Abstract
Questionnaires over a 9-year study period (2002–2010) were used to characterize cannabis, stimulant, and alcohol use among 3099 HIV-infected men participating in the Veterans Aging Cohort Study (VACS) to determine whether use of these substances is associated with changes in the VACS Index, a validated prognostic indicator for all-cause mortality. At baseline, 18% of participants reported no substance use in the past year, 24% lower risk alcohol use only, 18% unhealthy alcohol use only, 15% cannabis use (with or without alcohol), and 24% stimulant use (with or without alcohol or cannabis). In adjusted longitudinal analyses, cannabis use [β=-0.97 (95% CI: −1.93, 0.00), p=0.048] was not associated with mortality risk, while stimulant use [1.08 (0.16, 2.00), p=0.021] was associated with an increased mortality risk, compared to lower risk alcohol use. Our findings show no evidence of a negative effect of cannabis use on mortality risk, while stimulant use was associated with increased mortality risk among HIV-infected men. Interventions to reduce stimulant use in this patient population may reduce mortality.
Keywords: alcohol, cannabis, drug use, men who have sex with men, HIV/AIDS
Introduction
Alcohol and drug use negatively affect HIV disease progression through mechanisms including poorer adherence to antiretroviral therapy (ART), higher rates of depressive symptoms, and physiological harms including immunosuppression, neurocognitive dysfunction, and a greater propensity for respiratory infections.1–9 Of public health concern is the fact that alcohol and drug use are both prevalent among HIV-infected individuals.10,11 Estimates for cannabis use in the past year among HIV-infected adults range from 24–56%; and a 2001 study using a nationally representative probability sample estimated that the prevalence of illicit drug use other than cannabis in the past year is approximately 40%.12–16
Particular types of drugs, such as cannabis, methamphetamine, and cocaine, may differentially impact HIV disease progression.11,17,18 For example, use of methamphetamine among HIV-infected men who have sex with men (MSM) has been shown to negatively impact ART adherence and is associated with significant medical morbidity, including cardiovascular disease and impaired cognitive function.19,20 In contrast, several studies have shown that cannabis use is not associated with poor viral suppression or other clinical outcomes in HIV-infected patients.21–23 However, one longitudinal study examining trajectories of cannabis use over 29 years found that having a detectable HIV viral load was associated with cannabis use for HIV-infected MSM who increased their cannabis use over the follow-up period, even after controlling for other types of substance use.24 In addition, alcohol use has been shown to impact both the clinical manifestations and management of HIV with potentially synergistic effects on HIV-related comorbidities.9 While a growing body of literature has established the adverse effects of substance use on medication adherence, HIV disease progression, and HIV transmission risk, it is unclear whether specific types of substance use impact mortality prognosis among HIV-infected people in the current antiretroviral treatment era.
To address this research gap, the current study characterized cannabis, stimulant, and alcohol use among HIV-infected men participating in the Veterans Aging Cohort Study (VACS) to determine whether these substances were associated with changes in the VACS Index, a prognostic indicator for all-cause mortality. The VACS Index has been validated in numerous European and North American cohorts and a 5-point increase in the VACS Index score has been shown to approximate a 20% increased risk of 5-year mortality.25–27 VACS Index scores combine commonly collected clinical biomarkers into a cumulative index ranging from 0–164; incorporating the following: age, CD4 cell count, HIV RNA, hemoglobin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), platelet count, creatinine, and hepatitis C serologic status.26 Our primary hypothesis was that the longitudinal association between substance use and mortality risk would differ by substance type. Specifically, we hypothesized that the impact of substance use on mortality risk over the study period would differ when examining the use of cannabis, stimulant, and alcohol as distinct substance use categories.
Material and methods
Study design and data sources
VACS is a longitudinal prospective cohort study conducted among mostly male HIV-infected veterans and age-, race-, and site-matched HIV uninfected controls attending eight US Veterans Administration (VA) medical facilities. From 2002 to 2010, participants were recruited from VA facilities in: Atlanta, Georgia; Baltimore, Maryland; New York (Bronx and Manhattan); Houston, Texas; Los Angeles, California; Pittsburgh, Pennsylvania; and Washington, D.C. VACS study design and methods have been described elsewhere.28,29
Given that the relationship between the type of substance use and mortality risk may differ by gender and by HIV-serostatus, we excluded female patients and HIV-negative patients participating in VACS. We also excluded individuals and/or observations missing information on covariates of interest (see below).
Self-administered questionnaires were scheduled approximately annually (a total of seven waves of data collection) and contained questions regarding sociodemographic characteristics, general health, sexual activity, and substance use. The VACS was approved by the institutional review boards at each participating VA medical center and affiliated academic institutions.
Study measures
The primary outcome of interest was repeated assessment of the VACS Index score. The VACS Index score was recorded at time of the baseline assessment and each of the follow-up study visits. The score is re-calculated each time a lab is updated, with components of the index carried forward for up to a year to calculate the score. The exposure of interest was use of cannabis, stimulants, and alcohol reported at the baseline and each of the follow-up assessments. Alcohol use in the past year was determined using a component of the Alcohol Use Disorders Identification Test (AUDIT-C) reading: “How often do you have a drink containing alcohol?”.30 Those replying “never” were categorized as abstaining from alcohol for the past year. Participants with an AUDIT-C score of 4 or above were categorized as engaging in unhealthy alcohol use.31 For participants missing responses to the AUDIT-C, we evaluated alcohol use in the past year using responses to two additional questions: “When was the last time you had a drink?” and “In the last 12 months have you had a drink containing alcohol?”. Participants who reported alcohol use but lacked AUDIT-C information were categorized as lower risk alcohol use. Those who skipped all alcohol-use related questions were categorized as abstaining from alcohol for the past year.32
Cannabis and stimulant use were evaluated at each assessment with the following question: “For each of the following drugs, please mark the box that best indicates how often in the past year you used each drug: marijuana or hashish; cocaine or crack; and stimulants (amphetamines, uppers, speed, crank, crystal meth, bam).” Response options for all substances included: have never tried, no use in the last year, less than once a month, 1–3 times a month, 1–3 times a week, 4–6 times a week, or every day. Endorsement of marijuana (i.e., cannabis) or hashish less than once a month to everyday was considered use of cannabis. As done in previous studies and due to considerable overlap in use of various kinds of stimulant drugs, any reported use of cocaine, crack, or other stimulant in the past year (less than once a month to everyday) was considered use of a stimulant.33,34
Time-updated, mutually exclusive substance use categories for the primary exposure were defined as: 1) no substance use (drugs or alcohol) in past year; 2) lower risk alcohol use only with a score <4 on the AUDIT-C questionnaire; 3) unhealthy alcohol use (AUDIT-C score ≥4) with no other substance use; 4) any reported cannabis use, with or without alcohol; and 5) any stimulant use, with or without alcohol or cannabis use.
Other covariates of interest were selected based on a priori hypotheses and informed by previous research on alcohol and drug use among HIV-infected individuals.10,11,18,34 Sociodemographic (e.g., age, minority status, educational attainment) as well as behavioral (e.g., smoking status, MSM status) characteristics were included as covariates based on their association with type and frequency of substance use in previous studies.10,11,18,34 MSM behavior was assessed at baseline. Specifically, respondents’ sexual behaviors were either classified as current MSM (at least one male sexual partner in the past twelve months), heterosexual (only female sexual partners in the past twelve months), or not sexually active (no sexual partners in the past twelve months). Other time-invariant sociodemographic variables assessed at baseline included: race/ethnicity (non-white or white), education level (high school or less, some college or greater), marital status (married/living with a partner, divorced/separated/widowed, never married), employment status (employed or self-employed, not employed or self-employed), and annual income (<$6,000, $6–11,999, $12–24,999, ≥$25,000). Time-varying covariates used responses from every available assessment and included smoking status (current smoker, former smoker, never smoker), any opioid use (medical or non-medical) in the past year, and any history of injection drug use. Opioid use was included as a covariate rather than part of the substance use categorization schema because past year use, and in particular frequent use, was not as common as the other reported substances and survey questioning did not differentiate between medical and non-medical opioid use.
In addition, we identified participants who died during the follow-up period (2002–2010) using data available through Veterans Health Administration’s patient files, the Beneficiary Identification Records Locating System (which tracks Veterans Health Administration death benefits), the Medicare Vital Status file, and the Social Security National Death Index. Participants were considered lost to follow-up (for reasons other than death) if their last assessment occurred more than two years prior to the end of the study period (i.e., their last assessment occurred before January 1, 2009).
Statistical analyses
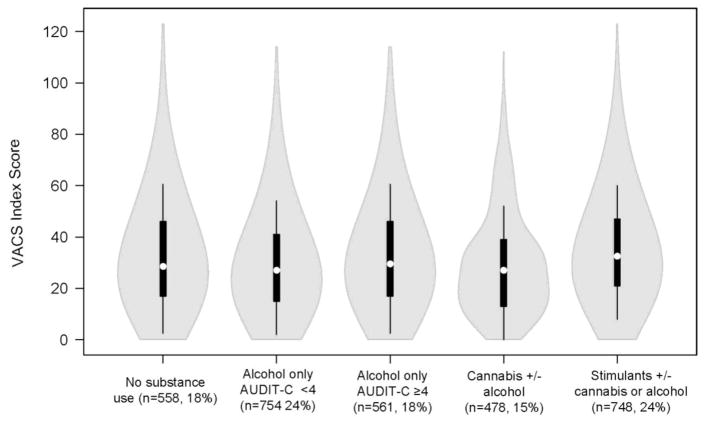
Initially, bivariate statistics were conducted to assess baseline cannabis, stimulant, and alcohol use by sociodemographic and behavioral characteristics using Pearson χ2 and Wilcoxon rank-sum tests. Violin plots using BoxPlotR (available at boxplot.tyerslab.com) were then used to plot the distribution of baseline VACS Index score by substance use category. Violin plots are box plots with the probability density incorporated within the figure.
Next, inverse probability weighted linear mixed effects models with a random intercept and slope were used to estimate the associations between substance use category and VACS Index score over time. Fitting a linear mixed effects model accounts for repeated measurements and yields parameters interpretable as the average difference in VACS Index score relative to the reference category.35,36 This method is particularly appropriate as the number of follow-up assessments per respondent ranged from 0–6, and linear mixed effects modeling has built-in flexibility that can incorporate imbalance in longitudinal data.35 Differences on measured factors between the respondents in the final study population and those excluded due to missing covariates or loss to follow-up were accounted for using inverse probability weights. Inverse probability weights were estimated as a function of substance use category at baseline and other baseline covariates using logistic regression models. Estimated inverse probability weights were subsequently evaluated based on their distributions (mean, range, minimum and maximum) and appeared to be well behaved, based on previously published guidelines.37,38
The lower risk alcohol-only group was chosen as the referent substance use category in response to evidence that those abstaining entirely from alcohol are a heterogeneous group comprising of lifetime abstainers, as well as individuals choosing to abstain due to health problems.39,40 A 2006 population-based study found that non-current drinkers reported poorer physical and mental health compared to life-time abstainers and current drinkers.40 Use of those abstaining from any substance use as the referent group could therefore bias results.
Multivariable models were then conducted to evaluate the independent effect of substance use category on VACS Index score, after adjusting for sociodemographic, behavioral, and other covariates. Covariates within the multivariable models were assessed for collinearity. Assessment of a substance use category and time interaction was also evaluated in multivariable analysis. Sensitivity analyses were conducted by excluding: 1) respondents who died during the follow-up period, 2) observations with VACS Index scores over 80, and 3) observations missing responses for the AUDIT-C score. In addition, we conducted two sensitivity analyses differing the categorization of substance use. One categorization used an AUDIT-C cut-off of 6, rather than 4, for unhealthy drinking and the other categorization accounted for frequency of cannabis and stimulant use. Statistical analyses performed using SAS statistical package (SAS Version 9.3, SAS Institute, Inc., Cary, NC).
Results
A total of 7,324 patients were enrolled in VACS between June 1st, 2002 and September 30th, 2010. We excluded 3,693 (50.4%) HIV-uninfected participants, 94 (2.6%) female participants, and 79 (2.2%) participants missing exposure information due to non-response to all three drug-related questions at baseline. In addition, we excluded 86 (2.5%) individuals missing VACS Index information and 273 (8.1%) participants missing data on covariates of interest, resulted in a total study sample of 3,099 HIV-infected male VACS participants. The average number of surveys per respondent was 3.98 (SD: 1.91), with a median of 4 (IQR: 2, 6), including baseline visit. A total of 12,339 person-visits were included within the analysis.
Substance Use Patterns
Sociodemographic, behavioral, and clinical characteristics by substance use category at baseline are presented in Table 1. Participant (N=3,099) median age was 49 (interquartile range: 44, 55), 79.6% were non-white, and 31.9% MSM. At baseline, 18.0% reported no substance use in the past year, 24.3% reported lower risk alcohol use only, 18.1% unhealthy alcohol use with no other substance use, 15.4% cannabis use (with or without alcohol), and 24.1% stimulant use (with or without alcohol and cannabis use). All measured sociodemographic and behavioral characteristics had statistically significant differences by substance use category (p<0.0001). Notably, a greater proportion of individuals reporting stimulant use also reported opioid (medical or non-medical) use in the past year compared to individuals within the other substance use categories. In addition, a greater proportion of individuals reporting stimulant use reported unhealthy alcohol use compared to individuals reporting cannabis use.
Table 1.
Baseline sociodemographic and behavioral characteristics associated with substance use category among HIV-infected male VACS participants (n= 3,099).a,b
| Substance-use category at baseline | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Total population (n=3,099, 100.0%) | No substance use (n=558, 18.0%) | Alcohol only, AUDIT-C <4 (n=754, 24.3%) | Alcohol only, AUDIT-C ≥4 (n=561, 18.1%) | Cannabis +/− alcohol(n=478, 15.4%) | Stimulants +/− cannabis or alcohol(n=748, 24.1%) | p- valuec | |
| VACS Index Score, median (IQR) | 28 (17, 45) | 28 (17, 46) | 27 (15, 41) | 30 (17, 46) | 27 (13, 39) | 32 (19, 47) | <0.0001 |
|
| |||||||
| Age, median (IQR) | 49 (44, 55) | 50 (45, 56) | 50 (43, 56) | 50 (44, 55) | 48 (42, 53) | 48 (44, 53) | <0.0001 |
|
| |||||||
| Smoking status, n (%) | |||||||
| Current smoker | 1,658 (53.5) | 220 (39.4) | 295 (39.1) | 292 (52.1) | 257 (53.8) | 594 (79.4) | <0.0001 |
| Former smoker | 731 (23.6) | 158 (28.3) | 204 (27.1) | 181 (32.3) | 117 (24.5) | 71 (9.5) | |
| Never smoker | 710 (22.9) | 180 (32.3) | 255 (33.8) | 88 (15.7) | 104 (21.8) | 83 (11.1) | |
|
| |||||||
| Any illicit opioid use in past year, n (%)d | 309 (10.0) | 23 (4.1) | 27 (3.6) | 24 (4.3) | 40 (8.4) | 195 (26.1) | <0.0001 |
|
| |||||||
| IDU ever, n (%) | 1,050 (33.9) | 203 (36.4) | 137 (18.2) | 202 (36.0) | 133 (27.8) | 375 (50.1) | <0.0001 |
|
| |||||||
| Unhealthy alcohol use AUDIT-C ≥4, n (%) | 1,091 (35.2) | n/a | 0 (0.0) | 561 (100.0) | 164 (34.5) | 366 (49.1) | n/a |
|
| |||||||
| MSM statuse | |||||||
| Current MSM | 988 (31.9) | 107 (19.2) | 268 (35.5) | 138 (24.6) | 225 (47.1) | 251 (33.6) | <0.0001 |
| Heterosexual | 1,166 (37.6) | 230 (41.2) | 227 (30.1) | 234 (41.7) | 139 (29.1) | 336 (44.9) | |
| Not sexually active | 944 (30.5) | 221 (39.6) | 259 (34.4) | 189 (33.7) | 114 (23.9) | 161 (21.5) | |
|
| |||||||
| Non-white race/ethnicity, n (%) | 2,467 (79.6) | 464 (83.2) | 558 (74.0) | 448 (79.9) | 338 (70.7) | 659 (88.1) | <0.0001 |
|
| |||||||
| High school education/less than high school, n (%) | 1,233 (39.8) | 234 (41.9) | 240 (31.8) | 250 (44.6) | 160 (33.5) | 349 (46.7) | <0.0001 |
|
| |||||||
| Marital status, n (%) | |||||||
| Married/living with partner | 720 (23.3) | 145 (26.0) | 191 (25.3) | 131 (23.4) | 135 (28.2) | 118 (15.8) | |
| Divorced, separate, or widowed | 1,257 (40.6) | 235 (42.1) | 286 (37.9) | 226 (40.3) | 154 (32.2) | 356 (47.6) | <0.0001 |
| Never married | 1,122 (36.2) | 178 (31.9) | 277 (36.7) | 204 (36.4) | 189 (39.5) | 274 (36.6) | |
|
| |||||||
| Unemployed/retired/other at baseline, n (%) | 2,074 (66.9) | 364 (65.2) | 443 (58.8) | 370 (66.0) | 310 (64.9) | 587 (78.5) | <0.0001 |
|
| |||||||
| Annual income at baseline, n (%) | |||||||
| <$6000 | 615 (19.9) | 95 (17.0) | 98 (13.0) | 114 (20.3) | 75 (15.7) | 233 (31.2) | <0.0001 |
| $6000–$11,999 | 955 (30.8) | 174 (31.2) | 185 (24.5) | 170 (30.3) | 148 (31.0) | 278 (37.2) | |
| $12,000–24,999 | 756 (24.4) | 141 (25.3) | 203 (26.9) | 147 (26.2) | 128 (26.8) | 137 (18.2) | |
| ≥$25,000 | 773 (24.9) | 148 (26.5) | 268 (35.5) | 130 (23.2) | 127 (26.6) | 100 (13.4) | |
Abbreviations: VACS= Veterans Aging Cohort Study; MSM=men who have sex with men; IQR= interquartile range; IDU=intravenous drug use; AUDIT= Alcohol Use Disorders Identification Test-Consumption questionnaire
Use or experiences in the past-year, reported at baseline.
Percentages calculated excluding missing responses.
Pearson χ2 and Wilcoxon rank-sum tests compared variables by substance-use category at baseline.
In response to the following question: “How often in the past year you used each drug - Opioids (heroin, morphine, codeine, opium)”
Respondents were categorized at baseline as current MSM (at least one male sexual partner in the past twelve months), heterosexual (only female sexual partners in past twelve months) or not sexually active (no sexual partners in past twelve months).
The distribution of baseline VACS Index scores differed by substance use category. Figure 1 presents violin plots of baseline VACS Index score distribution by substance use category at baseline. Overall, participants using stimulants and participants with unhealthy alcohol use had higher VACS index scores than other groups (Figure 1, Table 1). Median VACS Index was 5 points higher for the stimulants group compared to the lower risk alcohol use group. The distribution of VACS Index scores (i.e. the shape around the boxplot) was similar across substance use categories with the exception of cannabis use, which appeared to have a slight bimodal distribution.
Figure 1.
Violin plots of baseline VACS Index score distributions by substance use category at baseline among HIV-infected male VACS participants (n=3,099).
Mortality and loss to follow-up
By the end of the follow-up period in 2010, 952 (30.7%) of HIV-infected VACS participants included within this analytic sample had died. Of the 393 participants with only a baseline visit recorded, 253 (64.4%) died. In total, 468 (15.1%) participants included in the final study sample were lost to follow-up for reasons other than death. We accounted for potential biases arising from mortality by conducting a series of sensitivity analyses (see below).
Multivariable Models
In a multivariable linear mixed effects model, factors independently associated with VACS Index score included substance use category, smoking status, history of injection drug use, MSM status, race/ethnicity, marital status, employment status, and annual income at baseline (Table 2). The time-varying exposure variable (i.e., substance use category) was continually updated throughout follow up and not limited to the baseline assessment. A substance use category and time interaction term was not statistically significant in multivariable analysis and subsequently omitted from multivariable models. In adjusted analyses, cannabis use [β=−0.97 (95%CL: −1.93, 0.00), p=0.048] was not independently associated with mortality risk, while stimulant use [1.08 (0.16, 2.00), p=0.021] was associated with an increased 5-year mortality risk, compared to lower risk alcohol use. Since a 5-point increase in the VACS Index score has been shown to approximate a 20% increased risk of 5-year mortality, stimulant use was associated with a 4.3%increase in 5-year mortality risk.25–27 While not statistically significant, unhealthy alcohol use appeared to have negative impact [β=0.52 (95%CL: −0.43, 1.47), p=0.281] on VACS Index score. Current MSM at baseline [−4.03 (−5.96, −2.10), p=<0.001] had a decreased mortality risk than heterosexual men (16.1% decreased 5-year mortality risk), while men who were not sexually active [4.87 (3.08, 6.66), p=<0.001] had an increased mortality risk compared to heterosexual men (19.5% 5-year increased mortality risk). A history of injection drug use [3.96 (2.50, 5.41), p=<0.001], non-white race/ethnicity [3.90 (2.04, 5.75), p=<0.001], being divorced, separated, or widowed at baseline [4.31 (2.64, 5.98), p=<0.001], or being unemployed [6.75 (5.04, 8.45), p=<0.001] were associated with increased mortality risk. Annual income at baseline was also associated—those making between $6,000–11,999 [3.63 (1.45, 5.80), p=0.001] and $12,000–24,999 [2.66 (0.55, 4.77), p=0.014] had higher VACS Index scores compared to those making $25,000 or more annually.
Table 2.
Inverse probability weighted longitudinal linear mixed effect models with adjusted factors associated with VACS Index score among HIV-infected male VACS participants (n=3,099).a
| β | 95% CI | p - value | |
|---|---|---|---|
| Intercept | 18.81 | 15.59, 22.04 | |
|
| |||
| Time varying characteristics | |||
| Substance-use category | <0.001 | ||
| No substance use | −0.61 | −1.39, 0.18 | 0.129 |
| Unhealthy alcohol use (AUDIT-C ≥4) | 0.52 | −0.43, 1.47 | 0.281 |
| Any cannabis +/− alcohol | −0.97 | −1.93, 0.00 | 0.048 |
| Any stimulant +/− alcohol or cannabis | 1.08 | 0.16, 2.00 | 0.021 |
| Alcohol only <4 on AUDIT-C (reference) | ref | ||
|
| |||
| Smoking status | 0.005 | ||
| Current smoker | −0.80 | −1.94, 0.35 | 0.172 |
| Former smoker | 0.67 | −0.41, 1.75 | 0.225 |
| Never smoker (reference) | ref | ||
|
| |||
| Any illicit opioid use in past year (yes vs. no) | 0.19 | −0.55, 0.94 | 0.612 |
|
| |||
| IDU ever (at baseline, updated through follow-up) | 3.96 | 2.50, 5.41 | <0.001 |
|
| |||
| Time invariant or baseline characteristics | |||
| MSM statusb | <0.001 | ||
| Current MSM | −4.03 | −5.96, −2.10 | <0.001 |
| No sexual activity in past twelve months | 4.87 | 3.08, 6.66 | <0.001 |
| Heterosexual (reference) | ref | ||
|
| |||
| Non-white race/ethnicity vs. white race/ethnicity | 3. 90 | 2.04, 5.75 | <0.001 |
|
| |||
| High school education/less than high school vs. some college or greater | 0.81 | −0.71, 2.33 | 0.296 |
|
| |||
| Marital status at baseline | <0.001 | ||
| Married/living with partner | 1.88 | −0.10, 3.86 | 0.063 |
| Divorced, separated, or widowed | 4.31 | 2.64, 5.98 | <0.001 |
| Never married (reference) | ref | ||
|
| |||
| Unemployed/retired/other vs. employed at baseline | 6.75 | 5.04, 8.45 | <0.001 |
|
| |||
| Annual income at baseline | 0.006 | ||
| <$6000 | 1.68 | −0.76, 4.12 | 0.176 |
| $6000–$11,999 | 3.63 | 1.45, 5.80 | 0.001 |
| $12,000–24,999 | 2.66 | 0.55, 4.77 | 0.014 |
| ≥$25,000 (reference) | ref | ||
Abbreviations: VACS= Veterans Aging Cohort Study; CL=Confidence Limits; MSM=men who have sex with men; IDU=intravenous drug user; AUDIT= Alcohol Use Disorders Identification Test-Consumption questionnaire; Ref=Reference value.
Adjusted for year of recruitment.
Respondents were categorized at baseline as current MSM (at least one male sexual partner in the past twelve months), heterosexual (only female sexual partners in past twelve months) or not sexually active (no sexual partners in past twelve months).
Sensitivity Analyses
Sensitivity analyses excluding participants who died during follow-up, observations with VACS Index scores over 80, and observations missing information on the AUDIT-C supported our main findings (Table 3). The impact of substance use category on VACS Index scores remained consistent; however, estimates were attenuated. When an AUDIT-C cut-off score of 6 was used rather than 4, unhealthy alcohol use had a greater negative impact [β=1.10, p=0.069] on VACS Index score. A sensitivity analysis accounting for frequency of cannabis and stimulant use supported main findings and demonstrated increased mortality risk with frequent (at least once a week) use of stimulants. Results are presented in Supplemental Table 1.
Table 3.
Sensitivity analyses using inverse probability weighted longitudinal linear mixed effect models with adjusted factors associated with VACS Index score among HIV-infected male VACS participants.a
| Excluding VACS Index Scores >80 (n= 3,053) | Excluding observations missing AUDIT-C scores (n= 3,040) | |||
|---|---|---|---|---|
|
| ||||
| β | p - value | β | p - value | |
| Intercept | 19.90 | 19.45 | ||
|
| ||||
| Time varying characteristics | ||||
|
| ||||
| Substance-use category | <0.001 | <0.001 | ||
| No substance use | −0.23 | 0.517 | −0.85 | 0.108 |
| Unhealthy alcohol use (AUDIT-C ≥4) | 0.09 | 0.846 | 0.36 | 0.488 |
| Any cannabis +/− alcohol | −1.03 | 0.019 | −1.39 | 0.010 |
| Any stimulant +/− alcohol or cannabis | 0.97 | 0.022 | 0.87 | 0.090 |
| Alcohol only <4 on AUDIT-C (reference) | ref | ref | ||
|
| ||||
| Smoking status | 0.020 | 0.202 | ||
| Current smoker | −0.91 | 0.082 | −0.35 | 0.598 |
| Former smoker | 0.20 | 0.689 | 0.56 | 0.382 |
| Never smoker (reference) | ref | ref | ||
|
| ||||
| Any illicit opioid use in past year (yes vs. no) | 0.26 | 0.453 | 0.34 | 0.441 |
|
| ||||
| IDU ever (at baseline, updated through follow-up) | 3.63 | <0.001 | 4.23 | <0.001 |
|
| ||||
| Time invariant or baseline characteristics | ||||
| MSM statusb | <0.001 | <0.001 | ||
| Current MSM | −3.93 | <0.001 | −4.18 | <0.001 |
| No sexual activity in past twelve months | 3.19 | <0.001 | 4.62 | <0.001 |
| Heterosexual (reference) | ref | ref | ||
|
| ||||
| Non-white race/ethnicity vs. white race/ethnicity | 3.11 | 0.002 | 3.51 | <0.001 |
|
| ||||
| High school education/less than high school vs. some college or greater | 0.59 | 0.393 | 0.92 | 0.244 |
|
| ||||
| Marital status at baseline | <0.001 | <0.001 | ||
| Married/living with partner | 1.56 | 0.081 | 1.75 | 0.088 |
| Divorced, separated, or widowed | 3.88 | <0.001 | 4.26 | <0.001 |
| Never married (reference) | ref | ref | ||
|
| ||||
| Unemployed/retired/other vs. employed at baseline | ||||
| 5.82 | <0.001 | 6.40 | <0.001 | |
|
| ||||
| Annual income at baseline | 0.007 | 0.011 | ||
| <$6000 | 1.72 | 0.117 | 1.59 | 0.209 |
| $6000–$11,999 | 3.28 | <0.001 | 3.41 | 0.002 |
| $12,000–24,999 | 2.27 | 0.017 | 2.72 | 0.013 |
| ≥$25,000 (reference) | ref | ref | ||
Abbreviations: VACS= Veterans Aging Cohort Study; MSM=men who have sex with men; IDU=intravenous drug user; AUDIT= Alcohol Use Disorders Identification Test-Consumption questionnaire; Ref=Reference value.
Adjusted for year of recruitment.
Respondents were categorized at baseline as current MSM (at least one male sexual partner in the past twelve months), heterosexual (only female sexual partners in past twelve months) or not sexually active (no sexual partners in past twelve months).
Discussion
The objective of this study was to determine whether use of alcohol, cannabis, and stimulants are associated with changes in the VACS Index, a validated prognostic indicator for all-cause mortality. We found no evidence of a negative effect of cannabis use on mortality risk among HIV-infected men in care. Although the effect size was small, stimulant use was associated with increased mortality risk (i.e., stimulant use was independently associated with a 1.08 increase in the VACS Index score compared to lower risk alcohol use, indicating a 4.3% increased risk of 5-year mortality). These findings suggest that interventions to reduce stimulant use in HIV-infected men may reduce mortality.
The legalization of cannabis or marijuana for medical or recreational use has renewed attention to potential harms associated with cannabis use.41 Studies of cannabis use among HIV-infected populations have yielded mixed results.24,34,42 In another study using the VACS cohort, Green et al. concluded that the participants who primarily used cannabis comprised a unique drug-using group due to the low prevalence of drug dependence and absence of negative consequences.34 Furthermore, cannabis may be used for medical purposes to alleviate symptoms related to HIV disease and/or ART (e.g. nausea, appetite stimulation).12,43 However, medical providers within the Veterans Health Administration system do not prescribe cannabis to treat HIV-related symptoms and we were unable to determine if participants used cannabis for this purpose, with or without a prescription. Nonetheless, further research is needed to determine whether recreational cannabis use has an impact on long-term mortality risk among HIV-infected patients.
Frequent stimulant use negatively impacted mortality risk for our study population. Supporting these findings, active cocaine use has been shown to predict poor ART adherence and viral rebound in HIV-infected patients.1,4,8 Concerns regarding potential interactions between cocaine and ART medications or interactive toxicity beliefs may lead to non-adherence and subsequent HIV-related disease sequelae.3 In addition, frequent use of stimulants might increase barriers to care engagement as well as ART adherence.44 Cocaine and use of other stimulants have been associated with decreased ART adherence in several studies of HIV-infected adults.1,4 Our study provides additional support for provision of treatment for stimulant use in HIV-infected patients.
Unhealthy alcohol use was prevalent within our study population. At the baseline assessment, over a third of our study population and half of those reporting stimulant use reported unhealthy alcohol use in the past year. Previous studies using the VACS cohort have found a high prevalence of unhealthy alcohol use and that alcohol use is associated with increased mortality risk and poorer HIV quality of care outcomes.45–47 Notably, HIV-infected individuals have an increased risk of mortality and physiologic injury at lower levels of alcohol use compared to uninfected individuals.46 Within our study, the increased mortality risk among individuals reporting stimulant use may be due, in part, to the prevalence of unhealthy alcohol use or a specific pattern of substance use which includes concurrent unhealthy alcohol and stimulant use.
Within our study, established social determinants of health (i.e., low income, unemployment, non-white race/ethnicity) had a greater impact on VACS Index score than any substance use category. Despite significant gains in improving survival for HIV-infected patients over the last two decades, disparities by race and socioeconomic status persist. Poverty-related stressors such as unstable housing, low health literacy, and food insecurity can negatively impact ART adherence and viral suppression.48–52 A recent longitudinal study with over 10,000 HIV-infected patients found that black men and women had significantly greater 10-year all-cause mortality risk compared to white men.53 Our study findings echo the need to identify modifiable factors through which to overcome observed disparities. An additional finding that warrants further research is the difference in mortality risk by MSM status. Within this analysis, men who reported MSM behavior at baseline had a decreased mortality risk compared to other HIV-infected men. Future research will explore the potential reasons for differences in mortality risk between MSM and heterosexual men within the cohort.
This study has some limitations that warrant mentioning. First, substance use categories were based on self-report and may be impacted by social desirability or recall bias. However, use of self-administered paper-based surveys rather than in-person interviews is likely to have decreased the impact of such bias.54 In addition, two of the substance use categories included multiple substances (i.e., cannabis with or without alcohol; stimulants with or without cannabis or alcohol) and this may have impacted findings. Second, biases related loss to follow-up and mortality can impact results from a large, ongoing cohort study such as VACS. However, inverse probability weighting to account for attrition and a sensitivity analysis evaluating the impact of mortality found that major findings were consistent. Finally, the study sample was restricted to male, HIV-infected U.S. veterans receiving care in the VA health care system which may limit generalizability to other HIV-infected populations, women, or veterans receiving care elsewhere. Nevertheless, at least two studies have found the VACS cohort comparable to other HIV-infected populations and combined the VACS cohort with other large HIV cohorts in Europe and North America for cross-cohort analyses.55,56
Despite these limitations, this study has several strengths. To the best of our knowledge, this is the first study to characterize substance use categories based on cannabis, stimulant, and alcohol use among HIV-infected men and evaluate the associated mortality risk. Longitudinal study design was employed with a median follow-up time of 6 years. Inverse probability weighting accounted for loss to follow-up and missing data within multivariable analyses. In addition, use of a time-varying substance use category variable and linear mixed effects modeling allowed for increased flexibility and the ability to discern associations that may not have been apparent using cross-sectional analyses.
Overall, cannabis use does not appear to negatively impact mortality prognosis while stimulant use is associated with greater mortality risk compared to lower risk alcohol use among HIV-infected men in care. Identifying the association of stimulant use with increased mortality risk can help inform targeted intervention and substance use treatment. Additionally, knowledge of the risks associated with frequent stimulant use may help motivate patients to decrease or discontinue use. However, sociodemographic characteristics appear to impact mortality risk to a greater degree than alcohol, cannabis, or stimulant use. Programs to lessen the impact of poverty and racial disparities continue to be vital components of improving the health of male veterans living with HIV/AIDS.
Supplementary Material
Sensitivity analyses with alternative substance-use categorization using inverse probability weighted longitudinal linear mixed effect models with adjusted factors associated with VACS Index score among HIV-infected male VACS participants (n=3,099).a
Acknowledgments
Funding: This work was supported by grants from the National Institute on Alcohol Abuse and Alcoholism (NIAAA: U24-AA022000, U10-AA013566, U01-AA020795, U01-AA020790, U24-AA020794, and P01-AA019072), the National Institute of Allergy and Infectious Diseases (P30-AI042853), and in kind by the U.S. Department of Veterans Affairs.
We would like to acknowledge the veterans who participate in the Veterans Aging Cohort Study (VACS) and the study coordinators and staff at each VACS site and at the West Haven Coordinating Center. The authors declare that they have no conflict of interest. This work was supported by grants from the National Institute on Alcohol Abuse and Alcoholism (NIAAA: U24-AA022000, U10-AA013566, U01-AA020795, U01-AA020790, U24-AA020794, and P01-AA019072), the National Institute of Allergy and Infectious Diseases (P30-AI042853), and in kind by the U.S. Department of Veterans Affairs.
Footnotes
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
Conflict of Interest: The authors declare that they have no conflict of interest.
Compliance with Ethical Standards
Disclosure of potential conflicts of interest: The authors declare that they have no conflict of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- 1.Arnsten JH, Demas PA, Grant RW, et al. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. J Gen Intern Med. 2002;17(5):377–381. doi: 10.1046/j.1525-1497.2002.10644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langebeek N, Gisolf EH, Reiss P, et al. Predictors and correlates of adherence to combination antiretroviral therapy (ART) for chronic HIV infection: a meta-analysis. BMC Med. 2014;12:142. doi: 10.1186/s12916-014-0142-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalichman SC, Kalichman MO, Cherry C, et al. Intentional Medication Nonadherence Because of Interactive Toxicity Beliefs Among HIV-Positive Active Drug Users. J Acquir Immune Defic Syndr. 2015;70(5):503–509. doi: 10.1097/QAI.0000000000000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hinkin CH, Barclay TR, Castellon SA, et al. Drug use and medication adherence among HIV-1 infected individuals. AIDS Behav. 2007;11(2):185–194. doi: 10.1007/s10461-006-9152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sullivan LE, Goulet JL, Justice AC, Fiellin DA. Alcohol consumption and depressive symptoms over time: a longitudinal study of patients with and without HIV infection. Drug Alcohol Depend. 2011;117(2–3):158–163. doi: 10.1016/j.drugalcdep.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kapadia F, Vlahov D, Donahoe RM, Friedland G. The role of substance abuse in HIV disease progression: reconciling differences from laboratory and epidemiologic investigations. Clin Infect Dis. 2005;41(7):1027–1034. doi: 10.1086/433175. [DOI] [PubMed] [Google Scholar]
- 7.Nath A, Hauser KF, Wojna V, et al. Molecular basis for interactions of HIV and drugs of abuse. J Acquir Immune Defic Syndr. 2002;31(Suppl 2):S62–69. doi: 10.1097/00126334-200210012-00006. [DOI] [PubMed] [Google Scholar]
- 8.Baum MK, Rafie C, Lai S, Sales S, Page B, Campa A. Crack-cocaine use accelerates HIV disease progression in a cohort of HIV-positive drug users. J Acquir Immune Defic Syndr. 2009;50(1):93–99. doi: 10.1097/QAI.0b013e3181900129. [DOI] [PubMed] [Google Scholar]
- 9.Williams EC, Hahn JA, Saitz R, Bryant K, Lira MC, Samet JH. Alcohol Use and Human Immunodeficiency Virus (HIV) Infection: Current Knowledge, Implications, and Future Directions. Alcohol Clin Exp Res. 2016;40(10):2056–2072. doi: 10.1111/acer.13204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chander G, Himelhoch S, Moore RD. Substance abuse and psychiatric disorders in HIV-positive patients: epidemiology and impact on antiretroviral therapy. Drugs. 2006;66(6):769–789. doi: 10.2165/00003495-200666060-00004. [DOI] [PubMed] [Google Scholar]
- 11.Mimiaga MJ, Reisner SL, Grasso C, et al. Substance use among HIV-infected patients engaged in primary care in the United States: findings from the Centers for AIDS Research Network of Integrated Clinical Systems cohort. Am J Public Health. 2013;103(8):1457–1467. doi: 10.2105/AJPH.2012.301162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furler MD, Einarson TR, Millson M, Walmsley S, Bendayan R. Medicinal and recreational marijuana use by patients infected with HIV. AIDS Patient Care STDS. 2004;18(4):215–228. doi: 10.1089/108729104323038892. [DOI] [PubMed] [Google Scholar]
- 13.Harris GE, Dupuis L, Mugford GJ, et al. Patterns and correlates of cannabis use among individuals with HIV/AIDS in Maritime Canada. Can J Infect Dis Med Microbiol. 2014;25(1):e1–7. doi: 10.1155/2014/301713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D'Souza G, Matson PA, Grady CD, et al. Medicinal and recreational marijuana use among HIV-infected women in the Women's Interagency HIV Study (WIHS) cohort, 1994–2010. J Acquir Immune Defic Syndr. 2012;61(5):618–626. doi: 10.1097/QAI.0b013e318273ab3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruce D, Harper GW, Fernandez MI The Adolescent Medicine Trials Network for HIVAI. Heavy Marijuana Use among Gay and Bisexual Male Emerging Adults Living with Hiv/Aids. J HIV AIDS Soc Serv. 2013;12(1):26–48. doi: 10.1080/15381501.2012.735171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bing EG, Burnam MA, Longshore D, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry. 2001;58(8):721–728. doi: 10.1001/archpsyc.58.8.721. [DOI] [PubMed] [Google Scholar]
- 17.White JM, Gordon JR, Mimiaga MJ. The Role of Substance Use and Mental Health Problems in Medication Adherence Among HIV-Infected MSM. LGBT Health. 2014;1(4):319–322. doi: 10.1089/lgbt.2014.0020. [DOI] [PubMed] [Google Scholar]
- 18.Skeer MR, Mimiaga MJ, Mayer KH, O'Cleirigh C, Covahey C, Safren SA. Patterns of substance use among a large urban cohort of HIV-infected men who have sex with men in primary care. AIDS Behav. 2012;16(3):676–689. doi: 10.1007/s10461-011-9880-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colfax G, Shoptaw S. The methamphetamine epidemic: implications for HIV prevention and treatment. Curr HIV/AIDS Rep. 2005;2(4):194–199. doi: 10.1007/s11904-005-0016-4. [DOI] [PubMed] [Google Scholar]
- 20.Reback CJ, Fletcher JB, Shoptaw S, Grella CE. Methamphetamine and other substance use trends among street-recruited men who have sex with men, from 2008 to 2011. Drug Alcohol Depend. 2013;133(1):262–265. doi: 10.1016/j.drugalcdep.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milloy MJ, Marshall B, Kerr T, et al. High-intensity cannabis use associated with lower plasma human immunodeficiency virus-1 RNA viral load among recently infected people who use injection drugs. Drug Alcohol Rev. 2015;34(2):135–140. doi: 10.1111/dar.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marcellin F, Lions C, Rosenthal E, et al. No significant effect of cannabis use on the count and percentage of circulating CD4 T-cells in HIV-HCV co-infected patients (ANRS CO13-HEPAVIH French cohort) Drug Alcohol Rev. 2016 doi: 10.1111/dar.12398. [DOI] [PubMed] [Google Scholar]
- 23.Okafor CN, Zhou Z, Burrell LE, 2nd, et al. Marijuana use and viral suppression in persons receiving medical care for HIV-infection. Am J Drug Alcohol Abuse. 2016:1–8. doi: 10.1080/00952990.2016.1191505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okafor CN, Cook RL, Chen X, et al. Trajectories of Marijuana Use among HIV-seropositive and HIV-seronegative MSM in the Multicenter AIDS Cohort Study (MACS), 1984–2013. AIDS Behav. 2016 doi: 10.1007/s10461-016-1445-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tate JP, Justice AC, Hughes MD, et al. An internationally generalizable risk index for mortality after one year of antiretroviral therapy. AIDS. 2013;27(4):563–572. doi: 10.1097/QAD.0b013e32835b8c7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Justice AC, Modur SP, Tate JP, et al. Predictive accuracy of the Veterans Aging Cohort Study index for mortality with HIV infection: a North American cross cohort analysis. J Acquir Immune Defic Syndr. 2013;62(2):149–163. doi: 10.1097/QAI.0b013e31827df36c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Justice AC, Freiberg MS, Tracy R, et al. Does an index composed of clinical data reflect effects of inflammation, coagulation, and monocyte activation on mortality among those aging with HIV? Clin Infect Dis. 2012;54(7):984–994. doi: 10.1093/cid/cir989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conigliaro J, Madenwald T, Bryant K, et al. The Veterans Aging Cohort Study: observational studies of alcohol use, abuse, and outcomes among human immunodeficiency virus-infected veterans. Alcohol Clin Exp Res. 2004;28(2):313–321. doi: 10.1097/01.alc.0000113414.73220.21. [DOI] [PubMed] [Google Scholar]
- 29.Justice AC, Dombrowski E, Conigliaro J, et al. Veterans Aging Cohort Study (VACS): Overview and description. Med Care. 2006;44(8 Suppl 2):S13–24. doi: 10.1097/01.mlr.0000223741.02074.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158(16):1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 31.Saitz R. Clinical practice. Unhealthy alcohol use. N Engl J Med. 2005;352(6):596–607. doi: 10.1056/NEJMcp042262. [DOI] [PubMed] [Google Scholar]
- 32.Broyles LM, Gordon AJ, Sereika SM, Ryan CM, Erlen JA. Do words matter? Incongruent responses to inconsistently worded AUDIT-C alcohol screening instruments. Substance abuse. 2011;32(4):202–209. doi: 10.1080/08897077.2011.600673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim SH, Ostrow D, Stall R, et al. Changes in stimulant drug use over time in the MACS: evidence for resilience against stimulant drug use among men who have sex with men. AIDS Behav. 2012;16(1):151–158. doi: 10.1007/s10461-010-9866-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Green TC, Kershaw T, Lin H, et al. Patterns of drug use and abuse among aging adults with and without HIV: a latent class analysis of a US Veteran cohort. Drug Alcohol Depend. 2010;110(3):208–220. doi: 10.1016/j.drugalcdep.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Finucane MM, Samet JH, Horton NJ. Translational methods in biostatistics: linear mixed effect regression models of alcohol consumption and HIV disease progression over time. Epidemiol Perspect Innov. 2007;4:8. doi: 10.1186/1742-5573-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38(4):963–974. [PubMed] [Google Scholar]
- 37.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168(6):656–664. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weuve J, Tchetgen Tchetgen EJ, Glymour MM, et al. Accounting for bias due to selective attrition: The example of smoking and cognitive decline. Epidemiology (Cambridge, Mass) 2012;23(1):119–128. doi: 10.1097/EDE.0b013e318230e861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsubono Y, Yamada S, Nishino Y, Tsuji I, Hisamichi S. Choice of comparison group in assessing the health effects of moderate alcohol consumption. JAMA. 2001;286(10):1177–1178. doi: 10.1001/jama.286.10.1177. [DOI] [PubMed] [Google Scholar]
- 40.Stranges S, Notaro J, Freudenheim JL, et al. Alcohol drinking pattern and subjective health in a population-based study. Addiction. 2006;101(9):1265–1276. doi: 10.1111/j.1360-0443.2006.01517.x. [DOI] [PubMed] [Google Scholar]
- 41.Volkow ND, Baler RD, Compton WM, Weiss SR. Adverse health effects of marijuana use. N Engl J Med. 2014;370(23):2219–2227. doi: 10.1056/NEJMra1402309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghosn J, Leruez-Ville M, Blanche J, et al. HIV-1 DNA levels in peripheral blood mononuclear cells and cannabis use are associated with intermittent HIV shedding in semen of men who have sex with men on successful antiretroviral regimens. Clin Infect Dis. 2014;58(12):1763–1770. doi: 10.1093/cid/ciu187. [DOI] [PubMed] [Google Scholar]
- 43.Corless IB, Lindgren T, Holzemer W, et al. Marijuana effectiveness as an HIV self-care strategy. Clin Nurs Res. 2009;18(2):172–193. doi: 10.1177/1054773809334958. [DOI] [PubMed] [Google Scholar]
- 44.Tucker JS, Orlando M, Burnam MA, Sherbourne CD, Kung FY, Gifford AL. Psychosocial mediators of antiretroviral nonadherence in HIV-positive adults with substance use and mental health problems. Health Psychol. 2004;23(4):363–370. doi: 10.1037/0278-6133.23.4.363. [DOI] [PubMed] [Google Scholar]
- 45.Korthuis PT, McGinnis KA, Kraemer KL, et al. Quality of HIV Care and Mortality Rates in HIV-Infected Patients. Clin Infect Dis. 2016;62(2):233–239. doi: 10.1093/cid/civ762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Justice AC, McGinnis KA, Tate JP, et al. Risk of mortality and physiologic injury evident with lower alcohol exposure among HIV infected compared with uninfected men. Drug Alcohol Depend. 2016;161:95–103. doi: 10.1016/j.drugalcdep.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Korthuis PT, Fiellin DA, McGinnis KA, et al. Unhealthy alcohol and illicit drug use are associated with decreased quality of HIV care. J Acquir Immune Defic Syndr. 2012;61(2):171–178. doi: 10.1097/QAI.0b013e31826741aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalichman SC, Grebler T. Stress and poverty predictors of treatment adherence among people with low-literacy living with HIV/AIDS. Psychosom Med. 2010;72(8):810–816. doi: 10.1097/PSY.0b013e3181f01be3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weiser SD, Frongillo EA, Ragland K, Hogg RS, Riley ED, Bangsberg DR. Food insecurity is associated with incomplete HIV RNA suppression among homeless and marginally housed HIV-infected individuals in San Francisco. J Gen Intern Med. 2009;24(1):14–20. doi: 10.1007/s11606-008-0824-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Waldrop-Valverde D, Osborn CY, Rodriguez A, Rothman RL, Kumar M, Jones DL. Numeracy skills explain racial differences in HIV medication management. AIDS Behav. 2010;14(4):799–806. doi: 10.1007/s10461-009-9604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Delpierre C, Cuzin L, Lauwers-Cances V, Datta GD, Berkman L, Lang T. Unemployment as a risk factor for AIDS and death for HIV-infected patients in the era of highly active antiretroviral therapy. Sex Transm Infect. 2008;84(3):183–186. doi: 10.1136/sti.2007.027961. [DOI] [PubMed] [Google Scholar]
- 52.Leaver CA, Bargh G, Dunn JR, Hwang SW. The effects of housing status on health-related outcomes in people living with HIV: a systematic review of the literature. AIDS Behav. 2007;11(6 Suppl):85–100. doi: 10.1007/s10461-007-9246-3. [DOI] [PubMed] [Google Scholar]
- 53.Lesko CR, Cole SR, Miller WC, et al. Ten-year Survival by Race/Ethnicity and Sex Among Treated, HIV-infected Adults in the United States. Clin Infect Dis. 2015;60(11):1700–1707. doi: 10.1093/cid/civ183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosen CS, Henson BR, Finney JW, Moos RH. Consistency of self-administered and interview-based Addiction Severity Index composite scores. Addiction. 2000;95(3):419–425. doi: 10.1046/j.1360-0443.2000.95341912.x. [DOI] [PubMed] [Google Scholar]
- 55.Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360(18):1815–1826. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.When To Start C, Sterne JA, May M, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373(9672):1352–1363. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sensitivity analyses with alternative substance-use categorization using inverse probability weighted longitudinal linear mixed effect models with adjusted factors associated with VACS Index score among HIV-infected male VACS participants (n=3,099).a