Short abstract
Background
The enhanced expression of cytokines in the pathological states suggests that they have important roles in the initiation or maintenance of disease states.
Findings: To determine the involvement of cytokines in chronic neuropathic pain, the expression of cytokines in the anterior cingulate cortex neurons in the ligation of the common peroneal nerve mice was investigated. We utilized a cytokine enzyme-linked immunosorbent assay plate array to detect 23 cytokines in total eight mice including a female, and no significant differences were found in those cytokines between the common peroneal nerve model and sham surgery mice. Quantification of TNF-α at protein level revealed the unvaried expression in the anterior cingulate cortex in both neuropathic pain and visceral pain, but enhanced expression in the insular cortex in the visceral pain. Furthermore, we found that the IL-Ira, a kind of IL-1 receptor antagonist, had no effect on the theta burst stimulation-induced long-term potentiation in the anterior cingulate cortex.
Conclusions
Cytokines are not involved in chronic neuropathic pain induced by nerve injury in the anterior cingulate cortex. Our findings suggested that cytokines may not be a viable drug target to treat chronic neuropathic pain in the anterior cingulate cortex.
Keywords: Cytokines, anterior cingulate cortex, IL-Ira, chronic pain, long-term potentiation
Short report
Cytokines are small signaling molecules, synthesized by almost all cells.1,2 Cytokines are commonly implicated in infection and also have critical roles in many biochemical processes such as cellular growth, differentiation, gene expression, migration, and immunity. It is known that cytokines play an important role in neuronal development and function, and they are closely related to inflammatory responses in the peripheral and central nervous systems. Recent data has revealed that cytokines also play a key role in the pathology of brain injury and neuropathic pain.3–5 It has been documented that several cytokines are increased in the dorsal root ganglia (DRG) and spinal cord after peripheral nerve damage,6,7 and inhibition of these processes produces antinociception.4 Furthermore, recent studies have indicated that inflammatory factors are upregulated in the prefrontal cortex of animals after peripheral inflammation.8–10
Human and animal studies demonstrate that the anterior cingulate cortex (ACC) is an important brain area for chronic pain and pain-related emotion.11–15 Long-term potentiation (LTP) in the ACC is a key cellular mechanism for cortical potentiation in chronic pain conditions.11,16 Neuropathic and visceral pains are major forms of chronic pain that are resistant to conventional medicine. It is unknown whether cytokines may be also activated in these chronic pain conditions. In the present study, we want to detect if cytokines in the pain-related cortical areas including ACC and/or insular cortex may be altered after peripheral injuries.
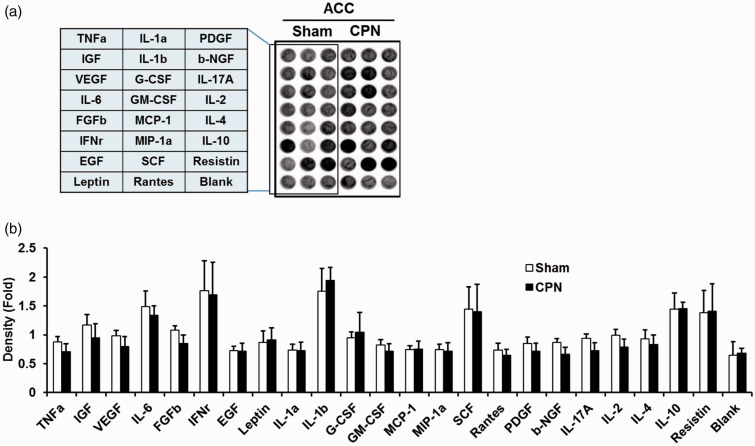
We employed a well-established animal model of a neuropathic pain we developed.13,17 At seven days after the injury, we found that animals showed significant mechanical allodynia as reported previously.18 After behavioral evaluation, we sacrificed animals and collected brain samples from ACC. Enzyme-linked immunosorbent assay (ELISA) array was used to determine the levels of 23 cytokines in the ACC (Figure 1(a)). Equal amount of samples from each group was added in the ELISA plates. As shown in Figure 1(b), we did not detect any significant changes among 23 cytokines in the ACC as compared with those of sham-treated mice (n = 8 mice). These results indicate that cytokines are not activated in the ACC after nerve injury. This is different from previous results reported in the spinal cord,6 DRG,7 and hippocampus.19
Figure 1.
Relative quantity of various cytokines in ACC from sham and CPN model mice. Cytokines analysis was determined in mice 7 days after nerve injury or sham surgery. (a) A typical image of cytokine ELISA plate array was shown, and total 23 cytokines were detected. (b) According to the manufacturer’s protocol, the cytokine assay was performed, and the ELISA plates were scanned by luminometer, the intensity of each well was detected by ImageJ, a relative quantity of cytokines was calculated and expressed in histogram (n = 8).
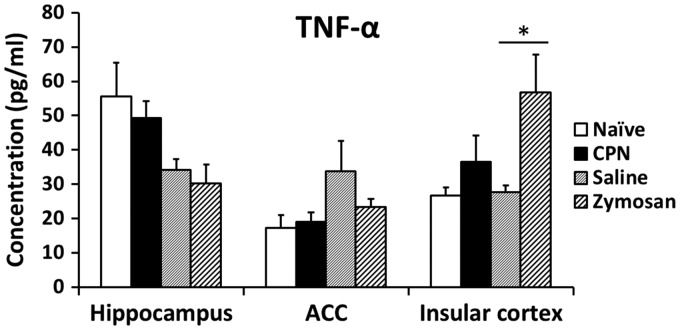
Among different cytokines, TNF-α has received the most attention. TNF-α plays an important role in triggering the activation of other cytokines in response to injury or inflammation and is critical for the development and maintenance of pain.4,9 We decided to further measure the level of TNF-α in the ACC as well as insular cortex in two pain conditions: neuropathic pain and visceral pain. We found that TNF-α was not changed in the ACC in either pain model (n = 3 mice, naïve: 17.2 ± 3.8 pg/ml, common peroneal nerve (CPN): 19.1 ± 2.7 pg/ml; saline: 33.6 ± 9.1 pg/ml, zymosan: 23.3 ± 2.4 pg/ml) neither was it changed in the hippocampus. However, TNF-α was increased in the insular cortex in visceral pain models compared with sham-treated groups (n = 3 mice, naïve: 26.7 ± 2.3 pg/ml, CPN: 36.4 ± 7.8 pg/ml; saline: 27.7 ± 1.9 pg/ml, zymosan: 56.7 ±11.0 pg/ml, p < 0.05 in comparison with saline) (Figure 2). These results show that TNF-α is associated with visceral pain in the insular cortex, but not in the ACC, and TNF-α may not participate in neuropathic pain in both ACC and insular cortex, indicating that TNF-α may have an effect on variant roles through different signaling pathway in different brain areas.
Figure 2.
Expression of TNF-α in different model in the ACC. The levels of cytokine TNF-α were detected in hippocampus, ACC and insular cortex of two animal models. Mice were treated with CPN or sham surgery for neuropathic pain, and treated with 0.1 ml zymosan or saline for visceral pain (n = 3).
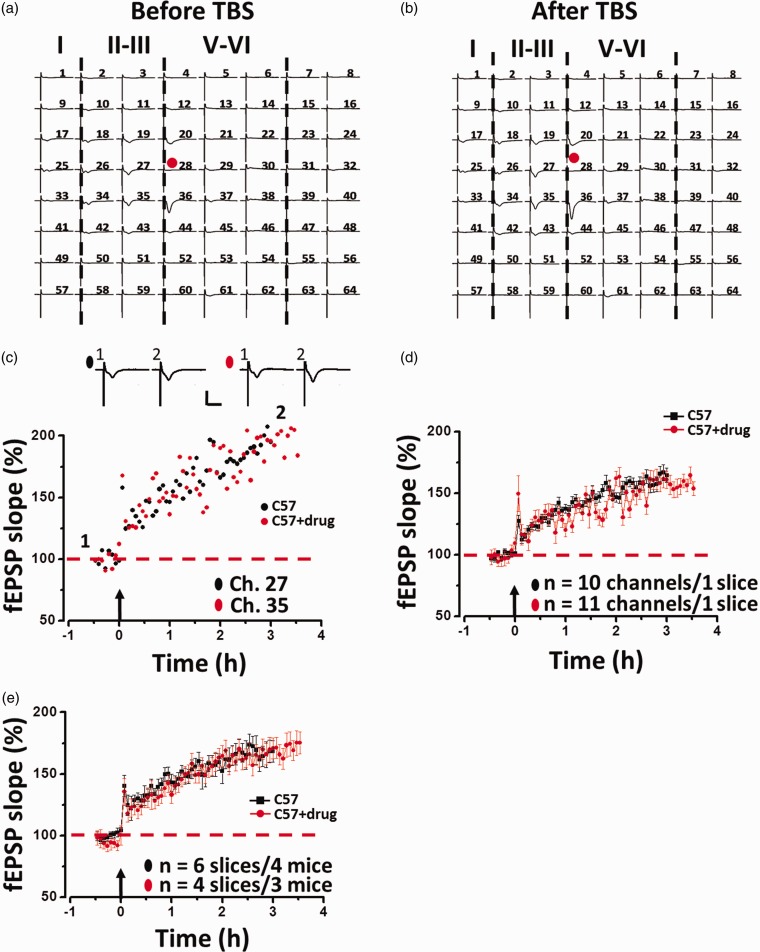
Interleukin-1 (IL-1) is also known to play a central role in inflammatory conditions and is associated with synaptic transmission. Previous reports showed that IL-1 receptor antagonist (IL-1ra), a specific blocker of IL-1, affects hippocampal LTP induction.20,21 To detect whether IL-Ira affects LTP induction in the ACC, IL-Ira (1 µg/ml) was bathed 1 h prior to the theta burst stimulation (TBS) delivery. The drug was washed out 30 min after TBS, and LTP was monitored for another 2 h. One representative 64-channel recording is shown in Figure 3(a) (before TBS) and Figure 3(b) (2 h after TBS). We found that TBS induced a long-lasting potentiation of fEPSP in the ACC. Analysis of one single channel (Ch. 27 and Ch. 35) in the superficial layer revealed that the response was potentiated to 193.5% and 196.8% of baseline at 2 h after TBS (Figure 3(c)). The averaged data from superficial channels for this slice are presented in Figure 3(d) (161.5 ± 5.8% of baseline, n= 10 channels; 160.8 ± 6.1% of baseline, n = 11 channels). Pooled data from a series of similar experiments are summarized in Figure 3(e). Bath infusion of IL-Ira showed no change in LTP induction in the ACC (168.0 ± 9.2% of baseline, n= 6 slices/4 mice; 170.8 ± 9.0% of baseline, n= 4 slices/3 mice; paired t-test). These results indicate that cytokine IL-1 is not affected on the LTP in ACC.
Figure 3.
Multi-channel recordings of post-LTP in the ACC of adult mouse. An overview of multi-site synaptic responses recorded at baseline (a) and 1 h after TBS (b) in one slice. After the stabilizing baseline responses for 1 h, a TBS protocol was delivered to the deep layer and LTP was then monitored for 2 h. The red filled circle denotes the stimulated channel (Ch. 28). Vertical lines indicate the layers of the ACC slice. (c) Results of one channel showing LTP in one slice for control (Ch. 27) and IL-Ira treated (Ch. 35) group, respectively. (d) Summary of averaged data of the same slice for control (10 channels) and IL-Ira-treated (11 channels) group. (e) Pooled data of LTP in the ACC for control (n = 6 slices/4 mice) and IL-Ira-treated (n = 4 slices/3 mice) groups.
In this present study, we found that the expression of 23 cytokines was not significantly changed in the ACC after peripheral nerve injury. These results are different from previous reports in the spinal cord and DRG.4,5,7 For example, it has been reported that TNF-α is up-regulated in both spinal cord and dorsal root ganglion after nerve injury.22,23 These reports suggest that upregulation of cytokines may be region related. In animal models of inflammatory pain, some reports showed that IL-8 is altered in prefrontal/ACC regions,8 while others found that TNF-α shows no changes in the somatosensory cortex.9 These findings also support that changes in cytokines may vary in different regions of the cortex. In our studies, we found TNF-α and IL-1 cytokines were not changed in ACC, but we noticed that the level of TNF-α cytokine was altered in the insular cortex after irritable bowel syndrome. Future studies are needed to explore the roles of cytokines in the insular cortex.
Previous studies indicate that inflammatory cytokines may contribute to synaptic potentiation or LTP;24,25 however, some studies failed to observe any significant role of certain cytokines. For example, IL-1ra inhibited LTP in the CA1 and dentate gyrus regions.21,26,27 But other studies found that IL-1ra enhanced LTP or had no effect on LTP.20,28 In the present study, we found that IL-1ra had no effect on the induction of LTP in the ACC. Together with results from the measurement of cytokines after nerve injury, our results indicate that cytokines likely do not play important roles in injury-related cortical potentiation in the ACC. The synaptic LTP is likely mediated through neuronal-related N-Methyl-D-aspartic acid receptor-dependent signaling pathways. However, we cannot rule out the possibility that some cytokines may be altered and cannot be detected by the current methods.
Materials and methods
Mice
Adult male and female C57BL/6 mice (7–8 weeks old) were used in this study. Mice were housed with food and water ad libitum under a 12-h light and dark cycle. All procedures and handling of animals were performed with permission according to the guidelines of Xi’an Jiaotong University.
Animal model
A model of neuropathic pain was induced by the ligation of the CPN as described previously.12 Briefly, the mice were anesthetized by intraperitoneal injection of 1% sodium pentobarbital (60 mg/kg). The CPN was visible between the anterior and posterior groups of muscles running almost transversely. The left CPN was slowly ligated with a chromic gut suture 5–0 (Ethicon) until dorsiflexor contraction of the foot was observed as twitching of the digits. Control mice were treated with sham surgery. The mechanical allodynia was tested on postsurgical day 1, 3, 5, and 7. The mice were used for analysis of cytokine protein expression.
Visceral pain was induced by the administration of zymosan as described previously.29 A volume of 0.1 ml zymosan suspension (30 mg/ml in saline; derived from Saccharomyces cerevisiae; Sigma, St. Louis, MO) was administered rectally via a 22-gauge, 24-mm-long stainless steel feeding needle into the colons of mice over a period of 2 min. Control mice were rectally administered with 0.1 ml saline. Zymosan or saline was given daily for three consecutive days.
Mechanical allodynia test
Mice were placed in a round container and allowed to acclimate for 30 min before testing. Mechanical allodynia was assessed on the basis of the hindpaw responsiveness to the application of von Frey filaments (Stoelting) to the point of bending. Positive responses include licking, biting, and sudden withdrawal of the hind paw. Experiments were performed to characterize the threshold stimulus. Mechanical pressure was innocuous from a 1.65 filament (force, 0.08 g) in naïve mice. This filament was then used to test the mechanical allodynia after nerve injury. Mechanical allodynia was tested five times with an interval of 10 min, and then the animals were tested again after 20 min.
Cytokine ELISA plate array assay
The cortical tissue was dissected on ice in cold artificial cerebrospinal fluid (ACSF) and homogenized in lysis buffer (10 mM sucrose, 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1% SDS, 1% Triton X-100, 1 mM DTT) containing a protease inhibitor cocktail and phosphatase inhibitor cocktails 2 and 3 (Sigma). Samples were centrifuged (10,000 g, 5 min, 4°C) to pellet the tissue debris, and then supernatants were collected and protein concentrations were normalized with the Bradford assay. Cytokine measurements were performed according to the manufacturer’s protocols (Mouse Cytokine ELISA Plate Array I, Signosis). TNF-α was measured by a commercial solution-based kit (TNF-α mouse ELISA Kit, Invitrogen). Briefly, supernatants were diluted by the provided diluent buffer to a final 10 μg/100 μl per well, and incubated for 2 h at room temperature with gentle shaking. After washing with assay wash buffer, 100 µl biotin-labeled antibody mixtures were added to each well and incubate for 1 h. The washing process was repeated three times and then 100 µl of streptavidin-HRP conjugate was added to each well and incubated for 30 min. Finally, 95 µl freshly prepared substrate solutions were added to each well and incubated for 2 min. The plate was placed in the luminometer and read with no filter position.
Brain slice preparation
The general procedures of ACC slice preparation are similar to previous description.30–32 Mice were anesthetized with gaseous isoflurane and were decapitated. The whole brain was rapidly removed and transferred to ice cold oxygenated (95% O2 and 5% CO2) ACSF containing (in mM) NaCl 124, KCl 2.5, NaH2PO4 1.0, MgSO4 1, CaCl2 2, NaHCO3 25, and glucose 10, pH 7.35–7.45. After cooling for about 2 min, needless parts of the brain were trimmed and the remaining brain block was glued onto the ice-cold stage of a vibrating tissue slicer (Leica VT1200S). Then four coronal ACC brain slices (300 µm) were cut and transferred to an incubation chamber with oxygenated ACSF at room temperature for at least 1 h.
Multi-channel field potential recordings
The general procedures of LTP recording are similar to previous description.33 After incubation, a 64-channel multi-electrode array system (MED64, Panasonic, Japan) was used for extracellular field potential recordings. One slice was positioned on the MED64 probe in such a way that the ACC area was entirely covered by the recording dish mounted on the stage of an inverted microscope (CKX41, Olympus). Once the slice was settled, a fine mesh anchor (Warner Instruments, Harvard) was carefully positioned to ensure slice stability during recording. The slice was continuously perfused with oxygenated, fresh ACSF at the rate of 2–3 ml/min with the aid of a peristaltic pump (Minipuls 3, Gilson) throughout the entire experimental period.
After 15–20 min recovery, one of the channels located in the deep layer (V-VI) of the ACC, from which the best synaptic responses can be induced in the surrounding channels, was chosen as the stimulation site. Monopolar and biphasic constant current pulses (10–20 µA, 0.2 ms) were applied to the stimulation site via the Mobius software. Field excitatory postsynaptic potential (fEPSP) evoked at both superficial layer (II–III) and deep layer of the ACC was amplified by a 64-channel amplifier. Channels in which field potentials can be induced were considered as active and their responses were sampled every 1 min. After the baseline responses were stabilized for at least 30 min, the IL-Ira (1µg/ml) was given 1 h before TBS.
Statistics
All data were presented as the mean ± S.E.M and no data were excluded. Unpaired t-test or one-way analysis of variance (Dunnett-t test was used for post hoc comparison) was used for statistical comparisons between CPN model and sham mice. The examples shown in all figures are representative and repeated at least three times. In all cases, p < 0.05 was considered statistically significant.
Authors’ contributions
JS Lu carried out the experiments, analyzed the data, and drafted the manuscript. Q Song and MM Zhang helped to finish the LTP and pain model experiments. M Zhuo designed the experiments and wrote the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from NSFC (81500945 to MMZ).
References
- 1.Dinarello CA. Proinflammatory cytokines. Chest 2000; 118: 503–508. [DOI] [PubMed] [Google Scholar]
- 2.Mousa A andBakhiet M.. Role of cytokine signaling during nervous system development. Int J Mol Sci 2013; 14: 13931–13957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carpentier PA andPalmer TD.. Immune influence on adult neural stem cell regulation and function. Neuron 2009; 64: 79–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark AK Old EA andMalcangio M.. Neuropathic pain and cytokines: current perspectives. J Pain Res 2013; 6: 803–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masuda T Tsuda M andInoue K.. Transcriptional regulation in microglia and neuropathic pain. Pain Manag 2016; 6: 91–94. [DOI] [PubMed] [Google Scholar]
- 6.Laughlin TM Bethea JR Yezierski RP andWilcox GL.. Cytokine involvement in dynorphin-induced allodynia. Pain 2000; 84: 159–167. [DOI] [PubMed] [Google Scholar]
- 7.Ohtori S Takahashi K Moriya H andMyers RR.. TNF-alpha and TNF-alpha receptor type 1 upregulation in glia and neurons after peripheral nerve injury – studies in murine DRG and spinal cord. Spine 2004; 29: 1082–1088. [DOI] [PubMed] [Google Scholar]
- 8.Cui GB An JZ Zhang N Zhao MG Liu SB andYi J.. Elevated interleukin-8 enhances prefrontal synaptic transmission in mice with persistent inflammatory pain. Mol Pain 2012; 8: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jia D Gao GD Liu Y He SM Zhang XN Zhang YF andZhao MG.. TNF-alpha involves in altered prefrontal synaptic transmission in mice with persistent inflammatory pain. Neurosci Lett 2007; 415: 1–5. [DOI] [PubMed] [Google Scholar]
- 10.Lu Y Zhu L andGao YJ.. Pain-related aversion induces astrocytic reaction and proinflammatory cytokine expression in the anterior cingulate cortex in rats. Brain Res Bull 2011; 84: 178–182. [DOI] [PubMed] [Google Scholar]
- 11.Bliss TV Collingridge GL Kaang BK andZhuo M.. Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nat Rev Neurosci 2016; 17: 485–496. [DOI] [PubMed] [Google Scholar]
- 12.Li XY Ko HG Chen T Descalzi G Koga K Wang H Kim SS Shang Y Kwak C Park SW Shim J Lee K Collingridge GL Kaang B-K andZhuo M.. Alleviating neuropathic pain hypersensitivity by inhibiting PKMzeta in the anterior cingulate cortex. Science 2010; 330: 1400–1404. [DOI] [PubMed] [Google Scholar]
- 13.Xu H Wu LJ Wang HS Zhang XH Vadakkan KI Kim SS Steenland HW andZhuo M.. Presynaptic and postsynaptic amplifications of neuropathic pain in the anterior cingulate cortex. J Neurosci 2008; 28: 7445–7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao MG Ko SW Wu LJ Toyoda H Xu H Quan J Li J Jia Y Ren M Xu ZC andZhuo M.. Enhanced presynaptic neurotransmitter release in the anterior cingulate cortex of mice with chronic pain. J Neurosci 2006; 26: 8923–8930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhuo M. Molecular mechanisms of pain in the anterior cingulate cortex. J Neurosci Res 2006; 84: 927–933. [DOI] [PubMed] [Google Scholar]
- 16.Zhuo M. Cortical excitation and chronic pain. Trends Neurosci 2008; 31: 199–207. [DOI] [PubMed] [Google Scholar]
- 17.Vadakkan KI Jia YH andZhuo M.. A behavioral model of neuropathic pain induced by ligation of the common peroneal nerve in mice. J Pain 2005; 6: 747–756. [DOI] [PubMed] [Google Scholar]
- 18.Zhang F Vadakkan KI Kim SS Wu LJ Shang Y andZhuo M.. Selective activation of microglia in spinal cord but not higher cortical regions following nerve injury in adult mouse. Mol Pain 2008; 4: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prieto GA andCotman CW.. Cytokines and cytokine networks target neurons to modulate long-term potentiation. Cytokine Growth Factor Rev 2017; 34: 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ross FM Allan SM Rothwell NJ andVerkhratsky A.. A dual role for interleukin-1 in LTP in mouse hippocampal slices. J Neuroimmunol 2003; 144: 61–67. [DOI] [PubMed] [Google Scholar]
- 21.Schmid AW Lynch MA andHerron CE.. The effects of IL-1 receptor antagonist on beta amyloid mediated depression of LTP in the rat CA1 in vivo. Hippocampus 2009; 19: 670–676. [DOI] [PubMed] [Google Scholar]
- 22.Hashizume H DeLeo JA Colburn RW andWeinstein JN.. Spinal glial activation and cytokine expression after lumbar root injury in the rat. Spine 2000; 25: 1206–1217. [DOI] [PubMed] [Google Scholar]
- 23.Schafers M Svensson CI Sommer C andSorkin LS.. Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J Neurosci 2003; 23: 2517–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.del Rey A Balschun D Wetzel W Randolf A andBesedovsky HO.. A cytokine network involving brain-borne IL-1beta, IL-1ra, IL-18, IL-6, and TNFα operates during long-term potentiation and learning. Brain Behav Immun 2013; 33: 15–23. [DOI] [PubMed] [Google Scholar]
- 25.Spedding M andGressens P.. Neurotrophins and cytokines in neuronal plasticity. Novartis Found Symp 2008; 289: 222–237. [DOI] [PubMed] [Google Scholar]
- 26.Loscher CE Mills KHG andLynch MA.. Interleukin-1 receptor antagonist exerts agonist activity in the hippocampus independent of the interleukin-1 type I receptor. J Neuroimmunol 2003; 137: 117–124. [DOI] [PubMed] [Google Scholar]
- 27.Schneider H Pitossi F Balschun D Wagner A del Rey A andBesedovsky HO.. A neuromodulatory role of interleukin-1 beta in the hippocampus. P Natl Acad Sci USA 1998; 95: 7778–7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edagawa Y Sato F Saito H Takeda T Shimizu N Narui T Shibata S andIto Y.. Dual effects of the lichen glucan PB-2, extracted from Flavoparmelia baltimorensis, on the induction of long-term potentiation in the dentate gyrus of the anesthetized rat: possible mediation via adrenaline beta- and interleukin-1 receptors. Brain Res 2005; 1032: 183–192. [DOI] [PubMed] [Google Scholar]
- 29.Zhang MM Liu SB Chen T Koga K Zhang T Li YQ andZhuo M.. Effects of NB001 and gabapentin on irritable bowel syndrome-induced behavioral anxiety and spontaneous pain. Mol Brain 2014; 7: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen T Lu JS Song Q Liu MG Koga K Descalzi G Li YQ andZhuo M.. Pharmacological rescue of cortical synaptic and network potentiation in a mouse model for fragile X syndrome. Neuropsychopharmacology 2014; 39: 1955–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang SJ Liu MG Chen T Ko HG Baek GC Lee HR Lee K Collingridge GL Kaang BK andZhuo M.. Plasticity of metabotropic glutamate receptor-dependent long-term depression in the anterior cingulate cortex after amputation. J Neurosci 2012; 32: 11318–11329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu MG andZhuo M.. No requirement of TRPV1 in long-term potentiation or long-term depression in the anterior cingulate cortex. Mol Brain 2014; 7: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song Q Zheng HW Li XH Huganir RL Kuner T Zhuo M andChen T.. Selective phosphorylation of ampa receptor contributes to the network of long-term potentiation in the anterior cingulate cortex. J Neurosci 2017; 37: 8534–8548. [DOI] [PMC free article] [PubMed] [Google Scholar]