Abstract
Background:
Low anti-tumor necrosis factor α (TNFα) serum concentrations may result in lack of treatment response in patients with inflammatory bowel disease. We determined the anti-TNFα drug concentrations in patients with inflammatory bowel disease and investigated whether or not subtherapeutic drug concentrations were associated with increased levels of disease activity.
Methods:
In a single-center cross-sectional study, we included patients with ulcerative colitis or Crohn’s disease who were receiving infliximab or adalimumab maintenance therapy. Demographic data, disease activity symptom scores (Partial Mayo Score, Harvey Bradshaw Index), inflammatory markers [C-reactive protein (CRP), fecal calprotectin], antidrug antibodies and serum drug concentrations were recorded. Therapeutic drug concentrations were defined as 3–8 mg/liter for infliximab and 5–12 mg/liter for adalimumab.
Results:
Of 210 patients included, 137 (65.2%) had Crohn’s disease. In the adalimumab group, subtherapeutic drug concentrations were measured in 16.7% of patients with ulcerative colitis and in 27.7% of patients with Crohn’s disease. In the infliximab group, subtherapeutic drug concentrations were found in 23% (ulcerative colitis) and 30.3% (Crohn’s disease) of patients. In Crohn’s disease, subtherapeutic adalimumab concentrations were associated with higher fecal calprotectin and CRP concentrations compared with therapeutic concentrations. Subtherapeutic infliximab concentrations in patients with Crohn’s disease were also associated with higher CRP concentrations compared with therapeutic concentrations.
Conclusions:
The prevalence of subtherapeutic drug levels ranged from 17% to 30%. In patients with Crohn’s disease, subtherapeutic serum drug concentrations were associated with significantly higher disease activity with both anti-TNFα agents. These findings were not observed in patients with ulcerative colitis.
Clinicaltrials.gov identifier [NCT02134054]
Keywords: antitumor necrosis factor α, inflammatory bowel disease, serum concentrations, subtherapeutic
Introduction
Biological therapy with anti-TNF α agents provides an important treatment option for patients with moderate to severe inflammatory bowel disease (IBD),1,2 improving their quality of life3,4 and reducing intestinal inflammatory activity.5,6
A major limitation of anti-TNFα treatment is lack of or loss of response.7–9 Primary nonresponse, defined as no response after induction therapy, may affect one third of patients.10 Secondary loss of response, defined as an initial treatment response after induction therapy that is subsequently lost during maintenance therapy, has been reported in 23–46% of patients, depending on the length of follow up.11,12
There is increasing evidence that an optimal anti-TNFα treatment response requires adequate drug concentrations in the blood. Concentrations can be lowered by pharmacokinetic as well as pharmacodynamic factors, and low drug concentrations can influence treatment responses.13 Secondary loss of response may be caused by formation of antidrug antibodies (immunogenicity). Neutralizing antidrug antibodies can prevent the anti-inflammatory effects of the drug, lead to increased drug clearance and lower serum drug concentrations.12 Alternatively, high intestinal inflammatory activity, as seen in severe ulcerative colitis (UC), may lead to higher drug consumption or increased drug clearance.14 Several other pharmacokinetic factors may also influence drug concentrations, and a situation where drug concentrations are too low to maintain an adequate treatment response may arise.12,15,16
Measurements of serum drug concentration offer the possibility of an individualized and optimized dosing regimen in patients on anti-TNFα therapy during induction or post induction17,18 and maintenance therapy.10 An attenuated effect from the anti-TNFα agent due to declining drug concentrations can be corrected to avoid further loss of response.19,20
For infliximab (IFX), nondetectable drug concentrations are associated with worse outcomes for both patients with UC and Crohn’s disease (CD) such as more severe endoscopic findings of inflammation, elevated C-reactive protein (CRP) and higher colectomy rates.21,22 For patients with CD receiving adalimumab (ADA), higher drug concentrations are associated with increased remission and mucosal healing rates23,24 and a reduced risk of drug discontinuation compared with patients with lower drug concentrations.25
Although measurements of serum drug concentration seem to improve the standard of care in patients receiving anti-TNFα treatments, optimal therapeutic drug intervals for the agents IFX and ADA have not been firmly established. It is unclear if ‘subtherapeutic’ drug concentrations influence disease activity compared with patients with adequate or ‘therapeutic’ concentrations.
In this study, we assessed the prevalence of subtherapeutic drug concentrations in patients with UC or CD receiving maintenance treatment with either IFX or ADA, and also evaluated our selected lower therapeutic limit. Furthermore, we explored whether subtherapeutic drug concentrations lead to higher concentrations of inflammatory markers or increased disease activity symptom scores. We also assessed the prevalence of antidrug antibodies and evaluated their influence on drug concentrations and disease activity.
Materials and methods
Patients and study design
Patients with either UC or CD, aged 16 years and above, from a cohort of outpatients with IBD receiving long-term anti-TNFα treatment, with a minimum of three doses of either IFX or ADA, were included in a cross-sectional study at the Unit of Gastroenterology, Department of Medicine, Stavanger University Hospital, Stavanger, Norway. The hospital catchment population is 360,000. All patients with IBD in need of anti-TNFα treatment in our catchment area are allocated to Stavanger University Hospital. This study can therefore be regarded as population based. The patients were included from 1 April to 30 September 2014. Exclusion criteria were inability to provide consent and inability to adhere to the study protocol.
Study visits
This study consisted of one study visit per patient. Patients treated with IFX were included at the time of scheduled infusion. Patients treated with ADA were included in connection with their already scheduled every 3-month regular ‘follow up’.
Symptom assessment
Symptomatic disease activity in patients with UC at each study visit was assessed by the Partial Mayo Score (PMS), a nine-point noninvasive score rating stool frequency, rectal bleeding, and the physicians global assessment of disease activity.26 The Harvey-Bradshaw Index (HBI) was assessed in patients with CD and consisted of the following clinical variables; wellbeing, abdominal pain, number of loose stools, abdominal mass, and complications.27 A PMS score of at least 2 or a HBI score of at least 5 was considered as active disease.
Inflammatory markers
CRP concentrations in blood were measured at each study visit and analyzed using the CRP VARIO 6K2641 on Architect c16000TM (Abbott Diagnostics, Lake Forrest, IL, USA). Fecal calprotectin was measured in all patients from the first morning defecation ±3 days of each study visit, and analyzed using the automated EliA Calprotectin 2 enzyme fluoroimmunoassay (Phadia/Thermo-Fischer, Uppsala, Sweden). Clinical response was defined as a fecal calprotectin less than 50 mg/kg and CRP less than 5 mg/liter.
Serum drug concentrations: assays and therapeutic ranges
Serum for concentrations of IFX was drawn 1 day prior to, or on the same day just before the next infusion (‘trough’), while the serum for concentrations of ADA was drawn independent of the injection time. All samples were analyzed using in-house assays where human recombinant TNF was immobilized on the solid phase. Both IFX and ADA in patient serum samples bind TNF, and a europium-labelled tracer binds the Fc domain of IFX/ADA. Assays for serum drug concentrations are fully automated on the AutoDELFIA (PerkinElmer, Waltham, MA, USA) immunoassay platform. Therapeutic drug concentrations were defined from 3–8 mg/liter for IFX and from 5–12 mg/liter for ADA.
Antidrug antibody measurements
Antidrug antibodies to IFX and ADA were analyzed using in-house inhibition assays that only measure neutralizing antibodies (antibodies that inhibit the TNF-binding capacity of the drugs). Antidrug antibodies were not analyzed in samples with serum concentrations of IFX or ADA greater than 5 mg/liter, since high drug concentrations interfere with the assays for antidrug antibodies. Assays for antidrug antibodies are fully automated on the AutoDELFIA (PerkinElmer) immunoassay platform. Antidrug antibody concentrations were dichotomized as detectable or nondetectable.
Statistical analysis
Normality of the data was tested using the Shapiro Wilk test. As all data were non-normally distributed, medians and ranges were reported and differences between groups were analyzed with the Mann-Whitney U test or χ2 test/Fischer’s exact test. For all analyses, p less than 0.05 was considered statistically significant. Corrections for multiple analyses were not performed.
The sample size estimation was based on the following assumption: if a therapeutic versus subtherapeutic drug concentration yields a difference in disease activity (CRP) of at least 50%, from 7 to 10 mg/liter (which is regarded as clinically relevant), a sample size of at least 16 persons in each group is required with a power of 80% and a significance level of 0.05 (CRP 7 mg/liter versus 10 mg/liter, SD ±3).
All analyses were conducted using SPSS, version 23 (IBM SPSS Statistics for Macintosh, IBM Corp., Armonk, NY, USA) or GraphPad Prism, version 7 (GraphPad Software, La Jolla, CA, USA).
Ethics
The study was approved by the regional ethics committee, REK Vest (2013/554/REK Vest), and was conducted in compliance with the principles expressed in the Declaration of Helsinki. All participating patients provided written informed consent. The study was registered at ClinicalTrials.gov [identifier: NCT02134054].
Results
Patients: baseline data
Out of 218 eligible patients receiving anti-TNFα treatment with either IFX or ADA for UC or CD, 210 were included. One patient was excluded due to treatment in a satellite outpatient clinic without trained study nurses, and six declined to participate in the study. The remaining patient was regarded as not eligible for participation due to mental illness.
In total, 73 patients (34.8%) had UC and 137 (65.2%) had CD. The median time since diagnosis was not different between disease groups (p = 0.1). Treatment duration was shorter in patients with UC versus CD (p = 0.003). Significantly fewer patients with UC than with CD had previously been exposed to anti-TNFα treatment (p = 0.011). Drug concentrations had been measured in around one fifth of patients in both groups during the 6 months before inclusion (Table 1).
Table 1.
Baseline characteristics.
| Variable | UC | CD |
|---|---|---|
| Patients, No. (%) | 73 (35%) | 137 (65%) |
| Age (years) | 42 (19–69) | 37 (17–78) |
| Male sex, No. (%) | 49 (67%) | 72 (53%) |
| Time since diagnosis (years) | 9 (1–36) | 12 (0–39) |
| Duration of treatment (months) | 30 (1–98) | 46 (1–64) |
| CRP (mg/liter) | 4 (1–46) | 5 (1–45) |
| F-calprotectin (mg/kg) | 167 (20–1250) | 147 (20–1250) |
| PMS/HBI | 1 (0–9) | 3 (0–11) |
| Disease distribution, No. (%) | ||
| Rectum | 6 (8%) | |
| Left-sided colon | 27 (37%) | |
| Extensive/total colon | 40 (55%) | |
| Upper GI | 3 (2%) | |
| Ileum | 54 (39%) | |
| Colon | 26 (19%) | |
| Ileocolon | 54 (40%) | |
| Perianal | 17 (12.4%) | |
| Anti-TNFα agent, No. (%) | ||
| Adalimumab | 24 (33%) | 103 (75%) |
| Infliximab | 49 (67%) | 34 (25%) |
| Prev. biol. exp., No. (%) | 16 (21.9%) | 54 (39.4%) |
| SDC 6 months prior to incl., No. (%) | 15 (20.5%) | 26 (19%) |
| Nonstandard (high) dosing, No. (%) | ||
| Adalimumab | 2 (8.3%) | 11 (10.9%) |
| Infliximab | 30 (61.2%) | 20 (58.8%) |
| Medication, No. (%) | ||
| 5-ASA | 57 (78%) | 5 (4%) |
| Corticosteroids | 8 (11%) | 4 (3%) |
| Antibiotics | 5 (7%) | 2 (2%) |
| Immunomodulators | 6 (8%) | 17 (12%) |
Values are absolute numbers or medians (ranges).
5-ASA, 5-aminosalicylic acid; CRP; C-reactive protein; GI, gastrointestinal; HBI, Harvey Bradshaw Index; PMS, Partial Mayo Score; SDC, serum drug concentration; TNF, tumor necrosis factor.
Serum drug concentration data were missing for four patients, CRP concentration for one patient, and fecal calprotectin concentration for 21 patients. HBI data were missing for two patients and PMS for five patients.
Serum drug concentrations
Infliximab
Forty-nine (67%) patients with UC and 34 (25%) with CD were treated with IFX. The median (range) drug concentration for patients treated with IFX did not differ between the disease groups [6.9 mg/liter (0–23.3) in patients with UC versus 4.5 mg/liter (0–23.3) in patients with CD, p = 0.12] (Figure 1A). The prevalence of subtherapeutic IFX concentrations was 23% and 30.3% in patients with UC and CD, respectively (p = 0.47). Antidrug antibodies towards IFX were present in 7 (16.6%) patients with UC and 1 (3.3%) patient with CD.
Figure 1.
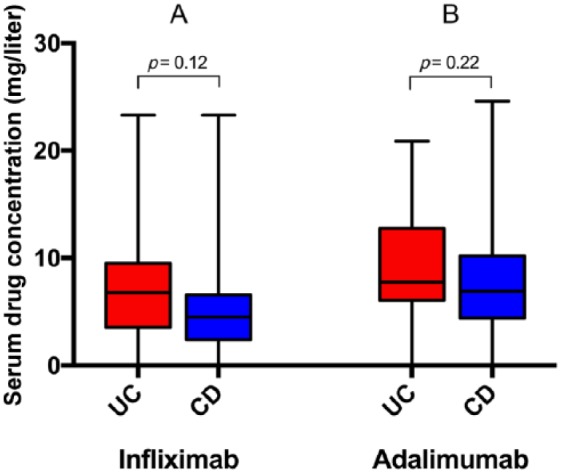
Serum drug concentrations in 48 patients with ulcerative colitis (UC) and 33 with Crohn’s disease (CD) treated with infliximab (A), and in 24 patients with UC and 101 with CD treated with adalimumab (B) and receiving at least three doses. Medians and ranges are shown.
Adalimumab
Twenty-four (33%) patients with UC and 103 (75%) with CD were treated with ADA.
The median drug concentration for ADA did not differ between the disease groups [7.8 mg/liter (0–20.9) in patients with UC versus 6.9 mg/liter (0–24.6) in patients with CD, p = 0.22] (Figure 1B). The prevalence of subtherapeutic ADA concentrations was 16.7% in patients with UC versus 27.7% in patients with CD (p = 0.22). Antidrug antibodies towards ADA were present in 0 and 7 (6.3%) patients with UC and CD, respectively.
Serum drug concentrations and inflammatory markers
Infliximab
For all patients, the median CRP concentration in the group with subtherapeutic drug levels was higher than in the group with therapeutic levels [2.8 mg/liter (1–43) versus 1.4 mg/liter (1–29), p = 0.001] (Figure 2A).
Figure 2.
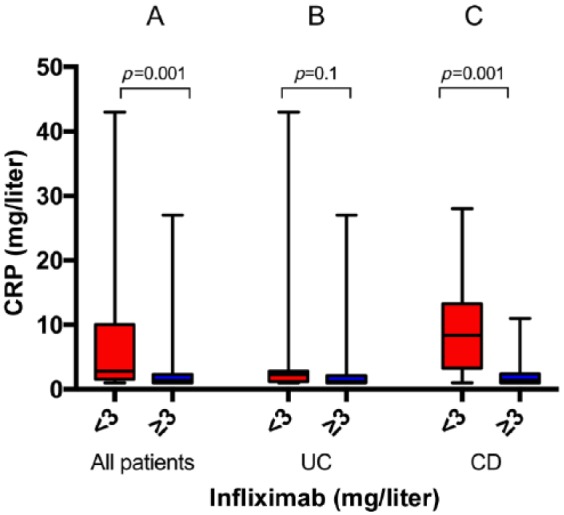
C-reactive protein (CRP) concentrations in patients with serum infliximab concentrations of less than 3 mg/liter versus at least 3 mg/liter in all patients (21 versus 60) (A), in patients with ulcerative colitis (UC) (11 versus 37) (B), and in patients with Crohn’s disease (CD) (10 versus 23) (C). Medians and ranges are shown.
This was also the case for the 33 patients with CD, where the median CRP concentration in the group with subtherapeutic drugs levels was 8.4 mg/liter (1–28) versus 1.4 mg/liter (1–11) in the group therapeutic levels (p = 0.001) (Figure 2C).
In the latter group, after excluding samples with nondetectable drug concentrations (<1.0 mg/liter, three patients), there was still a difference [8.1 mg/liter (1–28) versus 1.4 (1–11), p = 0.014].
There was no difference for patients with UC (Figure 2B).
There was no difference in median fecal calprotectin concentrations between patients with subtherapeutic versus therapeutic drug concentrations in either group [for UC 30 mg/kg (20–1250) versus 44 mg/kg (20–1158), p = 0.717 and for CD 181 mg/kg (20–1250) versus 30 mg/kg (20–554), p = 0.135].
Adalimumab
Patients with subtherapeutic drug concentrations had higher CRP concentrations than patients with therapeutic drug concentrations [4.4 mg/liter (1–38) versus 2.2 mg/liter (1–46), p = 0.008]. Similarly, in the 101 (81.5%) patients with CD in this group, the median CRP was elevated in the group with subtherapeutic versus therapeutic drug concentrations [4.7 mg/liter (1–38) versus 2.3 mg/liter (1–45), p = 0.003] (Figure 3C).
Figure 3.
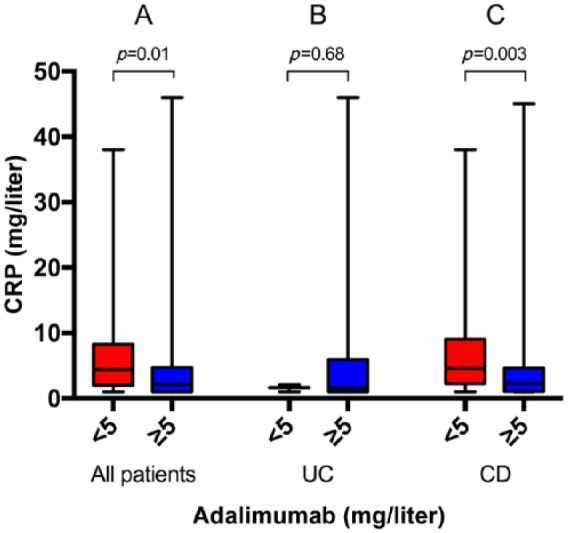
C-reactive protein (CRP) concentrations in patients with serum adalimumab concentrations of less than 5 mg/liter versus at least 5 mg/liter in all patients (31 versus 93) (A), in patients with ulcerative colitis (UC) (3 versus 20) (B), and in patients with Crohn’s disease (CD) (28 versus 73) (C). Medians and ranges are shown.
After removing samples with nondetectable drug concentrations (eight patients) in the CD group, the difference in CRP persisted [4.5 mg/liter (1–38) versus 2.3 mg/liter (1–45), p = 0.005].
There was no difference in CRP for patients with UC (Figure 3B).
Similar to CRP, the median fecal calprotectin for all patients was higher in the group with subtherapeutic drug concentrations than in the group with therapeutic drug concentrations [112 mg/kg (20–1250) versus 60 mg/kg (20–740), p = 0.014] (Figure 4A). Also, in the 91 (82.0%) patients with CD in this group, the median fecal calprotectin was increased in the group with subtherapeutic drug concentrations compared with the group with therapeutic levels [170 mg/kg (20–1250) versus 60 mg/kg (20–509), p = 0.004] (Figure 4C).
Figure 4.
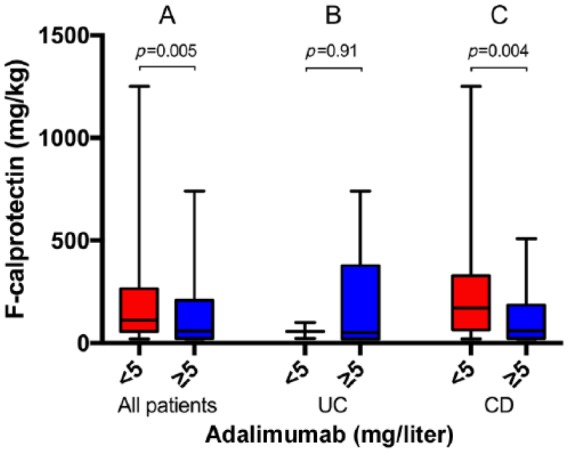
Fecal calprotectin concentrations in patients with serum adalimumab concentrations of less than 5 mg/liter versus at least 5 mg/liter in all patients (25 versus 86) (A), in patients with ulcerative colitis (UC) (3 versus 17) (B), and in patients with Crohn’s disease (CD) (22 versus 69) (C). Medians and ranges are shown.
This finding was still significant after removing samples with nondetectable drug concentrations (six patients) [181 mg/kg (20–1250) versus 60 mg/kg (20–509), p = 0.009].
Similar to CRP, there was no significant difference in fecal calprotectin in the UC group, 57 mg/kg (23–100) versus 50 mg/kg (20–740), p = 0.91 (Figure 4B).
Disease activity indexes
Based on PMS and HBI, 80.6% of patients with UC and 78.0% of patients with CD were in clinical remission.
There were no differences in disease activity symptom scores (HBI, PMS) between the groups with subtherapeutic and therapeutic drug concentrations in either of the drug treatment or disease groups.
Antidrug antibodies
Infliximab
Median serum drug concentration was significantly lower in patients in the antidrug antibody-positive group (eight patients, 9.9%) versus the antibody negative group [0 mg/liter (0–2.3) versus 6.4 mg/liter (0–23.3), p < 0.0001]. Only one patient in the antidrug antibody-positive group had detectable serum drug concentrations.
Adalimumab
None of the 7 (5.6%) patients who were antidrug antibody positive had detectable serum drug concentrations, and the median serum drug concentration was significantly lower in this group compared with patients in the antidrug antibody-negative group [0 mg/liter versus 7.2 mg/liter (0–24.6), p < 0.0001].
CRP and fecal calprotectin
No significant differences in median CRP or fecal calprotectin concentrations were observed except for in patients with UC treated with IFX. In this subgroup, the median CRP concentration was increased in the antidrug antibody-positive versus antibody-negative group [2.6 mg/liter (1.2–43.0) versus 1.2 mg/liter (1.0–27.0), p = 0.028] (Figure 5B).
Figure 5.
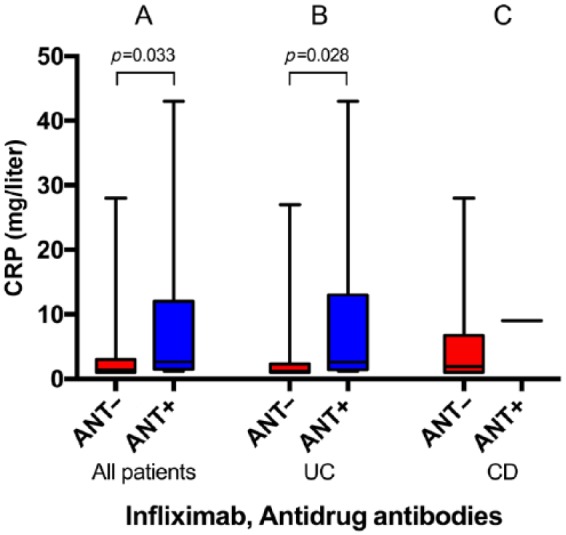
C-reactive protein (CRP) concentrations in patients with negative versus positive antidrug antibodies against infliximab in all patients (73 versus 8) (A), in patients with ulcerative colitis (UC) (41 versus 7) (B), and in patients with Crohn’s disease (CD) (32 versus 1) (C). Medians and ranges are shown.
Concomitant immunomodulator therapy, nonstandard (high) dosing and prior drug concentration measurements
There were no differences in IFX or ADA concentration, CRP or fecal calprotectin on concomitant immunomodulator therapy. In addition, high-dose anti-TNFα adjustments (IFX >5 mg/kg or <8-week interval, ADA >40 mg or <2-week interval) or drug concentration measurements prior to inclusion did not influence the results.
Subtherapeutic drug concentrations and loss of treatment response
In total, 53 (25.7%) patients had subtherapeutic drug levels. In this subgroup, 39 (73.6%) had a loss of clinical treatment response, whereas 14 (26.4%) had no loss of response, constituting 6.8% of all patients in the study.
Discussion
In this cross-sectional study of outpatients receiving maintenance treatment with IFX or ADA, the main findings were that 17–30% of patients had subtherapeutic serum drug concentrations. In patients with CD, but not in those with UC, subtherapeutic drug concentrations were associated with increased inflammatory activity as indicated by increased CRP and fecal calprotectin concentrations. Antidrug antibodies were present almost three times more frequently in patients with UC than in patients with CD, and CRP concentrations were significantly elevated in patients with UC who had antidrug antibodies towards IFX. Moreover, the presence of antidrug antibodies was associated with lower serum drug concentrations for both anti-TNFα agents.
The drug-concentration measurements were performed in patients who had been on long-term maintenance therapy and who had received anti-TNFα treatment for a median of 30 and 46 months, and had considerable disease duration. The participants were thus expected to be long-term drug responders, with adequate therapeutic drug concentrations. Nevertheless, we revealed that roughly one out of four patients had subtherapeutic serum drug concentrations. This is in line with findings from previous studies.28–30
Nondetectable drug concentrations of IFX have been associated with increased inflammation as assessed by endoscopy and CRP concentrations, and these patients experience higher surgery rates compared with those with detectable drug concentrations.21,22 In addition, patients with high ADA serum drug concentrations have been linked to less risk of drug discontinuation than those with lower serum drug concentrations.25 In the current study, this association was further explored by excluding cases with nondetectable drug concentrations. Interestingly, we found that subtherapeutic but measurable drug concentrations were still associated with higher inflammatory activity in patients with CD receiving either of the anti-TNFα agents. Elevated CRP has previously been associated with low28,31,32 or nondetectable drug concentrations.22 Also, fecal calprotectin concentrations were increased in patients with lower IFX drug concentrations.33,34 Our results indicate that for patients with CD, even those with serum drug concentrations that were only slightly insufficient, there is still a need for treatment optimization or dose increases. These findings also suggest that serum drug measurements are useful even in long-term responders receiving these agents.35 Increased concentrations of inflammatory markers linked to lower drug concentrations were detected even when one in five patients had measured anti-TNFα drug concentrations during the previous 6 months before the study, and may have had their drug dose adjusted.
The concept of a ‘therapeutic window’ of drug concentrations has been increasingly acknowledged in recent years.36 However, the exact upper and lower limits are not yet confirmed. This may partly be due to differences in methods and analyses used. Although the results from different assays are comparable, systematic differences have been demonstrated that may lead to differences in selected therapy ranges.37 Based on clinical experience using the current drug assay, the therapeutic range was pragmatically defined as 3–8 mg/liter for IFX and 5–12 mg/liter for ADA. As the chosen lower cutoff concentrations demonstrated significant differences in inflammatory disease activity, these limits seem useful for future studies using our method of analyzing drug concentrations. Less is known about the influence of supratherapeutic concentrations on disease activity. A previous study indicated that reducing the IFX dose above a chosen upper cutoff of 7 mg/liter did not affect patients’ outcomes after 1 year of follow up.29 However, this was not the aim of our study and was not investigated further.
Formation of antidrug antibodies has been linked to decreased serum drug concentrations and higher rates of loss of response38,39 and surgery.40 In our study, the prevalence of detectable antidrug antibodies was low. This could be expected because the study population consisted of long-term treatment responders, possibly leading to a selection of patients not developing antidrug antibodies. In line with previous studies, the formation of antidrug antibodies was associated with lower serum drug concentrations, both for IFX and ADA.31,40 This is an expected finding, since we only measured antidrug antibodies in samples with drug concentrations less than 5 mg/liter. However, as the assay is drug sensitive and only measures neutralizing antibodies, we would probably not have seen more antidrug antibodies even if samples with higher drug concentration had been included. Also, in patients with UC treated with IFX, detection of antidrug antibodies was associated with increased CRP concentrations. This is supported by previous findings showing that antidrug antibodies towards anti-TNFα-inhibitors were associated with a shorter duration of response and increased mucosal inflammation.41
There was a striking difference in findings for patients with CD as opposed to those with UC in our study. In patients with UC, there were no associations between drug concentrations and inflammatory activity. This may indicate that in the UC cohort, a subgroup of patients were in stable, probably long-term remission. Because about 80% of the patients with UC in our study were in clinical remission (based on PMS), their noninflammatory state may not depend on sufficient anti-TNFα serum drug concentrations. Consequently, the subgroup of study patients in remission (based on symptoms, CRP, and fecal calprotectin) and demonstrating subtherapeutic drug concentrations could be suitable for anti-TNFα treatment discontinuation. In fact, successful anti-TNFα drug withdrawal was shown in patients with long-term remission who had nondetectable drug concentrations.41 Our findings therefore support a deescalate or stop treatment strategy that has been proposed in patients with UC who have a durable remission.42
There was no association between clinical symptoms and serum drug concentrations in our study. This could be due to the study population or study design, with a large fraction of patients being in remission. However, objective disease activity markers showed differences, suggesting that symptom scoring may not be sufficiently sensitive in this setting of patients mostly in remission.
There are limitations to this study. First, the cross-sectional study design represents a limitation. We did not perform a colonoscopy on the study participants to assess an endoscopic grade of inflammation. As a result, we cannot present the rate of true mucosal healing in the cohort. Second, some of the subgroups in the study were small, limiting the statistical power of the analyses. Third, about one fifth of the patients had measured serum drug concentrations within the previous 6 months; thus, therapy may have been optimized before the study and this may have influenced the results. Serum ADA concentrations were not measured as trough, but randomly during the treatment interval as suggested by a previous trial.43 However, it is possible that ‘strict’ trough measurements of ADA shortly before the next dosage could have affected the results in our study. Finally, we did not explore other therapeutic ranges using our method of drug concentration analysis.
A strength of the study is that it represents an unselected population-based cohort of IBD patients treated with IFX and ADA.
In conclusion, in patients with CD on ADA or IFX maintenance therapy, subtherapeutic serum drug concentrations are associated with increased signs of inflammatory activity. Routine serum drug concentration measurements should be performed even in patients in long-term remission because about one in four may have insufficient drug concentrations requiring clinical decision making. In patients with UC on long-term maintenance therapy who are in remission, nondetectable drug concentrations may indicate that anti-TNFα therapy could be discontinued.
Supplemental Material
Supplemental material, Supplementary_Material for Subtherapeutic concentrations of infliximab and adalimumab are associated with increased disease activity in Crohn’s disease by Arne Carlsen, Roald Omdal, Kristian Øgreid Leitao, Kjetil Isaksen, Anne Kristine Hetta, Lars Normann Karlsen, Lars Aabakken, Nils Bolstad, David Warren, Knut E.A. Lundin and Tore Grimstad in Therapeutic Advances in Gastroenterology
Acknowledgments
We thank study nurse Merethe Lie Seglem, Head Nurse Inger Johanne Bø and all the nurses and doctors of the IBD group at the Unit of Gastroenterology, Department of Medicine, Stavanger University Hospital, for their efforts during study visits. Guarantor of the article: Arne Carlsen; study concept and design: Arne Carlsen, Tore Grimstad, Roald Omdal, Lars Normann Karlsen, and Lars Aabakken; acquisition of data: all authors; analysis and interpretation: Arne Carlsen, Tore Grimstad, and Roald Omdal; drafting of the manuscript: all authors; remission and approval of final manuscript: all authors.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: Arne Carlsen has served as speaker for Takeda AS and Tillotts Pharma AB. Knut Lundin has served as speaker for Takeda AS and MSD. Tore Grimstad has served as speaker for Ferring Pharmaceuticals, served as consultant for Takeda AS and Janssen, and has received unrestricted research grants from AbbVie AS and Tillotts Pharma AB. All other authors declare that there is no conflict of interests.
Supplementary Material: Supplementary material is available for this article online.
ORCID iD: Arne Carlsen  https://orcid.org/0000-0002-6005-6394
https://orcid.org/0000-0002-6005-6394
Contributor Information
Arne Carlsen, Stavanger University Hospital, PO Box 8100, 4068 Stavanger, Norway.
Roald Omdal, Unit of Clinical Immunology, Department of Medicine, Stavanger University Hospital, Stavanger, Norway.
Kristian Øgreid Leitao, Unit of Gastroenterology, Department of Medicine, Stavanger University Hospital, Stavanger, Norway.
Kjetil Isaksen, Unit of Gastroenterology, Department of Medicine, Stavanger University Hospital, Stavanger, Norway.
Anne Kristine Hetta, Unit of Gastroenterology, Department of Medicine, Stavanger University Hospital, Stavanger, Norway.
Lars Normann Karlsen, Unit of Gastroenterology, Department of Medicine, Stavanger University Hospital, Stavanger, Norway.
Lars Aabakken, Department of Gastroenterology, Oslo University Hospital, Oslo, Norway.
Nils Bolstad, Department of Medical Biochemistry, Oslo University Hospital, Oslo, Norway.
David Warren, Department of Medical Biochemistry, Oslo University Hospital, Oslo, Norway.
Knut E.A. Lundin, Department of Gastroenterology, Oslo University Hospital, Oslo, Norway
Tore Grimstad, Unit of Gastroenterology, Department of Medicine, Stavanger University Hospital, Stavanger, Norway.
References
- 1. Dignass A, Van Assche G, Lindsay JO, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: current management. J Crohns Colitis 2010; 4: 28–62. [DOI] [PubMed] [Google Scholar]
- 2. Dignass A, Lindsay JO, Sturm A, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis 2012; 6: 991–1030. [DOI] [PubMed] [Google Scholar]
- 3. Feagan BG, Reinisch W, Rutgeerts P, et al. The effects of infliximab therapy on health-related quality of life in ulcerative colitis patients. Am J Gastroenterol 2007; 102: 794–802. [DOI] [PubMed] [Google Scholar]
- 4. Louis E, Lofberg R, Reinisch W, et al. Adalimumab improves patient-reported outcomes and reduces indirect costs in patients with moderate to severe Crohn’s disease: results from the CARE trial. J Crohns Colitis 2013; 7: 34–43. [DOI] [PubMed] [Google Scholar]
- 5. Cholapranee A, Hazlewood GS, Kaplan GG, et al. Systematic review with meta-analysis: comparative efficacy of biologics for induction and maintenance of mucosal healing in Crohn’s disease and ulcerative colitis controlled trials. Aliment Pharmacol Ther 2017; 45: 1291–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005; 353: 2462–2476. [DOI] [PubMed] [Google Scholar]
- 7. Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology 2007; 132: 52–65. [DOI] [PubMed] [Google Scholar]
- 8. Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet 2002; 359: 1541–1549. [DOI] [PubMed] [Google Scholar]
- 9. Molnar T, Farkas K, Nyari T, et al. Frequency and predictors of loss of response to infliximab or adalimumab in Crohn’s disease after one-year treatment period: a single center experience. J Gastrointestin Liver Dis 2012; 21: 265–269. [PubMed] [Google Scholar]
- 10. Steenholdt C, Bendtzen K, Brynskov J, et al. Optimizing treatment with TNF inhibitors in inflammatory bowel disease by monitoring drug levels and antidrug antibodies. Inflamm Bowel Dis 2016; 22: 1999–2015. [DOI] [PubMed] [Google Scholar]
- 11. Roda G, Jharap B, Neeraj N, et al. Loss of response to anti-TNFs: definition, epidemiology, and management. Clin Transl Gastroenterol 2016; 7: e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yanai H, Hanauer SB. Assessing response and loss of response to biological therapies in IBD. Am J Gastroenterol 2011; 106: 685–698. [DOI] [PubMed] [Google Scholar]
- 13. Tracey D, Klareskog L, Sasso EH, et al. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther 2008; 117: 244–279. [DOI] [PubMed] [Google Scholar]
- 14. Brandse JF, van den Brink GR, Wildenberg ME, et al. Loss of infliximab into feces is associated with lack of response to therapy in patients with severe ulcerative colitis. Gastroenterology 2015; 149: 350–355e352. [DOI] [PubMed] [Google Scholar]
- 15. Brandse JF, Mould D, Smeekes O, et al. A real-life population pharmacokinetic study reveals factors associated with clearance and immunogenicity of infliximab in inflammatory bowel disease. Inflamm Bowel Dis 2017; 23: 650–660. [DOI] [PubMed] [Google Scholar]
- 16. Dotan I, Ron Y, Yanai H, et al. Patient factors that increase infliximab clearance and shorten half-life in inflammatory bowel disease: a population pharmacokinetic study. Inflamm Bowel Dis 2014; 20: 2247–2259. [DOI] [PubMed] [Google Scholar]
- 17. Papamichael K, Van Stappen T, Vande Casteele N, et al. Infliximab concentration thresholds during induction therapy are associated with short-term mucosal healing in patients with ulcerative colitis. Clin Gastroenterol Hepatol 2016; 14: 543–549. [DOI] [PubMed] [Google Scholar]
- 18. Papamichael K, Baert F, Tops S, et al. Post-induction adalimumab concentration is associated with short-term mucosal healing in patients with ulcerative colitis. J Crohns Colitis 2017; 11: 53–59. [DOI] [PubMed] [Google Scholar]
- 19. Duveau N, Nachury M, Gerard R, et al. Adalimumab dose escalation is effective and well tolerated in Crohn’s disease patients with secondary loss of response to adalimumab. Dig Liver Dis 2017; 49: 163–169. [DOI] [PubMed] [Google Scholar]
- 20. Taxonera C, Iglesias E, Munoz F, et al. Adalimumab maintenance treatment in ulcerative colitis: outcomes by prior anti-TNF use and efficacy of dose escalation. Dig Dis Sci 2017; 62: 81–490. [DOI] [PubMed] [Google Scholar]
- 21. Seow CH, Newman A, Irwin SP, et al. Trough serum infliximab: a predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut 2010; 59: 49–54. [DOI] [PubMed] [Google Scholar]
- 22. Maser EA, Villela R, Silverberg MS, et al. Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn’s disease. Clin Gastroenterol Hepatol 2006; 4: 1248–1254. [DOI] [PubMed] [Google Scholar]
- 23. Zittan E, Kabakchiev B, Milgrom R, et al. Higher adalimumab drug levels are associated with mucosal healing in patients with Crohn’s disease. J Crohns Colitis 2016; 10: 510–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yarur AJ, Jain A, Hauenstein SI, et al. Higher adalimumab levels are associated with histologic and endoscopic remission in patients with Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis 2016; 22: 409–415. [DOI] [PubMed] [Google Scholar]
- 25. Karmiris K, Paintaud G, Noman M, et al. Influence of trough serum levels and immunogenicity on long-term outcome of adalimumab therapy in Crohn’s disease. Gastroenterology 2009; 137: 1628–1640. [DOI] [PubMed] [Google Scholar]
- 26. Lewis JD, Chuai S, Nessel L, et al. Use of the noninvasive components of the mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis 2008; 14: 1660–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet 1980; 315: 514. [DOI] [PubMed] [Google Scholar]
- 28. Vande Casteele N, Khanna R, Levesque BG, et al. The relationship between infliximab concentrations, antibodies to infliximab and disease activity in Crohn’s disease. Gut 2015; 64: 1539–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology 2015; 148: 1320–1329.e1323. [DOI] [PubMed] [Google Scholar]
- 30. Vaughn BP, Martinez-Vazquez M, Patwardhan VR, et al. Proactive therapeutic concentration monitoring of infliximab may improve outcomes for patients with inflammatory bowel disease: results from a pilot observational study. Inflamm Bowel Dis 2014; 20: 1996–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Imaeda H, Takahashi K, Fujimoto T, et al. Clinical utility of newly developed immunoassays for serum concentrations of adalimumab and anti-adalimumab antibodies in patients with Crohn’s disease. J Gastroenterol 2014; 49: 100–109. [DOI] [PubMed] [Google Scholar]
- 32. Mazor Y, Almog R, Kopylov U, et al. Adalimumab drug and antibody levels as predictors of clinical and laboratory response in patients with Crohn’s disease. Aliment Pharmacol Ther 2014; 40: 620–628. [DOI] [PubMed] [Google Scholar]
- 33. Imaeda H, Bamba S, Takahashi K, et al. Relationship between serum infliximab trough levels and endoscopic activities in patients with Crohn’s disease under scheduled maintenance treatment. J Gastroenterol 2014; 49: 674–682. [DOI] [PubMed] [Google Scholar]
- 34. Marits P, Landucci L, Sundin U, et al. Trough s-infliximab and antibodies towards infliximab in a cohort of 79 IBD patients with maintenance infliximab treatment. J Crohns Colitis 2014; 8: 881–889. [DOI] [PubMed] [Google Scholar]
- 35. Papamichael K, Chachu KA, Vajravelu R, et al. Improved long-term outcomes of patients with inflammatory bowel disease receiving proactive compared with reactive monitoring of serum concentrations of infliximab. Clin Gastroenterol Hepatol 2017; 15: 1580–1588.e1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ungar B, Levy I, Yavne Y, et al. Optimizing anti-TNF-alpha therapy: serum levels of infliximab and adalimumab are associated with mucosal healing in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2016; 14: 550–557.e552. [DOI] [PubMed] [Google Scholar]
- 37. Steenholdt C, Ainsworth MA, Tovey M, et al. Comparison of techniques for monitoring infliximab and antibodies against infliximab in Crohn’s disease. Ther Drug Monit 2013; 35: 530–538. [DOI] [PubMed] [Google Scholar]
- 38. Nanda KS, Cheifetz AS, Moss AC. Impact of antibodies to infliximab on clinical outcomes and serum infliximab levels in patients with inflammatory bowel disease (IBD): a meta-analysis. Am J Gastroenterol 2013; 108: 40–47; quiz 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Roblin X, Rinaudo M, Del Tedesco E, et al. Development of an algorithm incorporating pharmacokinetics of adalimumab in inflammatory bowel diseases. Am J Gastroenterol 2014; 109: 1250–1256. [DOI] [PubMed] [Google Scholar]
- 40. Zitomersky NL, Atkinson BJ, Fournier K, et al. Antibodies to infliximab are associated with lower infliximab levels and increased likelihood of surgery in pediatric IBD. Inflamm Bowel Dis 2015; 21: 307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yarur AJ, Deshpande AR, Sussman DA, et al. Tu1147 serum adalimumab levels and antibodies correlate with endoscopic intestinal inflammation and inflammatory markers in patients with inflammatory bowel disease. Gastroenterology 2013; 144: S774–S775. [Google Scholar]
- 42. Ben-Horin S, Chowers Y, Ungar B, et al. Undetectable anti-TNF drug levels in patients with long-term remission predict successful drug withdrawal. Aliment Pharmacol Ther 2015; 42: 356–364. [DOI] [PubMed] [Google Scholar]
- 43. Ungar B, Yavzori M, Picard O, et al. P526 Sampling not at trough: adalimumab serum drug and antibody levels remain relatively stable in between injections. ECCO 2016 (Poster Presentations: Clinical: Therapy & observation (2016)), 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Material for Subtherapeutic concentrations of infliximab and adalimumab are associated with increased disease activity in Crohn’s disease by Arne Carlsen, Roald Omdal, Kristian Øgreid Leitao, Kjetil Isaksen, Anne Kristine Hetta, Lars Normann Karlsen, Lars Aabakken, Nils Bolstad, David Warren, Knut E.A. Lundin and Tore Grimstad in Therapeutic Advances in Gastroenterology


