Short abstract
Background
Offset analgesia is a disproportionate decrease of pain perception following a slight decrease of noxious thermal stimulus and attenuated in patients with neuropathic pain. We examined offset analgesia in patients with heterogeneous chronic pain disorders and used functional magnetic resonance imaging to explore modification of cerebral analgesic responses in comparison with healthy controls.
Results
We recruited seventeen patients with chronic pain and seventeen age-, sex-matched healthy controls. We gave a noxious thermal stimulation paradigm including offset analgesia and control stimuli on the left volar forearm, while we obtained a real-time continuous pain rating and a whole-brain functional magnetic resonance imaging. Baseline, first plateau (5 s), increment (5 s), and second plateau (20 s) temperatures of offset analgesia stimulus were set at 32°C, 46°C, 47°C, and 46°C, respectively. Control stimulus included 30-s 46°C stimulus or only the first 10 s of offset analgesia stimulus. We evaluated magnitude of offset analgesia, analyzed cerebral activation by thermal stimulation, and further compared offset analgesia-related activation between the groups. Magnitude of offset analgesia was larger in controls than in patients (median: 28.9% (interquartile range: 11.0–56.0%) vs. 19.0% (4.2–48.7%), p = 0.047). During the second plateau, controls showed a larger blood oxygenation level-dependent activation than patients at the putamen, anterior cingulate, dorsolateral prefrontal cortices, nucleus accumbens, brainstem, and medial prefrontal cortex (p < 0.05), which are known to mediate either of descending pain modulation or reward responses. Offset analgesia-related activity at the anterior cingulate cortex was negatively correlated with neuropathic component of pain in patients with chronic pain (p = 0.004).
Conclusions
Attenuation of offset analgesia was associated with suppressed activation of the descending pain modulatory and reward systems in patients with chronic pain, at least in the studied cohort. The present findings might implicate both behavioral and cerebral plastic alterations contributing to chronification of pain.
Clinical trial registry: The Japanese clinical trials registry (UMIN-CTR, No. UMIN000011253; http://www.umin.ac.jp/ctr/)
Keywords: Humans, chronic pain, magnetic resonance imaging, pain perception, analgesia
Introduction
Offset analgesia (OA) is a disproportionate decrease of pain perception following a slight decrease of noxious thermal stimulation on the skin.1 It has been assumed to be associated with temporal sharpening, i.e. top-down inhibitory filtering in the time domain, of nociceptive information.2,3 In other words, OA might “serve to enhance temporal contrasts during dynamic changes of noxious stimulus intensity,” facilitating escape behavior to guard against injurious stimuli.1 Recent studies reported attenuation of OA response in patients with chronic pain (CP) disorders such as neuropathic pain4 and fibromyalgia.5 We have also found that CP showed attenuation of OA and delayed pain perception, which distinguished patients from healthy controls (HCs).6 Pathophysiological significance of such attenuation of OA in chronic pain states, however, remains unresolved.
Cerebral mechanisms of OA were addressed by earlier functional magnetic resonance imaging (fMRI) studies, where OA was found to be associated with activation of the descending pain modulatory system,2,3 a type of endogenous analgesia, at the periaqueductal gray (PAG), rostral ventromedial medulla, and locus ceruleus.2,7,8 Dysfunction of the descending pain modulatory system could possibly be among the central mechanisms of the development of chronic pain.9 Since those earlier neuroimaging findings were limited to healthy volunteer subjects, the cerebral correlates of OA in CP have not been explored. On the other hand, offset of pain stimulus was also found to be associated with activation of the reward system, especially the nucleus accumbens (NAc), in an fMRI study using noxious thermal stimulation.10 Therefore, the reward system might also be associated with OA.
In the present study, we examined OA responses in patients with various chronic pain disorders, as well as in age- and sex-matched HC subjects, and also looked into cerebral activations during OA using simultaneous fMRI. We analyzed differences in cerebral activations during OA between the patients and HCs to uncover cerebral correlates of modified OA in chronic pain. We hypothesized that CP might show modified activity at areas involved in the descending pain modulatory and reward systems on the occurrence of OA. We further tested a hypothesis that these pathophysiologic alterations might correlate with some indicators of OA profiles, demographics, or various psychophysical scores of CP. From those findings, we aimed to delineate roles for descending pain modulatory and reward systems in mediating OA responses and in chronification of pain.
Methods
Subjects
The study protocol was approved by the local institutional review board at Tokyo Medical and Dental University (No. 1525) and registered at the Japanese clinical trials registry (UMIN-CTR, No. UMIN000011253; http://www.umin.ac.jp/ctr/). We obtained written informed consent from all the study participants.
We recruited 19 CPs at the outpatient care clinic of Tokyo Medical and Dental University Hospital of Medicine during the period from February 2014 to June 2016. Inclusion criteria were: (1) patients with complex regional pain syndrome (CRPS), chronic low back pain (CLBP), fibromyalgia, or other chronic pain disorders; (2) age between 20 and 75 years; (3) right-handedness; and (4) duration of persistent pain > three months. Exclusion criteria were: (1) ferromagnetic metal or electric appliances within the body; (2) tattoo; (3) pregnancy; (4) claustrophobia; (5) dementia or other psychiatric disorders; and (6) poor imaging quality due to head motion larger than half of a voxel. Two CP data sets were excluded for poor imaging quality, resulting in 17 CPs (7 males, 10 females; age: 42.5 ± 10.8 years (mean ± SD); age range: 21–60 years), whose data were finally analyzed.
Seventeen HC subjects (8 males, 9 females; age: 41.8 ± 12.5 years (mean ± SD); age range: 24–65 years) were also recruited during the same period with the same inclusion and exclusion criteria except for suffering from pain by posters or word of mouth. Efforts were made to match age and gender between the patients and controls. No data were excluded in the HC group. Details of demographics are shown in Table 1.
Table 1.
Demographic and psychophysical details of subjects.
|
HC (n = 17) |
CP (n = 17) |
||||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | p | |
| Sex | 8M 9F | 7M 10F | 0.73 | ||||
| Age (years) | 41.8 | 12.5 | (24–65) | 42.5 | 10.8 | (21–60) | .86 |
| Weight (kg) | 59.8 | 12.9 | (38–84) | 59.9 | 13.2 | (48–96) | 0.98 |
| Height (cm) | 165.7 | 10.0 | (151–183) | 162.1 | 8.2 | (151–177) | 0.26 |
| BMI | 21.5 | 2.4 | (15.6–25.1) | 22.6 | 3.0 | (19.5–30.6) | 0.2 |
| Pain duration (years) | – | – | – | 9.3 | 7.8 | (0.58–30) | |
| VAS (mm) | 0.1 | 0.2 | (0–1) | 53.4 | 27.4 | (6–100) | <0.0005 |
| Threshold at VAS = 60 (°C) | 46.7 | 1.4 | (45.0–50.2) | 44.9 | 2.4 | (38.9–48.9) | 0.015 |
| PD-Qb | 0.08 | 0.08 | (0–1) | 17.8 | 8.2 | (0–29) | <0.0005 |
| PCSa | 0 | 0 | (0) | 32.7 | 12.0 | (0–47) | <0.0005 |
| BDI | 3.2 | 4.8 | (0–18) | 17.9 | 12.8 | (0–42) | <0.0005 |
| SF-MPQ | 0.2 | 0.5 | (0–2) | 15.3 | 9.1 | (3–35) | <0.0005 |
HC: healthy control; CP: patients with chronic pain; BMI: body mass index; VAS: visual analog scale; PD-Q: PainDETECT questionnaire; PCS: Pain Catastrophizing Scale; BDI: Beck Depression Inventory; SF-MPQ: Short-Form McGill Pain Questionnaire.
an = 11 for HC.
bn = 12 for HC.
Based on many earlier neuroimaging studies on pain perception in general as well as our own experience, within- or between-group contrasts were successfully detected with numbers of subjects around 10 in each group.11–15 We therefore chose a number of subjects at 17 for each group.
Experimental procedures
Subjects were asked not to ingest alcohol or caffeine 24 h before the experiment. On the day of experiment, each subject arrived at the laboratory at around 3 p.m. After receiving an instruction of experimental flow in detail, they signed an informed consent form and filled psychophysical questionnaires, followed by an off-line practice session of thermal pain stimulation. Then, at around 5 p.m., each subject underwent a series of multimodal MRI including pain stimulus fMRI, resting-state fMRI, diffusion tensor imaging (DTI), and anatomical imaging.
Psychophysics
From each subject, we recorded various psychophysical parameters with questionnaires including the current pain intensity in visual analogue scale (VAS), thermal pain threshold for VAS = 60, PainDETECT questionnaire (PD-Q),16 Pain Catastrophizing Scale (PCS),17 Beck Depression Inventory (BDI),18 and Short-Form McGill Pain Questionnaire (SF-MPQ).19 Duration of pain and current therapeutic regimen were also recorded. All the questionnaires were given in Japanese versions. It took about 20 min on average to fill all the questionnaires.
Pain stimulation
We used a Peltier-type thermal stimulator (PATHWAY™, Medoc Ltd, Ramat Yishai, Israel) equipped with an MRI-compatible 2.7 cm-diameter “CHEPS™” thermode with a 10-m cable, and a computerized VAS recorder (CoVAS™, Medoc, Israel), ranging from 0 mm (no pain) to 100 mm (worst pain imaginable), which allowed for a continuous quantification of pain intensity. The thermode was set on the left volar forearm of each subject. A threshold temperature of VAS = 60 mm was recorded, and a prescan pain stimulus session was given for each subject to get accustomed to study environment.
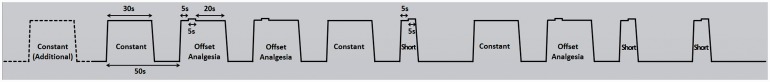
We programmed three kinds of thermal pain stimulation blocks on PATHWAY™ with the baseline fixed at 32°C (Figure 1): (1) OA: a total of 30-s block consisting of a series of 5-s 46°C (T1), 5-s 47°C (T2), and 20-s 46°C stimuli (T3); (2) Constant: a 30-s 46°C block; and (3) Short: a total of 10-s block consisting of T1 + T2 only.1,3,20 The ramp rates of stimulation temperature were +26°C/s (increase) and −6°C/s (decrease). The Constant and Short blocks were designed to be control stimuli in contrast to OA blocks and to avoid the effects of expectation.
Figure 1.
Offset analgesia paradigm during fMRI. Three kinds of noxious thermal pain stimulus blocks were designed with the baseline fixed at 32°C, each kind of stimuli given three times in a pseudorandom order. The starting time of each block was precisely controlled by TTL (transistor-transistor logic) output for triggering thermal stimulator synchronized with fMRI scanner. The ramp rates of stimulation temperature were +26°C/s (increase) and −6°C/s (decrease). (1) Offset analgesia (OA): a total of 30-s stimuli consisted of a series of 5-s 46°C, 5-s 47°C, and 20-s 46°C stimuli; (2) Constant: a total of 30-s 46°C stimulus; (3) Short: a total of 10-s stimuli consisted of 5-s 46°C, 5-s 47°C stimuli only. One additional Constant block (shown in dotted line) was given at the beginning of the stimulation session.
fMRI: functional magnetic resonance imaging.
Practice, thermal thresholds, and off-line OA paradigm
Each subject underwent an off-line practice session to get acclimatized to pain stimulation. They indicated pain ratings by a 100-mm VAS continuously with CoVAS™.
We gave a three-block stimulation to each subject in the order of Constant–OA–Constant with 20-s 32°C baseline interval between blocks. They were asked to rate perceived pain continuously with a slide bar on CoVAS™.
We recorded a threshold temperature of VAS = 60 mm for each subject by “level” mode of PATHWAY™. In brief, we slowly raised the thermode temperature from 32°C with a ramp-up rate of +0.5°C/s until a subject pressed a button on feeling pain sensation at the VAS of 60 mm. An average threshold for each subject was calculated from three trials.
We gave each subject off-line trial paradigms to record response characteristics including the magnitude of OA. We performed a 9-min trial paradigm including a mixture of OA, Constant, Short, and OA with variable durations of T1 and T2. Each of those stimulus blocks were repeated three times in a pseudorandom order with 20-s, 32°C interstimulus intervals as baseline, whose results has been reported elsewhere.6
OA paradigm during fMRI
The noxious thermal pain stimulation blocks, OA, Constant, and Short, were given three times each in a pseudorandom order, with one additional Constant block at the beginning of the stimulation session to make subjects accustomed to a sudden temperature change, resulting in a 10-block stimulation session (Figure 1). The start of stimulation paradigm was digitally synchronized with fMRI scanning sequences, lasting for 8 min and 28 s.
MRI acquisition
We used a 3.0 Tesla MRI scanner (Signa™ HDxt3.0T Optima Edition, GE Healthcare, USA) and an eight-channel birdcage head coil. Four dummy volumes at the beginning of each functional scan were automatically discarded to reduce potential saturation effects from B0 field inhomogeneity.
OA-related fMRI acquisition
We gave an OA paradigm of pain stimulation to each subject in the scanner while performing blood oxygenation level-dependent (BOLD) contrast-sensitive fMRI of the whole brain: a gradient-echo echo-planar sequence with the following parameters: repetition time (TR) = 2000 ms, echo time (TE) = 25 ms, flip angle (FA) = 80°, matrix = 64 × 64, field of view (FOV) = 192 × 192 mm, 38 contiguous axial slices, 3-mm slice thickness with no gap, resulting in a voxel size of 3 × 3 × 3 mm3, number of volumes = 254, scan time = 8 min and 28 s. Each subject viewed a screen of a personal computer via MRI-compatible goggles (Resonance Technology, Northridge, CA, USA), showing a graphical VAS scale of CoVAS™, with the pain stimulation thermode attached on the left volar forearm, and were asked to indicate the current intensity of pain continuously throughout the OA paradigm by manipulating the response box (CoVAS™) with the right hand. Both the OA paradigm on PATHWAY™ and a continuous pain rating were driven in synchrony with the acquisition of MR images.
Resting-state fMRI and DTI acquisition
We performed resting-state fMRI with the same parameters as above for 5 min, and DTI for 5 min and 52 s, whose results will be reported elsewhere.
Anatomical MRI acquisition
Finally, a high-resolution, T1-weighted, three-dimensional anatomical image of the whole brain was obtained with the following parameters: TR = 7.8 ms, TE = 3 ms, inversion time = 400 ms, FA = 12°, FOV = 256 × 205 mm, slice thickness = 1 mm, 137 contiguous sagittal slices, resulting in a voxel size of 1 × 1 × 1 mm3 (a total scan time of 6 min and 37 s).
Preprocessing of MR images
Functional and anatomical images were preprocessed and statistically analyzed with BrainVoyager QX™ 2.8.4 (BrainInnovations, Maastricht, the Netherlands).21
Functional data preprocessing included removal of the first 15 volumes of Constant block and 5 volumes of the former half of subsequent baseline; slice scan timing correction; head motion correction by trilinear and sinc interpolation; and removal of linear and nonlinear trends by temporal filtering. Any data with head motion ≥half a voxel size (1.5 mm) or 1° in rotation in any direction were excluded from analysis.
Each preprocessed functional image was coregistered to an anatomical image of the same subject and normalized into the stereotactic space of Talairach.22 For display purposes, a pair of three-dimentional meshes of cerebral hemispheres was made from the anatomical image of one HC.
Functional data analysis
Functional data were analyzed with multisubject general linear model (GLM) convolved with hemodynamic response function. To guarantee high signal-to-noise ratios and statistical robustness, we excluded voxels with exceptionally low signal intensities (arbitrary signal strength < 1000) especially in the orbitofrontal area23 and enabled mask restriction based on standardized brain areas averaged from all the subjects.
First, thermal pain-related brain activations and deactivations, common to both groups and all the three types of thermal pain stimuli compared with baseline (pain > baseline), were analyzed using BrainVoyager QX by a random-effects GLM analysis with false discovery rate: q = 0.05, p < 0.0076. Clusters larger than 300 mm3 were chosen as regions of interest (ROIs), which were localized with a stereotaxic atlas of Talairach.22
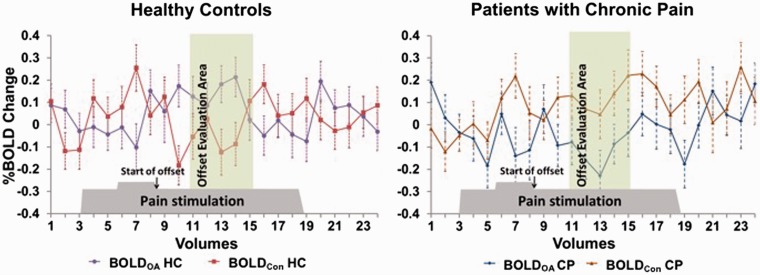
Second, differences in the OA-associated activation between HC and CP were analyzed. In brief, we executed a fixed-effects GLM comparison between the middle 10-s time frame (5 volumes) of T3 epoch of OA, after a 6-s hemodynamic delay from the onset of OA, and the corresponding 10-s epoch (5 volumes) of Constant blocks; and further made a between-group comparison (HC > CP). This epoch of OA block was considered to coincide with endogenous analgesic effect and associated brain activity causing OA,5 whereas brain activity during Constant blocks served as controls without OA occurrence. Those comparisons were described as [(HCOA − HCCon) − (CPOA − CPCon)], where each abbreviation meaning BOLD signals of HC during OA (HCOA), Constant (HCCon), those of CP during OA (CPOA), and Constant (CPCon), respectively. Epochs for this comparison are illustrated in Figure 2.
Figure 2.
Comparison of OA and Constant BOLD signals between healthy controls and patients with chronic pain at the brainstem. We compared BOLD signals during OA at the brainstem selectively during the 10-s “offset evaluation area,” beginning 6-s (hemodynamic delay) after the end of 1°C-increment (start of offset), with those during Constant stimulus. In HC (left panel), four out of five BOLD signals during OA (purple line) were larger than those during Constant (red line). In CP (right panel), all the BOLD signals during OA (blue line) were smaller than those of Constant (orange line). Error bars represents standard deviations.
OA: offset analgesia; HC: healthy control; CP: patients with chronic pain; BOLD: blood oxygenation level-dependent; Con: constant paradigm.
Because of low statistical robustness from limited time windows of comparison, we had to permit a relatively lower statistical threshold at p < 0.05, uncorrected. Clusters larger than 50 mm3 were chosen as ROIs, which were localized with a stereotaxic atlas of Talairach.22 Furthermore, BOLD amplitudes of resultant clusters were computed from each individual data for further analysis as described below.
Statistical analysis
Demographic and psychophysical data analysis
Demographic and psychophysical data of all subjects were statistically analyzed using SPSS (Version 20.0; IBM Corporation, Chicago, IL, USA). An unpaired t test was performed between HC and CP regarding age, weight, height, body mass index (BMI), VAS, threshold for VAS = 60, and other psychophysical variables of the PD-Q, PCS, BDI, and SF-MPQ, after a Shapiro–Wilk normality test confirming normal distribution in most data. Gender difference was examined by a chi-square test. p < 0.05 was considered statistically significant. Medication of patients was summarized into seven categories without further statistical analysis.
Definition of OA and BOLD signal parameters and their statistics
We defined OA-related and BOLD signal parameters as in Table 2 and made a further analysis on the relationships among magnitude of OA (%ΔOA), OA-related BOLD signals (ΔBOLD), and various behavioral parameters. Specifically, we computed average BOLD amplitudes at each ROI of each individual subject during the middle 10-s epoch of T3 OA (BOLDOA) and during the corresponding Constant blocks (BOLDCon), and defined the difference, [BOLDOA − BOLDCon], as ΔBOLD.
Table 2.
Definitions of OA and BOLD signal parameters.
| Parameters | Definition |
|---|---|
| VASMax T2 | Maximum VAS value during T2 of OA blocks |
| VASMin T3 | Minimum VAS value during the first 10 s of T3 of OA blocks |
| ΔOA | VASMax T2 − VASMin T3, magnitude of OA in VAS |
| %ΔOA | (ΔOA/VASMax T2) × 100%, standardized value of ΔOA |
| BOLDOA | An average BOLD signal during the middle 10 s of T3 of OA block |
| BOLDCon | An average BOLD signal during the Constant block corresponding to BOLDOA |
| ΔBOLD | BOLDOA − BOLDCon |
OA: offset analgesia; BOLD: blood oxygenation level-dependent; VAS: visual analog scale; T2: a 5-s 47°C stimulus during an OA block; T3: a 20-s 46°C stimulus during an OA block; Con: a Constant block.
Statistical analyses were performed using SPSS. Values of %ΔOA, showing nonnormal distribution by a Shapiro–Wilk normality test, were compared between HC and CP with a Mann–Whitney’s U test at a significance threshold of p < 0.05.
Values of ΔBOLD, showing normal distribution by a Shapiro–Wilk normality test except for NAc, were compared between HC and CP with a Mann–Whitney’s U test for NAc or an unpaired t test for the rest, at a significance threshold of p < 0.05. Data with and without homogeneous variance (a Levene’s test) were handled with an unpaired t test.
Correlation analysis
A Pearson’s correlation analysis was performed comprehensively among %ΔOA, ΔBOLD, and all the psychophysical variables using SPSS at a significance threshold of p < 0.05.
Results
Demographics and psychophysical data of subjects
The present study shows the primary analysis of simultaneous fMRI data during thermal stimulation including OA paradigms. Part of demographic and psychophysical data of subjects were analyzed and published in a separate paper using only off-line OA data obtained before the MRI experiment.6
There were no significant differences between HC and CP with respect to age (p = 0.86), sex (p = 0.73), weight (p = 0.98), height (p = 0.26), and BMI (p = 0.20). All the subjects in both groups were Asians. However, there were significant differences between the two groups in current pain intensity (VAS; p < 0.0005), threshold for VAS = 60 (p = 0.015), PD-Q (p < 0.0005), PCS (p < 0.0005), BDI (p < 0.0005), and SF-MPQ (p < 0.0005) (Table 1). In HC, five values of PD-Q and six of PCS were found larger than 0 despite the absence of pain, i.e. VAS of “0” for current pain intensity, which were likely caused by misinterpretation of questionnaires by subjects (e.g. describing nonexistent, imagined pain from past experience). Because those values did not reflect actual responses to current pain, they were removed from analysis, resulting in 12 and 11 values used for statistical analysis of PD-Q and PCS, respectively.
The CP group consisted of seven patients with fibromyalgia, two with CLBP, two with complex regional pain syndrome (CRPS), two with limb pain, one with phantom limb pain, one with sciatic neuralgia, and one with migraine. Their medications at the time of experiment were also documented (Table 3).
Table 3.
Diseases and medications of patients with chronic pain.
| Disease (single) | N |
| Fibromyalgia | 7 |
| Chronic low back pain | 2 |
| Complex regional pain syndrome | 2 |
| Limb pain | 2 |
| Phantom limb pain | 1 |
| Cervical spondylosis | 1 |
| Sciatic neuralgia | 1 |
| Migraine | 1 |
| Medication (multiple) | |
| Tricyclic antidepressant drugs | 13 |
| Anticonvulsant and antiepileptic drugs | 12 |
| Nonsteroidal anti-inflammatory drugs | 6 |
| Anti-anxiety drugs | 5 |
| Tramadol | 3 |
| Serotonin-noradrenalin reuptake inhibitors | 3 |
OA response
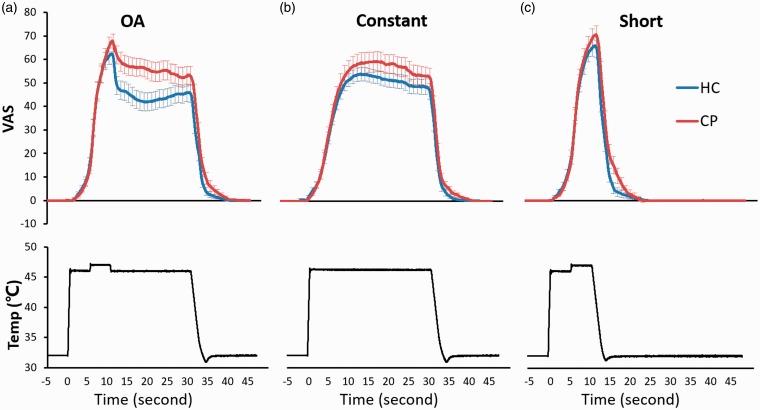
We observed a rapid, disproportionate decrease of pain rating on T3 epoch in both groups. %ΔOA was significantly larger in HC (28.9% (11.0–56.0%), median (interquartile range)) than in CP (19.0% (4.2–48.7%)) (p = 0.047) (Figure 3; Table 4).
Figure 3.
Stimulation temperature, time, and pain ratings. Pain ratings of HC (blue line) and CP (red line) during OA (upper panel of A), Constant (upper panel of B), and Short (upper panel of C) are shown together with stimulation temperature (black line) of OA (lower panel of A), Constant (lower panel of B), and Short (lower panel of C). Error bars represent standard errors of the mean.
OA: offset analgesia; HC: healthy control; CP: patients with chronic pain; VAS: visual analogue scale; Temp: stimulation temperature.
Table 4.
Individual %ΔOA values and statistical results.
| HC | %ΔOA1 | %ΔOA2 | %ΔOA3 | CP | %ΔOA1 | %ΔOA2 | %ΔOA3 |
|---|---|---|---|---|---|---|---|
| 1 | 58.97% | 19.12% | 19.05% | 1 | 1.11% | 5.49% | 79.55% |
| 2 | 98.65% | 56.94% | 98.72% | 2 | 0.00% | 0.00% | 0.00% |
| 3 | 100.00% | 50.00% | 28.57% | 3 | 69.84% | 57.40% | 65.20% |
| 4 | 9.21% | 14.81% | 8.22% | 4 | 100.00% | 52.00% | 70.00% |
| 5 | 76.36% | 55.10% | 100.00% | 5 | 27.00% | 19.00% | 53.00% |
| 6 | 67.06% | 34.88% | 66.67% | 6 | 1.35% | 1.30% | 8.50% |
| 7 | 51.22% | 46.67% | 52.17% | 7 | 20.00% | 30.40% | 18.29% |
| 8 | 47.78% | 43.96% | 54.02% | 8 | 4.71% | 10.00% | 8.00% |
| 9 | 11.70% | 2.04% | 0.00% | 9 | 63.04% | 100.00% | 87.90% |
| 10 | 21.98% | 28.89% | 11.36% | 10 | 7.90% | 19.28% | 11.76% |
| 11 | 88.64% | 44.19% | 50.00% | 11 | 33.85% | 13.30% | 14.30% |
| 12 | 10.94% | 10.45% | 7.46% | 12 | 22.86% | 0.00% | 40.43% |
| 13 | 13.73% | 3.50% | 1.80% | 13 | 0.00% | 0.00% | 0.00% |
| 14 | 100.00% | 100.00% | 100.00% | 14 | 48.28% | 49.21% | 11.63% |
| 15 | 6.25% | 3.23% | 3.57% | 15 | 23.53% | 24.32% | 13.89% |
| 16 | 42.86% | 28.57% | 23.81% | 16 | 0.00% | 3.70% | 0.00% |
| 17 | 13.40% | 11.11% | 8.33% | 17 | 65.00% | 39.00% | 20.00% |
| Median of HC | 28.89% | Median of CP | 19.00% | ||||
| 25% percentile | 11.02% | 25% percentile | 4.20% | ||||
| 75% percentile | 56.02% | 75% percentile | 48.74% | ||||
| p value | 0.047 | ||||||
HC: healthy control; CP: patients with chronic pain; OA: offset analgesia.
Numbers in the HC and CP columns indicate designated numbers following order of participation. A between-group comparison was made with a Mann–Whitney’s U test.
Thermal pain-related activation
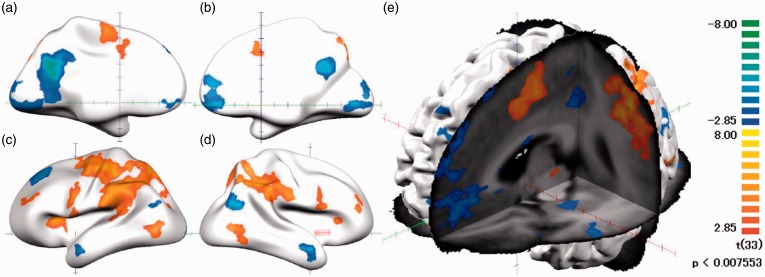
In the first-level random-effects GLM analysis that combined all the pain stimulus blocks of both groups (HC + CP), we observed robust positive and negative activations in multiple brain areas. Positive activations were seen in seven clusters and negative activations in six clusters. The distribution of positive clusters represented a typical pattern of pain-related cerebral activation, while major part of negative clusters belonged to the default mode network (DMN) (Figure 4; Table 5).
Figure 4.
Thermal pain-related activation. Activated brain regions during all three kinds of thermal pain stimulation compared with baseline shown in red to yellow and blue to green color on the brain mesh. Statistical strengths (t values) are color-coded as on the right bar. (a) Internal surface of the left hemisphere, (b) internal surface of the right hemisphere, (c) lateral surface of the left hemisphere, (d) lateral surface of the right hemisphere, and (e) three-sectional view of the left hemisphere.
Table 5.
Pain-related cerebral activation, Pain > Baseline, of all the subjects (HC + CP).
| Name of ROIs | Side |
Peak coordinates |
t | p | Size (mm3) | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Positive activation | |||||||
| Superior parietal lobule/Angular gyrus/Supramarginal gyrus | R | 42 | −46 | 43 | 8.14 | <0.000001 | 21634 |
| Premotor cortex and SMA | L/R | 3 | 5 | 49 | 7.83 | <0.000001 | 54720 |
| Primary sensory and motor cortex/Angular gyrus/Supramarginal gyrus/Associative visual cortex | L | ||||||
| Anterior insular/Pars opercularis/Premotor cortex | R | 54 | 8 | 4 | 7.65 | <0.000001 | 4703 |
| L | −30 | 17 | 10 | 7.33 | <0.000001 | 9024 | |
| Thalamus/Red Nucleus/PAG | R | 6 | −19 | −2 | 5.52 | 0.000004 | 777 |
| dlPFC | R | 24 | 47 | 22 | 4.65 | 0.000051 | 1616 |
| L | −27 | 38 | 22 | 4.81 | 0.000033 | 929 | |
| Fusiform gyrus | R | 51 | −55 | −8 | 5.18 | 0.000011 | 1851 |
| L | −45 | −67 | 4 | 5.35 | 0.000007 | 2310 | |
| Thalamus/Midbrain | L | −12 | −16 | 10 | 5.01 | 0.000018 | 894 |
| Negative activation | |||||||
| MTG | R | 54 | −4 | −11 | −5.86 | 0.000001 | 1337 |
| L | −54 | −1 | −14 | −4.99 | 0.000019 | 769 | |
| Hippocampus | R | 30 | −16 | −11 | −4.52 | 0.000076 | 400 |
| Frontal eye fields | R | 21 | 26 | 49 | −3.91 | 0.000433 | 996 |
| Angular gyrus | R | 42 | −61 | 22 | −5.26 | 0.000009 | 1382 |
| L | −39 | −67 | 25 | −5.14 | 0.000012 | 1238 | |
| MPFC | L/R | 0 | 44 | 1 | −6.02 | 0.000001 | 19279 |
| Ventral PCC/PCu/V2 | L/R | 0 | −61 | 28 | −7.86 | <0.000001 | 31215 |
ROI: region of interest; aINS: anterior insula; SMA: supplementary motor area; PAG: periaqueductal gray; dlPFC: dorsolateral prefrontal cortex; M1: primary motor; S1: primary sensory; MTG: middle temporal gyrus; MPFC: medial prefrontal cortex; PCC: posterior cingulate cortex; PCu: precuneus; V2: secondary visual cortex; L: left; R: right; HC: healthy control; CP: patients with chronic pain.
Statistical strength, false discovery rate (FDR): q = 0.05, p < 0.0076, cluster size > 300 mm3. Coordinates were based on the stereotaxic atlas of Talairach.
On the other hand, a between-group comparison (HC > CP) across all the pain stimulus blocks did not show any robust, meaningful contrasts (data not shown). We therefore chose to focus on the specific time window that coincided with OA and made a between-condition (OA > Con) and between-group (HC > CP) comparison of BOLD signals as a secondary analysis described below.
Comparisons of OA-associated activation between HCs and CP
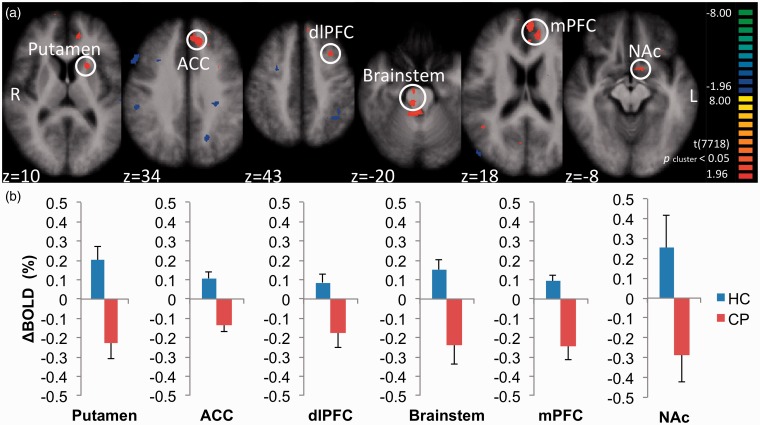
After a fixed-effects GLM comparison of the BOLD signals at [(HCOA − HCCon) − (CPOA − CPCon)] during OA, we obtained 10 positive clusters (Table 6). Those clusters were located at the left putamen, left anterior cingulate cortex (ACC), left dorsolateral prefrontal cortex (dlPFC), bilateral brainstem, bilateral medial prefrontal cortex (mPFC), left NAc, bilateral ACC, left pars orbitalis (BA 47), right angular gyrus, and bilateral dorsal posterior cingulate cortices. Among those, the six more robust clusters (t > 3.0) were selected as ROIs for calculation of BOLD contrasts (ΔBOLD) in each individual (Figure 5(a)). Whereas ΔBOLD was positive at all the six clusters in HC, it was all negative in CP (Figure 5(b); Table 7).
Table 6.
Resultant regions of interest from subtraction analysis [(HCOA − HCCon) − (CPOA − CPCon)].
| Name of ROIs | Side |
Peak coordinates |
t | p | Size (mm3) | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Putamen | L | −21 | 14 | 7 | 3.64 | 0.0003 | 256 |
| ACC | L | −12 | 35 | 34 | 3.49 | 0.0005 | 1275 |
| dlPFC | L | −30 | 11 | 37 | 3.41 | 0.0006 | 255 |
| Brainstem | L/R | 6 | −25 | −20 | 3.23 | 0.0012 | 227 |
| mPFC | L/R | 15 | 44 | 1 | 3.15 | 0.0017 | 706 |
| NAc | L | −12 | 8 | −8 | 3.05 | 0.0023 | 71 |
| ACC | L/R | 6 | 29 | 13 | 2.96 | 0.0030 | 297 |
| Pars orbitalis | L | −30 | 26 | −5 | 2.88 | 0.0039 | 104 |
| Angular gyrus | R | 42 | −49 | 19 | 2.59 | 0.0095 | 88 |
| dPCC | R | 6 | −64 | 19 | 2.59 | 0.0096 | 112 |
| L | −9 | −58 | 25 | 2.45 | 0.0142 | 133 | |
ROI: region of interest; HC: healthy control; CP: patients with chronic pain; OA: offset analgesia; Con: constant paradigm; ACC: anterior cingulate cortex; mPFC: medial prefrontal cortex; dlPFC: dorsolateral prefrontal cortex; NAc: nucleus accumbens; dPCC: dorsal posterior cingulate cortex; L: left; R: right.
Statistical threshold, p < 0.05; cluster size > 50 mm3. Coordinates were based on the stereotaxic atlas of Talairach.
Figure 5.
Brain areas that showed larger OA-related BOLD signals in healthy controls than in patients with chronic pain. We performed a fixed-effects general linear model analysis on BOLD signal contrasts, [(HCOA − HCCon) − (CPOA − CPCon)], and indicated brain areas with positive and negative contrasts. From this subtraction analysis, we found that principal regions concerned with descending pain modulatory and reward systems were activated in HC but not in CP. (a) Six most robust positive clusters, color-coded in red to yellow, were selected as ROIs (white circle), including the left putamen, left ACC, left dlPFC, bilateral brainstem, bilateral mPFC, and left NAc. (b) Mean OA-related ΔBOLD of six ROIs shown in Figure 5(a) between HC (blue bar) and CP (red bar). Error bars represent standard errors of the mean.
ROI: region of interest; HC: healthy control; CP: patients with chronic pain; ACC: anterior cingulate cortex; mPFC: medial prefrontal cortex; dlPFC: dorsolateral prefrontal cortex; NAc: nucleus accumbens; BOLD: blood oxygenation level-dependent.
Table 7.
Between-group comparisons of ΔBOLD at six significant ROIs.
| Name of ROIs |
Levene’s test |
Group |
ΔBOLD |
t | df | p | ||
|---|---|---|---|---|---|---|---|---|
| F value | p | Mean (%) | SE (%) | |||||
| Putamen | 0.190 | 0.665 | HC | 0.204 | 0.071 | 3.899 | 32 | <0.0005* |
| CP | −0.225 | 0.084 | ||||||
| ACC | 0.157 | 0.695 | HC | 0.107 | 0.032 | 5.51 | 32 | <0.0005* |
| CP | −0.138 | 0.030 | ||||||
| dlPFC | 0.606 | 0.442 | HC | 0.085 | 0.045 | 2.905 | 32 | 0.007* |
| CP | −0.175 | 0.078 | ||||||
| Brainstem | 4.205 | 0.049* | HC | 0.153 | 0.052 | 3.522 | 24.34 | 0.002* |
| CP | −0.240 | 0.099 | ||||||
| mPFC | 6.764 | 0.014* | HC | 0.094 | 0.030 | 4.340 | 21.44 | <0.0005* |
| CP | −0.242 | 0.072 | ||||||
|
Median (%) |
Interquartile range |
p | ||||||
| NAc | – | – | HC | 0.054 | −0.052 to 0.276 | 0.008† | ||
| CP | −0.175 | −0.467 to 0.052 | ||||||
Only data at the NAc showed nonnormal distribution. Statistical analysis was accordingly performed with a Mann–Whitney’s U test † for NAc or an unpaired t test * for the rest. Inhomogeneity of variance was found for Brainstem and mPFC data sets after a Levene’s test.
BOLD: blood oxygenation level-dependent; ROI: region of interest; HC: healthy control; CP: patients with chronic pain; SE: standard error of the mean; df: degree of freedom; ACC: anterior cingulate cortex; dlPFC: dorsolateral prefrontal cortex; mPFC: medial prefrontal cortex; NAc: nucleus accumbens.
BOLDOA and BOLDCon of the brainstem ROI were selected as representatives to illustrate the different activation pattern between HC and CP (Figure 2). During the 10-s “Offset Evaluation Area,” four out of five data points of mean BOLDOA from HC were larger than BOLDCon of HC, while all the data points of mean BOLDOA from CP were much smaller than BOLDCon of CP, indicating OA-related activity that was enhanced in HC but suppressed in CP.
Comprehensive correlation analyses among %ΔOA, psychophysical parameters, and OA-related cerebral activations (ΔBOLD)
Standardized magnitude of OA, %ΔOA, did not show any significant correlations with ΔBOLD at any of the six ROIs. It did not show any significant correlations with any of psychophysical parameters (Table 8).
Table 8.
Correlations among %ΔOA, ΔBOLD, and psychophysical parameters in HC and CP.
| Brain activity | Name of ROIs | Group |
%ΔOA |
PD-Q |
PCS |
BDI |
SF-MPQ |
VAS |
Duration |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | p | R | p | R | p | R | p | R | p | R | p | R | p | |||
| ΔBOLD | Putamen | HC | 0.250 | 0.333 | −0.135 | 0.676 | – | – | 0.561 | 0.019* | 0.238 | 0.358 | – | – | – | – |
| CP | −0.156 | 0.550 | −0.343 | 0.178 | −0.184 | 0.481 | −0.317 | 0.215 | 0.001 | 0.998 | 0.160 | 0.540 | −0.237 | 0.359 | ||
| ACC | HC | −0.216 | 0.405 | −0.086 | 0.790 | – | – | 0.241 | 0.352 | 0.089 | 0.733 | – | – | – | – | |
| CP | −0.053 | 0.840 | −0.660 | 0.004* | −0.217 | 0.402 | −0.136 | 0.603 | −0.192 | 0.461 | −0.139 | 0.594 | 0.086 | 0.742 | ||
| dlPFC | HC | −0.140 | 0.593 | −0.135 | 0.676 | – | – | −0.163 | 0.533 | −0.067 | 0.799 | – | – | – | – | |
| CP | −0.007 | 0.980 | −0.023 | 0.931 | −0.009 | 0.973 | 0.081 | 0.756 | 0.249 | 0.335 | 0.271 | 0.293 | −0.071 | 0.787 | ||
| Brainstem | HC | −0.208 | 0.423 | 0.147 | 0.648 | – | – | −0.187 | 0.473 | −0.068 | 0.795 | – | – | – | – | |
| CP | −0.068 | 0.794 | −0.364 | 0.151 | −0.188 | 0.471 | −0.267 | 0.300 | −0.209 | 0.421 | −0.292 | 0.256 | 0.027 | 0.918 | ||
| mPFC | HC | −0.249 | 0.334 | 0.090 | 0.781 | – | – | 0.061 | 0.815 | 0.028 | 0.916 | – | – | – | – | |
| CP | −0.021 | 0.937 | −0.388 | 0.124 | 0.019 | 0.941 | 0.059 | 0.822 | −0.071 | 0.785 | 0.137 | 0.601 | −0.321 | 0.209 | ||
| NAc | HC | −0.296 | 0.249 | −0.124 | 0.700 | – | – | −0.006 | 0.983 | 0.058 | 0.824 | – | – | – | – | |
| CP | 0.182 | 0.485 | −0.360 | 0.156 | −0.256 | 0.321 | −0.295 | 0.250 | −0.140 | 0.592 | −0.122 | 0.640 | 0.154 | 0.556 | ||
| %ΔOA | CP | – | −0.083 | 0.751 | 0.223 | 0.389 | 0.279 | 0.278 | 0.247 | 0.340 | 0.178 | 0.493 | −0.079 | 0.762 | ||
OA: offset analgesia; BOLD: blood oxygen level-dependent; HC: healthy control; CP: patients with chronic pain; ROI: region of interest; R: correlation coefficient; PD-Q: PainDETECT questionnaire; PCS: Pain Catastrophizing Scale; BDI: Beck Depression Inventory; SF-MPQ: Short-Form McGill Pain Questionnaire; VAS: visual analogue scale; ACC: anterior cingulate cortex; dlPFC: dorsolateral prefrontal cortex; mPFC: medial prefrontal cortex; NAc: nucleus accumbens.
*Statistically significant at p < 0.05.
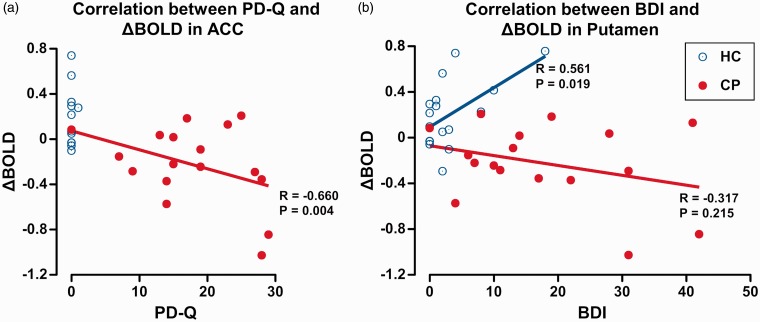
On the other hand, we found a significant negative correlation between PD-Q values and ΔBOLD of ACC in CP with a Pearson correlation coefficient R = −0.660, p = 0.004. We also found a significant positive correlation between BDI values and ΔBOLD of putamen in HC (R = 0.561, p = 0.019). The other psychophysical parameters did not show any significant correlations with ΔBOLD at any of the six ROIs (Figure 6; Table 8).
Figure 6.
Correlations between psychophysical parameters and OA-related brain activation. (a) A significant correlation was observed between PD-Q and ΔBOLD of ACC in CP with a Pearson correlation coefficient of R = −0.660, p = 0.004. (b) A significant correlation was observed between BDI and ΔBOLD of putamen in HC with a Pearson correlation coefficient of R = 0.561, p = 0.019.
OA: offset analgesia; PD-Q: PainDETECT questionnaire; BOLD: blood oxygenation level-dependent; ACC: anterior cingulate cortex; BDI: Beck Depression Inventory; HC: healthy control; CP: patients with chronic pain.
Discussion
OA was attenuated in CP
As far as we know, this is the first study that measured OA and performed fMRI simultaneously both in CP and HCs. During OA, we found a disproportionate reduction of pain ratings during T3 in both groups. CP showed a significantly smaller %ΔOA, by approximately 34%, than HC. These results might imply a disruption in temporal sharpening mechanisms of nociceptive information24 and dysfunction of endogenous analgesic mechanism in CP.2 It accords with earlier reports on reduced OA magnitude in patients with neuropathic pain,4 fibromyalgia,5 and cerebellar infarction.25
It also accords with our preceding report on off-line (out-of-scanner) measurement of OA, in which CP showed 43% reduction of OA compared with HC.6 The extent of OA attenuation was relatively smaller in the present study than in the off-line study in both HC and CP. Those two reports represent the first studies that showed an attenuation of OA in a more general population of CP from various etiologies.
Considering the recent “temporal contrast enhancement” model,26 CP showed a larger pain augmentation on 1°C-increment but a smaller pain decrease on 1°C-decrement (Figure 3). The former might have resulted from lower pain threshold to thermal stimulus (Table 1) and the latter from an obtunded “supra-threshold step-down” response in CP. An increase in the “step-down” temperature26 or that in the T2 duration6 could have resulted in larger OA responses both in HC and CP. Such an OA-enhanced design of study might potentially give more pronounced contrasts both in magnitude of OA and brain activity.
Thermal pain stimulation induced a typical pain-related activation and deactivation of the DMN
The present thermal pain stimulation resulted in activation of multiple brain areas concerned with processing of acute experimental pain, which included primary sensory, motor, anterior insular cortices, and thalamus,15 as well as mesolimbic structures and the PAG. The deactivated areas were mainly the DMN, a group of brain regions that are unanimously deactivated during task or stimulus and return to baseline at rest.27 Although the above analysis did not distinguish among different stimulation patterns, it showed that the present stimulation paradigm efficiently activated discrete, multiple brain areas within the pain-related cerebral network.
Attenuation of OA in CP was associated with dysfunction of both the descending pain modulatory and reward systems
We found that attenuation of OA magnitude in CP coincided with reduced BOLD signals at the putamen, ACC, dlPFC, brainstem, mPFC, and NAc. Whereas HC showed positive activation, CP showed negative activation at those areas. Those areas partially overlapped with the pattern of µ-opioid receptor-mediated placebo-induced activation at the ACC, dlPFC, insular cortex, and NAc,28 which supports similarity in brain responses between OA and placebo analgesia, both being considered involving the descending pain modulatory system.29 The descending pain modulatory system is a well-characterized network, across the cerebral cortex and the spinal cord, that regulates nociceptive processing and produces either facilitation or inhibition.9 The ACC and dlPFC are considered among cortical centers of the descending pain modulatory system that trigger the inhibitory signals to the brainstem, PAG, which further sends inhibitory signals down to the dorsal horn of the spinal cord.9 Attenuation of OA-related BOLD signals in those areas might indicate dysfunction of the descending pain modulatory system in CP.
On the other hand, attenuation of OA-related activity at the putamen, NAc, and mPFC might indicate dysfunction of the reward system in CP. The putamen belongs to the basal ganglia, encodes somatic nociceptive information to be prepared for defense and avoidance behavior,30 and affects reinforcement learning.31 The NAc also belongs to the basal ganglia and is involved in processing of aversion and reward.32 A negative and positive activations of NAc were observed at pain onset and offset, respectively, in an earlier fMRI study with prolonged painful stimulus.32 NAc activation could be a marker for analgesic or reward effect32 and predicted reward magnitude at stimulus offset.10 Alterations in the endogenous μ-opioid system in NAc, involved in pain modulation and reward, were related to clinical pain in patients with trigeminal neuropathic pain.33
The mPFC is a region involved in negative emotions and persistence of emotion after the offset of a nociceptive input.34 A recent animal fMRI study revealed that mPFC modulated expression of reward-seeking behavior.35 Activation of mPFC was strongly related to intensity of chronic back pain.36 The gray matter density in the bilateral dlPFC was reduced and negatively correlated with mPFC activity in CP.37 Greater NAc-mPFC functional connectivity predicted pain persistence,38 suggesting the involvement of corticostriatal network in the generation of chronic pain.
ΔBOLD at the ACC was negatively correlated with neuropathic component of pain
Standardized magnitude of OA, %ΔOA, did not show any significant correlations with OA-related BOLD signals at any of six ROIs. It might imply that the extent of OA is not determined by activity at specific, single brain area, but by interactions among multiple areas concerned with various functions including perception, modulation, and reward-related processing of pain. A further analysis using functional and anatomical connectivity analysis, using the rest of our multimodal MRI data sets, might potentially reveal such interactions.
OA-related BOLD signals at the ACC showed a strong negative correlation with PD-Q, an estimate for neuropathic component of pain in patients. A high PD-Q score is considered to imply high intensity of pain, more severe comorbidity, and lower quality of life.16 ACC belongs to the medial nociceptive system that mediates an affective component of pain39 and is also among cortical centers to drive descending pain modulatory system.9 Therefore, abnormal ACC activity, in association with high PD-Q scores, might reflect transition from sensory to emotional dominant phase of chronic pain,40 which underlies mechanisms of pain chronification.41
Attenuation of ACC activity during OA, in association with severity of pain, accords with our previous findings that mechanical pain stimulus caused negative activation at ACC in association with severity of pain (SF-MPQ).14 In contrast to positivity, negative BOLD signals are considered to reflect decreased neuronal firing in those regions.42 Decreased neuronal activity at ACC might possibly imply decreased descending pain modulatory activity originating from this region, which is also considered closely associated with pain chronification.14
On the other hand, OA-related BOLD signals at putamen were found positively correlated with BDI, an index for depressive mood, only in HC, whereas ΔBOLD was mostly negative without any correlation with BDI in CP. None of HC subjects were diagnosed as depression. Although pain-related activation at putamen might potentially be affected by mood disorder,43 significance of the present correlation remains unclear. Nevertheless, putamen is known to play a pivotal role in pain processing and to show altered responses in various chronic pain conditions.44
Limitations
First, statistical methods to detect differences in OA-specific brain activity in the whole brain between HC and CP were not robust enough to assume intersubject variability and to survive multiple comparisons. We attempted to compensate for statistical weakness from restriction to an OA-specific epoch, 10 s, by choosing a fixed-effects approach for group analysis, provided officially by the BrainVoyager QX statistical package, that increased measurement time points and hence sensitivity to detect between-group subtle differences in BOLD signals (http://brainvoyager.com/bv/doc/UsersGuide/StatisticalAnalysis/FixedEffectsAnalysis/FixedEffectsGroupAnalysis.html). Therefore, the present results were applicable only to the studied cohort and might not be applicable to general population of chronic pain. Although activation clusters did not survive multiple comparisons, a spatial extent threshold at > 50 mm3 efficiently showed 10 major significant clusters as shown in Table 3, from which we selected only 6 with t values > 3 for further ROI analysis. We believe that such strategy helped screening for significant ROIs, without any a priori hypothesis, showing different brain activities during OA between HC and CP, which indeed revealed contrasting activities between the groups as shown in Table 4 and Figure 2. Nevertheless, we will need a further increase in number of subjects and more elaborated methods in data preprocessing and statistics to obtain more robust results generalizable to CP.
Second, the stimulation temperature used in this study was 1°C higher than one study7 but lower than most earlier OA studies involving mostly Western populations.1–3,8,20,24,45,46 In a pilot study, we found that only when T1 = 46°C and T2 = 47°C, subjects were able to rate pain in VAS scores between 50 and 60, whereas most subjects did not tolerate any temperatures ≥48°C. All the patients and HCs were Asians. Such lower tolerance to thermal pain stimulation might have come from racial differences in experimental pain sensitivity.47 Another work from our laboratory also showed smaller pain thresholds of Asian subjects compared with earlier studies on Western populations.6 Those results implied remarkable between-race differences in pain perception thresholds and provided characteristics of OA responses in Asian populations.
Third, OA magnitude during fMRI was unexpectedly smaller than during off-line sessions. Large noise, restriction of body movement, and narrow visual fields by goggles might have distracted subjects from pain perception, resulting in less dynamic recordings of pain,48 and might have affected PAG activation.49
Fourth, manipulation of the response box (COVAS™) might have contaminated cerebral activations with hand movement-related activities. We did observe activations at the left primary sensorimotor cortex that could have been associated with such motion as well as pain stimulus. However, activations at the motor-related areas have often been observed in previous functional neuroimaging studies of experimental pain.12,13,15 Of note, OA-related activations did not overlap with any of such common pain-related activations in the current study, but rather observed in brain areas associated with emotions, descending modulation of pain, and reward-related responses (Tables 5 and 6). Hand motion-related activation, if any, should likely have been eliminated after subtraction analysis.
Lastly, we only described the medication used by CP but did not evaluate possible confounding effects of medication on the OA responses and BOLD signals. Such variability in medication, as well as various etiologies of chronic pain, might have resulted in large interindividual variability. In a future study, we plan to enroll a larger number of patients and matched controls, which might enable characterizing OA and associated brain responses for each etiology of chronic pain. We also plan to perform a longitudinal study to evaluate efficacy of treatment on an individual basis, which might reveal dynamic alterations of the descending pain modulatory and reward systems in association with chronification and cure of pain.
In summary, we found that CP, at least in the studied cohort, showed attenuated response of OA as well as associated reduction of OA-related activation in specific brain areas using fMRI, in comparison with HCs. Those brain areas included major structures of the descending pain modulatory and reward systems. Reduction of OA-related activity at the ACC was associated with enhanced neuropathic component of pain in CP. Dysfunction of those systems, revealed by OA measurement, might be associated with chronification of pain, which should warrant further replication studies.
Acknowledgments
The authors thank Prof. Shigehito Sawamura, Department of Anesthesia, Teikyo University School of Medicine, Itabashi City, Tokyo, Japan, for providing PATHWAYTM. The authors also thank Dr. Koshi Makita, a former Professor and Chairman, Department of Anesthesiology, Tokyo Medical and Dental University Graduate School of Medical and Dental Sciences, Tokyo, Japan, for his departmental support of the present study. The authors would also like to thank all the staff of the Department of Radiology, Tokyo Medical and Dental University Hospital of Medicine, Bunkyo City, Tokyo, Japan, for their efficient technical support during fMRI procedures.
Author Contributions
Study design: SZ and JK; study conduct and data collection: SZ, TL, HK, EI, TO, and JK; data analysis: SZ, TL, and JK; writing paper: SZ and JK.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by Grants-in-Aid for Scientific Research (No. JP 26460695 to JK) from the Japan Society for the Promotion of Science, Tokyo, Japan.
References
- 1. Grill JD andCoghill RC.. Transient analgesia evoked by noxious stimulus offset. J Neurophysiol 2002; 87: 2205–2208. [DOI] [PubMed] [Google Scholar]
- 2.Yelle MD Oshiro Y Kraft RA and Coghill RC. Temporal filtering of nociceptive information by dynamic activation of endogenous pain modulatory systems. J Neurosci 2009; 29: 10264–10271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yelle MD Rogers JM andCoghill RC.. Offset analgesia: a temporal contrast mechanism for nociceptive information. Pain 2008; 134: 174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niesters M Hoitsma E Sarton E Aarts L and Dahan A. Offset analgesia in neuropathic pain patients and effect of treatment with morphine and ketamine. Anesthesiology 2011; 115: 1063–1071. [DOI] [PubMed] [Google Scholar]
- 5.Oudejans LC Smit JM van Velzen M Dahan A and Niesters M. The influence of offset analgesia on the onset and offset of pain in patients with fibromyalgia. Pain 2015; 156: 2521–2527. [DOI] [PubMed] [Google Scholar]
- 6.Kobinata H Ikeda E Zhang S Li T Makita K and Kurata J. Disrupted offset analgesia distinguishes patients with chronic pain from healthy controls. Pain 2017; 158: 1951–1959. [DOI] [PubMed] [Google Scholar]
- 7.Derbyshire SW andOsborn J.. Offset analgesia is mediated by activation in the region of the periaqueductal grey and rostral ventromedial medulla. Neuroimage 2009; 47: 1002–1006. [DOI] [PubMed] [Google Scholar]
- 8.Nahman-Averbuch H Martucci KT Granovsky Y Weissman-Fogel I Yarnitsky D and Coghill RC. Distinct brain mechanisms support spatial vs temporal filtering of nociceptive information. Pain 2014; 155: 2491–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tracey I andMantyh PW.. The cerebral signature for pain perception and its modulation. Neuron 2007; 55: 377–391. [DOI] [PubMed] [Google Scholar]
- 10.Baliki MN Geha PY Apkarian AV and Sarton E. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron 2010; 66: 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi Y Kurata J Sekiguchi M Kokubun M Akaishizawa T Chiba Y Konno S and Kikuchi S. Augmented cerebral activation by lumbar mechanical stimulus in chronic low back pain patients: an FMRI study. Spine (Phila Pa 1976) 2009; 34: 2431–2436. [DOI] [PubMed] [Google Scholar]
- 12.Kurata J Thulborn KR andFirestone LL.. The cross-modal interaction between pain-related and saccade-related cerebral activation: a preliminary study by event-related functional magnetic resonance imaging. Anesth Analg 2005; 101: 449–456. [DOI] [PubMed] [Google Scholar]
- 13.Kurata J Thulborn KR Gyulai FE and Firestone LL. Early decay of pain-related cerebral activation in functional magnetic resonance imaging: comparison with visual and motor tasks. Anesthesiology 2002; 96: 35–44. [DOI] [PubMed] [Google Scholar]
- 14.Matsuo Y Kurata J Sekiguchi M Yoshida K Nikaido T and Konno S. Attenuation of cortical activity triggering descending pain inhibition in chronic low back pain patients: a functional magnetic resonance imaging study. J Anesth 2017; 31: 523–530. [DOI] [PubMed] [Google Scholar]
- 15.Peyron R Laurent B andGarcia-Larrea L.. Functional imaging of brain responses to pain. A review and meta-analysis (2000). Neurophysiol Clin 2000; 30: 263–288. [DOI] [PubMed] [Google Scholar]
- 16.Freynhagen R Baron R Gockel U and Tölle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin 2006; 22: 1911–1920. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan MJL Bishop SR andPivik J.. The pain catastrophizing scale: development and validation. Psychol Assess 1995; 7: 524–532. [Google Scholar]
- 18.Beck AT Ward CH Mendelson M Mock J and Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry 1961; 4: 561–571. [DOI] [PubMed] [Google Scholar]
- 19.Melzack R. The short-form McGill Pain Questionnaire. Pain 1987; 30: 191–197. [DOI] [PubMed] [Google Scholar]
- 20.Martucci KT Yelle MD andCoghill RC.. Differential effects of experimental central sensitization on the time-course and magnitude of offset analgesia. Pain 2012; 153: 463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goebel R Esposito F andFormisano E.. Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: from single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum Brain Mapp 2006; 27: 392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talairach J andTournoux P.. Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishers, 1988. [Google Scholar]
- 23.Alkire MT White NS Hsieh R and Haier RJ. Dissociable brain activation responses to 5-Hz electrical pain stimulation: a high-field functional magnetic resonance imaging study. Anesthesiology 2004; 100: 939–946. [DOI] [PubMed] [Google Scholar]
- 24.Martucci KT Eisenach JC Tong C and Coghill RC. Opioid-independent mechanisms supporting offset analgesia and temporal sharpening of nociceptive information. Pain 2012; 153: 1232–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruscheweyh R Kuhnel M Filippopulos F Blum B Eggert T and Straube A. Altered experimental pain perception after cerebellar infarction. Pain 2014; 155: 1303–1312. [DOI] [PubMed] [Google Scholar]
- 26.Petre B Tetreault P Mathur VA Schurgin MW Chiao JY Huang L and Apkarian AV. A central mechanism enhances pain perception of noxious thermal stimulus changes. Sci Rep 2017; 7: 3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raichle ME MacLeod AM Snyder AZ Powers WJ Gusnard DA and Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A 2001; 98: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zubieta JK Bueller JA Jackson LR Scott DJ Xu Y Koeppe RA Nichols TE and Stohler CS. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J Neurosci 2005; 25: 7754–7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wager TD Rilling JK Smith EE Sokolik A Casey KL Davidson RJ Kosslyn SM Rose RM and Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science 2004; 303: 1162–1167. [DOI] [PubMed] [Google Scholar]
- 30.Bingel U Gläscher J Weiller C and Büchel C. Somatotopic representation of nociceptive information in the putamen: an event-related fMRI study. Cereb Cortex 2004; 14: 1340–1345. [DOI] [PubMed] [Google Scholar]
- 31.Navratilova E andPorreca F.. Reward and motivation in pain and pain relief. Nat Neurosci 2014; 17: 1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Becerra L andBorsook D.. Signal valence in the nucleus accumbens to pain onset and offset. Eur J Pain 2008; 12: 866–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DosSantos MF Martikainen IK Nascimento TD Love TM Deboer MD Maslowski EC Monteiro AA Vincent MB Zubieta JK and DaSilva AF. Reduced basal ganglia μ-opioid receptor availability in trigeminal neuropathic pain: a pilot study. Mol Pain 2012; 8: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiatry 2002; 51: 68–80. [DOI] [PubMed] [Google Scholar]
- 35.Ferenczi EA Zalocusky KA Liston C Grosenick L Warden MR Amatya D Katovich K Mehta H Patenaude B Ramakrishnan C Kalanithi P Etkin A Knutson B Glover GH and Deisseroth K. Prefrontal cortical regulation of brainwide circuit dynamics and reward-related behavior. Science 2016; 351: aac9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baliki MN Chialvo DR Geha PY Levy RM Harden RN Parrish TB and Apkarian AV. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci 2006; 26: 12165–12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Apkarian AV Sosa Y Sonty S Levy RM Harden RN Parrish TB and Gitelman DR. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci 2004; 24: 10410–10415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baliki MN Petre B Torbey S Herrmann KM Huang L Schnitzer TJ Fields HL and Apkarian AV. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci 2012; 15: 1117–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Treede RD Kenshalo DR Gracely RH and Jones AK. The cortical representation of pain. Pain 1999; 79: 105–111. [DOI] [PubMed] [Google Scholar]
- 40.Wu Q Inman RD andDavis KD.. Neuropathic pain in ankylosing spondylitis: a psychophysics and brain imaging study. Arthritis Rheum 2013; 65: 1494–1503. [DOI] [PubMed] [Google Scholar]
- 41.Hashmi JA Baliki MN Huang L Baria AT Torbey S Hermann KM Schnitzer TJ and Apkarian AV. Shape shifting pain: chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain 2013; 136: 2751–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shmuel A Augath M Oeltermann A and Logothetis NK. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat Neurosci 2006; 9: 569–577. [DOI] [PubMed] [Google Scholar]
- 43.Geuze E Westenberg HG Jochims A de Kloet CS Bohus M Vermetten E and Schmahl C. Altered pain processing in veterans with posttraumatic stress disorder. Arch Gen Psychiatry 2007; 64: 76–85. [DOI] [PubMed] [Google Scholar]
- 44.Borsook D Upadhyay J Chudler EH and Becerra L. A key role of the basal ganglia in pain and analgesia–insights gained through human functional imaging. Mol Pain 2010; 6: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Honigman L Yarnitsky D Sprecher E and Weissman-Fogel I. Psychophysical testing of spatial and temporal dimensions of endogenous analgesia: conditioned pain modulation and offset analgesia. Exp Brain Res 2013; 228: 493–501. [DOI] [PubMed] [Google Scholar]
- 46.Naugle KM Cruz-Almeida Y Fillingim RB and Riley JL. Offset analgesia is reduced in older adults. Pain 2013; 154: 2381–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim HJ Yang GS Greenspan JD Downton KD Griffith KA Renn CL Johantgen M and Dorsey SG. Racial and ethnic differences in experimental pain sensitivity: systematic review and meta-analysis. Pain 2017; 158: 194–211. [DOI] [PubMed] [Google Scholar]
- 48.Kucyi A Salomons TV andDavis KD.. Mind wandering away from pain dynamically engages antinociceptive and default mode brain networks. Proc Natl Acad Sci USA 2013; 110: 18692–18697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tracey I Ploghaus A Gati JS Clare S Smith S Menon RS and Matthews PM. Imaging attentional modulation of pain in the periaqueductal gray in humans. J Neurosci 2002; 22: 2748–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]