Abstract
Hydrogels are three-dimensional polymeric networks filled with water and mimic tissue environments. Therefore, they are considered optimal to deliver cells and engineer damaged tissues. The hydrogel networks have been significantly modified to endow biochemical functionality with adhesive ligands, growth factors, or degradable sites that are helpful to drive proper cell functions. Recently, some of the biophysical properties of hydrogels have emerged as key players in dictating cell fate. Beyond static stiffness, time-dependent stress/strain changes in the interaction with cells and the cell-mediated degradation and matrix synthesis have been demonstrated to shape cell status and tissue repair process. We highlight here the emerging biophysical properties of hydrogels that can motivate tissue engineers to design and develop hydrogels optimally for tissue regeneration.
Keywords: Hydrogels, biophysical parameters, stiffness, tissue regeneration, cell fate
General properties and modifications of hydrogels
Hydrogels are in principle three-dimensional (3D) polymeric networks that are filled with water.1 The water content reaches as high as 90%–99% while it depends on the polymer concentration. This fact explains high hydrophilicity of hydrogels and the ability to safely incorporate biological entities (proteins and cells) without an aggregation. The mechanical behavior of hydrogels is typically viscoelastic which is associated with the water and the movement of polymer networks in fluid.2
Typical composition of hydrogels varies from synthetic (e.g. polyethylene glycol (PEG), polyacrylamide (PAA), polydimethylsiloxane (PDMS)) to natural polymers (e.g. collagen, gelatin, alginate, hyaluronic acid (HA), and chitosan).3 The gelation of hydrogels is enabled by either physical or chemical cross-link methods.4 Physical gelation is possible through weak interactions between polymer networks,2,5,6 whereas chemical cross link forms strong bonds between polymer chains.7,8 While the physically cross-linked hydrogels are easily relaxed when stressed, the chemically cross-linked hydrogels resist permanent deformation. Although many natural polymers are gelled through physical interactions upon pH or temperature change,9,10 chemical cross-link methods (e.g. UV curing) are often introduced.11 As to the type of hydrogels and their properties, readers are recommended to the work by Caliari and Burdick.12
Among other properties, stiffness of a hydrogel is considered a key parameter that determines cell fate. Starting from pioneering works by Engler et al.13 in 2006 where a lineage specification of mesenchymal stem cells (MSCs) was reported to be dictated by the elasticity of PAA hydrogels, many groups have demonstrated the essential role of stiffness played in various cell types,14,15 including pluripotent stem cells,16 neural stem cells,17 hematopoietic stem cells,18 and cancer cells.19 In those studies, one key issue is how to design hydrogels with varying stiffness levels independently of other hydrogel parameters, such as ligand density and network porosity,20 which can help understanding the cellular phenomena affected solely from the matrix stiffness.
Because hydrogels are used for biomedical applications, a lot of effort has been given to improve the biological interactions. Incorporation of adhesive ligands (e.g. Arg-Gly-Asp (RGD) peptide, fibronectin, and laminin) is a common way to improve cell adhesion and spreading, particularly for most synthetic hydrogels21,22 and some natural hydrogels (e.g. alginate).5,23 Cells pooled in a hydrogel recognize the adhesion motifs and then settle down, extend, and migrate along the polymer networks.24,25 Sometimes, signaling molecules (e.g. bone morphogenetic proteins (BMPs), transforming growth factor-beta 1 (TGFb1), neurotrophic factors) are conjugated with hydrogels to drive cells to perform specific functions such as osteogenic,26,27 chondrogenic,28,29 or neuronal differentiation.30,31
Degradable hydrogels are preferred for tissue engineering, which can ultimately be replaced by a growing tissue. Therefore, studies have also focused on controlling the degradation rate.32 Degradation is possible either hydrolytically or enzymatically. One promising approach of a controlled degradation is to incorporate enzymatically cleavable sites (e.g. matrix metalloproteinase (MMP) cleavage site) within polymer networks.33 The degradation eventually leads to changes in hydrogel properties with time including mechanical viscoelasticity.
Findings and emerging issues in hydrogels with cellular interactions
Due to the nature of mimicking 3D tissue environments, hydrogels have been extensively studied in interpreting the cell interactions with matrices.34 Initial studies have investigated the cell behaviors on two-dimensional (2D) hydrogel conditions.13 Stiffness, ligand density, and network porosity are relatively easy to tailor and considered decisive factors for cell behaviors. Although there has been an argument on which parameter is the most influential on the lineage specification of stem cells,20,35 the stiffness independently tailored from other parameters was found by far to be a key effector.
Even with the significant body of findings in cellular behaviors related with hydrogels over the past 20 years, the investigations in 3D hydrogel conditions date only back to 2010. Mooney’s group was the first that examined the stem cell behaviors in 3D hydrogels with variation in stiffness (2.5–110 kPa).14 They demonstrated some intriguing cellular behaviors in 3D hydrogels; cells did not spread actively even they underwent osteogenic differentiation, which being somewhat different from the findings in 2D hydrogels, highlighting the decoupling of cell shape and lineage commitment of stem cells in 3D hydrogel conditions. The findings thus suggest the need of cell studies in 3D hydrogels to better mimic the in vivo tissues and the biological phenomena there.
The static stiffness value has been a key parameter in hydrogels until the advent of stress relaxation in 2015.6 Chaudhuri et al.6 underscored the effects of time-dependent stress change (stress relaxation) on the fibroblast responses (particularly spreading) on 2D alginate-based hydrogels. When hydrogels were physically (ionically) cross-linked, they experienced substantial degree of stress relaxation (tens of % change in stress within tens to hundreds of seconds) under a constant strain. This stress relaxation behavior can mimic the hydrogel viscoelastic responses to cellular pushing-and-pulling forces and thus is considered dynamic in the interaction with cells. Interestingly, the chemically (covalently) cross-linked hydrogels that are not stress relaxing allowed cells to spread very limitedly. Given that the stiffness of stress-relaxing hydrogels decreases with time and cells recognize the decreasing stiffness, such findings in stress-relaxing hydrogels are somewhat contradictory to our expectation; cells are known to spread better on stiffer gels as proven in many hydrogels like PAA and PEG.13,15 However, cells seem to respond to the stress-relaxing hydrogels not just by simply gathering the information of decreasing stiffness; rather, they prefer to shape and remodel the hydrogels by clustering adhesive ligands and developing cytoskeletons.
Following the 2D study, the same group investigated the MSC differentiation in 3D alginate-based hydrogels that were tailored with varying stress-relaxing behaviors by means of different cross-linking methods.5 When the hydrogels were stress relaxing more rapidly, the osteogenic differentiation of cells was more stimulated; on the other hand, the adipogenic commitment was not critically dependent on the stress relaxation. The stress-relaxing hydrogels could allow cellular rearrangement of focal adhesions and thus shaping and spreading, which was eventually helpful for the osteogenic differentiation. This study highlights that a proper tuning of the stress relaxation of hydrogels is a key strategy to provide osteogenically favorable conditions of stem cells and bone tissue engineering. Motivated by the studies, the stress relaxation effect on chondrocyte behaviors was further investigated using 3D alginate hydrogels.36 The chondrocytes in rapidly relaxing hydrogels could produce cartilage extracellular matrix (ECM) significantly, whereas those in slowly relaxing hydrogels suffered limited volume expansion due to elastic stresses of the surrounding matrix, leading to cartilage degeneration. The study is helpful for interpreting the cellular phenomena in cartilage degradative in vivo conditions and suggests that the stress relaxation of hydrogels may be a key design parameter for cartilage tissue engineering.
Independently from the stress relaxation studies, degradation issue has become emerged very recently. Hydrogels mostly undergo certain degradation hydrolytically and/or enzymatically with time, thus the degradation-mediated cellular response is of interest to the hydrogel tissue engineers. Burdick’s group illuminated this issue by designing elegant hydrogels made of methacrylated hyaluronic acid (MeHA).33 The hydrogels were engineered to be nondegradable or proteolytically degradable to examine the degradation-dependent MSC behaviors. Interestingly, the cells in the nondegradable hydrogels did not conform to an osteogenic differentiation even for a wide range of stiffness values (4–95 kPa); however, when the hydrogels were degradable, cells could sense the stiffness and differentiate into an osteogenic lineage. The cellular differentiation is closely related with the degradation-mediated matrix remodeling and the interactive cellular spreading and mechanotransductory signaling. This study highlights again the importance of cell-induced matrix reorganization which is enabled by the matrix degradation, and this is considered to share the key molecular mechanisms in common with the stress-relaxing gels where the degradation issue has not been counted though.
Even the hydrogels exhibit 3D tissue–like properties, not all of them can replicate the microscale fibrous geometry that is a typical ECM microstructure of many tissues, including skin, muscle, vessels, and uncalcified bone, and the fibrous structure characterizes the biophysical properties. Chen and Burdick’s groups aimed to unravel the effects of an anisotropic fibrous structure which is different from isotropic hydrogels.37 UV-curable methacrylated dextran fibers were tailored to have different stiffness levels and dextran hydrogels were also prepared similarly, aiming to decouple the effects of stiffness and fiber structure. MSCs cultured on the fibers were shown to behave comparably to collagen matrix exhibiting similar cell shaping, extension, and matrix contraction, which, however, were not readily observable in hydrogels even tailored with similar elasticity. While they stressed the importance of the fiber dimensionality that can mimic the native tissue ECM, more notable events are that cells on fibers reorganized the networks and redistributed the focal adhesions and thus conformed accordingly. The phenomena are thus considered to be pretty much similar to those noticed in the stress-relaxing or degrading hydrogels aforementioned.
Taken the above recent key findings together, the matrix-mediated cellular events seem to share in common in a sense that cells prefer the matrix that enables reorganization, and this is possible by a matrix degradation (in degradable hydrogels) or a network debonding (in physically cross-linked hydrogels) all of which are ultimately associated with the dynamic change in viscoelastic behaviors of hydrogels such as stress relaxation, and the events are not limited to hydrogels but extend to the ECM mimic fibrous networks. Our future understanding of the behaviors of stem cells delivered through hydrogels or tissue-resident cells around hydrogels may thus need special considerations of this point. This emerging biophysical aspect of hydrogels highlighted here may help understanding the in vitro and in vivo phenomena related with cell-laden hydrogels and to motivate further design and development of hydrogels optimized for tissue repair and regeneration.
Concluding remarks
Hydrogels mimic 3D tissue environments. Thus, one side of studies has utilized hydrogels to understand the cell interactions with the tissue equivalent matrices. While many properties of hydrogels have been found to be important in dictating cell fate, the change in viscoelastic properties through dynamic interactions with cells has recently shown a key element that governs cell behaviors. The phenomena underscored here are dynamic and involve continual changes along different time frames that caused by mutual interactions of a matrix with cells. The cell-forced deformation, cell-carved degradation, and cell-secreted new ECM formation start to rebuild our understanding of hydrogel-related cellular events and our future design strategies to tissue engineering hydrogels (as depicted in Figure 1).
Figure 1.
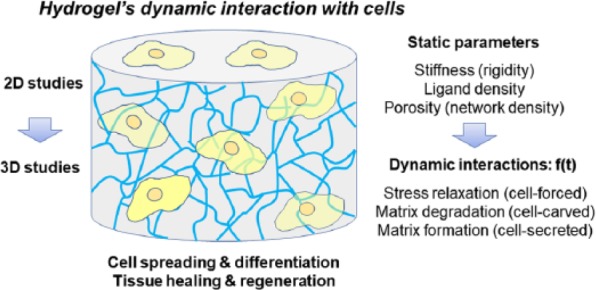
Schematic diagram showing the key parameters in hydrogel–cells’ dynamic interactions. Beyond the static physical–chemical properties of hydrogels (e.g. stiffness, ligand density, porosity), dynamic changes (stress relaxation, matrix degradation and matrix formation) with time (f(t)), induced by the interactive cells, are considered as critical factors in determining cell fate and tissue regeneration.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Research Foundation of Republic of Korea (No. NRF-2018R1A2B3003446).
References
- 1. Zhang YS, Khademhosseini A. Advances in engineering hydrogels. Science 2017; 356(6337): 3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nam S, Hu KH, Butte MJ, et al. Strain-enhanced stress relaxation impacts nonlinear elasticity in collagen gels. Proc Nat Acad Sci 2016; 113: 5492–5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li J, Mooney DJ. Designing hydrogels for controlled drug delivery. Nat Rev Materials 2016; 1: 16071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sureerat K, Younghyen J, Hansoo P. Crosslinking method of hyaluronic-based hydrogel for biomedical applications. J Tissue Eng 2017; 8: 2041731417726464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chaudhuri O, Gu L, Klumpers D, et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat Mater 2016; 15(3): 326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chaudhuri O, Gu L, Darnell M, et al. Substrate stress relaxation regulates cell spreading. Nat Commun 2015; 6: 6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guvendiren M, Burdick JA. Stiffening hydrogels to probe short- and long-term cellular responses to dynamic mechanics. Nat Commun 2012; 3: 792. [DOI] [PubMed] [Google Scholar]
- 8. Trappmann B, Baker BM, Polacheck WJ, et al. Matrix degradability controls multicellularity of 3D cell migration. Nat Commun 2017; 8(1): 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rosales AM, Anseth KS. The design of reversible hydrogels to capture extracellular matrix dynamics. Nat Rev Mater 2016; 1: 15012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Singh NK, Lee DS. In situ gelling pH- and temperature-sensitive biodegradable block copolymer hydrogels for drug delivery. J Control Release 2014; 193: 214–227. [DOI] [PubMed] [Google Scholar]
- 11. Yue K, Trujillo-de Santiago G, Alvarez MM, et al. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015; 73: 254–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Caliari SR, Burdick JA. A practical guide to hydrogels for cell culture. Nat Methods 2016; 13(5): 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Engler AJ, Sen S, Sweeney HL, et al. Matrix elasticity directs stem cell lineage specification. Cell 2006; 126(4): 677–689. [DOI] [PubMed] [Google Scholar]
- 14. Huebsch N, Arany PR, Mao AS, et al. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater 2010; 9(6): 518–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rennerfeldt DA, Renth AN, Talata Z, et al. Tuning mechanical performance of poly(ethylene glycol) and agarose interpenetrating network hydrogels for cartilage tissue engineering. Biomaterials 2013; 34(33): 8241–8257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Caiazzo M, Okawa Y, Ranga A, et al. Defined three-dimensional microenvironments boost induction of pluripotency. Nat Mater 2016; 15(3): 344–352. [DOI] [PubMed] [Google Scholar]
- 17. Leipzig ND, Shoichet MS. The effect of substrate stiffness on adult neural stem cell behavior. Biomaterials 2009; 30(36): 6867–6878. [DOI] [PubMed] [Google Scholar]
- 18. Choi JS, Mahadik BP, Harley BAC. Engineering the hematopoietic stem cell niche: frontiers in biomaterial science. Biotechnol J 2015; 10(10): 1529–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cavo M, Fato M, Peñuela L, et al. Microenvironment complexity and matrix stiffness regulate breast cancer cell activity in a 3D in vitro model. Sci Rep 2016; 6: 35367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wen JH, Vincent LG, Fuhrmann A, et al. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nat Mater 2014; 13(10): 979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Connelly JT, Garcia AJ, Levenston ME. Interactions between integrin ligand density and cytoskeletal integrity regulate BMSC chondrogenesis. J Cell Physiol 2008; 217(1): 145–154. [DOI] [PubMed] [Google Scholar]
- 22. Vega SL, Kwon MY, Song KH, et al. Combinatorial hydrogels with biochemical gradients for screening 3D cellular microenvironments. Nat Commun 2018; 9(1): 614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials 1999; 20(1): 45–53. [DOI] [PubMed] [Google Scholar]
- 24. Lukas K, Sandra G, Marc W, et al. Methacrylated gelatin/hyaluronan-based hydrogels for soft tissue engineering. J Tissue Eng 2017; 8: 2041731417744157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Satoshi M, James SF, Chuan L, et al. Administration of cells with thermosensitive hydrogel enhances the functional recovery in ischemic rat heart. J Tissue Eng 2016; 7: 2041731416646676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Park SH, Kwon JS, Lee BS, et al. BMP2-modified injectable hydrogel for osteogenic differentiation of human periodontal ligament stem cells. Sci Rep 2017; 7(1): 6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Benoit DSW, Collins SD, Anseth KS. Multifunctional hydrogels that promote osteogenic hMSC differentiation through stimulation and sequestering of BMP2. Adv Funct Mater 2007; 17(13): 2085–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bian L, Zhai DY, Tous E, et al. Enhanced MSC chondrogenesis following delivery of TGF-β3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials 2011; 32(27): 6425–6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sridhar BV, Doyle NR, Randolph MA, et al. Covalently tethered TGF-β1 with encapsulated chondrocytes in a PEG hydrogel system enhances extracellular matrix production. J Biomed Mater Res A 2014; 102(12): 4464–4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kandalam S, Sindji L, Delcroix GJ-R, et al. Pharmacologically active microcarriers delivering BDNF within a hydrogel: novel strategy for human bone marrow-derived stem cells neural/neuronal differentiation guidance and therapeutic secretome enhancement. Acta Biomater 2017; 49: 167–180. [DOI] [PubMed] [Google Scholar]
- 31. Lindsey S, Piatt JH, Worthington P, et al. Beta hairpin peptide hydrogels as an injectable solid vehicle for neurotrophic growth factor delivery. Biomacromolecules 2015; 16(9): 2672–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kloxin AM, Kasko AM, Salinas CN, et al. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science 2009; 324(5923): 59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Khetan S, Guvendiren M, Legant WR, et al. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat Mater 2013; 12(5): 458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dhaval K, Rajesh V. Bi-functional oxidized dextran–based hydrogel inducing microtumors: an in vitro three-dimensional lung tumor model for drug toxicity assays. J Tissue Eng 2017; 8: 2041731417718391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Trappmann B, Gautrot JE, Connelly JT, et al. Extracellular-matrix tethering regulates stem-cell fate. Nat Mater 2012; 11(7): 642–649. [DOI] [PubMed] [Google Scholar]
- 36. Lee H-P, Gu L, Mooney DJ, et al. Mechanical confinement regulates cartilage matrix formation by chondrocytes. Nat Mater 2017; 16(12): 1243–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Baker BM, Trappmann B, Wang WY, et al. Cell-mediated fibre recruitment drives extracellular matrix mechanosensing in engineered fibrillar microenvironments. Nat Mater 2015; 14(12): 1262–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]


