Abstract
Background:
The rapid increase in prescribing and use of opioids for noncancer pain has coincided with an increase in opioid-related adverse drug events (ADEs). The objective of our study was to describe ADEs related to tapentadol and oxycodone/naloxone spontaneously reported to the Australian Therapeutic Goods Administration (TGA).
Methods:
Public case detail reports for tapentadol (September 2013–March 2017) and oxycodone/naloxone (April 2011–March 2017) were sourced from the TGA. The total number of public case detail reports for tapentadol were 104 and 249 for oxycodone/naloxone. Demographic characteristics of patients, concomitant medications, causality assessment and outcome were described for each opioid according to the Medical Dictionary for Regulatory Activities (MedDRA) system organ class.
Results:
The most prevalent ADEs for tapentadol were nervous system disorders (n = 52, 50%), psychiatric (n = 34, 32.7%), gastrointestinal (n = 18, 17.3%), and general disorders and administration site conditions (n = 21, 20.2%). Sixteen (23.2%) of 69 nervous system disorders reaction terms were consistent with serotonin syndrome of which 14 (87.5%) involved documented coadministration with another serotonergic medication. The most prevalent ADEs for oxycodone/naloxone were psychiatric disorders (n = 78, 31.3%), gastrointestinal (n = 73, 29.3%), general disorders and administration site conditions (n = 87, 35%), and nervous system disorders (n = 62, 24.9%). There were 40 (16%) public case detail reports for oxycodone/naloxone with the MedDRA reaction terms ‘drug withdrawal syndrome’ and ‘withdrawal syndrome’.
Conclusion:
The profiles of spontaneous ADE reports for tapentadol and oxycodone/naloxone are largely consistent with their premarketing randomized controlled studies and profiles of opioids in general. Further research into the risk of serotonin syndrome with tapentadol use is warranted. The ADEs suggest clinicians should be cautious when switching patients to oxycodone/naloxone from other opioids.
Keywords: adverse drug events, opioid, oxycodone-naloxone, serotonin syndrome, substance withdrawal syndrome, tapentadol
Introduction
The rapid increase in prescribing and use of opioids for chronic noncancer pain has coincided with an increase in opioid-related harms.1,2 Tapentadol (Palexia, Grunenthal, Germany) and controlled release oxycodone/naloxone (Targin, Mundipharma, UK) are two recently marketed opioid analgesics in Australia.3,4 The number of tapentadol dispensings subsidized by the Pharmaceutical Benefits Scheme (PBS) increased from over 55,000 in 2014 to over 250,000 in 2015. Oxycodone/naloxone PBS subsidized dispensings increased from over 200,000 in 2012 to over 1.7 million in 2015.5 Although there has been considerable uptake in the use of these two opioids since registration, there are limited published data on postmarketing adverse drug events (ADEs). Tapentadol and oxycodone/naloxone are reimbursed through Australia’s PBS for the management of chronic severe disabling pain unresponsive to nonopioid analgesics.6 Tapentadol, a centrally acting μ-opioid receptor agonist and noradrenaline reuptake inhibitor,7–9 was first approved for marketing by the Therapeutic Goods Administration (TGA) in 2011. Oxycodone/naloxone was first marketed in Australia in 2010. Combining oxycodone with naloxone, a peripherally acting μ-opioid receptor antagonist, was designed to reduce the incidence of opioid-induced constipation.10,11
The most recent Australian Public Assessment Report (AusPAR) for tapentadol highlights the potential for serotonin syndrome.4 We are aware of three previous case reports of serotonin syndrome with tapentadol, although all three cases involved coadministration of tapentadol with other serotonergic agents including venlafaxine, duloxetine and amitriptyline.12–14 A systematic review of four randomized controlled trials conducted over a 3- to 12-week period suggested the addition of naloxone to oxycodone reduces the incidence of opioid-induced constipation without causing a reversal of analgesia.15 However, in order for naloxone to undergo first pass metabolism and avoid the systemic circulation, normal hepatic function is required.16,17 For this reason, recent clinical practice guidelines suggest avoiding oxycodone/naloxone in people with hepatic impairment as it may reduce analgesia or lead to opioid withdrawal in people who are opioid tolerant.18,19
There are a limited number of published studies on ADEs related to tapentadol and controlled release oxycodone/naloxone. A recent systematic review comparing tapentadol and oxycodone/naloxone showed that the most commonly reported ADEs for both medications were dizziness, headache and fatigue.20 Furthermore, ADEs involving the gastrointestinal tract (nausea, vomiting and constipation) were reported for both opioids at similar rates to those reported in premarketing trials and AusPAR.3,4,9,20,21 However, less common and rare adverse events are often identified by postmarketing surveillance data. The ADE profile of tapentadol and oxycodone/naloxone may be different in routine practice than in the clinical trials because people prescribed these medications in routine practice may have different characteristics to the clinical trial participants. In Australia, there are currently no published studies of spontaneous ADE reports linked to these two newly marketed opioids. Therefore, the objective of this study was to describe spontaneous adverse event reports related to tapentadol and oxycodone/naloxone in Australia. We compared spontaneously reported ADEs with those reported in the literature, and focused on ADEs not confirmed or well established in previous literature.
Methodology
Data source
Data were sourced from the Australian TGA Pharmacovigilance and Special Access Branch for tapentadol between September 2013 and March 2017 and oxycodone/naloxone between April 2011 and March 2017. These dates capture all the ADEs spontaneously reported to the TGA for each opioid. The Australian Adverse Drug Reactions Database maintained by the TGA holds details of Australian reports of suspected reactions to drugs received since 1 November 1972. It is mandatory for pharmaceutical companies to report ADEs, while health professionals and consumers are recommended to report ADEs. ADEs are reported via an electronic system where the reporter completes a standardized form. ADEs are indexed in the Database of Adverse Event Notifications (DAEN) using the Medical Dictionary for Regulatory Activities (MedDRA) classification system. MedDRA reaction terms are assigned by TGA medical officers.
Data extraction
Information on each patient’s age, sex, causality of the ADE, opioid used, type of reaction [using the MedDRA classification system], onset of reaction, reporter, concomitant medications (includes concomitant medications, tapentadol and oxycodone/naloxone) and outcome were extracted by two independent investigators. Any discrepancies were resolved with a third investigator. Duplicates were removed.
Data analysis
Data are described using means with standard deviations for continuous variables and proportions for categorical variables. MedDRA reaction terms were grouped according to the system organ class. For each opioid the four most commonly reported MedDRA system organ classes were identified.
Ethical review
The Monash University Human Research Ethics Committee deemed the study to be exempt from needing ethics approval.
Results
In total, 106 public case detail reports were retrieved for tapentadol between September 2013 and March 2017 and 250 cases for oxycodone/naloxone between April 2011 and March 2017. Of these, three public case detail reports were duplicated for tapentadol and one case detail was a group summary case report for oxycodone/naloxone. Therefore, the total number of public case detail reports for tapentadol were 104 and 249 for oxycodone/naloxone. Characteristics of public case detail reports for both opioids are reported in Table 1. The mean age of patients with a reported ADE was 54.2 years for tapentadol and 62.8 years for oxycodone/naloxone. There were more ADE reports for women than men (69.4% tapentadol, 59.4% oxycodone/naloxone). The most common reporter was the pharmaceutical company (47.2% tapentadol, 54.4% oxycodone/naloxone). The total mean number of medications used by patients was 2.67 for tapentadol and 3.57 for oxycodone/naloxone. Most patients in both groups were documented to have recovered from the ADE (tapentadol 87%, oxycodone/naloxone 76.4%).
Table 1.
Characteristics of cases in tapentadol and oxycodone/naloxone related adverse drug reaction reports.
| Characteristic | Tapentadol N = 104 |
Oxycodone/naloxone N = 249 |
|---|---|---|
| Female sex, n (%) | 68 (69.4) | 145 (59.4) |
| Missing sex | 8 | 6 |
| Age, mean (SD) | 54.2 (17.1) | 62.8 (17.8) |
| Missing age | 35 | 61 |
| Causality, n (%) | ||
| possible | 104 (100) | 248 (99.6) |
| probable | – | 1 (0.4) |
| Reporter type, n (%) | ||
| Drug company | 50 (47.2) | 136 (54.4) |
| Pharmacist | 24 (22.6) | 34 (13.6) |
| Hospital | 13 (12.3) | 49 (19.6) |
| Doctor | 11 (10.4) | 10 (4) |
| Coroner court | 4 (3.8) | – |
| Public | 3 (2.8) | 13 (5.2) |
| Nurse | 1 (0.9) | – |
| Specialist | – | 7 (2.8) |
| Not recorded | – | 1 (0.4) |
| Outcome, n (%) | ||
| Recovered | 47 (87) | 139 (76.4) |
| Death | 5 (9.3) | 4 (2.2) |
| Not yet recovered | 2 (3.7) | 25 (13.7) |
| Recovering | – | 13 (7.1) |
| Unrelated death | – | 1 (0.4) |
| Missing outcome | 52 | 68 |
| Number of medications, mean (SD) | 2.67 (2.8) | 3.57 (3.6) |
SD, standard deviation.
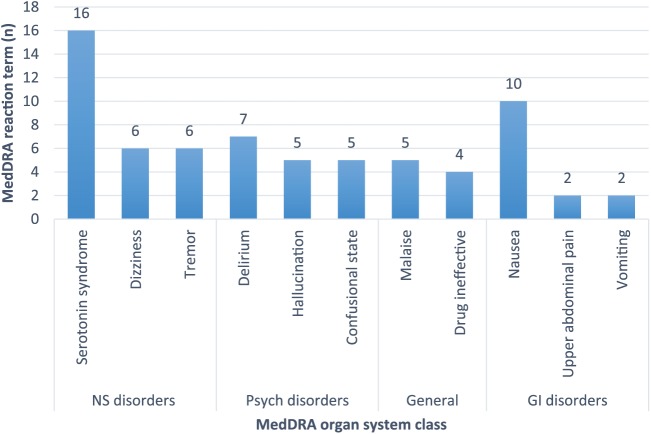
The most prevalent ADEs for tapentadol were nervous system disorders (n = 52, 50%), psychiatric (n = 34, 32.7%), gastrointestinal (n = 18, 17.3%), and general disorders and administration site conditions (n = 21, 20.2%) (Table 2). Sixteen (23.2%) of 69 nervous system disorders reaction terms were consistent with serotonin syndrome, and of these, 14 (87.5%) patients were reported to be taking another serotonergic drug in conjunction with tapentadol. Concomitant medications implicated in serotonin syndrome included tramadol, duloxetine, venlafaxine, desvenlafaxine, amitriptyline, sertraline, and escitalopram. The two remaining cases where serotonin syndrome was reported did not have specific details regarding concomitant medication use or patient characteristics. The most common psychiatric disorders reported for tapentadol were delirium (n = 7, 18.4%), hallucination (n = 5, 13.1%), and confusional state (n = 5, 13.1%). For general disorders and administration site conditions the most frequent reports were malaise (n = 5, 20.8%) and drug was considered ineffective (n = 4, 16.6%). The most common gastrointestinal ADEs reported were nausea (n = 10, 41.6%), upper abdominal pain (n = 2, 8.3%), and vomiting (n = 2, 8.3%). Figure 1 displays the two most common reaction terms of the four most common MedDRA organ system class of tapentadol.
Table 2.
Characteristics of the adverse drug reaction reports on the four most common MedDRA organ system class of tapentadol and oxycodone/naloxone.
| Nervous system disorders | Psychiatric disorders | General disorders and administration site conditions | Gastrointestinal disorders | |
|---|---|---|---|---|
| Tapentadol | n = 52 (50%) (69 reaction terms)* | n = 34 (32.7%) (38 reaction terms)* | n = 21 (20.2%) (24 reaction terms)* | n = 18 (17.3%) (24 reaction terms)* |
| Serotonin toxicity, n = 16 (23.2)**
Dizziness, n = 6 (8.7) Tremor, n = 6 (8.7) Other, n = 41 (59.4) |
Delirium, n = 7 (18.4) Hallucination, n = 5 (13.1) Confusional state, n = 5 (13.1) Other, n = 21 (68.4) |
Malaise, n = 5 (20.8) Drug ineffective, n = 4 (16.6) Other, n = 15 (41.6) |
Nausea, n = 10 (41.6) Abdominal pain upper, n = 2 (8.3) Vomiting, n = 2 (8.3) Other, n = 10 (41.6) |
|
| Age, mean | 51.4 | 51.7 | 46.3 | 43.3 |
| Missing age | 22 | 11 | 7 | 5 |
| Female sex, n | 36 | 19 | 13 | 12 |
| Missing sex | 2 | 2 | 2 | 2 |
| Causality possible, n | 52 | 34 | 21 | 18 |
| Outcome, n | ||||
| Recovered | 25 | 22 | 15 | 10 |
| Not yet recovered | 2 | 0 | 0 | 0 |
| Death | 1 | 0 | 0 | 1 |
| Outcome missing | 24 | 12 | 6 | 7 |
| Oxycodone/Naloxone | n = 62 (24.9%) (84 reaction terms)* | n = 78 (31.3%) (129 reaction terms)* | n = 87 (35%) (108 reaction terms)* | n = 73 (29.3%) (116 reaction terms)* |
| Dizziness, n = 9 (10.7) Somnolence, n = 8 (9.5) Tremor, n = 8 (9.5) Other, n = 59 (70.2) |
Hallucinations, n = 22 (17) Delirium, n = 12 (9.3) Withdrawal syndrome, n = 12 (9.3) Other, n = 83 (64.3) |
Drug withdrawal syndrome, n = 28 (26) Drug ineffective, n = 9 (8.3) Other, n = 71 (65.8) |
Nausea, n = 24 (20.7) Diarrhoea, n = 23 (19.9) Other, n = 69 (59.5) |
|
| Age, mean | 64.1 | 64.4 | 58.4 | 61.0 |
| Missing age | 7 | 18 | 24 | 19 |
| Female sex, n | 41 | 42 | 56 | 51 |
| Missing sex | 0 | 1 | 0 | 1 |
| Causality possible, n | 62 | 78 | 87 | 73 |
| Outcome, n | ||||
| Recovered | 32 | 45 | 48 | 42 |
| Not yet recovered | 6 | 10 | 8 | 11 |
| Recovering | 2 | 4 | 4 | 0 |
| Death | 2 | 1 | 2 | 1 |
| Unrelated death | 0 | 0 | 1 | 1 |
| Outcome missing | 20 | 18 | 24 | 18 |
Serotonin toxicity, n = 14 involved the use of one or more serotonergic agents (e.g. tramadol, duloxetine, venlafaxine, amitriptyline, sertraline, desvenlafaxine, escitalopram).
More than 1 reaction term can appear per report.
MedDRA, Medical Dictionary for Regulatory Activities.
Figure 1.
The two most common reaction terms of the four most common Medical Dictionary for Regulatory Activities (MedDRA) organ system class of tapentadol. GI, gastrointestinal; NS, nervous system.
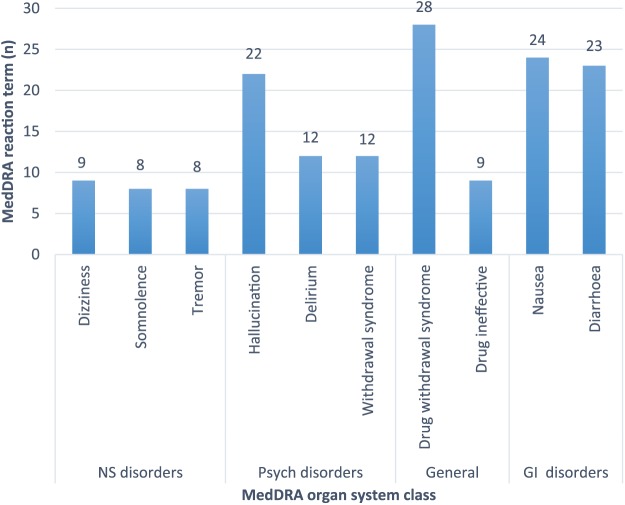
The most prevalent ADEs for oxycodone/naloxone were psychiatric disorders (n = 78, 31.3%), gastrointestinal (n = 73, 29.3%), general disorders and administration site conditions (n = 87, 35%), and nervous system (n = 62, 24.9%) (Table 2). The most common psychiatric disorders reported were hallucination (n = 22, 17%), delirium (n = 12, 9.3%), and withdrawal syndrome (n = 12, 9.3%). The most frequently reported reaction terms for gastrointestinal disorders were nausea (n = 24, 20.7%) and diarrhoea (n = 23, 19.9%). For nervous system disorders the most commonly reported ADEs were dizziness (n = 9, 10.7%), somnolence (n = 8, 9.5%), and tremor (n = 8, 9.5%). The most frequently reported general disorders and administration site conditions were drug withdrawal syndrome (n = 28, 26%) and that the drug was considered ineffective (n = 9, 8.3%). Figure 2 displays the two most common reaction terms of the four most common MedDRA organ system class of oxycodone/naloxone. There were 28 (26%) public case details reporting the reaction of drug withdrawal syndrome related to oxycodone/naloxone use. Further, there were 12 (9.3%) cases reporting withdrawal syndrome. These two MedDRA reaction terms were used in 40 (16%) public case detail reports. Of these, 25 (62.5%) public case detail reports documented use of another opioid prior to the prescriber changing the therapy to oxycodone/naloxone. There were seven cases of injecting oxycodone/naloxone that resulted in drug withdrawal syndrome/withdrawal syndrome with one of these public case details also documenting prior opioid use. The remaining nine public case detail reports did not have documentation of prior opioid use. Across the 249 reports we identified nine documented public case details suspecting or confirming degrees of hepatic impairment and raised liver enzymes. Four of these cases were implicated in reports of withdrawal reactions.
Figure 2.
The two most common reaction terms of the four most common Medical Dictionary for Regulatory Activities (MedDRA) organ system class of oxycodone/naloxone. GI, gastrointestinal; NS, nervous system.
Discussion
Our study identified that serotonin syndrome was the most frequently reported ADE with tapentadol, and ‘drug withdrawal syndrome’ and ‘withdrawal syndrome’ collectively were the most frequently reported ADE for oxycodone/naloxone. These ADEs are less well known and described in previous literature than other reported ADEs in our study. Other reported ADEs in our study are similar to those reported in the product information (PI) for each opioid. The most recent PI for tapentadol lists dizziness, somnolence, headache, nausea and vomiting as the most commonly reported ADEs in clinical trials.22 Oxycodone/naloxone PI includes nausea, vomiting, headache, somnolence, constipation, hyperhidrosis, and fatigue as the most commonly reported ADEs.23 ADEs related to oxycodone/naloxone were reported for people of higher average age than in tapentadol-related ADEs, while more ADEs were reported for women than men for both opioids.
The mean age of people reported with an ADE using tapentadol was 54 years and in people using oxycodone/naloxone was 63 years. This is somewhat consistent with the mean ages of participants in clinical trials. In tapentadol trials the mean age of participants was 62 years and the mean age of participants in oxycodone/naloxone trials was 56 years.3,4 The proportion of female sex was higher in both tapentadol and oxycodone/naloxone reports. This is consistent with the overall prescribing of opioids being higher in the female population.24
Sixteen cases of serotonin syndrome related to tapentadol use were reported to the TGA. In 12 cases tapentadol was coadministered with one other serotonergic medication and in two cases with two other serotonergic medications. Additionally, there were two cases of serotonin syndrome in which there was no documented coadministration with other serotonergic medications. Three published case reports of suspected serotonin syndrome associated with tapentadol were published in prior literature.12–14 In all three case reports tapentadol was coadministered with medications known to contribute to serotonin syndrome.12–14 We are not aware of any published cases of serotonin syndrome in which tapentadol was the sole causative agent.25 Interestingly, the most recent AusPAR for tapentadol published in February 2011 from the TGA indicates a theoretical risk of serotonin syndrome with other serotonergic medications, however a causal relationship has not been established.4 Furthermore, the US Food and Drug Administration has issued warnings about the risk of serotonin syndrome when tapentadol is used with other serotonergic medication.26 Tapentadol has been shown to have two mechanisms of action through the μ-opioid receptor and also has activity on noradrenaline receptors inhibiting reuptake of noradrenaline.7,27 Current literature suggests tapentadol has minimal effects on serotonin reuptake inhibition.27,28 Although it has been reported that tapentadol has minimal activity on serotonin receptors, our study highlights the possibility that tapentadol may contribute to serotonin syndrome when used alone or concomitantly with other serotonergic agents. However, further investigation is needed to determine whether tapentadol can cause serotonin syndrome as the sole causative agent.
There were 40 public case detail reports in total with the MedDRA reaction terms ‘drug withdrawal syndrome’ and ‘withdrawal syndrome’ for oxycodone/naloxone. In clinical trials there was a low incidence of drug withdrawal symptoms when patients were switched from other opioids to oxycodone/naloxone. Our study highlights the possibility of drug withdrawal syndromes when patients are switched from other opioids. We identified 25 withdrawal reactions resulting from a switch from other opioids to oxycodone/naloxone. The PI for Targin states that patients undergoing long-term opioid therapy may initially experience withdrawal symptoms if the prescriber changes therapy to oxycodone/naloxone.23,29 Of the 40 public case detail reports who had a withdrawal reaction we identified four cases of hepatic impairment and or raised liver enzymes. This is consistent with the literature which has reported cases of drug withdrawal with oxycodone/naloxone when used in patients with hepatic impairment.16,17,30 Liver problems can result in lowered first pass metabolism of naloxone, making it more systemically bioavailable thus precipitating withdrawal symptoms.16,17 The PI for Targin states that oxycodone/naloxone should be used with caution in patients with mild hepatic impairment and is contraindicated in patients with moderate to severe hepatic impairment.23 Patients with hepatic impairment may be more likely to experience withdrawal syndrome and therefore clinicians should exercise care and monitor patients when prescribing oxycodone/naloxone in hepatic impairment.19 There were seven cases of injecting oxycodone/naloxone that resulted in drug withdrawal syndrome/withdrawal syndrome. There are previous reports of withdrawal symptoms arising from misuse of oxycodone/naloxone formulations.17 Intravenously injecting oxycodone/naloxone results in the naloxone component acting on opioid receptors in the peripheral and central nervous systems precipitating drug withdrawal in opioid-tolerant people.17
Strengths and limitations
The strength of our study is that we included all adverse event reports in Australia for tapentadol and oxycodone/naloxone recorded up until March 2017. Spontaneous adverse event reporting is voluntary for patients and health care professionals whereas it is mandatory for pharmaceutical companies. Health care professionals may have been more likely to report serious rather than nonserious adverse events. This means that ADEs reported are unlikely to be representative of all potential ADEs that occur in clinical practice. As with other analyses of spontaneous ADE reports, it was not possible to estimate the incidence of ADEs associated with tapentadol and oxycodone/naloxone. Additionally, ADE reporting may have incomplete reporting/documentation of concomitant medications. This means that serotonin syndrome reported due to tapentadol use as a sole agent cannot be confirmed. Further, withdrawal reactions occurring when oxycodone/naloxone is used without switching from prior opioids cannot be confirmed due to the potential of incomplete reporting. Additional follow-up information for these cases was not available. Information such as time to onset of reaction, rechallenge data and duration of treatment were not available for most cases so we were not able to report these data.
Conclusion
Our study emphasizes the need for routine monitoring of ADEs for people using tapentadol or oxycodone/naloxone. Our study highlights the need for caution and ongoing monitoring when prescribing tapentadol in patients already taking serotoninergic medications. Clinicians should also exercise caution when switching from another opioid to oxycodone/naloxone or when prescribing oxycodone/naloxone to patients with hepatic impairment. This is important to avoid drug withdrawal syndrome and reduced analgesia.
Footnotes
Funding: JI was supported by a National Health and Medical Research Council Fellowship (grant number 1072137). SL was supported through an Australian Government Research Training Program Scholarship.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Carmen Abeyaratne, Centre for Medicine Use and Safety, Faculty of Pharmacy and Pharmaceutical Sciences, Monash University, 381 Royal Parade, Parkville, Victoria, 3052, Australia.
Samanta Lalic, Centre for Medicine Use and Safety, Faculty of Pharmacy and Pharmaceutical Sciences, Monash University, Parkville, Victoria, Australia; Pharmacy Department Austin Health, Melbourne, Australia.
J. Simon Bell, Centre for Medicine Use and Safety, Faculty of Pharmacy and Pharmaceutical Sciences, Monash University, Parkville, Victoria, Australia; Department of Epidemiology and Preventive Medicine, School of Public Health and Preventive Medicine, Monash University, Melbourne, Australia.
Jenni Ilomäki, Centre for Medicine Use and Safety, Faculty of Pharmacy and Pharmaceutical Sciences, Monash University, Parkville, Victoria, Australia; Department of Epidemiology and Preventive Medicine, School of Public Health and Preventive Medicine, Monash University, Melbourne, Australia.
References
- 1. Roxburgh A, Bruno R, Larance B, et al. Prescription of opioid analgesics and related harms in Australia. Med J Aust 2011; 195: 280–284. [DOI] [PubMed] [Google Scholar]
- 2. Blanch B, Pearson SA, Haber PS. An overview of the patterns of prescription opioid use, costs and related harms in Australia. Br J Clin Pharmacol 2014; 78: 1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Australian Public Assessment Report. AusPAR: oxycodone hydrochloride/naloxone hydrochloride (Targin). Submission no: PM-2008–2938–1, https://search.tga.gov.au/s/search.html?collection=tga-websites-web&query=pdf%20auspar%20auspar%20targin%20pdf (2010, accessed 12 August 2017).
- 4. Australian Public Assessment Report. AusPAR: tapentadol (Palexia SR). Submission no: PM-2009–02489–3–1, https://www.tga.gov.au/sites/default/files/auspar-palexia-sr.pdf (2011, accessed 25 August 2017).
- 5. Pharmaceutical Benefits Scheme. Australian statistics of medicines, http://www.pbs.gov.au/info/statistics/asm/australian-statistics-on-medicines (accessed 27 October 2017).
- 6. Pharmaceutical Benefits Scheme. Australian government department of health pharmaceutical benefits scheme, http://www.pbs.gov.au/ (accessed 20th August 2017).
- 7. Langford RM, Knaggs R, Farquhar-Smith P, et al. Is tapentadol different from classical opioids? A review of the evidence. Br J Pain 2016; 10: 217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baron R, Jansen JP, Binder A, et al. Tolerability, safety, and quality of life with tapentadol prolonged release (PR) compared with oxycodone/naloxone PR in patients with severe chronic low back pain with a neuropathic component: a randomized, controlled, open-label, phase 3b/4 trial. Pain Pract 2016; 16: 600–619. [DOI] [PubMed] [Google Scholar]
- 9. Wiffen PJ, Derry S, Naessens K, et al. Oral tapentadol for cancer pain. Cochrane Database Syst Rev 2015: Cd011460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kang JH, Lee GW, Shin SH, et al. Opioid withdrawal syndrome after treatment with low-dose extended-release oxycodone and naloxone in a gastric cancer patient with portal vein thrombosis. J Pain Symptom Manage 2013; 46: e15–e17. [DOI] [PubMed] [Google Scholar]
- 11. Ueberall MA, Mueller-Schwefe GHH. Efficacy and tolerability balance of oxycodone/naloxone and tapentadol in chronic low back pain with a neuropathic component: a blinded end point analysis of randomly selected routine data from 12-week prospective open-label observations. J Pain Res 2016; 9: 1001–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Walczyk H, Liu CH, Alafris A, et al. Probable tapentadol-associated serotonin syndrome after overdose. Hosp Pharm 2016; 51: 320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Franco DM, Ali Z, Levine B, et al. Case report of a fatal intoxication by Nucynta. Am J Forensic Med and Pathol 2014; 35: 234–236. [DOI] [PubMed] [Google Scholar]
- 14. Mancano M. ISMP adverse drug reactions. Hosp Pharm 2013; 48: 542–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ford AC, Brenner DM, Schoenfeld PS. Efficacy of pharmacological therapies for the treatment of opioid-induced constipation: systematic review and meta-analysis. Am J Gastroenterol 2013; 108: 1566–1574. [DOI] [PubMed] [Google Scholar]
- 16. Lau F, Gardiner M. Oxycodone/naloxone: an unusual adverse drug reaction. Aust Fam Physician 2017; 46: 42–43. [PubMed] [Google Scholar]
- 17. Wong A, Macleod D, Robinson J, et al. Oxycodone/naloxone preparation can cause acute withdrawal symptoms when misused parenterally or taken orally. Clin Toxicol 2015; 53: 815–818. [DOI] [PubMed] [Google Scholar]
- 18. Aged Care Companion Online. AMH aged care companion (online). Adelaide: Australian Medicines Handbook Online; Available from: https://agedcare.amh.net.au/ (2017, accessed 29 August 2017) [Google Scholar]
- 19. Ward A, Del Campo M, Hauser K. Complications with oxycodone and naloxone. Aust Presc 2017; 40: 156–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thakur D, Dickerson S, Kumar Bhutani M, et al. Impact of prolonged-release oxycodone/naloxone on outcomes affecting patients’ daily functioning in comparison with extended-release tapentadol: a systematic review. Clin Ther 2015; 37: 212–224. [DOI] [PubMed] [Google Scholar]
- 21. Baron R, Likar R, Martin-Mola E, et al. Effectiveness of tapentadol prolonged release (PR) compared with oxycodone/naloxone PR for the management of severe chronic low back pain with a neuropathic component: a randomized, controlled, open-label, phase 3b/4 study. Pain Pract 2016; 16: 580–599. [DOI] [PubMed] [Google Scholar]
- 22. TGA eBS. Palexia ® SR product information, https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2017-PI-01558–1 (2017, accessed 1 September 2017).
- 23. TGA eBS. Product information Targin® modified release tablets, https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2012-PI-01399–3 (2017, accessed 20 August 2017).
- 24. Hollingworth SA, Gray PD, Hall WD, et al. Opioid analgesic prescribing in Australia: a focus on gender and age. Pharmacoepidemiol Drug Saf 2015; 24: 628–636. [DOI] [PubMed] [Google Scholar]
- 25. Schmidt P. Safety information on Serotonin syndrome for Tapentadol IR and Tapentadol ER administration. In: AAPM 32nd annual meeting, Palm Springs, California, 18–21 February 2016, paper no. 212. [Google Scholar]
- 26. Food and Drug Administration. Drug Safety Communication: FDA warns about several safety issues with opioid pain medicines; requires label changes, https://www.fda.gov/Drugs/DrugSafety/ucm489676.htm (2016, accessed 7 August 2017).
- 27. Vadivelu N, Timchenko A, Huang Y, et al. Tapentadol extended-release for treatment of chronic pain: a review. J Pain Res 2011; 4: 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Raffa RB, Buschmann H, Christoph T, et al. Mechanistic and functional differentiation of tapentadol and tramadol. Exp Opin Pharmacother 2012; 13: 1437–1449. [DOI] [PubMed] [Google Scholar]
- 29. NPS Radar. Oxycodone-with-naloxone controlled-release tablets (Targin) for chronic severe pain, https://www.nps.org.au/radar/articles/oxycodone-with-naloxone-controlled-release-tablets-targin-for-chronic-severe-pain (accessed 24 August 2017).
- 30. Leppert W. Role of oxycodone and oxycodone/naloxone in cancer pain management. Pharmacol Rep 2010; 62: 578–591. [DOI] [PubMed] [Google Scholar]