Short abstract
Cotton rat (Sigmodon hispidus) is a useful experimental rodent for the study of human infectious diseases. We previously clarified that cotton rats, particularly females, developed chronic kidney disease characterized by cystic lesions, inflammation, and fibrosis. The present study investigated female-associated factors for chronic kidney disease development in cotton rats. Notably, female cotton rats developed separation of the pelvic symphysis and hypertrophy in the vaginal parts of the cervix with age, which strongly associated with pyometra. The development of pyometra closely associated with the deterioration of renal dysfunction or immunological abnormalities was indicated by blood urea nitrogen and serum creatinine or spleen weight and serum albumin/globulin ratio, respectively. These parameters for renal dysfunction and immunological abnormalities were statistically correlated. These phenotypes found in the female reproductive organs were completely inhibited by ovariectomy. Further, the female cotton rats with pyometra tended to show more severe chronic kidney disease phenotypes and immunological abnormalities than those without pyometra; these changes were inhibited in ovariectomized cotton rats. With regard to renal histopathology, cystic lesions, inflammation, and fibrosis were ameliorated by ovariectomy. Notably, the immunostaining intensity of estrogen receptor α and estrogen receptor β were weak in the healthy kidneys, but both estrogen receptors were strongly induced in the renal tubules showing cystic changes. In conclusion, the close correlations among female reproductive organ-associated abnormalities, immunological abnormalities, and renal dysfunction characterize the chronic kidney disease features of female cotton rats. Thus, the cotton rat is a unique rodent model to elucidate the pathological crosstalk between chronic kidney disease and sex-related factors.
Impact statement
The increasing number of elderly individuals in the overall population has led to a concomitant age-related increase in chronic kidney disease. Moreover, the global prevalence of patients with chronic kidney disease is gradually increasing, which poses a serious public health problem. The limited number of spontaneous chronic kidney disease animal models, which resemble chronic kidney disease pathogenesis in elderly individuals, is a major limitation in the development of experimental and curative medicines for chronic kidney disease. This pathological study clarified that sex-related factors, including hormones, and abnormalities of the female reproductive system, such as pyometra, are closely associated with chronic kidney disease development by using cotton rats (Sigmodon hispidus). Further, ovariectomy inhibited the phenotypes of the female reproductive system, immunological abnormalities, and chronic kidney disease. Thus, this laboratory rodent serves as a novel and useful spontaneous chronic kidney disease model to elucidate the candidate disease factors and the pathogenesis of chronic kidney disease both in human and experimental medicine.
Keywords: Cotton rat, chronic kidney disease, sex hormone, pyometra, ovariectomy, histopathology
Introduction
The cotton rat (Sigmodon hispidus) is an experimental rodent originating from the southern United States. Many studies have reported that this rodent is associated with an increased susceptibility to pathogenic human viruses, protozoans, metazoans, and bacteria, such as Leishmania, Echinococcus, and respiratory disease viruses.1–3 In addition, unique disease phenotypes were identified in cotton rats including fragile tails, stomach cancers, and cardiomyopathy.4 In our previous studies, we have also identified their unique phenotypes such as pharyngeal pouch remnants and female-dominant chronic kidney disease (CKD).5,6 Therefore, we expect that the clarification of disease pathogenesis found in cotton rats is a reasonably important process to better understand similar diseases both in human and veterinary medicine.
In particular, CKD is a serious problem in human and veterinary medicine because several humans and animals are diagnosed with CKD because of the accelerated aging of society.7,8 Several systemic factors also affect CKD development such as genetic factors, obesity, hypertension, infections, or autoimmune disease-related conditions.9 Notably, some types of CKD show sex-related differences, which have been closely associated with systemic conditions, but in general, men tend to show a more rapid progression of CKD than women.10 Characteristically, CKD caused by an autoimmune disorder, including lupus nephritis, shows a female-dominant progression in human and animal disease models.11–13 Female sex-hormone, especially estrogen, has an exacerbating effect on lupus nephritis in human and mouse models; however, estrogen has a mainly protective effect on renal lesions such as glomerulosclerosis and interstitial fibrosis, as found in Dahl salt-sensitive rats with ovariectomy (OVX).13 In the field of veterinary medicine, pyometra-associated nephritis was frequently found in companion dogs.14
Notably, in cotton rats, females show more severe CKD features than males, especially inflammatory cell infiltrations and dilations of distal tubules have been shown to be significantly different between sexes.5 Furthermore, spontaneous gastric adenocarcinoma was mainly observed in female cotton rats (23.6%) compared with male cotton rats (0.71%).15 Therefore, cotton rats would represent a useful animal model to clarify sex-related mechanisms of disease progression. An important key factor of this difference might be caused by female sex hormones, but the underlying mechanisms are yet to be fully understood. In this study, we focused on the renal pathogenesis of female cotton rats and suggested that female sex hormones have an important influence on the progression of CKD in this rodent model.
Materials and methods
Animals
Animal experimentation was performed according to the guidelines of the Hokkaido Institute of Public Health (approval no.: K27–03). Female cotton rats (aged 1–17 months) were maintained as the HIS/Hiph strain through continuous inbreeding under conventional conditions at the Hokkaido Institute of Public Health (Sapporo, Japan). We divided the examined cotton rats into young (aged 1–3 months) and adult (aged 5–17 months) groups according to our previous study.5 In the young group, the cotton rats showed no renal dysfunction and injury,5 and therefore were used as healthy controls (Cont) in the pathological analysis. The adult group was subdivided into two groups, those with or without pyometra in the pathological analysis. In some female cotton rats of the young and adult groups, OVX was performed under anesthesia as described in our previous study,16 and they were examined at four and two months after OVX, respectively. With cotton rats under deep anesthesia with isoflurane, blood was collected from the vena cava, and then they were euthanized by cutting the abdominal aorta. The extracted serum was used for serological analysis. The kidney, female reproductive organs, and bone marrow were fixed using 10% neutral buffered formalin for histopathological analyses.
Blood examination
Hematological analysis was performed to determine the number of white blood cells (WBCs) by using a KX-21NV instrument (Sysmex; Kobe, Japan). For the serological tests, the levels of blood urea nitrogen (BUN) and creatinine (Cr) were analyzed using a Fuji Dri-Chem 7000v analyzer (Fujifilm; Tokyo, Japan). The ratio of albumin to globulin (A/G) was measured using a commercial kit (A/G B-Test Wako; Wako; Osaka, Japan).
Histopathological analysis
Paraffin-embedded uterus and bone marrow from femoral bone sections were stained with hematoxylin and eosin (HE). Paraffin-embedded kidney sections were stained with periodic acid Schiff (PAS) for histopathological analysis. Immunohistochemistry for α-smooth muscle actin (αSMA), CD3, and calbindin-D28k was performed to detect myofibroblasts, pan T-cells, and distal tubules, respectively. Further, the localization of estrogen receptors α (ERα) and β (ERβ) was also examined. Details of the staining conditions and primary antibodies used in the study are listed in Table 1. In brief, the sections were deparaffinized, heated, and incubated with primary and secondary antibodies according to a previously published streptavidin-biotin method.5 The color was developed by incubating the sections in a 3,3′-diaminobenzidine tetrahydrochloride-H2O2 solution.
Table 1.
Primary antibodies used for immunohistochemistry.
| Antibody | Source | Dilution | Antigen retrieval |
|---|---|---|---|
| Rabbit anti-CD3 | SP7, Nichirei (Tokyo, Japan) | 1:200 | 20 mM TB (pH 9.0), 105°C, 20 min |
| Rabbit anti-αSMA | ab5694, Abcam (Cambridge, UK) | 1:1000 | 10 mM CB (pH 6.0), 105°C, 20 min |
| Rabbit anti-calbindin-D28k | E10342, Spring Bioscience (Pleasanton, CA, USA) | 1:500 | 10 mM CB (pH 6.0), 105°C, 20 min |
| Rabbit anti-ERα | 1D5, Nichirei (Tokyo, Japan) | Prediluted | VT, 90°C, 30 min |
| Rabbit anti-ERβ | PA1-311, Thermo Fisher Scientific (Waltham, MA, USA) | 1:10000 | VT, 90°C, 30 min |
αSMA: α-smooth muscle actin. ER: estrogen receptor, CB: citrate buffer, TB: Tris-HCl buffer, VT: Histo VTone (Nacalai tesque, Kyoto, Japan).
Histological and histometric examinations were conducted in a blinded manner. Digital images of the tubulointerstitium were prepared using the BZ-X710 inverted microscope (Keyence, Osaka, Japan), and histometric analysis of images was performed using BZ-H3A application software and hybrid Cell Count software (BZ-H3C, Keyence). From PAS-stained sections, the relative area of the tubular lumen in the outer medulla (%) was analyzed. For immunostained sections, the αSMA+ area (%) and CD3+ cell infiltration (number/µm2) were also assessed. The outer diameter of the vaginal parts of the cervix was measured by the digital caliper.
Statistical analyses
The results are expressed as means ± standard errors or Box-and-whisker plots. The Mann–Whitney U test was used to compare data between two groups (P < 0.05). The Kruskal–Wallis test was used to compare data between three groups. Multiple comparisons were performed using Dunnett's test to compare multiple parameters with the Cont group (P < 0.05). For the comparison of groups with pyometra, those without pyometra, and the OVX groups, Scheffé's method was used (P < 0.05). Spearman’s correlation test (P < 0.05) was used to analyze the correlation between two values.
Results
Reproductive organ-associated abnormalities in female cotton rats
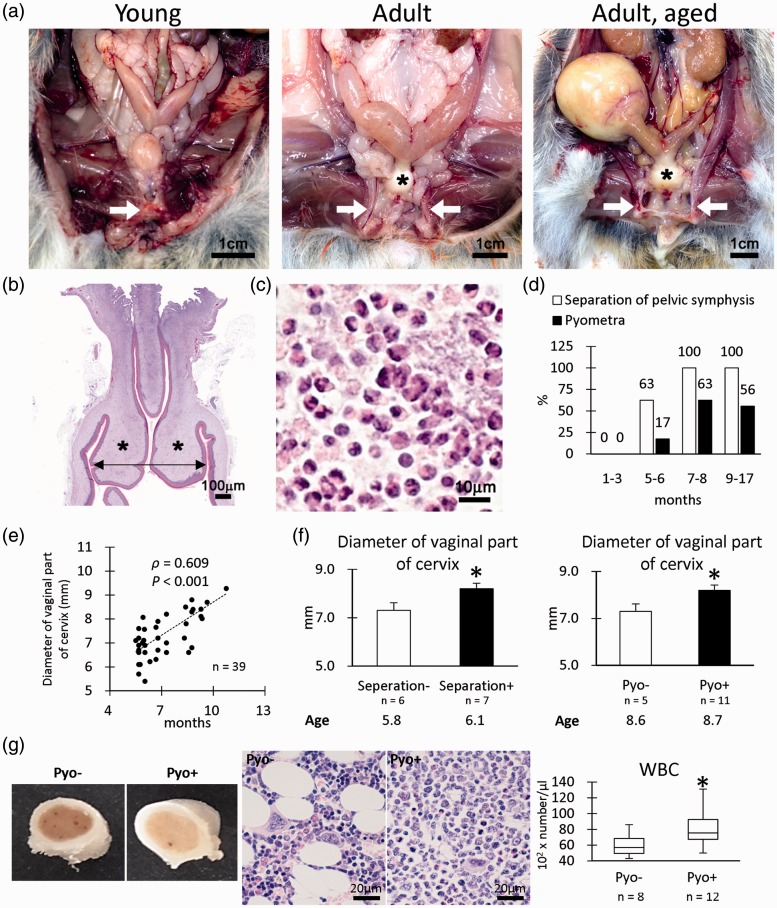
The gross anatomical analysis revealed that some adult female cotton rats developed a separation of the pelvic symphysis and pyometra (Figure 1(a)). In these female cotton rats, the vaginal parts of the cervix showed hypertrophy (Figure 1(a) and (b)), and numerous neutrophils were observed in the lumen of the uterus (Figure 1(c)). The separation of the pelvic symphysis was 63% at 5–6 months of age and reached 100% at the age of 7–8 months or higher (Figure 1(d)), while the incidence of pyometra was 17, 63, and 56% at 5–6, 7–8, and 9–17 months of age, respectively (Figure 1(d)). Furthermore, the diameters of the vaginal parts of the cervix showed a significant positive correlation with age (Figure 1(e)). The age-matched individuals having pelvic separations or pyometra showed increased diameters of the vaginal parts of the cervix (Figure 1(f)). To evaluate the effect of pyometra on immunological status, bone marrow and circulating WBC morphologies were examined. The bone marrow of cotton rats with pyometra showed more white color compared with the bone marrow of cotton rats without pyometra (Figure 1(g)). Histologically, the bone marrow cells as well as neutrophils were increased in the pyometra group compared with the group without pyometra. Blood WBC number also significantly increased in the pyometra group, indicating a systemic inflammatory condition.
Figure 1.
Pathological features of female reproduction system. (a) Gross anatomical features of female cotton rats. Pelvic symphysis is clearly observed in the young group (arrow), but they are separated in the adult group (arrows). Hypertrophy of the vaginal parts of the cervix is also remarkable in the adult group (asterisks). In the adult group, pyometra is evident in some cotton rats. (b) Histology of the vaginal parts of the cervix. Hematoxylin and eosin (HE) staining. Hypertrophy of the lamina propria is observed (asterisks). We measured outer diameters (arrow) of the vaginal parts of the cervix in graph e. (c) Inflammatory cells observed in the lumen of the uterus with pyometra. HE staining. Numerous neutrophils are noted. (d) Incidence of separation of pelvic symphysis and pyometra. (e) The correlation between diameter of the vaginal part of the cervix and age. Spearman’s correlation test (*, P < 0.05). (f) Diameter of the vaginal part of the cervix. Numerical results are expressed as means ± standard errors. Mann–Whitney U test (*, P < 0.05). (g) Gross and histopathology of bone marrow. The bone marrow samples of females with pyometra show more white blood cells, especially neutrophils, than those of females without pyometra. White blood cell (WBC). Box-and-whisker plot. Mann–Whitney U test (*, P < 0.05). (A color version of this figure is available in the online journal.)
Table 2 shows the incidence of the separation of the pelvic symphysis with or without pyometra. Notably, some female cotton rats exhibiting a separation of the pelvic symphysis did not develop pyometra (10/22, 45%), but all female cotton rats exhibiting pyometra showed pelvic separation (12/12, 100%). These data suggested that the separation of the pelvic symphysis occurred earlier than pyometra. Table 2 also shows the incidence of the separation of the pelvic symphysis with or without OVX treatment. Notably, no OVX-treated female cotton rats exhibited a separation of the pelvic symphysis (0/4, 0%). These data indicate that OVX prevents the separation of the pelvic symphysis.
Table 2.
Appearance of separation of pelvic symphysis affected by pyometra and ovariectomy.
| Group | Age (months) |
Separation of pelvic symphysis |
Total number | |
|---|---|---|---|---|
| - | + | |||
| Pyometra - | 10.5 | 6 | 10 | 16 |
| Pyometra + | 10.7 | 0 | 12 | 12 |
| Intact | 6.3 | 6 | 14 | 20 |
| OVX | 6.4 | 4 | 0 | 4 |
Note: Ovariectomy (OVX) was performed in the young group, and they were examined during their adult period (4 months after OVX).
Effects of pyometra and OVX on systemic immunological abnormalities and renal dysfunction in female cotton rats
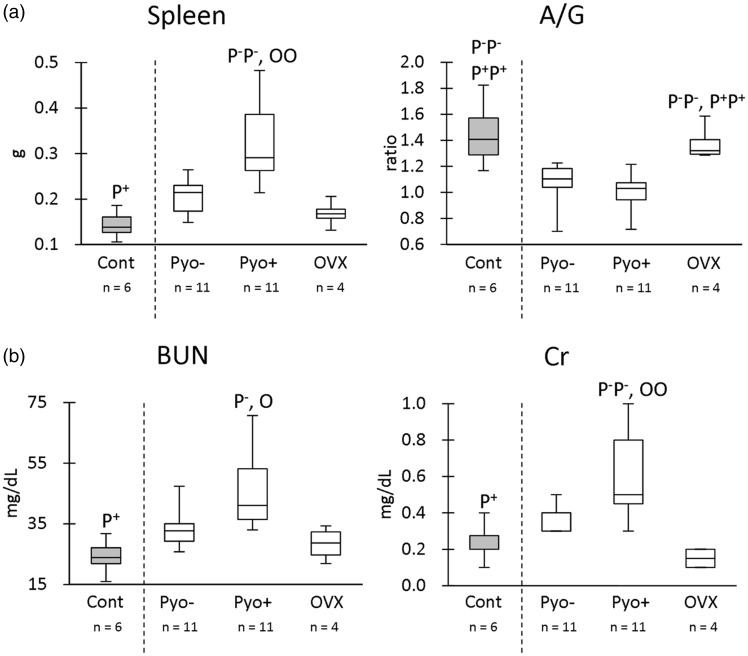
As shown in Figure 2, we evaluated indices for systemic immunological status (spleen weights, A/G ratio) and renal function (BUN, serum Cr). The young group (mean, 1.9 months) was defined as the Cont group to be compared with other groups because they had normal values for these indices.5 Further, adult groups were divided into groups without pyometra (Pyo-, mean, 9.8 months), with pyometra (Pyo+, mean, 11.5 months), and OVX (mean, 12.2 months). There was no significant difference in the ages examined among adults without pyometra, adults with pyometra, or the OVX groups by Kruskal–Wallis test (P > 0.05).
Figure 2.
Indices for immunological and renal function. (a) Spleen weight and serum albumin/globulin (A/G) ratio. (b) Blood urea nitrogen (BUN) and serum creatinine (Cr). Box-and-whisker plot. The Kruskal–Wallis test was used to compare data among groups. Multiple comparisons were performed using Dunnett's test to compare the parameters with the healthy control (Cont) group. For the comparison of groups with pyometra (Pyo+), those without pyometra (Pyo−), and ovariectomy (OVX), Scheffé's method was used. P−, P+, and O denote the significant difference with Pyo−, Pyo+, and OVX groups, respectively (P < 0.05). P−P−, P+P+, and OO denote the highly significant difference with Pyo−, Pyo+, and OVX groups, respectively (P < 0.01).
With regard to spleen weights, the Pyo+ group showed significantly higher values than the Cont group (Figure 2(a)). In multiple comparisons, the Pyo+ group showed significantly higher values than Pyo- and OVX groups. For the A/G ratio, both Pyo- and Pyo+ groups showed significantly lower values than the Cont group (Figure 2(a)). In multiple comparisons, both Pyo- and Pyo+ groups showed significantly lower A/G ratio than the OVX group. With regard to the renal function indices (Figure 2(b)), the Pyo+ group showed significantly higher BUN and Cr values than the Cont group (Figure 2(a)). In multiple comparisons, the Pyo+ group showed significantly higher BUN and Cr values than Pyo- and OVX groups. These data indicated that pyometra was associated with a progression of renal dysfunction and an altered systemic immunological status, which was ameliorated by OVX.
Effects of pyometra and OVX on renal histopathology in female cotton rats
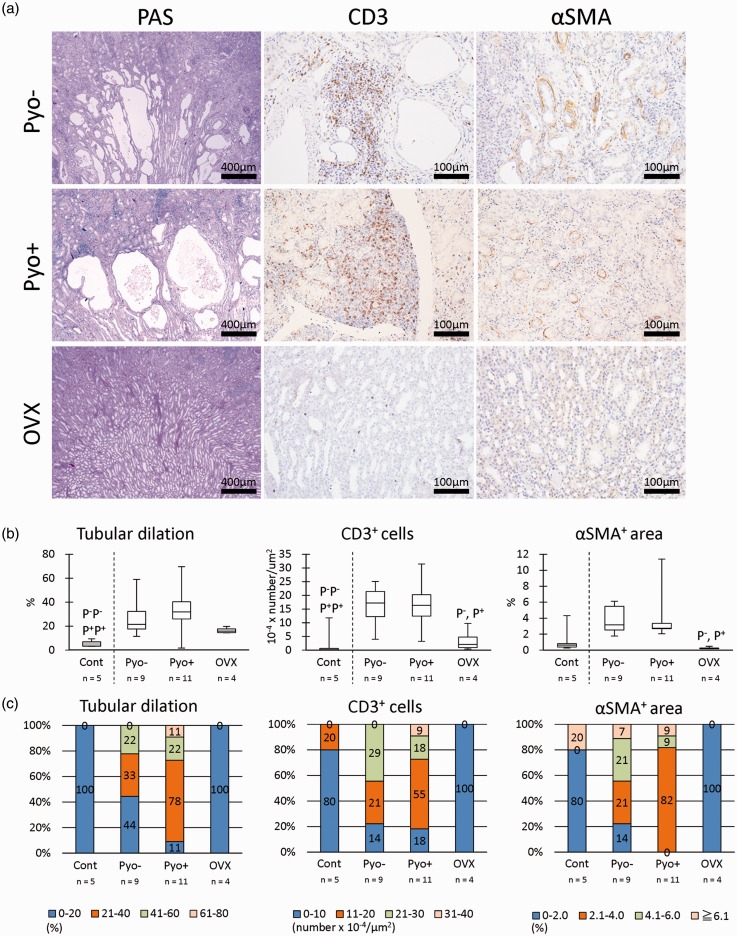
CKD of cotton rats is characterized by the dilation of distal tubules, infiltration of CD3+ T cells, and αSMA+ myofibroblasts.5 In our histopathological analysis, these CKD features were ameliorated in the OVX groups compared with other adult groups, and the Pyo+ group tended to show a more severe histopathology than the Pyo− group (Figure 3(a)).
Figure 3.
Renal histopathology. (a) Histochemistry analysis by using periodic acid Schiff (PAS) and immunohistochemistry of CD3 and α-smooth muscle actin (SMA). Regardless of the presence or absence of pyometra (Pyo−, Pyo+) and dilated distal tubules, numerous T-cells and myofibroblasts are observed. However, these lesions were mild in ovariectomy (OVX) groups. (b) Histoplanimetry of the tubular lumen, CD3+ cell number, and αSMA+ area. Box-and-whisker plot. The Kruskal–Wallis test was used to compare data among groups. Multiple comparisons were performed by using Dunnett's test to compare the parameters with the healthy control (Cont) group. For the comparison of Pyo+, Pyo−, and OVX groups, Scheffé's method was used. P−, P+, and O denote the significant differences in Pyo−, Pyo+, and OVX groups, respectively (P < 0.05). P−P−, P+P+, and OO denote the highly significant differences in Pyo−, Pyo+, and OVX groups, respectively (P < 0.01). (c) Population analysis of histoplanimetry of the tubular lumen, CD3+ cell number, and αSMA+ area. (A color version of this figure is available in the online journal.)
In terms of the tubular dilation index (Figure 3(b)), the Pyo− and Pyo+ groups showed significantly higher values than the Cont group, especially in the latter the difference was highly significant (P < 0.01). In multiple comparisons, the OVX groups tended to show lower values than Pyo− and Pyo+ groups, but this difference was not significant. In the cell infiltration index, Pyo− and Pyo+ groups showed significantly higher values than the Cont group, and in multiple comparisons, OVX showed significantly lower values than Pyo− and Pyo+ groups. With regard to the renal fibrosis index, the Pyo− and Pyo+ groups tended to show higher values than the Cont group, but the difference was not statistically significant, while in multiple comparison, the OVX groups showed significantly lower values than the Pyo− and Pyo+ groups.
The population showing a more severe histology was remarkably different among groups (Figure 3(b)). In particular, more cotton rats in the Pyo+ group showed more severe histopathological scores than those in the other groups in terms of the dilation of distal tubules, infiltration of CD3+ T cells, and αSMA+ myofibroblasts. Notably, the OVX groups had lower scores for these three parameters than the other groups. These histopathological data indicated that OVX clearly inhibited the progression of CKD, and pyometra was closely associated with the increase of the population showing severe CKD rather than each histopathological severity of CKD.
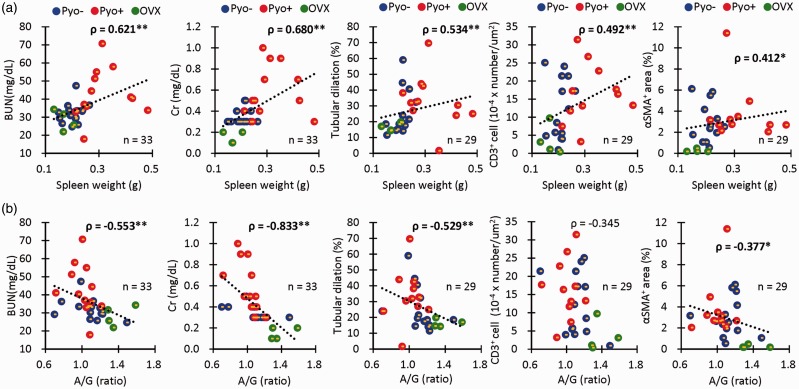
Correlation between systemic immunological abnormalities and CKD in female cotton rats
Figure 4 shows the statistical correlations between systemic immunological abnormalities (spleen weight, A/G ratio), and renal pathology (BUN, serum Cr, histopathological indices) between the Pyo− Pyo+, and OVX groups. Spleen weights showed a significant positive correlation with BUN, serum Cr, dilation of distal tubules, and infiltration of CD3+ T cells and αSMA+ myofibroblasts (Figure 4(a)). The A/G ratio showed a significant negative correlation with BUN, serum Cr, dilation of distal tubules, and the infiltration of αSMA+ myofibroblasts (Figure 4(b)). These data clearly demonstrated the close correlations between systemic immunological abnormalities and CKD in female cotton rats.
Figure 4.
Correlation between immunological abnormalities and CKD. (a) Correlations between spleen weights and indices for renal pathology. (b) Correlations between the serum albumin/globulin ratio and indices for renal pathology. Spearman’s correlation test (*, P < 0.05; **, P < 0.01). (A color version of this figure is available in the online journal.)
Localization of ERs in the kidney of female cotton rats
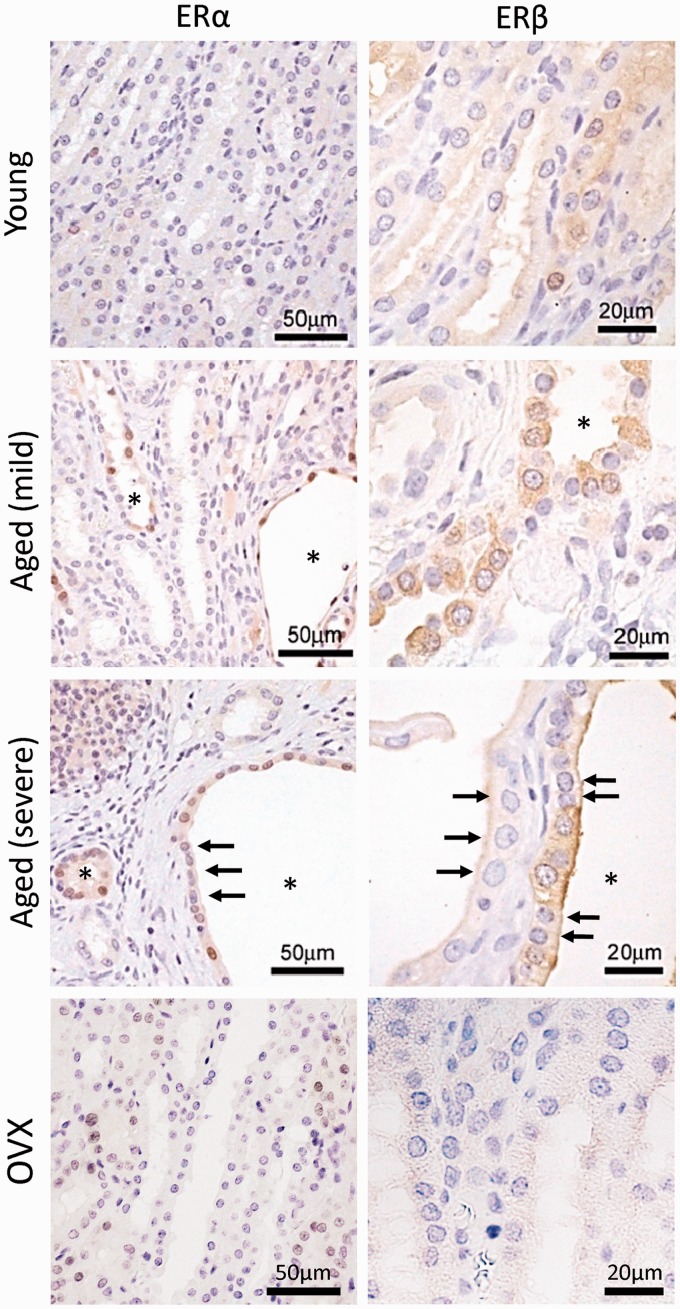
We examined the expression of ERs in the kidneys of cotton rats (Figure 5). In the young group, faint positive reactions for both ERα and ERβ were detected in the kidneys. However, the positive reaction intensity increased with age. In particular, dilated or cystic renal tubules showed strong positive reactions, which were confirmed to be distal tubules in our previous study.5 The intracellular localizations of ERα and ERβ were the nucleus and cytoplasm, respectively. For ERβ, the positive reaction in the apical part of renal epithelial cells was stronger in the older groups, and epithelial cells negative for both ERα and ERβ were also detected in the dilated tubules (Figure 5, arrows). Notably, these positive reactions were weaker in the OVX group.
Figure 5.
Localization of estrogen receptors. Estrogen receptors α (ERα) and ERβ are examined through immunohistochemistry. The young group showed low intensity for both receptors. The dilated renal tubules (asterisks) show strong positive reactions, and ERα and ERβ localize to the nucleus and cytoplasm of epithelial cells, respectively. Dilated renal tubules also contain the negative cells (arrows). These reactions are faint in ovariectomy (OVX) groups. (A color version of this figure is available in the online journal.)
Discussion
We previously found that cotton rats, especially females, developed CKD characterized by severe tubulointerstitial lesions including dilation of the distal tubules, immune cell infiltrations, and increased myofibroblasts.5 Although this previous study did not focus on the female-specific characteristics of other organs, we clarified that some female cotton rats developed pyometra with age in the present study. CKD tended to progress with age (as shown in Figure 2(b) and Figure 3) regardless of the presence or absence of pyometra. Therefore, these data suggest that the appearance of pyometra was not a mandatory requirement for the initiation of CKD in cotton rats.
In general, compared with women, men show a higher prevalence of primary diseases of CKD, such as glomerulonephritis and membranous nephropathy, and a more rapid progression of membranous nephropathy, IgA nephropathy, and polycystic kidney disease.10 In contrast, female-dominant progression was observed in some types of CKD, in particular CKD with systemic disorders, such as lupus nephritis, were more frequently observed in females than in males of human and mouse models due to the effects of sex hormones.11,12 The lupus nephritis-like glomerular lesions were not observed in the cotton rats,5 while the systemic immunological parameters, especially the A/G ratio, were altered in female cotton rats even though they did not show any pyometra. Therefore, sex hormone- and/or sex chromosome-associated systemic or intrarenal immunological changes were also associated with CKD development in female cotton rats.
From the present study, the separation of pelvic symphysis was identified first, and then hypertrophy of the vaginal parts of the cervix and pyometra consequently developed in female cotton rats. Pelvic symphysis fibrocartilage in mice expressed both the ERs,17 and estrogens have been reported to influence the collagen remodeling via relaxin in fibrocartilage tissue.18 Therefore, the separation of pelvic symphysis in cotton rats would be affected by estrogen. Pyometra was often observed in companion dogs due to the abnormalities of the uterus such as endometrial hypertrophy and bacterial infection, and estrogen treatment has been shown to increased its risk.19 In the preliminary study, we could not find any significant correlation between the separation of pelvic symphysis or pyometra and parturition (n = 28–37, Chi-square test). In cotton rats, the altered bacterial or immunological environment due to the closed the vaginal parts of the cervix would contribute to the development of septic pyometra. Notably, examination of renal biopsy specimens of dogs with pyometra revealed a high prevalence of mild tubulointerstitial nephritis with renal dysfunction, but few specific glomerular lesions.14 Indeed, pyometra groups deteriorated the indices of immunological status as well as renal dysfunction in the cotton rats. Therefore, we consider that the presence of pyometra is an exacerbating factor in the CKD of female cotton rats. Thus, the correlations among sex hormone, pelvic symphysis separation, and pyometra were strong but might be further investigated by examining the effect of surgical separation of the pelvic symphysis in younger cotton rats on the development of pyometra, with and without OVX. This would help to determine whether the pyometra requires endocrine abnormalities or is simply an effect of widening the pelvic cavity.
OVX inhibited the development of CKD, systemic immune abnormalities, and the separation of pelvic symphysis in female cotton rats. These data from the OVX groups indicated that these pathological events were strongly regulated by sex hormones. The absence of pyometra in the OVX groups would contribute to the amelioration of the systemic conditions, and these immunological changes may also indirectly affect the alleviation of CKD in cotton rats. Although it was unclear whether the inhibitory effects of sex hormones on tubulointerstitial lesions were direct or indirect, the dilation of the distal tubules seemed to be directly regulated by estrogen because of the expression of ERs in epithelial cells with the development of cystic lesions. In human cystic kidney disease, the epithelial cells of hepatic cysts express ER, and estrogen has been reported to accelerate the progression of cysts20; thus, increased estrogen levels could be responsible for ER-mediated proliferation of tubular epithelial cells.21 In rats, ERα and ERβ are more predominant in the kidney of females and males, respectively.22 Furthermore, ER expression was found to be relatively low in distal tubular segments in mice,23 but estrogen has been reported to alter the function of ion pump/channels.24,25 Interestingly, female cotton rats of young and OVX groups showed a low expression of ERs in the kidneys, but older female rats had higher ER expression in dilated distal tubules. Therefore, age- or CKD event-induced ER expression would be an important mechanism mediating the progression of tubular cystic lesions via morpho-functional alternations of distal tubular epithelial cells such as proliferation or ion transporting.
Sex hormones have complex effects in kidney disease. In general, estrogen has renoprotective effects. For example, ovariectomized glomerulosclerosis-prone ROP Os/+ mice developed severer glomerular lesions and renal dysfunction than age-matched female controls.26 Estrogen therapy after OVX has also been reported in the kidney of post-menopausal rats.27 Other adverse effects of estrogen on the kidney have also been reported in addition to lupus nephritis. In hyperlipidemic analbuminemic rats, females are more prone to develop renal injury than males, while OVX tends to decrease triglyceride levels and prevent renal disease.28 Furthermore, oral but not transvaginal estrogen therapy in postmenopausal women is associated with a loss of kidney function.29 While treatment of streptozotocin-induced diabetic rats with estrogen decreased tubulointerstitial fibrosis,30 the diabetic Cohen rats with OVX showed significantly decreased incidence of nephropathy, and estrogen treatment of the ovariectomized animal increased the rate of nephropathy.31 Notably, in Cohen rats, no difference was found between the ovariectomized and intact diabetic females with regard to blood glucose, insulin, or cholesterol levels, but a significant difference was found in their estrogen levels,31 suggesting a direct effect of estrogen on the kidneys. The effects of estrogen on the renin-angiotensin system, a crucial cascade for the development of kidney diseases, are also well-known, in particular the functions of renin, angiotensin-converting enzyme, angiotensin, and its receptor were affected.32 These reports indicate the estrogen effects on kidney disease differ according to the pathological features of kidney diseases due to systemic conditions and/or genetic backgrounds of animals or humans. In these events, the induction of intrarenal ERs, as found in the distal tubules of cotton rats, would be also important. Intermediate mesoderm develops into the urogenital system including kidneys and gonads. Therefore, kidney composing cells might naturally have the ability to alter the expression of sex-hormone receptor as gonadal cells, according to physiological and/or pathological status.
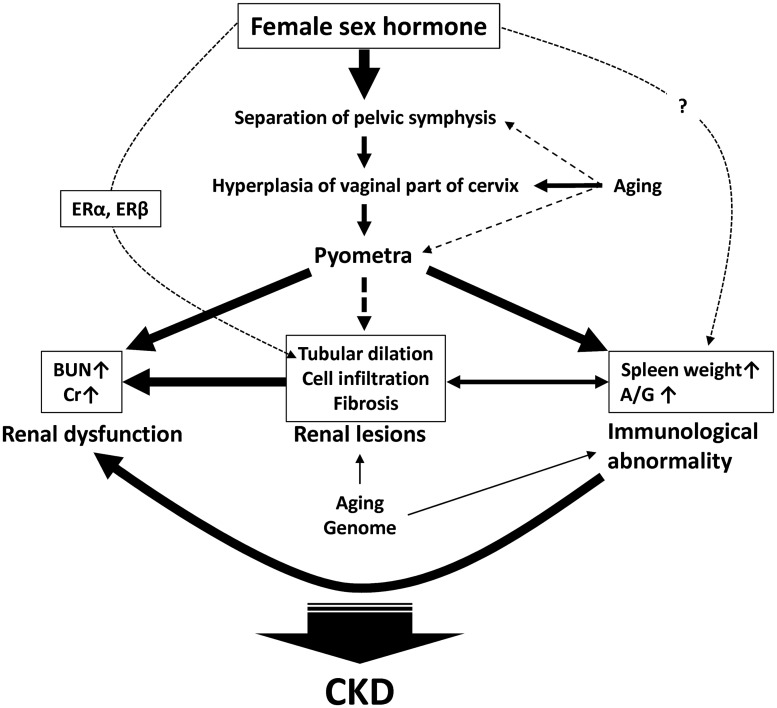
We have summarized the pathology of female cotton rats based on the findings of the present study (Figure 6). Firstly, female sex hormones and aging strongly affect the abnormalities of the female reproductive system and finally contribute to pyometra development. The development of pyometra directly correlated with the elevation of BUN, serum Cr, spleen weight, and the A/G ratio, and may consequently have a direct effect on renal lesions. Furthermore, aging and genome are important factors for the developments of renal lesions and immunological abnormalities, and estrogen via inducible ERs in distal tubules also participates in the tubular dilation process. Thus, the close correlations among female reproductive organ-associated abnormalities, immunological abnormalities, and renal dysfunction are characteristics of CKD pathogenesis in female cotton rats. However, future studies evaluating male sex hormones, which have an adverse effect on kidney diseases10 are also needed to better clarify the CKD pathogenesis in cotton rats.
Figure 6.
Scheme of close pathological correlations between chronic kidney disease and reproductive organ-associated abnormalities in female cotton rats. ER: estrogen receptor. BUN: blood urea nitrogen. Cr: serum creatinine. CKD: chronic kidney disease. A/G: serum albumin/globulin ratio. The widths of arrows reflect the decree of contribution in each process.
Thus, cotton rat is a unique and useful experimental rodent model to elucidate the pathological crosstalk between CKD and sex-related factors. On the other hand, the basic information of cotton rats such as sex-related characteristics is still scarce from juvenile to aged periods. In future, it would be worth to clarify the effects of sex hormone on anatomical and physiological differences of reproductive organs between male and female by using this animal model.
Acknowledgments
The animal experiments were supported by Saori Nakamura and Shinobu Sato in Sankyo Labo Service Corporation, Inc., Sapporo, Hokkaido, Japan.
Authors’ contributions
OI, TN, TI, HK, and YK conceived and designed the experiments. OI and TN performed the experiments. DN, SN, SS, and KY maintained the animals. OI, TN, TI, TH, YS, and YHAE analyzed the data. OI, TN, and YK wrote the manuscript.
Declaration of Conflicting Interests
This manuscript has not been published or presented elsewhere in part or in entirety and is not under consideration by another journal. All authors have approved the manuscript and agree with submission to your esteemed journal. The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This work was partially supported by JSPS KAKENHI (grant nos.: 15H05634 and 15K14873).
References
- 1.Azazy AA, Chance ML, Devaney E. A time-course study of circulating antigen and parasite-specific antibody in cotton rats infected with Leishmania donovani. Ann Trop Med Parasitol 1997; 91:153–62 [DOI] [PubMed] [Google Scholar]
- 2.Blanco JC, Boukhvalova MS, Perez DR, Vogel SN, Kajon A. Modeling human respiratory viral infections in the cotton rat (Sigmodon hispidus). J Antivir Antiretrovir 2014; 6:40–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kroeze WK, Tanner CE. Echinococcus multilocularis: responses to infection in cotton rats (Sigmodon hispidus). Int J Parasitol 1985; 15:233–8 [DOI] [PubMed] [Google Scholar]
- 4.Faith RE, Montgomery CA, Durfee WJ, Aguilar-Cordova E, Wyde PR. The cotton rat in biomedical research. Lab Anim Sci 1997; 47:337–45 [PubMed] [Google Scholar]
- 5.Ichii O, Nakamura T, Irie T, Kouguchi H, Nakamura D, Nakamura S, Sato S, Yokoyama K, Horino T, Sunden Y, Elewa YH, Kon Y. Female cotton rats (Sigmodon hispidus) develop chronic anemia with renal inflammation and cystic changes. Histochem Cell Biol 2016; 146:351–62 [DOI] [PubMed] [Google Scholar]
- 6.Nakamura T, Ichii O, Irie T, Mizoguchi T, Shinohara A, Kouguchi H, Sunden Y, Otsuka-Kanazawa S, Elewa YHA, Koshimoto C, Nagasaki K, Kon Y. Cotton rats (Sigmodon hispidus) possess pharyngeal pouch remnants originating from different primordia. Histol Histopathol. Epub ahead of print 21 November 2017. doi: 10.14670/HH-11-946. [DOI] [PubMed] [Google Scholar]
- 7.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298:2038–47 [DOI] [PubMed] [Google Scholar]
- 8.Bartges JW. Chronic kidney disease in dogs and cats. Vet Clin North Am Small Anim Pract 2012; 42:669–92 [DOI] [PubMed] [Google Scholar]
- 9.Levey AS, Coresh J. Chronic kidney disease. Lancet 2012; 379:165–80 [DOI] [PubMed] [Google Scholar]
- 10.Silbiger SR, Neugarten J. The impact of gender on the progression of chronic renal disease. Am J Kidney Dis 1995; 25:515–33 [DOI] [PubMed] [Google Scholar]
- 11.Inman RD. Immunologic sex differences and the female predominance in systemic lupus erythematosus. Arthritis Rheum 1978; 21:849–52 [DOI] [PubMed] [Google Scholar]
- 12.Ichii O, Konno A, Sasaki N, Endoh D, Hashimoto Y, Kon Y. Onset of autoimmune glomerulonephritis derived from the telomeric region of MRL-chromosome 1 is associated with the male sex hormone in mice. Lupus 2009; 18:491–500 [DOI] [PubMed] [Google Scholar]
- 13.Ichii O, Konno A, Sasaki N, Endoh D, Hashimoto Y, Kon Y. Autoimmune glomerulonephritis induced in congenic mouse strain carrying telomeric region of chromosome 1 derived from MRL/MpJ. Histol Histopathol 2008; 23:411–22 [DOI] [PubMed] [Google Scholar]
- 14.Stone EA, Littman MP, Robertson JL, Bovée KC. Renal dysfunction in dogs with pyometra. J Am Vet Med Assoc 1988; 193:457–64 [PubMed] [Google Scholar]
- 15.Maric C, Sandberg K, Hinojosa-Laborde C. Glomerulosclerosis and tubulointerstitial fibrosis are attenuated with 17 beta-estradiol in the aging Dahl salt sensitive rat. J Am Soc Nephrol 2004; 15:1546–56 [DOI] [PubMed] [Google Scholar]
- 16.Nakamura T, Ichii O, Irie T, Hosotani M, Dantsuka A, Nakamura S, Sato S, Satozaki K, Koguchi H, Yoshiyasu T, Nagasaki K, Kon Y. Usefulness of an anesthetic mixture of medetomidine, midazolam, and butorphanol in cotton rats (Sigmodon hispidus). Jpn J Vet Res 2016; 64:273–6 [PubMed] [Google Scholar]
- 17.Wang W, Hayami T, Kapila S. Female hormone receptors are differentially expressed in mouse fibrocartilages. Osteoarthr Cartil 2009; 17:646–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samuel CS, Butkus A, Coghlan JP, Bateman JF. The effect of relaxin on collagen metabolism in the nonpregnant rat pubic symphysis: the influence of estrogen and progesterone in regulating relaxin activity. Endocrinology 1996; 137:3884–90 [DOI] [PubMed] [Google Scholar]
- 19.Richard W, Couto CG. The disease of vagina and uterus. In: Hasegawa A and Tsujimoto H (eds) Small animal internal medicine. 3rd ed. (in Japanese). Tokyo: Interzoo, 2005, pp.913–917.
- 20.Alvaro D, Onori P, Alpini G, Franchitto A, Jefferson DM, Torrice A, Cardinale V, Stefanelli F, Mancino MG, Strazzabosco M, Angelico M, Attili A, Gaudio E. Morphological and functional features of hepatic cyst epithelium in autosomal dominant polycystic kidney disease. Am J Pathol 2008; 172:321–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Concolino G, Lubrano C, Ombres M, Santonati A, Flammia GP, Di Silverio F. Acquired cystic kidney disease: the hormonal hypothesis. Urology 1993; 41:170–5 [DOI] [PubMed] [Google Scholar]
- 22.Sabolić I, Asif AR, Budach WE, Wanke C, Bahn A, Burckhardt G. Gender differences in kidney function. Pflugers Arch 2007; 455:397–429 [DOI] [PubMed] [Google Scholar]
- 23.Grimont A, Bloch-Faure M, Abida B, El, Crambert G. Mapping of sex hormone receptors and their modulators along the nephron of male and female mice. FEBS Lett 2009; 583:1644–8 [DOI] [PubMed] [Google Scholar]
- 24.Dick IM, Liu J, Glendenning P, Prince RL. Estrogen and androgen regulation of plasma membrane calcium pump activity in immortalized distal tubule kidney cells. Mol Cell Endocrinol 2003; 212:11–8 [DOI] [PubMed] [Google Scholar]
- 25.Verlander JW, Tran TM, Zhang L, Kaplan MR, Hebert SC. Estradiol enhances thiazide-sensitive NaCl cotransporter density in the apical plasma membrane of the distal convoluted tubule in ovariectomized rats. J Clin Invest 1998; 101:1661–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elliot SJ, Karl M, Berho M, Potier M, Zheng F, Leclercq B, Striker GE, Striker LJ. Estrogen deficiency accelerates progression of glomerulosclerosis in susceptible mice. Am J Pathol 2003; 162:1441–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mercantepe T, Unal D, Selli J, Mercantepe F, Unal B, Karabiyik TN. Protective effects of estrogen and bortezomib in kidney tissue of post-menopausal rats: an ultrastructural study. Ren Fail 2016; 38:1129–35 [DOI] [PubMed] [Google Scholar]
- 28.Joles JA, van Goor H, Koomans HA. Estrogen induces glomerulosclerosis in analbuminemic rats. Kidney Int 1998; 53:862–8 [DOI] [PubMed] [Google Scholar]
- 29.Ahmed SB, Culleton BF, Tonelli M, Klarenbach SW, Macrae JM, Zhang J, Hemmelgarn BR. Oral estrogen therapy in postmenopausal women is associated with loss of kidney function. Kidney Int 2008; 74:370–6 [DOI] [PubMed] [Google Scholar]
- 30.Mankhey RW, Wells CC, Bhatti F, Maric C. 17 beta-estradiol supplementation reduces tubulointerstitial fibrosis by increasing MMP activity in the diabetic kidney. Am J Physiol Regul Integr Comp Physiol 2007; 292:R7697. [DOI] [PubMed] [Google Scholar]
- 31.Rosenmann E, Yanko L, Cohen A. Female sex hormone and nephropathy in Cohen diabetic rat (genetically selected sucrose-Fed). Horm Metab Res 1984; 16:11–6 [DOI] [PubMed] [Google Scholar]
- 32.Paul M, Poyan Mehr A, Kreutz R, Taugner R. Physiology of local renin-angiotensin systems. Physiol Rev 2006; 86:747–803 [DOI] [PubMed] [Google Scholar]