Short abstract
Background
Following peripheral nerve chronic constriction injury, the accumulation of the α2δ–1 auxiliary subunit of voltage-gated Ca2+ channels in primary afferent terminals contributes to the onset of neuropathic pain. Overexpression of α2δ–1 in Xenopus oocytes increases the opening properties of Cav1.2 L-type channels and allows Ca2+ influx at physiological membrane potentials. We therefore posited that L-type channels play a role in neurotransmitter release in the superficial dorsal horn in the chronic constriction injury model of neuropathic pain.
Results
Whole-cell recording from lamina II neurons from rats, subject to sciatic chronic constriction injury, showed that the L-type Ca2+ channel blocker, nitrendipine (2 µM) reduced the frequency of spontaneous excitatory postsynaptic currents. Nitrendipine had little or no effect on spontaneous excitatory postsynaptic current frequency in neurons from sham-operated animals. To determine whether α2δ–1 is involved in upregulating function of Cav1.2 L-type channels, we tested the effect of the α2δ–1 ligand, gabapentin (100 µM) on currents recorded from HEK293F cells expressing Cav1.2/β4/α2δ–1 channels and found a significant decrease in peak amplitude with no effect on control Cav1.2/β4/α2δ–3 expressing cells. In PC-12 cells, gabapentin also significantly reduced the endogenous dihydropyridine-sensitive calcium current. In lamina II, gabapentin reduced spontaneous excitatory postsynaptic current frequency in neurons from animals subject to chronic constriction injury but not in those from sham-operated animals. Intraperitoneal injection of 5 mg/kg nitrendipine increased paw withdrawal threshold in animals subject to chronic constriction injury.
Conclusion
We suggest that L-type channels show an increased contribution to synaptic transmission in lamina II dorsal horn following peripheral nerve injury. The effect of gabapentin on Cav1.2 via α2δ–1 may contribute to its anti-allodynic action.
Keywords: Allodynia, pharmacology, neuropathic pain, dihydropyridine, gabapentin, anti-allodynic, chronic constriction injury, central sensitization, calcium channel blocker, substantia gelatinosa
Introduction
The auxiliary alpha-2-delta (α2δ) subunits of voltage-gated Ca2+ channels control the trafficking, localization, and biophysical properties of the pore-forming α1-subunits.1–3 The α2δ–1 subunit also serves as the binding partner of the gabapentinoid drugs, pregabalin (PGB), and gabapentin (GBP),4,5 which are first-line treatments in the management of neuropathic pain.6
In experimental animal models, allodynia and hyperalgesia can be induced by chronic constriction injury (CCI) of the sciatic nerve.7,8 This leads to a decrease in paw withdrawal threshold (PWT) to tactile stimulation by von Frey filaments that correlates with increased excitability and altered expression of ion channels in dorsal root ganglia (DRG) neurons9–17 and with increased excitatory synaptic transmission in the superficial dorsal horn.3,18–21 This latter process of “central sensitization” is initiated in part by upregulation of expression of α2δ–1 in primary afferent terminals22–27 and leads to increased spontaneous neurotransmitter release.23
Recent experiments in Xenopus oocytes showed that overexpression of α2δ–1 influenced the properties of the voltage sensors of co-expressed Cav1.2 L-type channels such that Ca2+ influx occurs at physiological membrane potentials.28 Since sensory neurons express dihydropyridine sensitive, L-type channels,10,29 we wondered whether the increase in α2δ–1 expression produced by nerve injury could lead to persistent Ca2+ influx in primary afferent terminals. This in turn may contribute to an increase in spontaneous transmitter release, which would be dependent on the activity of L-type Ca2+ channels. To test this possibility, we examined the effect of the L-type Ca2+ channel blocker, nitrendipine on spontaneous transmitter release in spinal lamina II of rats subject to sciatic CCI. Nitrendipine was chosen by virtue of its especially high affinity for its binding site in rat brain (Ki = 55 pM compared to nifedipine Ki = 384 pM).30 In humans, nitrendipine is three times more potent than nifedipine in reducing peripheral vascular resistance, and arterial blood pressure, and in increasing leg blood flow.31
To determine whether augmentation of L-type channel function is contingent on α2δ–1, we applied GBP to HEK293F cells expressing Cav1.2/α2δ–1 and observed a reduction in peak amplitude of calcium current. GBP binds with high affinity to the α2δ-1 and α2δ-2 subunits but not to α2δ-3 subunits32 and failed to affect calcium current in Cav1.2/α2δ–3 expressing cells. We further confirmed these results on native L-type channels in PC-12 cells and observed that GBP reduced the peak amplitude of the dihydropyridine-sensitive current.
We also found that intraperitoneal injection of 5 mg/kg nitrendipine increased PWT in animals subject to CCI. Taken together, our findings suggest that L-type Cav 1.2 channels show an increased contribution to synaptic transmission in lamina II dorsal horn following peripheral nerve injury and that an effect of GBP on Cav1.2 via α2δ–1 may contribute to its anti-allodynic action.
Methods
Nerve injury surgery and assessment of mechanical allodynia
All procedures were approved by the University of Alberta Animal Welfare Committee in accordance with Canadian Council on Animal Care (CCAC) guidelines. Male Sprague–Dawley rats (19–23-day old) were subject to CCI of the sciatic nerve using polyethylene cuffs8 as described previously.18 Surgery was performed under isoflurane anesthesia (5% induction and 2% maintenance). For sham surgery, the sciatic nerve was exposed but not manipulated. Ten to 14 days after surgery, animals were assessed for the presence of allodynia using von Frey filaments as previously described.18 Animals exhibiting a PWT <6 g were assumed to be expressing allodynia, a sign of neuropathic pain.
Spinal cord slice preparation and electrophysiology
Rats were euthanized with an intraperitoneal injection of urethane (1.5 g/kg). Following cessation of respiration and loss of nociceptive reflexes (paw pinch with forceps), the spinal cord was removed by laminectomy, and 300 µm transverse spinal cord slices prepared on a vibratome as previously described.18,21 Whole-cell patch-clamp recordings were made under infrared differential-interference optics in neurons from slices from CCI or sham-operated animals. Recordings were made from lamina II (substantia gelatinosa) neurons ipsilateral to the sciatic injury.21 Substantia gelatinosa was identified by its translucent appearance in the slice. For recording, slices were superfused at room temperature (∼22°C) with 95% O2–5% CO2 saturated aCSF which contained (in mM): 127 NaCl, 2.5 KCl, 1.2 NaH2PO4, 26 NaHCO3, 1.3 MgSO4, 2.5 CaCl2, 25 D-glucose, pH 7.4. Recording pipettes had resistances of 5–10 MΩ when filled with an internal solution containing (in mM) 130 potassium gluconate, 1 MgCl2, 2 CaCl2, 10 HEPES, 10 EGTA, 4 Mg-ATP, 0.3 Na-GTP, pH 7.2, 290–300 mOsm. Recordings were made using an NPI SEC-05LX amplifier in discontinuous, single-electrode voltage-clamp or current clamp mode. Data were only collected from neurons that exhibited clear overshooting action potentials >60 mV in amplitude. Spontaneous excitatory postsynaptic currents (sEPSC) were recorded at −70 mV. Mini Analysis Program (Synaptosoft, Decatur, GA, USA) was used to distinguish sEPSC from baseline noise. All detected events were then re-examined and visually accepted or rejected based on visual examination. Acceptable events had a sharp onset and exponential offset, a total duration of <50 ms, and an amplitude at least double the baseline noise.
EPSCs were evoked at 0.05 Hz using a teflon-coated nichrome stimulating electrode (7620, A-M Systems, Carlsborg, WA, USA) on the dorsal root or near the dorsal root entry zone to activate primary afferent fibers. The voltage-gated ion channel blocker QX-314 (5 mM) was included in the internal solution to prevent action potential discharge while recording evoked EPSPs (eEPSCs) in the presence of 10 µM bicuculline and 1 µM strychnine. Stimulus intensity for eEPSCs was between 2 and 40 V and the stimulus duration was 100 or 400 µs. Measurements were made from six averaged responses.
Drugs and chemicals
All chemicals used in slice preparation and whole-cell electrophysiology were from Sigma (St. Louis, MO, USA), except GBP, which was from TCI America (Portland OR, USA) and nitrendipine, which was from Tocris Bioscience Minneapolis (MN, USA). For IP injections, 18 mg of nitrendipine was dissolved in 1 ml of dimethyl sulfoxide (DMSO). This was further diluted by the addition of 5 ml of deionized water and the solution made up to 20 ml by the addition of 14 ml saline (0.9% NaCl). This yielded a suspension of 0.9 mg/ml of nitrendipine. Animals received IP injections of 5 mg/kg nitrendipine. Control vehicle solution contained a mixture of DMSO, water, and saline in the ratio 1:5:14.
Cell culture, transfection, and electrophysiology
PC12 cells (ATCC CRL-1721) were grown on Bovine Collagen Type I-coated flasks in F-12K (Invitrogen 21127022) medium supplemented with 2.5% fetal bovine serum (FBS, GIBCO 12483–020) and 15% horse serum (Invitrogen 26050–088) at 37°C in a humidified atmosphere with 5% CO2–95% air. Whole-cell electrophysiological recordings were performed on cells plated on Poly-L-Lysine-coated glass coverslips 48–54 h after seeding.
HEK293F (Invitrogen 11625–019) cells were maintained in Dulbecco’s Modified Eagle’s Medium (Invitrogen 12800–017) supplemented with 10% FBS and 1% non-essential amino acids (Invitrogen 11140). Cells were plated on Poly-L-Lysine-coated glass coverslips and transfected after 24 h using TurboFect transfection reagent (Fisher Cat R0531), following the procedure recommended by the manufacturer. Plasmids encoding rat Cav1.2 α1 subunit and ancillary subunits β4, α2δ1, or α2δ3 were combined at equimolar ratio and co-transfected with enhanced green fluorescent protein (EGFP) as a reporter. A total of 1.1 µg or 1.6 µg cDNA per 35 mm dish was used for the transfection mix with α2δ1 or α2δ3, respectively. Current recordings were performed 18–24 h after transfection.
Whole-cell patch-clamp recordings from PC12 or HEK cells were obtained using a 200B Axopatch amplifier and data were acquired with a Digidata 1332A system (Molecular Devices, CA) controlled by pCLAMP 9 software. Data were low-pass filtered at 2 kHz using the Bessel filter of the amplifier and sampled at 10 kHz. Experiments were performed at room temperature (20–22°C). Borosilicate glass (Sutter BF150–86-10) patch pipettes were made with a horizontal puller P97 (Sutter Instrument Co) and polished with a microforge (Narishige MF-900). Electrodes had a resistance of 2.8–3.5 MΩ when filled with the following internal solution (mM): 105 Cesium methanesulphonate (CH3CsO3S), 25 TEA-Cl, 10 HEPES, 11 EGTA, 1 CaCl2, 5 Mg-ATP, 3 Tris2-Phosphocreatine, 3 Na2-Phosphocreatine, and 0.4 Tris-GTP (pH 7.2; 290 mOsm). The composition of the bathing external solution for recordings from cells expressing channels associated with α2δ1 ancillary subunit was (in mM) 88 CsCl, 40 TEA-Cl, 10 HEPES, 10 Glucose, 1 MgCl2, and 5 BaCl2. Osmolality was adjusted to 300 mOsm and pH at 7.4. Native macroscopic currents from PC12 cells and from recombinant Cav1.2 co-transfected with α2δ3 ancillary subunit were recorded with an extracellular solution containing (in mM) 95 CsCl, 30 TEA-Cl, 10 HEPES, 10 Glucose, 1 MgCl2, and 20 BaCl2 (300 mOsm; pH 7.4). S-(-)-Bay K8644 (Tocris 1546) stock solution was prepared in DMSO and stored in aliquots at −20°C; 100 nM working solutions were made fresh in external recording solution (final concentration of vehicle was 0.025%). ω-Conotoxin GVIA and ω-Agatoxin VIA (Alomone Labs) were reconstituted in sterile PBS and stored at −20°C; appropriate volume was added directly into external solution to a final concentration of 1 µM and 200 nM, respectively. GBP (TCI Chemicals, Portland, OH, USA) 100 mM stock solution was prepared in nanopure H2O and diluted into external solution to a final concentration of 100 µM. Perfusion rate was 1.2 mL/min. No corrections were made for liquid junction potentials.
To examine the effect of GBP on recombinant channels in HEK cells, barium currents were evoked by 120 ms repetitive step depolarizations from −100 mV to 0 mV (α2δ1) or from −100 mV to +10 mV (α2δ3) at 0.1 Hz. Current amplitudes were divided by the average value obtained during the perfusion with external control solution and the fraction of remaining (unblocked) current (I/IControl) was plotted against time. The steady state value of unblocked current after 3 min of GBP perfusion is reported as fraction of inhibition (IGBP/IControl). Current–voltage relationship for channels expressing α2δ1 subunit was studied with 180 ms pulses from −60 mV to +30 mV at 5 mV increments (Vh= −100 mV). Fitting of peak current values obtained with increasing depolarizations was performed using the modified Boltzmann equation:
where IBa is the peak current at test potential Vm, IBa,Max is the maximum peak current observed, Er is the apparent reversal potential, V50 is the midpoint voltage for activation, and k is the slope factor.
The effect of GBP on neuronal L-type currents was studied by examining native barium currents from undifferentiated PC12 cells, evoked by 200 ms voltage ramps from −100 to +100 mV applied every 10 s. Current amplitude at the peak of the resultant IV curve was measured in the presence of ω-Conotoxin GVIA and ω-Agatoxin VIA as control value, and compared to the values obtained after the consecutive application of S-(-)-Bay K8644 and GBP. Nitrendipine (1 µM) was applied to demonstrate that GBP affects the dihydropyridine-sensitive current component.
mRNA expression analysis using quantitative real-time PCR
PC12 cell pellets were homogenized in the presence of TRI-Reagent (Ambion) and total RNA isolated using the MagMAXTM-96 for Microarrays preparation kit (Life Technologies, AM1839). Total cDNA was synthesized from total RNA using a high capacity cDNA reverse transcription kit (Applied Biosystems, 4368814). Gene-specific qPCR reactions utilized the KAPA Probe Fast Universal qPCR Kit (KK4702) and TaqManTM probes from Life Technologies and Integrated DNA Technologies (IDT). Quantitative PCR reactions were performed on a Bio-Rad CFX Real-Time Systems using cycling conditions of 95°C for 3 min then 95°C for 15 s followed by 60°C for 45 s for 40 cycles. Specific TaqManTM probes used in this study: rCav1.1(Rn01490941_m1), rCav1.2(Rn00709287_m1), rCav1.3(Rn00692157_m1), rCav2.1(Rn00563825_m1), rCav2.2(Rn00595911_m1), rCav2.3(Rn00494444_m1), rA2D1(Rn01442581_m1), rA2D2 (Rn00457825_m1), and rA2D3(Rn00598241_m1) from Life Technologies, and Glyceraldehyde 3-phosphate dehydrogenase (rGAPDH) (Rn.PT.56a.35727291) from IDT.
Statistical analysis
The Kolmogorov–Smirnov two-sample test (K-S test) was used to compare distributions of sEPSC amplitudes and inter-event intervals.33 All other data were compared using the two-tailed t-test or by one-way analysis of variance (ANOVA) where appropriate. Significance was attributed if p < 0.05. Mini Analysis program (Synaptosoft, Decatur, GA, USA) was used to generate cumulative probability plots. All other graphing and statistical analyses were performed using Origin (v8.6 or 2018, Origin Lab., Northampton, MA, USA).
Results
Nitrendipine reduces the frequency of lamina II sEPSCs in animals subject to CCI
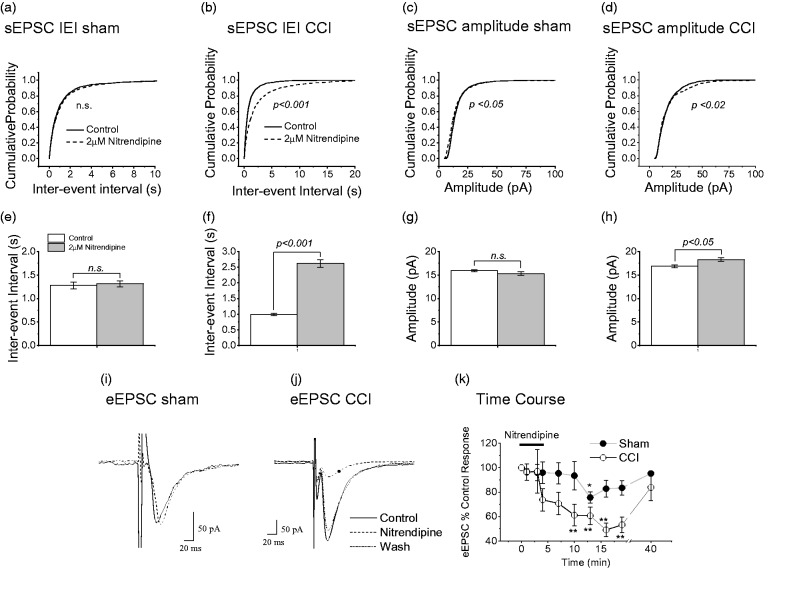
As already mentioned, peripheral nerve injury increases the excitability of the superficial dorsal horn.3,18–21 To assess the role of presynaptic L-type Ca2+ channels in this process, we examined the effect of nitrendipine (2 µM) on spontaneous excitatory postsynaptic currents (sEPSCs) in lamina II neurons of rats subject to sciatic CCI. Nitrendipine was highly effective in reducing frequency (increasing inter-event interval, IEI) of sEPSCs in rats subject to CCI (Figure 1(b); p < 0.001; K-S test: Figure 1(d); p < 0.001; t-test). By contrast in sham-operated rats, cumulative probability plots show that nitrendipine was without effect on IEI (Figure 1(a); p > 0.15; K-S test) of sEPSCs in lamina II neurons. There was also no significant effect of nitrendipine on average sEPSC IEI (Figure 1(c); p > 0.05; t-test).
Figure 1.
Effects of nitrendipine on sEPSC and eEPSCs recorded from lamina II neurons of CCI and sham-operated rats. Cumulative probability plots for effect of nitrendipine on inter-event interval (IEI) of sEPSCs from sham-operated animals (a) and those subject to CCI (b). (c and d) Same data replotted as mean IEI. Cumulative probability plots for effect of nitrendipine on amplitude of sEPSCs from sham-operated animals (e) and those subject to CCI (f). (g and h) same data replotted as mean IEI. For shams, IEI data for 1629 events and nitrendipine data for 1398 events in 24 neurons. For CCI, IEI data for 1853 events and nitrendipine data for 1379 events in 27 neurons. For shams, amplitude data for 1561 events and nitrendipine data for 1415 events in 24 neurons. For CCI, amplitude data for 1898 events and nitrendipine data for 1389 events in 27 neurons. Note that p values were derived from K-S test for cumulative probability graphs and paired t-test for the bar graphs. **p < 0.001 and *p < 0.05 compared to pre-drug amplitude (paired t-test). In each case, recordings were obtained from only one neuron per slice. Sample recordings of eEPSCs following DRZ stimulation from sham-operated animals (i) and those subject to CCI (j), before, during, and after superfusion of 2 µM nitrendipine. (k) Time course of effect of nitendipine on eEPSC amplitude in 13 neurons from sham-operated animals and 12 neurons form animals subject to CCI. Error bars = SEM. **p < 0.001 and *p < 0.05 compared to pre-drug amplitude (paired t-test). CCI: chronic constriction injury; eEPSC: evoked excitatory postsynaptic currents.
Nitrendipine slightly decreased sEPSC amplitude in sham-operated rats according to a cumulative probability plot (Figure 1(e); p < 0.05; K-S test), but the averaged amplitude was unaffected (Figure 1(g); p > 0.05; t-test). Somewhat unexpectedly, nitrendipine produced a slight increase in sEPSC amplitude in neurons from animals subject to CCI and this was significant according to K-S statistics (Figure 1(f); p < 0.02) and from consideration of average event amplitude (Figure 1(h); p < 0.05; t-test).
Nitrendipine reduces the amplitude of lamina II eEPSCs in animals subject to CCI
In neurons from sham-operated animals, superfusion of nitrendipine (2 µM) caused a small reduction in the amplitude of evoked EPSCs (eEPSCs) by an average of 14.85 ± 5.53% (n = 13, p < 0.05, paired t-test). This reduction is in agreement with earlier reports on effect of L-type channel blocker on evoked EPSCs in the superficial dorsal horn neurons.34,35 By contrast, in rats subject to CCI, nitrendipine caused a much larger reduction of 40.23 ± 6.77% (n = 12, p < 0.005, paired t-test) in the amplitude of eEPSCs. Sample traces are illustrated in Figure 1(i) and (j). Figure 1(k) illustrates the time course of the effect of nitrendipine on eEPSCs in CCI and sham-operated rats. The time course of action of nitrendipine varied from cell to cell. In general, the effect took about 10–15 min to develop and the washout time was 30 min or longer. Although we cannot rule out the possibility that some of the recorded events were polysynaptic, the eEPSCs observed under the conditions of our experiments were of short duration and monophasic and thus may have been predominantly monosynaptic.
Effects of nitrendipine on mechanical allodynia
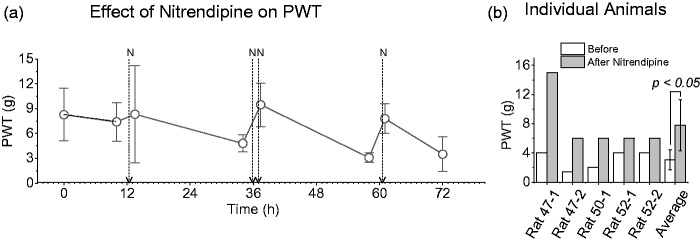
Following CCI, animals expressing a PWT of <6 g were defined as allodynic. PWT were measured 30 min after an IP injection of 5 mg/kg nitredipine. Pharmacokinetic studies of nitrendipine in rats are scarce but literature suggests that plasma concentration peaks at 1.2 h following intravenous, oral, or intraduodenal administration.36 The changes in PWT in response to a series of nitrendipine injections are shown in Figure 2(a). Injections given on day 1 increased PWT strongly in some animals and weakly in others. On day 2, two injections of 5 mg/kg were administered 60 min apart and PWT addressed 30 min after the second injection. On day 3, animals received a single injection of nitrendipine and all five animals responded with an increase in PWT. The progressive improvement in effect may reflect drug accumulation following repeated injections. The elimination half time in rats is 57 h.36 Data for each animal are shown in Figure 2(b). Vehicle injections failed to affect PWT of the ipsilateral limb (Figure 2(a)). The PWT for the paw contralateral to CCI was >15 g and therefore any non-specific effect of nitrendipine on could not be determined.
Figure 2.
Effects of nitrendipine on PWT in animals subject to CCI. (a) Averaged data from five animals, arrows labeled N indicate times of IP injections of 5 mg/kg nitrendipine. Dashed line with gray symbols illustrates minimal effect of vehicle injections (two animals) (b) Data from each animal at the 36 h time point to illustrate the variation in nitrendipine effectiveness. PWT: paw withdrawal threshold.
Effect of α2δ–1 on properties of exogenous L-type channel complexes in HEK cells
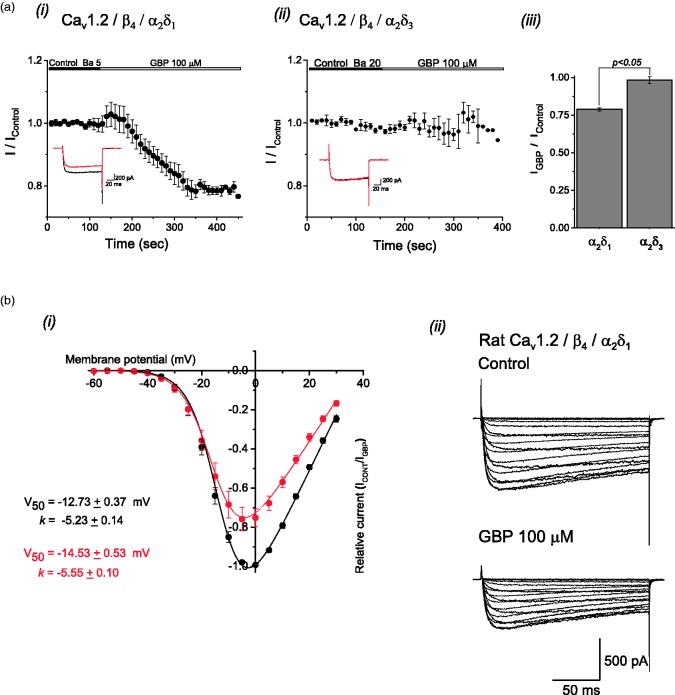
The above results suggest that L-type Ca2+ channels contribute to neurotransmitter release in nerve injured animals. Since Savalli et al. (2016) observed a potentiation of specifically Cav1.2 L-type channel current following α2δ–1 overexpression in Xenopus oocytes, we tested whether GBP (100 µM) has a direct effect on Cav1.2-mediated currents in the presence of α2δ–1. We used HEK293 cells expressing Cav1.2/β4/α2δ–1 and examined peak amplitude of calcium current before and after acute application of GBP (100 µM). Since GBP does not bind to the α2δ–3 subunit,31 we performed experiments on HEK293 cells expressing Cav1.2/ β4/α2δ–3 as a control. GBP (100 µM) produced a significant reduction (Figure 3(a, i), p < 0.05, ANOVA) of Cav1.2/ β4/α2δ–1 peak calcium currents with no significant effect on the peak amplitude on Cav1.2/ β4/α2δ–3 currents (Figure 3(a, ii), p > 0.05, ANOVA). This relative effect is summarized in Figure 3(a, iii) showing the effect of GBP after a 3 min application. The average inhibition of peak calcium current by GBP in Cav1.2/ β4/α2δ–1 cells was ∼25% (p < 0.05, ANOVA) as shown by current traces in Figure 3(b, ii). There were no significant effects of GBP on voltage dependence of activation (p > 0.05, ANOVA) as shown in Figure 3(b, i) by comparing the values of fitted parameters corresponding to the continuous lines obtained by the Boltzmann function before (V50 = −12.73 ± 0.37 mV, k = −5.23 ± 0.14) and after (V50 = −14.53 ± 0.53 mV, k = −5.55 ± 0.10) GBP application.
Figure 3.
Gabapentin block of L-type Cav1.2 channel currents expressed in HEK293F cells. (a) Time course of inhibitory effect of 100 µM GBP on Ba2+ currents recorded from HEK cells expressing Cav1.2 channels in combination with either α2δ–1 (i) or α2δ–3 (ii) auxiliary subunits. The averaged fraction of peak current amplitude is plotted against time. Cells were perfused with GBP during the periods indicated by horizontal bars. Inserts correspond to representative macroscopic currents obtained before (black) and after (red) the application of GBP. (iii) The fraction of inhibition after 3 min of drug application is shown as a bar graph. Number of cells examined are indicated in brackets; the asterisk indicates a statistical significance p< 0.05. (b) Normalized current-voltage curves (i) obtained for the recombinant Cav1.2 channel in the presence (red symbols) and in the absence (black symbols) of 100 µM GBP. The values of fitted parameters corresponding to the continuous lines obtained by Boltzmann function fits are shown. Application of GBP produced an average current inhibition of ∼25% with no significant changes in the voltage dependence parameters. Data are plotted as mean ± SEM (n = 5 for each data point). (ii) Typical traces from a representative cell show the effect of GBP on the I-V relationship with no apparent modification of current kinetics.
Gabapentin reduces the native dihydropyridine-sensitive current in PC-12 cells
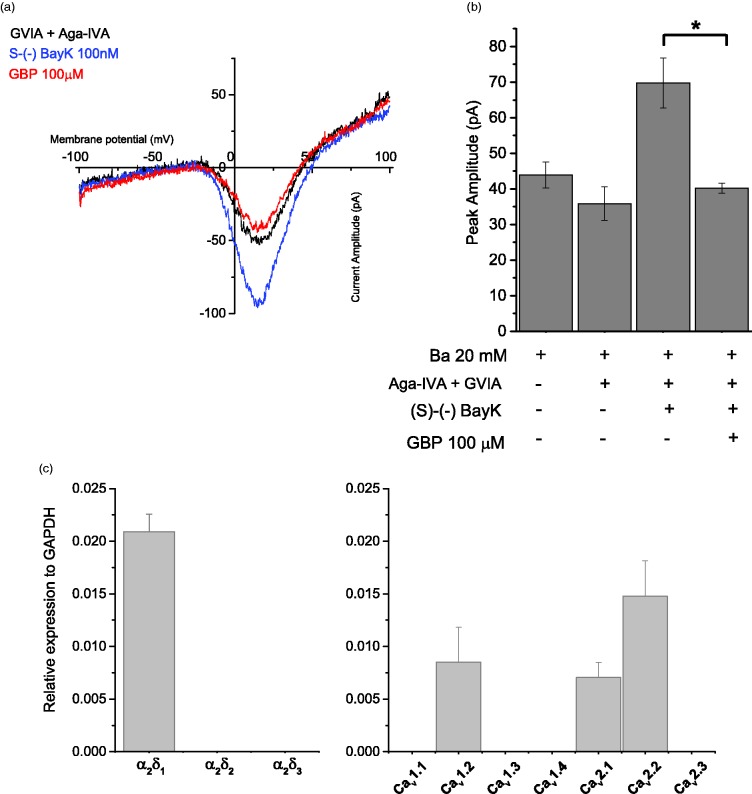
To further confirm that GBP inhibits L-type channels, we examined native L-type Ca2+currents expressed in undifferentiated pheochromocytoma cells (PC-12 cells). PC-12 cells have long been studied as a model system of neuronal physiology and biochemistry and the effects of pharmacological agents due to their similarity to sympathetic neurons upon differentiation.37 Undifferentiated PC-12 cells mainly express N- and L-type Ca2+ channels although studies have also reported the presence of an ω-Agatoxin-VIA-sensitive (P/Q-type) component.38 In order to isolate the L-type current in undifferentiated PC-12 cells, it was necessary to remove both N-type and P/Q-type currents with ω-Conotoxin GVIA (1 µM) and ω-Agatoxin VIA (200 nM; Figure 4(b)). Further, in order to obtain a large enough L-type current from which to establish any effects of GBP, we also applied the L-type agonist S-(S)-BayK8644 (100 nM) (Figure 4(b)). The resulting whole current represents a dihydropyridine-sensitive inward current as it was blocked by nitrendipine (1 µM) (Figure 4(c)). GBP (100 µM) significantly inhibited the peak amplitude of this current by approximately 40% (Figure 4(c), p < 0.05, ANOVA).
Figure 4.
Gabapentin inhibits a dihydropyridine-sensitive inward current in undifferentiated PC12 cells. a. Current-voltage relation of barium currents were examined in the presence of 1 mM ω-Conotoxin GVIA and 200nM ω-Agatoxin VIA to eliminate the contribution of native non-L-type channels. Currents were elicited by voltage ramps from −100 to +100mV at 1mV/ms, and cells were exposed to 100nM of the agonist (S)-(-)-BayK 8644 prior to the application of 100 µM GBP. b. The bar graph shows the average of peak amplitude values (n = 5) obtained in cells perfused with external recording solution (Ba2+ 20mM), compared to that obtained after the consecutive addition of peptide toxins, BayK 8644 and GBP. Data are plotted as Mean ± S.E.M. The asterisk indicates the change in current amplitude after perfusion with GBP is statistically significant at p < 0.05. c. Endogenous expression of ancillary α2δ subunits (Left) and HVA α1 subunits (Right) in PC12 cells was quantified by RT-PCR. Expression levels were calculated relative to the “housekeeping gene” GAPDH (Glyceraldehyde 3-phosphate dehydrogenase). Mean values ± S.D. of three samples.
In order to confirm the expression of selected Ca2+ channel complex proteins, we performed qRT-PCR on RNA isolated from PC-12 cells. Figure 4(c) confirms the endogenous expression of the Cav1.2 L-type channel together with the Cav2.2 (N-type) and Cav2.1 (P/Q-type) channels. Figure 4(d) demonstrates that PC-12 cells also express significant amounts of the α2δ–1, α2δ–2, and α2δ–3 proteins in support of the data from HEK293F cells showing that GBP requires the α2δ–1 subunit in order to elicit an inhibitory effect on L-type currents.
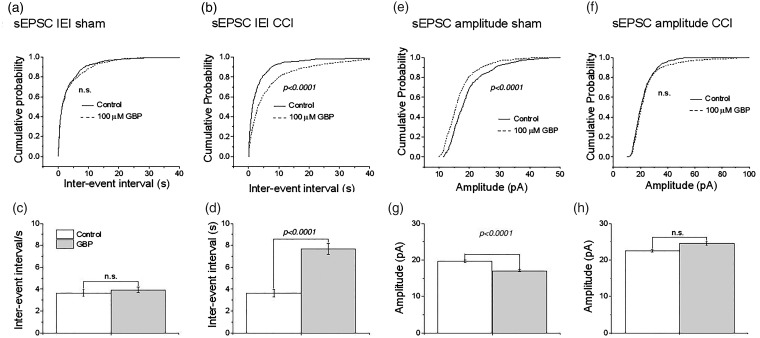
Gabapentin reduces sEPSC frequency in CCI but not sham-operated rats
To test whether the effectiveness of nitrendipine after CCI corresponds to increased expression of α2δ–1 in primary afferent neurons as reported in Luo et al.,24,25 we next examined the effect of the α2δ–1 ligand GBP at a clinically relevant concentration (100 µM).39 GBP did not alter IEI in neurons from sham-operated animals (Figure 5(a) and (c); p > 0.05) but profoundly decreased it in neurons from animals subject to CCI (Figure 5(b) and (d); p < 0.0001). GBP produced a small but significant reduction in the sEPSC amplitude in neurons from sham-operated animals (Figure 5(e) and (g); p < 0.001), but there was no significant effect on neurons from animals subject to CCI (Figure 5(f) and (h)). A comparison of the effects of nitrendipine and GBP on sEPSCs in CCI and sham-operated rats is shown in Table 1.
Figure 5.
Effects of GBP on sEPSCs recorded from lamina II neurons of CCI and sham-operated rats. Cumulative probability plots for effect of 100 µM GBP on (a) inter-event interval (IEI) of sEPSCs in sham-operated animal and (b) in animals subject to CCI. (c and d) same data replotted as bar graphs. Cumulative probability plots for effect of 100 µM GBP on (e) amplitude of sEPSCs in sham-operated animal and (f) in animals subject to CCI. (g and h) same data replotted as bar graphs. For sham-operated rats, control data for 515 events and GBP data for 625 events in 7 neurons from 5 rats. For CCI ,control data for 886 events and GBP data for 721 events in 9 neurons from 8 rats. p-values were derived from K-S test for cumulative probability graphs and paired t-test for the bar graphs. **p < 0.001 and *p < 0.05 compared to pre-drug amplitude (paired t-test). In each case, recordings were obtained from only one neuron per slice. sEPSC: spontaneous excitatory postsynaptic currents; CCI: chronic constriction injury.
Table 1.
Comparison of effects of nitrendipine and gabapentin on sEPSCs recorded from CCI and sham-operated rats.
| NTR (2 µM) | GBP (100 µM) | |
|---|---|---|
| CCI sEPSC frequency | ↓ 62.23% | ↓ 52.8% |
| CCI sEPSC amplitude | ↑ 8.38% | Not significant |
| Sham sEPSC frequency | Not significant | Not significant |
| Sham sEPSC amplitude | Not significant | ↓ 13.4% |
Note: L-type channel blockade using 2 µM NTR produces similar effects on sEPSCs as a clinically relevant concentration (100 µM) of α2δ–1 ligand GBP suggesting that GBP may produce effects on sEPSCs by binding to α2δ–1 associated with L-type channels. The effects of GBP are exclusively on frequency in CCI rats suggesting that GBP acts presynaptically. GBP: gabapentin; CCI: chronic constriction injury; sEPSCs: spontaneous excitatory postsynaptic currents; NTR: nitrendipine.
Discussion
We tested the hypothesis that the increased expression of α2δ–1 at nerve terminals5 after nerve injury5 alters the properties of L-type Cav1.2 channels such that influx of Ca2+ occurs at physiological membrane potential.27 This notion is supported by the observation that both GBP and nitrendipine produced a substantial increase in the IEI of sEPSCs in neurons from nerve injured animals (Figure 1(b) and (d) and Figure 5(b) and (d), Table 1), whereas in sham-operated animals, both nitrendipine and GBP were without effect (Figure 1(a) and (c) and Figure 5(a) and (c)). This is also supported by a recent report that intrathecal administration of the L-type blocker nifedipine reversed tactile allodynia in mice engineered to overexpress α2δ–1.40 It is also consistent with our observation that nitrendipine exerted an antiallodynic effect in nerve-injured animals (Figure 2).
Following CCI, nitrendipine acts predominantly presynaptically in lamina II as effects on sEPSC amplitude were small (Figures 1(f) and (h)), whereas those on sEPSC frequency were highly significant (Figure 1(b) and (d)). The observed slight increase in sEPSC amplitude in animals subject to CCI was unexpected (Figure 1(f) and (h)). One possibility is that there exists a weak blockade of postsynaptic dendritic K+ channels by nitrendipine,41,42 and that a resulting increase in space constant may increase the amplitude of events recorded in the soma. It remains to be determined why this effect was observed in animals subject to CCI (Figure 1(f) and (h)) but not from sham-operated animals (Figure 1(e) and (g)).
Given the ubiquitous distribution of L-type Ca2+ channels in the nervous system,43 it is possible that some of the anti-allodynic actions of nitredipine are exerted at thalamic or cortical levels or perhaps by actions on descending pain control mechanisms. We think this rather unlikely as no overt CNS effects are observed in patients receiving nitrendipine for the management of cardiovascular disorders.
Like nitrendipine, the effects of GBP were predominantly presynaptic as there was a large increase in IEI in neurons from animals subject to CCI (Figure 5(b) and (d)) but not in neurons from sham-operated animals (Figure 5(a) and (c)). This agrees with literature showing that α2δ–1 upregulation occurs in primary afferent neurons after CCI but not postsynaptically in the dorsal horn.24,25 There is no obvious explanation for the observation that GBP reduces sEPSC amplitude in neurons from sham-operated animals (Figure 5(e) and (g)) but not in those subject to CCI (Figure 5(f) and (h)).
Generally, N-type Ca2+ channels play a predominant role in neurotransmitter release from primary afferent terminals.34,35,44 Thus, our observation that nitrendipine increases the IEI of sEPSC and the amplitude of eEPSCs after CCI is intriguing as it suggests that L-type channels contribute to this process when nerves are injured and α2δ–1 is upregulated. This may be explicable in terms of the findings of Savalli et al. (2016) who showed that α2δ–1 upregulation alters voltage sensitivity of Cav1.2 channels. This may indicate that they are more likely to be activated by presynaptic action potentials and is supported by the classical observation that in DRG neurons N-type Ca2+ channels begin to activate around −20 mV and with depolarization to approximately −10 mV being required to open L-type channels.28 Such activation would be more likely to participate in the release process as α2δ–1 indiscriminately recruits voltage gated Ca2+ channels to release sites.45
Using both the HEK293F expression system and PC-12 cells, we have shown that acute application of a clinically relevant concentration of GBP produces a robust inhibition of L-type currents. In HEK293F cells, this depression is dependent on the co-expression of α2δ–1 (Figure 3(a), (i)) but is not observed when α2δ–3 is expressed (Figure 3(a), (ii)) as these subunits do not bind GBP.31 In rats subject to peripheral nerve injury, we have noted that GBP reduces the amplitude and frequency of sEPSCs recorded in excitatory neurons46 which receive input from C-fibers.47 GBP is less effective at synapses onto inhibitory neurons which receive input from both non-nociceptive Aβ-fibers48 and unmyelinated fibers.49 If the effects of GBP involve an action on Cav1.2 L-type channels as a result of inhibition of α2δ–1 function, its lack of effect on input to inhibitory neurons and preferential effect on excitatory neurons may reflect the relative paucity of L-type Ca2+ channels on large, non-nociceptive DRG neurons compared to small, nociceptive DRG neurons.10,50 It should also be noted that long-term application of GBP is more effective in suppressing high-voltage activated ICa in small DRG neurons compared to large neurons,39 suggesting that α2δ–1 may be preferentially expressed in nociceptive neurons which, as mentioned above, predominate the excitatory synaptic input to excitatory neurons.
While cardiovascular actions of dihydropyridines may limit their use in the treatment of neuropathic pain, their use as adjunct agents has been suggested.51 There is also increasing interest in the possible use of broad spectrum dihydropyridine-related Ca2+ channel blockers such as M4 that inhibits Cav1.2 (L-type), Cav 2.2 (N-type), and the Cav 3.2 and 3.3 T-type channels.52
Authors’ contributions
SRAA designed aspects of the study, carried out experiments with GBP, and wrote first draft of manuscript. SRAA and EG designed and carried out experiments on PC12 and HEK cells and contributed to writing final manuscript. KJ performed transfections and cell culture of HEK and PC-12 cells. JRT performed qPCR experiments and analysis. SB designed, carried out, and interpreted experiments with nitrendipine in spinal cord. TJ designed, executed, and interpreted behavioral experiments with nitrendipine. TPS provided CIHR funding and laboratory facilities, supervised the work of SRAA, EG, JRT, and KJ, and contributed to experimental design. PAS provided NSERC funding and laboratory facilities, supervised the work of SRAA, SB, and TJ, contributed to experimental design, edited and wrote final manuscript. All authors reviewed drafts of manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by grants from the Natural Sciences and Engineering Research Council (NSERC- Canada; PAS) and the Canadian Institutes of Health Research (CIHR, T.P.S.; #10677). TPS is also supported by the Canada Research Chair in Biotechnology and Genomics-Neurobiology.
References
- 1.Dolphin AC. Calcium channel auxiliary alpha2delta and beta subunits: trafficking and one step beyond. Nat Rev Neurosci 2012; 13: 542–555. [DOI] [PubMed] [Google Scholar]
- 2.Dolphin AC. Voltage-gated calcium channels and their auxiliary subunits: physiology and pathophysiology and pharmacology. J Physiol 2016; 594: 5369–5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alles SRA andSmith PA.. The etiology and pharmacology of neuropathic pain. Pharmacol Rev 2018; 70: 315–347. [DOI] [PubMed] [Google Scholar]
- 4.Gee NS Brown JP Dissanayake VU Offord J Thurlow R andWoodruff GN.. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the alpha2delta subunit of a calcium channel. J Biol Chem 1996; 271: 5768–5776. [DOI] [PubMed] [Google Scholar]
- 5.Bauer CS Nieto-Rostro M Rahman W Tran-Van-Minh A Ferron L Douglas L Kadurin I Sri Ranjan Y Fernandez-Alacid L Millar NS Dickenson AH Lujan R andDolphin AC.. The increased trafficking of the calcium channel subunit alpha2delta-1 to presynaptic terminals in neuropathic pain is inhibited by the alpha2delta ligand pregabalin. J Neurosci 2009; 29: 4076–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finnerup NB Sindrup SH andJensen TS.. The evidence for pharmacological treatment of neuropathic pain. Pain 2010; 150: 573–581. [DOI] [PubMed] [Google Scholar]
- 7.Kim KJ Yoon YW andChung JM.. Comparison of three rodent models of neuropathic pain. Exp Brain Res 1997; 113: 200–206. [DOI] [PubMed] [Google Scholar]
- 8.Mosconi T andKruger L.. Fixed-diameter polyethylene cuffs applied to the rat sciatic nerve induce a painful neuropathy: ultrastructural morphometric analysis of axonal alterations. Pain 1996; 64: 37–57. [DOI] [PubMed] [Google Scholar]
- 9.Abdulla FA andSmith PA.. Axotomy and autotomy-induced changes in the excitability of rat dorsal root ganglion neurons. J Neurophysiol 2001; 85: 630–643. [DOI] [PubMed] [Google Scholar]
- 10.Abdulla FA andSmith PA.. Axotomy- and autotomy-induced changes in Ca2+and K+ channel currents of rat dorsal root ganglion neurons. J Neurophysiol 2001; 85: 644–658. [DOI] [PubMed] [Google Scholar]
- 11.Abdulla FA andSmith PA.. Changes in Na+ channel currents of rat dorsal root ganglion neurons following axotomy and axotomy-induced autotomy. J Neurophysiol 2002; 88: 2518–2529. [DOI] [PubMed] [Google Scholar]
- 12.Cummins TR andWaxman SG.. Downregulation of tetrodotoxin resistant sodium currents and upregulation of rapidly repriming tetrodotoxin sensitive sodium current in small sensory neurons after nerve injury. J Neurosci 1997; 17: 3503–3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Everill B Cummins TR Waxman SG andKocsis JD.. Sodium currents of large (Abeta-type) adult cutaneous afferent dorsal root ganglion neurons display rapid recovery from inactivation before and after axotomy. Neuroscience 2001; 106: 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sleeper AA Cummins TR Dib-Hajj SD Hormuzdiar W Tyrrell L Waxman SG andBlack JA.. Changes in expression of two tetrodotoxin-resistant sodium channels and their currents in dorsal root ganglion neurons after sciatic nerve injury but not rhizotomy. J Neurosci 2000; 20: 7279–7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zamponi GW Lewis RJ Todorovic SM Arneric SP andSnutch TP.. Role of voltage-gated calcium channels in ascending pain pathways. Brain Res Rev 2009; 60: 84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waxman SG andZamponi GW.. Regulating excitability of peripheral afferents: emerging ion channel targets. Nat Neurosci 2014; 17: 153–163. [DOI] [PubMed] [Google Scholar]
- 17.Wall PD andDevor M.. Sensory afferent impulses result from dorsal root ganglia as well as from the periphery in normal and nerve-injured rats. Pain 1983; 17: 321–339. [DOI] [PubMed] [Google Scholar]
- 18.Balasubramanyan S Stemkowski PL Stebbing MJ andSmith PA.. Sciatic chronic constriction injury produces cell-type specific changes in the electrophysiological properties of rat Substantia Gelatinosa neurons. J Neurophysiol 2006; 96: 579–590. [DOI] [PubMed] [Google Scholar]
- 19.Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature 1983; 306: 686–688. [DOI] [PubMed] [Google Scholar]
- 20.Sandkuhler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev 2009; 89: 707–758. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y Balasubramanyan S Lai AY Todd KG andSmith PA.. Effects of sciatic nerve axotomy on excitatory synaptic transmission in rat Substantia Gelatinosa. J Neurophysiol 2009; 102: 3203–3215. [DOI] [PubMed] [Google Scholar]
- 22.Zhou C andLuo ZD.. Electrophysiological characterization of spinal neuron sensitization by elevated calcium channel alpha-2-delta-1 subunit protein. Eur J Pain 2014; 18: 649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou C andLuo ZD.. Nerve injury-induced calcium channel alpha-2-delta-1 protein dysregulation leads to increased pre-synaptic excitatory input into deep dorsal horn neurons and neuropathic allodynia. Eur J Pain 2015; 19: 1267–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo ZD Chaplan SR Higuera ES Sorkin LS Stauderman KA Williams ME andYaksh TL.. Upregulation of dorsal root ganglion (alpha)2(delta) calcium channel subunit and its correlation with allodynia in spinal nerve-injured rats. J Neurosci. 2001; 21: 1868–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo ZD Calcutt NA Higuera ES Valder CR Song YH Svensson CI andMyers RR.. Injury type-specific calcium channel alpha 2 delta-1 subunit up-regulation in rat neuropathic pain models correlates with antiallodynic effects of gabapentin. J Pharmacol Exp Ther 2002; 303: 1199–1205. [DOI] [PubMed] [Google Scholar]
- 26.Boroujerdi A Zeng J Sharp K Kim D Steward O andLuo ZD.. Calcium channel alpha-2-delta-1 protein upregulation in dorsal spinal cord mediates spinal cord injury-induced neuropathic pain states. Pain 2011; 152: 649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li CY Song YH Higuera ES andLuo ZD.. Spinal dorsal horn calcium channel α2δ-1 subunit upregulation contributes to peripheral nerve injury-induced tactile allodynia. J Neurosci 2004; 24: 8494–8499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Savalli N Pantazis A Sigg D Weiss JN Neely A andOlcese R.. The alpha2delta-1 subunit remodels CaV1.2 voltage sensors and allows Ca2+ influx at physiological membrane potentials. J Gen Physiol 2016; 148: 147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fox AP Nowycky MC andTsien RW.. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurons. J Physiol 1987; 394: 149–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gould RJ Murphy KM andSnyder SH.. [3H]nitrendipine-labeled calcium channels discriminate inorganic calcium agonists and antagonists. Proc Natl Acad Sci U S A 1982; 79: 3656–3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.EichelbaumMikus M Mast G Fischer V Kuhlmann C andMachleidt UC.. Pharmacokinetics and pharmacodynamics of nitrendipine in healthy subjects and patients with kidney and liver disease. J Cardiovasc Pharmacol 1988; 12: S6–S10. [DOI] [PubMed] [Google Scholar]
- 32.Marais E Klugbauer N andHofmann F.. Calcium channel alpha(2)delta subunits-structure and Gabapentin binding. Mol Pharmacol 2001; 59: 1243–1248. [DOI] [PubMed] [Google Scholar]
- 33.Prescott SA andde Koninck Y.. Four cell types with distinctive membrane properties and morphologies in lamina I of the spinal dorsal horn of the adult rat. J Physiol 2002; 539: 817–836. [Database][Mismatch] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bao J Li JJ andPerl ER.. Differences in Ca2+ channels governing generation of miniature and evoked excitatory synaptic currents in spinal laminae I and II. J Neurosci 1998; 18: 8740–8750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heinke B Balzer E andSandkuhler J.. Pre- and postsynaptic contributions of voltage-dependent Ca2+ channels to nociceptive transmission in rat spinal lamina I neurons. Eur J Neurosci 2004; 19: 103–111. [DOI] [PubMed] [Google Scholar]
- 36.Krause HP Ahr HJ Beermann D Siefert HM Suwelack D andWeber H.. The pharmacokinetics of nitrendipine. I. Absorption, plasma concentrations, and excretion after single administration of [14C]nitrendipine to rats and dogs. Arzneimittelforschung 1988; 38: 1593–1599. [PubMed] [Google Scholar]
- 37.Shafer TJ andAtchison WD.. Transmitter, ion channel and receptor properties of pheochromocytoma (PC12) cells: a model for neurotoxicological studies. Neurotoxicology 1991; 12: 473–492. [PubMed] [Google Scholar]
- 38.LiuFelix HR Gurnett CA Waard MD Witcher DR andCampbell KP.. Expression and subunit interaction of voltage-dependent Ca2+ channels in PC12 cells. J Neurosci 1996; 16: 7557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Biggs JE Boakye PA Ganesan N Stemkowski PL Lantero A Ballanyi K andSmith PA.. Analysis of the long-term actions of gabapentin and pregabalin in dorsal root ganglia and substantia gelatinosa. J Neurophysiol 2014; 112: 2398–2412. [DOI] [PubMed] [Google Scholar]
- 40.Chang E Chen X Kim M Gong N Bhatia S andLuo ZD.. Differential effects of voltage-gated calcium channel blockers on calcium channel alpha-2-delta-1 subunit protein-mediated nociception. Eur J Pain 2015; 19: 639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hatano N Ohya S Muraki K Giles W andImaizumi Y.. Dihydropyridine Ca2+ channel antagonists and agonists block Kv4.2, Kv4.3 and Kv1.4 K+ channels expressed in HEK293 cells. Br J Pharmacol 2003; 139: 533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fagni L Bossu JL andBockaert J.. Inhibitory effects of dihydropyridines on macroscopic K+ currents and on the large-conductance Ca(2+)-activated K+ channel in cultured cerebellar granule cells. Pflugers Arch 1994; 429: 176–182. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka O Sakagami H andKondo H.. Localization of MRNAs of voltage-dependent Ca(2+)-channels: four subtypes of alpha 1- and beta-subunits in developing and mature rat brain. Brain Res Mol Brain Res 1995; 30: 1–16. [DOI] [PubMed] [Google Scholar]
- 44.Motin L andAdams DJ.. Omega-conotoxin inhibition of excitatory synaptic transmission evoked by dorsal root stimulation in rat superficial dorsal horn. Neuropharmacology 2008; 55: 860–864. [DOI] [PubMed] [Google Scholar]
- 45.Hoppa MB Lana B Margas W Dolphin AC andRyan TA.. Alpha2delta expression sets presynaptic calcium channel abundance and release probability. Nature 2012; 486: 122–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alles SRA Bandet MV Eppler K Winship IR Noh MC Baker G Ballanyi K andSmith PA.. Acute anti-allodynic action of gabapentin in dorsal horn and primary somatosensory cortex: correlation of behavioural and physiological data. Neuropharmacology 2017; 113A: 576–590. [DOI] [PubMed] [Google Scholar]
- 47.Peirs C andSeal RP.. Neural circuits for pain: recent advances and current views. Science 2016; 354: 578–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Daniele CA andMacDermott AB.. Low-threshold primary afferent drive onto gabaergic interneurons in the superficial dorsal horn of the mouse. J Neurosci 2009; 29: 686–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng J Lu Y andPerl ER.. Inhibitory neurones of the spinal substantia gelatinosa mediate interaction of signals from primary afferents. J Physiol 2010; 588: 2065–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scroggs RS andFox AP.. Calcium current variation between acutely isolated adult rat dorsal root ganglion neurons of different size. J Physiol 1992; 445: 639–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lipman AG. Analgesic drugs for neuropathic and sympathetically maintained pain. Clin Geriatr Med 1996; 12: 501–515. [PubMed] [Google Scholar]
- 52.Gadotti VM Bladen C Zhang FX Chen L Gunduz MG Simsek R Safak C andZamponi GW.. Analgesic effect of a broad-spectrum dihydropyridine inhibitor of voltage-gated calcium channels. Pflugers Arch 2015; 467: 2485–2493. [DOI] [PubMed] [Google Scholar]