Short abstract
Maternal nutrition during gestation, especially dietary protein intake, is a key determinant in embryonic survival, growth, and development. Low maternal dietary protein intake can cause embryonic losses, intra-uterine growth restriction, and reduced postnatal growth due to a deficiency in specific amino acids that are important for cell metabolism and function. Of note, high maternal dietary protein intake can also result in intra-uterine growth restriction and embryonic death, due to amino acid excesses, as well as the toxicity of ammonia, homocysteine, and H2S that are generated from amino acid catabolism. Maternal protein nutrition has a pronounced impact on fetal programming and alters the expression of genes in the fetal genome. As a precursor to the synthesis of molecules (e.g. nitric oxide, polyamines, and creatine) with cell signaling and metabolic functions, L-arginine (Arg) is essential during pregnancy for growth and development of the conceptus. With inadequate maternal dietary protein intake, Arg and other important amino acids are deficient in mother and fetus. Dietary supplementation of Arg during gestation has been effective in improving embryonic survival and development of the conceptus in many species, including humans, pigs, sheep, mice, and rats. Both the balance among amino acids and their quantity are critical for healthy pregnancies and offspring.
Impact statement
This review aims at: highlighting adverse effects of elevated levels of ammonia in mother or fetus on embryonic/fetal survival, growth, and development; helping nutritionists and practitioners to understand the mechanisms whereby elevated levels of ammonia in mother or fetus results in embryonic/fetal death, growth restriction, and developmental abnormalities; and bringing, into the attention of nutritionists and practitioners, the problems of excess or inadequate dietary intake of protein or amino acids on pregnancy outcomes in animals and humans. The article provides new, effective means to improve embryonic/fetal survival and growth in mammals.
Keywords: Protein, fetus, growth, placenta, reproduction, nutrition
Introduction
Maternal nutrition during gestation plays a critical role in fetal survival, growth, and development.1 Therefore, both maternal undernutrition and overnutrition can be detrimental to the developing fetus. Specifically, insufficient or excessive maternal dietary protein intake can cause lifelong consequences for the neonate due to fetal programming. Fetal programming refers to the heritable changes in gene expression without alterations in DNA sequences within the genome.1 Malnutrition alters expression of the fetal genome, leading to metabolic disorders, organ dysfunction, hormone imbalances, and cell signaling defects.
Amino acids (AAs) are essential for protein synthesis and other nitrogenous substances such as catecholamines, creatine, dopamine, nitric oxide (NO), polyamines, and thyroid hormones.2 Additionally, certain AAs are responsible for regulating cell signaling and metabolic pathways. Low maternal dietary protein intake is linked to intrauterine growth restriction (IUGR) as well as reduced postnatal growth and feed efficiency.1 The placenta requires adequate levels of AAs for proper growth and development to supply enough nutrients to the fetus. With low dietary protein intake, the limited supply of AAs to the placenta results in placental insufficiency and consequently IUGR.2 High maternal dietary protein intake is also linked to IUGR and can cause fetal or neonatal death due to ammonia toxicity (Figure 1). Like low dietary protein intake, high protein intake results in AA excesses during pregnancy.1 In all species studied, including swine, cattle, and rodents, high concentrations of ammonia in plasma increase embryonic death.1 Ammonia is a product of AA catabolism, and high dietary protein intake leads to toxic levels of ammonia in the plasma.3 Excess production of other metabolites of AAs, such as homocysteine, H2S and indoles, may also impair embryonic/fetal survival and growth.4,5
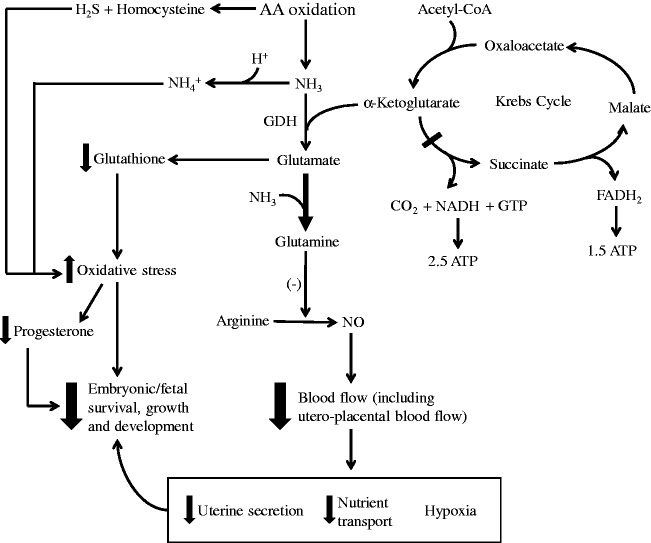
Figure 1.
Ammonia toxicity decreases embryonic and fetal survival, growth, and development. Ammonia, H2S, and homocysteine are products from AA oxidation. High levels of ammonia are toxic to the developing embryo/fetus due to (1) oxidative stress resulting from ammonia pulling glutamate away from glutathione synthesis to produce glutamine, which is an inhibitor of nitric oxide (NO) generation from arginine. H2S and homocysteine also contribute to oxidative stress, which reduces progesterone production and release by the ovaries. (2) Ammonia binds a proton to form ammonium ion, which increases intracellular pH. (3) Ammonia draws α-ketoglutarate from the Krebs cycle to form glutamate, therefore inhibiting the Krebs cycle and reducing ATP production. (4) Increased production of glutamine from ammonia inhibits NO production from arginine by endothelial cells, which decreases blood flow (including utero-placental blood flow) and nutrient transport, leading to a decrease in uterine secretion as well as hypoxia in the conceptus. These consequences result in decreased embryonic/fetal survival, growth, and development. GDH: glutamate dehydrogenase.
There is growing interest in the functional roles of certain AAs in mammalian pregnancies.2 One of these AAs is L-arginine (Arg), which has been properly recognized as a “conditionally essential AA” in the diet, especially for embryonic growth and survival.6 Arg is a precursor to biologically important substances such as polyamines, ornithine, proline, glutamate, agmatine, homoarginine, creatine, and NO.3 Arg is also required for hepatic urea synthesis to remove ammonia from the liver and blood. Of note, this AA regulates protein synthesis in the skeletal muscle and placenta by activating mechanistic target of rapamycin (mTOR) signaling, stimulating the secretion of growth hormone and insulin, and enhancing anti-oxidative signaling and the cellular redox state.3 Results of recent studies indicate that Arg enhances placental angiogenesis and growth to improve blood flow across the placenta, thereby increasing nutrient transfer from the mother to her fetus.1,2 NO and polyamines are also necessary for implantation, and they are known to regulate steroid hormone synthesis and stimulate cell proliferation and migration in the conceptus (embryo and its associated extra-embryonic membranes).2 Furthermore, through multiple cell signaling pathways, Arg increases development of fetal brown adipose tissue.3 Because of these beneficial effects of Arg during gestation, dietary supplementation of Arg has been found to improve reproductive performance and increase embryonic survival and growth.7 For dietary supplementation, Arg is administered as the neutral salt Arg-HCl to maintain an acid-base balance.6
The major objective of this article is to highlight impacts of maternal protein intake on survival, growth, and development of fetuses in humans, farm animals, and rodents. Wherever data are available, roles of specific AAs, such as Arg and L-glutamine (Gln), in healthy pregnancy are discussed. Findings from diverse animal species are expected to guide optimal nutritional support of gestating mammals.
Humans
Maternal undernutrition and overnutrition in humans are known to cause an imbalance of nutrients such as AAs (either deficiencies or excesses), elevated concentrations of cortisol in blood, and oxidative stress.8,9 The negative effects of this malnutrition include impairment of offspring growth and development, maternal insulin resistance, cretinism, IUGR, birth defects, cognitive and behavioral defects, postpartum complications, pre-eclampsia and eclampsia, anemia, preterm birth, maternal hemorrhaging and additional long-term effects for both mother and offspring.8 Additionally, low maternal dietary protein intake resulted in the greatest abdominal adiposity in fetuses compared to intermediate and high protein intake regardless of carbohydrate and fat intake.10–12 IUGR increases neonatal mortality, and surviving IUGR infants experience an increased risk of developing metabolic disorders, hormonal imbalances, abnormal development, and cardiovascular disorders into adulthood.8 Studies of maternal malnutrition during the Dutch famine in 1944 and 1945 showed that global undernutrition during the second and third trimester caused reduced weight, length, and head circumference of newborn babies.8 However, animal studies have shown that maternal undernutrition, specifically deficiencies in protein and AAs, during early gestation is more harmful than during late gestation.1 Due to the rapidly changing demands of the fetus and mother for nutrients, dietary protein and AA requirements vary at different stages of gestation. Recent studies report that protein synthesis increases by 15% in the second trimester and 25% in the third trimester, but AA catabolism and urea synthesis decrease in pregnant women, indicating that there is a preservation of protein during the period of high demand for AAs during pregnancy.9 Elango and Ball9 determined the protein requirements for pregnant women to be 1.2 g/kg of body weight/day during early gestation and 1.52 g/kg of body weight/day during late gestation. These requirements are equivalent to 14% of energy intake for early gestation and 17.5% of energy intake for late gestation. The current estimated average requirement (EAR) and recommended dietary allowance (RDA) for protein are 0.88 g/kg of body weight/day and 1.1 g/kg of body weight/day, respectively, throughout all gestational stages, which are significantly lower than the requirements determined by Elango and Ball.9 These new requirements were determined using the indicator AA oxidation (IAAO) method rather than the nitrogen balance method, which tends to underestimate protein requirements.3 Whether the IAAO method overestimates dietary protein requirements for humans and other mammals is unknown. Pregnancy outcomes of women as well as offspring growth and health10–12 should be the functional criteria with which to define their dietary AA requirements.
Low maternal dietary protein intake means that specific AAs are deficient in both mother and fetus.2 A deficiency in Arg can cause preterm labor by stimulating the uterine myometrium due to the reduced bioavailability of NO.8 NO deficiency is also linked to pre-eclampsia, which causes proteinuria and high blood pressure in pregnant women.8 Based on numerous animal studies, Arg supplementation is believed to be safe for humans.6 Dietary supplementation of 3 g of Arg daily for four weeks to women with pre-eclampsia reduced blood pressure, improved fetal health and growth, and also beneficially prolonged pregnancy.8 In addition, daily intravenous infusion of 20 g Arg for seven days during late gestation (week 33) increased birth weight by 6.4% in IUGR babies.7 Furthermore, Arg supplementation also decreases placental apoptosis and improves development of IUGR fetuses.7 Figure 2 summarizes mechanisms responsible for adverse consequences of maternal protein malnutrition and deficiencies of AAs (e.g., Arg and Gln) on fetal growth and development.
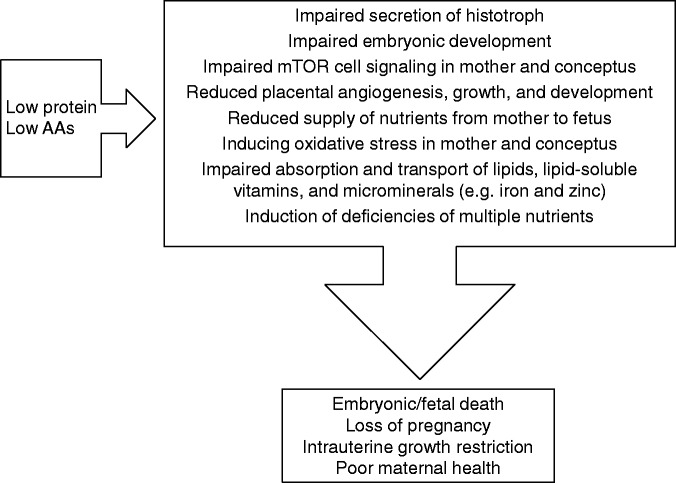
Figure 2.
Adverse consequences of low maternal dietary intake of protein or amino acids (AAs) during pregnancy. Low maternal dietary intake of protein and AAs leads to many negative effects, such as impaired secretion of histotroph; impaired embryonic development; impaired mechanistic target of rapamycin (mTOR) cell signaling in mother and conceptus; reduced placental angiogenesis, growth and development; reduced supply of nutrients from mother to fetus; inducing oxidative stress in mother and conceptus; impaired absorption and transport of lipids, lipid-soluble vitamins and microminerals (e.g. iron and zinc); and induction of deficiencies of multiple nutrients. These consequences result in embryonic/fetal death, loss of pregnancy, intrauterine growth restriction and poor maternal health.
High maternal dietary protein intake is detrimental due to excesses and imbalances of AAs.3 Protein supplements used during pregnancy must be balanced in order to avoid an AA imbalance. With high maternal concentrations of AAs, competition for AA transporters results in reduced placental transport and umbilical uptake of AAs.2 Like low protein intake, high protein intake also leads to lower birth weights indicating that moderate maternal dietary protein intake is optimal for fetal growth and survival.8 Reduced placental transport of AAs due to ammonia toxicity and impaired blood flow contributes to reduced protein synthesis in the fetus and lower birth weights. The ratio of protein to carbohydrates or fat in maternal diets also impacts fetal body composition and fetal adiposity.12 Increased abdominal visceral fat has been associated with a greater percentage of dietary energy as protein (>20%) rather than starch.10 A study conducted by Switkowski et al.13 found that concentrations of insulin-like growth factor (IGF)-II, IGF binding protein (IGFBP)-3, and insulin in umbilical cord blood were reduced with a higher maternal dietary protein intake. IGF-II activity is influenced by nutrient availability and is responsible for regulating placental and embryonic growth and nutrient transfer to the fetus. IGFBPs may also have an impact on fetal growth in response to nutrient availability by restricting IGF bioactivity. Decreased insulin in response to excessive protein consumption may also impair IGF-I and IGF-II production by cells of the fetus.13 Table 1 summarizes the studies reporting the adverse effects of excess or inadequate maternal intake of dietary protein on human fetal growth and development.
Table 1.
Adverse consequences of excessive or inadequate intake of dietary protein or amino acids (AAs) in humans and animals.
| 1. Excessive protein/AA intake in humans | References |
| Intrauterine growth restriction | 8 |
| Reduced placental transport and umbilical uptake of AAs | 8 |
| Low birth weights | 1 |
| Increased abdominal visceral fat | 12 |
| Decreased insulin-like growth factor-II activity | 13 |
| 2. Excessive protein/AA intake in animals | |
| Reduced skeletal muscle fiber size and number in neonates | 1 |
| Ammonia toxicity | 1,6 |
| Intrauterine growth restriction | 2 |
| Increased liver weight | 14 |
| Reduced thymus gland | 15 |
| Reduced bone weight | 14 |
| Changes in expression of genes involved in metabolism | 16 |
| Reduced development of hypothalamic-pituitary-gonadal axis | 17 |
| Decrease in pH of maternal blood | 18,19 |
| Low birth weights | 20,21 |
| Increase in oxytocin-stimulated release of prostaglandin F2α | 8 |
| Elevated blood pressure in offspring | 22 |
| Decreased litter size | 2 |
| Increased maternal kidney mass | 14 |
| Reduced food intake of offspring | 19 |
| Altered glucose metabolism | 22 |
| 3. Inadequate protein/AA intake in humans | |
| Increased abdominal adiposity in offspring | 12 |
| Intrauterine growth restriction | 8 |
| Preterm labor | 8 |
| Pre-eclampsia | 8 |
| 4. Inadequate protein/AA intake in animals | |
| Intrauterine growth restriction | 1,20 |
| Reduced skeletal muscle fiber number and muscle mass | 1 |
| Increased proportion of body fat in offspring | 23 |
| Changes in expression of genes involved in metabolism | 16 |
| Increased concentration of testosterone in maternal plasma | 17 |
| Increased postnatal blood pressure of offspring | 22 |
| Reduced fetal arterial blood pressure | 14 |
| Reduced meat quality | 24 |
| Low birth weights | 1,20 |
| Increased risk of dystocia, weak labor | 25 |
| Reduced gestation length | 8 |
| Increased oxidative stress | 3 |
| Decreased immune function of offspring | 15 |
| Increased fetal exposure to maternal cortisol | 26 |
| Altered glucose metabolism | 14 |
Swine
Pigs experience high rates of embryonic loss and neonatal deaths, which are greatly influenced by maternal nutrition.27 For example, 14-18% crude protein (CP) diets are commonly fed to gestating gilts and sows on many farms worldwide; however, 14–18% CP is considered high dietary protein intake during pregnancy and is detrimental to embryonic development.1 This level of dietary protein creates a toxic environment for the fetus due to high levels of ammonia and possibly other metabolites in the plasma.7 Also, high dietary protein intake reduces skeletal muscle fiber size and number in newborn piglets.27 For this reason, it is recommended that gestating gilts are fed ∼50% of their ad libitum feed intake.1 When sows are fed >50% of ad libitum feed intake, there is a significant increase in embryonic loss resulting from gaining excessive subcutaneous white adipose tissue (WAT).1 Fifty percent of ad libitum feed intake equates to 2 to 2.2 kg of feed per day consisting of 12% CP. Consequently, specific AAs are deficient in a 12% CP diet for gestating swine.1 Interestingly, 24% of newborn piglets from crossbred sows fed a 12% CP diet are IUGR neonates, weighing less than 1.1 kg at birth. IUGR piglets have an extremely high risk of mortality (76%) before weaning.27
Arginine is an example of an AA deficient in a 12% CP gestation diet that is important for proper placental growth.7 Also, glutamate, glycine, and cysteine are required for the synthesis of glutathione, which is an essential antioxidant.3 Since increasing dietary CP above 12% results in high embryonic mortality, supplementing certain AAs to the basal diet may help to overcome AA deficiencies.2 Placental angiogenesis occurs rapidly between days 20 and 40 of gestation in pigs; therefore, dietary Arg supplementation during this period could improve the growth and development of the placental vasculature by increasing NO production.28 Supplementation of a 2 kg corn-soybean meal-based diet (containing 12% CP) with 0.4% or 0.8% Arg to gilts between days 14 and 25 of gestation increased placental growth, the number and diameter of placental blood vessels, and the number of viable fetuses by two per litter.2,28 Also, multiple studies conducted in different countries have shown that Arg supplementation between day 30 of gestation and farrowing increases litter size and litter birth weight.2,7 Arg supplementation during late gestation (days 90 to 114) also increased the birth weights of live piglets.2 Thus, gestating swine have requirements for dietary Arg (Table 2). However, it should be borne in mind that dietary supplementation with >2% Arg may cause antagonism between Arg and Lys, as well as a lysine deficiency and harmful levels of ammonia,3 and consequently none of the beneficial effects seen with lower doses of Arg may be observed.
Table 2.
NRC-recommended minimal content of arginine in diets for pigs and Texas A&M University-recommended optimal content of arginine in diets for pigs.
| Nursery piglets (5 kg BW) | Weanling piglets (10 kg BW) | Growing-Finishing pigs (20–100 kg BW) | Gestating pigs (140 kg BW at breeding) | Lactating sows | |
|---|---|---|---|---|---|
| NRC (1998)a | 0.59 | 0.54 | 0.37 (20 kg BW)0.19 (100 kg BW) | 0.0 | 0.0 |
| NRC (2012)b | 0.75 | 0.68 | 0.62 (20 kg BW)0.38 (100 kg BW) | 0.36 (d 0–90)0.47 (d 90–114) | 0.60 (parity 1)c0.54 (parity 2)c |
| TAMU (2014)d | 1.19 | 1.01 | 0.83 (20 kg BW)0.64 (100 kg BW) | 1.03 (d 0–90)1.03 (d 0–90) | 1.37 |
Note: Values are % of total diet, unless indicated otherwise.
BW: body weight.
aNational Research Council (1998), Nutrient Requirements of Swine, minimal content of arginine in diets.
bNational Research Council (2012), Nutrient Requirements of Swine, minimal content of arginine in diets.
cStandardized ileal digestible value.
dWu.29 Texas A&M University-recommended arginine content in swine diets, optimal content of true digestible arginine in diets.
A study conducted by Rehfeldt et al.30 indicated the negative impacts of maternal undernutrition and overnutrition on postnatal growth in IUGR piglets. Specifically, the gestating gilts fed a high protein diet (30% CP) produced piglets with IUGR. At postnatal day 83 of age, these piglets had increased brain weights and decreased thymus and bone weights, compared to the offspring of the gilts fed the control diet (12.1% CP). As an organ of the immune system, a reduced thymus gland may be associated with decreased immune function.8 At postnatal day 188 of age, these piglets had increased liver weights compared to the control group, which may be lead to metabolic dysfunction when they are used for breeding.30 The gestating gilts fed a low protein diet (6.5% CP) also produced piglets with IUGR, but those piglets showed compensatory gain that was maintained from postnatal days 83 and 188 of age.30 However, these piglets had a larger proportion of body fat, as well as decreases in the numbers of skeletal muscle fibers, skeletal muscle mass, and total muscular DNA, compared to the control piglets.30 Of particular interest, Altmann et al.16 found that both low and high protein diets fed to gestating sows affected the expression of metabolic genes including glucocorticoid receptor NR3C1, peroxisome proliferator-activated receptor alpha (PPARα), insulin receptor (INSR), PPAR gamma coactivator 1-alpha (PGC1α), 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR), and cytochrome P450 2C34 (CYP2C34). Fetal expression of NR3C1 was increased as a consequence of increased glucocorticoid activity in sows fed a low protein diet, which also affected expression of INSR. PPARα, PGC1α, and HMGCR expression in fetuses from sows fed either a high or low protein diet differed from control-fed sows, possibly resulting in changes in lipid metabolism in the offspring.
In feeding gestating swine, a reduced amount of rations is intended to prevent excess accumulation of white adipose tissue in the body.1 This common practice results in inadequate provision of AAs, including Arg and Gln, as noted previously. Gln is a major metabolic fuel for the small intestine of sows, and its uptake from the uterine artery by the fetus is the greatest among all AAs.2 Thus, the concentration of Gln in maternal plasma was reduced by 42% in late gestation (e.g. Day 110) compared to early gestation (e.g. Day 10).31 We found that supplementing gilts with 1% Gln to a corn- and soybean meal-based diet (containing 12.2% crude protein and 1.2% Gln) between Days 90 and 114 of gestation reduced the incidence of IUGR piglets by 39%, variation in piglet birth weights by 33%, and pre-weaning mortality of live-born piglets by 46%.31 Furthermore, supplementing a corn- and soybean meal-based diet (containing 0.70% Arg and 1.22% Gln) with 0.4% Arg plus 0.6% Gln between Days 30 and 114 of gestation in gilts reduced variation in birth weights of live-born piglets by 24% and the proportion of live-born piglets with birth weights of 0.6 to 1.29 kg by 22%, while increasing the number of live-born piglets by 1.4 per litter and the litter birth weight of live-born piglets by 15%.27 Taken together, these results indicate the importance of adequate provision of functional AAs in improving pregnancy outcomes in swine.
Ruminants
Maternal undernutrition and overnutrition have detrimental effects on gestating ruminants.25 In cows, high and low protein diets have varying effects during different stages of gestation.25 Mossa et al.32 found that maternal undernutrition during the first trimester of gestation caused higher concentrations of testosterone in maternal blood, smaller ovarian reserves, enlarged aortas, and higher blood pressure in female calves. Deficiencies in specific AAs decrease angiogenesis, which may explain the increased blood pressure in female calves with enlarged aortas that were born from nutrient-restricted dams. Low maternal dietary protein intake (7% CP) resulted in offspring with semitendinosus muscles that had a higher shear force and lower collagen content, indicating an adverse effect of maternal malnutrition on the meat quality of male offspring.24 Restriction of maternal protein intake during the second trimester decreased birth weights of calves even when maternal weight gain was positive during that period.33 High maternal dietary protein intake during the first trimester can negatively affect development of the hypothalamic-pituitary-gonadal axis and consequently decrease reproductive performance in bull calves.17 In cattle, 75% of the increase in fetal weight occurs during the third trimester of gestation.33 Maternal undernutrition during the third trimester can negatively impact birth weight and postnatal growth, as well as fetal myogenesis and adipogenesis.24 For the cow, low dietary protein intake during the third trimester may result in weak labor, increased risk of dystocia, and increased perinatal death, all of which put the survival of the offspring at risk. Throughout the cow/calf production cycle, average dietary CP content ranges from 6.0% to 10.3%, which cannot provide the animals with sufficient AAs.17,24,32,33 With an increase in the use of corn byproducts, which have a CP concentration of 26–30% (DM basis), in the rations of dairy cows and feedlot beef cattle, maternal dietary protein intakes are being substantially exceeded during early, mid, and late gestation.34
According to Kwon et al.,35 undernutrition of pregnant ewes in the first half of gestation and throughout gestation reduces concentrations of Arg-family AAs, branched-chain AAs, and polyamines in maternal and fetal plasma and fetal fluids, thus causing IUGR. Serine was decreased to the greatest extent in fetal fluids, which could affect the synthesis of important biological substances including glucose, DNA, and protein.3 As little as a 15% reduction in maternal nutrient intake during early gestation reduces fetal arterial blood pressure, gestation length and pituitary, and adrenal responses of the fetus during late-gestation.8,36 These adverse effects are believed to be the result of an accelerated maturation of the hypothalamic-pituitary-adrenal axis from maternal undernutrition.17 Of particular interest, a 15% reduction in maternal nutrient intake during late gestation can lead to impaired relaxation of arterial blood vessels.8,36 After parturition, the offspring experienced increased blood pressure, indicating the effects of maternal undernutrition on early postnatal cardiovascular function.8,36 Of note, low maternal dietary protein intake (7.5% CP) in goats for 55 days during late gestation decreased the activity of enzymes associated with the antioxidant defense system, which leads to increased production of reactive oxygen species and free radicals.37 Reduced capacity for removing free radicals and ROS leads to oxidative stress, cell damage, and impairment of physiological function.
High maternal dietary protein intake (44% CP) is associated with high embryonic mortality due, at least in part, to a decrease in pH of maternal blood.18,19 When protein and AAs are degraded in the body, sulfuric acid produced from methionine and cysteine can decrease pH in the blood.3 The fetuses that survived had lower body weights than those from dams fed a lower protein diet (24% CP).18 Meza-Herrera et al.18 also found that sheep with a low body condition score that were fed the high protein diet exhibited a higher rate of embryonic mortality and a greater release of oxytocin-stimulated prostaglandin F2α (PGF2α). Furthermore, the oxidation of sulfur-containing AAs generates elevated levels of homocysteine and H2S that cause oxidative stress in cells. Biochemical mechanisms responsible for adverse consequences of high maternal protein intake on fetal growth and development are summarized in Figure 3.
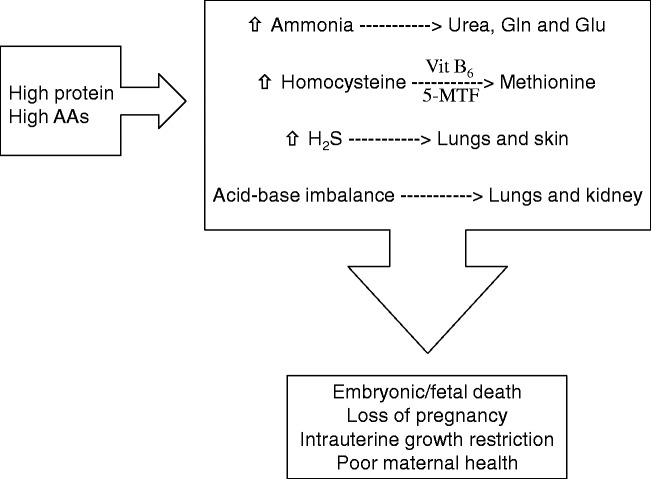
Figure 3.
Adverse consequences of high maternal dietary intake of protein or amino acids (AAs) during pregnancy. High maternal dietary intake of protein and AAs causes an increase in ammonia, homocysteine and hydrogen sulfide (H2S) and an acid-base imbalance. Under normal conditions (represented by a dashed arrow), ammonia is catabolized into urea, glutamine and glutamate, homocysteine is recycled into methionine, hydrogen sulfide is excreted through the lungs and skin, and acid-base homeostasis is regulated by the lungs and kidneys. However, excessive intake of protein or AAs dysregulates these processes and results in embryonic/fetal death, loss of pregnancy, intrauterine growth restriction, and poor maternal health. 5-MTF: N5-methyl-tetrahydrofolate; Vit B6: vitamin B6.
Like in other species, Arg supplementation to pregnant ewes improves embryonic survival because Arg is important for survival, growth, and development of the conceptus.7 Since dietary Arg is actively degraded in the rumen, intravenous administration is appropriate for Arg supplementation in ruminants. We found that intravenous administration of Arg to nutrient-restricted ewes from day 60 of gestation to parturition prevented IUGR and increased the birth weights of lambs compared to nutrient-restricted ewes receiving saline infusions.20,21 Fetal survival increased by 59% with intravenous administration of Arg between days 100 and 121 of gestation. Unlike porcine placentae, ovine placentomes have high arginase activity, which catabolizes Arg into ornithine and subsequently into polyamines and proline.38 Interestingly, Arg is conserved as its precursor, citrulline, in ovine conceptuses to minimize degradation of Arg by placentomal arginase.
Rodents
Maternal undernutrition and overnutrition have been studied extensively in rats and mice and have provided a basis for further research with farm animals and humans.23,39 Low maternal dietary protein intake impacts fetal body composition, body weight, metabolism, and hormonal balances.40 A decrease in skeletal muscle mass, but an increase in adipose tissue during compensatory growth has been observed in gestating rats fed a low protein diet.15 Also affecting the immune system, low maternal dietary protein intake (4% CP) reduced concentrations of protein, albumin, and gamma-globulin in plasma of neonatal rat pups compared to pups from a control fed dam (10% CP).15 Impairment of cardiovascular function in offspring as evidenced by increased blood pressure and dysregulation of circadian rhythms of blood pressure and heart rate can also result from a low maternal protein diet.14 Low maternal dietary protein intake decreases the activity of placental 11 β-hydroxysteroid dehydrogenase (11 β-HSD), thereby increasing fetal exposure to maternal cortisol.8 Exposure of glucocorticoids to the fetus affects growth, blood pressure, and glucose metabolism.22 The risk of insulin resistance throughout postnatal life is increased in offspring from a protein-restricted dam and represents a lifelong consequence of maternal undernutrition.40 Concentrations of corticosterone in plasma of offspring are also affected by maternal protein restriction. Interestingly, compared to the control group, lower concentrations of corticosterone are detected in male offspring of dams with low dietary protein intake which may lead to risk taking behaviors, while higher concentrations of corticosterone in female offspring may lead to anxious behaviors.41 Later in life, adult offspring from protein-restricted dams have a higher risk of metabolic disorders because of defects in mTOR activation and abnormal pancreatic development. Cell signaling via mTOR plays a crucial role in regulating energy homeostasis and protein synthesis.3 Thus, low maternal dietary intake decreases the proliferation and number of fetal pancreatic β-cells, as well as the insulin content of fetal pancreatic islet cells, while increasing the apoptosis of those cells.26 In the protein-deficient dams, the fetal pancreas also experiences decreased vascularization. Thus, multiple mechanisms are responsible for hormonal imbalances and IUGR in AA-deficient fetuses.
Either a low or a high maternal dietary protein intake impairs hemodynamics in rodents. For example, elevated blood pressure occurred in rat offspring born from protein-deficient dams when they were four weeks of age and continued throughout adulthood.19 Kucia et al.39 determined that a high maternal protein diet (40% CP) decreased litter size and litter mass at birth in mice. High protein diets in rodents provide less energy than isoenergetic control diets due to the high consumption of energy to dispose of excess ammonia via the synthesis of urea. Maternal kidney mass is greater in both mice and rats with high dietary protein intakes.39 High maternal dietary protein intake (55% CP) also decreases body weight and food intake of the offspring.19 These rat pups displayed higher concentrations of glucose in plasma in a fasting state and higher concentrations of insulin in plasma in a postprandial state, showing an effect on glucose metabolism and possible signs of insulin resistance.
Arg supplementation in pregnant rats during the first seven days of gestation or throughout gestation increases the number of implantation sites and litter size by approximately 3 via the PI3K/PKB/mTOR/NO signaling pathway.42 In mice, dietary Arg supplementation for 14 days pre-mating increased postnatal weight gain and decreased abortion rates when exposed to porcine circovirus type 2 that normally causes embryonic death.43 This provides another line of evidence for beneficial roles of Arg in improving maternal and fetal health. Thus, Arg is a dietarily essential AA for gestating rodents.29,44,45
Conclusion
Regulation of maternal dietary protein intake during pregnancy is essential for proper embryonic survival, growth, and development. Specific AAs are required for certain processes involved in pregnancy including implantation, placental growth and angiogenesis, and the transfer of nutrients from mother to fetus. Both low and high maternal dietary protein intake cause an imbalance of AAs, which may lead to embryonic loss as well as impaired growth and development of the conceptus. Surviving IUGR neonates likely face lifelong adverse consequences as a result of maternal malnutrition. To overcome the harmful consequences of maternal protein malnutrition, dietary supplementation with Arg and Gln during specific stages of gestation can help fulfill the nutritional requirements of both mother and fetus. In numerous animal and human studies, dietary Arg supplementation during pregnancy improved embryonic survival and growth by increasing placental angiogenesis and blood flow, as well as promoting embryonic protein synthesis. Understanding the role of maternal dietary protein intake can have great economical benefits in livestock production by increasing reproductive success and litter size. Additionally, for humans, adequate maternal dietary protein intake can improve reproductive success and result in healthier mothers and infants.
Acknowledgments
We thank our colleagues and students for their important contributions to research in our laboratories.
Authors’ contributions
GW conceived this project. CMH, FWB, GAJ, and GW wrote the manuscript. All authors contributed to the discussion and revision of the article. GW had the primary responsibility for the content of the paper. All authors read and approved this manuscript.
Declaration of Conflicting Interests
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the review.
Funding
This work was supported by Agriculture and Food Research Initiative Competitive Grants (2014–67015-21770, 2015–67015-23276 and 2016–67015-24958) from the USDA National Institute of Food and Agriculture, and Texas A&M AgriLife Research (H-8200).
References
- 1.Ji Y, Wu Z, Dai Z, Wang X, Li J, Wang B, Wu G. Fetal and neonatal programming of postnatal growth and feed efficiency in swine. J Anim Sci Biotechnol 2017; 8:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu G, Bazer FW, Johnson GA, Herring C, Seo H, Dai Z, Wang J, Wu Z, Wang X. Functional amino acids in the development of the pig placenta. Mol Reprod Dev 2017; 84:879–82 [DOI] [PubMed] [Google Scholar]
- 3.Wu G. Amino acids: biochemistry and nutrition. Boca Raton, USA: CRC Press, 2013 [Google Scholar]
- 4.Wu G. Dietary protein intake and human health. Food Funct 2016; 7:1251–65 [DOI] [PubMed] [Google Scholar]
- 5.Taylor CE. A novel treatment for “morning sickness”: nausea of pregnancy could be induced by excess sulfite which molybdenum can help alleviate. Med Hypotheses 2016; 95:31–3 [DOI] [PubMed] [Google Scholar]
- 6.Wu Z, Hou Y, Hu S, Bazer FW, Meininger CJ, McNeal CJ, Wu G. Catabolism and safety of supplemental L-arginine in animals. Amino Acids 2016; 48:1541–52 [DOI] [PubMed] [Google Scholar]
- 7.Wu G, Bazer FW, Satterfield MC, Li X, Wang X, Johnson GA, Burghardt RC, Dai Z, Wang J, Wu Z. Impacts of arginine nutrition on embryonic and fetal development in mammals. Amino Acids 2013; 45:241–56 [DOI] [PubMed] [Google Scholar]
- 8.Wu G, Imhoff-Kunsch B, Girard AW. Biological mechanisms for nutritional regulation of maternal health and fetal development. Paediatr Perinatal Epidemiol ▪: 26:4–26 [DOI] [PubMed] [Google Scholar]
- 9.Elango R, Ball RO. Protein and amino acid requirements during pregnancy. Adv Nutr 2016; 7:8395–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Switkowski KM, Jacques PF, Must A, Kleinman KP, Gillman MW, Oken E. Maternal protein intake during pregnancy and linear growth in the offspring. Am J Clin Nutr 2016; 104:1128–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stephens TV, Woo H, Innis SM, Elango R. Healthy pregnant women in Canada are consuming more dietary protein at 16- and 36- week gestation than currently recommended by the Dietary Reference Intakes, primarily from dairy food sources. Nutr Res 2014; 34:569–76 [DOI] [PubMed] [Google Scholar]
- 12.Blumfield ML, Hure AJ, MacDonald-Wicks LK, Smith R, Simpson SJ, Giles WB, Raubenheimer D, Collins CE. Dietary balance during pregnancy is associated with fetal adiposity and fat distribution. Am J Clin Nutr 2012; 96:1032–41 [DOI] [PubMed] [Google Scholar]
- 13.Switkowski KM, Jacques PF, Must A, Hivert MF, Fleisch A, Gillman MW, Rifas-Shiman S, Oken E. Higher maternal protein intake during pregnancy is associated with lower cord blood concentrations of insulin-like growth factor (IGF)-II, IGF binding protein 3, and insulin, but not IGF-I, in a cohort of women with high protein intake. J Nutr 2017; 147:1392–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langley-Evans SC. Developmental programming of health and disease. Proc Nutr Soc 2006; 65:97–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher RE, Steele M, Karrow NA. Fetal programming of the neuroendocrine-immune system and metabolic disease. J Pregnancy 2012; 2012:792934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altmann S, Murani E, Schwerin M, Metges CC, Wimmers K, Ponsuksili S. Dietary protein restriction and excess of pregnant German Landrace sows changes in hepatic gene expression and promoter methylation of key metabolic genes in the offspring. J Nutr Biochem 2013; 24:484–95 [DOI] [PubMed] [Google Scholar]
- 17.LeMaster CT, Taylor RK, Ricks RE, Long NM. The effects of late gestation maternal nutrient restriction with or without protein supplementation on endocrine regulation of newborn and postnatal beef calves. Theriogenology 2017; 87:64–71 [DOI] [PubMed] [Google Scholar]
- 18.Meza-Herrera CA, Ross TT, Hallford DM, Hawkins DE, Gonzalez-Bulnes A. High periconceptional protein intake modifies uterine and embryonic relationships increasing early pregnancy losses and embryo growth retardation in sheep. Reprod Dom Anim 2010; 45:723–8 [DOI] [PubMed] [Google Scholar]
- 19.Jahan-Mihan A, Rodriguez J, Christie C, Sadeghi M, Zerbe T. The role of maternal dietary proteins in development of metabolic syndrome in offspring. Nutrients 2015; 7:9185–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lassala A, Bazer FW, Cudd TA, Datta S, Keisler DH, Satterfield MC, Spencer TE, Wu G. Parenteral administration of L-arginine prevents fetal growth restriction in undernourished ewes. J Nutr 2010; 140:1242–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lassala A, Bazer FW, Cudd TA, Datta S, Keisler DH, Satterfield MC, Spencer TE, Wu G. Parenteral administration of L-arginine enhances fetal survival and growth in sheep carrying multiple pregnancies. J Nutr 2011; 141:849–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gluckman PD, Hanson MA. The developmental origins of the metabolic syndrome. Trends Endocrinol Metab 2004; 15:183–7 [DOI] [PubMed] [Google Scholar]
- 23.Desclée de Maredsous C, Oozeer R, Barbillon P, Mary-Huard T, Delteil C, Blachier F, Tomé D, van der Beek EM, Davila AM. High-protein exposure during gestation or lactation or after weaning has a period-specific signature on rat pup weight, adiposity, food intake, and glucose homeostasis up to 6 weeks of age. J Nutr 2016; 146:21–9 [DOI] [PubMed] [Google Scholar]
- 24.Alvarenga TIRC, Copping KJ, Han X, Clayton EH, Meyer RJ, Rodgers RJ, McMillen IC, Perry VEA, Geesink G. The influence of peri-conception and first trimester dietary restriction of protein in cattle on meat quality traits of entire male progeny. Meat Sci 2016; 121:141–7 [DOI] [PubMed] [Google Scholar]
- 25.Bollwein H, Janett F, Kaske M. Impact of nutritional programming on the growth, health and sexual development of bull calves. Dom Anim Endocrin 2016; 56:S180–90 [DOI] [PubMed] [Google Scholar]
- 26.Blondeau B, Lesage J, Czernichow P, Dupouy JP, Breant B. Glucocorticoids impair fetal beta-cell development in rats. Am J Physiol Endocrinol Metab 2001; 281:E592–9 [DOI] [PubMed] [Google Scholar]
- 27.Wu G, Bazer FW, Burghardt RC, Johnson GA, Kim SW, Li XL, Satterfield MC, Spencer TE. Impacts of amino acid nutrition on pregnancy outcome in pigs: mechanisms and implications for swine production. J Anim Sci 2010; 88:E195–204 [DOI] [PubMed] [Google Scholar]
- 28.Li XL, Bazer FW, Johnson GA, Burghardt RC, Frank JW, Dai ZL, Wu G. Dietary supplementation with L-arginine between days 14 and 25 of gestation enhances embryonic development and survival in gilts. Amino Acids 2014; 46:375–84 [DOI] [PubMed] [Google Scholar]
- 29.Wu G. Dietary requirements of synthesizable amino acids by animals: a paradigm shift in protein nutrition. J Anim Sci Biotechnol 2014; 5:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rehfeldt C, Stabenow B, Pfuhl R, Block J, Nürnberg G, Otten W, Metges CC, Kalbe C. Effects of limited and excess protein intakes of pregnant gilts on carcass quality and cellular properties of skeletal muscle and subcutaneous adipose tissue in fattening pigs. J Anim Sci 2012; 90:184–96 [DOI] [PubMed] [Google Scholar]
- 31.Wu G, Bazer FW, Johnson GA, Knabe DA, Burghardt RC, Spencer TE, Li XL, Wang JJ. Important roles for L-glutamine in swine nutrition and production. J Anim Sci 2011; 89:2017–30 [DOI] [PubMed] [Google Scholar]
- 32.Mossa F, Carter F, Walsh SW, Kenny DA, Smith GW, Ireland JLH, Hildebrandt TB, Lonergan P, Ireland JJ, Evans ACO. Maternal undernutrition in cows impairs ovarian and cardiovascular systems in their offspring. Bio Reprod 2013; 88:1–9 [DOI] [PubMed] [Google Scholar]
- 33.Micke GC, Sullivan TM, Soares Magalhaes RJ, Rolls PJ, Norman ST, Perry VEA. Heifer nutrition during early- and mid-pregnancy alters fetal growth trajectory and birth weight. Anim Reprod Sci 2010; 117:▪–110 [DOI] [PubMed] [Google Scholar]
- 34.Wilson TB, Long NM, Faulkner DB, Shike DW. Influence of excessive dietary protein intake during late gestation on drylot beef cow performance and progeny growth, carcass characteristics, and plasma glucose and insulin concentrations. J Anim Sci 2016; 94:2035–46 [DOI] [PubMed] [Google Scholar]
- 35.Kwon H, Ford SP, Bazer FW, Spencer TE, Nathanielsz PW, Nijland MJ, Hess BW, Wu G. Maternal nutrient restriction reduces concentrations of amino acids and polyamines in ovine maternal and fetal plasma and fetal fluids. Biol Reprod 2004; 71:901–8 [DOI] [PubMed] [Google Scholar]
- 36.Cleal JK, Poore KR, Newman JP, Noakes DE, Hanson MA, Green LR. The effect of maternal undernutrition in early gestation on gestation length and fetal and postnatal growth in sheep. Pediatr Res 2007; 62:422–7 [DOI] [PubMed] [Google Scholar]
- 37.He ZX, Sun ZH, Tan ZL, Tang SX, Zhou CS, Han XF, Wang M, Wu DQ, Kang JK, Beauchemin KA. Effects of maternal protein or energy restriction during late gestation on antioxidant status of plasma and immune tissues in postnatal goats. J Anim Sci 2012; 90:4319–26 [DOI] [PubMed] [Google Scholar]
- 38.Kwon H, Wu G, Bazer FW, Spencer TE. Developmental changes in polyamine levels and synthesis in the ovine conceptus. Biol Reprod 2003; 69:1626–34 [DOI] [PubMed] [Google Scholar]
- 39.Kucia M, Langhammer M, Görs S, Albrecht E, Hammon HM, Nürnberg, Metges CC. High-protein diet during gestation and lactation affects mammary gland mRNA abundance, milk composition and pre-weaning litter growth in mice. Animal 2011; 5:268–77 [DOI] [PubMed] [Google Scholar]
- 40.Wu G, Bazer FW, Cudd TA, Meininger CJ, Spencer TE. Maternal nutrition and fetal development. J Nutr 2004; 134:2169–72 [DOI] [PubMed] [Google Scholar]
- 41.Babenko O, Kovalchuk I, Metz GAS. Stress-induced perinatal and transgenerational epigenetic programming of brain development and mental health. Neurosci Biobehav Rev 2015; 48:70–91 [DOI] [PubMed] [Google Scholar]
- 42.Zeng XF, Wang FL, Fan X, Yang W, Zhou B, Li PF, Yin YL, Wu G, Wang JJ. Dietary arginine supplementation during early pregnancy enhances embryonic survival in rats. J Nutr 2008; 138:1421–5 [DOI] [PubMed] [Google Scholar]
- 43.Ren W, Yin YL, Liu G, Yu X, Li Y, Yang G, Li T, Wu G. Effect of dietary arginine supplementation on reproductive performance of mice with porcine circovirus type 2 infection. Amino Acids 2012; 42:2089–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hou YQ, Yin YL, Wu G. Dietary essentiality of “nutritionally nonessential amino acids” for animals and humans. Exp Biol Med 2015; 240:997–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hou YQ, Wu G. Nutritionally nonessential amino acids: a misnomer in nutritional sciences. Adv Nutr 2017; 8:137–9 [DOI] [PMC free article] [PubMed] [Google Scholar]