Abstract
Background:
Although many hospitalized neuroscience patients have physical and occupational therapy (rehabilitation) needs, patients with none or minimal physical impairments frequently receive rehabilitation consultation, diverting from patients with greatest need.
Methods:
A multidisciplinary team on the general and cerebrovascular neurology acute inpatient services mapped the rehabilitation consultation process, resulting in multiple implemented interventions including physician education on appropriate acute rehabilitation consultations, modification of multidisciplinary rounds, and discussion of patient rehabilitation needs throughout hospitalization. Nurses used the same functional impairment measurement tool used by physical and occupational therapists, the Activity Measure for Post-Acute Care Inpatient Short Forms (Basic Mobility and Activity domains).
Results:
The rate for initial rehabilitation consults for patients with no limitations in mobility or activity during the 6-month baseline period was 12%, which was decreased to 7% and 10% during the 6-month intervention and sustain periods, respectively (P < .001). The baseline rate for patients with no limitations receiving both physical therapy and occupational therapy consultations was 62% and was decreased to 21% and 39% in the intervention and sustain periods, respectively (P < .001). Rehabilitation sessions per hospital day increased for patients with high functional impairments, from 0.52 at baseline to 0.64 in the intervention and 0.66 in the sustain periods (P = .02), which equated to 1 more rehabilitation visit per patient hospitalization.
Conclusions:
A multifaceted intervention led to improved utilization of acute inpatient rehabilitation consultation while increasing the frequency of rehabilitation treatment for patients with highest functional impairment.
Keywords: neurology, stroke, rehabilitation, quality
Introduction
As is true elsewhere in medicine, value of neurological care delivery is determined by the quality of care delivered, among other outcomes, with respect to the costs of care delivery.1 Impairments in patient mobility and activity can predict hospital quality metrics such as prolonged length of hospital stay and readmission,2,3 while utilization of acute inpatient physical therapy following acute stroke is not only associated with a lower than expected cost of care but also increased probability of discharge home.4 A key issue is reliably identifying those acute inpatients without mobility or activity impairments to eliminate wasteful physical and occupational therapy (rehabilitation) consultation requests and better direct rehabilitation resources to inpatients with rehabilitation needs.
As part of an institution-level Choosing Wisely effort.5 The Johns Hopkins Hospital Departments of Physical Medicine & Rehabilitation (PM&R) and Neurology collaborated to improve utilization of rehabilitation consultation for neurology inpatients. Choosing Wisely is an initiative of the American Board of Internal Medicine, with the stated purpose of “advancing a national dialogue on avoiding wasteful or unnecessary medical tests, treatments and procedures.”5 Over 70 specialty and subspecialty societies have developed evidence-based recommendations through this initiative, including the American Academy of Neurology and American Academy of Physical Medicine and Rehabilitation. Our concern was rehabilitation consultation overutilization for those patients with no impairment in activity or mobility, with consequent diminished acute inpatient rehabilitation treatment frequency for those with greatest need. We set out to better understand our rehabilitation consultation processes, with the goal of developing interventions and processes to better direct rehabilitation resources among our acute neurological inpatient population.
Methods
The study was approved by the institutional review board of Johns Hopkins School of Medicine with approval for nurse participation through the Johns Hopkins Hospital Department of Nursing. A waiver of informed consent was granted since the intervention was part of a quality improvement project, and data were collected retrospectively.
Setting
This study was conducted on 2 mixed neurology and neurosurgery acute care units (total of 64 beds) at the Johns Hopkins Hospital, an academic medical center. The cerebrovascular and general neurology inpatient services are academic, with rotating medical students, resident physicians, and attending faculty as well as an advanced clinical practice provider. The cerebrovascular service additionally includes cerebrovascular fellowship trainees. Multidisciplinary care is delivered by the neurology physician teams, nurses, clinical support staff, physical therapists, occupational therapists, speech language pathologists, dietitians, and pharmacists.
Intervention Development
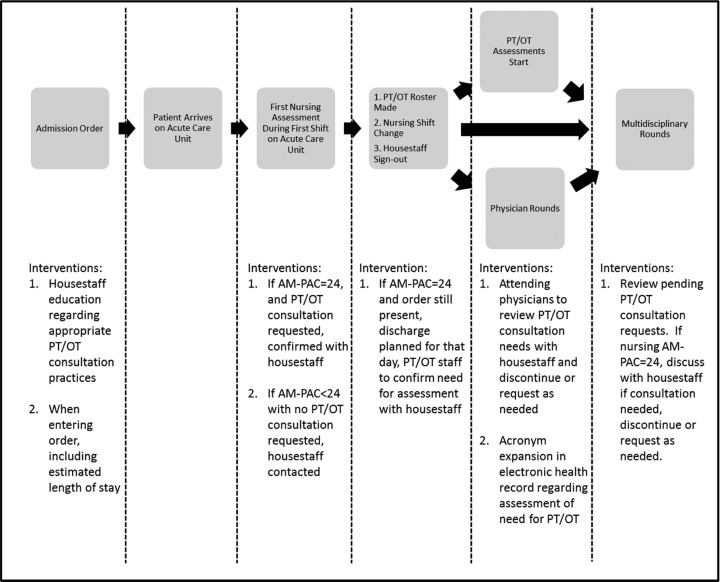
A team composed of nursing staff, physical and occupational therapists, cerebrovascular and general neurology attending physicians, PM&R physician and administrative leaders, hospital patient safety and quality improvement coordinators, and neurosciences departmental leadership was formed to examine the process for rehabilitation consultation. Applying Lean Six Sigma methodology, a process map for rehabilitation consultation was made through intervention team meetings and meetings with residents, nurses, physical, and occupational therapists.6 This process was mapped from resident admission order entry to initial nursing assessment, physical and occupational therapist daily coverage preparation, physician team rounding, and multidisciplinary care coordination rounding where all disciplines are present to discuss patient care (Figure 1).
Figure 1.
Rehabilitation therapy request process map and interventions.
Interventions were developed to directly impact physician rehabilitation consultation practices, nursing assessment of patient mobility and activity limitations, physical and occupational therapist daily coverage preparation procedures, physician team rounding and documentation, and multidisciplinary care coordination rounds (Figure 1). Physical and occupational therapists were invited to resident orientation to present on appropriate rehabilitation consultation practices. Emphasis was placed on specificity of rehabilitation consultation, with consideration for each patient’s need for physical or occupational therapy, and to request either as needed rather than indiscriminate requesting of both for patients they felt had acute inpatient rehabilitation therapy needs. Residents were also educated to enter specific indications for rehabilitation consultation requests and patient anticipated date of discharge at the time of consultation order entry in the electronic provider order entry and health record system (EHR).
Nursing staff were trained to assess patient limitations in activity and mobility using the Activity Measure for Post-Acute Care (AM-PAC) Inpatient Activity Short Form and Inpatient Mobility Short Form. The AM-PAC is a set of instruments developed by the Boston University Health and Disability Research Institute.7 The AM-PAC was designed to measure functional outcomes across acute and postacute settings, diagnoses, and conditions to examine functional outcomes as patients move across the care continuum.8-10 The AM-PAC inpatient short forms for activity and mobility consist of 6 questions each,7 and they were adopted by the Johns Hopkins Department of PM&R for use by occupational and physical therapists to assess patient limitations across the institution.
Physical and occupational therapists trained neuroscience nursing staff in the completion of AM-PAC activity and mobility short-form assessments. Nursing staff were educated to complete initial AM-PAC activity and mobility short-form assessments within the first 8-hour shift for all acute inpatients. If a patient was scored to have no activity or mobility limitations (a raw score of 24) on the respective AM-PAC short form, and rehabilitation consultation requests had been submitted for the same patients, nurses were trained to contact the residents to confirm the necessity and indication for consultation. Finally, nursing staff were trained in documentation of the AM-PAC short-form scores for care provider review in the EHR (Figure 1).
With the nursing-determined AM-PAC activity and mobility short-form scores entered during the first shift of patient admission and the anticipated date of discharge entered by the admitting resident in the EHR, physical and occupational therapists scheduled consultations and treatments during daily organization. Priority was given for patients anticipated to be discharged the same day as consultation. Therapists also confirmed with residents the necessity of physical therapy consultation for patients with AM-PAC mobility short-form scores of 24 as well as for occupational therapy consultation for patients with AM-PAC activity short-form scores of 24.
Resident and attending physicians were encouraged to specifically discuss patient rehabilitation consultation needs during physician team rounds, adding and cancelling consultation requests as appropriate based upon team assessment and despite patient AM-PAC short-form scores. Standard text describing patient assessment for rehabilitation need was developed to meet quality assurance documentation standards. This standard text was added to the EHR as an acronym expansion tool for incorporation in clinical documentation as appropriate. Resident and attending physicians were trained on use of the acronym expansion tool during resident and division meetings (Figure 1).
Finally, multidisciplinary staff members were educated on the meaning of both AM-PAC short-form scores. Nursing AM-PAC activity and mobility short-form scores were incorporated in multidisciplinary care coordination rounding discussions. The necessity of rehabilitation consultations for patients with AM-PAC activity or mobility short-form scores of 24 was discussed during multidisciplinary rounds, with consultations cancelled or added as appropriate (Figure 1).
Patient Inclusion Criteria
All cerebrovascular and general neurology patients greater than 18 years of age and admitted to a neurosciences acute care unit from January 1, 2014, to December 31, 2015, for whom a rehabilitation consultation request was made were included.
Study Design and Analysis
The intervention group was a prospectively collected sample of consecutive patients during the 6-month period of January 1, 2015, to June 30, 2015. The sustain group was a prospectively collected sample of consecutive patients during the period of July 1, 2015, to December 31, 2015. Of note, no additional interventions (eg, educational sessions) occurred during the sustain period. The intervention and sustain groups were compared to a retrospective baseline cohort of consecutive patients during the 6-month period of January 1, 2014, through June 30, 2014, the period prior to formation of the multidisciplinary team.
Data were collected from administration, clinical, and quality improvement databases as well as the EHR. Demographic data included patient age, gender, race, insurance payer, and observed length of stay. Prespecified outcomes measured included the number of physical and occupational therapy consultation requests, visits, and treatments per consultation as well as injurious falls on the study units.
Functional impairment categories were based on standardized AM-PAC tertiles: high is <35.3, middle is 35.3 to 40.8, and low is ≥42.1; no impairment was defined as the highest standardized score on the inpatient AM-PAC mobility short form of 61.1 with a raw score of 24 or inpatient AM-PAC activity short form of 57.7 with a raw score of 24. Of note, patients with no impairment were included in the low functional impairment category for tertile comparisons. To compare proportions of rehabilitation visits (physical and occupational therapy) across time periods, a χ2 test was used. To compare the number of rehabilitation visits (physical and occupational therapy) per patient day across time periods for each impairment group, separate bivariate linear regression models were used. Finally, rate of injurious falls (the number of injurious falls by total patient days) between the time periods was compared using an exact Poisson method.11Data were analyzed with R (version 3.3.1; http://www.r-project.org). Two-tailed statistical significance was assessed at the P < .05 level.
Results
Patients
During the baseline period, 389 acute neurological inpatients were seen in rehabilitation consultation and received therapy treatment. The intervention period included 321 inpatients and the sustain period included 307 inpatients. The baseline, intervention, and sustain groups were well matched in terms of age, race, payer, diagnoses, and observed length of stay (Table 1).
Table 1.
Patient Characteristics for Each Time Period.a
| Patient Characteristics | Time Period | |||
|---|---|---|---|---|
| Baseline (January 1, 2014 to June 30, 2014), n = 389 | Intervention (January 1, 2015 to June 30, 2015), n = 321 | Sustain (July 1, 2015 to December 31, 2015), n = 307 | P Valueb | |
| Age | 54.5 (17.5) | 55.7 (17.7) | 54.9 (18.3) | .76 |
| Male | 192 (49%) | 135 (42%) | 122 (40%) | .01 |
| Race | .006 | |||
| Caucasian | 197 (51%) | 159 (50%) | 136 (44%) | |
| Black | 154 (40%) | 127 (40%) | 116 (38%) | |
| Other | 38 (10%) | 35 (11%) | 55 (18%) | |
| Payer | .13 | |||
| Medicare | 157 (40%) | 123 (38%) | 102 (33%) | |
| Medicaid | 74 (19%) | 60 (19%) | 60 (20%) | |
| Other | 158 (41%) | 138 (43%) | 145 (47%) | |
| Length of stay | 8.7 (9.5) | 8.4 (8.4) | 9.1 (10.3) | .58 |
| Diagnoses | .34 | |||
| Progressive diseasesc | 88 (23%) | 59 (18%) | 63 (21%) | |
| Stroke | 79 (20%) | 60 (19%) | 71 (23%) | |
| Infectious disease | 43 (11%) | 35 (11%) | 26 (8%) | |
| Seizure disorder | 31 (8%) | 31 (10%) | 33 (11%) | |
| Craniotomy and maxillofacial surgery | 13 (3%) | 11 (3%) | 16 (5%) | |
| General medical | 12 (3%) | 17 (5%) | 8 (3%) | |
| Spinal surgery | 10 (3%) | 10 (3%) | 14 (5%) | |
| Headache disorder | 6 (2%) | 6 (2%) | 2 (1%) | |
| Other | 106 (27%) | 109 (34%) | 82 (27%) | |
Abbreviation: AHRQ, Agency for Healthcare Research and Quality.
aContinuous variables are presented as mean (standard deviation), and dichotomous variables are presented as n (%)
bCompared using bivariate linear regression for continuous variables and χ2 for categorical variables, comparing baseline and sustain time periods.
cProgressive diseases include multiple sclerosis, amyotrophic lateral sclerosis, Parkinson, and chronic inflammatory demyelinating polyneuropathy.
Among the 133 patients with no functional impairments evaluated, 38 (29%) had a primary diagnosis of a progressive disease, such as multiple sclerosis, amyotrophic lateral sclerosis, Parkinson disease, or chronic inflammatory demyelinating polyneuropathy, 12 (9%) had a stroke, 12 (9%) had an infectious disease, 8 (6%) had a seizure, 7 (5%) had a general medical problem, 5 (4%) had a headache disorder, 4 (3%) had a spinal surgery, 2 (2%) had a craniotomy and maxillofacial surgery, and 45 (34%) had another diagnosis. Of these patients with no functional impairments, 118 (89%) were discharged home without additional services, 12 (9%) were discharged with home care services, 1 (1%) was discharged to skilled nursing facility, and 2 (2%) were discharged to an inpatient psychiatry unit. Their mean length of stay was 4.0 (standard deviation: 2.7) days.
Rehabilitation Consultation Utilization
During the baseline period, 12% (87/706) of initial rehabilitation visits were for patients with no limitations in activity or mobility (Table 2A). The proportion of initial rehabilitation visits for patients with no limitations decreased in both the intervention (55/584, 7%) and sustain periods (57 of 558, 10%), which represented a relative 42% and 17% decrease, respectively (P < .001). A similar pattern was observed in terms of total rehabilitation visits for patients with no activity or mobility impairment, with 6% (101/1603) in the baseline period, which decreased to 3% (43/1548) in the intervention period and 4% (59 of 1537) in the sustain periods, a relative decrease of 50% and 33%, respectively (P < .001; Table 2B).
Table 2.
Changes in Initial Rehabilitation (Physical and Occupational Therapy) and All Rehabilitation Visits by Functional Impairment Category and Project Time Period.a,b
| A. Initial rehabilitation visits | Time Period | ||||
|---|---|---|---|---|---|
| Baseline (n = 706) | Intervention (n = 584) | Sustain (n = 558) | Baseline Versus Intervention | Baseline Versus Sustain | |
| Functional impairment | P Valuec | P Valuec | |||
| Impairment category | .004 | <.001 | |||
| Low | 414 (59%) | 251 (43%) | 255 (46%) | ||
| Middle | 112 (16%) | 136 (23%) | 146 (26%) | ||
| High | 180 (25%) | 197 (34%) | 157 (28%) | ||
| No impairment | 87 (12%) | 40 (7%) | 54 (10%) | .009 | <.001 |
| B. All Rehabilitation Visits | |||||
| Functional Impairment | Baseline (n = 1603) | Intervention (n = 1548) | Sustain (n = 1537) | P Valuec | P Valuec |
| Impairment category | <.001 | <.001 | |||
| Low | 672 (42%) | 413 (27%) | 456 (30%) | ||
| Middle | 313 (20%) | 415 (27%) | 450 (29%) | ||
| High | 618 (39%) | 720 (47%) | 631 (41%) | ||
| No impairment | 101 (6%) | 43 (3%) | 59 (4%) | .03 | <.001 |
Abbreviations: AM-PAC, Activity Measure for Post-Acute Care.
aFunctional Impairment Categories are based on standardized AM-PAC tertiles: high is <35.3; middle is 35.3 to 40.8; low is ≥42.1.
bNo impairment is defined as the highest standardized score on the inpatient AM-PAC mobility short form of 61.1 or raw score of 24 or AM-PAC activity short form of 57.7 or raw score of 24.
cCalculated using χ2 test.
Notably, dual consultation for both physical and occupational therapy for patients with no impairment decreased from a baseline rate of 62% to 21% in the intervention period and 39% in the sustain period, a relative decrease of 66% and 37%, respectively (P < .001).
Rehabilitation Treatment Frequency
When rehabilitation consultations were requested, the mean number of treatment sessions per hospital day for the no impairment as well as the low and middle impairment patient tertiles did not change significantly across study periods. However, for the high impairment tertile of patients, the treatment intensity increased from 0.52 at baseline to 0.64 in the intervention period and sustained at 0.66 (P < .05). This equated to 1 more rehabilitation treatment session per patient hospitalization in the high impairment tertile group (Table 3).
Table 3.
Mean Number of Rehabilitation (Physical and Occupational Therapy) Treatment Sessions per Hospital Day by Functional Status Category and Project Time Period.a,b
| Time Period | |||||
|---|---|---|---|---|---|
| Functional impairment | Baseline Versus Intervention | Baseline Versus Sustain | |||
| Baseline (n = 389) | Intervention (n = 321) | Sustain (n = 307) | P Valuec | P Valuec | |
| Impairment category | |||||
| Low | 0.66 | 0.66 | 0.64 | .93 | .68 |
| Middle | 0.69 | 0.75 | 0.66 | .03 | .14 |
| High | 0.52 | 0.64 | 0.66 | .05 | .02 |
| No impairment | 0.57 | 0.58 | 0.53 | .91 | .77 |
Abbreviation: AM-PAC, Activity Measure for Post-Acute Care.
aFunctional impairment categories are based on standardized AM-PAC tertiles: high is <35.3, middle is 35.3 to 40.8, low is ≥42.1.
bNo impairment is defined as the highest standardized score on the inpatient AM-PAC of 61.1.
cCalculated using linear regression models for each impairment category.
Falls
Injurious fall rates per patient days during the intervention and sustain periods were 13 in 9777 and 8 in 9742, respectively, which were not higher than the baseline period (10 falls in 10 224 patient days; P = .81 and P = .89, respectively).
Discussion
Here, we present a multifaceted, multidisciplinary quality improvement project which increased care value by improving utilization patterns for acute inpatient rehabilitation consultation. The proportion of initial treatment visits for patients with no limitations in activity or mobility was not only reduced during the intervention period but also sustained during the 6 months following, reflecting maintained changes in process and behavior. Also, rehabilitation consultation practices changed, with a significant decrease in the proportion of dual physical and occupational therapy requests for those with no functional impairment. Finally, the number of rehabilitation therapy treatments for those with highest impairment was increased in intervention period relative to baseline and sustained.
Value of care can be improved by reducing costs through cost and waste reduction programs, initiatives to better target treatments and procedures to specific at-risk populations, and process improvement projects.1,12 The American Board of Internal Medicine’s Choosing Wisely campaign was undertaken to advance a national dialogue to improve care value by avoiding wasteful or unnecessary treatments and procedures.5 Institutions such as ours have encouraged departments to develop projects to increase care value. By better directing the resources of acute in-hospital rehabilitation consultations and treatments from those with no limitations in mobility or activity to those in need, further care value for this acute inpatient neurology population was created. Not only was it created, but as the observations of the sustain period demonstrate, it was maintained relative to the baseline period.
Impairments in mobility and activity are not uncommon among neurological patients. These impairments impact patient experience of disease, and their degrees are measured and followed through the course of disease and treatment using tools such as the Kurtzke Expanded Disability Status Scale in multiple sclerosis.13 In ischemic and hemorrhagic stroke, the assessment for the need for physical and occupational therapy for patients after stroke is considered a measure of care quality,14 with utilization of physical therapy following acute stroke being associated with less than expected cost of care and increased probability of discharge home.4 The same is true in neurodegenerative diseases such as Parkinson and Alzheimer, as requesting and performing occupational and physical therapy treatments are measures of care quality.15,16
We reason that this approach can be adopted in other acute inpatient populations and clinical settings because of the nature of the intervention. The approach consisted of training residents in appropriate rehabilitation consultation practices and ingraining discussions of activity and mobility in multidisciplinary care discussions. This training was included as inpatient physicians submit consultation requests, often with input from other members of the care team. Importantly, nurses utilized the same functional assessment tools that have been traditionally used by physical and occupational therapists. This approach involved training nurses in the administration and scoring of the AM-PAC activity and mobility short forms, and their assessments were available for physician and therapist review in the EHR.
The use of the AM-PAC short forms facilitated nurse, physician, and therapist discussion of patient rehabilitation needs. In the hospital setting, it is important to have different disciplines caring for the same patients using a common language to describe patient activity and mobility. By utilizing nursing evaluation of patient activity and mobility early in the hospitalization, the resource of rehabilitation consultation and treatment was directed away from those with no impairment, with the associated increased number of treatments for those with greatest impairment, and without an increase in rates of injurious falls.
This study is limited in its generalizability as it was performed at a single tertiary academic medical center and involved only an acute inpatient neurology patient population. In addition, it did not control for fluctuations in the number of physical and occupational therapists available during the baseline, intervention, and sustain periods. Physical and occupational therapy staff on the neuroscience units consisted of teams of therapists who provide consultation and treatment services across the Johns Hopkins Hospital. Since availability of rehabilitation staff depends on the needs of the entire hospital, it was not possible to calculate the full-time equivalents available to the neuroscience units specifically during the study periods. A third limitation is that only patients for whom an acute inpatient rehabilitation consultation was made were included here. It is possible that patients who needed rehabilitation services during hospitalization did not receive it, thus potentially limiting our ability to discern whether the intervention led to less rehabilitation consultations for patients in need. However, this seems less likely given the several assessments of patient therapy needs performed here. Anecdotally, there were instances where nursing AM-PAC scores prompted rehabilitation consultation; however, these data were not formally collected and we are unable to comment further. A fourth limitation is that through the course of this study, nurses were monitored for completion and documentation of assessments; however, the time necessary for nurse completion of AM-PAC assessments and the concordance between nurse and therapist AM-PAC assessments were not studied. This study has prompted subsequent reliability and validity studies of mobility measures between therapists and nurses at our institution. Finally, it would be interesting to note the impact of these interventions on other quality metrics, such as other hospital-acquired harms and discharge disposition, measures not investigated here.
Conclusion
By addressing the various aspects of acute inpatient rehabilitation therapy consultation utilization, patterns of overutilization were reduced for acute neurology inpatients with no functional impairment as captured by validated mobility and activity assessment tools. In addition, the frequency of rehabilitation therapy treatment was increased for those with greatest impairment. These effects were not only seen during a focused quality improvement initiative but sustained well after. Future prospective studies across other medical and surgical populations and institutions are warranted to confirm this observation.
Footnotes
Authors’ Notes: Dr Probasco participated in study conception and design, data collection, data analysis, manuscript drafting, and manuscript revision. Ms Lavezza participated in study conception and design, data collection, and manuscript revision. Ms Shakes participated in study conception and design, data collection, and manuscript revision. Ms Feurer participated in data collection and manuscript revision. Ms Russell participated in study conception and design, data collection, and manuscript revision. Ms Sporney participated in study conception and design. Ms Burnett participated in study conception and design and manuscript revision. Ms Maritim participated in data collection and manuscript revision. Dr Urrutia participated in study conception and design, data collection, and manuscript revision. Dr Puttgen participated in study conception and design, data collection, and manuscript revision. Mr Friedman participated in study conception and design and manuscript revision. Dr Hoyer participated in study conception and design, data collection, and manuscript revision.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr Probasco reports receiving personal compensation as Editor-in-Chief of NEJM Journal Watch Neurology. Dr Urrutia reports receiving grant funding from Genentech.
References
- 1. Porter ME. What is value in health care? N Engl J Med. 2010;363(26):2477–2481. [DOI] [PubMed] [Google Scholar]
- 2. Schluep M, Bogousslavsky J, Regli F, Tendon M, Prod’hom LS, Kleiber C. Justification of hospital days and epidemiology of discharge delays in a department of neurology. Neuroepidemiology. 1994;13(1-2):40–49. [DOI] [PubMed] [Google Scholar]
- 3. Hoyer EH, Needham DM, Atanelov L, Knox B, Friedman M, Brotman DJ. Association of impaired functional status at hospital discharge and subsequent rehospitalization. J Hosp Med. 2014;9(5):277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Freburger JK. Analysis of the relationship between the utilization of physical therapy services and outcomes for patients with acute stroke. Phys Ther. 1999;79(10):906–918. [PubMed] [Google Scholar]
- 5. ABIM Foundation. Choosing wisely [online]. http://www.choosingwisely.org/. Accessed August 28, 2017.
- 6. George ML, Rowlands D, Price M, Maxey J. The Lean Six Sigma Pocket Toolbook. New York, NY: McGraw Hill; 2005. [Google Scholar]
- 7. Jette A, Haley SM, Coster W, Ni PS. AM-PAC Short Forms for Inpatient and Outpatient Settings Instruction Manual, Version 4. Boston, MA: Boston University; 2014. [Google Scholar]
- 8. Jette DU, Stilphen M, Ranganathan VK, Passek S, Frost FS, Jette AM. Interrater reliability of AM-PAC “6-clicks” basic mobility and daily activity short forms. Phys Ther. 2015;95(5):758–766. [DOI] [PubMed] [Google Scholar]
- 9. Jette DU, Stilphen M, Ranganathan VK, Passek SD, Frost FS, Jette AM. AM-PAC “6-Clicks” functional assessment scores predict acute care hospital discharge destination. Phys Ther. 2014;94(9):1252–1261. [DOI] [PubMed] [Google Scholar]
- 10. Jette DU, Stilphen M, Ranganathan VK, Passek SD, Frost FS, Jette AM. Validity of the AM-PAC “6-Clicks” inpatient daily activity and basic mobility short forms. Phys Ther. 2014;94(3):379–391. [DOI] [PubMed] [Google Scholar]
- 11. Fay MP. Confidence intervals that match Fisher’s exact or Blaker’s exact tests. Biostatistics. 2010;11(2):373–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moriates C, Mourad M, Novelero M, Wachter RM. Development of a hospital-based program focused on improving healthcare value. J Hosp Med. 2014;9(10):671–677. [DOI] [PubMed] [Google Scholar]
- 13. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology.1983;33(11):1444–1452. [DOI] [PubMed] [Google Scholar]
- 14. Poisson SN, Josephson SA. Quality measures in stroke. Neurohospitalist. 2011;1(2):71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cheng EM, Tonn S, Swain-Eng R, Factor SA, Weiner WJ, Bever CT., Jr Quality improvement in neurology: AAN Parkinson disease quality measures: report of the Quality Measurement and Reporting Subcommittee of the American Academy of Neurology. Neurology. 2010;75(22):2021–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Odenheimer G, Borson S, Sanders AE, et al. Quality improvement in neurology: dementia management quality measures. Neurology. 2013;81(17):1545–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]