Abstract
Helicobacter pylori treatment may be viewed as an uncertain situation, where current knowledge is insufficient to provide evidence-based recommendations for all possible scenarios. Evidence suggests that, under uncertainty conditions, a few simple rules of thumb tend to work better than complex algorithms. Overall, five evidence-based rules of thumb are suggested: (1) Use four drugs; (2) Use maximal acid inhibition; (3) Treat for 2 weeks; (4) Do not repeat antibiotics after treatment failure; and (5) If your treatment works locally, keep using it. These simple rules of thumb may help the reader to select the best alternative for a given patient, choosing between the heterogeneous recommendations provided by the many different consensus conferences on H. pylori treatment recently published.
Keywords: Helicobacter pylori, treatment, uncertainty
Introduction
Gigerenzer1 recently suggested that the confusion between uncertainty and risk is one of the major causes of erroneous decisions in medicine. Risk can be measured and adequately estimated only in controlled environments such as tossing a coin or playing a casino game. By contrast, most real-life situations such as health decisions, economic predictions or human relationships are situations of uncertainty. Situations of uncertainty depend on many different variables that fulfill two conditions: the variables can affect the outcome, and they cannot be measured precisely. In this context, complex algorithms or complicated attempts to calculate risk may lead to biased appreciations and erroneous decisions; in fact, a few simple rules of thumb tend to work much better for uncertain situations.
Helicobacter pylori, one of the most prevalent human infections, is the main cause of peptic ulcer and gastric cancers and is a major public health problem.2,3 It has been clearly demonstrated that cure of the infection also leads to cure of the peptic ulcer and may prevent gastric cancer development.4 Unfortunately, trials so far have failed to identify a single gold standard treatment for H. pylori infection, since cure rates for most treatments vary from study to study. The variability in the responses to treatment has been attributed to antibiotic resistances. Regrettably, antibiotic resistances in an individual patient are difficult (and expensive) to assess and in clinical practice are rarely obtained. Furthermore, not all the possible antibiotic combinations in all the possible conditions have been tested by randomized controlled trials. Therefore, the information provided by the scientific evidence is very useful but limited, and needs to be generalized to a wide spectrum of clinical situations.
In addition to antibiotic resistances, response to treatment can be influenced by many other factors that are difficult to measure. First, it is often impossible to predict a priori whether a patient will develop adverse reactions to antibiotics so severe as to require treatment discontinuation. Second, patients have beliefs and economic and cultural constraints which may negatively influence adherence to treatment. Finally, patients present idiosyncratic differences which may affect the outcome of the H. pylori treatment. In this regard, Furuta and colleagues5 clearly demonstrated that slow metabolizers with reduced cytochrome 2P19 activity achieve far higher levels of antibiotics, more intense acid inhibition, and better cure rates. As the determination of cytochrome activity is complex and has been performed only in the setting of clinical trials, this information is never available to clinicians.
In consequence, when treating an individual patient, clinicians face a situation of uncertainty; they rarely know whether the infecting H. pylori strain is resistant to antibiotics, and, if it is, to which antibiotics. Furthermore, it is impossible to determine the potential effect of the other known and unknown variables that might influence the efficacy of the treatment. In this case, using a few simple rules of thumb may be a better approach than complicated treatment algorithms. The rules of thumb we propose here are not new; they are based on the available evidence, and have been tested in earlier meta-analyses. Some of them have been already proposed as methods to optimize H. pylori treatment,6 and they underlie the recommendations of the Spanish Consensus, the Toronto Consensus, the Maastricht Consensus and the American College of Gastroenterology Consensus,7–10 even though they are never explicitly mentioned.
Although these consensus statements are similar overall, the specific recommendations can vary significantly and some of them are highly complex.7–10 A few clear, simple rules of thumb may help the clinician to choose wisely between divergent recommendations. The ones we propose for current H. pylori treatment are shown in Table 1.
Table 1.
Rules of thumb for optimizing Helicobacter pylori treatment.
| 1. Use four drugs 2. Use maximal acid inhibition 3. Treat for 2 weeks 4. Do not repeat antibiotics after treatment failure 5. If your treatment works locally, keep using it. |
Rule of thumb 1: use four drugs
Triple therapy combining a proton pump inhibitor (PPI), clarithromycin and amoxicillin has been the treatment of choice for a long time. Recent trials and meta-analyses, however, have shown that it may fail in approximately 20–30% of patients, and, in clinical practice, this rate may be even higher.11 Many new treatments have been evaluated in the search for better cure rates. Of all of them, quadruple therapies have been the most successful alternatives. Overall, three main groups of quadruple therapies have been assessed in the literature: (a) adding metronidazole to classical clarithromycin-containing triple therapy, (b) classical PPI-bismuth-metronidazole-tetracycline quadruple therapy, and (c) adding bismuth to triple therapy.
(a) Adding metronidazole to triple therapy
Therapies using a PPI, metronidazole, clarithromycin and metronidazole have been extensively evaluated. A total of three main therapies have been assessed, all containing a PPI, clarithromycin metronidazole and amoxicillin. Concomitant therapy uses all four drugs for 10–14 days; hybrid therapy consists in a PPI plus amoxicillin for 10–14 days, adding clarithromycin and metronidazole for the last 5–7 days, and sequential treatment gives a PPI for 10–14 days, administering amoxicillin in the first 5–7 days and clarithromycin and metronidazole for the second half of the treatment. Meta-analyses have found these quadruple therapies to be superior to triple therapy, except possibly sequential treatment, when compared with 14-day triple therapy.12,13 The superiority of one quadruple therapy over another is controversial. The meta-analyses published14,15 found no differences in tolerability or efficacy between the three types of nonbismuth quadruple therapies; however, the Toronto Consensus specifically performed an updated meta-analysis which showed that cure rates are better with concomitant than with sequential therapy.8 Additionally, the Spanish consensus suggested that concomitant therapy may be better in patients infected with antibiotic-resistant strains.7 Cure rates of around 90% with concomitant therapy have consistently been reported in most published studies and this approach has also proved effective in primary care.16 For all these reasons, concomitant therapy may be a first-line treatment of choice.
(b) The combination of a PPI, bismuth, metronidazole and tetracycline
This is usually termed ‘classical’ or ‘bismuth’ quadruple therapy, has long been used for H. pylori treatments. It has been shown to be superior to triple therapy in most recent meta-analyses.17–19 The combination seems to work acceptably even in the presence of ‘in vitro’ resistance to metronidazole. In the meta-analysis by Venerito and colleagues19 classical quadruple therapy achieved a 92% cure rate in patients carrying sensitive strains compared with 84% in patients infected by a resistant strain.
In many western countries a 3-in-1 pill is available, combining metronidazole, tetracycline and bismuth. This presentation simplifies dosing for patients and allows the use of this quadruple therapy in countries where tetracyclines or bismuth are not available. This combination has also been shown to achieve cure rates of over 90% in most published studies.16 In fact, nearly all the consensus statements recommend classical quadruple therapy as first-line treatment.7–10,16
(c) Adding bismuth to triple therapy
Bismuth is a very effective drug for treating H. pylori infection and no resistances have been described. However, as it is not absorbed, it is not effective against intracellular or pericellular bacteria, and it must be used in combination with additional drugs to effectively eradicate the infection. In an excellent review, Dore and colleagues20 showed that adding bismuth to triple therapy increases cure rates by improving the efficacy against resistant strains. Other than in classical quadruple therapy, bismuth has only rarely been added to triple therapy in first-line H. pylori treatment.20 In second-line therapy, however, combination treatment using triple therapy including a PPI, amoxicillin and levofloxacin plus bismuth was one of the few treatments that achieved cure rates over 90% in trials performed in western patients since 2010.21
Rule of thumb 2: use maximal acid inhibition
Acid inhibition is a key component of H. pylori treatment. In addition to a possible antibacterial effect of the PPIs, acid inhibition increases luminal concentrations of antibiotics by decreasing their acid-related degradation. Moreover, it is believed that H. pylori needs an acidic environment to live due to its production of NH3 creating a basic milieu which needs to be neutralized by acid. High gastric pH may, furthermore, allow H. pylori to enter a replicative state, thus becoming susceptible to amoxicillin and clarithromycin.22 Sugimoto and colleagues23 analyzed 24-h gastric pH during triple therapy with lansoprazole, clarithromycin and amoxicillin. Cure rates were closely related to acid inhibition: mean gastric pH was 6.4 in patients who were cured and 5.2 in those who were not. The infection was cured in all patients who attained a pH above 4 for more than 90% of the time, even in the presence of clarithromycin-resistant strains.
Acid inhibition in response to PPI is determined by the capacity of the individuals to metabolize the drug, which is determined by the cytochrome 2P19 polymorphisms. Extensive metabolizers, including most of the White population, require higher PPI doses to adequately control gastric pH.24,25 Many meta-analyses have shown that increasing acid inhibition raises cure rates with H. pylori triple therapy.7–10,26 Among conventional PPIs, esomeprazole 40 mg pill twice a day combines a simple dosage with a powerful acid inhibition.27
Recently, vonoprazan, a novel potassium-competitive acid blocker, has been used in H. pylori treatment. Like PPIs, vonoprazan inhibits the gastric proton pump, although it has a more potent, rapid and sustained acid-inhibitory effect.28 A recent meta-analysis suggests that this potent acid inhibition may increase the efficacy of clarithromycin-including triple therapy, mainly by increasing cure rates in patients infected with clarithromycin-resistant strains.29 Although further evidence is needed, this new drug combined with quadruple therapies or long treatments (see below) may increase cure rates to nearly 100%, even in western populations. It may also allow the reduction of the number of drugs or the length of treatment, although this hypothesis has still to be tested.
Rule of thumb 3: treat for 2 weeks
Since the first meta-analysis was published30 it has been clearly established that increasing the length of triple therapy from 7 to 14 days increases cure rates by 5–10%. A recent Cochrane review has confirmed these findings.31 The meta-analysis, however, highlights the need for evidence of this effect in other settings such as, for example, quadruple therapies. Although no randomized trials have been performed, a recent review showed that lengthening concomitant quadruple therapy increases cure rates.13 Furthermore, 14-day concomitant therapies consistently achieved cure rates of around 90%, whereas 10-day therapies were somewhat less reliable.16
Rule of thumb 4: do not repeat antibiotics after treatment failure
After a failed first treatment, the remaining H. pylori will show very high resistances to some (though not all) of the antibiotics administered. Due to the specific characteristics of the bacteria, resistance to amoxicillin, tetracycline and (probably) rifabutin is extremely rare, even after treatment failure including those antibiotics. By contrast, resistances to clarithromycin, quinolones and metronidazole approach 100% after treatment failure. As the efficacy of clarithromycin-containing regimens is strongly affected by clarithromycin resistance, repeating this drug in rescue treatments is discouraged.8 The same goes for quinolones: after failure of a first treatment, secondary resistance rates are very high and cure rates when repeating the drug very low; so, this antibiotic should not be repeated after treatment failure.8 With regard to metronidazole, some articles suggest that when using sufficiently long treatments and high doses, in vitro metronidazole resistance has a limited impact in the efficacy of H. pylori treatments.32 In a recent multicenter study, however, cure rates of a 14-day, high-dose, rescue triple metronidazole-amoxicillin-PPI therapy were as low as 37% in patients with previous metronidazole administration.33 Therefore, we suggest repeating this antibiotic only when it is indispensable and in the setting of 14-day quadruple therapies.34 Finally, the acquisition of resistance to amoxicillin and tetracycline is remarkably rare and these antibiotics can be used more than once in the same patients without a significant reduction in efficacy.7–10 The same applies to bismuth: although its antibacterial activity mechanism remains uncertain, no in vitro resistance to this drug has been described.20 Therefore, bismuth, amoxicillin and tetracycline can be used more than once in the same patients, as they remain active despite previous treatment failure. We should consider these data when choosing a rescue therapy among the various consensus recommendations.7–10,16
Rule of thumb 5: if your treatment works locally, keep using it
No worldwide gold standard treatment has been defined; most studies use combinations of the abovementioned drugs. However, recommendations should be locally adapted. For example, the strict antibiotic policies applied in some Nordic countries may keep resistances low35 and allow the continued use of triple therapies. As long as monitoring of cure rates confirm high effectiveness, there is no reason to change to more complicated schedules.
In some areas of the world, alternative antibiotics have been used and have shown excellent efficacy against H. pylori. Nitazoxanide and furazolidone obtained excellent results in several adequately designed studies. In settings with high resistances to ‘legacy’ antibiotics, for example, Iran or China, or in the treatment of first-line failures in the United States, these drugs have reliably achieved excellent cure rates.36–38
Finally, in certain conditions, treatments may never reach cure rates above 90%. Any treatment may be deemed as acceptable in practice when no better cure rates have been reported locally with any other therapy. In fact, it remains unclear whether consensus-recommended schedules will achieve cure rates above 90% overall. This is especially the case for rescue treatments. So, although considerable emphasis has been placed on the need to cure over 90% of infections, and on ruling out any treatment that does not reach this threshold,6,39 achieving very high cure rates is difficult and 100% cure rates are virtually impossible (indeed, studies reporting 100% cure rates should be treated with suspicion). The most important reason for this statement is that the tests used to assess cure are not perfect: false positive results range from 0 to 5% for the urea breath test using citric acid40 and from 5 to 15% when using the urea breath test without citric acid, or the stool test.41 Therefore, even if we tested a perfect ‘100% cure’ treatment, the cure rates observed would range between 85 and 95% and would only exceptionally reach 100%, with false positive test results accounting for most of the ‘failures’.
Furthermore, during a systematic search for ongoing meta-analyses, we found only two published studies in western patients that have reported second-line therapies with cure rates of 90% or above since 2010.42,43 Schedules achieving over 90% cure rates for third or fourth-line therapies may be even rarer.
In fact, many of the current consensus recommendations suggest that the scientific community may have overoptimistic expectations for H. pylori treatments, especially in the case of rescue therapies. As an example, a very recent study reported an 86% per protocol cure rate in a third-line treatment in clinical practice.44 Although very few previous reports had achieved such good figures in a third-line treatment, the effectiveness of the results was surprisingly labeled as ‘limited’.
General recommendations for first, second and third-line therapies
Bearing in mind all the consensus recommendations and the rules of thumb mentioned above, the current suggestions for first, second, third and fourth-line therapies appear to be the following (Figure 1; Table 2):
Figure 1.
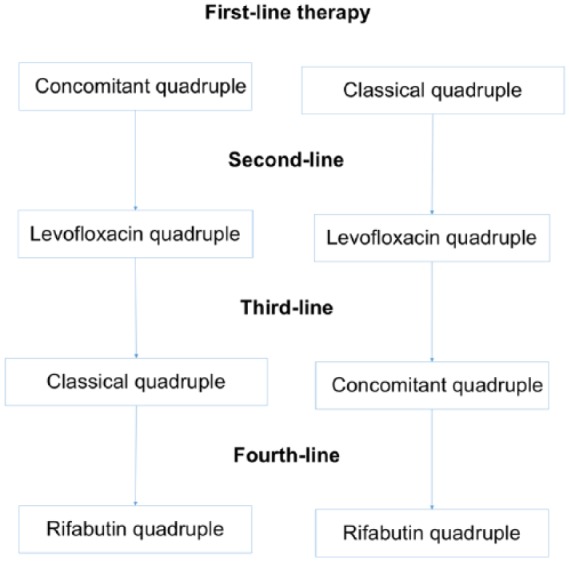
Summary of the first, second, third and fourth-line recommended treatments.
Table 2.
Recommended therapies.
| Classical quadruple therapy | |
| High-dose PPI/12 h and either 3-in-1 capsules 3/6 h or 4/8 h or Metronidazole 500 mg/8 h Tetracycline 500 mg/6 h Bismuth salt every 6–12/h |
10–14 days |
| Concomitant quadruple therapy | |
| High-dose PPI/12 h Amoxicillin1 g/12 h Clarithromycin 500 mg/12 h Metronidazole 500 mg/12 h |
14 days |
| Levofloxacin quadruple therapy | |
| High-dose PPI/12 h Levofloxacin 500 mg/24 h Amoxicillin 1 g/12 h Bismuth salt/12 h |
14 days |
| Quadruple rifabutin therapy | |
| High-dose PPI/12 h Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Bismuth salt/12 h |
14 days |
PPI, proton pump inhibitor.
First-line treatment
Currently, triple therapy with a PPI, clarithromycin and amoxicillin is not recommended because of the variability of its efficacy, which in most cases does not reach 80%. Exceptionally, it could be maintained in the few privileged areas where resistances are still low, provided that monitoring of cure rates continues to show excellent results. Regarding the recommendation in some consensus statements to adapt treatment to the local resistance pattern8–10 these resistances are rarely (if ever) adequately known, because: (a) resistances have been reported to vary year to year, and (b) they may change markedly from country to country (or even from county to county).45 So the decision to use triple therapies should be based not on published resistance data but according to the previous local experience. Furthermore, as stated, it is of the utmost importance to monitor cure rates in order to confirm that triple therapy continues to be effective.
The two most recommended first-line treatments are concomitant quadruple therapy for 14 days or classic quadruple bismuth therapy for 10–14 days. Both treatments have demonstrated efficacy equal to or greater than 90% in well-designed studies. Where available, classical quadruple therapy requiring only two drugs, a PPI plus a triple-drug pill including metronidazole, tetracycline and bismuth, is a convenient, easy-to-explain alternative. Its disadvantages are (a) the lack of global availability, (b) the limited number of studies evaluating this schedule in many geographical areas, (c) the four-times-a-day administration schedule (although some studies suggest that using it thrice a day with meals may favor adherence, without reducing efficacy),46 and (d) its cost, which may be higher than those of quadruple concomitant treatment (although this may vary markedly from country to country).
Concomitant quadruple therapy, on the other hand, is given twice a day and may be slightly more effective than the 10-day classic quadruple therapy. In addition, it has demonstrated its efficacy in many clinical practice situations.13,47 Its disadvantages are its longer duration and the fact that each of its components must be prescribed separately, which makes the treatment more difficult to prescribe and explain.
Adverse effects are moderate and appear to be similar with both quadruple treatments. According to current data, it seems reasonable to recommend the two at the same level as first-line treatment. As previously stated, the recommendation is to use a PPI at high doses every 12 h and to administer 14-day treatments if possible (see below).
Second-line treatment
A quadruple regimen with high-dose PPIs, levofloxacin, amoxicillin, and bismuth is recommended as rescue therapy after a concomitant regimen or classical quadruple treatment failure (Figure 1; Table 2). Triple therapy with a PPI, amoxicillin and levofloxacin results in insufficient cure rates, with an average figure of 74%. Therefore, although the number of studies is limited, it is reasonable to recommend a 14-day quadruple regimen with high-dose PPIs, levofloxacin, amoxicillin and bismuth as rescue therapy. A well-designed multicenter observational study demonstrated cure rates above 90%44 and a second study in China achieved lower but still acceptable cure rates.48 These results are consistent with those of a previous study showing that the addition of bismuth to triple treatments with levofloxacin or clarithromycin improves their cure rate by approximately 10%.20
Rescue treatment after two treatment failures
Given the high efficacy of the previous treatments, rescue therapy is needed only in exceptional cases. After the administration of two consecutive treatments with cure rates over 90%, a failure rate of below 1% may be expected. Therefore, in a patient with two previous H. pylori treatment failures, adherence to treatment should be thoroughly evaluated. The indication of eradication treatment should also be reconsidered, the risks and benefits of a third treatment carefully discussed with the patient, and his/her willingness to receive a tough third treatment should be carefully assessed. If the patient and the doctor finally agree to a third treatment, the recommended therapies (in this case, closely coinciding with the Toronto recommendations8) are the following:
If the initial therapy was quadruple concomitant and the second quadruple with levofloxacin and bismuth, classical quadruple therapy is recommended as rescue.
If the initial therapy was classical quadruple and the second quadruple with levofloxacin and bismuth, quadruple concomitant is recommended as rescue.
Finally, if a patient has received concomitant therapy as first-line and classical quadruple as rescue, a quadruple therapy including levofloxacin and bismuth is recommended.
Fourth-line treatment
After three treatment failures, treatment of infection should only be persisted with in patients with a very clear indication, as ulcer (especially after bleeding) or mucosa-associated lymphoid tissue lymphoma, or in patients who are highly motivated to try a fourth treatment and have been fully informed of the situation. After three failures, especially with the highly effective therapies that are currently recommended, the probability of either a false positive result of the post-treatment test or a low adherence to previous schedules increases markedly. Therefore, the possibility of confirming the presence of infection with an additional diagnostic test should be evaluated. Furthermore, assessing both adherence to previous treatments and the expected adherence to a new one is even more important than after a second-line treatment failure. If the patient finally chooses to receive treatment, the recommended regimen is 14 days of high-dose PPI, amoxicillin, rifabutin, and bismuth (Figure 1; Table 2).
Should I ever follow all the previous rules of thumb?
Everything in science is open to discussion and modifiable. It is likely that future developments may make some of the current rules of thumb unnecessary.
It is a real possibility that therapies that use vonoprazan (and possibly nitazoxanide) will achieve near 100% cure rates without the need for a fourth drug or with shorter treatments. Evidence on this is still needed, but it is likely to emerge in the near future. So, the second rule of thumb, use maximal acid inhibition, may overcome in part the need for four-drug or 14-day therapies. Vonoprazan may allow to introduce another rule of thumb that we have been forced to ignore in the current situation, that is ‘keep the treatment as simple as possible’.
In this sense, the rules of thumb are complementary, but their individual effect on cure rates decreases when the other rules of thumb are applied. For example, using either four drugs or 14-day therapies may increase first-line triple therapy cure rates from 80% to nearly 90%. The individual effect of using a second rule of thumb (either extending the treatment from 7 to 14 days or using a quadruple therapy) will be lower, and will increase the cure rates from 90 to 95%. Therefore, the margin of improvement for the remaining rule of thumb (for example, using high-dose PPI) will be low, perhaps 2–4%. This has many practical implications that may affect the use of these rules.
Costs are an important issue in medicine, which may change markedly from country to country and may modify medical decisions. As an example, in Spain, esomeprazole 40 mg is relatively expensive (25€ for H. pylori treatment) whereas the same dose of omeprazole costs 4€. It is unclear whether the increased cost, especially in first-line treatments, justifies the 2–4% expected rise in cure rates for 10–14-day quadruple therapies. However, as cure rates of rescue therapies fall steadily and the associated costs of the failures rise for second, third and fourth-line treatments, the potential increases in cure rates probably justify the costs of fully applying all the rules of thumb in this setting.
Similarly, in most cases, the 3-in-1 pill containing classical quadruple therapy is currently sold in packs designed for a 10-day treatment. If the length of classical quadruple treatment is increased to 14 days, then two packs of the drug are needed, thus doubling the costs. As most of the evidence provided with this combination is for 10-day therapy and cure rates have been satisfactory, using this combination for 10 days may be an acceptable strategy, especially for first-line therapy. Nevertheless, a presentation for 14 days is eagerly awaited.
Final consideration: why not use antibiotic sensitivity determination?
Both culture and molecular methods for detecting resistances require endoscopy. Although treating patients according to susceptibility testing increases cure rates when compared with empirical triple therapies,49 no comparison between susceptibility-guided treatment and adequately devised empirical quadruple therapies has been published. In addition, the economic burden of using endoscopy for culture in an infection affecting roughly 50% of the world’s population is probably unacceptable. Furthermore, although this has not been accurately estimated, the proportion of patients who will not agree to an invasive procedure is probably significant. Additional drawbacks are the fact that culture is not successful in all patients and that molecular methods may miss approximately 10% of resistances (and are unable to determine metronidazole resistance). Finally, resistances to amoxicillin, tetracycline and rifabutin are so rare that it is unlikely that their determination would be cost-effective.
Sensitivity determination is recommended by many of the consensus statements after second, third or fourth-line therapy failure.9 Evidence supporting this approach, however, is lacking.50 Cure rates of sensitivity-guided therapy in third or fourth-line therapies have been rather low, except when, in addition to the antibiotic sensitivity information, the rules of thumb described for the design of the treatment are used.50,51 Furthermore, as stated, the cost-effectiveness of resistance determination when using only antibiotics with extremely low resistance rates (such as rifabutin and amoxicillin) for the fourth-line rescue therapy remains to be determined.
Conclusion
Dealing with uncertainty in the treatment of H. pylori infection requires a few simple rules of thumb that allow to generalize the available evidence to clinical situations in which the data are still incomplete. Using quadruple therapies, high doses of PPIs and 14-day schedules have proved the most successful approaches for achieving optimal H. pylori cure rates. In rescue therapy especially, it may be preferable to use these rules of thumb, just adding the one of avoiding repeated antibiotics if possible, rather than limit the treatment to the published schedules, for which we already know that cure rates are suboptimal. These rules of thumb may be also useful in the design of new alternative treatment schedules for this infectious disease.
Acknowledgments
I thank Michael Maudsley for his help with the English.
Footnotes
Funding: The author received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The author declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The author has given lectures and received research grants from Allergan.
ORCID iD: Xavier Calvet  https://orcid.org/0000-0002-6278-9663
https://orcid.org/0000-0002-6278-9663
References
- 1. Gigerenzer Gerd. Risk Savvy: How To Make Good Decisions. Penguin Books Ltd. Kindle Edition. [Google Scholar]
- 2. Go MF. Review article: natural history and epidemiology of Helicobacter pylori infection. Aliment Pharmacol Ther 2002; 16(Suppl. 1): 3–15. [DOI] [PubMed] [Google Scholar]
- 3. McColl K. Helicobacter pylori infection. N Engl J Med 2010; 362: 1597–1604. [DOI] [PubMed] [Google Scholar]
- 4. Gisbert JP, Calvet X, Cosme A, et al. Long-term follow-up of 1,000 patients cured of Helicobacter pylori infection following an episode of peptic ulcer bleeding. Am J Gastroenterol 2012; 107: 1197–1204. [DOI] [PubMed] [Google Scholar]
- 5. Furuta T, Shirai N, Takashima M, et al. Effect of genotypic differences in CYP2C19 on cure rates for Helicobacter pylori infection by triple therapy with a proton pump inhibitor, amoxicillin, and clarithromycin. Clin Pharmacol Ther 2001; 69: 158–168. [DOI] [PubMed] [Google Scholar]
- 6. Gisbert JP, McNicholl AG. Optimization strategies aimed to increase the efficacy of H. pylori eradication therapies. Helicobacter. Epub ahead of print 2 May 2017. DOI: 10.1111/hel.12392. [DOI] [PubMed] [Google Scholar]
- 7. Gisbert JP, Molina-Infante J, Amador J, et al. IV Spanish Consensus Conference on Helicobacter pylori infection treatment. Gastroenterol Hepatol 2016; 39: 697–721. [DOI] [PubMed] [Google Scholar]
- 8. Fallone CA, Chiba N, van Zanten SV, et al. The Toronto Consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology 2016; 151: 51–69. [DOI] [PubMed] [Google Scholar]
- 9. Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection: the Maastricht V/Florence Consensus Report. Gut 2017; 66: 6–30. [DOI] [PubMed] [Google Scholar]
- 10. Chey WD, Leontiadis GI, Howden CW, et al. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol 2017; 112: 212–239. [DOI] [PubMed] [Google Scholar]
- 11. Gisbert JP, Calvet X. Review article: the effectiveness of standard triple therapy for Helicobacter pylori has not changed over the last decade, but it is not good enough. Aliment Pharmacol Ther 2011; 34: 1255–1268. [DOI] [PubMed] [Google Scholar]
- 12. Nyssen OP, McNicholl AG, Megraud F, et al. Sequential versus standard triple first-line therapy for Helicobacter pylori eradication. Cochrane Database Syst Rev 2016; 28: CD009034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gisbert JP, Calvet X. Update on non-bismuth quadruple (concomitant) therapy for eradication of Helicobacter pylori. Clin Exp Gastroenterol 2012; 5: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Song ZQ, Zhou LY. Hybrid, sequential and concomitant therapies for Helicobacter pylori eradication: a systematic review and meta-analysis. World J Gastroenterol 2016; 22: 4766–4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. He L, Deng T, Luo H. Meta-analysis of sequential, concomitant and hybrid therapy for Helicobacter pylori eradication. Intern Med 2015; 54: 703–710. [DOI] [PubMed] [Google Scholar]
- 16. Jordi Sánchez-Delgado J, García-Iglesias P, Titó LL, et al. Document de posicionament: Actualització en el Tractament de la Infecció per Helicobacter pylori, http://www.scdigestologia.org/docs/docs_posicionament/14_ACTUALITZACIO_TRACTAMENT_INFECCIO_HELICOBACTERPYLORI.pdf (2017, accessed August 2017).
- 17. Gené E, Calvet X, Azagra R, et al. Triple vs. quadruple therapy for treating Helicobacter pylori infection: a meta-analysis. Aliment Pharmacol Ther 2003; 17: 1137–1143. [DOI] [PubMed] [Google Scholar]
- 18. Luther J, Higgins PD, Schoenfeld PS, et al. Empiric quadruple vs. triple therapy for primary treatment of Helicobacter pylori infection: systematic review and meta-analysis of efficacy and tolerability. Am J Gastroenterol 2010; 105: 65–73. [DOI] [PubMed] [Google Scholar]
- 19. Venerito M, Krieger T, Ecker T, et al. Meta-analysis of bismuth quadruple therapy versus clarithromycin triple therapy for empiric primary treatment of Helicobacter pylori infection. Digestion 2013; 88: 33–45. [DOI] [PubMed] [Google Scholar]
- 20. Dore MP, Lu H, Graham DY. Role of bismuth in improving Helicobacter pylori eradication with triple therapy. Gut 2016; 65: 870–878. [DOI] [PubMed] [Google Scholar]
- 21. Gisbert JP, Romano M, Gravina AG, et al. Helicobacter pylori second-line rescue therapy with levofloxacin- and bismuth-containing quadruple therapy, after failure of standard triple or non-bismuth quadruple treatments. Aliment Pharmacol Ther 2015; 41: 768–775. [DOI] [PubMed] [Google Scholar]
- 22. Labenz J. Current role of acid suppressants in Helicobacter pylori eradication therapy. Best Pract Res Clin Gastroenterol 2001; 15: 413–431. [DOI] [PubMed] [Google Scholar]
- 23. Sugimoto M, Furuta T, Shirai N, et al. Evidence that the degree and duration of acid suppression are related to Helicobacter pylori eradication by triple therapy. Helicobacter 2007; 12: 317–323. [DOI] [PubMed] [Google Scholar]
- 24. Horai Y, Kimura M, Furuie H, et al. Pharmacodynamic effects and kinetic disposition of rabeprazole in relation to CYP2C19 genotypes. Aliment Pharmacol Ther 2001; 15: 793–803. [DOI] [PubMed] [Google Scholar]
- 25. Hunfeld NG, Mathot RA, Touw DJ, et al. Effect of CYP2C19*2 and *17 mutations on pharmacodynamics and kinetics of proton pump inhibitors in Caucasians. Br J Clin Pharmacol 2008; 65: 752–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Villoria A, García P, Calvet X, et al. Meta-analysis: high-dose proton pump inhibitors vs. standard dose in triple therapy for Helicobacter pylori eradication. Aliment Pharmacol Ther 2008; 28: 868–877. [DOI] [PubMed] [Google Scholar]
- 27. Kirchheiner J, Glatt S, Fuhr U, et al. Relative potency of proton pump inhibitors: comparison of effects on intragastric pH. Eur J Clin Pharmacol 2009; 65: 19–31. [DOI] [PubMed] [Google Scholar]
- 28. Sakurai Y, Mori Y, Okamoto H, et al. Acid-inhibitory effects of vonoprazan 20 mg compared with esomeprazole 20 mg or rabeprazole 10 mg in healthy adult male subjects: a randomised open-label cross-over study. Aliment Pharmacol Ther 2015; 42: 719–730. [DOI] [PubMed] [Google Scholar]
- 29. Jung YS, Kim EH, Park CH. Systematic review with meta-analysis: the efficacy of vonoprazan-based triple therapy on Helicobacter pylori eradication. Aliment Pharmacol Ther 2017; 46: 106–114. [DOI] [PubMed] [Google Scholar]
- 30. Calvet X, García N, López T, et al. A meta-analysis of short versus long therapy with a proton pump inhibitor, clarithromycin and either metronidazole or amoxycillin for treating Helicobacter pylori infection. Aliment Pharmacol Ther 2000; 14: 603–609. [DOI] [PubMed] [Google Scholar]
- 31. Yuan Y, Ford AC, Khan KJ, et al. Optimum duration of regimens for Helicobacter pylori eradication. Cochrane Database Syst Rev 2013; 12: CD008337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Laine L, Hunt R, El-Zimaity H, et al. Bismuth-based quadruple therapy using a single capsule of bismuth biskalcitrate, metronidazole, and tetracycline given with omeprazole versus omeprazole, amoxicillin, and clarithromycin for eradication of Helicobacter pylori in duodenal ulcer patients: a prospective, randomized, multicenter, North American trial. Am J Gastroenterol 2003; 98: 562–567. [DOI] [PubMed] [Google Scholar]
- 33. Puig I, Gonzalez-Santiago JM, Molina-Infante J, et al. Fourteen-day high-dose esomeprazole, amoxicillin and metronidazole as third-line treatment for Helicobacter pylori infection. Int J Clin Pract. Epub ahead of print 4 September 2017. DOI: 10.1111/ijcp.13004. [DOI] [PubMed] [Google Scholar]
- 34. Muller N, Amiot A, Le Thuaut A, et al. Rescue therapy with bismuth-containing quadruple therapy in patients infected with metronidazole-resistant Helicobacter pylori strains. Clin Res Hepatol Gastroenterol 2016; 40: 517–524. [DOI] [PubMed] [Google Scholar]
- 35. Megraud F, Coenen S, Versporten A, et al. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut 2013; 62: 34–42. [DOI] [PubMed] [Google Scholar]
- 36. Shehata MA, Talaat R, Soliman S, et al. Randomized controlled study of a novel triple nitazoxanide (NTZ)-containing therapeutic regimen versus the traditional regimen for eradication of Helicobacter pylori infection. Helicobacter. Epub ahead of print 19 May 2017. DOI: 10.1111/hel.12395. [DOI] [PubMed] [Google Scholar]
- 37. Basu PP, Rayapudi K, Pacana T, et al. A randomized study comparing levofloxacin, omeprazole, nitazoxanide, and doxycycline versus triple therapy for the eradication of Helicobacter pylori. Am J Gastroenterol 2011; 106: 1970–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mohammadi M, Attaran B, Malekzadeh R, et al. Furazolidone, an underutilized drug for H. pylori eradication: lessons from Iran. Dig Dis Sci 2017; 62: 1890–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Graham DY. Helicobacter pylori eradication therapy research: ethical issues and description of results. Clin Gastroenterol Hepatol 2010; 8: 1032–1036. [DOI] [PubMed] [Google Scholar]
- 40. Gisbert JP, Pajares JM. Review article: 13C-urea breath test in the diagnosis of Helicobacter pylori infection: a critical review. Aliment Pharmacol Ther 2004; 20: 1001–1017. [DOI] [PubMed] [Google Scholar]
- 41. Calvet X, Sánchez-Delgado J, Montserrat A, et al. Accuracy of diagnostic tests for Helicobacter pylori: a reappraisal. Clin Infect Dis 2009; 48: 1385–1391. [DOI] [PubMed] [Google Scholar]
- 42. Gisbert JP, Romano M, Gravina AG, et al. Helicobacter pylori second-line rescue therapy with levofloxacin- and bismuth-containing quadruple therapy, after failure of standard triple or non-bismuth quadruple treatments. Aliment Pharmacol Ther 2015; 41: 768–775. [DOI] [PubMed] [Google Scholar]
- 43. Fiorini G, Vakil N, Zullo A, et al. Culture-based selection therapy for patients who did not respond to previous treatment for Helicobacter pylori infection. Clin Gastroenterol Hepatol 2013; 11: 507–510. [DOI] [PubMed] [Google Scholar]
- 44. Rodríguez de Santiago E, Martín de Argila C, Marcos Prieto HM, et al. Limited effectiveness with a10-day bismuth-containing quadruple therapy (Pylera(®)) in third-line recue treatment for Helicobacter pylori infection: a real-life multicenter study. Helicobacter. Epub ahead of print 3 August 2017. DOI: 10.1111/hel.12423. [DOI] [PubMed] [Google Scholar]
- 45. Molina-Infante J, Gisbert JP. Update on the efficacy of triple therapy for Helicobacter pylori infection and clarithromycin resistance rates in Spain (2007–2012). Gastroenterol Hepatol 2013; 36: 375–381. [DOI] [PubMed] [Google Scholar]
- 46. Calvet X, Ducons J, Guardiola J, et al. One-week triple vs. quadruple therapy for Helicobacter pylori infection: a randomized trial. Aliment Pharmacol Ther 2002; 16: 1261–1267. [DOI] [PubMed] [Google Scholar]
- 47. McNicholl AG, Marin AC, Molina-Infante J, et al. Randomised clinical trial comparing sequential and concomitant therapies for Helicobacter pylori eradication in routine clinical practice. Gut 2014; 63: 244–249. [DOI] [PubMed] [Google Scholar]
- 48. Song Z, Zhou L, Zhang J, et al. Levofloxacin, bismuth, amoxicillin and esomeprazole as second-line Helicobacter pylori therapy after failure of non-bismuth quadruple therapy. Dig Liver Dis 2016; 48: 506–511. [DOI] [PubMed] [Google Scholar]
- 49. López-Góngora S, Puig I, Calvet X, et al. Systematic review and meta-analysis: susceptibility-guided versus empirical antibiotic treatment for Helicobacter pylori infection. J Antimicrob Chemother 2015; 70: 2447–2455. [DOI] [PubMed] [Google Scholar]
- 50. Puig I, López-Góngora S, Calvet X, et al. Systematic review: third-line susceptibility-guided treatment for Helicobacter pylori infection. Therap Adv Gastroenterol 2016; 9: 437–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tay CY, Windsor HM, Thirriot F, et al. Helicobacter pylori eradication in Western Australia using novel quadruple therapy combinations. Aliment Pharmacol Ther 2012; 36: 1076–1083. [DOI] [PubMed] [Google Scholar]


