Short abstract
Resveratrol has been showed to relieve neuropathic pain through its anti-inflammatory effects on the peripheral nerve system. However, it is not clear whether resveratrol, especially when administered systemically, is effective in alleviating the peripheral neuropathy-induced imbalance between pro- and anti-inflammatory responses in the central nervous system. To test this, we used a rat neuropathic pain model resulting from chronic constriction injury of the sciatic nerve. Resveratrol (200 mg/kg) or vehicle (dimethylsulfoxide) were administered intraperitoneally once daily for 14 consecutive days after chronic constriction injury. We found that resveratrol attenuated mechanical allodynia and thermal hyperalgesia in rats with chronic constriction injury. After 14 days of resveratrol treatment, expression of several anti-inflammatory cytokine receptors, including IL-1RA and IL-1R2, was increased in the dorsal spinal cord of rats with chronic constriction injury, and IL-4Rα was increased in dorsal spinal cord neurons. Knockdown of IL-4Rα in a neuronal cell line reversed the resveratrol-induced upregulation of IL-1RA and IL-1R2. These results indicate that resveratrol enhances IL-4 receptor-mediated anti-inflammatory responses in the spinal cord and thus might contribute to the alleviation of central sensitization following peripheral nerve injury.
Keywords: Resveratrol, nerve injury, central sensitization, pro-inflammatory response, anti-inflammatory response
Introduction
Inflammatory mechanisms in the peripheral nervous system and central nervous system (CNS) play important roles in the development of neuropathic pain. In response to nerve injury, infiltration of inflammatory cells and activation of resident immune cells lead to the production and secretion of various inflammatory mediators.1,2 These mediators, including cytokines, can be broadly categorized into two main families based on their effects on inflammation; namely, pro-inflammatory (e.g., tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, and IL-6) and anti-inflammatory (e.g., IL-4 and IL-10) mediators.3,4 After peripheral nerve damage, pro- and anti-inflammatory responses are activated in the injured nerve, the dorsal root ganglion, and in the spinal cord, but the pro-inflammatory response prevails, thereby contributing to peripheral and central sensitization.5–7 Restoring the balance between pro- and anti-inflammatory mediators has been shown to be effective in treating neuropathic pain.
Resveratrol (3,5,4′-trihydroxystilbene, Res) is a natural polyphenolic compound with many beneficial properties, including anti-inflammatory, anti-oxidative stress, and anti-tumorigenic activities.8–10 Res also has neuroprotective effects and improves the pathological and behavioral outcomes of various types of nerve injury, including stroke,11 traumatic brain injury,12 spinal cord injury,13 and peripheral nerve injury.14 A number of mechanisms have been proposed to explain Res-induced neuroprotection, many of which invoke its anti-inflammatory effects.15,16 For example, Res controls pain by attenuating peripheral sensitization via repression of inflammatory responses in the nerve or local tissue.17,18 Res has also been shown to inhibit the expression of pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6, and to promote the expression of the anti-inflammatory cytokine IL-10 in the peripheral nerve system of animals suffered from neuropathic pain.19,20 The anti-inflammatory effect of Res in the spinal cord also involves inhibition of glial activation.16 However, it remains unclear whether Res influences the balance between pro- and anti-inflammatory responses in the spinal cord, which is an important contributing factor in central sensitization.
We previously demonstrated that intrathecal administration of Res attenuates neuronal hypersensitivity in the rat spinal cord.21 However, drugs are more commonly administered systemically in the clinical setting. Therefore, in this study, we used a rat chronic constriction injury (CCI) model to test the effects of intraperitoneally (i.p.) administered Res on neuropathic pain. We measured the expression of a panel of pro- and anti-inflammatory cytokines and their receptors in the dorsal spinal cord to investigate the ability of Res to regulate the balance between pro- and anti-inflammatory responses to spinal cord injury.
Materials and methods
Animals
Male Sprague Dawley rats (200–250 g) were maintained in a quiet living environment at constant temperature (23°C ± 2°C) on a 12-h light/dark cycle. Food and water were available ad libitum. All experiments were implemented according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Chinese Council’s Guide for the Care and Use of Laboratory Animals. Every effort was made to minimize animal suffering.
Chronic constriction injury model
CCI was performed according to the protocol of Bennett and Xie.22 Animals were anesthetized with 1% pentobarbital sodium (50 mg/kg, i.p. injection). After blunt separation of the biceps femoris, the left sciatic nerve was exposed. Four ligatures (4–0) were loosely tied around the nerve at 1-mm intervals from the proximal spinal side to the bifurcation. The ligatures were tightened until they just induced a slight tremor of the operative limb. All nerve ligations were conducted by the same operator. For the sham group, the sciatic nerve was exposed for the same duration without ligation.
Behavioral testing
The mechanical withdrawal threshold (MWT) and thermal withdrawal latency (TWL) were measured on the plantar surface of a paw on the day before surgery and on days 3, 7, 11, and 14 post-surgery. Von Frey filaments (North Coast Medical, San Jose, CA) were used to examine MWT.23 Rats were placed on a metal mesh floor, caged with a plastic box, and then left for 30 min to habituate before testing. Von Frey filaments were used to stimulate the plantar skin of the left hindpaw until paw withdrawal was observed. The measurements were repeated three times at 5-min intervals and the average minimum force (g) that initiated a withdrawal response was recorded. A plantar algesimeter (Tes7370, Ugo Basile, Comerio, Italy) was used to measure TWL. Rats were placed on a transparent glass plate in a plastic cage and habituated to the test environment for 30 min. A radiant heat instrument was placed underneath the glass and focused on the plantar skin of the left paw. TWL was defined as the time between the start of heat stimulation and paw withdrawal. The measurements were repeated three times at 5-min intervals and the average duration (s) was recorded.
Cell culture
The rat neuronal cell line PC12 (Cellbank, Shanghai, China) was maintained at 37°C in a 5% CO2 incubator in complete medium consisting of RPMI 1640 medium (Thermo Fisher Scientific, Waltham, MA), 10% heat-inactivated fetal bovine serum (Thermo Fisher Scientific), and 1% penicillin and streptomycin (Thermo Fisher Scientific). The medium was changed every 2 days. To determine the optimal Res dose, cells were treated with 10, 50, or 100 μM Res (Sigma-Aldrich, Santa Clara, CA) for 24 h,24 and IL-4Rα messenger RNA (mRNA) levels were measured. Substantial upregulation was seen in the presence of 50 μM Res, which was taken as the optimal dose for further experiments. The same volume of 0.1% dimethylsulfoxide (DMSO) in saline solution was used as vehicle. Expression of IL-4Rα was silenced by transfection of cells with siRNA against rat IL-4Rα (siRNA-1479 sense GCCUUCAGAAAGUGGGCAATT and antisense UUGCCCACUUUCUGAAGGCTT) or with a non-targeting negative control (NC) siRNA (sense UUCUUCGAACGUGUCACGUTT and antisense ACGUGACACGUUCGGCGGAGAATT).
Eight groups of cells were tested in this study: blank control (no treatment, addition of medium alone), mock control (cells treated with transfection reagent without siRNA), si-NC, si-IL-4Rα, Res, Res + si-IL-4Rα, Res + si-NC, and Res + mock. Cells were transfected with 50 nM siRNA, based on the manufacturer’s recommendation, using riboFect CP reagent (RiboBio, Guangzhou, China) for 24 h. For the Res + mock, Res + si-IL-4Rα, and Res + si-NC groups, Res (50 μm) was added to the cells before transfection. Cells were cultured for 24 h, and total RNA and proteins were extracted for analysis.
Drug administration
Res (Sigma-Aldrich) was dissolved in 2% DMSO and saline solution and injected (i.p. 200 mg/kg) into rats once daily for 14 days starting on the day of surgery. This dose was selected based on a literature review,25 as well as previous studies which examined the effect of i.p. treatment of resveratrol on spinal pathology.13,26 The control groups were administered the same volume of 2% DMSO in saline.
Real-time quantitative polymerase chain reaction
Real-time quantitative polymerase chain reaction (RT-qPCR) analysis was performed to quantify expression of inflammatory cytokines and receptors. On the 14th day post-surgery, rats were placed under deep anesthesia and euthanized. The ipsilateral dorsal part of the lumbar spinal cord was harvested and stored at −80°C until use for RNA or protein extraction. Total RNA was extracted using E.Z.N.A. Total RNA Kit II (Omega, Stanford, CT), and cDNA was synthesized using a reverse transcription kit (Takara Bio Inc., Otsu, Japan) and amplified using corresponding primers (Table 1). RT-qPCR was conducted with an ABI Prism, 7900 Sequence Detection System (Applied Biosystems, Foster City, CA) using the following thermal cycling conditions: 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, 60°C for 29 s, and 72°C for 24s. β-Actin was probed as an endogenous control. Relative expression of the target genes compared with β-actin was calculated using the comparative threshold cycle (ΔΔCt) method.6
Table 1.
Primers for real-time quantitative polymerase chain reaction.
| Gene | Oligonucleotides | (5′ to 3′) |
|---|---|---|
| β-actin | Forward | TCGTGCGTGACATTAAAGAG |
| Reverse | ATTGCCGATAGTGATGACCT | |
| TNF-α | Forward | CCCAGACCCTCACACTCAGAT |
| Reverse | TTGTCCCTTGAAGAGAACCTG | |
| TNFR1 | Forward | CTTTCTAAGCGGAAATGAG |
| Reverse | TCGGCACAGTAGACTGA | |
| TNFR2 | Forward | GATGTTAGGACTGGCGA |
| Reverse | CGCTGTGACTCTTGCT | |
| IL-1β | Forward | CACCTTCTTTTCCTTCATCTTTG |
| Reverse | GTCGTTGCTTGTCTCTCCTTGTA | |
| IL-1RA | Forward | CTTATTGCCTCTGCCCTCTG |
| Reverse | TGATTGGTCTGGACTGTGGA | |
| IL-1R1 | Forward | AGGGACAGACCTGTGATTA |
| Reverse | TTCCAGTAGACAAGGTCGG | |
| IL-1R2 | Forward | CAGCCAAGAAACTGCAAGGT |
| Reverse | CGGGAGACTCAGAAACCCTT | |
| IL-4 | Forward | GGTCACAGAAAAAGGGACTCCAT |
| Reverse | GCTCGTTCTCCGTGGTGTTC | |
| IL-4Rα | Forward | GCAGACACATACTGGCTGGA |
| Reverse | CATTGGTGTGGAGTGTGAGG | |
| IL-10 | Forward | TAAGGGTTACTTGGGTTGC |
| Reverse | TATCCAGAGGGTCTTCAGC | |
| IL-10R | Forward | CTGGTCACCCTGCCATTGAT |
| Reverse | AGGCATGGCTAAAATACAAAGAAAC |
Immunofluorescence microscopy
On the 14th day post-surgery, rats were placed under deep anesthesia and infused with phosphate-buffered saline (PBS, 1 M, pH 7.4) and 4% paraformaldehyde. The lumbar spinal cord was harvested, frozen, and cut into 10-μm transverse sections, and the sections were placed on glass slides. Slides were washed with PBS three times, each for 10 min, and blocked with 5% donkey serum in PBS for 1 h. The slides were then incubated with primary antibodies: rabbit anti-IL-4Rα (1:200; Santa Cruz Biotechnology, Dallas, TX), mouse anti-NeuN (1:400; Cell Signaling Technology, Danvers, MA), goat anti-Iba-1 (1:400; Abcam, Cambridge, UK), and mouse anti-glial fibrillary acidic protein (GFAP) (1:400; Novus Biologicals, Littleton, CO) for 12 h at 4°C. The slides were washed with PBS and incubated with Dylight 488 donkey anti-rabbit IgG (1:400; Jackson ImmunoResearch Laboratories, West Grove, PA), Cy3 donkey anti-goat IgG (1:400; Abcam), or Dylight 549 donkey anti-mouse IgG (1:400; Jackson ImmunoResearch Laboratories) for 2 h at 4°C. The slides were washed, sealed with a coverslip using 4′,6-diamino-2-phenylindole Fluoromount-G (AmyJet Scientific, Wuhan, China), and visualized with a fluorescence microscope (Carl Zeiss, Oberkochen, Germany). Images were acquired with a digital camera (Carl Zeiss) under the same light intensity for each stain.
Western blot analysis
The ipsilateral dorsal part of the lumbar spinal cords was collected from animals on the 14th day after CCI. Spinal tissue and PC12 cells were lysed in radioimmunoprecipitation assay buffer and centrifuged at 14,000 × g for 30 min at 4°C. Proteins were electrophoresed in 10% polyacrylamide gels and transferred to polyvinylidene difluoride membranes (Merck Millipore, Billerica, MA). The membranes were blocked with 5% bovine serum albumin for 1 h and then incubated with rabbitpolyclonal anti-IL-4Rα (1:200; Santa Cruz Biotechnology) or rabbit anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1:5000; Cell Signaling Technology) for 24 h at 4°C. The membrane was washed and incubated with horseradish peroxidase-conjugated anti-rabbit antibody (1:4000; ConWin Biotech, Beijing, China) for 2 h at room temperature. Immunoreactive bands were visualized using super ECL detection reagent (Merck Millipore). ImageJ software (National Institutes of Health, Bethesda, MD) was used to analyze band densities.
Statistical analysis
Data are presented as the mean ± standard error of the mean. Two-way analysis of variance with repeated measures followed by Dunnett’s test was used to compare behavioral responses. All other data were analyzed using the Kruskal–Wallis test followed by Dunn’s test for comparison of multiple groups, and the Mann–Whitney U test for comparison of two groups. p < 0.05 was defined as statistically significant.
Results
CCI induces neuropathic pain and upregulates pro- and anti-inflammatory signaling in the dorsal spinal cord
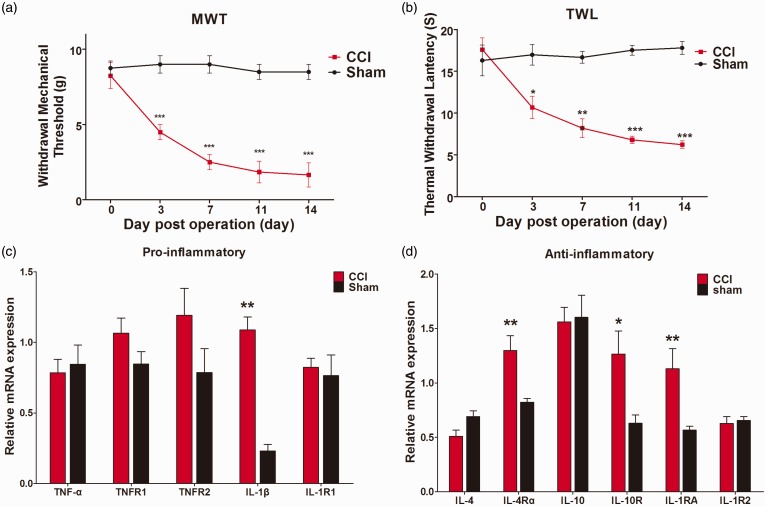
CCI induces obvious neuropathic pain in rats, manifesting as reductions of both MWT and TWL from 3 days to 14 days after surgery (compared with sham-operated rats, MWT p < 0.001 on days of 3, 7, 11, and 14 post-surgery, TWL p < 0.05 on days of 3 and p < 0.001 on days of 7, 11, and 14 post-surgery (Figure 1(a) and (b))).
Figure 1.
Evaluation of neuropathic pain and expression of pro- and anti-inflammatory cytokines and receptors in dorsal spinal cord after chronic constriction injury or sham surgery. (a) and (b) Mechanical withdrawal threshold and thermal withdrawal latency in the ipsilateral hindpaw were measured 1 day before and on days 3, 7, 11, and 14 after chronic constriction injury or sham surgery. (c) and (d) RT-qPCR analysis of mRNA expression of pro-inflammatory (TNF-α, TNFR1, TNFR2, IL-1β, and IL-1R1) and anti-inflammatory (IL-4, IL-4Rα, IL-10, IL-10R, IL-1RA, and IL-1R2) cytokines and receptors in the rat dorsal spinal cord after chronic constriction injury or sham surgery. Data are represented as mean ± SEM. *p < 0.05. **p < 0.01, ***p < 0.001 for chronic constriction injury + Vehicle group versus Sham + Vehicle group (n = 8).
We performed RT-qPCR analysis to examine mRNA levels of various cytokines and receptors in the ipsilateral dorsal spinal cord on day 14 after CCI or sham surgery. The pro-inflammatory genes examined were TNF-α and its receptors TNFR1 and TNFR2, and IL-1β and its receptor IL-1R1, and the anti-inflammatory genes were IL-4 and its receptor IL-4Rα, IL-10 and its receptor IL-10R, the IL-1R antagonist (IL-1RA), and the IL-1 decoy receptor IL-1R2. We found that CCI dramatically increased expression of the pro-inflammatory cytokine IL-1β (p < 0.01) (Figure 1(c)), consistent with our previous work.6 Alternatively, several anti-inflammatory factors were also enhanced; namely, IL-4Rα (p < 0.01), IL-1RA (p < 0.01), and IL-10R (p < 0.05), where especially the latter was highly amplified (Figure 1(d)). These results suggest that sciatic nerve injury activates both pro- and anti-inflammatory signaling in the spinal cord.
Res attenuates the duration of neuropathic pain and further enhances the anti-inflammatory signaling in the spinal cord
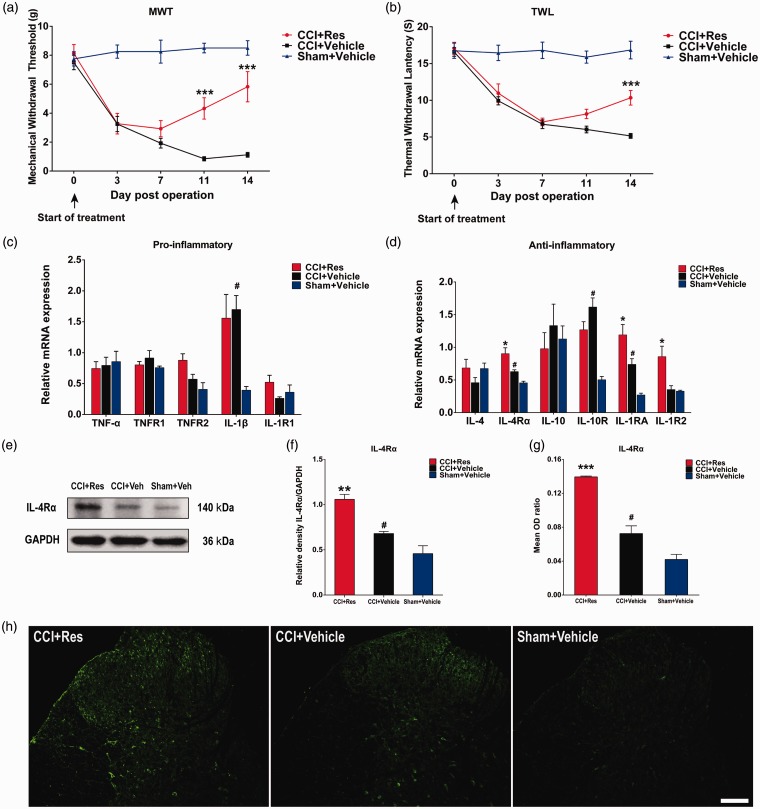
After CCI or sham surgery, rats were treated by i.p. injection with Res (200 mg/kg) or 2% DMSO (vehicle) in saline once daily for 14 days. As shown in Figure 2(a) and (b), the CCI + vehicle rat group showed significantly greater sensitivity to mechanical and thermal stimulation than did the sham + vehicle group (p < 0.001). Compared with vehicle treatment, i.p. injection of Res significantly increased tolerance in the MWT test on days 11 and 14 post-surgery (p < 0.001) (Figure 2(a)) and in the TWL test on day 14 post-surgery (p < 0.001) (Figure 2(b)). These results indicate that treatment with Res attenuated the duration of CCI-induced neuropathic pain.
Figure 2.
Effects of Res treatment on neuropathic pain and expression of pro- and anti-inflammatory cytokines and receptors in dorsal spinal cord of chronic constriction injury rats. Res (200 mg/kg) or DMSO (vehicle) in saline solution were intraperitoneally injected once daily for 14 days starting on the day of chronic constriction injury or sham surgery. (a) and (b) Mechanical withdrawal threshold and thermal withdrawal latency in the ipsilateral hindpaw were measured 1 day before and on days 3, 7, 11, and 14 after surgery (n = 12). (c) and (d) RT-qPCR analysis of mRNA expression of pro-inflammatory (TNF-α, TNFR1, TNFR2, IL-1β, and IL-1R1) and anti-inflammatory (IL-4, IL-4Rα, IL-10, IL-10R, IL-1RA, and IL-1R2) cytokines and receptors in rats subjected to chronic constriction injury or sham surgery and treated with Res or vehicle (n = 8). (e) and (f) Western blot (e) and densitometric quantification (f) of IL-4Rα protein expression (n = 8). GAPDH was probed as a loading control. (g) and (h) Quantified immunofluorescence intensity (g) and images (h) of IL-4Rα staining in the ipsilateral dorsal spinal cord (n = 4). Data are represented as mean ± SEM. #p < 0.05 for CCI + Vehicle group versus Sham + Vehicle group; *p < 0.05, **p < 0.01, ***p < 0.001 for CCI + Res group versus CCI + Vehicle group. Scale bar = 100 μm.
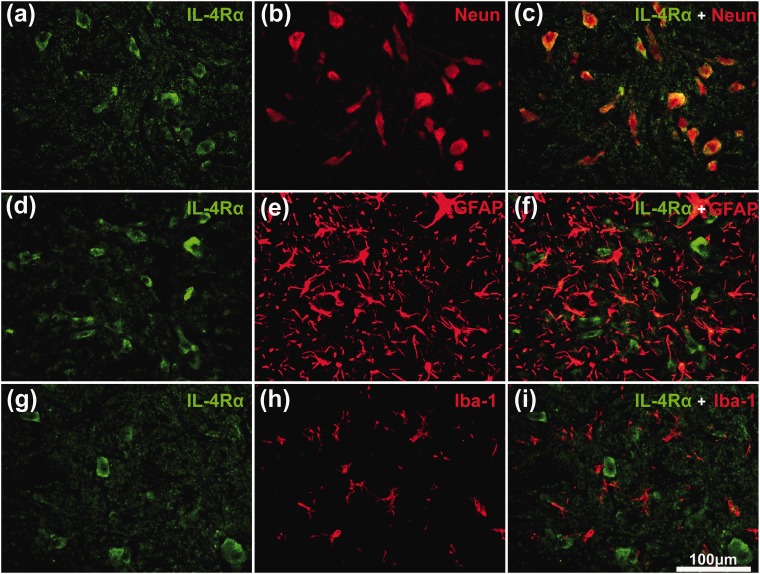
Treatment of CCI rats with Res further upregulated IL-4Rα, IL-1RA, and IL-1R2 mRNA levels compared with vehicle treatment (p < 0.05) (Figure 2(d)), whereas IL-1β mRNA levels were unaffected by Res (Figure 2(c)). Since IL-1RA and IL-1R2 mRNA levels can be regulated by IL-4R signaling, we also examined the expression of IL-4Rα protein in spinal cord by western blotting and immunofluorescence staining. Consistent with the mRNA results, IL-4Rα protein levels were increased after CCI (p < 0.05), and the expression was further elevated by Res treatment (p < 0.01) (Figure 2(e) and (f)). Immunofluorescence staining revealed that IL-4Rα expression was mainly increased in the superficial dorsal horn, indicating a potential relationship between IL-4Rα and nociceptive signaling (Figure 2(g) and (h)). To identify the cell type(s) expressing IL-4Rα after the treatment of Res, we performed double immunofluorescence staining for IL-4Rα and NeuN (neuronal marker), GFAP (astrocyte marker), or Iba-1 (microglial marker) (Figure 3(g) to (i)).
Figure 3.
Double immunofluorescence staining for IL-4Rα with NeuN, GFAP, or Iba-1 in the dorsal spinal cord of Res-treated chronic constriction injury rats. Sections were stained with antibodies to IL-4Rα (green) alone (a), (d) and (g), or together with a neuronal marker (b, NeuN), an astrocyte marker (e, GFAP), or a microglial marker (h, Iba-1) (red). The merged images (c), (f), and (i) show that IL-4Rα is mostly localized in neurons (c). Scale bar = 100 μm.
IL-4Rα knockdown reverses Res-activated transcription of anti-inflammatory genes
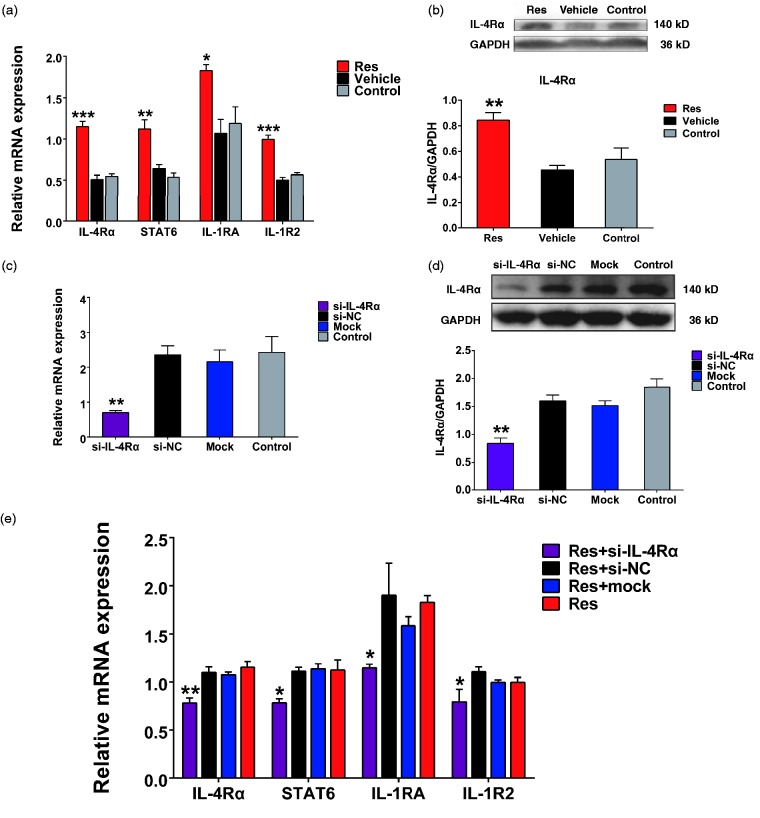
To investigate the relationship between IL-4Rα, IL-1RA, and IL-1R2 expression further, we examined the effects of small interfering RNA (siRNA)-mediated silencing of IL-4Rα expression in PC12 cells, a rat neuronal cell line. In preliminary experiments, we performed a dose-response analysis of Res effects on IL-4Rα expression and found marked upregulation of IL-4Rα mRNA in cells treated with 50 μM Res compared with vehicle (p < 0.001) (Figure 4(a)). Therefore, we compared the effects of 50 μM Res in PC12 cells on the mRNA expression of IL-4Rα, IL-1RA, IL-1R2 as well as STAT6, a transcription factor which is required for gene transcription responding to IL-4 signaling.27,28 Notably, all these genes were significantly upregulated by treatment with Res compared with vehicle (p < 0.01 for STAT6, p < 0.05 for IL-1RA, p < 0.001 for IL-4Rα and IL-1R2) (Figure 4(b)). Similarly, IL-4Rα protein expression was upregulated by Res treatment (p < 0.01) (Figure 4(c)).
Figure 4.
Effect of IL-4Rα knockdown in Res-treated PC12 cells. (a) Dose-response analysis on the mRNA expression of IL-4Rα in PC12 cells treated 10 μM, 50 μM, and 100 μM Res, medium (control), or DMSO (vehicle). ***p < 0.001 for 50 μM Res group versus vehicle group (n = 3) (b) RT-qPCR analysis of IL-4Rα, IL-1RA, and IL-1R2 mRNA levels in PC12 cells treated 50 μM Res. *p < 0.05, ***p < 0.001 for Res group versus vehicle group (n = 3). (c) Western blot analysis (upper) and densitometric quantification (lower) of IL-4Rα protein expression in cells treated 50 μM Res. GAPDH was probed as a loading control **p < 0.01 for Res group versus vehicle group (n = 4). (d) and (e) Cells were untreated, mock-transfected, or transfected with negative control or IL-4Rα–specific siRNA for 24 h and then tested for mRNA (d) and protein (e) expression of IL-4Rα. Data are represented as mean ± SEM. **p < 0.01 for si-IL-4Rα group versus si-NC group (n = 3). (f) RT-qPCR analysis of IL-4Rα, IL-1RA, and IL-1R2 mRNA levels in PC12 cells 24 h after treatment as in (d) and (e) with 50 μM Res added before transfection. Data are represented as mean ± SEM. *p < 0.05 and **p < 0.01 for Res + si-IL-4Rα group versus Res + si-NC group (n = 3).
Next, cells were transfected with IL-4Rα-targeting or non-targeting NC siRNAs. We verified that IL-4Rα expression was specifically knocked down with high efficiency. The IL-4Rα mRNA and protein levels were reduced over 70% and 50%, respectively, compared with si-NC-transfected cells (p < 0.01) (Figure 4(d) and (e)). Importantly, mRNA levels of STAT6 as well as IL-1RA and IL-1R2 in Res-treated cells were decreased by transfection with si-IL-4Rα compared with si-NC (p < 0.05) (Figure 4(f)). These results indicated that knockdown of IL-4Rα inhibits Res-activated transcription of anti-inflammatory genes.
Discussion
In this study, we demonstrated that Res treatment significantly alleviates neuropathic pain in rats subjected to CCI, a model of sciatic nerve injury. Res treatment also enhanced IL-4Rα receptor-mediated signaling in dorsal spinal cord neurons, leading to increased expression of IL-1RA and IL-1R2 mRNA. Thus, Res-induced upregulation of these anti-inflammatory molecules might contribute to the ability of Res to reduce central sensitization following peripheral nerve injury.
Pro-inflammatory response in the spinal cord contributes to central sensitization after CCI
Consistent with our previous work,6 we found that the pro-inflammatory cytokine IL-1β is increased in the dorsal spinal cord of rats on day 14 after CCI. Considerable evidence suggests that IL-1β expression in the CNS induces hyperalgesia in animal models.29–31 However, we also found increased mRNA expression of the anti-inflammatory receptors IL-1RA, IL-4Rα, and IL-10R in the dorsal spinal cord following CCI. These results indicate that both pro- and anti-inflammatory cytokine signaling are activated in the spinal cord after CCI, but the pro-inflammatory response may dominate and contribute to central sensitization.32
Res may inhibit IL-1β-mediated inflammation by enhancing the expression of IL-1RA and IL-1R2
Daily injections of CCI rats with Res alleviated pain-related behavior on days 11 and 14 after surgery, indicating that systemic Res treatment can attenuate the duration of neuropathic pain. Although Res has previously been shown to have anti-inflammatory effects in the peripheral nervous system,19 our study shows that Res also restores the balance between pro- and anti-inflammatory responses in the CNS following peripheral nerve injury. Although Res cannot suppress upregulation of IL-1β in the dorsal spinal cord, it can block its effect.33,34 Current data suggest that all known actions of IL-1 are mediated via a single cell surface receptor, IL-1R1.35,36 We demonstrated that treatment with Res increased IL-1RA and IL-1R2 mRNA levels in the rat dorsal spinal cord. IL-1RA binds more efficiently to IL-1R1 than does IL-1β and is thus able to block IL-1R1–IL-1β interactions.37 IL-1R2 is structurally similar to IL-1R1 and acts as a decoy receptor to block IL-1 signaling.38,39 By enhancing the expression of IL-1RA and IL-1R2, Res may inhibit IL-1β-mediated inflammation40,41 in the spinal cord and thus attenuate central sensitization following peripheral nerve injury.42
Activation of IL-4R signaling contributes to Res-mediated upregulation of IL-1RA and IL-1R2
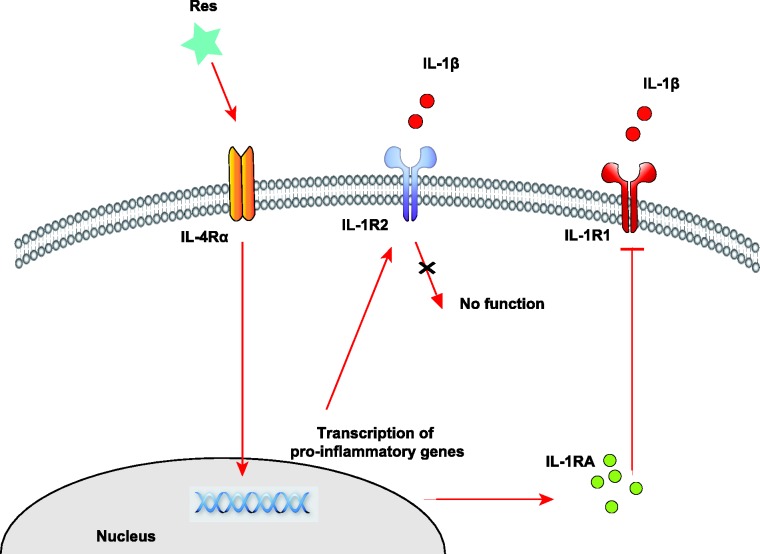
Res-mediated upregulation of IL-4Rα, IL-1RA and IL-1R2 may due to the activation of intracellular signal transduction pathways. In the cellular level, Res works as a nutrient signaling molecular that activates a number of stimulus-responsive transcription factors such as STAT6, CREB, AP-1, Egr-1, Elk-1, and Nrf2.43,44 Gene transcription of IL-4Rα, IL-1RA, and IL-1R2 has been shown to be resulted from these activations.27,45 Besides, the upregulation of IL-1RA and IL-1R2 requires IL-4R signaling. Although we did not detect a change in IL-4 mRNA levels on day 14 of Res treatment, IL-4Rα expression was significantly increased in the dorsal spinal cord neurons, suggesting that there is indeed an increased response to IL-4.46 Moreover, IL-4 signaling is known to increase both IL-1RA and IL-1R2 mRNA expression.47–49 We found not only that IL-4Rα, IL-1RA, and IL-1R2 are all elevated in the spinal cord of Res-treated rats, but also that IL-4Rα knockdown in PC12 neurons reversed the Res-induced upregulation of IL-1RA and IL-1R2. These results suggest that Res might act on the IL-4Rα to facilitate its anti-inflammatory effects (Figure 5).
Figure 5.
Schematic representation of Res-mediated anti-inflammatory effects in neurons. Res enhances the transcription of IL-1RA and IL-1R2 via IL-4Rα signaling. IL-1RA prevents IL-1β from interacting with IL-1R1, and IL-1R2 acts as a decoy target for IL-1 signaling.
Conclusion
Systemic administration of Res attenuated neuropathic pain in the CCI rat model. Res may alleviate central sensitization by enhancing IL-4R–mediated anti-inflammatory signaling in the dorsal spinal cord.
Acknowledgments
The authors thank Anne M. O’Rourke, PhD, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Author Contributions
CH conceived and designed the experiments. MX and ZC performed the experiments. ZD analyzed the data. CH, YW, and QG wrote the paper.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Natural Science Foundation of China (81771207, 81370027, 81571081, and 81400916).
References
- 1.Moalem G andTracey DJ.. Immune and inflammatory mechanisms in neuropathic pain. Brain Res Rev 2006; 51: 240–264. [DOI] [PubMed] [Google Scholar]
- 2.Scholz J andWoolf CJ.. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci 2007; 10: 1361–1368. [DOI] [PubMed] [Google Scholar]
- 3.O’Shea JJ andMurray PJ.. Cytokine signaling modules in inflammatory responses. Immunity 2008; 28: 477–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang F Wang H Wang X Jiang G Liu H Zhang G Wang H Fang R Bu X Cai S andDu J.. TGF-beta induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget 2016; 7: 52294–52306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milligan ED Langer SJ Sloane EM He L Wieseler-Frank J O’Connor K Martin D Forsayeth JR Maier SF Johnson K Chavez RA Leinwand LA andWatkins LR.. Controlling pathological pain by adenovirally driven spinal production of the anti-inflammatory cytokine, interleukin-10. Eur J Neurosci 2005; 21: 2136–2148. [DOI] [PubMed] [Google Scholar]
- 6.Xu F, Huang J, He Z, Chen J, Tang X, Song Z, Guo Q, Huang C. Microglial polarization dynamics in dorsal spinal cord in the early stages following chronic sciatic nerve damage. Neurosci Lett 2016; 617: 6–13. [DOI] [PubMed] [Google Scholar]
- 7.Ren K andDubner R.. Interactions between the immune and nervous systems in pain. Nat Med 2010; 16: 1267–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baur JA andSinclair DA.. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov 2006; 5: 493–506. [DOI] [PubMed] [Google Scholar]
- 9.Liu CW Sung HC Lin SR Wu CW Lee CW Lee IT Yang YF Yu IS Lin SW Chiang MH Liang CJ andChen YL.. Resveratrol attenuates ICAM-1 expression and monocyte adhesiveness to TNF-alpha-treated endothelial cells: evidence for an anti-inflammatory cascade mediated by the miR-221/222/AMPK/p38/NF-kappaB pathway. Sci Rep 2017; 7: 44689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Venkatadri R Muni T Iyer AK Yakisich JS andAzad N.. Role of apoptosis-related miRNAs in resveratrol-induced breast cancer cell death. Cell Death Dis 2016; 7: e2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Girbovan C Morin L andPlamondon H.. Repeated resveratrol administration confers lasting protection against neuronal damage but induces dose-related alterations of behavioral impairments after global ischemia. Behav Pharmacol 2012; 23: 1–13. [DOI] [PubMed] [Google Scholar]
- 12.Singleton RH Yan HQ Fellows-Mayle W andDixon CE.. Resveratrol attenuates behavioral impairments and reduces cortical and hippocampal loss in a rat controlled cortical impact model of traumatic brain injury. J Neurotrauma 2010; 27: 1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ates O Cayli S Altinoz E Gurses I Yucel N Kocak A Yologlu S andTurkoz Y.. Effects of resveratrol and methylprednisolone on biochemical, neurobehavioral and histopathological recovery after experimental spinal cord injury. Acta Pharmacol Sinica 2006; 27: 1317–1325. [DOI] [PubMed] [Google Scholar]
- 14.Bagriyanik HA Ersoy N Cetinkaya C Ikizoglu E Kutri D Ozcana T Kamanga LG andKiray M.. The effects of resveratrol on chronic constriction injury of sciatic nerve in rats. Neurosci Lett 2014; 561: 123–127. [DOI] [PubMed] [Google Scholar]
- 15.Fang L Gao H Zhang W Zhang W andWang Y.. Resveratrol alleviates nerve injury after cerebral ischemia and reperfusion in mice by inhibiting inflammation and apoptosis. Int J Clin Exp Med 2015; 8: 3219–3226. [PMC free article] [PubMed] [Google Scholar]
- 16.Yang YJ Hu L Xia YP Jiang CY Miao C Yang CQ Yuan M andWang L.. Resveratrol suppresses glial activation and alleviates trigeminal neuralgia via activation of AMPK. J Neuroinflamm 2 016; 13: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh AK andVinayak M.. Anti-nociceptive effect of resveratrol during inflammatory hyperalgesia via differential regulation of pro-inflammatory mediators. Phytother Res 2016; 30: 1164–1171. [DOI] [PubMed] [Google Scholar]
- 18.Sharma S Kulkarni SK andChopra K.. Effect of resveratrol, a polyphenolic phytoalexin, on thermal hyperalgesia in a mouse model of diabetic neuropathic pain. Fundam Clin Pharmacol 2007; 21: 89–94. [DOI] [PubMed] [Google Scholar]
- 19.Tao L Ding Q Gao C andSun X.. Resveratrol attenuates neuropathic pain through balancing pro-inflammatory and anti-inflammatory cytokines release in mice. Int Immunopharmacol 2016; 34: 165–172. [DOI] [PubMed] [Google Scholar]
- 20.Wu B Ma Y Yi Z Liu S Rao S Zou L Wang S Xue Y Jia T Zhao S Shi L Li L Yuan H andLiang S.. Resveratrol-decreased hyperalgesia mediated by the P2X7 receptor in gp120-treated rats. Mol Pain 2017; 13: 1744806917707667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He X Ou P Wu K Huang C Wang Y Yu Z andGuo Q.. Resveratrol attenuates morphine antinociceptive tolerance via SIRT1 regulation in the rat spinal cord. Neurosci Lett 2014; 566: 55–60. [DOI] [PubMed] [Google Scholar]
- 22.Bennett GJ andXie YK.. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988; 33: 87–107. [DOI] [PubMed] [Google Scholar]
- 23.Chaplan SR Bach FW Pogrel JW Chung JM andYaksh TL.. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Meth 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 24.Hayakawa N Shiozaki M Shibata M Koike M Uchiyama Y Matsuura N andGotow T.. Resveratrol affects undifferentiated and differentiated PC12 cells differently, particularly with respect to possible differences in mitochondrial and autophagic functions. Eur J Cell Biol 2013; 92: 30–43. [DOI] [PubMed] [Google Scholar]
- 25.Tome-Carneiro J Larrosa M Gonzalez SA Tomas BFA Garcia-Conesa MT andEspin JC.. Resveratrol and clinical trials: the crossroad from in vitro studies to human evidence. CPD 2013; 19: 6064–6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu C Shi Z Fan L Zhang C Wang K andWang B.. Resveratrol improves neuron protection and functional recovery in rat model of spinal cord injury. Brain Res 2011; 1374: 100–109. [DOI] [PubMed] [Google Scholar]
- 27.Ohmori Y Smith MF JrandHamilton TA.. IL-4-induced expression of the IL-1 receptor antagonist gene is mediated by STAT6. J Immunol 1996; 157: 2058–2065. [PubMed] [Google Scholar]
- 28.Takeda K Tanaka T Shi W Matsumoto M Minami M Kashiwamura S Nakanishi K Yoshida N Kishimoto T andAkira S.. Essential role of Stat6 in IL-4 signalling. Nature. 1996; 380: 627–630. [DOI] [PubMed] [Google Scholar]
- 29.Ren K andTorres R.. Role of interleukin-1beta during pain and inflammation. Brain Res Rev 2009; 60: 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gui WS Wei X Mai CL Murugan M Wu LJ Xin WJ Zhou LJ andLiu XG.. Interleukin-1beta overproduction is a common cause for neuropathic pain, memory deficit, and depression following peripheral nerve injury in rodents. Mol Pain 2016; 12: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu T Jiang CY Fujita T Luo SW andKumamoto E.. Enhancement by interleukin-1beta of AMPA and NMDA receptor-mediated currents in adult rat spinal superficial dorsal horn neurons. Mol Pain 2013; 9: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Austin PJ andMoalem-Taylor G.. The neuro-immune balance in neuropathic pain: involvement of inflammatory immune cells, immune-like glial cells and cytokines. Journal of Neuroimmunology 2010; 229: 26–50. [DOI] [PubMed] [Google Scholar]
- 33.Shakibaei M John T Seifarth C andMobasheri A.. Resveratrol inhibits IL-1 beta-induced stimulation of caspase-3 and cleavage of PARP in human articular chondrocytes in vitro. Ann New York Acad Sci 2007; 1095: 554–563. [DOI] [PubMed] [Google Scholar]
- 34.Shakibaei M Csaki C Nebrich S andMobasheri A.. Resveratrol suppresses interleukin-1beta-induced inflammatory signaling and apoptosis in human articular chondrocytes: potential for use as a novel nutraceutical for the treatment of osteoarthritis. Biochem Pharmacol 2008; 76: 1426–1439. [DOI] [PubMed] [Google Scholar]
- 35.Garlanda C Dinarello CA andMantovani A.. The interleukin-1 family: back to the future. Immunity 2013; 39: 1003–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glaccum MB Stocking KL Charrier K Smith JL Willis CR Maliszewski C Livingston DJ Peschon JJ andMorrissey PJ.. Phenotypic and functional characterization of mice that lack the type I receptor for IL-1. J Immunol 1997; 159: 3364–3371. [PubMed] [Google Scholar]
- 37.Boraschi D Bossu P Macchia G Ruggiero P andTagliabue A.. Structure–function relationship in the IL-1 family. Front Biosci 1996; 1: d270–d308. [DOI] [PubMed] [Google Scholar]
- 38.Peters VA Joesting JJ andFreund GG.. IL-1 receptor 2 (IL-1R2) and its role in immune regulation. Brain Behav Immun 2 013; 32: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colotta F Re F Muzio M Bertini R Polentarutti N Sironi M Giri JG Dower SK Sims JE andMantovani A.. Interleukin-1 type II receptor: a decoy target for IL-1 that is regulated by IL-4. Science 1993; 261: 472–475. [DOI] [PubMed] [Google Scholar]
- 40.Horai R Saijo S Tanioka H Nakae S Sudo K Okahara A Ikuse T Asano M andIwakura Y.. Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist-deficient mice. J Exp Med 2000; 191: 313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin P Palmer G Vigne S Lamacchia C Rodriguez E Talabot-Ayer D Rose-John S Chalaris A andGabay C.. Mouse neutrophils express the decoy type 2 interleukin-1 receptor (IL-1R2) constitutively and in acute inflammatory conditions. J Leukocyte Biol 2013; 94: 791–802. [DOI] [PubMed] [Google Scholar]
- 42.George A Buehl A andSommer C.. Tumor necrosis factor receptor 1 and 2 proteins are differentially regulated during Wallerian degeneration of mouse sciatic nerve. Exp Neurol 2005; 192: 163–166. [DOI] [PubMed] [Google Scholar]
- 43.Yang X Xu S Qian Y andXiao Q.. Resveratrol regulates microglia M1/M2 polarization via PGC-1alpha in conditions of neuroinflammatory injury. Brain Behav Immun 2017; 64: 162–172. [DOI] [PubMed] [Google Scholar]
- 44.Thiel G andRossler OG.. Resveratrol regulates gene transcription via activation of stimulus-responsive transcription factors. Pharmacol Res 2017; 117: 166–176. [DOI] [PubMed] [Google Scholar]
- 45.Waqas SFH Hoang AC Lin YT Ampem G Azegrouz H Balogh L Thuroczy J Chen JC Gerling IC Nam S Lim JS Martinez-Ibanez J Real JT Paschke S Quillet R Ayachi S Simonin F Schneider EM Brinkman JA Lamming DW Seroogy CM andRoszer T.. Neuropeptide FF increases M2 activation and self-renewal of adipose tissue macrophages. J Clin Invest 2017; 127: 2842–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang JM andAn J.. Cytokines, inflammation, and pain. Int Anaesthesiol Clin 2007; 45: 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Orino E Sone S Nii A andOgura T.. IL-4 up-regulates IL-1 receptor antagonist gene expression and its production in human blood monocytes. J Immunol 1992; 149: 925–931. [PubMed] [Google Scholar]
- 48.Pousset F Cremona S Dantzer R Kelley KW andParnet P.. IL-10 and IL-4 regulate type-I and type-II IL-1 receptors expression on IL-1 beta-activated mouse primary astrocytes. J Neurochem 2001; 79: 726–736. [DOI] [PubMed] [Google Scholar]
- 49.Busch-Dienstfertig M andGonzalez RS.. IL-4, JAK-STAT signaling, and pain. Jak-Stat 2013; 2: e27638. [DOI] [PMC free article] [PubMed] [Google Scholar]