Abstract
Multiple sclerosis (MS) is an autoimmune disease of the central nervous system, in which myelin and oligodendrocytes are the main targets recognized by inflammatory CD4+ T cells reactive to myelin peptides. Regulatory CD4+ T (Treg) cells normally keep homeostasis of the immune system by inhibiting detrimental effects of inflammatory T cells. However, Treg cells are reduced in patients with MS for unknown reason. This commentary highlights a novel function of circulating exosomes to inhibit the differentiation of Treg cells in MS. Our recent work has demonstrated that the circulating exosomes, a member of extracellular vesicles, of patients with MS exert this effect by transferring let-7i to naive CD4+ T cells. The transferred let-7i subsequently causes a decreased expression of insulin like growth factor 1 receptor (IGF1R) and transforming growth factor β receptor 1 (TGFBR1), leading to the inhibition of Treg cell differentiation. Thus, extrinsic microRNAs transferred by exosomes might have an active role in triggering autoimmune diseases. We hypothesize that extracellular vesicles including exosomes can be a communication tool between the gut microbiota and the host immune system. Further research in this area will expand the knowledge about the precise mechanism of autoimmune diseases and can lead to a new therapeutic approach.
Keywords: Multiple sclerosis, exosome, miRNA, let-7i, Treg cell
Comment on: Kimura K, Hohjoh H, Fukuoka M, Sato W, Oki S, Tomi C, Yamaguchi H, Kondo T, Takahashi R, Yamamura T. Circulating exosomes suppress the induction of regulatory T cells via let-7i in multiple sclerosis. Nat Commun. 2018 Jan 2;9(1):17. doi: 10.1038/s41467-017-02406-2. PubMed PMID: 29295981; PubMed Central PMCID: PMC5750223.
Multiple sclerosis (MS) is an autoimmune disease, which typically affects young adults at around 30 years of age.1 Accumulating neurologic deficits are a major problem in MS, which makes normal daily living difficult in the late stage. More precise understanding of the pathogenesis is needed for the development of a new therapeutic approach. Inflammatory CD4+ T cells, such as T helper 1 (TH1) and TH17 cells, have a pivotal function in MS pathogenesis.1 However, regulatory CD4+ T (Treg) cells intrinsically control the excessive activity of these inflammatory cells and thus suppress the development of autoimmune diseases.2,3 It has been recognized that Treg cells are decreased in frequency and functionally impaired in patients with MS, although the mechanism is still unclear.4,5
Recently, we found a novel pathogenic mechanism for MS, involving the exosomes in the plasma.6 Exosomes are extracellular vesicles smaller than 150 nm in diameter.7 They are secreted by various kinds of cells, delivered throughout the body in the circulation, and then taken up by target cells. Exosomes contain microRNAs (miRNA), proteins, and lipids, which could be delivered to target cells. Among these exosomal contents, miRNAs are unique in that they directly regulate translation of messenger RNAs (mRNA). MicroRNAs are small noncoding RNAs, which are involved in posttranscriptional regulation of gene expression. The critical role of miRNAs in autoimmunity has been formally proven in a number of studies. Dicer1 and Drosha sequentially process primary miRNAs (pri-miRNA) into mature miRNAs, and Dicer1−/− and Drosha−/− mice develop spontaneous autoimmunity.8 The differentiation of TH17 cells is promoted by miR-21, and deletion of this miRNA results in resistance to experimental autoimmune encephalomyelitis (EAE) in mice, a model of MS.9 Mir-183-96-182 cluster enhances pathogenicity of TH17 cells.10 The suppressive activity of Treg cells is impaired in the presence of interleukin (IL)-6. This phenomenon was found to be at least partially mediated by miR-17 induced by IL-6.11 Moreover, miRNA-containing exosomes secreted by Treg cells contribute to their suppressive function on proliferation and cytokine secretion of TH1 cells.12
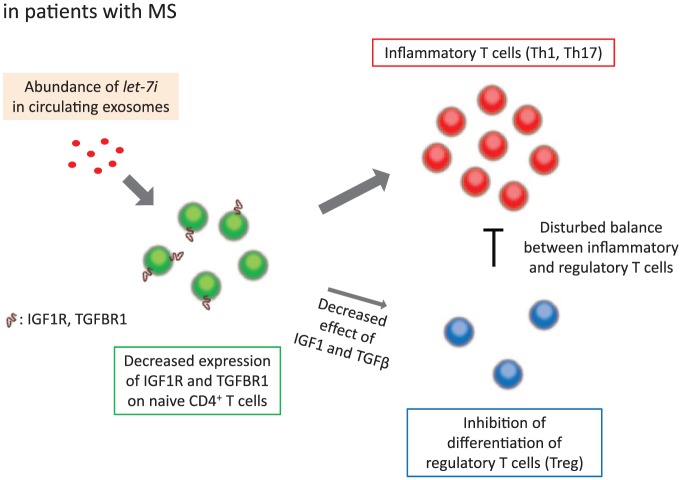
Expression profile of miRNAs is known to be altered in immune cells, brain, blood, and cerebrospinal fluid in patients with MS, as compared with healthy controls.13 Several studies reported that some miRNAs in the circulation could be used as markers for diagnosis or for evaluation of disease stage and severity in MS.14 Among miRNAs detected in the circulation, exosomal miRNAs are especially important and should be differentiated from others because they could function in target cells. In the field of cancer, for example, extracellular vesicles secreted from breast cancer cells reportedly trigger the destruction of blood-brain barrier via miR-181c and promote brain metastasis.15 We and others reported that the expression profile of miRNAs in the circulating exosomes is dysregulated in patients with MS.6,16,17 Furthermore, we clarified that the differentially expressed miRNAs are involved in the disease pathogenesis.6 In our own research, we first found that the frequency of Treg cells (IFN-γ−IL-17A−Foxp3+CD4+ T cells) among CD4+ T cells was lower after culture in the presence of exosomes from patients with MS (MS-exo) compared with those from healthy controls (HC-exo). Transfection of let-7i, one of the upregulated miRNAs in MS-exo, also decreased Treg cell frequency, and the effect of MS-exo disappeared when T cells were treated with let-7i inhibitor prior to culture. Further analysis revealed that let-7i decreased the expression of insulin like growth factor 1 receptor (IGF1R) and transforming growth factor β receptor 1 (TGFBR1) on naive CD4+ T cells and then inhibited their differentiation into Treg cells. The expression of these receptors was decreased on circulating naive CD4+ T cells in patients with MS and positively correlated with the frequency of Treg cells in the blood. Moreover, the frequency of Treg cells was lower in the group with a higher amount of exosomal let-7i. Collectively, these results indicate that circulating exosomes have a key role in homeostasis of Treg cells via let-7i-IGF1R/TGFBR1 axis and contribute to the pathogenesis of MS (Figure 1).6
Figure 1.
Circulating exosomes inhibit the differentiation of Treg cells in MS. Let-7i is upregulated in exosomes from patients with MS and in turn decreases the expression of IGF1R and TGFBR1 on naive CD4+ T cells. This results in lower frequency of Treg cells in MS. IGF1R indicates insulin like growth factor 1 receptor; TGFBR1, transforming growth factor β receptor 1; Treg cells, regulatory T cells.
Several studies experimentally proved pathogenic function for some cell-intrinsic miRNAs that were altered in patients with MS.13 Although whole circulating miRNAs and exosomal miRNAs had been reported as biomarkers, the possible pathogenic function of these extracellular miRNAs had not been studied before our study. Target prediction and pathway analysis are frequently used to assess the function of miRNAs. However, the profile of expressed genes is totally different depending on types of cells, and consequently the gene set affected by a certain miRNA is also different. Therefore, the actual function can be known only by experiments manipulating the amount of miRNAs in the cells of interest. Regarding let-7i, which was found to be upregulated in circulating exosomes in MS,6 it could affect dendritic cells and indirectly decrease Treg cells.18 Let-7 family could also promote differentiation of IFN-γ–producing NKT1 cells19 and inhibit production of IL-10 by T cells.20 Further studies are needed to verify these possibilities.
Although a critical function of exosomes has been shown in various diseases, there remain several major problems. The precise origin and target of exosomes are key determinants of their function in vivo. However, it is difficult to address this question once exosomes are released in the circulation. Interestingly, miRNAs with specific sequence motifs are likely to be sorted into exosomes,21 which results in differential miRNA profiles between the parent cells and the secreted exosomes. Therefore, the parent cells cannot be suggested simply by similarity of their miRNA profile with that of exosomes. As for targets of exosomes, the type of integrins on exosomes from metastatic tumor cells are involved in organ tropism of parent tumor cells.22 However, several pathways other than integrin interaction are assumed, and there is no efficient way to detect target cells of exosomes. Some genetic modification approaches are now being used to detect intercellular transfer of RNAs and proteins.23 For example, transgenic mice, which express CRE recombinase in the parent cells and have a reporter gene with floxed STOP codon in the cells of interest, can be used to search for extracellular vesicle–mediated transfer in vivo.23 However, such approaches are not easily applicable to human studies. Methodological breakthrough would enable high-resolution analysis of a single exosome particle and contribute to solving these problems, just like flow cytometry for single-cell analysis.
Notably, microvesicles, which are another type of extracellular vesicles typically larger than exosomes, also contain miRNAs and could function as an intercellular mediator.7 They are generated by direct outward budding and fission of the plasma membrane, in contrast to exosomes, which are first formed as intraluminal vesicles comprising multivesicular endosomes (MVE) and then secreted after fusion of MVEs with the plasma membrane.7 Several mRNAs and proteins other than miRNAs also participate in intercellular communications mediated by extracellular vesicles including exosomes. For example, ovarian cancer cells produce extracellular vesicles containing MMP1 mRNA and they are involved in peritoneal dissemination.24 B cells secrete exosomes loaded with major histocompatibility complex class II, which present antigens to corresponding T cells.25 Comprehensive analysis of various kinds of extracellular vesicles and their contents should further clarify the pathogenesis of disabling diseases such as MS.
Increasing amounts of evidence suggests that gut microbiota has a fundamental role in cancer, neurodegenerative diseases, and autoimmune diseases. We reported that the severity of EAE in mice is changed by modifying gut flora,26 and that the microbiome is altered in patients with MS.27 Regarding extracellular vesicles, Bacteroides fragilis delivers immunomodulatory molecules packaged in outer membrane vesicles to intestinal dendritic cells and induces Treg cells.28 In contrast, host intestinal epithelial cells are known to modulate gut microbiota by secreting miRNAs, which enter commensal bacteria and directly regulate bacterial gene expression.29 Some of these miRNAs are included in the secreted extracellular vesicles.29 Besides bacteria, parasite infection in the gut could ameliorate the disease course of MS.30 Previous studies showed that nematode in the intestine secretes vesicles that contain miRNAs and then modulates the function of host immune cells in mice.31 Interestingly, some nematode miRNAs are found in the circulation of infected mice.31 They could affect various kinds of cells including immune cells throughout the body. Extracellular vesicles may be critically involved in host-gut microbiota interaction. Further studies are needed to investigate the relevance in MS.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: KK, HH and TY drafted the manuscript.
References
- 1. Dendrou CA, Fugger L, Friese MA. Immunopathology of multiple sclerosis. Nat Rev Immunol. 2015;15:545–558. [DOI] [PubMed] [Google Scholar]
- 2. Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7–13. [DOI] [PubMed] [Google Scholar]
- 3. Kimura K, Nakamura M, Sato W, et al. Disrupted balance of T cells under natalizumab treatment in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2016;3:e210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Venken K, Hellings N, Broekmans T, Hensen K, Rummens JL, Stinissen P. Natural naive CD4+CD25+CD127low regulatory T cell (Treg) development and function are disturbed in multiple sclerosis patients: recovery of memory Treg homeostasis during disease progression. J Immunol. 2008;180:6411–6420. [DOI] [PubMed] [Google Scholar]
- 5. Dominguez-Villar M, Baecher-Allan CM, Hafler DA. Identification of t helper type 1-like, Foxp3+ regulatory T cells in human autoimmune disease. Nat Med. 2011;17:673–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kimura K, Hohjoh H, Fukuoka M, et al. Circulating exosomes suppress the induction of regulatory T cells via let-7i in multiple sclerosis. Nat Comm. 2018;9:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles [published online ahead of print January 17, 2018]. Nat Rev Molec Cell Biol. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 8. O’Connell RM, Rao DS, Chaudhuri AA, Baltimore D. Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol. 2010;10:111–122. [DOI] [PubMed] [Google Scholar]
- 9. Murugaiyan G, da Cunha AP, Ajay AK, et al. MicroRNA-21 promotes Th17 differentiation and mediates experimental autoimmune encephalomyelitis. J Clin Invest. 2015;125:1069–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ichiyama K, Gonzalez-Martin A, Kim BS, et al. The microRNA-183-96-182 cluster promotes T helper 17 cell pathogenicity by negatively regulating transcription factor Foxo1 expression. Immunity. 2016;44:1284–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang HY, Barbi J, Wu CY, et al. MicroRNA-17 modulates regulatory T cell function by targeting co-regulators of the Foxp3 transcription factor. Immunity. 2016;45:83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Okoye IS, Coomes SM, Pelly VS, et al. MicroRNA-containing T-regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells. Immunity. 2014;41:89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang Q, Xiao B, Ma X, et al. MicroRNAs associated with the pathogenesis of multiple sclerosis. J Neuroimmuno. 2016;295-296:148–161. [DOI] [PubMed] [Google Scholar]
- 14. Gandhi R, Healy B, Gholipour T, et al. Circulating microRNAs as biomarkers for disease staging in multiple sclerosis. Ann Neurol. 2013;73:729–740. [DOI] [PubMed] [Google Scholar]
- 15. Tominaga N, Kosaka N, Ono M, et al. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nat Comm. 2015;6:6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Selmaj I, Cichalewska M, Namiecinska M, et al. Global exosome transcriptome profiling reveals biomarkers for multiple sclerosis. Ann Neurol. 2017;81:703–717. [DOI] [PubMed] [Google Scholar]
- 17. Ebrahimkhani S, Vafaee F, Young PE, et al. Exosomal microRNA signatures in multiple sclerosis reflect disease status. Sci Reports. 2017;7:14293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang M, Liu F, Jia H, et al. Inhibition of microRNA let-7i depresses maturation and functional state of dendritic cells in response to lipopolysaccharide stimulation via targeting suppressor of cytokine signaling 1. J Immunol. 2011;187:1674–1683. [DOI] [PubMed] [Google Scholar]
- 19. Pobezinsky LA, Etzensperger R, Jeurling S, et al. Let-7 microRNAs target the lineage-specific transcription factor PLZF to regulate terminal NKT cell differentiation and effector function. Nat Immunol. 2015;16:517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Swaminathan S, Suzuki K, Seddiki N, et al. Differential regulation of the let-7 family of microRNAs in CD4+ T cells alters IL-10 expression. J Immunol. 2012;188:6238–6246. [DOI] [PubMed] [Google Scholar]
- 21. Villarroya-Beltri C, Gutierrez-Vazquez C, Sanchez-Cabo F, et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Comm. 2013;4:2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hoshino A, Costa-Silva B, Shen TL, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tkach M, Thery C. Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016;164:1226–1232. [DOI] [PubMed] [Google Scholar]
- 24. Yokoi A, Yoshioka Y, Yamamoto Y, et al. Malignant extracellular vesicles carrying MMP1 mRNA facilitate peritoneal dissemination in ovarian cancer. Nat Comm. 2017;8:14470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Raposo G, Nijman HW, Stoorvogel W, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yokote H, Miyake S, Croxford JL, Oki S, Mizusawa H, Yamamura T. NKT cell-dependent amelioration of a mouse model of multiple sclerosis by altering gut flora. Am J Pathol. 2008;173:1714–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miyake S, Kim S, Suda W, et al. Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to Clostridia XIVa and IV clusters. PLoS ONE. 2015;10:e0137429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chu H, Khosravi A, Kusumawardhani IP, et al. Gene-microbiota interactions contribute to the pathogenesis of inflammatory Bowel disease. Science. 2016;352:1116–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu S, da Cunha AP, Rezende RM, et al. The host shapes the gut microbiota via fecal microRNA. Cell Host Microbe. 2016;19:32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Correale J, Farez M. Association between parasite infection and immune responses in multiple sclerosis. Ann Neurol. 2007;61:97–108. [DOI] [PubMed] [Google Scholar]
- 31. Buck AH, Coakley G, Simbari F, et al. Exosomes secreted by nematode parasites transfer small RNAs to mammalian cells and modulate innate immunity. Nat Comm. 2014;5:5488. [DOI] [PMC free article] [PubMed] [Google Scholar]