Sleep and sleep-like states have been identified in all mammalian species, other vertebrates such as birds,1–4 and invertebrates (e.g. fruit fly or Drosophila melanogaster)5. The key features of sleep are that it is a reversible quiescent state, during which there is decreased sensitivity to environmental stimuli, and is homeostatically regulated. Conservation of sleep during evolution points to its importance in the survival of all species. Indeed, experimental sleep deprivation leads to death.6
The lowest morbidity/mortality for human beings occurs with sleep duration ranges from 7 to 9 hours per 24-hour period7–11 (Figure 1). “Sleep deficiency” includes: 1) sleep deprivation or insomnia; 2) sleep fragmentation due to abnormal breathing disorders or periodic leg movements; and 3) sleep circadian misalignment caused by shift work or other rhythm-related disorders.11
Figure 1. Mortality hazard ratios according to sleep duration for men and women.
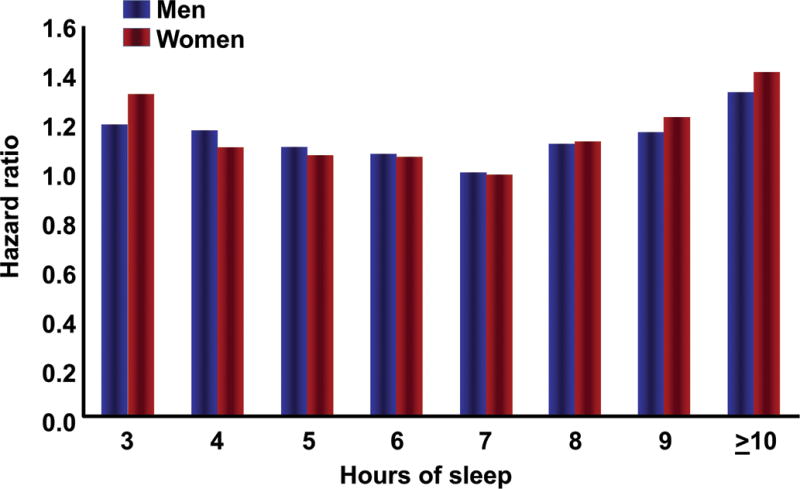
Data collected from 636,095 men and 480,841 women in the Cancer Prevention Study II (1982-1988). Both men and women with usual sleep duration of 7 hours had the best survival. Participants reporting sleep durations ≤ 6 hours and ≥ 8 hours had significantly increased mortality hazard.7 Reproduced, with permission, from Luyster et al.11
We spend one-third of our life sleeping;11–14 yet, fundamental questions as to the function of sleep and its regulation remain unanswered. Despite evidence that pregnancy is associated with sleep disturbances,15–23 the assessment of sleep quality and quantity during pregnancy and its consequences has not received the attention it deserves. Indeed, assessment of sleep has not been an integral part of prenatal care, even though many sleep disorders that affect pregnant women (e.g. obstructive apnea) are amenable to treatment, and therapy can have benefits.24–28
Evidence has emerged suggesting that sleep disorders may be associated with complications of pregnancy,22,29 such as gestational diabetes,21,22,30–33 gestational hypertension,22,31,34–39 preeclampsia,22,31,34,37,39–48 fetal growth disorders,22,39,42,49,50 preterm birth,32,47,49 and stillbirth.51 Animal studies indicate that maternal sleep deprivation may have long-term effects in the offspring.52,53
Two systematic reviews and meta-analyses (one of which is published in this issue of the Journal54 provide evidence of an association between sleep-disordered breathing and adverse pregnancy outcome.54,55 Moreover, recent ground-breaking studies about the effect of sleep on the brain have made the subject topical.
The purpose of sleep
Sleep is thought to be essential to clear the brain of metabolic waste products, which accumulate during wakefulness.56 All tissues produce metabolic waste during the course of normal activity – in the brain, this has particular significance. Neurons produce β-amyloid, which is excreted into the extracellular space.56,57 When this protein accumulates, it undergoes structural changes and can result in protofibrils and fibrils, which confer local toxicity.58,59 The sleep-wake state plays a key role in the clearance of β-amyloid sleep-deprivation results in accumulation of amyloid, and chronic sleep restriction leads to the formation of an amyloid plaque that is characteristic of Alzheimer’s disease.60,61
A fundamental question is how this clearance takes place. The brain was thought not to have a lymphatic system; hence, the question of how the organ disposed of metabolic waste.62 The answer came with the discovery of a draining system called “glymphatics,”56 considered by Maiken Nedergaard to be the “garbage truck of the brain” (Figure 2).62 The breakthrough is that, during natural sleep or anesthesia, there was a 60% increase in the interstitial space, and increased clearance of β-amyloid; hence, the proposal that the purpose of sleep is to remove neurotoxic waste products formed while we are awake (Figure 3).63 The potential implications are major: sleep deprivation is a risk factor for neurodegenerative diseases.64–66
Figure 2. Pathway to clear cellular waste from the brain using the glymphatic system.
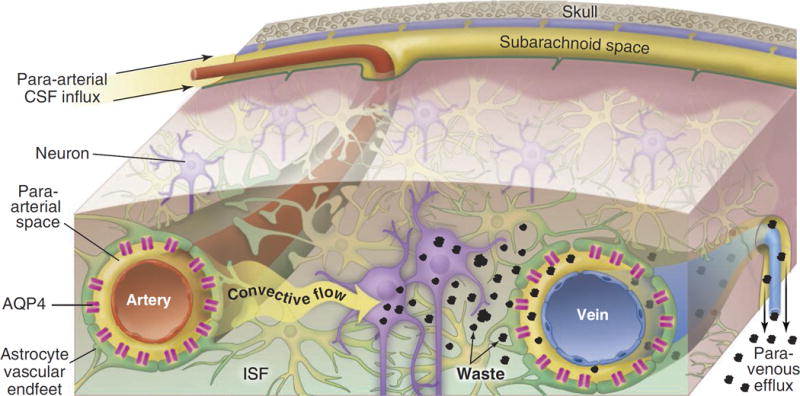
Cerebrospinal fluid (CSF) passes through the para-arterial space surrounding the arteries (top part of the Figure). This space is bound by the non-luminal surface of the blood vessel and the apical processes of astrocytes. Aquaporin-4 (AQP4) is a water channel that facilitates convective flow out of the para-arterial space into the interstitial space. CSF exchanges with interstitial fluid (ISF) and generates a convective flow that clears the waste using a paravenous route. Reproduced with permission from the American Association for the Advancement of Science, from Nedergaard.62
Figure 3. Cerebral spinal fluid influx in a murine model.
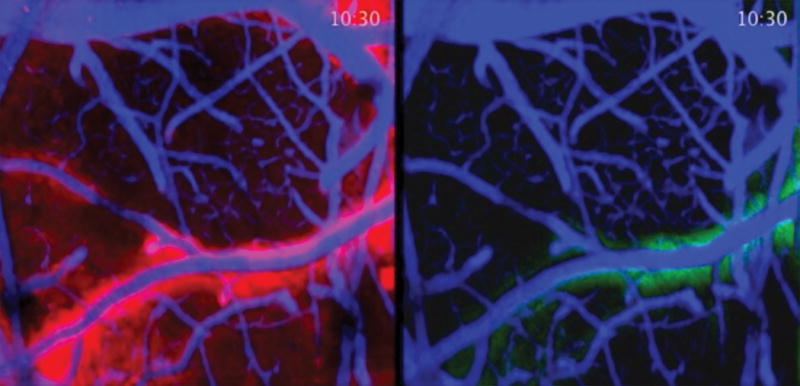
Difference of cerebral spinal fluid influx in the brain of a sleeping (left) and awake (right) mouse. Fluorescent dye was injected to enable the study of cerebral spinal fluid dynamics in a live mouse using 2-photon imaging. Red represents greater flow in a sleeping animal. Green indicates decreased flow in the awake animal. Images provided by Drs. Maiken Nedergaard and Lulu Xie of the Division of Glial Disease and Therapeutics, Center for Translational Medicine, Department of Neurosurgery, University of Rochester Medical Center. The work was funded by the National Institutes of Health/National Institute of Neurological Disorders and Stroke.
Changes in the patterns of sleep in society: sleep debt
The mean duration of sleep has changed over the last century: in 1910, it was 9 hours; in 1975, 7.5 hours; and in 2000, 6.9 hours.67,68 Occupational and social demands of modern life have been implicated. Although people in many developed countries (except those committed to napping as a cultural practice) believe that one must sleep for 8 consecutive hours, there is experimental and historical evidence that the current habit reflects an adaptation. In 1992, Thomas Wehr69 reported an experiment in which people were placed into darkness for 14 hours every day for a month. By the fourth week, people developed a pattern of sleep characterized by a “first sleep” for 4 hours, then waking for 1-2 hours, and subsequently had a “second sleep” for 4 hours.69 This biphasic pattern is considered the natural tendency for humans, and is consistent with the historical record.70,71 Prior to the availability of artificial light, the hours after the “first sleep” were not spent in solitude, but were often social events. The initial impetus to shift to a sleep pattern of 8 consecutive hours starting later in the evening is attributed to street and domestic lighting, which extended the period of wakefulness.
Sleep during pregnancy
Observational studies based on self-reports, as well as a limited number of polysomnography studies, have documented a high number of sleep disturbances during pregnancy, including snoring,19,31,44,46,72–75 non-refreshing sleep,76 insomnia,73,77 periodic leg movement during sleep,19,72,73,78,79 and sleep-disordered breathing.80,81 A critical appraisal of the evidence is beyond the scope of this article – the interested reader is referred to the original sources.
Sleep and pregnancy: two challenges to the respiratory system
Sleep is a rest period for most organ systems, including musculoskeletal, gastrointestinal, cardiovascular.14,82–84 In contrast, sleep challenges the respiratory system in multiple ways. For example, during sleep, the upper airways become narrow and lung volume smaller.82–84 These changes are more pronounced during rapid eye movement, when there is decreased muscular tone.
Pregnancy is a physiologic challenge to virtually every organ system (e.g., cardiovascular, metabolic, immune) and, in particular, the respiratory system.85 The upper airways narrow due to vascular congestion, mucosal edema, and decreased lung volume.20,44,74,75,86,87 Elevation of the diaphragm, especially in the third trimester, leads to a reduction in functional residual capacity.20,75,85 Thus, the combination of sleep and pregnancy represents a magnified “stress test” to the respiratory system, which can unmask the propensity to develop “sleep-disordered breathing.” This term encompasses obstructive sleep apnea, periodic episodes of hypoxia, central apnea, and sleep hypopnea. Although the mechanisms of disease, consequences, and treatment of each of these conditions may differ, the term “sleep-disordered breathing” has been widely used, because respiratory therapy (such as nasal continuous positive airway pressure [CPAP]) is effective for many of these conditions.
The diagnosis of sleep disorders
Methods of varying complexity are used to diagnose sleep disorders. Most studies during pregnancy have used a symptom-based approach, relying on either the patient or partner’s report of snoring, apnea, or excessive leg activity.31,88,89 Several questionnaires are available (e.g. Berlin or Hawaii questionnaire, Epworth sleepiness scale).31,34,35,37,42,44,46,48,49,88,90,91 The gold standard for the diagnosis of sleep disorders is polysomnography, in which the patient is studied and monitored continuously in a sleep laboratory. This modality consists of a comprehensive recording of biophysiologic parameters by electroencephalography, electrooculography, electromyography, electrocardiography, nasal and oral airflow as well as pulse oximetry.92,93
The different approaches to the diagnosis of sleep disorders have varying accuracies when compared to polysomnography; hence, the method of diagnosis can become a confounder when assessing the relationship between sleep disorders and pregnancy complications.94
Are sleep disorders during pregnancy associated with gestational diabetes?
In this issue of the American Journal of Obstetrics and Gynecology, Pamidi et al.54 report a systematic review and meta-analysis indicating that maternal sleep-disordered breathing is associated with gestational diabetes (adjusted odds ratio [aOR], 1.86; 95% confidence interval [CI], 1.30-2.45). This association relied on 4 studies in which sleep disorders were diagnosed based on symptoms.21,30–32
In October 2013, Luque-Fernandez et al.55 reported a meta-analysis of sleep-disordered breathing and gestational diabetes based on a total of 9,795 pregnant women, and reported that sleep-disordered breathing was associated with > three-fold risk of gestational diabetes with a pooled body mass index (BMI) aOR of 3.06 (95% CI, 1.89-4.96). In the same month, Reutrakul et al.33 reported a study using polysomnography and noted that women with gestational diabetes had a lower total sleep time, a higher apnea hypopnea index, and a greater frequency of obstructive sleep apnea than pregnant women with a normal glucose tolerance test (73% vs. 27%, P = 0.01). This association remained significant after adjustment for pre-pregnancy BMI (OR, 6.6; 95% CI, 1.15-37.96).
Experimental evidence in human beings that sleep deprivation leads to carbohydrate intolerance
The key observations include the following:
Partial sleep deprivation in healthy non-pregnant individuals during a single night (only 4 hours of sleep) reduces insulin sensitivity by 19-25%.95
Sleep restriction to 5 hours per night for 1 week in healthy men reduces insulin sensitivity by 11%.96
Progressive sleep restriction in non-pregnant women is associated with increased energy intake and net weight gain.97
In pregnant women, glucose concentrations after a 50-g oral glucose tolerance test decreased 4% for each hour of reduced sleep time.32
In pregnant women, the mean glucose concentration varies as a function of hours of sleep30 (Figure 4).
Figure 4. Maternal mean plasma glucose concentrations after a 50-g glucose challenge.
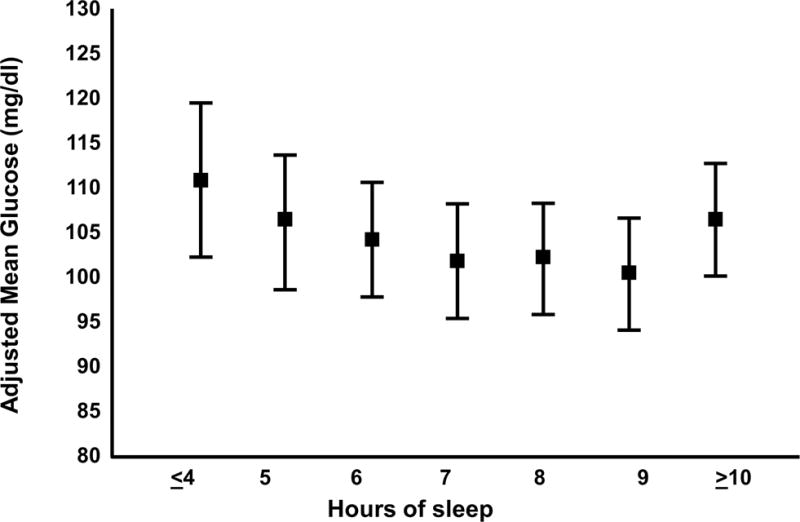
Means are adjusted for maternal age and race/ethnicity. Error bars are SE. Open access article. Qiu et al.30
The precise molecular mechanisms linking sleep deprivation with carbohydrate intolerance are complex, and involve hypoxia-induced inflammation, activation of the sympathetic nervous system with up-regulation of counter-regulatory hormones, and high circulating concentrations of leptin (which leads to insulin resistance).98
Sleep disorders (including pregnancy-onset snoring) and gestational hypertension/preeclampsia
Pamidi et al. reported that maternal sleep-disordered breathing is associated with an increased risk of gestational hypertension or preeclampsia.54 This conclusion was derived from 12 studies of symptom-based assessment of obstructive sleep apnea (OR, 3.11; 95% CI, 2.28-4.25), as well as 6 studies utilizing polysomnography (OR, 2.25; 95% CI, 1.13-4.52).
Snoring (the vibration of respiratory structures resulting from turbulent flow when the upper airway is narrowed during sleep)99 is more common in pregnant than in non-pregnant women (14-23% vs. 4%).42,100 The Nurse’s Health Study reported that snoring increases the risk of hypertension, independent of age and BMI in non-pregnant women.101 O’Brien et al.102 investigated the clinical significance of pregnancy-onset snoring in a prospective cohort study of 1,719 pregnant women (34% of patients had chronic snoring, and 25% pregnancy-onset snoring). New-onset (but not chronic) snoring was associated with gestational hypertension (OR, 2.36; 95% CI, 1.48-3.77; P < 0.001) and preeclampsia (OR, 1.59; 95% CI, 1.06-2.37; P = 0.024) after adjustment for confounders. The authors estimated that if snoring plays a causal role in hypertensive disorders of pregnancy, 18.7% of gestational hypertension and 11.6% of preeclampsia could be ameliorated by eliminating pregnancy-onset snoring. Interestingly, snoring was not implicated in gestational diabetes.102 An important contribution by O’Brien et al. is that two questions about snoring and the timing of its onset could be a strategy to identify patients at risk for hypertensive disorders of pregnancy in a clinical setting.102 The healthcare implications of this have been the subject of correspondence in this Journal.103,104
The precise mechanisms linking sleep disorders and gestational hypertension/preeclampsia remain to be elucidated. Sleep deprivation is associated with intravascular inflammation, which is also an important feature of preeclampsia.105–107 Specifically, poor sleep quality during pregnancy is associated with higher serum concentrations of inflammatory cytokines and chemokines, such as tumor necrosis factor-alpha,108 interleukin (IL)-8,109 and IL-6,109 all of which have been observed in patients with preeclampsia. Intravascular inflammation leading to endothelial cell dysfunction is a common pathway of multiple mechanisms of disease responsible for preeclampsia.110,111
Is there a role for CPAP in preeclampsia?
In a series of fascinating studies using polysomnography, Edwards et al. first demonstrated marked alterations in the sleep architecture of patients with preeclampsia compared to that of normal pregnant women.41 A key finding was that patients with preeclampsia had a significantly increased percentage of time spent in slow-wave sleep (21 ± 2 vs. 43 ± 3; p < 0.001).
What is slow-wave sleep and is it important? Also known as “deep sleep,” slow-wave sleep is characterized by epochs (30 seconds of sleep) that consist of ≥20% of slow-wave (delta) sleep (also known as stage-3 sleep).14 Slow-wave sleep is considered important for the consolidation of new memories112 and is also involved in the secretion of growth hormone.113 Women with preeclampsia spend more time in deep sleep. One possible explanation is that cytokines, such as IL-1 and tumor necrosis factor-alpha, can increase slow-wave sleep,41 as they are elevated in the maternal circulation of those with preeclampsia.105–107 Cerebral edema, which can be observed in some patients with preeclampsia, can also play a role.41
In normal pregnancy, blood pressure is the highest during the daytime. In 1976, Redman et al. reported that, in preeclampsia, there was a reversal diurnal blood pressure rhythm (i.e. the nocturnal blood pressure was higher than at daytime).114,115 Such reversal had also been reported with snoring and obstructive sleep apnea.116–119 Therefore, Edwards et al. proposed that nasal CPAP may improve blood pressure in women with preeclampsia and reported that autosetting nasal CPAP administered through sleep at night resulted in a marked reduction in blood pressure (before treatment, mean systolic 146 mm Hg, and mean diastolic 92 mm Hg; after treatment, mean systolic 128 mm Hg, and mean diastolic 73 mm Hg).120 The authors concluded that partial upper airway obstruction during sleep in women with preeclampsia was associated with an elevation in blood pressure, which could be treated with nasal CPAP.120
Next, a randomized clinical trial tested the effect of nocturnal nasal CPAP on maternal cardiac output.45 Twenty-four women with preeclampsia were randomly allocated to receive nasal CPAP or no treatment. Patients allocated to CPAP had a reduction in total peripheral vascular resistance and improvement in cardiac output. It is noteworthy that all of these observations were made in women with preeclampsia without requiring the diagnosis of a specific sleep disorder prior to respiratory treatment.45 In a different study, nasal CPAP was shown to increase fetal movements in patients studied with polysomnography and continuous ultrasound.28 These studies have been the subject of an insightful analysis by O’Brien et al.121
Poyares et al. recently reported a randomized study in which women with preexisting hypertension (receiving treatment) and snoring were allocated to either standard of care or nasal CPAP in the first eight weeks of pregnancy.24 Patients in the control group had a progressive increase in blood pressure which required treatment with α-methyldopa. In contrast, women allocated to CPAP had decreases in blood pressure and doses of anti-hypertensive medications.
Sleep disorders and fetal growth restriction
The evidence of whether sleep disorders increase the risk of fetal growth restriction has been contradictory. A recent prospective observational study using serial fetal biometry in women who had confirmed obstructive sleep apnea by polysomnography found that the rate of impaired fetal growth (defined as a fall in the customized centile < 33% between 33 weeks and term) was significantly greater in patients with obstructive sleep apnea than in the control group (43% vs. 11%; P = 0.04). After adjustment for BMI, the association between obstructive sleep apnea and fetal growth restriction was borderline significant (OR, 5.3; 95% CI, 0.93-30.34; P = 0.06).122 Despite the sample size, a strength of the study rests on the use of a longitudinal approach, which is more sensitive than a cross-sectional approach for the characterization of growth.
Maternal sleep position and late stillbirth
Women are advised to sleep on their left side during pregnancy based on the concept that the supine position can lead to compression of the inferior vena cava and reduce venous return and uterine blood flow. One study using xenon found that the intervillous blood flow was lower in the supine position than in the left tilt position (113 ± 48 vs. 141 ± 48 mL/min/dL; P < 0.01).123 Whether this has any real consequences on the outcome of pregnancy has not been established. A controversial observation has been reported recently in a case-control study in which women who slept on their back or on their right side the previous night (before fetal death) had a higher rate of late stillbirth than women who slept on their left side (aOR for back sleeping, 2.54; 95% CI, 1.04-6.18).124 An editorial commenting on this article concluded that the study should be considered hypothesis-generating rather than hypothesis-testing.125 The puzzling observation was that women who got up to go to the toilet once or less on the last night before the diagnosis of stillbirth were more likely to have a late fetal death than those who got up more frequently. Chappell and Smith125 proposed that this may be explained by reverse causation. Compromised babies have reduced frequency of fetal movements in the days leading to fetal death, and thus, mothers may sleep for longer periods of time because of decreased fetal activity.125 Therefore, the longer sleep would not be the cause, but the consequence of decreased fetal movement. A recent cross-sectional study conducted in Ghana about sleep quality and practices during pregnancy (based on a postpartum questionnaire) found that women who slept in the supine position had an increased risk of stillbirth over those who did not [15.8% (3/19) vs. 3% (6/197); OR, 8.0; 95% CI, 1.5-43.2; P = 0.016).51 Interestingly, stillbirth was less frequent in women who snored than in those who did not snore (3.8% vs. 4.2%), although this was not statistically significant (p=1.0).51 Prospective studies would be required to determine if an association between sleep position during pregnancy is associated with fetal death. These associations require replication and further study.
Could there be long-term consequences of maternal sleep deprivation in the offspring?
Recent experimental evidence from animals suggests that sleep deprivation during pregnancy may have long-lasting effects on the offspring. One study examined the effect of sleep restriction on pregnant rats, and observed the sexual behavior of the offspring.53 Male offspring were reported to have a lower motivation for sex (longer latency to first mount and a reduced number of mounts within a 30-minute period of observation) than males born to non-sleep-deprived mothers. In contrast, female offspring of sleep-deprived mothers had enhanced proceptivity (hopping, darting, and ear-wiggling) as well as disrupted sexual behavior characterized by mounting of the males during the period of observation, when compared to females in the control group. There were also changes in sexual behavior in male and female offspring when the fathers were exposed to sleep deprivation. This study raises interesting questions about the long-term behavioral consequences and the mechanisms by which they arise in the offspring of parents with sleep deprivation.
Finally, investigators attempted to model chronic intermittent hypoxia, which resembles repetitive obstructive sleep apnea in pregnant rats.126 Offspring of mothers exposed to intermittent hypoxia had asymmetric growth restriction at birth, but in adulthood, body weight was greater than in the controls. Moreover, exposed offspring had greater body fat deposition, hyperglycemia, and higher levels of insulin.126 Whether the experimental model mirrors obstructive sleep apnea of pregnancy remains to be determined.
Conclusion
Pregnant women have an increased frequency of sleep disturbances, and such disorders have been associated with pregnancy complications. Assessment of the quality and quantity of sleep has not been part of routine prenatal care. Whether the association between sleep disturbances and adverse pregnancy outcome is causal needs to be established (using a longitudinal approach, determining a dose response gradient between the severity of the sleep disorder and subsequent pregnancy complications, and generation of large datasets of observational as well as interventional studies). Practical methods to characterize sleep architecture in pregnancy and define pregnancy-specific screening tools are also needed.104 The diagnosis and optimal treatment of sleep disorders has potential benefits for: 1) reducing pregnancy complications in the index pregnancy; 2) health of the mother after pregnancy; and 3) short- and long-term consequences for the offspring. Pregnancy may be an ideal time to screen for sleep disturbances and to implement treatment that may have preventive implications and long-term benefits for public health.
Acknowledgments
This work was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services.
Footnotes
Disclosure: The authors report no conflicts of interest.
References
- 1.Zepelin H, Rechtschaffen A. Mammalian sleep, longevity, and energy metabolism. Brain Behav Evol. 1974;10:425–70. doi: 10.1159/000124330. [DOI] [PubMed] [Google Scholar]
- 2.Campbell SS, Tobler I. Animal sleep: a review of sleep duration across phylogeny. Neurosci Biobehav Rev. 1984;8:269–300. doi: 10.1016/0149-7634(84)90054-x. [DOI] [PubMed] [Google Scholar]
- 3.Rechtschaffen A. Current perspectives on the function of sleep. Perspect Biol Med. 1998;41:359–90. doi: 10.1353/pbm.1998.0051. [DOI] [PubMed] [Google Scholar]
- 4.McNamara P, Barton RA, Nunn CL. Evolution of sleep. New York, NY: Cambridge University Press; 2009. [Google Scholar]
- 5.Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287:1834–7. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- 6.Rechtschaffen A, Bergmann BM. Sleep deprivation in the rat by the disk-over-water method. Behav Brain Res. 1995;69:55–63. doi: 10.1016/0166-4328(95)00020-t. [DOI] [PubMed] [Google Scholar]
- 7.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–6. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 8.Ferrie JE, Shipley MJ, Cappuccio FP, et al. A prospective study of change in sleep duration: associations with mortality in the Whitehall II cohort. Sleep. 2007;30:1659–66. doi: 10.1093/sleep/30.12.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hublin C, Partinen M, Koskenvuo M, Kaprio J. Sleep and mortality: a population-based 22-year follow-up study. Sleep. 2007;30:1245–53. doi: 10.1093/sleep/30.10.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grandner MA, Hale L, Moore M, Patel NP. Mortality associated with short sleep duration: the evidence, the possible mechanisms, and the future. Sleep Med Rev. 2010;14:191–203. doi: 10.1016/j.smrv.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luyster FS, Strollo PJ, Jr, Zee PC, Walsh JK. Sleep: a health imperative. Sleep. 2012;35:727–34. doi: 10.5665/sleep.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chokroverty S. An overview of normal sleep. In: Chokroverty S, editor. Sleep disorders medicine: basic science, technical considerations, and clinical aspects. Philadelphia, PA: Saunders Elsevier; 2009. [Google Scholar]
- 13.Carskadon MA, Dement WC. Normal human sleep: an overview. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. Toronto, Ontario, Canada: Elsevier; 2011. [Google Scholar]
- 14.Rowley JA, Safwan Badr M. Normal sleep. In: Safwan Badr M, editor. Essentials of sleep medicine: an approach for clinical pulmonology. New York, NY: Springer; 2012. [Google Scholar]
- 15.Santiago JR, Nolledo MS, Kinzler W, Santiago TV. Sleep and sleep disorders in pregnancy. Ann Intern Med. 2001;134:396–408. doi: 10.7326/0003-4819-134-5-200103060-00012. [DOI] [PubMed] [Google Scholar]
- 16.Sahota PK, Jain SS, Dhand R. Sleep disorders in pregnancy. Curr Opin Pulm Med. 2003;9:477–83. doi: 10.1097/00063198-200311000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Pien GW, Schwab RJ. Sleep disorders during pregnancy. Sleep. 2004;27:1405–17. doi: 10.1093/sleep/27.7.1405. [DOI] [PubMed] [Google Scholar]
- 18.Driver HS, Sloan EP. Women’s sleep. In: Chokroverty S, editor. Sleep disorders medicine: basic science, technical considerations, and clinical aspects. Philadelphia, PA: Saunders Elsevier; 2009. [Google Scholar]
- 19.Facco FL, Kramer J, Ho KH, Zee PC, Grobman WA. Sleep disturbances in pregnancy. Obstet Gynecol. 2010;115:77–83. doi: 10.1097/AOG.0b013e3181c4f8ec. [DOI] [PubMed] [Google Scholar]
- 20.Izci Balserak B, Kathryn L. Sleep disturbances and sleep-related disorders in pregnancy. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. Toronto, Ontario, Canada: Elsevier; 2011. [Google Scholar]
- 21.Facco FL, Grobman WA, Kramer J, Ho KH, Zee PC. Self-reported short sleep duration and frequent snoring in pregnancy: impact on glucose metabolism. Am J Obstet Gynecol. 2010;203:142.e1–5. doi: 10.1016/j.ajog.2010.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Facco FL, Liu CS, Cabello AA, Kick A, Grobman WA, Zee PC. Sleep-disordered breathing: a risk factor for adverse pregnancy outcomes? Am J Perinatol. 2012;29:277–82. doi: 10.1055/s-0031-1295658. [DOI] [PubMed] [Google Scholar]
- 23.Facco FL, Lappen J, Lim C, Zee PC, Grobman WA. Preeclampsia and sleep-disordered breathing: a case-control study. Pregnancy Hypertens. 2013;3:133–9. doi: 10.1016/j.preghy.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poyares D, Guilleminault C, Hachul H, et al. Pre-eclampsia and nasal CPAP, part 2: hypertension during pregnancy, chronic snoring, and early nasal CPAP intervention. Sleep Med. 2007;9:15–21. doi: 10.1016/j.sleep.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 25.Caughey AB, Lee KA. CPAP for the prevention of pre-eclampsia: is this a clinically promising intervention? Sleep Med. 2007;9:1–2. doi: 10.1016/j.sleep.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 26.Guilleminault C, Palombini L, Poyares D, Takaoka S, Huynh NT, El-Sayed Y. Pre-eclampsia and nasal CPAP, part 1. Early intervention with nasal CPAP in pregnant women with risk factors for pre-eclampsia: preliminary findings. Sleep Med. 2007;9:9–14. doi: 10.1016/j.sleep.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 27.Bourjeily G, Barbara N, Larson L, He M. Clinical manifestations of obstructive sleep apnea in pregnancy: more than snoring and witnessed apneas. J Obstet Gynaecol. 2012;32:434–8. doi: 10.3109/01443615.2012.658892. [DOI] [PubMed] [Google Scholar]
- 28.Blyton DM, Skilton MR, Edwards N, Hennessy A, Celermajer DS, Sullivan CE. Treatment of sleep disordered breathing reverses low fetal activity levels in preeclampsia. Sleep. 2013;36:15–21. doi: 10.5665/sleep.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Facco FL. Sleep-disordered breathing and pregnancy. Semin Perinatol. 2011;35:335–9. doi: 10.1053/j.semperi.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 30.Qiu C, Enquobahrie D, Frederick IO, Abetew D, Williams MA. Glucose intolerance and gestational diabetes risk in relation to sleep duration and snoring during pregnancy: a pilot study. BMCWomensHealth. 2010;10:17. doi: 10.1186/1472-6874-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bourjeily G, Raker CA, Chalhoub M, Miller MA. Pregnancy and fetal outcomes of symptoms of sleep-disordered breathing. Eur Respir J. 2010;36:849–55. doi: 10.1183/09031936.00021810. [DOI] [PubMed] [Google Scholar]
- 32.Reutrakul S, Zaidi N, Wroblewski K, et al. Sleep disturbances and their relationship to glucose tolerance in pregnancy. Diabetes Care. 2011;34:2454–7. doi: 10.2337/dc11-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reutrakul S, Zaidi N, Wroblewski K, et al. Interactions between pregnancy, obstructive sleep apnea, and gestational diabetes mellitus. J Clin Endocrinol Metab. 2013;98:4195–202. doi: 10.1210/jc.2013-2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calaora-Tournadre D, Ragot S, Meurice JC, et al. Obstructive sleep apnea syndrome during pregnancy: prevalence of main symptoms and relationship with pregnancy-induced hypertension and intrauterine growth retardation [in French] Rev Med Interne. 2006;27:291–5. doi: 10.1016/j.revmed.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 35.Perez-Chada D, Videla AJ, O’Flaherty ME, et al. Snoring, witnessed sleep apneas and pregnancy-induced hypertension. Acta Obstet Gynecol Scand. 2007;86:788–92. doi: 10.1080/00016340701281919. [DOI] [PubMed] [Google Scholar]
- 36.Champagne K, Schwartzman K, Opatrny L, et al. Obstructive sleep apnea and its association with gestational hypertension. Eur Respir J. 2009;33:559–65. doi: 10.1183/09031936.00122607. [DOI] [PubMed] [Google Scholar]
- 37.Ursavas A, Karadag M, Nalci N, Ercan I, Gozu RO. Self-reported snoring, maternal obesity and neck circumference as risk factors for pregnancy-induced hypertension and preeclampsia. Respiration. 2008;76:33–9. doi: 10.1159/000107735. [DOI] [PubMed] [Google Scholar]
- 38.Reid J, Skomro R, Cotton D, et al. Pregnant women with gestational hypertension may have a high frequency of sleep disordered breathing. Sleep. 2011;34:1033–8. doi: 10.5665/SLEEP.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen YH, Kang JH, Lin CC, Wang IT, Keller JJ, Lin HC. Obstructive sleep apnea and the risk of adverse pregnancy outcomes. Am J Obstet Gynecol. 2012;206:136.e1–5. doi: 10.1016/j.ajog.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 40.Genova G, Guddo F, Vita C, Arena N, Morello V, Tomasino RM. Argyrophilic nucleoproteins of the cervical epithelium in HPV infection and intraepithelial neoplasia [in Italian] Pathologica. 1991;83:461–6. [PubMed] [Google Scholar]
- 41.Edwards N, Blyton CM, Kesby GJ, Wilcox I, Sullivan CE. Preeclampsia is associated with marked alterations in sleep architecture. Sleep. 2000;23:619–25. [PubMed] [Google Scholar]
- 42.Franklin KA, Holmgren PA, Jonsson F, Poromaa N, Stenlund H, Svanborg E. Snoring, pregnancy-induced hypertension, and growth retardation of the fetus. Chest. 2000;117:137–41. doi: 10.1378/chest.117.1.137. [DOI] [PubMed] [Google Scholar]
- 43.Connolly G, Razak AR, Hayanga A, Russell A, McKenna P, McNicholas WT. Inspiratory flow limitation during sleep in pre-eclampsia: comparison with normal pregnant and nonpregnant women. Eur Respir J. 2001;18:672–6. doi: 10.1183/09031936.01.00053501. [DOI] [PubMed] [Google Scholar]
- 44.Izci B, Riha RL, Martin SE, et al. The upper airway in pregnancy and pre-eclampsia. Am J Respir Crit Care Med. 2003;167:137–40. doi: 10.1164/rccm.200206-590OC. [DOI] [PubMed] [Google Scholar]
- 45.Blyton DM, Sullivan CE, Edwards N. Reduced nocturnal cardiac output associated with preeclampsia is minimized with the use of nocturnal nasal CPAP. Sleep. 2004;27:79–84. doi: 10.1093/sleep/27.1.79. [DOI] [PubMed] [Google Scholar]
- 46.Izci B, Martin SE, Dundas KC, Liston WA, Calder AA, Douglas NJ. Sleep complaints: snoring and daytime sleepiness in pregnant and preeclamptic women. Sleep Med. 2005;6:163–9. doi: 10.1016/j.sleep.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 47.Louis JM, Auckley D, Sokol RJ, Mercer BM. Maternal and neonatal morbidities associated with obstructive sleep apnea complicating pregnancy. Am J Obstet Gynecol. 2010;202:261.e1–5. doi: 10.1016/j.ajog.2009.10.867. [DOI] [PubMed] [Google Scholar]
- 48.Higgins N, Leong E, Park CS, Facco FL, McCarthy RJ, Wong CA. The Berlin questionnaire for assessment of sleep disordered breathing risk in parturients and non-pregnant women. Int J Obstet Anesth. 2011;20:22–5. doi: 10.1016/j.ijoa.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 49.Micheli K, Komninos I, Bagkeris E, et al. Sleep patterns in late pregnancy and risk of preterm birth and fetal growth restriction. Epidemiology. 2011;22:738–44. doi: 10.1097/EDE.0b013e31822546fd. [DOI] [PubMed] [Google Scholar]
- 50.Odibo AO, Rada CC, Cahill AG, et al. First-trimester serum soluble fms-like tyrosine kinase-1, free vascular endothelial growth factor, placental growth factor and uterine artery Doppler in preeclampsia. J Perinatol. 2013;33:670–4. doi: 10.1038/jp.2013.33. [DOI] [PubMed] [Google Scholar]
- 51.Owusu JT, Anderson FJ, Coleman J, et al. Association of maternal sleep practices with pre-eclampsia, low birth weight, and stillbirth among Ghanaian women. Int J Gynaecol Obstet. 2013;121:261–5. doi: 10.1016/j.ijgo.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomal JT, Palma BD, Ponzio BF, et al. Sleep restriction during pregnancy: hypertension and renal abnormalities in young offspring rats. Sleep. 2010;33:1357–62. doi: 10.1093/sleep/33.10.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alvarenga TA, Aguiar MF, Mazaro-Costa R, Tufik S, Andersen ML. Effects of sleep deprivation during pregnancy on the reproductive capability of the offspring. Fertil Steril. 2013;100:1752–7. doi: 10.1016/j.fertnstert.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 54.Pamidi S, Pinto LM, Marc I, Benedetti A, Schwartzman K, Kimoff RJ. Maternal sleep-disordered breathing and adverse pregnancy outcomes: a systematic review and metaanalysis. Am J Obstet Gynecol. 2014;210:87–8. doi: 10.1016/j.ajog.2013.07.033. [DOI] [PubMed] [Google Scholar]
- 55.Luque-Fernandez MA, Bain PA, Gelaye B, Redline S, Williams MA. Sleep-disordered breathing and gestational diabetes mellitus: a metaanalysis of 9,795 participants enrolled in epidemiological observational studies. Diabetes Care. 2013;36:3353–60. doi: 10.2337/dc13-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4:147ra11. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bateman RJ, Munsell LY, Morris JC, Swarm R, Yarasheski KE, Holtzman DM. Human amyloid-beta synthesis and clearance rates as measured in cerebrospinal fluid in vivo. Nat Med. 2006;12:856–61. doi: 10.1038/nm1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martins IC, Kuperstein I, Wilkinson H, et al. Lipids revert inert Abeta amyloid fibrils to neurotoxic protofibrils that affect learning in mice. EMBO J. 2008;27:224–33. doi: 10.1038/sj.emboj.7601953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walker LC, Diamond MI, Duff KE, Hyman BT. Mechanisms of protein seeding in neurodegenerative diseases. JAMA Neurol. 2013;70:304–10. doi: 10.1001/jamaneurol.2013.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frost B, Diamond MI. Prion-like mechanisms in neurodegenerative diseases. Nat Rev Neurosci. 2010;11:155–9. doi: 10.1038/nrn2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mucke L, Selkoe DJ. Neurotoxicity of amyloid beta-protein: synaptic and network dysfunction. Cold Spring Harb Perspect Med. 2012;2:a006338. doi: 10.1101/cshperspect.a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nedergaard M. Neuroscience: garbage truck of the brain. Science. 2013;340:1529–30. doi: 10.1126/science.1240514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–7. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chokroverty S. Sleep and neurodegenerative diseases. Semin Neurol. 2009;29:446–67. doi: 10.1055/s-0029-1237124. [DOI] [PubMed] [Google Scholar]
- 65.Raggi A, Ferri R. Sleep disorders in neurodegenerative diseases. Eur Neurol. 2010;17:1326–38. doi: 10.1111/j.1468-1331.2010.03034.x. [DOI] [PubMed] [Google Scholar]
- 66.Osorio RS, Pirraglia E, Aguera-Ortiz LF, et al. Greater risk of Alzheimer’s disease in older adults with insomnia. J Am Geriatr Soc. 2011;59:559–62. doi: 10.1111/j.1532-5415.2010.03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kripke DF, Simons RN, Garfinkel L, Hammond EC. Short and long sleep and sleeping pills: is increased mortality associated? Arch Gen Psychiatry. 1979;36:103–16. doi: 10.1001/archpsyc.1979.01780010109014. [DOI] [PubMed] [Google Scholar]
- 68.Johnson EO. Sleep in America: 2000, Results from the National Sleep Foundation’s 2000 Omnibus sleep poll. Washington, DC: The National Sleep Foundation; 2000. Available at: http://www.sleepfoundation.org/atf/cf/fF6BF2668-A1B4-4FE8-8D1A-A5D39340D9CBg/2000_poll.pdf. Accessed Nov. 22, 2013. [Google Scholar]
- 69.Wehr TA. In short photoperiods, human sleep is biphasic. J Sleep Res. 1992;1:103–7. doi: 10.1111/j.1365-2869.1992.tb00019.x. [DOI] [PubMed] [Google Scholar]
- 70.Ekirch AR. At day’s close. New York, NY: WW Norton and Company; 2005. [Google Scholar]
- 71.Koslofsky C. Evening’s empire. Cambridge, England, United Kingdom: Cambridge University Press; 2011. [Google Scholar]
- 72.Mindell JA, Jacobson BJ. Sleep disturbances during pregnancy. J Obstet Gynecol Neonatal Nurs. 2000;29:590–7. doi: 10.1111/j.1552-6909.2000.tb02072.x. [DOI] [PubMed] [Google Scholar]
- 73.Hedman C, Pohjasvaara T, Tolonen U, Suhonen-Malm AS, Myllyla VV. Effects of pregnancy on mothers’ sleep. Sleep Med. 2002;3:37–42. doi: 10.1016/s1389-9457(01)00130-7. [DOI] [PubMed] [Google Scholar]
- 74.Izci B, Vennelle M, Liston WA, Dundas KC, Calder AA, Douglas NJ. Sleep-disordered breathing and upper airway size in pregnancy and post-partum. Eur Respir J. 2006;27:321–7. doi: 10.1183/09031936.06.00148204. [DOI] [PubMed] [Google Scholar]
- 75.Bourjeily G, Ankner G, Mohsenin V. Sleep-disordered breathing in pregnancy. Clin Chest Med. 2011;32:175–89. x. doi: 10.1016/j.ccm.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 76.Neau JP, Texier B, Ingrand P. Sleep and vigilance disorders in pregnancy. Eur Neurol. 2009;62:23–9. doi: 10.1159/000215877. [DOI] [PubMed] [Google Scholar]
- 77.Baratte-Beebe KR, Lee K. Sources of midsleep awakenings in childbearing women. Clin Nurs Res. 1999;8:386–97. doi: 10.1177/10547739922158377. [DOI] [PubMed] [Google Scholar]
- 78.Hertz G, Fast A, Feinsilver SH, Albertario CL, Schulman H, Fein AM. Sleep in normal late pregnancy. Sleep. 1992;15:246–51. doi: 10.1093/sleep/15.3.246. [DOI] [PubMed] [Google Scholar]
- 79.Manconi M, Govoni V, De Vito A, et al. Restless legs syndrome and pregnancy. Neurology. 2004;63:1065–9. doi: 10.1212/01.wnl.0000138427.83574.a6. [DOI] [PubMed] [Google Scholar]
- 80.Pien GW, Fife D, Pack AI, Nkwuo JE, Schwab RJ. Changes in symptoms of sleep-disordered breathing during pregnancy. Sleep. 2005;28:1299–305. doi: 10.1093/sleep/28.10.1299. [DOI] [PubMed] [Google Scholar]
- 81.Edwards N, Blyton DM, Hennessy A, Sullivan CE. Severity of sleep-disordered breathing improves following parturition. Sleep. 2005;28:737–41. doi: 10.1093/sleep/28.6.737. [DOI] [PubMed] [Google Scholar]
- 82.Hornyak M, Cejnar M, Elam M, Matousek M, Wallin BG. Sympathetic muscle nerve activity during sleep in man. Brain. 1991;114:1281–95. doi: 10.1093/brain/114.3.1281. [DOI] [PubMed] [Google Scholar]
- 83.Chokroverty S. Physiologic changes in sleep. In: Chokroverty S, editor. Sleep disorders medicine: basic science, technical considerations, and clinical aspects. Philadelphia, PA: Saunders Elsevier; 2009. [Google Scholar]
- 84.Chokroverty S. Overview of sleep and sleep disorders. Indian J Med Res. 2010;131:126–40. [PubMed] [Google Scholar]
- 85.Okun ML, Roberts JM, Marsland AL, Hall M. How disturbed sleep may be a risk factor for adverse pregnancy outcomes. Obstet Gynecol Surv. 2009;64:273–80. doi: 10.1097/OGX.0b013e318195160e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Camann WR, Ostheimer GW. Physiological adaptations during pregnancy. Int Anesthesiol Clin. 1990;28:2–10. doi: 10.1097/00004311-199002810-00002. [DOI] [PubMed] [Google Scholar]
- 87.Pilkington S, Carli F, Dakin MJ, et al. Increase in Mallampati score during pregnancy. Br J Anaesth. 1995;74:638–42. doi: 10.1093/bja/74.6.638. [DOI] [PubMed] [Google Scholar]
- 88.Bourjeily G, El Sabbagh R, Sawan P, et al. Epworth sleepiness scale scores and adverse pregnancy outcomes. Sleep Breath. 2013;17:1179–86. doi: 10.1007/s11325-013-0820-9. [DOI] [PubMed] [Google Scholar]
- 89.Bourjeily G, Raker C, Chalhoub M, Miller M. Excessive daytime sleepiness in late pregnancy may not always be normal: results from a cross-sectional study. Sleep Breath. 2013;17:735–40. doi: 10.1007/s11325-012-0753-8. [DOI] [PubMed] [Google Scholar]
- 90.Yin TT, Williams N, Burton C, et al. Hypertension, fetal growth restriction and obstructive sleep apnea in pregnancy. Eur J Obstet Gynecol Reprod Biol. 2008;141:35–8. doi: 10.1016/j.ejogrb.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 91.Ayrim A, Keskin EA, Ozol D, Onaran Y, Yiidirim Z, Kafali H. Influence of self-reported snoring and witnessed sleep apnea on gestational hypertension and fetal outcome in pregnancy. Arch Gynecol Obstet. 2011;283:195–9. doi: 10.1007/s00404-009-1327-2. [DOI] [PubMed] [Google Scholar]
- 92.Patil SP. What every clinician should know about polysomnography. Respir Care. 2010;55:1179–95. [PubMed] [Google Scholar]
- 93.Chervin RD. Use of clinical tools and tests in sleep medicine. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. Toronto, Ontario, Canada: Elsevier; 2011. [Google Scholar]
- 94.Facco FL, Ouyang DW, Zee PC, Grobman WA. Development of a pregnancy-specific screening tool for sleep apnea. J Clin Sleep Med. 2012;8:389–94. doi: 10.5664/jcsm.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Donga E, van Dijk M, van Dijk JG, et al. A single night of partial sleep deprivation induces insulin resistance in multiple metabolic pathways in healthy subjects. J Clin Endocrinol Metab. 2010;95:2963–8. doi: 10.1210/jc.2009-2430. [DOI] [PubMed] [Google Scholar]
- 96.Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes. 2010;59:2126–33. doi: 10.2337/db09-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bosy-Westphal A, Hinrichs S, Jauch-Chara K, et al. Influence of partial sleep deprivation on energy balance and insulin sensitivity in healthy women. Obes Facts. 2008;1:266–73. doi: 10.1159/000158874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.O’Keeffe M, St-Onge MP. Sleep duration and disorders in pregnancy: implications for glucose metabolism and pregnancy outcomes. Int J Obes (Lond) 2013;37:765–70. doi: 10.1038/ijo.2012.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dalmasso F, Prota R. Snoring: analysis, measurement, clinical implications and applications. Eur Respir J. 1996;9:146–59. doi: 10.1183/09031936.96.09010146. [DOI] [PubMed] [Google Scholar]
- 100.Loube DI, Poceta JS, Morales MC, Peacock MD, Mitler MM. Self-reported snoring in pregnancy: association with fetal outcome. Chest. 1996;109:885–9. doi: 10.1378/chest.109.4.885. [DOI] [PubMed] [Google Scholar]
- 101.Hu FB, Willett WC, Colditz GA, et al. Prospective study of snoring and risk of hypertension in women. Am J Epidemiol. 1999;150:806–16. doi: 10.1093/oxfordjournals.aje.a010085. [DOI] [PubMed] [Google Scholar]
- 102.O’Brien LM, Bullough AS, Owusu JT, et al. Pregnancy-onset habitual snoring, gestational hypertension, and preeclampsia: prospective cohort study. Am J Obstet Gynecol. 2012;207:487.e1–9. doi: 10.1016/j.ajog.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Reichmann JP. Pregnancy-onset habitual snoring, gestational hypertension, and preeclampsia: prospective cohort study. Am J Obstet Gynecol. 2013;208:507. doi: 10.1016/j.ajog.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 104.O’Brien LM. Reply: to PMID 22999158. Am J Obstet Gynecol. 2013;208:507–8. doi: 10.1016/j.ajog.2013.01.030. [DOI] [PubMed] [Google Scholar]
- 105.Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol. 1998;179:80–6. doi: 10.1016/s0002-9378(98)70254-6. [DOI] [PubMed] [Google Scholar]
- 106.Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180:499–506. doi: 10.1016/s0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- 107.Gervasi MT, Chaiworapongsa T, Pacora P, et al. Phenotypic and metabolic characteristics of monocytes and granulocytes in preeclampsia. Am J Obstet Gynecol. 2001;185:792–7. doi: 10.1067/mob.2001.117311. [DOI] [PubMed] [Google Scholar]
- 108.Okun ML, Coussons-Read ME. Sleep disruption during pregnancy: how does it influence serum cytokines? J Reprod Immunol. 2007;73:158–65. doi: 10.1016/j.jri.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 109.Okun ML, Luther JF, Wisniewski SR, Wisner KL. Disturbed sleep and inflammatory cytokines in depressed and nondepressed pregnant women: an exploratory analysis of pregnancy outcomes. Psychosom Med. 2013;75:670–81. doi: 10.1097/PSY.0b013e31829cc3e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1989;161:1200–4. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- 111.Rodgers GM, Taylor RN, Roberts JM. Preeclampsia is associated with a serum factor cytotoxic to human endothelial cells. Am J Obstet Gynecol. 1988;159:908–14. doi: 10.1016/s0002-9378(88)80169-8. [DOI] [PubMed] [Google Scholar]
- 112.Rasch B, Born J. About sleep’s role in memory. Physiol Rev. 2013;93:681–766. doi: 10.1152/physrev.00032.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Van Cauter E, Latta F, Nedeltcheva A, et al. Reciprocal interactions between the GH axis and sleep. Growth Horm IGF Res. 2004;14(Suppl):S10–7. doi: 10.1016/j.ghir.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 114.Redman CW, Beilin LJ, Bonnar J. Reversed diurnal blood pressure rhythm in hypertensive pregnancies. Clin SciMol Med Suppl. 1976;3:687–89s. doi: 10.1042/cs051687s. [DOI] [PubMed] [Google Scholar]
- 115.Beilin LJ, Deacon J, Michael CA, et al. Diurnal rhythms of blood pressure, plasma renin activity, angiotensin II and catecholamines in normotensive and hypertensive pregnancies. Clin Exp Hypertens B. 1983;2:271–93. doi: 10.3109/10641958309006086. [DOI] [PubMed] [Google Scholar]
- 116.Imai Y, Abe K, Munakata M, et al. Circadian blood pressure variations under different pathophysiological conditions. J Hypertens Suppl. 1990;8:S125–32. [PubMed] [Google Scholar]
- 117.Sforza E, Parchi P, Contin M, Cortelli P, Lugaresi E. Do autonomic cardiovascular reflexes predict the nocturnal rise in blood pressure in obstructive sleep apnea syndrome? Blood Press. 1994;3:295–302. doi: 10.3109/08037059409102277. [DOI] [PubMed] [Google Scholar]
- 118.Lapinski M, Przybylowski T, Lewandowski J, et al. Diurnal blood pressure rhythm and urinary catecholamine excretion in obstructive sleep apnea and essential hypertension. J Hypertens Suppl. 1993;11:S292–3. [PubMed] [Google Scholar]
- 119.Bayliss DA, Millhorn DE. Central neural mechanisms of progesterone action: application to the respiratory system. J Appl Physiol (1985) 1992;73:393–404. doi: 10.1152/jappl.1992.73.2.393. [DOI] [PubMed] [Google Scholar]
- 120.Edwards N, Blyton DM, Kirjavainen T, Kesby GJ, Sullivan CE. Nasal continuous positive airway pressure reduces sleep-induced blood pressure increments in preeclampsia. Am J Respir Crit Care Med. 2000;162:252–7. doi: 10.1164/ajrccm.162.1.9905006. [DOI] [PubMed] [Google Scholar]
- 121.O’Brien LM. Positive airway pressure as a therapy for preeclampsia? Sleep. 2013;36:5–6. doi: 10.5665/sleep.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fung AM, Wilson DL, Lappas M, et al. Effects of maternal obstructive sleep apnea on fetal growth: a prospective cohort study. PloS One. 2013;8:e68057. doi: 10.1371/journal.pone.0068057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kauppila A, Koskinen M, Puolakka J, Tuimala R, Kuikka J. Decreased intervillous and unchanged myometrial blood flow in supine recumbency. Obstet Gynecol. 1980;55:203–5. [PubMed] [Google Scholar]
- 124.Stacey T, Thompson JM, Mitchell EA, Ekeroma AJ, Zuccollo JM, McCowan LM. Association between maternal sleep practices and risk of late stillbirth: a case-control study. BMJ. 2011;342:d3403. doi: 10.1136/bmj.d3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chappell LC, Smith GC. Should pregnant women sleep on their left? BMJ. 2011;342:d3659. doi: 10.1136/bmj.d3659. [DOI] [PubMed] [Google Scholar]
- 126.Iqbal W, Ciriello J. Effect of maternal chronic intermittent hypoxia during gestation on offspring growth in the rat. Am J Obstet Gynecol. 2013;209:564.e1–9. doi: 10.1016/j.ajog.2013.08.027. [DOI] [PubMed] [Google Scholar]


