Abstract
Objective
Intra-amniotic infection/inflammation are major causes of spontaneous preterm labor and delivery. However, diagnosis of intra-amniotic infection is challenging because most are subclinical and amniotic fluid (AF) cultures take several days before results are available. Several tests have been proposed for the rapid diagnosis of microbial invasion of the amniotic cavity (MIAC) or intra-amniotic inflammation. The aim of this study was to examine the diagnostic performance of the AF Mass Restricted (MR) score in comparison with interleukin-6 (IL-6) and matrix metalloproteinase-8 (MMP-8) for the identification of MIAC or inflammation.
Methods
AF samples were collected from patients with singleton gestations and symptoms of preterm labor (n = 100). Intra-amniotic inflammation was defined as >100 white blood cells/mm3 (WBCs) in AF; MIAC was defined as a positive AF culture. AF IL-6 and MMP-8 were determined using ELISA. The MR score was obtained using the Surface-Enhanced Laser Desorption Ionization Time of Flight (SELDI-TOF) mass spectrometry. Sensitivity and specificity were calculated and logistic regression models were fit to construct receiver-operating characteristic (ROC) curves for the identification of each outcome. The McNemar’s test and paired sample non-parametric statistical techniques were used to test for differences in diagnostic performance metrics.
Results
(1) The prevalence of MIAC and intra-amniotic inflammation was 34% (34/100) and 40% (40/100), respectively; (2) there were no significant differences in sensitivity of the three tests under study (MR score, IL-6 or MMP-8) in the identification of either MIAC or intra-amniotic inflammation (using the following cutoffs: MR score >2, IL-6 >11.4 ng/mL, and MMP-8 >23 ng/mL); (3) there was no significant difference in the sensitivity among the three tests for the same outcomes when the false positive rate was fixed at 15%; (4) the specificity for IL-6 was not significantly different from that of the MR score in identifying either MIAC or intra-amniotic inflammation when using previously reported thresholds; and (5) there were no significant differences in the area under the ROC curve when comparing the MR score, IL-6 or MMP-8 in the identification of these outcomes.
Conclusions
IL-6 and the MR score have equivalent diagnostic performance in the identification of MIAC or intra-amniotic inflammation. Selection from among these three tests (MR score, IL-6 and MMP-8) for diagnostic purposes should be based on factors such as availability, reproducibility, and cost. The MR score requires a protein chip and a SELDI-TOF instrument which are not widely available or considered “state of the art”. In contrast, immunoassays for IL-6 can be performed in the majority of clinical laboratories.
Keywords: Biomarker, chorioamnionitis, IL-6, MMP-8, pregnancy, preterm birth, preterm labor, proteomics, reproducibility
Introduction
Preterm birth is the leading cause of perinatal morbidity and mortality worldwide [1–4]. Two-thirds of all preterm births occur after the spontaneous onset of labor with either intact or ruptured membranes [2,5]. Microbial invasion of the amniotic cavity (MIAC) is frequently observed in patients with preterm labor and intact membranes [6–22] and, rarely, in patients with indicated preterm delivery (such as preeclampsia [23] and small-for-gestational-age [24]). Moreover, there is considerable evidence that MIAC is causally linked with spontaneous preterm labor and delivery [25–28].
Under normal circumstances, bacteria are not present in the amniotic cavity [29–32]. Microorganisms can gain access to the amniotic cavity in patients with preterm labor (PTL) and intact membranes [6–22], preterm prelabor rupture of membranes (PROM) [33–41], a short cervix [42–44], cervical insufficiency [45–49], PTL in twin gestations [50–52], vaginal bleeding in the third trimester [53], placenta previa [54,55] or in selected cases of fetal death [56–60].
The presence of microorganisms in amniotic fluid (AF) can be detected using cultivation and/or molecular techniques [15,16,21,22,29,61–66]. When bacteria are present in the amniotic cavity in the absence of an inflammatory response, the condition is referred to as MIAC. Once MIAC elicits a localized inflammatory response, the condition is known as intra-amniotic infection [8,14,20,31,38,61,67–73]. Intraamniotic infection and MIAC are largely subclinical, and only a small fraction of patients have evidence of clinical chorioamnionitis [1,12,16,17,43,68,74–76]. Despite being clinically silent, microorganisms may gain access to the fetus and generate a fetal inflammatory response, which is characterized by an elevation of fetal circulating cytokines [77–79], multi-systemic involvement [80–120] and the impending onset of labor [121]. Some patients have intraamniotic inflammation in the absence of demonstrable MIAC for bacteria or viruses – these cases of “sterile” intraamniotic inflammation appear to be associated with adverse pregnancy outcome, yet the cause of the inflammatory process remains to be determined, and may be attributed to “danger signals” or damage-associated molecular patterns (DAMPs) [122–124].
The diagnosis of MIAC, intra-amniotic inflammation, and intra-amniotic infection (which requires a combination of MIAC and intra-amniotic inflammation) is challenging because most infections are subclinical [1,12,16,17,43,68, 74–76]. The “gold standard” for diagnosis of MIAC is the demonstration of microbial growth in AF, which is normally sterile [29–32]; however, AF culture results may take several days to be informative. Therefore, diagnosis has relied per force on tests aimed at detecting an intra-amniotic inflammatory process.
We advocated tests used in other body fluids (e.g. cerebrospinal fluid), such as the Gram stain [125–130], AF glucose [126–137] and AF white blood cell (WBC) count [126–128,130,138,139] for the rapid assessment of the presence of bacteria and/or inflammation. Subsequently, other methods were used, including the detection of microbial products, such as endotoxin [25,140–142], the acridine orange stain [143], leukocyte esterase [138,139,144] and gas liquid chromatography of microbial metabolites [145,146]. Immunoassays have also been used for the detection of proteins produced in response to microorganisms or during the course of inflammation, such as interleukin 6 (IL-6) [32,127,137,147–162], other cytokines and chemokines [26,27,77,78,163–188], and rapid tests for matrix metalloproteinase-8 (MMP-8) [160,162,189–194].
In 2005, a method to diagnose intra-amniotic inflammation based on mass spectrometry was reported and referred to as the Mass Restricted (MR) score [195,196]. The MR score was based on the detection of four peaks on mass spectrometry at 3378.2, 3449.7, 10 471.7 and 10 874.4 Daltons. These peaks correspond to four proteins, respectively: neutrophil defensins-1 and −2, and calgranulins A and C [195,196]. The MR score equals the number of these four peaks observed on the mass spectrometry tracing of a particular AF sample, and ranges from 0 to 4; 0 when none of the peaks are present, and 4 when all peaks are present. An MR score of 3 or higher has been proposed as evidence of intra-amniotic inflammation [195,196].
The MR score was formulated and tested in 101 stored AF samples collected in our unit, as previously described [195,196]. These results were subsequently confirmed using fresh, rather than stored, AF samples in a follow-up study of 169 consecutive women with singleton pregnancies and PTL or preterm PROM [197]. The authors compared the performance of the MR score with IL-6 and MMP-8 as well as other biomarkers for the detection of intra-amniotic inflammation, and claimed that their study “clearly demonstrates the superiority. . .of the MR score in comparison with any other clinical test. . .” in identifying intra-amniotic inflammation, defined as an AF WBC >100 cells/mm3, and MIAC (a positive AF culture for microorganisms) [197]. The sensitivity and specificity of the MR score, IL-6, and MMP-8 for the identification of MIAC and intra-amniotic inflammation reported by the authors are shown in Table 1.
Table I.
Performance of IL-6, MMP-8, and the MR score for the detection of intra-amniotic inflammation and infection (defined as AF WBC count >100 cells/mm3 and a positive AF culture, respectively) as originally reported in reference [197].
| Biomarker | Intra-amniotic inflammation | Intra-amniotic infection | ||
|---|---|---|---|---|
| Sensitivity (%) [95% CI] | Specificity (%) [95% CI] | Sensitivity (%) [95% CI] | Specificity (%) [95% CI] | |
| MR score ≥ 3 | 93.0 [84.1–97.4] | 92.4 [89.2–94.0] | 79.5 [68.4–87.8] | 85.6 [81.7–88.5] |
| IL-6 >11.4 ng/mL | 45.0 [34.5–52.4] | 94.8 [91.2–97.4] | 45.2 [36.6–52.9] | 61.9 [44.7–77.3] |
| MMP-8 >23 ng/mL | 92.9 [82.5–97.5] | 66.7 [62.9–68.3] | 90.7 [79.9–96.2] | 65.0 [61.3–67.0] |
MR score: Mass restricted score; IL-6: Interleukin 6; MMP-8: Matrix metalloproteinase 8. Source reference [197].
This study was conducted to examine the diagnostic performance of the MR score in comparison with IL-6, and MMP-8 in the identification of MIAC and intra-amniotic inflammation using the original AF samples examined in developing the MR score [195,196]. This was possible because the original study of the MR score was conducted in collaboration with our unit, and we measured IL-6 and MMP-8 concentrations in 100 of the 101 stored AF samples. Comparing the diagnostic performance of IL-6 and MMP-8 with that of the MR score using these samples has two unique advantages. First, it eliminates potential confounding factors related to technique - that is, potential differences in expertise, subjective evaluation of peaks, and the technique used for mass spectrometry. Second, because the MR score was defined based on these samples, this data set should maximize the diagnostic performance of the MR score. Therefore, the aim of this study was to examine the diagnostic performance of the AF MR score in comparison with that of IL-6 and MMP-8 for the diagnosis of MIAC and inflammation.
Methods
Study population
This study included a total of 100 AF samples remaining out of the total 101 samples used for the study originally reported by Buhimschi et al. [195,196] that were selected from the bank of biological samples of Wayne State University, the Detroit Medical Center, and the Perinatology Research Branch of the Eunice Kennedy Shriver National Institutes of Child Health and Human Development (NICHD). Samples were collected by ultrasound guided transabdominal amniocentesis in patients with symptoms of PTL or preterm PROM. PTL was diagnosed in the presence of regular uterine contractions (at least three in 30 minutes) and documented cervical changes that required admission to the hospital before 37 weeks of gestation. The collection of these samples and the method of storage have been previously described in detail [195,196].
Originally, 77 samples were used to define the MR score [195,196]. These AF samples were selected on the basis of known outcomes (spontaneous PTL or symptoms of PTL but who delivered at term). Subsequently, an additional 24 AF samples, bringing the total to 101 samples, were selected to examine the diagnostic performance of the MR score for the identification of MIAC and inflammation. Mass spectrometry tracings were obtained for these samples using SELDITOF (Ciphergen, Fremont, CA) between May 2001 and April 2002.
The current study was conducted to compare the diagnostic performance of the MR score to that of IL-6 and MMP-8 for the identification of MIAC and intra-amniotic inflammation, and for the diagnosis of delivery before 34 weeks of gestation. The rationale for the selection of such outcome was based on the severity and frequency of adverse outcomes from spontaneous preterm birth in preterm infants (<34 weeks of gestation) [198,199]. The IL-6 and MMP-8 assays of the 100 AF samples were conducted in October 2002 in preparation for the presentation of the results in abstract form at the annual meeting of the Society for Maternal Fetal Medicine in 2003 [195]. Intra-amniotic inflammation was defined as an AF WBC count >100 cells/mm3; MIAC was defined as a positive AF culture. Demographic and clinical information about the mothers and neonates was extracted from medical records.
Immunoassays for IL-6 and MMP-8
Determination of IL-6 concentrations was performed using a commercially available enzyme-linked immunosorbent assay (ELISA) (R & D Systems, Minneapolis, MN) with a sensitivity of 2.3 pg/mL. Inter- and intra-assay coefficients of variations were 9.02% and 7.24%, respectively. MMP-8 concentrations were determined with the use of a commercially available ELISA (Amersham Pharmacia Biotech, Inc. Arlington Heights, IL). The sensitivity of the assay in our laboratory was 0.06 ng/mL; inter- and intra-assay coefficients of variation were 4.6% and 3.7%, respectively.
Statistical analysis
Sensitivity and specificity were calculated and logistic regression models were fit to construct ROC curves portraying the respective diagnostic performance of each of the three markers (MR score, IL-6 and MMP-8) in identifying each of the three selected obstetrical complications (MIAC, intra-amniotic inflammation and preterm delivery before 34 weeks of gestation). Sensitivity and specificity were calculated using thresholds previously reported [195,196] and, separately, sensitivity using cutoffs determined at a fixed false-positive rate of 15%. An additional threshold (IL-6 >2.6 ng/mL) based on previous publications was used to calculate sensitivity and specificity for IL-6 in identifying preterm delivery before 34 weeks of gestation [194,200]. The McNemar’s test and paired sample non-parametric statistical techniques were used to examine differences in diagnostic performance comparing the MR score to IL-6 and MMP-8 for the identification of selected outcomes. A 5% threshold for type I error was used to determine statistical significance. Statistical analyses were performed using SAS version 9.3 (SAS, Cary, NC).
Results
Demographic and clinical characteristics of the study population are presented in Table 2. Forty percent (40/100) of the AF samples showed intra-amniotic inflammation based on AF WBC count >100 cells/mm3. A positive AF culture for microorganisms was present in 34% (34/100) of the AF samples. Sixty-two (62%) mothers delivered prior to 34 weeks of gestation: 40 delivered spontaneously, and 22 were delivered for maternal and/or fetal indications.
Table II.
Demographic and clinical characteristics of the study population, and results of the amniotic fluid analysis
| Clinical characteristics | Patients who delivered at term (≥37 weeks) | Patients who delivered preterm (<37 weeks) | |||
|---|---|---|---|---|---|
| WBC (−) AFC (−) (n=29) |
WBC (+) AFC (+) (n=27) |
WBC (+) AFC (−) (n=13) |
WBC (−) AFC (+) (n=7) |
WBC (−) AFC (−) (n=24) |
|
| Age (years) ł | 25.9 (6.9) | 26.7 (6.1) | 23.2 (4.4) | 28.9 (4.6) | 24.0(5.5) |
| Ethnicity, n (%, African-American) ∞ | 23 (79.3) | 24 (88.9) | 10 (76.9) | 7 (100) | 23 (95.8) |
| Parity§ | 1 [0–5] | 1 [0–7] | 1 [0–5] | 2 [0–6] | 1 [0–9] |
| GA at amniocentesis (weeks) ł | 29.3 (3.3) | 26.5 (4.2) | 27.0 (2.2) | 31.9 (1.5) | 30.0 (3.5) |
| GA at delivery (weeks) ł | 38.6 (1.3) | 26.7 (4.2) | 27.8 (2.2) | 33.0 (1.2)* | 31.1(3.7)* |
| PPROM, n (%)∞ | 0 (0) | 12 (44.4%)* | 4 (30.8%)* | 6 (85.7%)* | 10 (41.7%)* |
| Indicated delivery, n (%)∞ | 3 (10.3) | 12 (44.4%)* | 1 (7.7%) | 4 (57.1%)* | 8 (33.3%)* |
| Spontaneous delivery, n (%)∞ | 26 (89.7) | 15 (55.6%)* | 12 (92. 3%) | 3 (42.9%)* | 16 (66.7%)* |
| Birthweight (g) ł | 3248.2 (411.7) | 1010 (566.2) | 1076.2 (381.3) | 1491.9 (325)* | 1776.3 (643.0)* |
| Amniotic fluid analysis | |||||
| WBC (cell/mm3) § | 5 [0–80] | 1000 [260–19200]* | 1000 [200–14800]* | 10 [3–88]* | 3 [0–62]* |
| Positive Gram stain, n (%) ∞ | 0 (0) | 17 (63.0)* | 1 (7.7) | 3 (42.9)* | 1 (4.2) |
| Glucose concentration <10 mg/dL, n (%) ∞ | 0 (0) | 16 (59.3)* | 6 (46.2)* | 0 (0) | 0 (0) |
| Storage IL-6 (years) § | 3.0 [1.5–9.0] | 2.5 [1.4–2.5] | 2.9 [1.6–3.6] | 3.0 [1.9–8.7] | 2.4 [1.2–9.7] |
| Storage MMP8 (years) § | 3.0 [0.7–10.2] | 2.5 [0.7–9.7] | 3.0 [1.1–9.0] | 3.1 [1.9–8.7] | 2.4 [1.3–9.7] |
| Placental pathology | |||||
| Histological chorioamnionitis, n (%) ∞ | 1 (6.3) | 25 (92.6)* | 13 (100)* | 2 (33.3) | 8 (36.4) |
GA=gestational age; PPROM=preterm prelabor rupture of membranes; WBC=white blood cell count (+WBC: WBC count > 100 cells/mm3); AFC=amniotic fluid cultures.
Missing placental pathology, n=16 (13 in term delivery group).
p < 0.05 versus “normal” group (ł Dunnet’s, ∞ Fisher’s exact, § Dunn’s tests).
Data presented as mean (SD).
Data presented as median [range].
Diagnostic performance in identifying intra-amniotic inflammation
Table 3 shows the sensitivity and specificity for each biomarker in identifying intra-amniotic inflammation using analyte cut-off values previously published (MR score >2, IL-6 >11.4 ng/mL and MMP-8 >23 ng/mL) [197]. There was no difference in sensitivity when comparing the MR score to IL-6 or MMP-8, both using previously identified cut-off values and when using thresholds selected based on a fixed false-positive rate of 15% (both p ≥1.0). There was also no difference in specificity comparing the MR score to IL-6 for the identification of intra-amniotic inflammation when using the previously identified thresholds, whereas MMP-8 had a lower specificity than either of these two markers (both p <0.001). ROC curves characterizing each marker’s performance in identifying intra-amniotic inflammation are shown in Figure 1. The areas under the ROC curves (AUCs) for IL-6 (0.98) and MMP-8 (0.97) were not statistically significantly different from that of the MR score (0.97) (each p ≥ 0.7).
Table III.
Diagnostic performance of IL-6, MMP-8 and the MR score for the identification of intra-amniotic inflammation
| Biomarker | Sensitivity (%) | 95% CI | Specificity (%) | 95% CI |
|---|---|---|---|---|
| MR score ≥ 3 | 95.0% (38/40) | 83.1–99.4 | 95.0% (57/60) | 86.1–99.0 |
| IL-6 >11.4 ng/mL | 95.0% (38/40) | 83.1–99.4 | 91.7% (55/60) | 81.6–97.2 |
| MMP-8 >23 ng/mL | 97.5% (39/40) | 86.8–99.9 | 70.0% (42/60) | 56.8–81.2 |
MR score: Mass restricted score; IL-6: Interleukin 6; MMP-8: Matrix metalloproteinase 8.
Figure 1.
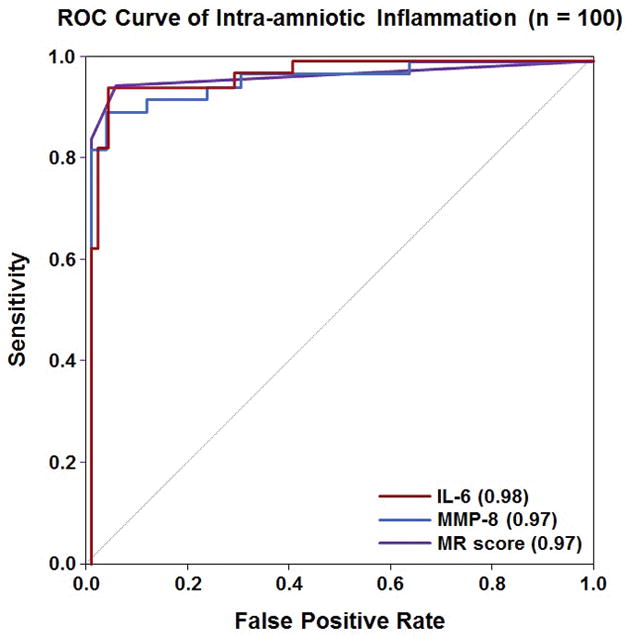
Receiver operating characteristic curve analysis for the use of IL-6, MMP-8 and the Mass Restricted (MR) score for the detection of intra-amniotic inflammation. The AUCs for using IL-6 or MMP-8 were not statistically significantly different from that of the MR score (each p ≥ 0.7).
Diagnostic performance in identifying MIAC
Table 4 shows the sensitivity and specificity for each marker in identifying MIAC using the previously identified thresholds (MR score >2, IL-6 >11.4 ng/mL and MMP-8 >23 ng/mL). There was no difference in sensitivity when comparing the MR score to IL-6 or MMP-8 for the identification of MIAC, either when using previously identified cut-off values (p>0.3) or when using separate thresholds selected based on a fixed false-positive rate of 15% (p >0.3). There was also no difference in specificity comparing the MR score to IL-6 for the identification of MIAC when using the previously identified thresholds, whereas MMP-8 had a lower specificity than either of these two markers (both p <0.01). ROC curves characterizing the performance of the MR score, IL-6, and MMP-8 in identifying MIAC are shown in Figure 2. The AUCs for IL-6 (0.83) and MMP-8 (0.85) were not statistically significantly different from that of the MR score (0.86; p = 0.3 and p = 0.8, respectively).
Table IV.
Diagnostic performance of IL-6, MMP-8 and the MR score to identify a positive amniotic fluid culture or microbial invasion of the amniotic cavity (MIAC)
| Biomarker | Sensitivity (%) | 95% CI | Specificity (%) | 95% CI |
|---|---|---|---|---|
| MR score ≥ 3 | 88.2% (30/34) | 72.6–96.7 | 83.3% (55/66) | 72.1–91.4 |
| IL-6 >11.4 ng/mL | 85.3% (29/34) | 68.9–95.1 | 78.8% (52/66) | 67.0–87.9 |
| MMP-8 >23 ng/mL | 94.1% (32/34) | 80.3–99.3 | 62.1% (41/66) | 49.3–73.8 |
MR score: Mass Restricted score; IL-6: Interleukin 6; MMP-8: Matrix metalloproteinase 8.
Figure 2.
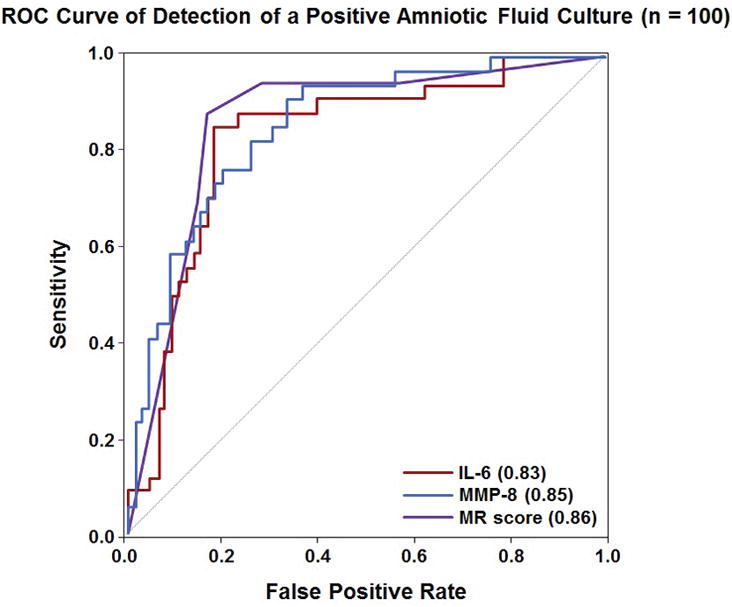
Receiver operating characteristic curve analysis for the use of IL-6, MMP-8 and the Mass Restricted (MR) score for the detection of a positive amniotic fluid culture. The AUCs for IL-6 and MMP-8 were not statistically significantly different from that of the MR score (p = 0.3 and p = 0.8, respectively).
Diagnostic performance in identifying preterm delivery before 34 weeks of gestation
Tables 5 and 6 show the sensitivity and specificity for the MR score, IL-6 and MMP-8 in identifying all deliveries before 34 weeks of gestation and spontaneous preterm delivery before 34 weeks of gestation, respectively. Using previously identified thresholds, there was no significant difference in sensitivity or specificity when comparing the MR score to IL-6 for the identification of all (p = 0.7 and p = 1.0, respectively) and, separately, for spontaneous deliveries, before 34 weeks (both p = 1.0). In contrast, the sensitivity for the MR score was significantly lower than that of MMP-8 (p ≤ 0.02), whereas the specificity of MMP-8 was significantly lower than that of both the MR score and IL-6 (both p < 0.05) for the identification of spontaneous, but not overall, deliveries before 34 weeks of gestation.
Table V.
Diagnostic performance of the MR score, IL-6 and MMP-8 for the identification of patients who delivered before 34 weeks of gestation (spontaneous and indicated)
| Delivery before 34 weeks of gestation | ||||
|---|---|---|---|---|
| Sensitivity | 95%CI | Specificity | 95%CI | |
| MR score ≥ 3 | 66.1% (41/62) | 53.0–77.7 | 100% (38/38) | 90.8–100 |
| IL-6 >2.6 ng/mL | 77.4% (48/62) | 65.0–87.1 | 89.5% (34/38) | 75.2–97.1 |
| IL-6 >11.4 ng/mL | 69.4% (43/62) | 56.4–80.4 | 100% (38/38) | 90.8–100 |
| MMP-8 >23 ng/mL | 83.9% (52/62) | 72.3–92.0 | 86.8% (33/38) | 71.9–95.6 |
MR score: Mass Restricted score; IL-6: Interleukin 6; MMP-8: Matrix metalloproteinase 8.
Table VI.
Diagnostic performance of the MR score, IL-6, and MMP-8 for spontaneous delivery <34 weeks of gestation
| Deliveries < 34 weeks | ||||
|---|---|---|---|---|
| Sensitivity | 95%CI | Specificity | 95%CI | |
| MR score ≥ 3 | 67.5% (27/40) | 50.9–81.4 | 76.7% (46/60) | 64.0–86.6 |
| IL-6 >2.6 ng/mL | 80% (32/40) | 64.4–91.0 | 66.7% (40/60) | 53.3–78.3 |
| IL-6 >11.4 ng/mL | 70% (28/40) | 53.5–83.4 | 75% (45/60) | 62.1–85.3 |
| MMP-8 >23 ng/mL | 85% (34/40) | 70.2–94.3 | 61.7% (37/60) | 48.2–73.9 |
MR score: Mass restricted score; IL-6: Interleukin 6; MMP-8: Matrix metalloproteinase 8.
There was also no significant difference in the sensitivity when comparing the MR score to either IL-6 or MMP-8 using cut-offs selected by fixing the false-positive rate at 15%, either for the identification of all or, separately, for spontaneous preterm delivery before 34 weeks of gestation (p >0.6). In addition, there was no difference in sensitivity comparing the MR score to IL-6 when using a separate threshold (IL-6 >2.6 ng/mL) for the identification of these complications. ROC curves characterizing the performance of the MR score, IL-6, and MMP-8 in identifying patients who delivered spontaneously prior to 34 weeks of gestation are shown in Figure 3. The AUC for the MR score (0.76) was not statistically different from those of IL-6 (0.80) or MMP-8 (0.79) in identifying this outcome (each p = 0.2); the same was true for the identification of all preterm deliveries before 34 weeks of gestation (each p >0.3).
Figure 3.
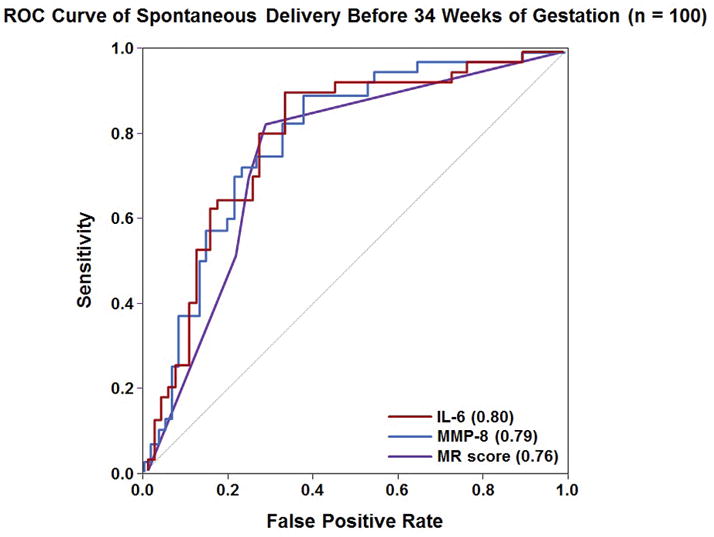
Receiver-operating characteristic curves for the use of IL-6, MMP-8 and the Mass Restricted (MR) score for the detection of intra-amniotic inflammation. The areas under the ROC curves for IL-6 and MMP-8 were not statistically significantly different from that of the MR score (each p = 0.2).
Discussion
Principal findings of this study
(1) There were no significant differences in sensitivity at a fixed false-positive rate of 15%, or AUC, in identifying either MIAC or intra-amniotic inflammation, or preterm delivery before 34 weeks of gestation, when comparing the MR score to AF concentrations of IL-6 or MMP-8; and (2) there were no significant differences in specificity in identifying either MIAC or intra-amniotic inflammation when comparing the MR score to AF concentrations of IL-6 using previously identified thresholds [197]. These findings contradict the claim that the MR score is “clearly superior” to any other clinical test for the diagnosis of MIAC and inflammation [197].
The frequency and clinical significance of microbial invasion of the amniotic cavity and intra-amniotic inflammation
A positive AF culture for bacteria has been reported in approximately 10% of patients with spontaneous PTL and intact membranes [14,15,21,130], 30–40% of patients with preterm PROM [37,41,127,201,202], 9% of patients with a short cervix [42–44], 51% of patients with acute cervical insufficiency [45–49], 10% of patients with PTL and twin gestations [50–52], and 14% of patients with idiopathic vaginal bleeding [53]. With the use of molecular microbiologic techniques, the frequency with which bacteria have been found in AF is even higher [19,21,22,66,203–214]. Moreover, the presence of microbial footprints detected with polymerase chain reaction, even in the absence of microbial growth in the laboratory, is associated with adverse pregnancy outcome [200].
Microbial invasion of the amniotic cavity and intra-amniotic inflammation are risk factors for impending preterm delivery and perinatal mortality and morbidity (e.g. otitis media [215], congenital pneumonia [216,217], admission to the neonatal intensive care unit [202,218], respiratory distress syndrome or chronic lung disease [218–220], congenital sepsis [19,218,219,221], cerebral palsy [112–114,117,118, 222–230] and necrotizing enterocolitis [147,163,231]). Moreover, intra-amniotic infection is associated with clinical chorioamnionitis [217,231–233] and puerperal endometritis [234], and may be complicated by maternal sepsis [235,236] and disseminated intravascular coagulation [237,238].
The early identification of MIAC has implications for the clinical management of the patient with PTL and preterm PROM. For example, in patients with PTL, intact membranes, and the presence of MIAC, tocolytics should not be administered, because they are ineffective and increase the risk of pulmonary edema [239]. After a positive diagnosis, antibiotic treatment can be initiated immediately, rather than waiting for delivery. A randomized clinical trial in which antimicrobial therapy began before delivery, rather than after, showed a decreased rate of neonatal sepsis with early treatment [240]. This trial was conducted in pregnancies near term and was discontinued on the recommendation of the Data and Safety Monitoring Board after the observation of an increased rate of adverse events (sepsis) when treatment was delayed [240]. A similar trial has not been conducted in cases of subclinical MIAC in preterm gestation. However, such a trial may not be ethically possible – it would be difficult to argue that the preterm fetus, generally considered as immunocompromised in comparison with the term fetus/neonate, would not be harmed by delayed treatment (see a detailed discussion of this issue in reference [42]).
Reproducibility in science and medicine
Replication is a cornerstone of scientific validity [241–244]. Many claims by prestigious laboratories and journals have not been subsequently replicated, despite several attempts [245,246]. The lack of replication and its implications are particularly worrisome when dealing with diagnostic tests or therapies intended for clinical applications in humans [247–249]. In the case of pregnancy, false-positive or false-negative results may lead to very serious consequences.
We were not able to replicate the claim that the MR score is superior to other tests (e.g. IL-6) for the detection of MIAC or intra-amniotic inflammation. The findings in this study suggest that IL-6 and the MR score had a higher specificity (although not sensitivity) than MMP-8 for detecting intra-amniotic inflammation (Table 3).
There was no difference in sensitivity when comparing the MR score to IL-6 in identifying the patient who will deliver before 34 weeks of gestation, regardless of the thresholds employed (e.g. those proposed by Buhimschi et al. [MR score >2, IL-6 >11.3 ng/mL], cut-offs selected based on a fixed false-positive rate of 15% or an IL-6 threshold proposed in prior studies [2.6 ng/mL]) [200].
IL-6 was reported to have a high sensitivity for the identification of MIAC in patients with PTL and intact membranes, with values ranging from 80% to 100% [151]. The cut-off of 11.3 ng/mL, established by our group two decades ago, was derived from an analysis of AF samples from 120 patients with spontaneous PTL and/or intact membranes, in which the prevalence of positive AF cultures was 9.2% (11/120) [151]. The analysis showed that, using a cut-off of 11.3 ng/mL, IL-6 was the most sensitive test (100%) for the detection of MIAC compared to glucose (81%), WBC count (63.6%), and Gram stain (63.6%); (p <0.01 for all) [151]. The diagnostic performance of 11.3 ng/mL as a cutoff of IL-6 to identify a positive AF culture was confirmed by our group in another set of patients [150], and subsequently, by other investigators [161,250,251]. The threshold of 2.6 ng/mL was also proposed by our unit to detect intra-amniotic inflammation after we observed that patients with concentrations of IL-6 above 2.6 ng/mL were at risk for preterm delivery, and frequently had evidence of acute chorioamnionitis and/or funisitis on placental examination [200]. Moreover, even with negative cultures, an elevated IL-6 concentration was associated with a short amniocentesis-to-delivery interval and a significantly higher rate of neonatal adverse outcomes [200]. Subsequently, the value of this cut-off as a marker of intra-amniotic inflammation has been confirmed by others [71,162,252].
Strengths and limitations of the study
The diagnostic performance of selected markers was compared using the same AF samples originally used to define the MR score, and, if anything, should have biased the diagnostic performance in favor of the MR score. Yet, the diagnostic performance of the MR score was similar to that of IL-6. In contrast to the findings of the MR score (which have not been independently reproduced), the claims about the sensitivity and specificity of IL-6 in the diagnosis of MIAC [147,151,200,253] have been independently confirmed [161,250,251,254].
Proteomics is used to identify candidate biomarkers in biological fluids and tissues [255–258]; yet, proteomics is largely a discovery (rather than diagnostic) tool. In general, a proteomics approach is used to discover differentially expressed proteins/peptides between diseased and non-diseased states, and targeted assays are designed to identify and quantify differentially-expressed biomarkers which can distinguish health from disease. The targeted assays implemented after discovery are generally immunoassays, because of the wide availability of these assay platforms (e.g. ELISAs). However, chemical assays using mass spectrometry to identify specific biomarkers are also possible. This was one of the hopes of using SELDI-TOF and the MR score for the rapid identification of intra-amniotic inflammation. Unfortunately, the diagnostic performance of the MR score is not superior to that of a single ELISA for IL-6, and the instrumentation to perform SELDI-TOF has not gained popularity in clinical laboratories. It has accordingly been largely abandoned in research laboratories in favor of more sensitive and accurate mass spectrometry techniques. Proteomic platforms have been used successfully to identify biomarkers for the adequate identification of spontaneous preterm birth and adverse pregnancy outcome using cervical/vaginal fluid [259–264], AF [265–269] and maternal serum [265,270,271].
Conclusions
Immunoassays for IL-6 or MMP-8 can be used to identify intra-amniotic inflammation and MIAC (which is frequently associated with intra-amniotic inflammation) with equivalent diagnostic performance to the MR score. There is no justification to use the MR score, which requires SELDITOF-technology which is not available in most clinical units. Proteomics may be used to discover new biomarkers of intra-amniotic inflammation, infection, or other disease states associated with preterm labor. Advances in mass spectrometry may render these approaches feasible (and even chemical assays of biomarkers possible) in the clinical setting, and this possibility requires future studies.
Footnotes
Disclosure: The authors report no conflicts of interest.
Declaration of interest: This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver, National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services (NICHD/NIH); and, in part, with Federal funds from NICHD, NIH under Contract No. HHSN275201300006C.
References
- 1.Muglia LJ, Katz M. The enigma of spontaneous preterm birth. N Engl J Med. 2010;362:529–35. doi: 10.1056/NEJMra0904308. [DOI] [PubMed] [Google Scholar]
- 2.Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. BJOG. 2006;113:17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iams JD, Romero R, Culhane JF, Goldenberg RL. Primary, secondary, and tertiary interventions to reduce the morbidity and mortality of preterm birth. Lancet. 2008;371:164–75. doi: 10.1016/S0140-6736(08)60108-7. [DOI] [PubMed] [Google Scholar]
- 4.Lawn JE, Cousens S, Zupan J. 4 million neonatal deaths: when? Where? Why? Lancet. 2005;365:891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 5.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bobitt JR, Ledger WJ. Unrecognized amnionitis and prematurity: a preliminary report. J Reprod Med. 1977;19:8–12. [PubMed] [Google Scholar]
- 7.Bobitt JR, Ledger WJ. Amniotic fluid analysis. Its role in maternal neonatal infection. Obstet Gynecol. 1978;51:56–62. [PubMed] [Google Scholar]
- 8.Miller JM, Jr, Pupkin MJ, Hill GB. Bacterial colonization of amniotic fluid from intact fetal membranes. Am J Obstet Gynecol. 1980;136:796–804. doi: 10.1016/0002-9378(80)90458-5. [DOI] [PubMed] [Google Scholar]
- 9.Wallace RL, Herrick CN. Amniocentesis in the evaluation of premature labor. Obstet Gynecol. 1981;57:483–6. [PubMed] [Google Scholar]
- 10.Bobitt JR, Hayslip CC, Damato JD. Amniotic fluid infection as determined by transabdominal amniocentesis in patients with intact membranes in premature labor. Am J Obstet Gynecol. 1981;140:947–52. doi: 10.1016/0002-9378(81)90090-9. [DOI] [PubMed] [Google Scholar]
- 11.Wahbeh CJ, Hill GB, Eden RD, Gall SA. Intra-amniotic bacterial colonization in premature labor. Am J Obstet Gynecol. 1984;148:739–43. doi: 10.1016/0002-9378(84)90558-1. [DOI] [PubMed] [Google Scholar]
- 12.Gravett MG, Hummel D, Eschenbach DA, Holmes KK. Preterm labor associated with subclinical amniotic fluid infection and with bacterial vaginosis. Obstet Gynecol. 1986;67:229–37. doi: 10.1097/00006250-198602000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Romero R, Mazor M, Wu YK, et al. Infection in the pathogenesis of preterm labor. Semin Perinatol. 1988;12:262–79. [PubMed] [Google Scholar]
- 14.Romero R, Sirtori M, Oyarzun E, et al. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol. 1989;161:817–24. doi: 10.1016/0002-9378(89)90409-2. [DOI] [PubMed] [Google Scholar]
- 15.Skoll MA, Moretti ML, Sibai BM. The incidence of positive amniotic fluid cultures in patients preterm labor with intact membranes. Am J Obstet Gynecol. 1989;161:813–6. doi: 10.1016/0002-9378(89)90407-9. [DOI] [PubMed] [Google Scholar]
- 16.Gibbs RS, Romero R, Hillier SL, et al. A review of premature birth and subclinical infection. Am J Obstet Gynecol. 1992;166:1515–28. doi: 10.1016/0002-9378(92)91628-n. [DOI] [PubMed] [Google Scholar]
- 17.Watts DH, Krohn MA, Hillier SL, Eschenbach DA. The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstet Gynecol. 1992;79:351–7. doi: 10.1097/00006250-199203000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Andrews WW, Hauth JC, Goldenberg RL, et al. Amniotic fluid interleukin-6: correlation with upper genital tract microbial colonization and gestational age in women delivered after spontaneous labor versus indicated delivery. Am J Obstet Gynecol. 1995;173:606–12. doi: 10.1016/0002-9378(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 19.Yoon BH, Romero R, Kim M, et al. Clinical implications of detection of Ureaplasma urealyticum in the amniotic cavity with the polymerase chain reaction. Am J Obstet Gynecol. 2000;183:1130–7. doi: 10.1067/mob.2000.109036. [DOI] [PubMed] [Google Scholar]
- 20.Jacobsson B, Mattsby-Baltzer I, Andersch B, et al. Microbial invasion and cytokine response in amniotic fluid in a Swedish population of women in preterm labor. Acta Obstet Gynecol Scand. 2003;82:120–8. doi: 10.1034/j.1600-0412.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 21.Yoon BH, Romero R, Lim JH, et al. The clinical significance of detecting Ureaplasma urealyticum by the polymerase chain reaction in the amniotic fluid of patients with preterm labor. Am J Obstet Gynecol. 2003;189:919–24. doi: 10.1067/s0002-9378(03)00839-1. [DOI] [PubMed] [Google Scholar]
- 22.DiGiulio DB, Romero R, Amogan HP, et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One. 2008;3:e3056. doi: 10.1371/journal.pone.0003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DiGiulio DB, Gervasi M, Romero R, et al. Microbial invasion of the amniotic cavity in preeclampsia as assessed by cultivation and sequence-based methods. J Perinat Med. 2010;38:503–13. doi: 10.1515/JPM.2010.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DiGiulio DB, Gervasi MT, Romero R, et al. Microbial invasion of the amniotic cavity in pregnancies with small-for-gestational-age fetuses. J Perinat Med. 2010;38:495–502. doi: 10.1515/JPM.2010.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romero R, Roslansky P, Oyarzun E, et al. Labor and infection. II. Bacterial endotoxin in amniotic fluid and its relationship to the onset of preterm labor. Am J Obstet Gynecol. 1988;158:1044–9. doi: 10.1016/0002-9378(88)90216-5. [DOI] [PubMed] [Google Scholar]
- 26.Romero R, Brody DT, Oyarzun E, et al. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol. 1989;160:1117–23. doi: 10.1016/0002-9378(89)90172-5. [DOI] [PubMed] [Google Scholar]
- 27.Romero R, Mazor M, Brandt F, et al. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol. 1992;27:117–23. doi: 10.1111/j.1600-0897.1992.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 28.Romero R, Gomez R, Chaiworapongsa T, et al. The role of infection in preterm labour and delivery. Paediatr Perinat Epidemiol. 2001;15:41–56. doi: 10.1046/j.1365-3016.2001.00007.x. [DOI] [PubMed] [Google Scholar]
- 29.Gray DJ, Robinson HB, Malone J, Thomson RB., Jr Adverse outcome in pregnancy following amniotic fluid isolation of Ureaplasma urealyticum. Prenat Diagn. 1992;12:111–7. doi: 10.1002/pd.1970120206. [DOI] [PubMed] [Google Scholar]
- 30.Seong HS, Lee SE, Kang JH, et al. The frequency of microbial invasion of the amniotic cavity and histologic chorioamnionitis in women at term with intact membranes in the presence or absence of labor. Am J Obstet Gynecol. 2008;199:375e371–5. doi: 10.1016/j.ajog.2008.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romero R, Lockwood CJ. Pathogenesis of Spontaneous Preterm Labor. In: Creasy RK, Resnik R, Iams JD, editors. Creasy and Resnik’s Maternal-Fetal Medicine: Principles and Practice. Philadelphia: Elsevier; 2009. pp. 521–43. [Google Scholar]
- 32.Gervasi MT, Romero R, Bracalente G, et al. Midtrimester amniotic fluid concentrations of interleukin-6 and interferon-gammainducible protein-10: evidence for heterogeneity of intra-amniotic inflammation and associations with spontaneous early (532 weeks) and late (432 weeks) preterm delivery. J Perinat Med. 2012;40:329–43. doi: 10.1515/jpm-2012-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garite TJ, Freeman RK, Linzey EM, Braly P. The use of amniocentesis in patients with premature rupture of membranes. Obstet Gynecol. 1979;54:226–30. [PubMed] [Google Scholar]
- 34.Miller JM, Jr, Hill GB, Welt SI, Pupkin MJ. Bacterial colonization of amniotic fluid in the presence of ruptured membranes. Am J Obstet Gynecol. 1980;137:451–8. doi: 10.1016/0002-9378(80)91126-6. [DOI] [PubMed] [Google Scholar]
- 35.Cotton DB, Hill LM, Strassner HT, et al. Use of amniocentesis in preterm gestation with ruptured membranes. Obstet Gynecol. 1984;63:38–43. [PubMed] [Google Scholar]
- 36.Broekhuizen FF, Gilman M, Hamilton PR. Amniocentesis for gram stain and culture in preterm premature rupture of the membranes. Obstet Gynecol. 1985;66:316–21. [PubMed] [Google Scholar]
- 37.Romero R, Quintero R, Oyarzun E, et al. Intraamniotic infection and the onset of labor in preterm premature rupture of the membranes. Am J Obstet Gynecol. 1988;159:661–6. doi: 10.1016/s0002-9378(88)80030-9. [DOI] [PubMed] [Google Scholar]
- 38.Asrat T, Nageotte MP, Garite TJ, et al. Gram stain results from amniocentesis in patients with preterm premature rupture of membranes–comparison of maternal and fetal characteristics. Am J Obstet Gynecol. 1990;163:887–9. doi: 10.1016/0002-9378(90)91089-u. [DOI] [PubMed] [Google Scholar]
- 39.Gomez R, Romero R, Edwin SS, David C. Pathogenesis of preterm labor and preterm premature rupture of membranes associated with intraamniotic infection. Infect Dis Clin North Am. 1997;11:135–76. doi: 10.1016/s0891-5520(05)70347-0. [DOI] [PubMed] [Google Scholar]
- 40.Jacobsson B, Mattsby-Baltzer I, Andersch B, et al. Microbial invasion and cytokine response in amniotic fluid in a Swedish population of women with preterm prelabor rupture of membranes. Acta Obstet Gynecol Scand. 2003;82:423–31. doi: 10.1034/j.1600-0412.2003.00157.x. [DOI] [PubMed] [Google Scholar]
- 41.DiGiulio DB, Romero R, Kusanovic JP, et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol. 2010;64:38–57. doi: 10.1111/j.1600-0897.2010.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gomez R, Romero R, Nien JK, et al. A short cervix in women with preterm labor and intact membranes: a risk factor for microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 2005;192:678–89. doi: 10.1016/j.ajog.2004.10.624. [DOI] [PubMed] [Google Scholar]
- 43.Hassan S, Romero R, Hendler I, et al. A sonographic short cervix as the only clinical manifestation of intra-amniotic infection. J Perinat Med. 2006;34:13–19. doi: 10.1515/JPM.2006.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vaisbuch E, Hassan SS, Mazaki-Tovi S, et al. Patients with an asymptomatic short cervix (5or¼15 mm) have a high rate of subclinical intraamniotic inflammation: implications for patient counseling. Am J Obstet Gynecol. 2010;202:433e431–8. doi: 10.1016/j.ajog.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romero R, Gonzalez R, Sepulveda W, et al. Infection and labor. VIII. Microbial invasion of the amniotic cavity in patients with suspected cervical incompetence: prevalence and clinical significance. Am J Obstet Gynecol. 1992;167:1086–91. doi: 10.1016/s0002-9378(12)80043-3. [DOI] [PubMed] [Google Scholar]
- 46.Mays JK, Figueroa R, Shah J, et al. Amniocentesis for selection before rescue cerclage. Obstet Gynecol. 2000;95:652–5. doi: 10.1016/s0029-7844(99)00633-x. [DOI] [PubMed] [Google Scholar]
- 47.Lee SE, Romero R, Park CW, et al. The frequency and significance of intraamniotic inflammation in patients with cervical insufficiency. Am J Obstet Gynecol. 2008;198:633e631–8. doi: 10.1016/j.ajog.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 48.Bujold E, Morency AM, Rallu F, et al. Bacteriology of amniotic fluid in women with suspected cervical insufficiency. J Obstet Gynaecol Can. 2008;30:882–7. doi: 10.1016/S1701-2163(16)32967-X. [DOI] [PubMed] [Google Scholar]
- 49.Oh KJ, Lee SE, Jung H, et al. Detection of ureaplasmas by the polymerase chain reaction in the amniotic fluid of patients with cervical insufficiency. J Perinat Med. 2010;38:261–8. doi: 10.1515/JPM.2010.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Romero R, Shamma F, Avila C, et al. Infection and labor. VI. Prevalence, microbiology, and clinical significance of intraamniotic infection in twin gestations with preterm labor. Am J Obstet Gynecol. 1990;163:757–61. doi: 10.1016/0002-9378(90)91063-i. [DOI] [PubMed] [Google Scholar]
- 51.Mazor M, Hershkovitz R, Ghezzi F, et al. Intraamniotic infection in patients with preterm labor and twin pregnancies. Acta Obstet Gynecol Scand. 1996;75:624–7. doi: 10.3109/00016349609054686. [DOI] [PubMed] [Google Scholar]
- 52.Yoon BH, Park KH, Koo JN, et al. Intramniotic infection of twin pregnancies with preterm labor. Am J Obstet Gynecol. 1997;176:S35. [Google Scholar]
- 53.Gomez R, Romero R, Nien JK, et al. Idiopathic vaginal bleeding during pregnancy as the only clinical manifestation of intrauterine infection. J Matern Fetal Neonatal Med. 2005;18:31–7. doi: 10.1080/14767050500217863. [DOI] [PubMed] [Google Scholar]
- 54.Park CW, Moon KC, Park JS, et al. The frequency and clinical significance of intra-uterine infection and inflammation in patients with placenta previa and preterm labor and intact membranes. Placenta. 2009;30:613–8. doi: 10.1016/j.placenta.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 55.Madan I, Romero R, Kusanovic JP, et al. The frequency and clinical significance of intra-amniotic infection and/or inflammation in women with placenta previa and vaginal bleeding: an unexpected observation. J Perinat Med. 2010;38:275–9. doi: 10.1515/JPM.2010.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thorp JM, Jr, Katz VL, Fowler LJ, et al. Fetal death from chlamydial infection across intact amniotic membranes. Am J Obstet Gynecol. 1989;161:1245–6. doi: 10.1016/0002-9378(89)90675-3. [DOI] [PubMed] [Google Scholar]
- 57.Goldenberg RL, Thompson C. The infectious origins of stillbirth. Am J Obstet Gynecol. 2003;189:861–73. doi: 10.1067/s0002-9378(03)00470-8. [DOI] [PubMed] [Google Scholar]
- 58.Blackwell S, Romero R, Chaiworapongsa T, et al. Maternal and fetal inflammatory responses in unexplained fetal death. J Matern Fetal Neonatal Med. 2003;14:151–7. doi: 10.1080/jmf.14.3.151.157. [DOI] [PubMed] [Google Scholar]
- 59.Horvath B, Yang M, Manning FA. Intrauterine fetal death caused by Haemophilus influenzae infection: a case report. J Reprod Med. 2008;53:55–6. [PubMed] [Google Scholar]
- 60.Vigliani M. Chorioamnionitis and intrauterine fetal death after second-trimester amniocentesis. Fetal Diagn Ther. 2009;26:216–8. doi: 10.1159/000257087. [DOI] [PubMed] [Google Scholar]
- 61.Cassell GH, Davis RO, Waites KB, et al. Isolation of Mycoplasma hominis and Ureaplasma urealyticum from amniotic fluid at 16–20 weeks of gestation: potential effect on outcome of pregnancy. Sex Transm Dis. 1983;10:294–302. [PubMed] [Google Scholar]
- 62.Horowitz S, Mazor M, Romero R, et al. Infection of the amniotic cavity with Ureaplasma urealyticum in the midtrimester of pregnancy. J Reprod Med. 1995;40:375–9. [PubMed] [Google Scholar]
- 63.Berg TG, Philpot KL, Welsh MS, et al. Ureaplasma/Mycoplasmainfected mniotic fluid: pregnancy outcome in treated and nontreated patients. J Perinatol. 1999;19:275–7. doi: 10.1038/sj.jp.7200185. [DOI] [PubMed] [Google Scholar]
- 64.Nguyen DP, Gerber S, Hohlfeld P, et al. Mycoplasma hominis in mid-trimester amniotic fluid: relation to pregnancy outcome. J Perinat Med. 2004;32:323–6. doi: 10.1515/JPM.2004.060. [DOI] [PubMed] [Google Scholar]
- 65.Rallu F, Morency AM, Laferriere C, Bujold E. Invasion of the amniotic cavity by an uncultured bacterium, a Gram-positive coccus. J Matern Fetal Neonatal Med. 2007;20:185–7. doi: 10.1080/14767050601135212. [DOI] [PubMed] [Google Scholar]
- 66.Rodriguez N, Fernandez C, Zamora Y, et al. Detection of Ureaplasma urealyticum and Ureaplasma parvum in amniotic fluid: association with pregnancy outcomes. J Matern Fetal Neonatal Med. 2011;24:47–50. doi: 10.3109/14767058.2010.482609. [DOI] [PubMed] [Google Scholar]
- 67.Prevedourakis CN, Strigou-Charalabis E, Kaskarelis DB. Bacterial invasion of amniotic cavity during pregnancy and labor. Obstet Gynecol. 1971;37:459–61. [PubMed] [Google Scholar]
- 68.Goldstein I, Zimmer EZ, Merzbach D, et al. Intraamniotic infection in the very early phase of the second trimester. Am J Obstet Gynecol. 1990;163:1261–3. doi: 10.1016/0002-9378(90)90703-a. [DOI] [PubMed] [Google Scholar]
- 69.Romero R, Mazor M, Morrotti R, et al. Infection and labor. VII. Microbial invasion of the amniotic cavity in spontaneous rupture of membranes at term. Am J Obstet Gynecol. 1992;166:129–33. doi: 10.1016/0002-9378(92)91845-2. [DOI] [PubMed] [Google Scholar]
- 70.Romero R, Nores J, Mazor M, et al. Microbial invasion of the amniotic cavity during term labor. Prevalence and clinical significance. J Reprod Med. 1993;38:543–8. [PubMed] [Google Scholar]
- 71.Gomez R, Ghezzi F, Romero R, et al. Premature labor and intraamniotic infection. Clinical aspects and role of the cytokines in diagnosis and pathophysiology. Clin Perinatol. 1995;22:281–342. [PubMed] [Google Scholar]
- 72.Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev. 2002;8:3–13. doi: 10.1002/mrdd.10008. [DOI] [PubMed] [Google Scholar]
- 73.Maxwell NC, Davies PL, Kotecha S. Antenatal infection and inflammation: what’s new? Curr Opin Infect Dis. 2006;19:253–8. doi: 10.1097/01.qco.0000224819.42729.2e. [DOI] [PubMed] [Google Scholar]
- 74.Hameed C, Tejani N, Verma UL, Archbald F. Silent chorioamnionitis as a cause of preterm labor refractory to tocolytic therapy. Am J Obstet Gynecol. 1984;149:726–30. doi: 10.1016/0002-9378(84)90111-x. [DOI] [PubMed] [Google Scholar]
- 75.Duff P, Kopelman JN. Subclinical intra-amniotic infection in asymptomatic patients with refractory preterm labor. Obstet Gynecol. 1987;69:756–9. [PubMed] [Google Scholar]
- 76.Blackwell SC, Berry SM. Role of amniocentesis for the diagnosis of subclinical intra-amniotic infection in preterm premature rupture of the membranes. Curr Opin Obstet Gynecol. 1999;11:541–7. doi: 10.1097/00001703-199912000-00001. [DOI] [PubMed] [Google Scholar]
- 77.Chaiworapongsa T, Romero R, Berry SM, et al. The role of granulocyte colony-stimulating factor in the neutrophilia observed in the fetal inflammatory response syndrome. J Perinat Med. 2011;39:653–66. doi: 10.1515/JPM.2011.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Savasan ZA, Chaiworapongsa T, Romero R, et al. Interleukin-19 in fetal systemic inflammation. J Matern Fetal Neonatal Med. 2012;25:995–1005. doi: 10.3109/14767058.2011.605917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stampalija T, Romero R, Korzeniewski SJ, et al. Soluble ST2 in the fetal inflammatory response syndrome: in vivo evidence of activation of the anti-inflammatory limb of the immune response. J Matern Fetal Neonatal Med. 2013;26(14):1384–93. doi: 10.3109/14767058.2013.784258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gomez R, Romero R, Ghezzi F, et al. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179:194–202. doi: 10.1016/s0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- 81.Yoon BH, Romero R, Kim KS, et al. A systemic fetal inflammatory response and the development of bronchopulmonary dysplasia. Am J Obstet Gynecol. 1999;181:773–9. doi: 10.1016/s0002-9378(99)70299-1. [DOI] [PubMed] [Google Scholar]
- 82.Gravett MG, Hitti J, Hess DL, Eschenbach DA. Intrauterine infection and preterm delivery: evidence for activation of the fetal hypothalamic-pituitary-adrenal axis. Am J Obstet Gynecol. 2000;182:1404–13. doi: 10.1067/mob.2000.106180. [DOI] [PubMed] [Google Scholar]
- 83.Dammann O, Phillips TM, Allred EN, et al. Mediators of fetal inflammation in extremely low gestational age newborns. Cytokine. 2001;13:234–9. doi: 10.1006/cyto.2000.0820. [DOI] [PubMed] [Google Scholar]
- 84.Witt A, Berger A, Gruber CJ, et al. IL-8 concentrations in maternal serum, amniotic fluid and cord blood in relation to different pathogens within the amniotic cavity. J Perinat Med. 2005;33:22–6. doi: 10.1515/JPM.2005.003. [DOI] [PubMed] [Google Scholar]
- 85.Gotsch F, Romero R, Kusanovic JP, et al. The fetal inflammatory response syndrome. Clin Obstet Gynecol. 2007;50:652–83. doi: 10.1097/GRF.0b013e31811ebef6. [DOI] [PubMed] [Google Scholar]
- 86.Madsen-Bouterse SA, Romero R, Tarca AL, et al. The transcriptome of the fetal inflammatory response syndrome. Am J Reprod Immunol. 2010;63:73–92. doi: 10.1111/j.1600-0897.2009.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kacerovsky M, Cobo T, Andrys C, et al. The fetal inflammatory response in subgroups of women with preterm prelabor rupture of the membranes. J Matern Fetal Neonatal Med. 2013;26:795–801. doi: 10.3109/14767058.2013.765404. [DOI] [PubMed] [Google Scholar]
- 88.Kramer BW, Moss TJ, Willet KE, et al. Dose and time response after intraamniotic endotoxin in preterm lambs. Am J Respir Crit Care Med. 2001;164:982–8. doi: 10.1164/ajrccm.164.6.2103061. [DOI] [PubMed] [Google Scholar]
- 89.Moss TJ, Nitsos I, Ikegami M, et al. Experimental intrauterine Ureaplasma infection in sheep. Am J Obstet Gynecol. 2005;192:1179–86. doi: 10.1016/j.ajog.2004.11.063. [DOI] [PubMed] [Google Scholar]
- 90.Goldenberg RL, Andrews WW, Goepfert AR, et al. The Alabama Preterm Birth Study: umbilical cord blood Ureaplasma urealyticum and Mycoplasma hominis cultures in very preterm newborn infants. Am J Obstet Gynecol. 2008;198:43e41–5. doi: 10.1016/j.ajog.2007.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moss TJ, Knox CL, Kallapur SG, et al. Experimental amniotic fluid infection in sheep: effects of Ureaplasma parvum serovars 3 and 6 on preterm or term fetal sheep. Am J Obstet Gynecol. 2008;198:122e121–8. doi: 10.1016/j.ajog.2007.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pacora P, Chaiworapongsa T, Maymon E, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med. 2002;11:18–25. doi: 10.1080/jmf.11.1.18.25. [DOI] [PubMed] [Google Scholar]
- 93.Leviton A, Hecht JL, Allred EN, et al. Persistence after birth of systemic inflammation associated with umbilical cord inflammation. J Reprod Immunol. 2011;90:235–43. doi: 10.1016/j.jri.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 94.Carroll SG, Nicolaides KH. Fetal haematological response to intra-uterine infection in preterm prelabour amniorrhexis. Fetal Diagn Ther. 1995;10:279–85. doi: 10.1159/000264244. [DOI] [PubMed] [Google Scholar]
- 95.Sampson JE, Theve RP, Blatman RN, et al. Fetal origin of amniotic fluid polymorphonuclear leukocytes. Am J Obstet Gynecol. 1997;176:77–81. doi: 10.1016/s0002-9378(97)80015-4. [DOI] [PubMed] [Google Scholar]
- 96.Kramer BW, Kallapur SG, Moss TJ, et al. Intra-amniotic LPS modulation of TLR signaling in lung and blood monocytes of fetal sheep. Innate Immun. 2009;15:101–7. doi: 10.1177/1753425908100455. [DOI] [PubMed] [Google Scholar]
- 97.Romero R, Savasan ZA, Chaiworapongsa T, et al. Hematologic profile of the fetus with systemic inflammatory response syndrome. J Perinat Med. 2011;40:19–32. doi: 10.1515/JPM.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yoon BH, Romero R, Jun JK, et al. An increase in fetal plasma cortisol but not dehydroepiandrosterone sulfate is followed by the onset of preterm labor in patients with preterm premature rupture of the membranes. Am J Obstet Gynecol. 1998;179:1107–14. doi: 10.1016/s0002-9378(98)70114-0. [DOI] [PubMed] [Google Scholar]
- 99.Kim YM, Romero R, Chaiworapongsa T, et al. Dermatitis as a component of the fetal inflammatory response syndrome is associated with activation of Toll-like receptors in epidermal keratinocytes. Histopathology. 2006;49:506–14. doi: 10.1111/j.1365-2559.2006.02542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kemp MW, Saito M, Nitsos I, et al. Exposure to in utero lipopolysaccharide induces inflammation in the fetal ovine skin. Reprod Sci. 2011;18:88–98. doi: 10.1177/1933719110380470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Romero R, Espinoza J, Goncalves LF, et al. Fetal cardiac dysfunction in preterm premature rupture of membranes. J Matern Fetal Neonatal Med. 2004;16:146–57. doi: 10.1080/14767050400009279. [DOI] [PubMed] [Google Scholar]
- 102.Di Naro E, Cromi A, Ghezzi F, et al. Fetal thymic involution: a sonographic marker of the fetal inflammatory response syndrome. Am J Obstet Gynecol. 2006;194:153–9. doi: 10.1016/j.ajog.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 103.Murphy DJ, Sellers S, MacKenzie IZ, et al. Case-control study of antenatal and intrapartum risk factors for cerebral palsy in very preterm singleton babies. Lancet. 1995;346:1449–54. doi: 10.1016/s0140-6736(95)92471-x. [DOI] [PubMed] [Google Scholar]
- 104.Watterberg KL, Demers LM, Scott SM, Murphy S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics. 1996;97:210–5. [PubMed] [Google Scholar]
- 105.Jobe AH, Newnham JP, Willet KE, et al. Effects of antenatal endotoxin and glucocorticoids on the lungs of preterm lambs. Am J Obstet Gynecol. 2000;182:401–8. doi: 10.1016/s0002-9378(00)70231-6. [DOI] [PubMed] [Google Scholar]
- 106.Speer CP. New insights into the pathogenesis of pulmonary inflammation in preterm infants. Biol Neonate. 2001;79:205–9. doi: 10.1159/000047092. [DOI] [PubMed] [Google Scholar]
- 107.Willet KE, Kramer BW, Kallapur SG, et al. Intra-amniotic injection of IL-1 induces inflammation and maturation in fetal sheep lung. Am J Physiol Lung Cell Mol Physiol. 2002;282:L411–20. doi: 10.1152/ajplung.00097.2001. [DOI] [PubMed] [Google Scholar]
- 108.Speer CP. Inflammation and bronchopulmonary dysplasia. Semin Neonatol. 2003;8:29–38. doi: 10.1016/s1084-2756(02)00190-2. [DOI] [PubMed] [Google Scholar]
- 109.Jobe AH, Ikegami M. Antenatal infection/inflammation and postnatal lung maturation and injury. Respir Res. 2001;2:27–32. doi: 10.1186/rr35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jobe AH. Antenatal associations with lung maturation and infection. J Perinatol. 2005;25:S31–5. doi: 10.1038/sj.jp.7211317. [DOI] [PubMed] [Google Scholar]
- 111.Kramer BW, Kallapur S, Newnham J, Jobe AH. Prenatal inflammation and lung development. Semin Fetal Neonatal Med. 2009;14:2–7. doi: 10.1016/j.siny.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yoon BH, Romero R, Yang SH, et al. Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am J Obstet Gynecol. 1996;174:1433–40. doi: 10.1016/s0002-9378(96)70585-9. [DOI] [PubMed] [Google Scholar]
- 113.Yoon BH, Jun JK, Romero R, et al. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol. 1997;177:19–26. doi: 10.1016/s0002-9378(97)70432-0. [DOI] [PubMed] [Google Scholar]
- 114.Yoon BH, Romero R, Kim CJ, et al. High expression of tumor necrosis factor-alpha and interleukin-6 in periventricular leukomalacia. Am J Obstet Gynecol. 1997;177:406–11. doi: 10.1016/s0002-9378(97)70206-0. [DOI] [PubMed] [Google Scholar]
- 115.Dammann O, Leviton A. Infection remote from the brain, neonatal white matter damage, and cerebral palsy in the preterm infant. Semin Pediatr Neurol. 1998;5:190–201. doi: 10.1016/s1071-9091(98)80034-x. [DOI] [PubMed] [Google Scholar]
- 116.Grether JK, Nelson KB. Maternal infection and cerebral palsy in infants of normal birth weight. JAMA. 1997;278:207–11. [PubMed] [Google Scholar]
- 117.Leviton A, Paneth N, Reuss ML, et al. Maternal infection, fetal inflammatory response, and brain damage in very low birth weight infants. Developmental Epidemiology Network Investigators. Pediatr Res. 1999;46:566–75. doi: 10.1203/00006450-199911000-00013. [DOI] [PubMed] [Google Scholar]
- 118.Moon JB, Kim JC, Yoon BH, et al. Amniotic fluid matrix metalloproteinase-8 and the development of cerebral palsy. J Perinat Med. 2002;30:301–6. doi: 10.1515/JPM.2002.044. [DOI] [PubMed] [Google Scholar]
- 119.Wharton KN, Pinar H, Stonestreet BS, et al. Severe umbilical cord inflammation-a predictor of periventricular leukomalacia in very low birth weight infants. Early Hu Dev. 2004;77:77–87. doi: 10.1016/j.earlhumdev.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 120.Hagberg H, Mallard C. Effect of inflammation on central nervous system development and vulnerability. Curr Opin Neurol. 2005;18:117–23. doi: 10.1097/01.wco.0000162851.44897.8f. [DOI] [PubMed] [Google Scholar]
- 121.Romero R, Gomez R, Ghezzi F, et al. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol. 1998;179:186–93. doi: 10.1016/s0002-9378(98)70271-6. [DOI] [PubMed] [Google Scholar]
- 122.Romero R, Espinoza J, Hassan S, et al. Soluble receptor for advanced glycation end products (sRAGE) and endogenous secretory RAGE (esRAGE) in amniotic fluid: modulation by infection and inflammation. J Perinat Med. 2008;36:388–98. doi: 10.1515/JPM.2008.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Romero R, Chaiworapongsa T, Alpay Savasan Z, et al. Damage-associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: a study of the alarmin HMGB1. J Matern Fetal Neonatal Med. 2011;24:1444–55. doi: 10.3109/14767058.2011.591460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Romero R, Chaiworapongsa T, Savasan ZA, et al. Clinical chorioamnionitis is characterized by changes in the expression of the alarmin HMGB1 and one of its receptors, sRAGE. J Matern Fetal Neonatal Med. 2012;25:558–67. doi: 10.3109/14767058.2011.599083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Romero R, Emamian M, Quintero R, et al. The value and limitations of the Gram stain examination in the diagnosis of intraamniotic infection. Am J Obstet Gynecol. 1988;159:114–9. doi: 10.1016/0002-9378(88)90503-0. [DOI] [PubMed] [Google Scholar]
- 126.Gauthier DW, Meyer WJ. Comparison of gram stain, leukocyte esterase activity, and amniotic fluid glucose concentration in predicting amniotic fluid culture results in preterm premature rupture of membranes. Am J Obstet Gynecol. 1992;167:1092–5. doi: 10.1016/s0002-9378(12)80044-5. [DOI] [PubMed] [Google Scholar]
- 127.Romero R, Yoon BH, Mazor M, et al. A comparative study of the diagnostic performance of amniotic fluid glucose, white blood cell count, interleukin-6, and gram stain in the detection of microbial invasion in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 1993;169:839–51. doi: 10.1016/0002-9378(93)90014-a. [DOI] [PubMed] [Google Scholar]
- 128.Garry D, Figueroa R, Aguero-Rosenfeld M, et al. A comparison of rapid amniotic fluid markers in the prediction of microbial invasion of the uterine cavity and preterm delivery. Am J Obstet Gynecol. 1996;175:1336–41. doi: 10.1016/s0002-9378(96)70051-0. [DOI] [PubMed] [Google Scholar]
- 129.Hussey MJ, Levy ES, Pombar X, et al. Evaluating rapid diagnostic tests of intra-amniotic infection: Gram stain, amniotic fluid glucose level, and amniotic fluid to serum glucose level ratio. Am J Obstet Gynecol. 1998;179:650–6. doi: 10.1016/s0002-9378(98)70059-6. [DOI] [PubMed] [Google Scholar]
- 130.Odibo AO, Rodis JF, Sanders MM, et al. Relationship of amniotic fluid markers of intra-amniotic infection with histopathology in cases of preterm labor with intact membranes. J Perinatol. 1999;19:407–12. doi: 10.1038/sj.jp.7200210. [DOI] [PubMed] [Google Scholar]
- 131.Romero R, Jimenez C, Lohda AK, et al. Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. Am J Obstet Gynecol. 1990;163:968–74. doi: 10.1016/0002-9378(90)91106-m. [DOI] [PubMed] [Google Scholar]
- 132.Kiltz RJ, Burke MS, Porreco RP. Amniotic fluid glucose concentration as a marker for intra-amniotic infection. Obstet Gynecol. 1991;78:619–22. [PubMed] [Google Scholar]
- 133.Kirshon B, Rosenfeld B, Mari G, Belfort M. Amniotic fluid glucose and intraamniotic infection. Am J Obstet Gynecol. 1991;164:818–20. doi: 10.1016/0002-9378(91)90522-s. [DOI] [PubMed] [Google Scholar]
- 134.Gonen R. Amniotic fluid glucose and intraamniotic infection: sensitivity, specificity, and predictive values. Am J Obstet Gynecol. 1992;166:1863–4. doi: 10.1016/0002-9378(92)91580-4. [DOI] [PubMed] [Google Scholar]
- 135.Dildy GA, Pearlman MD, Smith LG, et al. Amniotic fluid glucose concentration: a marker for infection in preterm labor and preterm premature rupture of membranes. Infect Dis Obstet Gynecol. 1994;1:166–72. doi: 10.1155/S1064744994000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Greig PC, Ernest JM, Teot L. Low amniotic fluid glucose levels are a specific but not a sensitive marker for subclinical intrauterine infections in patients in preterm labor with intact membranes. Am J Obstet Gynecol. 1994;171:365–70. doi: 10.1016/s0002-9378(94)70036-2. discussion 370–361. [DOI] [PubMed] [Google Scholar]
- 137.Gonzalez-Bosquet E, Cerqueira MJ, Dominguez C, et al. Amnioticfluid glucose and cytokines values in the early diagnosis of amniotic infection in patients with preterm labor and intact membranes. J Matern Fetal Med. 1999;8:155–8. doi: 10.1002/(SICI)1520-6661(199907/08)8:4<155::AID-MFM3>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 138.Romero R, Emamian M, Wan M, et al. The value of the leukocyte esterase test in diagnosing intra-amniotic infection. Am J Perinatol. 1988;5:64–9. doi: 10.1055/s-2007-999657. [DOI] [PubMed] [Google Scholar]
- 139.Egley CC, Katz VL, Herbert WN. Leukocyte esterase: a simple bedside test for the detection of bacterial colonization of amniotic fluid. Am J Obstet Gynecol. 1988;159:120–2. doi: 10.1016/0002-9378(88)90504-2. [DOI] [PubMed] [Google Scholar]
- 140.Romero R, Kadar N, Hobbins JC, Duff GW. Infection and labor: the detection of endotoxin in amniotic fluid. Am J Obstet Gynecol. 1987;157:815–9. doi: 10.1016/s0002-9378(87)80061-3. [DOI] [PubMed] [Google Scholar]
- 141.Cox SM, MacDonald PC, Casey ML. Assay of bacterial endotoxin (lipopolysaccharide) in human amniotic fluid: potential usefulness in diagnosis and management of preterm labor. Am J Obstet Gynecol. 1988;159:99–106. doi: 10.1016/0002-9378(88)90501-7. [DOI] [PubMed] [Google Scholar]
- 142.Fidel PL, Jr, Romero R, Wolf N, et al. Systemic and local cytokine profiles in endotoxin-induced preterm parturition in mice. Am J Obstet Gynecol. 1994;170:1467–75. doi: 10.1016/s0002-9378(94)70180-6. [DOI] [PubMed] [Google Scholar]
- 143.Romero R, Emamian M, Quintero R, et al. Diagnosis of intraamniotic infection: the acridine orange stain. Am J Perinatol. 1989;6:41–5. doi: 10.1055/s-2007-999542. [DOI] [PubMed] [Google Scholar]
- 144.Hoskins IA, Johnson TR, Winkel CA. Leukocyte esterase activity in human amniotic fluid for the rapid detection of chorioamnionitis. Am J Obstet Gynecol. 1987;157:730–2. doi: 10.1016/s0002-9378(87)80039-x. [DOI] [PubMed] [Google Scholar]
- 145.Gravett MG, Eschenbach DA, Speigel-Brown CA, Holmes KK. Rapid diagnosis of amniotic-fluid infection by gas-liquid chromatography. N Engl J Med. 1982;306:725–8. doi: 10.1056/NEJM198203253061207. [DOI] [PubMed] [Google Scholar]
- 146.Romero R, Scharf K, Mazor M, et al. The clinical value of gasliquid chromatography in the detection of intra-amniotic microbial invasion. Obstet Gynecol. 1988;72:44–50. [PubMed] [Google Scholar]
- 147.Yoon BH, Romero R, Kim CJ, et al. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol. 1995;172:960–70. doi: 10.1016/0002-9378(95)90028-4. [DOI] [PubMed] [Google Scholar]
- 148.Santhanam U, Avila C, Romero R, et al. Cytokines in normal and abnormal parturition: elevated amniotic fluid interleukin-6 levels in women with premature rupture of membranes associated with intrauterine infection. Cytokine. 1991;3:155–63. doi: 10.1016/1043-4666(91)90037-e. [DOI] [PubMed] [Google Scholar]
- 149.Romero R, Sepulveda W, Kenney JS, et al. Interleukin 6 determination in the detection of microbial invasion of the amniotic cavity. Ciba Found Symp. 1992;167:205–20. doi: 10.1002/9780470514269.ch13. discussion 220–203. [DOI] [PubMed] [Google Scholar]
- 150.Romero R, Yoon BH, Kenney JS, et al. Amniotic fluid interleukin-6 determinations are of diagnostic and prognostic value in preterm labor. Am J Reprod Immunol. 1993;30:167–83. doi: 10.1111/j.1600-0897.1993.tb00618.x. [DOI] [PubMed] [Google Scholar]
- 151.Romero R, Yoon BH, Mazor M, et al. The diagnostic and prognostic value of amniotic fluid white blood cell count, glucose, interleukin-6, and gram stain in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 1993;169:805–16. doi: 10.1016/0002-9378(93)90009-8. [DOI] [PubMed] [Google Scholar]
- 152.Hillier SL, Witkin SS, Krohn MA, et al. The relationship of amniotic fluid cytokines and preterm delivery, amniotic fluid infection, histologic chorioamnionitis, and chorioamnion infection. Obstet Gynecol. 1993;81:941–8. [PubMed] [Google Scholar]
- 153.Greig PC, Ernest JM, Teot L, et al. Amniotic fluid interleukin-6 levels correlate with histologic chorioamnionitis and amniotic fluid cultures in patients in premature labor with intact membranes. Am J Obstet Gynecol. 1993;169:1035–44. doi: 10.1016/0002-9378(93)90050-s. [DOI] [PubMed] [Google Scholar]
- 154.Gravett MG, Witkin SS, Haluska GJ, et al. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol. 1994;171:1660–7. doi: 10.1016/0002-9378(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 155.Negishi H, Yamada H, Mikuni M, et al. Correlation between cytokine levels of amniotic fluid and histological chorioamnionitis in preterm delivery. J Perinat Med. 1996;24:633–9. doi: 10.1515/jpme.1996.24.6.633. [DOI] [PubMed] [Google Scholar]
- 156.Hsu CD, Meaddough E, Hong SF, et al. Elevated amniotic fluid nitric oxide metabolites and interleukin-6 in intra-amniotic infection. J Soc Gynecol Investig. 1998;5:21–4. doi: 10.1016/s1071-5576(97)00099-3. [DOI] [PubMed] [Google Scholar]
- 157.Kara M, Ozden S, Arioglu P, Cetin A. The significance of amniotic fluid interleukin-6 levels in preterm labour. Aust N Z J Obstet Gynaecol. 1998;38:403–6. doi: 10.1111/j.1479-828x.1998.tb03097.x. [DOI] [PubMed] [Google Scholar]
- 158.Greci LS, Gilson GJ, Nevils B, et al. Is amniotic fluid analysis the key to preterm labor? A model using interleukin-6 for predicting rapid delivery. Am J Obstet Gynecol. 1998;179:172–8. doi: 10.1016/s0002-9378(98)70269-8. [DOI] [PubMed] [Google Scholar]
- 159.Hsu CD, Meaddough E, Aversa K, et al. Elevated amniotic fluid levels of leukemia inhibitory factor, interleukin 6, and interleukin 8 in intra-amniotic infection. Am J Obstet Gynecol. 1998;179:1267–70. doi: 10.1016/s0002-9378(98)70144-9. [DOI] [PubMed] [Google Scholar]
- 160.Figueroa R, Garry D, Elimian A, et al. Evaluation of amniotic fluid cytokines in preterm labor and intact membranes. J Matern Fetal Neonatal Med. 2005;18:241–7. doi: 10.1080/13506120500223241. [DOI] [PubMed] [Google Scholar]
- 161.Massaro G, Scaravilli G, Simeone S, et al. Interleukin-6 and Mycoplasma hominis as markers of preterm birth and related brain damage: our experience. J Matern Fetal Neonatal Med. 2009;22:1063–7. doi: 10.3109/14767050903026473. [DOI] [PubMed] [Google Scholar]
- 162.Kim SM, Romero R, Lee J, et al. The frequency and clinical significance of intra-amniotic inflammation in women with preterm uterine contractility but without cervical change: do the diagnostic criteria for preterm labor need to be changed? J Matern Fetal Neonatal Med. 2012;25:1212–21. doi: 10.3109/14767058.2011.629256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hitti J, Tarczy-Hornoch P, Murphy J, et al. Amniotic fluid infection, cytokines, and adverse outcome among infants at 34 weeks’ gestation or less. Obstet Gynecol. 2001;98:1080–8. doi: 10.1016/s0029-7844(01)01567-8. [DOI] [PubMed] [Google Scholar]
- 164.Romero R, Durum SK, Dinarello CA. Interleukin-1: A signal for the initiation of labor in chorioamnionitis. Society for Gynecologic Investigation (33rd Annual Meeting); Toronto, Ontario. 1986. p. 71. [Google Scholar]
- 165.Romero R, Wu YK, Brody DT, et al. Human decidua: a source of interleukin-1. Obstet Gynecol. 1989;73:31–4. [PubMed] [Google Scholar]
- 166.Romero R, Parvizi ST, Oyarzun E, et al. Amniotic fluid interleukin-1 in spontaneous labor at term. J Reprod Med. 1990;35:235–8. [PubMed] [Google Scholar]
- 167.Liechty KW, Koenig JM, Mitchell MD, et al. Production of interleukin-6 by fetal and maternal cells in vivo during intraamniotic infection and in vitro after stimulation with interleukin-1. Pediatr Res. 1991;29:1–4. doi: 10.1203/00006450-199101000-00001. [DOI] [PubMed] [Google Scholar]
- 168.McDuffie RS, Jr, Sherman MP, Gibbs RS. Amniotic fluid tumor necrosis factor-alpha and interleukin-1 in a rabbit model of bacterially induced preterm pregnancy loss. Am J Obstet Gynecol. 1992;167:1583–8. doi: 10.1016/0002-9378(92)91745-v. [DOI] [PubMed] [Google Scholar]
- 169.Baggia S, Gravett MG, Witkin SS, et al. Interleukin-1 beta intraamniotic infusion induces tumor necrosis factor-alpha, prostaglandin production, and preterm contractions in pregnant rhesus monkeys. J Soc Gynecol Investig. 1996;3:121–6. doi: 10.1177/107155769600300304. [DOI] [PubMed] [Google Scholar]
- 170.Casey ML, Cox SM, Beutler B, et al. Cachectin/tumor necrosis factor-alpha formation in human decidua. Potential role of cytokines in infection-induced preterm labor. J Clin Invest. 1989;83:430–6. doi: 10.1172/JCI113901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Romero R, Manogue KR, Mitchell MD, et al. Infection and labor. IV. Cachectin-tumor necrosis factor in the amniotic fluid of women with intraamniotic infection and preterm labor. Am J Obstet Gynecol. 1989;161:336–41. doi: 10.1016/0002-9378(89)90515-2. [DOI] [PubMed] [Google Scholar]
- 172.Romero R, Mazor M, Sepulveda W, et al. Tumor necrosis factor in preterm and term labor. Am J Obstet Gynecol. 1992;166:1576–87. doi: 10.1016/0002-9378(92)91636-o. [DOI] [PubMed] [Google Scholar]
- 173.Baumann P, Romero R, Berry S, et al. Evidence of participation of the soluble tumor necrosis factor receptor I in the host response to intrauterine infection in preterm labor. Am J Reprod Immunol. 1993;30:184–93. doi: 10.1111/j.1600-0897.1993.tb00619.x. [DOI] [PubMed] [Google Scholar]
- 174.Laham N, Brennecke SP, Bendtzen K, Rice GE. Tumour necrosis factor alpha during human pregnancy and labour: maternal plasma and amniotic fluid concentrations and release from intrauterine tissues. Eur J Endocrinol. 1994;131:607–14. doi: 10.1530/eje.0.1310607. [DOI] [PubMed] [Google Scholar]
- 175.Laham N, Van Dunne F, Abraham LJ, et al. Tumor necrosis factorbeta in human pregnancy and labor. J Reprod Immunol. 1997;33:53–69. doi: 10.1016/s0165-0378(97)01012-7. [DOI] [PubMed] [Google Scholar]
- 176.Arntzen KJ, Kjollesdal AM, Halgunset J, et al. TNF, IL-1, IL-6, IL-8 and soluble TNF receptors in relation to chorioamnionitis and premature labor. J Perinat Med. 1998;26:17–26. doi: 10.1515/jpme.1998.26.1.17. [DOI] [PubMed] [Google Scholar]
- 177.MacDonald PC, Koga S, Casey ML. Decidual activation in parturition: examination of amniotic fluid for mediators of the inflammatory response. Ann N Y Acad Sci. 1991;622:315–30. doi: 10.1111/j.1749-6632.1991.tb37877.x. [DOI] [PubMed] [Google Scholar]
- 178.Romero R, Ceska M, Avila C, et al. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am J Obstet Gynecol. 1991;165:813–20. doi: 10.1016/0002-9378(91)90422-n. [DOI] [PubMed] [Google Scholar]
- 179.Saito S, Kasahara T, Kato Y, et al. Elevation of amniotic fluid interleukin 6 (IL-6), IL-8 and granulocyte colony stimulating factor (G-CSF) in term and preterm parturition. Cytokine. 1993;5:81–8. doi: 10.1016/1043-4666(93)90027-3. [DOI] [PubMed] [Google Scholar]
- 180.Cherouny PH, Pankuch GA, Romero R, et al. Neutrophil attractant/activating peptide-1/interleukin-8: association with histologic chorioamnionitis, preterm delivery, and bioactive amniotic fluid leukoattractants. Am J Obstet Gynecol. 1993;169:1299–303. doi: 10.1016/0002-9378(93)90297-v. [DOI] [PubMed] [Google Scholar]
- 181.Dudley DJ, Hunter C, Varner MW, Mitchell MD. Elevation of amniotic fluid interleukin-4 concentrations in women with preterm labor and chorioamnionitis. Am J Perinatol. 1996;13:443–7. doi: 10.1055/s-2007-994385. [DOI] [PubMed] [Google Scholar]
- 182.Pacora P, Romero R, Maymon E, et al. Participation of the novel cytokine interleukin 18 in the host response to intra-amniotic infection. Am J Obstet Gynecol. 2000;183:1138–43. doi: 10.1067/mob.2000.108881. [DOI] [PubMed] [Google Scholar]
- 183.Athayde N, Romero R, Maymon E, et al. A role for the novel cytokine RANTES in pregnancy and parturition. Am J Obstet Gynecol. 1999;181:989–94. doi: 10.1016/s0002-9378(99)70337-6. [DOI] [PubMed] [Google Scholar]
- 184.Shobokshi A, Shaarawy M. Maternal serum and amniotic fluid cytokines in patients with preterm premature rupture of membranes with and without intrauterine infection. Int J Gynaecol Obstet. 2002;79:209–15. doi: 10.1016/s0020-7292(02)00238-2. [DOI] [PubMed] [Google Scholar]
- 185.Keelan JA, Yang J, Romero RJ, et al. Epithelial cell-derived neutrophil-activating peptide-78 is present in fetal membranes and amniotic fluid at increased concentrations with intra-amniotic infection and preterm delivery. Biol Reprod. 2004;70:253–9. doi: 10.1095/biolreprod.103.016204. [DOI] [PubMed] [Google Scholar]
- 186.Chaiworapongsa T, Romero R, Espinoza J, et al. Macrophage migration inhibitory factor in patients with preterm parturition and microbial invasion of the amniotic cavity. J Matern Fetal Neonatal Med. 2005;18:405–16. doi: 10.1080/14767050500361703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Esplin MS, Romero R, Chaiworapongsa T, et al. Monocyte chemotactic protein-1 is increased in the amniotic fluid of women who deliver preterm in the presence or absence of intra-amniotic infection. J Matern Fetal Neonatal Med. 2005;17:365–73. doi: 10.1080/14767050500141329. [DOI] [PubMed] [Google Scholar]
- 188.Maymon E, Ghezzi F, Edwin SS, et al. The tumor necrosis factor alpha and its soluble receptor profile in term and preterm parturition. Am J Obstet Gynecol. 1999;181:1142–8. doi: 10.1016/s0002-9378(99)70097-9. [DOI] [PubMed] [Google Scholar]
- 189.Maymon E, Romero R, Chaiworapongsa T, et al. Amniotic fluid matrix metalloproteinase-8 in preterm labor with intact membranes. Am J Obstet Gynecol. 2001;185:1149–55. doi: 10.1067/mob.2001.118165. [DOI] [PubMed] [Google Scholar]
- 190.Yoon BH, Oh SY, Romero R, et al. An elevated amniotic fluid matrix metalloproteinase-8 level at the time of mid-trimester genetic amniocentesis is a risk factor for spontaneous preterm delivery. Am J Obstet Gynecol. 2001;185:1162–7. doi: 10.1067/mob.2001.117678. [DOI] [PubMed] [Google Scholar]
- 191.Angus SR, Segel SY, Hsu CD, et al. Amniotic fluid matrix metalloproteinase-8 indicates intra-amniotic infection. Am J Obstet Gynecol. 2001;185:1232–8. doi: 10.1067/mob.2001.118654. [DOI] [PubMed] [Google Scholar]
- 192.Biggio JR, Jr, Ramsey PS, Cliver SP, et al. Midtrimester amniotic fluid matrix metalloproteinase-8 (MMP-8) levels above the 90th percentile are a marker for subsequent preterm premature rupture of membranes. Am J Obstet Gynecol. 2005;192:109–13. doi: 10.1016/j.ajog.2004.06.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Nien JK, Yoon BH, Espinoza J, et al. A rapid MMP-8 bedside test for the detection of intra-amniotic inflammation identifies patients at risk for imminent preterm delivery. Am J Obstet Gynecol. 2006;195:1025–30. doi: 10.1016/j.ajog.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 194.Kim KW, Romero R, Park HS, et al. A rapid matrix metalloproteinase-8 bedside test for the detection of intraamniotic inflammation in women with preterm premature rupture of membranes. Am J Obstet Gynecol. 2007;197:292e291–5. doi: 10.1016/j.ajog.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 195.Buhimschi IA, Christner R, Buhimschi CS, et al. Proteomic analysis of preterm parturion: a novel method of identifying the patient at risk of impending preterm delivery. Am J Obstet Gynecol. 2003;187:S55. [Google Scholar]
- 196.Buhimschi IA, Christner R, Buhimschi CS. Proteomic biomarker analysis of amniotic fluid for identification of intra-amniotic inflammation. BJOG. 2005;112:173–81. doi: 10.1111/j.1471-0528.2004.00340.x. [DOI] [PubMed] [Google Scholar]
- 197.Buhimschi CS, Bhandari V, Hamar BD, et al. Proteomic profiling of the amniotic fluid to detect inflammation, infection, and neonatal sepsis. PLoS Med. 2007;4:e18. doi: 10.1371/journal.pmed.0040018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Green NS, Damus K, Simpson JL, et al. Research agenda for preterm birth: recommendations from the March of Dimes. Am J Obstet Gynecol. 2005;193:626–35. doi: 10.1016/j.ajog.2005.02.106. [DOI] [PubMed] [Google Scholar]
- 199.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371:261–9. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- 200.Yoon BH, Romero R, Moon JB, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185:1130–6. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 201.Armer TL, Duff P. Intraamniotic infection in patients with intact membranes and preterm labor. Obstet Gynecol Surv. 1991;46:589–93. doi: 10.1097/00006254-199109000-00002. [DOI] [PubMed] [Google Scholar]
- 202.Shim SS, Romero R, Hong JS, et al. Clinical significance of intraamniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 2004;191:1339–45. doi: 10.1016/j.ajog.2004.06.085. [DOI] [PubMed] [Google Scholar]
- 203.Blanchard A, Hentschel J, Duffy L, et al. Detection of Ureaplasma urealyticum by polymerase chain reaction in the urogenital tract of adults, in amniotic fluid, and in the respiratory tract of newborns. Clin Infect Dis. 1993;17:S148–53. doi: 10.1093/clinids/17.supplement_1.s148. [DOI] [PubMed] [Google Scholar]
- 204.Jalava J, Mantymaa ML, Ekblad U, et al. Bacterial 16S rDNA polymerase chain reaction in the detection of intra-amniotic infection. Br J Obstet Gynaecol. 1996;103:664–9. doi: 10.1111/j.1471-0528.1996.tb09835.x. [DOI] [PubMed] [Google Scholar]
- 205.Markenson GR, Martin RK, Tillotson-Criss M, et al. The use of the polymerase chain reaction to detect bacteria in amniotic fluid in pregnancies complicated by preterm labor. Am J Obstet Gynecol. 1997;177:1471–7. doi: 10.1016/s0002-9378(97)70093-0. [DOI] [PubMed] [Google Scholar]
- 206.Hitti J, Riley DE, Krohn MA, et al. Broad-spectrum bacterial rDNA polymerase chain reaction assay for detecting amniotic fluid infection among women in premature labor. Clin Infect Dis. 1997;24:1228–32. doi: 10.1086/513669. [DOI] [PubMed] [Google Scholar]
- 207.Oyarzun E, Yamamoto M, Kato S, et al. Specific detection of 16 micro-organisms in amniotic fluid by polymerase chain reaction and its correlation with preterm delivery occurrence. Am J Obstet Gynecol. 1998;179:1115–9. doi: 10.1016/s0002-9378(98)70115-2. [DOI] [PubMed] [Google Scholar]
- 208.Bearfield C, Davenport ES, Sivapathasundaram V, Allaker RP. Possible association between amniotic fluid microorganism infection and microflora in the mouth. BJOG. 2002;109:527–33. doi: 10.1111/j.1471-0528.2002.01349.x. [DOI] [PubMed] [Google Scholar]
- 209.Gerber S, Vial Y, Hohlfeld P, Witkin SS. Detection of Ureaplasma urealyticum in second-trimester amniotic fluid by polymerase chain reaction correlates with subsequent preterm labor and delivery. J Infect Dis. 2003;187:518–21. doi: 10.1086/368205. [DOI] [PubMed] [Google Scholar]
- 210.Perni SC, Vardhana S, Korneeva I, et al. Mycoplasma hominis and Ureaplasma urealyticum in midtrimester amniotic fluid: association with amniotic fluid cytokine levels and pregnancy outcome. Am J Obstet Gynecol. 2004;191:1382–6. doi: 10.1016/j.ajog.2004.05.070. [DOI] [PubMed] [Google Scholar]
- 211.Gardella C, Riley DE, Hitti J, et al. Identification and sequencing of bacterial rDNAs in culture-negative amniotic fluid from women in premature labor. Am J Perinatol. 2004;21:319–23. doi: 10.1055/s-2004-831884. [DOI] [PubMed] [Google Scholar]
- 212.Yi J, Yoon BH, Kim EC. Detection and biovar discrimination of Ureaplasma urealyticum by real-time PCR. Mol Cell Probes. 2005;19:255–60. doi: 10.1016/j.mcp.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 213.Miralles R, Hodge R, McParland PC, et al. Relationship between antenatal inflammation and antenatal infection identified by detection of microbial genes by polymerase chain reaction. Pediatr Res. 2005;57:570–7. doi: 10.1203/01.PDR.0000155944.48195.97. [DOI] [PubMed] [Google Scholar]
- 214.Leon R, Silva N, Ovalle A, et al. Detection of Porphyromonas gingivalis in the amniotic fluid in pregnant women with a diagnosis of threatened premature labor. J Periodontol. 2007;78:1249–55. doi: 10.1902/jop.2007.060368. [DOI] [PubMed] [Google Scholar]
- 215.De Felice C, De Capua B, Costantini D, et al. Recurrent otitis media with effusion in preterm infants with histologic chorioamnionitis–a 3 years follow-up study. Early Hum Dev. 2008;84:667–71. doi: 10.1016/j.earlhumdev.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 216.Aziz N, Cheng YW, Caughey AB. Neonatal outcomes in the setting of preterm premature rupture of membranes complicated by chorioamnionitis. J Matern Fetal Neonatal Med. 2009;22:780–4. doi: 10.3109/14767050902922581. [DOI] [PubMed] [Google Scholar]
- 217.Newton ER. Chorioamnionitis and intraamniotic infection. Clin Obstet Gynecol. 1993;36:795–808. doi: 10.1097/00003081-199312000-00004. [DOI] [PubMed] [Google Scholar]
- 218.Yoon BH, Romero R, Park JS, et al. Microbial invasion of the amniotic cavity with Ureaplasma urealyticum is associated with a robust host response in fetal, amniotic, and maternal compartments. Am J Obstet Gynecol. 1998;179:1254–60. doi: 10.1016/s0002-9378(98)70142-5. [DOI] [PubMed] [Google Scholar]
- 219.Lahra MM, Beeby PJ, Jeffery HE. Intrauterine inflammation, neonatal sepsis, and chronic lung disease: a 13-year hospital cohort study. Pediatrics. 2009;123:1314–9. doi: 10.1542/peds.2008-0656. [DOI] [PubMed] [Google Scholar]
- 220.Kallapur SG, Kramer BW, Jobe AH. Ureaplasma and BPD. Semin Perinatol. 2013;37:94–101. doi: 10.1053/j.semperi.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Gibbs RS, Duff P. Progress in pathogenesis and management of clinical intraamniotic infection. Am J Obstet Gynecol. 1991;164:1317–26. doi: 10.1016/0002-9378(91)90707-x. [DOI] [PubMed] [Google Scholar]
- 222.Dammann O, Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res. 1997;42:1–8. doi: 10.1203/00006450-199707000-00001. [DOI] [PubMed] [Google Scholar]
- 223.O’Shea TM, Klinepeter KL, Meis PJ, Dillard RG. Intrauterine infection and the risk of cerebral palsy in very low-birthweight infants. Paediatr Perinat Epidemiol. 1998;12:72–83. [PubMed] [Google Scholar]
- 224.Wu YW, Colford JM., Jr Chorioamnionitis as a risk factor for cerebral palsy: A meta-analysis. JAMA. 2000;284:1417–24. doi: 10.1001/jama.284.11.1417. [DOI] [PubMed] [Google Scholar]
- 225.Yoon BH, Romero R, Park JS, et al. Fetal exposure to an intraamniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol. 2000;182:675–81. doi: 10.1067/mob.2000.104207. [DOI] [PubMed] [Google Scholar]
- 226.Dammann O, Kuban KC, Leviton A. Perinatal infection, fetal inflammatory response, white matter damage, and cognitive limitations in children born preterm. Ment Retard Dev Disabil Res Rev. 2002;8:46–50. doi: 10.1002/mrdd.10005. [DOI] [PubMed] [Google Scholar]
- 227.Yoon BH, Park CW, Chaiworapongsa T. Intrauterine infection and the development of cerebral palsy. BJOG. 2003;110:124–7. doi: 10.1016/s1470-0328(03)00063-6. [DOI] [PubMed] [Google Scholar]
- 228.Grether JK, Nelson KB, Walsh E, et al. Intrauterine exposure to infection and risk of cerebral palsy in very preterm infants. Arch Pediatr Adolesc Med. 2003;157:26–32. doi: 10.1001/archpedi.157.1.26. [DOI] [PubMed] [Google Scholar]
- 229.Elovitz MA, Mrinalini C, Sammel MD. Elucidating the early signal transduction pathways leading to fetal brain injury in preterm birth. Pediatr Res. 2006;59:50–5. doi: 10.1203/01.pdr.0000191141.21932.b6. [DOI] [PubMed] [Google Scholar]
- 230.Berger A, Witt A, Haiden N, et al. Intrauterine infection with Ureaplasma species is associated with adverse neuromotor outcome at 1 and 2 years adjusted age in preterm infants. J Perinat Med. 2009;37:72–8. doi: 10.1515/JPM.2009.016. [DOI] [PubMed] [Google Scholar]
- 231.Bracci R, Buonocore G. Chorioamnionitis: a risk factor for fetal and neonatal morbidity. Biol Neonate. 2003;83:85–96. doi: 10.1159/000067956. [DOI] [PubMed] [Google Scholar]
- 232.Gibbs RS. Chorioamnionitis and bacterial vaginosis. Am J Obstet Gynecol. 1993;169:460–2. doi: 10.1016/0002-9378(93)90341-f. [DOI] [PubMed] [Google Scholar]
- 233.Martinelli P, Sarno L, Maruotti GM, Paludetto R. Chorioamnionitis and prematurity: a critical review. J Matern Fetal Neonatal Med. 2012;25:29–31. doi: 10.3109/14767058.2012.714981. [DOI] [PubMed] [Google Scholar]
- 234.Keski-Nisula L, Kirkinen P, Katila ML, et al. Amniotic fluid U. urealyticum colonization: significance for maternal peripartal infections at term. Am J Perinatol. 1997;14:151–6. doi: 10.1055/s-2007-994117. [DOI] [PubMed] [Google Scholar]
- 235.Barton JR, Sibai BM. Severe sepsis and septic shock in pregnancy. Obstet Gynecol. 2012;120:689–706. doi: 10.1097/AOG.0b013e318263a52d. [DOI] [PubMed] [Google Scholar]
- 236.Surgers L, Valin N, Carbonne B, et al. Evolving microbiological epidemiology and high fetal mortality in 135 cases of bacteremia during pregnancy and postpartum. Eur J Clin Microbiol Infect Dis. 2013;32:107–13. doi: 10.1007/s10096-012-1724-5. [DOI] [PubMed] [Google Scholar]
- 237.Plachouras N, Sotiriadis A, Dalkalitsis N, et al. Fulminant sepsis after invasive prenatal diagnosis. Obstet Gynecol. 2004;104:1244–7. doi: 10.1097/01.AOG.0000141650.01076.98. [DOI] [PubMed] [Google Scholar]
- 238.Fernandez-Perez ER, Salman S, Pendem S, Farmer JC. Sepsis during pregnancy. Crit Care Med. 2005;33:S286–93. doi: 10.1097/01.ccm.0000182479.63108.cd. [DOI] [PubMed] [Google Scholar]
- 239.Hatjis CG, Swain M. Systemic tocolysis for premature labor is associated with an increased incidence of pulmonary edema in the presence of maternal infection. Am J Obstet Gynecol. 1988;159:723–8. doi: 10.1016/s0002-9378(88)80041-3. [DOI] [PubMed] [Google Scholar]
- 240.Gibbs RS, Dinsmoor MJ, Newton ER, Ramamurthy RS. A randomized trial of intrapartum versus immediate postpartum treatment of women with intra-amniotic infection. Obstet Gynecol. 1988;72:823–8. doi: 10.1097/00006250-198812000-00001. [DOI] [PubMed] [Google Scholar]
- 241.Russell JF. If a job is worth doing, it is worth doing twice. Nature. 2013;496:7. doi: 10.1038/496007a. [DOI] [PubMed] [Google Scholar]
- 242.Jaeschke R, Guyatt G, Sackett DL. Users’ guides to the medical literature. III. How to use an article about a diagnostic test. A. Are the results of the study valid? Evidence-Based Medicine Working Group. JAMA. 1994;271:389–91. doi: 10.1001/jama.271.5.389. [DOI] [PubMed] [Google Scholar]
- 243.Enhancing reproducibility. Nat Methods. 2013;10:367. doi: 10.1038/nmeth.2471. [DOI] [PubMed] [Google Scholar]
- 244.Shafer SL, Dexter F. Publication bias, retrospective bias, and reproducibility of significant results in observational studies. Anesth Analg. 2012;114:931–2. doi: 10.1213/ANE.0b013e31824a0b5b. [DOI] [PubMed] [Google Scholar]
- 245.Tzoulaki I, Liberopoulos G, Ioannidis JP. Assessment of claims of improved prediction beyond the Framingham risk score. JAMA. 2009;302:2345–52. doi: 10.1001/jama.2009.1757. [DOI] [PubMed] [Google Scholar]
- 246.Tsilidis KK, Papatheodorou SI, Evangelou E, Ioannidis JP. Evaluation of excess statistical significance in meta-analyses of 98 biomarker associations with cancer risk. J Natl Cancer Inst. 2012;104:1867–78. doi: 10.1093/jnci/djs437. [DOI] [PubMed] [Google Scholar]
- 247.Nierenberg AA, Feinstein AR. How to evaluate a diagnostic marker test. Lessons from the rise and fall of dexamethasone suppression test. JAMA. 1988;259:1699–702. [PubMed] [Google Scholar]
- 248.Ioannidis JP. Biomarker failures. Clin Chem. 2013;59:202–4. doi: 10.1373/clinchem.2012.185801. [DOI] [PubMed] [Google Scholar]
- 249.Rajasekharan S, Bar-Or A. From bench to MS bedside: challenges translating biomarker discovery to clinical practice. J Neuroimmunol. 2012;248:66–72. doi: 10.1016/j.jneuroim.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 250.Coultrip LL, Lien JM, Gomez R, et al. The value of amniotic fluid interleukin-6 determination in patients with preterm labor and intact membranes in the detection of microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 1994;171:901–11. doi: 10.1016/s0002-9378(94)70057-5. [DOI] [PubMed] [Google Scholar]
- 251.Aguin E, Aguin T, Cordoba M, et al. Amniotic fluid inflammation with negative culture and outcome after cervical cerclage. J Matern Fetal Neonatal Med. 2012;25:1990–4. doi: 10.3109/14767058.2012.667177. [DOI] [PubMed] [Google Scholar]
- 252.Lee J, Oh KJ, Yang HJ, et al. The importance of intra-amniotic inflammation in the subsequent development of atypical chronic lung disease. J Matern Fetal Neonatal Med. 2009;22:917–23. doi: 10.1080/14767050902994705. [DOI] [PubMed] [Google Scholar]
- 253.Gomez R, Romero R, Galasso M, et al. The value of amniotic fluid interleukin-6, white blood cell count, and gram stain in the diagnosis of microbial invasion of the amniotic cavity in patients at term. Am J Reprod Immunol. 1994;32:200–10. doi: 10.1111/j.1600-0897.1994.tb01115.x. [DOI] [PubMed] [Google Scholar]
- 254.Combs CA, Gravett MG, Garite TJ, et al. Intraamniotic inflammation may be more important than the presence of microbes as a determinant of perinatal outcome in preterm labor. Am J Obstet Gynecol. 2013;208:S44. [Google Scholar]
- 255.Blackstock WP, Weir MP. Proteomics: quantitative and physical mapping of cellular proteins. Trends Biotechnol. 1999;17:121–7. doi: 10.1016/s0167-7799(98)01245-1. [DOI] [PubMed] [Google Scholar]
- 256.Banks RE, Dunn MJ, Hochstrasser DF, et al. Proteomics: new perspectives, new biomedical opportunities. Lancet. 2000;356:1749–56. doi: 10.1016/S0140-6736(00)03214-1. [DOI] [PubMed] [Google Scholar]
- 257.Klein LL, Freitag BC, Gibbs RS, et al. Detection of intra-amniotic infection in a rabbit model by proteomics-based amniotic fluid analysis. Am J Obstet Gynecol. 2005;193:1302–6. doi: 10.1016/j.ajog.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 258.Huang DJ, Nelson MR, Holzgreve W. Maldi-TOF mass spectrometry for trisomy detection. Methods Mol Biol. 2008;444:123–32. doi: 10.1007/978-1-59745-066-9_9. [DOI] [PubMed] [Google Scholar]
- 259.Gravett MG, Thomas A, Schneider KA, et al. Proteomic analysis of cervical-vaginal fluid: identification of novel biomarkers for detection of intra-amniotic infection. J Proteome Res. 2007;6:89–96. doi: 10.1021/pr060149v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Pereira L, Reddy AP, Jacob T, et al. Identification of novel protein biomarkers of preterm birth in human cervical-vaginal fluid. J Proteome Res. 2007;6:1269–76. doi: 10.1021/pr0605421. [DOI] [PubMed] [Google Scholar]
- 261.Dasari S, Pereira L, Reddy AP, et al. Comprehensive proteomic analysis of human cervical-vaginal fluid. J Proteome Res. 2007;6:1258–68. doi: 10.1021/pr0605419. [DOI] [PubMed] [Google Scholar]
- 262.Shah SJ, Yu KH, Sangar V, et al. Identification and quantification of preterm birth biomarkers in human cervicovaginal fluid by liquid chromatography/tandem mass spectrometry. J Proteome Res. 2009;8:2407–17. doi: 10.1021/pr8010342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Hitti J, Lapidus JA, Lu X, et al. Noninvasive diagnosis of intraamniotic infection: proteomic biomarkers in vaginal fluid. Am J Obstet Gynecol. 2010;203:32e31–8. doi: 10.1016/j.ajog.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Lee DC, Hassan SS, Romero R, et al. Protein profiling underscores immunological functions of uterine cervical mucus plug in human pregnancy. J Proteomics. 2011;74:817–28. doi: 10.1016/j.jprot.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Gravett MG, Novy MJ, Rosenfeld RG, et al. Diagnosis of intraamniotic infection by proteomic profiling and identification of novel biomarkers. JAMA. 2004;292:462–9. doi: 10.1001/jama.292.4.462. [DOI] [PubMed] [Google Scholar]
- 266.Michaels JE, Dasari S, Pereira L, et al. Comprehensive proteomic analysis of the human amniotic fluid proteome: gestational agedependent changes. J Proteome Res. 2007;6:1277–85. doi: 10.1021/pr060543t. [DOI] [PubMed] [Google Scholar]
- 267.Bujold E, Romero R, Kusanovic JP, et al. Proteomic profiling of amniotic fluid in preterm labor using two-dimensional liquid separation and mass spectrometry. J Matern Fetal Neonatal Med. 2008;21:697–713. doi: 10.1080/14767050802053289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Romero R, Kusanovic JP, Gotsch F, et al. Isobaric labeling and tandem mass spectrometry: a novel approach for profiling and quantifying proteins differentially expressed in amniotic fluid in preterm labor with and without intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med. 2010;23:261–80. doi: 10.3109/14767050903067386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Fotopoulou C, Kyeyamwa S, Linder M, et al. Proteomic analysis of midtrimester amniotic fluid to identify novel biomarkers for preterm delivery. J Matern Fetal Neonatal Med. 2012;25:2488–93. doi: 10.3109/14767058.2012.712565. [DOI] [PubMed] [Google Scholar]
- 270.Pereira L, Reddy AP, Alexander AL, et al. Insights into the multifactorial nature of preterm birth: proteomic profiling of the maternal serum glycoproteome and maternal serum peptidome among women in preterm labor. Am J Obstet Gynecol. 2010;202:555e1–10. doi: 10.1016/j.ajog.2010.02.048. [DOI] [PubMed] [Google Scholar]
- 271.Esplin MS, Merrell K, Goldenberg R, et al. Proteomic identification of serum peptides predicting subsequent spontaneous preterm birth. Am J Obstet Gynecol. 2011;204:391e391–8. doi: 10.1016/j.ajog.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]


