Abstract
Immune checkpoint inhibition against advance malignancies was named breakthrough discovery by the science magazine in 2013. In numerous clinical studies, monoclonal antibodies against the immune checkpoints, CTLA4, PD1 and PD1 ligand PDL1 have shown promising tumor response in different type of metastatic malignancies. The adverse events are autoimmune-related. The endocrine disorders, hypophysitis and thyroiditis are among the most common side effects associated with immune checkpoint inhibition treatment. Hypophysitis, a very rare endocrine disorder occurs in about one tenth of the patients receiving anti-CTLA4 treatment. Thyroiditis, on the other hand, is more commonly seen in patients receiving anti-PD1 treatment. In addition, both thyroiditis and hypophysitis are common in patients receiving combination treatment with anti-CTLA4 and anti-PD1 treatment. The time to onset of hypophysitis and thyroiditis is short. Most of the endocrine disorders occur within 12 weeks after initiation of the immune checkpoint inhibition therapy. Hypophysitis can manifest as total anterior pituitary hormone deficiency or isolated pituitary hormone deficiency. Diabetes insipidus is rare. TSH and gonadotropin deficiencies may be reversible but ACTH deficiency appears permanent. Thyroiditis can present as hypothyroidism or thyrotoxicosis followed by hypothyroidism. Hypothyroidism appears irreversible. Early identifying the onset of hypophysitis and thyroiditis and proper management of these endocrine disorders will improve the quality of the life and the outcome of this novel immunotherapy.
Keywords: Cancer, Hypophysitis, Immune checkpoint inhibition, Immune-related adverse events, Thyroiditis
Introduction
Immune system regulation is through immune checkpoints expressing on the T lymphocytes to stimulate or inhibit T cell activity.1 Alternation of the checkpoint function results in disruption of the balance between co-stimulator and inhibitory signaling in T cells leading to change T cell activity. For instance, knockout cytotoxic T-lymphocyte-associated antigen 4 (CTLA4), an inhibitory immune checkpoint, in mice caused T cell activation and lymphoproliferative disorders.2 CTLA4 null mice die at an age of 2–3 weeks due to massive lymphoproliferation. In contrast, the phenotype of program death-1 (PD-1) deletion mice appears more mild. The mice developed and grew normally.3 Although the thymus was apparently normal, PD-1 deletion mice had splenomegaly. Biochemical tests show increased levels of subset of immunoglobulins, IgG2b, IgA and most strikingly IgG3. The phenotype of PD-1 deletion mice supports the role of PD1 in the negative regulation for subset of B cell proliferation and differentiation including class switching.3
Over a century's efforts4 searching for immunotherapy to augment our own immune system to fight against cancer have finally reach a breakthrough discovery when a humanized monoclonal antibody against immune checkpoint CTLA4, ipilimumab demonstrated effective and durable anticancer activity in patients with metastatic melanoma.5 In 2011, FDA has approved ipilimumab as the first immune checkpoint inhibitor to treat metastatic melanoma. In 2013, the Science Magazine name cancer immunotherapy “the breakthrough of the year”.6 Following the success of anti-CTLA4 therapy in melanoma, the clinical trials exploring the anticancer efficacy by anti-PD1 and anti-PDL1 demonstrated promising outcome. In 2014 and 2015, two anti-PD1 agents nivolumab and pembrolizumab received FDA approval to treat metastatic melanoma and other metastatic malignancy. More clinical trials are undergoing to explore the combination anti-PD1 and anti-CTLA4 therapy. Not surprisingly, combination therapy resulted in higher tumor respond rate.7
Immune checkpoint blockade therapy represents a major success in cancer therapy, yet this novel treatment is associated with a unique spectrum of adverse events that are mostly immune-related adverse events (irAEs). Among irAEs, immune-related endocrinopathies including hypophysitis and thyroid disorders are common.8 Early recognition and proper management of these endocrinopathies are important for the oncologists, endocrinologists and other clinicians to safely use these immune checkpoint inhibitors. The goal of this review is to describe the clinical manifestations and management of hypophysitis and thyroid disorders associated with anti-CTLA4 and anti-PD1 as monotherapy or combination therapy. The rare endocrine disorders such as autoimmune diabetes as well as hypercalcemia will be briefly discussed.
Hypophysitis
Hypophysitis, the inflammation of the pituitary, emerged to be one of the most common irAEs in patient receiving anti-CTLA4 treatment. The incidence of hypophysitis ranged from 0 to 17% in earlier studies.9 Recent cohort studies from our institution,10 Massachusetts General Hospital,11 and Memorial Sloan Kettering Cancer Center12 show consistent high incidence (8–13%). The higher incidence reported in recent studies suggests the increased awareness of this rare disease that occurs in 1 per 9 million per year13 in general population. The incidence of hypophysitis is low in patients receiving anti-PD1 treatment, less than 1% in most of the clinical studies.8, 14 On the other hand, the incidence of hypophysitis in the combination therapy is higher8 or comparable to the incidence in patients receiving anti-CTLA4 treatment.12 Unlike sporadic hypophysitis, anti-CTLA4-related hypophysitis is more commonly reported in male patients. In our study, the incidence of hypophysitis in patients received ipilimumab treatment was 16% in male and 8.7% in female respectively.10 A higher male to female ratio (11:8) was reported in a different study.12 The mechanism underlying anti-CTLA4-related hypophysitis remains to be elucidated but a recent study displayed that pituitary glands expressed CTLA-4, particularly in a subset of prolactin- and thyrotropin-secreting cells. These cells became the site of complement activation, featuring deposition of C3d and C4d components and an inflammatory cascade similar to that seen in type II hypersensitivity.15
Since anti-CTLA4-related hypophysitis is a manageable adverse event, early identification of this potential life threatening condition warrants timely initiation of the proper management to improve the outcome and quality of life in patients receiving anti-CTLA4 treatment and developed hypophysitis. The symptoms of hypophysitis are nonspecific. Fatigue and headache are most common symptoms as initial manifestation of hypophysitis.11 The median onset of hypophysitis after initiation of anti-CTLA4 treatment is 8–9 weeks.10, 11, 12, 16 Because anti-CTLA4 treatment is usually given every 3 weeks, most anti-CTLA4-related hypophysitis occur after 2–3 cycles of the treatments.
Pituitary is the master gland that secrets hormones to regulate the downstream endocrine organ function and the hormone production from the targeted endocrine organs. Anterior pituitary secrets corticotropin (ACTH) to regulate cortisol production from the adrenal glands, thyrotropin (TSH) to regulate thyroxine production from thyroid, gonadotropins (LH and FSH) to regulate the function of gonads, and growth hormone (GH) to regulate muscle, adipose and bone metabolism; prolactin to regulate milk production. Posterior pituitary produce arginine vasopressin to regulate fluid volume homeostasis and oxytocin to regulate labor process. Anti-CTLA4-related hypophysitis mainly causes anterior pituitary hormone deficiency,10, 16 while posterior hormone deficiency as manifestation of diabetes insipidus is rarely reported. Ipilimumab-related hypophysitis can manifest as either isolated or pan-anterior pituitary hormone deficiency.9, 10, 16, 17 Among the anterior pituitary hormones, ACTH and TSH deficiencies are most common.10, 16, 17 The mechanisms of selective damage to subgroups of pituitary cells remain to be decoded but the high incidence of destructive injury to pituitary corticotrophs and thyrotrophs secondary to anti-CTLA4-related hypophysitis underscores the importance of vigilant monitor of adrenal and thyroid functions after the patients are initiated with anti-CTLA4 therapy. It is important to note that in anti-CTLA4-related hypophysitis, some anterior pituitary hormone deficiency may recover while central adrenal insufficiency appears permanent.10 Hyponatremia is common in patients developed anti-CTLA4-related hypophysitis,10, 16 which usually resolved after replacement of hormones for adrenal and thyroid hypofunction.10, 11 Pituitary enlargement in brain MRI is appreciated in majority of the patients who developed hypophysitis.10, 16 Besides hypophysitis, the differentials for an enlarged pituitary include metastasis to the pituitary. While a pituitary biopsy can help to make the diagnosis but there has been no reported pituitary biopsy performed in patients who developed anti-CTLA4-related pituitary enlargement. The biopsy is usually not indicated because most of the enlarged pituitary normalized in size in a few weeks.16 Indeed, the dynamic changes of the pituitary morphology as shown in Fig. 1 supports an inflammatory process rather than metastatic disease.
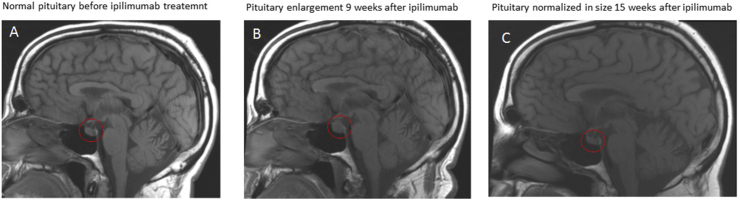
Fig. 1.
Dynamic change of pituitary size in brain MRI. A 55-year old male with history of metastatic melanoma presented with headache and fatigue. He had history of hypothyroidism. He had been on stable levothyroxine dose for 1 year. He was on a clinical trial of ipilimumab, an anti-CTLA4 monoclonal antibody, therapy. He received the ipilimumab infusion every 3 weeks. After 3 infusions of ipilimumab, he noted headache and fatigue. He denied palpitations, tremors and heat intolerance. His TSH and free T4 were normal 1 week prior to his first ipilimumab infusion. After 3 ipilimumab infusions, his TSH was very low, but his free T4 is normal. He is compliant with his levothyroxine. Both his morning cortisol and ACTH levels were very low. His MRI of pituitary showed pituitary enlargement (B). Prior to the initiation of ipilimumab treatment, his pituitary MRI was normal (A). He was started with hydrocortisone replacement. Six weeks after replacement of hydrocortisone for his central adrenal insufficiency, his pituitary normalized in size (C).
The core of the management strategy is the hormone replacement in anti-CTLA4-related hypophysitis. Although high dose of glucocorticoid is recommended as a standard treatment for anti-CTLA4-related hypophysitis in several literatures,18, 19, 20 we did not find that high dose glucocorticoid improved the outcome of anti-CTLA4-related hypophysitis in our recent cohort study.10 We recommend replacement dose of glucocorticoid in most patients with anti-CTLA4-related hypophysitis and central adrenal insufficiency. We reserve the high dose glucocorticoid treatment for patients with severe hyponatremia, severe illness such as sepsis and shock, or patient is undergoing major surgery. Headache, a common manifestation, can be severe. Usually, glucocorticoid replacement relieves the headaches in several days. High dose glucocorticoid is indicated in patients with persistent severe headache. Replace thyroid hormone in patients who develop central hypothyroidism after non-thyroidal illness is ruled out. Initiate testosterone replacement in male patients with persistent low testosterone if not contraindicated. Replacement estrogen in female hypogonadotropic hypogonadism is controversial for the concern that estrogen increases coagulopathies and is contraindicated in several malignancies such as breast cancer. Obviously, growth hormone replacement is contraindicated in patients with active malignancies. Hyponatremia usually resolves shortly after hormone replacement.
Thyroid disorders
Anti-PD1 and anti-PDL1 are second generation of immune checkpoint inhibitors. The mechanisms that PD1 regulates immune response is different from that of CTLA4 although both negatively regulate T-cell activity. CTLA4 is expressed exclusively on T cells where it counteracts the activity of the T cell co-stimulatory receptor, CD28.21 CD28 mediated intracellular signaling cascades strongly augments T-cell receptor signaling to activate T cells but it does not affect T cell activation unless the T-cell receptor is first engaged by cognate antigen presented by antigen-presenting cells. CTLA4 attenuate CD28 mediated T-cell activation by outcompeting CD28 in binding with the shared ligands: CD80 and CD86,22, 23 the ligands from antigen presenting cells that bind to and activate T-cell through CD28 signaling. PD1, like CTLA4, expresses on the membrane of T-cells. Unlike CTLA4, which negatively regulates antigen-presenting cell-mediated T-cell activity, PD1 inhibits T-cells activity in peripheral tissues. The two ligands for PD1 are PD1 ligand 1 (PDL1) and PD1 ligand 2 (PDL2), while both ligands express in the peripheral tissues.24, 25, 26 During an inflammatory response, PD1 ligands from peripheral tissues interact with PD1 to limit autoimmunity.24, 27, 28, 29 The interaction between PD1 and its ligands plays a key role in immune tolerance within the tumor microenvironment30, 31 that helps the tumor cells to escape immune attack. Briefly, PD1 expression is induced when T cells are activated. When PD1 binds with its ligands, it inhibits T cell activation.32 Interestingly, the incidence and pattern of PD1 or PDL1 blockade-associated endocrinopathies are different from those of CTLA4 blockade. Anti-PD1 treatment is associated with higher incidence of thyroid disorder that anti-CTLA4 treatment, yet combination therapy with anti-PD1 and anti-CTLA4 induced highest incidence (15%) of thyroid disorder.7 Intriguingly, in a phase 2 clinical trial of anti-PDL1 (Atezolizumab), there were no hypophysitis or thyroid disorders identified.33
Immune checkpoint blockade-associated thyroid disorders could manifest as different types of thyroid disorders including thyrotoxicosis followed by hypothyroidism34 and Fig. 2, thyrotoxicosis followed by euthyroidism,35 hypothyroidism,36 Graves hyperthyroidism37 or Graves ophthalmopathy.35 Rarely, immune checkpoint blockade treatment may induce life threatening thyroid-storm.38 Thyrotoxicosis is usually transient and followed by the development of hypothyroidism as discussed above. Hypothyroidism is permanent in most of the patients who received anti-PD1 monotherapy or combination therapy with anti-PD1 and anti-CTLA4 requiring long-term levothyroxine replacement. On the other hand, anti-CTLA4 therapy-related thyroiditis could be reversible.35 Based on current available data from clinical study34 and our own practice, immune checkpoint blockade-induced thyroid disorders are thyroiditis in most of the cases. The management of thyroid disorder include close monitor the thyroid function after onset of thyrotoxicosis. Treat the symptoms of thyrotoxicosis with beta-blocker, i.e. propranolol in patients with low risks for cardiovascular events. High dose glucocorticoid reserves for the patients with advanced age, severe thyrotoxicosis, thyroid storm, and the patients with high risks for arrhythmia, and coronary artery disease.
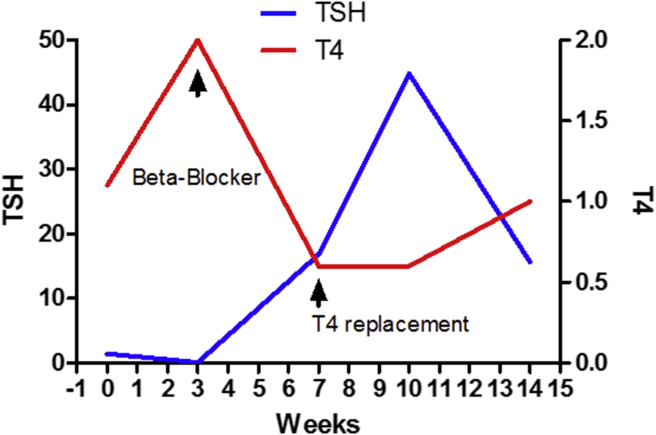
Fig. 2.
Timeline of thyroid function in a patient who developed anti-PD1-related thyroiditis. A 37-year-old female with history of advanced melanoma who received pembrolizumab (anti-PD1) treatment and developed thyroid disorders. Prior to the initiation of anti-PD1 treatment, she had no history of thyroid disorder. She did well with the first treatment. The infusion of pembrolizumab was given every 3 weeks. Prior to her second infusion of pembrolizumab, her routine blood tests revealed high T4 and suppressed TSH. She reported palpitation, hyperdefecation and 2–3 lbs weight loss over the past 3 weeks. On exam, her heart rate was 99 beats per minutes. She has not history of cardiovascular disease. She was started with propranolol 10 mg orally three times daily. Four weeks later, her repeated tests for thyroid function show overt hypothyroidism. She reported gained back her weight and endorsed fatigue. Propranolol was discontinued. She was started with levothyroxine replacement. Her autoantibody tests were negative for both thyroid peroxidase antibody (TPO) and thyroid-stimulating globulin (TSI). She became clinically and biochemically euthyroid a few weeks later after a proper replacement of levothyroxine.
Other rare immune checkpoint-inhibition-related endocrine disorders
A fulminant type 1-like diabetes was reported after the patient treated with anti-PD1 pembrolizumab.39 This female patient presented with severe hyperglycemia and diabetic ketoacidosis as well as elevated lipase 2 weeks after treated with a second infusion of pembrolizumab. Subsequent test shows negative autoantibodies (anti-GAD and anti-IA2). A larger case series reported 5 cases of immune checkpoint inhibition-related type 1-like diabetes.40 Among these 5 patients, 3 had positive anti-GAD65 while the rest of 2 patients had negative anti-GAD65. Interestingly, 3 of 5 patients had HLA A2.1+ and DR4+ genotype, 4 of 5 patients had HLA DR4+ and 4 of 5 had A2.1+ respectively. The results suggest a correlation between HLA subtype and immune checkpoint-inhibition-related type 1-like diabetes. A grade 4 hypercalcemia was reported in a large clinical trial but no detail description was given.41
In conclusion, immune checkpoint inhibition therapy has shown effective and durable tumor responses in different type of cancers. Endocrine disorders, especially hypophysitis and thyroiditis are among most common immune-related adverse events-induced by immune checkpoint blockade. With the widespread use of this novel immunotherapy against the malignancies, significant amount of the patients will develop endocrine disorders. Early identification and proper management of the endocrine disorders will improve the outcome of the immune checkpoint inhibition therapy.
Conflicts of interest
There is no conflict of interest.
Acknowledgement
This review is supported by NICHD/NIH K08 HD070957.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khattri R., Auger J.A., Griffin M.D., Sharpe A.H., Bluestone J.A. Lymphoproliferative disorder in CTLA-4 knockout mice is characterized by CD28-regulated activation of Th2 responses. J Immunol. 1999;162(10):5784–5791. [PubMed] [Google Scholar]
- 3.Nishimura H., Minato N., Nakano T., Honjo T. Immunological studies on PD-1 deficient mice: implication of PD-1 as a negative regulator for B cell responses. Int Immunol. 1998;10(10):1563–1572. doi: 10.1093/intimm/10.10.1563. [DOI] [PubMed] [Google Scholar]
- 4.Parish C.R. Cancer immunotherapy: the past, the present and the future. Immunol Cell Biol. 2003;81(2):106–113. doi: 10.1046/j.0818-9641.2003.01151.x. [DOI] [PubMed] [Google Scholar]
- 5.Hodi F.S., O'Day S.J., McDermott D.F. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342(6165):1432–1433. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 7.Larkin J., Chiarion-Sileni V., Gonzalez R. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boutros C., Tarhini A., Routier E. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016;13(8):473–486. doi: 10.1038/nrclinonc.2016.58. [DOI] [PubMed] [Google Scholar]
- 9.Dillard T., Yedinak C.G., Alumkal J., Fleseriu M. Anti-CTLA-4 antibody therapy associated autoimmune hypophysitis: serious immune related adverse events across a spectrum of cancer subtypes. Pituitary. 2010;13(1):29–38. doi: 10.1007/s11102-009-0193-z. [DOI] [PubMed] [Google Scholar]
- 10.Min L., Hodi F.S., Giobbie-Hurder A. Systemic high-dose corticosteroid treatment does not improve the outcome of ipilimumab-related hypophysitis: a retrospective cohort study. Clin Cancer Res. 2015;21(4):749–755. doi: 10.1158/1078-0432.CCR-14-2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faje A.T., Sullivan R., Lawrence D. Ipilimumab-induced hypophysitis: a detailed longitudinal analysis in a large cohort of patients with metastatic melanoma. J Clin Endocrinol Metab. 2014;99(11):4078–4085. doi: 10.1210/jc.2014-2306. [DOI] [PubMed] [Google Scholar]
- 12.Ryder M., Callahan M., Postow M.A., Wolchok J., Fagin J.A. Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: a comprehensive retrospective review from a single institution. Endocr Relat Cancer. 2014;21(2):371–381. doi: 10.1530/ERC-13-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buxton N., Robertson I. Lymphocytic and granulocytic hypophysitis: a single centre experience. Br J Neurosurg. 2001;15(3):242–245. doi: 10.1080/02688690120057664. discussion 245–246. [DOI] [PubMed] [Google Scholar]
- 14.Topalian S.L., Hodi F.S., Brahmer J.R. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwama S., De Remigis A., Callahan M.K., Slovin S.F., Wolchok J.D., Caturegli P. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med. 2014;6(230) doi: 10.1126/scitranslmed.3008002. 230ra245. [DOI] [PubMed] [Google Scholar]
- 16.Faje A. Immunotherapy and hypophysitis: clinical presentation, treatment, and biologic insights. Pituitary. 2016;19(1):82–92. doi: 10.1007/s11102-015-0671-4. [DOI] [PubMed] [Google Scholar]
- 17.Min L., Vaidya A., Becker C. Association of ipilimumab therapy for advanced melanoma with secondary adrenal insufficiency: a case series. Endocr Pract. 2012;18(3):351–355. doi: 10.4158/EP11273.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Postow M.A., Callahan M.K., Wolchok J.D. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33(17):1974–1982. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrews S., Holden R. Characteristics and management of immunerelated adverse effects associated with ipilimumab, a new immunotherapy for metastatic melanoma. Cancer Manag Res. 2012;4:299–307. doi: 10.2147/CMAR.S31873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corsello S.M., Salvatori R., Barnabei A., De Vecchis L., Marchetti P., Torino F. Ipilimumab-induced endocrinopathies: when to start corticosteroids (or not) Cancer Chemother Pharmacol. 2013;72(2):489–490. doi: 10.1007/s00280-013-2213-y. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz R.H. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 1992;71(7):1065–1068. doi: 10.1016/s0092-8674(05)80055-8. [DOI] [PubMed] [Google Scholar]
- 22.Linsley P.S., Greene J.L., Brady W., Bajorath J., Ledbetter J.A., Peach R. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity. 1994;1(9):793–801. doi: 10.1016/s1074-7613(94)80021-9. [DOI] [PubMed] [Google Scholar]
- 23.Schneider H., Downey J., Smith A. Reversal of the TCR stop signal by CTLA-4. Science. 2006;313(5795):1972–1975. doi: 10.1126/science.1131078. [DOI] [PubMed] [Google Scholar]
- 24.Freeman G.J., Long A.J., Iwai Y. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong H., Zhu G., Tamada K., Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5(12):1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 26.Latchman Y., Wood C.R., Chernova T. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2(3):261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 27.Keir M.E., Liang S.C., Guleria I. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203(4):883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okazaki T., Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19(7):813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 29.Keir M.E., Butte M.J., Freeman G.J., Sharpe A.H. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong H., Strome S.E., Salomao D.R. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 31.Blank C., Brown I., Peterson A.C. PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res. 2004;64(3):1140–1145. doi: 10.1158/0008-5472.can-03-3259. [DOI] [PubMed] [Google Scholar]
- 32.Taube J.M., Anders R.A., Young G.D. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4(127) doi: 10.1126/scitranslmed.3003689. 127ra137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenberg J.E., Hoffman-Censits J., Powles T. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387(10031):1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orlov S., Salari F., Kashat L., Walfish P.G. Induction of painless thyroiditis in patients receiving programmed death 1 receptor immunotherapy for metastatic malignancies. J Clin Endocrinol Metab. 2015;100(5):1738–1741. doi: 10.1210/jc.2014-4560. [DOI] [PubMed] [Google Scholar]
- 35.Min L., Vaidya A., Becker C. Thyroid autoimmunity and ophthalmopathy related to melanoma biological therapy. Eur J Endocrinol. 2011;164(2):303–307. doi: 10.1530/EJE-10-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Min L., Hodi F.S. Anti-PD1 following ipilimumab for mucosal melanoma: durable tumor response associated with severe hypothyroidism and rhabdomyolysis. Cancer Immunol Res. 2014;2(1):15–18. doi: 10.1158/2326-6066.CIR-13-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Azmat U., Liebner D., Joehlin-Price A., Agrawal A., Nabhan F. Treatment of ipilimumab induced Graves' disease in a patient with metastatic melanoma. Case Rep Endocrinol. 2016;2016:2087525. doi: 10.1155/2016/2087525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McMillen B., Dhillon M.S., Yong-Yow S. A rare case of thyroid storm. BMJ Case Rep. 2016;2016 doi: 10.1136/bcr-2016-214603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaudy C., Clevy C., Monestier S. Anti-PD1 pembrolizumab can induce exceptional fulminant type 1 diabetes. Diabetes Care. 2015;38(11):e182–183. doi: 10.2337/dc15-1331. [DOI] [PubMed] [Google Scholar]
- 40.Hughes J., Vudattu N., Sznol M. Precipitation of autoimmune diabetes with anti-PD-1 immunotherapy. Diabetes Care. 2015;38(4):e55–57. doi: 10.2337/dc14-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Postow M.A., Chesney J., Pavlick A.C. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]