Abstract
Hyperamylinemia is a condition that accompanies obesity and precedes type II diabetes, and it is characterized by above-normal blood levels of amylin, the pancreas-derived peptide. Human amylin oligomerizes easily and can deposit in the pancreas [1], brain [2], and heart [3], where they have been associated with calcium dysregulation. In the heart, accumulating evidence suggests that human amylin oligomers form moderately cation-selective [4, 5] channels that embed in the cell sarcolemma (SL). The oligomers increase membrane conductance in a concentration-dependent manner [5], which is correlated with elevated cytosolic Ca2+. These findings motivate our core hypothesis that non-selective inward Ca2+ conduction afforded by human amylin oligomers increase cytosolic and SR Ca2+ load, which thereby magnifies intracellular Ca2+ transients. Questions remain however regarding the mechanism of amylin-induced Ca2+ dysregulation, including whether enhanced SL Ca2+ influx is sufficient to elevate cytosolic Ca2+ load [6], and if so, how might amplified Ca2+ transients perturb Ca2+-dependent cardiac pathways. To investigate these questions, we modified a computational model of cardiomyocytes Ca2+ signaling to reflect experimentally-measured changes in SL membrane permeation and decreased sarcoplasmic/endoplasmic reticulum calcium ATPase (SERCA) function stemming from acute and transgenic human amylin peptide exposure. With this model, we confirmed the hypothesis that increasing SL permeation alone was sufficient to enhance Ca2+ transient amplitudes. Our model indicated that amplified cytosolic transients are driven by increased Ca2+ loading of the sarcoplasmic reticulum (SR) and the greater fractional release may contribute to the Ca2+-dependent activation of calmodulin. Importantly, elevated Ca2+ in the SR and dyadic space collectively drive greater fractional SR Ca2+ release for human amylin expressing rats (HIP) and acute amylin-exposed rats (+Amylin) mice, which contributes to the inotropic rise in cytosolic Ca2+ transients. These findings suggest that increased membrane permeation induced by oligomeratization of amylin peptide in cell sarcolemma contributes to Ca2+ dysregulation in pre-diabetes.
Keywords: cardio myocytes, amylin Ca2+ leak, Ca2+ transients, pre-diabetic rats, Ca2+ dysregulation
1 Introduction
Amylin, a 3.9 kilodalton peptide produced by the pancreatic β cells [7], is secreted along with insulin into the blood stream [8]. Human amylin is amyloidogenic, i.e. at high concentrations it aggregates into amyloid and fibrils. In contrast, the amylin isoform found in rodents is non-amyloidogenic [6]. Secretion of amylin (and insulin) is increased in individuals with insulin resistance, a metabolic abnormality that precedes the onset of type II diabetes. Enhanced amylin secretion leads to accumulation of amylin aggregates in the pancreas [9] and other organs, including the heart [6]. These amylin deposits have been shown to induce diastolic dysfunction [6], hypertrophy, and dilation [10]. While studies correlating human amylin oligomerization in tissue with the onset of pathological states typical of diabetic cardiomyopathy [11] are beginning to emerge [3], the molecular mechanisms linking amylin insult with cellular dysfunction remain incompletely understood. Gaining momentum, however, is the notion that amylin oligomers in cardiac tissue may disrupt normal calcium homeostasis [6], stemming from amylin’s moderately cation-selective conductance properties [4, 5, 12]. While this conductance is small relative to predominant sarcolemmal Ca2+ currents including the L-type calcium channel (LCC) and Na+/Ca2+ exchanger (NCX), it nevertheless exhibits largely unexplained effects on perturbing intracellular Ca2+ signals and recruiting Ca2+-dependent pathways associated with pathological, hypertrophic remodeling [13].
In the healthy heart, the Ca2+-dependent excitation-contraction (EC) coupling cycle begins with a depolarizing action potential (AP) that modulates sarcolemma (SL) Ca2+ fluxes, including contributions from LCC and NCX [14]. Ca2+ entry via LCC and NCX triggers [15] sarcoplasmic reticulum (SR) Ca2+ release via ryanodine receptors (RyRs), leading to a rapid increase in intracellular Ca2+(Ca2+ transient) that ultimately activates and regulates competent myocyte contraction [14]. The cycle completes as SR Ca2+ uptake via the sarcoplasmic/endoplasmic reticulum calcium ATPase (SERCA), as well sarcolemmal Ca2+ extrusion via NCX and the sarcolemmal Ca2+ ATPase, collectively restore diastolic Ca2+ levels. Recently, we reported that this process is perturbed in hearts from rats that express human amylin in the pancreas (HIP), as well as in isolated cardiomyocytes acutely exposed to the peptide (+Amylin conditions) [6]. In both cases, measurements of a passive, trans-sarcolemmal Ca2+ leak from isolated myocytes were faster relative to control [6], which suggested that amylin oligomers insert into the membrane to facilitate a non-selective Ca2+ current that correlated with increased cytosolic Ca2+ transients. Furthermore, in HIP rat myocytes SERCA function was impaired, and the hypertrophic remodeling associated with nuclear factor of activated T-cells (NFAT) and histone deacetylase (HDAC) pathways were activated. Both properties are strongly associated with the progression toward heart failure [13]. In this study, therefore, we seek to clarify whether and through which mechanisms the human amylin-induced sarcolemmal Ca2+ leak leads to myocyte Ca2+ dysregulation.
Cardiac computational models are routinely used for exploring intracellular mechanisms of Ca2+ signaling and their dysregulation in cardiac tissue [16–22]. We extended one such model, the Shannon-Bers model of ventricular myocyte Ca2+ dynamics [23], to unravel the influence of amylin in the HIP phenotype. Specifically, the revised model reflects our experimentally-measured changes in SL membrane Ca2+ permeation as well as decreased SERCA function consistent with acutely-exposed myocytes and transgenic human amylin rats [6]. We find that increased a sarcolemmal Ca2+ background current (’leak’) arising from human amylin oligomerization was sufficient to reproduce enhanced Ca2+ transients previously measured in HIP rats [6]. These simulations implicate increased SR loading and fractional SR Ca2+ release work in tandem to magnify Ca2+ transient amplitude for the amylin phenotypes, which in turn elevates cytosolic Ca2+ load. Accompanying enhanced SR Ca2+ loading is an increased fractional release, stemming from both SR and dyadic cleft Ca2+ content. Finally, we show higher propensities for CaM activation under conditions of elevated diastolic Ca2+, which we speculate may trigger the CaM-dependent NFAT remodeling pathway. These findings lead to our hypothesized model of amylin-induced Ca2+ dysregulation summarized in Fig. 1.
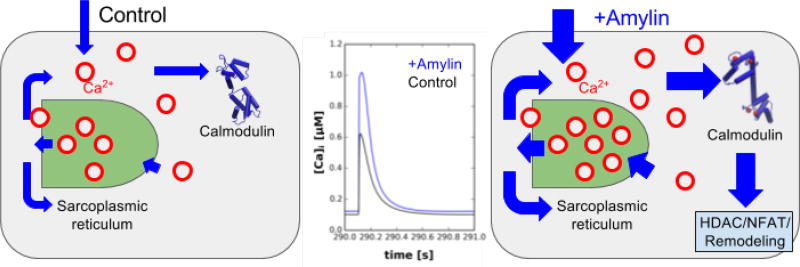
Figure 1.
Hypothesized model. Increased sarcolemmal Ca2+ in acute amylin-exposed rats (+Amylin) increases sarcoplasmic reticulum Ca2+ loading, amplifies intracellular Ca2+ transients and increases the Ca2+-bound state of proteins including calmodulin (CaM). Blue arrows represent Ca2+ fluxes.
2 Materials and Methods
2.1 Experimental animals
N=12 Sprague-Dawley rats were used in this study. All animal experiments were performed conform to the NIH guide for the care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committee at University of Kentucky. Ventricular myocytes were isolated by perfusion with collagenase on a gravity-driven Langendorff apparatus [6].
2.2 Measurements of Ca2+ transients and passive, trans-sarcolemmal sarcolemmal Na+ and Ca2+ leaks
Myocytes were plated on laminin-coated coverslips, mounted on the stage of a fluorescence microscope and loaded with either Fluo4-AM (10 µmol/L, for 25 min) for Ca2+ transient recordings or Fura2-AM (10 µmol/L, for 25 min) for measurements of sarcolemmal Ca2+ leak. Ca2+ transients were elicited by stimulation with external electrodes at a frequency of 1 Hz. The passive trans-sarcolemmal Ca2+ leak was measured as the initial rate of Ca2+ decline upon reducing external Ca2+ from 1 to 0 mM. In these experiments, Ca2+ fluxes to and from the SR were blocked by pre-treating the cells with 10 µM thapsigargin for 10 min whereas the NCX and sarcolemmal Ca2+- ATPase were abolished by using 0 Na+/0 Ca2+ solution (Na+ replaced with Li+) and adding 20 µM carboxyeosin, respectively. Sarcolemmal Ca2+ leak was measured in control cells and in myocytes incubated with human amylin (50 µM for 2 hrs) in the absence and presence of the membrane sealant poloxamer 188 (P188, 50 µM) or epoxyeicosatrienoic acid (14,15-EET, 5 M). Data for HIP rats are from Despa et al. [6].
Na+ influx was measured as the initial rate of the increase in intracellular Na+ concentration ([Na+]i) immediately following Na+/K+ ATPase (NKA) inhibition with 10 mM ouabain. As described previously [24], [Na+]i was measured using the fluorescent indicator SBFI (TefLabs). The SBFI ratio was calibrated at the end of each experiment using divalent-free solutions with 0, 10, or 20 mmol/L of extracellular Na+ in the presence of 10 µmol/L gramicidin and 100 µmol/L strophanthidin.
2.3 Statistical analysis
Data are expressed as mean ± SEM. Statistical discriminations were performed using (1) 2-tailed unpaired Student t-test when comparing 2 groups and 1-way ANOVA when comparing multiple groups Statistical analysis was done in GraphPad Prism version 5.0 for Windows (GraphPad Software, La Jolla, CA). P<0.05 was considered significant.
2.4 Simulation and analysis protocols
2.4.1 Summary of Shannon-Bers-Morotti rat Ca2+ handling model
To examine the relationship between increased sarcolemmal Ca2+ entry and elevated Ca2+ transients reported in rats [6], we adapted a rabbit ventricular myocyte model of Ca2+ signaling to reflect handling terms specific to rodents. This choice was based on the initial lack of rat-specific Ca2+ handling models available in the literature. Recent computational models of rat cardiomyocyte Ca2+ handling have been published [25, 26], and are in qualitative agreement with our implementation, as we will discuss below. Myocytes from rats and mice have similar rates of Ca2+ relaxation via SERCA, NCX, and minor contributors such as sarcolemmal Ca2+-ATPase and mitochondrial Ca2+ uptake (92, 8 and 1%, versus 90.3, 9.2 and 0.5%, respectively) [27, 28]. Accordingly, mouse-specific parameters and potassium current changes were introduced into the Shannon-Bers rabbit cardiomyocyte Ca2+ model [23] according to Morotti et al. [29] (summarized in Supplement). The resulting model is hereafter referred to as the Shannon-Bers-Morroti (SBM) model. Model equations, ’state’ names, current names and initial conditions are provided in the supplement. As noted in [29], four predominant changes in potassium channels were included: 1) the transient outward potassium current expression for rabbits was replaced with fast component (itof) for mice, 2) the slowly activating delayed rectifier current was substituted with a slowly inactivating delayed rectifier current (iKs), 3) a non-inactivating potassium steady-state current (iss) was added 4) the inward rectifier potassium current (iK1) was reduced. Other distinctions between the two species are the elevated intracellular sodium load and sodium ion current in murine versus rabbit species, which we optimized to match experimental data collected in this study. In Fig. S2–Fig. S4, we compare metrics such as Ca2+ transients, action potentials, potassium currents and prominent Na+/Ca2+ currents for rabbits versus mice predicted using the ’Numerical model of Ca2+ handling’ described below, for which we report excellent agreement with data from Morotti et al. [29].
The prominent adjustment required to then match rat Ca2+ collected at 310 K (98.3° F) from Gattoni et al. [25] was optimization of SERCA Vmax (Fig. S5) via a genetic algorithm (GA) we describe in the Supplement. The decision to use the Gattoni Ca2+ transient data was based on the common temperature of 310K used in their data and the fitting of the Shannon-Bers model, whereas transients reported by Despa et al were measured at 298K (76.7° F). Thus, all simulations were conducted at 310 K. After fitting the Ca2+ transient, NKA Vmax was varied to maintain intracellular Na+ content at 12 mM, as was measured in this study Fig. S16.
In rat myocytes incubated with human amylin (+Amylin), amylin oligomer deposits was correlated with a roughly 73% higher rate of sarcolemmal Ca2+ leak (see Figure 3D of Despa et al. [6]). We indicate in the supplement that amylin does not significantly impact LCC current in our experiments (see Sect. S.3.1 and Fig. S15), thus the computational model assumed amylin only impacts sarcolemmal Ca2+ leak when acutely applied to isolated myocytes. Therefore, to reflect increased SL leak due for the +Amylin condition, we increased by 73% (see Table S3) the Ca2+ conductance term, GCa, in the Shannon-Bers leak model described by Eq. 1
| (1) |
where ρi represents sarcolemmal leak density of ion i, Gi is the max conductance for ion i, V is voltage, and Ei is the Nernst potential of ion i. It is important to emphasize that the leak model assumed in the Shannon-Bers model balances Ca2+ entry (via LCC and NCX) with extrusion mechanisms to ensure physiologically-reasonable resting diastolic Ca2+ levels [23]. For simplicity, we utilized Eq. 1 to account for the additional trans-sarcolemmal Ca2+ leak due to amylin oligomerization, though we discuss in Limitations how this model might be improved. Although amylin pores exhibit poor cation selectivity [5], we maintained GNa at baseline values, given that we observed no detectable change in Na+ load (Fig. S16, discussed in Sect. S.3.2). This required a slight reoptimization of NKA max to remain in agreement with the consistent 12 mM loading reported in Fig. S16. Specifically, the GA determined that increasing NKA Vmax by 14% was required to maintain normal Na+ levels (see Table S3), which interestingly concurs with a study indicating amylin-agonized NKA function in skeletal muscle [30]. For the human amylin transgenic rat model (HIP), the SL leak rate is unknown, therefore we fit GCA to reproduce the approximately 40% higher Ca2+ transients observed in the Despa et al. rats paced at 0.5 Hz (Fig 5 of [6]). It was also observed that Ca2+ transient decay time increased by nearly 30% in HIP rats relative to control [6], for which the GA determined that a reduction in SERCA Vmax by 38% was necessary.
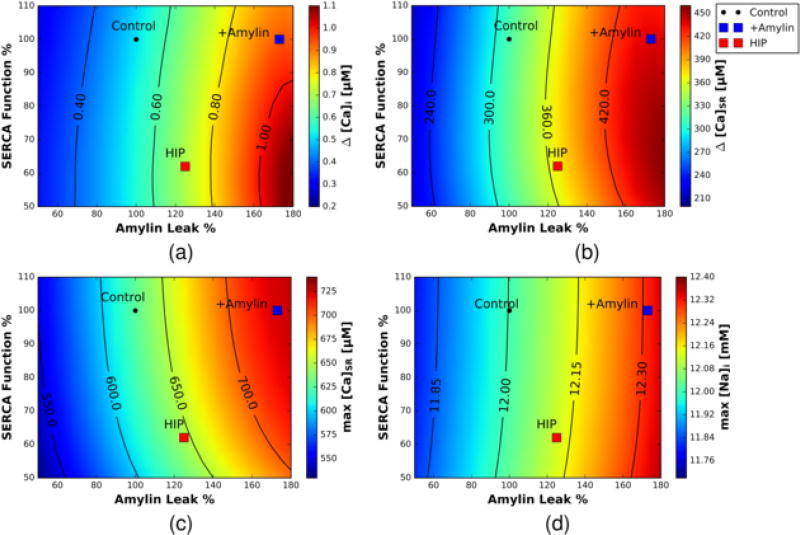
Figure 3.
Predicted Ca2+ transients and loads as a function of SERCA Vmax activity (% of control) and SL Ca2+ leak (% of control). a) intracellular Ca2+, b) SR Ca2+ transient c) maximum SR Ca2+ load and d) sodium load. A black circle is representative of the Control case, a blue square is representative of the +Amylin case, and a red square is representative of the HIP case.
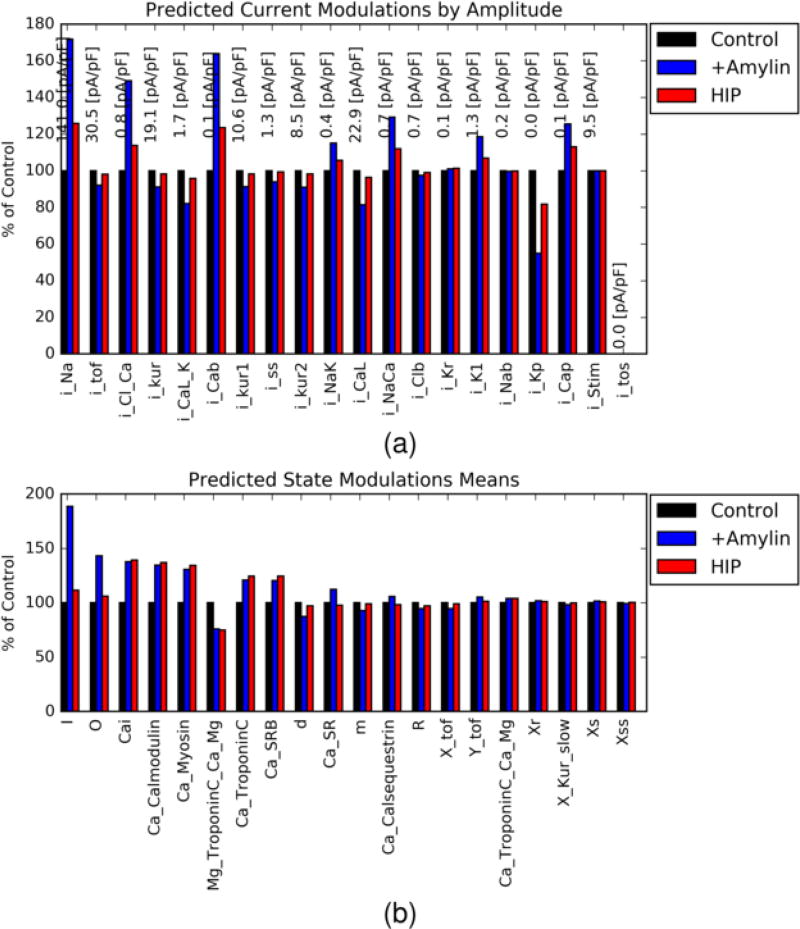
Figure 5.
Percent change in SBM-predicted a) ion current amplitudes and b) model state variables for +Amylin (blue) and HIP (red) configurations relative to control (black, normalized to 100%). A list of current labels is provided in the supplement Table S2
2.4.2 Numerical model of Ca2+ handling
The Shannon-Bers cell ML model was converted into a Python module via the Generalized ODE Translator gotran (https://bitbucket.org/johanhake/gotran) to make use of our Python-based routines for simulation and analyses. The mouse-specific alterations summarized in the previous section were implemented into the resulting module. In our numerical experiments, the SBM model was numerically integrated by the scipy function odeint, which utilizes the LSODA algorithm for stiff ordinary differential equations [31]. The numerical model was integrated using a timestep of 0.1 ms for a total simulation time of up to 5 minutes. These simulations provide as output the time-dependent values of the SBM ’states’, such as intracellular Ca2+ load or the action potential, as well as ’currents’ that include major Ca2+, Na+, K+, and Cl−-conducting proteins. Model fitting proceeded by a genetic algorithm (reviewed in [32]) that iteratively improved parameter values, such as LCC Ca2+ conductance, sarcolemmal Ca2+ leak, and NKA conductance over several generations of ’progeny’ (Fig. S6). Experimentally-measured outputs, such as Ca2+ transient decay time and amplitude, were measured for each of the progeny; those that reduced output error relative to the experimentally-measured equivalent with stored for future generations (see Sect. S.3.5 for more details). To validate our implementation, we present comparisons of action potentials, intracellular Ca2+ and Na+ transients, as well as ionic currents for rabbit versus murine cardiac ventricular myocytes (see Sect. S.2), for which we report good agreement across these model outputs.
We additionally stimulated the model at several frequencies ranging from 0.1 to 2.0 Hz, to ensure that our model predictions were consistent with transient data recorded by Despa et al. [6]. Sensitivity analyses were additionally performed to determine relative correlations between model input parameters and predicted outputs (see Supplement). Data processing was performed using scipy and the ipython notebooks, with exception to the sensitivity analyses described in the supplement. Source code will be provided at HTTPS://BITBUCKET.ORG/HUSKEYPM/WHOLECELL. Since the initiation of this project, an additional rat cardiomyocyte model has been published. This model is based on the 2001 Pandit model of the rat LV cardiomyocyte [33] and includes recalibrated Ca2+ fluxes parameters to provide more consistent fitting with experimental data. We discuss later that the Devenyi et al [26] model reproduces trends discussed in this paper, which we obtained using the above described SBM formulation.
2.4.3 Analyses
To examine potential mechanisms that link increased SL Ca2+ permeation to elevated Ca2+ transients, we present a simple method, State Decomposition Analysis (SDA), that monitors and identifies prominent changes in key ’state’ variables (including the action potential, SR-load, channel gate probabilities among others) as well as ion channel currents, relative to control conditions. The key benefit of this approach is the automated identification of modulated EC coupling components that can motivate model refinements and additional experiments. The SDA method consists of the following steps: 1) numerically solve the time-dependent ordinary differential equations (ODE)s governing all components (the state variables) of the EC coupling model for trial and control parameter configurations 2) ’score’ the time-dependent state values according to metrics like amplitude 3) calculate percent differences between trial and control state variable scores 4) rank order states by either the percent difference with a reference state or by the amplitudes in the reference.
3 Results
3.1 Effects of human amylin on intracellular Ca2+ transients in rat cardiac ventricular myocytes
Accumulation of human amylin aggregates in rat cardiomyocyte SL was previously correlated with increased rates of sarcolemmal Ca2+ leak and amplified Ca2+ transient amplitudes [6]. However, the mechanism linking sarcolemmal Ca2+ leak and Ca2+ transient amplitudes is not established. We first validate the hypothesis that amylin oligomerization has the primary effect of elevating sarcolemmal Ca2+ leak. This was evaluated by measuring the effect of human amylin on the Ca2+ leak and Ca2+ transients in the absence and in the presence of poloxamer 188 (P188), a surfactant that stabilizes lipid bilayers and seals sarcolemmal lesions [34] through hydrophobic interactions [35]. As in previous reports, human amylin significantly increased both passive SL Ca2+ leak and Ca2+ transient amplitude (Fig. S1A). When amylin was applied in the presence of P188, however, SL Ca2+ leak and transient amplitudes were statistically comparable to control (Fig. S1). Similar behavior was observed upon co-incubation of amylin with epoxyeicosatrienoic acids (EET), which have anti-aggregation effects and reduce amylin oligomerization at the SL [10], though they are reported to have opposing effects on the regulation inward Ca2+ entry via voltage-gated and L-type Ca2+ channels [36, 37]. These results support the hypothesis that amylin primarily acts to increase trans-sarcolemmal Ca2+ conductance potentially through poration of the membrane, without significant recruitment of transmembrane Ca2+ channels or transporters.
To investigate the mechanism linking membrane poration via human amylin to amplified Ca2+ transients, we numerically solved the SBM whole-cell model at 1 Hz pacing under control conditions and with 73% increased SL leak (+Amylin) in accordance with the experimental results of Despa et al. [6]. Our simulations confirmed that Ca2+ transients for the +Amylin configuration were higher than control (65% in Fig. 2), consistent with increases observed in experimental data collected in this study (Fig. S1b), as well as Fig. 3C of [6]). We further modeled the HIP rats examined in [6] by additionally considering decreased SERCA function, which together with SL Ca2+ leak rate, we fitted to reproduce the experimentally measured relative increase in Ca2+ transient amplitudes and decay. We note here that the absolute rates of the Ca2+ declines in our model (solved at 310K) were consistent with experimental data recorded at 310K by Gattoni et al [25] (see Fig. S5), but were faster than those obtained at 298K by Despa et al [6]. Similar to +Amylin the HIP model predicted elevated Ca2+ transient amplitudes that were higher than control (approximately 30%).
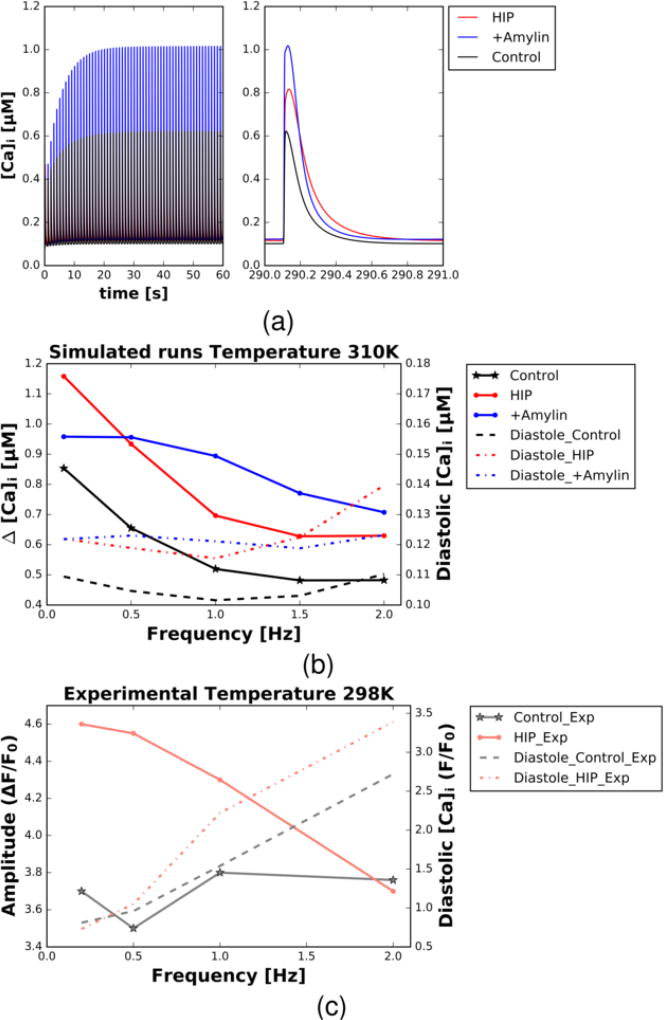
Figure 2.
(a) Intracellular Ca2+ transients (concentration versus time) predicted using the Shannon-Bers-Morroti (SBM) Ca2+ cycling model following 300s of 1.0 Hz pacing. Transients are reported for model conditions representing control (black), acute amylin-exposed rats (+Amylin, blue) and human amylin transgenic (HIP, red). (b,c) Predicted intracellular Ca2+ transient amplitude (ΔCa [uM], left axis, solid) and diastolic Ca2+ load (right axis, dashed) versus pacing frequency [Hz] for control (black) and HIP (red) conditions. Data are provided based on SBM model predictions at 310 K (b) and data collected by Despa et al at 298 K [6] (c).
In contrast to the +Amylin configuration, however, the HIP model presented 27% slower diastolic relaxation and a 33% increase in diastolic intracellular Ca2+ load relative to control, as would be expected with reduced SERCA function [38]. Intracellular Ca2+, Ca2+ SR, intracellular Na+, and AP are shown for +Amylin and HIP relative to control in Fig. S7–Fig. S8. We further note that the enhancement of Ca2+ transient amplitudes for +Amylin/HIP rats relative to control diminished with increased pacing (up to 2 Hz), in accordance with experimental findings (see Fig. 2). Ca2+ transient relaxation rates remained unchanged over this range, as our model does not currently include factors governing frequency dependent acceleration of relaxation, such as the involvement of Ca2+/calmodulin-dependent protein kinase II (CaMKII) [39].
3.2 Effects of acute amylin-induced modulation of sarcolemmal ion handling
The rate of Ca2+ entry directly due to amylin oligomerization in the sarcolemma is small relative to contributions from the prominent sarcolemmal Ca2+ currents, namely LCC and NCX. Hence, the SL leak alone is insufficient to directly account for the observed increase in Ca2+ amplitude for the amylin models on a beat-to-beat basis. This implicates other indirect mechanisms in driving the increased Ca2+ transients observed in HIP and +Amylin rats. Since the majority of the Ca2+ released during a single beat originates in the SR [14], we hypothesized that the increased intracellular Ca2+ transient amplitudes for the amylin-incubated myocytes and HIP rats stemmed from elevated SR Ca2+ loading owing to increased sarcolemmal Ca2+ leak. Under these conditions, we would expect that Ca2+ transient amplitudes should scale proportionally with SL leak rates. Therefore, we examined how the control model responded to variations SL Ca2+ leak (Amylin Leak %), as well in SERCA function. These effects are summarized in Fig. 3a–c, for which we report predicted cytosolic Ca2+ transients (a., ΔCai), SR Ca2+ transients (b., ΔCa_SR) and diastolic SR Ca2+ loads (c., max Ca_SR). These data indicate that under the parameters considered in this study, the SR Ca2+ load is positively correlated with increasing sarcolemmal Ca2+ leak and to a lesser extent, SERCA function. More importantly, the increased sarcolemmal Ca2+ leak assumed for +Amylin and HIP relative to control largely accounted for the elevated Ca2+ transients and SR load in our model. In other words, SERCA appeared to play less of a role in tuning the Ca2+ transient over the Vmax and SL leak values we considered, as the reduced SERCA Vmax for HIP relative to +Amylin maintained enhanced, albeit modestly reduced, Ca2+ transients and load.
It is important, though, to distinguish the modest changes in SERCA function we considered from complete SERCA knockout, for which mice subject to the latter conditions develop heart failure, manifesting in reduced twitch and caffeine-induced Ca2+ transients, as well as Na+ dysregulation [40]. Similarly, it has been reported [41] that thapsigargin dose-dependent reductions in SERCA activity as measured by Ca2+ transient decline rate correlate with reduced Ca2+ transient amplitudes, and SR load to some extent. Indeed, we find that more significant reductions in Vmax (beyond a 50% reduction) attenuate the Ca2+ transient, but in our model this would present a Ca2+ decay rate incongruent with data used for fitting.
These results raise the question of why the modest modulation of SERCA Vmax seems to have lesser impact on Ca2+ transient amplitude than sarcolemmal Ca2+ leak rates (Fig. 3). First, assuming normal sarcolemmal Ca2+ leak, modestly reduced Vmax will expectedly slow the rate of replenishing the SR diastolic Ca2+ load; however, the time interval for 1 Hz pacing rate was still sufficiently long for cytosolic and SR Ca2+ to approach steady state (e.g. when in our model). As pacing frequency is increased, the time interval during which to reestablish diastolic SR Ca2+ load is diminished. When the SL Ca2+ leak is increased by way of amylin oligomerization, the total Ca2+ content of the cell is increased, which manifests in both increased cytosolic and SR Ca2+ load. Both Ca2+ pools are coupled to the cleft (junctional) Ca2+ compartment, which in part controls ryanodine receptor (RyR) gating by favoring the RyR open state at higher cleft Ca2+ concentrations. We support this assertion in Sect. S.3.7, wherein we demonstrate that both higher SR and cleft Ca2+ independently lead to increased intracellular Ca2+ transient amplitudes, although SR Ca2+ load has the largest effect. In other words, higher cleft Ca2+ for HIP despite nearly normal SR load is still sufficient to increase Ca2+ transient amplitudes, which we rationalize below is due to increased SR fractional Ca2+ release. Nevertheless, the amylin-induced Ca2+ transient enhancement diminished with increased pacing and nearly approached control transient amplitudes at 2 Hz (see Fig. 2) Further, since the decline in transient amplitude with pacing was faster for HIP relative to +Amylin, this expectedly suggests that amylin’s inotropic effects are at least partially modulated by the efficiency of SERCA Ca2+ handling.
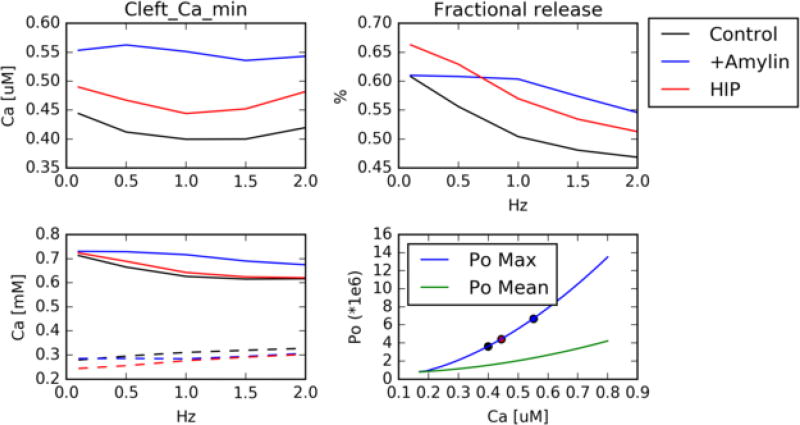
It is apparent from our data that modest changes in SR load permit significant changes in Ca2+ transient amplitudes, as has been demonstrated by Bode et al in thapsigargin-treated rat ventricular myocytes [41]. To investigate the mechanism of this effect in our models of human amylin-induced Ca2+ leak, we report in Fig. 4 for control, HIP and +Amylin conditions the SR Ca2+ load, cleft Ca2+(’junctional’ Ca2+ localized to the dyadic cleft, which triggers RyR activation [42]), fractional Ca2+ release
| (2) |
where and are the diastolic and systolic SR Ca2+ loads, respectively), the RyR open probability with respect to cleft Ca2+. These data indicate that cleft Ca2+ is elevated for +Amylin and HIP relative to control, and this trend appears to be insensitive to pacing. Additionally, we report that SR Ca2+ load is marginally elevated for +Amylin and HIP relative to control, but all conditions present loads that monotonically decrease with pacing frequency. As a result, fractional Ca2+ release for the amylin-treated cases is generally larger than that which is observed for control. These data therefore suggest that 1) higher cleft Ca for HIP/+Amylin prime RyR activation for SR Ca release and 2) faster pacing reduces the SR load available for RyR Ca2+ release, which manifests in increased fractional release for +Amylin/HIP that is frequency-dependent. This interpretation is supported by our predictions of RyR open probability (Po) means and maxima, which demonstrate that Po monotonically increase with cleft Ca2+ over the ranges predicted by our control and HIP/+Amylin conditions. Further, we discuss in Sect. S.3.7 and Fig. S17 that peak cytosolic Ca2+ transient amplitudes, which are indicative of fractional SR release, are positively correlated with increases in both SR Ca2+ diastolic load as well as cleft Ca2+. In other words, the increased fractional release exhibited in our +Amylin and HIP models likely arises from both increased SR Ca2+ load as well as higher cleft Ca2+ relative to control.
Figure 4.
(Upper left) ’Junctional’ Ca2+ localized to the LCC/RyR dyadic space as a function of pacing for control (black), +Amylin (blue) and HIP (red) rat cardiac ventricular myocytes. upper right) fractional release as measured by Eq. 2. bottom left) Diastolic (solid) and systolic (dashed) SR Ca content. bottom right) Mean/maximum ryanodine open probability with respect to junctional Ca2+, assuming constant SR content. Junctional Ca2+ characteristic of the control, +Amylin and HIP are marked with symbols.
It was expected that amylin-driven increases in cytosolic and SR Ca2+ loading would culminate in the modulation of multiple downstream Ca2+-dependent signaling pathways [24]. In this regard, we leveraged the computational model to systematically probe the response of its outputs, such as the activity of various Ca2+ handling components, to changes in model inputs including SL Ca2+ leak. Accordingly, we depict in Fig. 5 relative changes in all ion channel amplitudes described in the SBM model for the +Amylin and HIP configurations, ranked by their absolute magnitudes. These data expectedly reflect increased sarcolemmal Ca2+ leak (iCaB) for +Amylin and HIP, as we assumed increased leak conductance parameters for both cases. Interestingly, iNa was predicted to increase for both cases relative to control, which in principle could influence the AP upstroke velocity [43]. However, the AP waveform is largely unchanged in the amylin cases relative to control, thus the predicted effects on iNa amplitude appear to be of little consequence (See Fig. S7d). Common to both HIP and +Amylin, we found modestly higher iNaCa and iCap relative to control, which reflect redistribution of sarcolemmal Ca2+ extrusion versus SR Ca2+ uptake. Similar redistributions are known to occur when SERCA function is reduced [44].
In Fig. 5 we depict the relative change in activity for the top twenty modulated model ’states’ upon increasing SL Ca2+ leak. Unique to +Amylin, and HIP to a lesser extent, were increases in the inactive (I) and open (O) states of the Ryanodine receptor model [45, 46] relative to control. These increases are consistent with elevated junctional (cleft) and SR Ca2+ that together both promote and terminate RyR opening. More importantly, the greater RyR open probability translates to an increased SR Ca2+ release flux and commensurate increase in cytosolic Ca2+ transients. Apparent to both +Amylin and HIP conditions are 30–75% increases in states representing intracellular Ca2+ and Ca2+-bound buffers, including CaM, Troponin C (TnC), and myosin, which can be expected with increased Ca2+ loading.
3.3 Sensitivity analyses
Following the protocol outlined in Sect. S.3.6, we determined the sensitivity of SBM model outputs including Ca2+ amplitude, cytosolic Na+, SR Ca2+, diastolic Ca2+, action potential duration (APD), and Ca2+ transient decay (τ) to the model parameters, by randomizing model parameters temperature, background Ca2+ leak, background Na+ leak, SERCA function, NKA function, and LCC Ca2+ permeability. An advantage of this approach is to potentially isolate a small number of contributions that disproportionally modulate a given physiological output, which can serve as a basis for experimental testing of novel model prediction as done recently by Devenyi et al [26]. Here we present Spearman correlations in Table S4 as a measure of the relative monotonicity between parameter/model output pairs. These statistical analyses revealed substantial correlations for background Ca2+ leak with APD (rs = −0.61, p < 0.01), SR Ca2+ (rs = 0.60, p < 0.01), Ca2+ amplitude (rs = 0.58, p < 0.01), diastolic Ca2+ (rs = 0.59, p < 0.01), cytosolic Na+ (rs = 0.41, p < 0.01), and Ca2+ transient decay (τ) (rs = −0.50, p < 0.01). These analyses confirm that an increase in background calcium leak correlates with increased cellular Ca2+ content, as well as increased SR Ca2+, Ca2+ amplitude, and diastolic Ca2+. Other notable associations were between: background Na+ leak and cytosolic Na+ (rs = 0.46, p < 0.01) and max NKA current and cytosolic Na+ (rs = −0.61, p < 0.01) (greater NKA activity reduces intracellular Na+ content). We emphasize that the correlations reported are inclusive of all sampled parameters. As an example, Ca2+ amplitude (the calcium transient amplitude) is controlled not only by the background calcium leak, but also other parameter inputs including LCC Ca2+ permeability. For this reason, the moderate correlation of background leak with calcium transient amplitude (rs = 0.58, p < 0.01) indicates that other parameters significantly contribute to the variation in Ca2+ amplitude. If we instead consider a dataset for which only background Ca2+ leak is modulated, the rs increases to > 0.95. Further details concerning correlation magnitudes and significances are reported in Sect. S.3.6.
3.4 Extension of findings to higher mammals
A distinctive feature of murine species is the dominant role of the SR in managing Ca2+ homeostasis, with nearly 90% of the intracellular Ca2+ transient originating from SR [14]. In contrast, in higher species, including humans, sarcolemmal derived Ca2+ plays a significantly larger role; in rabbits, for instance, inward sarcolemmal Ca2+ currents account for roughly 40% of the intracellular Ca2+ transient [14]. As a proof of principle, we augmented the original Shannon-Bers (SB) formulation of cardiac Ca2+ cycling in rabbits [23] with increased sarcolemmal Ca2+ leak. In Fig. S9, we demonstrate similar trends of increased cytosolic and SR Ca2+ load under conditions of increased sarcolemmal Ca2+ leak.
4 Discussion
4.1 Shannon-Bers-Morotti myocyte model
We revised the Shannon-Bers model of rabbit ventricular myocyte Ca2+ cycling [23] to reflect Ca2+ handling in murine species, as a close approximation to the human amylin transgenic/amylin-exposed rats used in [6]. The predominant changes implemented in our model primarily entailed increasing the rates of SR Ca2+ uptake and release to mirror the larger role of SR Ca2+ handling in murine relative to higher order animals, as well as modulating potassium channel current profiles. The SBM model captured key distinguishing features of murine cardiomyocyte Ca2+ handling, including shorter AP and Ca2+ transient duration relative to rabbit, as well as a greater role of Ca2+ release and uptake via the SR, as opposed to NCX [47]. When we included sarcolemmal Ca2+ leak data from Despa et al. [6] appropriate for the +Amylin and HIP phenotypes in rats, as well as reduced SERCA Ca2+ uptake rates for HIP, the computational model reproduced the altered Ca2+ transient amplitudes across a broad range of pacing intervals. With this model, we conclude that
increased rates of Ca2+ influx through the sarcolemma, for instance as a result of amylin-induced membrane poration, promotes the amplification of cytosolic Ca2+ transients.
the increase in Ca2+ transient amplitude arises due to greater SR Ca2+ load relative to control
elevated cytosolic Ca2+ load stemming from higher rates of sarcolemmal Ca2+ influx (+Amylin), and especially when SERCA function is reduced (HIP), significantly increases the proportion of Ca2+-bound proteins. Of these proteins, CaM activation in particular may trigger remodeling via the calcineurin/NFAT pathway [48](see Fig. 1).
the concerted relationship between amylin-induced increased sarcolemmal Ca2+ leak, intracellular Ca2+ transients, and SR loading gives rise to similar Ca2+ transient amplification in the Shannon-Bers model of EC coupling in rabbit [23], which suggests similar mechanisms of dysregulation in pre-diabetes may manifest in higher order mammals.
Since the initiation of this project, an additional rat cardiomyocyte model has been published (Devenyi et al [26]). This model is based on the 2001 Pandit model of the rat ventricular cardiomyocyte [33] and included optimized Ca2+ fluxes parameters to further refine its reproduction of experimental data, including the Ca2+ transient. We discuss in the next section that the Devenyi et al [26] model reproduces trends discussed in this paper, which we obtained using the above described SBM formulation.
4.2 Enhanced SL Ca2+ fluxes are sufficient to elevate cytosolic Ca2+ load in absence of altered SR Ca2+ handling
Recently, it was established that pre-diabetic rats transgenic for human amylin peptide presented a high density of oligomerized amylin deposits in ventricular tissue [6]. Cells containing these deposits were additionally found to have greater sarcolemmal Ca2+ leak rates and amplified Ca2+ transients. These effects on sarcolemmal Ca2+ conductance and transient amplitudes were recapitulated in isolated myocytes that were incubated with human amylin, which suggested that the phenotypical changes likely precede any significant changes in protein expression that might otherwise produce similar effects. Further, disruption of amylin oligomers via increasing eicosanoid serum levels (EET) [10] and the application of membrane sealant P188 (Fig. S1) were both found to restore normal Ca2+ handling. These experiments together firmly establish the link between oligomer-induced membrane poration and Ca2+ dysregulation. Similarly, in our implementation of the Morotti-Shannon-Bers Ca2+ cycling model, we found that amplified Ca2+ transients could be induced solely by increasing the sarcolemmal Ca2+ conductance parameter (see Eq. 1). Indeed, the trend of increasing Ca2+ transient amplitude with increased sarcolemmal Ca2+ leak was also captured in the Devenyi et al [26] model (Fig. S14), which suggests the effect is not limited to our choice of EC coupling model.
The enhancement of intracellular Ca2+ transient amplitudes by amylin bears similarity to agonism of the sarcolemmal Ca2+ channels LCC and P2X. It is well-established, for instance, that activation of LCC via β-adrenergic receptor (βAR) agonists promote larger Ca2+ transients that are accompanied by elevated SR Ca2+ load [14], which has also recently been demonstrated for human amylin expressed in rat hippocampal neurons [49]. Further, P2X receptor activation has comparable effects on Ca2+ transients and SR load [50], albeit without the multifarious changes in Ca2+ handling associated with βAR stimulation. In fact, though our characterization of LCC in amylin-incubated revealed no significant changes in its current/voltage profile relative to control, our modeling results indicate similar amplification of Ca2+ transients can be obtained by increasing the peak LCC Ca2+ conductance term (see). While we defer the topic of SR load to later in the Discussion, our simulations present strong evidence that increased inward sarcolemmal Ca2+ alone is sufficient to explain amylin dose-dependent effects on Ca2+ transients in Despa et al. [6].
For pacing intervals at 1 Hz and greater, our predictions of the control Ca2+ transient using the SBM model (see Fig. 2) follow a neutral transient amplitude/frequency relationship, as is frequently exhibited in mice [51] and the Despa et al. rat control data [6]. Further, the computational model captures the negative Ca2+ transient relationships with pacing frequency reflected in the Despa et al. HIP rat data, including the diminishing difference in transient amplitude relative to control. The decline in transient amplitude for HIP can be ascribed to the inability to maintain elevated SR load as pacing increases, given the reduced SERCA activity evident for these rats [6]. Our data also reflect a negative transient amplitude/frequency relationship for the +Amylin conditions, which may arise because the model does not reflect phosphorylation-dependent effects on relaxation, including CaMKII activation [52]. Nevertheless, given that our model captures the predominant changes in Ca2+ handling exhibited in +Amylin and HIP pre-diabetic rats [6] chiefly through modulating sarcolemmal Ca2+ leak, our simulations support the hypothesis that increased SL Ca2+ entry alone, without recruiting cation-specific channels like L-type calcium channel (LCC), promotes the development of enhanced Ca2+ transients (see Fig. 1).
4.3 Contributions of SR loading to amylin phenotype
We demonstrated in Fig. S17 a positive correlation of increasing Ca2+ SL leak rates with elevated SR Ca2+ loading and transients, respectively, with preserved SERCA function. This configuration is analogous to the +Amylin conditions assumed in this study. Therefore, the predicted amplification of the cytosolic Ca2+ transients appears to be driven by Ca2+-loading of the SR, which in turn affords greater RyR Ca2+ flux per release event. We note that diastolic SR Ca2+ load was modestly increased relative to control under the +Amylin conditions (see Fig. S8) The increased SR load appeared to be of little consequence to spontaneous Ca2+ release associated with SR Ca2+ overload [53], as steady-state behavior was maintained through several minutes of simulated pacing without evidence of delayed after-depolarizations. These results concur with those of Campos et al., for which computational studies of rabbit ventricular myocytes indicated considerable tolerance to SR Ca2+ overload before abnormal AP behavior was evident [54]. Further, our hypothesis is congruent with a study examining triggering of the SL Ca2+ channel P2X4, which was found to yield both elevated Ca2+ transients and SR Ca2+ load [50].
An interesting finding from our simulations, is that both +Amylin and HIP rats presented amplified intracellular Ca2+ transients, despite the latter having predicted diastolic SR Ca2+ loads that were commensurate with the control (see Fig. S8). The notion that diastolic SR Ca2+ loads are comparable for HIP and control has precedent, as insignificant changes in SR load relative to control were reported in Despa et al. [6]. We demonstrate that the higher diastolic cytosolic Ca2+ exhibited in HIP amplifies RyR release, as measured by SR fractional release, prominently through elevated Ca2+ in the dyadic cleft, which would ultimately yield larger Ca2+ transients despite unchanged SR Ca2+ load.
Our model indicates that the increased Ca2+ transient amplitudes arise in part due to higher relative fractional release rates. Firstly, all conditions considered reported reduced fractional release at higher pacing in line with experimental findings from Antoons et al [51], for which they found 50% fractional release at 1 Hz, versus 40 percent at 2 Hz. Second, our model reflects that fractional Ca2+ release is increased for the +Amylin and HIP phenotypes relative to control, which we attribute to increased Ca2+ cleft Ca2+. These predictions bear resemblance to transgenic LCC overexpression models, for which higher amplitude iCaL supports increased fractional release [55]. An important distinction here, however, is that the increase in fractional release for LCC overexpression is likely due to elevated cleft Ca2+ only during electrical stimulation, whereas for our +Amylin/HIP models elevated cleft Ca2+ persists during diastole. This behavior is also analogous to effects of CaMKII activation on promoting RyR SR Ca2+ leak, for which higher fractional release rates [56] with preserved SR Ca2+ loads are reported [57]. Progression toward transverse tubule (TT) remodeling may further exacerbate this effect [58] though such remodeling is not evident in +Amylin/HIP rats [6]. At a minimum, given the preponderance of data suggesting that CaMKII activity is enhanced in heart failure (HF), the effects of human amylin on increasing fractional release could synergize SR Ca2+ dysregulation.
4.4 Implications of elevated cytosolic Ca2+ load
An interesting consequence of elevated Ca2+ transients and in the case of HIP, increased diastolic Ca2+ load, is the potential for activating Ca2+-dependent pathways that are normally quiescent during normal Ca2+ handling. We observed in Fig. 5 for instance, that greater levels of Ca2+-bound CaM and TnC are evident relative to control. Under normal conditions, Ca2+ activation of TnC is the critical substrate for force development in contractile tissue [59], while CaM in part regulates normal force-frequency relationships and responses to β-adrenergic stimulation [60]. However, it is also implicated in the activation of pathways associated with remodeling and failure [13]. In particular, activation of the CaM-regulated CaMKII is attributed to cardiac remodeling via the histone deacetylase (HDAC) pathway. Concurrently, activation of the phosphatase calcineurin via CaM is known to promote transcriptional changes by way of NFAT activation [61], which together contribute to the hypertrophic response to dysregulated Ca2+ handling [62]. Indeed, in pre-diabetic HIP rats there was evidence that CaMKII-HDAC and calcineurin-NFAT remodeling were simultaneously activated [6]. In Sect. S.4, we present computational evidence based on the calmodulin/calcineurin/NFAT computational model from Cooling et al [63] that increased intracellular Ca2+ transients analogous to those predicted for HIP and +Amylin conditions, as well as increased diastolic Ca2+ load consistent with HIP, increase total nuclear NFAT concentration (see Fig. S19. In this regard, while the increased Ca2+ transients stemming from amylin oligomerization may initially have beneficial inotropic effects, activation of CaM and its dependent hypertrophic pathways may contribute to diastolic dysfunction. While this model has not been fitted to quantitatively reproduce changes in nuclear NFAT reported in [6], the predictions nevertheless qualitatively indicate +Amylin and HIP induced Ca2+ dysregulation can activate a transcriptional pathway associated with hypertrophy, consistent with experiment.
Additionally, the positive correlation between sarcolemmal Ca2+ leak and Na+ load we identified via our sensitivity analysis parallels findings from Louch et al indicating sodium accumulation upon SERCA knock-out [40, 64]. In mice, they found that seven week old SERCA KO mice presented elevated Na+ among other signs of cardiac failure, which in part was attributed to increased NCX Na+/Ca2+ exchange. Our model demonstrates that persistent increased SL Ca2+ leak alone may raise cytosolic Ca2+ and Na+ levels, in a manner that may activate the NFAT/HDAC remodeling pathways associated with cardiac dysfunction.
4.5 Limitations
Our model was based on a rather modest set of changes in Ca2+, Na+ and K+ handling to a rabbit ventricular cardiomyocyte formulation. Further refinement of rat electrophysiology [65, 66] and implementation of a recent rat Ca2+ handling model [25], could provide improved predictive power for our model of amylin-induced dysregulation. In the greater context of diabetes, it is likely that the Ca2+ dysregulation and subsequent activation of CaMKII sets forth a cascade of maladaptive events that drive heart failure. As such, our simulation results could be improved by including the impact of altered protein kinase A (PKA) and CaMKII activity on Ca2+ handling. Here, tuning the full Morotti model [29], which explicitly considers PKA and CaMKII signaling, to reflect excitation-contraction coupling in rats may be appropriate. Moreover, while the computational model used in this study assumes distinct intracellular compartments, such as the subsarcolemmal and cleft spaces, it is well-established that spatially heterogeneous factors, including transverse tubule organization and RyR subcellular distribution, profoundly influence EC coupling (reviewed in [67]). An intriguing hypothesis is that amylin may distribute non-uniformly in the cell sarcolemma and thereby disproportionately impact local Ca2+ homeostasis. Given the similar sizes of amylin and insulin, as well as the precedent for insulin signaling within skeletal TT networks [68, 69], in principle amylin could distribute within analogous invaginations in cardiac myocytes. There they might interfere with normal Ca2+ homeostasis in the critical dyadic compartments that dictate EC coupling. Describing these Ca2+ signaling against a backdrop of potential alterations in TT microstructure and redistribution of Ca2+ handling proteins could benefit from three-dimensional modeling.
5 Conclusions
Our predictions of elevated calcium transients under enhanced SL Ca2+ leak (via amylin oligomers) relative to control are in qualitative agreement with findings from Despa et al. [6]. Further, these simulations suggest a potential mechanism linking human amylin infiltration of cardiac sarcolemma, amplification of intracellular Ca2+ transients and potential activation of CaM-dependent remodeling pathways; namely, amylin-induced increases in SL Ca2+ leak potentially dually elevate Ca2+ load in the cytosol and sarcoplasmic reticulum. Increased sarcoplasmic reticulum Ca2+ content facilitates Ca2+ release, while elevated cytosolic Ca2+ levels enhance SR fractional Ca2+ release, as well as the activation of Ca2+-dependent proteins, including CaM. The latter effect may potentially contribute to the CaM-dependent activation of NFAT/HDAC pathways reported in [6]. Given that human amylin oligomers have been shown to deposit in cell types including cardiac, neuronal, microglia, and beta cells [2, 6, 9, 49], the effects of amylin-induced Ca2+ dysregulation may generalize to a variety of pathologies in higher animals.
Supplementary Material
Highlights.
Rats expressing or subject to human amylin peptide, exhibit moderate Ca2+ dysregulation including larger cytosolic Ca2+ transients and activation of hypertrophic remodeling pathways.
Our computational model of Ca2+ handling in rat cardiac ventricular myocytes implicate greater sarcoplasmic reticulum (SR) Ca2+ load and increased fractional Ca2+ release gives rise to amylin-induced Ca2+ dysregulation.
Elevated cytosolic Ca2+ is accompanied by increased Ca2+ binding to calmodulin (CaM), a substrate for activation of the calcineurin/nuclear factor of activated T-cells (NFAT) remodeling pathway.
Similar results were found for a rabbit model, suggesting human amylin may have analogous effects in higher order mammals.
Acknowledgments
PKH dedicates this study to Anushka P. Michailova, who tragically passed away in the spring of 2014. Research reported in this publication was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences (NIGMS) of the National Institutes of Health (NIH) under grant number P20GM103527, NIH/NIGMS award R35GM124977 and NIH National Heart Lung and Blood Institute award R56HL131782. This work was also supported by the National Institutes of Health (R01HL118474 to FD and R01HL109501 to SD) and The National Science Foundation (CBET 1357600 to FD).
Abbreviations
- SL
sarcolemma
- SR
saroplasmic reticulum
- SERCA
sarcoplasmic/endoplasmic reticulum calcium ATPase
- NCX
Na+/Ca2+ exchanger
- EC
excitation-contraction
- AP
action potential
- LCC
L-type calcium channel
- HIP
human amylin transgenic
- NFAT
Nuclear factor of activated T-cells
- HDAC
histone deacetylases
- CaM
calmodulin
- P188
poloxamer 188
- NKA
Na+/K+ ATPase
- L-type Ca2+ channel current (iCa)
L-type Ca2+ current
- SBM
Shannon-Bers-Morroti
- itof
fast component outward potassium current
- iKs
slowly inactivating delayed rectifier current
- iss
non-inactivating potassium steady-state current
- iK1
inward rectifier potassium current
- GA
genetic algorithm
- V
voltage
- SDA
state decomposition analysis
- ODE
ordinary differential equations
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
- SB
Shannon-Bers
- TnC
troponin C
- TRPV4
transient receptor potential cation channel subfamily V member
- βAR
β-adrenergic receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Westermark P, Andersson A, Westermark GT. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiological reviews. 2011;91:795–826. doi: 10.1152/physrev.00042.2009. [DOI] [PubMed] [Google Scholar]
- 2.Verma N, Ly H, Liu M, Chen J, Zhu H, Chow M, Hersh LB, Despa F. Intraneuronal Amylin Deposition, Peroxidative Membrane Injury and Increased IL-1β Synthesis in Brains of Alzheimer’s Disease Patients with Type-2 Diabetes and in Diabetic HIP Rats. Journal of Alzheimer’s Disease. 2016;53:259–272. doi: 10.3233/JAD-160047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu M, Verma N, Peng X, Srodulski S, Morris A, Chow M, Hersh LB, Chen J, Zhu H, Netea MG, Margulies KB, Despa S, Despa F. Hyperamylinemia Increases IL-1β Synthesis in the Heart via Peroxidative Sarcolemmal Injury. Diabetes. 2016;65:2772–83. doi: 10.2337/db16-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sciacca MFM, Kotler SA, Brender JR, Chen J, Lee D-k, Ramamoorthy A. Two-step mechanism of membrane disruption by Aβ through membrane fragmentation and pore formation. Biophysical journal. 2012;103:702–10. doi: 10.1016/j.bpj.2012.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mirzabekov TA, Lin MC, Kagan BL. Pore formation by the cytotoxic islet amyloid peptide amylin. Journal of Biological Chemistry. 1996;271:1988–1992. doi: 10.1074/jbc.271.4.1988. [DOI] [PubMed] [Google Scholar]
- 6.Despa S, Margulies KB, Chen L, Knowlton AA, Havel PJ, Taegtmeyer H, Bers DM, Despa F. Hyperamylinemia contributes to cardiac dysfunction in obesity and diabetes: a study in humans and rats. Circulation Research. 2012;110:598–608. doi: 10.1161/CIRCRESAHA.111.258285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper GJ, Willis AC, Clark A, Turner RC, Sim RB, Reid KB. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:8628–32. doi: 10.1073/pnas.84.23.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pieber TR, Stein DT, Ogawa A, Alam T, Ohneda M, McCorkle K, Chen L, McGarry JD, Unger RH. Amylin-insulin relationships in insulin resistance with and without diabetic hyperglycemia. The American journal of physiology. 1993;265:E446–53. doi: 10.1152/ajpendo.1993.265.3.E446. [DOI] [PubMed] [Google Scholar]
- 9.Haataja L, Gurlo T, Huang CJ, Butler PC. Islet amyloid in type 2 diabetes, and the toxic oligomer hypothesis. Endocrine reviews. 2008;29:303–16. doi: 10.1210/er.2007-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Despa S, Sharma S, Harris TR, Dong H, Li N, Chiamvimonvat N, Taegtmeyer H, Margulies KB, Hammock BD, Despa F. Cardio protection by Controlling Hyperamylinemia in a ”Humanized” Diabetic Rat Model. Journal of the American Heart Association. 2014;3:e001015–e001015. doi: 10.1161/JAHA.114.001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bugger H, Abel ED. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia. 2014;57:660–671. doi: 10.1007/s00125-014-3171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schauerte JA, Wong PT, Wisser KC, Ding H, Steel DG, Gafni A. Simultaneous Single-Molecule Fluorescence and Conductivity Studies Reveal Distinct Classes of Aβ Species on Lipid Bilayers. Biochemistry. 2010;49:3031–3039. doi: 10.1021/bi901444w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roderick HL, Higazi DR, Smyrnias I, Fearnley C, Harzheim D, Bootman MD. Calcium in the heart: when it’s good, it’s very very good, but when it’s bad, it’s horrid. Biochemical Society transactions. 2007;35:957–961. doi: 10.1042/BST0350957. [DOI] [PubMed] [Google Scholar]
- 14.Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. Vol. 1. Kluwer Academic Publishers: Kluwer Academic Publishers; 2001. [Google Scholar]
- 15.Litwin SE, Li J, Bridge JHB. Na-Ca Exchange and the Trigger for Sarcoplasmic Reticulum Ca Release: Studies in Adult Rabbit Ventricular Myocytes. Biophysical Journal. 1998;75:359–371. doi: 10.1016/S0006-3495(98)77520-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gong JQ, Shim JV, Núñez-Acosta E, Sobie EA. I love it when a plan comes together: Insight gained through convergence of competing mathematical models. Journal of Molecular and Cellular Cardiology. 2017;102:31–33. doi: 10.1016/j.yjmcc.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hake J, Kekenes-Huskey P, McCulloch A. Computational modeling of subcellular transport and signaling. Current Opinion in Structural Biology. 2014;25:92–97. doi: 10.1016/j.sbi.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winslow RL, Trayanova N, Geman D, Miller MI. Computational Medicine: Translating Models to Clinical Care. Science Translational Medicine. 2012;4:158rv11–158rv11. doi: 10.1126/scitranslmed.3003528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L, Niederer SA, Idigo W, Zhang YH, Swietach P, Casadei B, Smith NP. A mathematical model of the murine ventricular myocyte: a data-driven biophysically based approach applied to mice overexpressing the canine NCX isoform. AJP: Heart and Circulatory Physiology. 2010;299:H1045–63. doi: 10.1152/ajpheart.00219.2010. [DOI] [PubMed] [Google Scholar]
- 20.Williams GS, Chikando AC, Tuan H-TM, Sobie EA, Lederer W, Jafri MS. Dynamics of Calcium Sparks and Calcium Leak in the Heart. Biophysical Journal. 2011;101:1287–1296. doi: 10.1016/j.bpj.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker M, Williams G, Kohl T, Lehnart S, Jafri M, Greenstein J, Lederer W, Winslow R. Superresolution Modeling of Calcium Release in the Heart. Biophysical Journal. 2014;107:3009–3020. doi: 10.1016/j.bpj.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kekenes-Huskey PM, Cheng Y, Hake JE, Sachse FB, Bridge JH, Holst MJ, McCammon JA, McCulloch AD, Michailova AP. Modeling effects of L-type ca(2+) current and na(+)-ca(2+) exchanger on ca(2+) trigger flux in rabbit myocytes with realistic T-tubule geometries. Frontiers in physiology. 2012;3:351. doi: 10.3389/fphys.2012.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shannon TR, Wang F, Puglisi J, Weber C, Bers DM. A mathematical treatment of integrated Ca dynamics within the ventricular myocyte. Biophysical Journal. 2004;87:3351–3371. doi: 10.1529/biophysj.104.047449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Despa S, Islam MA, Weber CR, Pogwizd SM, Bers DM. Intracellular Na(+) concentration is elevated in heart failure but Na/K pump function is unchanged. Circulation. 2002;105:2543–8. doi: 10.1161/01.cir.0000016701.85760.97. [DOI] [PubMed] [Google Scholar]
- 25.Gattoni S, Røe ÅT, Frisk M, Louch WE, Niederer SA, Smith NP. The calcium-frequency response in the rat ventricular myocyte: an experimental and modelling study. The Journal of Physiology. 2016;594:4193–4224. doi: 10.1113/JP272011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Devenyi RA, Sobie EA. There and back again: Iterating between population-based modeling and experiments reveals surprising regulation of calcium transients in rat cardiac myocytes. Journal of Molecular and Cellular Cardiology. 2016;96:38–48. doi: 10.1016/j.yjmcc.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li L, Chu G, Kranias EG, Bers DM. Cardiac myocyte calcium transport in phospholamban knockout mouse: relaxation and endogenous CaMKII effects. The American journal of physiology. 1998;274:H1335–H1347. doi: 10.1152/ajpheart.1998.274.4.H1335. [DOI] [PubMed] [Google Scholar]
- 28.Bassani JW, Bassani RA, Bers DM. Relaxation in rabbit and rat cardiac cells: species-dependent differences in cellular mechanisms. The Journal of Physiology. 1994;476:279–293. doi: 10.1113/jphysiol.1994.sp020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morotti S, Edwards AG, McCulloch AD, Bers DM, Grandi E. A novel computational model of mouse myocyte electrophysiology to assess the synergy between Na+ loading and CaMKII. The Journal of physiology. 2014;592:1181–97. doi: 10.1113/jphysiol.2013.266676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clausen T. Effects of amylin and other peptide hormones on Na +-K +transport and contractility in rat skeletal muscle. The Journal of Physiology. 2004;527:121–130. doi: 10.1111/j.1469-7793.2000.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petzold L. Automatic Selection of Methods for Solving Stiff and Non-stiff Systems of Ordinary Differential Equations. SIAM Journal on Scientific and Statistical Computing. 1983;4:136–148. [Google Scholar]
- 32.Srinivas M, Patnaik LM. Genetic Algorithms: A Survey. Computer. 1994;27:17–26. [Google Scholar]
- 33.Pandit SV, Clark RB, Giles WR, Demir SS. A Mathematical Model of Action Potential Heterogeneity in Adult Rat Left Ventricular Myocytes. Biophysical Journal. 2001;81:3029–3051. doi: 10.1016/S0006-3495(01)75943-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ng R, Metzger JM, Clain DR, Faulkner JA. Poloxamer 188 reduces the contraction-induced force decline in lumbrical muscles from mdx mice. American journal of physiology. Cell physiology. 2008;295:C146–50. doi: 10.1152/ajpcell.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collins JM, Despa F, Lee RC. Structural and functional recovery of electropermeabilized skeletal muscle in-vivo after treatment with surfactant poloxamer 188. Biochimica et biophysica acta. 2007;1768:1238–46. doi: 10.1016/j.bbamem.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fang X, Weintraub NL, Stoll LL, Spector AA. Epoxyeicosatrienoic Acids Increase Intracellular Calcium Concentration in Vascular Smooth Muscle Cells. Hypertension. 1999;34:1242–1246. doi: 10.1161/01.hyp.34.6.1242. [DOI] [PubMed] [Google Scholar]
- 37.Chen J, Capdevila JH, Zeldin DC, Rosenberg RL. Inhibition of cardiac L-type calcium channels by epoxyeicosatrienoic acids. Molecular pharmacology. 1999;55:288–95. doi: 10.1124/mol.55.2.288. [DOI] [PubMed] [Google Scholar]
- 38.Pereira L, Matthes J, Schuster I, Valdivia HH, Herzig S, Richard S, Gomez AM. Mechanisms of [Ca2+](i) transient decrease in cardiomyopathy of db/db type 2 diabetic mice. Diabetes. 2006;55:608–615. doi: 10.2337/diabetes.55.03.06.db05-1284. [DOI] [PubMed] [Google Scholar]
- 39.DeSantiago J, Maier LS, Bers DM. Frequency-dependent acceleration of relaxation in the heart depends on CaMKII, but not phospholamban. Journal of molecular and cellular cardiology. 2002;34:975–984. doi: 10.1006/jmcc.2002.2034. [DOI] [PubMed] [Google Scholar]
- 40.Li L, Louch WE, Niederer SA, Aronsen JM, Christensen G, Sejersted OM, Smith NP. Sodium Accumulation in SERCA Knockout-Induced Heart Failure. Biophysical Journal. 2012;102:2039–2048. doi: 10.1016/j.bpj.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bode EF, Briston SJ, Overend CL, O’Neill SC, Trafford AW, Eisner DA. Changes of SERCA activity have only modest effects on sarcoplasmic reticulum Ca 2+ content in rat ventricular myocytes. The Journal of Physiology. 2011;589:4723–4729. doi: 10.1113/jphysiol.2011.211052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neco P, Rose B, Huynh N, Zhang R, Bridge JHB, Philipson KD, Goldhaber JI. Sodium-Calcium Exchange Is Essential for Effective Triggering of Calcium Release in Mouse Heart. Biophysical Journal. 2010;99:755–764. doi: 10.1016/j.bpj.2010.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lowe JS, Palygin O, Bhasin N, Hund TJ, Boyden PA, Shibata E, Anderson ME, Mohler PJ. Voltage-gated Nav channel targeting in the heart requires an ankyrin-G dependent cellular pathway. The Journal of cell biology. 2008;180:173–86. doi: 10.1083/jcb.200710107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bovo E, de Tombe PP, Zima AV. The Role of Dyadic Organization in Regulation of Sarcoplasmic Reticulum Ca2+ Handling during Rest in Rabbit Ventricular Myocytes. Biophysical Journal. 2014:1–8. doi: 10.1016/j.bpj.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stern MD, Song LS, Cheng H, Sham JS, Yang HT, Boheler KR, Ríos E. Local control models of cardiac excitation-contraction coupling. A possible role for allosteric interactions between ryanodine receptors. The Journal of general physiology. 1999;113:469–89. doi: 10.1085/jgp.113.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stern MD, Pizarro G, Ríos E. Local control model of excitation contraction coupling in skeletal muscle. The Journal of general physiology. 1997;110:415. doi: 10.1085/jgp.110.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 48.Wilkins BJ, Molkentin JD. Calcium calcineurin signaling in the regulation of cardiac hypertrophy. Biochemical and Biophysical Research Communications. 2004;322:1178–1191. doi: 10.1016/j.bbrc.2004.07.121. [DOI] [PubMed] [Google Scholar]
- 49.Zhang N, Yang S, Wang C, Zhang J, Huo L, Cheng Y, Wang C, Jia Z, Ren L, Kang L, Zhang W. Multiple target of hAmylin on rat primary hippocampal neurons. Neuropharmacology. 2017;113:241–251. doi: 10.1016/j.neuropharm.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 50.Shen J-B. Extracellular ATP-stimulated current in wild-type and P2X4 receptor transgenic mouse ventricular myocytes: implications for a cardiac physiologic role of P2X4 receptors. The FASEB Journal. 2006;20:277–284. doi: 10.1096/fj.05-4749com. [DOI] [PubMed] [Google Scholar]
- 51.Antoons G, Mubagwa K, Nevelsteen I, Sipido KR. Mechanisms underlying the frequency dependence of contraction and [Ca(2+)](i) transients in mouse ventricular myocytes. The Journal of physiology. 2002;543:889–98. doi: 10.1113/jphysiol.2002.025619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bassani RA, Mattiazzi A, Bers DM. CaMKII is responsible for activity-dependent acceleration of relaxation in rat ventricular myocytes. The American journal of physiology. 1995;268:H703–12. doi: 10.1152/ajpheart.1995.268.2.H703. [DOI] [PubMed] [Google Scholar]
- 53.Eisner DA, Venetucci LA, Trafford AW. Life, sudden death, and intracellular calcium. Circulation Research. 2006;99:223–224. doi: 10.1161/01.RES.0000236790.08267.74. [DOI] [PubMed] [Google Scholar]
- 54.Campos FO, Shiferaw Y, Prassl AJ, Boyle PM, Vigmond EJ, Plank G. Stochastic spontaneous calcium release events trigger premature ventricular complexes by overcoming electrotonic load. Cardiovascular research. 2015;107:175–183. doi: 10.1093/cvr/cvv149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Song L-S, Guia A, Muth J, Rubio M, Wang S-Q, Xiao R-P, Josephson I, Lakatta E, Schwartz A, Cheng H. Ca2+ Signaling in Cardiac Myocytes Overexpressing the {alpha}1 Subunit of L-Type Ca2+ Channel. Circulation Research. 2002;90:174. doi: 10.1161/hh0202.103230. [DOI] [PubMed] [Google Scholar]
- 56.Kohlhaas M, Zhang T, Seidler T, Zibrova D, Dybkova N, Steen A, Wagner S, Chen L, Heller Brown J, Bers DM, Maier LS. Increased Sarcoplasmic Reticulum Calcium Leak but Unaltered Contractility by Acute CaMKII Overexpression in Isolated Rabbit Cardiac Myocytes. Circulation Research. 2006;98 doi: 10.1161/01.RES.0000200739.90811.9f. [DOI] [PubMed] [Google Scholar]
- 57.Guo T, Zhang T, Ginsburg KS, Mishra S, Brown JH, Bers DM. CaMKIIδC slows [Ca]i decline in cardiac myocytes by promoting Ca sparks. Biophysical Journal. 2012;102:2461–2470. doi: 10.1016/j.bpj.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nivala M, Song Z, Weiss JN, Qu Z. T-tubule disruption promotes calcium alternans in failing ventricular myocytes: Mechanistic insights from computational modeling. Journal of molecular and cellular cardiology. 2015;79:32–41. doi: 10.1016/j.yjmcc.2014.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gordon AMAM, Homsher EE, Regnier MM. Regulation of contraction in striated muscle. Physiological Reviews. 2000;80:853–924. doi: 10.1152/physrev.2000.80.2.853. [DOI] [PubMed] [Google Scholar]
- 60.Maier LS, Bers DM. Calcium, calmodulin, and calcium-calmodulin kinase II: heartbeat to heartbeat and beyond. Journal of molecular and cellular cardiology. 2002;34:919–39. doi: 10.1006/jmcc.2002.2038. [DOI] [PubMed] [Google Scholar]
- 61.Heineke J, Ritter O. Cardiomyocyte calcineurin signaling in subcellular domains: from the sarcolemma to the nucleus and beyond. Journal of molecular and cellular cardiology. 2012;52:62–73. doi: 10.1016/j.yjmcc.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 62.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annual review of physiology. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 63.Cooling MT, Hunter P, Crampin EJ. Sensitivity of NFAT cycling to cytosolic calcium concentration: Implications for hypertrophic signals in cardiac myocytes. Biophysical Journal. 2009;96:2095–2104. doi: 10.1016/j.bpj.2008.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Louch WE, Hougen K, Mørk HK, Swift F, Aronsen JM, Sjaastad I, Reims HM, Roald B, Andersson KB, Christensen G, Sejersted OM. Sodium accumulation promotes diastolic dysfunction in end-stage heart failure following <i>Serca2</i> knockout. The Journal of Physiology. 2010;588:465–478. doi: 10.1113/jphysiol.2009.183517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Demir SS. Computational modeling of cardiac ventricular action potentials in rat and mouse: review. The Japanese journal of physiology. 2004;54:523–30. doi: 10.2170/jjphysiol.54.523. [DOI] [PubMed] [Google Scholar]
- 66.Hintz KK, Norby FL, Duan J, Cinnamon Ma, Doze Va, Ren J. Comparison of cardiac excitation-contraction coupling in isolated ventricular myocytes between rat and mouse., Comparative biochemistry and physiology. Part A. Molecular & integrative physiology. 2002;133:191–8. doi: 10.1016/s1095-6433(02)00177-0. [DOI] [PubMed] [Google Scholar]
- 67.Louch WE, Sejersted OM, Swift F. There Goes the Neighborhood: Pathological Alterations in T-Tubule Morphology and Consequences for Cardiomyocyte Ca2+ Handling. Journal of Biomedicine and Biotechnology. 2010;2010:1–18. doi: 10.1155/2010/503906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lauritzen HPMM, Ploug T, Prats C, Tavaré JM, Galbo H. Imaging of insulin signaling in skeletal muscle of living mice shows major role of T-tubules. Diabetes. 2006;55:1300–6. doi: 10.2337/db05-1216. [DOI] [PubMed] [Google Scholar]
- 69.Shorten PR, McMahon CD, Soboleva TK. Insulin Transport within Skeletal Muscle Transverse Tubule Networks. Biophysical Journal. 2007;93:3001–3007. doi: 10.1529/biophysj.107.107888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dybkova N, Sedej S, Napolitano C, Neef S, Rokita AG, Hünlich M, Brown JH, Kockskämper J, Priori SG, Pieske B, Maier LS. Overexpression of CaMKIIδc in RyR2R4496C+/ Knock-In Mice Leads to Altered Intracellular Ca2+ Handling and Increased Mortality. Journal of the American College of Cardiology. 2011;57:469–479. doi: 10.1016/j.jacc.2010.08.639. [DOI] [PubMed] [Google Scholar]
- 71.Despa S, Bers DM. Na transport in the normal and failing heart - remember the balance. Journal of molecular and cellular cardiology. 2013;61:2–10. doi: 10.1016/j.yjmcc.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Altamirano J, Li Y, DeSantiago J, Piacentino V, Houser SR, Bers DM. The inotropic effect of cardioactive glycosides in ventricular myocytes requires Na + -Ca 2 + exchanger function. The Journal of Physiology. 2006;575:845–854. doi: 10.1113/jphysiol.2006.111252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Swift F, Birkeland JAK, Tovsrud N, Enger UH, Aronsen JM, Louch WE, Sjaastad I, Sejersted OM. Altered Na+/Ca2+-exchanger activity due to downregulation of Na+/K+-ATPase alpha2-isoform in heart failure. Cardiovascular Research. 2008;78:71–78. doi: 10.1093/cvr/cvn013. [DOI] [PubMed] [Google Scholar]
- 74.Sipido KR, Maes M, Van de Werf F. Low efficiency of Ca2+ entry through the Na(+)-Ca2+ exchanger as trigger for Ca2+ release from the sarcoplasmic reticulum. A comparison between L-type Ca2+ current and reverse-mode Na(+)-Ca2+ exchange. Circulation research. 1997;81:1034–44. doi: 10.1161/01.res.81.6.1034. [DOI] [PubMed] [Google Scholar]
- 75.Sobie EA, Cannell MB, Bridge JHB. Allosteric Activation of Na+-Ca2+ Exchange by L-Type Ca2+ Current Augments the Trigger Flux for SR Ca2+ Release in Ventricular Myocytes. Biophysical Journal. 2008;94:L54–L56. doi: 10.1529/biophysj.107.127878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lambert R, Srodulski S, Peng X, Margulies KB, Despa F, Despa S. Intracellular Na + Concentration ([Na +] i) Is Elevated in Diabetic Hearts Due to Enhanced Na + Glucose Cotransport. Journal of the American Heart Association. 2015;4:e002183. doi: 10.1161/JAHA.115.002183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim S, Rhim H. Effects of amyloid-β peptides on voltage-gated L-type CaV1.2 and CaV1.3 Ca2+ channels. Molecules and Cells. 2011;32:289–294. doi: 10.1007/s10059-011-0075-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rencher AC, William FC. Methods of multivariate analysis. (Third) 2012 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.