Short abstract
Background
Multiple sclerosis is an inflammatory, neurodegenerative disease of the central nervous system for which therapeutic mesenchymal stem cell transplantation is under study. Published experience of culture-expanding multiple sclerosis patients’ mesenchymal stem cells for clinical trials is limited.
Objective
To determine the feasibility of culture-expanding multiple sclerosis patients’ mesenchymal stem cells for clinical use.
Methods
In a phase I trial, autologous, bone marrow-derived mesenchymal stem cells were isolated from 25 trial participants with multiple sclerosis and eight matched controls, and culture-expanded to a target single dose of 1–2 × 106 cells/kg. Viability, cell product identity and sterility were assessed prior to infusion. Cytogenetic stability was assessed by single nucleotide polymorphism analysis of mesenchymal stem cells from 18 multiple sclerosis patients and five controls.
Results
One patient failed screening. Mesenchymal stem cell culture expansion was successful for 24 of 25 multiple sclerosis patients and six of eight controls. The target dose was achieved in 16–62 days, requiring two to three cell passages. Growth rate and culture success did not correlate with demographic or multiple sclerosis disease characteristics. Cytogenetic studies identified changes on one chromosome of one control (4.3%) after extended time in culture.
Conclusion
Culture expansion of mesenchymal stem cells from multiple sclerosis patients as donors is feasible. However, culture time should be minimized for cell products designated for therapeutic administration.
Keywords: Multiple sclerosis, clinical trial, mesenchymal stem cell, culture expansion, cytogenetic analysis
Introduction
Multiple sclerosis (MS) is a chronic, immune-mediated demyelinating and neurodegenerative disease of the central nervous system. Current therapies reduce the accumulation of focal inflammatory damage but do not directly promote repair, representing a major unmet need.1 There is interest in the therapeutic potential of mesenchymal stem cell (MSC) transplantation in MS.2–6 MSCs are non-hematopoietic stem cells with potential to differentiate into several cell types,7 broad immunomodulatory effects8 and ability to promote repair by direct cell replacement in some tissues or indirectly by secreting numerous trophic factors.9,10
The safety and efficacy of MSC transplantation are currently under investigation in MS. Many practical questions remain unanswered, including whether sufficient cells can be culture-expanded reliably from MS donors to allow autologous transplantation.11 In other indications, such as graft versus host disease, allogeneic donors have been used;12 however, autologous cells represent a simpler approach. While growth characteristics of MSCs from healthy donors are well documented,13,14 data are limited concerning growth profiles of MSCs harvested from MS donors and whether donor demographic or clinical characteristics affect proliferation rate, total cell yield, and the consistency of cell expansion.
Ex vivo culture expansion may increase the risk of cytogenetic alterations.15 While the specific cause is unclear, genomic instability may accumulate and chromosomal abnormalities that may be precursors to neoplastic development may spontaneously occur.16 Although not seen in all studies of culture-expanded human MSCs,16–19 chromosomal aberrations have been reported after prolonged culture following senescent crisis.20–25 Some authors have advocated including karyotypic analysis of culture-expanded cells in release criteria for therapeutic cell products.21
Here, we describe the methods and results of bone marrow harvest, MSC isolation, culture expansion, and cytogenetic analysis of cell products from a recent phase I study of autologous MSC transplantation in MS.26
Materials and methods
Study design
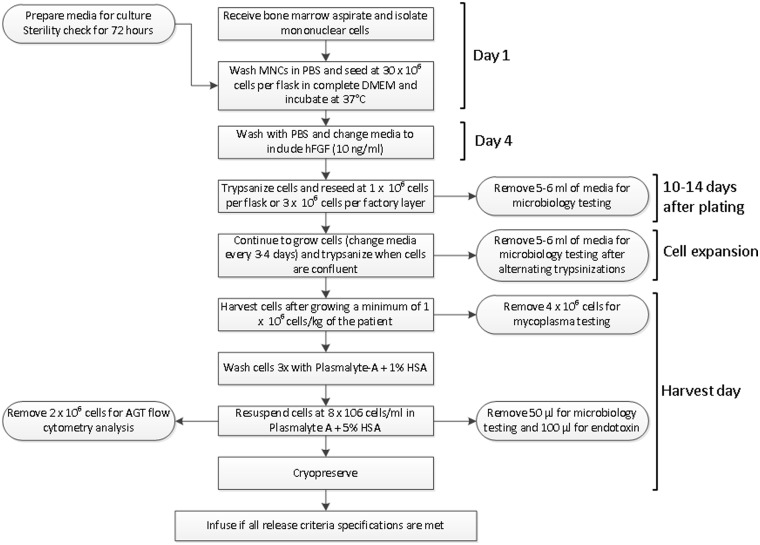
The study was a single arm, pre-post comparison trial to assess the feasibility, safety, and tolerability of single dose autologous MSC transplantation in patients with relapsing forms of MS (NCT00813969).26 Prior to study initiation, the protocol and informed consent document were approved by US Food and Drug Administration (FDA) (IND BB-13917) and the Cleveland Clinic and University Hospitals Cleveland Medical Center (UHCMC) institutional review boards. All participants gave informed consent before any study procedures. Cell production (Figure 1) was carried out in a good manufacturing practice-compliant facility that processes biologics regulated under section 351 of the Public Health Service Act and operates under foundation for accreditation of cellular therapy cell production guidelines and FDA phase I clinical trial guidelines. MSCs were harvested from bone marrow, expanded in culture, and administered by intravenous (IV) infusion.27 The target infused dose of MSCs was 1–2 × 106 cells/kg body weight.
Figure 1.
Cellular product manufacturing process. Standard process flow of mesenchymal stem cell expansion ex vivo, as reviewed and approved by the US Food and Drug Administration prior to study start.
Participants
Twenty five participants, 11 with relapsing–remitting and 14 with secondary-progressive MS, age 46.4±5.1 years, Expanded Disability Status Scale28 3.0–6.5, were recruited. Participants were required to have documented disease activity and afferent visual system involvement. Eight age and sex-matched healthy controls were also recruited for blood and bone marrow donation.
Bone marrow aspiration
Participants were admitted to the Dahms Clinical Research Unit at UHCMC. Bone marrow was aspirated from the posterior iliac crest under local anesthetic (bupivacaine, 0.25%, subcutaneously) with 2 mg morphine sulfate IV if needed for additional pain control; 20–50 ml bone marrow was briskly aspirated into a syringe containing preservative-free heparin (1000 μ/ml).
Culture expansion
MS and control/hMSC medium
Expansion of MS MSCs for clinical use was performed in a biological safety cabinet in an ISO 7 clean room suite. The marrow cell suspension was loaded onto a Percoll gradient (1.073 g/ml), at a 1:1 volume ratio and centrifuged at 900g for 30 minutes. The mononuclear cells (MNCs) were washed with Dulbecco’s phosphate-buffered saline (DPBS) then 30 × 106 cells were plated in 175 cm2 flasks containing hMSC medium (Dulbecco’s modified Eagle’s medium low glucose, 10% fetal bovine serum (FBS), antibiotic/antimycotic, Glutamax) and incubated at 37°C and 5% carbon dioxide. Medium was replaced 72 hours later, then every 3–4 days. Human fibroblast growth factor (R&D Systems) (10 ng/ml) was added to the hMSC medium at the initial medium change and throughout the rest of the expansion. When 95–100% confluent, cells were passaged into new culture flasks at 6 × 103/cm2 for expansion until the clinical dose was achieved. For passaging, cells were washed in DPBS and detached using porcine trypsin at 37°C for 5 minutes. Cells were counted by hemacytometer, centrifuged and plated or harvested as needed. Identical procedures were used to isolate MSCs from control donors aside from using a standard laminar flow tissue culture hood. Control MSCs were grown until they reached the same passage as their matched MSC participant, aliquotted and cryopreserved.
Control/Mosaic XF medium
The FDA encouraged development of a culture protocol without FBS. Thus marrow from the initial five control donors was divided prior to MNC isolation to allow parallel MSC expansion in either hMSC media or Mosaic XF medium (Becton Dickinson), a serum/xeno-free medium. For Mosaic XF expansion, the marrow suspension was diluted 1:1 in PBS and the mixture was loaded onto a Ficoll-Paque gradient (VWR Scientific) at a 2:1 ratio and centrifuged for 30 minutes at 540g. MNCs were washed with DPBS. For cell culture using the Mosaic medium, flasks were coated with a collagen/fibronectin blend up to passage 1, and fibronectin alone thereafter. Cells were initially plated at 30 × 103 cells/cm2 in a 175 cm2 flask containing Mosaic XF medium and processed as described above, but using this medium. Three control donors were aspirated after the manufacturer terminated production of this medium, so their aspirates were isolated and expanded as described for trial participants.
Preparation of cell product for autologous infusion
Once the target dose was reached, cells were washed in DPBS, detached using porcine trypsin at 37°C, and washed three times with Plasma-Lyte + 1% human serum albumin (HSA). Viability was determined by Trypan Blue exclusion. If ≥70%, cell concentration was adjusted to 8 × 106 cells/ml in Plasma-Lyte A + 5% HSA. Cells needed for release testing were removed; the remainder of the cell suspension was mixed 1:1 with a Plasma-Lyte A + 5% HSA/10% dimethyl sulfoxide freezing solution, placed into a Cryocyte bag (Baxter Scientific), and cryopreserved using a controlled-rate freezing chamber. Cells were stored in a temperature-monitored liquid nitrogen freezer until release criteria were confirmed.
Release testing
Cell product release criteria prior to infusion included viability (>70% by Trypan Blue exclusion), sterility (microbiological testing), product identity (immunophenotyping), as well as the absence of endotoxin and mycoplasma contamination.
Microbiological testing
Samples of culture media (5 ml/flask, pooled) were collected prior to every other cell passage and at harvest and analyzed in the UHCMC Microbiology Laboratory as per USP71 standards by membrane filtration and inoculation of thioglycollate broth and soybean-casein digest medium. Cultures were continued for 14 days with daily assessments. For infusion, the thawed cell product was withdrawn from the Cryocyte bag into a 50 ml syringe; 5 ml of sterile DPBS were injected into the bag, and a small aliquot was removed for viability testing. The remaining wash was transferred to a blood collection tube (Becton Dickinson) and sent to the Cleveland Clinic Microbiology Laboratory to screen for fungus and aerobic and anaerobic bacteria.
Product identity (immunophenotyping)
Product identity and purity were determined by cell surface marker expression by flow cytometry. hMSCs were identified by dual positive staining of 90% or more of the cells positive for CD10529,30 and CD73,29,30 and less than 5% positive for markers CD45 and CD14.30
Endotoxin
Endotoxin testing was performed on an aliquot of the final product prior to cryopreservation, using Endosafe-PTS and LAL (0.05 EU/ml sensitivity) cartridges (Charles River Laboratories). The endotoxin limit was 5 EU/kg of patient/total product.
Mycoplasma
Mycoplasma testing was performed on an aliquot of the final product prior to cryopreservation by Bionique Testing Laboratories, Inc. (Saranac Lake, NY, USA), using their M700 method.
PBMC isolation
Whole blood was collected from trial participants and controls, diluted in an equal volume of PBS (1:1 ratio), and loaded onto a Ficoll-Paque gradient (VWR Scientific) at a 2:1 blood:Ficoll ratio. Gradients were centrifuged for 30 minutes at 540g. Peripheral blood mononuclear cells (PBMCs) were aspirated and washed in PBS prior to cryopreservation.
DNA copy number analysis
As culture expansion has been linked to the possible introduction of cytogenetic changes in MSC populations, molecular cytogenetic analyses were performed on MSCs from the 18 clinical trial participants and five controls with sufficient available samples, expanded by an identical protocol in hMSC media. DNA was isolated from cryopreserved cell pellets using the QIAamp DNA mini-prep kit (Qiagen, Boston, MA, USA). DNA concentration was determined using a Nanodrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). DNA from each sample was hybridized to the HumanCytoSNP-12 DNA Analysis BeadChip (Illumina, San Diego, CA, USA). Illumina’s Infinium assay was performed by the Tecan eight-tip robot housed in the Cleveland Clinic Genomics Core. Hybridized chips were scanned using the iScan system. Data were analyzed using Genome Studio. The DNA copy number profile of the PBMC sample was considered to reflect the donor’s germline state; deviations detected in MSCs were considered to have developed during MSC isolation and culture expansion.
Statistical analysis
Scatterplots and boxplots are used for graphical description. To determine if MSC growth differed between subject subgroups or culture protocols, we linearly regressed the log cell counts of each cell product on time, and compared the estimated slopes between groups using Student’s independent samples t test, or the Mann–Whitney Wilcoxon test if substantial within-group skewing was observed.
Results
Twenty five MS patients were enrolled in the clinical trial.26 MSCs from one participant grew slowly and ceased proliferating after 25 days, failing to reach the target dose. This participant declined repeat bone marrow aspiration, withdrew from the trial and was replaced. Table 1 summarizes demographic and cell production characteristics of the clinical trial participants.
Table 1.
Summary of participant characteristics and cell production endpoints.
| Study ID | MS type | Age | Sex | Marrow volume aspirated (ml) | Initial MNC yield at harvest (×106) | Final MSC yield (×106) | Cells for Infusion (×106) | Actual dose (×106/kg) | Terminal passage number | Days in Culture |
|---|---|---|---|---|---|---|---|---|---|---|
| MSC-001 | SP | 45 | M | 52.0 | 391.6 | 586.0 | 182.8 | 2.00 | 2 | 34 |
| MSC-002 | RR | 39 | F | 69.3 | 273.35 | 194.0 | 144.0 | 2.01 | 1 | 21 |
| MSC-003 | SP | 44 | F | 63.0 | 151.8 | 137.5 | 124.5 | 1.57 | 3 | 41 |
| MSC-004 | SP | 55 | F | 72.1 | 317.07 | 110.0 | 81.2 | 1.48 | 3 | 34 |
| MSC-005 | SP | 41 | F | 77.0 | 377.14 | 131.0 | 109.2 | 1.95 | 3 | 49 |
| MSC-006 | SP | 43 | M | 81.0 | 526.14 | 250.0 | 174.6 | 1.89 | 2 | 24 |
| MSC-007 | SP | 39 | F | 70.0 | 579.6 | 475.0 | 89.2 | 2.00 | 1 | 18 |
| MSC-008 | RR | 42 | F | 74.2 | 262.35 | 502.0 | 133.6 | 2.00 | 1 | 20 |
| MSC-009 | RR | 47 | M | 68.6 | 591.1 | 263.0 | 172.8 | 1.99 | 1 | 20 |
| MSC-010 | SP | 54 | M | 84.0 | 364.8 | 231.0 | 183.6 | 2.00 | 3 | 34 |
| MSC-011 | RR | 47 | F | 86.0 | 1127 | 346.0 | 135.2 | 1.95 | 1 | 16 |
| MSC-012 | RR | 43 | F | 81.6 | 369.36 | 0.3 | N/Aa | N/Aa | N/Aa | 28 |
| MSC-014 | SP | 52 | M | 76.3 | 173.8 | 304.0 | 156.2 | 1.97 | 1 | 23 |
| MSC-015 | RR | 49 | F | 85.6 | 765.18 | 222.0 | 213.8 | 1.97 | 3 | 34 |
| MSC-016 | SP | 44 | F | 78.4 | 656.65 | 311.0 | 193.2 | 1.97 | 1 | 24 |
| MSC-017 | RR | 35 | F | 72.8 | 349.6 | 233.0 | 113.0 | 2.00 | 1 | 21 |
| MSC-018 | SP | 48 | M | 86.4 | 780 | 226.0 | 196.4 | 2.04 | 1 | 23 |
| MSC-019 | SP | 49 | M | 114.4 | 914.76 | 222.0 | 169.0 | 2.00 | 1 | 20 |
| MSC-020 | RR | 46 | F | 84.0 | 217.6 | 247.0 | 164.8 | 1.92 | 2 | 23 |
| MSC-021 | SP | 49 | F | 78.4 | 316.4 | 332.0 | 119.0 | 1.90 | 2 | 42 |
| MSC-022 | SP | 50 | F | 73.5 | 220.73 | 86.0 | 80.0 | 1.27 | 3 | 62 |
| MSC-023 | RR | 53 | M | 72.1 | 755.55 | 386.0 | 250.4 | 2.01 | 3 | 38 |
| MSC-024 | RR | 43 | F | 76.0 | 987.16 | 181.0 | 123.2 | 1.91 | 2 | 41 |
| MSC-025 | SP | 55 | F | 65.8 | 596.24 | 157.0 | 132.0 | 1.99 | 2 | 30 |
| MSC-026 | RR | 47 | F | 65.8 | 422.94 | 434.0 | 224.0 | 1.96 | 2 | 27 |
| n | 25 | 25 | 25 | 25 | 24 | 24 | 24 | 25 | ||
| Mean | 46.36 | 76.3 | 499.52 | 262.7 | 152.74 | 1.91 | 1.88 | 29.96 | ||
| SD | 5.22 | 11.4 | 267.46 | 137.4 | 45.18 | 0.19 | 0.85 | 11.28 | ||
| Median | 47.00 | 76.0 | 391.60 | 233.0 | 150.1 | 1.92 | 2.00 | 25.50 |
aCulture failure.
Study ID: de-identified code assigned to each study participant. MS type: MS disease course of participant at study entry: RR: relapsing–remitting MS; SP: secondary progressive MS; Age: age of participant at study entry; Sex: sex of participant at study entry; Marrow volume aspirated: total volume, in ml, of bone marrow aspirated; Initial yield at harvest: number of mononuclear cells initially isolated from aspirated bone marrow; Final yield: total number of MSCs harvested at time of termination of culture expansion; Cryopreserved for infusion: total number of MSCs cryopreserved specifically for infusion into the study participant; Target number calculated as 2.0 x 106 cells/kg participant body weight as measured at screening study visit; actual number cryopreserved varied depending on yield of final culture; Actual dose: dose of MSCs infused expressed as total number of MSCs/kg body weight of study participant as measured at the baseline study visit; Terminal passage number: passage number of MSC culture at time of harvest; Days in culture: number of days from initial plating of mononuclear cells isolated from bone marrow aspiration to day of harvest of terminal culture.
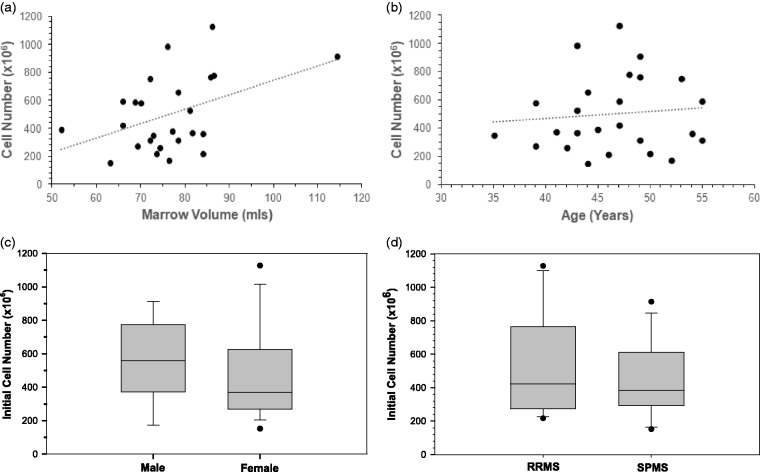
The number of nucleated cells isolated from the bone marrow aspirate varied widely across participants, from 150 to 1125 × 106 cells. The total number of nucleated cells isolated correlated moderately with aspiration volume, as might be anticipated (Figure 2(a)), but did not vary statistically significantly by participant age, sex, or MS course (Figure 2(b) to (d)).
Figure 2.
Mononuclear cell isolation from bone marrow aspirates. (a) As anticipated, the number of mononuclear cells (MNCs) isolated was moderately positively correlated with the volume of marrow drawn (r=0.44). (b) Number of MNCs isolated was not notably correlated with participant age (r=0.09, P=0.76). (c) Number of MNCs isolated from bone marrow aspirates was not significantly related to sex of participants. Two-tailed t-test, P=0.43. (d) Number of MNCs isolated from bone marrow aspirates was not significantly related to multiple sclerosis disease status of participants. Two-tailed t-test, P=0.36.
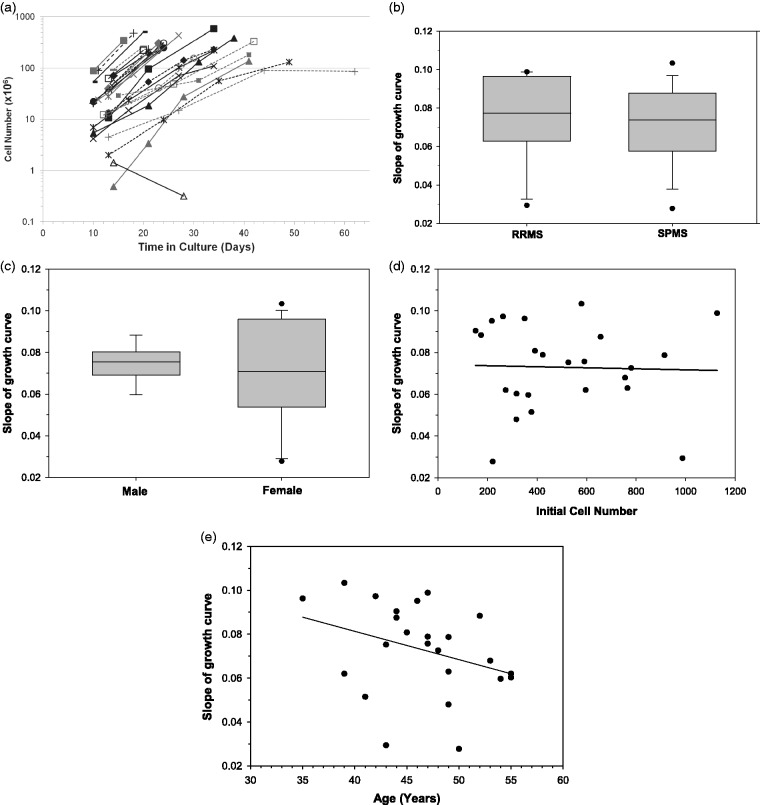
Culture time for the MS MSCs, excluding the participant with morphological changes and abnormal growth, ranged from 16 to 62 days. Twenty-one per cent of the 25 participants received within ±5.5% of the target dose of 2 × 106 cells/kg. The growth profiles of cell products of three participants were less robust. These participants’ MSCs were harvested once the number exceeded the protocol-specified minimum of 1 × 106 cells/kg required for transplantation. The log scale proliferation curves of the cell products (Figure 3(a)) demonstrate variability in both times and passage numbers required to achieve the target dose, as well as the general consistency of growth rates, except for the one culture failure, which was the only culture with a negative growth rate.
Figure 3.
Growth profiles of multiple sclerosis (MS) mesenchymal stem cell (MSC) cultures. (a) Summary of growth curves for MSC cultures for each study participant who underwent a bone marrow aspiration. Logarithmic y-axis used to highlight the consistency of growth rates across a majority of the MSC cultures. (b) Mean slope of MSC culture growth curves did not significantly differ by participant MS disease type. Two-tailed t-test, P=0.98. (c) Mean slope of MSC culture growth curves did not significantly differ by participant sex. Two-tailed t-test, P=0.50. (d) Slope of MSC culture growth curve was not significantly associated with initial mononuclear cell number (r=0.03, P=0.86). (e) Slope of MSC culture growth curve was modestly negatively and non-significantly correlated with participant age at study entry (r=–0.33, P=0.57).
There were no significant relationships between growth rate and MS disease course, sex, or number of nucleated cells in the bone marrow aspirate (Figure 3(b) to (d)). Culture growth rate was modestly, non-significantly, negatively correlated with patient age (Figure 3(e)).
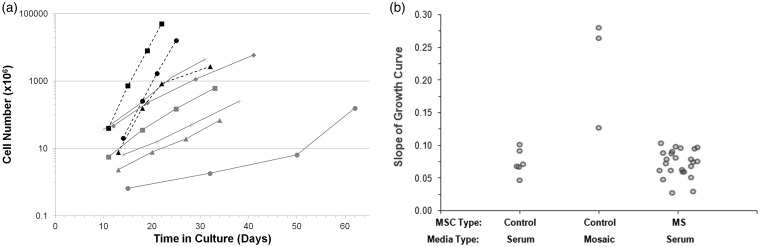
The demographics and culture characteristics of control donors are summarized in Table 2. Cells in two control cultures expanding in hMSC medium stopped proliferating and were considered culture failures. In addition, two cultures from MS participants expanding in Mosaic medium became contaminated and were terminated, leaving only one remaining matched MS–control comparison. The growth curves of the successful control cultures are displayed in Figure 4(a). The growth rates of the three control cultures expanded in Mosaic medium exceeded, and two of three more than doubled, those of all MS participant and control cultures expanded in hMSC medium (Figure 4(b)).
Table 2.
Summary of control donor characteristics and cell production endpoints.
| ID | Age | Sex | Trial participant match | Marrow volume (ml) | MNC number plated in MSC media (×106) | MNC number plated in Mosaic media (×106) | Final MSC yield (×106) | Days in Culture |
|---|---|---|---|---|---|---|---|---|
| C-001 | 54 | F | MSC-015 | 5.9 | 39.6 | N/Aa | N/Aa | |
| 5.9 | 30.6 | 16284.3 | 25 | |||||
| C-002 | 34 | F | MSC-017 | 14.0 | 168.0 | N/Aa | N/Aa | |
| 14.0 | 103.5 | 51120.7 | 22 | |||||
| C-003 | 59 | F | MSC-004 | 16.8 | 50.0 | 158.7 | 62 | |
| 16.8 | 109.1 | N/Ab | N/Ab | |||||
| C-004 | 54 | M | MSC-014 | 17.0 | 112.1 | 621.7 | 33 | |
| 17.0 | 147.3 | N/Ab | N/Ab | |||||
| C-005 | 53 | F | MSC-011 | 20.5 | 62.4 | 68.97 | 34 | |
| 10.0 | 29.7 | 2748.3 | 32 | |||||
| C-006 | 43 | F | MSC-007 | 32.5 | 316.8 | 5968.3 | 41 | |
| C-007 | 44 | F | MSC-020 | 20.0 | 342.0 | 4488.6 | 31 | |
| C-008 | 45 | F | MSC-008 | 20.0 | 450.0 | 254.4 | 38 |
aCulture failure.
bCulture contamination.
Study ID: de-identified code assigned to each study participant; Age: age of participant at study entry; Sex: sex of participant at study entry; Trial participant match: study ID of MS trial participant to which the control donor was matched; Marrow volume aspirated: total volume, in ml, of bone marrow aspirated; Initial yield of MNCs at harvest: number of mononuclear cells initially isolated from aspirated bone marrow; MNC number plated in MSC media: number of MNCs plated for expansion in MSC media; MNC number plated in Mosaic media: number of MNCs plated for expansion in Mosaic media; Final MSC yield: total number of MSCs harvested at time of termination of culture expansion; Terminal passage number: passage number of MSC culture at time of harvest; Days in culture: number of days from initial plating of mononuclear cells isolated from bone marrow aspiration to day of harvest of terminal culture.
Figure 4.
Growth profiles of control mesenchymal stem cell (MSC) cultures. (a) Summary of growth curves for MSC cultures for each control donor who underwent a bone marrow aspiration. Mononuclear cells isolated from the aspirates were grown in either MSC media (containing fetal bovine serum) or Mosaic media, a xeno-free alternative. Each data point represents the cell count at the time of a cell passage or harvest. Grey, solid lines = MSC media. Black, dotted lines = Mosaic media. (b) Comparison of the slope (growth rate) of each of three groups of MSCs expanded in culture. Multiple sclerosis trial participant MSCs expanded in serum-containing MSC media (n=24), control MSCs expanded in serum-containing MSC media (n=6) and control MSCs expanded in xeno-free Mosaic media (n=3).
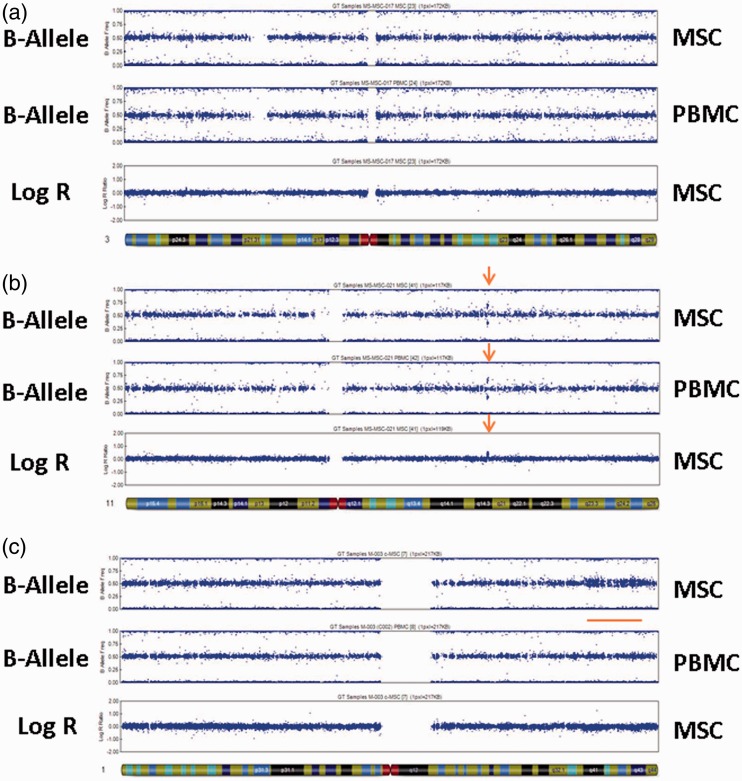
Allele and Log R analyses of the single nucleotide polymorphism (SNP) data were performed on all chromosomes. Figure 5(a) depicts the B-allele and Log R plots from one participant for which chromosome 3 showed no variations from normal copy number. Figure 5(b) represents the profile of chromosome 11 from another participant, in which a small amplification was detected in the B-allele and Log R profiles (red arrows) in both PBMCs and culture-expanded MSCs, indicating that it was present in the donor’s germline DNA and was not culture-induced.
Figure 5.
Representative single nucleotide polymorphism (SNP) array profiles. A normal profile is expected to show heterozygous SNPs tightly clustered around a B-allele frequency of 0.5 and Log R ratio of 0. Deviations from this indicate the presence of a deletion or duplication. (a) B-allele and Log R plots of SNP profile for chromosome 3, isolated from mesenchymal stem cell (MSC) and peripheral blood mononuclear cell (PBMC) samples from a trial participant. No variations from normal copy number are noted. (b) B-allele and Log R plots of SNP profile for chromosome 11, isolated from MSC and PBMC samples from a trial participant. Duplication of a small region of DNA was identified in both MSC and PBMC samples, and is likely a normal copy number polymorphism (red arrows). (c) B-allele and Log R plots of SNP profile for chromosome 1, isolated from MSC and PBMC samples from a control donor. A slight variation in copy number from what is normal was identified in a region of DNA in MSC but not PBMC samples, indicative of a deletion that is present in about 10–20% of the MSCs (red bar).
A single genetic change not present in the paired PBMCs was identified in one control MSC sample (red bar). Figure 5(c) shows this modest copy number change in the distal portion of the q arm of chromosome 1. The Log R profile shows a slight decrease in copy number, indicating that the cytogenetic change is a deletion in approximately 20% of cells. These MSCs had undergone a 62-day culture expansion, longer than any other sample (mean 27 days).
Discussion
MSC transplantation is under study as a potential MS therapy. The number of MSCs that can be directly isolated from bone marrow aspirates is well below the cell number believed to have therapeutic value, necessitating either infusion of a mixed cell population or culture-expansion.31 In this phase I trial of MSC transplantation in MS, 24 of the 25 MSC culture expansions (96%) were successfully completed.26 Three of the 24 successful culture expansions (12.5%) were harvested after a decrease in growth rate, before the target number of 2 × 106 cells/kg weight was reached but well within the protocol-specified dose range. As with the single MS culture failure, these growth decelerations were unexplained.
The proliferation rates for most cultures were similar; however, they differed in the numbers of days and passages required to achieve the absolute numbers of cells required for the protocol-specified infusion dose based on kg body weight. There were no evident relationships of growth rate and success in achieving the required dose to participant sex, MS disease characteristics, or the numbers of cells initially isolated from the bone marrow aspiration. In this small study, we saw no indication of any difference in culture expansion as a function of clinical features, suggesting it may be feasible to expand in culture MSCs from individuals with MS, regardless of disease course. Consistent with other reports, the growth rates of MS MSCs in culture declined modestly, although non-significantly, with participant age,32 a relationship of potential importance if reproduced in larger studies. Growth rates varied within a limited range with few exceptions (Figure 3(a)), so that the number of cells noted at initial passage was reasonably indicative of the final yield.
Strict release criteria were employed to ensure that the cell products were viable (both at the time of cryopreservation and infusion), sterile (no detectable bacteria, fungus, or mycoplasma), endotoxin-free, and phenotypically pure (as determined by cell surface markers). All 24 successful cultures fulfilled the release criteria. To inform future studies using this cell production method, we assessed the cytogenetic stability of the expanded cell products using modern molecular cytogenetic techniques. SNP profiles of paired MSC and PBMC samples from 18 of 24 trial participants and five healthy controls identified only a single cytogenetic change likely to have developed during MSC culture expansion. While this result is reassuring, it occurred in the cell sample cultured for the longest time, and suggests screening for cytogenetic changes in cultures that extend beyond three passages and/or 60 days. If more cells per dose or multiple doses are required for a protocol, extended culture time may be required. Incorporating cytogenetic analysis into the release criteria should be considered in these cases to minimize the chance of infusing MSCs that have acquired transformative changes in culture.
The FDA encouraged the use of serum-free culture media for future studies. Therefore, we cultured expanded MSCs from control donors in standard hMSC medium and Mosaic XF, a serum-free, xeno-free product. Growth of the three uncontaminated MSC cultures in the Mosaic medium was faster and resulted in a better final yield than any of the cultures in hMSC medium. Unfortunately, before these studies were completed, the manufacturer permanently discontinued production of Mosaic XF, preventing further investigation. These results, while preliminary, are encouraging and suggest that improvements in the media formulations used in MSC manufacturing procedures may improve both the growth rate and proliferation capacity of MSCs, while removing the potential safety issues with FBS.
This study demonstrates that culture expansion of MSCs for a single infusion is feasible. However, three MS MSC cultures exhibited slow growth, and a cytogenetic abnormality was noted in control MSCs with extended time in culture. With accumulating evidence that more than one dose of MSCs may be needed to show therapeutic benefit,2,3 more efficient MSC culture expansion methods for MSCs are required. Our limited preliminary data suggest that serum-free media may provide increased efficiency and safety for future MSC culture for clinical use.
Acknowledgements
The authors would like to thank Cynthia Mackey, Vinette Zinkand and the staff of the Dahms Clinical Research Unit, Seidman Cancer Center for their assistance with the study. Preliminary results of this study were presented at the 2014 Joint ACTRIMS-ECTRIMS Meeting in Boston, MA, USA, 10–13 September 2014.
Author contribution
Study conception or design: SM Planchon, JA Cohen, PB Imrey, J Reese Koç, HM Lazarus, KM Drake and MA Aldred. Study supervision or coordination: all authors. Analysis or interpretation of data: all authors. Statistical analysis: SM Planchon, PB Imrey. Drafting and revising the manuscript for content: all authors.
Conflict of Interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JA Cohen reports personal compensation for consulting for Adamas, Merck, Mallinkrodt, Novartis and Receptos and as co-editor of the Multiple Sclerosis Journal – Experimental, Translational and Clinical. SM Planchon, KT Lingas, JR Koc, BM Hooper, B Maitra, RM Fox, PB Imrey, KM Drake, MA Aldred and HM Lazarus, report no conflicts of interest.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this study was provided by grants from the Department of Defense (W81XWH-10-1-0270), NIH (R01 NS074787), National Multiple Sclerosis Society (PP-1752) and Cleveland Clinic Research Programs Committee (2012-1017) to JAC.
References
- 1.Freedman MS. Present and emerging therapies for multiple sclerosis. Continuum (Minneapolis, Minn) 2013; 19: 968–991. [DOI] [PubMed] [Google Scholar]
- 2.Auletta JJ, Bartholomew AM, Maziarz RT, et al. The potential of mesenchymal stromal cells as a novel cellular therapy for multiple sclerosis. Immunotherapy 2012; 4: 529–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen JA. Mesenchymal stem cell transplantation in multiple sclerosis. J Neurol Sci 2013; 333: 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freedman MS, Bar-Or A, Atkins HL, et al. The therapeutic potential of mesenchymal stem cell transplantation as a treatment for multiple sclerosis: consensus report of the International MSCT Study Group. Mult Scler (Houndmills, Basingstoke, England) 2010; 16: 503–510. [DOI] [PubMed] [Google Scholar]
- 5.Slavin S Kurkalli BG andKarussis D.. The potential use of adult stem cells for the treatment of multiple sclerosis and other neurodegenerative disorders. Clin Neurol Neurosurg 2008; 110: 943–946. [DOI] [PubMed] [Google Scholar]
- 6.Uccelli A Laroni A andFreedman MS.. Mesenchymal stem cells for the treatment of multiple sclerosis and other neurological diseases. Lancet Neurol 2011; 10: 649–656. [DOI] [PubMed] [Google Scholar]
- 7.Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 2002; 418: 41–49. [DOI] [PubMed] [Google Scholar]
- 8.Aggarwal S andPittenger MF.. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005; 105: 1815–1822. [DOI] [PubMed] [Google Scholar]
- 9.Meirelles Lda S, Fontes AM, Covas DT, et al. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev 2009; 20: 419–427. [DOI] [PubMed] [Google Scholar]
- 10.Dong F andCaplan AI.. Cell transplantation as an initiator of endogenous stem cell-based tissue repair. Curr Opin Organ Transplant 2012; 17: 670–674. [DOI] [PubMed] [Google Scholar]
- 11.Ferreira RJ, Irioda AC, Cunha RC, et al. Controversies about the chromosomal stability of cultivated mesenchymal stem cells: their clinical use is it safe? Curr Stem Cell Res Ther 2012; 7: 356–363. [DOI] [PubMed] [Google Scholar]
- 12.Munneke JM, Spruit MJ, Cornelissen AS, et al. The potential of mesenchymal stromal cells as treatment for severe steroid-refractory acute graft-versus-host disease: a critical review of the literature. Transplantation 2016; 100: 2309–2314. [DOI] [PubMed] [Google Scholar]
- 13.Wagner W, Wein F, Seckinger A, et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol 2005; 33: 1402–1416. [DOI] [PubMed] [Google Scholar]
- 14.Jones EA, Kinsey SE, English A, et al. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum 2002; 46: 3349–3360. [DOI] [PubMed] [Google Scholar]
- 15.Bentivegna A, Miloso M, Riva G, et al. DNA methylation changes during in vitro propagation of human mesenchymal stem cells: implications for their genomic stability? Stem Cells Intl 2013; 2013: 192425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernardo ME, Zaffaroni N, Novara F, et al. Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res 2007; 67: 9142–9149. [DOI] [PubMed] [Google Scholar]
- 17.Bernardo ME, Avanzini MA, Perotti C, et al. Optimization of in vitro expansion of human multipotent mesenchymal stromal cells for cell-therapy approaches: further insights in the search for a fetal calf serum substitute. J Cell Physiol 2007; 211: 121–130. [DOI] [PubMed] [Google Scholar]
- 18.Caisander G, Park H, Frej K, et al. Chromosomal integrity maintained in five human embryonic stem cell lines after prolonged in vitro culture. Chromosome Res: an international journal on the molecular, supramolecular and evolutionary aspects of chromosome biology. 2006; 14: 131–137. [DOI] [PubMed] [Google Scholar]
- 19.Zhang ZX, Guan LX, Zhang K, et al. Cytogenetic analysis of human bone marrow-derived mesenchymal stem cells passaged in vitro. Cell Biol Intl 2007; 31: 645–648. [DOI] [PubMed] [Google Scholar]
- 20.Breitbach M, Bostani T, Roell W, et al. Potential risks of bone marrow cell transplantation into infarcted hearts. Blood 2007; 110: 1362–1369. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Huso DL, Harrington J, et al. Outgrowth of a transformed cell population derived from normal human BM mesenchymal stem cell culture. Cytotherapy 2005; 7: 509–519. [DOI] [PubMed] [Google Scholar]
- 22.Miura M, Miura Y, Padilla-Nash HM, et al. Accumulated chromosomal instability in murine bone marrow mesenchymal stem cells leads to malignant transformation. Stem cells (Dayton, Ohio) 2006; 24: 1095–1103. [DOI] [PubMed] [Google Scholar]
- 23.Pan Q, Fouraschen SM, de Ruiter PE, et al. Detection of spontaneous tumorigenic transformation during culture expansion of human mesenchymal stromal cells. Exp Biol Med (Maywood, NJ) 2014; 239: 105–115. [DOI] [PubMed] [Google Scholar]
- 24.Tolar J, Nauta AJ, Osborn MJ, et al. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells (Dayton, Ohio) 2007; 25: 371–379. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Zhang Z, Chi Y, et al. Long-term cultured mesenchymal stem cells frequently develop genomic mutations but do not undergo malignant transformation. Cell Death Dis 2013; 4: e950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen JA, Imrey PB, Planchon SM, et al. Pilot trial of intravenous autologous culture-expanded mesenchymal stem cell transplantation in multiple sclerosis. Mult Scler (Houndmills, Basingstoke, England) 2017; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lazarus HM, Haynesworth SE, Gerson SL, et al. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant 1995; 16: 557–564. [PubMed] [Google Scholar]
- 28.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983; 33: 1444–1452. [DOI] [PubMed] [Google Scholar]
- 29.Ramos TL, Sanchez-Abarca LI, Muntion S, et al. MSC surface markers (CD44, CD73, and CD90) can identify human MSC-derived extracellular vesicles by conventional flow cytometry. Cell Commun Signal 2016; 14: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobs SA, Roobrouck VD, Verfaillie CM, et al. Immunological characteristics of human mesenchymal stem cells and multipotent adult progenitor cells. Immunol Cell Biol 2013; 91: 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scolding N. Adult stem cells and multiple sclerosis. Cell Prolif 2011; 44 (Suppl 1): 35–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu H Xia X andLi B.. Mesenchymal stem cell aging: mechanisms and influences on skeletal and non-skeletal tissues. Exp Biol Med (Maywood, NJ) 2015; 240: 1099–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]