Short abstract
Primary sensory neurons in the dorsal root ganglia and trigeminal ganglia are responsible for sensing mechanical and thermal stimuli, as well as detecting tissue damage. These neurons express ion channels that respond to thermal, mechanical, or chemical cues, conduct action potentials, and mediate transmitter release. These neurons also express a large number of G-protein coupled receptors, which are major transducers for extracellular signaling molecules, and their activation usually modulates the primary transduction pathways. Receptors that couple to phospholipase C via heterotrimeric Gq/11 proteins and those that activate adenylate cyclase via Gs are considered excitatory; they positively regulate somatosensory transduction and they play roles in inflammatory sensitization and pain, and in some cases also in inducing itch. On the other hand, receptors that couple to Gi/o proteins, such as opioid or GABAB receptors, are generally inhibitory. Their activation counteracts the effect of Gs-stimulation by inhibiting adenylate cyclase, as well as exerts effects on ion channels, usually resulting in decreased excitability. This review will summarize knowledge on Gi-coupled receptors in sensory neurons, focusing on their roles in ion channel regulation and discuss their potential as targets for analgesic and antipruritic medications.
Keywords: dorsal root ganglion neuron, GABAB receptor, Gi-coupled, G-protein coupled receptor, opioid receptor, trigeminal ganglion neuron
Introduction
Chronic pain is an unsolved medical problem,1 causing immense suffering to millions of people worldwide. The annual costs of chronic pain have been estimated to be hundreds of billions of dollars in the United States alone in medical costs and in lost productivity.2,3 The mainstream therapy against severe pain is opioids, which activate receptors that couple to inhibitory heterotrimeric G-proteins in the Gi/o family. Opioids, while efficient against severe pain, have significant side effects, such as tolerance, sedation, respiratory depression, physical dependence, and addiction. The lack of optimal therapies against chronic pain is thought to be a major contributor to the recent opioid epidemic.4 Most of the effects of opioids leading to addiction are likely caused by activation of receptors in the central nervous system (CNS). DRG neurons are the primary sensory neurons detecting thermal and mechanical stimuli; their peripheral processes and cell bodies are located outside the CNS. These neurons express opioid receptors, as well as a large number of other GPCRs that activate the Gi/o pathway. Selectively activating some of these receptors, in principle, can be utilized to develop novel therapeutic approaches that are devoid of side effects caused by receptor activation in the CNS.
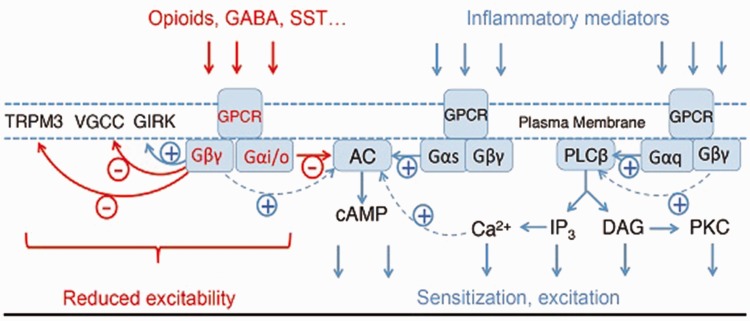
The three major classes of heterotrimeric G-proteins, defined by their alpha subunits, are Gs, Gi/o, and Gq/11 (Figure 1); the physiological roles of the forth class G12/13 are much less understood. The classical view of G-protein activation is that under resting conditions, Gα and Gβγ subunits tightly associate with each other and they are inactive. Upon receptor stimulation, Gα binds GTP, dissociates from Gβγ, and the two subunits bind to different effecors, until Gα hydrolyses GTP and re-associates with Gβγ, which terminates the biological effect. A more nuanced recent model postulates that Gα and Gβγ are associated with effectors in the resting state, and they activate them via a conformational switch or partial dissociation, see later at G-protein activated Inwardly Rectifying K+ (GIRK) channels section. G-protein signaling is modulated by many regulatory proteins5 including regulators of G-protein signaling6,7 and the G-protein coupled receptor kinase β-arrestin system.8 Various agonists of the same receptor do not necessarily couple with the same efficiency to downstream targets, a concept called biased agonism.9 For example, for μ-opioid receptors (μOR), the balanced agonist DAMGO activates both G-protein signaling and recruitment of β-arrestin, whereas other agonists, such as the recently described PZM21, activate G-proteins but induce negligible recruitment of β-arrestin.10
Figure 1.
Signaling by GPCRs, abbreviations are explained in the main text.
Receptors coupling to Gαs stimulate adenylate cyclase (AC), and thus the formation of cAMP. Activation of Gs-coupled receptors in DRG neurons, such as prostaglandin D receptors, leads to increased excitability, which contributes to inflammatory sensitization and pain.11 Downstream effectors of cAMP include protein kinase A, exchange proteins activated by cAMP, and hyperpolarization-activated cyclic nucleotide-gated ion channels, all expressed in DRG neurons.
Activation of Gq-coupled receptors stimulates phospholipase Cβ (PLCβ) enzymes, leading to the hydrolysis of the plasma membrane phospholipid phosphatidylinositol 4,5-bisphosphate.12 This results in the formation of the two classical second messengers inositol 1,4,5-trisphopshate, which releases Ca2+ from intracellular stores, and diacylglycerol, which activates protein kinase C. Activation of Gq-coupled receptors by inflammatory mediators, such as bradykinin, or extracellular ATP in DRG neurons plays an important role in inflammatory hypersensitivity.11,13 Downstream targets of the Gq pathway include protein kinase C-mediated sensitization of the heat and capsaicin-sensitive Transient Receptor Potential Vanilloid 1 (TRPV1) channels14 and voltage-gated Na+ channels.15 Activation of Gq-coupled receptors may also lead to direct excitation and pain.16 It was shown, for example, that bradykinin induces acute nociceptive signals by inhibiting M-type K+ channels as well as activating Ca2+-activated Cl− channels in DRG neurons.17 Another set of Gq-coupled receptors highly expressed in DRG neurons is the Mas-related G-protein coupled receptor (Mrgpr) family. While the functions and physiological activators of these receptors are not fully elucidated, some of them serve as itch receptors. The MrgprA3 (human MrgprX1) is responsible for chloroquine-induced itch,18 while the MrgprD receptor is activated by β-alanine, and it is responsible for the itch evoked by this compound.19
The physiological roles of Gα12/13 proteins, the fourth class of Gα proteins, are much less understood. They may activate small G-proteins;20 their expression levels in DRG neurons at the RNA level are lower than that of Gq, Gs, or Gi/o,21 and very little if any knowledge is available on their roles in these cells.
Gi-coupled receptors, such as opioid, GABAB, and somatostatin (SST) receptors, are generally considered inhibitory, and their activation reduces hypersensitivity and pain.22 The Gαi family consists of four members in mammals, Gαi1, Gαi2, Gαi3, and Gαo. Gβγ was originally considered an inactive scaffold molecule, but now it is very well accepted to act as an effector stimulating or inhibiting various signaling enzymes and ion channels. While all Gα proteins associate with Gβγ subunits, documented effects of Gβγ are most pronounced when Gi-coupled receptors are stimulated (see possible explanation under the section on GIRK channels).
DRG neurons are pseudounipolar cells; their cell bodies are located in the intervertebral foramen (opening). These neurons have a long peripheral process reaching from the ganglion to the periphery innervating not only the skin but also internal organs, as well as the bones and muscles. A shorter central process forms a synapse with secondary neurons in the dorsal horn of the spinal cord, thus transmitting the stimulus to the CNS. The equivalent primary sensory neurons innervating the orofacial region are located in the trigeminal ganglia (TG). Gi-coupled receptors are often presynaptic, and some of them are located in the central processes of DRG or TG neurons, and their activation reduces transmitter (glutamate) release. Many of the Gi-coupled receptors, however, are also found on the cell bodies and on the peripheral sensory processes, where they can inhibit the generation of receptor potential. Much of the electrophysiological characterization of native sensory ion channels is based on measurements performed on isolated and cultured cell bodies of DRG neurons. Due to this fact, it is often difficult to tell if a regulatory effect described in isolated DRG neurons takes place physiologically on the central, the peripheral, or both processes. When drugs are administered locally, such as in the hind paw in rodent models, the assumption is that they mainly exert their effects at the peripheral termini, unless they are injected at concentrations high enough to reach distant targets via the bloodstream. When drugs are injected intrathecaly, they exert effects both at the central termini of DRG neurons and on secondary neurons in the spinal cord. Systemically injected drugs reach both central and peripheral targets, unless they do not cross the blood brain and blood spinal-cord barrier,23 in that case, they reach the peripheral processes and potentially the cell bodies in the DRG,24 but not the central termini.
DRG neurons are also notoriously heterogeneous, a detailed description of the different cell types can be found in a recent review.25 Briefly, larger cells generate myelinated fibers mediating light discriminatory touch (Aβ) and proprioception (Aα). Medium-sized and small neurons give rise to lightly myelinated Aδ fibers and non-myelinated C-fibers, which mediate thermosensation, pain, and itch. These latter neurons have been divided into peptidergic and non-peptidergic neurons, depending on the expression of various markers, such as CGRP, Substance P, and IB4. A recent article divided mouse DRG neurons into 11 groups based on single cell RNA sequencing and principle component analysis of ∼900 cells.26 Further resources based on their data are available at http://linnarssonlab.org/drg/. See also Table 1 for expression of selected Gi-coupled receptors and sensory ion channels in the different cell populations. Additional single cell RNA sequencing articles have been also published for DRG neurons27,28 and for TG neurons.29 As this technology advances, it is likely that we will have higher coverage data available in the near future as it has happened for other organs such as the brain30 and the kidney.31
Table 1.
Expression of various Gi-coupled receptors, Gαi subunits, and some sensory TRP channels in mouse DRG neurons.
| whole | purified | TRPV1 | TRPV1 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | DRG | DRG neuron | lineage | depleted | NF1 | NF2 | NF3 | NF4 | NF5 | NP1 | NP2 | NP3 | PEP1 | PEP2 | TH |
| Gabbr1 | 148.86 | 175.317 | 133.64 | 79.23 | 0.323 | 0.354 | 0.417 | 0.273 | 0.385 | 0.440 | 0.375 | 0.250 | 0.281 | 0.588 | 0.391 |
| Gabbr2 | 44.778 | 53.985 | 48.63 | 35.09 | 0.129 | 0.167 | 0 | 0.045 | 0.154 | 0.136 | 0.063 | 0.083 | 0.031 | 0.059 | 0.172 |
| Oprm1 | 4.6222 | 5.31885 | 7.75 | 2.98 | 0 | 0 | 0 | 0.045 | 0 | 0.056 | 0.125 | 0.250 | 0.047 | 0.118 | 0.004 |
| Oprd1 | 4.8355 | 2.22282 | 1.74 | 5.89 | 0 | 0.063 | 0.250 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Oprk1 | 0.9234 | 0.833171 | 1.29 | 0.96 | 0 | 0.104 | 0.083 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Oprl1 | 8.7985 | 3.96903 | 3.15 | 7.61 | 0.129 | 0.208 | 0.083 | 0.136 | 0.154 | 0.008 | 0 | 0 | 0.063 | 0 | 0.052 |
| Sstr1 | 1.358 | 1.1629 | 1.81 | 0.39 | 0.065 | 0 | 0 | 0.136 | 0.115 | 0 | 0.031 | 0 | 0.031 | 0 | 0.021 |
| Sstr2 | 2.8183 | 3.29239 | 11.17 | 0.30 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.083 | 0.156 | 0 | 0 |
| Sstr4 | 1.5131 | 0.859453 | 1.86 | 0.47 | 0 | 0 | 0 | 0 | 0 | 0.008 | 0 | 0 | 0 | 0.059 | 0.004 |
| Grm2 | 0.0389 | 0.0933552 | 0.05 | 0.03 | 0 | 0 | 0 | 0 | 0.077 | 0.008 | 0 | 0 | 0 | 0 | 0 |
| Grm3 | 0.2625 | 0.182401 | 0.78 | 0.18 | 0 | 0.021 | 0 | 0 | 0 | 0 | 0 | 0 | 0.031 | 0 | 0 |
| Grm4 | 10.154 | 1.40604 | 1.32 | 14.79 | 0.355 | 0.167 | 0.333 | 0.136 | 0.154 | 0.008 | 0 | 0 | 0 | 0.235 | 0.004 |
| Grm7 | 25.656 | 32.1534 | 24.73 | 8.02 | 0.032 | 0 | 0 | 0.045 | 0.038 | 0.120 | 0.125 | 0 | 0.141 | 0.118 | 0.013 |
| Grm8 | 9.3652 | 2.30104 | 1.40 | 6.52 | 0 | 0.063 | 0.583 | 0.318 | 0.192 | 0.016 | 0.031 | 0 | 0 | 0.294 | 0.039 |
| Adora1 | 34.493 | 43.8114 | 26.44 | 15.89 | 0.452 | 0.354 | 0.500 | 0.227 | 0.192 | 0.456 | 0.125 | 0.167 | 0.094 | 0.294 | 0.506 |
| Npy1r | 8.3534 | 6.94382 | 17.33 | 2.40 | 0 | 0.042 | 0 | 0.091 | 0 | 0 | 0 | 0 | 0.328 | 0 | 0.021 |
| Npy2r | 8.2259 | 16.8471 | 14.64 | 1.37 | 0 | 0.021 | 0 | 0 | 0 | 0 | 0.094 | 0.833 | 0.031 | 0.294 | 0 |
| Htr1a | 2.8533 | 4.61 | 6.05 | 0.55 | 0 | 0.021 | 0 | 0.045 | 0 | 0 | 0.063 | 0.250 | 0.078 | 0.059 | 0 |
| Htr1b | 6.6005 | 2.62054 | 6.43 | 9.09 | 0 | 0 | 0 | 0 | 0.038 | 0 | 0 | 0 | 0 | 0.059 | 0.004 |
| Htr1d | 18.537 | 6.73259 | 3.88 | 24.09 | 0.677 | 0.688 | 0.917 | 0.273 | 0.500 | 0.016 | 0.031 | 0.083 | 0.047 | 0.059 | 0.258 |
| Htr1f | 2.4569 | 4.09502 | 5.62 | 2.35 | 0 | 0.250 | 0 | 0.182 | 0.154 | 0 | 0.094 | 0.833 | 0 | 0 | 0 |
| Cnr1 | 12.014 | 5.04595 | 4.65 | 7.31 | 0.000 | 0.271 | 0 | 0 | 0.154 | 0.032 | 0 | 0 | 0 | 0.118 | 0.000 |
| Cnr2 | 0.0557 | 0.0823272 | 0.02 | 0.11 | 0.000 | 0.000 | 0 | 0 | 0.000 | 0.000 | 0 | 0 | 0 | 0.000 | 0.004 |
| Gnai1 | 59.773 | 25.0302 | 20.43 | 46.27 | 0.484 | 0.500 | 0.750 | 0.636 | 0.808 | 0.088 | 0.188 | 0.083 | 0.109 | 0.529 | 0.223 |
| Gnai2 | 117.32 | 158.576 | 133.48 | 56.09 | 0.355 | 0.188 | 0.167 | 0.136 | 0.077 | 0.584 | 0.469 | 0.333 | 0.422 | 0.412 | 0.472 |
| Gnai3 | 30.677 | 34.3675 | 24.53 | 16.11 | 0.097 | 0.063 | 0 | 0.045 | 0.038 | 0.216 | 0.250 | 0.167 | 0.188 | 0.059 | 0.197 |
| Gnao1 | 305.81 | 558.236 | 395.80 | 99.89 | 0.194 | 0.021 | 0.083 | 0 | 0 | 0.760 | 0.813 | 0.417 | 0.313 | 0.353 | 0.245 |
| TRPV1 | 44.431 | 65.284 | 151.22 | 1.34 | 0 | 0 | 0 | 0.045 | 0 | 0.032 | 0.281 | 0.583 | 0.313 | 0.059 | 0 |
| TRPA1 | 18.674 | 34.2468 | 23.57 | 0.95 | 0 | 0 | 0 | 0 | 0 | 0.512 | 0.219 | 0.167 | 0.063 | 0 | 0.176 |
| TRPM8 | 10.388 | 7.30955 | 15.20 | 0.73 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.063 | 0 | 0 |
| TRPM3 | 9.8029 | 9.43739 | 6.31 | 3.86 | 0 | 0 | 0 | 0 | 0 | 0.104 | 0.031 | 0 | 0.078 | 0 | 0 |
Gene names for Gi-coupled receptors and Gα subunits: Gabbr1&2: GABAB receptor 1&2; Oprm1: μOR; Oprd1: δOR; Oprk1: κOR; Sstr1,2 & 3: SST receptor 1,2,3; Grm2,3,4,7,8: metabotropic glutamate receptors 2,3,4,7,8; Adora1: adenosine receptor 1, Npy1r: NPY receptor 1; Npy2r: NPY receptor 2, Htr1a,b,d,f: 5HT receptors 1a, 1b, 1d, 1f; Cnr1,2 Cannabinoid receptors 1,2; Gnai1,2,3: Gαi1,2,3; Gnao1: Gαo. Columns Whole DRG and purified DRG neurons enriched in small neurons are from Thakur et al.41 expression levels are expressed as FPKM. TRPV1 lineage and TRPV1 depleted are from Goswami et al.,21 from FACS isolated TRPV1 lineage DRG neurons, and from DRGs where this lineage was ablated, values are expressed as RPKM. NF1-NF5 are five different populations of neurofilament positive neurons, NP1-NP3 are three populations of non-peptidergic neurons, PEP1–2 are peptidergic neurons, and TH is tyrosine hydroxylase positive neurons from Usoskin et al.,26 based on single cell RNA sequencing of mouse DRG neurons, from external resource table available at http://linnarssonlab.org/drg/. Numbers note the fraction of cells that had detectable RNA for the given gene.
Targets of Gi-coupled receptors
Here, we will briefly discuss three classical targets of Gi signaling and a recently discovered new one. Other targets will be discussed at the parts dedicated to individual receptors. For example, the heat and capsaicin-sensitive TRPV1 is affected by GABAB receptors via a G-protein independent manner;32 this effect is not shared by other Gi-coupled receptors, thus we will discuss it at the GABAB receptors section.
Adenylate cyclase
The letter “i” in Gαi stands for “inhibitory” because receptors coupled to Gαi proteins inhibit AC, as opposed to “stimulatory” Gαs proteins. Gs-coupled receptors, such as prostaglandin D receptors, generally increase excitability of DRG neurons; thus contribute to inflammatory hypersensitivity. Concurrent activation of Gi-coupled receptors, in principle, counteracts this effect by decreasing cAMP levels. There are nine mammalian membrane bound AC isoforms. AC5 and AC6 are inhibited by all Gαi isoforms via protein–protein interactions, while AC1 is inhibited by Gαo, and Gβγ may also contribute to inhibition of these AC isoforms. AC2, AC4, and AC7 on the other hand are potentiated by Gβγ subunits in the presence of Gs stimulation (Figure 1).33 Further complicating this picture is the finding that sustained stimulation of Gi-coupled receptors paradoxically potentiates cAMP production, especially after cessation of the stimulus.34 This may underlie hypersensitivity upon repeated application of Gi-coupled agonists such as morphine and adenosine.35,36 Also, increased cytoplasmic Ca2+ stimulates several isoforms of AC (Figure 1), while inhibits others, providing a cross talk from Gq-coupled receptors.33
GIRK (Kir3.x) channels
GIRK channels are stimulated by activation of Gi-coupled cell surface receptors, leading to hyperpolarization and thus decreased excitability. GIRKs are members of the inwardly rectifying K+ (Kir) family of ion channels37; four subunits, Kir3.1, Kir3.2, Kir3.3, and Kir3.4, form homo- or hetero-tetramers to produce functional GIRK channels. The GIRK1/GIRK4 (Kir3.1/Kir3/4) combination forms the classical cardiac K+ channel activated by acetylcholine and contributes to slowing the heart rate, while GIRK2 is generally expressed in the nervous system. Activation of various Gi-coupled receptors including GABAB and SST receptors have been shown to activate GIRK currents in rat DRG neurons,38,39 and mRNA has been detected for all four Kir subunits in those cells.38 Another study reported that GIRK channels were present in rat and human DRG neurons, but they were absent in mouse DRG neurons. In vivo nociceptor-specific transgenic expression GIRK2 in mouse DRG neurons using Nav1.8 promoter restored peripheral analgesia induced by the μOR agonist DAMGO.40 Unbiased RNA sequencing in mouse DRG neurons detected low levels of GIRK channel expression, with the exception of GIRK1 (KCNJ3),41 and single cell RNA sequencing of mouse DRG neurons showed significant enrichment of GIRK2 in the tyrosine-hydroxylase positive subpopulation.26
GIRK channel activation is mediated by direct interactions between Gβγ and the channel.42,43 Interestingly, the channels are activated by Gi-coupled receptors, but not by Gq- or Gs-coupled receptors, even in heterologous expression systems. The mechanism of this selectivity has been a subject of intensive research; it cannot be explained by different subunit composition, because all Gβ-s (Gβ1–4) with the exception of Gβ5 activate GIRK channels,44 and no clear differences were identified in subunit composition of Gβ and Gγ associating with different Gα-s. The most likely explanation is that the Gαi-Gβγ complex associates with high affinity with GIRK channels in resting cells, and upon receptor activation, a local conformation switch, similar to a clamshell opening, rather than full dissociation of Gβγ from Gαi, activates the channel. This model is based largely on fluorescent resonance energy transfer measurements between the channel, receptor, and the G-proteins.45,46 The key findings supporting this model are that upon receptor stimulation, fluorescent resonance energy transfer may increase or decrease between Gβ and the channel,46 and between Gβ and Gαi,45 depending on the location of the CFP and YFP tags on the individual proteins. It remains to be seen if other effectors of Gβγ show similar mechanism.
Voltage-gated Ca2+ channels
N-type (Cav2.2, CACNA1B) and P/Q-type (Cav2.1, CACNA1A) voltage-gated Ca2+ channels (VGCC) are also classical targets of Gβγ released from Gαi.47 N-type channels are usually found presynaptically, where they play an important role in initiating neurotransmitter release. Inhibition of N-type channels by Gi-coupled receptors reduces transmitter release, an effect expected to take place in the central process in the context of DRG neurons. Indeed, inhibition of N-type Ca2+ channels by several Gi-coupled receptors including GABAB receptors and opioid receptors have been reported in DRG neurons.48
DRG neurons also express low-voltage activated Ca2+ channel (T-type), and the GABAB receptor agonist baclofen has been shown to inhibit both low- and high-voltage activated Ca2+ channels in DRG neurons.49 T-type channels (Cav3.2) are expressed both in the soma and the in the peripheral nerve termini and play important roles in initiating the receptor potential in response to mechanical stimuli.50,51
Transient receptor potential melastatin 3
Transient receptor potential melastatin 3 (TRPM3) channels are activated by heat,52 and chemical agonists such as pregnenolone sulphate (PregS)53 and the synthetic agonist CIM0216.54 These channels are expressed in small nociceptive DRG neurons, and their genetic deletion in mice reduces sensitivity to noxious heat.52 Recent reports from three different laboratories identified TRPM3 as a novel target of Gβγ upon activation of Gi-coupled receptors in DRG neurons using a wide range of overlapping techniques.55–57 Activation of μ-opioid,56,57 GABAB,55–57 SST,55,57 or Neuropeptide Y (NPY) receptors57 inhibited Ca2+ signals evoked by PregS in DRG neurons. Activation of recombinant GABAB, M2 muscarinic, and Dopamine 2 receptors also inhibited TRPM3 expressed in HEK cells, and the effect on M2 receptor activation was inhibited by co-expressing the Gβγ binding C-terminal fragment of the β-adrenergic receptor kinase.55 Co-expressing Gβ1γ2 in HEK cells inhibited PregS-induced Ca2+ signals and currents, but various Gαi/o isoforms including the constitutively active Gα1-Q204L had no effect.56 Similarly, co-expressing Gβ1γ2 in Xenopus oocytes inhibited PregS-induced currents, but none of the tested Gαi/o isoforms had a significant effect.55 The effect of Gβγ likely proceeds via direct protein–protein interaction as application of purified Gβγ, but not Gαi2 inhibited TRPM3 currents in excised inside out patches55,57 and TRPM3 co-immunoprecipitated with Gβ.55,57 Nocifensive responses evoked by hind paw injection of either CIM0216 or PregS were inhibited by co-injection of baclofen,55,57 DAMGO,56 morphine,57 or NPY.57 On the other hand, Ca2+ responses in DRG neurons evoked by agonists of other sensory TRP channels TRPV1,56 TRPA1,55,56 and TRPM855 were not affected by Gi-coupled receptor activation, and accordingly, nocifensive responses to the TRPV1 agonist capsaicin were not inhibited by co-injection of DAMGO,56 and baclofen did not inhibit nocifensive responses to the TRPA1 agonist mustard oil.55 Overall, the three articles described here convincingly demonstrate that TRPM3 is a bona fide novel ion channel target of Gβγ in DRG neurons, see also discussion by Csanady.58
Other targets
Downstream targets of Gβγ also include phosphoinositide 3-kinase-γ (PI3Kγ) and mitogen-activated protein kinases.59,60 While there is an extensive literature on mitogen-activated protein kinases in DRG neurons, most studies focused on its role in inflammatory hypersensitivity, and little is known if they play any roles in signaling by Gαi/o-coupled receptors.61 Similarly, PI3K enzymes have been studied largely in the context of hypersensitivity, NGF-signaling62 and inflammation,63 and little is known about their role in Gαi/o-coupled receptor signaling.
β-arrestin 1 (arrestin 2) and β-arrestin 2 (arrestin 3) were originally identified to bind to phosphorylated GPCRs and induce their desensitization and internalization8; their roles, however, are emerging as independent signaling mediators.8,64 Arrestins have been extensively studied in the context of opioid receptor signaling, and new biased opioid receptor agonists with minimal arrestin recruitment are being developed with the hope of minimizing the side effects of these drugs.10 Relatively little is known about the roles of arrestins in DRG neurons. It has been shown that δ-opioid receptor (δOR) signaling to VGCC was enhanced in β-arrestin1 knockout mice, and the behavioral effects of δOR agonists were enhanced in the absence of β-arrestin1.65 The authors concluded that these effects are due to δOR activation of cofilin through Rho-associated coiled-coil containing protein kinase, LIM domain kinase, and β-arrestin1 to regulate actin polymerization.65 Another article found that the high-internalizing δOR agonist (SNC80) preferentially recruited β-arrestin 1, and genetic deletion of β-arrestin 1 induced a significant increase in the potency of SNC80 to inhibit mechanical pain and decreased acute tolerance. In contrast, the low-internalizing δOR agonists (ARM390) preferentially recruited β-arrestin 2 with unaltered behavioral effects in β-arrestin 2 knockout animals.66
There are several less common targets of Gi signaling; some of them with relevance to DRG neurons are discussed below. Substance P released from nociceptive nerve endings is generally thought to be pro-nociceptive, but acute antinociceptive effects of this peptide have also been described.67 Substance P activates Neurokinin receptors (NK1–3), which are generally thought to couple to Gq and activate PLC, but they may also couple to Gi. Substance P has been shown to inhibit T-type VGCC68 and potentiate M-type K+ channels69 in DRG neurons, both of which reduce excitability. These effects were mediated by production of reactive oxygen species, and they were eliminated by overnight pertussis toxin (PTX) treatment showing the involvement of Gi signaling.
While Gi-coupled receptors are generally inhibitory, there are examples where pro-nocicepitive mediators increase excitability with the involvement of Gi-coupled receptors. Three examples are listed below on tetrodotoxin-resistant voltage-gated Na+ channels Nav1.8 and Nav1.9. The pro-inflammatory prostaglandin PGE2 has been reported to potentiate Nav1.9 currents in mouse DRG neurons, and PTX inhibited the effect, pointing to the role of Gi signaling.70 The chemokine CCL2 potentiated Nav1.8 channels in rat DRG neurons; the effect was blocked by PTX and gallein, suggesting the involvement of Gi signaling and Gβγ.71 The chemokine CXCL12 increased the activity of Nav1.8 and Nav1.9 currents in rat DRG neurons; PTX and the PI3K inhibitor LY294002 eliminated the effect on Nav1.9, but not on Nav1.8.72
Gi-coupled receptors in DRG neurons
DRG neurons express a number of different Gi-coupled receptors. We compiled RNA expression levels for Gi-coupled receptors, Gαi subunits, and some selected sensory ion channels from three different publications based on RNA sequencing of mouse DRG neurons (Table 1). The first two columns show data from Thakur et al.,41 who performed RNA sequencing on whole mouse DRG, as well as purified DRG neurons enriched in small nociceptive neurons. As can be seen in Table 1, RNA levels for many neuron-specific receptors and ion channels show some enrichment in purified neurons (e.g., TRPV1, TRPA1, and NPY2-receptors), while some transcript levels drop significantly (e.g., Grm4), indicating that they are mainly expressed in non-neuronal cells. The “TRPV1 lineage” and “TRPV1 depleted” data are from Goswami et al.,21 who used FACS sorted DRG neurons from a TRPV1 cre-based reporter mouse, which labels all TRPV1-expressing neurons and neurons that expressed the channel developmentally. The column “TRPV1 depleted” denotes DRG tissue depleted of the TRPV1-lineage by Cre-mediated excision of a floxed transcriptional stop-codon preceding the DTA coding sequence.21 We also included data from a single cell RNA sequencing article;26 the numbers for each subset of cells (NF1–5, NP1–3, PEP1–2, and TH) show the fraction of cells where transcripts were detected for a given gene.
We chose to present these data as they were obtained in an unbiased fashion, and the results for the two cell population-based RNA sequencing papers were expressed in comparable units, RPKM (Reads Per Kilobase Million) or FPKM (Fragments Per Kilobase Million). The single cell RNA sequencing data provides some estimate on the expression levels in different cell populations. The limitations of these data also need to be acknowledged. RNA levels do not necessarily correlate well with protein expression levels, and single cell RNA sequencing with relatively low cell number can result substantial false negative rate. Also note that all data in Table 1 are from mice, and other species may show different expression levels of some of these proteins.
Opioid receptors
Morphine and other opioid receptor agonists are mainstream therapy against severe pain. Most clinically relevant effects and many side effects of opioids are mediated by Gi-coupled μOR. The two other opioid receptor subtypes δOR and κ-opioid receptors (κOR) also couple to Gi/o, and have been studied as alternative targets for analgesics.73 Specific activation of both δOR and κOR has also been reported to induce analgesic effects, but κOR activation has been associated with dysphoria, while δOR activation has been reported to have anxiolytic and antidepressant effects.74 The nociceptin receptor or opioid receptor like 1 shares homology with opioid receptors; it is activated by its endogenous ligand nociceptin, but not by most opioid drugs.75
Opioid receptors are expressed both centrally, in the brain and spinal cord, as well as peripherally in cell bodies and peripheral processes of DRG neurons. DRG neurons express all three opioid receptors and opioid receptor like 1 at different levels and cellular distribution41 (Table 1). Both locally administered morphine and opioid receptor agonists such as the μOR agonist DAMGO, which do not cross the blood brain barrier, have been shown to have analgesic effects.76,77 The idea of peripherally acting opioids targeting DRG neurons, potentially devoid of central side effects, such euphoria and tolerance, have been raised, but so far, there are no clinically useful antinociceptive drugs available.76,78 Loperamide or Imodium is a peripherally acting μOR agonist, used as an over the counter antidiarrheal medication.79 Loperamide has no antinociceptive effect when taken orally, but it was reported to alleviate painful symptoms of oral or skin ulcers when applied topically.79 The main reason for the lack of the analgesic effect of oral loperamide is that it does not reach the systemic circulation, due to its almost complete degradation by the liver.79 Loperamide has been shown to have analgesic effect when injected subcutaneously80,81 or applied topically.82
A recent review on δOR in primary sensory neurons provides a thorough description of the roles of δOR as well as μOR in DRG neurons.83 Briefly, most research in DRG neurons focused on μOR and δOR, and experiments based on immunocytochemistry suggested that μOR and δOR are expressed in an overlapping set of cells.84 A more recent study by Scherrer et al.85 using a δOR-GFP reporter mouse line showed that μOR and δOR are expressed in different cell populations; δOR were restricted to medium-to-large myelinated NF200 expressing cells and non-peptidergic IB4 positive smaller neurons. μOR on the other hand was mainly expressed in small, peptidergic TRPV1- and substance P-positive neurons.85 These data are also consistent with the distribution of RNA expression of these receptors in a recent single cell RNA sequencing article26 (see also Table 1). Consistent with μOR and δOR being expressed in different cell populations, selective activation of μOR or δOR also had functionally distinct effects. Intrathecal administration of the μOR selective agonist DAMGO decreased sensitivity to noxious heat, without significant effect on mechanical pain; the δOR-specific SNC80 on the other hand significantly attenuated mechanical pain, without having an effect on heat sensitivity.85
The debate on whether or not μOR and δOR are co-expressed in the same DRG neurons however is not yet settled. Recent studies demonstrated the coexistence of μORs and δORs in small DRG neurons using single-cell PCR, in situ hybridization, immunostaining, and electrophysiology.86 Heteromers of μOR and δOR were shown in DRG neurons using antibodies that recognize those heteromers.87 Finally, facilitation of the degradation of μOR-δOR heteromers by δOR agonists have been shown to be alleviated by disrupting heteromer formation.88
A recent paper showed that nociceptor-specific deletion of μOR had no effect on morphine-induced analgesia, but eliminated both tolerance and opioid-induced hyperalgesia.89 The same study also showed that methylnaltrexone bromide, a peripherally restricted μOR antagonist, was sufficient to abrogate tolerance and hyperalgesia induced by morphine, without diminishing its antinociceptive effect. These data raise doubt about the usefulness of peripherally acting μOR agonists as analgesics. As mentioned earlier, it was suggested that in mice, analgesic effect of the peripherally acting μOR agonist DAMGO required transgenic expression of GIRK2 in DRG neurons.40
Significant recent efforts used innovative approaches to target peripheral opioid receptors for pain relief.90 A recent article reported a peripherally acting μOR agonist, which acts selectively at the site of injury. Spahn et al.91 synthesized a fentanyl analog that only activates μOR at low pH, which is characteristic of inflamed and injured tissues, and they showed that the compound reduced inflammatory hyperalgesia to both thermal and mechanical stimuli in rats. Another recent article reported covalently attaching morphine to hyperbranched polyglycerol by a cleavable linker, which prevents blood-brain barrier permeation and selectively releases morphine in injured tissue. This conjugated morphine produced analgesia in inflamed rat paws without major side effects.92
While RNA levels for κOR are lower than those of other opioid receptors (Table 1) both κOR expression84 and inhibitory effects of κOR agonists on VGCC93 have been reported in DRG neurons. As mentioned earlier, DRG neurons play important roles not only in pain, but also in itch, which in chronic forms is a significant medical problem. Pruritus, or itch, is one of the side effects of activation of μOR,73 but activation of κOR has the opposite effect. The κOR agonist nalfurafine,73 as well as two different peripherally acting κOR agonists, asimadoline and CR845, were shown to be effective against itch.94 CR845 showed promising results in phase II clinical trials against pruritus associated with chronic kidney disease in hemodialysis patients.95
DRG neurons also express ORL-1 nociceptin receptors (Table 1), and a recent study using a mouse line in which the ORL-1 protein was tagged with GFP found that 43% of DRG neurons were GFP-positive. GFP was expressed both in small and large neurons, with a slight dominance (58%) of neurofilament positive myelinated neurons.96 Nociceptin receptors were reported to inhibit N-type VGCC in DRG neurons in a tonic, agonist independent manner.97 Nociceptin receptors were also reported to be expressed in human DRG neurons, and the same study showed that their activation reduced capsaicin-induced Ca2+ signals in rat DRG neurons.98
Overall, there are conflicting data on the efficiency of stimulating peripheral opioid receptors in alleviating pain in mice, and there are clear receptor subtype specific effects. Peripheral κOR-s on the other hand are promising targets against itch in humans.
GABAB receptors
GABAB receptors are obligate heteromers of GABAB1 and GABAB2 subunits; the presence of both subunits is required for functional G-protein signaling for the following two reasons. First, the GABA binding site is on GABAB1 receptors and the Gi-activating domain is on the GABAB2 subunit. Second, GABAB1 subunits have an ER retention signal, which prevents trafficking of the subunit in the absence of GABAB2 receptors, which masks this signal when they form a dimer with GABAB1.99
GABAB receptors are the highest expressing GPCRs in DRG neurons on the RNA level41 (Table 1). The only widely available GABAB agonist baclofen is used clinically as a central muscle relaxant; its effect is attributed to inhibiting neurotransmitter release onto motoneurons in the ventral horn of the spinal cord.100 The use of systemic baclofen is limited by its severe side effects at higher doses such as drowsiness, mental confusion, and even coma,101 which is not surprising, given the abundance of these receptors in the CNS.99 Systemic side effects can be limited by administering baclofen intrathecally, which is often done to reduce spasticity in various conditions. Baclofen is also used to treat pain conditions, as an adjuvant therapy, but its effect is mainly attributed to acting as a central muscle relaxant.
As discussed earlier, GABAB receptor activation by baclofen was shown to activate GIRK channels,38 inhibit VGCC,49 and inhibit the heat-activated TRPM3 channels55–57 in DRG neurons; these effects are mediated by the Gβγ arm of classical heterotrimeric G-protein signaling. All of these mechanisms, in principle, may mediate antinociceptive effects.
GABAB receptors can also be activated by α-conotoxins. These toxins are generally considered to be inhibitors of nicotinic acetylcholine receptors, but some of them such as Vc1.1 and RgIA also inhibit N-type VGCC via activation of GABAB receptors102 reviewed in Adams et al.103 Accordingly, intramuscular injection of Vc1.1 was shown to induce a long-lasting reversal of mechanical allodynia, which was prevented by the GABAB receptor antagonist, SCH50911.104
Activation of GABAB receptors in DRG neurons by baclofen was recently shown to inhibit the sensitized state of TRPV1, but not the basal heat or capsaicin activation of TRPV1. The effect was independent of Gβγ signaling; it was mediated by direct protein–protein interaction between GABAB1 receptors and TRPV1.32 While GABAB2 receptors were not detected in the protein complexes of TRPV1 and GABAB1 receptors in DRG neurons, GABAB2 receptors were required for the effect of baclofen both in a heterologous expression system and in DRG neurons. Baclofen was effective when injected locally, showing the presence of the receptors in the peripheral processes, and GABA was shown to be released from nociceptive nerve terminals, suggesting an autocrine feedback mechanism.32 The growing evidence that these receptors have important antinociceptive effects in the periphery, raise the possibility that peripherally acting GABAB receptor agonists can be developed as novel analgesics with less side effects.
SST receptors
SST receptors are expressed not only in DRG neurons but also centrally, as well as in inflammatory cells, and can affect nociception and inflammation; the topic is reviewed in literature.105,106 Briefly, it has been shown that SST is released from activated capsaicin-sensitive nerve endings, and it can exert both local and systemic anti-nociceptive and anti-inflammatory effects.105,107 Intraplantar injection of SST reduced mechanical allodynia in a rat inflammatory pain model.108 The SST receptor agonist octreotide inhibited formalin-induced nociceptive behaviors when injected locally, and it also reduced the responses of C-fibers to bradykinin-induced excitation and sensitization to heat.109 It was also shown that intraplantar injection of octreotide inhibited capsaicin-induced nocifensive responses in rats, and it also inhibited capsaicin-induced nerve activity in the skin-nerve preparation.110 Furthermore, intra-articular injection of SST was shown to inhibit knee pain in humans.111 The SST4 receptor agonist J-2156 was shown to inhibit capsaicin-induced Ca2+ signals in rat DRG neurons,112 as well as activate GIRK channels and inhibit VGCC.39 SST4 receptor deficient mice showed increased mechanical hyperalgesia after carrageenan-induced inflammation, and the antinociceptive effect of the SSTR4 agonist J-2156 was absent in these animals.113 Lipopolysaccharide-induced airway inflammation and bronchoconstriction were also markedly enhanced in SSTR4 knockout animals, pointing to the important role of these receptors in inflammatory cells.113 SST was also shown recently to inhibit Ca2+ signals induced by the TRPM3 agonist PregS in a subset of mouse DRG neurons.55,56 Targeting SST receptors for pain control is complicated by the fact that activation of these receptors have significant other effects, including inhibition of insulin release and inhibition of exocrine secretion and motor activity of the gastrointestinal tract, which may be overcome by developing subtype specific agonists.105
Metabotropic glutamate receptors
Metabotropic glutamate receptors (mGlur-s) can function either as homodimers, or as heterodimers.114 They are divided into group I receptors (mGluR1 and 5) which signal via Gαq and group II (mGluR2 and 3), and group III (4,6,7, and 8), which signal via Gαi.115 Group I mGluRs, similar to other PLC-coupled receptors, have been shown to be present on peripheral terminals of DRG neurons and play roles in inflammatory hyperalgesia,116 reviewed in study by Neugebauer.117
There are several articles showing antinociceptive effects of the activation of peripheral group II Gi/o-coupled mGluR-s. Subcutaneous injection of a selective group II mGluR agonist (APDC) into the plantar surface of the hind paw inhibited prostaglandin E2 (PGE2)-induced thermal hyperalgesia in mice.118 The same study also showed that in cultured DRG neurons, APDC blocked PGE2-induced potentiation of capsaicin-induced Ca2+ responses, which was abolished when neurons were pretreated with PTX. Another article from the same group showed that subcutaneous injection of group II mGluR agonists into the plantar surface of the mouse hind paw did not alter basal mechanical thresholds, but inhibited PGE2- or carrageenan-induced mechanical allodynia.119 Group II metabotropic glutamate receptor agonists also inhibited forskolin-induced potentiation of tetrodotoxin-resistant sodium currents in mouse DRG neurons.120 Finally, it was shown that membrane hyperexcitability in mouse and human DRG neurons exposed to PGE2 was prevented by the group II mGluR agonist APDC.121
While several studies focused on group II mGluR-s, on the RNA level, group III mGluR-s show substantially higher expression in mouse DRG neurons (Table 1). Recent studies also demonstrated potential antinociceptive roles of this group; mGluR8 was found to be present in peripheral nociceptive terminals, and ipsilateral, but not contralateral hind paw injection of the group III mGluR agonist L-AP-4 inhibited nociceptive behavioral responses to capsaicin in rats.122 Local L-AP-4 injection also attenuated forskolin-induced thermal hyperalgesia.122 It was also shown that mGluR7 was expressed in small peptidergic and large rat DRG neurons.123 Nerve ligation experiments in the same study also showed that mGluR7 was anterogradely transported from the cell body to the peripheral site, and after peripheral nerve injury, mGluR7 expression was down-regulated. It was also shown that inhibiting peripheral group II/III mGluR-s by intraplantar injection of various antagonists increased capsaicin-induced nociceptive behaviors and nociceptor activity,124 indicating peripheral glutamate release. On the other hand, the mGluR group III agonist L-AP4 did not have a significant effect on TRPM3 activity, as assessed by PregS-induced Ca2+ signals,57 while agonists of many other Gαi/o-coupled receptors showed robust inhibition.55–57
Overall, both excitatory group I and inhibitory groupII/III mGluR-s are expresed at peripheral nerve terminals, but the opposing effects of the two different receptor groups makes the effects of a potential peripheral glutamate release complex. Neverthelesss, in principle, both group I antagonsist and group II/III agonists may induce beneficial antinociceptive effects.117
Adenosine receptors
ATP is released from many cell types and acts as a paracrine signal; it activates both metabotropic (P2X) and ionotropic (P2Y) receptors. Activation of both P2X and P2Y receptors in DRG neurons is generally excitatory. Secreted ATP becomes dephosphorylated rapidly to adenosine by ectoenzymes.125 Adenosine receptors are distinct from purinergic receptors and couple to different G-proteins.126 Adenosine 1 receptors (A1R, adora1) couple to Gi/o-proteins, and they are the most abundant adenosine receptors in DRG neurons; however, Gs-coupled Adenosine 2A receptors (A2AR) are also expressed in DRG neurons at lower levels21,41 (see also Table 1). Adenosine release has been detected in response to capsaicin and formalin from nociceptive nerve fibers.127 Due to the presence of receptors with different signal transduction pathways, as well as to the fact that A1R may also couple to Gq, the local effects of adenosine can be quite complex, both pro- and antinociceptive effects have been observed, reviewed in study by Sawynok and Liu.126 The presence of various adenosine receptors on many other cell types including immune and vascular cells makes the overall effects of pharmacological modulation of this pathway quite complex.128
NPY receptors
NPY is a 36 amino acid peptide; it has five receptors Y1R–Y5R, all couple to Gαi/o proteins. DRG neurons express Y1R and Y2R (Table 1). NPY was shown to inhibit VGCC in rat DRG neurons129,130 and it also inhibited depolarization-induced Ca2+ signals and release of substance P from DRG neurons.130 While both nociceptive and antinociceptive effects of NPY have been described, in general, it is believed that this peptide is mainly antinociceptive.131 Two independent mouse lines with genetic deletion of Y1R have been generated, and the two studies largely agree that the knockout mice display hyperalgesia to mechanical and thermal stimuli.132,133 NPY receptors are also expressed in the dorsal horn, and the analgesic effects of NPY may be due to activation of spinal receptors.134 Consistent with the main role of central NPY receptors, it was shown that intrathecal, but not local administration of NPY reduced guarding behavior in a rat model of plantar incision pain.135 As mentioned earlier, application of NPY or peptide YY inhibited PregS-induced activation of TRPM3 in mouse DRG neurons,55,57 and local injection of peptide YY inhibited nocifensive responses evoked by the TRPM3 agonist PregS.57
Serotonin receptors
The neurotransmitter serotonin (5-hydroxytryptamine, 5-HT) is part of the inflammatory soup that sensitizes nociceptors. It binds to a variety of receptors including ionotropic 5-HT3 receptors, and a variety of GPCRs coupling to Gq (5HT2), Gs (5HT4,6,7), and Gi/o (5HT1,5).136 Many of these receptors are expressed in DRG and TG neurons, and the overall effect of serotonin is complex, but pro-algesic effects likely dominate. Ionotropic HT3a receptors, for example, are expressed in the central termini and play roles in central sensitization to painful stimuli.137 Injecting serotonin or a 5HT2 receptor agonist in the hind paw of mice evoked hyperalgesia to mechanical stimuli indicating the presence of stimulatory 5HT2 receptors in the peripheral nerve termini.138 Serotonin also induced action potentials and potentiated TRPV1 currents in isolated DRG neurons through 5HT2C receptors.139 Sumatriptam, a drug, which is used to treat migraine headaches,140 selectively activates Gi/o-coupled 5HT1B and 5HT1D receptors, which are expressed in DRG neurons (Table 1). It is not clear to what extent direct effects of sumatriptam on TG neurons contribute to its beneficial effects,140 but the drug was shown to inhibit TRPV1 activity in TG neurons.141 Serotonin application was shown to potentiate calcium signals and CGRP release induced by capsaicin in TG neurons, but sumatriptam had an inhibitory effect, showing opposing effects of activating different 5HT receptors expressed in those neurons.142 Sumatriptam was also shown to induce hyperalgesic priming in rats, which may explain the clinical finding that the drug may contribute to migraine chronification.36
Designer receptors exclusively activated by designer drugs
Designer receptors exclusively activated by designer drugs (DREADDs) are mutated GPCRs that do not respond to endogenous ligands, but can be activated by synthetic compounds. Most of them are based on muscarinic acetylcholine receptors; they are activated by the inert clozapine derivative clozapine-N-oxide (CNO).143 DREADDs based on other receptors are also available, and are being developed.144 By expressing various forms of these receptors in specific cell types, the effect of activating Gi-, Gq-, or Gs-coupled receptors can be studied by applying their chemical activator. Together with optogenetic approaches,145 DREADDs, in principle, are promising selective tools to study the effects of activation or inhibition of specific neuronal populations in various pain conditions. DREADDs can be expressed in vivo either by crossing mice expressing cre-dependent DREADDs with cell-type specific cre-mice146 or by injecting DREADD-expressing viral particles.
Expressing inhibitory DREADDs in DRG neurons is a compelling strategy to achieve pain relief. Currently, there are two published articles using this strategy.
Iyer et al.147 showed that viral expression of the hM4-based Gi-coupled DREADD in small-diameter nociceptors enabled chemogenetic increase of mechanical and thermal nociception thresholds. In the same article, the authors found that transdermal illumination in mice expressing an inhibitory channelrhodopsin inhibited pain.
Another article however raised doubts about the peripheral Gi-coupled DREADD-based approach to inhibit pain. Saloman et al.148 expressed the Gi-coupled hM4Di receptor in nociceptive DRG neurons expressing the heat- and capsaicin-sensitive TRPV1 ion channel, by crossing TRPV1-cre mice with floxed hM4Di expressing mice. As expected, injection of CNO produced a significant increase in the heat threshold in these animals. Consistent with TRPV1 positive cells being largely insensitive to mechanical stimuli, mechanical sensitivity was not affected by CNO. Surprisingly, however, expression of these receptors induced significant changes in the absence of CNO, including changes in voltage-gated Na+ and Ca2+ currents, as well as an increase in the expression of Nav1.7 channels. Expression of the Gi-coupled DREADD also reduced the effectiveness of stimulating endogenous μOR by DAMGO on PGE2-induced inflammatory thermal hyperalgesia. The authors concluded that while DREADDs are useful tools, they need additional refinement, especially for potential clinical use. Recognizing the imperfections in currently available DREADDs, novel receptors and compounds are being developed.149,150
Additional caution on using these designer receptors have been raised by a recent paper showing that CNO is converted to clozapine in vivo, and the latter is responsible for activating them.151 Clozapine is an atypical antipsychotic medication; its mechanism of action is not fully understood, but it inhibits certain dopamine and serotonin receptors. The doses required in vivo activation of DREADDs were below that required to exert effects in animals not expressing DREADDs, suggesting that this compound can be more useful than CNO for in vivo use.151
Optogenetic approaches
Optogenetic approaches classically use light-activated ion channels to study the effects of activating or inhibiting specific neurons and have been used in pain research, see Copits et al.145 for review. In addition to light-activated ion channels, various GPCRs have also been engineered to become light sensitive. Among Gi-coupled receptors, a photoactivatable μOR was created by splicing together the transmembrane and extracellular parts of the light-activated GPCR rhodopsin, and the intracellular loops and C-terminus of μ-opioid receptor.152 This opto-μOR was virally expressed in isolated DRG neurons, where they were shown to increase the phosphorylation of extracellular signaling-regulated kinase. Opto-μOR and other light inducible Gi-coupled receptor constructs are promising tools to study the effects of Gi-coupled receptor activation in DRG neurons.
Conclusions
Activation of cell surface receptors coupling to Gαi proteins in DRG neurons generally inhibits various processes involved in initiation of painful signals, and therefore, in principle, they can be targets for novel antinociceptive drugs. Several factors complicate this seemingly simple idea. First, DRG neurons are highly heterogeneous, and the expression patterns of the various receptors are different; therefore, the activation of distinct Gαi/o-coupled receptors is likely to affect different cell types. Second, repeated application of Gi-coupled receptor agonists may induce hyperalgesia.153 Third, the signaling mechanisms induced by different receptor agonists may not be identical, leading to diversity of the effects. Fourth, expression at the central versus peripheral terminal may induce distinct effects. Clearly, further research is needed to understand the effects of the activation of individual receptors and to explore the potential of targeting these receptors for pain relief. In additions to pain, activation of some of these receptors may also relieve itch, and peripherally acting κOR agonists are currently in clinical trials against uremic pruritus.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Work in the Rohacs lab has been supported from NIH grants NS055159 and GM093290 and a grant from the New Jersey Health Foundation.
References
- 1.Basbaum AI Bautista DM Scherrer Gand Julius. Cellular and molecular mechanisms of pain. Cell 2009; 139: 267–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woodard GE Jardin I Berna-Erro A Salido GM andRosado JA. Chronic pain syndromes, mechanisms, and current treatments. Prog Mol Biol Transl Sci 2015; 131: 565–611. [DOI] [PubMed] [Google Scholar]
- 3.Turk DC. Clinical effectiveness and cost-effectiveness of treatments for patients with chronic pain. Clin J Pain 2002; 18: 355–365. [DOI] [PubMed] [Google Scholar]
- 4.Skolnick P andVolkow ND.. Re-energizing the development of pain therapeutics in light of the opioid epidemic. Neuron 2016; 92: 294–297. [DOI] [PubMed] [Google Scholar]
- 5.Magalhaes AC Dunn H andFerguson SS.. Regulation of GPCR activity, trafficking and localization by GPCR-interacting proteins. Br J Pharmacol 2012; 165: 1717–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woodard GE, Jardin I, Berna-Erro A, Salido GM and Rosado JA. Regulators of G-protein-signaling proteins: negative modulators of G-protein-coupled receptor signaling. Int Rev Cell Mol Biol 2015; 317: 97–183. [DOI] [PubMed] [Google Scholar]
- 7.Kach J Sethakorn N andDulin NO.. A finer tuning of G-protein signaling through regulated control of RGS proteins. Am J Physiol Heart Circ Physiol 2012; 303: H19–H35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeWire SM Ahn S Lefkowitz RJ andShenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol 2007; 69: 483–510. [DOI] [PubMed] [Google Scholar]
- 9.Violin JD Crombie AL Soergel DG andLark MW. Biased ligands at G-protein-coupled receptors: promise and progress. Trends Pharmacol Sci 2014; 35: 308–316. [DOI] [PubMed] [Google Scholar]
- 10.Manglik A Lin H Aryal DK McCorvy JD Dengler D Corder G Levit A Kling RC Bernat V Hubner H Huang XP Sassano MF Giguere PM Lober S Da D Scherrer G Kobilka BK Gmeiner P Roth BLand Shoichet BK. Structure-based discovery of opioid analgesics with reduced side effects. Nature 2016; 537: 185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linley JE Rose K Ooi Land Gamper N. Understanding inflammatory pain: ion channels contributing to acute and chronic nociception. Pflugers Arch - Eur J Physiol 2010; 459: 657–669. [DOI] [PubMed] [Google Scholar]
- 12.Kamato D Mitra P Davis F Osman N Chaplin R Cabot PJ Afroz R Thomas W Zheng W Kaur H Brimble Mand Little PJ. Proteins: molecular pharmacology and therapeutic potential. Cell Mol Life Sci 2017; 74: 1379–1390. Gαq [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rohacs T. Phosphoinositide signaling in somatosensory neurons. Adv Biol Regul 2016; 61: 2–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X Li L andMcNaughton PA.. Proinflammatory mediators modulate the heat-activated ion channel TRPV1 via the scaffolding protein AKAP79/150. Neuron 2008; 59: 450–461. [DOI] [PubMed] [Google Scholar]
- 15.Cang CL Zhang H Zhang YQand Zhao ZQ. PKC epsilon-dependent potentiation of TTX-resistant Nav1.8 current by neurokinin-1 receptor activation in rat dorsal root ganglion neurons. Mol Pain 2009; 5: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petho G andReeh PW.. Sensory and signaling mechanisms of bradykinin, eicosanoids, platelet-activating factor, and nitric oxide in peripheral nociceptors. Physiol Rev 2012; 92: 1699–1775. [DOI] [PubMed] [Google Scholar]
- 17.Liu B Linley JE Du X Zhang X Ooi L Zhang Hand Gamper N. The acute nociceptive signals induced by bradykinin in rat sensory neurons are mediated by inhibition of M-type K+ channels and activation of Ca2+-activated Cl- channels. J Clin Invest 2010; 120: 1240–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Q Tang Z Surdenikova L Kim S Patel KN Kim A Ru F Guan Y Weng HJ Geng Y Undem BJ Kollarik M Chen ZF anderson DJand Dong X. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell 2009; 139: 1353–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Q Sikand P Ma C Tang Z Han L Li Z Sun S LaMotte RHand Dong X. Mechanisms of itch evoked by beta-alanine. J Neurosci 2012; 32: 14532–14537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neves SR Ram PT andIyengar R.. G protein pathways. Science 2002; 296: 1636–1639. [DOI] [PubMed] [Google Scholar]
- 21.Goswami SC Mishra SK Maric D Kaszas K Gonnella GL Clokie SJ Kominsky HD Gross JR Keller JM Mannes AJ Hoon MAand Iadarola MJ. Molecular signatures of mouse TRPV1-lineage neurons revealed by RNA-Seq transcriptome analysis. J Pain 2014; 15: 1338–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stone LS andMolliver DC.. In search of analgesia: emerging roles of GPCRs in pain. Mol Interv 2009; 9: 234–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartanusz V Jezova D Alajajian Band Digicaylioglu M. The blood-spinal cord barrier: morphology and clinical implications. Ann Neurol 2011; 70: 194–206. [DOI] [PubMed] [Google Scholar]
- 24.Sapunar D Kostic S Banozic Aand Puljak L. Dorsal root ganglion – a potential new therapeutic target for neuropathic pain. J Pain Res 2012; 5: 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Pichon CE andChesler AT.. The functional and anatomical dissection of somatosensory subpopulations using mouse genetics. Front Neuroanat 2014; 8: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Usoskin D Furlan A Islam S Abdo H Lonnerberg P Lou D Hjerling-Leffler J Haeggstrom J Kharchenko O Kharchenko PV Linnarsson Sand Ernfors P. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci 2015; 18: 145–153. [DOI] [PubMed] [Google Scholar]
- 27.Li CL Li KC Wu D Chen Y Luo H Zhao JR Wang SS Sun MM Lu YJ Zhong YQ Hu XY Hou R Zhou BB Bao L Xiao HS and Zhang X. Somatosensory neuron types identified by high-coverage single-cell RNA-sequencing and functional heterogeneity. Cell Res 2016; 26: 83–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu G Huang K Hu Y Du G Xue Z Zhu Xand Fan G. Single-cell RNA-seq reveals distinct injury responses in different types of DRG sensory neurons. Sci Rep 2016; 6: 31851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen MQ Wu Y Bonilla LS von Buchholtz LJ and Ryba NJP. Diversity amongst trigeminal neurons revealed by high throughput single cell sequencing. PLoS One 2017; 12: e0185543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ofengeim D Giagtzoglou N Huh D Zou Cand Yuan J. Single-cell RNA sequencing: unraveling the brain one cell at a time. Trends Mol Med 2017; 23: 563–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park J Shrestha R Qiu C Kondo A Huang S Werth M Li M Barasch Jand Susztak K. Comprehensive single cell RNAseq analysis of the kidney reveals novel cell types and unexpected cell plasticity. bioRxiv 2017; 203125. [Google Scholar]
- 32.Hanack C Moroni M Lima WC Wende H Kirchner M Adelfinger L Schrenk-Siemens K Tappe-Theodor A Wetzel C Kuich PH Gassmann M Roggenkamp D Bettler B Lewin GR Selbach Mand Siemens J. GABA blocks pathological but not acute TRPV1 pain signals. Cell 2015; 160: 759–770. [DOI] [PubMed] [Google Scholar]
- 33.Sunahara RK andTaussig R.. Isoforms of mammalian adenylyl cyclase: multiplicities of signaling. Mol Interv 2002; 2: 168–184. [DOI] [PubMed] [Google Scholar]
- 34.Brust TF Conley JM andWatts VJ.. Galpha(i/o)-coupled receptor-mediated sensitization of adenylyl cyclase: 40 years later. Eur J Pharmacol 2015; 763: 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Araldi D Ferrari LF andLevine JD.. Repeated Mu-opioid exposure induces a novel form of the hyperalgesic priming model for transition to chronic pain. J Neurosci 2015; 35: 12502–12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Araldi D Ferrari LF andLevine JD.. Gi-protein-coupled 5-HT1B/D receptor agonist sumatriptan induces type I hyperalgesic priming. Pain 2016; 157: 1773–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hibino H Inanobe A Furutani K Murakami S Findlay Iand Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev 2010; 90: 291–366. [DOI] [PubMed] [Google Scholar]
- 38.Gao XF Zhang HL You ZD Lu CLand He C. G protein-coupled inwardly rectifying potassium channels in dorsal root ganglion neurons. Acta Pharmacol Sin 2007; 28: 185–190. [DOI] [PubMed] [Google Scholar]
- 39.Gorham L Just S andDoods H.. Somatostatin 4 receptor activation modulates G-protein coupled inward rectifying potassium channels and voltage stimulated calcium signals in dorsal root ganglion neurons. Eur J Pharmacol 2014; 736: 101–106. [DOI] [PubMed] [Google Scholar]
- 40.Nockemann D Rouault M Labuz D Hublitz P McKnelly K Reis FC Stein Cand Heppenstall PA. The K+channel GIRK2 is both necessary and sufficient for peripheral opioid-mediated analgesia. EMBO Mol Med 2013; 5: 1263–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thakur M Crow M Richards N Davey GI Levine E Kelleher JH Agley CC Denk F Harridge SDand McMahon SB. Defining the nociceptor transcriptome. Front Mol Neurosci 2014; 7: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Logothetis DE Kurachi Y Galper J Neer EJand Clapham DE. The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature 1987; 325: 321–326. [DOI] [PubMed] [Google Scholar]
- 43.Whorton MR andMacKinnon R.. X-ray structure of the mammalian GIRK2-betagamma G-protein complex. Nature 2013; 498: 190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mirshahi T Robillard L Zhang H Hebert TEand Logothetis DE. Gbeta residues that do not interact with Galpha underlie agonist-independent activity of K+ channels. J Biol Chem 2002; 277: 7348–7355. [DOI] [PubMed] [Google Scholar]
- 45.Bunemann M Frank M andLohse MJ.. Gi protein activation in intact cells involves subunit rearrangement rather than dissociation. Proc Natl Acad Sci USA 2003; 100: 16077–16082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riven I Iwanir S andReuveny E.. GIRK channel activation involves a local rearrangement of a preformed G protein channel complex. Neuron 2006; 51: 561–573. [DOI] [PubMed] [Google Scholar]
- 47.Currie KP (2010) G protein modulation of CaV2 voltage-gated calcium channels. Channels 2010; 4: 497–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bourinet E Altier C Hildebrand ME Trang T Salter MWand Zamponi GW. Calcium-permeable ion channels in pain signaling. Physiol Rev 2014; 94: 81–140. [DOI] [PubMed] [Google Scholar]
- 49.Huang D Huang S Peers C Du X Zhang Hand Gamper N. GABAB receptors inhibit low-voltage activated and high-voltage activated Ca2+ channels in sensory neurons via distinct mechanisms. Biochem Biophys Res Commun 2015; 465: 188–193. [DOI] [PubMed] [Google Scholar]
- 50.Rose KE Lunardi N Boscolo A Dong X Erisir A Jevtovic-Todorovic Vand Todorovic SM. Immunohistological demonstration of Cav3.2 T-type voltage-gated calcium channel expression in soma of dorsal root ganglion neurons and peripheral axons of rat and mouse. Neuroscience 2013; 250: 263–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Francois A Schuetter N Laffray S Sanguesa J Pizzoccaro A Dubel S Mantilleri A Nargeot J Noel J Wood JN Moqrich A Pongs Oand Bourinet E. The low-threshold calcium channel Cav3.2 determines low-threshold mechanoreceptor function. Cell Rep 2015; 10: 370–382. [DOI] [PubMed] [Google Scholar]
- 52.Vriens J Owsianik G Hofmann T Philipp SE Stab J Chen X Benoit M Xue F Janssens A Kerselaers S Oberwinkler J Vennekens R Gudermann T Nilius Band Voets T. TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron 2011; 70: 482–494. [DOI] [PubMed] [Google Scholar]
- 53.Wagner TF Loch S Lambert S Straub I Mannebach S Mathar I Dufer M Lis A Flockerzi V Philipp SEand Oberwinkler J. Transient receptor potential M3 channels are ionotropic steroid receptors in pancreatic beta cells. Nat Cell Biol 2008; 10: 1421–1430. [DOI] [PubMed] [Google Scholar]
- 54.Held K Kichko T De Clercq K Klaassen H Van Bree R Vanherck JC Marchand A Reeh PW Chaltin P Voets Tand Vriens J. Activation of TRPM3 by a potent synthetic ligand reveals a role in peptide release. Proc Natl Acad Sci USA 2015; 112: E1363–E1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Badheka D Yudin Y Borbiro I Hartle CM Yazici A Mirshahi Tand Rohacs T. Inhibition of transient receptor potential melastatin 3 ion channels by G-protein βγ subunits. Elife 2017; 6: e26147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dembla S Behrendt M Mohr F Goecke C Sondermann J Schneider FM Schmidt M Stab J Enzeroth R Leitner MG Nunez-Badinez P Schwenk J Nurnberg B Cohen A Philipp SE Greffrath W Bunemann M Oliver D Zakharian E Schmidt Mand Oberwinkler J. Anti-nociceptive action of peripheral mu-opioid receptors by G-beta-gamma protein-mediated inhibition of TRPM3 channels. Elife 2017; 6: e26280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Quallo T Alkhatib O Gentry C Andersson DAand Bevan S. G protein betagamma subunits inhibit TRPM3 ion channels in sensory neurons. Elife 2017; 6: e26138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Csanady L. A new target for G protein signaling. Elife 2017; 6: e31106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khan SM Sleno R Gora S Zylbergold P Laverdure JP Labbe JC Miller GJand Hebert TE. The expanding roles of Gbetagamma subunits in G protein-coupled receptor signaling and drug action. Pharmacol Rev 2013; 65: 545–577. [DOI] [PubMed] [Google Scholar]
- 60.Clapham DE andNeer EJ.. G protein beta gamma subunits. Annu Rev Pharmacol Toxicol 1997; 37: 167–203. [DOI] [PubMed] [Google Scholar]
- 61.Obata K andNoguchi K.. MAPK activation in nociceptive neurons and pain hypersensitivity. Life Sci 2004; 74: 2643–2653. [DOI] [PubMed] [Google Scholar]
- 62.Zhang X Huang J andMcNaughton PA.. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J 2005; 24: 4211–4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leinders M Koehrn FJ Bartok B Boyle DL Shubayev V Kalcheva I Yu NK Park J Kaang BK Hefferan MP Firestein GSand Sorkin LS. Differential distribution of PI3K isoforms in spinal cord and dorsal root ganglia: potential roles in acute inflammatory pain. Pain 2014; 155: 1150–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu CH Gong Z Liang ZL Liu ZX Yang F Sun YJ Ma ML Wang YJ Ji CR Wang YH Wang MJ Cui FA Lin A Zheng WS He DF Qu CX Xiao P Liu CY Thomsen AR Joseph Cahill T III Kahsai AW Yi F Xiao KH Xue T Zhou Z Yu Xand Sun JP. Arrestin-biased AT1R agonism induces acute catecholamine secretion through TRPC3 coupling. Nat Comms 2017; 8: 14335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mittal N Roberts K Pal K Bentolila LA Fultz E Minasyan A Cahill C Pradhan A Conner D DeFea K Evans Cand Walwyn W. Select G-protein-coupled receptors modulate agonist-induced signaling via a ROCK, LIMK, and beta-arrestin 1 pathway. Cell Rep 2013; 5: 1010–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pradhan AA Perroy J Walwyn WM Smith ML Vicente-Sanchez A Segura L Bana A Kieffer BLand Evans CJ. Agonist-specific recruitment of arrestin isoforms differentially modify delta opioid receptor function. J Neurosci 2016; 36: 3541–3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lin CC Chen WN Chen CJ Lin YW Zimmer Aand Chen CC. An antinociceptive role for substance P in acid-induced chronic muscle pain. Proc Natl Acad Sci USA 2012; 109: E76–E83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang D Huang S Gao H Liu Y Qi J Chen P Wang C Scragg JL Vakurov A Peers C Du X Zhang Hand Gamper N. Redox-dependent modulation of T-type Ca2+ channels in sensory neurons contributes to acute anti-nociceptive effect of substance. Antioxid Redox Signal 2016; 25: 233–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Linley JE Ooi L Pettinger L Kirton H Boyle JP Peers Cand Gamper N. Reactive oxygen species are second messengers of neurokinin signaling in peripheral sensory neurons. Proc Natl Acad Sci USA 2012; 109: E1578–E1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rush AM andWaxman SG.. PGE2 increases the tetrodotoxin-resistant Nav1.9 sodium current in mouse DRG neurons via G-proteins. Brain Res 2004; 1023: 264–271. [DOI] [PubMed] [Google Scholar]
- 71.Belkouch M Dansereau MA Reaux-Le Goazigo A Van Steenwinckel J Beaudet N Chraibi A Melik-Parsadaniantz Sand Sarret P. The chemokine CCL2 increases Nav1.8 sodium channel activity in primary sensory neurons through a Gbetagamma-dependent mechanism. J Neurosci 2011; 31: 18381–18390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qiu F Li Y Fu Q Fan YY Zhu C Liu YHand Mi WD. Stromal cell-derived factor 1 increases tetrodotoxin-resistant sodium currents Nav1.8 and Nav1.9 in rat dorsal root ganglion neurons via different mechanisms. Neurochem Res 2016; 41: 1587–1603. [DOI] [PubMed] [Google Scholar]
- 73.Gunther T Dasgupta P Mann A Miess E Kliewer A Fritzwanker S Steinborn Rand Schulz S. Targeting multiple opioid receptors – improved analgesics with reduced side effects? Br J Pharmacol 2017.Epub ahead of print 5 April 2017. DOI: 10.1111/bph.13809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gendron L Cahill CM von Zastrow M Schiller PWand Pineyro G. Molecular pharmacology of delta-opioid receptors. Pharmacol Rev 2016; 68: 631–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meunier J Mouledous L andTopham CM.. The nociceptin (ORL1) receptor: molecular cloning and functional architecture. Peptides 2000; 21: 893–900. [DOI] [PubMed] [Google Scholar]
- 76.Smith HS. Peripherally-acting opioids. Pain Physician 2008; 11: S121–S132. [PubMed] [Google Scholar]
- 77.Sawynok J. Topical and peripherally acting analgesics. Pharmacol Rev 2003; 55: 1–20. [DOI] [PubMed] [Google Scholar]
- 78.Sehgal N Smith HS andManchikanti L.. Peripherally acting opioids and clinical implications for pain control. Pain Physician 2011; 14: 249–258. [PubMed] [Google Scholar]
- 79.Regnard C Twycross R Mihalyo Mand Wilcock A. Loperamide. J Pain Symptom Manage 2011; 42: 319–323. [DOI] [PubMed] [Google Scholar]
- 80.Labuz D Mousa SA Schafer M Stein Cand Machelska H. Relative contribution of peripheral versus central opioid receptors to antinociception. Brain Res 2007; 1160: 30–38. [DOI] [PubMed] [Google Scholar]
- 81.Liang L Zhao JY Gu X Wu S Mo K Xiong M Marie Lutz B Bekker Aand Tao YX. G9a inhibits CREB-triggered expression of mu opioid receptor in primary sensory neurons following peripheral nerve injury. Mol Pain 2016; 12: 1744806916682242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nozaki-Taguchi N andYaksh TL.. Characterization of the antihyperalgesic action of a novel peripheral mu-opioid receptor agonist–loperamide. Anesthesiology 1999; 90: 225–234. [DOI] [PubMed] [Google Scholar]
- 83.Francois A andScherrer G.. Delta opioid receptor expression and function in primary afferent somatosensory neurons. Handb Exp Pharmacol. 2017. Epub ahead of print 10 October 2017. DOI: 10.1007/164_2017_58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ji RR Zhang Q Law PY Low HH Elde Rand Hokfelt T. Expression of mu-, delta-, and kappa-opioid receptor-like immunoreactivities in rat dorsal root ganglia after carrageenan-induced inflammation. J Neurosci 1995; 15: 8156–8166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Scherrer G Imamachi N Cao YQ Contet C Mennicken F O?Donnell D Kieffer BLand Basbaum AI. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell 2009; 137: 1148–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang HB Zhao B Zhong YQ Li KC Li ZY Wang Q Lu YJ Zhang ZN He SQ Zheng HC Wu SX Hokfelt TG Bao Land Zhang X. Coexpression of delta- and mu-opioid receptors in nociceptive sensory neurons. Proc Natl Acad Sci USA 2010; 107: 13117–13122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gupta A Mulder J Gomes I Rozenfeld R Bushlin I Ong E Lim M Maillet E Junek M Cahill CM Harkany Tand Devi LA. Increased abundance of opioid receptor heteromers after chronic morphine administration. Sci Signal 2010; 3: ra54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.He SQ Zhang ZN Guan JS Liu HR Zhao B Wang HB Li Q Yang H Luo J Li ZY Wang Q Lu YJ Bao Land Zhang X. Facilitation of mu-opioid receptor activity by preventing delta-opioid receptor-mediated codegradation. Neuron 2011; 69: 120–131. [DOI] [PubMed] [Google Scholar]
- 89.Corder G Tawfik VL Wang D Sypek EI Low SA Dickinson JR Sotoudeh C Clark JD Barres BA Bohlen CJand Scherrer G. Loss of mu opioid receptor signaling in nociceptors, but not microglia, abrogates morphine tolerance without disrupting analgesia. Nat Med 2017; 23: 164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Del Vecchio G Spahn V andStein C.. Novel opioid analgesics and side effects. ACS Chem Neurosci 2017; 8: 1638–1640. [DOI] [PubMed] [Google Scholar]
- 91.Spahn V Del Vecchio G Labuz D Rodriguez-Gaztelumendi A Massaly N Temp J Durmaz V Sabri P Reidelbach M Machelska H Weber Mand Stein C. A nontoxic pain killer designed by modeling of pathological receptor conformations. Science 2017; 355: 966–969. [DOI] [PubMed] [Google Scholar]
- 92.Gonzalez-Rodriguez S Quadir MA Gupta S Walker KA Zhang X Spahn V Labuz D Rodriguez-Gaztelumendi A Schmelz M Joseph J Parr MK Machelska H Haag Rand Stein C. Polyglycerol-opioid conjugate produces analgesia devoid of side effects. Elife 2017; 6: e27081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moises HC Rusin KI andMacdonald RL.. Mu- and kappa-opioid receptors selectively reduce the same transient components of high-threshold calcium current in rat dorsal root ganglion sensory neurons. J Neurosci 1994; 14: 5903–5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cowan A Kehner GB andInan S.. Targeting itch with ligands selective for kappa opioid receptors. Handb Exp Pharmacol 2015; 226: 291–314. [DOI] [PubMed] [Google Scholar]
- 95.Spencer RHMC Oberdick MS Stauffer JW andMenzaghi F.. Randomized, placebo-controlled study on the efficacy of CR845 in reducing CKD-associated pruritus in hemodialysis patients. J Am Soc Nephrol 2017; 28: 629A. [Google Scholar]
- 96.Ozawa A Brunori G Mercatelli D Wu J Cippitelli A Zou B Xie XS Williams M Zaveri NT Low S Scherrer G Kieffer BLand Toll L. Knock-in mice with NOP-eGFP receptors identify receptor cellular and regional localization. J Neurosci 2015; 35: 11682–11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Beedle AM McRory JE Poirot O Doering CJ Altier C Barrere C Hamid J Nargeot J Bourinet Eand Zamponi GW. Agonist-independent modulation of N-type calcium channels by ORL1 receptors. Nat Neurosci 2004; 7: 118–125. [DOI] [PubMed] [Google Scholar]
- 98.Anand P Yiangou Y Anand U Mukerji G Sinisi M Fox M McQuillan A Quick T Korchev YEand Hein P. Nociceptin/orphanin FQ receptor expression in clinical pain disorders and functional effects in cultured neurons. Pain 2016; 157: 1960–1969. [DOI] [PubMed] [Google Scholar]
- 99.Padgett CL andSlesinger PA.. GABAB receptor coupling to G-proteins and ion channels. Adv Pharmacol 2010; 58: 123–147. [DOI] [PubMed] [Google Scholar]
- 100.Bowery NG Bettler B Froestl W Gallagher JP Marshall F Raiteri M Bonner TIand Enna SJ. International union of pharmacology. XXXIII. Mammalian gamma-aminobutyric acid(B) receptors: structure and function. Pharmacol Rev 2002; 54: 247–264. [DOI] [PubMed] [Google Scholar]
- 101.Caron E Morgan R andWheless JW.. An unusual cause of flaccid paralysis and coma: baclofen overdose. J Child Neurol 2014; 29: 555–559. [DOI] [PubMed] [Google Scholar]
- 102.Callaghan B Haythornthwaite A Berecki G Clark RJ Craik DJand Adams DJ. Analgesic alpha-conotoxins Vc1.1 and Rg1A inhibit N-type calcium channels in rat sensory neurons via GABAB receptor activation. J Neurosci 2008; 28: 10943–10951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Adams DJ Callaghan B andBerecki G.. Analgesic conotoxins: block and G protein-coupled receptor modulation of N-type (Cav2.2) calcium channels. Br J Pharmacol 2012; 166: 486–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Klimis H Adams DJ Callaghan B Nevin S Alewood PF Vaughan CW Mozar CAand Christie MJ. A novel mechanism of inhibition of high-voltage activated calcium channels by alpha-conotoxins contributes to relief of nerve injury-induced neuropathic pain. Pain 2011; 152: 259–266. [DOI] [PubMed] [Google Scholar]
- 105.Pinter E Helyes Z andSzolcsanyi J.. Inhibitory effect of somatostatin on inflammation and nociception. Pharmacol Ther 2006; 112: 440–456. [DOI] [PubMed] [Google Scholar]
- 106.Szolcsanyi J Pinter E Helyes Zand Petho G. Inhibition of the function of TRPV1-expressing nociceptive sensory neurons by somatostatin 4 receptor agonism: mechanism and therapeutical implications. CTMC 2011; 11: 2253–2263. [DOI] [PubMed] [Google Scholar]
- 107.Szolcsanyi J Helyes Z Oroszi G Nemeth Jand Pinter E. Release of somatostatin and its role in the mediation of the anti-inflammatory effect induced by antidromic stimulation of sensory fibres of rat sciatic nerve. Br J Pharmacol 1998; 123: 936–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Corsi MM Ticozzi C Netti C Fulgenzi A Tiengo M Gaja G Guidobono Fand Ferrero ME. The effect of somatostatin on experimental inflammation in rats. Anesth Analg 1997; 85: 1112–1115. [DOI] [PubMed] [Google Scholar]
- 109.Carlton SM Du J Davidson E Zhou Sand Coggeshall RE. Somatostatin receptors on peripheral primary afferent terminals: inhibition of sensitized nociceptors. Pain 2001; 90: 233–244. [DOI] [PubMed] [Google Scholar]
- 110.Carlton SM Zhou S Du J Hargett GL Ji G and Coggeshall RE. Somatostatin modulates the transient receptor potential vanilloid 1 (TRPV1) ion channel. Pain 2004; 110: 616–627. [DOI] [PubMed] [Google Scholar]
- 111.Silveri F Morosini P Brecciaroli Dand Cervini C. Intra-articular injection of somatostatin in knee osteoarthritis: clinical results and IGF-1 serum levels. Int J Clin Pharmacol Res 1994; 14: 79–85. [PubMed] [Google Scholar]
- 112.Gorham L Just S andDoods H.. Somatostatin 4 receptor activation modulates TRPV1 currents in dorsal root ganglion neurons. Neurosci Lett 2014; 573: 35–39. [DOI] [PubMed] [Google Scholar]
- 113.Helyes Z Pinter E Sandor K Elekes K Banvolgyi A Keszthelyi D Szoke E Toth DM Sandor Z Kereskai L Pozsgai G Allen JP Emson PC Markovics Aand Szolcsanyi J. Impaired defense mechanism against inflammation, hyperalgesia, and airway hyperreactivity in somatostatin 4 receptor gene-deleted mice. Proc Natl Acad Sci USA 2009; 106: 13088–13093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pin JP andBettler B.. Organization and functions of mGlu and GABAB receptor complexes. Nature 2016; 540: 60–68. [DOI] [PubMed] [Google Scholar]
- 115.Julio-Pieper M Flor PJ Dinan TGand Cryan JF. Exciting times beyond the brain: metabotropic glutamate receptors in peripheral and non-neural tissues. Pharmacol Rev 2011; 63: 35–58. [DOI] [PubMed] [Google Scholar]
- 116.Bhave G Karim F Carlton SMand Gereau RW IV. Peripheral group I metabotropic glutamate receptors modulate nociception in mice. Nat Neurosci 2001; 4: 417–423. [DOI] [PubMed] [Google Scholar]
- 117.Neugebauer V. Metabotropic glutamate receptors–important modulators of nociception and pain behavior. Pain 2002; 98: 1–8. [DOI] [PubMed] [Google Scholar]
- 118.Yang D andGereau RWT.. Peripheral group II metabotropic glutamate receptors (mGluR2/3) regulate prostaglandin E2-mediated sensitization of capsaicin responses and thermal nociception. J Neurosci 2002; 22: 6388–6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yang D andGereau RWT.. Peripheral group II metabotropic glutamate receptors mediate endogenous anti-allodynia in inflammation. Pain 2003; 106: 411–417. [DOI] [PubMed] [Google Scholar]
- 120.Yang D andGereau RWT.. Group II metabotropic glutamate receptors inhibit cAMP-dependent protein kinase-mediated enhancemednt of tetrodotoxin-resistant sodium currents in mouse dorsal root ganglion neurons. Neurosci Lett 2004; 357: 159–162. [DOI] [PubMed] [Google Scholar]
- 121.Davidson S Golden JP Copits BA Ray PR Vogt SK Valtcheva MV Schmidt RE Ghetti A Price TJand Gereau RW IV. Group II mGluRs suppress hyperexcitability in mouse and human nociceptors. Pain 2016; 157: 2081–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Govea RM Zhou S andCarlton SM.. Group III metabotropic glutamate receptors and transient receptor potential vanilloid 1 co-localize and interact on nociceptors. Neuroscience 2012; 217: 130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li JY Wang X Ji PT Li XF Guan GH Jiang XS Zhou GS Hua Fand Wang N. Peripheral nerve injury decreases the expression of metabolic glutamate receptor 7 in dorsal root ganglion neurons. Neurosci Lett 2012; 531: 52–56. [DOI] [PubMed] [Google Scholar]
- 124.Carlton SM Zhou S Govea Rand Du J. Group II/III metabotropic glutamate receptors exert endogenous activity-dependent modulation of TRPV1 receptors on peripheral nociceptors. J Neurosci 2011; 31: 12727–12737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fredholm BB AP IJ Jacobson KA Linden Jand Muller CE. International union of basic and clinical pharmacology. LXXXI. Nomenclature and classification of adenosine receptors–an update. Pharmacol Rev 2011; 63: 1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sawynok J andLiu XJ.. Adenosine in the spinal cord and periphery: release and regulation of pain. Prog Neurobiol 2003; 69: 313–340. [DOI] [PubMed] [Google Scholar]
- 127.Liu XJ White TD andSawynok J.. Involvement of primary sensory afferents, postganglionic sympathetic nerves and mast cells in the formalin-evoked peripheral release of adenosine. Eur J Pharmacol 2001; 429: 147–155. [DOI] [PubMed] [Google Scholar]
- 128.Sawynok J. Adenosine receptor targets for pain. Neuroscience 2016; 338: 1–18. [DOI] [PubMed] [Google Scholar]
- 129.Ewald DA Pang IH Sternweis PCand Miller RJ. Differential G protein-mediated coupling of neurotransmitter receptors to Ca2+ channels in rat dorsal root ganglion neurons in vitro. Neuron 1989; 2: 1185–1193. [DOI] [PubMed] [Google Scholar]
- 130.Walker MW Ewald DA Perney TMand Miller RJ. Neuropeptide Y modulates neurotransmitter release and Ca2+ currents in rat sensory neurons. J Neurosci 1988; 8: 2438–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hokfelt T Brumovsky P Shi T Pedrazzini Tand Villar M. NPY and pain as seen from the histochemical side. Peptides 2007; 28: 365–372. [DOI] [PubMed] [Google Scholar]
- 132.Naveilhan P Hassani H Lucas G Blakeman KH Hao JX Xu XJ Wiesenfeld-Hallin Z Thoren Pand Ernfors P. Reduced antinociception and plasma extravasation in mice lacking a neuropeptide Y receptor. Nature 2001; 409: 513–517. [DOI] [PubMed] [Google Scholar]
- 133.Shi TJ Li J Dahlstrom A Theodorsson E Ceccatelli S Decosterd I Pedrazzini Tand Hokfelt T. Deletion of the neuropeptide Y Y1 receptor affects pain sensitivity, neuropeptide transport and expression, and dorsal root ganglion neuron numbers. Neuroscience 2006; 140: 293–304. [DOI] [PubMed] [Google Scholar]
- 134.Smith PA, Moran TD, Abdulla F, Tumber KK and Taylor BK. Spinal mechanisms of NPY analgesia. Peptides 2007; 28: 464–474. [DOI] [PubMed] [Google Scholar]
- 135.Yalamuri SM Brennan TJ andSpofford CM.. Neuropeptide Y is analgesic in rats after plantar incision. Eur J Pharmacol 2013; 698: 206–212. [DOI] [PubMed] [Google Scholar]
- 136.Alexander SP Mathie A andPeters JA.. Guide to receptors and channels (GRAC), 5th edition. Br J Pharmacol 2011; 164: S1–S324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kim YS Chu Y Han L Li M Li Z LaVinka PC Sun S Tang Z Park K Caterina MJ Ren K Dubner R Wei Fand Dong X. Central terminal sensitization of TRPV1 by descending serotonergic facilitation modulates chronic pain. Neuron 2014; 81: 873–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lin SY Chang WJ Lin CS Huang CY Wang HFand Sun WH. Serotonin receptor 5-HT2B mediates serotonin-induced mechanical hyperalgesia. J Neurosci 2011; 31: 1410–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Salzer I Gantumur E Yousuf Aand Boehm S. Control of sensory neuron excitability by serotonin involves 5HT2C receptors and Ca2+-activated chloride channels. Neuropharmacology 2016; 110: 277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Goadsby PJ Holland PR Martins-Oliveira M Hoffmann J Schankin Cand Akerman S. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev 2017; 97: 553–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Evans MS Cheng X Jeffry JA Disney KEand Premkumar LS. Sumatriptan inhibits TRPV1 channels in trigeminal neurons. Headache 2012; 52: 773–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Loyd DR Weiss G Henry MAand Hargreaves KM. Serotonin increases the functional activity of capsaicin-sensitive rat trigeminal nociceptors via peripheral serotonin receptors. Pain 2011; 152: 2267–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Armbruster BN Li X Pausch MH Herlitze Sand Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci USA 2007; 104: 5163–5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Roth BL. DREADDs for neuroscientists. Neuron 2016; 89: 683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Copits BA Pullen MY andGereau RWT.. Spotlight on pain: optogenetic approaches for interrogating somatosensory circuits. Pain 2016; 157: 2424–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhu H Aryal DK Olsen RH Urban DJ Swearingen A Forbes S Roth BLand Hochgeschwender U. Cre-dependent DREADD (Designer receptors exclusively activated by designer drugs) mice. Genesis 2016; 54: 439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Iyer SM Vesuna S Ramakrishnan C Huynh K Young S Berndt A Lee SY Gorini CJ Deisseroth Kand Delp SL. Optogenetic and chemogenetic strategies for sustained inhibition of pain. Sci Rep 2016; 6: 30570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Saloman JL Scheff NN Snyder LM Ross SE Davis BMand Gold MS. Gi-DREADD expression in peripheral nerves produces ligand-dependent analgesia, as well as ligand-independent functional changes in sensory neurons. J Neurosci 2016; 36: 10769–10781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Vardy E, Robinson JE, Li C, Olsen RH, DiBerto JF, Giguere PM, Sassano FM, Huang XP, Zhu H, Urban DJ, White KL, Rittiner JE, Crowley NA, Pleil KE, Mazzone CM, Mosier PD, Song J, Kash TL, Malanga CJ, Krashes MJ and Roth BL. A new DREADD facilitates the multiplexed chemogenetic interrogation of behavior. Neuron 2015; 86: 936–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chen X Choo H Huang XP Yang X Stone O Roth BLand Jin J. The first structure-activity relationship studies for designer receptors exclusively activated by designer drugs. ACS Chem Neurosci 2015; 6: 476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gomez JL Bonaventura J Lesniak W Mathews WB Sysa-Shah P Rodriguez LA Ellis RJ Richie CT Harvey BK Dannals RF Pomper MG Bonci Aand Michaelides M. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science 2017; 357: 503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Barish PA Xu Y Li J Sun J Jarajapu YPand Ogle WO. Design and functional evaluation of an optically active mu-opioid receptor. Eur J Pharmacol 2013; 705: 42–48. [DOI] [PubMed] [Google Scholar]
- 153.Araldi D Ferrari LF andLevine JD.. Adenosine-A1 receptor agonist induced hyperalgesic priming type II. Pain 2016; 157: 698–709. [DOI] [PMC free article] [PubMed] [Google Scholar]