Short abstract
Ca2+ release from the endoplasmic reticulum is an important component of Ca2+ signal transduction that controls numerous physiological processes in eukaryotic cells. Release of Ca2+ from the endoplasmic reticulum is coupled to the activation of store-operated Ca2+ entry into cells. Store-operated Ca2+ entry provides Ca2+ for replenishing depleted endoplasmic reticulum Ca2+ stores and a Ca2+ signal that regulates Ca2+-dependent intracellular biochemical events. Central to connecting discharge of endoplasmic reticulum Ca2+ stores following G protein-coupled receptor activation with the induction of store-operated Ca2+ entry are stromal interaction molecules (STIM1 and STIM2). These highly homologous endoplasmic reticulum transmembrane proteins function as sensors of the Ca2+ concentration within the endoplasmic reticulum lumen and activators of Ca2+ release-activated Ca2+ channels. Emerging evidence indicates that in addition to their role in Ca2+ release-activated Ca2+ channel gating and store-operated Ca2+ entry, STIM1 and STIM2 regulate other cellular signaling events. Recent studies have shown that disruption of STIM expression and function is associated with the pathogenesis of several diseases including autoimmune disorders, cancer, cardiovascular disease, and myopathies. Here, we provide an overview of the latest developments in the molecular physiology and pathophysiology of STIM1 and STIM2.
Impact statement
Intracellular Ca2+ signaling is a fundamentally important regulator of cell physiology. Recent studies have revealed that Ca2+-binding stromal interaction molecules (Stim1 and Stim2) expressed in the membrane of the endoplasmic reticulum (ER) are essential components of eukaryote Ca2+ signal transduction that control the activity of ion channels and other signaling effectors present in the plasma membrane. This review summarizes the most recent information on the molecular physiology and pathophysiology of stromal interaction molecules. We anticipate that the work presented in our review will provide new insights into molecular interactions that participate in interorganelle signaling crosstalk, cell function, and the pathogenesis of human diseases.
Keywords: STIM1, STIM2, store-operated calcium entry, calcium signaling, endoplasmic reticulum, signal transduction
Introduction
Calcium (Ca2+) is a universal intracellular signaling molecule that controls many cellular processes by acting over a wide spatio-temporal range within multiple subcellular compartments including the cytosol, mitochondria, endoplasmic reticulum (ER), and nucleus. Ca2+ signals consist of time-dependent changes in the concentration of Ca2+ in the cytosol ([Ca2+]c) and subcellular organelles that are generated by the mobilization of Ca2+ into the cytosol via entry through Ca2+-permeable ion channels localized in the plasma membrane (PM) and the ER membrane. A major Ca2+ entry pathway in non-excitable and excitable cells is store-operated Ca2+ entry (SOCE) by which Ca2+ influx across the PM is activated by a decrease in the Ca2+ concentration within the lumen of the endoplasmic reticulum ([Ca2+]ER). Since Dr. James Putney first proposed in 1986 that lowering [Ca2+]ER activated Ca2+ channels in the PM, investigators have focused on identifying the molecular basis of store-operated channels (SOCs), the signaling mechanisms involved in SOC activation and inactivation, and the cellular functions controlled by SOCE.
SOC current can be conducted by several types of ion channels. The most well-characterized SOC is the Ca2+ release-activated Ca2+ (CRAC) channel. Although the biophysical properties of CRAC channels in a wide range of cell types were defined by numerous investigators in the 1990s, the molecular constituents controlling the activation and regulation of these channels were unknown for many years. In 2005 and 2006, results from studies in independent laboratories revealed two proteins necessary for SOCE: stromal interaction molecule 1 (STIM1) and Orai1.1–6 STIM1, a type I single-pass ER transmembrane protein that is activated consequent to depletion of ER Ca2+ stores, was found to be essential for CRAC channel gating.3–5 The Orai1 protein was found to form the ion-conducting pore subunit of CRAC channels.4–6 The current consensus model of SOCE suggests that STIM1 functions as the main sensor of [Ca2+]ER stores and activator of Orai1. Compared to STIM1 and Orai1, relatively little is known about the roles of STIM1 and Orai1 homologues, namely STIM2, Orai2 and Orai3, in SOCE and other cellular functions.7
In this review, we focus on the molecular physiology and pathophysiology of STIM1 and STIM2. After a brief review of cellular Ca2+ signal transduction, we will summarize recent advances in our understanding of STIM proteins with a particular emphasis on STIM2, the lesser studied of the two STIM proteins. Following a discussion of their structure-function properties, we will describe the role of STIM in regulating SOCE and other cellular functions. Finally, we will discuss the pathophysiological implications of disrupted STIM-dependent signaling in cancer, metabolic disease, immunological disorders, and other diseases.
An overview of intracellular Ca2+ homeostasis and signaling
Intracellular Ca2+ homeostasis is a fundamentally important property of all cells that is crucial for regulating a wide range of cell functions and cell viability, and is precisely regulated by Ca2+ entry into and out of the cytosol. In resting, unstimulated cells, [Ca2+]c is maintained at a low level (50–200 nm) relative to the [Ca2+] in the extracellular space (1–2 mM) by the actions of Ca2+-ATPases and counter-ion exchangers that remove Ca2+ from the cytosol. Subsequent to cellular stimulation, these same Ca2+ handling mechanisms participate in the regulation of dynamic changes in Ca2+ signals and rapidly restore [Ca2+]c to pre-stimulus, basal levels, since prolonged elevation of [Ca2+]c is detrimental to cell viability.
Spatial and temporal changes in [Ca2+]c produced after exposure of cells to hormones, neurotransmitters, growth factors, and mechanostimulation are essential signals in eukaryotic cells that regulate cellular growth and proliferation, differentiation, gene expression, motility, secretion, and cell survival.8,9 Following stimulation, [Ca2+]c increases consequent to release of Ca2+ through PM and ER (or sarcoplasmic reticulum (SR) in myocytes) Ca2+-permeable channels. Ca2+ signals also can be affected by Ca2+ released from mitochondria, Golgi apparatus, and acidic Ca2+ stores.10–17 The increases in [Ca2+]c exhibit temporally distinct patterns that can be sustained, transient, or oscillatory. Dynamic changes in [Ca2+]c are shaped by proteins that transfer Ca2+ across the PM to the extracellular space and sequester Ca2+ into membrane-bound organelles such as the ER/SR, mitochondria, and Golgi apparatus. During the past four decades, the molecular biology, physiology and pathophysiology of Ca2+ transport mechanisms including plasma membrane Ca2+-ATPases (PMCAs), sarcoendoplasmic reticulum Ca2+-ATPases (SERCAs), secretory-pathway Ca2+-transport ATPases (SPCAs), the mitochondrial voltage-dependent anion transporter (VDAC), and mitochondrial Ca2+ uniporter (MCU) have been well characterized. Detailed information about recent advances in our knowledge of these Ca2+ handling mechanisms and other aspects of intracellular Ca2+ signal transduction is available elsewhere.9,18,19
Store-operated Ca2+ entry—The nexus of PM and ER Ca2+ signaling
SOCE is a primary source for intracellular Ca2+ signaling in non-excitable cells such as fibroblasts, cells of the immune system, and hepatocytes. It is also present in excitable cells including endocrine and neuroendocrine cells, neuronal cells, cardiomyocytes, and vascular smooth muscle cells. A unique property of this Ca2+ influx mechanism is that it is controlled by the concentration of Ca2+ sequestered within the ER/SR.20 Activation of SOCE is triggered by cellular stimuli that cause a decrease in [Ca2+]ER. This can occur, for example, in cells after the application of G protein-coupled or tyrosine kinase receptors agonists that stimulate phospholipase C activity, inositol 1,4,5-trisphosphate (IP3) production, and IP3 receptor (IP3R)-mediated release of Ca2+ from the ER. After more than 30 years of study, we now know that SOCE is critically important in the maintenance of cytosolic and ER Ca2+ homeostasis, as well as the regulation of spatial and temporal changes in [Ca2+]c, protein biosynthesis, gene expression, and cell viability.21–27
The biophysical properties of SOCE were established during the early 1990s. Patch-clamp electrophysiology was used to first characterize CRAC currents in mast cells and T cells.28,29 CRAC currents have been detected in a wide range of cell types from immune cells, to hepatocytes, fibroblasts, and intestinal epithelial cells, suggesting broad functional importance.30 The Ca2+ current through these channels, ICRAC, exhibits strong inward rectification, a positive reversal potential, low single-channel conductance and is voltage-independent.28,29 Furthermore, ICRAC is highly selective for Ca2+ over other divalent and monovalent cations.29 The small pore size, ∼3.9 Å, of the CRAC channels contributes to its high Ca2+ selectivity.31 CRAC channels also exhibit Ca2+-dependent fast inactivation. The channels are inactivated by local negative feedback from Ca2+ entering the channel, but is unaffected by Ca2+ release from intracellular stores.32
The molecular basis of SOCE was discovered in 2005 and 2006.1–6 These studies revealed that STIM1 functioned as the sensor of ER/SR Ca2+ stores and an activator of SOCE,1–3 while Orai1 formed the ion conducting, pore subunit of the CRAC channel.4,33 STIM1 and Orai1 are both necessary and sufficient for SOCE.34,35 Depletion of the ER Ca2+ stores causes a destabilizing conformational change of STIM1 to reveal the Orai1-activating domain and initiate oligomerization and translocation of STIM1 molecules to ER-PM junctions.36,37 This allows STIM1 interaction with PM Ca2+ channel Orai1 and activation of SOCE.23
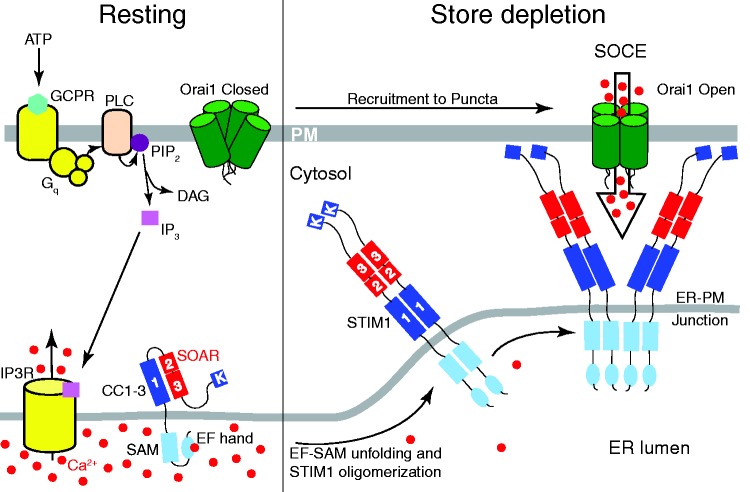
Figure 3.
Consensus model of SOCE. When the ER Ca stores are full at rest, Ca2+ is bound to the STIM1 EF-hand. In the Ca2+-bound mode, the EF-hand has a stabilizing interaction with the SAM domain, maintaining monomeric distribution of the STIM1 molecule. When an agonist, such as ATP, binds to and activates a G-coupled protein receptor (GCPR), phospholipase C (PLC) cleaves phosphatidylinositol 4,5-bisphosphate (PIP2), generating inositol trisphosphate (IP3) and diacylglycerol (DAG). IP3 diffuses through the cytosol, then binds to and opens IP3Rs in the ER membrane, resulting in discharge of the ER Ca2+ stores. Following ER Ca2+ store-depletion, Ca2+ dissociates from the EF-hand, initiating a destabilization-coupled STIM1 oligomerization process. Conformational changes in the EF-hand cause EF-SAM unfolding, destabilization, and subsequent aggregation of multiple STIM molecule EF-SAM domains. This oligomerization in the luminal domains also triggers conformational changes throughout the C-terminal domain of STIM1. At rest the coiled-coil (CC) domains are folded, but oligomerization results in an unfolded, extended conformation of the CC domains. Finally, STIM1 multimerizes and moves to ER-PM junctions, where it recruits and opens Orai1 channels to facilitate SOCE. Note: STIM2 is not pictured in this model as the role of STIM2 in SOCE is still unclear. Likewise, variants of the STIM proteins are not illustrated as they appear to play cell-specific roles as discussed in the text. (A color version of this figure is available in the online journal.)
Orai1, a member of the Orai family of ion channel proteins, was initially identified through genome-wide RNAi screens in Drosophila S2 cells,5,6,38 while human Orai1 was identified by linkage analysis of immune cells isolated from severe combined immunodeficient patients.38 CRAC channels consist of six Orai1 subunits. Each subunit has four transmembrane domains and intracellular N- and C- termini.4,6,39–41 Although not well studied, Orai2 and Orai3 also form SOCs and may form heteromultimeric channels.42–44 The C-termini of each Orai homologue contains coiled-coil domains. The coiled-coil region of Orai1 is necessary for binding STIM1 following depletion of intracellular Ca2+ stores.45,46 Additionally, a conserved region within the N-terminus of Orai1 is required for SOC channel opening.46 More detailed information about the molecular biology, protein biochemistry, and biophysical properties of the Orai ion channels can be found in several recently published review articles.47–50
Although SOCE is mediated by STIM1 interaction with Orai1 at ER-PM junctions, other regulators of SOCE have been identified. Many of these regulators act directly on STIM1 and/or Orai1, while others regulate SOCE indirectly. Proteins that directly regulate SOCE by binding STIM1 and/or Orai1 include CRACR2A, SARAF, junctate, α-SNAP, STIMATE, and junctophilin-4 (Table 1).51–57 Junctate and junctophilin-4 are components of the ER-PM junctions that facilitate STIM1 recruitment, clustering, and interaction with Orai1 at the ER-PM junctions.52,56 STIMATE and CRACR2A are also positive regulators of SOCE. Through direct interactions with STIM1 and/or Orai1, these proteins facilitate STIM1 clustering in plasma membrane-associated ER membrane (PAM) nanodomains.51,54 α-SNAP is also a positive regulator of SOCE, but it interacts with existing STIM1-Orai1 clusters to facilitate a molecular rearrangement necessary for the activation of SOCE.57 In contrast, SARAF, a negative regulator of SOCE, directly interacts with STIM1 to facilitate slow Ca2+-dependent inactivation of SOCE.53
Table 1.
STIM1 interacting proteins.
| Binding Protein | Identity of binding protein | Cell type/in vitro method | Reference |
|---|---|---|---|
| Cytoskeletal/cell surface | |||
| EB1, EB2, and EB3 | Microtubule binding proteins | HEK 293, HeLa | 58 |
| PI(4,5)P2 | Membrane phospholipid | Lipid-binding assay | 59 |
| GluA1 and GluA2 | AMPA receptor subunit | Hippocampal neurons, rat cortical neurons | 60,61 |
| PMCA | PM ATPase that pumps Ca2+ out of the cell | HEK293 | 62 |
| SOCE proteins | |||
| STIM2 | ER Ca2+ sensor | HEK 293, HEK 293T, Human platelets, HeLa, NIH 3T3 | 63–65 |
| Orai1 | CRAC channel | Human Platelets, rat cortical neurons, mouse dendritic cells, HEK 293, MEG-01, HeLa, parathyroid adenoma tissues, Xenopus oocytes | 63,66–72 |
| Orai2 | Store-operated ion channel | Mouse dendritic cells, HEK293 | 44,66 |
| Orai3 | Store-operated ion channel | HEK293 | 44 |
| TRPC1, TRPC4, TRPC5 | Non-selective cation channels | Human platelets, parathyroid adenoma tissues, SY5Y neuroblastoma cells, HEK293 | 71,73–76 |
| Nuclear transport | |||
| Exportin1 | Nuclear export | HEK 293T | 62,77 |
| Transportin1 | Nuclear import | HEK 293T | 77 |
| ER proteins | |||
| IP3R1 and IP3R3 | ER Ca2+ channels | BAECs, MDCK, Xenopus oocytes | 78,79,72 |
| SERCA3, SERCA2b | ER Ca2+ ATPase | Human Platelets, Xenopus oocytes | 62,63 |
| Calnexin | ER chaperone/MAM protein | HEK293T | 77 |
| Calsequestrin1 | Ca2+ buffering | HEK293 | 80 |
| SARAF | Negative regulator of SOCE | MEG-01 | 81 |
| STIMATE (TMEM110) | ER multi-transmembrane protein | HEK293 | 54 |
| TMEM203 | ER resident regulator of Ca2+ homeostasis | HEK293 | 82 |
| SPPL3 | ER membrane protein of the aspartyl protease family | HEK293T | 83 |
| Junctate | Ca2+ sensing ER protein | HeLa | 52 |
| Others | |||
| SUR1 | KATP channel subunit | HEK293T, mouse islets | 84 |
| Cav1.2 and Cav1.3 | Voltage dependent Ca2+ channels | HEK293, HEK293T, SY5Y neuroblastoma cells, hippocampal neurons | 74,85,86 |
| Calmodulin | Ca2+ binding protein | CaM binding assay, isothermal titration calorimetry | 59,87 |
| AKAP | Scaffold protein | Hippocampal neurons | 60 |
| rPKA and cPKA | PKA subunits | Hippocampal neurons | 60 |
| PRKAA2 | Energy sensor | HEK 293T | 88 |
| CRACR2A | Cytosolic Ca2+ sensing protein | HeLa | 51 |
| POST (TMEM110) | ER-resident membrane protein | HEK 293 | 62 |
| Junctophilin-4 | Contribute to the formation of junctional membrane complexes | HEK 293 | 56 |
| α-SNAP | Cytosolic protein involved in intracellular trafficking and fusion of vesicles | HEK 293 | 57 |
| Golli | Myelin basic protein | HeLa | 89 |
Other proteins indirectly affect SOCE, including mitsugumin 29 (MG29), RAS association domain family 4 (RASSF4), and the ubiquitin-proteasome system (UPS).90–92 MG29, a synaptophysin-related protein expressed in skeletal muscle is localized in SR-PM junctions and is required for maintenance of SR-PM junctions. The role of MG29 in SOCE was revealed in knockout mice studies. Decreased SOCE was observed in skeletal myocytes isolated from mice in which the expression of MG29 gene expression was silenced.90,93 The MG29 null cells, however, did not exhibit any change in STIM1 and Orai1 expression levels.90,93 Collectively, this suggests MG29 may play a role in facilitating SOCE in skeletal muscle by maintaining the integrity of the SR-PM junctions where STIM1 and Orai1 interact. RASSF4 was identified as a positive regulator of SOCE in a siRNA screen of HeLa cells.91 Further experiments showed that RASSF4 regulates SOCE by affecting the translocation of STIM1 to ER-PM junctions. Rather than recruiting STIM1 via direct interactions, RASSF4 affects steady state PM phosphatidylinositol 4,5-bisphosphate (PIP2) levels, thereby regulating targeting of STIM1 to the ER-PM junctions.91 Finally, the UPS has also been shown to affect SOCE by regulating STIM1 surface expression.92 In HEK 293 cells treated with proteosome inhibitors, STIM1 surface expression and SOCE were significantly increased. Additionally, overexpression of an E3 ubiquitin ligase resulted in decreased STIM1 surface expression levels. This suggests a novel role for the UPS in regulation of STIM1 localization and SOCE.
The process of SOCE whereby depletion of intracellular Ca2+ stores in the ER activates Ca2+ influx across the PM has been under investigation for more than 30 years. This has allowed for the identification of STIM proteins as Ca2+ sensors within the ER that activate PM channels of the Orai family. Since their discovery, recent experiments have allowed for the characterization of this process, as well as ways in which it is regulated as described above. We will continue this review with an in depth analysis of STIM1 and STIM2. We will discuss their roles in SOCE and other signaling pathways as well as the pathophysiology associated with these proteins and altered SOCE.
Molecular biology of STIM
STIM1 (initially named GOK) was identified in 1996 as a novel human gene cloned from chromosome region 11p15.5. This chromosomal region was known to be involved in a number of tumors and childhood malignancies.94 Early work suggested STIM1 was a tumor suppressor gene as in vitro studies revealed a growth-inhibitory function of STIM1 in rhabdoid tumor and rhabdomyosarcoma cell lines.95 The mouse homologue of STIM1, originally called SIM, was identified independently from a screen of stromal cell cDNA for proteins that recognized pre-B cells and promoted their proliferation.96 Murine Stim1 maps to a distal region of mouse chromosome 7 and its amino acid sequence shares 96% identity with human STIM1.97
A screen of gene databases in 2001 for STIM1-related sequences led to the discovery of STIM2, a STIM1 homologue present in vertebrates.98 In the same study, a single STIM homologue, D-STIM, was identified in Drosophila melanogaster and Caenorhabditis elegans. Human Stim2 was mapped to chromosome 4p15.1, mouse Stim2 is located on chromosome 5, and D-Stim is on band 14A of the X chromosome.98 cDNA and amino acid database alignment studies revealed that the genomic organization of STIM1 and STIM2 is highly conserved, suggesting that the two proteins evolved from a common ancestor.98 Human and mouse STIM1 and STIM2 are each encoded by 12 exons spaced across more than 120 kb.95 On the other hand, D-Stim is more compact; it is encoded by seven exons spaced over 4.2 kb.98
STIM1 and STIM2 are expressed at varying levels in a wide range of cell and tissue types.98,99 While STIM1 is more abundant than STIM2 in platelets, macrophages, and T cells,100–103 STIM1 and STIM2 are expressed at relatively equal levels in skeletal muscle, liver, and spleen.100 In contrast, STIM2 is more highly expressed than STIM1 in dendritic cells, neurons, and the brain.66,98,100,104 The STIM proteins also have varied expression levels during different stages of development in some tissues. For example, STIM1 is expressed in neonatal cardiomyocytes, but absent in adult cardiomyocytes.105 Other cell types in which both STIM1 and STIM2 are expressed include beta cells, intestinal epithelial cells, endothelial progenitor cells, and pituitary gonadotrophs.84,106,107
The majority of STIM1 is localized in the ER membrane; however, STIM1 is also expressed in the PM1,3,98,99,108–111 In contrast, STIM2 is located in the membranes of the ER and acidic organelles.3,63,98,99,110 However, a small population of pre-STIM2 escapes ER targeting and is present in the cytosol proximal to the PM, where it constitutively interacts with Orai1.112
Splice variations of Stim1 transcripts produce two isoforms of the protein: a long version (STIM1L) as well as the dominant shorter version (STIM1). STIM1L contains an extra 106-amino acid sequence in the cytosolic C-terminus of STIM1. While STIM1 is ubiquitously expressed in human tissues, STIM1L is expressed exclusively in skeletal muscle and neonatal heart.105,113 STIM1L plays an important functional role in myocytes as a fast activator of SOCE. Actin binding localizes STIM1L in clusters with Orai1 at the PM for rapid activation of SOCE following depletion of SR Ca2+.113
Unlike STIM1, three distinct forms of STIM2 generated by post-translational modifications have been identified.112 In HEK 293 cells, the majority of STIM2 exists as an ER membrane protein produced after cleavage of a 101-amino acid signal peptide at the N-terminus. Another smaller population, referred to as preSTIM2, escapes ER targeting and remains in the cytosol with the signal peptide intact. A third variant of STIM2 results from cleavage of the signal peptide into a smaller signal peptide fragment (SPF). These isoforms play distinct functional roles in cells. STIM2 senses and responds to changes in [Ca2+]ER to activate SOCE, while pre-STIM2 associates with the PM and regulates store-independent Ca2+ entry. In contrast, the SPF of STIM2 functions as a transcriptional regulator.112 Since the initial discovery and description of STIM2 isoforms, we still do not fully understand their physiological roles. Further investigation into the expression and function of these variants in other types of cells could yield insightful information that will further define the multiple roles of STIM2 in cellular physiology.
More recently, two independent studies have reported expression of a splice variant of STIM2, termed STIM2β, in CD8+ and CD4+ T-cells, Jurkat cells, human monocytes, and C2C12 cells .114,115 STIM2β is highly conserved and expressed in many tissue types. STIM2β contains an additional eight amino acids in the channel-activating domain encoded by exon 9. Unlike STIM2, STIM2β inhibits Orai1-mediated SOCE. Although STIM2β localization and puncta formation resembles STIM2, Orai1 recruitment into puncta formed by STIM2β is significantly reduced. STIM2β interaction with Orai1 is mediated by heterodimerization with STIM1 or STIM2. Although the underlying mechanism of STIM2β inhibition of Orai1 is unclear, it may be mediated by a sequence-specific inhibitory allosteric and Ca2+-dependent interaction with Orai1.114
STIM: Structure/function considerations
STIM proteins are type 1A single-pass transmembrane proteins located in the ER (Figure 1). STIM1 is 685 amino acids long. The nucleotide sequence of STIM2 encodes a 746-amino acid protein that is post-translationally processed into a 732-residue protein. D-STIM, the smallest of the STIM proteins, contains 570 amino acids.98 The STIM proteins are largely conserved and share high homology through most of their length, but differ considerably in the distal C-terminal end. At the N-terminus, STIM proteins contain a pair of conserved cysteine residues, a Ca2+-binding helix-loop-helix EF-hand domain, and a sterile α-motif (SAM) (Figure 2). Also conserved in the three STIM proteins is an N-linked glycosylation site at the N-terminus of the SAM domain. STIM1 contains a second N-linked glycosylation site within the SAM domain that is absent in STIM2 and D-STIM. The transmembrane region is highly conserved and contains a single cysteine residue in each STIM protein. STIM proteins have a series of α-helices, forming coiled-coil domains that mediate protein–protein interactions. Distal to the coiled-coil domains, STIM1 has a serine- and proline-rich region, whereas STIM2 contains a histidine- and proline-rich domain (Figure 2). Other than a similar lysine-rich tail at the C-terminus, the amino acid sequences of STIM1 and STIM2 diverge beyond the coiled-coil domains.
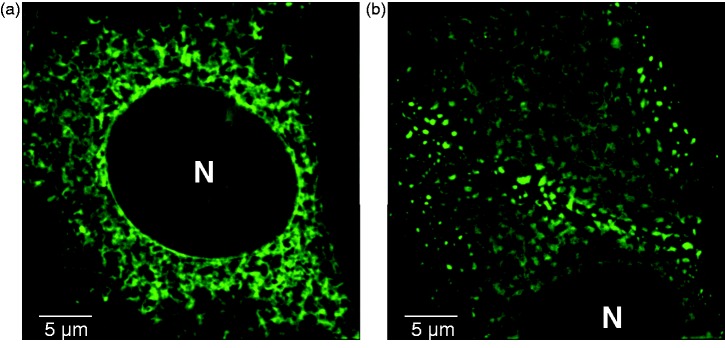
Figure 1.
STIM1 and STIM2 have a reticular ER distribution. NIH 3T3 cells transiently expressing STIM1-YFP (a) or STIM2-YFP (b). Images shown are a z-projection of a z-stack acquired on a Nikon’s N-SIM super resolution microscope. N: nucleus. (A color version of this figure is available in the online journal.)
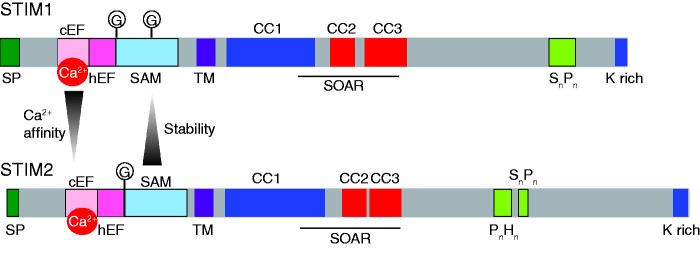
Figure 2.
Comparison of the structure of human STIM1 and STIM2, highlighting each functional domain. SP: signal peptide; cEF: classical EF hand; hEF: hidden EF hand; SAM: sterile α motif; G: N-glycosylation site; TM: transmembrane domain; CC: coiled-coil domain; SOAR: STIM-Orai activating region; SnPn: serine proline rich domain; PnHn: proline histidine rich domain; K rich: lysine rich domain. As indicated by the gradient arrows, the STIM2 EF-hand has a lower Ca2+ affinity but its EF-SAM domain is more stable than that of STIM1. (A color version of this figure is available in the online journal.)
The molecular basis of STIM1-mediated SOCE activation following ER Ca2+ depletion was discovered in HeLa cells using site-directed mutagenesis, nuclear magnetic resonance, and Ca2+ imaging.116 STIM1 is maintained in an inactive conformation through hydrophobic interactions between the Ca2+-bound EF-hand regions and the SAM domain. After ER Ca2+ levels decrease, Ca2+ dissociates from the EF-hand. The EF-hand motif is essential for STIM function as an ER Ca2+ sensor (Table 2). The Ca2+ binding domain of the EF-hand enables STIM proteins to sense changes in [Ca2+]ER, which, under resting conditions, ranges between 0.2 and 0.6 mM.117 The Ca2+-binding domain comprised a canonical Ca2+-binding EF-hand (cEF), a non-canonical EF-hand (ncEF), and a SAM domain. The cEF binds a single Ca2+ ion; the ncEF hand does not bind Ca2+.116 The SAM domains in STIM1 and STIM2 are highly conserved, exhibiting more than 85% sequence similarity. The SAM functions as a protein interaction domain and is composed of five α-helices that closely associate with the Ca2+-bound EF-hand to stabilize the STIM proteins as monomers (Table 2). As [Ca2+]ER decreases, Ca2+ dissociates from the EF-hand. This results in a partial unfolding of the EF-SAM complex, which leads to oligiomerization of the STIM proteins.36,64,116
Table 2.
Function of STIM1 and STIM2 protein domains.
| Domain | Function |
|---|---|
| EF-hand | ER Ca2+ sensor |
| SAM | Protein interaction domain. When ER Ca2+ stores are full, maintains STIM in folded conformation Upon Ca2+ store-depletion, facilitates STIM oligomerization |
| CC1-CC3 | ER retention and stability Under-store replete conditions, inhibitory helix in CC1 keeps STIM in inactive conformation Under store-deplete conditions, elongates to facilitate interaction with Orai1 |
| SOAR (within CC2-CC3) | Minimal region required for Orai1 activation |
| K-rich | Initial recruitment of STIM to ER-PM junction STIM1 gating of TRPC channels Electrostatic interactions with PM phospholipids |
Although binding assays have shown that the STIM1 and STIM2 EF-SAM domains exhibit similar Ca2+ binding affinities (Kd ∼0.2–0.6 mM and ∼0.5 mM, respectively), STIM2 has a greater sensitivity to smaller decreases in [Ca2+]ER than STIM1.118,119 The STIM2 EF-SAM domain is more stable than that of STIM1 in the absence of Ca2+ (Figure 2). While STIM1 EF-SAM partially unfolds upon dissociation of Ca2+, STIM2 EF-SAM maintains a highly folded secondary structure.119 How the EF-SAM domain of STIM2 participates in regulation of SOCE is unclear. Although the STIM2 EF-SAM regions share 88% sequence similarity to that of STIM1, detailed structural information about the STIM2 EF-SAM domain is limited. Biophysical studies of recombinant human STIM proteins indicate that the STIM2 SAM domain is more structurally stable than the STIM1 SAM and does not readily aggregate.119 This suggests that loss of the EF-SAM interaction does not lead to oligomerization of STIM2 molecules to the same extent as STIM1.
The cytoplasmic region of STIM proteins is composed of three conserved coiled-coil (CC) domains located within a larger ezrin/radixin/moesin (ERM) homology domain. ERM proteins are a family of proteins that mediate attachment of cytoskeletal proteins to the PM.120 Structurally, ERM proteins exist in a conformationally inactive form. Intramolecular interactions within the ERM complex block PM and cytoskeletal protein binding sites. A conformational change that displaces the electrostatic intramolecular interactions opens up the protein binding sites, yielding an active form of the ERM protein.121–123 ERM domains have been identified in several proteins located at the PM, including focal adhesion kinase, talin, and protein tyrosine phosphatases. They play an important role in mediating protein–protein and protein–phospholipid interactions that facilitate the anchorage of protein complexes at specific membrane sites.124 We currently do not understand how the ERM complex affects STIM localization and function.
The CC domains of STIM1 contain the CRAC activation domain (CAD)125 or STIM1-Orai1-activating region (SOAR) that is required for Orai1 activation (Table 2).126 The SOAR region is highly conserved and spans amino acids 344–442 in human STIM1. Direct binding of the SOAR domain to an α-helical region of the Orai1 C-terminus is required for full channel activation and SOCE. When expressed on its own, SOAR binding to the C-terminus of Orai1 is sufficient for activation126; however, when the full-length protein is transiently expressed in cells, binding of SOAR to the N-terminus of Orai1 is also required for channel activation.125 Co-immunoprecipitation assays have shown that the SOAR domain directly binds to both the C- and N-termini of Orai1. Deletion of the Orai1 C-terminus prevents STIM1 clustering and SOCE, whereas deletion of the N-terminus of Orai1 did not prevent clustering but abolished SOCE.125 Dimerization of STIM1 molecules following Ca2+ depletion and EF-SAM destabilization is also facilitated by interactions of the STIM SOAR domain.127–129 The cytosolic coiled-coil domains (CC1, CC2, and CC3) of STIM1 have been implicated as important facilitators of STIM1 oligomerization in response to ER Ca2+ store depletion. Under store-replete conditions, the SOAR domain is inactive due to electrostatic interactions between an inhibitory helix in CC1 and the SOAR domain spanning CC2 and CC3.130,131 Consequent to a decrease in [Ca2+]ER, unfolding and dimerization of EF-SAM domain cause a conformational change in the cytosolic terminal, removing inhibition of CC1, allowing unfolding and elongation of the C-terminus. STIM1 unfolding reveals a polybasic domain in SOAR that interacts with Orai channels and the PM.37
Although human STIM2 SOAR (SOAR2) shares 76% sequence identity and 91% similarity with that of STIM1 (SOAR1),132 variations in amino acid sequence result in discrete structural and functional differences between STIM1 and STIM2. X-ray crystallography and single-wavelength anomalous diffraction of human SOAR1 indicate that each SOAR1 monomer is made up of a group of four α-helices (Sα1-Sα4), with two long α-helices (Sα1 and Sα4) at the N- and C- terminal regions flanking two short α-helices (Sα2 and Sα3).128 A cluster of positively charged residues in Sα1 of SOAR1 is important for binding of STIM1 to Orai1.133 Additionally, a phenylalanine (F394) in Sα2 of SOAR1 is critical for STIM1 interaction with and activation of Orai. The positively charged residues are conserved in SOAR2. However, the replacement of F394 in SOAR1 with a leucine residue in SOAR2 may contribute to the decreased ability of STIM2 to interact with and activate Orai1.
The CC domains have also been shown to be important for ER retention and stability of STIM1 (Table 2).77 Mutation analysis of STIM1 showed that mutants lacking the CC domains were either expressed on the cell surface or secreted, while mutants with an intact CC domain remained localized to the ER. STIM1 lacks the KKXX ER-retention sequence present in STIM2, so it is uncertain if the CC domain plays a similar localizing function in STIM2.134 Mutational analysis also showed the CC domains is important for stabilizing the STIM proteins. The half-life of mutants lacking the CC domain is shorter than that of the full protein or mutant containing the CC domain.77
STIM proteins also have a polybasic lysine-rich (K-rich) domain (Table 2). Although not sufficient on its own for activation of Orai, the K-rich domain facilitates the initial recruitment of STIM1 to ER-PM junctions (Figure 3).125,126 The K-rich domain of STIM1 also participates in the regulation of TRPC channel gating 135 and may be important for mediating the electrostatic interactions that enables the binding of STIM proteins to PIP2.59 STIM1 and STIM2 exhibit different affinities for PIP2, with STIM2 having a greater affinity for the plasma membrane phospholipid than STIM1. Tetramers of STIM1 bind PIP2, whereas dimers of STIM2 are sufficient for PIP2 binding. A conserved cysteine residue located immediately upstream of the K-rich domain in STIM2 may contribute to the difference in lipid binding between the STIM isoforms. This cysteine residue is essential for dimerization of STIM2. Furthermore, STIM1 has two conserved proline residues within its K-rich domain that are not present in STIM2. The absence of proline in STIM2 allows the formation of a short α-helix; a feature of a similar lipid-binding signal sequence in yeast. When the proline residues in STIM1 K-rich domains are mutated, there is a two-fold increase in lipid affinity, suggesting that the absence of proline in STIM2 could account for its increased lipid affinity.59 The functional consequence of increased affinity of STIM2 over STIM1 for PM lipids remains to be determined.
In summary, STIM1 and STIM2 are conserved and share high homology throughout the length of the molecule. Common to both isoforms are protein domains required for STIM function, namely a Ca2+-binding EF-hand motif, a SAM domain that stabilizes STIM proteins and regulates protein–protein interactions, three coiled-coil domains containing an Orai activating region, and the K-rich domain that facilitates protein anchoring to the PM during STIM activation (Table 2). Although each of domains exists in both STIM1 and STIM2, there are a few amino acid changes that contribute to different binding affinities and activation kinetics between STIM1 and STIM2. These differences may partially explain the diversity of the molecular functions regulated by STIM1 and STIM2 that we describe below.
Posttranslational regulation of STIM
STIM proteins undergo posttranslational modifications and interact with other regulatory proteins. Studies using Calyculin A, a serine/threonine phosphatase inhibitor, have revealed that STIM1 is phosphorylated in K562 cells.99 Phosphorylation of STIM1 at Ser575, Ser608, and Ser621 by extracellular signal-regulated kinases 1/2 (ERK1/2) regulates SOCE in HEK293 cells.136 ERK1/2 phosphorylation of STIM1 is inversely related to [Ca2+]ER: STIM1 phosphorylation increases after ER Ca2+ store depletion, but decreases upon store refilling.137 Phosphorylation of STIM1 at tyrosine residues has also been shown to be important for activation of SOCE in human platelets.138 These studies show that inhibition of phosphorylation decreases the formation of STIM1 puncta and STIM1-Orai1 interactions, and reduces SOCE.
N-linked glycosylation has been implicated in the regulation STIM1. A STIM1 mutant in which two consensus glycosylation site arginine residues (N) were mutated to glutamine (Q) N131Q/N171Q (QQ) failed to rescue SOCE in neuroblastoma × glioma hybrid cells.139 Alternatively, when this mutant was expressed in HEK293 cells, SOC currents were unaffected; however, arachidonic-acid-regulated Ca2+-selective (ARC) channel currents were significantly reduced due to the inability of the STIM1 QQ mutant to be expressed in the PM.109 Interestingly, other specific glycosylation site mutant combinations have differing effects. N131D/N171Q (DQ) resulted in a gain of function mutant as opposed to loss of function seen in the QQ mutant.140 The DQ mutant increased the rate of STIM1 translocation to the ER-PM interface and reduced current latency. Furthermore, when co-expressed with Orai1, STIM1 DQ decreases Orai1 expression, alters STIM1:Orai1 stoichiometry and affects Ca2+ homeostasis.140 Although STIM2 contains one of two glycosylation sites found in STIM1, the role of glycosylation in STIM2 function has not yet been defined.
Direct interactions between STIM and other proteins can influence STIM function. STIM1 and STIM2 both contain a calmodulin (CaM) binding site in the K-rich domain that may serve as an additional mechanism by which Ca2+ regulates STIM proteins. STIM binds CaM in a Ca2+-dependent manner. In the presence of Ca2+, STIM1 and STIM2 bind CaM with high affinity (Kd = 0.8 and 0.9 µM, respectively). When Ca2+ is not bound, however, STIM1 and STIM2 bind to CaM with much lower affinity (Kd of 55 and 150 µM, respectively).87 Although the functional significance of CaM-binding remains uncertain, recent evidence suggests that binding of Ca2+/CaM to STIM2 competitively inhibits interactions of the STIM2 K-rich domain with liposomes and PIP2 in the PM.59 This interaction may prevent Ca2+ overload by reducing STIM2-mediated ER-PM contacts when [Ca2+]c is elevated, and consequently diminish the store-operated Ca2+ current.
Interactions between STIM1 and other proteins also can contribute to reducing SOCE. Two Ca2+-dependent modes of SOCE inactivation have been reported: fast inactivation and slow inactivation. Fast inactivation is Ca2+-dependent and mediated through a CRAC modulatory domain in the C-terminus of STIM1, glutamate residues in the Orai C-terminus, and CaM. 141–144 Slow Ca2+-dependent inactivation is mediated by SOCE-associated regulatory factor (SARAF), an ER resident protein.53 Like STIM proteins, SARAF is a single-pass ER membrane protein. The ER lumenal domain of SARAF regulates its inhibitory action mediated by the cytosolic domain that inactivates SOCE through interactions with the C-terminal domain of STIM1.53
Another mechanism of STIM regulation by protein-protein interactions involves STIM-activating enhancer encoded by TMEM110 (STIMATE), an ER-resident protein. Proteomic analysis and genetic manipulation identified STIMATE as a positive regulator of Ca2+ entry via a direct interaction with STIM1.54,55 The C-terminus of STIMATE binds to the coiled coil 1 (CC1) domain in STIM1, causing disruption of the auto-inhibitory STIM1 CC1-SOAR domain interaction and promotes translocation of STIM1 to ER-PM junctions. The interaction of STIMATE with STIM2 has not yet been investigated.
STIM 2 in SOCE and Ca2+ homeostasis
It has been well documented that STIM1 is essential for SOCE (Figure 3).1–3 Under resting conditions, when the ER Ca2+ stores are full, Ca2+ is bound to the STIM EF-hand and STIM1 is distributed evenly in the ER membrane. Consequent to store depletion, Ca2+ dissociation from STIM1 results in partial unfolding, destabilization, and oligomerization of STIM1 molecules. This is followed by translocation of STIM1 to ER-PM junctions where the STIM oligomers bind to Orai1, forming distinct puncta.2,127,145 Interaction of the SOAR domain of STIM1 with Orai1 opens the channels and activates SOCE.67,125,126 In contrast, the role STIM2 in SOCE remains unclear. Studies have reported that STIM2 activates SOCE in mast cells, Jurkat T cells, and HEK 293 cells.1,68,146,147 However, stable overexpression of STIM2 was found to inhibit SOCE when stably overexpressed in HEK293, PC12, A7r5, and Jurkat T cells.110,148 The underlying mechanisms that account for the different roles of STIM2 in the regulation of SOCE have not been defined. One possible explanation may be differences in tissue culture conditions used to grow the cells. In the experiments that found stable overexpression of STIM2 inhibited SOCE, the cells were maintained in a selective media containing geneticin (G418).110 G418 is an aminoglycoside antibiotic that inhibits STIM2-mediated SOCE.68 This suggests that the inhibition of SOCE observed in these experiments could be a result of direct effects of G418 on SOCE. In support of this idea, experiments in which STIM2 was transiently overexpressed in the absence of G418 showed a modest increase in SOCE.64 Another possibility is an adaptive mechanism in which extended STIM2 overexpression gradually downregulates SOCE. This is consistent with a study that has shown prolonged overexpression of STIM2 significantly decreased maximal SOCE.64
Knockdown studies have also provided contradictory information about the role of STIM2 in SOCE. Silencing of STIM2 in murine T cells, cortical neurons, and mast cells significantly reduced SOCE.100,146,149 Knockdown of STIM2 in HeLa cells, B cells, MDA-MB-231 cells, and mouse embryonic fibroblasts (MEFs) resulted in a reduction of SOCE, but much smaller than that observed after STIM1 was knocked down.3,103,150,151 In contrast, in Xenopus oocytes, STIM2, but not STIM1, played a predominant role in SOCE.152 Alternatively, silencing of STIM2 expression in HEK293, neutrophil-like HL-60 cells, rat pulmonary arterial smooth muscle, airway myocytes, and CD4 T+ cells did not affect SOCE.1,103,148,153–155 In mouse neurons, where STIM2 is the dominant isoform, there was a significant reduction of SOCE following knockout of STIM2.100 However, in arterial smooth muscle cells where STIM2 is expressed at much lower levels than STIM1, there was no effect of STIM2 knockdown on SOCE.154 The differences presented for a role of STIM2 in SOCE may reflect cell type, experimental approach, or the methodologies used for genomic editing. Additionally, it may be difficult to accurately assess STIM2 function using siRNA due to variable degrees of Stim2 knockdown.
Although the role of STIM2 may be cell type-specific, it may also depend on the ratio of STIM1 and STIM2 protein expression levels (STIM1:STIM2) within cells. An investigation of HEK cells transiently overexpressing STIM2 found that transfection with 2 µg Stim2 cDNA inhibited SOCE, but no effect was observed in cells transfected with 0.5 µg Stim2 cDNA.148 In the same study, it was discovered that STIM2 activated SOCE when co-overexpressed with Orai1. These findings suggest that the STIM1:STIM2 ratio may affect signaling. This phenomenon may be in part caused by a signal-trap mechanism found in Chinese hamster ovary (CHO) and HEK293 cells.156 In these cell types, Orai1 undergoes constant recycling with only about 40% localized in the PM at any given point in time. After ER Ca2+ store depletion, STIM1 traps Orai1 and prevents its recycling away from the PM, and results in an enrichment of Orai1 at the PM. However, in cells in which STIM1 is highly overexpressed, Orai1 becomes trapped within STIM1 clusters intracellularly and does not translocate to the PM after discharge of ER Ca2+ stores. The trapping of Orai1 leads to a reduction in SOCE.
Basal Ca2+ homeostasis is critical to maintain, as deviations in baseline Ca2+ can interfere with ER and mitochondrial function as well as receptor-mediated Ca2+ signaling. Aside from a role in SOCE, some studies have shown STIM2 regulates Ca2+ homeostasis. siRNA screening of the human signaling proteome identified STIM2 as a strong positive regulator of basal [Ca2+].95 While STIM2 knockdown in HeLa, HUVEC, and HEK293T cells resulted in decreased resting [Ca2+] in the ER and cytosol, cells in which human STIM2 was over-expressed exhibited increased basal [Ca2+]c.64 This is in contrast to knockdown of STIM1 which did not significantly lower [Ca2+]c and had a much smaller effect on [Ca2+]ER than STIM2. This observation was later confirmed in HEK293 cells, human myoblasts, human breast cancer cells, and in murine neuronal cells.100,113,148,151 STIM2 is partially active in unstimulated cells under resting conditions, which may account for its role as a regulator of Ca2+ homeostasis. Unlike STIM2 which is fully activated by low agonist concentrations and low levels of ER Ca2+ store depletion, STIM1 is activated by high agonist concentrations and full store-depletion in mast cells, Jurkat T cells, and HEK293 cells.146 Thus, STIM2 would respond to passive leakage of Ca2+ from the ER to activate SOCE and allow for Ca2+ entry and refilling of the ER stores, while STIM1 would remain inactive until the cell was exposed to a higher level of stimulus-induced decrease in [Ca2+]ER.
Functional versatility of STIM proteins
Studies of the interactions between STIM1, STIM2, and other proteins have provided strong support for the hypothesis that STIM1 and STIM2 play multifunctional roles in cells (Tables 1 and 3). STIM1 regulation of Ca2+ signaling is neither limited to its actions on SOCs nor is it dependent on ER Ca2+ store depletion. In addition to Orai and TRPC channels, STIM1 regulates ARC channels independent of [Ca2+]ER.109 Regulation of ARC channels by STIM1 is thought to be mediated through interactions with STIM1 expressed in the PM and not in the ER membrane. Control of ARC channels appears unique to a subpopulation of STIM1 protein that is expressed in the PM, and does not involve STIM2 since biotinylation assays indicate that STIM2 is not localized within the PM.110 STIM1 has also been shown to inhibit voltage-dependent Ca2+ channels (VDCCs) in rat neurons, A7r5 cells, mouse HL-1 cardiomyocytes, and HEK293 cells.85,86,157 The inhibitory effect of STIM1 on VDCC, L-type, alpha 1c subunit (Cav1.2) depends on binding of the SOAR domain with the C-terminus of Cav1.2. STIM1 interaction also has been shown to stimulate long-term internalization of the VDCCs from the PM of rat hippocampal neurons.86
Table 3.
STIM2 interacting proteins.
| Binding Protein | Identity of binding protein | Cell type/in vitro method | Reference |
|---|---|---|---|
| Cytoskeletal/cell surface | |||
| EB1 and EB3 | Microtubule binding proteins | HEK293, HeLa | 58 |
| PI(4,5)P2 | Membrane phospholipid | Lipid-binding assay | 59 |
| GluA1 and GluA2 | AMPA receptor subunit | Hippocampal neurons, rat cortical neurons | 60,61 |
| Spectrin α and β chains | Scaffold protein | MIN6,NIH 3T3 | *** |
| SOCE proteins | |||
| STIM1 | ER Ca2+ sensor | HEK293, HEK293T, Human platelets, HeLa, NIH 3T3 | 64,65,98,112 |
| Orai1 | CRAC channel | Human Platelets, rat cortical neurons, mouse dendritic cells, HEK293 | 63,66,68,69 |
| Orai2 | Store-operated Ion Channel | Mouse dendritic cells | 66 |
| TRPC3 | Non-selective cation channel | HUVEC | |
| Nuclear transport | |||
| Exportin1 | Nuclear export | HEK293T | 77 |
| Transportin1 | Nuclear import | HEK293T | 77 |
| ER Proteins | |||
| IP3R1 | ER Ca2+ channel | BAECs | 79 |
| SERCA3 | ER Ca2+ ATPase | Human Platelets | 63 |
| Calnexin | ER chaperone/MAM protein | HEK293T | 77 |
| GRP78/BiP | ER chaperone | NIH 3T3 | *** |
| Others | |||
| Calmodulin | Ca2+ binding protein | CaM binding assay, isothermal titration calorimetry | 59,87 |
| AKAP | Scaffold protein | Hippocampal neurons | 60 |
| rPKA and cPKA | PKA subunits | Hippocampal neurons | 60 |
| PRKAA2 | Energy sensor | HEK293T | 88 |
| Liprin-alpha-1, 2, and 3 | Focal adhesion regulator | MIN6, NIH 3T3 | *** |
| Heat Shock Cognate 71 kDa protein | Molecular chaperone | NIH 3T3 | *** |
| Rab11 family-interacting protein | Endosomal recycling | NIH 3T3 | *** |
***Nelson HA and Roe MW: unpublished observations.
More recently, we found that STIM1 plays a role in regulating ATP-sensitive potassium (KATP) channels and SOC channels in MIN6 cells, an insulin-secreting β-cell line derived from mouse insulinoma.84 Co-immunoprecipitation studies demonstrated that STIM1 directly interacts with the nucleotide binding fold-1 (NBF1) of the sulfonylurea receptor 1 (SUR1) subunit of the KATP channels expressed in MIN6 cells and mouse islets of Langerhans. Since KATP channels are critically involved in the control of pancreatic β-cell membrane potential and stimulus-secretion coupling, our findings suggest that STIM1 couples PM ion channel activity and [Ca2+]ER to regulate membrane excitability and β-cell function.84
In addition to regulating several different Ca2+ entry pathways, STIM1 has also been shown to play a role in the efflux of cytosolic Ca2+ through interactions with PMCA and SERCA2b.21,158 In Jurkat T cells, immunoprecipitation of endogenous PMCA pulled down endogenous STIM1, demonstrating a direct physical interaction.21 Interaction of STIM1 with PMCA decreased PMCA mediated Ca2+ efflux. In Jurkat cells, when extracellular Ca2+ is removed, basal [Ca2+]c is restored following a two-phase (fast then slow) exponential decay pattern. While STIM1 overexpression significantly reduced the slow phase of Ca2+ clearance, STIM2 overexpression had no effect on Ca2+ clearance rates. The STIM1 cytosolic C-terminus encompassing the proline-rich domain appears to be responsible for the interaction with PMCA and the attenuation of Ca2+ efflux mediated by PMCA. Although STIM2 contains an analogous proline-rich domain, whether STIM2 also binds PMCA remains unknown. While the STIM1-PMCA interaction reduces Ca2+ efflux, an interaction of STIM1 with SERCA2b potentiates Ca2+ clearance from the cytosol into the ER in HeLa cells during SOCE activation.158 Taken together, these studies suggest STIM1 shapes dynamic changes in [Ca2+]c through its interactions with Ca2+-ATPases and by regulation of SOCE.21,158
STIM1 has also been implicated in the regulation of other second messenger signaling pathways in cells. In HEK 293 cells, Orai1, STIM1, and adenylyl cyclase type 8 (AC8) were found to be co-localized in lipid raft domains of the PM.159 When STIM1 and Orai1 are co-expressed in HEK293 cells, AC8 activity increases proportionally to the increase in SOCE. Inhibitors of SOCE reduced the effects on AC8 activity in HEK293 cells overexpressing AC8 and in non-manipulated MIN6 cells.159 This work suggests SOCE mediated by STIM1 regulates Ca2+-dependent activation of AC8. Alternatively, in NCM460 cells, ER Ca2+ depletion, independent of any changes in [Ca2+]c, leads to the recruitment of ACs to PAM domains and therefore increased cAMP accumulation and protein kinase A (PKA) activation.160 Furthermore, STIM1 translocation was required for linking reduced ER Ca2+ to increased AC activity. These findings suggest the existence of a store-operated cAMP signaling pathway regulated by STIM1. Interestingly, recent work has shown that cAMP can induce STIM1 translocation, but not Orai1 clustering or SOCE in pancreatic islet cells.161 This suggests a bi-directional control of signaling pathways between STIM1 and cAMP.
STIM1 may also function as a sensor of cellular stressors. Studies of DT40 cells indicate that STIM1 functions as a sensor of oxidative stress.162 Oxidant (buthionine sulfoximine)-induced S-glutathionylation at cysteine 56 near the EF-hand decreases Ca2+ binding, activating STIM1 and SOCE independent of ER Ca2+ content. Consequently, [Ca2+]c is increased at rest and after cellular activation. This elevation of cytosolic Ca2+ leads to impaired mitochondrial function and induction of apoptotic cell death.162 Recent studies of HEK293 and HeLa cells indicate that STIM1 participates in cellular responses to heat stress.163 Using fluorescence confocal imaging, investigators found that heating HeLa cells from 23.5°C to 50°C induced GFP-STIM1 puncta formation without ER Ca2+ store depletion. Upon cooling, the number of STIM1 puncta was reduced by 80%, but did not return to baseline. During the cooling period, Orai1-mediated Ca2+ influx was initiated, resulting in a large rise in [Ca2+]c. Physiologically, relevant temperature changes have also been shown to modulate STIM1-dependent nuclear factor of activated T-cells (NFAT) activation and gene expression in Jurkat cells.163
STIM proteins also have important functional roles beyond Ca2+ signaling. Glutathione S-transferase (GST)-pull down and immunoprecipitation assays revealed an interaction of both STIM1 and STIM2 with microtubule plus end tracking proteins EB1 and EB3.58,164 Some investigators have proposed that microtubules facilitate SOCE by organizing STIM1 localization and movement.165 Other studies have shown that the interaction of STIM1 with EB1 was dispensable for SOCE, but important in the formation of contacts between the ER and microtubules that participate in dynamic changes in ER spatial organization and remodelling.58 This may have important implications in regulating the transition from interphase to mitosis or meiosis. During interphase, STIM1 binds to EB1 via a T-R-I-P sequence located in the distal end of its C-terminus, maintaining proper ER spatial arrangement.164,166 Phosphorylation of STIM1 near the T-R-I-P domain during mitosis inhibits the STIM1-EB1 interaction, causing dissociation of the ER from the microtubules. This allows exclusion of the ER from the mitotic spindle and proper separation of organelles during cell division. Interestingly, phosphorylation of STIM1 in this region was also shown to inhibit SOCE during mitosis, suggesting phosporylation may play a role in differential regulation of STIM1 function.167 Furthermore, the effects of phosphorylation on STIM1-EB1 interactions and SOCE are independent. This was demonstrated as loss of the STIM1-EB1 interaction failed to block SOCE,58 while a phospho-deficient STIM1 mutant restored SOCE independent of EB1.166 It is uncertain whether STIM2 has similar functional interactions with microtubule plus end tracking proteins. STIM2 does associate with EB1 and EB3 in B16F1mouse melanoma cells, albeit to a lesser extent than STIM1.58 The consequences of such interaction have not been studied.
A broader range of STIM1 functions were suggested following the identification of Partner of STIM1 (POST).62 POST is a 10-transmembrane–spanning segment adaptor protein located in the ER and PM. Although POST-Orai1 binding is independent of ER Ca2+ store depletion, lowering [Ca2+]ER triggers binding of POST to STIM1 and POST movement within the ER towards the cell membrane where it inhibits PMCA activity, and may thereby sustain elevated [Ca2+]c. POST also has been shown to facilitate interactions between STIM1 and PMCA, SERCA2, Na+/K+-ATPase, and the nuclear transporters, importin-β, and exportin. PMCA, SERCA, and Na+/K+-ATPase are all regulators of intracellular Ca2+. STIM1 interaction with these transporters may serve to modulate their activity and coordinate [Ca2+]c. The proposed interaction between STIM1 and the nuclear transport proteins was further confirmed in an independent study that used co-immunoprecipitation in combination with mass spectrometry to identify exportin1, transportin1, and calnexin as both STIM1- and STIM2-associated molecules in HEK293T and HeLa cells.77 An interaction with importin, exportin, and transportin following reduction in [Ca2+]ER suggests the possibility that nuclear import/export can be directly modulated by store depletion. The significance of STIM interactions with calnexin and nuclear transport factors remains unclear, but future studies may reveal more complex interactions and functional consequences of these associations.
STIM1 has also been shown to interact with type 3 IP3 receptor (IP3R3) in mouse pancreatic acini and Madin-Darby Canine Kidney Epithelial (MDCK) cells, and IP3R1 in bovine aortic endothelial cells (BAECs).78,79,168 STIM2 was found to bind with IP3R1 in BAECs.79 The functional consequences of these interactions are not well characterized. In murine pancreatic acini and Drosophila melanogaster neurons, the association of IP3R3 and STIM1 forms part of a signaling complex at the ER-PM junctions that stabilizes the STIM1-Orai1 interaction to facilitate SOCE.79,169 It is likely that the interaction of STIM1 and STIM2 with IP3R contributes to the regulation of IP3R function. In BAECs, STIM1 positively regulated Ca2+ release stimulated by ATP or bradykinin, whereas an increased interaction between STIM1 and IP3R3 mediated by polycistin1 and the PI3K/AKT pathway was shown to decrease Ca2+ release in MDCK cells.79,169
While most studies looking to identify STIM associated proteins have focused on STIM1, a study in adult rat brains and hippocampal neurons investigated STIM2 specifically.60 This study showed STIM2, but not STIM1, is important for regulation of dendritic spine formation in rat neurons.60 In addition to regulating spine morphogenesis, STIM2 also was found to participate in the regulation of synaptic transmission as well as synaptic delivery and removal of α-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid receptors (AMPARs).60,170 AMPARs are nonselective transmembrane cation channels allowing the passage of Na+ and K+, and are the primary mediators of excitatory neurotransmission in the brain. AMPAR is composed of four subunits (GluA1–4). GluA1 has two well-defined phosphorylation sites in the cytoplasmic tail that regulate activity of the AMPAR; Ser-831 and Ser-845. In nervous tissues, co-immunoprecipitation experiments revealed STIM2 interacts with GluA1, AKAP, and both the regulatory and catalytic subunits of PKA.60 STIM2 facilitates phosphorylation of GluA1 by recruiting PKA to the AMPAR independent of SOCE. More interestingly, it was shown that cAMP initiates STIM2 translocation to ER-PM junctions where it acts in a signaling complex with PKA to phosphorylate GluA1 and enhance cell surface delivery of the AMPAR.60 Although this function was thought to be a unique property of STIM2, other co-immunoprecipitation studies have demonstrated that both STIM1 and STIM2 interact with GluA1 and GluA2 in rat cortical neurons.61 The STIM-AMPAR subunit interaction was important for amplifying the SOCE response via Ca2+ entry through AMPARs. Although STIM1 interacted with AMPARs in a SOCE-dependent context, STIM1 was not important for dendritic spine morphogenesis, suggesting a novel role as a central organizer of excitatory synapses unique to STIM2 in neural cells.
We have used co-immunoprecipitation to pull down STIM2 and mass spectrophotometry to identify novel STIM2 binding proteins from mouse insulinoma (MIN6) and NIH 3T3 fibroblast cell lysates (Table 3). Alpha and beta chain spectrin were both pulled down with STIM2 in MIN6 and NIH 3T3 cells. Spectrin is a cytoskeletal protein that forms a scaffold important for maintaining PM and cytoskeletal integrity. The STIM2-spectrin interaction has not been explored, but could be important for anchoring STIM2 at ER-PM junctions. Liprins 1, 2, and 3 were also pulled down with STIM2 in both the NIH 3T3 and MIN6 cells. Liprins interact with the LAR family of transmembrane protein tyrosine phosphatases and may facilitate their localization to focal adhesions. The functional significance of a STIM2-Liprin interaction remains to be determined. Inducible heat shock cognate 71 kDa protein (also termed heat shock 70 kDa protein 8, Hsc70 or Hsp73) and Rab11 family-interacting protein also were pulled down by STIM2 in NIH 3T3 cell lysates. An interesting and potentially important STIM2 binding partner we identified in NIH 3T3 cells was GRP78, another member of the heat shock protein 70 family. This protein, also known as binding immunoglobulin protein (BiP) is located in the ER lumen where it regulates folding and assembly of proteins in the ER as well as participates in ER stress signaling through the unfolded protein response signaling pathways. Future investigations of the STIM2-GRP78 interaction could provide exciting new insights into the function of STIM2 in ER stress and cell death.
In summary, recent studies have demonstrated that STIM1 and STIM2 are multifunctional signaling effectors that participate in a wide array cellular signaling pathways. STIM1 has been implicated in Ca2+ clearance and various transport mechanisms to coordinate [Ca2+]c. 21,62,77,158 Not only does STIM1 activate Orai1, but STIM1 also regulates KATP channels and ARC channels.84,110 STIM1 may play a role in cAMP and IP3R signaling pathways.78,79,159,160 STIM1 also functions as a sensor of various kinds of cellular stress, interacts with the microtubule cytoskeleton and is involved in remodeling of the ER.58,162,163,166 Finally, although not as thoroughly studied as STIM1, emerging evidence indicates that STIM2-mediated signaling plays an important role as a central organizer of excitatory synapses in neural cells.60
Pathophysiology of STIM
Ca2+ entry through SOCs controls a diverse range of cellular functions, such as exocytosis, cell proliferation, gene transcription, and cell death. It is not surprising therefore that several human diseases have been associated with disruption of Stim1 and Stim2 expression. A growing body of evidence indicates that aberrant SOCE contributes to the pathogenesis of immunodeficiency disorders, cancer, neurodegeneration, cardiovascular disease, musculoskeletal disorders, and diabetes.
Immune system diseases associated with disruption of STIM1 and STIM2
Although abnormal SOC activity may result from absence or mutation in the channel protein, Orai1, studies have shown mutations in STIM1 contribute to abnormal SOCE and the pathogenesis of disease. For example, a nonsense mutation in Stim1 was discovered in a patient with complex immunodeficiency disorder (CID).171 The mutation introduced a premature stop codon in Stim1, resulting in a truncated STIM1 lacking the SAM domain. Examination of fibroblasts isolated from the individual with CID showed loss of STIM1 expression and decreased SOCE. Expression of wild type (WT) human STIM1 restored normal patterns of Ca2+ entry following ER Ca2+ store discharge. Expression of WT STIM2 also increased SOCE, although to a smaller extent than with that of WT STIM1. It is interesting to note that mRNA levels of Stim2 were not altered in the CID patient fibroblasts. This raises the likelihood that the level of endogenous STIM2 is not sufficient to compensate for the loss of STIM1; however, when overexpressed, STIM2 can partially compensate for the loss of STIM1 function. Severe immunodeficiency was also observed in another cohort of patients possessing a mutation that caused reduced Stim1 mRNA expression and absence of STIM1 protein expression. 172,173 The association between STIM mutations and immunodeficiency can be explained by the resulting defects in SOCE as this pathway of Ca2+ entry controls T cell proliferation, cytokine production and expression, regulatory B cell and mast cell function, and neutrophil phagocytosis.
SOCE and the STIM molecules are critical components of Ca2+ signals that regulate cellular processes in many types of immune cells. Mast cells from mice lacking STIM1 exhibit impaired Ca2+ influx and reduced activation of transcription factors NF-κB and NFAT. Furthermore, following IgE stimulation, STIM1-deficient mast cells had significantly less degranulation and cytokine production, demonstrating an important role for STIM1 mediated SOCE in mast cell activation and anaphylaxis.175 T cells isolated from mice lacking STIM1 or STIM2 had reduced SOCE, decreased NFAT translocation to the nucleus, and lower cytokine production.103 Stim1/Stim2 double-knockout mice displayed a lymphoproliferative phenotype and a reduced number of regulatory T cells. Other studies have found impaired T cell receptor mediated activation of T cells in the absence of SOCE in patients lacking Orai1 or STIM1.171,176 SOCE has also been shown to play a key role in the function of natural killer (NK) cells; cytotoxic lymphocytes important for viral and antitumor immunity. NK cells deficient for Orai1 and STIM1 exhibited reduced cytokine production, defects in the exocytosis of cytotoxic granules, and an inability to lyse tumor targets in vitro.173,177 In addition to NK cell deficits, NKT cell development is impaired in STIM1- STIM2-deficient mice.178
While absent or reduced SOCE can cause immunodeficiency, overactive SOCs are also linked to the pathogenesis of human disease. One such example is the contribution of SOCE to airway inflammation and bronchoconstriction. A 3,5-bistrifluoromethylpyrazole derivative, BTP2, blocks SOCs and interleukin (IL)-2 production in T cells. When BTP2 was orally administered in sensitized guinea pigs, it reduced asthmatic bronchoconstriction.179 Additionally, in sensitized Brown Norway rats, BTP2 reduced antigen-induced eosinophil infiltration and IL-4 and leukotriene production in airways. These studies raise the possibility that SOCE contributes to the development of bronchial asthma via Ca2+ influx activation of T cell cytokine production and release of inflammatory mediators.179–181 Increases in SOCE are also thought to enhance proliferation of T cells. This may account for the elevated cytokine production in intestinal mucosa associated with inflammatory bowel disease.182–184 A more detailed review of the roles of STIM1 and STIM2 in immune diseases is available elsewhere.174
Abnormal SOCE and its role in the pathogenesis of cancer
STIM1, Orai1, and SOCE have also been implicated in carcinogenesis. Studies have indicated that cancer cell proliferation, apoptosis, metastasis, and tumor neovascularization are affected by disruption of SOCE and other components of intracellular Ca2+ signal transduction.185 Several forms of cancer are associated with altered Ca2+ homeostasis and abnormal expression of Ca2+ channels and other Ca2+ regulatory proteins.186
Initially, STIM1 was identified as a potential tumor suppressor in rhabdomyosarcomas and rhabdoid tumors.95 While STIM1 is highly expressed in skeletal myocytes, Stim1 mRNA has not been detected in rhabdomyosarcoma or rhaboid tumor cell lines. Furthermore, when STIM1 is overexpressed in cells derived from rhaboid tumors or rhabdomyosarcomas lacking STIM1 expression, the cells die. Collectively, these data suggest loss of STIM1 expression is critical for the establishment or progression of these tumors.
In contrast, a tumor growth promoting role for STIM1 was found in patients with cervical cancer and breast cancer.151,187 In a study of 24 cervical cancer patients, expression of STIM1 directly correlated with tumor size and clinical outcome.187 Increased expression of STIM1 correlated with larger primary tumors, greater metastasis, and a decreased five-year survival rate. Similarly, there was greater tumor growth and decreased patient survival in breast cancer patients with high Stim1 mRNA expression levels compared to controls.151 In rat and human glioblastoma cells, knockdown of STIM1 or Orai1 inhibited tumor cell proliferation and promoted apoptosis, suggesting a role for SOCE in glioblastoma formation.26 Stim2 expression was also found to be increased in a genome-wide gene expression analysis of 20 patients with primary glioblastoma.188
Others studies have provided additional support for the hypothesis that STIM1 and STIM2 participate in tumorogenesis by showing that SOCE is important for the vascularization in tumors by controlling the production of vascular endothelial growth factor (VEGF).187 Knockdown of STIM1 in human cervical cancer cells impairs VEGF secretion and in SCID mice injected with SiHA tumor cells, SOCE inhibitors 2-aminoethoxydiphenyl borate (2-APB), and SKF 96365 reduced neovascularization.187 In human umbilical vein endothelial cells (HUVEC), RNAi silencing of Stim1, Stim2, or Orai1 reduced both VEGF-mediated Ca2+ influx and cell proliferation.189 Furthermore, in vitro treatment of human endothelial progenitor cells with BTP2, an inhibitor of SOCE, decreased VEGF-induced cytoplasmic Ca2+ oscillations, cell proliferation, and tubologenesis.190
A role for STIM1 and Orai1 in tumor metastasis has also been proposed. Migration of tumors from the primary site to secondary locations is the leading cause of death of cancer patients. It has been shown that Orai1 and STIM1 play a critical role in tumor cell migration in vitro and tumor cell metastasis in mice.191 Application of SKF96365, an inhibitor of SOCE, as well as lowering the expression level of Orai1 and Stim1 by RNA interference, reduced cancer cell migration and tumor metastasis. This suggests the loss of SOCE caused by reduction of STIM1 or Orai1 expression is an important molecular signaling mechanism that could be exploited to develop new therapeutic approaches that reduce tumor migration and metastasis. However, caution must be taken when using such an approach since SOCE is also critical for antitumor immunity.192 In mice with a T cell specific deletion of both Stim1 and Stim2, cytotoxic lymphocytes were unable to prevent tumor cell engraftment. Specifically, STIM1 and STIM2 were required for production of interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α), degranulation, and Fas ligand (FasL)-mediated tumor cell killing. This indicates that SOCE is important for antitumor immune responses and raises the possibility that inhibition of CRAC channels in cancer therapy has the potential to decrease the body’s own ability to inhibit tumorgenesis.
While the STIM1-Orai1 pathway has been extensively implicated in the pathogenesis of cancer as described above, some studies have suggested that Orai3 plays a key regulatory role in cell proliferation and cancer progression. Orai3 has been found to be overexpressed in breast, prostate, and lung cancers.193–196 Interestingly, Orai3 is implicated in estrogen receptor (ER)(+) breast tumors specifically, while the canonical STIM1/Orai1 pathway appears to predominate in ER(−) breast cancers cells.195 Orai3-mediated SOCE is required for cell cycle progression, particularly through G1 and G1/S transition phases. 194,197,198 Knockdown of Orai3 expression in an ER(+) breast cancer cell line (MCF7) inhibited cell cycle progression, induced apoptosis, and inhibited tumorigenesis.194,198 Taken together, these studies suggest Orai3 is a specific ER-regulated Ca2+ channel associated with uncontrolled cell growth and tumorigenesis. Similarly, in lung cancer tissue, Orai3 expression was elevated, correlating with increased cell proliferation and high tumor grade.196 In human prostate cancer cell lines, Orai3, but not Orai1 is significantly overexpressed.193 In in vitro models used to mimick this change in Orai expression, Orai3 overexpression favored heteromerization with Orai1 to form an ER Ca2+ store-independent Ca2+ channel. The Orai3-Orai1 channels promote cell proliferation and reduce the number of Orai1-based SOCs, which are implicated in supporting vulnerability to apoptosis. In vivo, Orai3 knockdown in prostate cancer tumors reduced tumor growth, while overexpression of Orai3 increased tumor volume.193 This suggests there may be a critical switch in cancer progression, shifting the Orai channels from Orai1 homomultimers to Orai3-Orai1 heteromultimers that promote cell growth and migration while preventing apoptosis.
Neurodegenerative diseases associated with disruption of STIM1 and STIM2
There is a growing body of evidence supporting a role of STIM1, STIM2, and SOCE in nervous system pathology, such as Alzheimer’s disease (AD), Huntington’s disease (HD), and ischemic damage of the brain.100,199–201 For instance, it is known that mushroom dendritic spine structures are essential for memory storage and the loss of mushroom spines may explain memory defects in Alzheimer's disease (AD). Interestingly, it was shown that STIM2 is important for stabilization of neuronal mushroom spines via a STIM2-mediated neuronal store-operated Ca2+ entry (nSOC) and Ca2+/calmodulin kinase II pathway.199 This suggests STIM2 downregulation is associated with spine loss and memory decline. Consistent with this, overexpression of STIM2 in two murine models of AD rescued nSOC and prevented mushroom spine loss.199,202 Furthermore, downregulation of STIM2 in familial Alzheimer’s disease (FAD) is thought to be linked to presenilins, endoprotease complexes that catalyze the intramembrane cleavage of integral membrane proteins.203 Mutations in presenilin 1 (PS1) are a major cause of FAD. Mutations in PS1 reduce STIM2 expression and diminish SOCE in human lymphocytes. In human neuroblastoma cells, expression of a mutant PS1 linked to AD was also shown to reduce SOCE.204 Surprisingly, STIM2 knockdown rescued SOCE in these cells, which markedly contrasts with the results from STIM2 overexpression in the murine models. In this case, it appears STIM2 may be acting as an inhibitor of SOCE. Taken together, this data suggest that the STIM2-mediated SOCE pathway in neurons may be a therapeutic target for the development of novel treatments of memory loss and AD.
STIM1 and STIM2 are also important in the regulation of synaptic plasticity.170,200,205 Long-term potentiation (LTP) and depression (LTD) are two widely studied models of synaptic plasticity implicated in information storage. Using a conditional knockout mouse models, deletion of both Stim1 and Stim2 enhanced LTP at CA3-CA1 hippocampal synapses.200 This enhancement was associated with increased PKA mediated phosphorylation of the AMPAR subunit GluA1, cAMP response element binding protein (CREB), and Cav1.2. Physiologically, this resulted in impaired spatial learning and memory. In a Stim2 conditional knockout mouse model, synaptic delivery and removal of AMPARs were impaired.170 Similarly, STIM1 was shown to regulate mGluR1/TRPC3-dependent slow excitatory synaptic potentials.205 Deletion of Stim1 in mouse cerebellar Purkinje neurons has also been shown to cause impairments in cerebellar motor behavior. Taken together, these studies suggest STIM1 and STIM2 are key regulators of synaptic plasticity, mediated through their regulation of AMPAR cycling and Ca2+ signaling.
STIM2 has also been show to play a critical role in hypoxic neuronal cell death.100 In neurons, STIM2 is the most abundant STIM isoform. SOCE was reduced in neurons from Stim2 deficient mice (Stim2−/−), but not in neurons isolated from Stim1 knockout mice. Under anoxic conditions in the brain, excess [Ca2+]c is thought to contribute to neuronal cell death. In Stim2−/− neurons, ischemia-induced Ca2+ accumulation was markedly reduced, suggesting decreased STIM2 may contribute to neuron survival. Indeed, when mice were subjected to ischemic conditions, Stim2−/− mice showed significantly less neuronal damage compared with wild type mice.100 This data suggest that in cells where STIM2 is the predominant isoform, it may play a critical role in SOCE and pathophysiological conditions associated with SOCE and Ca2+ homeostasis.
Involvement of STIM1 and STIM2 in disorders of the cardiovascular system
Cardiac hypertrophy is an abnormal enlargement or thickening of the heart muscle that can result from increase in cardiomyocyte size or other changes in heart muscle machinery. Hypertrophic growth often accompanies various forms of heart disease that can lead to death. Orai1, STIM1, TRPC1, and therefore, SOCE play an important role in cardiac hypertrophy in mouse and rat cardiomyocytes.105,206–208 Despite this data, the role of SOCE in adult cardiomyocytes is unclear. STIM1 expression and SOCE are absent in adult mouse cardiomyocytes, present in neonatal cardiomyocytes, suggesting a developmental regulation.105 Interestingly, STIM1 expression was induced in adult cardiomyocytes by pathological stress to trigger SOCE and hypertrophy.
Hemostasis following blood vessel damage is the first stage of wound healing and depends on the coordinated activation of platelets. Ca2+ is a critical second messenger, controlling a variety of platelet responses, including integrin activation, secretion, shape change, and procoagulant activity.209,210 SOCE mediated by Orai1 and STIM1 appears to be the main mechanism for Ca2+-dependent platelet activation and thrombosis.210–216 A gain-of-function (GoF) STIM1 mutation in mice resulting in constitutively active STIM1 was lethal in utero in mice homozygous for the mutation due to uncontrolled bleeding.214 Mice heterozygous for the mutation was viable, but had extremely low platelet. Low platelet counts were attributed to SOCE induced pre-activation of the platelets, leading to enhanced platelet clearance. GoF mutations in STIM1 have also been described as the causative agent in patients suffering from York platelet syndrome or Stormorken syndrome— diseases that are characterized by increased bleeding due to thrombocytopenia and platelet defects.217–219
Loss-of-function (LoF) mutations or conditional knockout of STIM1 or Orai1 expression in different mouse models exhibit significant defects in SOCE in platelets.211–213,215 Although these platelets with impaired SOCE showed relatively minor defects in integrin activation and granule release, they had a defect in their ability to switch to a pro-coagulant state. As a direct result of the role of STIM1 and Orai1 in promoting platelet activation and blood clotting, STIM1 or Orai1 deficiency results in resistance to pulmonary and arterial thrombosis.211,213 Indeed, when STIM1 was conditionally knocked out of the platelets in a mouse model, thrombi were instable and fibrin formation at sites of vascular injury was slowed.211 Interestingly, this phenotype was not associated with significantly increased bleeding risk, suggesting STIM1 and Orai1 may be novel targets for anti-platelet therapy for the treatment of thrombosis-associated diseases.
SOCE has also been implicated in vascular remodeling in patients with pulmonary arterial hypertension (PAH).220 PAH is a progressive and fatal disease for which there are some treatments, but no cures. Excessive proliferation of pulmonary arterial smooth muscle cells (PASMC) is a common causative agent in the development and progression of PAH. Expression of STIM2, Orai2, and transient receptor potential channel 6 (TRPC6) is upregulated in PASMC with a proliferative phenotype compared to those with a contractile phenotype.220 Increased [Ca2+]c is a key signal initiating the switch from a contractile to proliferative phenotype in PASMC. The data indicate that upregulation of STIM2 and Orai2 in PASMC results in enhanced SOCE, and therefore an increase in proliferating PASMC. Furthermore, clinically, STIM2 and Orai2 expression levels have been found to be elevated in PASMC from patients with PAH compared to normal controls. This study identifies potential new molecular targets for the treatment of PAH and raises the possibility that by downregulating STIM2 or Orai2, PASMC proliferation and progression of PAH could be prevented or reduced.
Musculoskeletal diseases associated with abnormal SOCE
Although Ca2+ influx via SOCE is not necessary for skeletal muscle fiber excitation-contraction coupling, it does appear important for fatigue resistance.221–223 In mouse and human myotubes, deletion of STIM1 or STIM2 reduced Ca2+ stores, impaired SOCE, and decreased fatigue resistance.221,223 Similar results were obtained in transgenic mice with skeletal myocyte-specific expression of a dominant-negative mutant of Orai1 (Orai1E108Q).224 Muscle fibers expressing Orai1E108Q fatigued more rapidly than controls upon repeated stimulation. Pharmacological inhibition of SOCE in the presence of extracellular Ca2+ has been shown to increase muscle fatigue.90 Furthermore, muscles isolated from Mitsugumin 29 (MG29) null mice were more susceptible to muscle fatigue and this susceptibility can be attributed to a dysfunction in SOCE.90 In sarcalumenin null mice, MG29 expression was significantly increased, resulting in enhanced SOCE and elevated muscle fatigue resistance.225
In addition to playing a role in skeletal muscle fatigue resistance, SOCE also serves as a signal to regulate muscle development and differentiation through Ca2+-dependent pathways.226,227 The expression of STIM1 and Orai1, and SOCE have been found to be significantly increased during differentiation of mouse and human myoblasts into myotubes in culture.228 While knockdown of STIM1 or Orai1 reduced SOCE and myoblast differentiation, overexpression of STIM1 and Orai1 increased and quickened myoblast differentiation.228 The roles STIM1 and SOCE myocyte development were further supported in experiments using conditional deletion of STIM1 in mouse skeletal muscle. This study revealed that STIM1-mediated SOCE was required for neonatal muscle growth and differentiation.229
These defects noted can also be directly linked to human skeletal muscle diseases. For example, LoF or null mutations in Orai1 and STIM1 are associated with global muscular hypotonia in most patients.38,174,230,231 Partial iris hypoplasia and/or mydriasis in some Orai1- and STIM1-deficient patients is yet another sign of muscular hypotonia and further underscores the importance of defects in SOCE in the development of myopathies in humans.200,230
More recently, mutations in the STIM1 intralumenal Ca2+-binding EF hand domain have been identified as a genetic cause of tubular-aggregate myopathy (TAM).232 TAM is characterized by the abnormal build-up of protein in type I and type II fibers that forms clumps of tube-like structures called tubular aggregates. The disease onset is in childhood or early adulthood and presents itself as slow and progressive lower-limb weakness. The TAM-related STIM1 mutants are constitutively active, forming clusters with Orai1 even when the stores are filled. This leads to higher basal [Ca2+] and dysregulated Ca2+ homeostasis in muscles from TAM patients.232
Increased Ca2+ influx and protease activity for which SOCE may be involved is also proposed to play a key role in Duchenne muscular dystrophy (DMD).233 In adult mdx mice, a well-characterized DMD animal model, Orai1 expression was significantly increased. Additionally, SOCE and [Ca2+]SR were elevated in the dystrophic muscles. RNAi mediated knockdown of Orai1 eliminated the differences in SOCE and [Ca2+]SR between mdx and control muscle fibers, suggesting Orai1-mediated SOCE contributes to the altered Ca2+ homeostasis and subsequent enhanced proteolytic activity in dystrophic muscles.
Disruption of SOCE in the pathogenesis of diabetic complications
Abnormal SOCE has also been identified in diabetes. For example, STIM1 is significantly reduced in mouse coronary endothelial cells (MCEC) isolated from diabetic mice.234 As a result of reduced STIM1 expression, diabetic MCECs exhibit reduced [Ca2+]ER and consequently, diminished endothelium-dependent coronary vasodilation. These changes could contribute to cardiovascular complications common in diabetic patients. Consequent to STIM1 overexpression in diabetic MCECs, [Ca2+]ER and endothelium-dependent relaxation of the coronary artery were restored to levels observed in control MCECs. The mechanism by which STIM1 is reduced in diabetic MCECs is unknown, but may be a secondary consequence of hyperglycemia, which has been shown to reduce STIM1 protein expression in control MCECs.234 Although the mechanisms are not completely understood, changes in SOCE may also be linked to various complications resulting from diabetes, such as diabetic cardiomyopathy, diabetic vasculopathy, or diabetic nephropathy. A more thorough discussion of diabetes-associated changes in SOCE and its role in disease complications is provided elsewhere.235
Conclusions and future directions
STIM proteins are highly versatile, multifunctional members of a family of Ca2+-binding proteins. The identification of the molecular basis of SOCE with STIM1 as the ER Ca2+ sensor and Orai1 as the channel pore has provided exciting new understanding of the roles of these proteins in Ca2+ signal transduction and contributed novel insights into the pathogenesis of human diseases. A rapidly growing body of evidence indicates that STIM1 and STIM2 dynamically control Ca2+ signal transduction by regulating several different channel proteins, including Orai1, TRPC1, ARC, T- and L-type voltage-gated Ca2+ channels, and KATP channels.84,86,109,157 Given that proteomic and biochemical analyses have revealed a broad array of potential and novel STIM1 and STIM2 binding proteins, future studies will be necessary to identify and characterize other cellular functions mediated by interaction of STIM1 and STIM2 with their binding partners.
Acknowledgements
We thank Dr. Colin A. Leech and Dr. Richard F. Kopp for many helpful comments and suggestions during the writing of this review.
Authors’ contributions
HAN wrote the manuscript. MWR participated in the design and review of the article.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This work was supported by the National Institute of Health [Grant number R01 DK092616].
References
- 1.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Veliçelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol 2005; 169:435–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 2005; 437:902–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liou J, Kim ML, WDH, Jones JT, Myers JW, Ferrell JE, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store- depletion-triggered Ca2+ influx. Curr Biol 2005; 15:1235–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature 2006; 443:230–3 [DOI] [PubMed] [Google Scholar]
- 5.Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet J-P. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science 2006; 312:1220–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang SL, Yeromin AV, Zhang XH-F, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD. Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc Natl Acad Sci U S A 2006; 103:9357–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoth M, Niemeyer BA. The neglected CRAC proteins: Orai2, Orai3, and STIM2. Curr Top Membr 2013; 71:237–71 [DOI] [PubMed] [Google Scholar]
- 8.Parekh AB, Putney JW. Store-operated calcium channels. Physiol Rev 2005; 85:757–810 [DOI] [PubMed] [Google Scholar]
- 9.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 2000; 1:11–21 [DOI] [PubMed] [Google Scholar]
- 10.Yang Z, Kirton HM, MacDougall DA, Boyle JP, Deuchars J, Frater B, Ponnambalam S, Hardy ME, White E, Calaghan SC, Peers C, Steele DS. The Golgi apparatus is a functionally distinct Ca2+ store regulated by the PKA and Epac branches of the β1-adrenergic signaling pathway. Sci Signal 2015; 8:ra101, http://stke.sciencemag.org/content/8/398/ra101 (accessed 4 May 2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L-H, Tian X-R, Jiang Z, Zeng L-W, He W-F, Hu Z-P. The Golgi apparatus: panel point of cytosolic Ca 2+ regulation. Neurosignals 2013; 21:272–84 [DOI] [PubMed] [Google Scholar]
- 12.Xue S, Nicoud MR, Cui J, Jovin DJA. High concentration of calcium ions in Golgi apparatus. Cell Res 1994; 4:97–108 [Google Scholar]
- 13.Medina DL, Di Paola S, Peluso I, Armani A, De Stefani D, Venditti R, Montefusco S, Scotto-Rosato A, Prezioso C, Forrester A, Settembre C, Wang W, Gao Q, Xu H, Sandri M, Rizzuto R, De Matteis MA, Ballabio A. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat Cell Biol 2015; 17:288–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCue HV, Wardyn JD, Burgoyne RD, Haynes LP. Generation and characterization of a lysosomally targeted, genetically encoded Ca2+-sensor. Biochem J 2013; 449:449–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Contreras L, Drago I, Zampese E, Pozzan T. Mitochondria: the calcium connection. Biochim Biophys Acta 2010; 1797:607–18 [DOI] [PubMed] [Google Scholar]
- 16.Ichas F, Jouaville LS, Mazat J-P. Mitochondria are excitable organelles capable of generating and conveying electrical and calcium signals. Cell 1997; 89:1145–53 [DOI] [PubMed] [Google Scholar]
- 17.Rizzuto R, De Stefani D, Raffaello A, Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol 2012; 13:566–78 [DOI] [PubMed] [Google Scholar]
- 18.Clapham DE. Calcium signaling. Cell 2007; 131:1047–58 [DOI] [PubMed] [Google Scholar]
- 19.Bootman MD, Lipp P, Berridge MJ. The organisation and functions of local Ca2+ signals. J Cell Sci 2001; 114:2213–22 [DOI] [PubMed] [Google Scholar]
- 20.Putney J. A model for receptor-regulated calcium entry. Cell Calcium 1986; 7:1–12 [DOI] [PubMed] [Google Scholar]
- 21.Ritchie MF, Samakai E, Soboloff J. STIM1 is required for attenuation of PMCA-mediated Ca2+ clearance during T-cell activation. EMBO J 2012; 31:1123–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis RS. Store-operated calcium channels: new perspectives on mechanism and function. Cold Spring Harb Perspect Biol 2011; 3: pii: a003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J Cell Biol 2006; 174:815–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kar P, Nelson C, Parekh AB. Selective activation of the transcription factor NFAT1 by calcium microdomains near Ca2+ release-activated Ca2+ (CRAC) channels. J Biol Chem 2011; 286:14795–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willoughby D, Wachten S, Masada N, Cooper DMF. Direct demonstration of discrete Ca2+ microdomains associated with different isoforms of adenylyl cyclase. J Cell Sci 2010; 123:107–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu H, Hughes JD, Rollins S, Chen B, Perkins E. Calcium entry via ORAI1 regulates glioblastoma cell proliferation and apoptosis. Exp Mol Pathol 2011; 91:753–60 [DOI] [PubMed] [Google Scholar]
- 27.Ong HL, Liu X, Sharma A, Hegde RS, Ambudkar IS. Intracellular Ca2+ release via the ER translocon activates store-operated calcium entry. Pf lügers Arch 2007; 453:797–808 [DOI] [PubMed] [Google Scholar]
- 28.Hoth M, Penner R. Calcium release-activated calcium current in rat mast cells. J Physiol 1993; 465:359–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature 1992; 355:353–6 [DOI] [PubMed] [Google Scholar]
- 30.Parekh AB. Functional consequences of activating store-operated CRAC channels. Cell Calcium 2007; 42:111–21 [DOI] [PubMed] [Google Scholar]
- 31.Yamashita M, Navarro-Borelly L, McNally BA, Prakriya M. Orai1 mutations alter ion permeation and Ca2+-dependent fast inactivation of CRAC channels: evidence for coupling of permeation and gating. J Gen Physiol 2007; 130:525–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fierro L, Parekh AB. Fast calcium-dependent inactivation of calcium release-activated calcium current (CRAC) in RBL-1 cells. J Membr Biol 1999; 168:9–17 [DOI] [PubMed] [Google Scholar]
- 33.Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature 2006; 443:226–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Putney JW. New molecular players in capacitative Ca2+ entry. J Cell Sci 2007; 120:1959–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hewavitharana T, Deng X, Soboloff J, Gill DL. Role of STIM and Orai proteins in the store-operated calcium signaling pathway. Cell Calcium 2007; 42:173–82 [DOI] [PubMed] [Google Scholar]
- 36.Liou J, Fivaz M, Inoue T, Meyer T. Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion. Proc Natl Acad Sci U S A 2007; 104:9301–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou Y, Srinivasan P, Razavi S, Seymour S, Meraner P, Gudlur A, Stathopulos PB, Ikura M, Rao A, Hogan PG. Initial activation of STIM1, the regulator of store-operated calcium entry. Nat Struct Mol Biol 2013; 20:973–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel S-H, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 2006; 441:179–85 [DOI] [PubMed] [Google Scholar]
- 39.Mignen O, Thompson JL, Shuttleworth TJ. Orai1 subunit stoichiometry of the mammalian CRAC channel pore. J Physiol 2008; 586:419–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Penna A, Demuro A, Yeromin AV, Zhang SL, Safrina O, Parker I, Cahalan MD. The CRAC channel consists of a tetramer formed by stim-induced dimerization of Orai dimers. Nature 2008; 456:116–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ji W, Xu P, Li Z, Lu J, Liu L, Zhan Y, Chen Y, Hille B, Xu T, Chen L. Functional stoichiometry of the unitary calcium-release-activated calcium channel. Proc Natl Acad Sci U S A 2008; 105:13668–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lis A, Peinelt C, Beck A, Parvez S, Monteilh-Zoller M, Fleig A, Penner R. CRACM1, CRACM2, and CRACM3 are store-operated Ca2+ channels with distinct functional properties. Curr Biol 2007; 17:794–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gwack Y, Srikanth S, Feske S, Cruz-Guilloty F, Oh-Hora M, Neems DS, Hogan PG, Rao A. Biochemical and functional characterization of Orai proteins. J Biol Chem 2007; 282:16232–43 [DOI] [PubMed] [Google Scholar]
- 44.Frischauf I, Muik M, Derler I, Bergsmann J, Fahrner M, Schindl R, Groschner K, Romanin C. Molecular determinants of the coupling between STIM1 and Orai channels differential activation of Orai1–3 channels by a STIM1 coiled-coil mutant. J Biol Chem 2009; 284:21696–2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muik M, Frischauf I, Derler I, Fahrner M, Bergsmann J, Eder P, Schindl R, Hesch C, Polzinger B, Fritsch R, Kahr H, Madl J, Gruber H, Groschner K, Romanin C. Dynamic coupling of the putative coiled-coil domain of ORAI1 with STIM1 mediates ORAI1 channel activation. J Biol Chem 2008; 283:8014–22 [DOI] [PubMed] [Google Scholar]
- 46.Li Z, Lu J, Xu P, Xie X, Chen L, Xu T. Mapping the interacting domains of STIM1 and Orai1 in Ca2+ release-activated Ca2+ channel activation. J Biol Chem 2007; 282:29448–56 [DOI] [PubMed] [Google Scholar]
- 47.Yeung PS-W, Yamashita M, Prakriya M. Pore opening mechanism of CRAC channels. Cell Calcium 2017; 63:14–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amcheslavsky A, Wood ML, Yeromin AV, Parker I, Freites JA, Tobias DJ, Cahalan MD. Molecular biophysics of Orai store-operated Ca2+ channels. Biophys J 2015; 108:237–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prakriya M. Store-operated Orai channels: structure and function. Curr Top Membr 2013; 71:1–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Putney JW, Steinckwich-Besançon N, Numaga-Tomita T, Davis FM, Desai PN, D’agostin DM, Wu S, Bird GS. The functions of store-operated calcium channels. Biochim Biophys Acta 2017; 1864:900–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Srikanth S, Jung H-J, Kim K-D, Souda P, Whitelegge J, Gwack Y. A novel EF-hand protein, CRACR2A, is a cytosolic Ca2+ sensor that stabilizes CRAC channels in T cells. Nat Cell Biol 2010; 12:436–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Srikanth S, Jew M, Kim K-D, Yee M-K, Abramson J, Gwack Y, Weiss A. Junctate is a Ca2+ -sensing structural component of Orai1 and stromal interaction molecule 1 (STIM1). Proc Natl Acad Sci U S A 2012; 109:8682–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palty R, Raveh A, Kaminsky I, Meller R, Reuveny E. SARAF inactivates the store operated calcium entry machinery to prevent excess calcium refilling. Cell 2012; 149:425–38 [DOI] [PubMed] [Google Scholar]
- 54.Jing J, He L, Sun A, Quintana A, Ding Y, Ma G, Tan P, Liang X, Zheng X, Chen L, Shi X, Zhang SL, Zhong L, Huang Y, Dong M-Q, Walker CL, Hogan PG, Wang Y, Zhou Y. Proteomic mapping of ER–PM junctions identifies STIMATE as a regulator of Ca2+ influx. Nat Cell Biol 2015; 17:1339–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Quintana A, Rajanikanth V, Farber-Katz S, Gudlur A, Zhang C, Jing J, Zhou Y, Rao A, Hogan PG. TMEM110 regulates the maintenance and remodeling of mammalian ER-plasma membrane junctions competent for STIM-ORAI signaling. Proc Natl Acad Sci U S A 2015; 112:E7083–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Woo JS, Srikanth S, Nishi M, Ping P, Takeshima H, Gwack Y, Weiss A. Junctophilin-4, a component of the endoplasmic reticulum–plasma membrane junctions, regulates Ca 2+ dynamics in T cells. Proc Natl Acad Sci U S A 2016; 113:2762–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miao Y, Miner C, Zhang L, Hanson PI, Dani A, Vig M. An essential and NSF independent role for α-SNAP in store-operated calcium entry. eLife 2013; 2:e00802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grigoriev I, Gouveia SM, van der Vaart B, Demmers J, Smyth JT, Honnappa S, Splinter D, Steinmetz MO, Putney JW, Hoogenraad CC, Akhmanova A. STIM1 is a MT-plus-end-tracking protein involved in remodeling of the ER. Curr Biol 2008; 18:177–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bhardwaj R, Müller H-M, Nickel W, Seedorf M. Oligomerization and Ca2+/calmodulin control binding of the ER Ca2+ -sensors STIM1 and STIM2 to plasma membrane lipids. Biosci Rep 2013; 33:833–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garcia-Alvarez G, Lu B, Yap KAF, Wong LC, Thevathasan JV, Lim L, Ji F, Tan KW, Mancuso JJ, Tang W, Poon SY, Augustine GJ, Fivaz M. STIM2 regulates PKA-dependent phosphorylation and trafficking of AMPARs. Mol Biol Cell 2015; 26:1141–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gruszczynska-Biegala J, Sladowska M, Kuznicki J. AMPA receptors are involved in store-operated calcium entry and interact with STIM proteins in rat primary cortical neurons. Front Cell Neurosci 2016; 10:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Krapivinsky G, Krapivinsky L, Stotz SC, Manasian Y, Clapham DE. POST, partner of stromal interaction molecule 1 (STIM1), targets STIM1 to multiple transporters. Proc Natl Acad Sci U S A 2011; 108:19234–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zbidi H, Jardin I, Woodard GE, Lopez JJ, Berna-Erro A, Salido GM, Rosado JA. STIM1 and STIM2 are located in the acidic Ca2+ stores and associates with Orai1 upon depletion of the acidic stores in human platelets. J Biol Chem 2011; 286:12257–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brandman O, Liou J, Park WS, Meyer T. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell 2007; 131:1327–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ong HL, Souza LB, De Zheng C, Cheng KT, Liu X, Goldsmith CM, Feske S, Ambudkar IS. STIM2 enhances receptor-stimulated Ca2+ signaling by promoting recruitment of STIM1 to the endoplasmic reticulum – plasma membrane junctions. Sci Signal 2015; 8:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bandyopadhyay BC, Pingle SC, Ahern GP. Store-operated Ca2+ signaling in dendritic cells occurs independently of STIM1. J Leukoc Biol 2011; 89:57–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Navarro-Borelly L, Somasundaram A, Yamashita M, Ren D, Miller RJ, Prakriya M. STIM1-Orai1 interactions and Orai1 conformational changes revealed by live-cell FRET microscopy. J Physiol 2008; 586:5383–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Parvez S, Beck A, Peinelt C, Soboloff J, Lis A, Monteilh-Zoller M, Gill DL, Fleig A, Penner R. STIM2 protein mediates distinct store-dependent and store-independent modes of CRAC channel activation. FASEB J 2008; 22:752–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gruszczynska-Biegala J, Kuznicki J. Native STIM2 and ORAI1 proteins form a calcium-sensitive and thapsigargin-insensitive complex in cortical neurons. J Neurochem 2013; 126:727–38 [DOI] [PubMed] [Google Scholar]
- 70.Jardin I, Lopez JJ, Salido GM, Rosado JA. Orai1 mediates the interaction between STIM1 and hTRPC1 and regulates the mode of activation of hTRPC1-forming Ca2+ channels. J Biol Chem 2008; 283:25296–304 [DOI] [PubMed] [Google Scholar]
- 71.Lu M, Brä Nströ MR, Berglund E, HÖGA, Bjö Rklund P, Westin G, Larsson C, Farnebo L-O, Forsberg L. Expression and association of TRPC subtypes with Orai1 and STIM1 in human parathyroid. J Mol Endocrinol 2010; 44:285–94 [DOI] [PubMed] [Google Scholar]
- 72.Courjaret R, Machaca K. Mid-range Ca2+ signalling mediated by functional coupling between store-operated Ca2+ entry and IP3-dependent Ca2+ release. Nat Comms 2014; 5:3916. [DOI] [PubMed] [Google Scholar]
- 73.López JJ, Salido GM, Pariente JA, Rosado JA. Interaction of STIM1 with endogenously expressed human canonical TRP1 upon depletion of intracellular Ca2+ stores. J Biol Chem 2006; 281:28254–64 [DOI] [PubMed] [Google Scholar]
- 74.Sun Y, Zhang H, Selvaraj S, Sukumaran P, Lei S, Birnbaumer L, Singh BB. Inhibition of L-Type Ca2+ channels by TRPC1-STIM1 complex is essential for the protection of dopaminergic neurons. J Neurosci 2017; 37:3364–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yuan JP, Kim MS, Zeng W, Shin DM, Huang GN, Worley PF, Muallem S. TRPC channels as STIM1-regulated SOCs. Channels 2009; 3:221–5 [DOI] [PubMed] [Google Scholar]
- 76.Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol 2007; 9:636–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saitoh N, Oritani K, Saito K, Yokota T, Ichii M, Sudo T, Fujita N, Nakajima K, Okada M, Kanakura Y. Identification of functional domains and novel binding partners of STIM proteins. J Cell Biochem 2011; 112:147–56 [DOI] [PubMed] [Google Scholar]
- 78.Santoso NG, Cebotaru L, Guggino WB, Polycystin 1, Stim1 2. interact with IP3R to modulate ER 2+ release through the PI3K/Akt pathway. Cell Physiol Biochem 2011; 27:715–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Béliveau É, Lessard V, Guillemette G. STIM1 positively regulates the Ca2+ release activity of the inositol 1,4,5-trisphosphate receptor in bovine aortic endothelial cells. PLoS One 2014; 9:e114718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang L, Zhang L, Li S, Zheng Y, Yan X, Chen M, Wang H, Putney JW, Luo D. Retrograde regulation of STIM1-Orai1 interaction and store-operated Ca2+ entry by calsequestrin. Sci Rep 2015; 5:11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Albarran L, Lopez JJ, Amor N, Ben Martin-Cano FE, Berna-Erro A, Smani T, Salido GM, Rosado JA. Dynamic interaction of SARAF with STIM1 and Orai1 to modulate store- operated calcium entry. Sci Rep 2016; 6:24452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shambharkar PB, Bittinger M, Latario B, Xiong Z, Bandyopadhyay S, Davis V, Lin V, Yang Y, Valdez R, Labow MA. TMEM203 is a novel regulator of intracellular calcium homeostasis and is required for spermatogenesis. PLoS One 2015; 10:e0127480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Makowski SL, Wang Z, Pomerantz JL. A protease-independent function for SPPL3 in NFAT activation. Mol Cell Biol 2015; 35:451–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Leech CA, Kopp RF, Nelson HA, Nandi J, Roe MW. Stromal interaction molecule 1 (STIM1) regulates ATP-sensitive Potassium (K ATP) and store-operated Ca 2+ channels in MIN6 β-Cells. J Biol Chem 2017; 292:2266–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang Y, Deng X, Mancarella S, Hendron E, Eguchi S, Soboloff J, Tang XD, Gill DL. The calcium store sensor, STIM1, reciprocally controls Orai and Cav1.2 channels. Science 2010; 330:105–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Park CY, Shcheglovitov A, Dolmetsch R. The CRAC channel activator STIM1 binds and inhibits L-type voltage-gated calcium channels. Science 2010; 330:101–5 [DOI] [PubMed] [Google Scholar]
- 87.Bauer MC, O’connell D, Cahill DJ, Linse S. Calmodulin binding to the polybasic C-termini of STIM proteins involved in store-operated calcium entry. Biochemistry 2008; 47:6089–91 [DOI] [PubMed] [Google Scholar]
- 88.Behrends C, Sowa ME, Gygi SP, Harper JW. Network organization of the human autophagy system. Nature 2010; 466:68–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Walsh CM, Doherty MK, Tepikin AV, Burgoyne RD. Evidence for an interaction between Golli and STIM1 in store-operated calcium entry. Biochem J 2010; 430:453–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pan Z, Yang D, Nagaraj RY, Nosek TA, Nishi M, Takeshima H, Cheng H, Ma J. Dysfunction of store-operated calcium channel in muscle cells lacking mg29. Nat Cell Biol 2002; 4:379–83 [DOI] [PubMed] [Google Scholar]
- 91.Chen Y-J, Chang C-L, Lee W-R, Liou J. RASSF4 controls SOCE and ER-PM junctions through regulation of PI(4,5)P2. J Cell Biol 2017; 216:2011–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Keil JM, Shen Z, Briggs SP, Patrick GN. Regulation of STIM1 and SOCE by the ubiquitin-proteasome system (UPS). PLoS One 2010; 5:e13465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhao X, Weisleder N, Thornton A, Oppong Y, Campbell R, Ma J, Brotto M. Compromised store-operated Ca2+ entry in aged skeletal muscle. Aging Cell 2008; 7:561–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Parker NJ, Begley CG, Smith PJ, Fox RM. Molecular cloning of a novel human gene (D11S4896E) at chromosomal region 11p15.5. Genomics 1996; 37:253–6 [DOI] [PubMed] [Google Scholar]
- 95.Sabbioni S, Barbanti-Brodano G, Croce CM, Negrini M. GOK: a gene at 11p15 involved in rhabdomyosarcoma and rhabdoid tumor development. Cancer Res 1997; 57:4493–7 [PubMed] [Google Scholar]
- 96.Oritani K, Kincade PW. Identification of stromal cell products that interact with pre-B cells. J Cell Biol 1996; 134:771–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Overall ML, Parker NJ, Scarcella DL, Smith PJ, Dziadek M. Murine Stim1 maps to distal Chromosome 7 and is not imprinted. Mamm Genome 1998; 9:657–9 [DOI] [PubMed] [Google Scholar]
- 98.Williams RT, Manji SS, Parker NJ, Hancock MS, Van Stekelenburg L, Eid JP, Senior PV, Kazenwadel JS, Shandala T, Saint R, Smith PJ, Dziadek M. A. Identification and characterization of the STIM (stromal interaction molecule) gene family: coding for a novel class of transmembrane proteins. Biochem J 2001; 357:673–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Manji SS, Parker NJ, Williams RT, van Stekelenburg L, Pearson RB, Dziadek M, Smith PJ. STIM1: a novel phosphoprotein located at the cell surface. Biochim Biophys Acta 2000; 1481:147–55 [DOI] [PubMed] [Google Scholar]
- 100.Berna-Erro A, Braun A, Kraft R, Kleinschnitz C, Schuhmann MK, Stegner D, Wultsch T, Eilers J, Meuth SG, Stoll G, Nieswandt B. STIM2 regulates capacitive Ca2+ entry in neurons and plays a key role in hypoxic neuronal cell death. Sci Signal 2009; 2:ra67. [DOI] [PubMed] [Google Scholar]
- 101.Gilio K, van Kruchten R, Braun A, Berna-Erro A, Feijge MAH, Stegner D, van der Meijden PEJ, Kuijpers MJE, Varga-Szabo D, Heemskerk JWM, Nieswandt B. Roles of platelet STIM1 and Orai1 in glycoprotein VI- and thrombin-dependent procoagulant activity and thrombus formation. J Biol Chem 2010; 285:23629–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gao Y, Hanley PJ, Rinné S, Zuzarte M, Daut J. Calcium-activated K+ channel (KCa3.1) activity during Ca2+ store depletion and store-operated Ca2+ entry in human macrophages. Cell Calcium 2010; 48:19–27 [DOI] [PubMed] [Google Scholar]
- 103.Oh-Hora M, Yamashita M, Hogan PG, Sharma S, Lamperti E, Chung W, Prakriya M, Feske S, Rao A. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat Immunol 2008; 9:432–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gruszczynska-Biegala J, Pomorski P, Wisniewska MB, Kuznicki J. Differential roles for STIM1 and STIM2 in store-operated calcium entry in rat neurons. PLoS One 2011; 6:e19285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Luo X, Hojayev B, Jiang N, Wang ZV, Tandan S, Rakalin A, Rothermel BA, Gillette TG, Hill JA. STIM1-dependent store-operated Ca2+ entry is required for pathological cardiac hypertrophy. J Mol Cell Cardiol 2012; 52:136–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jaladanki RN, Rathor N, Zou T, Liu L, Xiao L, Wang J-Y. STIM2 inhibits intestinal epithelial restitution by repressing stim1-activated ca2+ influx after wounding. FASEB J 2011; 25:10672 [Google Scholar]
- 107.Dragoni S, Laforenza U, Bonetti E, Reforgiato M, Poletto V, Lodola F, Bottino C, Guido D, Rappa A, Pareek S, Tomasello M, Guarrera MR, Cinelli MP, Aronica A, Guerra G, Barosi G, Tanzi F, Rosti V, Moccia F. Enhanced expression of Stim, Orai, and TRPC transcripts and proteins in endothelial progenitor cells isolated from patients with primary myelofibrosis. PLoS One 2014; 9:e91099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Williams RT, Senior PV, Van Stekelenburg L, Layton JE, Smith PJ, Dziadek MA. Stromal interaction molecule 1 (STIM1), a transmembrane protein with growth suppressor activity, contains an extracellular SAM domain modified by N-linked glycosylation. Biochim Biophys Acta 2002; 1596:131–7 [DOI] [PubMed] [Google Scholar]
- 109.Mignen O, Thompson JL, Shuttleworth TJ. STIM1 regulates Ca2+ entry via arachidonate-regulated Ca2+-selective (ARC) channels without store depletion or translocation to the plasma membrane. J Physiol 2007; 579:703–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Soboloff J, Spassova M. A, Hewavitharana T, He LP, Xu W, Johnstone LS, Dziadek M. A, Gill DL. STIM2 is an inhibitor of STIM1-mediated store-operated Ca2+ entry. Curr Biol 2006; 16:1465–70 [DOI] [PubMed] [Google Scholar]
- 111.Spassova MA, Soboloff J, He L-P, Xu W, Dziadek MA, Gill DL. STIM1 has a plasma membrane role in the activation of store-operated Ca2+ channels. Proc Natl Acad Sci U S A 2006; 103:4040–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Graham SJL, Dziadek MA, Johnstone LS. A cytosolic STIM2 preprotein created by signal peptide inefficiency activates ORAI1 in a store-independent manner. J Biol Chem 2011; 286:16174–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Darbellay B, Arnaudeau S, Bader CR, Konig S, Bernheim L. STIM1L is a new actin-binding splice variant involved in fast repetitive Ca2+ release. J Cell Biol 2011; 194:335–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rana A, Yen M, Sadaghiani AM, Malmersjö S, Park CY, Dolmetsch RE, Lewis RS. Alternative splicing converts STIM2 from an activator to an inhibitor of store-operated calcium channels. J Cell Biol 2015; 209:653–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Miederer A-M, Alansary D, Schwär G, Lee P-H, Jung M, Helms V, Niemeyer BA. A STIM2 splice variant negatively regulates store-operated calcium entry. Nat Comms 2015; 6:6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stathopulos PB, Zheng L, Li G-Y, Plevin MJ, Ikura M. Structural and mechanistic insights into STIM1-mediated initiation of store-operated calcium entry. Cell 2008; 135:110–22 [DOI] [PubMed] [Google Scholar]
- 117.Barrero MJ, Montero M, Alvarez J. Dynamics of [Ca2+] in the endoplasmic reticulum and cytoplasm of intact HeLa cells. A comparative study. J Biol Chem 1997; 272:27694–9 [DOI] [PubMed] [Google Scholar]
- 118.Stathopulos PB, Li G-Y, Plevin MJ, Ames JB, Ikura M. Stored Ca2+ depletion-induced oligomerization of stromal interaction molecule 1 (STIM1) via the EF-SAM region: an initiation mechanism for capacitive Ca2+ entry. J Biol Chem 2006; 281:35855–62 [DOI] [PubMed] [Google Scholar]
- 119.Zheng L, Stathopulos PB, Li G-Y, Ikura M. Biophysical characterization of the EF-hand and SAM domain containing Ca2+ sensory region of STIM1 and STIM2. Biochem Biophys Res Commun 2008; 369:240–6 [DOI] [PubMed] [Google Scholar]
- 120.Chishti AH, Kim AC, Marfatia SM, Lutchman M, Hanspal M, Jindal H, Liu SC, Low PS, Rouleau GA, Mohandas N, Chasis JA, Conboy JG, Gascard P, Takakuwa Y, Huang SC, Benz EJ, Bretscher A, Fehon RG, Gusella JF, et al. The FERM domain: a unique module involved in the linkage of cytoplasmic proteins to the membrane. Trends Biochem Sci 1998; 23:281–2 [DOI] [PubMed] [Google Scholar]
- 121.Hamada K, Shimizu T, Yonemura S, Tsukita S, Tsukita S, Hakoshima T. Structural basis of adhesion-molecule recognition by ERM proteins revealed by the crystal structure of the radixin-ICAM-2 complex. EMBO J 2003; 22:502–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jung JR, Kim H, Jeun S-S, Lee JY, Koh E-J, Ji C. The phosphorylation status of merlin is important for regulating the Ras-ERK pathway. Mol Cells 2005; 20:196–200 [PubMed] [Google Scholar]
- 123.Ceccarelli DFJ, Song HK, Poy F, Schaller MD, Eck MJ. Crystal structure of the FERM domain of focal adhesion kinase. J Biol Chem 2006; 281:252–9 [DOI] [PubMed] [Google Scholar]
- 124.Mangeat P, Roy C, Martin M. ERM proteins in cell adhesion and membrane dynamics. Trends Cell Biol 1999; 9:187–92 [DOI] [PubMed] [Google Scholar]
- 125.Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE, Lewis RS. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell 2009; 136:876–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yuan JP, Zeng W, Dorwart MR, Choi Y-J, Worley PF, Muallem S. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat Cell Biol 2009; 11:337–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Baba Y, Hayashi K, Fujii Y, Mizushima A, Watarai H, Wakamori M, Numaga T, Mori Y, Iino M, Hikida M, Kurosaki T. Coupling of STIM1 to store-operated Ca2+ entry through its constitutive and inducible movement in the endoplasmic reticulum. Proc Natl Acad Sci U S A 2006; 103:16704–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yang X, Jin H, Cai X, Li S, Shen Y. Structural and mechanistic insights into the activation of Stromal interaction molecule 1 (STIM1). Proc Natl Acad Sci U S A 2012; 109:5657–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Covington ED, Wu MM, Lewis RS. Essential role for the CRAC activation domain in store-dependent oligomerization of STIM1. Mol Biol Cell 2010; 21:1897–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Korzeniowski MK, Manjarrés IM, Varnai P, Balla T. Activation of STIM1-Orai1 involves an intramolecular switching mechanism. Sci Signal 2010; 3:ra82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Muik M, Fahrner M, Schindl R, Stathopulos P, Frischauf I, Derler I, Plenk P, Lackner B, Groschner K, Ikura M, Romanin C. STIM1 couples to ORAI1 via an intramolecular transition into an extended conformation. EMBO J 2011; 30:1678–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Stathopulos PB, Ikura M. Structure and function of endoplasmic reticulum STIM calcium sensors. Curr Top Membr 2013; 71:59–93 [DOI] [PubMed] [Google Scholar]
- 133.Wang X, Wang Y, Zhou Y, Hendron E, Mancarella S, Andrake MD, Rothberg BS, Soboloff J, Gill DL. Distinct Orai-coupling domains in STIM1 and STIM2 define the Orai-activating site. Nat Commun 2014; 5:3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Soboloff J, Spassova MA, Dziadek MA, Gill DL. Calcium signals mediated by STIM and Orai proteins–a new paradigm in inter-organelle communication. Biochim Biophys Acta 2006; 1763:1161–8 [DOI] [PubMed] [Google Scholar]
- 135.Zeng W, Yuan JP, Kim MS, Choi YJ, Huang GN, Worley PF, Muallem S. STIM1 gates TRPC channels, but not Orai1, by electrostatic interaction. Mol Cell 2008; 32:439–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pozo-Guisado E, Campbell DG, Deak M, Alvarez-Barrientos A, Morrice NA, Alvarez IS, Alessi DR, Martín-Romero FJ. Phosphorylation of STIM1 at ERK1/2 target sites modulates store-operated calcium entry. J Cell Sci 2010; 123:3084–93 [DOI] [PubMed] [Google Scholar]
- 137.Pozo-Guisado E, Casas-Rua V, Tomas-Martin P, Lopez-Guerrero AM, Alvarez-Barrientos A, Martin-Romero FJ. Phosphorylation of STIM1 at ERK1/2 target sites regulates interaction with the microtubule plus-end binding protein EB1. J Cell Sci 2013; 126:3170–80 [DOI] [PubMed] [Google Scholar]
- 138.López E, Salido GM, Rosado J. A, Berna-Erro A. Unraveling STIM2 function. J Physiol Biochem 2012; 68:619–33 [DOI] [PubMed] [Google Scholar]
- 139.Csutora P, Peter K, Kilic H, Park KM, Zarayskiy V, Gwozdz T, Bolotina VM. Novel role for STIM1 as a trigger for calcium influx factor production. J Biol Chem 2008; 283:14524–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kilch T, Alansary D, Peglow M, DK, Rychkov G, Rieger H, Peinelt C, Niemeyer BA. Mutations of the Ca2+ -sensing stromal interaction molecule STIM1 regulate Ca2+ influx by altered oligomerization of STIM1 and by destabilization of the Ca2+ channel Orai1. J Biol Chem 2012; 288:1653–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Derler I, Fahrner M, Muik M, Lackner B, Schindl R, Groschner K, Romanin C. A Ca2+ release-activated Ca2+ (CRAC) modulatory domain (CMD) within STIM1 mediates fast Ca2+-dependent inactivation of ORAI1 channels. J Biol Chem 2009; 284:24933–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lee KP, Yuan JP, Zeng W, So I, Worley PF, Muallem S. Molecular determinants of fast Ca2+-dependent inactivation and gating of the Orai channels. Proc Natl Acad Sci U S A 2009; 106:14687–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Litjens T, Harland ML, Roberts ML, Barritt GJ, Rychkov GY. Fast Ca2+-dependent inactivation of the store-operated Ca2+ current (ISOC) in liver cells: a role for calmodulin. J Physiol 2004; 558:85–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mullins FM, Park CY, Dolmetsch RE, Lewis RS. STIM1 and calmodulin interact with Orai1 to induce Ca2+-dependent inactivation of CRAC channels. Proc Natl Acad Sci U S A 2009; 106:15495–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Luik RM, Wang B, Prakriya M, Wu MM, Lewis RS. Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature 2008; 454:538–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Thiel M, Lis A, Penner R. STIM2 drives Ca2+ oscillations through store-operated Ca2+ entry caused by mild store depletion. J Physiol 2013; 591:1433–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kar P, Bakowski D, Di Capite J, Nelson C, Parekh AB. Different agonists recruit different stromal interaction molecule proteins to support cytoplasmic Ca2+ oscillations and gene expression. Proc Natl Acad Sci U S A 2012; 109:6969–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bird GS, Hwang S-Y, Smyth JT, Fukushima M, Boyles RR, Putney JW. STIM1 is a calcium sensor specialized for digital signaling. Curr Biol 2009; 19:1724–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Schuhmann MK, Stegner D, Berna-Erro A, Bittner S, Braun A, Kleinschnitz C, Stoll G, Wiendl H, Meuth SG, Nieswandt B. Stromal interaction molecules 1 and 2 are key regulators of autoreactive T cell activation in murine autoimmune central nervous system inflammation. J Immunol 2010; 184:1536–42 [DOI] [PubMed] [Google Scholar]
- 150.Matsumoto M, Fujii Y, Baba A, Hikida M, Kurosaki T, Baba Y. The calcium sensors STIM1 and STIM2 control B cell regulatory function through interleukin-10 production. Immunity 2011; 34:703–14 [DOI] [PubMed] [Google Scholar]
- 151.McAndrew D, Grice DM, Peters AA, Davis FM, Stewart T, Rice M, Smart CE, Brown MA, Kenny PA, Roberts-Thomson SJ, Monteith GR. ORAI1-mediated calcium influx in lactation and in breast cancer. Mol Cancer Ther 2011; 10:448–60 [DOI] [PubMed] [Google Scholar]
- 152.Serrano-Flores B, Garay E, Vázquez-Cuevas FG, Arellano RO. Differential role of STIM1 and STIM2 during transient inward (Tin) current generation and the maturation process in the Xenopus oocyte. BMC Physiol 2014; 14:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Peel SE, Liu B, Hall IP. A key role for STIM1 in store operated calcium channel activation in airway smooth muscle. Respir Res 2006; 7:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lu W, Wang J, Peng G, Shimoda LA, Sylvester JT. Knockdown of stromal interaction molecule 1 attenuates store-operated Ca2+ entry and Ca2+ responses to acute hypoxia in pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol 2009; 297:L17–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bréchard S, Plançon S, Melchior C, Tschirhart EJ. STIM1 but not STIM2 is an essential regulator of Ca2+ influx-mediated NADPH oxidase activity in neutrophil-like HL-60 cells. Biochem Pharmacol 2009; 78:504–13 [DOI] [PubMed] [Google Scholar]
- 156.Hodeify R, Selvaraj S, Wen J, Arredouani A, Hubrack S, Dib M, Al-Thani SN, McGraw T, Machaca K. A STIM1-dependent “trafficking trap” mechanism regulates Orai1 plasma membrane residence and Ca2+ influx levels. J Cell Sci 2015; 128:3143–54 [DOI] [PubMed] [Google Scholar]
- 157.Nguyen N, Biet M, Simard E, Béliveau E, Francoeur N, Guillemette G, Dumaine R, Grandbois M, Boulay G. STIM1 participates in the contractile rhythmicity of HL-1 cells by moderating T-type Ca2+ channel activity. Biochim Biophys Acta 2013; 1833:1294–303 [DOI] [PubMed] [Google Scholar]
- 158.Manjarrés IM, Rodríguez-García A, Alonso MT, García-Sancho J. The sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) is the third element in capacitative calcium entry. Cell Calcium 2010; 47:412–8 [DOI] [PubMed] [Google Scholar]
- 159.Martin ACL, Willoughby D, Ciruela A, Ayling L-J, Pagano M, Wachten S, Tengholm A, Cooper DMF. Capacitative 2+ entry via Orai1 and stromal interacting molecule 1 (STIM1) regulates adenylyl cyclase type 8. Mol. Pharmacol 2009; 75:830–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lefkimmiatis K, Srikanthan M, Maiellaro I, Moyer MP, Curci S, Hofer AM. Store-operated cyclic AMP signalling mediated by STIM1. Nat Cell Biol 2009; 11:433–42 [DOI] [PubMed] [Google Scholar]
- 161.Tian G, Tepikin AV, Tengholm A, Gylfe E. cAMP induces stromal interaction molecule 1 (STIM1) puncta but neither Orai1 protein clustering nor store-operated Ca2+ entry (SOCE) in islet cells. J Biol Chem 2012; 287:9862–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hawkins BJ, Irrinki KM, Mallilankaraman K, Lien Y-C, Wang Y, Bhanumathy CD, Subbiah R, Ritchie MF, Soboloff J, Baba Y, Kurosaki T, Joseph SK, Gill DL, Madesh M. S-glutathionylation activates STIM1 and alters mitochondrial homeostasis. J Cell Biol 2010; 190:391–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Xiao B, Coste B, Mathur J, Patapoutian A. Temperature-dependent STIM1 activation induces Ca2+ influx and modulates gene expression. Nat Chem Biol 2011; 7:351–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Honnappa S, Gouveia SM, Weisbrich A, Damberger FF, Bhavesh NS, Jawhari H, Grigoriev I, van Rijssel FJA, Buey RM, Lawera A, Jelesarov I, Winkler FK, Wüthrich K, Akhmanova A, Steinmetz MO. An EB1-binding motif acts as a microtubule tip localization signal. Cell 2009; 138:366–76 [DOI] [PubMed] [Google Scholar]
- 165.Smyth JT, DeHaven WI, Bird GS, Putney JW. Role of the microtubule cytoskeleton in the function of the store-operated Ca2+ channel activator STIM1. J Cell Sci 2007; 120:3762–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Smyth JT, Beg AM, Wu S, Putney JW, Rusan NM. Phosphoregulation of STIM1 leads to exclusion of the endoplasmic reticulum from the mitotic spindle. Curr Biol 2012; 22:1487–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Smyth JT, Petranka JG, Boyles RR, DeHaven WI, Fukushima M, Johnson KL, Williams JG, Putney JW. Phosphorylation of STIM1 underlies suppression of store-operated calcium entry during mitosis. Nat Cell Biol 2009; 11:1465–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hong JH, Li Q, Kim MS, Shin DM, Feske S, Birnbaumer L, Cheng KT, Ambudkar IS, Muallem S. Polarized but differential localization and recruitment of STIM1, Orai1 and TRPC channels in secretory cells. Traffic 2011; 12:232–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chakraborty S, Deb BK, Chorna T, Konieczny V, Taylor CW, Hasan G. Mutant IP3 receptors attenuate store-operated Ca2+ entry by destabilizing STIM–Orai interactions in Drosophila neurons. J Cell Sci 2016; 129:3903–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yap KAF, Shetty MS, Garcia-Alvarez G, Lu B, Alagappan D, Oh-Hora M, Sajikumar S, Fivaz M. STIM2 regulates AMPA receptor trafficking and plasticity at hippocampal synapses. Neurobiol Learn Mem 2017; 138:54–61 [DOI] [PubMed] [Google Scholar]
- 171.Picard C, McCarl C-A, Papolos A, Khalil S, Lüthy K, Hivroz C, LeDeist F, Rieux-Laucat F, Rechavi G, Rao A, Fischer A, Feske S. STIM1 mutation associated with a syndrome of immunodeficiency and autoimmunity. N Engl J Med 2009; 360:1971–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Byun M, Abhyankar A, Lelarge V, Plancoulaine S, Palanduz A, Telhan L, Boisson B, Picard C, Dewell S, Zhao C, Jouanguy E, Feske S, Abel L, Casanova J-L. Whole-exome sequencing-based discovery of STIM1 deficiency in a child with fatal classic Kaposi sarcoma. J Exp Med 2010; 207:2307–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fuchs S, Rensing-Ehl A, Speckmann C, Bengsch B, Schmitt-Graeff A, Bondzio I, Maul-Pavicic A, Bass T, Vraetz T, Strahm B, Ankermann T, Benson M, Caliebe A, Fölster-Holst R, Kaiser P, Thimme R, Schamel WW, Schwarz K, Feske S, et al. Antiviral and regulatory T cell immunity in a patient with stromal interaction molecule 1 deficiency. J Immunol 2012; 188:1523–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Shaw PJ, Feske S. Physiological and pathophysiological functions of SOCE in the immune system. Front Biosci 2012; 4:2253–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Baba Y, Nishida K, Fujii Y, Hirano T, Hikida M, Kurosaki T. Essential function for the calcium sensor STIM1 in mast cell activation and anaphylactic responses. Nat Immunol 2008; 9:81–8 [DOI] [PubMed] [Google Scholar]
- 176.Beyersdorf N, Braun A, Vogtle T, Varga-Szabo D, Galdos RR, Kissler S, Kerkau T, Nieswandt B. STIM1-independent T cell development and effector function in vivo. J Immunol 2009; 182:3390–7 [DOI] [PubMed] [Google Scholar]
- 177.Maul-Pavicic A, Chiang SCC, Rensing-Ehl A, Jessen B, Fauriat C, Wood SM, Sjoqvist S, Hufnagel M, Schulze I, Bass T, Schamel WW, Fuchs S, Pircher H, McCarl C-A, Mikoshiba K, Schwarz K, Feske S, Bryceson YT, Ehl S. ORAI1-mediated calcium influx is required for human cytotoxic lymphocyte degranulation and target cell lysis. Proc Natl Acad Sci U S A 2011; 108:3324–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Oh-Hora M, Komatsu N, Pishyareh M, Feske S, Hori S, Taniguchi M, Rao A, Takayanagi H. Agonist-selected T cell development requires strong T cell receptor signaling and store-operated calcium entry. Immunity 2013; 38:881–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yoshino T, Ishikawa J, Ohga K, Morokata T, Takezawa R, Morio H, Okada Y, Honda K, Yamada T. YM-58483, a selective CRAC channel inhibitor, prevents antigen-induced airway eosinophilia and late phase asthmatic responses via Th2 cytokine inhibition in animal models. Eur J Pharmacol 2007; 560:225–33 [DOI] [PubMed] [Google Scholar]
- 180.Ishikawa J, Ohga K, Yoshino T, Takezawa R, Ichikawa A, Kubota H, Yamada T. A pyrazole derivative, YM-58483, potently inhibits store-operated sustained Ca2+ influx and IL-2 production in T lymphocytes. J Immunol 2003; 170:4441–9 [DOI] [PubMed] [Google Scholar]
- 181.Ohga K, Takezawa R, Yoshino T, Yamada T, Shimizu Y, Ishikawa J. The suppressive effects of YM-58483/BTP-2, a store-operated Ca2+ entry blocker, on inflammatory mediator release in vitro and airway responses in vivo. Pulm Pharmacol Ther 2008; 21:360–9 [DOI] [PubMed] [Google Scholar]
- 182.Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology 1998; 115:182–205. [DOI] [PubMed] [Google Scholar]
- 183.Schwarz A, Tutsch E, Ludwig B, Schwarz EC, Stallmach A, Hoth M. Ca2+ signaling in identified T-lymphocytes from human intestinal mucosa. Relation to hyporeactivity, proliferation, and inflammatory bowel disease. J Biol Chem 2004; 279:5641–7 [DOI] [PubMed] [Google Scholar]
- 184.Ng SW, di Capite J, Singaravelu K, Parekh AB. Sustained activation of the tyrosine kinase Syk by antigen in mast cells requires local Ca2+ influx through Ca2+ release-activated Ca2+ channels. J Biol Chem 2008; 283:31348–55 [DOI] [PubMed] [Google Scholar]
- 185.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100:57–70 [DOI] [PubMed] [Google Scholar]
- 186.Monteith GR, McAndrew D, Faddy HM, Roberts-Thomson SJ. Calcium and cancer: targeting Ca2+ transport. Nat Rev Cancer 2007; 7:519–30 [DOI] [PubMed] [Google Scholar]
- 187.Chen Y-F, Chiu W-T, Chen Y-T, Lin P-Y, Huang H-J, Chou C-Y, Chang H-C, Tang M-J, Shen M-R. Calcium store sensor stromal-interaction molecule 1-dependent signaling plays an important role in cervical cancer growth, migration, and angiogenesis. Proc Natl Acad Sci U S A 2011; 108:15225–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ruano Y, Mollejo M, Ribalta T, Fiaño C, Camacho FI, Gómez E, de Lope AR, Hernández-Moneo J-L, Martínez P, Meléndez B.Identification of novel candidate target genes in amplicons of Glioblastoma multiforme tumors detected by expression and CGH microarray profiling. Mol Cancer 2006; 5:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Abdullaev IF, Bisaillon JM, Potier M, Gonzalez JC, Motiani RK, Trebak M. Stim1 and Orai1 MEDIATE CRAC currents and store-operated calcium entry important for endothelial cell proliferation. Circ Res 2008; 103:1289–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Dragoni S, Laforenza U, Bonetti E, Lodola F, Bottino C, Berra-Romani R, Carlo Bongio G, Cinelli MP, Guerra G, Pedrazzoli P, Rosti V, Tanzi F, Moccia F. Vascular endothelial growth factor stimulates endothelial colony forming cells proliferation and tubulogenesis by inducing oscillations in intracellular Ca2+ concentration. Stem Cells 2011; 29:1898–907 [DOI] [PubMed] [Google Scholar]
- 191.Yang S, Zhang JJ, Huang X-Y. Orai1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell 2009; 15:124–34 [DOI] [PubMed] [Google Scholar]
- 192.Weidinger C, Shaw PJ, Feske S. STIM1 and STIM2-mediated Ca2+ influx regulates antitumour immunity by CD8+ T cells. EMBO Mol Med 2013; 5:1311–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Dubois C, Vanden Abeele F, Lehen’kyi V, Gkika D, Guarmit B, Lepage G, Slomianny C, Borowiec AS, Bidaux G, Benahmed M, Shuba Y, Prevarskaya N. Remodeling of channel-forming ORAI proteins determines an oncogenic switch in prostate cancer. Cancer Cell 2014; 26:19–32 [DOI] [PubMed] [Google Scholar]
- 194.Faouzi M, Hague F, Potier M, Ahidouch A, Sevestre H, Ouadid-Ahidouch H. Down-regulation of Orai3 arrests cell-cycle progression and induces apoptosis in breast cancer cells but not in normal breast epithelial cells. J Cell Physiol 2011; 226:542–51 [DOI] [PubMed] [Google Scholar]
- 195.Motiani RK, Abdullaev IF, Trebak M. A novel native store-operated calcium channel encoded by Orai3. J Biol Chem 2010; 285:19173–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ay A-S, Benzerdjerb N, Sevestre H, Ahidouch A, Ouadid-Ahidouch H. Orai3 constitutes a native store-operated calcium entry that regulates non small cell lung adenocarcinoma cell proliferation. [Languino LR, ed]. PLoS One 2013; 8:e72889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Faouzi M, Kischel P, Hague F, Ahidouch A, Benzerdjeb N, Sevestre H, Penner R, Ouadid-Ahidouch H. ORAI3 silencing alters cell proliferation and cell cycle progression via c-myc pathway in breast cancer cells. Biochim Biophys Acta 2013; 1833:752–60 [DOI] [PubMed] [Google Scholar]
- 198.Motiani RK, Zhang X, Harmon KE, Keller RS, Matrougui K, Bennett JA, Trebak M. Orai3 is an estrogen receptor α-regulated Ca2+ channel that promotes tumorigenesis. FASEB J 2013; 27:63–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sun S, Zhang H, Liu J, Popugaeva E, Xu N-J, Feske S, White CL, Bezprozvanny I. Reduced synaptic STIM2 expression and impaired store-operated calcium entry cause destabilization of mature spines in mutant presenilin mice. Neuron 2014; 82:79–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Garcia-Alvarez G, Shetty MS, Lu B, Yap KAF, Oh-Hora M, Sajikumar S, Bichler Z, Fivaz M. Impaired spatial memory and enhanced long-term potentiation in mice with forebrain-specific ablation of the Stim genes. Front Behav Neurosci 2015; 9:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wu J, Shih H-P, Vigont V, Hrdlicka L, Diggins L, Singh C, Mahoney M, Chesworth R, Shapiro G, Zimina O, Chen X, Wu Q, Glushankova L, Ahlijanian M, Koenig G, Mozhayeva GN, Kaznacheyeva E, Bezprozvanny I. Neuronal store-operated calcium entry pathway as a novel therapeutic target for Huntington’s disease treatment. Chem Biol 2011; 18:777–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zhang H, Wu L, Pchitskaya E, Zakharova O, Saito T, Saido T, Bezprozvanny I. Neuronal store-operated calcium entry and mushroom spine loss in amyloid precursor protein knock-in mouse model of Alzheimer’s disease. J Neurosci 2015; 35:13275–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Bojarski L, Pomorski P, Szybinska A, Drab M, Skibinska-Kijek A, Gruszczynska-Biegala J, Kuznicki J. Presenilin-dependent expression of STIM proteins and dysregulation of capacitative Ca2+ entry in familial Alzheimer’s disease. Biochim Biophys Acta 2009; 1793:1050–7 [DOI] [PubMed] [Google Scholar]
- 204.Ryazantseva M, Skobeleva K, Kaznacheyeva E. Familial Alzheimer’s disease-linked presenilin-1 mutation M146V affects store-operated calcium entry: does gain look like loss? Biochimie 2013; 95:1506–9 [DOI] [PubMed] [Google Scholar]
- 205.Hartmann J, Karl R, Alexander RPD, Adelsberger H, Brill MS, Rühlmann C, Ansel A, Sakimura K, Baba Y, Kurosaki T, Misgeld T, Konnerth A. STIM1 controls neuronal Ca2+ signaling, mGluR1-dependent synaptic transmission, and cerebellar motor behavior. Neuron 2014; 82:635–44 [DOI] [PubMed] [Google Scholar]
- 206.Hulot J-S, Fauconnier J, Ramanujam D, Chaanine A, Aubart F, Sassi Y, Merkle S, Cazorla O, Ouille A, Dupuis M, Hadri L, Jeong D, Muhlstedt S, Schmitt J, Braun A, Benard L, Saliba Y, Laggerbauer B, Nieswandt B, et al. Critical role for stromal interaction molecule 1 in cardiac hypertrophy. Circulation 2011; 124:796–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Voelkers M, Salz M, Herzog N, Frank D, Dolatabadi N, Frey N, Gude N, Friedrich O, Koch WJ, Katus HA, Sussman MA, Most P. Orai1 and Stim1 regulate normal and hypertrophic growth in cardiomyocytes. J Mol Cell Cardiol 2010; 48:1329–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ohba T, Watanabe H, Murakami M, Sato T, Ono K, Ito H. Essential role of STIM1 in the development of cardiomyocyte hypertrophy. Biochem Biophys Res Commun 2009; 389:172–6 [DOI] [PubMed] [Google Scholar]
- 209.White GC, Scarborough DE, Brinkhous KM. Morphological study of early phases of platelet adhesion to foreign surfaces: effect of calcium. Ann N Y Acad Sci 1983; 416:351–62. [DOI] [PubMed] [Google Scholar]
- 210.Bevers EM, Comfurius P, Zwaal RF. Changes in membrane phospholipid distribution during platelet activation. Biochim Biophys Acta 1983; 736:57–66 [DOI] [PubMed] [Google Scholar]
- 211.Ahmad F, Boulaftali Y, Greene TK, Ouellette TD, Poncz M, Feske S, Bergmeier W. Relative contributions of stromal interaction molecule 1 and CalDAG-GEFI to calcium-dependent platelet activation and thrombosis. J Thromb Haemost 2011; 9:2077–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Bergmeier W, Oh-Hora M, McCarl C-A, Roden RC, Bray PF, Feske S. R93W mutation in Orai1 causes impaired calcium influx in platelets. Blood 2009; 113:675–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Braun A, Varga-Szabo D, Kleinschnitz C, Pleines I, Bender M, Austinat M, Bosl M, Stoll G, Nieswandt B. Orai1 (CRACM1) is the platelet SOC channel and essential for pathological thrombus formation. Blood 2009; 113:2056–63 [DOI] [PubMed] [Google Scholar]
- 214.Grosse J, Braun A, Varga-Szabo D, Beyersdorf N, Schneider B, Zeitlmann L, Hanke P, Schropp P, Mühlstedt S, Zorn C, Huber M, Schmittwolf C, Jagla W, Yu P, Kerkau T, Schulze H, Nehls M, Nieswandt B. An EF hand mutation in Stim1 causes premature platelet activation and bleeding in mice. J Clin Invest 2007; 117:3540–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Varga-Szabo D, Braun A, Kleinschnitz C, Bender M, Pleines I, Pham M, Renné T, Stoll G, Nieswandt B. The calcium sensor STIM1 is an essential mediator of arterial thrombosis and ischemic brain infarction. J Exp Med 2008; 205:1583–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Tolhurst G, Carter RN, Amisten S, Holdich JP, Erlinge D, Mahaut-Smith MP. Expression profiling and electrophysiological studies suggest a major role for Orai1 in the store-operated Ca2+ influx pathway of platelets and megakaryocytes. Platelets 2008; 19:308–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Misceo D, Holmgren A, Louch WE, Holme PA, Mizobuchi M, Morales RJ, De Paula AM, Stray-Pedersen A, Lyle R, Dalhus B, Christensen G, Stormorken H, Tjønnfjord GE, Frengen E. A dominant STIM1 mutation causes Stormorken syndrome. Hum Mutat 2014; 35:556–64 [DOI] [PubMed] [Google Scholar]
- 218.Morin G, Bruechle NO, Singh AR, Knopp C, Jedraszak G, Elbracht M, Brémond-Gignac D, Hartmann K, Sevestre H, Deutz P, Hérent D, Nürnberg P, Roméo B, Konrad K, Mathieu-Dramard M, Oldenburg J, Bourges-Petit E, Shen Y, Zerres K, et al. Gain-of-function mutation in STIM1 (P.R304W) is associated with Stormorken syndrome. Hum Mutat 2014; 35:1221–32 [DOI] [PubMed] [Google Scholar]
- 219.Markello T, Chen D, Kwan JY, Horkayne-Szakaly I, Morrison A, Simakova O, Maric I, Lozier J, Cullinane AR, Kilo T, Meister L, Pakzad K, Bone W, Chainani S, Lee E, Links A, Boerkoel C, Fischer R, Toro C, White JG, Gahl WA, Gunay-Aygun M. York platelet syndrome is a CRAC channelopathy due to gain-of-function mutations in STIM1. Mol Genet Metab 2015; 114:474–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Fernandez RA, Wan J, Song S, Smith KA, Gu Y, Tauseef M, Tang H, Makino A, Mehta D, Yuan JX-J. Upregulated expression of STIM2, TRPC6, and Orai2 contributes to the transition of pulmonary arterial smooth muscle cells from a contractile to proliferative phenotype. Am J Physiol Cell Physiol 2015; 308:C581–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Stiber J, Hawkins A, Zhang Z-S, Wang S, Burch J, Graham V, Ward CC, Seth M, Finch E, Malouf N, Williams RS, Eu JP, Rosenberg P. STIM1 signalling controls store-operated calcium entry required for development and contractile function in skeletal muscle. Nat Cell Biol 2008; 10:688–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Launikonis BS, Murphy RM, Edwards JN. Toward the roles of store-operated Ca2+ entry in skeletal muscle. Pflugers Arch 2010; 460:813–23 [DOI] [PubMed] [Google Scholar]
- 223.Darbellay B, Arnaudeau S, Ceroni D, Bader CR, Konig S, Bernheim L. Human muscle economy myoblast differentiation and excitation-contraction coupling use the same molecular partners, STIM1 and STIM2. J Biol Chem 2010; 285:22437–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wei-LaPierre L, Carrell EM, Boncompagni S, Protasi F, Dirksen RT. Orai1-dependent calcium entry promotes skeletal muscle growth and limits fatigue. Nat Comms 2013; 4:2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zhao X, Yoshida M, Brotto L, Takeshima H, Weisleder N, Hirata Y, Nosek TM, Ma J, Brotto M. Enhanced resistance to fatigue and altered calcium handling properties of sarcalumenin knockout mice. Physiol Genomics 2005; 23:72–8 [DOI] [PubMed] [Google Scholar]
- 226.Stiber JA, Rosenberg PB. The role of store-operated calcium influx in skeletal muscle signaling. Cell Calcium 2011; 49:341–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Kiviluoto S, Decuypere J-P, De Smedt H, Missiaen L, Parys JB, Bultynck G. STIM1 as a key regulator for Ca2+ homeostasis in skeletal-muscle development and function. Skelet Muscle 2011; 1:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Darbellay B, Arnaudeau S, König S, Jousset H, Bader C, Demaurex N, Bernheim L. STIM1- and Orai1-dependent store-operated calcium entry regulates human myoblast differentiation. J Biol Chem 2009; 284:5370–80 [DOI] [PubMed] [Google Scholar]
- 229.Li T, Finch EA, Graham V, Zhang Z-S, Ding J-D, Burch J, Oh-Hora M, Rosenberg P. STIM1-Ca2+ signaling is required for the hypertrophic growth of skeletal muscle in mice. Mol Cell Biol 2012; 32:3009–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.McCarl C-A, Picard C, Khalil S, Kawasaki T, Röther J, Papolos A, Kutok J, Hivroz C, LeDeist F, Plogmann K, Ehl S, Notheis G, Albert MH, Belohradsky BH, Kirschner J, Rao A, Fischer A, Feske S. ORAI1 deficiency and lack of store-operated Ca2+ entry cause immunodeficiency, myopathy, and ectodermal dysplasia. J Allergy Clin Immunol 2009; 124:1311–8.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Feske S. ORAI1 and STIM1 deficiency in human and mice: roles of store-operated Ca 2+ entry in the immune system and beyond. Immunol Rev 2009; 231:189–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Böhm J, Chevessier F, De Paula AM, Koch C, Attarian S, Feger C, Hantaï D, Laforêt P, Ghorab K, Vallat J-M, Fardeau M, Figarella-Branger D, Pouget J, Romero NB, Koch M, Ebel C, Levy N, Krahn M, Eymard B, et al. Constitutive activation of the calcium sensor STIM1 causes tubular-aggregate myopathy. Am J Hum Genet 2013; 92:271–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zhao X, Moloughney JG, Zhang S, Komazaki S, Weisleder N. Orai1 mediates exacerbated Ca2+ entry in dystrophic skeletal muscle. Plos One 2012; 7:e49862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Estrada IA, Donthamsetty R, Debski P, Zhou M-H, Zhang SL, Yuan JX-J, Han W, Makino A. STIM1 restores coronary endothelial function in type 1 diabetic mice. Circ Res 2012; 111:1166–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Chaudhari S, Ma R. Store-operated calcium entry and diabetic complications. Exp Biol Med 2016; 241:343–52 [DOI] [PMC free article] [PubMed] [Google Scholar]