Short abstract
Low linoleic acid concentration is a common finding in patients with cystic fibrosis and associated with severe clinical phenotype. Low docosahexaenoic and arachidonic acids are more inconsistently found in patients, but arachidonic/docosahexaenoic ratio is usually high. In animal models with cftr mutations or KO animals for the cftr gene, linoleic acid deficiency has not been consistently reported and some report docosahexaenoic deficiency as the major fatty acid abnormality. We hereby describe fatty acid profile in a severe clinical cystic fibrosis phenotype in mice with a duplication of exon 3 generated in the cystic fibrosis gene of C57B1/6J mice (cftrm1Bay allele). In 43/50 animals, plasma phospholipid fatty acids were repeatedly analyzed (mean three times/animal) covering ages between 7 and 235 days. Linoleic acid concentrations were significantly lower in cftr−/− mice compared to heterozygotes (P = 0.03) and wild type mice (P < 0.001). Females had significantly lower linoleic acid than males, not related to age. Arachidonic acid did not differ but docosahexaenoic acid was higher in cftr−/− than in wild type mice (P < 0.001). The arachidonic/docosahexaenoic acid ratio did not differ but arachidonic/linoleic acid ratio was higher in cftr−/− mice compared to wild type mice (P = 0.007). Similar to clinical studies, type of mutation is important for lipid abnormality with low linoleic acid most consistently found in the animals. Rodents differ in metabolism by synthesizing docosahexaenoic acid more efficiently comparing to humans, suggesting greater influence by diet. Precaution seems important when comparing animal and humans.
Impact statement
In translational research, animal models are important to investigate the effect of genetic mutations in specific diseases and their metabolism. Special attention has to be given to differences in physiology and metabolism between species and humans, which otherwise can hazard the conclusions. Our work illustrates that the different synthesis capacity in mice and humans for DHA would explain different results in different models for cystic fibrosis and different influences of diets. To avoid disappointing clinical results, these facts have to be considered before extensive clinical studies are started based on results from single animal studies.
Keywords: CFTR, transgenic mice, genetics, arachidonic acid, oleic acid, heterozygotes, homozygotes
Introduction
Cystic fibrosis (CF) is generally characterized by pancreatic insufficiency and pulmonary disease although careful examinations reveal abnormalities in many more tissues, also when not presenting with clinical symptoms.1–5 Low linoleic acid (LA, 18:2n-6) concentration has been described in CF since more than 50 years6 and slight abnormalities have also been described in heterozygotes and in patients with CF without pancreatic insufficiency.7,8 Mutations associated with more severe phenotype seem to be associated with more expressed LA deficiency.9,10
Before the gene was discovered, linking the disease to mutations related to a defective chloride channel, the cystic fibrosis transmembranous conductance regulator (CFTR), research in animals and patients suggested that much of the clinical symptomatology would be related to LA deficiency.11–15 This was further suggested to be associated with an increased arachidonic (AA, 20:4n-6) release, which by increasing the turnover was reflected in the low serum levels of LA16–20 and high eicosanoid metabolites.21 Low LA concentrations are associated with increase of Δ5- and Δ6 desaturase expressions22 supported by findings of inverse associations between LA and AA.23–25 Docosahexaenoic acid (DHA, 22:6n-3) was first reported low in tissues in CF at autopsy.26 In patients with CF plasma DHA has been reported low,8,10,27,28 or not different from controls,7,9,26,29 and the most common abnormality related to DHA was an increased ratio between AA and DHA.9,27,30 In patients with CF related liver disease, the concentrations of both LA and DHA have been reported low.31,32
In the 90s, new interest was directed to the lipid abnormality when a study of cftr−/− mice showed that lung, pancreas, and ileum had low DHA concentrations related to morphology and that these changes could be improved by high dose DHA supplementation.33 This turned all interest to this fatty acid resulting in many clinical studies supplementing DHA to patients with CF, but the results were mainly disappointing regarding any influence on the symptomatology.34 Later a long-term study of similar cftr−/− mouse strain could not repeat the beneficial effect of DHA supplementation, but showed a protection of liver abnormality.35
The development of murine models opened a possibility to study organ pathophysiology in CF, but the investigations have shown different results depending on models with null alleles or those with more or less CFTR function related to mutation strategy.36–39 Much of the differences might also be related to the different mouse strains and to species and illustrate that the basic metabolism may have an impact on the model. Some models are more suitable for studying special symptomatology36,39 and very seldom fatty acid concentrations are documented. In some mouse models, no fatty acid abnormality was reported40 or only a low AA/DHA ratio.35 In some studies, LA was low without a decrease or even with an increase of DHA.41 Analyses of different phospholipids in different tissues showed decrease in plasma and pancreas of molecular species containing AA and increase in pancreas for those containing DHA.42 However, some studies of cftr−/− mice and cell culture studies based on CFTR sense/antisense cells reported low DHA concentration as the major finding43(Table 1).
Table 1.
Results of fatty acid analysis in reported CF mouse strains or cells compared to the present study.
| Laboratory origin | Basic mouse strain | Mutation | Tissue | Fatty acid analysis | Reference |
|---|---|---|---|---|---|
| Erasmus Medical Center, Rotterdam | C57B1/6J/129 | ΔF508/ΔF508, cftr−/−tm1CAMExon 10 | Different tissues | No different conc.; no abn. transformation(13C-labels) | 40 |
| Animal Facility, Univ Louvain | 129/FVB | Homolog recombF508del cftrtm1EUR | Different tissues | LA low, AA high, DHA higha | 41 |
| Beth Israel Deaconess Med Center (Jackson Lab) | UNC(C57) | Exon 10 cftr−/−UNCInframe stop | Different tissues | AA high DHA lowLA not reported | 33 |
| Dr Pamela Davis, Cleveland–Am type culture coll (Manassas, VA) | Bronchial epithelial cells (16HBE) –IB3-1 and C38 cells | First 131 nucleotides of CFTR in sense antisenseF508/W1282X and C38 corrected with WT CFTR | Cell analyses | LA low DHA lowLA low DHA low | 43 |
| Jackson Laboratory., Bar Harbor, ME | C57B1/6J | Exon 10 cftrtm1UNCInframe stop | Plasma and red blood cell membranes | Ratio AA/DHA lowLA not reported | 35 |
| Edinburg/Hannover | ZTM:MF-1 | cftrtm1HGU/tm1HGU Exon 10 insertion | Different tissues | Plasma: PC 16:0/20:4, PC 18:0/20:4, 16:0/22:6 low Pancreas: PE, PS, PI 18:0/22:6high and 18:0/20:4 low | 42 |
| Baylor College of Medicine | C57B1/6J | Dupl exon 3 cftr−/−m1Bay | Plasma phospholipids | LA low, DHA high | Present |
Note: Only significant differences are reported.
aOnly in duodenum-jejunum.
LA: linoleic acid; AA: arachidonic acid; DHA: docosahexaenoic acid; PC: phosphatidylcholine; PE: phosphatidylethanolamine; PS: phosphatidylserine; PI: phosphatidylinositol.
Most transgenic mouse studies are performed by manipulations of exon 10, corresponding to the dF508 mutations in humans. However, several mutations related to severe clinical diagnosis of CF are associated with exon 3, like 394delTT, E60X, and R75Q.44 In patients with CF, a similar fatty acid profile is obtained in patients with mutations dF508 and 394delTT.9 The aim of this paper was to investigate if a similar fatty acid profile was obtained in a transgenic mice strain with duplication of exon 3 as previously described in those with mutations dF508.
Material and methods
Animals
Mice with clinically severe phenotype of CF, characterized by severe intestinal obstruction causing 40% death within one week of life, were analyzed for plasma phospholipid fatty acids. The mice had a duplication of exon 3 generated in the CF gene of C57B1/6J mice (cftrm1Bay allele) and are previously described.45 The CF mice showed a high mortality, and expressed no cftr mRNA expression (<1–2%). They had to be fed a liquid diet to survive due to severe intestinal obstructions. They were kept under constant conditions of humidity, temperature, and light. They were divided by sex and pair-fed in cages ad libitum (homozygote, heterozygote together with wild type (WT)) to equal as much as possible the feeding between the different genotypes and ages.
Fifty mice were included in the study; 15 wild type, 13 heterozygotes and 22 homozygotes for the mutation. Females constituted 7, 6, and 10 animals and males 8, 7, and 12, respectively. Plasma samples were obtained in the morning by tail bleeding, in three animals only once and in all others at a mean of three times per animal (range 2–6). The samples were obtained between 7 and 235 days of age, median serial time was 48, 39, and 38 days for WT, heterozygotes, and homozygotes, respectively.
The animals were treated according to ethical rules and the North American legislation and regulation of the use of live animals for scientific research.
Fatty acid analysis
Blood samples were collected from the tip of the tails in the animals in each cage at the same time. Plasma were kept frozen at −70°C and transported to the laboratory in carbon ice until analyses as previously described.46 Lipids were extracted from the plasma with chloroform–methanol 2:1 (v/v) containing 0.01% butylated hydroxytoluene. The lipids were fractionated on a single SEP-PAK aminopropyl cartridge (Waters Corp., Milford, MA, USA) and the fraction of phospholipids was transmethylated in methanolic-HCL-3N at 90°C over 4 h. The fatty acid methyl esters (FAME) were extracted with n-hexane and, thereafter, washed with water until neutral, dried with MgSO4, and then dried with nitrogen. The FAME were separated by capillary gas–liquid chromatography in a Hewlett-Packard, 6890 gas chromatograph equipped with a 30 m × 0.25-mm SP-2380 column, film thickness 20 μm. Helium at 2.0 ml/min was used as carrier gas, and a split less injection was used. The injector and detector temperatures were 300°C and 250°C, respectively. The column oven temperature was programmed from 50°C to 230°C at a heating rate of 20°C/min up to 180°C and, thereafter, 2°C/min. The separation was recorded with HP GC Chem Station software (HP GC, Wilmington, DE). C21:1 was used as internal standard and the FAME identified by comparison with retention times of pure reference substances (Sigma Aldrich Sweden AB, Stockholm, Sweden).
Statistical analysis
We used a three-level mixed model to account for the fact that mice were pair-fed within cages and also the fact that several measurements were taken on the same mouse. Restricted maximum likelihood was used to calculate parameter estimates and standard errors.47 Our outcome variables were LA, AA, DHA, and oleic acid (18:1n-9, OA) and the ratios AA/DHA, AA/LA, and OA/LA.
We created bar charts illustrating the predicted values for the different fatty acids in the mice at the median age at measurement, 118 days (Supplementary Tables 1 and 2). To model the cftr gene, we used three categories, one for WT, one for the recessive homozygotes, and one for the heterozygotes. WT was used as the reference category. Adjustments were made for sex and age (in loge days), and we also allowed for an interaction between sex and age. The significance of sex and age in the models was evaluated by testing the coefficient of both main term and interaction term simultaneously (Figure 1). The level of significance was chosen as alpha = 0.05.
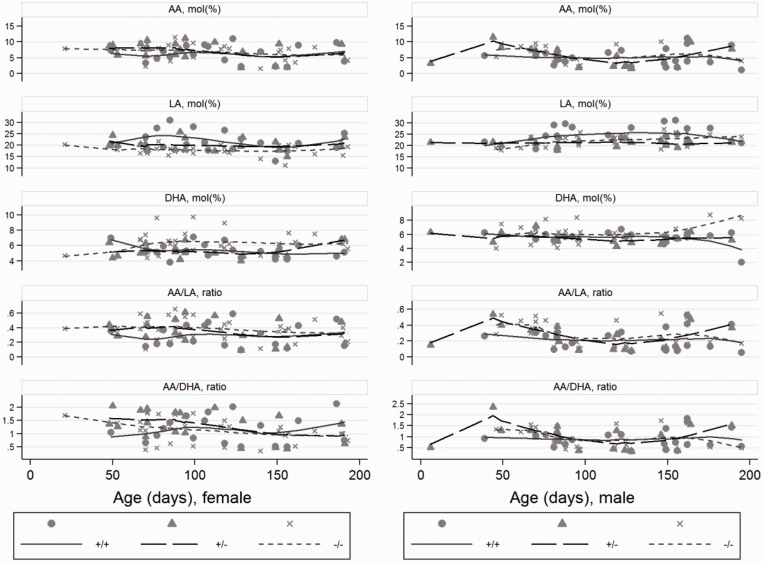
Figure 1.
The longitudinal trend of serum phospholipid concentrations based on individual mice and separated by alleles and sex for wild type (+/+), heterozygotes (+/−) and homozygotes (−/−) for the cftrm1Bay allele. Arachidonic acid (AA); docosahexaenoic acid (DHA); linoleic acid (LA) and ratios.
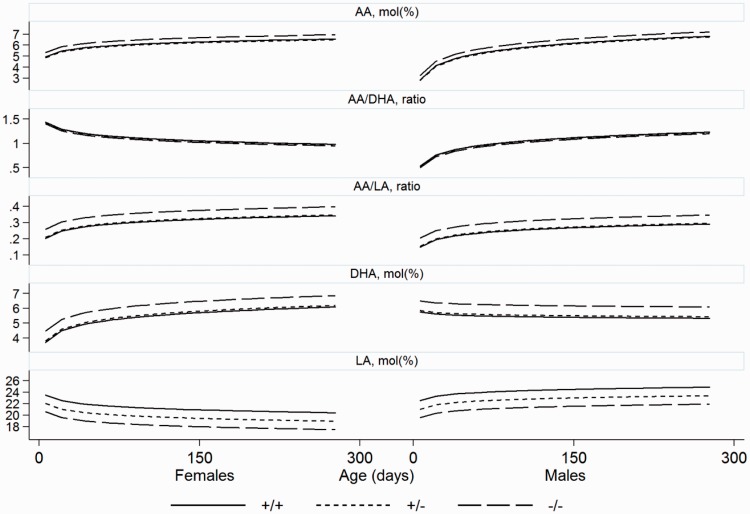
We also indicated all measurements in a population based model (Figure 2) by generating locally weighted scatterplot smoothing (LOWESS)48 with a bandwidth of 0.8. These figures thus show the trend in the population over time. Smoothing was performed separately for each combination of allele and gender. Thus, Figure 1 is based on the individual depicted values with adapted curves illustrating differences by age but showing similar results as the other model (Figure 2), which illustrates the trend of the population over time.
Figure 2.
The longitudinal trend of serum phospholipid concentrations based on population of mice and separated by alleles and sex for wild type (+/+), heterozygotes (+/−) and homozygotes (−/−) for the cftrm1Bay allele. Arachidonic acid (AA); docosahexaenoic acid (DHA); linoleic acid (LA) and ratios.
Results
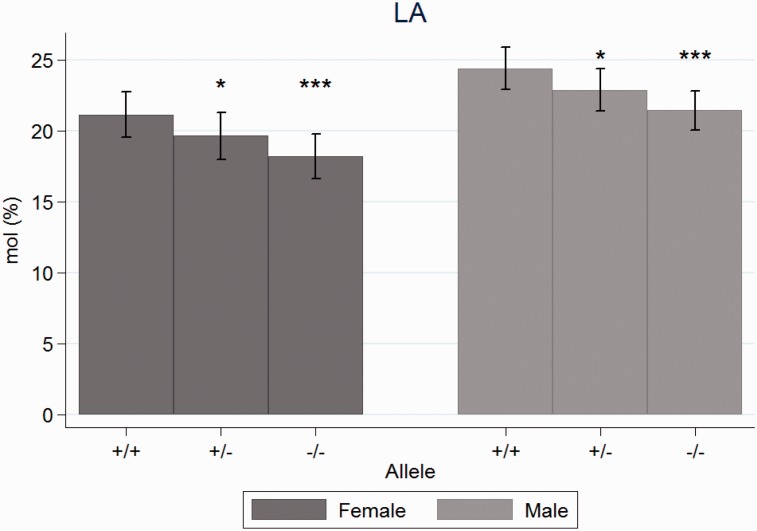
The concentration of LA was significantly higher for the WT compared to the heterozygotes (P = 0.03) and the cftr−/− mice (P < 0.001). LA was also higher for males than females (P = 0.006) (Figure 3). LA did not change significantly with age (P = 0.32) (Figures 1 and 2).
Figure 3.
Mean concentrations of linoleic acid in plasma phospholipids at day 118 in wild type (+/+), heterozygotes (+/−) and homozygotes (−/−) for the cftrm1Bay allele, divided by sex. * Indicates P < 0.05 and *** P< 0.001 compared to wild type. The differences were significant also between gender.
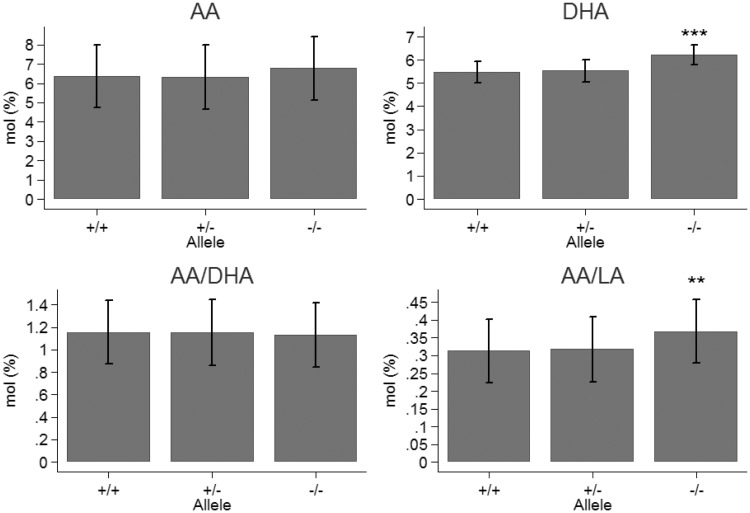
The concentration of AA increased significantly with age (P = 0.004) without difference between sex (P = 0.54) (Figures 1 and 2). There were no significant differences between alleles (P = 0.34) (Figure 4). The AA/LA-ratio was significantly higher for cftr−/− mice compared to the WT (P = 0.006) (Figures 1, 2, and 4). The difference between heterozygotes and WT was not significant (P = 0.65), and neither between sex (P = 0.65), nor age (P = 0.06).
Figure 4.
Mean concentrations arachidonic acid (AA), docosahexaenoic acid (DHA) and the ratios AA/DHA and AA/linoleic acid (LA) in plasma phospholipids at day 118 in wild type mice (+/+), heterozygotes (+/−) and homozygotes (−/−) for the cftrm1Bay allele. Significant differences to wild type and heterozygotes were only found for DHA (P < 0.001) and the ratio AA/LA (P < 0.01).
The concentration of DHA was significantly higher for cftr−/−mice compared to the WT (P < 0.001) (Figure 4). The difference between heterozygotes and WT was not significant (P = 0.66). No differences were found regarding sex (P = 0.17) or age (P = 0.15) (Figures 1 and 2). The AA/DHA-ratio increased with age for males, but decreased with age for females (Figures 1 and 2). This interaction between age and sex was significant (P = 0.008). The ratio did not differ significantly between alleles (P = 0.88) (Figure 4).
The concentration of OA decreased significantly with age (P = 0.004), but did not differ between sex (P = 0.30). The heterozygote had significantly higher values than the WT (P = 0.015) (Supplementary Table 3), but the difference between homozygotes and WT was not significant (P = 0.19). The ratio OA/LA was significantly higher in both heterozygotes and homozygotes compared to WT (P = 0.03 and P = 0.001, respectively).
Supplementary Figure 1 illustrates the results of animals in different cages. LA was constantly low and DHA normal or high in homozygotes in comparison with the WT and heterozygotes.
Discussion
This study shows that mice with a severe genotype/phenotype showed low LA concentrations, similar to patients with CF. Contrary to patients with CF, these cftr−/− mice had a normal or high DHA concentration and did not have increased AA/DHA ratio in serum phospholipids, which is commonly seen in patients with CF. One possible explanation to this discrepancy might be if AA is excessively liberated from membranes16 and transformed to eicosanoids.21,49,50 A compensatory upregulation of the enzyme systems would also increase transformation to DHA, which is considered more efficient in rodents than in humans.51 The difference in DHA with time between male and female is in agreement with the well-known increased capacity of female to synthesize DHA.52 The high mortality of the mice made consistent individual longitudinal studies impossible, but the pair feeding strengthened the results since the differences between the WT, heterozygotes, and CF mice remained the same (Supplementary Figure 1).
The fatty acid abnormality differs in different CF mouse strains with different mutations, but our study corroborates an earlier study showing low LA and high DHA concentration in another mouse model.41 This illustrates that differences in fatty acid analyses might not only be related to the basic strain for the CFTR knockout models or in which strain the cftr mutation is inserted, the latter might theoretically have some CFTR function although usually clinically equal to KO animals. Furthermore, there is not a full homology between the mouse cftr and the human CFTR and also tissue distribution differs.53 The study of Mimoun et al.41 illustrates the impact of dietary fat composition given to the mice on the body tissue composition as well as on survival. Mice in contrast to humans can better synthezise DHA51 and therefore diets containing alpha-linoleic acid or other omega-3 fatty acids can influence the difference between results. In the study showing the beneficial effect of DHA in the cftr −/− mice,33 Peptamen® was used, the fat content of which according to manufacturer’s information mainly consists of medium chain fatty acids and for essential fatty acid supply only soybean oil, which is mainly rich in omega-6 fatty acids. It cannot therefore be excluded that these mice had an initial deficiency of DHA which explained the beneficial effect of the very high dose supplementation. It illustrates why comparison between different models is difficult and the difference in DHA concentrations in different mice models may be related to both background strain, kind of and locus of mutation and diets (Table 1). A limitation of our study is that we did not have the fatty acid composition of the diet. Since we kept animals with different genotypes in cages with supply of the same diet, the consistent difference in fatty acid profile between the genotypes in the cages would indicate no impairment of the cftr−/− mice to synthesize DHA.
The finding that OA was not increased in the homozygotes was unexpected since it is a common feature in patients with CF with low LA concentrations, usually interpreted as compensatory, since LA is important for membranes.7,54,55 One explanation to the found difference might be the high DHA concentration, since it is well known that high concentration of polyunsaturated fatty acids, especially the long-chain polyunsaturated fatty acids, inhibits Δ9- desaturase, stearoyl-CoA desaturase.56 Such explanation is supported by the lack of difference in DHA concentration between WT and heterozygotes, which thus would explain the significantly higher OA concentration in the latter despite a decrease of LA.
The differences in different models of transgenic CF mice might illustrate the divergence sometimes seen in phenotype/symptomatology between siblings with CF carrying the same mutations and might reflect presence of modifying genes. It might also reflect different dietary intake. Epigenetic factors may as well be of importance, but that has not been studied in the context of CF.57 The study suggests that low DHA might not be a characteristic finding in the CF model, but as in humans be mainly dependent on diet; in rodent not only DHA but also its substrates. It is a limitation that we could not illustrate that due to impossibility to analyze the food. In conclusion, our study confirms a low LA concentration in CF but also the difference in metabolism between mice and humans, which has to be considered when comparing mouse models with human patients.
Supplemental Material
Supplemental material, Supplementary Table1 for Low linoleic and high docosahexaenoic acids in a severe phenotype of transgenic cystic fibrosis mice by Birgitta Strandvik, Wanda K O´ Neal, Mohamed A Ali and Ulf Hammar in Experimental Biology and Medicine
Supplemental Material
Supplemental material, Supplementary Table2 for Low linoleic and high docosahexaenoic acids in a severe phenotype of transgenic cystic fibrosis mice by Birgitta Strandvik, Wanda K O´ Neal, Mohamed A Ali and Ulf Hammar in Experimental Biology and Medicine
Supplemental Material
Supplemental material, Supplementary Table3 for Low linoleic and high docosahexaenoic acids in a severe phenotype of transgenic cystic fibrosis mice by Birgitta Strandvik, Wanda K O´ Neal, Mohamed A Ali and Ulf Hammar in Experimental Biology and Medicine
Supplemental Material
Supplemental material, Supplementary Figure1 for Low linoleic and high docosahexaenoic acids in a severe phenotype of transgenic cystic fibrosis mice by Birgitta Strandvik, Wanda K O´ Neal, Mohamed A Ali and Ulf Hammar in Experimental Biology and Medicine
Acknowledgements
The authors are grateful to Berit Holmberg for excellent technical assistance.
Authors’ contributions
Participating in research design: BS and WKO. Conducted experiments: WKO and BS.
Performed data analysis: BS, WKO, MAA, UH. Contributing to the writing of the manuscript: BS, WKO, UH.
Declaration of Conflicting Interests
The authors have no conflict of interest to declare with respect to research, authorship and publication of this article.
Funding
This work was supported by Swedish Research Council (4995), Erica Lederhausen´s Foundation and The Royal Society of Arts and Sciences in Gothenburg.
References
- 1.O'Sullivan BP, Freedman SD. Cystic fibrosis. Lancet 2009; 373:1891–04 [DOI] [PubMed] [Google Scholar]
- 2.Van Devanter DR, Kahle JS, O´Sullivan AK, Sikirica S, Hodkins PS. Cystic fibrosis in young children: a review of disease manifestation, progression, and response to early treatment. J Cyst Fibr 2016; 15:147–57 [DOI] [PubMed] [Google Scholar]
- 3.Strandvik B, Berg U, Kallner A, Kusoffsky E. Effect on renal function of essential fatty acid supplementation in cystic fibrosis. J Pediatr 1989; 115:242–50 [DOI] [PubMed] [Google Scholar]
- 4.Arborg B, Eklund A, Norman A, Strandvik B. Urinary bile acid excretion in correlation to liver histopathology in cystic fibrosis. Scand J Gastroenterol 1980; 15:73–80 [DOI] [PubMed] [Google Scholar]
- 5.Lindblad A, Hultcrantz R, Strandvik B. Bile-duct destruction and collagen deposition: a prominent ultrastructural feature of the liver in cystic fibrosis. Hepatology 1992; 16:372–81 [DOI] [PubMed] [Google Scholar]
- 6.Kuo PT, Huang NN, Bassett DR. The fatty acid composition of the serum chylomicrons and adipose tissue of children with cystic fibrosis of the pancreas. J Pediatr 1962; 60:394–403 [DOI] [PubMed] [Google Scholar]
- 7.Christophe AB, Warwick WJ, Holman RT. Serum fatty acid profiles in cystic fibrosis patients and their parents. Lipids 1994; 29:569–75 [DOI] [PubMed] [Google Scholar]
- 8.Coste TC, Deumer G, Reychler G, Lebecque P, Wallemacq P, Leal T. Influence of pancreatic status and sex on polyunsaturated fatty acid profiles in cystic fibrosis. Clin Chem 2008; 54:388–95 [DOI] [PubMed] [Google Scholar]
- 9.Strandvik B, Gronowitz E, Enlund F, Martinsson T, Wahlström J. Essential fatty acid deficiency in relation to genotype in patients with cystic fibrosis. J Pediatr 2001; 139:650. [DOI] [PubMed] [Google Scholar]
- 10.Van Biervliet S, Vanbillemont G, Van Biervliet J-P, Declercq D, Robberecht E, Christophe A. Relation between fatty acid composition and clinical status or genotype in cystic fibrosis patients. Ann Nutr Metab 2007; 51:541–9 [DOI] [PubMed] [Google Scholar]
- 11.Hjelte L, Ahrén B, Andrén-Sandberg Å, Böttcher G, Strandvik B. Pancreatic function in the essential fatty acid deficient rat. Metab Clin Exp 1990; 39:871–5 [DOI] [PubMed] [Google Scholar]
- 12.Hjelte L, Larsson M, Alvestrand A, Malmborg AS, Strandvik B. Renal function in rats with essential fatty acid deficiency. Clin Sci 1990; 79:299–305 [DOI] [PubMed] [Google Scholar]
- 13.Strandvik B. Relation between essential fatty acid metabolism and gastrointestinal symptoms in cystic fibrosis. Acta Paediatr 1989; 363:58–63 [DOI] [PubMed] [Google Scholar]
- 14.Strandvik B. Long chain fatty acid metabolism and essential fatty acid deficiency with special emphasis on cystic fibrosis In: Bracco U andDeckelbaum RJ (eds) Polyunsaturated fatty acids in human nutrition. New York: Nestlé ‘workshop Ser Raven Press Ltd; , 1992, Vol.28, pp.159–67 [Google Scholar]
- 15.Van Egmond AWA, Kosorok MR, Koscik R, Laxova A, Farrell PM. Effect of linoleic acid intake on growth of infants with cystic fibrosis. Am J Clin Nutr 1996; 63:746–52 [DOI] [PubMed] [Google Scholar]
- 16.Carlstedt-Duke J, Brönnegard M, Strandvik B. Pathological regulation of arachidonic acid release in cystic fibrosis: the putative basic defect. Proc Natl Acad Sci U S A 1986; 83:9202–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levistre R, Lemnaouar M, Rybkine T, Bereziat G, Masliah J. Increase of bradykinin-stimulated arachidonic acid release in a deltaF508 cystic fibrosis epithelial cell line. Biochim Biophys Acta 1993; 1181:233–9 [DOI] [PubMed] [Google Scholar]
- 18.Berguerand M, Klapisz E, Thomas G, Humbert L, Jouniaux A-M, Olivier JL, Béréziat G, Masliah J. Differential stimulation of cytosolic phospholipase A2 by bradykinin in human cystic fibrosis cell lines. Am J Respir Cell Mol Biol 1997; 17:481–90 [DOI] [PubMed] [Google Scholar]
- 19.Miele L, Cordella-Miele E, Xing M, Frizzell R, Mukherjee AB. Cystic fibrosis gene mutation (deltaF508) is associated with an intrinsic abnormality in Ca2+-induced arachidonic acid release by epithelial cells. DNA Cell Biol 1997; 16:749–59 [DOI] [PubMed] [Google Scholar]
- 20.Bhura-Bandali FN, Suh M, Man SF, Clandinin MT. The deltaF508 mutation in the cystic fibrosis transmembrane conductance regulator alters control of essential fatty acid utilization in epithelial cells. J Nutr 2000; 130:2870–5 [DOI] [PubMed] [Google Scholar]
- 21.Strandvik B, Svensson E, Seyberth HW. Prostanoid biosynthesis in patients with cystic fibrosis. Prostagland Leukotr Ess Fatty Acids 1996; 55:419–25 [DOI] [PubMed] [Google Scholar]
- 22.Cho HP, Nakanmura M, Clarke SD. Cloning, expression, and fatty acid regulation of the human Δ5 desaturase. J Biol Chem 1999; 274:37335–9 [DOI] [PubMed] [Google Scholar]
- 23.Adam O, Tesche A, Wolfram G. Impact of linoleic acid intake on arachidonic acid formation and eicosanoid biosynthesis in humans. Prostagland Leukotr Ess Fatty Acids 2008; 79:177–81 [DOI] [PubMed] [Google Scholar]
- 24.Rett BS, Whelan J. Increasing dietary linoleic acid does not increase tissue arachidonic acid content in adults consuming Western-type diets: a systematic review. Nutr Metab 2011; 8:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strandvik B, Holmberg B. Plasma phospholipid arachidonic acid is inversely correlated to the linoleic acid concentration also in cystic fibrosis. J Cyst Fibr 2011; 10:S 73 [Google Scholar]
- 26.Underwood BA, Denning CR, Navab M. Polyunsaturated fatty acids and tocopherol levels in patients with cystic fibrosis. Ann N Y Acad Sci 1972; 203:237–47 [DOI] [PubMed] [Google Scholar]
- 27.Freedman SD, Blanco PG, Zaman MM, Shea JC, Ollero M, Hopper IK, Weed DA, Gelrud A, Regan MM, Laposata M, Alvarez JG, O´Sullivan BP. Association of cystic fibrosis with abnormalities in fatty acid metabolism. N Engl J Med 2004; 350:560–9 [DOI] [PubMed] [Google Scholar]
- 28.Thompson GN. Relationships between essential fatty acid levels, pulmonary function and fat absorption in pre-adolescent cystic fibrosis children with good clinical scores. Eur J Pediatr 1989; 148:327–9 [DOI] [PubMed] [Google Scholar]
- 29.Lepage G, Levy E, Ronco N, Smith L, Galéano N, Roy CC. Direct transesterification of plasma fatty acids for the diagnosis of essential fatty acid deficiency in cystic fibrosis. J Lipid Res 1989; 30:1483–90 [PubMed] [Google Scholar]
- 30.Walkowiak J, Lisowska A, Blaszcynski M, Przyslawski J, Walczak M. Polyunsaturated fatty acids in cystic fibrosis are related to nutrition and clinical expression of the disease. J Pediatr Gastroenterol Nutr 2007; 45:488–90 [DOI] [PubMed] [Google Scholar]
- 31.Lindblad A, Glaumann H, Strandvik B. Natural history of liver disease in cystic fibrosis. Hepatology 1999; 30:1151–8 [DOI] [PubMed] [Google Scholar]
- 32.Van Biervliet S, Van Biervliet JP, Robberecht E, Christophe A. Fatty acid composition of serum phospholipids in cystic fibrosis (CF) patients with or without CF related liver disease. Clin Chem Lab Med 2010; 48:1751–5 [DOI] [PubMed] [Google Scholar]
- 33.Freedman SD, Katz MH, Parker EM, Laposata M, Urman MY, Alvarez JG. A membrane lipid imbalance plays a role in the phenotypic expression of cystic fibrosis in cftr-/- mice. Proc Natl Acad Sci U S A 1999; 96:13995–4000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oliver C, Watson H. Omega-3 fatty acids for cystic fibrosis. Cochrane Database Syst Rev 2016; 1:CD002201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beharry S, Ackerley C, Corey M, Kent G, Heng YM, Christensen H, Luk C, Yantiss RK, Nasser IA, Zaman M, Freedman SD, Durie PR. Long-term docosahexaenoic acid therapy in a congenic murine model of cystic fibrosis. Am J Physiol Gastrointest Liver Physiol 2007; 292:G839–48 [DOI] [PubMed] [Google Scholar]
- 36.Davidson DJ, Rolfe M. Mouse models of cystic fibrosis. Trends Genet 2001; 17:S29–37 [DOI] [PubMed] [Google Scholar]
- 37.Guilbault C, Saeed Z, Downey GP, Radzioch D. Cystic fibrosis mouse models. Am J Respir Cell Mol Biol 2007; 36:1–7 [DOI] [PubMed] [Google Scholar]
- 38.Wilke M, Buijs-Offerman RM, Aarbiou J, Colledge WH, Sheppard DN, Touqui L, Bot A, Jorna H, de Jonge HR, Scholte BJ. Mouse models of cystic fibrosis: phenotypic analysis and research applications. J Cyst Fibr 2011; 10:S152–71 [DOI] [PubMed] [Google Scholar]
- 39.Rosen BH, Chanson M, Gawenis LR, Liu J, Sofoluwe A, Zoso A, Engelhardt JF. Animal and model systems for studying cystic fibrosis. J Cyst Fibros 2017; 19:pii: S1569-1993(17)30880-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Werner A, Bongers MEJ, Bijvelds MJ, de Jonge HR, Verkade HJ. No indications for altered essential fatty acid metabolism in two murine models for cystic fibrosis. J Lipid Res 2004; 45:2277–86 [DOI] [PubMed] [Google Scholar]
- 41.Mimoun M, Coste TC, Lebacq J, Lebecque P, Wallemacq P, Leal T, Armand M. Increased tissue arachidonic acid and reduced linoleic acid in a mouse model of cystic fibrosis are reversed by supplemental glycerophospholipids enriched in docosahexaenoic acid. J Nutr 2009; 139:2358–64 [DOI] [PubMed] [Google Scholar]
- 42.Dombrowsky H, Clark GT, Rau GA, Bernhard W, Postle AD. Molecular species compositions of lung and pancreas phospholipids in the cftr tm1HGU/tm1HGU cystic fibrosis mouse. Pediatr Res 2003; 53:447–54 [DOI] [PubMed] [Google Scholar]
- 43.Alturkmani R, Katrangi W, Cluette-Brown JE, Zaman MM, Laposata M, Freedman SD. Cell culture models demonstrate that CFTR dysfunction leads to defective fatty acid composition and metabolism. J Lipid Res 2008; 49:1692–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strandvik B, Björck E, Fallström M, Gronowitz E, Thountzouris J, Lindblad A, Markiewicz D, Wahlström J, Tsui LC, Zielenski J. Spectrum of mutation in the CFTR gene of patients with classical and atypical forms of cystic fibrosis from southwestern Sweden: identification of 12 novel mutations. Gen Test 2001; 5:235–42 [DOI] [PubMed] [Google Scholar]
- 45.O´Neal WK, Hasty P, McCray PB, Jr, Casey B, Rivera-Pérez J, Welsh MJ, Beaudet AL, Bradley A. A severe phenotype in mice with a duplication of exon 3 in the cystic fibrosis locus. Hum Mol Genet 1993; 2:1561–9 [DOI] [PubMed] [Google Scholar]
- 46.Korotkova M, Gabrielsson B, Hanson LA, Strandvik B. Maternal essential fatty acid deficiency depresses serum leptin levels in suckling rat pups. J Lipid Res 2001; 42:359–65 [PubMed] [Google Scholar]
- 47.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics 1982; 38:963–74 [PubMed] [Google Scholar]
- 48.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc 1979; 74:829–36 [Google Scholar]
- 49.Jabr S, Gartner S, Milne GL, Roca-Ferrer J, Casas J, Moreno A, Gelpí E, Picado C. Quantification of major urinary metabolites of PGE2 and PGD2 in cystic fibrosis: correlation with disease severity. Prostagland Leukot Ess Fatty Acids 2013; 89:121–6 [DOI] [PubMed] [Google Scholar]
- 50.O'Connor MG, Thomsen K, Brown RF, Laposata M, Seegmiller A. Elevated prostaglandin E metabolites and abnormal plasma fatty acids at baseline in pediatric cystic fibrosis patients: a pilot study. Prostagland Leukotr Ess Fatty Acids 2016; 113:46–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pauter A, Olsson P, Asadi A, Herslöf B, Csikasz RI, Zadravec D, Jacobsson A. Elovl2 ablation demonstrates that systemic DHA is endogenously produced and is essential for lipid homeostasis in mice. J Lipid Res 2014; 55:718–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burdge GC, Wootton SA. Conversion of α-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br J Nutr 2002; 88:411–20 [DOI] [PubMed] [Google Scholar]
- 53.Ellsworth RE, Jamison CD, Touchman JW, Chissoe SL, Braden Maduro VV, Bouffard GG, Dietrich NL, Beckstrom-Sternberg SM, Iyer LM, Weintraub LA, Cotton M, Courtney L, Edwards J, Maupin R, Ozersky P, Rohlfing T, Wohldmann P, Miner T, Kemp K, Kramer J, Korf I, Pepin K, Antonacci-Fulton L, Fulton RS, Minx P, Hillier LW, Waterston RH, Miller W, Green ED. Comparative genomic sequence analysis of the human and mouse cystic fibrosis transmembrane conductance regulator genes. Proc Natl Acad Sci U S A 2000; 97:1172–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Farrell PM, Mischler EH, Engle MJ, Brown DJ, Lau S-M. Fatty acid abnormalities in cystic fibrosis. Pediatr Res 1985; 19:105–9 [DOI] [PubMed] [Google Scholar]
- 55.Gronowitz E, Mellström D, Strandvik B. Serum phospholipid fatty acid pattern is associated with bone mineral density in children, but not adults, with cystic fibrosis. BJN 2006; 95:1159–65 [DOI] [PubMed] [Google Scholar]
- 56.Ntambi JM. Regulation of stearoyl-CoA desaturase by polyunsaturated fatty acids and cholesterol. J Lipid Res 1999; 40:1549–58 [PubMed] [Google Scholar]
- 57.Sirinupong N, Yang Z. Epigenetics in cystic fibrosis: epigenetic targeting of a genetic disease. Curr Drug Targets 2015; 16:976–87 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary Table1 for Low linoleic and high docosahexaenoic acids in a severe phenotype of transgenic cystic fibrosis mice by Birgitta Strandvik, Wanda K O´ Neal, Mohamed A Ali and Ulf Hammar in Experimental Biology and Medicine
Supplemental material, Supplementary Table2 for Low linoleic and high docosahexaenoic acids in a severe phenotype of transgenic cystic fibrosis mice by Birgitta Strandvik, Wanda K O´ Neal, Mohamed A Ali and Ulf Hammar in Experimental Biology and Medicine
Supplemental material, Supplementary Table3 for Low linoleic and high docosahexaenoic acids in a severe phenotype of transgenic cystic fibrosis mice by Birgitta Strandvik, Wanda K O´ Neal, Mohamed A Ali and Ulf Hammar in Experimental Biology and Medicine
Supplemental material, Supplementary Figure1 for Low linoleic and high docosahexaenoic acids in a severe phenotype of transgenic cystic fibrosis mice by Birgitta Strandvik, Wanda K O´ Neal, Mohamed A Ali and Ulf Hammar in Experimental Biology and Medicine