Short abstract
Background
Intense nociceptive signaling arising from ongoing injury activates primary afferent nociceptive systems to generate peripheral sensitization. ERK1/2 phosphorylation in dorsal root ganglion can be used to visualize intracellular signal activity immediately after noxious stimulation. The aim of this study was to investigate spatiotemporal characteristics of ERK1/2 phosphorylation against tissue injury in the primary afferent neurons.
Methods
Plantar incisions were made in the hind paws of Sprague-Dawley rats (n =150). Levobupivacaine was injected into the plantar aspect of the paws and ankles, Mitogen-activated protein kinase kinase (MEK) inhibitor was injected into the paw, and carbenoxolone, dual inhibitor of the gap junction and pannexin channel, was intraperitoneally injected. Pain hypersensitivity was investigated by a behavioral study, while phosphorylated ERK1/2 was detected in dorsal root ganglion and hind paw using immunohistochemistry and Western blot.
Results
Phosphorylated ERK1/2 was induced in dorsal root ganglion (26.8 ± 2.9% at baseline, 65.6 ± 3.6% at 2 min, and 26.3 ± 3.4% at 2 h) after the incision. NF-200 positive A-fiber neurons and satellite glial cells were positive for phosphorylated ERK1/2. Injury-induced pain hypersensitivity was abolished by MEK inhibitor. Levobupivacaine treatment inhibited phosphorylated ERK1/2 induction, carbenoxolone treatment inhibited glial phosphorylated ERK1/2 at 2 min after the injury, and carbenoxolone inhibited pain hypersensitivity and neuronal phosphorylated ERK1/2 at 1 h after the injury.
Conclusion
ERK1/2 phosphorylation in A-fiber neurons and satellite glial cells immediately after injury contributes to the generation of pain hypersensitivity. Signal communication between neurons and satellite glial cells expands the duration of neuronal ERK1/2 phosphorylation and pain hypersensitivity at 1 h after tissue injury.
Keywords: Tissue injury, hyperalgesia, A-fiber, satellite glial cell
Introduction
The mechanism responsible for the development of postsurgical pain involves a unique feature that differs from the pain that occurs after tissue inflammation or nerve injury.1 Intense nociceptive signaling arising from an ongoing injury and signaling that follows an immune reaction both activate the primary afferent nociceptive systems to generate peripheral sensitization. We previously reported that p38MAPK is phosphorylated in a TNF-α dependent manner in the dorsal root ganglion (DRG) and contributes to the development of acute2 and chronic3 postsurgical pain which confirms that one of the important features of the immune reaction is to act as a driver of the peripheral sensitization.4
Blunt nociceptive stimulation due to tissue injury is another component that is associated with peripheral sensitization. Kawamata et al.5 observed the rapid generation of a hyperalgesic area around the incision site in humans. As local anesthetics prevent the generation of this hyperalgesia, this demonstrates the important role that the signal transduction of a noxious sensation plays in the development of immediate hyperalgesia. However, the neuronal response that occurs immediately after a tissue injury has yet to be documented.
ERK1/2 is one of the major MAPK family members that are involved in organizing the cellular activity in response to extracellular stimulation.6 ERK1/2 is activated in the DRG immediately after nerve injury,7 capsaicin injection, and electrical nerve stimulation.8 Activity-dependent ERK1/2 phosphorylation contributes to the development of peripheral sensitization9 and abnormal pain.10
Neuronal cell bodies and glial cells are clustered in the DRG. Satellite glial cells (SGCs) around the neuron actively regulate neuronal functions.11 Signals are transduced from neuron to SGCs by calcium influx via the gap junction or ATP release from pannexin channel.12 Cytokines or other inflammatory mediators are released from SGC and reciprocally activate DRG neurons.12 Coupled activation of the DRG neurons facilitated by SGCs is responsible for the generation of pain hypersensitivity.13
In the present study, we hypothesized that the neurons and glial cells undergo activation of the signal transduction immediately after tissue injury and that this should be able to be visualized by monitoring the ERK1/2 phosphorylation. Therefore, we used phosphorylated ERK1/2 (pERK1/2) as a molecular marker, and then attempted to identify the spatiotemporal cellular response against the tissue injury in primary afferent neurons. Local anesthetics or a dual inhibitor of the gap junction and pannexin channel were used to determine the role of the noxious signal transduction or neuron-glial communication for the alteration of the cellular activity in the primary afferent neuron.
Materials and methods
Animals and treatments
All of the experimental procedures included in this study were approved by the Kyoto Prefectural University of Medicine Animal Care Committee and were performed in accordance with the guidelines of the National Institutes of Health and The International Association for the Study of Pain. Male Sprague-Dawley rats (200–250g; Shimizu Laboratory Supplies Co. Ltd., Kyoto, Japan) were housed in groups of three per cage under a 12-h light/dark cycle. Surgeries and drug injections were performed using 2% v/v of isoflurane anesthesia. Sample sizes for each of the experiments were determined based on the results obtained in our previous studies.3
Experiment 1: Expression of pERK1/2 after the plantar incision
All rats underwent plantar incisions as previously described.2 Briefly, a 1-cm longitudinal incision was made through the skin, fascia, and plantaris muscle of the left hind paw using a number 11 surgical blade. The skin was closed with a 5–0 nylon suture. The left L5 DRG and plantar tissue of the left hind paw were removed at 2 min, 1 h, and 2 h after the procedure (n = 5 for each time point). Based on our preliminary experiments, a 90-s period from the beginning of dissection was required in order to obtain a sample. Therefore, we started the dissection procedure 30 s after the end of the plantar incision in order to ensure that we would be able to obtain the DRG by 2 min after the procedure. Naive animals were used as controls. Immunohistochemistry and Western blotting were performed using the DRG and plantar tissue samples. After each of the rats were administered a capsaicin injection on the plantar surface, the DRG specimen was collected 2 min later.
Experiment 2: Effect of the Mitogen-activated protein kinase kinase inhibitor on the hypersensitivity after the incision
Rats were randomly assigned to a control or Mitogen-activated protein kinase kinase (MEK) inhibitor group (n = 5 for each). Rats in both groups underwent plantar incisions as described above. One hour prior to the incision, rats in the MEK inhibitor group received an intraplantar injection of U0126 (MEK inhibitor, Promega, Fitchburg, WI, 0.1 mg/kg dissolved in 100 µL of 10% DMSO in 0.1 M phosphate buffered saline) in the ipsilateral hind paw. Rats in the control group were injected with the same amount of vehicle. Behavioral testing was performed at 1, 2, and 4 h after the incision. Separately, plantar tissue of the ipsilateral hind paw and left L5 DRG was removed at 2 min after the end of the procedure from rats in both groups (n = 5 for each).
Experiment 3: Effect of regional analgesia on the pERK1/2 expression
Rats were randomly assigned to either the control or regional analgesia groups (n = 5 for each group). Rats in regional analgesia group received levobupivacaine (2.5 mg/0.5 mL) injections into the paw plantar (0.3 mL) as well as the medial and lateral aspect of the ankle (0.1 mL each) 5 min prior to the incision.14 Rats in control group received same amount of saline injection. Rats in both groups underwent plantar incisions as described above. Separately, the left L5 DRG was removed at 2 min after the procedure from rats of both groups (n = 5 for each group).
Experiment 4: Effect of carbenoxolone on the pain behavior and pERK1/2 expression
Rats were randomly assigned to either the carbenoxolone (CBX) or control groups. The CBX group received an intraperitoneal injection of CBX (Sigma, St. Louis, MO, 100 mg/kg, dissolved in saline), while the control group received an injection of vehicle. In both groups, the plantar incisions were made at 1 h after the injection. DRG was removed at 2 min and 1 h after the incision, and then subjected to immunohistochemistry (n = 5 for each group, respectively). Separately, rats were randomly assigned to either the CBX or control groups (n = 5 for each group). Behavioral testing was performed at 1, 2, and 4 h after the above procedure.
Behavioral assessment
All behavioral experiments were performed by a single experimenter (YY) in a blinded manner. Mechanical sensitivity was assessed by determining the withdrawal threshold against von Frey stimulation, as previously described.14 Briefly, rats were placed in a clear plastic chamber on an elevated wire grid. After acclimatization, the withdrawal response was determined using a mechanical stimulation induced by a calibrated von Frey monofilament set (Muromachi Kikai Co., Ltd., Tokyo, Japan). Each von Frey filament was applied at the incision point of the left paw. The lowest force that evoked a clear withdrawal response at least twice during 10 applications was accepted as the threshold. Cut off value was set as 50 mN in order to avoid tissue damage.
Immunohistochemistry
Rats were transcardially perfused with 0.9% NaCl followed by 10% neutralized formalin (Wako Pure Chemical Industries, Ltd., Osaka, Japan). L5 DRG and plantar tissue were immersed in 20% sucrose in 0.1 M phosphate-buffer (PB, pH 7.4) at 4°C overnight. Sections (10 µm for DRG and 30 µm for plantar tissue) were cut using a cryostat (Leica Biosystems, Nussloch, Germany). Sections were incubated with Blocking One P (Nacalai Tesque Inc., Kyoto, Japan) at room temperature for 30 min, followed by incubation at 4°C for three days with rabbit anti-pERK1/2 (1:4000, Merck Millipore, Billerica, MA, USA), in 0.1% tween 20 in 0.1 M tris-buffered saline (TBS, pH 7.4) containing 1% blocking reagent. After washing with phosphate buffered saline, the sections were incubated overnight at 4°C with rhodamine-conjugated anti-rabbit secondary antibody (1:200, Merck Millipore) in 0.1 M TBS.
For double-labeling immunohistochemistry, all sections were incubated with rabbit anti-p-ERK1/2 and mouse anti-Neurofilament-200 (1:4000, NF-200, Sigma), goat anti-transient receptor potential V1 (1:100, TRPV1, Santa Cruz, Dallas, TX), guinea pig anti-3-phosphoglycerate dehydrogenase (1:400, 3PGDH, Frontier Institute, Sapporo, Japan), or mouse anti-Protein Gene Product 9.5 (1:400, PGP9.5, Abcam, Cambridge, England) in 0.1 M TBS containing 1% blocking reagent at 4°C for three days. Sections were then incubated with rhodamine-conjugated anti-rabbit secondary antibody and FITC-conjugated anti-mouse secondary antibody (1:100, Merck Millipore, for NF-200 and PGP9.5) or FITC-conjugated anti-goat secondary antibody (1:100, Merck Millipore, for TRPV1) or FITC-conjugated anti-guinea pig secondary antibody (1:1000, Merck Millipore, for 3PGDH). Sections were washed and visualized using a fluorescence microscope with a digital camera system (Nikon, Tokyo, Japan).
Cell count
Immunohistochemistry images were analyzed by Image-J (NIH, Bethesda, MD) on a Macintosh computer system. In order to investigate pERK1/2 expression in the DRG neuron, the pERK1/2 or DAPI positive nuclei in the DRG neurons were identified based on the nuclear size (>50 µm2). Our preliminary experiments identified that the signal selection based on these criteria could successfully identify the neuronal pERK1/2/DAPI signal while excluding the glial signal. The number of pERK1/2 or DAPI positive neurons that were four sections apart (at least 100 µm) was counted, with the fraction then calculated for a single rat. In order to investigate the pERK1/2 expression in the SGCs, we first identified the 3PGDH positive area in the DRG section. We then applied the 3PGDH positive area profile to the pERK1/2 image of the same section in order to obtain the pERK1/2 expression within the 3PGDH positive area. Areas for the pERK1/2 or 3PGDH for the four sections that were at least 100 µm apart were measured and used to calculate the fraction for a single rat.
Western blotting
L5 DRG was homogenized in a homogenate buffer (20 mM Tris-HCl (pH 8.0) containing 137 mM NaCl, 2 mM EDTA, 10% glycerol, 1% TritonX-100, 1 µM PMSF, 10 µg/mL leupeptin and 10 µg/mL pepstatin A) with protease inhibitor (Thermo Fisher Scientific, Waltham, MA). The protein concentration of each homogenate was determined by the Bradford reagent (BioRad, Hercules, CA). After separating 50 µg of the cell lysate on 8.0% SDS-PAGE, the specimens were immunoblotted onto nitrocellulose membranes. The membranes were incubated overnight at 4°C with rabbit anti-p-ERK1/2 (1:1000; Cell Signaling Technology Danvers, MA) or rabbit anti-GAPDH (1:20000; Cell Signaling Technology), followed by incubation with horseradish peroxidase (HRP)-conjugated secondary antibody. Visualization was performed by using the Enhanced Chemiluminescence Select Western Blotting Detection Kit (GE Healthcare, Chicago, IL) and Hyperfilm (GE Healthcare). The intensity of the selected band was captured and analyzed using Image J software (NIH) on a Macintosh computer.
Results
Experiment 1: Expression of pERK1/2 after the plantar incision
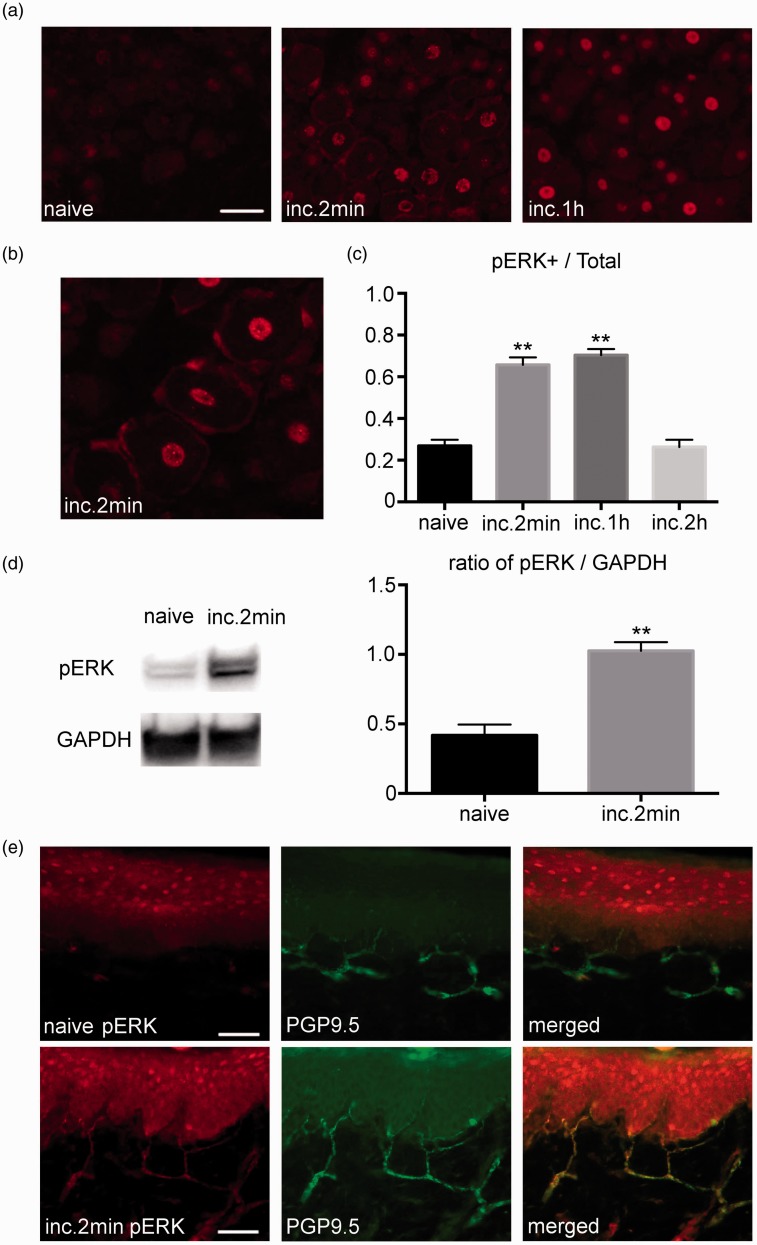
Figure 1(a) shows the expression of the pERK1/2 in the DRG and plantar tissue after the incision. A robust induction of the pERK1/2 expression was observed in the DRG after the incision. Neurons and SGCs were positive for pERK1/2. Among the 118 of p-ERK1/2 positive satellite cells, 117 cells were existed around the neuron positive for p-ERK1/2 (Figure 1(b)). The number of pERK1/2 positive neurons significantly increased at 2 min and at 1 h after the incision (26.8 ± 2.9% at baseline, 65.6 ± 3.6% at 2 min, and 26.3 ± 3.4% at 2 h; Figure 1(c)). Western blotting demonstrated that the amount of pERK1/2 significantly increased after the incision, which confirmed the immunohistochemistry data (Figure 1(d)). In the plantar tissue, pERK1/2 was induced in the PGP9.5 positive nerve endings after the incision (Figure 1(e)).
Figure 1.
Expression of pERK1/2 in the DRG and plantar tissue after the plantar incision. (a) Weak pERK1/2 signals were detected in the DRG of the naive group. Robust pERK1/2 expression was detected in the DRG after the incision. Scale bar = 50 µm. (b) Magnified image of the DRG at 2 min after the incision. pERK1/2 was visualized in the neurons and glial cells. (c) There was a significant increase in the number of neurons that express pERK1/2 at 2 min and at 1 h after the incision. The number of pERK1/2 positive neurons returned to baseline at 2 h after the incision. n=5 for each group. ** p<0.01 versus the naive group. (d) There was a significant increase in the amount of pERK1/2 in the DRG measured by Western blotting after the incision. n=5 for each group. ** p<0.01 versus the naive group. (e) Double-labeling immunohistochemistry for pERK1/2 and PGP9.5 in the plantar tissue. pERK1/2 expression was not observed in the naive group. Strong pERK1/2 expression was detected in the PGP9.5 positive nerve endings at 2 min after the incision. DRG: dorsal root ganglion; pERK1/2: phosphorylated ERK1/2.
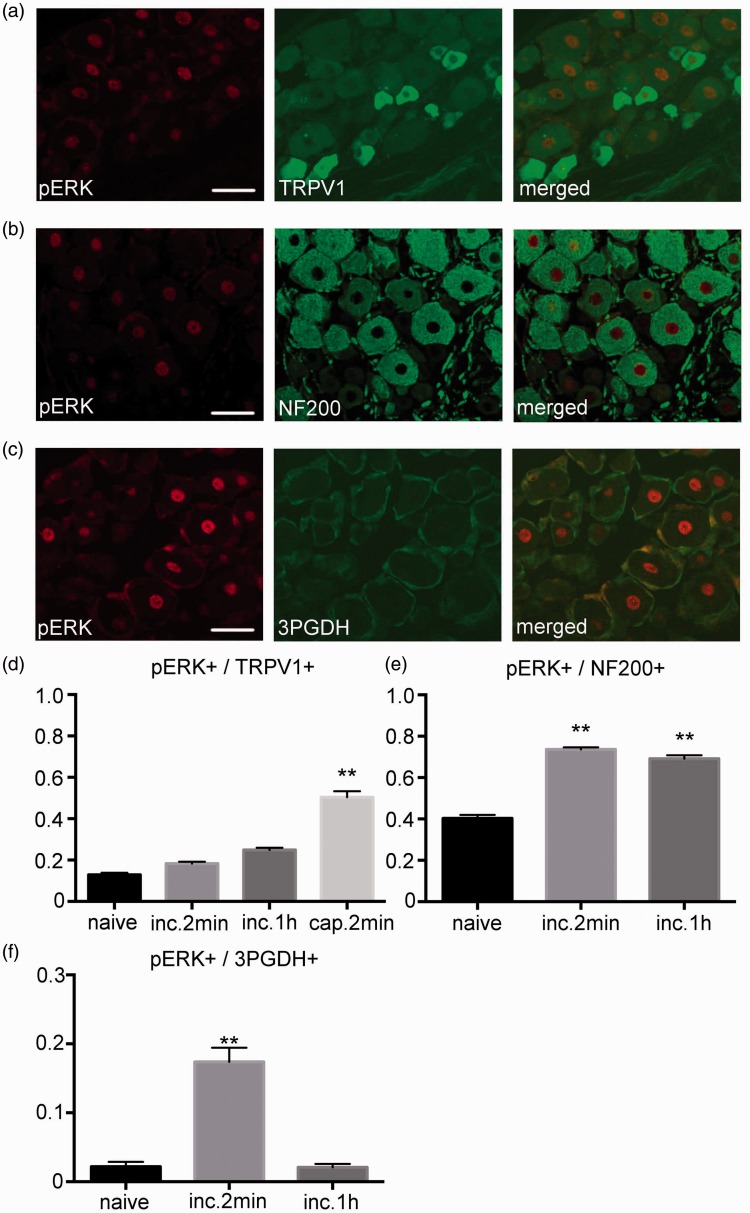
Figure 2 shows the results of the double-labeling immunohistochemistry for pERK1/2 and the cell markers. A small fraction of TRPV1 expressing neurons were positive for pERK1/2 after the incision. There was no increase in the number of pERK1/2 positive neurons within the TRPV1 positive neuronal profile after the incision. After the capsaicin injection, there was a significant increase in the pERK1/2 positive neurons within the TRPV1 positive profile (Figure 2(a) and (d)). In addition, there were a considerable number of NF-200 expressing neurons found to be positive for pERK1/2 after the incision. There was a significant increase in the number of pERK1/2 positive neurons within the NF-200 positive neuronal profile at 2 min and at 1 h after the incision (Figure 2(b) and (e)). pERK1/2 expression was also observed within the 3PGDH positive SGCs. Although there was a significant increase in the pERK1/2 positive area within the 3PGDH positive area profile at 2 min after the incision, it returned to baseline at 1 h after the incision (Figure 2(c) and (f)).
Figure 2.
Distribution of pERK1/2 in the DRG. (a) Double-labeling immunohistochemistry for pERK1/2 and TRPV1 2 min after the incision. A small fraction of the TRPV1 positive neurons expressed pERK1/2 after the incision. Scale bar = 50 µm. (b) Double-labeling immunohistochemistry for pERK1/2 and NF200 at 2 min after the incision. A considerable number of NF200 positive neurons expressed pERK1/2 after the incision. Scale bar = 50 µm. (c) Double-labeling immunohistochemistry for pERK1/2 and 3PGDH 2 min after the incision. pERK1/2 expression was detected in the 3PGDH positive satellite glial cells (SGCs) in the DRG. Scale bar = 50 µm. (d) Although there was no increase in the expression of pERK1/2 in the TRPV1 positive neuron after the incision, there was an increase after the capsaicin injection. n=5 for each group. **: p<0.01 versus the naive group. (e) There was a significant increase in the expression of pERK1/2 in the NF200 positive neuron at 2 min and at 1 h after the incision. n=5 for each group. **: p<0.01 versus the naive group. (f) Fraction of pERK1/2 positive cell area in the 3PGDH positive cell area. There was a significant increase in the pERK1/2 positive area at 2 min after the incision, with a reduction back to baseline values at 1 h after the incision. n=5 for each group. **: p<0.01 versus naive. DRG: dorsal root ganglion; pERK1/2: phosphorylated ERK1/2.
Experiment 2: Effect of MEK inhibitor on the hypersensitivity after the incision
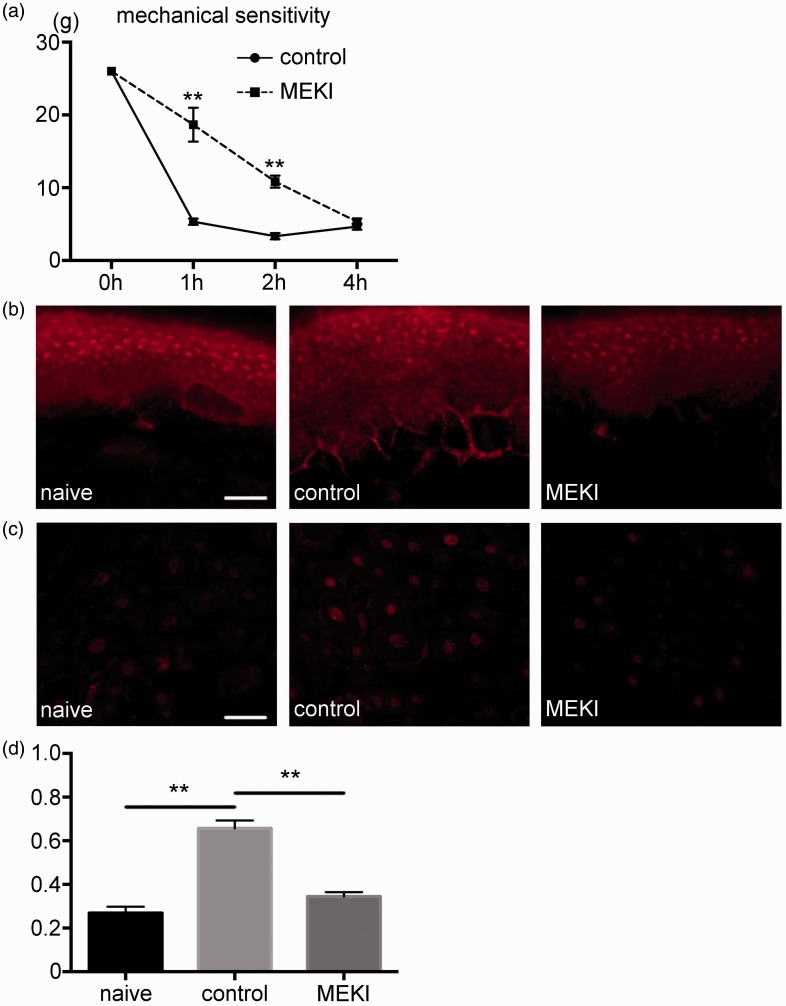
In the control group, there was a reduction in the mechanical threshold against the von Frey stimulation after the incision. Mechanical threshold in the MEK inhibitor group was significantly higher compared to the control group at 1 and 2 h after the incision (Figure 3(a)). Immunohistochemistry examination of the plantar tissue demonstrated that there was an induction of the pERK1/2 expression in the control group, whereas its expression was not detected in the MEK inhibitor group (Figure 3(b)). In the DRG, robust induction of pERK1/2 expression after the incision was suppressed in the MEK inhibitor group (Figure 3(c)). The number of pERK1/2 positive neurons was significantly smaller in MEK inhibitor group compared to the control group (Figure 3(d)).
Figure 3.
Inhibition of pERK1/2 reduced pain hypersensitivity after the plantar incision. (a) Behavioral testing results. In the control group, plantar incision induced a decline of the threshold against mechanical stimulation after the incision. The mechanical threshold was significantly higher in the MEK inhibitor group at 1 and 2 h after the incision, as compared to the control group. n=5 for each group. **: p<0.01. versus control group. (b) The effect of MEK inhibitor on pERK1/2 expression in the plantar tissue. pERK1/2 expression in the nerve endings of the plantar tissue was detected in the control group but not in the MEK inhibitor group. Scale bar = 50 µm. (c) The effect of MEK inhibitor on pERK1/2 expression in the DRG. pERK1/2 expression in the DRG reduced in the MEK inhibitor group compared to the control group. Scale bar = 50 µm. (d) The number of pERK1/2 positive neurons was significantly smaller in MEK inhibitor group compared to the control group. **: p<0.01. MEKI: MEK inhibitor; DRG: dorsal root ganglion; pERK1/2: phosphorylated ERK1/2.
Experiment 3: Effect of regional analgesia on the pERK1/2 expression
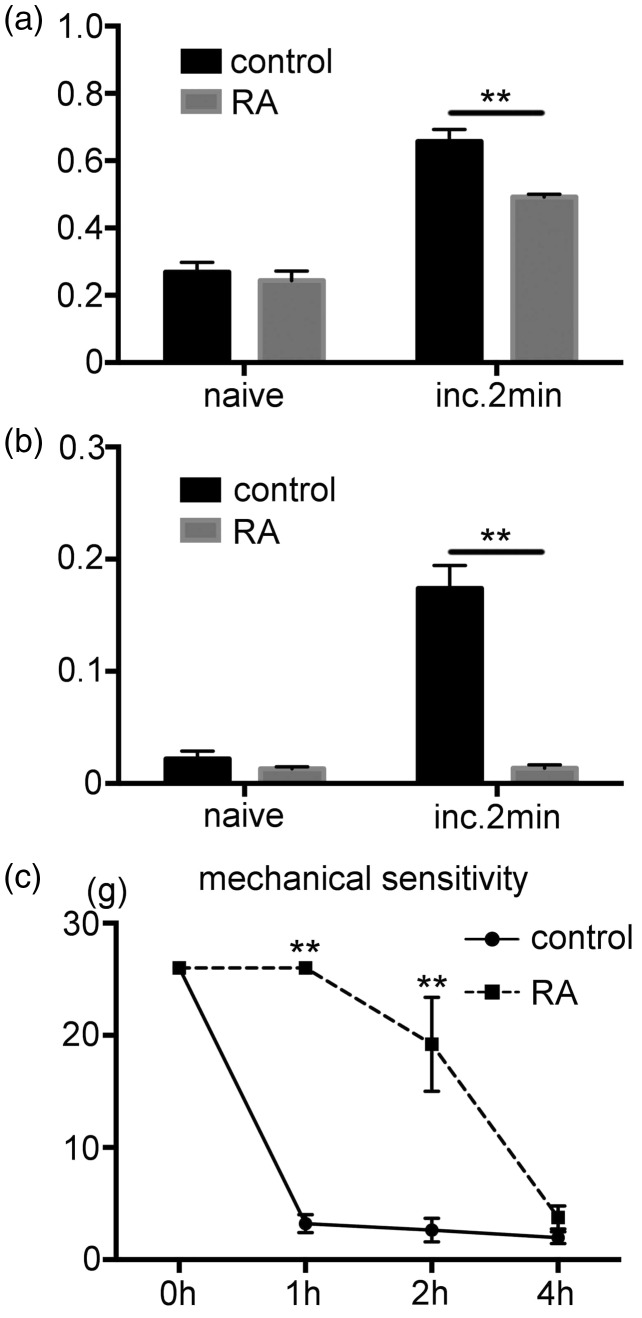
There was a significant increase in the pERK1/2 expression in the DRG neurons after the incision in the control group. This induction was suppressed by the regional analgesia. The number of pERK1/2 expressions was significantly lower in the regional analgesia group compared to the control (Figure 4(a)). Reginal analgesia also effected on the pERK1/2 expression in the SGCs. The pERK1/2 positive area within the 3PGDH positive profile was significantly lower in the regional analgesia group compared to the control group (Figure 4(b)). Results for the behavioral experiments demonstrated that the regional analgesia suppressed reduction of the withdrawal threshold against the von Frey stimulation (Figure 4(c)).
Figure 4.
Effect of regional analgesia by levobupivacaine on the expression of pERK1/2 after the incision. (a) Effect of regional analgesia on the pERK1/2 expression in the DRG neurons. There was a significant increase in the fraction of the pERK1/2 positive DRG neurons after the incision in the control group. In the regional analgesia group, pERK1/2 expression at 2 min after the incision was significantly lower than control group. n=5 for each group. ** p<0.01. (b) Effect of regional analgesia on the pERK1/2 expression in SGCs. There was a significant increase in the fraction of the pERK1/2 positive area in the 3PGDH positive area at 2 min after the incision in the control group. In the regional analgesia group, there was a significantly lower pERK1/2 expression at 2 min after the incision as compared to the control. n=5 for each group. ** p<0.01. (c) Behavioral testing demonstrates the effect of regional analgesia. The control group showed a significant decline of the mechanical threshold after the incision. There was a significantly higher mechanical threshold of the regional analgesia versus the control group at 1 and 2 h after the incision. n=5 for each group. **: p<0.01 versus the control group. RA, regional analgesia. DRG: dorsal root ganglion; SGCs: satellite glial cells; pERK1/2: phosphorylated ERK1/2.
Experiment 4: Effect of CBX on the pain behavior and pERK1/2 expression
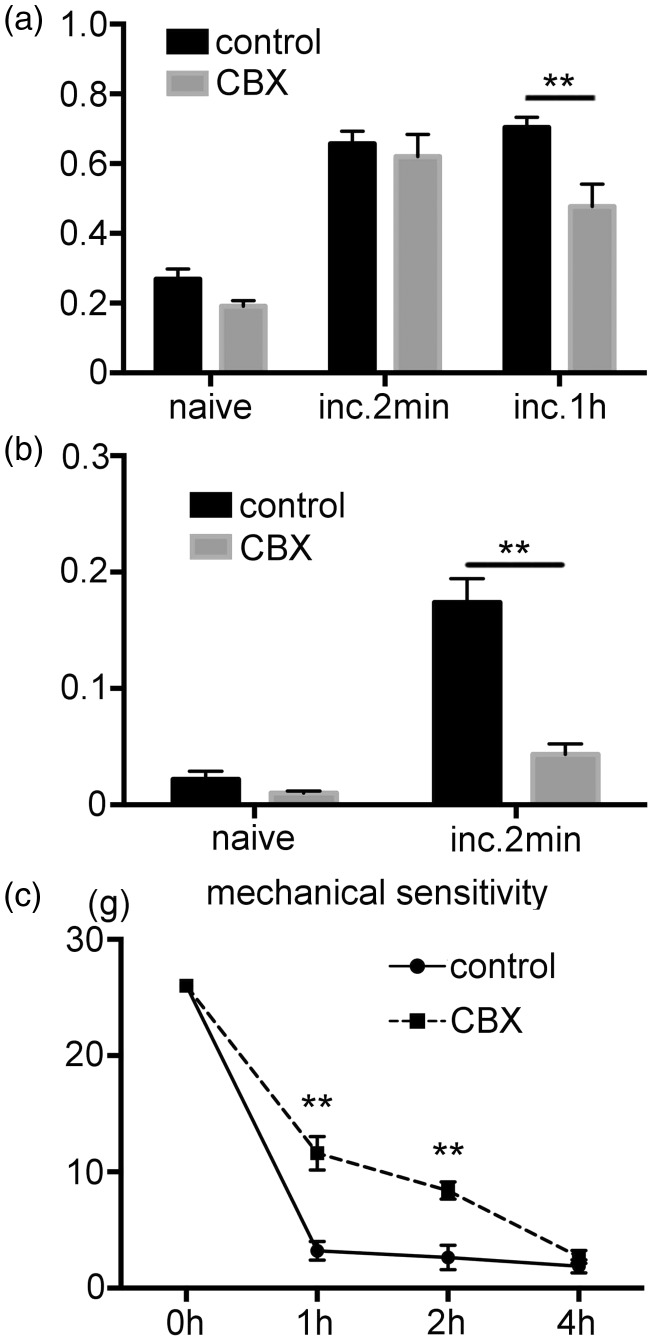
CBX treatment effected on the pERK1/2 expression in the DRG neurons and SGCs. In the DRG neurons, pERK1/2 expression 2 min after the incision was similar between control and CBX groups, whereas pERK1/2 reduced in CBX group 1 h after the incision, demonstrating rapid resolution of neuronal pERK1/2 in CBX group (Figure 5(a)). In the SGCs, the pERK1/2 positive area was significantly lower in the CBX versus the control group 2 min after the incision (Figure 5(b)). Results for the behavioral experiments demonstrated that there was a significantly higher withdrawal threshold against the von Frey stimulation in the CBX versus the control group (Figure 5(c)).
Figure 5.
The effect of CBX on the pain hypersensitivity and pERK1/2 expression after the incision. (a) Effect of the CBX treatment on the pERK1/2 expression in the DRG neurons. There was a significant increase in the fraction of the pERK1/2 positive DRG neuron at 2 min and 1 h after the incision in the control group. pERK1/2 expression in the CBX group at 2 min after the incision was similar to that observed for the control group. At 1 h after the incision, the pERK1/2 expression in the CBX group was significantly lower than the control group. n=5 for each group. ** p<0.01. (b) Effect of the CBX treatment on the pERK1/2 expression in the SGCs. In the control group, there was a significantly increased fraction of the pERK1/2 positive area in 3PGDH positive area at 2 min after the incision. In the CBX group, there was a significantly lower fraction of the pERK1/2 positive area compared to the control group at 2 min after the incision. n=5 for each group. ** p<0.01. (c) Behavioral testing demonstrates the effect of the CBX on the pain hypersensitivity after the incision. Compared to the vehicle group, the pain threshold was significantly higher in the CBX group at 1 and 2 h after the incision. n=5 for each group. **: p<0.01 versus the control group. CBX: carbenoxolone; DRG: dorsal root ganglion; SGCs: satellite glial cells; pERK1/2: phosphorylated ERK1/2.
Discussion
In the present study, we observed that (1) pERK1/2 induced in the DRG neurons and SGCs contributes to the generation of pain hypersensitivity after the tissue injury, (2) regional analgesia inhibited neuronal- and glial pERK1/2 expression, (3) CBX inhibited pERK1/2 expression in the SGCs and induces rapid resolution of neuronal pERK1/2 induction.
Visualization results for the ERK1/2 phosphorylation in the DRG showed that there were two novel characteristics of the signal transduction after the tissue injury. These included the selective activation in the A-fiber neurons and communication between the neuron–glial interaction, both of them are responsible for pain hypersensitivity immediately after the tissue injury. We identified the induction of pERK1/2 in the DRG and nerve terminals in the peripheral tissue in response to the tissue injury. The pERK1/2 increased at 2 min after the incision, which suggests that the activity-dependent phosphorylation of the ERK1/215 as a consequence of the robust activation of the primary afferent neurons during the injury.16 Inhibition of the pERK1/2 expression by regional analgesia confirms this mechanism. Phosphorylation of ERK in nerve terminal and DRG contribute to the generation of injury-induced pain hypersensitivity, since pain inhibition by the MEK inhibitor was associated with the reduction of ERK phosphorylation in nerve terminal and the DRG. The relationship between nerve ending and cell body in terms of pERK1/2 induction and their relative contribution for the generation of pain hypersensitivity should be determined in the future study. The pERK1/2 expression retuned to baseline at 2 h after the injury, and the MEK inhibitor reduced pain hypersensitivity at 1 and 2 h after the injury. These results imply that ERK1/2 phosphorylation contributes to the development, but not the maintenance of the pain hypersensitivity after tissue injury.
After the incision, pERK1/2 expression was preferentially detected within the NF-200 positive A-fiber neurons. It is unlikely that we failed to detect pERK1/2 in C-fiber neuron after the incision, based on our observation that capsaicin injection induced pERK1/2 in the TRPV1 positive C-fiber neurons. Similar distribution was observed after the sciatic nerve axotomy.17 pERK1/2 induction in A-fiber neurons after the nerve injury is associated with the nerve injury induced activation of spontaneous activity in A-fiber neurons.18 Compared to the nerve injury, less is known about the neuronal cell activation against ongoing tissue injury. Recent electrophysiological studies identified fast conducting myelinated high threshold mechanoreceptors (AHTMR)19 to be responsible for mechanical nociception.20 Our observation suggested that pERK1/2 is likely to contribute to the immediate sensitization of AHTMR after the tissue injury.21 One day after the incision, both A-fiber and C-fiber were become sensitized.22 We previously demonstrated p38MAPK phosphorylation in C-fiber neurons after the tissue injury.2 Multiple signaling cascade including p38MAPK and ERK1/2 might underlie the sensitization of primary afferent after the tissue injury.
Most of the activated glia cells were existed around the activated neurons, demonstrating direct signal transition from neuron to glia. Conduction blockade by the regional analgesia inhibited pERK1/2 induction in the SGCs confirming peripherally arising noxious signal reached to the neuron activate glia cells. Functional communication between the neuron and glia in the DRG recently becomes evident.12 Calcium ion influx via gap junctions23,24 or ATP release from the pannexin channel25,26 transmit signals from neuron to glia and facilitate coupled activation of the DRG neurons.13 As we expected, inhibition of pannexin or gap junction by the CBX reduced the glial pERK1/2 expression. In addition, CBX induced rapid resolution of the neuronal pERK1/2 and suppressed pain hypersensitivity after the incision. Activated SGCs release various kinds of substances that lead to neuronal cell activation in paracrine manner.13,27,28 Reciprocal activation of neuron and SGCs contribute to the development of the peripheral sensitization after the tissue injury.
In conclusion, ERK1/2 phosphorylation in A-fiber neurons immediately after tissue injury contributes to the generation of pain hypersensitivity after the tissue injury. Communication between the neuron and SGCs expands the duration of the ERK1/2 phosphorylation and tissue injury-induced pain hypersensitivity.
Author contributions
SY performed experiments, wrote the paper, and designed experiments; YH, HT, YM, AY, YY, and MM designed and performed experiments; TS wrote the paper; FA designed experiments and wrote the paper.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Fumimasa Amaya was supported by a grant-in-aid from the Japan Society for the Promotion of Science (JSPS, Tokyo, Japan, KAKENHI).
References
- 1.Brennan TJ. Pathophysiology of postoperative pain. Pain 2011; 152: S33–S40. DOI: S0304-3959(10)00690-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mizukoshi K Sasaki M Izumi Y Miura M Watanabe M andAmaya F.. Activation of p38 mitogen-activated protein kinase in the dorsal root ganglion contributes to pain hypersensitivity after plantar incision. Neuroscience 2013; 234: 77–87. DOI: S0306-4522(13)00003-1 [pii] [DOI] [PubMed] [Google Scholar]
- 3.Matsuda M Oh-Hashi K Yokota I Sawa T andAmaya F.. Acquired exchange protein directly activated by cyclic adenosine monophosphate activity induced by p38 mitogen-activated protein kinase in primary afferent neurons contributes to sustaining postincisional nociception. Anesthesiology 2017; 126: 150–162. DOI: 10.1097/ALN.0000000000001401. [DOI] [PubMed] [Google Scholar]
- 4.Julius D andBasbaum AI.. Molecular mechanisms of nociception. Nature 2001; 413: 203–210. DOI: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 5.Kawamata M Watanabe H Nishikawa K Takahashi T Kozuka Y Kawamata T Omote K andNamiki A.. Different mechanisms of development and maintenance of experimental incision-induced hyperalgesia in human skin. Anesthesiology 2002; 97: 550–559. [DOI] [PubMed] [Google Scholar]
- 6.Johnson GL andLapadat R.. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002; 298: 1911–1912. DOI: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 7.Zhuang ZY Gerner P Woolf CJ andJi RR.. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain 2005; 114: 149–159. DOI: 10.1016/j.pain.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 8.Dai Y Iwata K Fukuoka T Kondo E Tokunaga A Yamanaka H Tachibana T Liu Y andNoguchi K.. Phosphorylation of extracellular signal-regulated kinase in primary afferent neurons by noxious stimuli and its involvement in peripheral sensitization. J Neurosci 2002; 22: 7737–7745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Obata K andNoguchi K.. MAPK activation in nociceptive neurons and pain hypersensitivity. Life Sci 2004; 74: 2643–2653. DOI: 10.1016/j.lfs.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Ji RR Gereau RWt Malcangio M andStrichartz GR.. MAP kinase and pain. Brain Res Rev 2009; 60: 135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohara PT Vit JP Bhargava A Romero M Sundberg C Charles AC andJasmin L.. Gliopathic pain: when satellite glial cells go bad. Neuroscientist 2009; 15: 450–463. DOI: 15/5/450 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang LY Gu Y andChen Y.. Communication between neuronal somata and satellite glial cells in sensory ganglia. Glia 2013; 61: 1571–1581. DOI: 10.1002/glia.22541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim YS Anderson M Park K Zheng Q Agarwal A Gong C Saijilafu Young L He S LaVinka PC Zhou F Bergles D Hanani M Guan Y Spray DC andDong X.. Coupled activation of primary sensory neurons contributes to chronic pain. Neuron 2016; 91: 1085–1096. DOI: 10.1016/j.neuron.2016.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamakita S Matsuda M Yamaguchi Y Sawa T andAmaya F.. Dexmedetomidine prolongs levobupivacaine analgesia via inhibition of inflammation and p38 MAPK phosphorylation in rat dorsal root ganglion. Neuroscience 2017; 361: 58–68. DOI: 10.1016/j.neuroscience.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Grewal SS York RD andStork PJ.. Extracellular-signal-regulated kinase signalling in neurons. Curr Opin Neurobiol 1999; 9: 544–553. DOI: 10.1016/S0959-4388(99)00010-0. [DOI] [PubMed] [Google Scholar]
- 16.Pogatzki EM Vandermeulen EP andBrennan TJ.. Effect of plantar local anesthetic injection on dorsal horn neuron activity and pain behaviors caused by incision. Pain 2002; 97: 151–161. DOI: S0304395902000143 [pii]. [DOI] [PubMed] [Google Scholar]
- 17.Obata K Yamanaka H Dai Y Tachibana T Fukuoka T Tokunaga A Yoshikawa H andNoguchi K.. Differential activation of extracellular signal-regulated protein kinase in primary afferent neurons regulates brain-derived neurotrophic factor expression after peripheral inflammation and nerve injury. J Neurosci 2003; 23: 4117–4126. DOI: 23/10/4117 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu CN Wall PD Ben-Dor E Michaelis M Amir R andDevor M.. Tactile allodynia in the absence of C-fiber activation: altered firing properties of DRG neurons following spinal nerve injury. Pain 2000; 85: 503–521. [DOI] [PubMed] [Google Scholar]
- 19.Boada MD Houle TT Eisenach JC andRirie DG.. Differing neurophysiologic mechanosensory input from glabrous and hairy skin in juvenile rats. J Neurophysiol 2010; 104: 3568–3575. DOI: 10.1152/jn.00415.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boada MD Martin TJ Peters CM Hayashida K Harris MH Houle TT Boyden ES Eisenach JC andRirie DG.. Fast-conducting mechanoreceptors contribute to withdrawal behavior in normal and nerve injured rats. Pain 2014; 155: 2646–2655. DOI: 10.1016/j.pain.2014.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boada MD Gutierrez S Giffear K Eisenach JC andRirie DG.. Skin incision-induced receptive field responses of mechanosensitive peripheral neurons are developmentally regulated in the rat. J Neurophysiol 2012; 108: 1122–1129. DOI: 10.1152/jn.00399.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pogatzki EM Gebhart GF andBrennan TJ.. Characterization of Adelta- and C-fibers innervating the plantar rat hindpaw one day after an incision. J Neurophysiol 2002; 87: 721–731. [DOI] [PubMed] [Google Scholar]
- 23.Huang TY Belzer V andHanani M.. Gap junctions in dorsal root ganglia: possible contribution to visceral pain. Eur J Pain 2010; 14: 49 e41–e11. DOI: 10.1016/j.ejpain.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Ledda M Blum E De Palo S andHanani M.. Augmentation in gap junction-mediated cell coupling in dorsal root ganglia following sciatic nerve neuritis in the mouse. Neuroscience 2009; 164: 1538–1545. DOI: 10.1016/j.neuroscience.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 25.Hanstein R Hanani M Scemes E andSpray DC.. Glial pannexin1 contributes to tactile hypersensitivity in a mouse model of orofacial pain. Sci Rep 2016; 6: 38266. DOI: 10.1038/srep38266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y Laumet G Chen SR Hittelman WN andPan HL.. Pannexin-1 upregulation in the dorsal root ganglion contributes to neuropathic pain development. J Biol Chem 2015; 290: 14647–14655. DOI: 10.1074/jbc.M115.650218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng CF Cheng JK Chen CY Lien CC Chu D Wang SY andTsaur ML.. Mirror-image pain is mediated by nerve growth factor produced from tumor necrosis factor alpha-activated satellite glia after peripheral nerve injury. Pain 2014; 155: 906–920. DOI: 10.1016/j.pain.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 28.Ghasemlou N Chiu IM Julien JP andWoolf CJ.. CD11b+Ly6G- myeloid cells mediate mechanical inflammatory pain hypersensitivity. Proc Natl Acad Sci U S A 2015; 112: E6808–E6817. DOI: 10.1073/pnas.1501372112. [DOI] [PMC free article] [PubMed] [Google Scholar]