D. The role of second transplants for leukemia. Best Pract Res Clin Haematol. 2016;29(4):359–64. doi: 10.1016/j.beha.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bejanyan N, Weisdorf DJ, Logan BR, et al. Survival of patients with acute myeloid leukemia relapsing after allogeneic hematopoietic cell transplantation: a center for international blood and marrow transplant research study. Biol Blood Marrow Transplant. 2015;21(3):454–9. doi: 10.1016/j.bbmt.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanakry JA, Gocke CD, Bolaños-Meade J, et al. Phase II Study of Nonmyeloablative Allogeneic Bone Marrow Transplantation for B Cell Lymphoma with Post-Transplantation Rituximab and Donor Selection Based First on Non-HLA Factors. Biol Blood Marrow Transplant. 2015;21(12):2115–22. doi: 10.1016/j.bbmt.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Distler E, Bloetz A, Albrecht J, et al. Alloreactive and leukemia-reactive T cells are preferentially derived from naive precursors in healthy donors: implications for immunotherapy with memory T cells. Haematologica. 2011;96(7):1024–32. doi: 10.3324/haematol.2010.037481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaffer BC, Hsu KC. How important is NK alloreactivity and KIR in allogeneic transplantation? Best Pract Res Clin Haematol. 2016;29(4):351–8. doi: 10.1016/j.beha.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]